Abstract
Background
Despite the well-known association between hypertensive disorders of pregnancy (HDP) and cardiovascular disease, there is limited data on which specific cardiovascular diagnoses have the greatest risk profiles during the first 24 months’ postpartum. Most existing data on hypertensive disorders of pregnancy and short-term cardiovascular disease risk is limited to the immediate postpartum period; however, it is critical to determine cardiovascular disease risk up to 24 months’ postpartum to inform cardiovascular disease screening protocols during the extended postpartum period.
Objective
We aimed to delineate the risk of significant cardiovascular diagnoses in the first 24 months’ postpartum among patients with HDP compared with patients without HDP.
Study Design
Our longitudinal population-based study included pregnant individuals with deliveries during 2007–2019 in the Maine Health Data Organization’s All Payer Claims Data. We excluded those with pre-existing cardiovascular disease, with multifetal gestations, or without continuous insurance during pregnancy. HDP and cardiovascular disease (categorized by specific condition: heart failure, ischemic heart disease, arrhythmia/cardiac arrest, cardiomyopathy, cerebrovascular disease/stroke, and new chronic hypertension) were identified by ICD 9/10 diagnosis codes. Cox proportional hazards models were used to estimate hazard ratios (HR), adjusting for potential confounding factors.
Results
Of the 119,422 pregnancies examined, the cumulative risk of cardiovascular disease within 24 months’ postpartum for those with HDP vs. without HDP was 0.6% vs. 0.2% for heart failure, 0.3% vs. 0.1% for ischemic heart disease, 0.2% vs. 0.2% for arrhythmia/cardiac arrest, 0.6% vs. 0.2% for cardiomyopathy, 0.8% vs. 0.4% for cerebrovascular disease/stroke, 1.6% vs. 0.7% for severe cardiac disease (composite outcome of heart failure, cerebrovascular disease/stroke, or cardiomyopathy), and 9.7% vs. 1.5% for new chronic hypertension. After adjustment for potential confounders, those with HDP had increased risk of heart failure, cerebrovascular disease, cardiomyopathy, and severe cardiac disease within the first 24 months’ postpartum (adjusted HR (aHR) 2.81 (95% CI 1.90–4.15), aHR 1.43 (95% CI 1.07–1.91), aHR 2.90 (95% CI 1.96–4.27), and aHR 1.90 (95% CI 1.54–2.30), respectively) as compared to those without HDP. In addition, those with HDP had an increased risk for new chronic hypertension diagnosed after 42 days’ postpartum, (aHR 7.29 (95% CI 6.57–8.09)). There was no association between HDP and ischemic heart disease (aHR 0.92 (95% CI 0.55–1.54) or cardiac arrest/arrhythmia (aHR 0.90 (95% CI 0.52–1.57). In addition, among women with HDP, the highest proportion of first cardiovascular disease diagnoses occurred during the first month postpartum for cardiomyopathy (44%), heart failure (39%), cerebrovascular disease/stroke (39%), and severe cardiac disease (41%).
Conclusion
Patients with HDP had an increased risk for development of new chronic hypertension, heart failure, cerebrovascular disease, and cardiomyopathy within 24 months’ postpartum. There was no association between HDP and ischemic heart disease or cardiac arrest/arrhythmia. Patients with HDP need targeted early postpartum interventions and increased monitoring over the first 24 months. This may preserve long term health and improve maternal and neonatal outcomes in a subsequent pregnancy.
Keywords: gestational hypertension, preeclampsia, eclampsia, postpartum screening, heart failure, cerebrovascular disease, cardiomyopathy, chronic hypertension, ischemic heart disease
Introduction
Cardiovascular disease (CVD) is the leading cause of death among women in the US, accounting for 1 in 5 deaths.1 It is well established that hypertensive disorders of pregnancy (HDP) confer a substantial increased risk of long-term cardiovascular morbidity and mortality decades after delivery.2–5 However, in the immediate postpartum period, most existing data on short-term cardiovascular disease risk is from 6 weeks’ postpartum up to 12 months’.6–12 There is very limited data on CVD risk up to 24 months’ postpartum.
Because pregnancy is a vascular stress test, the initial 24 months’ postpartum offers a unique window of opportunity to identify women at greatest risk, and implement life-saving cardiovascular screening and prevention.6,7 In order to reduce cardiovascular morbidity and mortality, identification of early risk factors and implementation of individualized prevention strategies during the postpartum period are critical.6,13–16 The American Heart Association (AHA) and American College of Obstetricians and Gynecologists (ACOG) issued urgent calls to action to address this staggering problem of cardiovascular morbidity and mortality for women.17–19
Unfortunately, the optimal postpartum screening strategy for women with HDP remains unclear. Currently, ACOG recommends women with HDP have long-term follow-up postpartum with more frequent visits with their primary care provider and a CVD risk assessment, which includes assessment of the effect of social determinants of health on cardiometabolic disease.20–23 Despite a few cardiovascular screening clinics with evaluations between 6–12 months’ postpartum, including the Mothers Program Maternal Health Clinic in Ontario, Canada, these CVD screening clinics are not considered standard of care in the United States.6,15,16,24 This is in part due to the lack of financial reimbursement for patients who lose healthcare insurance after 6 weeks’ postpartum.18 In addition, it is unclear the optimal timing for CVD risk assessment postpartum due to the lack of identification of specific CVD risks within 24 months’ postpartum and which CVD conditions occur soonest after delivery. Most prior studies have focused either on 1–2 cardiovascular diseases such as heart failure or coronary artery disease or a composite of many cardiovascular diseases.2–5 In order to effectively screen for cardiovascular morbidity and mortality among women, we must first be able to identify the specific CVD diagnoses that occur as early as 24 months’ postpartum.19
Therefore, we sought to delineate the risk and timing of onset of key cardiovascular diagnoses in the first 24 months’ postpartum among patients with HDP compared with patients without HDP.
Materials and Methods
We performed a longitudinal population-based study of multiparous and nulliparous individuals with singleton livebirth or stillbirth gestations to determine the risk of significant cardiovascular diagnoses in the first 24 months’ postpartum, comparing patients with HDP to those without HDP. Data from the Maine Health Data Organization’s All Payer Claims Data (APCD) was used and included pregnant individuals with deliveries in Maine that were paid for by either private or public insurers.25 The largest possible sample size in our database, 13 years of deliveries, was utilized in order to maximize power. The All Payer Claims Data includes both facility and professional claims for hospital inpatient and outpatient encounters and office visits.25 The International Classification of Diseases, Clinical Modification (ICD-CM) diagnosis and procedure codes, current procedural terminology (CPT) codes, and the Medicare Severity Diagnosis Related Group (MS-DRG) classification system were used to identify deliveries during the study period. APCD must meet internal quality standards before being released for analysis.25
HDP included one or more of the following diagnoses: gestational hypertension, preeclampsia without severe features, preeclampsia with severe features, superimposed preeclampsia with or without severe features, or eclampsia.20 Claims met the criteria for HDP if there were 2 or more outpatient visits (to avoid patients presenting for rule-out HDP) with non-severe HDP diagnosis codes or at least 1 inpatient visit with non-severe or severe HDP diagnosis codes from 20 weeks of gestation until 42 days’ postpartum. HDP were defined using a previously published method using ICD-9 codes.26 The ICD-9 codes were then cross-walked to the corresponding ICD-10 diagnosis codes (Appendix 1).
The primary outcome was cardiovascular disease, categorized into 6 sub-categories: heart failure, ischemic heart disease, cerebrovascular disease/stroke, arrhythmia/cardiac arrest, cardiomyopathy, and new chronic hypertension. In addition, given the anticipated rarity of some of the CVD outcomes, a composite outcome of severe cardiac disease was developed a priori that included heart failure, cerebrovascular disease/stroke, and cardiomyopathy. We used previously published cross-walked ICD 9/10 code lists (Cartus et al; Ackerman et al) and added “not otherwise specified” cardiovascular disease diagnosis codes based on review by Maternal Fetal Medicine physician study authors (C.A.B, H.S.L.) (Appendix 1).27,28 They were defined from the delivery date until 24 months’ postpartum.
In contrast, new chronic hypertension was defined only from 43 days’ postpartum until 24 months’ postpartum because hypertension within 42 days of delivery is considered a HDP.29,30 The Centers for Disease Control and Prevention code list was used to define new chronic hypertension.31
Socioeconomic factors and access to care were estimated by linking the All Payer Claims Data to publicly available community-level information. ZIP-code level data on the median percentage of residents living below federal poverty level, of non-White race/ethnicity, and who were adults with less than college educational attainment were identified from the American Community Survey 5-year ZIP-code files.32 To assess population-level access to care measures, information on the number of general practice and medical specialties physicians per capita from the Area Health Resources Files were linked by county Federal Information Processing Standard code.33 Insurance status was assessed using the All Payer Claims Data eligibility file. People were classified as Medicaid insured if they were enrolled in Medicaid during their delivery month.34 Otherwise, they were classified as insured by commercial insurance or Medicare based on delivery month enrollment information.
Cox proportional hazard models were used to estimate hazard ratios and 95% confidence intervals for the time to first diagnosis for each of the 6 cardiovascular conditions in the first 24 months’ postpartum. Models were adjusted for potential confounding factors, including maternal age at time of delivery, pre-pregnancy depression (diagnosed from one year prior to conception to 6 weeks of gestation), pre-existing chronic hypertension (diagnosed from one year prior to conception to 20 weeks of gestation), pre-pregnancy diabetes, obesity, smoking, nulliparity, pregnancy number in dataset (as a proxy for parity), year of delivery, Medicaid coverage during pregnancy, county-level measures, ZIP-code level measures (including percentage of population with non-White race/ethnicity), prenatal depression (diagnosed from 6 weeks’ gestation until delivery), and gestational diabetes. Each model excluded records with any diagnosis before pregnancy of the cardiovascular conditions being examined postpartum, and records with gestational age <20 weeks (i.e. miscarriages erroneously coded as livebirths or stillbirths), non-Maine residence, multifetal gestation, those without evidence of insurance in the month of delivery, those with implausible time to next pregnancy (<60 days), and those without health insurance during pregnancy and through the first 2 months’ postpartum. We excluded multifetal pregnancies because of their increased risk of pregnancy complications such as HDP and cardiovascular complications, including cardiac arrest, compared to their singleton counterparts.35,36 Those with pre-existing chronic hypertension were excluded from models examining new postpartum chronic hypertension, but for the other CVD outcomes, persons with pre-existing chronic hypertension or other cardiovascular conditions during pregnancy were not excluded in the primary analysis. Observations were censored upon loss of health insurance coverage, start of next pregnancy, or at 24 months, whichever came first. As a sensitivity analysis, models were rerun after stratification by pre-existing chronic hypertension to see if the effect of HDP on CVD outcomes differed by this key pre-existing medical condition. To visualize the survival curves, we used inverse-probability-of-treatment-weighted Cox proportional hazard models.37 All time-to-event analyses used month since delivery as the time scale.
Results
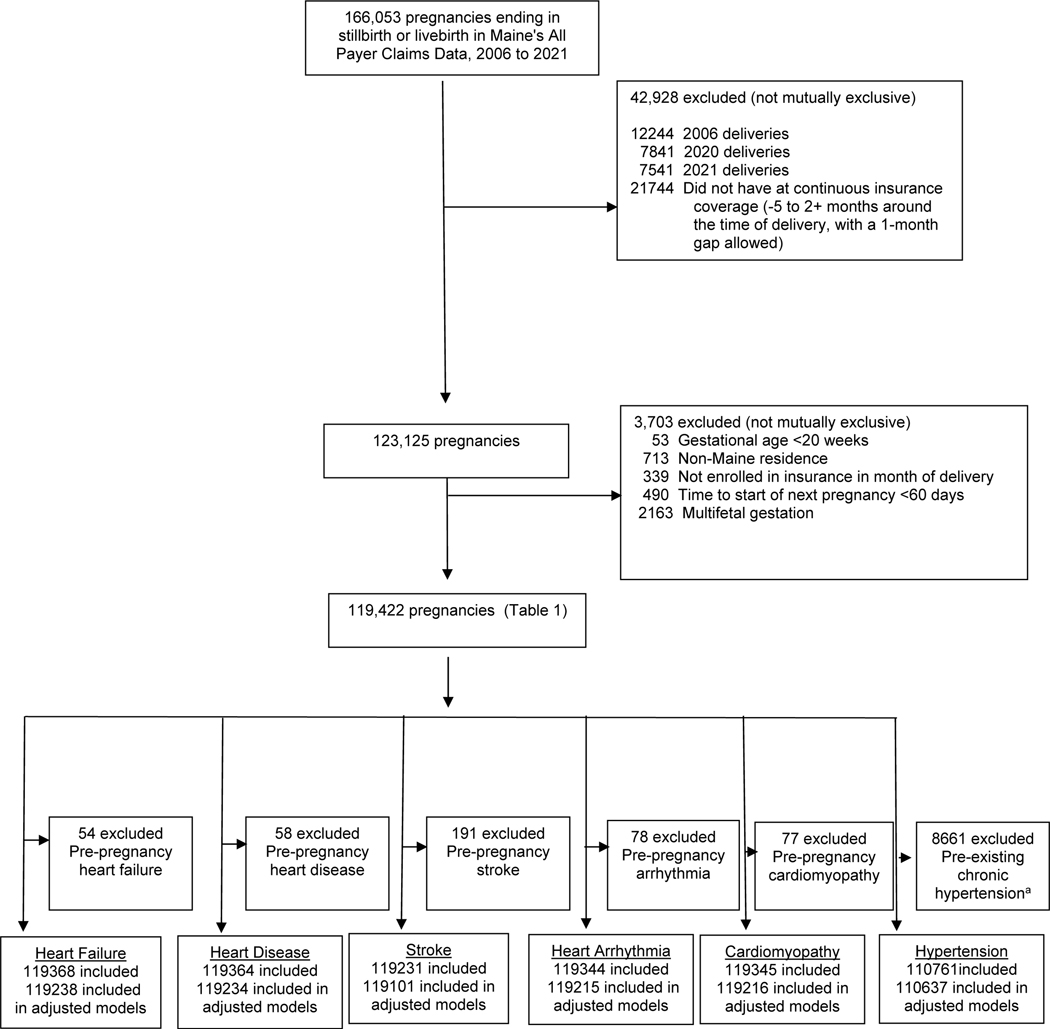
Out of the 166,053 unique pregnancies in the Maine Health Data Organization’s All Payer Claims Data from 2006 to 2021, we included 119,422 pregnancies (Figure 1). The prevalence of HDP was 12.4% (Table 1). Preterm delivery, cesarean section, pre-existing diabetes, obesity, and pre-existing chronic hypertension were more commonly observed in the HDP group. The cumulative risk of cardiovascular disease within 24 months’ postpartum for those with HDP vs. without HDP was 0.6% vs. 0.2% for heart failure, 0.3% vs. 0.1% for ischemic heart disease, 0.2% vs. 0.2% for arrhythmia/cardiac arrest, 0.6% vs. 0.2% for cardiomyopathy, 0.8% vs. 0.4% for cerebrovascular disease/stroke; 1.6% vs. 0.7% for severe cardiac disease; and 9.7% vs. 1.5% for new chronic hypertension (Table 2).
Figure 1: Analytic Sample Identification.
aPre-existing chronic hypertension was defined as hypertension from one year prior to conception to 20 weeks of gestation
Table 1:
Characteristics of deliveries in Maine 2007–2019
| Total | No hypertensive disorder of pregnancy | Hypertensive disorder of pregnancy | |||
|---|---|---|---|---|---|
| N | N | Column % | N | Column % | |
|
| |||||
| Total | 119422 | 104785 | 100.0 | 14637 | 100.0 |
| Row % | 87.6 | 12.4 | |||
| Maternal age at delivery | |||||
| Missing | 50 | 42 | 0.0 | a | a |
| 15 to 19 | 7392 | 6548 | 6.3 | 844 | 5.8 |
| 20 to 24 | 28161 | 24903 | 23.8 | 3258 | 22.3 |
| 25 to 29 | 35761 | 31396 | 30.0 | 4365 | 29.8 |
| 30 to 34 | 30257 | 26487 | 25.3 | 3770 | 25.8 |
| 35+ | 17801 | 15409 | 14.7 | 2392 | 16.3 |
| Stillbirth | 626 | 563 | 0.5 | 63 | 0.4 |
| Gestational age at delivery | |||||
| At least 37 weeks | 110450 | 97570 | 93.1 | 12880 | 88.0 |
| 20 to <37 weeks | 8972 | 7215 | 6.9 | 1757 | 12.0 |
| Cesarean Section | 34452 | 28610 | 27.3 | 5842 | 39.9 |
| Delivery number in dataset | |||||
| 1 | 79992 | 68943 | 65.8 | 11049 | 75.5 |
| 2 or more | 39430 | 35842 | 34.2 | 3588 | 24.5 |
| Insurance coverage | |||||
| Medicaid | 66014 | 58588 | 55.9 | 7426 | 50.7 |
| Private | 53120 | 45953 | 43.9 | 7167 | 49.0 |
| Medicare | 288 | 244 | 0.2 | 44 | 0.3 |
| Pre-existing diabetes | 3373 | 2490 | 2.4 | 883 | 6.0 |
| Obesity | 7165 | 5510 | 5.3 | 1655 | 11.3 |
| Pre-pregnancy heart failure b | 54 | 44 | 0.0 | 10 | 0.1 |
| Pre-pregnancy heart disease b | 58 | 46 | 0.0 | 12 | 0.1 |
| Pre-pregnancy stroke b | 191 | 158 | 0.2 | 33 | 0.2 |
| Pre-pregnancy arrhythmia b | 78 | 60 | 0.1 | 18 | 0.1 |
| Pre-pregnancy cardiomyopathy b | 77 | 60 | 0.1 | 17 | 0.1 |
| Pre-existing chronic hypertensionb | 8661 | 3323 | 3.2 | 5338 | 36.5 |
Data source: Maine Health Data Organization’s All Payer Claims Data
Observations were excluded from Cox regression modeling of the cardiovascular conditions being examined postpartum.
Data counts between 1 and 9 suppressed from presentation.
Table 2:
Cumulative risk of diagnosis in the first 24 months’ postpartum for specific
| Total | Number of events postpartum | %a | HDP | Non-HDP | %b among exposed | %b among unexposed | |
|---|---|---|---|---|---|---|---|
| Heart failure | 119368 | 202 | 0.2 | 14627 | 104741 | 0.6 | 0.2 |
| Ischemic heart disease | 119364 | 121 | 0.2 | 14625 | 104739 | 0.3 | 0.1 |
|
Cerebrovascular
disease/stroke |
119231 | 443 | 0.5 | 14604 | 104627 | 0.8 | 0.4 |
|
Arrhythmia/cardiac
arrest |
119344 | 151 | 0.2 | 14619 | 104725 | 0.2 | 0.2 |
| Cardiomyopathy | 119345 | 213 | 0.2 | 14620 | 104725 | 0.6 | 0.2 |
| Severe cardiac disease (heart failure, stroke, or cardiomyopathy) | 119124 | 741 | 0.8 | 14582 | 104542 | 1.6 | 0.7 |
| New chronic hypertension (43+ days after delivery) b | 110761 | 1662 | 2.1 | 9299 | 101462 | 9.7 | 1.5 |
Data source: Maine Health Data Organization’s All Payer Claims Data
HDP=hypertensive disorder of pregnancy
Cumulative risk by 24 months. Censoring events were loss of health insurance coverage or start of the next pregnancy, whichever was earlier. Each model excluded records with any diagnosis before pregnancy of the cardiovascular conditions being examined postpartum, and records with gestational age (<20 weeks), non-Maine residence, multifetal gestation, those with implausible time to next pregnancy (<60 days), and those without health insurance during pregnancy through the first 2 months’ postpartum.
Hypertension diagnoses in the first 42 days’ postpartum were not included as chronic hypertension outcomes, as these were included in the definition of hypertensive disorders of pregnancy.
Unadjusted hazard ratios for diagnoses of CVD within the first 24 months’ postpartum for people with vs. without HDP were 4.40 (95% CI 3.32–5.84) for heart failure, 2.34 (95% CI 1.56–3.52) for ischemic heart disease, 2.06 (95% CI 1.64–2.58) for cerebrovascular disease/stroke, 1.21 (95% CI 0.77–1.91) for arrythmia/cardiac arrest, 4.13 (95% CI 3.11–5.48) for cardiomyopathy, and 2.69 (95% CI 2.28–3.16) for the composite outcome of severe cardiac disease. After adjusting for potential confounders, those with HDP had increased risks of heart failure (adjusted hazard ratio (aHR) 2.81 (95% CI 1.90–4.15)), cerebrovascular disease/stroke (aHR 1.43 (95% CI 1.07–1.91)), cardiomyopathy (2.90 (95% CI 1.96–4.27)), and severe cardiac disease (aHR 1.90 (95% CI 1.54–2.36)) within the first 24 months’ postpartum, as compared to those without HDP (Table 3, Figure 2). There was no association between HDP and ischemic heart disease ((aHR 0.92 (95% CI 0.55–1.54)) or cardiac arrest/arrhythmia ((aHR 0.90 (95% CI 0.52–1.51)). The unadjusted hazard ratio for new diagnoses of chronic hypertension within the first 24 months’ postpartum for people with vs. without HDP was 7.30 (95% CI 6.61–8.06); after adjusting for potential confounders, the hazard ratio changed little (aHR 7.29 (95% CI 6.57–8.09)).
Table 3:
Risk of CVD disease in first 24 months’ postpartum for women with hypertensive disorders of pregnancy (HDP), deliveries in Maine 2007–2019, n=119,422
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) a | |
|---|---|---|
|
| ||
| Heart failure | ||
| No-HDP | Reference | Reference |
| HDP | 4.40 ( 3.32, 5.84) | 2.81 ( 1.90, 4.15) |
| Ischemic heart disease | ||
| No-HDP | Reference | Reference |
| HDP | 2.34 ( 1.56, 3.52) | 0.92 ( 0.55, 1.54) |
| Cerebrovascular disease/stroke | ||
| No-HDP | Reference | Reference |
| HDP | 2.06 ( 1.64, 2.58) | 1.43 ( 1.07, 1.91) |
| Arrhythmia/cardiac arrest | ||
| No-HDP | Reference | Reference |
| HDP | 1.21 ( 0.77, 1.91) | 0.90 ( 0.52, 1.57) |
| Cardiomyopathy | ||
| No-HDP | Reference | Reference |
| HDP | 4.13 ( 3.11, 5.48) | 2.90 ( 1.96, 4.27) |
| Severe cardiac disease (heart failure, stroke, or cardiomyopathy) | ||
| No-HDP | Reference | Reference |
| HDP | 2.69 (2.28, 3.16) | 1.90 (1.54, 2.36) |
| New chronic hypertension (43+ days after delivery)b | ||
| No-HDP | Reference | Reference |
| HDP | 7.30 ( 6.61, 8.06) | 7.29 ( 6.57, 8.09) |
Data source: Maine Health Data Organization’s All Payer Claims Data
CI=confidence interval; CVD= cardiovascular disease; HR= hazard ratio; HDP=hypertensive disorders of pregnancy
Adjusted for maternal age at time of delivery (continuous), pre-pregnancy depression (yes/no), pre-existing chronic hypertension (yes/no; for chronic hypertension models, these observations were excluded), pre-pregnancy diabetes (yes/no), obesity (yes/no), smoking (yes/no), nulliparity (yes/no), pregnancy number in dataset (continuous), year of delivery (continuous), Medicaid coverage during pregnancy (months covered, continuous), county-level measures (ratio of population to primary care physicians [continuous]; percentage uninsured adults [continuous]; percentage smoking adults [continuous]; percentage of adults with obesity [continuous]), ZIP-code level measures (percentage of non-white residents [continuous], percentage of adults with no Bachelor’s degree [continuous], percentage of persons living below poverty level [continuous]), prenatal depression (yes/no), and gestational diabetes (yes/no).
Hypertension diagnoses in the first 42 days’ postpartum were not included as chronic hypertension outcomes, as these were included in the definition of hypertensive disorders of pregnancy.
Figure 2: Weighted adjusted.
a cumulative hazard curves and 95% confidence interval bands for diagnosis of cardiovascular disease in the first 24 months’ postpartum among people with and without hypertensive disorders of pregnancy, deliveries in Maine 2007–2019, n= 119,422
aAdjusted for maternal age at time of delivery, pre-pregnancy depression, pre-existing chronic hypertension (for chronic hypertension models, these observations were excluded), pre-pregnancy diabetes, obesity, smoking, nulliparity, pregnancy number in dataset, year of delivery, Medicaid coverage during pregnancy, county-level measures, ZIP-code level measures, prenatal depression, and gestational diabetes.
Month 0 = month of delivery
After rerunning the analyses stratified by pre-existing chronic hypertension, all of the previously established associations between HDP and CVD persisted for those without pre-existing chronic hypertension; however, no significant associations were found for those with pre-existing chronic hypertension (Supplemental Table 2)
Among people with HDP, the highest proportion of first cardiovascular disease diagnoses occurred during the first month postpartum for cardiomyopathy (44%), heart failure (39%), cerebrovascular disease/stroke (39%), and severe cardiac disease (41%) (Supplemental Figure 1). However, for ischemic heart disease, arrhythmia/cardiac arrest, and new chronic hypertension (after the first 42 days’ postpartum) the month of first diagnosis was distributed fairly uniformly across the first 24 months’ postpartum.
Comment
Principal Findings:
Postpartum patients with a HDP have a significantly increased risk of most but not all cardiovascular diseases within the first 24 months’ postpartum, even after adjusting for potential confounders. Specifically, those with a HDP have nearly three times the risk for heart failure and cardiomyopathy within 24 months’ postpartum than their normotensive counterparts. Overall, of the six CVD outcomes we examined, the strongest association was for HDP and chronic hypertension. Patients with HDP were over 7 times as likely to have new chronic hypertension within 24’ months postpartum compared to those without HDP. These associations between HDP and CVD persisted even in the population with no pre-existing chronic hypertension. Among women with HDP, the highest proportion of first diagnoses for cardiomyopathy (44%), heart failure (39%), cerebrovascular disease/stroke (39%), and severe cardiac disease (41%) occurred during the first month postpartum. There was no association between HDP and ischemic heart disease or arrhythmia/cardiac arrest.
Results in the Context of What is Known:
Our findings of the association of HDP with CVD within 24 months’ postpartum are in alignment with previously published literature on the risks of CVD up to 12 months’ postpartum. When examining the risk of new chronic hypertension, a prior prospective cohort found that those with HDP have significantly increased odds of new chronic hypertension within 6–12 months postpartum (aOR 4.60).6 Other studies also support our finding of a strong association of HDP with a composite marker of severe cardiac disease (chronic hypertension, cerebrovascular disease/stroke, or cardiomyopathy) from as soon as 1 year postpartum through up to 40 years after delivery.2,4–6,15,38–43 In addition, our results have biologic plausibility based on previous literature on the cardiovascular abnormalities that occur during pregnancies complicated by a HDP. The maternal heart undergoes profound remodeling, including progressive increase in left ventricular mass, which is often a predictor of cardiovascular risk in the non-pregnant patient.44
Our findings of no associations between HDP and ischemic heart disease and HDP and arrythmia/cardiac arrest are somewhat unexpected. Prior studies found that individuals with preeclampsia have an increased risk of ischemic heart disease 11–27 years after delivery.38,45,46 This discrepancy in findings can be explained by the physiology of ischemic heart disease, which is typically dependent on the development of atherosclerotic plaques and takes years to build to a clinically significant level.19 There are few studies that have examined the association between HDP and arrhythmia/cardiac arrest. Small retrospective studies report a modest association between HDP with arrhythmia, but they included follow-up of up to 40 years since delivery.47 Our study is unique in that it examines these rare CVD outcomes within the immediate 24 months’ postpartum, which is a more clinically relevant time period because screenings and interventions could prevent downstream adverse cardiovascular events.
Clinical Implications:
Women’s cardiovascular health is a staggering public health problem because it leads to substantial premature and preventable morbidity and mortality. It is understudied, underdiagnosed, and undertreated.17 The first step in prioritizing women’s cardiovascular research and clinical care is to identify which specific cardiovascular diagnoses have the greatest risk profile postpartum for those with HDP 48 This can inform individualized postpartum screening and intervention program that is timely and targeted to the most clinically relevant cardiovascular diseases.14,49 The results of our study can inform this initiative because we found that HDP patients are at the highest risk for heart failure, stroke, cardiomyopathy, and new chronic hypertension in the first 24 months’ postpartum.
Therefore, while the well-known association between HDP and future CVD is indeed supported by our data, we present novel findings showing this association does not apply to all types of cardiovascular disease during the first 24 months’ postpartum. Future studies are needed to inform how to tailor postpartum cardiovascular screenings and interventions to specifically reduce the risk of these outcomes. Screenings could include a comprehensive history and physical exam, serum testing with cholesterol evaluation, diabetes evaluation, and possibly brain natriuretic peptide levels, cardiac imaging, and cardiology referral. In addition, since the highest proportion of first postpartum cardiovascular diagnoses for cardiomyopathy (44%), heart failure (39%), cerebrovascular disease/stroke (39%) occurred during month 0 postpartum (the month of delivery), patients with HDP may benefit from increased surveillance immediately postpartum and the use of a lower threshold for cardiac imaging and cardiology consultation. These screening and primary prevention interventions may preserve long-term cardiovascular health and improve maternal and neonatal outcomes in a subsequent pregnancy for patients with a HDP.
New chronic hypertension was distributed fairly uniformly across the first 24 months’ postpartum; this suggests that care specific to pregnancy complications should not be confined to the traditional 42-day postpartum period. Patients need access to care and resources that extend beyond 42 days’ postpartum. Our results provide evidence that supports advocacy efforts to extend insurance coverage and offer additional health resources for 24 months postpartum for those with pregnancy complications.50 Likewise, providers must focus on improving the transition of care to primary care physicians and/or cardiologists after the immediate postpartum period.
Research Implications:
Our study presents novel findings that HDP is not universally associated with all CVD subtypes. While many prior studies used a composite CVD outcome to examine the association between placental syndromes (including HDP) and CVD, 3,51 our findings suggest that HDP is not associated with ischemic heart disease or arrythmia/cardiac arrest in the initial 24 months’ postpartum. These CVD subtypes may not be appropriate to include in composite CVD measures. Future study in other more diverse populations are needed for confirmation.
As mentioned, few prior studies have examined the association of HDP with individual CVD diagnoses, highlighting the research implications of our study’s findings. While one prior study using birth certificate data found an 8-fold increased incidence of heart failure and 14-fold increased incidence of stroke for patients with preeclampsia, their outcomes included only a few major cardiovascular events and utilized composite outcomes that occurred during pregnancy through 36 months’ postpartum.52 Further research with more granularity into the types of cardiovascular diseases and their interplay with pregnancy is critical in order to clarify the causal pathway between HDP and CVD and inform evidence-based clinical recommendations.
Strengths and Limitations:
Our study had several strengths. It focuses on a highly clinically relevant question that can inform targeted screening and primary preventive strategies postpartum in order to mitigate premature cardiovascular morbidity and mortality for women. It is unique among the large growing body of literature on preeclampsia and cardiovascular disease in that it not only has a higher level of granularity in terms of cardiovascular disease but that it also focuses on the immediate 24 months after delivery. In addition, the Maine All Payer Claims Data is comprehensive because it includes public and private payer’s facility and professional claims from an entire state across 16 years, and allows for longer term follow-up beyond the traditional 42 days’ postpartum, regardless of change in insurance provider.
Limitations of our study include that it was limited to one state, so generalizability to other areas within the US may be limited. However, our prevalence of HDP is similar to national estimates.49,53 While our database is comprehensive because it uses all payer claims and includes a longer duration of postpartum follow-up, it does not include race and ethnicity data. This is unfortunate, because it is well known that racial and ethnic disparities are a major component of cardiovascular morbidity and mortality among women.48,54 We did not differentiate new CVD events that occurred only after delivery from ongoing CVD conditions that first occurred during pregnancy or the delivery hospitalization. In addition, we did not have an exclusion or washout period postpartum, which could have further allowed us to better identify truly new cardiovascular disease diagnoses. In addition, since we utilized claims data, there is a possibility of residual confounding due to inaccurate or missing diagnoses codes for medical conditions, e.g. obesity;55 and we used population-level measures instead of individuallevel measures to define certain confounders (e.g. access to care), which are less accurate measures. While we used previously published algorithms to identify HDP based on ICD codes, these codes were not validated for our specific cohort against medical records.26 Finally, although we did not see crossing curves in our visual assessment of Cox proportional hazards models (for our significant findings), there did appear to be some variation in the magnitude of hazard ratios over time, suggesting that modification of our Cox model may have useful.
Conclusions:
Patients with HDP have a substantially increased risk for heart failure, cerebrovascular disease/stroke, cardiomyopathy, and new chronic hypertension within 24 months’ postpartum. While prior literature has found an association between HDP and ischemic heart disease in the long term, our study does not show an association between HDP and ischemic heart disease in the first 24 months’ postpartum. Our results have the potential to inform lifesaving targeted postpartum intervention strategies that focus specifically on the cardiovascular risks that are increased postpartum. Further research to confirm these findings and development of multidisciplinary, individualized postpartum cardiovascular screening strategies are crucial to save our patients from premature, preventable morbidity and mortality.
Supplementary Material
Supplement Figure 1: Distribution of first diagnosis of cardiovascular disease within 24 months’ postpartum among people with and without hypertensive disorders of pregnancy, deliveries in Maine 2007–2019, n= 119,422
CONDENSATION:
Patients with hypertensive disorders of pregnancy have an increased risk for heart failure, cerebrovascular disease, cardiomyopathy, and new chronic hypertension in the first 24 months’ postpartum.
AJOG AT A GLANCE:
- Why was this study conducted?
- To identify the risk of significant cardiovascular diagnoses in the first 24 months’ postpartum among patients with hypertensive disorders of pregnancy
- To determine when the highest incidences of cardiovascular disease diagnoses occur within 24 months’ postpartum
- What are the key findings?
- Those with hypertensive disorders of pregnancy have a significantly increased risk of postpartum diagnoses of heart failure, cerebrovascular disease, and cardiomyopathy. Most of the diagnoses occurred within the first month postpartum.
- Those with hypertensive disorders of pregnancy have a significantly increased risk of new chronic hypertension within 24 months’ postpartum.
- What does this study add to what is already known?
- Since most existing data on hypertensive disorders of pregnancy and short-term cardiovascular disease risk is limited to the immediate postpartum period and focuses on overall CVD risk, we present novel findings on the association between hypertensive disorders of pregnancy and specific cardiovascular diseases during the first 24 months’ postpartum.
Acknowledgements:
We thank Mariah Pfeiffer and Catherine Gelsinger for their work as graduate research assistants of this project while they were Master of Public Health students at the University of Southern Maine. They received no financial compensation. We thank the Maine Health Data Organization, which is responsible for the State of Maine’s All Payer Claims Data. We used the Maine Health Data Organization’s All Payer Claims Data as authorized under Data Request Number 2021040501.
FUNDING
The research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R15HD101793. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
Kristin Palmsten receives research contracts from AbbVie, GSK, and Sanofi that are unrelated to this study.
The remaining authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.About Multiple Causes of Death, 1999–2020. Center for Disease Control and Prevention, National Center for Health Statistics. February 21, 2022. https://www.cdc.gov/heartdisease/women.htm [Google Scholar]
- 2.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. Jan 2013;28(1):1–19. doi: 10.1007/s10654-013-9762-6 [DOI] [PubMed] [Google Scholar]
- 3.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. Feb 2017;10(2)doi: 10.1161/circoutcomes.116.003497 [DOI] [PubMed] [Google Scholar]
- 4.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. Nov 2008;156(5):918–30. doi: 10.1016/j.ahj.2008.06.042 [DOI] [PubMed] [Google Scholar]
- 5.Grandi SM, Vallée-Pouliot K, Reynier P, et al. Hypertensive Disorders in Pregnancy and the Risk of Subsequent Cardiovascular Disease. Paediatr Perinat Epidemiol. Sep 2017;31(5):412–421. doi: 10.1111/ppe.12388 [DOI] [PubMed] [Google Scholar]
- 6.Ackerman-Banks CM, Grechukhina O, Spatz E, et al. Seizing the Window of Opportunity Within 1 Year Postpartum: Early Cardiovascular Screening. J Am Heart Assoc. Apr 19 2022;11(8):e024443. doi: 10.1161/jaha.121.024443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol. Oct 2016;215(4):484.e1–484.e14. doi: 10.1016/j.ajog.2016.05.047 [DOI] [PubMed] [Google Scholar]
- 8.Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. Jul 1 2014;180(1):41–4. doi: 10.1093/aje/kwu118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park Y, Cho GJ, Kim LY, Lee TS, Oh MJ, Kim YH. Preeclampsia Increases the Incidence of Postpartum Cerebrovascular Disease in Korean Population. J Korean Med Sci. Feb 5 2018;33(6):e35. doi: 10.3346/jkms.2018.33.e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholten RR, Hopman MT, Sweep FC, et al. Co-occurrence of cardiovascular and prothrombotic risk factors in women with a history of preeclampsia. Obstet Gynecol. Jan 2013;121(1):97–105. doi: 10.1097/aog.0b013e318273764b [DOI] [PubMed] [Google Scholar]
- 11.Smith GN, Pudwell J, Walker M, Wen SW. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can. Sep 2012;34(9):830–835. doi: 10.1016/s1701-2163(16)35381-6 [DOI] [PubMed] [Google Scholar]
- 12.Smith GN, Walker MC, Liu A, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol. Jan 2009;200(1):58.e1–8. doi: 10.1016/j.ajog.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 13.Brown HL, Smith GN. Pregnancy Complications, Cardiovascular Risk Factors, and Future Heart Disease. Obstet Gynecol Clin North Am. Sep 2020;47(3):487–495. doi: 10.1016/j.ogc.2020.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Smith GN, Louis JM, Saade GR. Pregnancy and the Postpartum Period as an Opportunity for Cardiovascular Risk Identification and Management. Obstet Gynecol. Oct 2019;134(4):851–862. doi: 10.1097/aog.0000000000003363 [DOI] [PubMed] [Google Scholar]
- 15.Cusimano MC, Pudwell J, Roddy M, Cho CK, Smith GN. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. Am J Obstet Gynecol. May 2014;210(5):438.e1–9. doi: 10.1016/j.ajog.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 16.Janmohamed R, Montgomery-Fajic E, Sia W, et al. Cardiovascular risk reduction and weight management at a hospital-based postpartum preeclampsia clinic. J Obstet Gynaecol Can. Apr 2015;37(4):330–337. doi: 10.1016/s1701-2163(15)30283-8 [DOI] [PubMed] [Google Scholar]
- 17.Vogel B, Acevedo M, Appelman Y, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. Jun 19 2021;397(10292):2385–2438. doi: 10.1016/s0140-6736(21)00684-x [DOI] [PubMed] [Google Scholar]
- 18.Mehta LS, Sharma G, Creanga AA, et al. Call to Action: Maternal Health and Saving Mothers: A Policy Statement From the American Heart Association. Circulation. Oct 12 2021;144(15):e251–e269. doi: 10.1161/cir.0000000000001000 [DOI] [PubMed] [Google Scholar]
- 19.Brown HL, Warner JJ, Gianos E, et al. Promoting Risk Identification and Reduction of Cardiovascular Disease in Women Through Collaboration With Obstetricians and Gynecologists: A Presidential Advisory From the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. Jun 12 2018;137(24):e843–e852. doi: 10.1161/cir.0000000000000582 [DOI] [PubMed] [Google Scholar]
- 20.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. Jan 2019;133(1):1. doi: 10.1097/aog.0000000000003018 [DOI] [PubMed] [Google Scholar]
- 21.ACOG Committee Opinion No. 736: Optimizing Postpartum Care. Obstet Gynecol. May 2018;131(5):e140–e150. doi: 10.1097/aog.0000000000002633 [DOI] [PubMed] [Google Scholar]
- 22.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2014;36(1):57–70. doi: 10.1093/epirev/mxt006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women−-2011 update: a guideline from the american heart association. Circulation. Mar 22 2011;123(11):1243–62. doi: 10.1161/CIR.0b013e31820faaf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GN. The Maternal Health Clinic: Improving women’s cardiovascular health. Semin Perinatol. Jun 2015;39(4):316–9. doi: 10.1053/j.semperi.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 25.Maine Health Data Organization. Accessed March 7, 2022, https://mhdo.maine.gov/rules.htm
- 26.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. Jama. Nov 12 2014;312(18):1897–904. doi: 10.1001/jama.2014.14825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartus AR, Jarlenski MP, Himes KP, James AE, Naimi AI, Bodnar LM. Adverse Cardiovascular Events Following Severe Maternal Morbidity. Am J Epidemiol. Jan 1 2022;191(1):126–136. doi: 10.1093/aje/kwab208 [DOI] [PubMed] [Google Scholar]
- 28.Ackerman CM, Platner MH, Spatz ES, et al. Severe cardiovascular morbidity in women with hypertensive diseases during delivery hospitalization. Am J Obstet Gynecol. Jun 2019;220(6):582.e1–582.e11. doi: 10.1016/j.ajog.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 29.Magee L, von Dadelszen P. Prevention and treatment of postpartum hypertension. Cochrane Database Syst Rev. Apr 30 2013;(4):Cd004351. doi: 10.1002/14651858.CD004351.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel A, Maski MR, Bajracharya S, et al. Epidemiology and Mechanisms of De Novo and Persistent Hypertension in the Postpartum Period. Circulation. Nov 3 2015;132(18):1726–33. doi: 10.1161/circulationaha.115.015721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Data on Selected Pregnancy Complications in the United States. Center for Disease Control and Prevention. Accessed January 12, 2002, https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications-data.htm. [Google Scholar]
- 32.American Community Survey. United States Census Bureau. https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml.
- 33.Area Health Resource File. Health Resources & Services Administration. data.hrsa.gov
- 34.Medicaid and CHIP Income Eligibility Limits for Pregnant Women as a Percent of the Federal Poverty Level. Kaiser Family Foundation. Accessed March 7, 2022, https://www.kff.org/health-reform/state-indicator/medicaid-and-chip-income-eligibility-limitsfor-pregnant-women-as-a-percent-of-the-federal-povertylevel/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D . [Google Scholar]
- 35.Narang K, Szymanski LM. Multiple Gestations and Hypertensive Disorders of Pregnancy: What Do We Know? Curr Hypertens Rep. Nov 18 2020;23(1):1. doi: 10.1007/s11906-020-011074 [DOI] [PubMed] [Google Scholar]
- 36.Sibai BM, Hauth J, Caritis S, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. Apr 2000;182(4):938–42. doi: 10.1016/s0002-9378(00)70350-4 [DOI] [PubMed] [Google Scholar]
- 37.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. Jul 2004;75(1):45–9. doi: 10.1016/j.cmpb.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 38.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj. Nov 10 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haas DM, Parker CB, Marsh DJ, et al. Association of Adverse Pregnancy Outcomes With Hypertension 2 to 7 Years Postpartum. J Am Heart Assoc. Oct 2019;8(19):e013092. doi: 10.1161/jaha.119.013092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Facca TA, Mastroianni-Kirsztajn G, Sabino ARP, et al. Pregnancy as an early stress test for cardiovascular and kidney disease diagnosis. Pregnancy Hypertens. Apr 2018;12:169–173. doi: 10.1016/j.preghy.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 41.Brown DW, Dueker N, Jamieson DJ, et al. Preeclampsia and the risk of ischemic stroke among young women: results from the Stroke Prevention in Young Women Study. Stroke. Apr 2006;37(4):1055–9. doi: 10.1161/01.STR.0000206284.96739.ee [DOI] [PubMed] [Google Scholar]
- 42.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. May 13 2014;63(18):1815–22. doi: 10.1016/j.jacc.2014.02.529 [DOI] [PubMed] [Google Scholar]
- 43.Bello N, Rendon ISH, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol. Oct 29 2013;62(18):1715–1723. doi: 10.1016/j.jacc.2013.08.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masini G, Foo LF, Tay J, et al. Preeclampsia has two phenotypes which require different treatment strategies. Am J Obstet Gynecol. Feb 2022;226(2s):S1006–s1018. doi: 10.1016/j.ajog.2020.10.052 [DOI] [PubMed] [Google Scholar]
- 45.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. Feb 1997;77(2):154–8. doi: 10.1136/hrt.77.2.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cassidy-Bushrow AE, Bielak LF, Rule AD, et al. Hypertension during pregnancy is associated with coronary artery calcium independent of renal function. J Womens Health (Larchmt). Oct 2009;18(10):1709–16. doi: 10.1089/jwh.2008.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garovic VD, White WM, Vaughan L, et al. Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J Am Coll Cardiol. May 12 2020;75(18):2323–2334. doi: 10.1016/j.jacc.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation. May 4 2021;143(18):e902–e916. doi: 10.1161/cir.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 49.Benschop L, Duvekot JJ, Roeters van Lennep JE. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. Aug 2019;105(16):1273–1278. doi: 10.1136/heartjnl-2018-313453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luther JP, Johnson DY, Joynt Maddox KE, Lindley KJ. Reducing Cardiovascular Maternal Mortality by Extending Medicaid for Postpartum Women. J Am Heart Assoc. Aug 3 2021;10(15):e022040. doi: 10.1161/jaha.121.022040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. Nov 19 2005;366(9499):1797–803. doi: 10.1016/s0140-6736(05)67726-4 [DOI] [PubMed] [Google Scholar]
- 52.Lin YS, Tang CH, Yang CY, et al. Effect of pre-eclampsia-eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol. Jan 15 2011;107(2):325–30. doi: 10.1016/j.amjcard.2010.08.073 [DOI] [PubMed] [Google Scholar]
- 53.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. May 2008;21(5):521–6. doi: 10.1038/ajh.2008.20 [DOI] [PubMed] [Google Scholar]
- 54.Shahul S, Tung A, Minhaj M, et al. Racial Disparities in Comorbidities, Complications, and Maternal and Fetal Outcomes in Women With Preeclampsia/eclampsia. Hypertens Pregnancy. Nov 2015;34(4):506–515. doi: 10.3109/10641955.2015.1090581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goff SL, Pekow PS, Markenson G, Knee A, Chasan-Taber L, Lindenauer PK. Validity of using ICD-9-CM codes to identify selected categories of obstetric complications, procedures and co-morbidities. Paediatr Perinat Epidemiol. Sep 2012;26(5):421–9. doi: 10.1111/j.1365-3016.2012.01303.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1: Distribution of first diagnosis of cardiovascular disease within 24 months’ postpartum among people with and without hypertensive disorders of pregnancy, deliveries in Maine 2007–2019, n= 119,422