Abstract
Centrally administered insulin stimulates the reward system to reduce appetite in response to food intake in animal studies. In humans, studies have shown conflicting results, with some studies suggesting that intranasal insulin (INI) in relatively high doses may decrease appetite, body fat, and weight in various populations. These hypotheses have not been tested in a large longitudinal placebo-controlled study. Participants in the Memory Advancement with Intranasal Insulin in Type 2 Diabetes (MemAID) trial were enrolled in this study. This study on energy homeostasis enrolled 89 participants who completed baseline and at least 1 intervention visit (42 women; age 65±9 years; 46 INI, 38 with type 2 diabetes) and 76 completed treatment (16 women, age 64±9; 38 INI, 34 with type 2 diabetes). The primary outcome was the INI effect on food intake. Secondary outcomes included the effect of INI on appetite and anthropometric measures, including body weight and body composition. In exploratory analyses, we tested the interaction of treatment with gender, body mass index (BMI), and diagnosis of type 2 diabetes. There was no INI effect on food intake or any of the secondary outcomes. INI also showed no differential effect on primary and secondary outcomes when considering gender, BMI, and type 2 diabetes. INI did not alter appetite or hunger nor cause weight loss when used at 40 I.U. intranasally daily for 24 weeks in older adults with and without type 2 diabetes.
Introduction
Insulin is a pancreatic hormone with important effects on metabolism. Insulin gains access to the central nervous system (CNS) by binding to insulin receptors in the endothelial cells of the blood-brain barrier (1) and with intranasal administration through extra-neuronal transport in the olfactory epithelium (2). Insulin receptors are broadly distributed in the CNS (olfactory bulbs, arcuate nucleus, hypothalamic paraventricular nucleus, suprachiasmatic and periventricular regions), suggesting that peripheral insulin acts on hypothalamic nuclei to control energy homeostasis (3). It has been proposed that states of peripheral insulin resistance such as obesity or type 2 diabetes may also disrupt the central effects of insulin possibly due to low levels of insulin delivered centrally (4) or due to insulin resistance in the brain (5). Systemic use of Insulin Detemir has shown the potential to acutely activate brain insulin signaling more than regular human insulin, and even overcome or restore central insulin resistance observed in humans with obesity, possibly due to acute increase in brain insulin concentrations (6). Of note, there seems to be a discrepancy between central and peripheral insulin effects; peripheral insulin administration may actually promote weight gain through anabolic effects on adipose tissue and/or by inducing hypoglycemia and thus stimulating hunger(7). In contrast, animal studies have shown that centrally administered insulin may act as an anorexigenic signal resulting in decreased food intake and body weight. The intracerebro-ventricular administration of an insulin-mimetic has reduced food intake and body weight in rats, altering the expression of hypothalamic genes known to regulate eating behavior (8), but no similar studies have been performed in humans. Intranasal insulin (INI) administration is an effective and safe method for direct drug delivery to the CNS (2) in humans. In previous clinical studies evaluating the effects of INI on energy homeostasis and food intake, results are conflicting. INI has inconsistently shown to decrease appetite, reduce food palatability, or increase every expenditure. The effects vary, and some are in the opposite direction, or vary according to sex and the population tested, i.e. reduction of body fat in men but not in women (9), reduced food intake in obese as compared to lean women (10), improvement of energy metabolism in healthy patients but not in patients with type 2 diabetes (11). A recent crossover placebo-controlled trial indicated that 160 I.U. of human insulin administered intranasally achieved reduction of intake and reward value of cookies, and appetite in women (10). We aimed to determine whether 40 I.U. of INI would have effects in terms of food intake and appetite levels as well as body composition (i.e. body weight, fat and lean mass) in men and women between 50 and 85 years old including persons with and without diabetes. Participants from the Memory Advancement by Intranasal Insulin in type 2 diabetes (MemAID) clinical trial were enrolled in this study. The MemAID trial (12) was a randomized, placebo-controlled, double blind study that examined the effects of daily administration of INI vs. placebo over 24 weeks, followed by 24 weeks of post-treatment follow-up. We hypothesize that INI will promote anorexigenic effects as evidenced by reduction in food intake which, sustained over time, could lead to weight loss. Furthermore, as a proof-of-concept, we expected to see differences in the effect of INI on energy homeostasis when adjusting or stratifying by gender and states reflecting underlying insulin resistance, such as obesity and diagnosis of type 2 diabetes in secondary exploratory analyses.
Research design and methods
Design
The MemAID study was a two-center, randomized, double-blind, parallel design placebo-controlled trial evaluating the long-term effects of 40 I.U. of human INI (Novolin® R Novo Nordisk Inc., Baksvaerd, Denmark, off label use (13)) or sterile saline once daily (12,14). Participants were randomized into four treatment arms (type 2 diabetes-INI, type 2 diabetes-Placebo, Control-INI, and Control-Placebo). The trial was conducted from October 6, 2015 to May 31, 2020 and it was approved by the US Food and Drug Administration (FDA; IND#107690) and registered on www.clinicaltrials.gov (NCT02415556). MemAID study was conducted at the Syncope and Falls in the Elderly (SAFE) Laboratory, Clinical Research Center (CRC) at Beth Israel Deaconess Medical Center (BIDMC), and at the Center for Clinical Investigation (CCI) at Brigham and Women’s Hospital (BWH). The protocol was approved by the Committee on Clinical Investigation at BIDMC and BWH, and all participants signed the informed consent. Participant enrollment in this study took place from March 2016 to March 2018.
Study Population
Participants between ages of 50 and 85 years able to walk for six minutes, used as a minimum functional capacity index, were screened. Inclusion criteria were participants with type 2 diabetes treated with non-insulin oral or injectable agents, participants without type 2 diabetes with fasting plasma glucose <126 mg/dL and HbA1c <6.5%, and being able to provide informed consent. In this study, 38 participants with type 2 diabetes and 51 participants without type 2 diabetes were included. Exclusion criteria were type 1 diabetes, insulin-treated type 2 diabetes, intolerance to insulin, history of severe hypoglycemia, diagnosis of dementia or mini-mental state examination ≤20, current recreational drug or alcohol abuse, acute medical condition that required either hospitalization or surgery within the past 6 months and liver or renal failure or transplant. Participants took part in an on-site screening session to ensure eligibility when informed consent was obtained. During this visit, participants underwent a comprehensive review of medical history and medications, physical examination, height, weight, waist and hip circumference measurements, and blood sampling. Throughout the duration of this study, participants consented to undergo measurements of body composition and caloric intake as part of MemAID study protocol.
Intervention and procedures
Volunteers were randomly allocated to receive 40 I.U. (0.4mL) of human insulin (rDNA origin, Novolin® R, Novo Nordisk, Bagsværd, Denmark) or placebo (0.4 mL bacteriostatic Sodium Chloride 0.9% solution) in a blinded fashion. The BIDMC and BWH research pharmacies performed sterility procedures, reconstituted study drug and di spensed Insulin and matching placebo to avoid distinguishable characteristics. Participants were instructed to self-administer INI/placebo intranasally, twice into each nostril, alternating sides once daily before breakfast for 24 weeks using the ViaNase™ electronic atomizer (Kurve Technology, Inc. Lynnwood, WA, USA)(14). This device dispenses 0.1 ml over 20 seconds in a single dose. Participants recorded their INI/Placebo intake routine. Medication vials were stored at approximately 2 to 8°C and were replaced every 10 to 12 days. Study compliance was assessed by self-reported device usage and by subtracting the amount of medication returned in a given study visit to the amount of medication given in the prior visit. This active period was followed by a 24 week observation follow up period on no active medication.
Four in-person assessments, eight weeks apart, were performed in the treatment and follow-up periods. In preparation for assessment visits, participants recorded their food and beverage intake specifying quantities, method of preparation, and brand names at the time of consumption for any 2 weekdays and 1 weekend day between study visits. Participants were asked to fast for at least eight hours before the study visit. During assessment visits, volunteers had vital signs measurement and underwent blood sampling. Subsequently, INI was administered and assessment of body composition, appetite feelings, anthropometric measurements and collection of food diaries were performed. Bioelectrical impedance analysis (BIA, 50 kHz) using the RJL Quantum II (RJL Systems, Clinton Township, Michigan) was used to measure lean mass (%), body fat (%), basal metabolic rate (BMR, kcal), and total body water (TBW, %). Participants rated how they felt in relation to hunger, satiety, nausea, desire to eat something salty, sweet, and savory, and the amount they could eat at the moment by marking a vertical line on a 10 cm visual analog scales (VAS). After administration of INI/placebo, continental breakfast was provided. VAS were completed three times per visit during the treatment period: 1) fasting before INI/placebo, 2) Fasting after INI/placebo (within five minutes of administration of study drug), 3) Postprandial after INI/placebo and two times per visit during the follow-up period: 1) fasting and 2) postprandial. Study staff measured waist circumference at the level of iliac crests and widest hip circumference. Caloric intake (kcal), total amount of food (gr), calories from fat, carbohydrate and protein (%) were analyzed from 3-day food diaries. For the purpose of this study, fasting serum glucose (mmol/l) and insulin (mU/l) levels were analyzed.
Randomization and group allocation
The randomization was done for each of the two sites (BIDMC, BWH). Within each site, a subject was randomized to one of the four treatment arms. Block randomization was used with three block sizes: 4, 8, and 12. The size of the block was randomly determined by uniform distribution (e.g. if the random variable is less than 1/3, block size of four was selected, between 1/3 and 2/3, block size of eight was selected, and greater than 2/3, block size of twelve was selected). We expected the distribution of subject-specific confounding variables to be similar across groups. The principal investigator, study physicians, study staff, participants, and their health care providers were blinded to the randomization. The principal investigator and the study physicians reviewed eligibility, adverse events, outcomes, and provide approval for enrollment. The study staff conducted the study procedures. The randomization code was generated by the study statistician (L.N.) and was given to BIDMC and BWH study pharmacists.
Statistical methods
Statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC). Data for continuous variables are presented as mean ± SD when normally distributed or median (interquartile range) when otherwise. Categorical variables are presented as numbers and/or percentages. We evaluated the differences at baseline between study groups by one-way ANOVA, Wilcoxon rank-sum or Fisher exact test. On treatment analysis included data from participants who completed baseline assessment and had data for outcomes of interest. Linear mixed-effects model was used to assess the treatment effect of INI vs. placebo in body composition, food intake, and anthropometric measurements over time as compared to baseline, adjusting by time effect (Table 2). Intention-to-treat analysis was done to confirm on-treatment analysis using the last observation carried forward method for all outcomes. Because we anticipated that gender and states of insulin resistance such as obesity and type 2 diabetes would play a role on the effect of INI on body composition and energy expenditure, we evaluated the interaction effect of treatment (INI/Placebo) with body mass index (BMI) categories, diagnosis of type 2 diabetes, and gender across time. To analyze appetite feelings, the mean hunger and satiety score were calculated by treatment group at week 0 (baseline), 8, 16, and 24 (treatment period) and the longitudinal trends of INI vs. Placebo group are displayed in Figure 2. Graphical comparisons of salty, sweet, and fatty food palatability, and amount can eat scores are available as supplementary material.
Table 2. On-treatment analysis of the longitudinal effect of INI vs. Placebo over 24 weeks anthropometric measurements, body composition, and food intake.
Linear mixed-effect models testing for differences between the effect of INI and Placebo over 24 weeks of treatment. Variance was estimated using restricted maximum likelihood technique and results are displayed as estimate (standard error of estimate). P-values are derived from the interaction between covariates, gender, diagnosis of diabetes, and BMI, and treatment effect. INI (intranasal insulin), SE (standard error), Body Mass Index (BMI).
| Outcomes | INI vs. Placebo | Interaction effect by | ||||
|---|---|---|---|---|---|---|
| Gender | Diagnosis of Diabetes | BMI | ||||
| n | Estimate (SE) | p-value | p-value | p-value | p-value | |
| Anthropometric measurements | ||||||
| Weight, kg | 89 | −5.0 (3.5) | 0.16 | 0.28 | 0.50 | - |
| BMI, kg/m2 | 89 | −1.5 (1.2) | 0.22 | 0.44 | 0.91 | - |
| Waist circumference, cm | 89 | −2.3 (3.2) | 0.48 | 0.29 | 0.67 | - |
| Body composition | ||||||
| Basal metabolic rate, kcal | 60 | −113.6 (71.4) | 0.12 | 0.67 | 0.46 | 0.45 |
| Fat mass, kg | 60 | −2.2 (2.8) | 0.44 | 0.96 | 0.43 | 0.23 |
| Lean mass, kg | 60 | −4.7 (3.2) | 0.14 | 0.73 | 0.63 | 0.89 |
| Total body water, % | 60 | −0.8 (1.8) | 0.65 | 0.72 | 0.79 | 0.48 |
| Food intake | ||||||
| Energy intake, kcal | 76 | −150.9 (122.7) | 0.22 | 0.25 | 0.31 | 0.49 |
| Calories from carbohydrates, kcal | 76 | −94.1 (69.6) | 0.18 | 0.75 | 0.40 | 0.84 |
| Calories from proteins, kcal | 76 | −37.9 (25.9) | 0.15 | 0.14 | 0.10 | 0.38 |
| Calories from fat, kcal | 76 | −15.2 (46.1) | 0.74 | 0.26 | 0.66 | 0.17 |
Figure 2.
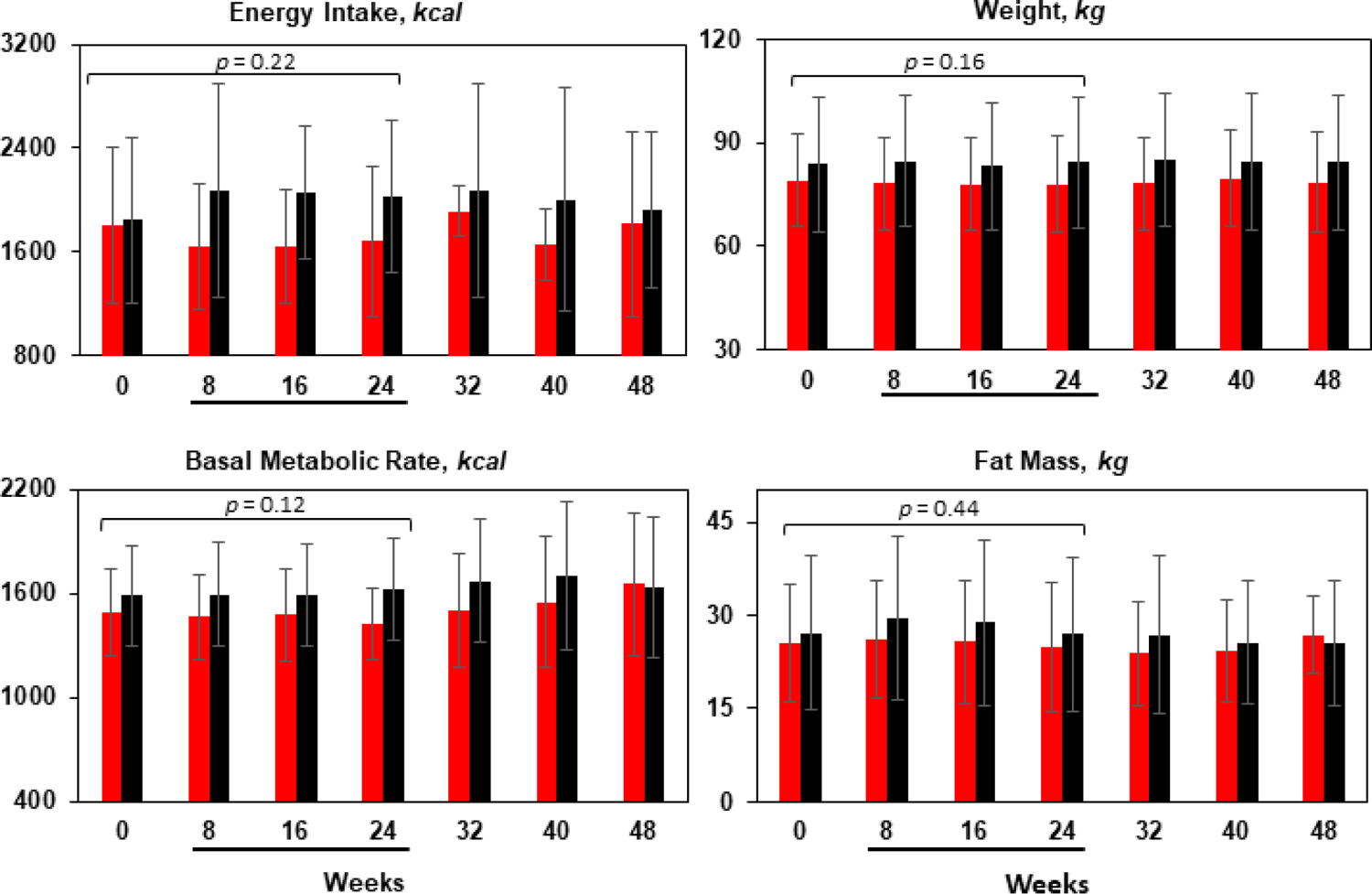
Mean and SD at each study visit for INI (red bars) and placebo (black bars). There was no differences between INI and Placebo groups on energy intake, basal metabolic rate, weight and fat mass over 24 weeks of treatment and 24 weeks of follow-up. P-values are derived from linear mixed-effect models testing differences between 40 I.U. of INI and placebo after 24 weeks of treatment adjusted by time. Underlined weeks in x-axis corresponds to weeks on treatment.
Results
Baseline characteristics of study participants
Of the 402 participants assessed for eligibility, 154 (38.3%) were randomized. A total of 89 participants, 46 assigned to INI (19 with type 2 diabetes) and 43 to placebo (19 with type 2 diabetes), completed baseline and at least one follow-up visit and were included in the final analysis. Of the 89 participants, 76 (38 INI, 38 placebo) completed treatment and 71 (35 INI, 36 placebo) completed follow-up (Figure 1, CONSORT). Age and gender were balanced across study groups, overall baseline mean age was 65.2 years (SD 9.2) and 52.8% were males. The majority of our cohort self-identified as white (75.3%), non-Hispanic (92.1%). At baseline, 42.7% of the cohort had diagnosis of type 2 diabetes and 35.6% were obese. Table 1 shows the baseline characteristics by treatment group and by diagnosis of type 2 diabetes. There were no differences in overall food intake among study groups at baseline. Participants fasted for 12.7 hours (SD 2.4) before appetite assessments, mean hunger score was 4.9 (SD 2.7) with no differences between INI and placebo groups. There were no differences in other body composition measurements such as fat mass, lean mass, TBW, and BMR in the whole cohort and within groups of participants with and without type 2 diabetes. No differences in BMI, weight, and waist circumference between INI and placebo group were seen across study groups.
Figure 1.
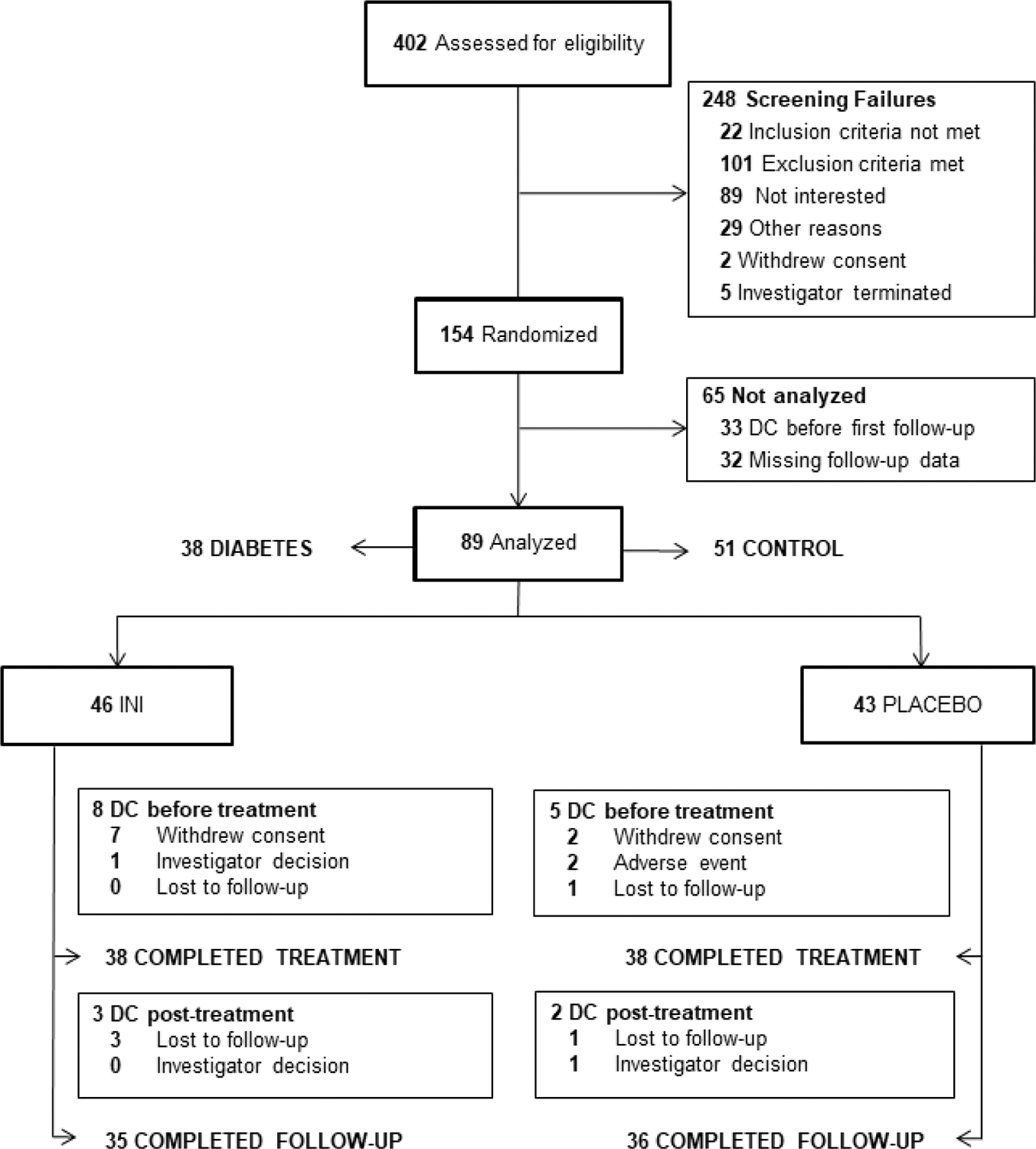
CONSORT diagram. INI, intranasal insulin; DC, discontinued.
Table 1. Baseline characteristics.
For continuous variables, data are means (standard deviation) or medians (interquartile range). For categorical variables, n was used. Comparisons between INI and Placebo groups in the whole cohort and among participants with and without diagnosis of Diabetes were not statistically significant. Differences were tested by Wilcoxon rank-sum test, analysis of variance, or Fisher exact test as appropriate to determine differences at baseline between INI and placebo within each group. Intranasal Insulin (INI), African-American (AA), Body Mass Index (BMI), Basal Metabolic Rate (BMR), Fasting Plasma Glucose (FPG), Fasting Plasma Insulin (FPI).
| Demographics | All Participants | With Diabetes | Without Diabetes | |||
|---|---|---|---|---|---|---|
| INI | Placebo | INI | Placebo | INI | Placebo | |
| n=46 | n=43 | n=19 | n=19 | n=27 | n=24 | |
| Age, years | 65.5 (8.8) | 64.7 (9.6) | 63.5 (7.3) | 62.9 (9.4) | 66.9 (9.7) | 66.2 (9.8) |
| Sex, n: Male, Female | 21, 25 | 26, 17 | 8, 11 | 13, 6 | 13, 14 | 13, 11 |
| Race, n: White, AA, other | 38, 7, 1 | 29, 10, 4 | 15, 4, 0 | 8, 8, 3 | 23, 3, 1 | 21, 2, 1 |
| Ethnicity, n: Hispanic, Not Hispanic | 2, 44 | 5, 38 | 1, 18 | 3, 16 | 1, 26 | 22, 2 |
| Anthropometric measurements | n=46 | n=43 | n=19 | n=19 | n=27 | n=24 |
| Weight, kg | 79.1 (13.4) | 83.9 (19.5) | 84.9 (13.6) | 92.2 (18.8) | 74.9 (11.8) | 77.2 (17.6) |
| BMI, kg/m2 | 28.5 (5.0) | 29.9 (6.6) | 31.2 (5.0) | 32.7 (7.5) | 26.5 (4.1) | 27.5 (4.6) |
| Waist circumference, cm | 101.1 (12.5) | 103.6 (18.1) | 109.7 (9.6) | 109.7 (17.0) | 95.1 (10.8) | 98.7 (17.8) |
| Hips circumference, cm | 105.8 (11.1) | 107.7 (11.9) | 114.5 (12.5) | 110.3 (13.3) | 102.5 (8.8) | 105.7 (10.5) |
| Body composition | n=29 | n=31 | n=15 | n=16 | n=14 | n=15 |
| Fat mass, kg | 25.5 (9.3) | 27.2 (12.3) | 27.3 (9.2) | 31.9 (12.8) | 23.7 (9.5) | 22.2 (9.8) |
| Lean mass, kg | 53.9 (12.1) | 58.7 (12.1) | 56.9 (13.9) | 62.2 (10.0) | 50.6 (9.3) | 54.9 (13.3) |
| Body water, % | 52.5 (45.0–56.4) | 54.1 (45.9–56.7) | 53.1 (14.9) | 53.2 (11.9) | 49.9 (11.1) | 54.9 (9.9) |
| BMR, kcal | 1494.5 (249.8) | 1589.3 (289.6) | 1572 (273.8) | 1703.6 (224.0) | 1410.7 (197.5) | 1467.3 (308.3) |
| Food intake | n=41 | n=35 | n=17 | n=14 | n=24 | n=21 |
| Energy intake, kcal | 1768.0 (1389.5–2077.0) | 1704.0 (1411.5–2074.0) | 1574 (500) | 1635.3 (478) | 1862 (671.3) | 1808.6 (701.9) |
| Calories from fat, kcal | 587.0 (489.5–823.9) | 628.0 (504.9–784.6) | 635.7 (286.9) | 653.7 (203.8) | 684.3 (222.0) | 631.7 (295.1) |
| Calories from carbohydrates, kcal | 795.9 (647.4–920.5) | 805.5 (592.3–1122.6) | 659.9 (238.8) | 807.2 (296.9) | 916.7 (384.2) | 926.7 (380.3) |
| Calories from protein, kcal | 285.5 (228.6–354.6) | 277.5 (214.7–355.7) | 299.0 (122.1) | 351.6 (140.7) | 319.0 (128.6) | 283.0 (138.9) |
| Appetite assessment | n=46 | n=42 | n=19 | n=19 | n=27 | n=23 |
| Hunger – fasting, score | 5.0 (2.6) | 4.8 (2.9) | 5.5 (2.3) | 4.9 (3.0) | 4.6 (2.8) | 4.7 (2.9) |
| Satiety – Postprandial, score | 6.7 (2.8) | 6.7 (2.8) | 6.5 (3.1) | 5.5 (3.4) | 6.8 (2.6) | 7.8 (1.5) |
| Hours of fasting | 12.8 (2.4) | 12.9 (2.5) | 12.6 (2.2) | 13 (1.9) | 13 (2.5) | 12.8 (2.9) |
| Metabolic profile | n=46 | n=43 | n=19 | n=19 | n=27 | n=24 |
| FPG, mg/dL | 101.0 (86.8–128.8) | 96.0 (89.0–110.0) | 12.2 (11) | 13.3 (16.9) | 92 (12) | 90 (12) |
| FPI, mU/L | 9.5 (6.3–16.5) | 10.0 (6.9–16.3) | 131 (73) | 117 (46) | 7.5 (4.4) | 7.7 (4.2) |
Longitudinal effects of INI on food intake, appetite, satiety, and food palatability
Participants in the INI group fasted for 12.5 ± 2.2 hours and 12.5 ± 2.4 hours in the placebo group, in average. Food intake (median (interquartile range)) was 1,599 (671) kcal in the INI group vs. 1,818 (948) kcal in the placebo group at baseline. During the intervention period, INI group consumed 1,594 (721) kcal vs. 1,962 (1,090) by the placebo group, which is 368 kcal less in the INI group, but this difference was not statistically significant (p=0.22). There were no changes in overall food intake nor in preference of macronutrients in the diet. Macronutrient palatability was similar between INI and placebo across the 24-week intervention period as reflected by meal composition in terms of calories consumed from carbohydrates, proteins and fat. Figure 2 displays the longitudinal effects of INI vs. placebo over 24 weeks of treatment and 24 weeks of follow-up on food intake, fat mass, BMR, and weight.
During assessment visits, breakfast induced a sharp decline in appetite score and an increase in satiety score. There were no immediate differences in perception of appetite feelings between INI and placebo group after study drug administration. Fasting INI administration did not alter appetite over time nor satiety ratings in relation to breakfast intake (Figure 3).
Figure 3. 3A) Hunger 3B) Satiety.
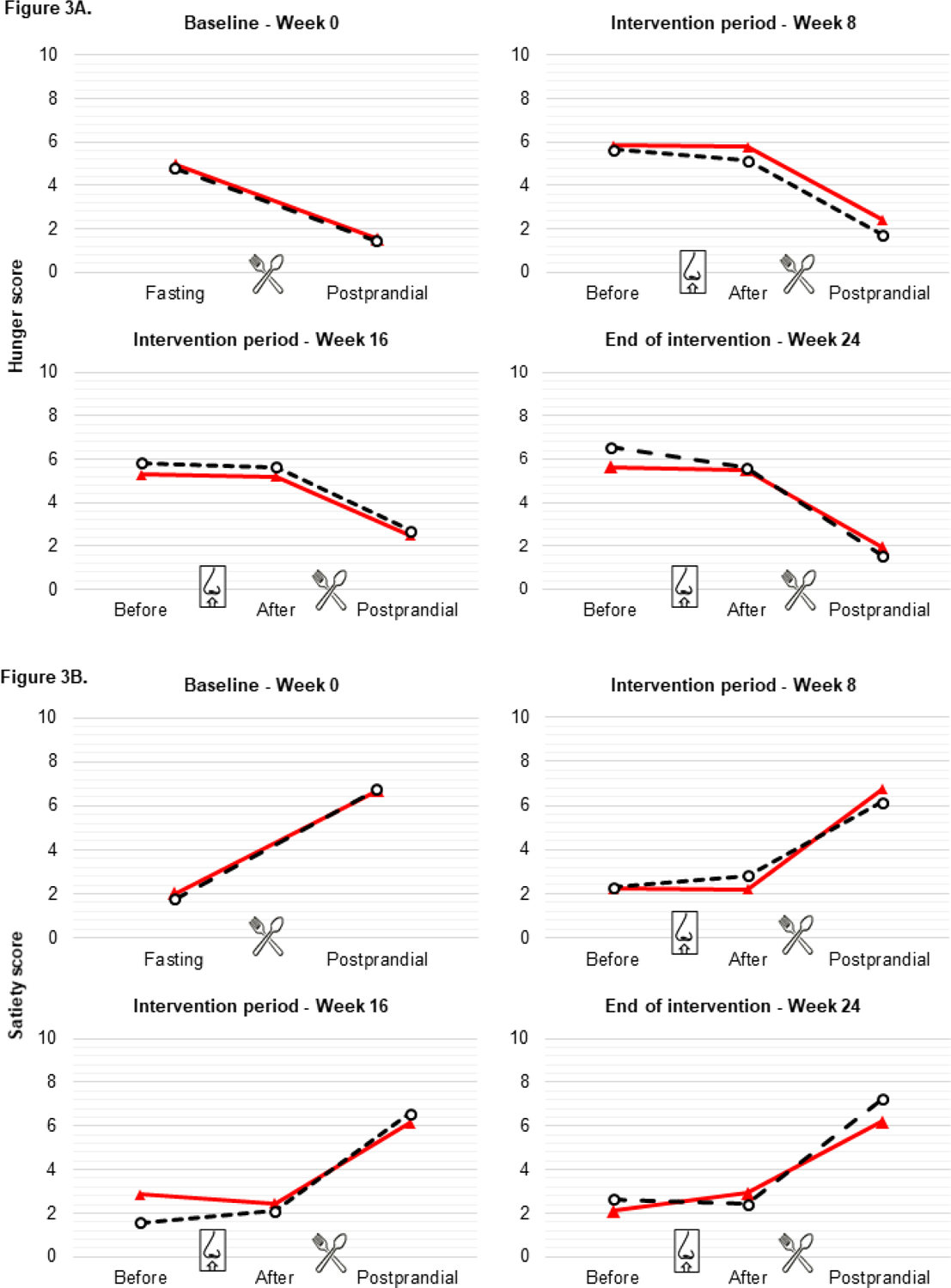
Values are mean scores of visual analogue scales (VAS) ratings. For panels at baseline (week 0), “Fasting” is before breakfast and “Postprandial” is after breakfast. For panels during intervention period (week 8 and week 16) and at end of intervention (week 24), “Before” is before breakfast and before INI/placebo; “After” is before breakfast and after INI/Placebo; “Postprandial” is after breakfast and after INI/Placebo. There was no difference between INI and placebo groups at any of these time points. Red line=INI; black dashed line= placebo.
Longitudinal effects of INI in weight and body composition
On-treatment analyses (Table 2) of participants with data at baseline showed no differences in weight, BMI or waist circumference between INI and placebo group. Estimated difference in body weight, through linear mixed effect models, was 5kg lower in INI group as compared to placebo during treatment period, but this difference was not statistically significant when adjusting by time effect. Average weight in the INI group was 1.2kg less at end of treatment compared to baseline and average weight in the placebo group was 0.6kg higher at end of treatment compared to baseline. Body composition measured by fat mass, lean mass, and TBW remained steady during treatment period, with no longitudinal differences in participants who received INI as compared to placebo. Participants underwent follow-up assessments for 24 weeks after treatment discontinuation. There were no differences in weight (p=0.14), BMI (p=0.19), BMR (p=0.96), fat mass (p=0.45), lean mass (p=0.13), and energy intake (p=0.23) between INI and Placebo groups during post-treatment follow-up.
Previous studies have shown mixed results of INI effects in men as compared to women and in normal weight populations as compared to overweight and obese. In our cohort, women had lower overall food intake and consumed less calories from carbohydrates, protein, and fat as compared to men. In addition, women had lower weight, lower BMR, lower fat mass, and lower TBW. As expected, participants with type 2 diabetes had higher weight, BMI, and fat mass. Higher lean mass and BMR were also observed in participants with type 2 diabetes. In addition, BMI was associated with BMR, fat mass, lean mass, and TBW. The interaction of INI or placebo effect with gender, BMI, and diagnosis of type 2 diabetes was not statistically significant for all outcomes: anthropometric measures, body composition, and food intake. Further on-treatment analyses showed no differences between Diabetes-INI vs. Diabetes-Placebo groups in weight, body composition or energy intake. When comparing INI vs. placebo within overweight/obese group, INI group weighted 8kg less than placebo group (p=0.02) at the end of the intervention period. However, the estimated weight difference at end of intervention from baseline was 0.16kg less in INI group vs. Placebo group. There were no differences in BMI, BMR, fat or lean mass, and food intake. Results from on-treatment analyses were confirmed when adjusting by missing data through intention-to-treat analysis using last observation carried forward (supplemental material).
Discussion
In this study, we report a null effect of long-term (24 weeks) daily administration of 40 I.U. of INI once daily on food intake, appetite, and weight in older adults. Similarly, there were no differences in the effect of INI when comparing men to women, obese to lean participants, and diagnosis of type 2 diabetes. The lack of INI effects on body weight, BMI, glucose and HbA1c is consistent as seen the whole cohort of the MemAID trial (12). To date, this study assesses the largest reported sample size and longest treatment duration allowing better understanding of the effect of INI on energy homeostasis, as well as providing new evidence for the possible usage of INI to treat obesity and metabolic disorders.
Existing data on the effect of INI on energy metabolism have been conflicting. In human studies, INI has shown to modify weight and food intake in particular populations and settings but its effect, if any, has been variable and primarily shown only in subgroups of study participants. For instance, three studies (10,15,16) with sizes ranging from 17 to 35 participants found that one time administration of 160 I.U. of INI regulates energy homeostasis centrally acutely in women: one showed modulation of intrinsic brain activity in the hypothalamus and orbitofrontal cortex, which is associated with the rewarding properties of food, in 17 healthy lean women (15). Other study found decreased appetite, food intake, and palatability after INI administration in 30 healthy women in postprandial state only (16) and another showed similar results but only in women with obesity, when comparing 17 obese vs. 35 lean women (10). When including men and testing the effect of INI as compared to women, existing evidence suggests that women do not benefit from 160 I.U. of INI in terms of body weight and energy consumption. One study showed that 160 I.U. of INI decreased food intake in 14 men but not in women(17), another showed that after 40 I.U. of INI 4 times daily for 8 weeks (160 IU daily) only men lose weight and reduced fat mass whereas women gained weight due to increased total body water. Of note, in this study that included 24 men vs. 16 women, hunger ratings decreased overtime but not acutely after INI use and there were no changes in energy expenditure overall (17).
In contrast, another study found that 40 I.U. of INI increases brain levels of adenosine triphosphate and phosphocreatine and lowered calorie intake in 15 healthy young men (18). There is also controversy on how humans with different degrees of peripheral insulin resistance respond to INI administration. Healthy overweight participants (30 young males) displayed reduced regional cerebral blood flow (CBF) after 160 I.U. of INI as compared to normal weight in regions involved in gustation, memory and reward in one study (19). Another study showed that 23 obese/overweight participants had increased CBF 30 minutes after 160 I.U. of INI use as compared to 25 lean participants but when adjusting for sex, only lean men showed a reduction of high-caloric food palatability as compared to the obese group (20). Two other studies found that 160 I.U. of INI induced lower food intake and body weight only in lean but not in overweight individuals, each with a sample size of 10 to 15 individuals (21,22). Recently, a functional MRI study measuring blood oxygen level-dependent (BOLD) signal in response to visual food cues after 160IU of INI showed food-cue reactivity in the amygdala and insular cortex with interactions between sex and obesity. Women showed higher BOLD activity than men in response to central insulin, and particularly normal weight men and women with overweight showed an increase in central insulin-induced BOLD response in the insula (23). In terms of age, 160 I.U. showed no difference of INI effect on appetite, food intake, and energy expenditure between young and older adults (24,25). Overall, sample sizes in these studies have ranged from 10 to 30 participants in each group of interest and the largest study included 54 participants in total. Notably effects on body metabolism were only observed at a dose 160IU of INI, a four times higher than in our study.
Herein, we report no decrease in food intake, fat mass, lean mass, and weight after chronic administration of 40 I.U. of human Insulin (Novolin®) and that any INI effect lacked statistical significance when comparing with placebo and adjusting for time in 89 participants. Furthermore, our study did not find a significant interaction between the central effect of INI with states of peripheral insulin resistance such as high BMI and diagnosis of type 2 diabetes, nor differences between INI and placebo within diabetes and overweight/obese groups. The effect of INI was tested over 24 weeks of treatment, when including 38 participants with overweight (BMI ≥ 25kg/m2), 32 participants with obesity (BMI ≥ 30 kg/m2), and 38 participants with diagnosis of type 2 diabetes. In addition, we did not observe differences among gender. In this present study, participants had access to food ad libitum as they were logging the amount and food kinds according to their likes at home; this allows the assessment of overall changes in eating habits while on INI therapy. We did not observe differences in hunger or satiety scores between INI and placebo group acutely, and no changes in appetite after 24 weeks of daily administration of 40 I.U. of INI as compared to baseline. The MemAID trial had a study compliance of 78% with participants self-reported medication device usage >65% (>109 days)(12).
Insulin receptors in the CNS are predominantly present in the olfactory bulb, cerebellum, and the arcuate nucleus of the hypothalamus which is known to be involved in neuroendocrine functions [4]. When adiposity signals reach the arcuate nucleus, pro-opiomelanocortin (POMC) neurons suppress food intake (27). Prior studies in rodents have shown that centrally administered insulin increases the expression of POMC by the activation of phosphoinositol-3 kinase pathway (28), which in turn may result in anorexigenic behavior. Brain insulin sensitivity correlates with adiposity as increased insulin peripherally results in chronic exposure of the hypothalamus to high insulin levels with subsequent over-activation of insulin receptors and development of hypothalamic insulin resistance (29). One of the key concepts that has gained attention as a potential way to overcome obesity is the conversion of white adipose tissue (WAT) to brown adipose tissue (BAT) and the role of CNS-mediated signals on this interaction. BAT requires high-energy substrate from the oxidation of fatty acids for thermogenesis whereas dysfunctional WAT (as in obesity) generates excess of free fatty acids, inflammation, and insulin resistance due to inadequately high lipolysis (30). In animal studies, the administration of Insulin in the CNS suppressed sympathetic nervous outflow to WAT (31) suppressing lipolysis, stimulated higher uptake of fatty acids, and WAT browning (32). Importantly, Brain insulin also showed to decrease food intake in baboons (33), chickens (34), and marmots (35) but mixed results in rats (8,36). In humans, some studies showed that INI might decrease lipolysis (37) and circulating free fatty acids (11,38,39), and cause acute reduction in hepatic lipid content after INI administration in men (11). Other studies showed no change in hepatic lipid content after 4 weeks of INI administration (40), and unchanged triglyceride-rich lipid particle production in the constant-fed state or clamp conditions (39). Ultimately, INI use has not resulted in fat mass reduction in most studies and when loss of fat mass and body weight was achieved, no concomitant change in energy expenditure was present (9). Animal models have been more conclusive and consistent than translational human models thus far, which suggests that physiologic mechanisms behind the effect of INI administration on metabolism in humans differ from animals and need further investigation.
A limiting effect that could play a role in the reproducibility of results across existing clinical trials in humans is the dosage of INI used. Most clinical trials assessing the effect of INI on energy homeostasis have been conducted using a single dose of 40 to 160 I.U. (15,16,18,20,24,25,37,41–45). Studies in mice have showed an elevation of reward thresholds by directly applying low doses of insulin in the ventral-tegmental area and a modulated food intake without affecting reward-related behavior at higher doses of insulin (46). In humans, the MemAID study evaluated the INI effect on resting-state functional connectivity (rsFC) in medio-prefrontal cortex (mPFC) through blood oxygen level dependent (BOLD) fMRI scans. We showed that after 24 weeks of treatment, 40 I.U. of INI increased resting state mPFC-postcentral rsFC and decreased mPFC-cerebellum rsFC suggesting the potential effect of INI on decision making and goal-oriented behaviors (12), confirming results of an improved connectivity between hippocampus and default mode network regions (47). A prior placebo-controlled fMRI study looked at the effect of 40, 100 and 160 I.U. of INI on the functional connectivity of the dopaminergic midbrain in 42 men (48). The connectivity between the dopaminergic midbrain and ventromedial prefrontal cortex (vmPFC) was modulated equally by doses of 40 and 160 I.U. in individuals with high insulin sensitivity and in those with low insulin sensitivity, the connectivity modulation was still significant but at a slower increase. Doses of 160 I.U. of INI have been associated with mild tingling and burning sensation in the nose and with changes in plasma glucose and insulin levels (24) indicating local and systemic effects. The results hereby presented are the product of daily administration of 40 I.U. of human insulin for 168 days. INI was well tolerated and all reported adverse events were self-resolving with no sequelae (12). The mechanism of why low and high doses of insulin seem to act equally when administered centrally remains to be understood.
In the elderly, similar insulin binding to adipocytes and monocytes than in nonelderly groups has been observed through euglycemic clamp monitoring, which suggests the presence of a post-receptor insulin action defect causing both a decrease in peripheral glucose disposal and a rightward shift in the dose-response curve of insulin action (49). Therefore, it could be argued that older age represents an even state of insulin resistance across our study population. The degree of peripheral insulin sensitivity might affect the response to central insulin, as is the case of a prior study where participants with higher insulin sensitivity showed an increase activation in the insular cortex in response to INI (23). This could be further assessed in future studies by contrasting BMI and type 2 diabetes among younger adults.
Strengths of this study are its large sample size that included a diverse pool of participants such as individuals with and without type 2 diabetes, with obesity, healthy lean, and both men and women. We also performed a detailed assessment of energy metabolism: intake through food diaries, expenditure by bioelectrical impedance, perception of appetite and satiety through VAS, and periodic body measures, all of them recorded at baseline, during intervention, and during post-intervention follow-up period.
A limitation of the present study is missing data reflected in the different samples sizes across groups depending upon the outcome under evaluation (Table 1.). Our study entailed a multipart protocol involving in-person visits every four weeks and completion of 24-hour food intake logs for a total of 21 days over 48 weeks of study participation, which hindered adherence to the protocol. We handled missing data by including intention-to-treat analysis through last observation carried forward as part of our analyses obtaining the same results than in on-treatment analyses. There was no estimation of the sample size needed to achieve statistical significance given lack of prior data testing the effect of INI at a dose of 40 IU that could be used to perform power calculations. An empirically chosen number of 34 subjects per group would provide an 80% power at the two-tailed p-value of 0.05 to demonstrate a 0.5 effect estimate i.e. ratio of mean difference between the groups over standard deviation. In contrast, an empirically chosen a number of 46 subjects per group which would provide a 90% power at the two tailed p-value of 0.05 to demonstrate a 0.5 effect estimate i.e. ratio of mean difference between the groups over standard deviation. Differences, albeit non-significant, in weight and energy intake in the INI as compared to placebo group might be of clinical relevance if future larger studies demonstrate significant effects with the doses and mode of administration used herein. However, given our sample size and characteristics of study participants, it remains uncertain if any outcome changes correspond to INI effect and these hypotheses remain to be fully explored by larger future studies A dose response curve of INI effects on body metabolism may be needed to determine if any effects could be seen at higher INI doses.
Conclusion
Research in animals has underscored the role of brain insulin on the regulation of appetite, but data in humans are inconsistent despite initial hopes that intranasal insulin would translate into lower food intake and ultimately weight loss. Using intranasal administration of insulin is a promising effective method of direct drug delivery to the CNS. In our study, 40 I.U. of INI did not regulate appetite or peripheral energy expenditure and could not correct insulin resistance in participants with type 2 diabetes. This influences the way we understand and approach INI for the treatment of obesity and provides further insight in the selection of individuals who would not respond to INI by regulating eating behavior and achieving weight loss. Future large and inclusive (i.e. women and men, as well as individuals with and without type 2 diabetes) studies looking into a dose-response curve of more than 40 I.U. and its effect on measures of energy homeostasis and fMRI in humans are needed. The scientific community should continue to dive in potential pathways and therapies involved with rewarding of food and change on dietary habits to continue to battle obesity. In addition, to identify different phenotypes of obesity and individualize therapies according to the degree of metabolic disease associated with obesity (50,51). The ultimate goal is to reduce the prevalence of obesity which has affected nearly 40% of adults in the US over the last decade and is a significant risk factor for poor health outcomes and higher mortality.
Supplementary Material
Acknowledgments
The authors thank MemAID Investigators, Beth Israel Deaconess Medical Center (BIDMC) and Brigham and Women’s Hospital (BWH) Clinical Research Center (CRC) nurses and staff for their contributions, time, and skills for completion of this study. The authors acknowledge contribution of Joanna Radziewska, MS, RND from BIDMC- CRC nutrition unit.
Conflict of interest
L. Aponte Becerra, V. Novak, and F. Khan report no disclosures relevant to the manuscript. L. Ngo provided consultation to the Radiological Society; to the Journal of Cardiovascular Magnetic Resonance; to Five Island Consulting LLC, Georgetown ME; and to Vinmec Inc. Hanoi, Vietnam between 2015 and 2020. C.S. Mantzoros reports over the past 3 years 3YRS CSM reports grants through his institution from Merck, Massachusetts Life Sciences Center and Beohringer Ingellheim, has been a shareholder of and has received grants through his Institution and personal consulting fees from Coherus Inc. and AltrixBio, he reports personal consulting fees from Novo Nordisk, reports personal consulting fees and support with research reagents from Ansh Inc., collaborative research support from LabCorp Inc., reports personal consulting fees from Genfit, Lumos, Amgen, Corcept, Intercept, 89 Bio, Madrigal and Regeneron, reports support (educational activity meals through his institution or national conferences) from Amarin, Novo Nordisk, Astra Zeneca, Boehringer Ingelheim and travel support and fees from TMIOA, Elsevier, the California Walnut Commission, College Internationale Researche Servier and the Cardio Metabolic Health Conference. None is related to the work presented herein.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIDDK) under Award Number R01DK103902 to Vera Novak (FDA IND 107690, clinical trials.gov registration NCT2415556), and with support from Harvard Catalyst - The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. This research study was supported with study drug from Novo-Nordisk Inc.; Bagsværd, Denmark through an independent ISS grant (ISS-001063) (to V.N), and Medtronic Inc., Northridge CA, USA by providing CGM monitoring devices and supplies through an independent grant NERP15-031 (to V.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, or its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baura GD, Foster DM, Porte D, Kahn SE, Bergman RN, Cobelli C, et al. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993. Oct;92(4):1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmid V, Kullmann S, Gfrörer W, Hund V, Hallschmid M, Lipp HP, et al. Safety of intranasal human insulin: A review. Diabetes Obes Metab. 2018. Jul;20(7):1563–77. [DOI] [PubMed] [Google Scholar]
- 3.Marks JL, Porte D, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990. Dec;127(6):3234–6. [DOI] [PubMed] [Google Scholar]
- 4.Kern W, Benedict C, Schultes B, Plohr F, Moser A, Born J, et al. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia. 2006. Nov;49(11):2790–2. [DOI] [PubMed] [Google Scholar]
- 5.Cetinkalp S, Simsir IY, Ertek S. Insulin resistance in brain and possible therapeutic approaches. Curr Vasc Pharmacol. 2014;12(4):553–64. [DOI] [PubMed] [Google Scholar]
- 6.Tschritter O, Hennige AM, Preissl H, Porubska K, Schäfer SA, Lutzenberger W, et al. Cerebrocortical beta activity in overweight humans responds to insulin detemir. PloS One. 2007. Nov 21;2(11):e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005. Feb;184(2):291–318. [DOI] [PubMed] [Google Scholar]
- 8.Air EL, Strowski MZ, Benoit SC, Conarello SL, Salituro GM, Guan XM, et al. Small molecule insulin mimetics reduce food intake and body weight and prevent development of obesity. Nat Med. 2002. Feb;8(2):179–83. [DOI] [PubMed] [Google Scholar]
- 9.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004. Nov;53(11):3024–9. [DOI] [PubMed] [Google Scholar]
- 10.Schneider E, Spetter MS, Martin E, Sapey E, Yip KP, Manolopoulos KN, et al. The effect of intranasal insulin on appetite and mood in women with and without obesity: an experimental medicine study. Int J Obes 2005. 2022. Apr 9; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gancheva S, Koliaki C, Bierwagen A, Nowotny P, Heni M, Fritsche A, et al. Effects of intranasal insulin on hepatic fat accumulation and energy metabolism in humans. Diabetes. 2015. Jun;64(6):1966–75. [DOI] [PubMed] [Google Scholar]
- 12.Novak V, Mantzoros CS, Novak P, McGlinchey R, Dai W, Lioutas V, et al. MemAID: Memory advancement with intranasal insulin vs. placebo in type 2 diabetes and control participants: a randomized clinical trial. J Neurol. 2022. Apr 28;269(9):4817–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novo Nordisk Medical, Inc Novolin R (insulin human injection) Prescribing Information and Safety. Updated 11/2019 [Internet]. [cited 2022 Aug 27]. Available from: https://www.novo-pi.com/novolinr.pdf
- 14.Galindo-Mendez B, Trevino JA, McGlinchey R, Fortier C, Lioutas V, Novak P, et al. Memory advancement by intranasal insulin in type 2 diabetes (MemAID) randomized controlled clinical trial: Design, methods and rationale. Contemp Clin Trials [Internet]. 2020. Feb 1 [cited 2020 Dec 3];89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kullmann S Frank S, Heni M, Ketterer C, Veit R, Häring H, Fritsche A, Preissl H. Intranasal insulin modulates intrinsic reward and prefrontal circuitry of the human brain in lean women. Neuroendocrinology. 2013;97:176–82. [DOI] [PubMed] [Google Scholar]
- 16.Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes. 2012;61:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008. Apr;93(4):1339–44. [DOI] [PubMed] [Google Scholar]
- 18.Jauch-Chara K, Friedrich A, Rezmer M, Melchert UH, G. Scholand-Engler H, Hallschmid M, et al. Intranasal Insulin Suppresses Food Intake via Enhancement of Brain Energy Levels in Humans. Diabetes. 2012. Sep;61(9):2261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingrove JO, O’Daly O, Forbes B, Swedrowska M, Amiel SA, Zelaya FO. Intranasal insulin administration decreases cerebral blood flow in cortico-limbic regions: A neuropharmacological imaging study in normal and overweight males. Diabetes Obes Metab. 2021;23(1):175–85. [DOI] [PubMed] [Google Scholar]
- 20.Kullmann S, Heni M, Veit R, Scheffler K, Machann J, Häring HU, et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care. 2015. Jun;38(6):1044–50. [DOI] [PubMed] [Google Scholar]
- 21.Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes 2005. 2008. Feb;32(2):275–82. [DOI] [PubMed] [Google Scholar]
- 22.Heni M, Wagner R, Kullmann S, Gancheva S, Roden M, Peter A, et al. Hypothalamic and Striatal Insulin Action Suppresses Endogenous Glucose Production and May Stimulate Glucose Uptake During Hyperinsulinemia in Lean but Not in Overweight Men. Diabetes. 2017. Jul;66(7):1797–806. [DOI] [PubMed] [Google Scholar]
- 23.Wagner L, Veit R, Fritsche L, Häring HU, Fritsche A, Birkenfeld AL, et al. Sex differences in central insulin action: Effect of intranasal insulin on neural food cue reactivity in adults with normal weight and overweight. Int J Obes. 2022. Sep;46(9):1662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santiago JCP, Hallschmid M. Central Nervous Insulin Administration before Nocturnal Sleep Decreases Breakfast Intake in Healthy Young and Elderly Subjects. Front Neurosci. 2017;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krug R, Benedict C, Born J, Hallschmid M. Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab. 2010. Dec;95(12):E468–472. [DOI] [PubMed] [Google Scholar]
- 26.Bouret SG, Draper SJ, Simerly RB. Formation of Projection Pathways from the Arcuate Nucleus of the Hypothalamus to Hypothalamic Regions Implicated in the Neural Control of Feeding Behavior in Mice. J Neurosci. 2004. Mar 17;24(11):2797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, et al. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATPchannel activation. BMC Neurosci. 2004. Dec 6;5(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003. Feb;52(2):227–31. [DOI] [PubMed] [Google Scholar]
- 29.Sipols AJ, Baskin DG, Schwartz MW. Effect of Intracerebroventricular Insulin Infusion on Diabetic Hyperphagia and Hypothalamic Neuropeptide Gene Expression. Diabetes. 1995. Feb 1;44(2):147–51. [DOI] [PubMed] [Google Scholar]
- 30.Scherer T, Sakamoto K, Buettner C. Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol. 2021. Aug;17(8):468–83. [DOI] [PubMed] [Google Scholar]
- 31.Scherer T, O’Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011. Feb 2;13(2):183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015. Jan 15;160(1–2):88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods SC, Lotter EC, McKay LD, Porte D. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979. Nov 29;282(5738):503–5. [DOI] [PubMed] [Google Scholar]
- 34.Honda K, Kamisoyama H, Saneyasu T, Sugahara K, Hasegawa S. Central administration of insulin suppresses food intake in chicks. Neurosci Lett. 2007. Aug 16;423(2):153–7. [DOI] [PubMed] [Google Scholar]
- 35.Florant GL, Singer L, Scheurink AJ, Park CR, Richardson RD, Woods SC. Intraventricular insulin reduces food intake and body weight of marmots during the summer feeding period. Physiol Behav. 1991. Feb;49(2):335–8. [DOI] [PubMed] [Google Scholar]
- 36.Jessen L, Clegg DJ, Bouman SD. Evaluation of the lack of anorectic effect of intracerebroventricular insulin in rats. Am J Physiol Regul Integr Comp Physiol. 2010. Jan;298(1):R43–50. [DOI] [PubMed] [Google Scholar]
- 37.Iwen KA, Scherer T, Heni M, Sayk F, Wellnitz T, Machleidt F, et al. Intranasal insulin suppresses systemic but not subcutaneous lipolysis in healthy humans. J Clin Endocrinol Metab. 2014. Feb;99(2):E246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes. 2015. Mar;64(3):766–74. [DOI] [PubMed] [Google Scholar]
- 39.Xiao C, Dash S, Stahel P, Lewis GF. Effects of Intranasal Insulin on Triglyceride-Rich Lipoprotein Particle Production in Healthy Men. Arterioscler Thromb Vasc Biol. 2017. Sep;37(9):1776–81. [DOI] [PubMed] [Google Scholar]
- 40.Scherer T, Wolf P, Smajis S, Gaggini M, Hackl M, Gastaldelli A, et al. Chronic Intranasal Insulin Does Not Affect Hepatic Lipids but Lowers Circulating BCAAs in Healthy Male Subjects. J Clin Endocrinol Metab. 2017. Apr 1;102(4):1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Raecke R, Yang H, Bruenner YF, Freiherr J. Intranasal Insulin Boosts Gustatory Sensitivity. J Neuroendocrinol. 2017. Jan;29(1). [DOI] [PubMed] [Google Scholar]
- 42.Heni M, Schöpfer P, Peter A, Sartorius T, Fritsche A, Synofzik M, et al. Evidence for altered transport of insulin across the blood-brain barrier in insulin-resistant humans. Acta Diabetol. 2014. Aug;51(4):679–81. [DOI] [PubMed] [Google Scholar]
- 43.Kullmann S, Heni M, Veit R, Scheffler K, Machann J, Häring HU, et al. Intranasal insulin enhances brain functional connectivity mediating the relationship between adiposity and subjective feeling of hunger. Sci Rep. 2017. May 9;7(1):1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedict C, Frey WH, Schiöth HB, Schultes B, Born J, Hallschmid M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp Gerontol. 2011. Mar;46(2–3):112–5. [DOI] [PubMed] [Google Scholar]
- 45.Guthoff M, Grichisch Y, Canova C, Tschritter O, Veit R, Hallschmid M, et al. Insulin modulates food-related activity in the central nervous system. J ClinEndocrinolMetab. 2010. Feb;95(1945–7197 (Electronic)):748–55. [DOI] [PubMed] [Google Scholar]
- 46.Bruijnzeel AW, Corrie LW, Rogers JA, Yamada H. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav Brain Res. 2011. Jun 1;219(2):254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Novak V, Novak P, Mantzoros C, Ngo Lh, Lioutas V, et al. 219-OR: Intranasal Insulin Enhanced Resting-State Functional Connectivity of Medio-Prefrontal Cortex in Type 2 Diabetes: A Substudy of the Memory Advancement with Type 2 Diabetes (MemAID) Randomized Control Trial. Diabetes. 2022. Jun 1;71(Supplement_1):219–OR.34753801 [Google Scholar]
- 48.Edwin Thanarajah S, Iglesias S, Kuzmanovic B, Rigoux L, Stephan KE, Brüning JC, et al. Modulation of midbrain neurocircuitry by intranasal insulin. NeuroImage. 2019. Jul 1;194:120–7. [DOI] [PubMed] [Google Scholar]
- 49.Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of Insulin Resistance in Aging. J Clin Invest. 1983. Jun;71(6):1523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathew H, Farr OM, Mantzoros CS. Metabolic health and weight: Understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism. 2016. Jan;65(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantzoros CS, Flier JS. Insulin resistance: the clinical spectrum. Adv Endocrinol Metab. 1995;6:193–232. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


