Abstract
Handedness has been studied for association with language‐related disorders because of its link with language hemispheric dominance. No clear pattern has emerged, possibly because of small samples, publication bias, and heterogeneous criteria across studies. Non‐right‐handedness (NRH) frequency was assessed in N = 2503 cases with reading and/or language impairment and N = 4316 sex‐matched controls identified from 10 distinct cohorts (age range 6–19 years old; European ethnicity) using a priori set criteria. A meta‐analysis (N cases = 1994) showed elevated NRH % in individuals with language/reading impairment compared with controls (OR = 1.21, CI = 1.06–1.39, p = .01). The association between reading/language impairments and NRH could result from shared pathways underlying brain lateralization, handedness, and cognitive functions.
Abbreviations
- ALSPC
Avon Longitudinal Study of Parents and Children
- CCC
Childrens Communication Checklist
- DLD
developmental language disorder
- GWAS
Genome‐wide association studies
- ICD
International Classification of Disease
- KEMH
King Edward Memorial Hospital
- LH
left‐handed
- NRH
non‐right‐handedness
- NTR
Netherlands Twin Register
- QHP
Quantitative Hand Preference
- RD
reading disability
- RH
right‐handedness
- SWRT
Single‐Word Reading Test
- TOLD‐2P
Test of Language Development 2:P
- TROG–2
Test for Reception of Grammar Version 2
- WAIT
Wechsler Individual Achievement Test Spelling Test
- WISC
Wechsler Intelligence Scale for Children
Handedness is the most obvious lateralized behavioral trait in humans. Most individuals preferentially use one hand versus the other one for most motor tasks, with a strong rightward bias. Across populations, only about 10% of people are left‐handed (LH; Papadatou‐Pastou et al., 2020), with males being ~23% more likely than females to prefer the left hand (Papadatou‐Pastou et al., 2008). Twin studies have estimated the heritability of handedness to be 0.25 (Medland et al., 2009). Hand activities are controlled by the contralateral brain hemisphere such that a right‐ or left‐hand preference implies a left‐ or right‐hemisphere dominance for motor control, respectively (McManus, 2022). The low frequency of left‐handedness across populations has motivated investigations of possible associated disadvantages. A higher rate of non‐right‐handedness (NRH), which includes left‐ and mixed‐handedness, has been reported for neuropsychiatric and neurodevelopmental conditions, such as schizophrenia, autism, and intellectual disability (Hirnstein & Hugdahl, 2014; Markou et al., 2017; Papadatou‐Pastou & Tomprou, 2015). The underlying hypothesis is that the genetic pathways required for establishing left–right brain asymmetries might also contribute to handedness and be involved in neurodevelopmental conditions (Corballis, 2021). It is important to emphasize that the majority of left‐handers are not affected by these conditions and therefore left‐handedness should not be equated to a pathological status.
A link between handedness and language abilities is of particular interest because of the known role of hemispheric lateralization underpinning both traits. Language processing is highly lateralized, involving circuits that reside typically in the left hemisphere, as demonstrated by patients who had language function compromised as the result of strokes affecting the left side of the brain (Kertesz & McCabe, 1977). Left‐handers are more likely than right‐handers to present atypical lateralization for language processing. Specifically, current estimates show that up to 30% of left‐handers present language dominance in the right hemisphere compared with only 5% of right‐handers, with substantial variability across studies (Carey & Johnstone, 2014; Knecht, 2001; Szaflarski et al., 2002; Whitehouse & Bishop, 2009; Woodhead et al., 2021). Such association is more evident in individuals with a very strong left‐hand preference (Mazoyer et al., 2014). Similarly, atypical lateralization for other cognitive domains is more likely to be observed in left‐ than right‐handers (McManus, 2022). Hemispheric dominance for manual praxis (i.e., skilled manual actions) usually resides in the left hemisphere but atypical lateralization has been observed in left‐handers (Vingerhoets, 2019). The pathways involved in and linking different types of asymmetries remain unclear (Fagard, 2013).
The hypothesis that a failure to establish cerebral asymmetries may lead to language disorders was first proposed by Orton who suggested that dyslexia resulted from a failure to establish a complete cerebral dominance (Orton, 1937). Subsequently, the Geschwind‐Galaburda Hypothesis (Galaburda et al., 1985) proposed that reduced hemispheric asymmetries increase the probability of being LH and of developing dyslexia (Galaburda et al., 1985). Annett's Right‐Shift theory also predicted a link between NRH and dyslexia determined by an “asymmetry gene” which would affect the typical left hemisphere lateralization for both language and handedness. This, and other single‐gene theories (McManus, 1985) are not supported by recent genomic studies which show that in most cases, handedness is influenced by the combined effects of variants in a large number of genes (Armour et al., 2014; Cuellar‐Partida et al., 2021; Schmitz et al., 2022). Genome‐wide association studies (GWAS) for handedness have identified some of these genes, some of which have also been implicated in neurodevelopmental conditions, including schizophrenia and dyslexia (Brandler et al., 2013; Brandler & Paracchini, 2014; Cuellar‐Partida et al., 2021; Wiberg et al., 2019).
Language‐associated disorders, including dyslexia (or reading disability, RD) and developmental language disorders (DLD previously referred to as specific language impairment) are reported in about 5%–10% of children, present higher prevalence in males and often co‐occur (Bishop & Snowling, 2004). In both conditions, genetic contributions play a role, with strongest risk factor being an affected first‐degree family member (Arnett et al., 2017; Erbeli et al., 2021; Katusic et al., 2001; Tomblin et al., 1997; Whitehouse, 2010). In twin studies, heritability for both RD and DLD has been reported to be as high as ~70% (Erbeli et al., 2021). Although rare monogenic forms of reading and language disorders have been reported, the majority of cases are polygenic with shared genetic factors contributing to both conditions, as shown by recent and well powered GWASs (Eising et al., 2022; Gialluisi et al., 2014, 2019, 2020). For example, the genetic correlation between single‐word reading (a task used to assess reading abilities) and nonword repetition (a measure of speech perception, phonological short‐term memory and articulation) was reported to be r = .7, p < .001 (Eising et al., 2022). Genetic studies have also demonstrated complex overlaps between genes contributing to neurodevelopmental disorders, handedness and left/right brain asymmetries. The most recent GWAS for dyslexia conducted in almost 52,000 cases and over 1 million controls reported a significant genetic correlation between dyslexia and ambidexterity (Doust et al., 2021). Genes associated with handedness have been shown to be associated with regional asymmetries of cortical surface areas, including those involved in language‐related circuitry (Sha, Pepe, et al., 2021). A GWAS for brain asymmetry highlighted the role of genes involved in autism and schizophrenia (Sha, Schijven, et al., 2021). Overall, these findings demonstrate with molecular data that brain asymmetries, handedness, and neurodevelopmental disorders, including language‐related conditions, are partially influenced by the same genes. Variants in these shared genes can increase the chances of both being LH and having a neurodevelopmental disorder. The cellular functions associated with the shared genes include cytoskeletal dynamics and the left–right patterning of visceral organs (Paracchini, 2021), supporting the hypotheses that behavioral and anatomical asymmetries might, at least partly, be influenced by the same factors (Brandler & Paracchini, 2014).
At the behavioral level, putative links between handedness and language conditions have been tested both across the normal range of variation observed in the general population as well as in cohorts clinically ascertained for RD or DLD. The literature surrounding a link between handedness and dyslexia is inconsistent, as determined by meta‐analyses (Bishop, 1990; Eglinton & Annett, 1994; Somers et al., 2015). In 1990, Bishop conducted a meta‐analysis of 25 studies examining a total of N = 14,159 individuals (Bishop, 1990). Overall, a non‐significant increase of NRH was found in individuals with dyslexia. However, the increase was statistically significant only when the largest study, which had a negative finding and weighted disproportionately on the overall analysis, was omitted. When reanalyzing the complete dataset with a different method, Eglinton and Annett reported a significant over‐representation of NRH among cases with dyslexia (Eglinton & Annett, 1994). In addition to the inconsistency resulting from different analytical methods, Bishop (1990) noted how the heterogeneous criteria used for handedness and dyslexia classification introduced biases in the analyses. For example, studies included in the meta‐analyses measured handedness either as quantitative indexes (Annett & Kilshaw, 1984) or as a category (Felton et al., 1987; Gross et al., 1978). Also, individuals were classified as reading impaired through highly heterogeneous criteria. A recent study compared the epidemiology of dyslexia using both the Statistical Manual in its 5th version (DSM‐5) and the 11th version of the International Classification of Diseases (ICD‐11) on the same sample of 25,000 French pupils. Left‐handedness was associated with dyslexia as defined by the DSM‐5 but not according to the ICD‐11 criteria (Di Folco et al., 2022).
A meta‐analysis for studies investigating potential links between handedness and language abilities found no significant effects in the entire dataset (N = 359,890 total individuals; Somers et al., 2015). No differences were detected between males and females. However, analysis in the subgroup of children (age < 16 years) showed a weak handedness effect with right‐handers performing better than non‐right‐handers on verbal skills. High heterogeneity was reported across the studies analyzed reflecting different criteria for group assignment. For example, handedness was assessed in different ways across studies, including self‐reported hand preference for writing (Crow et al., 1998; Gordon & Kravetz, 1991; Kocel, 1977; Peters et al., 2006), different questionnaires (Coulson & Lovett, 2004; Hicks & Beveridge, 1978; Tremblay et al., 2004) and quantitative indexes derived from performance tests like the pegboard task (Annett & Turner, 1974). Inconsistent findings continue to be observed in more recent literature. A small study of 45 individuals with dyslexia and 90 controls found a significant increase of left‐handedness, measured with the Edinburgh Inventory, in the cases (Vlachos et al., 2013). A right‐hand advantage was also reported in a larger study of about 5000 children from the Longitudinal Study of Australian Children (Johnston et al., 2009). LH, and especially mixed‐handed children, tended to perform worse on a broad range of cognitive skills, including reading, writing, and receptive language. This handedness effect was more marked in boys. A similar trend was observed for receptive language, but not for expressive language, implying that NRH‐associated effects might differ between language sub‐domains. Another study with a focus on language abilities found no handedness differences between typically developing (N = 156) and children with DLD (N = 107; Wilson & Bishop, 2018). In this study, handedness was measured with the Edinburgh Handedness Inventory and the Quantitative Hand Preference (QHP) tasks (see Bishop et al., 1996). The QHP assessment did not show a correlation between handedness and language scores in a general population sample of 569 children (Pritchard et al., 2019).
The inconsistency across results conducted for both reading and language impairment may be due to the different criteria and designs used across studies. Meta‐analyses are a valid approach to extract the most consistent patterns from published studies, although it must be acknowledged that this approach is affected by potential publications biases.
We invited cohorts from the GenLang consortium (https://www.genlang.org/) to participate in this confirmatory study. GenLang is an international collaboration that facilitates large‐scale meta‐analyses in relation to speech, language, reading and related skills. The association between hand preference and language/reading abilities has not been investigated before in these cohorts. Thanks to the availability of raw data, we were able to apply criteria set a priori for defining reading and language impairments to reduce heterogeneity across cohorts. Handedness categories were defined as non‐right (NRH) or right‐handedness (RH) based on the preferred hand for writing or drawing. We report handedness frequency in 10 different cohorts (N = 2503 cases with reading and/or language impairment). Eight of these cohorts met the inclusion criteria and entered the meta‐analysis (N = 1994 cases).
MATERIALS AND METHODS
Study design
This study aims to test whether hand preference is associated with language and reading abilities by comparing the frequency of RH and NRH in cases and controls. We used datasets available through the GenLang Consortium because of their focus on reading and language measures (Table 1). Assignment to case and control groups was based on an existing clinical diagnosis or was derived from psychometric tests (Table S1). In the latter case, assignment to the case group was determined by a score 1 SD or more below the mean on standardized tests for assessing reading or language performance. Participants presenting low scores on performance IQ (i.e., 1 SD below the mean, unless otherwise specified) were excluded to ensure that poor language/reading skills were not secondary manifestations of other neurological or intellectual problems. Children scoring poorly on both language and reading measures were classified as comorbid. Assignment to the control group was based on scores equal to or above the mean of the same reading and language tests, unless otherwise specified. As a result, individuals that scored between the cut‐off criteria for cases and control assignment were excluded from the analysis, ensuring that the controls had no reading or language difficulties. The control groups were individually sex‐matched with the cases to avoid potential bias introduced by the higher prevalence of language disorder and left‐handedness in males. Handedness was defined as the preferred hand for writing and classified as two categories: right‐hand (RH) or non‐right‐hand (NRH) preference. The NRH group included participants who preferred the left‐hand or with no preference (often referred to as ambidextrous). The ambidextrous group was too small to be analyzed separately. This strategy avoided the heterogeneity introduced by the use of different instruments (e.g., different questionnaires or performance test) and classifications (e.g., left/right, right/no‐right, left/mixed/right and left/non‐left) reported in the literature. Controls were not available for three clinical cohorts (UK Dyslexia [UKDYS], Manchester Language Study (MLS) and the Multicenter Study Marburg/Würzburg cohort). For the two UK cohorts, controls were derived from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort which used directly comparable assessment.
TABLE 1.
Summary of the cohorts involved in the study.
| Cohort | Country | Total N a | Cohort type | Phenotype | References |
|---|---|---|---|---|---|
| ALSPAC cohort | UK | ~13,000 | Epidemiological, singletons | Reading, language | Boyd et al. (2013) |
| Iowa Cohort | USA | ~7000 | Epidemiological, singletons | Language | Tomblin et al. (1997) |
| Netherlands Twin Register cohort | Netherlands | ~60,000 | Epidemiological, twins | Reading | Ligthart et al. (2019) |
| The Raine Study | Australia | ~2900 | Epidemiological, singletons | Language | Newnham et al. (1993), Straker et al. (2017) |
| Twins Early Development Study cohort | England and Wales | ~13,000 | Epidemiological, twins | Reading, language | Haworth et al. (2013) |
| Manchester Language Study | UK | ~240 | Clinical, singletons | Language | Conti‐Ramsden et al. (1997) |
| Multicenter Study Marburg/Würzburg cohort | Germany | ~400 | Clinical, singletons and families | Reading | Schulte‐Körne et al. (1996) |
| Toronto Cohort | Canada | ~860 | Clinical, families | Reading | Price et al. (2020) |
| UK Dyslexia Cohort | UK | ~1300 | Clinical, singletons and families | Reading | Scerri et al. (2017) |
| York cohort | UK | ~260 | Clinical, families | Reading, language | Nash et al. (2013) |
Refers to the total number of probands in the initial cohorts.
The third cohort was collected in Germany and could not be matched with suitable controls. This cohort and another (Netherlands Twin Register cohort [NTR]) did not meet the required inclusions and exclusions criteria and therefore were not included in the meta‐analysis. Nevertheless, their handedness frequencies are presented in Table 2. We compared the mean values of possible confounding factors (i.e. performance IQ, total IQ, and birth weight) for the cases stratified by their handedness status (Table S2). We observed no differences for these potential confounders between RH and NRH cases and therefore did not correct our analyses for such factors.
TABLE 2.
Non‐right‐handedness frequencies.
| Cohort name | Cohort type | Phenotype | N cases | N controls | %NRH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NRH (males) | RH | Males % | NRH | RH | Males e % | Cases | Controls | |||
| (males) | (males) | (males) | ||||||||
| ALSPAC Language | Epidemiological | Language | 27 (15) | 214 (127) | .59 | 112 (69) | 749 (450) | .60 | .11 | .13 |
| ALSPAC Reading | Epidemiological | Reading | 30 (22) | 168 (101) | .62 | 112 (69) | 749 (450) | .60 | .15 | .13 |
| IOWA cohort | Epidemiological | Language | 22 (16) | 182 (105) | .59 | 56 (35) | 610 (360) | .59 | .11 | .08 |
| NTR cohort a | Epidemiological | Reading | 31 (18) | 203 (97) | .49 | 136 (66) | 914 (450) | .49 | .13 | .13 |
| The Raine Study | Epidemiological | Language | 21 (15) | 136 (87) | .65 | 49 (37) | 389 (248) | .65 | .13 | .11 |
| TEDS Reading | Epidemiological | Reading | 29 (8) | 163 (84) | .48 | 143 (60) | 1031 (431) | .42 | .15 | .13 |
| TEDS Language | Epidemiological | Language | 34 (11) | 187 (75) | .39 | 143 (60) | 1031 (431) | .42 | .15 | .12 |
| Manchester Language Study b | Clinical | Language | 34 (28) | 133 (103) | .78 | 93 (69) | 586 (450) | .76 | .20 | .14 |
| Multicenter Study Marburg/Würzburg c | Clinical | Reading | 22 (19) | 255 (189) | .75 | NA | NA | NA | .08 | NA |
| Toronto cohort | Clinical | Reading | 28 (16) | 207 (137) | .65 | 7 (4) | 50 (33) | .65 | .12 | .12 |
| UKDYS b | Clinical | Reading | 40 (24) | 262 (181) | .68 | 98 (69) | 667 (450) | .68 | .13 | .13 |
| York Reading | Clinical | Reading | 11 (8) | 25 (18) | .72 | 13 (11) | 57 (37) | .69 | .30 | .18 |
| York Language | Clinical | Language | 9 (8) | 30 (18) | .67 | 13 (11) | 57 (37) | .69 | .23 | .18 |
| Total | 2503 | 4,316 d | ||||||||
Note: The table includes the comorbid individuals in the language impairment group.
Abbreviations: ALSPAC, Avon Longitudinal Study of Parents and Children; NA, not available; NRH, non‐right‐handers; RH, right‐handedness; TEDS, Twins Early Development Study; UKDYS, UK Dyslexia.
This cohort was not included in the meta‐analysis because it lacked IQ data required for group assignment. The NRH frequency is reported.
These cohorts used overlapping controls from the ALSPAC cohort.
This cohort was not included in the meta‐analysis because of the lack of comparable controls.
Refers to the number of unique controls. Overlapping controls were analyzed for the ALSPAC, Manchester Language Study and the UKDYS cohorts.
Sex‐matching for the ALSPAC, TEDS, and York cohort was done combining the reading and language‐impaired cases.
Overall, this study addresses a long‐standing research question addressing previous limitations, for example, small samples, publication bias and heterogeneity, which affected previous literature.
Given this is a secondary data analysis study, full compliance to the Society for Research in Child Development Sociocultural Policy was not possible.
Individual cohorts
ALSPAC cohort
The ALSPAC is a longitudinal cohort representing the general population living in the Bristol area. The ALSPAC cohort consists of pregnant women in the Avon County, UK, with expected dates of delivery from April 1, 1991 to December 31, 1992 (Boyd et al., 2013; Fraser et al., 2013). The initial number of pregnancies enrolled was 14,541. All children, from age 7, were invited annually for assessments on a wide range of physical, behavioral, and neuropsychological traits, including cognitive (reading ‐and language‐related) measures. Attendance at the annual assessment determined the availability of data for the measures used in this study.
For this study, participants were assigned to the language impairment or reading impairment groups as described previously (Scerri et al., 2011). Briefly, children were excluded if they had (i) a performance IQ score ≤ 85 (Wechsler Intelligence Scale for Children [WISC‐III]; Wechsler et al., 1992), (ii) presence of autistic features based on a Childrens Communication Checklist (CCC) score below −3 SD (Bishop, 1998) (iii) missing data on all relevant phenotypes. Participants were assigned to the reading impairment group when scoring below 1 SD on age‐adjusted single‐word reading at age 7 and age 9 (WORD; Rust et al., 1993). Participants were assigned to the language impairment group when meeting at least two out of four of the following criteria: (i) an overall CCC score below 1 SD from the mean; (ii) an age‐adjusted non‐word repetition score below 1 SD from the mean (Gathercole et al., 1994); (iii) a listening and comprehension test score below 1 SD from the mean (age‐adjusted WOLD; Rust, 1996); (iv) reporting the need for speech/language therapy via a questionnaire. In the case of siblings and twin pairs meeting, the criteria for the impairment group, one child for each nuclear family was selected randomly or based on completeness of the data. Participants were classified as comorbid when meeting the criteria for both reading and language impairments. Assignment to the control group was determined by scores above −0.25 SD from the mean on all the quantitative tests used to assess language and reading impairments as well as no reports of needs for speech/language therapy.
In total, 439 cases (191 language‐impaired, 198 reading impaired, 50 comorbid) and 1138 controls were identified. The control group resulted in 861 individuals after sex‐matching. The cut‐off at −0.25 SD was chosen following a simulation analysis (see Supplementary Material; Table S3) showing that N > 1000 controls are necessary to reduce fluctuations in NRH frequency when randomly sex‐matching (N simulation = 1000). Setting the cut‐off above the mean of all tests would have resulted in a smaller sample (N = 592), leading to larger fluctuations of NRH. We also used a simulation to test for potential biases introduced by the use of a single set of controls for comparing both the reading and language impairment groups. No inflation was detected (Supplementary Methods). The same observation applies to the Twins Early Development Study (TEDS) and York cohorts.
Handedness was assessed as the self‐reported preferred hand for writing at age 7 and coded as a binary variable (“Right” or “Left”). The study website contains details of all the data through a fully searchable data dictionary (http://www.bristol.ac.uk/alspac/researchers/our‐data/).
Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the local research ethics committees (http://www.bristol.ac.uk/alspac/researchers/research‐ethics/). Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time.
Iowa cohort
The Iowa cohort is a cross‐sectional epidemiological study of early language ability in 5‐ and 6‐year‐old children (Tomblin et al., 1997). A total of 7218 children were screened for language ability with a 40‐item subset of the Test of Language Development 2:P (TOLD‐2P; Newcomer & Hammill, 1988). Inclusion criteria for entering the study included being monolingual English speakers without hearing loss. The 26.2% of children who failed the test during the language ability screening were selected to compose approximately half of the final cohort. The other half was randomly selected from the children who passed the screening test. In total, the cohort included 1929 children. A more comprehensive battery of language assessments—consisting of the five principal subtests of the TOLD‐2P, and a discourse task with both narrative comprehension and production components (Culatta et al., 1983)—was used to derive a composite language score (age 5–6). For this study, participants were excluded if they had a performance IQ score below 85 (WISC‐IV; Wechsler, 2012). Participants scoring below 1 SD and above the mean on the composite score were assigned to the case and control group, respectively. A total of 204 cases and 666 sex‐matched controls were selected. Handedness was defined as left‐ or right‐hand used to draw a picture, as assessed by the child's examiner.
Analysis of the Iowa cohort was approved under the University of Iowa IRB #201406727, which covers secondary data analysis of the data originally collected under the University of Iowa IRB #200511767 under which all subjects (or legal guardians) provided informed consent/assent, as appropriate.
NTR cohort
The NTR is a national register including more than 120,000 twins and their relatives (Ligthart et al., 2019). The twins were assessed repeatedly using a range of cognitive and behavioral tasks at regular intervals. Teachers provided test scores on the nationally standardized tests that form the Dutch Pupil Monitoring System. Reading ability (or decoding fluency) was assessed with a single‐word reading test by asking children to read aloud as many words as possible from a word list within 1 min. Children were tested at school in Grades 1–6, with up to three word‐reading fluency lists, administered by the teacher to children individually (Verhoeven, 1995; Verhoeven & van Leeuwe, 2009).
For the current study, the score at the latest measurement was used. Children were excluded for (i) not attending mainstream education programs, or (ii) missing data. Participants were defined as cases if they scored in the bottom 10th percentile based on the national norms in Dutch education (equivalent to 1.28 SD below the mean), which was the closest cut‐off that could be applied to conform to our criteria. Individuals scoring above the mean of the national norms were assigned to the control group. A total of 234 individuals with reading impairment and 1050 sex‐matched controls were selected. Because of the lack of IQ data, this cohort was not included in the meta‐analyses.
Handedness was recorded in questionnaires for the mothers as hand preference for “drawing on a piece of paper” at age 5. Answer options were right‐, left‐ or no‐preference. The left‐ and no‐preference were merged in the NRH category.
Ethical approval was granted by the Vrije Universiteit Amsterdam's Medical Ethics Committee (NTR/25‐05‐2007). Data were collected following parental consent.
The Raine Study
The Raine Study is a prospective pregnancy cohort that recruited 2900 women between 1989 and 1991 (Newnham et al., 1993; Straker et al., 2017). Recruitment took place at Western Australia's major perinatal center, King Edward Memorial Hospital (KEMH), and nearby private practices.
The mothers (Gen1) completed questionnaires regarding their children (Gen2) who underwent physical examinations at ages 1, 2, 3, 6, 8, 10, 14, 17, 20, and 22 years. The data used for this study were from the assessment at 10 years of age. Participants were excluded if they had a performance IQ score below 1 SD from the mean assessed through the Raven Coloured Progressive Matrices test (Raven et al., 1996). Total standard scores of the CELF‐3 (Semel et al., 1995) were used for group assignments. Participants were assigned to the case group when scoring equal or below 1 SD from the mean, and to the control group when scoring above the mean. This resulted in N = 157 language‐impaired cases and N = 438 sex‐matched controls. Hand preference for writing was self‐reported and recorded in the McCarron Assessment of Neuromuscular Development (McCarron, 1997).
The study was approved by the Human Ethics Committee at KEMH, Princess Margaret Hospital for Children, the University of Western Australia and the Health Department of Western Australia.
TEDS cohort
The TEDS is a longitudinal study of a cohort of twins from over 13,000 families born in England and Wales between 1994 and 1996 (Haworth et al., 2013; Rimfeld et al., 2019). The cohort includes a broad range of phenotypic data, including language and reading skills and handedness. The TEDS website includes a complete data dictionary https://www.teds.ac.uk/datadictionary/home.html, which details exclusions based on medical and perinatal factors, missing data, and other factors. For this study, participants were excluded if they had a performance IQ score that was below 1 SD based on Raven Matrices and Picture Completion tests. Individuals were assigned to the language impairment group when scoring 1 SD below a language composite score mean (Hayiou‐Thomas et al., 2021). Briefly, the composite score was based on a battery of audio‐streamed, web‐administered measures including vocabulary (WISC‐III‐PI; Kaplan, 1999), syntax (Listening Grammar; Test of Adolescent & Adult Language‐3; Hammill et al., 1994), non‐literal semantics, and understanding of inferences (Test of Language Competence‐Level 2; Wiig & Secord, 1985) administered at age 12. Previous analysis showed substantial phenotypic and genetic overlap among these four measures (Dale et al., 2010). The four tests were standardized and averaged.
Participants were assigned to the reading impairment group if they scored 1 SD below the mean of a reading fluency composite score (Hayiou‐Thomas et al., 2021). Briefly, children completed an online adaptation of the Woodcock‐Johnson III Reading Fluency test (Woodcock et al., 2001). In addition, the Test of Word Reading Efficiency (TOWRE Form B; Torgesen et al., 1999) was included in a test booklet sent to families by mail and administered to each twin separately by telephone. Previous work with the TEDS sample established strong concurrent validity for telephone administration of the TOWRE (Dale et al., 2005). The tests were standardized and averaged.
Participants scoring 1 SD below the mean for both the language and reading composite scores were assigned to the comorbid group. Participants scoring above −0.25 SD from the mean of both composite tests were assigned to the control group. One child per twin pair was selected at random if both twins had the relevant phenotypes. A total of 413 cases (N = 192 cases with reading impairment; N = 152 cases with language impairment, N = 69 comorbid) and 1174 sex‐matched controls were identified.
The primary measure of handedness was self‐reported at 16 years. It included a question asking the preferred hand used for writing (left, right, mixed). The TEDS study received ethical approval from the King's College London Ethics Committee.
Manchester Language Study cohort
The MLS followed 242 children with language impairment (Conti‐Ramsden et al., 1997). Probands were recruited at age 7 from 118 language units attached to English mainstream schools (Conti‐Ramsden et al., 1997; Conti‐Ramsden & Botting, 1999). Participants were contacted and reassessed again at ages 8 (N = 232), 11 (N = 200), 14 (N = 113), 16 (N = 139), and 24 (N = 84) years old. All children attended a language unit for at least 50% of the week, and as such, met the criteria for a language impairment diagnosis. Children with other neurological difficulties, hearing impairment, a diagnosis of autism or a general learning disability were excluded. Participants were excluded when they had a Raven matrices performance score IQ that was more than 1 SD below the mean. A total of 167 cases were selected for the current study. Handedness was assessed at age 8 as self‐reported hand preference (“are you left‐ or right‐handed?”). If data were not available at age 8 (N = 26), reports from age 14 were used. Hand preference was consistent in 97% of the participants who had data at both time points. Controls were not available for the MLS cohort, and therefore were derived from the ALSPAC control group resulting in N = 679 after sex‐matching.
Ethical approval was given by The University of Manchester Research Ethics Committee, UK. Parents or legal guardians provided informed consent for all participants up to the age of 16 years.
Multicenter study Marburg/Würzburg cohort
The Marburg/Würzburg cohort is a family‐based cohort that focuses on the genetic basis of reading impairment (Schulte‐Körne et al., 1996). Participants were excluded if they had (i) Nonverbal IQ < 85 (Culture Fair Intelligence Test; Weiß, 1998), (ii) presence of visual or auditory impairments, (iii) inadequate schooling and absences for more than 6 weeks per school year, (iv) first language other than German, (v) diagnosis of attention deficit hyperactivity disorder (ADHD), and (vi) presence of psychiatric disorders, seizure disorder, and use of medication affecting the central nervous system. The study enrolled 403 probands between 8 and 19 years old (grades 2 to 11). Probands were assessed on a large cognitive battery including reading and arithmetic skills, and neurophysiological correlates (ERP studies) associated with language and reading processing. Of the 403 participants, 277 scored more than 1 SD below the mean on single‐word reading (see Schulte‐Körne et al., 1996) meeting the criteria for assignment to the reading impairment group. Handedness was measured by a questionnaire including 10 items describing a specific activity (e.g., writing, throwing a ball, brushing teeth). Participants reported which hand they used for the specific activity based on a four‐point rating scale (1 = always left, 2 = mostly left, 3 = mostly right, 4 = always right). For the current study, only hand preference for writing was considered. Answers 1 and 2 were coded as “non‐right” and answers 3 and 4 were coded as “right.” No controls assessed with comparable measures were available, and therefore, this cohort was not included in the meta‐analyses. Ethical approval was obtained from the ethics committees of the Universities of Marburg and Würzburg.
Toronto cohort
Children between the ages of 6 and 16 years who struggled primarily with reading acquisition were recruited from the Toronto area and across Ontario (Couto et al., 2008; Elbert et al., 2011; Price et al., 2020; Tran et al., 2014). Siblings in the same age range with or without reading difficulties were also invited to participate.
Individuals were excluded for a performance IQ < 80 (WISC‐III) on either Verbal Comprehension or Perceptual Reasoning on the WISC‐IV. Three main reading subtests were used to determine reading impairment: (i) Word Identification and (ii) Word Attack from the Woodcock Reading Mastery Tests Revised (Woodcock, 1987) and (iii) Reading subtest of the Wide Range Achievement Test (WRAT‐3; Wilkinson, 1993) Individuals were assigned to the reading impairment group if they scored at least 1.5 SD below the mean on 2 out of 3 reading measures or at least 1 SD below the mean on all three measures. Controls were defined as scoring above the mean on all three measures. A total of 235 cases and 57 sex‐matched controls were included in the analyses. If families included multiple children meeting these criteria, one child was selected at random.
Right‐ and left‐hand preference was determined by a psychometrist as the child wrote to complete the WISC‐IV Coding test. The participants provided verbal or written consent and the parents provided written consent. The study was approved by the Hospital for Sick Children and University Health Network Research Ethics Boards.
UKDYS cohort
The UKDYS cohort includes nuclear families and singletons recruited to study the genetics of dyslexia (Scerri et al., 2010, 2017). The family cohort was recruited by research clinics in Oxford and Reading and included 689 siblings from 409 families. The singleton cohort was recruited in clinics in Oxford, Reading and Aston, and included 676 children. The age at assessment ranged from 7 years to 18 years.
For this study, individuals were excluded when presenting performance IQ scores <85 (WISC‐III) and were assigned to the case group if they scored at least 1 SD below the mean on the British Abilities Scales single‐word reading test (Thomson, 1982). Handedness was defined as self‐reported hand preference for writing (“Right” or “Left”). In total, 302 children met the criteria for reading impairment. Controls were derived from the main ALSPAC control group (N = 765 sex‐matched controls).
Ethical approvals for the Oxford family and case/control cohorts were granted by the Oxfordshire Psychiatric Research Ethics Committee (OPREC O01.02). Ethical approval for the Aston cohort was granted by the Aston University Ethics Committee.
York cohort
The York cohort comprises 260 children who were followed longitudinally in a study of language and reading disorders (Nash et al., 2013). Children were assessed on a battery of cognitive, language, and reading tests approximately annually between the ages of 3½ and 9 years. Assignment to the reading and language impairment group was based on the assessment at age 8–9 years old (described fully in Snowling et al., 2019). Children with performance IQ < 85 (WISC‐IV) were excluded. For this study, a reading impairment outcome was defined based on a score 1 SD or more below the mean, on a reading composite measure of the Single‐Word Reading Test (SWRT 6–16; Foster, 2007) and the Wechsler Individual Achievement Test Spelling Test (WIAT–II; Wechsler, 2005). A language impairment outcome was defined based on a score 1 SD or more below the mean, on a composite language measure of Expressive Vocabulary (CELF–4 UK; Wiig et al., 2006), Test for Reception of Grammar Version 2 (TROG–2; Bishop, 2003), and Formulated Sentences (CELF–4). According to these criteria, 36 children had reading impairment, 20 children had language impairment, and 19 children showed comorbidity for both conditions. Participants scoring above the mean for both the reading and language composite scores were sex‐matched to the combined cases, resulting in N = 70 controls. Handedness was defined as self‐reported hand preference for writing collected at age 8 years as “Right” or “Left” categories.
Ethical approval for the study was provided by the University of York, Department of Psychology's Ethics Committee and the NHS Research Ethics Committee. Parents provided written informed consent for their child to be involved.
Statistical analyses
Handedness frequency was compared between cases and controls using random‐effect meta‐analyses with and without moderators for impairment type (language/reading impairment) and cohort type (clinical/epidemiological). The number of individuals with comorbidities was too small to be analyzed separately and was therefore combined with the language impairment group. The analysis was also run including individuals with comorbidities in the reading impairment group (see Supplementary Material).
Meta‐analyses were conducted using the rma function in the R package metafor (test = “knha,” Balduzzi et al., 2019; R Core Team, 2019) under REML random effect model. The presence of heterogeneity between groups was explored using the Cochran's Q test and the I 2 index. The summary data for all cohorts and the code to run the analysis are available at https://github.com/fabbondanza/GenLang_hand_preference_meta_analysis.
RESULTS
We investigated the frequency of NRH in individuals with reading or language impairment (N total cases = 2503) from 10 cohorts (Table 2). Overall, the NRH frequency ranged from 8% in the Multicenter Study Marburg/Würzburg cohort (N cases = 277) to 30% in the York reading cohort (N cases = 36). In the controls, NRH ranged from 8% (IOWA, N controls = 666) to 18% (York, N controls = 70). When excluding the York cohort, which appeared to be an outlier for both cases and controls and had a small sample size, the NRH frequency ranged from 8%–20% in the cases and 8%–14% in the controls.
Meta‐analysis
The NTR cohort and the Multicenter Study Marburg/Würzburg cohort were excluded from the meta‐analysis because of the lack of IQ data or suitable controls, respectively. We meta‐analyzed data from 8 cohorts, including 4 clinical and 4 epidemiological cohorts.
We observed an increase of NRH in the case group (OR = 1.21, CI = 1.06–1.39, t = 3.16, p = .01; Figure 1). Egger's t test showed no evidence of funnel plot asymmetry (t = 0.563, p = .59, df = 9). We observed no evidence of heterogeneity (Q (10) = 6.27, p = .79, τ 2 = .01, I 2 = 0%; see Figure S1 for funnel plot). When the MLS and UKDys which lacked independent controls were removed, the results remained comparable to those observed in the full datasets (OR = 1.19, CI = 1.03–1.38, t = 2.78, p = .02, Figure S2). Inclusion of the comorbid individuals as part of the reading impairment group made no major difference (Figures S3 and S4). The lowest OR (0.84) was observed for an epidemiological cohort, while the highest ORs (1.93) were observed in clinical cohorts. However, a formal analysis did not reveal a moderator effect of cohort type (clinical vs. epidemiological; p = .21) or type of impairment (reading vs. language; p = .59).
FIGURE 1.
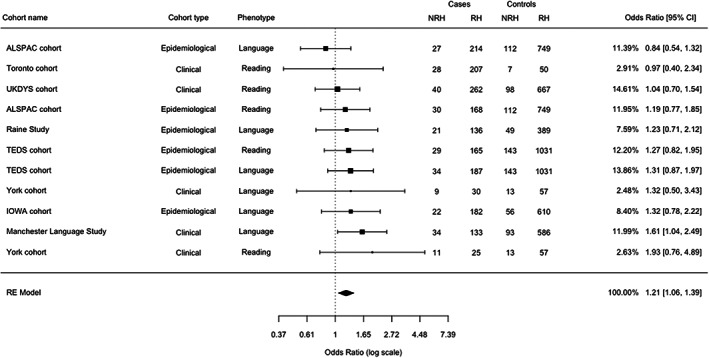
Meta‐analysis of non‐right‐handedness (NRH) frequency in individuals with language/reading impairments. The forest plot shows the results of the meta‐analysis run under a random effect (RE) model. The OR estimates are shown with the 95% confidence interval and the weights (in percentages) on the overall result of OR = 1.21, CI = 1.06–1.39 (t = 3.16, p = .01). See Figure S1 for the corresponding funnel plot. ALSPAC, Avon Longitudinal Study of Parents and Children; RH, right‐handedness; TEDS, Twins Early Development Study; UKDYS, UK Dyslexia.
DISCUSSION
We investigated the prevalence of NRH in individuals with reading and language impairments in a total of 2503 cases from 10 cohorts. NRH frequency tended to be elevated and presented a wider range of variation in the cases (8%–30%) compared with the controls (8%–18%). The upper range of variation was observed in the York cohort for both cases and controls, possibly suggesting a bias introduced by how NRH was assessed. However, the NRH prevalence in this cohort could have also been inflated random variations associated with the small sample size and the high rate of males. The second‐highest level of NRH was observed in the MLS (20%), a clinical cohort collected for language impairment. The high rate of NRH likely reflects a genuine association with a particularly severe language phenotype considering that the MLS was recruited following very stringent inclusion criteria. The lowest level of NRH in cases (8%) was observed in the Multicenter Study Marburg/Würzburg cohorts which lacked internal controls and therefore could not be evaluated for a potential assessment bias.
The meta‐analysis was conducted in the eight cohorts that met the inclusion criteria (N cases = 1994). Overall, we observed a higher rate of NRH in individuals with language/reading impairment compared with controls (OR = 1.21, CI = 1.06–1.39, t = 3.16, p = .01). The availability of raw data allowed us to apply similar criteria for group definition, yet it is worth noting that all cohorts analyzed here were originally recruited for different types of studies and designs. Nevertheless, no moderator effects were detected for impairment (reading vs. language) or cohort type (epidemiological vs. clinical). No changes in the results were observed when the comorbid groups, which were too small to be analyzed individually, were included in either the language or the reading impaired groups. A similar but attenuated trend was observed after removing the UKDYS and Manchester Language cohorts, thus ruling out a possible bias introduced by the lack of independent controls. The removal of the York cohort, which represented an outlier, also led to a similar but attenuated trend (OR = 1.19, CI = 1.03–1.38, p = .02; Figure S5). Although we analyzed almost 2000 cases, the sample sizes of the individual cohorts were too small to test subgroups selected for phenotype severity or disorder subtype, when considering the small effect size observed in the whole sample. A systematic assessment of handedness in larger cohorts of individuals, recruited and assessed with the same criteria for reading or language impairment, will be necessary to differentiate potential group‐specific effects and to evaluate differences between clinical and population‐based cohorts.
We acknowledge that the use of overlapping controls derived from the ALSPAC cohort and used for the UKDYS and Manchester Language cohort is not ideal, as non‐independent datasets might lead to biases (Noble et al., 2017). When the UKDYS and the Manchester Language cohorts were excluded, the results were comparable (same direction, but attenuated strength; OR = 1.19, CI = 1.03–1.38, p = .02) to the full dataset. An alternative option could have been the use of non‐overlapping controls from ALSPAC. However, a simulation analysis showed that the use of smaller subsets of independent controls would increase the fluctuation of NRH and thus increase the noise in the analysis. Cultural factors, such as stigma against left‐handedness, are known to vary to some extent with ethnicity and generations, but this is not a concern for our study. The children analyzed have similar birth years and large studies in the UK Biobank have not identified geographical factors that influence handedness prevalence within England (de Kovel et al., 2019). However, it is worth noting that our study is limited to cohorts of White European ancestry and therefore generalizability of our results will require analysis in other populations.
Previous meta‐analyses of the literature have been inconclusive (Bishop, 1990; Eglinton & Annett, 1994; Somers et al., 2015) and studies that applied different definitions of dyslexia found inconsistent results in the same dataset (Di Folco et al., 2022). When applying the DSM‐5, which is more closely in line with the criteria adopted here, Di Folco and colleagues found an association between handedness and dyslexia that was very similar to our study (OR = 1.24, p = .003). The effect disappeared when applying the ICD‐11 definition which is based on IQ discrepancy. Di Folco and colleagues concluded that the original effect was not specific to reading but mediated by IQ. Such a conclusion was supported by the observation that “non‐right‐handers scored on average 2 IQ points lower than right‐handers.” When comparing IQ between NRH and RH cases in the present study, we observed no significant differences with the exception of the UKDYS cohort (uncorrected p = .03). Our observation is in line with meta‐analyses investigating the associations between handedness and cognitive abilities, which reported that right‐handers had only marginally higher scores compared with left‐handers (Ntolka & Papadatou‐Pastou, 2017).
Some potential issues affecting the reliability of our data could have been introduced by the assessment of handedness at a young age. Hand preference can fluctuate in the early years of development but is well established by the time a child is 3 years old (McManus et al., 1988). In all our cohorts, handedness data were collected when children were at least 5 years old, and therefore after the handedness direction is fully established, as demonstrated also by the high correlation of assessments conducted at different time points (e.g., ALSPAC: r = .95 CI = [0.93, 0.97], p < 2.2 × 10−16, Schmitz et al., 2022).
In summary, our study investigates an old question with new data addressing issues that affected the previous literature, including small samples, heterogeneous criteria, and publication bias. The findings support an association, albeit small in size, between NRH and language/reading impairment, expanding the range of neurodevelopmental traits (e.g., autism and schizophrenia) known to be associated with handedness. From these data, it is not possible to infer any cause/effect directionality between brain asymmetries, disorders, and handedness but provide an important foundation for theoretical framework. Our results are in line with the evidence emerging from genetic studies supporting the role of shared genes and biological pathways contributing to both lateralization and neurodevelopmental disorders.
FUNDING INFORMATION
Silvia Paracchini and Filippo Abbondanza are funded by the Royal Society (UF150663; RGF\EA\180141). The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and will serve as guarantors for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website: http://www.bristol.ac.uk/alspac/external/documents/grant‐acknowledgements.pdf. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Elsje van Bergen was supported by NWO VENI fellowship 451‐15‐017. Support for the Toronto cohort collection was provided by grants from the Canadian Institutes of Health Research (MOP‐133440). K.M.P. was supported by the Hospital for Sick Children Research Training Program (Restracomp). Simon Fisher is funded by the Max Planck Society. Dorothy Bishop is funded by European Research Council Advanced Grant 694189. Andrew Whitehouse is supported by an Investigator Grant from the National Health and Medical Research Council (1173896). The Raine Study was supported by the National Health and Medical Research Council of Australia (grant numbers 572613, 403981, 1059711), and the Canadian Institutes of Health Research (grant number MOP‐82893). The Multicenter Study Marburg/Würzburg cohort was funded by the Deutsche Forschungsgemeinschaft (DFG).
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
The authors are grateful to all participants taking part in the different studies and the research teams involved in collecting the data. Specifically, we thank all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses; the Raine Study participants and their families for their ongoing participation in the study and the Raine Study staff for their dedicated commitment to coordination and data collection. We gratefully acknowledge the ongoing contribution of the participants in the NTR, including twins, their families and teachers. The authors are grateful to the Raine Study participants and their families, and to the Raine Study team for cohort coordination and data collection. The authors gratefully acknowledge the NHMRC for their long‐term funding to the study over the last 30 years and also the following institutes for providing funding for Core Management of the Raine Study: The University of Western Australia (UWA), Curtin University, Women and Infants Research Foundation, Telethon Kids Institute, Edith Cowan University, Murdoch University, The University of Notre Dame Australia and The Raine Medical Research Foundation. This work was supported by resources provided by the Pawsey Supercomputing Center with funding from the Australian Government and the Government of Western Australia.
Abbondanza, F. , Dale, P S. , Wang, C A. , Hayiou‐Thomas, M E. , Toseeb, U. , Koomar, T S. , Wigg, K G. , Feng, Y. , Price, K M. , Kerr, E N. , Guger, S L. , Lovett, M W. , Strug, L J. , van Bergen, E. , Dolan, C V. , Tomblin, J B. , Moll, K. , Schulte‐Körne, G. , Neuhoff, N. … Paracchini, S. (2023). Language and reading impairments are associated with increased prevalence of non‐right‐handedness. Child Development, 94, 970–984. 10.1111/cdev.13914
REFERENCES
- Annett, M. , & Kilshaw, D. (1984). Lateral preference and skill in dyslexics: Implications of the right shift theory. Journal of Child Psychology and Psychiatry., 25, 357–377. 10.1111/j.1469-7610.1984.tb00158.x [DOI] [PubMed] [Google Scholar]
- Annett, M. , & Turner, A. (1974). Laterality and the growth of intellectual abilities. British Journal of Educational Psychology, 44, 37–46. 10.1111/j.2044-8279.1974.tb00764.x [DOI] [PubMed] [Google Scholar]
- Armour, J. A. , Davison, A. , & McManus, I. C. (2014). Genome‐wide association study of handedness excludes simple genetic models. Heredity, 112, 221–225. 10.1038/hdy.2013.93hdy201393[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett, A. B. , Pennington, B. F. , Peterson, R. L. , Willcutt, E. G. , DeFries, J. C. , & Olson, R. K. (2017). Explaining the sex difference in dyslexia. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58, 719–727. 10.1111/jcpp.12691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi, S. , Rücker, G. , & Schwarzer, G. (2019). How to perform a meta‐analysis with R: A practical tutorial. Evidence‐Based Mental Health, 22, 153–160. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, D. V. (1990). Handedness and developmental disorders. MacKeith Press. [Google Scholar]
- Bishop, D. V. (1998). Development of the Children's Communication Checklist (CCC): A method for assessing qualitative aspects of communicative impairment in children. Journal of Child Psychology and Psychiatry, 39, 879–891. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9758196 [PubMed] [Google Scholar]
- Bishop, D. V. (2003). Test for reception of grammar, version 2 (TROG—2). Psychological Corporation. [Google Scholar]
- Bishop, D. V. , Ross, V. A. , Daniels, M. S. , & Bright, P. (1996). The measurement of hand preference: A validation study comparing three groups of right‐handers. British Journal of Psychology, 87, 269–285. 10.1111/j.2044-8295.1996.tb02590.x [DOI] [PubMed] [Google Scholar]
- Bishop, D. V. , & Snowling, M. J. (2004). Developmental dyslexia and specific language impairment: Same or different? Psychological Bulletin, 130, 858–886. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15535741 [DOI] [PubMed] [Google Scholar]
- Boyd, A. , Golding, J. , Macleod, J. , Lawlor, D. A. , Fraser, A. , Henderson, J. , Molloy, L. , Ness, A. , Ring, S. , & Smith, G. D. (2013). Cohort profile: The ’children of the 90 s'‐The index offspring of the Avon Longitudinal Study of Parents and Children. International Journal of Epidemiology, 42, 111–127. 10.1093/ije/dys064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandler, W. , Morris, A. P. , Evans, D. M. , Scerri, T. S. , Kemp, J. P. , Timpson, N. J. , St Pourcain, B. , Da Smith, G. , Ring, S. M. , Stein, J. , Monaco, A. P. , Talcott, J. B. , Fisher, S. E. , Webber, C. , & Paracchini, S. (2013). Common variants in left/right asymmetry genes and pathways are associated with relative hand skill. PLoS Genetics, 9, e1003751. 10.1371/journal.pgen.1003751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandler, W. , & Paracchini, S. (2014). The genetic relationship between handedness and neurodevelopmental disorders. Trends in Molecular Medicine, 20, 83–90. 10.1016/j.molmed.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, D. P. , & Johnstone, L. T. (2014). Quantifying cerebral asymmetries for language in dextrals and adextrals with random‐effects meta analysis. Frontiers in Psychology, 5, 1128. 10.3389/fpsyg.2014.01128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti‐Ramsden, G. , & Botting, N. (1999). Characteristics of children attending language units in England: A national study of 7‐year‐olds. International Journal of Language & Communication Disorders, 34, 359–366. 10.1080/136828299247333 [DOI] [PubMed] [Google Scholar]
- Conti‐Ramsden, G. , Crutchley, A. , & Botting, N. (1997). The extent to which psychometric tests differentiate subgroups of children with SLI. Journal of Speech, Language, and Hearing Research, 40, 765–777. 10.1044/jslhr.4004.765 [DOI] [PubMed] [Google Scholar]
- Corballis, M. C. (2021). How asymmetries evolved: Hearts, brains, and molecules. Symmetry, 13, 914. 10.3390/SYM13060914 [DOI] [Google Scholar]
- Coulson, S. , & Lovett, C. (2004). Handedness, hemispheric asymmetries, and joke comprehension. Cognitive Brain Research, 19, 275–288. 10.1016/j.cogbrainres.2003.11.015 [DOI] [PubMed] [Google Scholar]
- Couto, J. M. , Gomez, L. , Wigg, K. , Cate‐Carter, T. , Archibald, J. , Anderson, B. , Tannock, R. , Kerr, E. N. , Lovett, M. W. , Humphries, T. , & Barr, C. L. (2008). The KIAA0319‐like (KIAA0319L) gene on chromosome 1p34 as a candidate for Reading disabilities. Journal of Neurogenetics, 22, 295–313. 10.1080/01677060802354328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow, T. J. , Crow, L. R. , Done, D. J. , & Leask, S. (1998). Relative hand skill predicts academic ability: Global deficits at the point of hemispheric indecision. Neuropsychologia, 36, 1275–1282. 10.1016/S0028-3932(98)00039-6 [DOI] [PubMed] [Google Scholar]
- Cuellar‐Partida, G. , Tung, J. Y. , Eriksson, N. , Albrecht, E. , Aliev, F. , Andreassen, O. A. , Barroso, I. , Beckmann, J. S. , Boks, M. P. , Boomsma, D. I. , Boyd, H. A. , Breteler, M. M. B. , Campbell, H. , Chasman, D. I. , Cherkas, L. F. , Davies, G. , de Geus, E. J. C. , Deary, I. J. , Deloukas, P. , … Medland, S. E. (2021). Genome‐wide association study identifies 48 common genetic variants associated with handedness. Nature Human Behaviour, 5, 59–70. 10.1038/s41562-020-00956-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culatta, B. , Page, J. L. , & Ellis, J. (1983). Story retelling as a communicative performance screening tool. Language, Speech, and Hearing Services in Schools, 14, 66–74. 10.1044/0161-1461.1402.66 [DOI] [Google Scholar]
- Dale, P. S. , Harlaar, N. , Hayiou‐Thomas, M. E. , & Plomin, R. (2010). The etiology of diverse receptive language skills at 12 years. Journal of Speech, Language, and Hearing Research, 53, 982–992. 10.1044/1092-4388(2009/09-0108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, P. S. , Harlaar, N. , & Plomin, R. (2005). Telephone testing and teacher assessment of reading skills in 7‐year‐olds: I. substantial correspondence for a sample of 5544 children and for extremes. Reading and Writing, 18, 385–400. 10.1007/s11145-004-8130-z [DOI] [Google Scholar]
- de Kovel, C. G. F. , Carrión‐Castillo, A. , & Francks, C. (2019). A large‐scale population study of early life factors influencing left‐handedness. Scientific Reports, 9, 584. 10.1038/s41598-018-37423-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Folco, C. , Guez, A. , Peyre, H. , & Ramus, F. (2022). Epidemiology of reading disability: A comparison of DSM‐5 and ICD‐11 criteria. Scientific Studies of Reading, 26, 337–355. 10.1080/10888438.2021.1998067 [DOI] [Google Scholar]
- Doust, C. , Fontanillas, P. , Eising, E. , Gordon, S. D. , Wang, Z. , Alagöz, G. , Molz, B. , Team, 23andMe Research, Consortium, Q. T. W. G. of the G , Pourcain, B. S. , Francks, C. , Marioni, R. E. , Zhao, J. , Paracchini, S. , Talcott, J. B. , Monaco, A. P. , Stein, J. F. , Gruen, J. R. , Olson, R. K. , … Luciano, M. (2021). Discovery of 42 genome‐wide significant loci associated with dyslexia. MedRxiv. 10.1101/2021.08.20.21262334 [DOI] [Google Scholar]
- Eglinton, E. , & Annett, M. (1994). Handedness and dyslexia: A meta‐analysis. Perceptual and Motor Skills, 79, 1611–1616. 10.2466/pms.1994.79.3f.1611 [DOI] [PubMed] [Google Scholar]
- Eising, E. , Mirza‐Schreiber, N. , de Zeeuw, E. L. , Wang, C. A. , Truong, D. T. , Allegrini, A. G. , Shapland, C. Y. , Zhu, G. , Wigg, K. G. , Gerritse, M. , Molz, B. , Alagöz, G. , Gialluisi, A. , Abbondanza, F. , Rimfeld, K. , van Donkelaar, M. , Liao, Z. , Jansen, P. R. , Andlauer, T. F. M. , … Fisher, S. E. (2022). Genome‐wide association analyses of individual differences in quantitatively assessed reading‐ and language‐related skills in up to 34,000 people. Proceedings of the National Academy of Sciences of the United States of America, 119, e2202764119. 10.1073/pnas.2202764119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert, A. , Lovett, M. W. , Cate‐Carter, T. , Pitch, A. , Kerr, E. N. , & Barr, C. L. (2011). Genetic variation in the KIAA0319 5′ region as a possible contributor to dyslexia. Behavior Genetics, 41, 77–89. 10.1007/S10519-010-9434-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbeli, F. , Rice, M. , & Paracchini, S. (2021). Insights into dyslexia genetics research from the last two decades. Brain Sciences, 12, 27. 10.3390/BRAINSCI12010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard, J. (2013). Early development of hand preference and language lateralization: Are they linked, and if so, how? Developmental Psychobiology, 55, 596–607. 10.1002/DEV.21131 [DOI] [PubMed] [Google Scholar]
- Felton, R. H. , Wood, F. B. , Brown, I. S. , Campbell, S. K. , & Harter, M. R. (1987). Separate verbal memory and naming deficits in attention deficit disorder and reading disability. Brain and Language, 31, 171–184. 10.1016/0093-934X(87)90067-8 [DOI] [PubMed] [Google Scholar]
- Foster, H. (2007). Single word reading test 6–16. NFER‐Nelson. [Google Scholar]
- Fraser, A. , Macdonald‐wallis, C. , Tilling, K. , Boyd, A. , Golding, J. , Davey Smith, G. , Henderson, J. , Macleod, J. , Molloy, L. , Ness, A. , Ring, S. , Nelson, S. M. , & Lawlor, D. A. (2013). Cohort profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. International Journal of Epidemiology, 42, 97–110. 10.1093/ije/dys066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda, A. M. , Sherman, G. F. , Rosen, G. D. , Aboitz, F. , Geschwind, N. , Aboitiz, F. , & Geschwind, N. (1985). Developmental dyslexia: Four consecutive patients with cortical anomalies. Annals of Neurology, 18, 222–233. 10.1002/ana.410180210 [DOI] [PubMed] [Google Scholar]
- Gathercole, S. E. , Willis, C. S. , Baddeley, A. D. , & Emslie, H. (1994). The Children's test of nonword repetition: A test of phonological working memory. Memory, 2, 103–127. 10.1080/09658219408258940 [DOI] [PubMed] [Google Scholar]
- Gialluisi, A. , Andlauer, T. F. M. , Mirza‐Schreiber, N. , Moll, K. , Becker, J. , Hoffmann, P. , Ludwig, K. U. , Czamara, D. , Pourcain, B. S. , Honbolygó, F. , Tóth, D. , Csépe, V. , Huguet, G. , Chaix, Y. , Iannuzzi, S. , Demonet, J. F. , Morris, A. P. , Hulslander, J. , Willcutt, E. G. , … Schulte‐Körne, G. (2020). Genome‐wide association study reveals new insights into the heritability and genetic correlates of developmental dyslexia. Molecular Psychiatry, 26, 3004–3017. 10.1038/s41380-020-00898-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialluisi, A. , Andlauer, T. F. M. M. , Mirza‐Schreiber, N. , Moll, K. , Becker, J. , Hoffmann, P. , Ludwig, K. U. , Czamara, D. , St Pourcain, B. , Brandler, W. , Honbolygó, F. , Tóth, D. , Csépe, V. , Huguet, G. , Morris, A. P. , Hulslander, J. , Willcutt, E. G. , DeFries, J. C. , Olson, R. K. , … Schulte‐Körne, G. (2019). Genome‐wide association scan identifies new variants associated with a cognitive predictor of dyslexia. Translational Psychiatry, 9, 77. 10.1038/s41398-019-0402-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialluisi, A. , Newbury, D. F. , Wilcutt, E. G. , Consortium, T. S. L. I. , & Luciano, M. (2014). Genome‐wide screening for DNA variants associated with reading and language traits. Genes, Brain and Behavior, 13, 686–701. 10.1111/gbb.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, H. W. , & Kravetz, S. (1991). The influence of gender, handedness, and performance level on specialized cognitive functioning. Brain and Cognition, 15, 37–61. 10.1016/0278-2626(91)90014-Y [DOI] [PubMed] [Google Scholar]
- Gross, K. , Rothenberg, S. , Schottenfeld, S. , & Drake, C. (1978). Duration thresholds for letter identification in left and right visual fields for normal and reading‐disabled children. Neuropsychologia, 16, 709–715. 10.1016/0028-3932(78)90005-2 [DOI] [PubMed] [Google Scholar]
- Hammill, D. D. , Brown, V. L. , Larsen, S. C. , & Wiederholt, J. L. (1994). Test of Adolescent and Adult Language—Third edition. Pro‐Ed. [Google Scholar]
- Haworth, C. M. A. , Davis, O. S. P. , & Plomin, R. (2013). Twins early development study (TEDS): A genetically sensitive investigation of cognitive and behavioral development from childhood to young adulthood. Twin Research and Human Genetics, 16, 117–125. 10.1017/thg.2012.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayiou‐Thomas, M. E. , Smith‐Woolley, E. , & Dale, P. S. (2021). Breadth versus depth: Cumulative risk model and continuous measure prediction of poor language and reading outcomes at 12. Developmental Science, 24, e12998. 10.1111/desc.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, R. A. , & Beveridge, R. (1978). Handedness and intelligence. Cortex, 14, 304–307. 10.1016/S0010-9452(78)80056-2 [DOI] [PubMed] [Google Scholar]
- Hirnstein, M. , & Hugdahl, K. (2014). Excess of non‐right‐handedness in schizophrenia: Meta‐analysis of gender effects and potential biases in handedness assessment. The British Journal of Psychiatry, 205, 260–267. 10.1192/BJP.BP.113.137349 [DOI] [PubMed] [Google Scholar]
- Johnston, D. W. , Nicholls, M. E. R. , Shah, M. , & Shields, M. A. (2009). Nature's experiment? Handedness and early childhood development. Demography, 46, 281–301. 10.1353/dem.0.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, E. (1999). WISC‐III PI. Psychological Corporation. [Google Scholar]
- Katusic, S. K. , Colligan, R. C. , Barbaresi, W. J. , Schaid, D. J. , & Jacobsen, S. J. (2001). Incidence of Reading disability in a population‐based birth cohort, 1976–1982, Rochester, Minn. Mayo Clinic Proceedings, 76, 1081–1092. 10.4065/76.11.1081 [DOI] [PubMed] [Google Scholar]
- Kertesz, A. , & McCabe, P. (1977). Recovery patterns and prognosis in aphasia. Brain, 100, 1–18. 10.1093/brain/100.1.1 [DOI] [PubMed] [Google Scholar]
- Knecht, S. (2001). Behavioural relevance of atypical language lateralization in healthy subjects. Brain, 124, 1657–1665. 10.1093/brain/124.8.1657 [DOI] [PubMed] [Google Scholar]
- Kocel, K. M. (1977). Cognitive abilities: Handedness, familial sinistrality, and sex. Annals of the New York Academy of Sciences. 10.1111/j.1749-6632.1977.tb41910.x [DOI] [PubMed] [Google Scholar]
- Ligthart, L. , Van Beijsterveldt, C. E. M. , Kevenaar, S. T. , De Zeeuw, E. , Van Bergen, E. , Bruins, S. , Pool, R. , Helmer, Q. , Van Dongen, J. , Hottenga, J. J. , Van'T Ent, D. , Dolan, C. V. , Davies, G. E. , Ehli, E. A. , Bartels, M. , Willemsen, G. , De Geus, E. J. C. , & Boomsma, D. I. (2019). The Netherlands Twin Register: Longitudinal research based on twin and twin‐family designs. Twin Research and Human Genetics., 22, 623–636. 10.1017/thg.2019.93 [DOI] [PubMed] [Google Scholar]
- Markou, P. , Ahtam, B. , & Papadatou‐Pastou, M. (2017). Elevated levels of atypical handedness in autism: Meta‐analyses. Neuropsychology review, 27(3), 258–283. 10.1007/s11065-017-9354-4 [DOI] [PubMed] [Google Scholar]
- Mazoyer, B. , Zago, L. , Jobard, G. , Crivello, F. , Joliot, M. , Perchey, G. , Mellet, E. , Petit, L. , & Tzourio‐Mazoyer, N. (2014). Gaussian mixture modeling of hemispheric lateralization for language in a large sample of healthy individuals balanced for handedness. PLoS One, 9, e101165. 10.1371/journal.pone.0101165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron, L. T. (1997). McCarron Assessment of Neuromuscular Development (3rd ed.). McCarron‐Dial Systems Inc. [Google Scholar]
- McManus, I. C. (1985). Handedness, language dominance and aphasia: A genetic model. Psychological Medicine. Monograph Supplement, 8, 1–40. http://www.ncbi.nlm.nih.gov/pubmed/3863155 [PubMed] [Google Scholar]
- McManus, I. C. (2022). Cerebral polymorphisms for lateralisation: Modelling the genetic and phenotypic architectures of multiple functional modules. Symmetry, 14, 7–82. [Google Scholar]
- McManus, I. C. , Sik, G. , Cole, D. R. , Mellon, A. F. , Wong, J. , & Kloss, J. (1988). The development of handedness in children. British Journal of Developmental Psychology, 6, 257–273. 10.1111/J.2044-835X.1988.TB01099.X [DOI] [Google Scholar]
- Medland, S. E. , Duffy, D. L. , Wright, M. J. , Geffen, G. M. , Hay, D. A. , Levy, F. , van‐Beijsterveldt, C. E. , Willemsen, G. , Townsend, G. C. , White, V. , Hewitt, A. W. , Mackey, D. A. , Bailey, J. M. , Slutske, W. S. , Nyholt, D. R. , Treloar, S. A. , Martin, N. G. , & Boomsma, D. I. (2009). Genetic influences on handedness: Data from 25,732 Australian and Dutch twin families. Neuropsychologia, 47, 330–337. 10.1016/j.neuropsychologia.2008.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash, H. M. , Hulme, C. , Gooch, D. , & Snowling, M. J. (2013). Preschool language profiles of children at family risk of dyslexia: Continuities with specific language impairment. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54, 958–968. 10.1111/jcpp.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer, P. , & Hammill, D. (1988). Test of language development—2 primary. Pro‐Ed. [DOI] [PubMed] [Google Scholar]
- Newnham, J. P. , Evans, S. F. , Michael, C. A. , Stanley, F. J. , & Landau, L. I. (1993). Effects of frequent ultrasound during pregnancy: A randomised controlled trial. Lancet, 342, 887–891. [DOI] [PubMed] [Google Scholar]
- Noble, D. W. A. , Lagisz, M. , O'dea, R. E. , & Nakagawa, S. (2017). Nonindependence and sensitivity analyses in ecological and evolutionary meta‐analyses. Molecular Ecology, 26, 2410–2425. 10.1111/MEC.14031 [DOI] [PubMed] [Google Scholar]
- Ntolka, E. , & Papadatou‐Pastou, M. (2017). Right‐handers have marginally higher IQ scores than left‐handers: Systematic review and meta‐analyses. Neuroscience & Biobehavioral Reviews, 84, 376–393. 10.1016/j.neubiorev.2017.08.007 [DOI] [PubMed] [Google Scholar]
- Orton, S. (1937). Reading, writing and speech problems in children. Norton. [Google Scholar]
- Papadatou‐Pastou, M. , Martin, M. , Munafò, M. R. , & Jones, G. V. (2008). Sex differences in left‐handedness: A meta‐analysis of 144 studies. Psychological Bulletin, 134, 677–699. 10.1037/a0012814 [DOI] [PubMed] [Google Scholar]
- Papadatou‐Pastou, M. , Ntolka, E. , Schmitz, J. , Martin, M. , Munafò, M. R. , Ocklenburg, S. , & Paracchini, S. (2020). Human handedness: A meta‐analysis. Psychological Bulletin, 146, 481–524. 10.1037/bul0000229 [DOI] [PubMed] [Google Scholar]
- Papadatou‐Pastou, M. , & Tomprou, D. M. (2015). Intelligence and handedness: Meta‐analyses of studies on intellectually disabled, typically developing, and gifted individuals. Neuroscience and Biobehavioral Reviews, 56, 151–165. 10.1016/j.neubiorev.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Paracchini, S. (2021). Recent advances in handedness genetics. Symmetry, 13. 10.3390/sym13101792 [DOI] [Google Scholar]
- Peters, M. , Reimers, S. , & Manning, J. T. (2006). Hand preference for writing and associations with selected demographic and behavioral variables in 255,100 subjects: The BBC internet study. Brain and Cognition, 62, 177–189. 10.1016/j.bandc.2006.04.005 [DOI] [PubMed] [Google Scholar]
- Price, K. M. , Wigg, K. G. , Feng, Y. , Blokland, K. , Wilkinson, M. , He, G. , Kerr, E. N. , Carter, T. C. , Guger, S. L. , Lovett, M. W. , Strug, L. J. , & Barr, C. L. (2020). Genome‐wide association study of word reading: Overlap with risk genes for neurodevelopmental disorders. Genes, Brain and Behavior, 19, e12648. 10.1111/gbb.12648 [DOI] [PubMed] [Google Scholar]
- Pritchard, V. E. , Malone, S. A. , Burgoyne, K. , Heron‐Delaney, M. , Bishop, D. V. , & Hulme, C. (2019). Stage 2 registered report: There is no appreciable relationship between strength of hand preference and language ability in 6‐ to 7‐year‐old children. Wellcome Open Research, 4, 81. 10.12688/wellcomeopenres.15254.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.r‐project.org/index.html [Google Scholar]
- Raven, J. C. , Court, J. H. , & Raven, J. (1996). Manual for Raven's standard progressive matrices and vocabulary scales. Oxford Psychologists Press. [Google Scholar]
- Rimfeld, K. , Malanchini, M. , Spargo, T. , Spickernell, G. , Selzam, S. , McMillan, A. , Dale, P. S. , Eley, T. C. , & Plomin, R. (2019). Twins early development study: A genetically sensitive investigation into behavioral and cognitive development from infancy to emerging adulthood. Twin Research and Human Genetics, 22, 508–513. 10.1017/thg.2019.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust, J. (1996). WOLD Wechsler objective language dimensions manual. The Psychological Corporation. [Google Scholar]
- Rust, J. , Golombok, S. , & Trickey, G. (1993). WORD: Wechsler objective reading dimensional manual. Psychological Corporation. [Google Scholar]
- Scerri, T. S. , Macpherson, E. , Martinelli, A. , Wa, W. C. , Monaco, A. P. , Stein, J. , Zheng, M. , Suk‐Han Ho, C. , McBride, C. , Snowling, M. , Hulme, C. , Hayiou‐Thomas, M. E. , Waye, M. M. Y. Y. , Talcott, J. B. , & Paracchini, S. (2017). The DCDC2 deletion is not a risk factor for dyslexia. Translational Psychiatry, 7, e1182. 10.1038/tp.2017.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri, T. S. , Morris, A. P. , Buckingham, L. L. , Newbury, D. F. , Miller, L. L. , Monaco, A. P. , Bishop, D. V. M. , & Paracchini, S. (2011). DCDC2, KIAA0319 and CMIP are associated with reading‐related traits. Biological Psychiatry, 70, 237–245. 10.1016/j.biopsych.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri, T. S. , Paracchini, S. , Morris, A. , MacPhie, I. L. , Talcott, J. , Stein, J. , Smith, S. D. , Pennington, B. F. , Olson, R. K. , deFries, J. C. , & Monaco, A. P. (2010). Identification of candidate genes for dyslexia susceptibility on chromosome 18. PLoS One, 5, e13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, J. , Zheng, M. , Lui, K. F. H. , McBride, C. , Ho, C. S.‐H. , & Paracchini, S. (2022). Quantitative multidimensional phenotypes improve genetic analysis of laterality traits. Translational Psychiatry, 12, 68. 10.1038/s41398-022-01834-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte‐Körne, G. , Deimel, W. , Müller, K. , Gutenbrunner, C. , & Remschmidt, H. (1996). Familial aggregation of spelling disability. Journal of Child Psychology and Psychiatry and Allied Disciplines, 37, 817–822. 10.1111/j.1469-7610.1996.tb01477.x [DOI] [PubMed] [Google Scholar]
- Semel, E. , Wiig, E. H. , & Secord, W. (1995). Clinical evaluation of language fundamentals (3rd ed.). The Psychological Corporation. [Google Scholar]
- Sha, Z. , Pepe, A. , Schijven, D. , Carrion‐Castillo, A. , Roe, J. M. , Westerhausen, R. , Joliot, M. , Fisher, S. E. , Crivello, F. , & Francks, C. (2021). Handedness and its genetic influences are associated with structural asymmetries of the cerebral cortex in 31,864 individuals. Proceedings of the National Academy of Sciences of the United States of America, 118. 10.1073/PNAS.2113095118/-/DCSUPPLEMENTAL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha, Z. , Schijven, D. , Carrion‐Castillo, A. , Joliot, M. , Mazoyer, B. , Fisher, S. E. , Crivello, F. , & Francks, C. (2021). The genetic architecture of structural left–right asymmetry of the human brain. Nature Human Behaviour, 5, 1226–1239. 10.1038/s41562-021-01069-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling, M. J. , Lervåg, A. , Nash, H. M. , & Hulme, C. (2019). Longitudinal relationships between speech perception, phonological skills and reading in children at high‐risk of dyslexia. Developmental Science., 22, e12723. 10.1111/desc.12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers, M. , Shields, L. S. , Boks, M. P. , Kahn, R. S. , & Sommer, I. E. (2015). Cognitive benefits of right‐handedness: A meta‐analysis. Neuroscience & Biobehavioral Reviews, 51, 48–63. 10.1016/J.NEUBIOREV.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Straker, L. , Mountain, J. , Jacques, A. , White, S. , Smith, A. , Landau, L. , Stanley, F. , Newnham, J. , Pennell, C. , & Eastwood, P. (2017). Cohort profile: The Western Australian pregnancy cohort (RAINE) study‐generation 2. International Journal of Epidemiology., 46, dyw308–d1385j. 10.1093/ije/dyw308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski, J. P. , Binder, J. R. , Possing, E. T. , McKiernan, K. A. , Ward, B. D. , & Hammeke, T. A. (2002). Language lateralization in left‐handed and ambidextrous people: fMRI data. Neurology, 59, 238–244. 10.1212/wnl.59.2.238 [DOI] [PubMed] [Google Scholar]
- Thomson, M. E. (1982). The assessment of children with specific reading difficulties (dyslexia) using the British ability scales. British Journal of Psychology, 73, 461–478. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7171921 [DOI] [PubMed] [Google Scholar]
- Tomblin, J. B. , Records, N. L. , Buckwalter, P. , Zhang, X. , Smith, E. , & O'Brien, M. (1997). Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language, and Hearing Research, 40, 1245–1260. 10.1044/jslhr.4006.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen, J. K. , Rashotte, C. A. , & Wagner, R. K. (1999). TOWRE: Test of word reading efficiency. Pro‐ed. [Google Scholar]
- Tran, C. , Wigg, K. G. , Zhang, K. , Cate‐Carter, T. D. , Kerr, E. , Field, L. L. , Kaplan, B. J. , Lovett, M. W. , & Barr, C. L. (2014). Association of the ROBO1 gene with reading disabilities in a family‐based analysis. Genes, Brain and Behavior, 13, 430–438. 10.1111/gbb.12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, T. , Monetta, L. , & Joanette, Y. (2004). Phonological processing of words in right‐ and left‐handers. Brain and Cognition, 55, 427–432. 10.1016/j.bandc.2004.02.068 [DOI] [PubMed] [Google Scholar]
- Verhoeven, L. (1995). Drie‐minuten‐toets. Cito. [Google Scholar]
- Verhoeven, L. , & van Leeuwe, J. (2009). Modeling the growth of word‐decoding skills: Evidence from dutch. Scientific Studies of Reading, 13, 205–223. 10.1080/10888430902851356 [DOI] [Google Scholar]
- Vingerhoets, G. (2019). Phenotypes in hemispheric functional segregation? Perspectives and challenges. Physics of Life Reviews, 30, 1–18. 10.1016/J.PLREV.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Vlachos, F. , Andreou, E. , Delliou, A. , & Agapitou, P. (2013). Dyslexia and hand preference in secondary school students. Psychology & Neuroscience, 6, 67–72. 10.3922/j.psns.2013.1.10 [DOI] [Google Scholar]
- Wechsler, D. (2005). Wechsler Individual Achievement Test Second UK edition (WIAT‐II UK). Psychological Corporation. [Google Scholar]
- Wechsler, D. (2012). Wechsler preschool and primary scale of intelligence—Fourth edition. The Psychological Corporation. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. , Golombok, S. , & Rust, J. (1992). WISC‐III UK: Wechsler Intelligence Scale for Children. The Psycological Corporation. [Google Scholar]
- Weiß, R. H. (1998). Grundintelligenztest Skala 2. CFT‐20 [Culture Fair Intelligence Test]. Hogrefe. [Google Scholar]
- Whitehouse, A. J. O. (2010). Is there a sex ratio difference in the familial aggregation of specific language impairment? A meta‐analysis. Journal of Speech, Language, and Hearing Research, 53, 1015–1025. 10.1044/1092-4388(2009/09-0078) [DOI] [PubMed] [Google Scholar]
- Whitehouse, A. J. O. , & Bishop, D. V. M. (2009). Hemispheric division of function is the result of independent probabilistic biases. Neuropsychologia, 47, 1938–1943. 10.1016/J.NEUROPSYCHOLOGIA.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg, A. , Ng, M. , Al Omran, Y. , Alfaro‐Almagro, F. , McCarthy, P. , Marchini, J. , Bennett, D. L. , Smith, S. , Douaud, G. , & Furniss, D. (2019). Handedness, language areas and neuropsychiatric diseases: Insights from brain imaging and genetics. Brain, 142, 2938–2947. 10.1093/brain/awz257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig, E. H. , & Secord, W. (1985). Test of language competence (TLC): Expanded edition. Psychological Corporation. [Google Scholar]
- Wiig, E. H. , Semel, E. , & Secord, W. A. (2006). Clinical Evaluation of Language Fundamentals 4th edition (CELF4). Pearson. [Google Scholar]
- Wilkinson, G. (1993). Wide Range Achievement Test WRAT. Wide Range Inc. [Google Scholar]
- Wilson, A. C. , & Bishop, D. V. M. (2018). Resounding failure to replicate links between developmental language disorder and cerebral lateralisation. PeerJ, 6, e4217. 10.7717/peerj.4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock, R. W. (1987). Woodcock reading mastery tests‐revised. American Guidance Service. [Google Scholar]
- Woodcock, R. W. , McGrew, K. S. , & Mather, N. (2001). Woodcock‐Johnson III tests of achievement. Riverside Publishing Company. [Google Scholar]
- Woodhead, Z. V. J. , Thompson, P. A. , Karlsson, E. M. , & Bishop, D. V. M. (2021). An updated investigation of the multidimensional structure of language lateralization in left‐ and right‐handed adults: A test–retest functional transcranial doppler sonography study with six language tasks. Royal Society Open Science, 8. 10.1098/RSOS.200696 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


