Abstract
Traffic noise and air pollution are environmental stressors found to increase risk for cardiovascular events. The burden of disease attributable to environmental stressors and cardiovascular disease globally is substantial, with a need to better understand the contribution of specific risk factors that may underlie these effects. Epidemiologic observations and experimental evidence from animal models and human controlled exposure studies suggest an essential role for common mediating pathways. These include sympathovagal imbalance, endothelial dysfunction, vascular inflammation, increased circulating cytokines, activation of central stress responses, including hypothalamic and limbic pathways, and circadian disruption. Evidence also suggests that cessation of air pollution or noise through directed interventions alleviates increases in blood pressure and/or intermediate surrogate pathways, supporting a causal link. In the second part of this review, we discuss the current understanding of mechanisms underlying and current gaps in knowledge and opportunities for new research.
Keywords: Environment, Air Pollution, Noise Pollution, Hypertension, Endothelial Dysfunction, Oxidative Stress
Introduction
Environmental risk factors such as noise and air pollution have been shown to have significant effects on atherosclerotic cardiovascular disease events, clearly supported by clinical/epidemiological studies that are discussed in detail in Part I of the joint reviews. There is a large overlap of pathophysiological mechanisms by which environmental exposures may cause this excess risk, one of which is increases in arterial blood pressure with potential for additive/synergistic damage1,2,3. These shared pathways include increased oxidative stress, systemic inflammation, and activation of central mechanisms including sympathetic activation that translate exposures to rapid increase in resistance vessel tone.1,2,4 Recent insights from controlled exposures to air pollution and noise and mitigation experiments, in both animals and humans, have suggested a direct effect of noise and air pollution on the pressor response, providing incontrovertible evidence for the direct impact of exposure on increased blood pressure. In Part II of this review, we provide evidence to date, on mechanistic links between exposure to noise and air pollution, including both animal and human studies that mimic ambient exposure environments, and provide a broad mechanistic framework of understanding of how environmental stressors may mediate susceptibility to hypertension.
Mechanisms of noise-induced arterial hypertension and cardiovascular disease
Human mechanistic studies
The most current noise reaction model suggests that a so called “indirect pathway” plays a central role in the initiation and progression of cardiovascular disease1. The “indirect pathway” refers to the non-auditory health effects of noise via chronic, low-level noise exposure vs. the “direct pathway” that is characterized by exposure to high noise levels associating with auditory effects such as tinnitus or hearing loss. The cognitive perception of noise, triggers a cortical activation and the release of stress hormones, leading to increased vascular/cerebral inflammation and oxidative stress. In the long run, this may result in the genesis of risk factors, such as diabetes, high cholesterol, hypercoagulable states and hypertension, subsequently manifesting as CVD.5 Noise disturbs sleep and communication, which may lead to stress reactions and thus increased risk of CVD, in particular to ischemic heart disease6 and as recently shown to arrhythmias such as atrial fibrillation7 and neurocognitive diseases1. The pathophysiologic pathways influenced by noise are multitudinous, although oxidative stress and inflammation as a consequence of chronic activation of stress pathways and disturbance of circadian rhythms are key elements of noise pathophysiology.1
Recently, Osborne et al established a cerebral link between noise stimulus, vascular inflammation and major adverse cardiovascular events (MACE).8 He demonstrated that transportation noise was associated with increased amygdalar activity (part of the limbic system and in general involved in stress perception and control of emotions), vascular inflammation and major adverse cardiovascular events (MACE).8 A HR of 1.341 (CI 1.147–1.567) for MACE was found per 5 dB(A) increase in noise exposure levels that remained robust to common multivariable adjustments. Accordingly, in a subsequent study, the authors could demonstrate that more stress resilience is associated with less amygdala activation and vascular inflammation and MACE.9
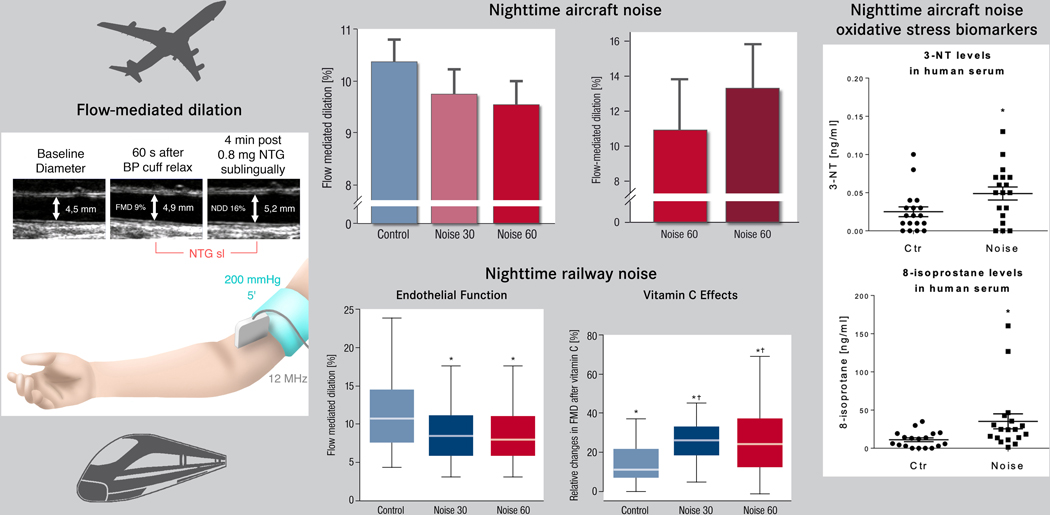
Field studies of transportation noise (aircraft and railway) have revealed that night-time noise exposure for just one night, was able to deteriorate sleep quality, to increase circulating stress hormones and blood pressure and to induce vascular (endothelial) dysfunction, a subclinical parameter for atherosclerosis.10,11 Importantly endothelial dysfunction was more pronounced in patients with established coronary artery disease5,12 and was partially improved by the acute administration of vitamin C (2g p.o.), suggesting a reduction of oxidative stress-related endothelial dysfunction10,11. Oxidative stress markers 3-nitrotyrosine and 8-isoprostane were upregulated in the noise-exposed subjects’ serum (Figure 1). The targeted proteomic analysis detected a significant increase in plasma proteins within redox, pro-thrombotic and pro-inflammatory pathways.11
Figure 1. Selected results from human field studies on nighttime aircraft/railway noise exposure for one night and endothelial function and oxidative stress markers.
Data on flow-mediated dilation after exposure to aircraft noise is based on 75 healthy non-smokers (FLUG study) 10, exposure to railway noise is based on 70 healthy non-smokers (ZUG study) 11 and all exposures were applied in a randomized cross-over design. Vitamin C effects were assessed in a subgroup of the cohort. Oxidative stress markers (3-nitroytosine [3-NT] and 8-isoprostane) were measured in the remaining samples of the FLUG study and published in 17. Data summarized from 10,11,17 with permission.
Animal mechanistic studies
Previous preclinical studies have established that chronic noise increases blood pressure in monkeys13 and rats14. These studies have typically involved very high sound pressure levels exceeding ≥100 dB(A). Very high levels of white noise for 2 and 4 weeks (100 dB(A), 4 h/d, 6 d/week) caused endothelial dysfunction in the aorta, higher vasoconstrictor response, and increased systolic blood pressure.15 Recently, in an animal model of aircraft noise, mice exposed to around-the-clock aircraft noise (Leq 72 dB(A), peak level 85 dB(A) for 24h/d for 1, 2, and 4d), increased blood pressure. Increase in stress hormones, vascular and cerebral oxidative stress [caused by the phagocytic NADPH oxidase (NOX2) and uncoupled nitric oxide synthase] increased inflammation due to immune cell infiltration, changes not observed in response to comparable sound pressure level of white noise (using similar average exposure levels, Leq), with the major difference that white noise was used as a continuous swoosh in contrast to the up and down of the aircraft noise pattern.16
Comparative Illumina sequencing of transcriptomes of aortic tissues from around-the-clock aircraft noise-treated animals displayed significant changes of genes in part responsible for regulating vascular function, circadian rhythm, vascular remodeling, and cell death.16,17 Importantly, aircraft noise exposure during sleep but not during the awake phase was more detrimental to the cardiovascular and cerebral system by triggering endothelial dysfunction, increases in circulating neurohormones and blood pressure, and oxidative stress in the vasculature and the brain.17 This was also supported by RNAseq data of aortic tissue, which showed a more obvious overlap in regulated genes between the sleep phase and around-the-clock noise exposure that was only visible by the trend in the awake phase noise exposure group, suggesting impaired circadian rhythms by sleep deprivation as a central pathomechanism of noise exposure.
Aircraft noise-induced vascular and cerebral damage was almost completely prevented by NOX2 deficiency 17 and by heme oxygenase-1 activation,18 pointing to the crucial role of inflammatory cells and of oxidative stress in mediating noise-induced cardiovascular and cerebral side effects. Transportation noise also induced a downregulation and uncoupling of nNOS, creating a neuroinflammatory phenotype and astrocyte activation along with enhanced cerebral reactive oxygen species (ROS) formation17, all of which may explain at least in part, the retarded cognitive development of children (memory and learning) and increased presence of dementia, in response to aircraft19 and road or railway noise.20
The molecular mechanisms of vascular dysfunction in response to aircraft noise i.e. inducing oxidative stress and inflammation are strikingly similar to mechanisms by which traditional cardiovascular risk factors induce endothelial/vascular dysfunction 1. This suggests that noise stress may accelerated process of vascular/cerebral atherosclerosis and neurodegenerative disease through shared molecular mechanisms with other traditional risk factors and raise the question of additive contributions of environmental risk factors to traditional risk factors such as hypertension, type 2 diabetes etc. Thus, it is not surprising that the cardiovascular side effects of aircraft noise, were exacerbated in mice with pre-existing hypertension.21 Noise also potentiated neuroinflammation and cerebral oxidative stress in this model.21
An increase in stress and vasoconstrictor hormones may provide a direct explanation for the observed dysregulation of vascular tone in response to noise. The consequences of nighttime noise include circadian disruption, fragmentation of sleep and/or abbreviated sleep periods and chronic stress, thereby increasing susceptibility to cardiovascular events.22
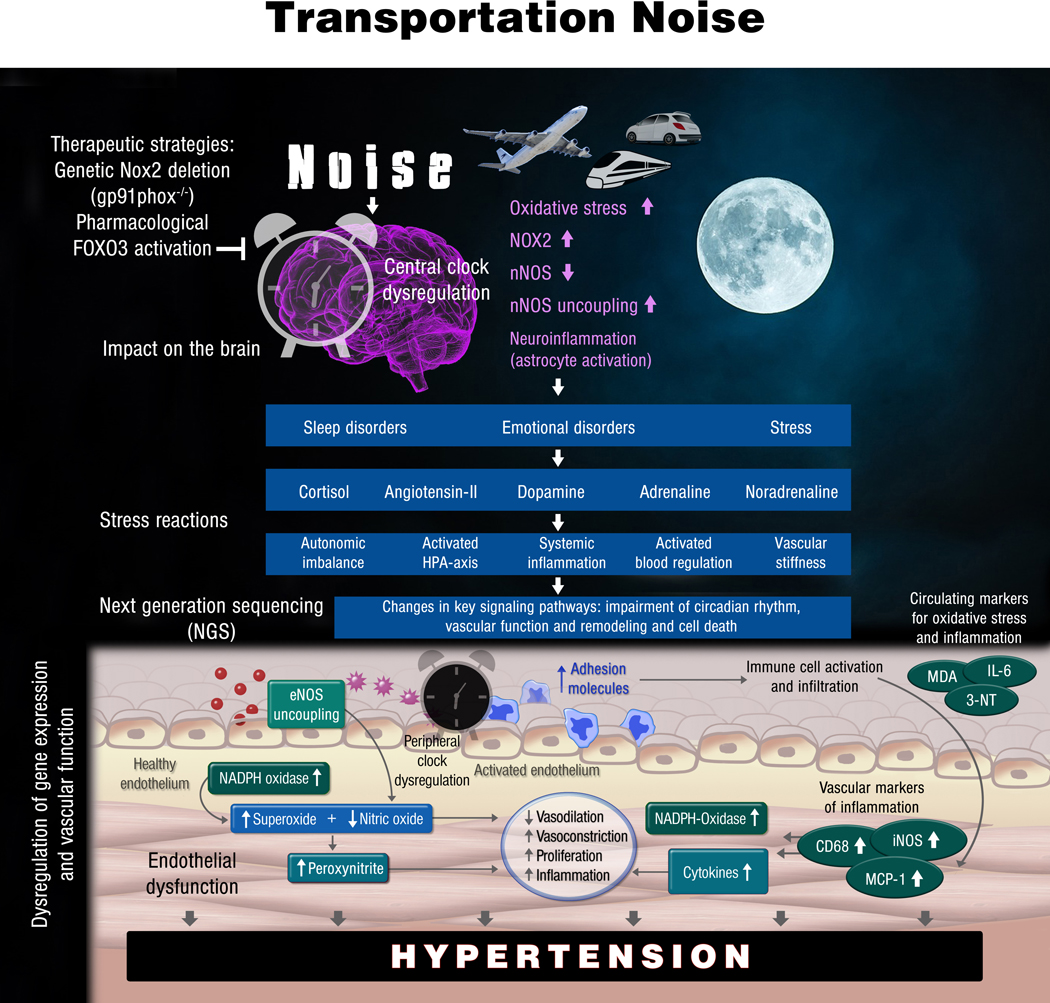
Noise-exposed animals have also increased circulating levels of the neurohormone of angiotensin-II.16,23 Prolonged exposure may initiate cerebral oxidative stress e.g. by enhanced angiotensin-II signaling and NOX-2 activation, all of which may trigger inflammation of the microvasculature of the brain.24 Activation of the sympathetic nervous system in animals through NADPH oxidase-induced oxidative stress provides the link between renin-angiotensin-aldosterone system (RAAS)-mediated NOX-2 activation and a subsequent catecholamine release and arterial hypertension.25,26 Catecholamines may initiate the formation of reactive oxygen species, either by feeding monoamine oxidase activity27 or by activating astrocytes, microglia, and NOX-2.28 In addition, aircraft noise increases the expression of endothelin-1 in vascular tissue, a potent vasoconstrictor, and activator of NOX-2 activity16,17,29, which is in part dependent on the RAAS.30 A summary of all relevant pathomechanistic pathways of noise-induced cardiovascular damage is shown in Figure 2.
Figure 2.
Pathophysiology of nighttime noise-induced arterial hypertension. Genetic Nox2 deficiency and pharmacological FOXO3 activation by bepridil prevented the adverse noise effects. 3-NT, 3-nitrotyrosine; CD68, macrosialin; eNOS, endothelial nitric oxide synthase; HPA, hypothalamic pituitary adrenal; IL-6, interleukin 6; iNOS, inducible nitric oxide synthase; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde. Figure adapted from reference 70 with permission.
Mechanisms of air pollution-induced arterial hypertension and cardiovascular disease
Human mechanistic evidence
In controlled studies in humans, acute exposure to PM2.5 and dilute diesel exhaust (ultrafine particles, UFP) results in rapid vascular dysfunction that manifests as conduit or microvascular endothelial dysfunction, or transient constriction of a peripheral conduit vessel, that is reversible.31 In some of these studies, concentrated PM2.5 exposure diminished conduit artery endothelial-dependent vasodilatation in a delayed fashion, post 24h (but not immediately).32 Not all studies demonstrating endothelial dysfunction in humans have shown increases in blood pressure.31 PM2.5 mass and TNF-α level post-exposure have both been associated with the degree of endothelial dysfunction, suggesting that systemic inflammation induced by particles and the degree of pollution are likely responsible.32 However these responses have not been consistently observed, suggesting that other complex factors including chemical composition could have a decisive impact on systemic responses.33 Blood pressure and arterial stiffness increased in response to systemic NO synthase inhibition in humans with intravenous L-NMMA (3mg/kg), following exposure to UFP, suggesting a greater generation of NO and preservation of systemic blood pressure with UFP exposure, which was unmasked by systemic NO synthase inhibition.34 Supplemental Table S1 compile all randomized controlled studies that have investigated blood pressures and/or alterations in vascular indexes in response to short-term exposure as well as the impact of interventions by air filtration.35 Part of these studies were also subject of a recent meta-analysis.36
The role of gaseous pollutants such as ozone on blood pressure are mixed. A panel study noted reduction in brachial artery diameter without changes in endothelial function, and a marginally significant reduction in diastolic blood pressure, with a lag period of 48h.37 This delayed temporal profile of reduction in blood pressure with ozone was similar to another panel study that also demonstrated that a 5-day mean increase in the ozone of 13.3 ppb, was associated with a 5 mmHg decrease in systolic blood pressure. These results suggest but are not definitive of a counteraction of increased blood pressure in response to PM2.5 noted in the same study. In a randomized crossover study, exposure to ozone for 3 h (0 ppb (filtered air), 70 ppb ozone, and 120 ppb ozone, alternating 15 min of moderate exercise with 15 min of rest did not have any effect on endothelial function or blood pressure.38 Other components of air pollution, such as volatile organic compounds such as acrolein and 1,3-butadiene have been associated with endothelial dysfunction and may contribute to elevated risk of hypertension in participants with an increased sympathetic tone, particularly in Black individuals.39
Short-term exposure to high levels of ultrafine, fine PM2.5 and coarse PM10 have been associated with increased blood pressure in humans, with evidence based on heart rate variability measures that suggest sympathetic activation.4,40 In humans, there is evidence of systemic permeation of particles, including into the CNS, based on post-mortem studies. Still, these are difficult to do because monitoring the particles in vivo is challenging, and post-mortem studies may be subject to pathologic artefacts.41,42 Most of the evidence in humans for sympathetic activation comes from extrapolation from secondhand smoke studies or indirect inference in relation to specific responses following air pollution exposure (e.g., acute vasoconstriction, increased heart rate and BP, and altered heart rate variability (Table S1).43 In a critical study, peripheral muscle sympathetic nerve activity (MSNA) measured by microneurography significantly increased within 30 minutes of diesel exhaust particle exposure when quantified in real‐time, during exposure, which was prevented by a facemask intervention44. Heart rate significantly increased, while there were trends for systolic and diastolic BP elevations, likely hemodynamic consequences of SNS activation. In a posthoc analysis of the MESA cohort, a 17 ppb higher annual NOx concentration was associated with 6.3% higher mean urinary epinephrine level; A 2 mg/m3 higher annual ambient PM2.5 concentration was associated with a 9.1% higher mean epinephrine and 4.4% higher dopamine levels. In contrast, short-term exposures were not associated with any of the catecholamines.45
The findings from randomized, double-blind trials, most notably using portable air cleaners, corroborate the epidemiology and bolster support for the causal linkage between PM2.5 and high BP.46 One recent meta-analysis in 10 trials (n=604) demonstrated that air cleaner usage resulted in 4 mmHg lower blood pressure over 2 weeks.47 This benefit was observed in highly polluted and relatively clean environments, further bolstering support for a monotonic relationship between PM exposure and higher BP levels, extending across the global ambient concentration range. These results generally suggest that personal strategies to lower air pollution risks through reducing blood pressure, may have great promise as preventive interventions (Supplemental Table S1).2,48
Mechanistic studies in Animals
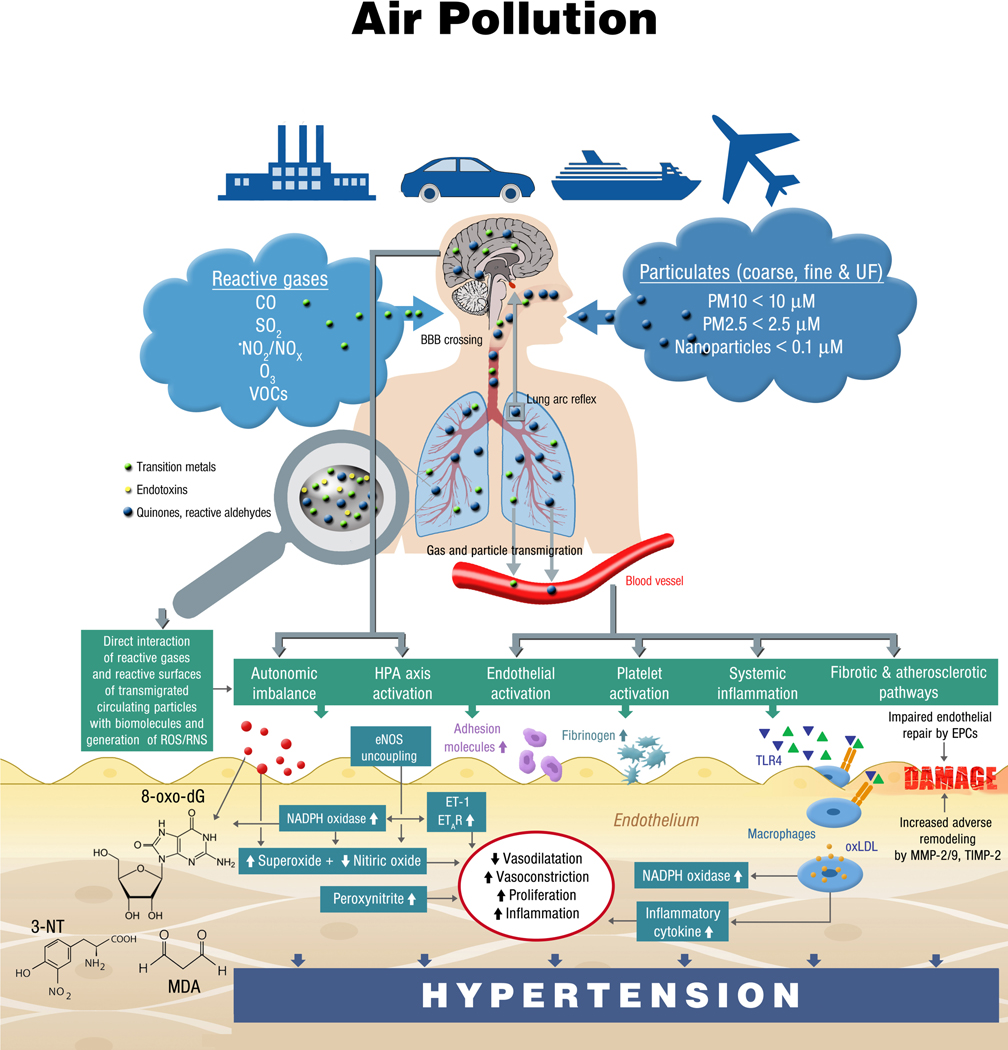
An increase in oxidative stress is one of the earliest pathophysiologic mechanisms in response to air pollution exposure and appears to be a critical initiating event. Given the central role of ROS in physiology, its contribution to disease in response to air pollution is somewhat complex and has been the subject of several reviews (summary in Figure 3).49–51 Receptors such as the transient receptor potential cation channel, subfamily A, member 1 (TRPA1) receptors in airway sensory neurons can also sense the environmental toxicants and aerogenic oxidants, resulting in neurogenic inflammation and facilitate rapid autonomic regulation of blood pressures, also with impact on rapid changes in blood pressure in humans.52,53 Direct translocation of particulate constituents and secondary damage-associated molecular patterns (DAMPs) and biologic intermediates play an important role. However, their role in inducing blood pressure changes remain unknown (summary in Figure 3).50,54–56 Air pollutants have been shown to permeate the central nervous system, inducing inflammation in several critical areas of the central nervous system responsible for blood pressure regulation and metabolic control in animal models.31,54
Figure 3.
Pathophysiology of air pollution-induced endothelial dysfunction, increased oxidative stress, inflammation, and subsequently arterial hypertension. Green and blue triangles are damage- and pathogen-associated molecular patterns (e.g., free DNA fragments, hyaluronan, 7-ketocholesterol, oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine, lipopolysaccharide) as well as soluble heavy/transition metals. HPA, hypothalamic pituitary adrenal; EPCs, endothelial progenitor cells; eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; ETAR, endothelin type A receptor; MMP, metalloproteinase; 8-oxo-dG, 8-oxo-deoxyguanosine; 3-NT, 3-nitrotyrosine; MDA, malondialdehyde; TIMP, tissue inhibitor of metalloproteinases; TLR4, toll-like receptor 4; VOCs, volatile organic compounds. Figure adapted from reference 31 with permission.
In both mice and rats, sub-acute and chronic exposure to air pollution alone and/or in conjunction with agents such as angiotensin II, resulted in increased superoxide (O2•−) and potentiation of vasoconstrictor responses and increase in blood pressure.57,58 Concentrated ambient PM2.5 exposure in C75/Bl6 mice, induced significant increases in urinary angiotensin II and aldosterone, along with the decrease of ACE2 and Ang (1–7) in kidney compared with FA-exposed mice.59 Several reports have found amplified endothelin-1/ETA-receptor signaling upon exposure to diesel exhaust which is consistent with the known involvement of NADPH oxidase-driven endothelin-1 promoter activation, and conversely, activation of NADPH oxidase and O2•− production by endothelin-1.60,61 Vascular O2•− production in response to chronic PM2.5 exposure, was abolished by NAD(P)H oxidase inhibitor apocynin and NOS inhibitor N-omega-nitro-L-arginine methyl ester (L-NAME), suggesting that reduction in NO bioavailability, due to NADPH oxidases and uncoupled eNOS respectively, may be an important mechanism inducing adverse vascular effects, akin to noise exposures.62,63 Increased microvascular adhesion of inflammatory monocytes in the adipose microcirculation has been noted with concentrated PM2.5 exposure, together with perivascular deposition of mononuclear cells, with deficiency of NOX2 and Tlr4 improving vascular responsiveness.64 Alterations in perivascular fat with infiltration of monocytes and inflammatory mediator release may affect vascular tone and affect pressor responses, also with a central role for NADPH oxidase.65
Recently an important link between chronic exposure to concentrated PM2.5 and circadian dysfunction in a manner identical to light exposure at night has been made. PM2.5 induced peripheral insulin resistance, circadian rhythm dysfunction, and metabolic and brown adipose tissue (BAT) dysfunction, akin to light at night.66 Transcriptomic analysis of liver and BAT revealed widespread but unique alterations in circadian genes, with evidence for differentially accessible promoters and enhancers of circadian genes in response to PM2.5 when examined by Assay for Transposase-Accessible Chromatin with high-throughput sequencing (ATAC-seq). The histone deacetylases 2, 3, and 4 were downregulated with PM2.5 exposure, with increased promoter occupancy by the histone acetyltransferase p300 as evidenced by chromatin immunoprecipitation (ChIP)-seq. These findings suggest a previously unrecognized role of PM2.5 in promoting CR disruption and metabolic dysfunction through epigenetic regulation of circadian targets. It is likely though unproven that, circadian disruption by PM2.5 exposure may indeed also induce changes in blood pressure and could represent a mechanism that is common to both noise and air pollution exposure.
Gaps of knowledge and future research tasks
There is a growing realization that co-exposures to noise and air pollution may represent an example of complex conjoint stressors that influence susceptibility to CVD through common mechanisms (Figure 2 and 3). A few epidemiological studies have indeed examined the effect of noise and air pollution or have attempted to control for the other exposure.67 These studies have suggested an association of both noise and air pollution after adjusting for the other exposure68 and even revealed additive risk for diabetes in one study by combined exposure to noise and air pollutants69. Preclinical studies on combined exposure to environmental pollutants and stressors are rare. One study in mice has suggested additive cardiovascular damage by activation of partially synergistic mechanisms upon co-exposure, via lung inflammation and brain stress response, respectively3. A number of important mechanistic questions in remain to be addressed, including the magnitude and time course of response of co-exposure, interactive effects of both factors on blood pressure and metabolic risk and duration of effect/time course of reversal. Importantly, integrative mechanisms including the link between exposures and activation of central mechanisms and circadian rhythm need to be addressed. Importantly the impact of mitigation measures including other of preventive measures on the pressor response is worth investigating. Integrating mechanistic studies with health impact assessment of noise and air pollution mitigation, in the context of climate interventions in cities may offer an extraordinary opportunity. In this regard the development of personal biometric technologies that provide measures of health in conjunction with granular data on environmental exposure, provide an unprecedented opportunity for research, and may allow an extraordinary broad understanding of conjoint impact of multiple environmental factors.
Supplementary Material
Acknowledgements
T.M. is a PI of the DZHK (German Center for Cardiovascular Research), partner site Rhein-Main, Mainz, Germany.
Sources of Funding
This study was supported by the Center for Translational Vascular Biology (CTVB) and funded by the ‘Stiftung Mainzer Herz’. Dr. Rajagopalan is supported by National Institutes of Health Grants 1R35ES031702 and R01ES017290.
Footnotes
Disclosure
None.
References
- 1.Munzel T, Sorensen M, Daiber A. Transportation noise pollution and cardiovascular disease. Nat Rev Cardiol. 2021;18:619–636. doi: 10.1038/s41569-021-00532-5 [DOI] [PubMed] [Google Scholar]
- 2.Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J. 2017;38:557–564. doi: 10.1093/eurheartj/ehw294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuntic M, Kuntic I, Krishnankutty R, Gericke A, Oelze M, Junglas T, Bayo Jimenez MT, Stamm P, Nandudu M, Hahad O, et al. Co-exposure to urban particulate matter and aircraft noise adversely impacts the cerebro-pulmonary-cardiovascular axis in mice. Redox Biol. 2023;59:102580. doi: 10.1016/j.redox.2022.102580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Kindi SG, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol. 2020;17:656–672. doi: 10.1038/s41569-020-0371-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sorensen M. Environmental Noise and the Cardiovascular System. J Am Coll Cardiol. 2018;71:688–697. doi: 10.1016/j.jacc.2017.12.015 [DOI] [PubMed] [Google Scholar]
- 6.Babisch W, Pershagen G, Selander J, Houthuijs D, Breugelmans O, Cadum E, Vigna-Taglianti F, Katsouyanni K, Haralabidis AS, Dimakopoulou K, et al. Noise annoyance--a modifier of the association between noise level and cardiovascular health? Sci Total Environ. 2013;452–453:50–57. doi: 10.1016/j.scitotenv.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 7.Hahad O, Beutel M, Gori T, Schulz A, Blettner M, Pfeiffer N, Rostock T, Lackner K, Sorensen M, Prochaska JH, et al. Annoyance to different noise sources is associated with atrial fibrillation in the Gutenberg Health Study. Int J Cardiol. 2018;264:79–84. doi: 10.1016/j.ijcard.2018.03.126 [DOI] [PubMed] [Google Scholar]
- 8.Osborne MT, Radfar A, Hassan MZO, Abohashem S, Oberfeld B, Patrich T, Tung B, Wang Y, Ishai A, Scott JA, et al. A neurobiological mechanism linking transportation noise to cardiovascular disease in humans. Eur Heart J. 2020;41:772–782. doi: 10.1093/eurheartj/ehz820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dar T, Osborne MT, Abohashem S, Abbasi T, Choi KW, Ghoneem A, Naddaf N, Smoller JW, Pitman RK, Denninger JW, et al. Greater Neurobiological Resilience to Chronic Socioeconomic or Environmental Stressors Associates With Lower Risk for Cardiovascular Disease Events. Circ Cardiovasc Imaging. 2020;13:e010337. doi: 10.1161/CIRCIMAGING.119.010337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt FP, Basner M, Kroger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A, et al. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J. 2013;34:3508–3514a. doi: 10.1093/eurheartj/eht269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herzog J, Schmidt FP, Hahad O, Mahmoudpour SH, Mangold AK, Garcia Andreo P, Prochaska J, Koeck T, Wild PS, Sorensen M, et al. Acute exposure to nocturnal train noise induces endothelial dysfunction and pro-thromboinflammatory changes of the plasma proteome in healthy subjects. Basic Res Cardiol. 2019;114:46. doi: 10.1007/s00395-019-0753-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M, Binder H, Gori T, Munzel T. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clinical research in cardiology : official journal of the German Cardiac Society. 2015;104:23–30. doi: 10.1007/s00392-014-0751-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson EA, Augenstein JS, Tanis DC, Augenstein DG. Noise raises blood pressure without impairing auditory sensitivity. Science. 1981;211:1450–1452. [DOI] [PubMed] [Google Scholar]
- 14.Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. Noise-induced hypertension and magnesium in rats: relationship to microcirculation and calcium. J Appl Physiol (1985). 1992;72:194–202. [DOI] [PubMed] [Google Scholar]
- 15.Wu CC, Chen SJ, Yen MH. Effects of noise on blood pressure and vascular reactivities. Clinical and experimental pharmacology & physiology. 1992;19:833–838. [DOI] [PubMed] [Google Scholar]
- 16.Munzel T, Daiber A, Steven S, Tran LP, Ullmann E, Kossmann S, Schmidt FP, Oelze M, Xia N, Li H, et al. Effects of noise on vascular function, oxidative stress, and inflammation: mechanistic insight from studies in mice. Eur Heart J. 2017;38:2838–2849. doi: 10.1093/eurheartj/ehx081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroller-Schon S, Daiber A, Steven S, Oelze M, Frenis K, Kalinovic S, Heimann A, Schmidt FP, Pinto A, Kvandova M, et al. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur Heart J. 2018;39:3528–3539. doi: 10.1093/eurheartj/ehy333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayo Jimenez MT, Frenis K, Kroller-Schon S, Kuntic M, Stamm P, Kvandova M, Oelze M, Li H, Steven S, Munzel T, et al. Noise-Induced Vascular Dysfunction, Oxidative Stress, and Inflammation Are Improved by Pharmacological Modulation of the NRF2/HO-1 Axis. Antioxidants (Basel). 2021;10. doi: 10.3390/antiox10040625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stansfeld SA, Berglund B, Clark C, Lopez-Barrio I, Fischer P, Ohrstrom E, Haines MM, Head J, Hygge S, van Kamp I, et al. Aircraft and road traffic noise and children’s cognition and health: a cross-national study. Lancet. 2005;365:1942–1949. doi: 10.1016/S0140-6736(05)66660-3 [DOI] [PubMed] [Google Scholar]
- 20.Cantuaria ML, Waldorff FB, Wermuth L, Pedersen ER, Poulsen AH, Thacher JD, Raaschou-Nielsen O, Ketzel M, Khan J, Valencia VH, et al. Residential exposure to transportation noise in Denmark and incidence of dementia: national cohort study. BMJ. 2021;374:n1954. doi: 10.1136/bmj.n1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steven S, Frenis K, Kalinovic S, Kvandova M, Oelze M, Helmstadter J, Hahad O, Filippou K, Kus K, Trevisan C, et al. Exacerbation of adverse cardiovascular effects of aircraft noise in an animal model of arterial hypertension. Redox Biol. 2020:101515. doi: 10.1016/j.redox.2020.101515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- 23.Wright JW, Dengerink HA, Miller JM, Goodwin PC. Potential role of angiotensin II in noise-induced increases in inner ear blood flow. Hear Res. 1985;17:41–46. doi: 10.1016/0378-5955(85)90128-5 [DOI] [PubMed] [Google Scholar]
- 24.Schiavone S, Jaquet V, Trabace L, Krause KH. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxidants & redox signaling. 2013;18:1475–1490. doi: 10.1089/ars.2012.4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye S, Zhong H, Yanamadala S, Campese VM. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension. 2006;48:309–315. doi: 10.1161/01.HYP.0000231307.69761.2e [DOI] [PubMed] [Google Scholar]
- 26.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283, 276p following 283. doi: 10.1161/HYPERTENSIONAHA.109.142646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neri M, Cerretani D, Fiaschi AI, Laghi PF, Lazzerini PE, Maffione AB, Micheli L, Bruni G, Nencini C, Giorgi G, et al. Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J Cell Mol Med. 2007;11:156–170. doi: 10.1111/j.1582-4934.2007.00009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HY, Lee JS, Kim HG, Kim WY, Lee SB, Choi YH, Son CG. The ethanol extract of Aquilariae Lignum ameliorates hippocampal oxidative stress in a repeated restraint stress mouse model. BMC Complement Altern Med. 2017;17:397. doi: 10.1186/s12906-017-1902-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deiuliis JA, Kampfrath T, Zhong J, Oghumu S, Maiseyeu A, Chen LC, Sun Q, Satoskar AR, Rajagopalan S. Pulmonary T cell activation in response to chronic particulate air pollution. Am J Physiol Lung Cell Mol Physiol. 2012;302:L399–409. doi: 10.1152/ajplung.00261.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension. 1997;30:29–34. [DOI] [PubMed] [Google Scholar]
- 31.Munzel T, Gori T, Al-Kindi S, Deanfield J, Lelieveld J, Daiber A, Rajagopalan S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur Heart J. 2018;39:3543–3550. doi: 10.1093/eurheartj/ehy481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, et al. Insights into the Mechanisms and Mediators of the Effects of Air Pollution Exposure on Blood Pressure and Vascular Function in Healthy Humans. Hypertension. 2009;54:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills NL, Robinson SD, Fokkens PH, Leseman DL, Miller MR, Anderson D, Freney EJ, Heal MR, Donovan RJ, Blomberg A, et al. Exposure to concentrated ambient particles does not affect vascular function in patients with coronary heart disease. Environ Health Perspect. 2008;116:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langrish JP, Unosson J, Bosson J, Barath S, Muala A, Blackwell S, Soderberg S, Pourazar J, Megson IL, Treweeke A, et al. Altered nitric oxide bioavailability contributes to diesel exhaust inhalation-induced cardiovascular dysfunction in man. J Am Heart Assoc. 2013;2:e004309. doi: 10.1161/JAHA.112.004309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Sun Z, Ruan Y, Yan J, Mukherjee B, Yang F, Duan F, Sun L, Liang R, Lian H, et al. Personal black carbon exposure influences ambulatory blood pressure: air pollution and cardiometabolic disease (AIRCMD-China) study. Hypertension. 2014;63:871–877. doi: 10.1161/HYPERTENSIONAHA.113.02588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faridi S, Brook RD, Yousefian F, Hassanvand MS, Nodehi RN, Shamsipour M, Rajagopalan S, Naddafi K. Effects of respirators to reduce fine particulate matter exposures on blood pressure and heart rate variability: A systematic review and meta-analysis. Environ Pollut. 2022;303:119109. doi: 10.1016/j.envpol.2022.119109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirowsky JE, Carraway MS, Dhingra R, Tong H, Neas L, Diaz-Sanchez D, Cascio W, Case M, Crooks J, Hauser ER, et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environmental Health. 2017;16:126–126. doi: 10.1186/s12940-017-0335-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich DQ, Balmes JR, Frampton MW, Zareba W, Stark P, Arjomandi M, Hazucha MJ, Costantini MG, Ganz P, Hollenbeck-Pringle D, et al. Cardiovascular function and ozone exposure: The Multicenter Ozone Study in oldEr Subjects (MOSES). Environ Int. 2018;119:193–202. doi: 10.1016/j.envint.2018.06.014 [DOI] [PubMed] [Google Scholar]
- 39.McGraw KE, Riggs DW, Rai S, Navas-Acien A, Xie Z, Lorkiewicz P, Lynch J, Zafar N, Krishnasamy S, Taylor KC, et al. Exposure to volatile organic compounds - acrolein, 1,3-butadiene, and crotonaldehyde - is associated with vascular dysfunction. Environ Res. 2021;196:110903. doi: 10.1016/j.envres.2021.110903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajagopalan S, Al-Kindi SG, Brook RD. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2054–2070. doi: 10.1016/j.jacc.2018.07.099 [DOI] [PubMed] [Google Scholar]
- 41.Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens PHB, Boere AJF, et al. Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano. 2017;11:4542–4552. doi: 10.1021/acsnano.6b08551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maher BA, Ahmed IA, Karloukovski V, MacLaren DA, Foulds PG, Allsop D, Mann DM, Torres-Jardon R, Calderon-Garciduenas L. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci U S A. 2016;113:10797–10801. doi: 10.1073/pnas.1605941113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brook RD, Rajagopalan S. Getting Sympathetic About Air Pollution Exposure. J Am Heart Assoc. 2021;10:e021675. doi: 10.1161/JAHA.121.021675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rankin GD, Kabele M, Brown R, Macefield VG, Sandstrom T, Bosson JA. Acute Exposure to Diesel Exhaust Increases Muscle Sympathetic Nerve Activity in Humans. J Am Heart Assoc. 2021;10:e018448. doi: 10.1161/JAHA.120.018448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hajat A, Diez Roux AV, Castro-Diehl C, Cosselman K, Golden SH, Hazlehurst MF, Szpiro A, Vedal S, Kaufman JD. The Association between Long-Term Air Pollution and Urinary Catecholamines: Evidence from the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect. 2019;127:57007. doi: 10.1289/EHP3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajagopalan S, Brauer M, Bhatnagar A, Bhatt DL, Brook JR, Huang W, Munzel T, Newby D, Siegel J, Brook RD, et al. Personal-Level Protective Actions Against Particulate Matter Air Pollution Exposure: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e411–e431. doi: 10.1161/CIR.0000000000000931 [DOI] [PubMed] [Google Scholar]
- 47.Walzer D, Gordon T, Thorpe L, Thurston G, Xia Y, Zhong H, Roberts TR, Hochman JS, Newman JD. Effects of Home Particulate Air Filtration on Blood Pressure: A Systematic Review. Hypertension. 2020;76:44–50. doi: 10.1161/HYPERTENSIONAHA.119.14456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajagopalan S, Brook RD. Personalizing your airspace and your health. J Am Coll Cardiol. 2015;65:2288–2290. doi: 10.1016/j.jacc.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 49.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–361. doi: 10.1038/nri3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyata R, van Eeden SF. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol Appl Pharmacol. 2011;257:209–226. doi: S0041-008X(11)00360-7 [pii] 10.1016/j.taap.2011.09.007 [doi] [DOI] [PubMed] [Google Scholar]
- 51.Gangwar RS, Bevan GH, Palanivel R, Das L, Rajagopalan S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020;34:101545. doi: 10.1016/j.redox.2020.101545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon SA, Liedtke W. How irritating: the role of TRPA1 in sensing cigarette smoke and aerogenic oxidants in the airways. J Clin Invest. 2008;118:2383–2386. doi: 10.1172/JCI36111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119:951–957. doi: 10.1289/ehp.1003200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao X, Zhong J, Brook RD, Rajagopalan S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxidants & redox signaling. 2018;28:797–818. doi: 10.1089/ars.2017.7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aragon M, Erdely A, Bishop L, Salmen R, Weaver J, Liu J, Hall P, Eye T, Kodali V, Zeidler-Erdely P, et al. MMP-9-Dependent Serum-Borne Bioactivity Caused by Multiwalled Carbon Nanotube Exposure Induces Vascular Dysfunction via the CD36 Scavenger Receptor. Toxicol Sci. 2016;150:488–498. doi: 10.1093/toxsci/kfw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao X, Zhong J, Maiseyeu A, Gopalakrishnan B, Villamena Fa, Chen L-C, Harkema JR, Sun Q, Rajagopalan S. CD36-Dependent 7-Ketocholesterol Accumulation in Macrophages Mediates Progression of Atherosclerosis in Response to Chronic Air Pollution Exposure. Circulation research. 2014:770–780. doi: 10.1161/CIRCRESAHA.115.304666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang J- SS, Zweier JL, Chen LC, Rajagopalan S, et al. Air Pollution Exposure Potentiates Hypertension Through Reactive Oxygen Species-Mediated Activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/atvbaha.108.166967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, Zhao J, Liu D, Morishita M, Sun Q, et al. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect. 2014;122:79–86. doi: 10.1289/ehp.1307151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du X, Zeng X, Zhang J, Pan K, Song L, Zhou J, Zhou L, Xie Y, Sun Q, Ge W, et al. Ambient fine particulate matter induced the elevation of blood pressure through ACE2/Ang(1–7) pathway: The evidence from urine metabolites. Ecotoxicol Environ Saf. 2020;203:111044. doi: 10.1016/j.ecoenv.2020.111044 [DOI] [PubMed] [Google Scholar]
- 60.Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, Li H, Lamas S, Munzel T. Targeting vascular (endothelial) dysfunction. Br J Pharmacol. 2017;174:1591–1619. doi: 10.1111/bph.13517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wenzel P, Kossmann S, Munzel T, Daiber A. Redox regulation of cardiovascular inflammation - Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic Biol Med. 2017;109:48–60. doi: 10.1016/j.freeradbiomed.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 62.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cherng TW, Paffett ML, Jackson-Weaver O, Campen MJ, Walker BR, Kanagy NL. Mechanisms of Diesel-Induced Endothelial Nitric Oxide Synthase Dysfunction in Coronary Arterioles. Environmental Health Perspectives. 2010;119:98–103. doi: 10.1289/ehp.1002286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, et al. Ambient Air Pollution Exaggerates Adipose Inflammation and Insulin Resistance in a Mouse Model of Diet-Induced Obesity. Circulation. 2009;119:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108:716–726. doi: 10.1161/CIRCRESAHA.110.237560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palanivel R, Vinayachandran V, Biswal S, Deiuliis JA, Padmanabhan R, Park B, Gangwar RS, Durieux JC, Ebreo Cara EA, Das L, et al. Exposure to Air Pollution Disrupts Circadian Rhythm through Alterations in Chromatin Dynamics. iScience. 2020;23:101728. doi: 10.1016/j.isci.2020.101728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heritier H, Vienneau D, Foraster M, Eze IC, Schaffner E, de Hoogh K, Thiesse L, Rudzik F, Habermacher M, Kopfli M, et al. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: a nationwide cohort study in Switzerland. Eur Heart J. 2019;40:598–603. doi: 10.1093/eurheartj/ehy650 [DOI] [PubMed] [Google Scholar]
- 68.Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook J, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J. 2017;38:550–556. doi: 10.1093/eurheartj/ehw269 [DOI] [PubMed] [Google Scholar]
- 69.Sorensen M, Poulsen AH, Hvidtfeldt UA, Brandt J, Frohn LM, Ketzel M, Christensen JH, Im U, Khan J, Munzel T, et al. Air pollution, road traffic noise and lack of greenness and risk of type 2 diabetes: A multi-exposure prospective study covering Denmark. Environ Int. 2022;170:107570. doi: 10.1016/j.envint.2022.107570 [DOI] [PubMed] [Google Scholar]
- 70.Munzel T, Kroller-Schon S, Oelze M, Gori T, Schmidt FP, Steven S, Hahad O, Roosli M, Wunderli JM, Daiber A, et al. Adverse Cardiovascular Effects of Traffic Noise with a Focus on Nighttime Noise and the New WHO Noise Guidelines. Annu Rev Public Health. 2020;41:309–328. doi: 10.1146/annurev-publhealth-081519-062400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.