Abstract
Objective:
To determine the effects of a preoperative, home-based exercise program on fitness and physical function in patients with pancreatic cancer.
Background:
We previously established a well-tolerated preoperative exercise program after finding a high frequency of sarcopenia and frailty in patients with pancreatic cancer.
Methods:
In this randomized, controlled trial (NCT03187951), patients with pancreatic cancer were randomized to Arm A: enhanced usual care or Arm B: prescribed aerobic and resistance exercise during neoadjuvant therapy. Patients received nutrition counseling and activity trackers. The primary endpoint was 6-minute walk distance (6MWD; ≥14 meters improvement was clinically meaningful). Secondary endpoints included additional physical function tests, health-related quality of life, and clinical outcomes.
Results:
151 patients were randomized. Objectively measured weekly activity (153.2±135.6 and 159.8±122.8 minutes in Arm A and B, respectively, P=0.62) and self-reported weekly moderate-to-strenuous physical activity (107.4±160.4 and 129.6±161.6 minutes in Arm A and Arm B, respectively, P=0.49) were similar, but weekly strength training sessions increased more in Arm B (by 1.8±1.8 vs. 0.1±2.4 sessions, P<0.001). 6MWD improved in both Arm A (mean change 18.6±56.8 m, P=0.01) and Arm B (27.3±68.1 m, P=0.002). Quality of life and clinical outcomes did not significantly differ between arms. Pooling patients in both study groups, exercise and physical activity were favorably associated with physical performance and clinical outcomes.
Conclusions:
In this randomized trial of prescribed exercise versus enhanced usual care during neoadjuvant therapy for pancreatic cancer, high volume of activity and increased exercise capacity were observed in both arms, highlighting the importance of activity among patients preparing for surgery.
MINI-ABSTRACT
In this randomized trial comparing prescribed exercise with enhanced usual care during neoadjuvant therapy for pancreatic cancer, there was a statistically and clinically significant improvement in the primary endpoint of 6-minute walk distance in both arms. Among all patients, activity and exercise were favorably associated with physical performance and clinical outcomes.
INTRODUCTION
Pancreatic cancer (PC) is the third leading cause of cancer-related deaths in the United States.1 Surgical resection of the primary tumor and regional lymph nodes is necessary but insufficient for cure. The longevity of patients who undergo surgery is prolonged by adjuvant chemotherapy,2 but systemic chemotherapy and/or (chemo)radiation is increasingly administered before pancreatectomy instead of after it.3, 4 National guidelines now recommend that neoadjuvant therapy be administered to all patients with borderline resectable cancer and at least some with resectable tumors.5
Patients with PC are generally older adults who frequently present with conditions such as cachexia, sarcopenia, and frailty, which increase risk for adverse disease and treatment outcomes. Among 142 patients with a variety of stages of newly diagnosed PC who we recently described, 56% were sarcopenic and 25% were frail. Frailty was associated with comorbidities and poor performance status and with poor overall survival following treatment with either curative or palliative intent.6 Other studies have shown that frailty is associated with increased risk of complications and mortality following major abdominal surgery.7, 8 Sarcopenia and frailty also increase chemotherapy-associated toxicity and reduce tolerance and adherence.9 Conversely, chemotherapy may reduce muscle mass, setting up a vicious cycle that can reduce both quantity and quality of life.10
In 2022, the American Cancer Society and the American Society of Clinical Oncology released guidelines recommending that oncology clinicians endorse regular aerobic and resistance exercise during treatment with curative intent.11, 12 Preliminary evidence suggests that exercise during cancer treatment can enhance the efficacy of cancer therapies.13 Among patients with PC, small studies suggest that exercise concurrent with treatment can improve quality of life and fitness and may improve postoperative outcomes such as the rate of delayed gastric emptying and duration of postoperative stay.14, 15 Among patients treated with neoadjuvant therapy for resectable PC, we have found that physical activity is associated with improved submaximal exercise capacity, maintenance of quality of life and physical function, and preservation of skeletal muscle mass.16-18
We have long hypothesized that exercise prescribed concurrent with neoadjuvant therapy can mitigate functional decline and improve physical function prior to pancreatectomy. To test this hypothesis, we conducted a randomized study of a low-cost, low-risk, home-based program of aerobic and resistance exercise compared to enhanced usual care.
METHODS
We conducted this randomized, controlled trial at The University of Texas MD Anderson Cancer Center, a comprehensive cancer center in Houston, Texas (ClinicalTrials.gov NCT03187951). Study procedures were approved by the Institutional Review Board (protocol #2017-0198) and conducted in accord with the ethical standards of the Helsinki Declaration of 1975. All patients who presented with PC between October 2017 and May 2021 were screened. Eligibility requirements included intended pancreatectomy for biopsy-confirmed PC; a treatment plan including preoperative chemotherapy and/or radiation for at least 6 weeks prior to anticipated pancreatectomy; English fluency; telephone or email access; and willingness to engage in follow-up calls every 2 weeks. Exclusion criteria included unstable cardiac or pulmonary disease, symptomatic cardiac disease (New York Heart Association functional class III or IV), acute musculoskeletal injury that affected exercise ability, poorly controlled pain (numeric rating ≥7 out of 10), or other disease that precluded unsupervised exercise.
Following recommendation and approval from medical or surgical oncologists, patients completed the Physical Activity Readiness Questionnaire19 and the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function 12a Short Form screener (“Can you walk 25 feet on a level surface, with or without support?”).20 Self-reported chest pain, loss of balance because of dizziness, loss of consciousness, or inability to walk 25 feet on a level surface were grounds for exclusion. Patients who reported musculoskeletal dysfunction that limited physical activity (PA) required clearance from a physical medicine and rehabilitation physician (ANH). Patients with poorly controlled hypertension required clearance by internal medicine prior to enrollment.
Stratification and Randomization
In this parallel arm study design, study participants were stratified prior to randomization based on their expected duration of preoperative treatment (i.e., chemotherapy versus chemoradiation as first treatment at enrollment) and their baseline physical activity using the Godin-Shephard Leisure Time Physical Activity Questionnaire (GSLTPAQ) score (physically active: scores ≥ 24 versus insufficiently active: scores < 24).21 Using the Pocock-Simon minimization method, participants were randomized 1:1 to enhanced usual care (Arm A) versus prescribed exercise (Arm B). Study participants and research staff were unblinded to the study arm.
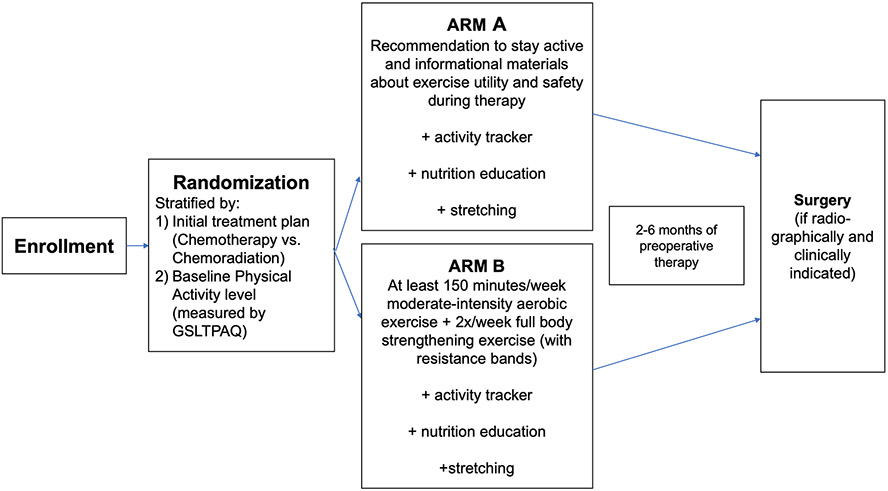
The study schema is depicted in Figure 1. All participants were encouraged to be physically active, and all received Fitbit Charge 2 activity trackers and instructions for setting up Fitbit accounts and syncing to their devices. To determine baseline nutritional status, all participants completed the Patient-Generated Subjective Global Assessment Short Form (PGSGAsf). Participants with a PGSGAsf score < 6 (low malnutrition risk) received general nutrition education materials from the research staff and were recommended to consume a high-protein snack/meal/shake (15-25 g) within 1 hour after strengthening exercises. Participants with a PGSGAsf score ≥ 6 (high malnutrition risk) received the same education materials, a complete nutritional assessment, and personalized recommendations by a registered clinical dietitian, including the recommendation to consume a high-protein snack/meal/shake (15-25 g) within 1 hour after strengthening exercises.
Figure 1.
Study schema including the preoperative period of the intervention.
Participants randomized to Arm A received an information packet consisting of a handout on the benefits of and precautions for exercise, a stretching guide to help them maintain flexibility during treatment, and a nutrition guide. They did not receive a specific exercise prescription.
Participants randomized to Arm B also received a set of resistance bands (stackable and color-coded with up to 75 pounds of resistance; Black Mountain Products, Spring Grove, IL) and were prescribed an exercise program we previously implemented in a single-arm trial.16-18 The program included stretching, moderate-intensity aerobic exercise, and resistance exercises to based on the American College of Sports Medicine (ACSM) guidelines for cancer survivors. When the program was designed, these guidelines recommended ≥ 150 minutes of moderate-intensity aerobic exercise weekly plus ≥ two resistance exercise sessions weekly.22 Updated guidelines now recommend ≥ 30 minutes of moderate-intensity aerobic exercise ≥ three times weekly, plus ≥ two resistance exercise sessions weekly.23 Arm B participants received in-person instruction from an ACSM-certified personal trainer, an instructional DVD that demonstrated set-up for all exercises, and written and photographic descriptions of the exercises. The participants were instructed to perform ≥ 30 minutes of moderate-intensity aerobic exercise (Borg Rate of Perceived Exertion, 12-13) ≥ 5 days per week. Additionally, they were instructed to engage in ≥ two resistance exercise sessions per week, which included ≥ one set of 10-15 repetitions of each of eight exercises designed to engage major muscle groups using body weight and resistance tubes. They were encouraged to gradually increase repetitions (goal 15), sets (goal 3), and resistance (upgraded to the next color resistance tube). The pragmatic nature of this trial allowed patients in both arms to participate regardless of the location of their hometown and neoadjuvant therapy administration.
Outcome measures
Outcome measures were obtained at the time of enrollment and at the preoperative clinic visit. The 6-minute walk distance (6MWD), conducted per the American Thoracic Society’s guidelines,24 measures submaximal exercise capacity and has been validated in patients with colorectal and lung cancer.25, 26 A change in 6MWD of 14 to 30 meters was considered clinically meaningful.27, 28 Lower limb performance was measured by the 5-time sit to stand test (5xSTS), wherein patients rise from sitting to standing five consecutive times.29 The arm curl test includes performing as many bicep curls in a 30-second period (8 and 5-pound dumbbell for men and women, respectively).30 Handgrip strength was measured via handheld dynamometry (Jamar hydraulic hand dynamometer).31 A 3-meter walk was used to measure gait speed.32
Patient-reported outcomes were evaluated using the following instruments. The validated, modified version of GSLTPAQ assessed duration and frequency of self-reported PA, allowing for computation of weekly mild, moderate, and strenuous PA minutes.33 We used the sum of weekly moderate and strenuous PA to determine moderate-to-strenuous PA. We also added a single, validated item from the Health Information National Trends Survey34 to assess weekly frequency of strengthening exercises, as previously employed in studies involving cancer survivors.35 Self-reported functional status was recorded via the PROMIS Physical Function 12a Short Form.20 Health-related quality of life was measured using the Functional Assessment of Cancer Therapy—Hepatobiliary questionnaire (FACT-Hep), a validated, consistent, and reliable tool comprising the FACT-General (FACT-G) subscale and the hepatobiliary subscale.36
As previously described, muscle mass was quantified using SliceOmatic version 5.0 software (TomoVision, Magog, Canada) to process computed tomography images of the abdomen and pelvis obtained for routine clinical care.16, 17 Both skeletal muscle index (SMI) and skeletal muscle density were measured. Sarcopenia was defined as SMI ≤ 38.9 cm2/m2 for women and SMI ≤ 55.4 cm2/m2 for men.37
We collected the following clinical and demographic characteristics and outcomes: age, sex, ethnicity, race, radiographic disease stage, Eastern Cooperative Oncology Group performance status, Adult Comorbidity Evaluation-27 score, follow-up duration for study participation, and treatment received during the study period. For participants who underwent surgical resection: hospital length of stay (in days), readmissions within 90 days following resection, and any Accordion grade 3 or higher complication within 90 days following resection were recorded.38
Objectively measured activity
Participants received instructions to connect their Fitbit to a study database (“Fitabase”, Small Step Labs, San Diego, CA) to which they synced throughout study participation. Participants’ Fitbit data (enrollment to preoperative follow-up) included: average daily steps; average weekly lightly active, fairly active, very active minutes, and sedentary minutes. Fairly active and very active minutes were added to compute weekly active minutes, as done in previous studies involving cancer survivors.39 We followed established protocols to include valid days of Fitbit wear (≥1000 steps/day)40 and valid weeks (≥4 valid days within 7 consecutive days).41 Potential weeks of wear was determined as: successive 7-day periods starting with the first valid wear day and ending with the day on which a participant either withdrew or presented for follow-up data collection. If 1-3 days of Fitbit wear remained between the end of the final 7-day period at the date of study withdrawal or follow-up, they were not included in analyses.
Statistical analysis
The primary endpoint was the change in the 6MWD between enrollment and the preoperative follow-up within each arm as well as the difference in the preoperative 6MWD between Arm A and Arm B.
With a total sample size of 128 (64 per arm) and assuming the mean 6MWD changes would be 0-20 meters and 30-50 meters, respectively, for Arm A and Arm B, we had 80% power to detect a difference of a 30-m change between the two arms prior to surgery, using a two-sample t-test and at a two-sided significance level of 0.05. The sample size/power calculation assumed a common standard deviation of 60. It was anticipated that 60% of enrolled patients would undergo surgery following preoperative therapy and 40% would not (e.g., due to disease progression). It was assumed that we would have preoperative 6MWD data in 60% of patients who did not undergo surgery. Therefore, we planned to enroll 152 patients to have 6MWD data collected from 128 patients at both the baseline and preoperative timepoints.
We used chi-square tests and independent t-tests or non-parametric alternatives to compare means or frequencies of all clinical, demographic, physical activity, exercise, nutrition and outcome variables between study groups. We used paired t-tests or non-parametric alternatives to assess changes in outcome variables within groups and independent t-tests or non-parametric alternatives to compare differences in change scores between groups. Finally, we pooled participants from both groups and used multiple linear regression models to determine associations between exercise and physical activity variables and changes in outcome measures. The baseline value or score for the outcome variable of interest was included as a covariate in each regression model. Logistic regression models were used to evaluate associations between exercise and physical activity variables and likelihood of surgical resection and, among the subsample of participants who underwent resection, dichotomous outcomes including readmission and occurrence of grade 3 complications. All statistical analyses were performed in SPSS version 26 (IBM Corp., Armonk, NY).
RESULTS
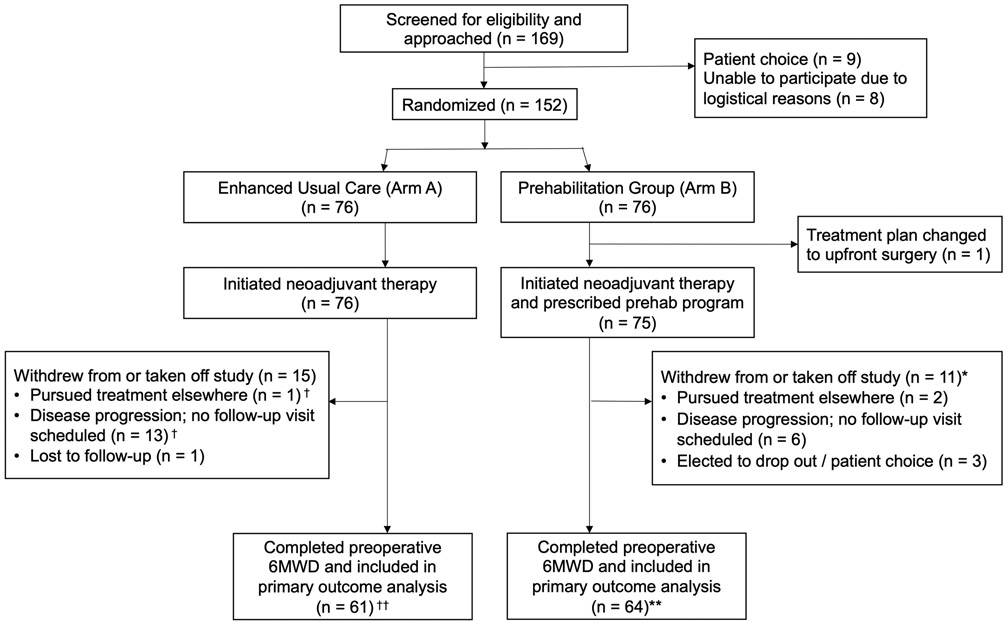
As presented in Figure 2, among 169 patients screened for eligibility, 152 patients were enrolled. 76 patients were randomized to arm A and 76 to arm B. However, one patient on Arm B was deemed ineligible shortly after enrollment because their treatment plan changed to surgery de novo due to gastric outlet obstruction. Table 1 describes baseline demographic, clinical, and PA behavioral characteristics of the 151 participants who comprised the final study sample.
Figure 2.
CONSORT diagram describing the trial recruitment. †Some had valid activity tracker data; included in related analysis (n = 9). ††Some missing valid activity tracker data; excluded from related analysis (n = 12). *Some had valid activity tracker data; included in related analysis (n = 7). **Some missing valid activity tracker data; excluded from related analysis (n = 16). Abbreviations: Prehab, prehabilitation; 6MWD, 6-minute walk distance.
Table 1.
Baseline demographic, clinical, and behavioral characteristics of the study sample (n=151).
| Characteristic | Arm A (n=76) |
Arm B (n=75) |
|---|---|---|
| Mean age at enrollment, years ± SD | 66.2 ± 8.2 | 66.1 ± 8.5 |
| Sex, n (%) | ||
| Female | 33 (43) | 26 (35) |
| Male | 43 (57) | 49 (65) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 11 (15) | 6 (8) |
| Non-Hispanic or Latino | 65 (85) | 69 (92) |
| Race, n (%) | ||
| American Indian or Alaska Native | 0 (0) | 1 (1) |
| Asian | 2 (3) | 2 (3) |
| Black or African American | 8 (10) | 6 (8) |
| White | 61 (80) | 65 (87) |
| Other | 5 (7) | 0 (0) |
| Unknown | 0 (0) | 1 (1) |
| Radiographic disease stage, n (%) | ||
| Potentially resectable | 40 (53) | 46 (61) |
| Borderline resectable | 29 (38) | 21 (28) |
| Locally advanced | 7 (9) | 8 (11) |
| Performance status, n (%) | ||
| 0 | 32 (42) | 36 (48) |
| 1 | 44 (58) | 38 (51) |
| 2 | 0 (0) | 1 (1) |
| Comorbidity score, n (%) | ||
| None | 13 (17) | 12 (16) |
| Mild | 34 (45) | 29 (39) |
| Moderate | 18 (24) | 22 (29) |
| Severe | 11 (15) | 12 (16) |
| Body mass index (BMI), kg/m2, mean ± SD | 28.5 ± 6.0 | 27.8 ± 5.1 |
| Sarcopenia, n (%) | 38 (54) | 33 (47) |
| Nutritional risk score, mean ± SD | 7.0 ± 5.1 | 7.2 ± 5.2 |
| Nutritional risk score ≥ 6, n (%) | 43 (57) | 43 (57) |
| Godin leisure-time exercise score, mean ± SD | 23.9 ± 21.1 | 22.1 ± 18.9 |
| Self-reported moderate-to-strenuous physical activity, weekly minutes, mean ± SD | 127.4 ± 218.8 | 132.1 ± 166.2 |
| Self-reported strength training at enrollment, weekly sessions, mean ± SD | 1.2 ± 2.0 | .6 ± 1.3 |
The mean (± SD) duration of the intervention was 22 ± 10.3 weeks for Arm A and 24 ± 12.2 weeks for Arm B (p = 0.39). On Arm A, 41 (54%) participants received chemotherapy only, 3 (4%) received chemoradiation only, and 32 (42%) received both. On Arm B, 39 (52%) received chemotherapy, 3 (4%) received chemoradiation, and 33 (44%) received both (P = 0.9). Supplementary Table 1 describes the baseline demographic, clinical, and PA behavioral characteristics among the 125 participants who completed the preoperative 6MWD and were included in the primary endpoint analysis.
Table 2 compares self-reported exercise and objectively measured PA between time points and between study arms for all participants with data available. Self-reported exercise variables did not significantly increase over the study period within either arm except for mean frequency of strength training in Arm B (0 .4 ± 1.0 sessions/week at baseline vs. 2.2 ± 1.8 sessions/week preoperatively, P < 0.001). The increase in strength training frequency was significantly higher in Arm B compared to Arm A (mean increase of 1.8 ± 1.8 sessions/week vs. 0.1 ± 2.4 sessions/week, P < 0.001). Objectively measured PA variables did not significantly differ between arms.
Table 2.
Self-reported and objectively measured activity during neoadjuvant therapy.
| Arm A | Arm B |
P-value for difference between Arms A and B** |
|
|---|---|---|---|
| Self-reported exercise (modified Godin questionnaire) † | |||
| n | 51 | 50 | |
| Mild-intensity exercise, mean weekly minutes ± SD | |||
| Baseline | 297.3 ± 699.5 | 135.0 ± 220.5 | 0.99 |
| Follow-up | 131.1 ± 212.3 | 155.6 ± 180.6 | 0.06 |
| Δ within group | −166.2 ± 727.8 | 20.6 ± 234.6 | 0.09 |
| P-value for Δ within group* | 0.65 | 0.23 | |
| Moderate-intensity exercise, mean weekly minutes ± SD | |||
| Baseline | 80.3 ± 112.9 | 126.2 ± 162.6 | 0.31 |
| Follow-up | 89.4 ± 124.6 | 115.7 ± 150.8 | 0.22 |
| Δ within group | 9.1 ± 147.8 | −10.5 ± 168.2 | 0.53 |
| P-value for Δ within group* | 0.98 | 0.95 | |
| Strenuous-intensity exercise, mean weekly minutes ± SD | |||
| Baseline | 33.2 ± 82.0 | 26.4 ± 60.4 | 0.75 |
| Follow-up | 17.9 ± 53.9 | 13.9 ± 27.9 | 0.40 |
| Δ within group | −15.3 ± 86.6 | −12.5 ± 54.2 | 0.85 |
| P-value for Δ within group* | 0.13 | 0.21 | |
| Moderate-to-strenuous exercise, mean weekly minutes ± SD | |||
| Baseline | 113.5 ± 141.3 | 152.6 ± 181.7 | 0.23 |
| Follow-up | 107.4 ± 160.4 | 129.6 ± 161.6 | 0.49 |
| Δ within group | −6.2 ± 172.2 | −23.0 ± 172.0 | 0.62 |
| P-value for Δ within group* | 0.29 | 0.49 | |
| Strength training, mean weekly sessions ± SD | |||
| Baseline | 1.4 ± 2.1 | 0.4 ± 1.0 | 0.003 |
| Follow-up | 1.5 ± 1.9 | 2.2 ± 1.8 | 0.06 |
| Δ within group | 0.1 ± 2.4 | 1.8 ± 1.8 | <0.001 |
| P-value for Δ within group* | 0.75 | <0.001 | |
| Activity tracker-measured physical activity during the preoperative period | |||
| n | 58 | 54 | |
| Valid wear day percentage, mean ± SD | 88.7 ± 13.0 | 86.1 ± 18.4 | 0.82 |
| Valid wear week percentage, mean ± SD | 90.8 ± 17.0 | 89.2 ± 19.2 | 0.95 |
| Daily steps, mean ± SD | 5349.4 ± 2226.5 | 5586.7 ± 2164.4 | 0.58 |
| Weekly sedentary minutes, mean ± SD | 6362.4 ± 1621.8 | 6427.3 ± 1191.6 | 0.86 |
| Weekly light activity minutes, mean ± SD | 1025.8 ± 448.5 | 920.4 ± 405.5 | 0.21 |
| Weekly fairly active minutes, mean ± SD | 75.3 ± 66.6 | 75.3 ± 65.9 | 0.87 |
| Weekly very active minutes, mean ± SD | 77.9 ± 85.4 | 84.5 ± 77.7 | 0.47 |
| Weekly active minutes, mean ± SD | 153.2 ± 135.6 | 159.8 ± 122.8 | 0.62 |
Wilcoxon signed rank test used due to non-normal variable distribution.
Wilcoxon signed rank test used for baseline and follow-up comparisons and activity-tracker variables due to non-normal variable distribution; independent t-test used for change score comparisons.
Follow-up time point assessed self-reported exercise throughout the study period.
Changes in primary and secondary outcome measures within study groups and differences between arms are reported in Tables 3 and 4. Participants in both arms had a statistically and clinically significant improvement in 6MWD (18.5 ± 56.7 meters vs. 27.3 ± 68.1 meters, P < 0.01). Participants in Arm B had a statistically significant improvement in 5xSTS time (P < 0.001) and 3-meter walk for gait speed (P = 0.04). Both arms had statistically significant improvement in arm curl repetitions (P = 0.002 for Arm A and P < 0.001 for Arm B). There were no statistically significant changes in self-reported physical functioning, health-related quality of life, or skeletal muscle index or density in either arm. None of the changes in outcome measures were significantly different between arms (all P > 0.05).
Table 3.
Changes in physical function outcomes within arms and between arms.
| Outcome | Arm A | Arm B |
P-value for difference between Arm A and Arm B |
|---|---|---|---|
| 6-minute walk distance (m), mean ± SD | |||
| n | 61 | 64 | |
| Baseline | 466.5 ± 102.0 | 478.1 ± 92.7 | 0.50 |
| Follow-up | 484.9 ± 109.6 | 505.4 ± 113.5 | 0.31 |
| Δ within group | 18.5 ± 56.7 | 27.3 ± 68.1 | 0.44 |
| P-value for Δ within group | 0.01 | 0.002 | |
| 5x sit-to-stand time (s), mean ± SD | |||
| n | 58 | 61 | |
| Baseline | 11.9 ± 3.9 | 11.2 ± 3.4 | 0.32 |
| Follow-up | 11.0 ± 5.3 | 9.7 ± 3.4 | 0.12 |
| Δ within group | −0.9 ± 3.3 | −1.5 ± 2.2 | 0.24 |
| P-value for Δ within group | 0.05 | <0.001 | |
| Handgrip strength (kg), mean ± SD | |||
| n | 61 | 64 | |
| Baseline | 32.3 ± 11.0 | 33.0 ± 11.3 | 0.72 |
| Follow-up | 32.0 ± 10.6 | 32.9 ± 11.5 | 0.64 |
| Δ within group | −0.3 ± 5.2 | −0.1 ± 4.4 | 0.80 |
| P-value for Δ within group | 0.64 | 0.86 | |
| Arm curl test repetitions, mean ± SD | |||
| n | 60 | 62 | |
| Baseline | 20.1 ± 5.2 | 18.6 ± 4.9 | 0.11 |
| Follow-up | 21.6 ± 6.2 | 21.4 ± 6.4 | 0.85 |
| Δ within group | 1.5 ± 3.6 | 2.8 ± 3.8 | 0.06 |
| P-value for Δ within group | 0.002 | <0.001 | |
| 3-meter walk test time (s), mean ± SD | |||
| n | 59 | 63 | |
| Baseline | 2.8 ± .7 | 2.7 ± .6 | 0.74 |
| Follow up | 2.7 ± .8 | 2.6 ± .5 | 0.55 |
| Δ within group | −0.1 ± .7 | −0.1 ± .5 | 0.76 |
| P-value for Δ within group | 0.20 | 0.04 |
Table 4.
Changes in patient-reported outcomes and body composition within arms and between arms.
| Outcome | Arm A | Arm B |
P-value for difference between Arm A and Arm B |
|---|---|---|---|
| Physical functioning (PROMIS score), mean ± SD | |||
| n | 44 | 45 | |
| Baseline | 47.6 ± 6.4 | 47.0 ± 6.1 | 0.67 |
| Follow-up | 47.2 ± 6.1 | 46.9 ± 7.7 | 0.84 |
| Δ within group | −0.4 ± 4.6 | −0.1 ± 5.3 | 0.79 |
| P-value for Δ within group | 0.56 | 0.87 | |
| Health-related quality of life (FACT-Hep score), mean ± SD | |||
| n | 45 | 46 | |
| Baseline | 139.6 ± 20.7 | 140.3 ± 20.5 | 0.86 |
| Follow-up | 141.6 ± 23.7 | 141.1 ± 23.7 | 0.93 |
| Δ within group | 2.0 ± 16.3 | 0.8 ± 19.6 | 0.75 |
| P-value for Δ within group | 0.41 | 0.78 | |
| Skeletal muscle index (cm2/m2), mean ± SD | |||
| Females | |||
| n | 26 | 22 | |
| Baseline | 41.1 ± 7.8 | 41.1 ± 5.1 | 0.99 |
| Follow-up | 41.0 ± 6.9 | 40.4 ± 6.5 | 0.76 |
| Δ within group | −0.04 ± 3.3 | −.6 ± 4.7 | 0.61 |
| P-value for Δ within group | 0.95 | 0.09 | |
| Males | |||
| n | 30 | 38 | |
| Baseline | 55.0 ± 10.3 | 50.8 ± 7.8 | 0.06 |
| Follow-up | 54.3 ± 8.6 | 49.5 ± 6.6 | 0.01 |
| Δ within group | −0.7 ± 5.9 | −1.3 ± 4.6 | 0.62 |
| P-value for Δ within group | 0.53 | 0.54 | |
| Skeletal muscle density (HU), mean ± SD | |||
| n | 56 | 60 | |
| Baseline | 38.0 ± 11.0 | 37.3 ± 7.7 | 0.71 |
| Follow-up | 38.4 ± 11.0 | 38.4 ± 8.8 | 0.99 |
| Δ within group | 0.5 ± 7.3 | 1.1 ± 7.2 | 0.63 |
| P-value for Δ within group | 0.64 | 0.24 |
Overall, participants’ mean nutritional risk score improved following neoadjuvant therapy from 6.9 ± 5.3 to 3.5 ± 4.1 (n = 99, P < 0.001). The improvement in nutritional risk score of participants at high malnutrition risk at baseline (n = 53, mean change −6.8 ± 6.3) was greater than that of participants at low malnutrition risk at baseline (n = 46, mean change 0.5 ± 3.8, P < 0.001).
Following neoadjuvant therapy, 37 (49%) patients on Arm A and 42 (56%) patients on Arm B underwent pancreatectomy (P = 0.4). There were no differences between arms in mean length of stay (5.7 vs. 6.0 days, P = 0.6), readmission (27% vs. 29%, P = 0.5), or grade 3 postoperative adverse events (16% vs. 14%, P = 0.5).
Associations between activity variables and changes in outcome measures for all study participants (with both study groups pooled) are reported in Table 5. Self-reported moderate-to-strenuous exercise was favorably associated with changes in 6MWD, handgrip strength, arm curl repetitions, self-reported physical functioning, and skeletal muscle index after adjustment for baseline values of the outcome measures. Self-reported moderate-to-strenuous exercise was also associated with higher likelihood of surgical resection and, among the subsample of participants who underwent surgical resection, lower likelihood of readmission. Weekly objectively measured activity was favorably associated with changes in handgrip strength, self-reported physical functioning, and skeletal muscle index. Daily steps were favorably associated with changes in 6MWD, 5xSTS, handgrip strength, gait speed, and self-reported physical functioning.
Table 5. Associations between physical activity and exercise variables and changes in outcome measures.
Linear or logistic († Y=1, N=0) regression models; the study groups were pooled and all adjusted for the baseline value of the outcome measure of interest. Abbreviations: 6MWD, six-minute walk distance (meters); 5xSTS (seconds), five-time sit-to-stand; grip, handgrip strength (kg); arm curls (repetitions); 3MWT, 3-meter walk test (seconds); SMI, skeletal muscle index (cm2/m2); SMD, skeletal muscle density ( Hounsfield units); LOS, length of stay (days).
| 6MWD | 5xSTS | Grip | Arm curls |
3MWT | PROMIS score |
FACT- Hep |
SMI | SMD | Surgical resection† |
LOS | Readmission† | Grade 3 complication(s)† |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Self-reported weekly moderate-to-strenuous exercise minutes | n | 100 | 96 | 100 | 98 | 99 | 89 | 91 | 91 | 91 | 101 | 62 | 62 | 62 |
| β ± SD | 0.1 ± 0.04 | −0.003 ± 0.001 | 0.006 ± 0.003 | 0.006 ± 0.002 | 0.000 ± 0.000 | 0.01 ± 0.004 | 0.02 ± 0.01 | 0.008 ± 0.003 | 0.003 ± 0.005 | 0.004 ± 0.002 | 0.003 ± 0.002 | −0.01 ± 0.4 | −0.002 ± 0.002 | |
| P | 0.01 | 0.09 | 0.03 | 0.02 | 0.4 | 0.006 | 0.10 | 0.004 | 0.6 | 0.03 | 0.1 | 0.01 | 0.4 | |
| Self-reported weekly strength training sessions | n | 100 | 96 | 100 | 98 | 99 | 89 | 91 | 91 | 91 | 101 | 62 | 62 | 62 |
| β ± SD | 0.4 ± 3.5 | −0.1 ± 0.1 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.02 ± 0.02 | −0.05 ± 0.3 | 1.4 ± 1.0 | 0.3 ± 0.3 | 0.2 ± 0.4 | 0.08 ± 0.1 | −0.1 ± 0.2 | −0.3 ± 0.2 | −0.1 ± 0.2 | |
| P | 0.5 | 0.4 | 0.3 | 0.09 | 0.3 | 0.9 | 0.2 | 0.3 | 0.7 | 0.5 | 0.5 | 0.1 | 0.9 | |
| Weekly objectively-measured active minutes | N | 96 | 92 | 95 | 94 | 93 | 73 | 74 | 90 | 90 | 112 | 59 | 59 | 59 |
| β ± SD | 0.08 ± 0.05 | −0.002 ± 0.002 | 0.009 ± 0.003 | 0.004 ± 0.003 | 0.000 ± 0.000 | 0.01 ± 0.004 | 0.01 ± 0.02 | 0.009 ± 0.004 | −0.001 ± 0.006 | 0.000 ± 0.001 | 0.003 ± 0.003 | −0.004 ± 0.003 | −0.004 ± 0.003 | |
| P | 0.09 | 0.4 | 0.02 | 0.2 | 0.7 | 0.02 | 0.4 | 0.02 | 0.9 | 0.8 | 0.3 | 0.1 | 0.3 | |
| Daily objectively-measured step count | n | 96 | 92 | 95 | 94 | 93 | 73 | 74 | 90 | 90 | 112 | 59 | 59 | 59 |
| β ± SD | 0.008 ± 0.003 | 0.000 ± 0.000 | 0.001 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.001 | 0.000 ± 0.000 | 0.001 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | |
| P | 0.01 | 0.04 | 0.009 | 0.2 | 0.07 | 0.001 | 0.4 | 0.09 | 0.1 | 0.4 | 0.1 | 0.08 | 0.1 |
DISCUSSION
In this pragmatic randomized clinical trial comparing prescribed exercise with enhanced usual care during neoadjuvant therapy for PC, there was a statistically and clinically significant improvement in the primary endpoint, 6-minute walk distance, in both arms. In a pooled analysis of all accrued participants, activity and exercise were favorably associated with multiple measures of physical performance as well as with receipt of pancreatectomy and likelihood of postoperative readmission.
Activity and exercise are increasingly recognized as critical to the health and well-being of people with cancer. Guidelines for cancer survivors published in 2019 recommend moderate-intensity aerobic training at least three times per week, for at least 30 minutes, with resistance training at least two times per week.23 Although these guidelines are meant to reduce anxiety, depression, and fatigue and improve quality of life and perceived physical function of people with cancer, adherence without intervention is woefully uncommon. For example, we found that only 24% of survivors who had previously undergone pancreatectomy for pancreatic or periampullary cancer were adherent to both of these aerobic or strengthening exercise guidelines, and 39% did not meet either of them.35 Despite active concurrent treatment for PC and high rates of pre-existing comorbidity and sarcopenia, the participants in this study maintained average weekly minutes of activity that exceeded those recommended in these guidelines. Furthermore, participants in Arm B performed resistance exercises at an average number of sessions greater than that recommended in these guidelines.
The data from our study suggest the clinical potential of a simple, home-based, low-cost exercise prescription in the preoperative setting. However, two important caveats exist with regard to this conclusion. First, the inaccuracy of self-reported measures of exercise notwithstanding,42 levels of self-reported aerobic exercise did not increase between baseline and follow-up within either arm. While activity trackers measured average levels of activity that were remarkable, the extent to which this activity actually reflects exercise prescribed in the program is therefore unclear. Second, there was substantial variability between participants’ self-reported exercise and objectively measured PA. We previously investigated interpersonal and environmental factors that might contribute to such variability and found that encouragement and support from family and friends was a key influence; barriers included treatment, weather, time, and resources.43 It may be that simple motivation from the healthcare team, combined with concerted efforts to reduce barriers—as opposed to a well-defined exercise prescription—may be all that is needed to drive outcome-changing behavior in this setting.
Indeed, participants in both arms of this study had statistically and clinically significant improvement in functional status, measured by 6-minute walk distance, as well as improvement in functional measures of upper- and lower-body muscle group function. And, a pooled analysis of all participants identified associations between activity and key physical and clinical outcomes including pancreatectomy and readmission. Physical function tests have been demonstrated to be prognostic among patients with cancer.32 Other studies have suggested that exercise can improve aerobic fitness and functional capacity44 and reduce length of stay45 and perioperative complications44, 46 among patients anticipated to undergo surgery for gastrointestinal cancers. Preoperative exercise should thus be viewed as a central component of enhanced recovery after major abdominal surgery protocols. We have incorporated preoperative exercise as a fundamental component of perioperative treatment pathways for all patients with PC.47
We also emphasize attention to preoperative nutrition, particularly for patients who present with malnutrition or who are at high risk for it. In this study, participants in both study arms at high risk for malnutrition received a complete nutritional assessment and personalized recommendations by a registered clinical dietician, with favorable results.
Strong influence exists from national organizations to incorporate exercise assessment and prescription into standard care for patients during and after cancer treatment.11, 12, 23 Our group has no equipoise regarding the favorable, yet largely unproven, effects of exercise in the preoperative setting. We therefore could not ethically randomize patients to a “no exercise” arm. The improvement in submaximal exercise capacity observed in both study arms may therefore be attributed to the fact that both arms received extrinsic motivation from the healthcare team to exercise, as well as activity monitors, which are known to provide motivation for PA.48 Nonetheless, as activity self-monitoring becomes more commonplace with commercially available trackers and smartphones, this “enhanced usual care” condition may actually represent a real-world control condition.
Other limitations of this study exist. For example, although the comprehensive exercise prescribed to participants in Arm B was developed by a physical medicine and rehabilitation physician and a doctorate researcher in kinesiology in alignment with national guidelines, it is certainly possible that it was suboptimally effective. We could have encouraged longer, continuous bouts of exercise, for example, or increased the monitoring of resistance training. Further, prior data suggest that adherence and results may be maximized using a supervised program, although a home-based program is easier to implement in our quaternary care center.23 Finally, as we clearly documented, some data for outcome measures are missing because patients did not return for in-person clinic follow-ups during the COVID-19 pandemic, pursued treatment elsewhere, or developed disease progression and opted to terminate follow-up. However, this was not a significant concern for the primary endpoint.
The strengths of this study must also be acknowledged. To our knowledge, this is the most robust randomized study of the effects of exercise concurrent with preoperative therapy specifically for PC. Patients with PC are a unique population who are commonly frail and sarcopenic.6 Exercise is hypothesized as critical for patients undergoing symptomatically taxing neoadjuvant chemotherapy and/or (chemo)radiation and is a necessary intervention to support rapid recovery after pancreatectomy. However, few trials have studied exercise in this setting. We also obtained blood and tissue samples from participants, which will be evaluated for the effects of preoperative exercise on tumor vasculature and immune cell function, as an extension of our previous analyses.49, 50
In conclusion, in this randomized trial comparing prescribed exercise with enhanced usual care during neoadjuvant therapy for pancreatic cancer, there was significant improvement in submaximal exercise capacity in both arms. Physical activity and exercise were favorably associated with improved fitness, physical function, and clinical outcomes, highlighting the importance of encouraging physical activity among all patients preparing for pancreatectomy.
Supplementary Material
Acknowledgements:
The manuscript was edited by Sarah Bronson, ELS, of the Research Medical Library at The University of Texas MD Anderson Cancer Center.
Funding:
This study was supported by the Knox Family Foundation; National Institutes of Health/National Cancer Institute (award number 5R21CA218732-02); Center for Energy Balance in Cancer Prevention & Survivorship, Duncan Family Institute for Cancer Prevention and Risk Assessment; Cancer Prevention & Research Institute of Texas Training Grant/MD Anderson Cancer Prevention Research Training Program RP170259, (PI: Shine Chang); TREC Training Workshop R25CA203650 (PI: Melinda Irwin); and the National Institutes of Health/National Cancer Institute (award number P30CA016672; used the Clinical Trials Support Resource and the Biostatistics Resource Group).
Data availability:
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
REFERENCES
- 1.National Cancer Institute Surveillance Epidemiology and End Results Program. Cancer Stat Facts: Pancreatic Cancer 2022. Available at: https://seer.cancer.gov/statfacts/html/pancreas.html. Accessed January 19, 2023, 2022.
- 2.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013; 310(14):1473–81. [DOI] [PubMed] [Google Scholar]
- 3.Van Eijck CHJ, Versteijne E, van Tienhoven G. Preoperative chemoradiotherapy to improve overall survival in pancreatic cancer: Long-term results of the multicenter randomized phase III PREOPANC trial. 2021. [Google Scholar]
- 4.Katz MHG, Shi Q, Meyers J, et al. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol 2022; 8(9):1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khorana AA, Mangu PB, Berlin J, et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016; 34(21):2541–56. [DOI] [PubMed] [Google Scholar]
- 6.Ngo-Huang A, Holmes HM, des Bordes JKA, et al. Association between frailty syndrome and survival in patients with pancreatic adenocarcinoma. Cancer Med 2019; 8(6):2867–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale W, Hemmerich J, Kamm A, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg 2014; 259(5):960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 9.Bozzetti F Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 2017; 28(9):2107–2118. [DOI] [PubMed] [Google Scholar]
- 10.Betge J, Schulte N, Belle S, et al. Neglected geriatric assessment and overtreatment of older patients with pancreatic cancer - Results from a prospective phase IV clinical trial. J Geriatr Oncol 2022; 13(5):662–666. [DOI] [PubMed] [Google Scholar]
- 11.Rock CL, Thomson CA, Sullivan KR, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA: A Cancer Journal for Clinicians 2022; 72(3):230–262. [DOI] [PubMed] [Google Scholar]
- 12.Ligibel JA, Bohlke K, May AM, et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J Clin Oncol 2022; 40(22):2491–2507. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Morielli AR, Heer E, et al. Effects of Exercise on Cancer Treatment Efficacy: A Systematic Review of Preclinical and Clinical Studies. Cancer Res 2021; 81(19):4889–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta P, Hodgman CF, Schadler KL, et al. Effect of exercise on pancreatic cancer patients during treatment: a scoping review of the literature. Support Care Cancer 2022; 30(7):5669–5690. [DOI] [PubMed] [Google Scholar]
- 15.Ausania F, Senra P, Meléndez R, et al. Prehabilitation in patients undergoing pancreaticoduodenectomy: a randomized controlled trial. Rev Esp Enferm Dig 2019; 111(8):603–608. [DOI] [PubMed] [Google Scholar]
- 16.Ngo-Huang A, Parker NH, Bruera E, et al. Home-Based Exercise Prehabilitation During Preoperative Treatment for Pancreatic Cancer Is Associated With Improvement in Physical Function and Quality of Life. Integr Cancer Ther 2019; 18:1534735419894061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker NH, Gorzelitz J, Ngo-Huang A, et al. The Role of Home-Based Exercise in Maintaining Skeletal Muscle During Preoperative Pancreatic Cancer Treatment. Integr Cancer Ther 2021; 20:1534735420986615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker NH, Ngo-Huang A, Lee RE, et al. Physical activity and exercise during preoperative pancreatic cancer treatment. Support Care Cancer 2019; 27(6):2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci 1992; 17(4):338–45. [PubMed] [Google Scholar]
- 20.Jensen RE, Potosky AL, Reeve BB, et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res 2015; 24(10):2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amireault S, Godin G. The Godin-Shephard leisure-time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills 2015; 120(2):604–22. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010; 42(7):1409–26. [DOI] [PubMed] [Google Scholar]
- 23.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 2019; 51(11):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002. Jul 1;166(1):111–7. [DOI] [PubMed] [Google Scholar]
- 25.Pecorelli N, Fiore JF Jr., Gillis C, et al. The six-minute walk test as a measure of postoperative recovery after colorectal resection: further examination of its measurement properties. Surg Endosc 2016; 30(6):2199–206. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt K, Vogt L, Thiel C, et al. Validity of the six-minute walk test in cancer patients. Int J Sports Med 2013; 34(7):631–6. [DOI] [PubMed] [Google Scholar]
- 27.Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 2017; 23(2):377–381. [DOI] [PubMed] [Google Scholar]
- 28.Carli F, Charlebois P, Stein B, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg 2010; 97(8):1187–97. [DOI] [PubMed] [Google Scholar]
- 29.Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills 2006; 103(1):215–22. [DOI] [PubMed] [Google Scholar]
- 30.Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2013; 53(2):255–67. [DOI] [PubMed] [Google Scholar]
- 31.Kilgour RD, Vigano A, Trutschnigg B, et al. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer 2013; 21(12):3261–70. [DOI] [PubMed] [Google Scholar]
- 32.Verweij NM, Schiphorst AH, Pronk A, et al. Physical performance measures for predicting outcome in cancer patients: a systematic review. Acta Oncol 2016; 55(12):1386–1391. [DOI] [PubMed] [Google Scholar]
- 33.Amireault S, Godin G, Lacombe J, et al. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol 2015; 15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finney Rutten LJ, Blake KD, Skolnick VG, et al. Data Resource Profile: The National Cancer Institute's Health Information National Trends Survey (HINTS). Int J Epidemiol 2020; 49(1):17–17j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker NH, Basen-Engquist K, Rubin ML, et al. Factors Influencing Exercise Following Pancreatic Tumor Resection. Annals of Surgical Oncology 2021; 28(4):2299–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol 2002; 20(9):2229–39. [DOI] [PubMed] [Google Scholar]
- 37.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008; 33(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz L, Bruno M, Parker NH, et al. Active Surveillance for Adverse Events Within 90 Days: The Standard for Reporting Surgical Outcomes After Pancreatectomy. Ann Surg Oncol 2015; 22(11):3522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brewer W, Swanson BT, Ortiz A. Validity of Fitbit’s active minutes as compared with a research-grade accelerometer and self-reported measures. & Exercise Medicine 2017; 3(1):e000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardcastle SJ, Jimenez-Castuera R, Maxwell-Smith C, et al. Fitbit wear-time and patterns of activity in cancer survivors throughout a physical activity intervention and follow-up: Exploratory analysis from a randomised controlled trial. PLoS One 2020; 15(10):e0240967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orstad SL, Gerchow L, Patel NR, et al. Defining Valid Activity Monitor Data: A Multimethod Analysis of Weight-Loss Intervention Participants’ Barriers to Wear and First 100 Days of Physical Activity. Informatics 2021; 8(2):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prince SA, Adamo KB, Hamel ME, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 2008; 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker NH, Lee RE, O'Connor DP, et al. Supports and Barriers to Home-Based Physical Activity During Preoperative Treatment of Pancreatic Cancer: A Mixed-Methods Study. J Phys Act Health 2019; 16(12):1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pang NQ, Tan YX, Samuel M, et al. Multimodal prehabilitation in older adults before major abdominal surgery: a systematic review and meta-analysis. Langenbecks Arch Surg 2022; 407(6):2193–2204. [DOI] [PubMed] [Google Scholar]
- 45.Nakajima H, Yokoyama Y, Inoue T, et al. Clinical Benefit of Preoperative Exercise and Nutritional Therapy for Patients Undergoing Hepato-Pancreato-Biliary Surgeries for Malignancy. Ann Surg Oncol 2019; 26(1):264–272. [DOI] [PubMed] [Google Scholar]
- 46.Berkel AEM, Bongers BC, Kotte H, et al. Effects of Community-based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery With High Risk for Postoperative Complications: Results of a Randomized Clinical Trial. Ann Surg 2022; 275(2):e299–e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denbo JW, Bruno M, Dewhurst W, et al. Risk-stratified clinical pathways decrease the duration of hospitalization and costs of perioperative care after pancreatectomy. Surgery 2018; 164(3):424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh B, Zopf EM, Howden EJ. Effect and feasibility of wearable physical activity trackers and pedometers for increasing physical activity and improving health outcomes in cancer survivors: A systematic review and meta-analysis. J Sport Health Sci 2022; 11(2):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Florez Bedoya CA, Cardoso ACF, Parker N, et al. Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci Rep 2019; 9(1):13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurz E, Hirsch CA, Dalton T, et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell 2022; 40(7):720–737.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.