Abstract
Objective:
To longitudinally evaluate differences in HIV viral suppression (< 200 c/mL) by intersections of race/ethnicity, gender, and psychosocial issues in people with HIV in the Los Angeles County Medical Care Coordination Program.
Design:
We analyzed 74,649 viral load measurements over 10,184 people with HIV enrolled in the Medical Care Coordination Program between January 1, 2013 and March 1, 2020.
Methods:
We fit Bayesian logistic hierarchical random effects models to test interactions between gender, race/ethnicity, and a psychosocial acuity score on viral suppression over time from 1 year prior to program enrollment to 24 months after enrollment.
Results:
The probability of viral suppression declined prior to enrollment, then increased and stabilized by 6 months after enrollment. Black/African American patients with low and moderate psychosocial acuity scores did not achieve the same increase in percentage of viral suppression as those in other racial/ethnic groups. Transgender women with high psychosocial acuity scores took longer (about 1 year) to achieve the same percentage of viral suppression as clients of other gender identities.
Conclusions:
Some racial/ethnic and gender disparities in viral suppression persisted after enrollment in the Los Angeles County Medical Care Coordination Program while accounting for psychosocial acuity score, which may be explained by factors not assessed in the program.
Keywords: HIV, viral suppression, race, gender, disparities, psychosocial health, public health programs
Introduction
In 2013, the Los Angeles County Department of Public Health, Division of HIV and STD Programs developed and implemented a countywide Medical Care Coordination (MCC) Program to provide medical and psychosocial case management services to its diverse community of people with HIV (PWH) with complex needs [1, 2]. Even when accounting for comorbidities, MCC has shown to improve the percentage of patients who achieve viral suppression (VS; <200 copies/mL) [3].
Despite the success of the Los Angeles County-based MCC and other similar programs such as the HIV Care Coordination Program from New York City that provide psychosocial support for PWH, those jurisdictions continue to see disparities in HIV care outcomes among diverse PWH [4–6]. In 2019, Black/African American PWH in Los Angeles County had lower levels of retention in care in the past 12 months (41%) and VS at most recent test (53%) compared to Latinx (48% retention , 61% VS) and White PWH (45% retention, 61% VS) [6]. Among cisgender men, cisgender women, and transgender PWH, retention in care and VS were similar, ranging from 44-48% and 58-60%, respectively.
It is important to consider racial, gender, or other disparities in HIV care continuum outcomes beyond the context of the individual [7, 8]. Experiences of racism, sexism, transphobia, and homophobia can be interpersonal (from other individuals) or structural (societal and institutional) in nature [8, 9] with consequences ranging from housing and financial instability, medical mistrust and challenges accessing equitable medical care, limited social support, risk behaviors, substance use, mental health issues, and legal issues [8, 10]. All of these may influence HIV care continuum outcomes such as VS due to barriers to accessing HIV care, challenges maintaining antiretroviral therapy (ART) adherence, and stress on the immune system [11–14].
As MCC aims to address the holistic psychosocial and medical health needs of PWH in Los Angeles County, it is unclear whether disparities in VS by race or ethnicity, gender identity, or intersections of race/ethnicity and gender are explained by differences in psychosocial issues upon enrollment in the program [15], or whether these disparities still persist after accounting for these psychosocial issues. To determine this, we report a longitudinal evaluation of the ongoing MCC Program that compares trajectories of VS by gender and race/ethnicity, while accounting for differences in psychosocial issues at enrollment.
Methods
Study Design
The present study is a longitudinal evaluation of public health program and surveillance data of 10,184 patients who enrolled in the Los Angeles County Department of Public Health’s MCC Program to receive coordinated HIV care and support services.
Program Description
Implemented in March 2013, the MCC Program is an ongoing safety net program provided at HIV clinics across Los Angeles County contracted by the Los Angeles County Department of Public Health and funded by the Ryan White Program. One or more MCC teams—consisting of a registered nurse, Master’s level social worker, case manager, and retention specialist–are co-located at each clinic to assess needs, tailor care plans, link to HIV medical care, provide brief interventions, and assist with navigating and making appointments for mental health care, substance use treatment, housing, and other psychosocial health services. Clinic patients who: 1) have been newly diagnosed with HIV in the past 6 months; 2) have not seen an HIV medical provider in 7 or more months; 3) are not on ART despite meeting current clinical guidelines for treatment; 4) are on ART but virally unsuppressed (≥200 copies/mL); or 5) were diagnosed with a sexually transmitted infection (STI) in the past 6 months are prioritized for MCC enrollment but patients can also be referred by their medical provider. More details on the MCC Program are in the Los Angeles County MCC Guidelines and published elsewhere [2, 16, 17].
Data Sources
Patient-level sociodemographic and assessment data came from HIV Casewatch, the local Ryan White Program reporting system for contracted agencies. Programmatic data were matched with data from the Los Angeles County HIV surveillance database, the Enhanced HIV/AIDS Reporting System (eHARS), to get HIV diagnosis dates and viral load (VL) reports collected as part of routine medical care. The matched dataset was de-identified prior to sharing with UCLA collaborators.
Sample and Time Frame
The study sample included 10,184 PWH who enrolled in MCC between January 1, 2013 and March 1, 2020. We analyzed 74,649 VS measurements reported from 12 months prior to the patient’s MCC enrollment date through 24 months after enrollment, excluding measurements occurring after March 1, 2020. Baseline VS is based on the VL most recently reported prior to MCC enrollment or at enrollment if a VL in the prior year does not exist.
Measures
Viral suppression (VS).
The outcome of our evaluation is VS, defined as a VL less than 200 copies per mL of blood. This threshold is shown to be clinically meaningful in preventing transmission and disease progression [18] and is adopted by the U.S. Ending the HIV Epidemic initiative as an indicator of progress toward its treatment goals [19].
Sociodemographic variables.
Ryan White Program clinics reported information in CaseWatch on race/ethnicity and gender identity during patient intake and registration. We coded race/ethnicity as White, Black/African American, Latinx, and Asian American/Pacific Islander (AAPI). Other racial/ethnic groups and those of mixed racial/ethnic identity did not have sufficient numbers for analysis.
Psychosocial acuity (PSA).
At the time of enrollment, MCC teams administer a standardized assessment on the severity of medical and psychosocial needs across 12 domains associated with poor HIV outcomes—health status, quality of life, ART access, ART adherence, housing, finances, medical care access, social support, risk behaviors, substance use (including alcohol), mental health, and legal needs. Overall and domain-specific acuity scores were calculated from patient responses to inform integrated care plans, delivery of brief interventions, and provision of support service referrals. Each domain classifies people into one of four levels from self-managed (lowest need) to moderate, high, and severe need. The MCC assessment tool and scoring is on the Los Angeles County Department of Public Health website [17].
For the purposes of this analysis in which VS is the outcome of interest, we revised the acuity calculation to exclude the clinical domains (health status, ART access, ART adherence), resulting in a psychosocial acuity (PSA) score encompassing 9 psychosocial domains and ranging from 9 (lowest need) to 72 (most complex need). Consistent with the original MCC acuity decision tree scoring, we assigned greater weight to domains most strongly associated with poor HIV outcomes: housing, substance use (including alcohol), and mental health levels are scored 1, 4, 9, or 16 from low to severe, while the domains quality of life, medical access, financial, legal needs, social support, and risk behaviors levels are scored on a scale of 1, 2, 3, or 4.
Statistical Analysis
For descriptive purposes, we summarized differences in baseline demographic variables by PSA median split (< 19 vs. ≥ 19) and by baseline VS, and report chi-square tests for independence of PSA and VS with demographic variables.
We fit Bayesian logistic semi-parametric hierarchical random effects models to analyze longitudinal VS varying jointly with time and PSA score and with: 1) no covariates, 2) with race/ethnicity, 3) with gender, 4) or with both [20]. Bayesian regression modeling was done in R 4.0.3 [21], using the brms package [22] to specify models which in turn calls Stan [23] to fit the models. We fit each model using Markov chain Monte Carlo (MCMC) in Stan with 3 chains, 1500 iterations warmup and 1500 samples per chain, for a total of 4500 posterior samples and calculated posterior means, standard deviations (SD), and 95% credible intervals (CI) for quantities of interest. The model included two correlated subject-specific random intercepts, one for the intercept prior to MCC enrollment and another for the intercept following MCC enrollment to accommodate the idea that a patient’s average VS would likely be quite different prior versus following MCC enrollment. We specified weakly informative priors. We used the Watanabe–Akaike information criterion (WAIC) [24] for model choice in model development, for example for picking knot frequency. We fit and report on 4 models: Model 1 was a base model of VS regressed on PSA and time without demographic variables; Model 2 included gender and the interaction of gender with PSA as predictors; Model 3 included race/ethnicity and its interaction with PSA but not gender; and Model 4 included gender, race/ethnicity, their interaction and all three interactions with PSA. Model 4 omitted all AAPI and all transgender women because the subgroups were too small to model interactions with PSA.
Time is measured from MCC enrollment; times before enrollment are negative, times after enrollment are positive. The time trend of VS was modeled semi-parametrically to avoid making restrictive assumptions about the time and PSA association with VS. We used natural cubic splines with knots (changepoints) every 3 months from 12 months prior to 24 months after MCC enrollment, with two additional knots at 1.5 months before and 1.5 months after MCC enrollment. The choice of knot locations was motivated by inspecting LOESS curves of VS as a function of time. Additionally, a jump in average VS at MCC enrollment, time = 0, was included to additionally accommodate the change in percentage of VS around MCC enrollment date. The effect of PSA was modeled semi-parametrically using a natural cubic spline with knots at the endpoints 9 and 72 and interior knots (changepoints) selected at the 5th (= 11), 25th (= 15), 50th (= 19), 75th (= 28), and 95th (= 44) percentiles of the PSA score.
We fit a two-dimensional LOESS curve of VS as a function of both time and PSA score and determined that there was an interaction between time and PSA. In our Bayesian models, the joint effect of time and PSA was modeled by the interaction between the time spline and the PSA spline [25]. An interaction was included between the jump at time zero and a piecewise linear function of PSA score with a single knot at PSA of 25.
For Models 2-4, each model is fit separately for each level of the demographic variables. Population probabilities of VS were calculated by Monte Carlo, integrating out a hypothetical subject’s random effects and all unknown parameters. Predicted VS probabilities were calculated for each week from 1 year prior to MCC enrollment to 18 months following MCC enrollment for each level of PSA score observed in the data and for each gender, race/ethnicity, or both combined included in the data set. The effect of PSA score was integrated out by taking a weighted average over PSA scores based on the empirical distribution of PSA score among all patients in the sample. The percentage of VS at time = 0 includes the effect of the jump, and percentage of VS at time = 0- (zero minus) omits the jump. We report posterior means (M) and 95% Bayesian Credible Intervals (CI) with 2.5% posterior percentage in either tail, and defined a differences in VS between groups as significantly negative (or positive) if the posterior probability of that difference was greater than 0.975.
We plotted time trends of population probabilities of VS for each demographic group over time at PSA archetypes representing low (PSA = 12, 10% quantile), moderate (PSA = 19, 50% quantile), and high (PSA = 38, 90% quantile) PSA scores.
This analysis was approved by the institutional review boards of the University of California, Los Angeles and the Los Angeles Department of Public Health.
Results
Baseline characteristics
The analytic sample has 8,979 (85.2%) cisgender men, 1,201 (11.8%) cisgender women, and 304 (3.0%) transgender women. Latinx patients comprise the largest racial/ethnic group (47.1%), followed by Black/African American (27.7%), White (21.2%), and Asian American/Pacific Islander patients (4.0%).
Table 1 displays MCC patient characteristics at baseline stratified by PSA median split (<19 vs. ≥19) and by VS status (< 200 c/mL vs. ≥200). Baseline VS slightly differed between cisgender men (51.5%), cisgender women (47.5%), and transgender women (51.0%). Latinx patients had the lowest rate of baseline VS (46.6%), followed by Black/African American (49.5%), AAPI (50.2%), and White patients (53.5%).
Table 1.
Participant characteristics at MCC enrollment, stratified by median split of psychosocial acuity and viral suppression
| Psychosocial Acuity (PSA) Score | Viral Load a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total (N = 10,184) | < 19 (n = 4,862) | ≥ 19 (n = 5,322) | < 200 c/mL (n = 4,992) | ≥ 200 c/mL (n = 5,192) | |||||
|
| |||||||||
| M (SD) | M (SD) | M (SD) | t | p | M (SD) | M (SD) | t | p | |
|
|
|||||||||
| Age (years) | 40.60 (12.15) | 41.30 (12.49) | 39.97 (11.81) | 5.50 | <0.001 | 43.05 (12.34) | 38.25 (11.50) | 20.33 | <0.001 |
| Years Living with HIV | 8.60 (8.31) | 8.64 (8.41) | 8.56 (8.23) | 0.51 | 0.611 | 10.48 (8.42) | 6.79 (7.79) | 22.90 | <0.001 |
| n (%) | n (%) | n (%) | χ2 | p | n (%) | n (%) | χ2 | p | |
|
|
|||||||||
| Gender | 33.69 | <0.001 | 6.78 | 0.034 | |||||
| Cisgender men | 8679 (85.2%) | 4095 (47.2%) | 4584 (52.8%) | 4212 (48.5%) | 4467 (51.5%) | ||||
| Cisgender women | 1201 (11.8%) | 652 (44.3%) | 549 (45.7%) | 631 (52.5%) | 570 (47.5%) | ||||
| Transgender women | 304 (3.0%) | 115 (37.8%) | 189 (62.2%) | 149 (49.0%) | 155 (51.0%) | ||||
| MCC Enrollment Year | 9.84 | 0.007 | 225.48 | <0.001 | |||||
| 2012-2014 | 1972 (19.4%) | 881 (54.7%) | 1091 (55.3%) | 1260 (36.1%) | 712 (63.9%) | ||||
| 2015-2017 | 4712 (46.3%) | 2302 (48.9%) | 2410 (51.1%) | 2433 (48.4%) | 2279 (51.6%) | ||||
| 2018-2020 | 3500 (34.4%) | 1679 (48.0%) | 1821 (52.0%) | 1499 (57.2%) | 2001 (42.8%) | ||||
| Place of Birth | 332.08 | <0.001 | 1.29 | 0.256 | |||||
| US | 6640 (65.2%) | 2732 (41.1%) | 3908 (58.9%) | 3227 (48.6%) | 3413 (51.4%) | ||||
| Not US | 3544 (34.8%) | 2130 (61.1%) | 1414 (39.9%) | 1765 (49.8%) | 1779 (50.2%) | ||||
| Education | 24.94 | <0.001 | 5.71 | 0.058 | |||||
| Unknown | 4325 (42.5%) | 2184 (50.5%) | 2141 (49.5%) | 2007 (46.4%) | 2318 (53.6%) | ||||
| Less than High School | 1876 (32.0%) | 770 (41.0%) | 1106 (59.0%) | 980 (52.2%) | 896 (47.8%) | ||||
| High School or Equivalent | 1600 (27.3%) | 753 (47.1%) | 847 (52.9%) | 775 (48.4%) | 825 (51.6%) | ||||
| Some College | 2383 (40.7%) | 1155 (48.5%) | 1228 (51.5%) | 1230 (52.6%) | 1153 (47.4%) | ||||
| Income | 330.32 | <0.001 | 6.78 | 0.009 | |||||
| Less than or Equal to FPL | 7204 (70.7%) | 3022 (41.9%) | 4182 (58.1%) | 3733 (48.2%) | 3471 (51.8%) | ||||
| Above FPL | 2980 (29.3%) | 1840 (61.7%) | 1140 (38.3%) | 1459 (51.0%) | 1521 (49.0%) | ||||
| MSM (cisgender men only) | <0.001 | 1.000 | 4.17 | 0.041 | |||||
| No | 1487 (17.1%) | 702 (47.3%) | 785 (52.7%) | 758 (51.0%) | 729 (49.0%) | ||||
| Yes | 7192 (82.9%) | 3393 (47.2%) | 3799 (52.8%) | 3454 (48.0%) | 3738 (52.0%) | ||||
| Race/Ethnicity | 126.41 | <0.001 | 28.60 | < 0.001 | |||||
| White | 2159 (21.2%) | 903 (47.2%) | 1256 (52.8%) | 1155 (53.5%) | 1004 (46.5%) | ||||
| Latinx | 4800 (47.1%) | 2490 (51.9%) | 2310 (48.1%) | 2239 (46.6%) | 2561 (53.4%) | ||||
| Black/African American | 2819 (27.7%) | 1213 (43.0%) | 1606 (57.0%) | 1394 (49.5%) | 1425 (50.5%) | ||||
| Asian American/ Pacific Islander | 406 (4.0%) | 256 (63.1%) | 150 (36.9%) | 204 (50.2%) | 202 (48.8%) | ||||
| Psychosocial Acuity (PSA) Scoreb | 34.14 | <0.001 | |||||||
| < 19 | 2531 (52.1%) | 2331 (47.9%) | |||||||
| ≥ 19 | 2461 (46.2%) | 2861 (53.8%) | |||||||
Baseline VS is defined as the most recent VL prior to MCC enrollment or at enrollment if a VL in the year before enrollment does not exist.
Psychosocial Acuity (PSA) Scores account for the following domains: housing, finances, medical care access, social support, risk behaviors, substance use, mental health, HIV care, and legal needs
A higher percentage of transgender women had a PSA score ≥ 19 (62.2%) compared to cisgender men (52.8%,) and cisgender women (45.6%,). A higher percentage of Black/African American (56.9%) and White patients (58.4%) had a PSA score ≥ 19 compared to Latinx (48.1%) and AAPI patients (36.9%).
Change in viral suppression by psychosocial acuity (Model 1)
The percentage of VS for all MCC patients decreased from 61% (95% Credible Interval (CI) [58%, 64%]) at 1 year prior to enrollment to 45% (95% CI [43%, 48%]) shortly before enrollment, a change in VS percentage of −16% (95% CI [−18%, −13%]). Immediately following MCC enrollment, the VS percentage increased to 66% (95% CI [63%, 69%]), an increase of 21% (95% CI [18%, 23%]). VS continued to increase for 6 months to 81% (95% CI [79%, 82%])—a further increase of 15% (95% CI [13%, 16%])—and remained stable thereafter.
The same qualitative trends in the overall relationship between time and VS are exhibited at fixed PSA scores 12 (10% quantile), 19 (50% quantile), and 38 (90% quantile), corresponding to low, moderate, and high PSA archetypes, respectively. For low, moderate, and high PSA, the percentage of VS from immediately prior to enrollment to 1 year after enrollment increased by 36% (95% CI [33%, 38%]), 38% (95% CI [35%, 40%]), and 28% (95% CI [25%, 31%]), respectively.
Change in viral suppression by gender (Model 2)
Cisgender men had the largest increase in the VS percentage from just before MCC enrollment to 1 year after (posterior mean (M) = 36%, 95% CI [34%, 39%]), followed by transgender women, then cisgender women (Table 2). The percentage of VS for all gender groups was lower with higher PSA scores (Figure 1). At high PSA, transgender women took longer than cisgender men and cisgender women to reach stable longer-term VS percentages after MCC enrollment, but the post-enrollment differences in VS by gender were not significant.
Table 2.
Change in viral suppression percentage from just before MCC enrollment to 1 year after MCC enrollment
| Model | Group | Change | 95% CI |
|---|---|---|---|
| 1 | All PWH in MCC | 35% | 33%, 38% |
| 2 | Cisgender men | 36% | 34%, 39% |
| Transgender women | 31% | 27%, 34% | |
| Cisgender women | 27% | 21%, 33% | |
| 3 | Asian American/Pacific Islander | 40% | 35%, 45% |
| Latinx | 39% | 36%, 42% | |
| White | 35% | 32%, 38% | |
| Black/African American | 29% | 26%, 31% | |
| 4 | Latinx cisgender men | 40% | 37%, 43% |
| White cisgender men | 35% | 32%, 38% | |
| Black/African American cisgender men | 29% | 26%, 32% | |
| White cisgender women | 32% | 25%, 40% | |
| Latinx cisgender women | 32% | 28%, 37% | |
| Black/African American cisgender women | 25% | 20%, 29% |
Figure 1.

(Model 2) Posterior mean viral suppression percentage from 1 year before MCC enrollment to 1.5 years after MCC enrollment, by gender for fixed low (12, left), moderate (19, middle) and high (38, right) PSA scores.
Change in viral suppression by race/ethnicity (Model 3)
AAPI (M = 40%, 95% CI [35%, 45%]) and Latinx patients (M = 39%, 95% CI [36%, 42%]) had the greatest increases in percentage of VS from just before MCC Program enrollment to 1 year after enrollment, followed by White patients (M = 35%, 95% CI [32%, 38%]) (Table 2). Latinx patients took longer to reach longer term VS percentages than AAPI and White patients. Black/African American patients had the lowest increase in VS percentage over this period (M = 29%, 95% CI [26%, 31%]).
The percentage of VS for all racial/ethnic groups was lower with higher PSA scores. The disparities in VS percentage in Black/African American patients compared to other racial/ethnic groups were most pronounced at moderate and low PSA scores and persisted through 18 months following MCC enrollment (Figure 2).
Figure 2.
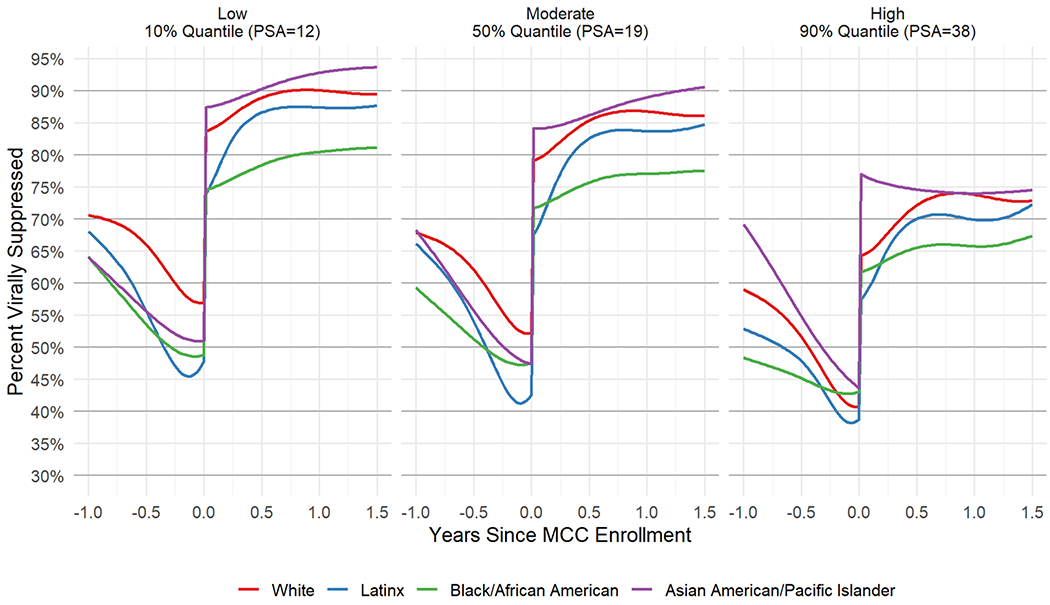
(Model 3) Posterior mean viral suppression percentage from 1 year before MCC enrollment to 1.5 years after MCC enrollment, by race/ethnicity for fixed low (12, left), moderate (19, middle) and high (38, right) PSA scores.
Change in viral suppression by intersection of gender and race/ethnicity (Model 4)
Among cisgender men, those who were Black/African American had the smallest increase in percentage of VS from before MCC enrollment to 1 year after (M = 29%, 95% CI [26%, 32%]), with White (M = 35%, 95% CI [32%, 38%]) and Latinx cisgender men (M = 40%, 95% CI [37%, 43%]) (Table 2) having much higher increases. For each PSA score, Black/African American cisgender men had a lower VS percentage than White and Latinx cisgender men after enrollment. This disparity was most pronounced in low and moderate PSA archetypes (Figure 3).
Figure 3.
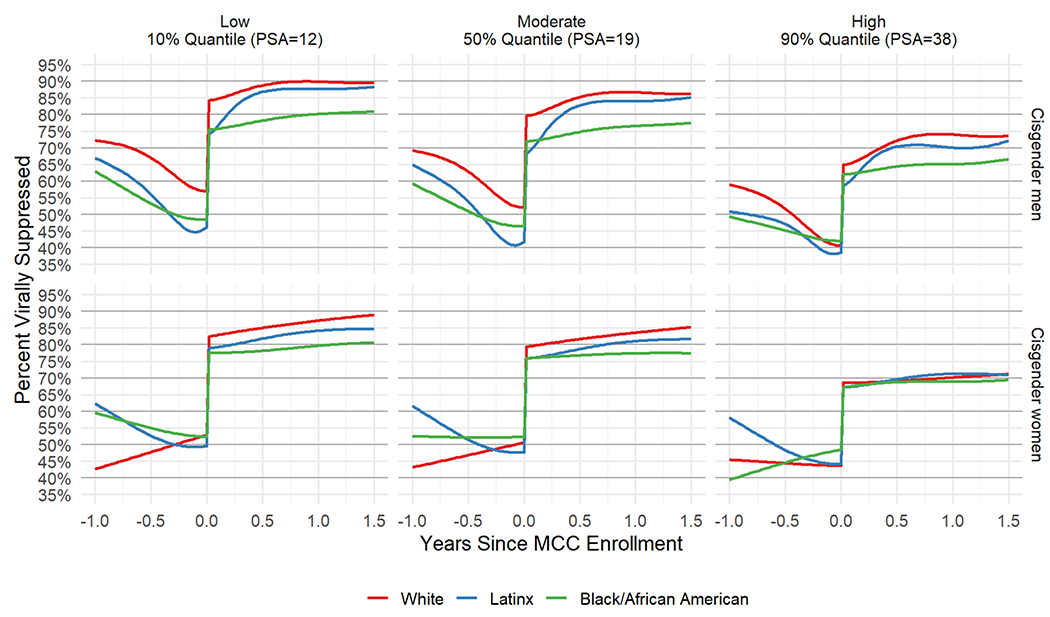
(Model 4) Posterior mean viral suppression percentage from 1 year before MCC enrollment to 1.5 years after MCC enrollment, by gender and race/ethnicity for fixed low (12, left), moderate (19, middle) and high (38, right) PSA scores.
Note: Transgender women and Asian American/Pacific Islander categories not included in this analysis due to small cell sizes.
Among cisgender women, Black/African American women had the smallest increase in VS percentage (M = 25%, 95% CI [20%, 29%]), followed by White (M = 32%, 95% CI [25%, 40%]) and Latinx cisgender women (M = 32%, 95% CI [28%, 37%]) (Table 2). At low PSA, Black/African American cisgender women had a smaller VS percentage (M = 80%, 95% CI [75%, 84%]) than White (M = 87%, 95% CI [81%, 92%]) and Latinx cisgender women (M = 84%, 95% CI [81%, 87%]) 12 months after MCC enrollment (Figure 3). At high PSA, the percentage of VS among cisgender women was similar between Black/African American, Latinx, and White cisgender women (about 70%) up to 12 months after enrollment. The Supplemental Table displays the mean VS percentages for the different demographic groups just before MCC enrollment, 6 months and 1 year after MCC enrollment by PSA archetype (low, moderate, and high) from Models 1-4.
Discussion
Our findings underscore that across the gender identities, racial/ethnic groups, and levels of PSA analyzed, all groups increased in the percentage of VS in MCC. Also noteworthy is that the VS percentage achieved 6 to 18 months following MCC enrollment exceed the VS percentage in any of the 12 months prior to MCC enrollment. As might be expected, a group with higher PSA scores consistently had lower percentages of VS before and after MCC enrollment than a comparable group with lower PSA scores.
Black/African American patients with low and moderate PSA did not achieve the same percentage of VS increase compared to other racial/ethnic groups. Unexpectedly, this racial/ethnic disparity was less pronounced with high PSA. One explanation is that patients across different racial/ethnic groups with high PSA are encountering similar barriers to engaging in care and adhering to treatment. Similar findings from New York City’s comprehensive HIV care program show that the association between race/ethnicity and low VS were less apparent when accounting for homelessness, substance use, and lack of insurance [15].
Transgender women with high PSA took longer to achieve VS compared to patients from other gender identity groups also with high PSA—about 1 year—though VS differences by gender were not significant. It is likely this analysis is underpowered given that there were only 304 transgender women in this sample [17]. Still, class differences and lack of resources can increase risk of experienced transmisogyny, drive medical mistrust, and hinder treatment among transgender women with HIV [26] and may explain the observed pattern.
When accounting for intersections of race/ethnicity, gender, and PSA together, we see that improvement in the percentage of VS after MCC enrollment was notably smaller in Black/African American cisgender men compared to White or Latinx cisgender men. Black/African American cisgender women also showed lower percentages of VS than White and Latinx cisgender women. Again, these racial disparities were less pronounced with higher PSA scores.
Given that some disparities were still identified after accounting for psychosocial issues (measured by PSA), there may be structural-level factors not assessed in the program that are worth exploring in future research. The link between structural factors and HIV disparities in minority or other marginalized communities is not unique to Los Angeles County, or the U.S. for that matter. The global response to HIV has had a history of unmet needs among racial/ethnic, sexual, and gender minorities [27–29]. This has often been driven by stigma, limited socioeconomic opportunity, and lack of available, culturally competent medical care [27–29]. Coordinated health care programs such as MCC are designed to tailor care toward the specific needs of diverse individuals and tend to be better suited for management of chronic conditions than routine care, especially in systems lacking universal healthcare coverage [30, 31].
There is also growing research interest around the impact of implicit bias, stigma, discrimination, and medical mistrust on HIV and other medical care engagement among people of color and transgender people [32–37]. Currently there are limited evidence-based interventions to address medical mistrust, but there are some recommended strategies: engaging clinicians to recognize implicit bias and communicate non-judgmentally; empowering PWH to channel mistrust towards taking control of their health; and supporting community members to become agents of change within the healthcare system and society [38]. New community-based cognitive behavioral therapy approaches have also shown some promise for addressing medical mistrust and increasing ART adherence [39].
Our analyses have some limitations. PWH who enroll in MCC are intentionally selected based on need, so those successfully maintaining VS are not prioritized for MCC and thus less likely to be in these analyses than PWH with greater challenges. This also means that these analyses do not have an equivalent comparison group of PWH not enrolled in MCC. The PSA score used in this analysis was based on items from the MCC assessment originally intended to identify service need and not validated or originally intended to estimate risk of unsuppressed VL. Still, including the PSA score made it possible to account for psychosocial factors in our models, and there were noted differences in the VS percentages between PSA archetypes (low, moderate, high). Lastly, tobacco use was not assessed at MCC enrollment and therefore could not be factored into the PSA score and analyses.
Future research is needed to integrate intersectional and syndemic frameworks to ascertain the impact of intersectional experiences (e.g., race and gender-based stigma) on comorbid health conditions among PWH in coordinated HIV care programs [40]. Integrating these frameworks together can help us understand whole person needs and interrelated health inequities, advance patient-centered care models, inform training of clinical and public health staff, and develop strategies to earn rapport with minority PWH.
Supplementary Material
References
- 1.Garland WH, Oksuzyan S, Mejia M, Kulkarni S. Medical Care Coordination Services for Persons Living with HIV in Los Angeles County: A Robust Strategy to Strengthen the HIV Care Continuum. In. Los Angeles, CA: Division of HIV and STD Programs, Los Angeles County Department of Public Health; 2017. [Google Scholar]
- 2.Division of HIV and STD Programs. HIV/AIDS Medical Care Coordination Service Guidelines. In. Los Angeles, CA: Los Angeles County Department of Public Health; 2018. [Google Scholar]
- 3.Li MJ, Su E, Garland WH, Oksuzyan S, Lee S-J, Kao UH, et al. Trajectories of Viral Suppression in People Living With HIV Receiving Coordinated Care: Differences by Comorbidities. JAIDS Journal of Acquired Immune Deficiency Syndromes 2020; 84(4):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson MM, Penrose K, Irvine MK, Robbins RS, Kulkarni S, Braunstein SL, et al. Impact of an HIV Care Coordination Program on Durable Viral Suppression. JAIDS J Acquired Immune Defic Syndromes 2019; 80(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimmel AD, Masiano SP, Bono RS, Martin EG, Belgrave FZ, Adimora AA, et al. Structural barriers to comprehensive, coordinated HIV care: geographic accessibility in the US South. AIDS Care 2018; 30(11):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Division of HIV and STD Programs. HIV Surveillance Annual Report, 2020. Los Angeles, CA: Los Angeles County Department of Public Health; 2021. [Google Scholar]
- 7.Jones CP. Invited Commentary: “Race,” Racism, and the Practice of Epidemiology. Am J Epidemiol 2001; 154(4):299–304. [DOI] [PubMed] [Google Scholar]
- 8.Doshi RK, Bowleg L, Blankenship KM. Tying structural racism to human immunodeficiency virus viral suppression. Clin Infect Dis 2021. [DOI] [PubMed] [Google Scholar]
- 9.Bailey ZD, Feldman JM, Bassett MT. How structural racism works—racist policies as a root cause of US racial health inequities. In: Mass Medical Soc; 2021. pp. 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson LE. Awakening to an Intersectional Reality: Ending the HIV Epidemic in the USA Starts with Reducing Inequities among Black MSM. J Urban Health 2020; 97(5):589–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passaro RC, Pandhare J, Qian H-Z, Dash C. The complex interaction between methamphetamine abuse and HIV-1 pathogenesis. J Neuroimmune Pharmacol 2015; 10(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MJ, Richter EI, Okafor CN, Kalmin MM, Dalvie S, Takada S, et al. Social Genomics of Methamphetamine Use, HIV Viral Load, and Social Adversity. Ann Behav Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li MJ, Su E, Garland WH, Oksuzyan S, Lee S-J, Kao UH, et al. Trajectories of viral suppression in people living with HIV receiving coordinated care: Differences by comorbidities. J Acquir Immune Defic Syndr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim S, Nash D, Hollod L, Harris TG, Lennon MC, Thorpe LE. Influence of jail incarceration and homelessness patterns on engagement in HIV care and HIV viral suppression among New York City adults living with HIV/AIDS. PLoS ONE 2015; 10(11):e0141912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feller DJ, Agins BD. Understanding determinants of racial and ethnic disparities in viral load suppression: a data mining approach. Journal of the International Association of Providers of AIDS Care (JIAPAC) 2017; 16(1):23–29. [DOI] [PubMed] [Google Scholar]
- 16.Division of HIV and STD Programs. Guidelines for the Provision of HIV/AIDS Medical Care Coordination Services in Los Angeles County. In. Los Angeles, CA: Los Angeles County Department of Public Health; 2017. pp. 77. [Google Scholar]
- 17.Division of HIV and STD Programs. Medical Care Coordination (MCC) Assessment. In. Los Angeles, CA: Los Angeles County Department of Public Health; 2020. [Google Scholar]
- 18.Eisinger RW, Dieffenbach CW, Fauci AS. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA 2019; 321(5):451–452. [DOI] [PubMed] [Google Scholar]
- 19.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic: A Plan for the United States. JAMA 2019; 321(9):844–845. [DOI] [PubMed] [Google Scholar]
- 20.Robert CP, Casella G. Monte Carlo Statistical Methods. 2 ed. New York, NY: Springer; 2010. [Google Scholar]
- 21.Stan Development Team. Stan modeling language users guide and reference manual - Version 2.29. In. New York, NY: Stan Development Team; 2022. [Google Scholar]
- 22.Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. Journal of statistical software 2017; 80:1–28. [Google Scholar]
- 23.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. In. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 24.Gelman A, Hwang J, Vehtari A. Understanding predictive information criteria for Bayesian models. Statistics and computing 2014; 24(6):997–1016. [Google Scholar]
- 25.Wood SN, Scheipl F, Faraway JJ. Straightforward intermediate rank tensor product smoothing in mixed models. Statistics and Computing 2013; 23(3):341–360. [Google Scholar]
- 26.Lacombe-Duncan A An Intersectional Perspective on Access to HIV-Related Healthcare for Transgender Women. Transgender Health 2016; 1(1):137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernando V, Alvárez-del Arco D, Alejos B, Monge S, Amato-Gauci AJ, Noori T, et al. HIV infection in migrant populations in the European Union and European Economic Area in 2007–2012: an epidemic on the move. JAIDS J Acquired Immune Defic Syndromes 2015; 70(2):204–211. [DOI] [PubMed] [Google Scholar]
- 28.Poteat TC, Keatley J, Wilcher R, Schwenke C. Evidence for action: a call for the global HIV response to address the needs of transgender populations. Journal of the International AIDS Society 2016; 19(3Suppl 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beyrer C, Baral SD, Collins C, Richardson ET, Sullivan PS, Sanchez J, et al. The global response to HIV in men who have sex with men. The Lancet 2016; 388(10040):198–206. [DOI] [PubMed] [Google Scholar]
- 30.Boyd CM, Lucas GM. Patient-centered care for people living with multimorbidity. Curr Opin HIV AIDS 2014; 9(4):419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busse R, Stahl J. Integrated care experiences and outcomes in Germany, the Netherlands, and England. Health Affairs 2014; 33(9):1549–1558. [DOI] [PubMed] [Google Scholar]
- 32.Winter S, Diamond M, Green J, Karasic D, Reed T, Whittle S, et al. Transgender people: health at the margins of society. The Lancet 2016; 388(10042):390–400. [DOI] [PubMed] [Google Scholar]
- 33.Eaton LA, Driffin DD, Kegler C, Smith H, Conway-Washington C, White D, et al. The role of stigma and medical mistrust in the routine health care engagement of black men who have sex with men. Am J Public Health 2015; 105(2):e75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsan M, Wanamaker M. TUSKEGEE AND THE HEALTH OF BLACK MEN. Q J Econ 2018; 133(1):407–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thrasher AD, Earp JA, Golin CE, Zimmer CR. Discrimination, distrust, and racial/ethnic disparities in antiretroviral therapy adherence among a national sample of HIV-infected patients. J Acquir Immune Defic Syndr 2008; 49(1):84–93. [DOI] [PubMed] [Google Scholar]
- 36.Galvan FH, Bogart LM, Klein DJ, Wagner GJ, Chen Y-T. Medical mistrust as a key mediator in the association between perceived discrimination and adherence to antiretroviral therapy among HIV-positive Latino men. J Behav Med 2017; 40(5):784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beach MC, Saha S, Korthuis PT, Sharp V, Cohn J, Wilson IB, et al. Patient–Provider Communication Differs for Black Compared to White HIV-Infected Patients. AIDS Behav 2011; 15(4):805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogart LM, Takada S, Cunningham WE. Medical Mistrust, Discrimination, and the Domestic HIV Epidemic. In: HIV in US Communities of Color. Ojikutu VES Bisola O. (editor). New York, NY: Springer; 2020. pp. 207–231. [Google Scholar]
- 39.Bogart LM, Barreras JL, Gonzalez A, Klein DJ, Marsh T, Agniel D, et al. Pilot Randomized Controlled Trial of an Intervention to Improve Coping with Intersectional Stigma and Medication Adherence Among HIV-Positive Latinx Sexual Minority Men. AIDS Behav 2021; 25(6):1647–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith LR, Patel VV, Tsai AC, Mittal ML, Quinn K, Earnshaw VA, et al. Integrating Intersectional and Syndemic Frameworks for Ending the US HIV Epidemic. Am J Public Health 2022; 112(S4):S340–S343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


