Abstract
The second year of life is a time when social communication skills typically develop, but this growth may be slower in toddlers with language delay. In the current study, we examined how brain functional connectivity is related to social communication abilities in a sample of 12–24 month-old toddlers including those with typical development (TD) and those with language delays (LD). We used an a-priori, seed-based approach to identify regions forming a functional network with the left posterior superior temporal cortex (LpSTC), a region associated with language and social communication in older children and adults. Social communication and language abilities were assessed using the Communication and Symbolic Behavior Scales (CSBS) and Mullen Scales of Early Learning. We found a significant association between concurrent CSBS scores and functional connectivity between the LpSTC and the right posterior superior temporal cortex (RpSTC), with greater connectivity between these regions associated with better social communication abilities. However, functional connectivity was not related to rate of change or language outcomes at 36 months of age. These data suggest an early marker of low communication abilities may be decreased connectivity between the left and right pSTC. Future longitudinal studies should test whether this neurobiological feature is predictive of later social or communication impairments.
Keywords: functional MRI, toddler, social communication, language, superior temporal sulcus, functional connectivity
1. Introduction
Social communication abilities, such as gestures, eye contact, and joint attention, are important aspects of prelinguistic communication that facilitate language acquisition in the first years of life. Prior to the use of words, toddlers respond to and initiate bids for joint attention with caregivers to share interest about an object or event (Bakeman & Adamson, 1984; Corkum & Moore, 1995; Tomasello, 1995). These behaviors are an important part of a toddler’s communicative repertoire, but also provide scaffolding for the emergence of spoken language (Mundy & Newell, 2007). Toddlers also use gestures before they use words, and then integrate and expand gesture use as their vocabulary increases in the second year of life (Blake, McConnell, Horton, & Benson, 1992; M. Carpenter, Nagell, Tomasello, Butterworth, & Moore, 1998; R. L. Carpenter, Mastergeorge, & Coggins, 1983).
Young children with language or other developmental delays may also exhibit delays or differences in prelinguistic social communication behaviors in the toddler years (Estes et al., 2015; Manwaring et al., 2019). For example, joint attention behaviors are reduced in toddlers later diagnosed with autism spectrum disorder (ASD) and predictive of both autism severity and delayed language development (Bottema-Beutel, 2016; Franchini et al., 2019; Mundy, Sullivan, & Mastergeorge, 2009). An important aspect of understanding the neurobiology of language impairments therefore involves identifying neural patterns associated with these early social communication behaviors during infancy and toddlerhood.
In adults, processes involved in social communication, including language, gesture, and joint attention, have neural bases that engage both shared and distinct regions and networks (Redcay & Saxe, 2013; Redcay, Velnoskey, & Rowe, 2016). Production and comprehension of spoken language rely on a distributed network including posterior temporal cortex and left inferior frontal gyrus (Basilakos, Smith, Fillmore, Fridriksson, & Fedorenko, 2018; Fedorenko, Nieto-Castañón, & Kanwisher, 2012; Hickok & Poeppel, 2007; Poeppel, Emmorey, Hickok, & Pylkkänen, 2012). The posterior superior temporal cortex (pSTC, Redcay et al., 2016; Willems, Ozyürek, & Hagoort, 2007), which includes the posterior superior temporal gyrus (pSTG), posterior superior temporal sulcus (pSTS), and portions of the temporo-parietal junction (TPJ), is engaged during the perception of nonverbal social communication signals such as gaze, gesture, and joint attention. The left pSTS is specifically engaged when both initiating and responding to joint attention (Redcay, Kleiner, & Saxe, 2012). The pSTS is also a part of the neural mirroring network, which is thought to be central to production and comprehension of gestures and encompasses ventral inferior frontal and posterior parietal regions with inputs to the region from bilateral pSTS (Mainieri, Heim, Straube, Binkofski, & Kircher, 2013; Schippers, Roebroeck, Renken, Nanetti, & Keysers, 2010). The pSTC has therefore been proposed to be a key integrative region between networks supporting language and social communication (Redcay, 2008; Yang, Rosenblau, Keifer, & Pelphrey, 2015).
Functional connectivity analyses of fMRI data investigate coherent fluctuations between regions of the brain and have been widely used to provide information on the role of a region from a network perspective (Lurie et al., 2020; van den Heuvel & Hulshoff Pol, 2010). Thus far, functional connectivity studies during both rest and social tasks support the potential role of the pSTC as a nexus for integration among functional networks associated with action perception and production, social cognition, and language (Carter & Huettel, 2013; Lahnakoski et al., 2012; Yang et al., 2015). For example, joint attention tasks increase functional connectivity between the pSTS and other social-cognitive regions (Redcay et al., 2012). Structural connectivity between the pSTC and regions associated with language and social cognition is also seen. For example, anatomical white matter tracts between these regions are thought to support semantics, syntax, pragmatics, and communicative intentions, respectively. Further, functional connectivity between pSTC (specifically temporoparietal regions) and inferior frontal regions is associated with social cognition (Grosse Wiesmann, Schreiber, Singer, Steinbeis, & Friederici, 2017; Wang, Metoki, Alm, & Olson, 2018). Thus, in adults, the pSTC (and specifically, the pSTS) demonstrates an important functional and structural role in social communication abilities of language, gesture, and joint attention, through both activation during these behaviors and connectivity to associated brain regions and networks.
Relatively less is known about how these neurobiological substrates of social communication develop, especially during the toddler years (Ilyka, Johnson, & Lloyd-Fox, 2021). From infant studies, we know that perception of communicative gestures engages a similar neurobiologic response in infants and adults. For example, similar to adults, 8- and 13-month-old infants who viewed others’ pointing that was incongruent compared to congruent with a target, demonstrated a P400 component over posterior superior temporal regions (Melinder, Konijnenberg, Hermansen, Daum, & Gredebäck, 2015). Infants, like adults also engage a dorsal medial prefrontal region when viewing bids for joint attention (Grossmann & Johnson, 2010; Grossmann, Lloyd-Fox, & Johnson, 2013; Schilbach et al., 2010). Posterior temporal regions and medial prefrontal regions also respond to other social communication stimuli such as communicative facial gestures in infants (Grossmann et al., 2008).
These posterior temporal regions also play a role in speech perception in toddlers. For example, a magnetoencephalography (MEG) study of 12- to 18-month-old infants demonstrated adult-like N400 responses to words that were incongruous with a picture compared to congruous, and these N400 responses were localized to temporal and frontal regions, similar to adults (Travis et al., 2011). Neuroimaging studies of speech processing during natural sleep have identified patterns of distributed activation at one year to more focal activation within superior temporal cortex between one and 3 years of age (Redcay, Haist, & Courchesne, 2008). Although limited, these data suggest similar regions are engaged in processing social and communicative stimuli in toddlers and adults, and these networks may undergo significant development in the toddler years. However, these studies did not examine how connectivity within social communication networks change with age and are related to social communication abilities beyond auditory speech processing.
One reason for these limited data is the difficulty in acquiring fMRI data with minimal motion artifact in toddlers. Resting-state functional connectivity MRI (rs-fcmri) provides a tool to examine functional network connectivity in infants and toddlers with relatively good spatial resolution (Cusack, Ball, Smyser, & Dehaene-Lambertz, 2016; Graham, Pfeifer, Fisher, Lin, et al., 2015; Redcay, Kennedy, & Courchesne, 2007). Functional networks at rest are thought to reflect the intrinsic functional organization of the brain. These intrinsic networks show striking overlap with co-activations and correspond to networks engaged by functional tasks (Smith et al., 2009). Although this large-scale network organization is present at birth or earlier (Cusack et al., 2016; Doria et al., 2010; Fransson et al., 2007) and persists during sleep or sedation (Fukunaga et al., 2006), individual differences in the strength of these connections and in network properties reflect individual differences in behavior (e.g., Finn et al., 2016; Vatansever et al., 2017; Xiao, Geng, Riggins, Chen, & Redcay, 2019). Between birth and 2 years of age, functional connectivity increases for long-range connections within functional networks and decreases elsewhere. These changes are most dramatic in the first year of life and become more stable in the second (Gao, Lin, Grewen, & Gilmore, 2017; Gao et al., 2004, 2009; Huang et al., 2013).
Many rs-fcmri studies of infants and toddlers have been limited to questions of age-related change in network organization, but the rs-fcmri method has also been used in combination with behavioral measures to examine the relation between networks and behavioral and cognitive outcomes in infants and toddlers (Alcauter et al., 2014; Ball et al., 2015; Bruchhage, Ngo, Schneider, D’Sa, & Deoni, 2020; Chen et al., 2021; Eggebrecht et al., 2017; Graham, Pfeifer, Fisher, Carpenter, & Fair, 2015; Graham, Pfeifer, Fisher, Lin, et al., 2015; Marrus et al., 2018). Of these, one study investigates the relation between functional connectivity and social communication behaviors and two studies investigate relations with language; Eggebrecht and colleagues used a data-driven approach to examine relations between large-scale brain networks and initiating joint attention (IJA) behaviors in a mix of infants at 12 or 24 months of age at high or low likelihood for developing ASD. They showed a differential relation between functional connectivity and joint attention abilities by age, such that different networks were correlated with joint attention at 12 months than at 24 months. In addition, they showed that the relation between connectivity and outcome depends on the network used. Specifically, greater connectivity between the visual network and posterior cingulate portion of the default mode network was associated with better joint attention, whereas greater connectivity between the visual network and dorsal attention network was associated with fewer instances of joint attention. Chen and colleagues investigated network homogeneity, which captures degree of local specialization of networks, and showed that in the toddler years, increased local specialization of the language network was associated with better language scores on the Mullen Scales (Chen et al., 2021). Bruchlage and colleagues showed that receptive and expressive language ability show overlapping but also independent associations with use of specific networks, and that the number of networks associated with language measures increased with age. These studies emphasize the important role of functional connectivity in the emergence of social communication and language skills in the toddler years. However, they do not investigate whether features of an individual child’s functional connectivity relate to later outcomes. Whether connectivity predicts outcomes is an essential next question, especially given the scaffolding effects of early social communication skills on spoken language outcomes in infants and toddlers (Doi, 2020). Understanding the relation between brain regions associated with social communication abilities and later language abilities is an important next step in the quest to understand mechanisms of language delay as well as biomarkers for specific outcomes.
In the current study, we investigated network connectivity in toddlers with language delay, including its relation with later language abilities, by analyzing rs-fcmri data from a combined sample of toddlers with typical development and language delay. As part of a larger study, toddlers participated in an MRI scan between 12 and 25 months of age and completed measures of social communication and language at 3–4 timepoints through 36 months of age. This longitudinal approach allowed us to examine how brain connectivity in the second year of life (Time 1) is related to both 1) concurrent measures of social communication as well as 2) language outcomes at 36 months of age. Measurement of these two different constructs allowed us to query the longitudinal relation between early social communication and later language abilities. Groups were combined to leverage maximum variability in language abilities and outcomes. We hypothesized that a) an a-priori selected seed region in the left pSTC would show connectivity to brain regions supporting language and social communication, and b) that this network would be related to concurrent social communication functioning. This hypothesis was based on the principle that resting-state networks reflect a history of co-activation and emergence of specialization (Johnson, 2011; Smith et al., 2009). Finally, given the role of social communication in scaffolding language development, we c) predicted that integrity of those network connections would predict better language outcomes.
2. Method
2.1. Participants
The data presented here were collected as part of a larger longitudinal study on language development in typically developing children and children with early language delays. The Institutional Review Board at the National Institutes of Health approved this study. Toddlers were initially evaluated at 12 or 18 months of age (+/− 3 months); follow-up visits to collect behavioral data were conducted at 18, 24, and 36 months. Toddlers were excluded if they were born preterm (<36 weeks), lived in households where English was not the primary language, or had a known genetic disorder or significant medical or motor impairment. Children were recruited to the Language Delay (LD) group if they had receptive and expressive language scores in the Very Low range (T scores ≤ 30) based on the Mullen Scales of Early Learning (MSEL, Mullen, 1995) at the time of initial evaluation (Time 1, 12–18 months). Typically developing (TD) toddlers had MSEL scores on all domains within normal limits (no more than 1.5 SDs below the mean).
An MRI scan was collected as part of the Time 1 (12–18 months) and was also attempted at the 24-month visit. A total of 62 toddlers were enrolled in the study in total, with 55 participating in an MRI visit. Of those 33 completed both a resting-state and a requisite structural scan at either Time 1 or the 24 months visit; therefore, all scans between 12–24 months were compiled in order to increase the sample size. Whereas a small subset of toddlers (n=9) completed a resting-state fMRI scan at 36 months, this study is focused on longitudinal prediction of language at 36 months and thus that data is excluded here. Of the 33 toddlers with MRI data between 12–24 months of age, one was excluded due to high levels of motion (see threshold for head motion in fMRI preprocessing section). The final sample consisted of 32 toddlers (12 recruited to the LD group and 20 recruited to the TD group) between 12–25 months of age at scan time. All of the LD toddlers in the current sample began the study at the 18-month visit. Of the 12 LD toddlers, 4 received a diagnosis of ASD at 36 months. See Table 1 for participant details. Each toddler had behavioral measures collected within 3 months (mean 1.7 (.85) months, range .27–3.5 months) of the MRI scan. The behavioral assessment closest to the time of the scan was chosen for the Time 1 analyses.
Table 1.
Participant Descriptives
| Combined Sample | Typically Developing | Language Delay | Statistic | p-value | |
|---|---|---|---|---|---|
| n | 32 | 20 | 12 | ||
| Scan age (months) | 18.70 (3.2) | 17.75 (3.5) | 20.25 (2.2) | t(30)=−2.5 | .019 |
| Sex M:F | 22:10 | 13:7 | 9:3 | Χ2(1,N=32)=.06 | .8 |
| Race White:Nonwhite | 22:10 | 16:4 | 6:6 | Χ2(1,N=32)=3.14 | .08 |
| Ethnicity Hispanic: Non | 4:28 | 2:18 | 2:10 | Χ2(1,N=32)=.30 | .58 |
| Motion (mean FD) | .06 (.03) | .06 (.04) | .06 (.03) | t(30)=.27 | .78 |
| Nonverbal DQ | 105.56 (19.9) | 115.64 (15) | 88.77 (15.3) | t(30)=4.9 | <.001 |
| Verbal DQ | 80.35 (28.6) | 100.7 (10.7) | 46.44 (9.9) | t(30)=14.3 | <.001 |
Note: Both Nonverbal and Verbal DQ calculated per Henry et al. (2018).
2.2. Behavioral assessments
The Communication and Symbolic Behavior Scales Developmental Profile – Behavior Sample (CSBS) is an observational assessment of early social communication, in which the child is presented with a series of temptations designed to elicit communication. The CSBS (Wetherby & Prizant, 2002) is comprised of three domains: Social, Speech, and Symbolic. The Social domain includes emotion and eye gaze, gestures, and communication (e.g., communicative rate, joint attention). The Speech domain includes the use of words and sounds with communicative intent. The Symbolic domain captures understanding of words and use of objects in a play context. Here, we evaluated only the Total as well as Social and Speech domains given that these best capture social communication acts, specifically the use of gestures, gaze, and words in a social communication context. CSBS data were collected at 6 month intervals through the 24 month visit (i.e., 12, 18, and 24 month timepoints). The CSBS is designed to evaluate social communication of children whose developmental level of functioning is ≤24 months and is normed through 24 months. Weighted raw scores, which were developed to adjust for differences in the ranges of possible scores across items on the CSBS, were used to best capitalize on variability across the sample. For the time 1 analyses, the CSBS collected closest to the date of scan was used.
The MSEL, collected at 12-, 18-, 24-, and 36-month visits, measures fine motor, visual reception, receptive language, and expressive language, and is used in the community for determining eligibility for early intervention, as well as for research. The fine motor scale evaluates the use of hands and fingers and manual dexterity. The visual reception domain assesses visual perceptual abilities. The receptive language scale assesses response to verbal directions, memory for general information and commands, and auditory concepts. The expressive language scale assesses verbal responses to tasks and vocalizations and utterances. Developmental Quotients (DQs), including Nonverbal (NVDQ) and Verbal (VDQ), are used to describe the sample (Henry, Farmer, Manwaring, Swineford, & Thurm, 2018). We used the Age Equivalent scores for both the Receptive and Expressive language scales at 36 months as an outcome measure, to reduce floor effects.
Toddlers with LD and TD differed significantly in their VDQ, NVDQ, and age at time of scan completion (See Table 1). Groups also differed significantly on their CSBS Total, Social, and Speech composite scores (Total: t(30)=−4.6, p<.001; Social: t(30)=−4.3, p<.001; Speech t(30)=−4.01, p<.001, see Figure 1s in Supplemental Materials ). Groups did not differ on overall motion during the scan.
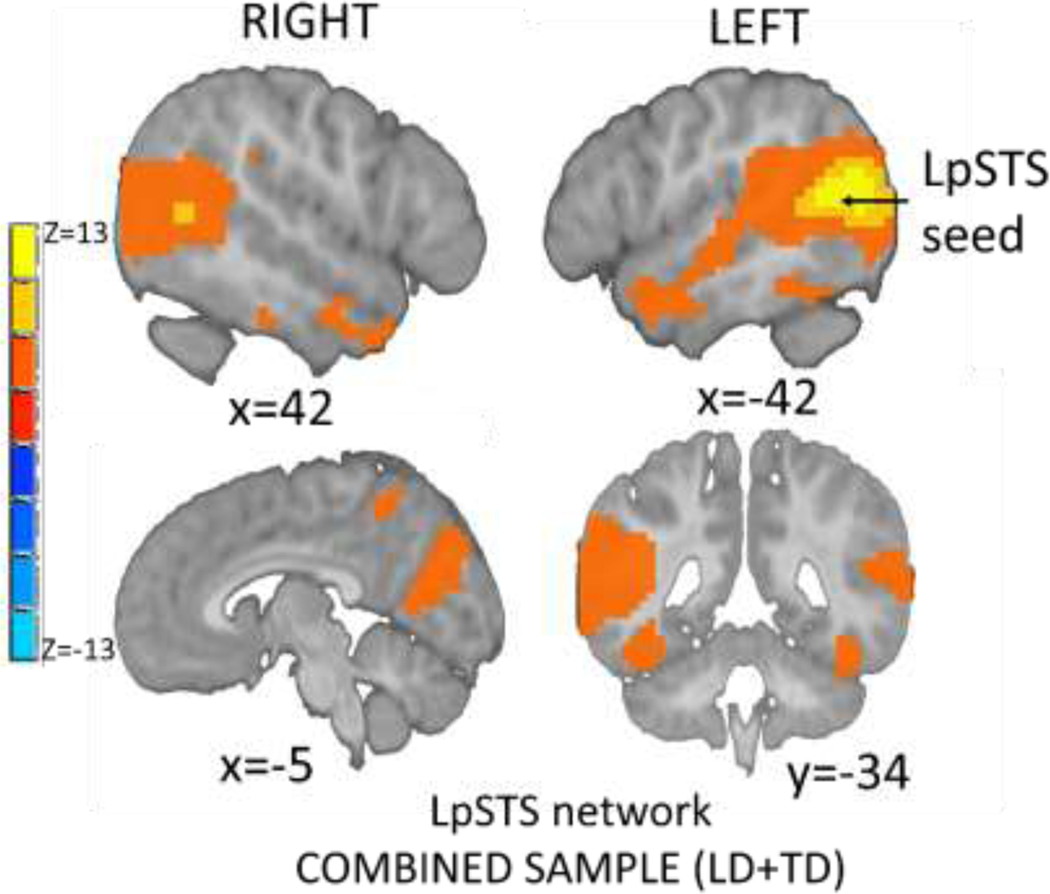
Figure 1. The LpSTC network.
Note: Regions demonstrating significant connectivity with the LpSTC seed are displayed on a pediatric template brain in MNI space (p <.05, corrected).
2.3. MRI data acquisition
MRI data were collected with a 32-channel coil on a GE 3.0-T scanner. All data were collected during natural sleep without sedation at the National Institute of Mental Health functional MRI facility. Whole-brain resting-state fMRI data were collected using a T2*-weighted gradient-echo echo-planner imaging sequence (TR 2 s, TE 30 ms, slice thickness 3 mm with 3 mm gap, voxel size 1.5 mm × 1.5 mm × 3.0 mm, voxel matrix 128 × 128 mm, flip angle 65°, field of view 192 mm, 36 slices, 240 volumes). As these data were considered a pilot study some adjustments to the functional MRI protocol were made as the study was ongoing. Specifically, for five scans, an additional 5 volumes (245 volumes) were collected. For another six, only 180 volumes were collected. The number of volumes collected did not vary by group (χ2 (2, 32) = .68, p>.05). For one participant, the TR was adjusted to 2.4 to provide greater whole-brain coverage (no. volumes = 240, duration = 9.6 minutes). Resting-state images were collected between 32 to 160 minutes after the toddler fell asleep (mean=98.2 minutes, SD=39 minutes). The following high-resolution structural images were acquired with a T1-weighted magnetization prepared rapid gradient echo sequence: TR 2 s; TE 30 ms; voxel size 1×0.75×0.75 mm; matrix 256×256 mm; flip angle 65°; field of view 230 mm.
2.4. fMRI preprocessing
Prior to preprocessing, the first 4 volumes were discarded for signal equilibration. The remaining images were preprocessed using DPABI (a toolbox for Data Processing & Analysis for Brain Imaging, version 2.3, Yan, Wang, Zuo, & Zang, 2016), which is based on Statistical Parametric Mapping (SPM12) (http://www.fil.ion.ucl.ac.uk/spm) and Resting-State fMRI Data Analysis Toolkit (REST) (Song et al., 2011, http://www.restfmri.net ). Slice timing was conducted by shifting slices from the rest of the signal time points to match the slice at the midpoint of each TR. The functional images obtained from the same participant were then realigned using a least squares approach and a 6 parameter (rigid body) spatial transformation. Both functional and T1 images were manually reoriented to adjust the position when needed for a better co-registration, and then the T1 image was realigned to the mean functional image of each participant. Spatial normalization were performed via the uniformed segmentation approach (Ashburner & Friston, 2005): 1) T1 images were segmented into white matter (WM), grey matter (GM), and cerebral spinal fluid (CSF) based on the age-specific atlas for the age range of 17–21 months (Fonov et al., 2011; http://www.bic.mni.mcgill.ca/ServicesAtlases/NIHPD-obj2); 2) T1 images were nonlinearly warped to the given atlas space. This atlas was selected because it was the one with an age range that included the largest number of participants in the sample. The resulting warp parameters were applied to functional images, and the functional images were resampled into 3*3*3 mm3. All functional images were smoothed with a 5-mm full-width-at-half-maximum Gaussian kernel. Nuisance covariates, including Friston 24-motion parameters (6 head motion parameters, 6 head motions one time point before, and the 12 corresponding squared items) (Friston, Williams, Howard, Frackowiak, & Turner, 1996) and the first 5 principal components extracted from subject-specific WM and CSF tissues using a component based noise correction method (CompCor) (Behzadi, Restom, Liau, & Liu, 2007), were regressed out. Finally, bandpass filtering (0.01–0.1 Hz) was applied to the data.
We calculated the framewise displacement (FD) following Jenkinson et al. (2002) to quantify the head motion of each volume. We used a threshold of mean FD ≤ 0.2 mm, with one participant excluded for excessive head motion (mean FD = 0.31 mm). The average mean FD for the remaining 32 participants was 0.06 ± 0.03 mm. There were no correlations between age and mean FD (r(30) = −.12, p = .52). Nevertheless, mean FD was included in the regression analyses to control for its potential effect.
2.5. Functional connectivity MRI analysis
LpSTC region of interest (ROI).
In order to maximize power in this small but valuable sample, we used an a-priori seed-based functional connectivity analysis approach, which was implemented using the functions in DPABI version 2.3 (Yan et al., 2016, described below). Given the support in the literature for the role of left superior temporal cortex in social communication abilities (Redcay, 2008), we thus selected a seed a-priori from which functional connectivity analyses were conducted. The specific seed (MNI coordinates: −50 −66 14) was chosen from a study of joint attention in adults (Redcay et al., 2012). Joint attention was chosen as it is a core social communication ability and related to language development. Although there are limitations to using a seed identified in an adult sample, there are not available functionally defined ROIs within the age group of the current sample. We also chose a functionally defined seed due to concerns with anatomically-defined seeds (i.e., the STS anatomical ROI is large and has multiple functional sub-divisions).
As the pediatric template used in the current study is smaller than the standard MNI-152 template, the MNI coordinates were scaled down: 0.8197528 in x direction, 0.809651 in y direction, and 0.7772786 in z direction (http://www.bic.mni.mcgill.ca/ServicesAtlases/NIHPD-obj2). The mean time series of the LpSTC were computed across subjects within a 6-mm-radius sphere centered around the LpSTC (pediatric coordinates: −41, −53, 11), and then connectivity between the time series of the seed region and those of the whole brain was calculated to generate the individual resting state functional connectivity map (r-map). Subsequently, we used Fisher’s r-to-z transformation to convert r-maps into z-maps to obtain normally distributed values of the connectivity values. The coordinates were then transformed back to MNI space by using the following scaling: 1.21988 in x direction, 1.23510 in y direction and 1.28654 in z direction (http://www.bic.mni.mcgill.ca/ServicesAtlases/NIHPD-obj2). We also conducted an exploratory analysis with the RpSTC seed (MNI coordinate: 50, −56, 10) identified in Redcay et al., (2012) following the same procedures as the LpSTC seed. No significant relations with RpSTC connectivity and concurrent CSBS scores were found.
2.6. Statistical analyses
We divided the analyses into Time 1 analyses (concurrent) and longitudinal analyses (predictive). Time 1 analyses involved first identifying the LpSTC network using the LpSTC region as a seed in a whole-brain analysis. This network was then used as a mask in the individual difference analysis so we could identify whether connectivity between regions of the LpSTC network was related to concurrent CSBS scores. We also used a whole-brain (unmasked) approach to allow for identification of regions beyond the LpSTC network where LpSTC connectivity was related to individual differences in CSBS scores. The longitudinal analyses determined the relation between the voxel-wise connectivity values (i.e., z values) with the LpSTC region that were related to CSBS at Time 1 as a predictor of both language trajectory (i.e., rate of change, (Age Equivalent1-Age Equivalent2)/(age1-age2)) and outcome (36 months) on the MSEL.
2.6.1. Time 1: MRI & associations with CSBS
2.6.1.1. Whole-brain analyses.
We conducted a whole-brain t-test using AFNI’s 3dttest++ to identify the LpSTC network (i.e., regions correlated with activity in the seed region), while controlling for age at scan, sex, and motion (mean FD). The resulting map was subsequently used as a mask to identify correlations within the “LpSTC network” (i.e., the mean LpSTC connectivity map in the combined sample). To examine individual differences in social communication abilities in this sample enriched for language variability, we conducted whole-brain regressions to examine the relation between LpSTC connectivity and CSBS scores (Total, and Social) in this combined sample of TD and LD toddlers. Note that the CSBS Total and Social scores showed a normal distribution across the combined sample whereas CSBS Speech did not (Shapiro-Wilk test for Total: W(32) =.961, p=.23; for Social: W(32)= .956, p=.202). Therefore, we removed the CSBS speech scores from Time 1 analyses. We did this both for whole-brain connectivity (unmasked) and within the LpSTC network (masked). Here, we controlled for age at scan, motion, sex, and number of MRI volumes collected (i.e., square root of TR). We did not include group as a covariate given that group was highly collinear with CSBS scores and age.
2.6.1.2. Multiple comparison corrections.
All whole-brain correlation maps were transformed into Z maps and corrected for family-wise error (FWE) rate through Monte Carlo simulations using 3dClustSim program in AFNI (Cox, 1996). This spatial cluster correction takes into account spatial autocorrelation by using the ‘–acf’ option in 3dClustSim (Cox et al., 2017). To identify the LpSTS network we used a liberal voxel-wise threshold of p = .005 which revealed a minimum cluster size of 135 for an overall alpha of p < .05. For the individual difference analyses we used a standard threshold of p=.001, resulting in a minimum cluster size of 41 voxels for the whole-brain (unmasked) analysis and 20 voxels for analyses constrained to the LpSTC network (i.e., masked).
2.6.2. Longitudinal analyses: MRI measures predicting MSEL
Longitudinal statistical analyses were conducted with R and SAS/STAT Version 9.3. In the exploratory data analysis stage, we used a Shapiro-Wilks test for normality to examine the distribution of potential language outcome measures (i.e., MSEL Expressive and Receptive Language Age Equivalents) across the full sample. Due to non-normality (right-skew) within the Expressive Language measures and given the high correlation between Expressive and Receptive domains we chose to focus only on the MSEL Receptive Language Age Equivalent.
Regions for which connectivity with LpSTC was related to CSBS scores (including CSBS Total and Social scores) at the time of scan were selected for the secondary analysis to determine whether connectivity in these regions was related to the rate of change over time or endpoint score (36 months) in the MSEL Receptive Language Age Equivalent. Note that we did not examine regions in which pSTC connectivity was related to CSBS Social scores because no region was significant after correction for multiple comparisons in the T1 analysis. Relations of CSBS Total and Social scores with pSTC connectivity identified the same RpSTC region and thus a single ROI was created at that peak coordinate (5 mm radius). This smaller radius was chosen over 6 mm due to the region’s proximity to white matter and wanting to minimize inclusion of non-neuronal noise within the estimate.
To account for clustering of observations within subject, a generalized (hierarchical) linear model with random subject-level intercept and time effects was used. The fixed effect of time (beginning at time 1 and continuing through outcome) was entered as continuous age in months, centered at 36 months, which was the relative endpoint. The model also included a three-way interaction between time, the fixed effects for group, and LpSTC-to-RpSTC connectivity (centered at the grand mean). We added group as a fixed effect in order to control for any effects of group membership on slopes. After adding these between-subject effects, the random slope of time was zero and was subsequently excluded from the model. The denominator degrees of freedom were adjusted using the Satterthwaite correction. Model assumptions were assessed via visual inspection of the conditional residuals. The three-way-interaction of group, time, and connectivity was decomposed to obtain the effects of interest, which were: the main effect of connectivity, which represents the correlation of early connectivity with Receptive Language age equivalent at 36 months, and the connectivity-by-time interaction within each group, which represents the correlation of early connectivity with the rate of change in Receptive Language age equivalent over the observation period. In alignment with the current recommendations of the American Statistical Association, we report uncorrected p-values alongside 95% confidence intervals for the parameters of interest (Wasserstein, Schirm, & Lazar, 2019).
3. Results
3.1. Time 1 network and correlations with CSBS
3.1.1. Mean LpSTS connectivity.
To identify the LpSTC network, we tested for mean effects of LpSTC connectivity in the combined sample by identifying mean effects while controlling for age at scan, sex, and head motion. We found LpSTC connectivity with the right hemisphere homologues (i.e., RpSTC) extending into anterior temporal lobe, precuneus, and posterior cingulate cortex (Figure 1).
3.1.2. CSBS relation to LpSTC connectivity.
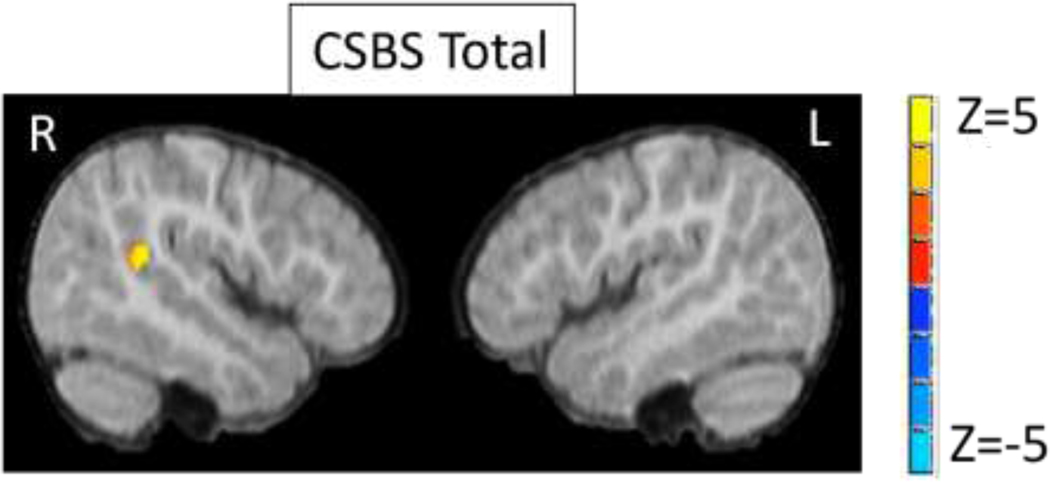
When examining connectivity within the LpSTC network (i.e., masked by the mean LpSTC connectivity), LpSTC connectivity to RpSTC, encompassing regions of STS and superior temporal gyrus, was significantly related to CSBS Total scores (Figure 2, Table 2) (p<.05 corrected within LpSTC network map). Specifically, greater connectivity between the LpSTC and RpSTC was associated with more advanced social communication abilities. No other regions reached significance for any of the CSBS measures. No relations between LpSTC connectivity and CSBS scores (Total or Social) were significant at the whole-brain level (p<.05, FWE corrected).
Figure 2. Regions showing significant correlations between LpSTC connectivity and social communication abilities on the CSBS.
Note: p<.05, corrected
Table 2.
Regions where LpSTC connectivity related to CSBS scores
| Measure | Region | MNI Coordinates | z-score | k | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| CSBS Total | RpSTC | 36 | −33 | 12 | 3.9 | 22 |
| CSBS Social | n.s. | n/a | n/a | n/a | n/a | n/a |
Note: LpSTC, left posterior superior temporal cortex. RpSTC, right posterior superior temporal cortex. Coordinates represent voxel with peak connectivity. k=cluster size.
3.2. Longitudinal analyses.
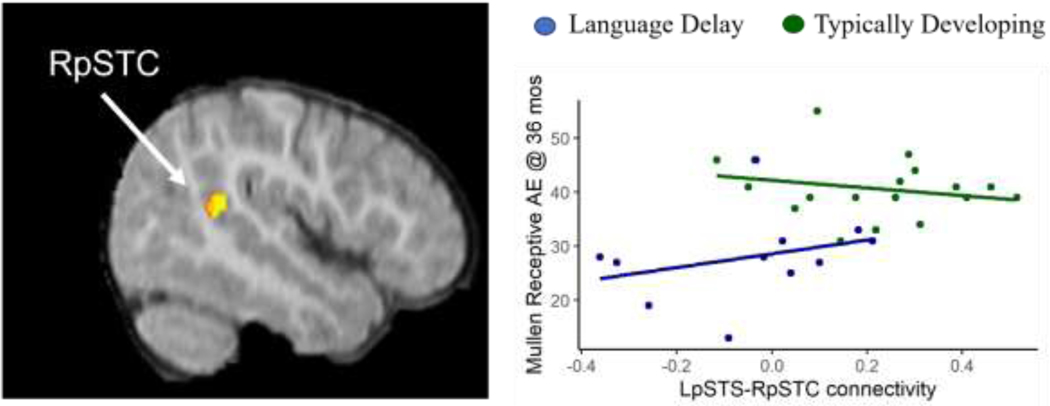
The 95% CI confidence intervals surrounding the parameters of interest in this exploratory analysis were very wide and contained zero. LpSTC-to-RpSTC connectivity was positively but not significantly related to rate of change in MSEL Receptive Language age equivalent in the LD group (B = 0.69 [−0.64, 2.01]), whereas the relationship within the TD group was evenly distributed around zero (B = 0.05 [−0.63, 0.73]) (Type III test of the difference, for the three-way interaction of group, time, and connectivity: F(28) = 0.80, p = −.38). LpSTC-to-RpSTC connectivity was positively but not significantly related to MSEL Receptive Language age equivalent at 36 months among the LD participants (B = 12.93 [−5.44, 31.41]), and negatively related among TD participants (B = −5.82 [−17.53, 5.88]) (Type III test of the difference, for the group-by-connectivity interaction: F(55) = – 3.05, p = .086) (Figure 3, Table 3).
Figure 3. No relation between LpSTC connectivity at Time 1 and longitudinal outcome measures.
LpSTS-RpSTC connectivity values are plotted against MSEL Receptive Age Equivalents. Fit lines are shown by group; note that the fit line for the combined groups is also not significant (see Table 3).
Table 3.
Selected results of mixed model predicting MSEL Receptive Language Age Equivalent at 36 months
| Parameter | Estimate | SE | 95% CI lower limit | 95% CI upper limit | DF | t | p |
|---|---|---|---|---|---|---|---|
| LpSTC-RpSTC connectivity with outcome at endpoint | 3.5797 | 5.3814 | −7.2051 | 14.3644 | 54.96 | 0.67 | 0.5087 |
| LpSTC-RpSTC connectivity with rate of change in outcome | 0.3699 | 0.3566 | −0.3611 | 1.1009 | 27.61 | 1.04 | 0.3086 |
3.3. Supplemental Analysis.
Given the limitations of an a-priori seed-based approach, we also conducted a supplemental analysis in which we used a data-driven ICA method to locate the LpSTC within the toddler data. Details on this method and results are provided in Supplementary Materials. In short, we found the same pattern, with increased LpSTS to RpSTS connectivity associated with higher CSBS Total scores. However, after correction for multiple comparisons, these correlations were not significant.
4. Discussion
In the present study, we examined how social communication abilities of toddlers related to functional connectivity with the posterior superior temporal sulcus, a region associated with social communication behaviors in adults, and whether these measures predicted language outcomes. To maximize variability in communicative abilities and outcome, we investigated these variables in a combined sample of toddlers with typical development and those with language delays. We first replicated existence of a functional network between the LpSTC and other important language regions in toddlers, then showed that functional connectivity in a specific part of this network was correlated with social communication abilities in toddlers. Finally, we showed that connectivity in this target region was not associated with later receptive language across the combined sample.
4.1. LpSTC network in toddlers
We show that in toddlers the LpSTC is functionally connected to anterior and posterior regions of the STS bilaterally, including left mid-STS. Additionally, LpSTC showed connectivity with the fusiform gyrus, precuneus, and posterior cingulate cortex. Regions of this LpSTC network are broadly associated with social perception (e.g., fusiform gyrus, bilateral superior temporal sulcus, Lahnakoski et al., 2012) and social cognition (e.g., precuneus and posterior cingulate, bilateral superior temporal sulcus, Schurz, Radua, Aichhorn, Richlan, & Perner, 2014) in adults. More specific to social communication, the STC is involved in perceiving communicative gestures and words and demonstrates functional connectivity with posterior temporal, prefrontal, and parietal regions during a joint attention task in adults (Redcay et al., 2012, 2016). Together, these data suggest continuity in the networks associated with social communication behaviors between toddlers and adults.
4.2. LpSTS to RpSTC connectivity related to social communication.
We found that variability in LpSTS to RpSTC connectivity was related to the CSBS Total scores in this sample. Given that the two groups varied on the CSBS at baseline, it is possible that bilateral posterior temporal connectivity within the LpSTC network may be picking up group differences between TD and LD rather than a neural correlate of a dimension of social communication per se. In fact, in a study of older children and adults, LpSTC connectivity at rest maximally discriminated ASD and TD groups compared to other regions (Plitt, Anne, & Martin, 2015). Although no relation was found with the CSBS social scores, we caution against interpreting differences in relations between the composites given the high correlations among these measures and the reduced variance within the social scores in TD toddlers which may have contributed to a lack of effect.
Analyzing data across groups with defined delays and those with typical development is consistent with previous studies investigating brain and behavior correlations in infants and toddlers by combining samples who are at a high or low likelihood of developing ASD (Eggebrecht et al., 2017; Marrus et al., 2018). This dimensional approach is argued to provide a better means to identify underlying mechanisms of atypical development in comparison to a categorical diagnostic approach (Insel & Cuthbert, 2010). One important distinction in this sample is that LD toddlers were recruited specifically based on language delay whereas TD toddlers were recruited for no evidence of developmental delay. This is in contrast to a standard dimensional approach (e.g., Research Domain Criteria or RDoC) in which participants are sampled broadly from the community and no exclusions are made based on diagnostic information, leading to a more continuous distribution.
Although posterior superior temporal interhemispheric connectivity has not previously been associated specifically with social communication development, previous work has demonstrated a role for temporal interhemispheric connectivity in typical and atypical language development. At birth, bilateral temporal connectivity is present when listening to speech (Perani et al., 2011). From work with older children and adults, bilateral temporal activation and connectivity is thought to support semantic and lexical processing (Bozic, Tyler, Ives, Randall, & Marslen-wilson, 2010) or integrating syntactic and prosodic computations during sentence processing (Friederici, 2011; Skeide & Friederici, 2016). This interhemispheric connectivity may play a particularly important role in language development (Friederici, 2011) and contribute to language impairments when disrupted (Northam et al., 2012). In one of the few studies to examine functional connectivity with fMRI in toddlers with ASD, Dinstein et al. (2011) demonstrated reduced interhemispheric connectivity between superior temporal and inferior frontal regions in toddlers with ASD compared to toddlers without ASD (Dinstein et al., 2011). Future work should first examine the role that this bilateral temporal connectivity plays in concurrent processing of social communication stimuli and then investigate how this pattern changes with age. One possibility is that interhemispheric connectivity may integrate linguistic or semantic information with social intentions, processes of which both rely on posterior temporal regions but may show hemispheric biases (Redcay, 2008).
4.3. Linking functional connectivity to early social communication
More broadly, these findings add to a small but growing body of literature relating functional connectivity measures in infants and toddlers to concurrent behavioral measures including motor development, cognitive development, working memory, emotionality, and joint attention (Alcauter et al., 2014; Ball et al., 2015; Bruchhage et al., 2020; Chen et al., 2021; Eggebrecht et al., 2017; Graham, Pfeifer, Fisher, Carpenter, et al., 2015; Graham, Pfeifer, Fisher, Lin, et al., 2015; Marrus et al., 2018). Most relevant to the current study is work examining how joint attention is related to network organization in toddlers (Eggebrecht et al., 2017). Eggebrecht et al. (2017) took a data-driven approach to examine how connectivity between large-scale brain networks related to joint attention abilities at 12 and 24 months in infants at high and low likelihood of developing ASD. They found broadly positive associations between anterior prefrontal networks at 24 months related to joint attention whereas a combination of positive and negative associations between visual and higher-order networks as well as somatomotor networks related to joint attention at 12 months. Most relevant to the current study is that a positive relation between visual processing regions and posterior regions of the default mode network (DMN) (encompassing the pSTS and precuneus regions in the current study) was associated with joint attention at 12 months. Interestingly, however, this relationship was specific to 12-month-olds. Eggebrecht et al.’s (2017) data-driven exploratory approach differs from our a priori hypothesis-driven approach that focuses on just a single network and can be a powerful tool to examine whole-brain organization. Data-driven approaches are better-suited to larger samples. Moving forward with both data-driven and hypothesis-driven approaches will be important in characterizing how functional network development is related to developmental changes.
4.4. Network correlates of social cognitive abilities do not predict language outcomes.
Given the role of social communication in preceding and scaffolding language development in the infant and toddler years, we predicted that the functional connectivity patterns associated with social communicative abilities would be predictive of language outcomes at 36 months. We did not find support for this hypothesis, and instead found that receptive and expressive language scores were not correlated with the strength of connections in regions associated with social communication between 12–24 months. We did see a trend supporting potentially different relations between network strength and later language outcomes by group (e.g., TD vs LD, F(55) = − 3.05, p = .086). Given our small sample size, it will be important for future studies to determine if strength of networks associated with social cognition predict language outcomes, possibly with that relationship varying by group.
4.5. Limitations
This first study to relate social communication abilities to posterior superior temporal functional connectivity in toddlers provides novel insights into functional brain development and its relation to social development. However, this study should be viewed as preliminary due to several limitations. First and foremost, the practical challenges in collecting functional connectivity MRI data from sleeping toddlers resulted in a relatively small sample to address questions of individual differences and prediction of change over time. Specifically, a larger group of LD toddlers would allow for a group comparison between LD and TD toddlers. This, in combination with our dimensional approach would have given a more thorough understanding of how variability in social communication and language outcomes are related to underlying brain differences. The limited sample also led us to include usable scans completed between 12–24 months, a time within which the infant brain changes significantly. Although we controlled for linear age at scan within our models, the cross sectional nature of the scans makes it difficult to account for developmental differences. Future studies can address this through multiple scans per toddler at more carefully specified timepoints, which will make it easier to relate brain structure to behavior and will also make it possible to account for nonlinear effects of age on the brain and behavior.
Second, these data were collected during natural sleep without monitoring for sleep stage. This limitation is pervasive in the field of infant imaging due to the challenges in using physiological monitoring to accurately infer sleep stage in a sleeping infant or toddler. Work in adults that has combined fMRI and EEG suggests that general patterns of functional connectivity within higher-order brain networks, including the DMN, are consistent between sleep and wake states but differences in connectivity strength may emerge with some evidence for reduced connectivity within regions of the DMN with increasingly deep sleep (Fukunaga et al., 2006; Graham, Pfeifer, Fisher, Lin, et al., 2015; Horovitz et al., 2009; Larson-prior et al., 2009). To mitigate this limitation, toddlers were always awake before the scan started so that resting-state data collection could occur at a relatively consistent interval across participants. Nonetheless, variability in sleep stage is likely present between toddlers and these differences in sleep stage may contribute to differences in functional connectivity. An important direction for future infant and toddler imaging research will be to develop novel methods to characterize sleep stage that do not require combining EEG with fMRI (e.g., Altmann et al., 2016).
Third, the LpSTS seed region was identified a-priori from a study of joint attention in adults. Although use of adult seed regions is common within fMRI studies of infants and toddlers (e.g., Alcauter et al., 2014; Fransson et al., 2007; Graham, Pfeifer, Fisher, Carpenter, et al., 2015; Graham, Pfeifer, Fisher, Lin, et al., 2015), it raises questions as to whether this seed would show similar functional properties as in the adult brain. Although this age range restricted our ability to measure the functional response of this region during a social communication task, we did see that regions functionally connected to this LpSTC seed region are consistent with activated during social and communicative processing in adults. Thus, these data provide some validity for the use of an adult seed. In addition, the LpSTC is but one of many regions in the brain shown to be related to social communication processes (Redcay & Saxe, 2013; Redcay et al., 2016), and as such future studies should investigate network properties of those regions and their relation with early development of social communication abilities.
4.6. Conclusion
Interhemispheric posterior superior temporal connectivity between the LpSTC and RpSTC is significantly related to social communication abilities in toddlers. Although this connectivity was related to concurrent social communication abilities, it did not predict receptive language outcome. Findings from this preliminary study provide an important stepping stone for future research incorporating longitudinal behavioral data on social and language development with resting-state functional connectivity measures.
Supplementary Material
Highlights.
Functional connectivity identifies putative social communication network in toddlers
Bilateral temporal connectivity may be an early marker of communication abilities
Bilateral posterior temporal connectivity did not predict later language scores
Acknowledgements
The authors thank the families and toddlers who participated in this study. We also thank the research staff for assistance with data collection and the NIH fMRI facility, particularly Vinai Roopchansingh for assistance with imaging protocols. Thank you to all the Intramural Research Training Awardees (IRTAs) who assisted in data collection. This research was supported by the Intramural Program of the National Institute of Mental Health of the National Institutes of Health, ZIA MH002868 (Study NCT01339767, 11-M-0144).
Footnotes
The authors have no competing interests to declare.
Author Statement:
Elizabeth Smith – Investigation, writing – original, review & editing
Yaqiong Xiao – Data curation, formal analysis, review & editing
Hua Xie – Formal analysis
Stacy S. Manwaring – Conceptualization, Investigation, writing – review & editing’
Cristan Farmer – Formal analysis
Lauren Thompson – Investigation, writing – review & editing
Precilla D’Souza - Investigation
Audrey Thurm – Conceptualization, investigation, supervision, funding Acquisition, project administration
Elizabeth Redcay – Conceptualization, formal analysis, supervision, writing – original draft, review & editing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
The imaging and behavioral data from this study are or will be available in the NIHM Data Archive, collection #2901 (https://nda.nih.gov/edit_collection.html?id=2901). Until the data are deposited, they may be accessed by reasonable request to the corresponding author.
References
- Alcauter S, Lin W, Smith XJK, Short SJ, Goldman BD, Reznick JS, … Gao W. (2014). Development of Thalamocortical Connectivity during Infancy and Its Cognitive Correlations. The Journal of Neuroscience, 34(27), 9067–9075. 10.1523/JNEUROSCI.0796-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann A, Schröter MS, Spoormaker VI, Kiem SA, Jordan D, Ilg R, … Sämann PG (2016). Validation of non-REM sleep stage decoding from resting state fMRI using linear support vector machines. NeuroImage, 125, 544–555. 10.1016/j.neuroimage.2015.09.072 [DOI] [PubMed] [Google Scholar]
- Ashburner J, & Friston K. (2005). Unified segmentation. NeuroImage, 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Bakeman R, & Adamson LB (1984). Coordinating joint attention to people and objects in mother-infant and peer-infant interaction. Child Development, 55(4), 1278–1289. [PubMed] [Google Scholar]
- Ball G, Pazderova L, Chew A, Tusor N, Merchant N, Arichi T, … Counsell SJ (2015). Thalamocortical Connectivity Predicts Cognition in Children Born Preterm. Cerebral Cortex, 25, 4310–4318. 10.1093/cercor/bhu331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilakos A, Smith KG, Fillmore P, Fridriksson J, & Fedorenko E. (2018). Functional Characterization of the Human Speech Articulation Network. Cerebral Cortex, 28, 1816–1830. 10.1093/cercor/bhx100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, & Conant L. (2009). Where Is the Semantic System ? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies, (December). 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake J, McConnell S, Horton G, & Benson N. (1992). The gestural repetoire and its evolution over the second year. Early Development and Parenting, 1(3), 127–136. [Google Scholar]
- Bottema-beutel K. (2016). Associations Between Joint Attention and Language in Autism Spectrum Disorder and Typical Development : A Systematic Review and Meta-Regression Analysis. Autism Research, 9, 1021–1035. 10.1002/aur.1624 [DOI] [PubMed] [Google Scholar]
- Bozic M, Tyler LK, Ives DT, Randall B, & Marslen-wilson WD (2010). Bihemispheric foundations for human speech comprehension. Proceedings of the National Academy of Sciences, 107(40), 17439–17444. 10.1073/pnas.1000531107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124(section III), 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Carpenter M, Nagell K, & Tomasello M. (1998). Social Cognition, Joint Attention, and Communicative Competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development, 63(4). [PubMed] [Google Scholar]
- Carpenter RL, Mastergeorge AM, & Coggins TE (1983). The Acquisition of Communicative Intentions in Infants Eight to Fifteen Months of Age. Language and Speech, 26(2), 101–116. [DOI] [PubMed] [Google Scholar]
- Carter RM, & Huettel SA (2013). A nexus model of the temporal – parietal junction. Trends in Cognitive Sciences, 17(7), 328–336. 10.1016/j.tics.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, & Bambini V. (2014). A model for Social Communication And Language Evolution and Development (SCALED). Current Opinion in Neurobiology, 28, 165–171. 10.1016/j.conb.2014.07.018 [DOI] [PubMed] [Google Scholar]
- Corkum V, & Moore C. (1995). Development of joint visual attention in infants. In Moore C. & Dunham PJ (Eds.), Joint attention: Its origins and role in development (pp. 61–83). Hillsdale, NJ: Laurence Erlbaum Associates. [Google Scholar]
- Cusack R, Ball G, Smyser CD, & Dehaene-lambertz G. (2016). A neural window on the emergence of cognition. Annals of the New York Academy of Sciences, 1369, 7–23. 10.1111/nyas.13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, & Courchesne E. (2011). Disrupted neural synchronization in toddlers with autism. Neuron, 70(6), 1218–1225. 10.1016/j.neuron.2011.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, & Turkheimer FE (2010). Emergence of resting state networks in the preterm human brain. 10.1073/pnas.1007921107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht AT, Elison JT, Feczko E, Todorov A, Wolff JJ, Kandala S, … Pruett JR (2017). Joint Attention and Brain Functional Connectivity in Infants and Toddlers, 1–12. 10.1093/cercor/bhw403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Zwaigenbaum L, Gu H, John TS, Paterson S, Elison JT, … Piven J. (2015). Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. Journal of Neurodevelopmental Disorders, 1–10. 10.1186/s11689-015-9117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Nieto-Castañón A, & Kanwisher N. (2012). Syntactic processing in the human brain: what we know, what we don’t know, and a suggestion for how to proceed. Brain and Language, 120(2), 187–207. 10.1016/j.bandl.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L, Marchman VA, Thal D, Dale PS, Reznick JS, & Bates E. (2006). The MacArthur-Bates Communicative Development Inventories: User’s guide and techincal manual. Baltimore, MD: Paul H. Brookes Publishing Co. [Google Scholar]
- Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, … Constable RT (2016). Functional connectome fingerprinting: Identifying individuals based on patterns of brain connectivity. Nature Neuroscience, 18(11), 1664–1671. 10.1038/nn.4135.Functional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, & Collins DL (2011). Unbiased average age-appropriate atlases for pediatric studies. NeuroImage, 54(1), 313–327. 10.1016/j.neuroimage.2010.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M, Hamodat T, Armstrong VL, Sacrey LR, Brian J, Bryson SE, & Garon N. (2019). Infants at Risk for Autism Spectrum Disorder: Frequency, Quality, and Variety of Joint Attention Behaviors. Journal of Abnormal Child Psychology, 47, 907–920. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, & Aden U. (2007). Resting-state networks in the infant brain. Proceedings of the National Academy of Sciences of the United States of America, 104(39), 15531–15536. 10.1073/pnas.0704380104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2011). The brain basis of language processing: from structure to function. Physiological Reviews, 91(4), 1357–1392. 10.1152/physrev.00006.2011 [DOI] [PubMed] [Google Scholar]
- Friston K, Williams S, Howard R, Frackowiak R, & Turner R. (1996). Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346–355. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, Gelderen P. Van, Zwart J. A. De, Leopold DA, & Duyn (2006). Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magnetic Resonance in Medicine, 24, 979–992. 10.1016/j.mri.2006.04.018 [DOI] [PubMed] [Google Scholar]
- Gao W, Lin W, Grewen K, & Gilmore JH (2017). Functional Connectivity of the Infant Human Brain : Plastic and Modifiable. The Neuroscientist, 23(2), 169–184. 10.1177/1073858416635986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, & Lin W. (2004). Brain Functional Networks in the Developing Brain Using Resting BOLD. [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, & Lin W. (2009). Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences of the United States of America, 106(16), 6790–6795. 10.1073/pnas.0811221106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Carpenter S, & Fair DA (2015). Early life stress is associated with default system integrity and emotionality during infancy. Journal of Child Psychology and Psychiatry, 11, 1212–1222. 10.1111/jcpp.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, & Fair DA (2015). The potential of infant fMRI research and the study of early life stress as a promising exemplar. Developmental Cognitive Neuroscience, 12, 12–39. 10.1016/j.dcn.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, & Johnson MH (2010). Selective prefrontal cortex responses to joint attention in early infancy. Biology Letters, 6(4), 540–543. 10.1098/rsbl.2009.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH, Lloyd-Fox S, Blasi A, Deligianni F, Elwell C, & Csibra G. (2008). Early cortical specialization for face-to-face communication in human infants. Proceedings. Biological Sciences / The Royal Society, 275(1653), 2803–2811. 10.1098/rspb.2008.0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Lloyd-fox S, & Johnson MH (2013). Brain responses reveal young infants ‘ sensitivity to when a social partner follows their gaze. Developmental Cognitive Neuroscience, 6, 155–161. 10.1016/j.dcn.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D. (n.d.). Towards a functional neuroanatomy of speech perception. [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(May), 393–402. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, & Duyn JH (2009). Decoupling of the brain’s default mode network during deep sleep. Proceedings of the National Academy of Sciences, 106(27), 11376–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ, … He Y. (2013). Development of Human Brain Structural Networks Through Infancy and Childhood. Cerebral Cortex (New York, N.Y. : 1991). 10.1093/cercor/bht335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, & Cuthbert B. (2010). Research Domain Criteria ( RDoC ): Toward a New Classification Framework for Research on Mental Disorders. American Journal of Psychiatry, 167(7), 748–751. [DOI] [PubMed] [Google Scholar]
- Lahnakoski JM, Glerean E, Salmi J, Iiro P, & Nummenmaa L. (2012). Naturalistic fMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Frontiers in Human Neuroscience, 6, 1–14. 10.3389/fnhum.2012.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, & Raichle ME (2009). Cortical network functional connectivity in the descent to sleep. Proceedings of the National Academy of Sciences, 106(11), 4489–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainieri a G., Heim S, Straube B, Binkofski F, & Kircher T. (2013). Differential role of the Mentalizing and the Mirror Neuron system in the imitation of communicative gestures. NeuroImage, 81, 294–305. 10.1016/j.neuroimage.2013.05.021 [DOI] [PubMed] [Google Scholar]
- Manwaring SS, Swineford L, Mead DL, Chih-Ching Y, Zhang Y, & Thurm A. (2019). The gesture-language association over time in toddlers with and without language delays. Autism & Developmental Language Impairments, 4, 1–15. 10.1177/239694151984554533912683 [DOI] [Google Scholar]
- Marrus N, Eggebrecht AT, Todorov A, Elison JT, Wolff JJ, Cole L, … Jr JRP (2018). Walking, Gross Motor Development, and Brain Functional Connectivity in Infants and Toddlers. Cerebral Cortex, 28, 750–763. 10.1093/cercor/bhx313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert F, Maryann P, Sallet J, Toni I, & Rushworth MFS (2012). On the relationship between the “default mode network” and the “social brain.” Frontiers in Human Neuroscience, 6(June), 1–9. 10.3389/fnhum.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melinder A, Konijnenberg C, Hermansen T, Daum M, & Gredeback G. (2015). The developmental trajectory of pointing perception in the first year of life. Experimental Brain Research, 233, 641–647. 10.1007/s00221-014-4143-2 [DOI] [PubMed] [Google Scholar]
- Mills D, Coffey-Corina S, & Neville H. (1997). Language comprehension and cerebral specialization from 13 to 20 months. Developmental Neuropsychology, 13(3), 397–445. [Google Scholar]
- Mills DL, Coffey-Corina S, & Neville H. (1993). Language acquisition and cerebral specialization in 20-month-old infants. Journal of Cognitive Neuroscience, 5(3), 317–344. [DOI] [PubMed] [Google Scholar]
- Mullen E. (1995). Mullen scales of early learning. Circle Pines, MN: American Guidance Services. [Google Scholar]
- Mundy P, & Newell L. (2007). Attention, Joint Attention, and Social Cognition. Current Directions in Psychological Science : A Journal of the American Psychological Society, 16(5), 269–274. 10.1111/j.1467-8721.2007.00518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sullivan L, & Mastergeorge A. (2009). A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Research : Official Journal of the International Society for Autism Research, 2(1), 2–21. 10.1002/aur.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam GB, Liegeois F, Tournier J, Croft LJ, Johns PN, Chong WK, … Baldeweg T. (2012). Interhemispheric temporal lobe connectivity. Brain, 135, 3781–3798. 10.1093/brain/aws276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Awander A, Spada D, & Baldoli C. (2011). Neural language networks at birth. Proceedings of the National Academy of Sciences, 108(45), 18566. 10.1073/pnas.1102991108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitt M, Anne K, & Martin A. (2015). Functional connectivity classi fi cation of autism identi fi es highly predictive brain features but falls short of biomarker standards. Neuroimage: Clinical, 7, 359–366. 10.1016/j.nicl.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D, Emmorey K, Hickok G, & Pylkkänen L. (2012). Towards a new neurobiology of language. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(41), 14125–14131. 10.1523/JNEUROSCI.3244-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E. (2008). The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neuroscience and Biobehavioral Reviews, 32(1), 123–142. 10.1016/j.neubiorev.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Redcay E, Haist F, & Courchesne E. (2008). Functional neuroimaging of speech perception during a pivotal period in language acquisition. Developmental Science, 2, 237–252. 10.1111/j.1467-7687.2008.00674.x [DOI] [PubMed] [Google Scholar]
- Redcay E, Kennedy DP, & Courchesne E. (2007). fMRI during natural sleep as a method to study brain function during early childhood. NeuroImage, 38(4), 696–707. 10.1016/j.neuroimage.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Redcay E, Kleiner M, & Saxe R. (2012). Look at this : the neural correlates of initiating and responding to bids for joint attention. Frontiers in Human Neuroscience, 6(June), 1–14. 10.3389/fnhum.2012.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Velnoskey KR, & Rowe ML (2016). Perceived Communicative Intent in Gesture and Language Modulates the Superior Temporal Sulcus. Human Brain Mapping, 37(10), 3444–3461. 10.1002/hbm.23251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, & Gabrieli J. (2006). Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience, 1(3), 229–234. 10.1093/scan/nsl034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach Leo, Eickhoff SB, Rotarska-Jagiela A, Fink GR, & Vogeley K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition, 17(2), 457–467. 10.1016/j.concog.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Schilbach Leonhard, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, … Vogeley K. (2010). Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. Journal of Cognitive Neuroscience, 22(12), 2702–2715. [DOI] [PubMed] [Google Scholar]
- Schilbach Leonhard, Wohlschlaeger AM, Kraemer NC Newen A, Shah NJ, Fink GR, & Vogeley K. (2006). Being with virtual others: Neural correlates of social interaction. Neuropsychologia, 44(5), 718–730. 10.1016/j.neuropsychologia.2005.07.017 [DOI] [PubMed] [Google Scholar]
- Schippers MB, Roebroeck A, Renken R, Nanetti L, & Keysers C. (2010). Mapping the information flow from one brain to another during gestural communication. Proceedings of the National Academy of Sciences, 107(20), 9388–9393. 10.1073/pnas.1001791107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, & Perner J. (2014). Fractionating theory of mind : A meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Skeide MA, & Friederici AD (2016). The ontogeny of the cortical language network. Nature Reviews Neuroscience, 17(5), 323–332. 10.1038/nrn.2016.23 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, … Beckmann CF (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. (1995). Joint attention as social cognition. In Moore C. & Dunham PJ (Eds.), Joint attention: Its origins and role in development (pp. 103–130). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Travis KE, Leonard MK, Brown TT, Jr DJH, Curran M, & Dale AM (2011). Spatiotemporal Neural Dynamics of Word Understanding in 12- to 18-Month-Old-Infants, (August), 1832–1839. 10.1093/cercor/bhq259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever D, Bzdok D, Wang H, Mollo G, Sormaz M, Murphy C, … Jefferies E. (2017). NeuroImage Varieties of semantic cognition revealed through simultaneous decomposition of intrinsic brain connectivity and behaviour. NeuroImage, 158(January), 1–11. 10.1016/j.neuroimage.2017.06.067 [DOI] [PubMed] [Google Scholar]
- Wang Y, Metoki A, Alm KH, & Olson IR (2018). White matter pathways and social cognition. Neuroscience and Biobehavioral Reviews, 90(September 2017), 350–370. 10.1016/j.neubiorev.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby AM, & Prizant B. (2002). Communication and Symbolic Behavior Scales - Developmental Profile. Baltimore, MD: Brookes Publishing Co. [Google Scholar]
- Wiesmann CG, Schreiber J, Singer T, Steinbeis N, & Friederici AD (2017). White matter maturation is associated with the emergence of Theory of Mind in early childhood. Nature Communications, 8, 14692. 10.1038/ncomms14692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Ozyurek A, & Hagoort P. (2007). When Language Meets Action: The Neural Integration of Gesture and Speech. Cerebral Cortex, 17(10), 2322–2333. 10.1093/cercor/bhl141 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Geng F, Riggins T, Chen G, & Redcay E. (2019). Neural correlates of developing theory of mind competence in early childhood. Neuroimage, 184, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wang X, Zuo X, & Zang Y. (2016). DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics, 14(3), 339–351. [DOI] [PubMed] [Google Scholar]
- Yang DY-J, Rosenblau G, Keifer C, & Pelphrey KA (2015). An integrative neural model of social perception, action observation, and theory of mind. Neuroscience and Biobehavioral Reviews, 51, 263–275. 10.1016/j.neubiorev.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The imaging and behavioral data from this study are or will be available in the NIHM Data Archive, collection #2901 (https://nda.nih.gov/edit_collection.html?id=2901). Until the data are deposited, they may be accessed by reasonable request to the corresponding author.