Abstract
There is growing concern from scientists, policy makers, and the public about the contamination of natural and indoor environments with plastics, particularly micro/nanoplastics. Typically, characterizing microplastics in environmental samples requires extensive sample processing to isolate particles, followed by spectroscopic methodologies to identify particle polymer composition. Spectroscopic techniques are limited in their ability to provide polymer mass or advanced chemical composition (e.g., chemical additive content), which are important for toxicological assessments. To achieve mass fraction quantification and chemical characterization of plastics in environmental samples, many researchers have turned to thermoanalytical spectrometric approaches, particularly pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS). Sample preparation for Py–GC/MS may be approached similarly to techniques needed for spectroscopic approaches (e.g., isolate particles on a filter), employ pressurized solvent extraction, or use ultrafiltration techniques to concentrate nanoplastics. Great strides have been made in using calibration curves to quantify plastics in complex matrices. However, the approaches to the pyrolysis thermal program, as well as calibrant and sample preparation, are inconsistent, requiring refinement and harmonization. This review provides a critical synthesis of previous Py–GC/MS work and highlights opportunities for novel and improved Py–GC/MS analysis of plastics in the future.
Keywords: Pyrolysis, Microplastics, Nanoplastics, Thermal desorption, Plastic pollution, Marine debris
Introduction
Plastic pollution is omnipresent across natural and indoor environments. This is particularly true of microplastics (1 μm–5 mm) and nanoplastics (< 1 μm), which are formed as larger plastics wear, weather, and fragment [1, 2]. There is a demand for analytical techniques to measure plastic pollution in a variety of matrices, which is a critical need for regulatory actions. Quantifying plastic in environmental samples requires polymer identification and the ability to count particles or measure the mass of each polymer type. Currently available methods are challenged by the fact that no two pieces of plastic in the environment are alike. Plastics vary by polymer type(s), chemical additive constituents, size, shape, color, density, molecular weight distribution, crystallinity, and more [3, 4]. Furthermore, despite their seemingly ubiquitous distribution, sample processing and analytical instrumentation capacities are likely leading to an underestimation of plastics in the environment, particularly in the smallest size ranges [5].
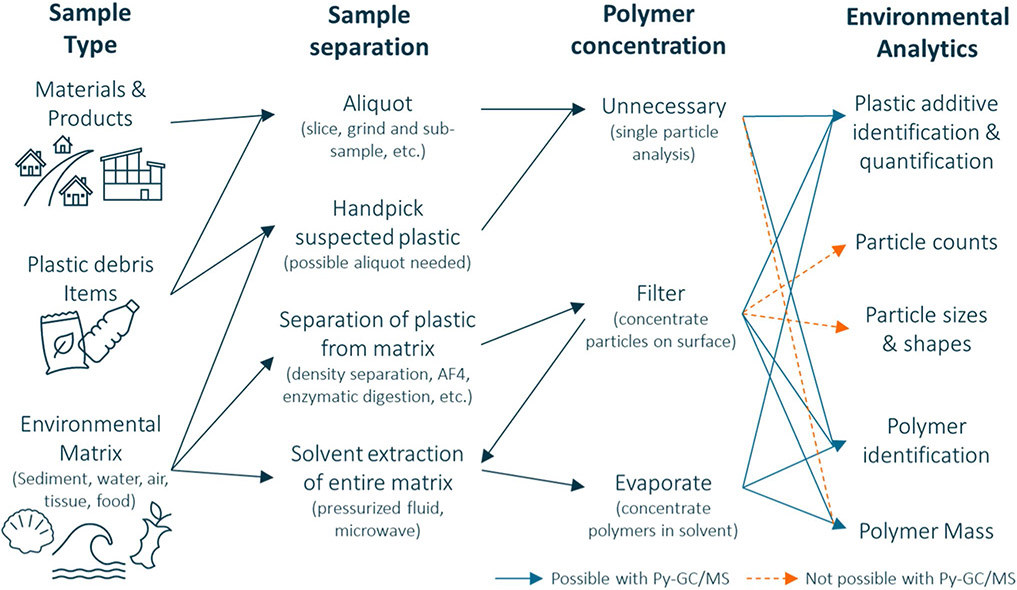
The analysis of plastics in any environmental sample hinges upon confirmation that a suspected plastic is a synthetic polymer (and not natural particulate), which is usually accomplished using chemical identification methods. Polymer identification can also be important in sourcing the debris by original product type. Commonly, spectroscopy (e.g., Raman and Fourier transform infrared (FT-IR)) is employed, providing a count of plastic particles by polymer type [6]. These data may be difficult to translate into risk assessments or policy, as they do not measure particle mass toward dose estimates [7]. Complementary mass-based approaches have not been as commonly used. Pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) has grown in popularity for the analysis of plastic debris in diverse environmental matrices over the past decade [8]. The analytics possible for plastics using Py–GC/MS, with associated sample types and preparation, is provided in Fig. 1. Considering its growing utility, this is a critical time to consider best practices for Py–GC/MS and harmonize approaches moving forward [9-11]. This review provides a synthesis of previous Py–GC/MS research for the analysis of plastic in the environment and recommendations for future work.
Fig. 1.
Schematic illustrating the utility of Py–GC/MS for different environmental analytics. The sample type, preparation, and necessity of polymer concentration leading to analysis detailed. Connecting polymer concentration and environmental analytics, solid blue lines indicate that the analysis is possible with Py–GC/MS while dashed orange lines are not. Of note, while Py–GC/MS does not measure particle size or shape, some size information is possible with sample sieving or sequential filtration in sample preparation
Approaches to pyrolysis–GC/MS
Pyrolysis instrumentation
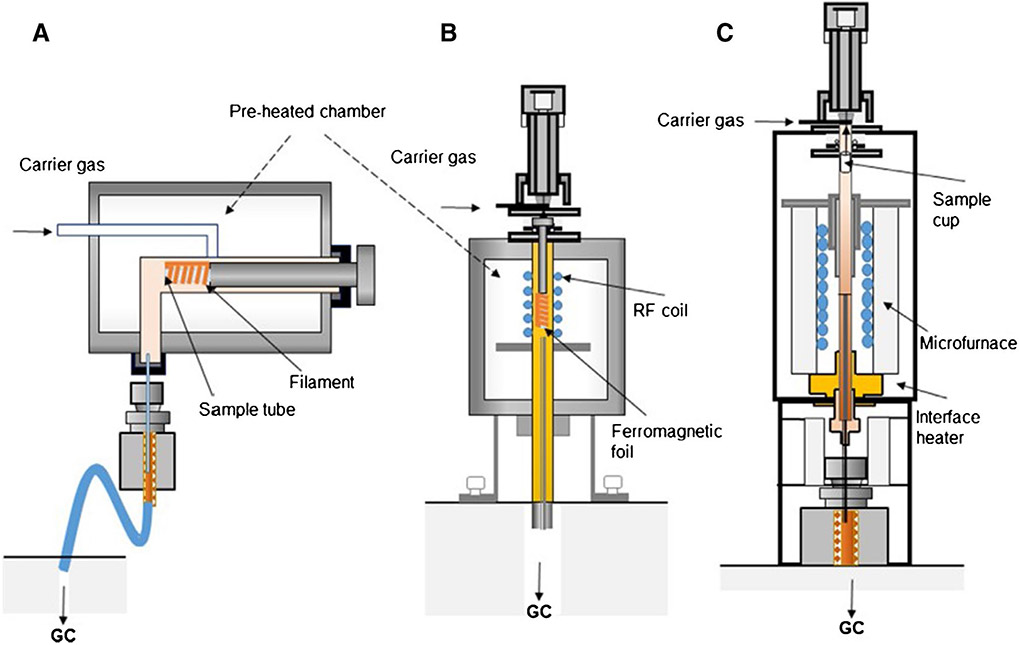
In general, Py–GC/MS is made possible by a sample furnace attached to a GC/MS inlet [12]. The pyrolysis mechanism can be categorized as pulse mode (a sample is introduced cold and then flashed at pyrolysis temperature) or continuous mode. Pulse mode systems use a heated filament or Curie-point pyrolysis, while continuous systems use furnaces or microfurnaces [13]. For either system, a small sample size and a heating area are required to ensure rapid, homogeneous pyrolysis and successful purging. The analysis of environmental plastics has been carried out with filament [14, 15], Curie point [16, 17], and microfurnace [18-34] pyrolysis (Fig. 2). Microfurnace pyrolysis (specifically vertical microfurnace) is the most common due to its ability to rapidly heat a sample (improving transfer onto the column and peak resolution) and allow different thermal schedules. Fischer and Scholz-Böttcher [19] illustrated the advantages of microfurnace over Curie-point pyrolysis, including a large sample volume capacity. Nonetheless, the overall sample size capacity for pyrolysis is generally small; for example, in microfurnace pyrolysis, only 0.1–0.5 mg is recommended, and a common sample cup volume is 80 μL. Sample overloading can lead to incomplete purging of the sample onto the column, yielding ghost peaks in subsequent runs [19, 33].
Fig. 2.
Common types of pyrolysis instrumentation, including filament (A), Curie point (B), and vertical microfurnace (C). Reproduced from Pico and Barcelo [13] with permission from the publisher (license number: 5461480936973)
Commonly, the pyrolysis unit is directly attached to the GC/MS for rapid and effective transfer. Sample heating and column evolution occurs via an inert carrier gas (commonly, helium) in the absence of oxygen. Rarely, off-line pyrolysis may be employed, which condenses pyrolyzates onto a solid-phase capture device which are resuspended in solvent prior to GC/MS [35, 36]. This technique can generate considerable variability, resulting from different behaviors between pyrolyzates and solid-phase capture devices [36]. An advantage, however, is that the solvent-suspended pyrolyzate can be retained for repeat or different analyses, possibly expanding beyond the GC-amenable window [37]. Online pyrolysis units (e.g., microfurnace) can also be attached to different analytical suites, such as time-of-flight MS [38] or tandem MS [39], but these can require increased data-processing time and training.
Evolved gas analysis Py–GC/MS
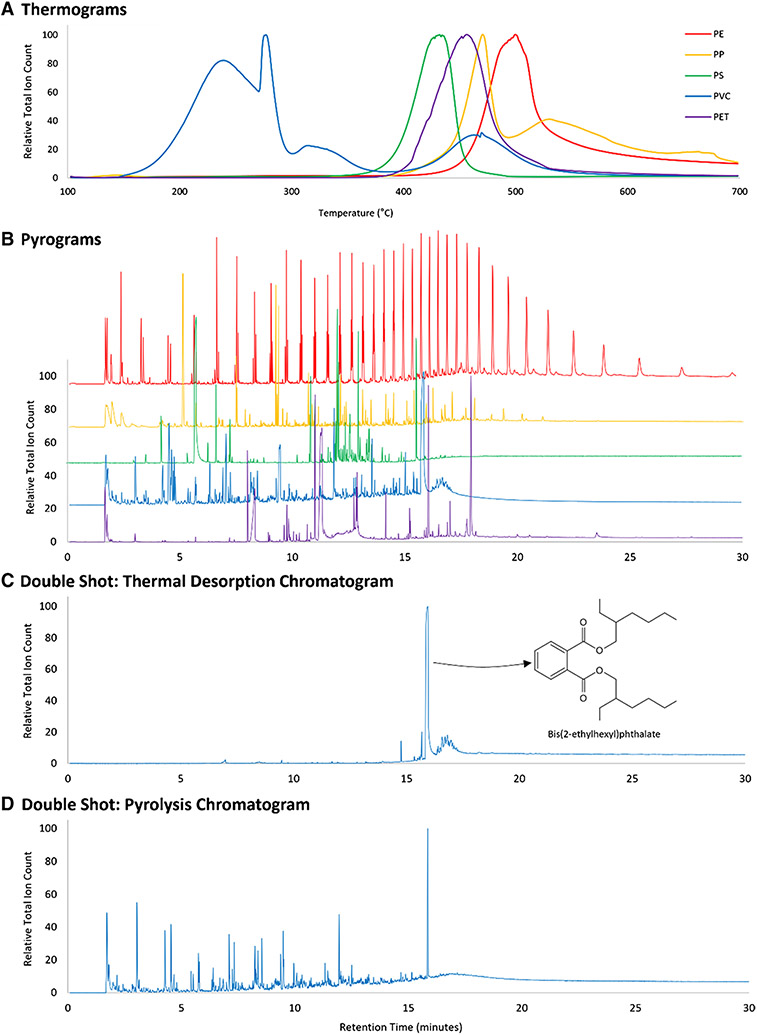
Evolved gas analysis (EGA) is the simplest of the pyrolysis approaches. EGA is commonly utilized to gain insight into the thermal deconstruction profile of a sample. During this analysis, the sample is slowly heated (e.g., 50 to 700 °C at 20 °C min−1) and volatilized material is simultaneously eluted through a short and narrow (e.g., 2.5 m, 0.15 mm) capillary tube without stationary phase. As the goal is to understand total thermal characteristics (i.e., and not presence/absence of specific compounds), the MS is run with a slower scan speed, yielding a smooth thermogram (temperature versus total ion count). Figure 3(A) shows EGA thermograms of five polymers from Hawaii Pacific University Center for Marine Debris Research (HPU CMDR) Polymer Kit 1.0 [40]. EGA thermograms are complimentary to other thermal analytical techniques, but cover a wider temperature range and provide mass spectra (as opposed to melt characteristics provided by differential scanning calorimetry (DSC), for example). EGA is particularly useful for individual samples, not complex mixtures. As such, EGA can be a valuable first step in determining temperature programs for double-shot and thermal slicing analysis.
Fig. 3.
Examples of Py–GC/MS analyses of five plastics from the HPU CMDR Polymer Kit 1.0, including polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), and polyethylene phthalate (PET). The figure includes evolved gas analysis thermograms (A), flash pyrolysis pyrograms (B; offset to improve readability), and double-shot PVC chromatograms from thermal desorption (C) and pyrolysis (D) zones, illustrating the separation of phthalate additive bis-2ethylhexyl phthalate in the thermal desorption zone. Analysis parameters are detailed in Tables S1-S3
Single-shot pyrolysis–GC/MS
In single-shot pyrolysis, a sample is flash pyrolyzed at a high temperature, ≥ 500 °C. This heating occurs as rapid as possible, inducing quasi-instantaneous and homogenous pyrolysis. The resulting gaseous products, or pyrolyzates, are formed via random chain scission, end-chain scission, and side-chain cleavage reactions. Pyrolyzates are typically deposited onto a separatory column (commonly 30 m, 0.25 mm internal diameter, 0.25 μm film thickness) connected to a quadrupole MS, although tandem MS has recently been used [39]. The resulting “pyrogram” details pyrolyzate concentration (i.e., total ion count) as a function of retention time (Fig. 3(B)). This pyrogram is like traditional GC/MS chromatograms but is specific to the flash pyrolysis products. Single-shot Py–GC/MS has been applied to the analysis of discrete plastic particles [18, 41-43] as well as complex environmental samples [14-16, 19-27, 44, 45].
Single-shot Py–GC/MS can vary by temperature. Generally, the aim is to pyrolyze a sample at a temperature that fully fragments the polymer, but does not degrade pyrolyzates [12]. Hermabessiere et al. [31] explored pyrolysis temperatures for several common plastic polymers. These authors found that PE, for example, had a maximum pyrolysis yield at 700 °C, above which detectability decreased. Recently, Okoffo et al. [29] found that many detector responses for some polymers reached their peak at 650 °C, after which the signal decreased. This is true for styrene as a pyrolyzate of PS, for example, which degrades above 650 °C [29, 31]. Most researchers employ moderate pyrolysis temperatures to avoid degrading pyrolyzates. Indeed, pyrolysis temperatures reported in the literature for the analysis of environmental plastics include 450 °C [46], 500 °C [30, 35, 36, 47, 48], 550 °C [33, 46, 49], 590 °C [16, 20, 20-22, 24-26, 36], 600 °C [14, 23, 28, 28, 32, 38, 42, 50], 650 °C [27, 29, 41, 45, 51], 700 °C [42, 52, 53], or 750 °C [15]. While the sensitivity of the results may vary for different polymers by temperature, this is a minimally consequential decision if a consistent temperature is used.
Double-shot pyrolysis–GC/MS
In a single-shot pyrolysis isothermal program, mobile (labile) components of a sample (i.e., plastic additives) are not separated from the more recalcitrant components (i.e., polymers). Double-shot Py–GC/MS (or TD–Py–GC/MS) employs two temperature programs. The first is thermal desorption (TD), where a sample is heated over a low temperature ramp (e.g., 100–300 °C at 20 °C min−1 and held for 1 min) and analyzed via GC/MS on a separatory column, yielding a TD chromatogram (Fig. 3(C)). Following TD, pyrolysis occurs according to single-shot parameters (e.g., flash pyrolysis at 550 °C; Fig. 3(D)). A growing number of studies have used TD–Py–GC/MS for the analysis of plastic debris, as there are multiple advantages [28, 29, 52-55].For individual plastic particles, double-shot can separate potential additives in the TD zone from polymeric pyrolyzates in the Py zone. This was demonstrated in 2013 by Fries et al. [52], and even earlier for presence of phthalates in recycled plastics [47] and other additives of plastics in environmental samples [54]. A recent study quantified phthalate additives (mass fraction) via thermal desorption of solvent-extracted beach sand [55], demonstrating the quantitative capacities beyond plastic polymers. A barrier to expanded additive identification in TD–Py–GC/MS is that thousands of plastic additives exist in commerce, many of which are not currently available in reference mass spectral libraries [56]. Further, GC/MS methods for the identification of additives are often directed toward one additive class (e.g., phthalates, antioxidants), and not several types of plastic additives simultaneously.
Beyond separating plastic additives, double-shot Py–GC/MS can be used to separate natural organic materials from polymer pyrolyzates, improving quantification. For example, Okoffo et al. [29] used TD–Py–GC/MS for the analysis of solvent-extracted sewage sludge samples. The authors report that adding TD reduced matrix interference from the natural organic matter present in the sample, but they did not analyze the TD chromatogram for additives. Similarly, analysis of microplastics in human blood by Leslie et al. employed TD–Py–GC/MS to reduce interference of unpolymerized monomers, additives, and adsorbed compounds, but the TD chromatogram was not analyzed [28]. Finally, double-shot analysis has been employed for analysis of changes in oxidation and pyrolysis cracking patterns in artificially weathered plastics [46]. A drawback of double-shot Py–GC/MS is that it is more time and resource intensive, taking twice as long to analyze a sample as single shot. In addition, while most polymers do not break down below 300 °C, there may be exceptions (e.g., PET [28]). Polymer fragmentation during TD could result in underestimated polymer concentrations, if sample and calibrant polymer thermal properties vary.
Thermal slicing pyrolysis–GC/MS
Some pyrolysis instruments are capable of advanced thermal programing, facilitating deeper exploration of the thermal properties and products of different materials. Thermal slicing Py–GC/MS analyzes a sample over more than two thermal ranges. This can be interchangeable with heart-cut Py–GC/MS, although heart-cut analysis may specifically refer to when a thermal range is not analyzed, accomplished by selective sampling [57]. Thermal slicing can be useful for samples where finer thermal resolution than TD–Py–GC/MS is informative. However, thermal slicing Py–GC/MS has not yet been employed, to our knowledge, for the analysis of plastics in environmental samples. Thermal slicing offers some potential benefits for advanced analysis of plastic materials, including information on structural changes and oxidation following weathering (as demonstrated in the analysis of weathered oil spill residues [58]) and distinction between labile adsorbed versus additive components.
Other Py–GC/MS adaptations
Thermochemolytic (or reactive) Py–GC/MS has been employed to improve the analysis of polymers with non-GC-amenable or polar pyrolyzates. When a thermochemolytic agent is added to a sample, esterification, transesterification, and methylation occurs, improving sensitivity for polyamides (PA; nylon), PET, polyurethane (PU), poly(methyl methacrylate) (PMMA), and others. This does not typically interfere with pyrolyzates of other polymers [16]. The most common derivatizing agent is tetramethylammonium hydroxide (TMAH) [16, 18-22, 24, 26-28, 30, 33, 36, 49]. TMAH is a very toxic and dangerous compound as it can cause chemical burns, respiratory failure, organ and central nervous system disruption, and even death; safer alternatives should be explored [59].
A less-often employed pyrolysis adaptation is cryogenic trapping Py–GC/MS, made possible with a micro-jet cryo trap accessory [58]. This cryo-focuses analytes at the start of the column, as opposed to direct elution as a sample is pyrolyzed. This can improve separation of compounds following an extended heating ramp, for example, the temperature ramp during the TD portion of double-shot Py–GC/MS or during thermal slicing Py–GC/MS. It can also improve capture of low molecular weight, volatile compounds. This has not been utilized for the analysis of environmentally sourced plastics, but has proven useful in the analysis of oil spill residues [58].
Pyrolysis GC/MS for environmental plastics analysis
Polymer identification
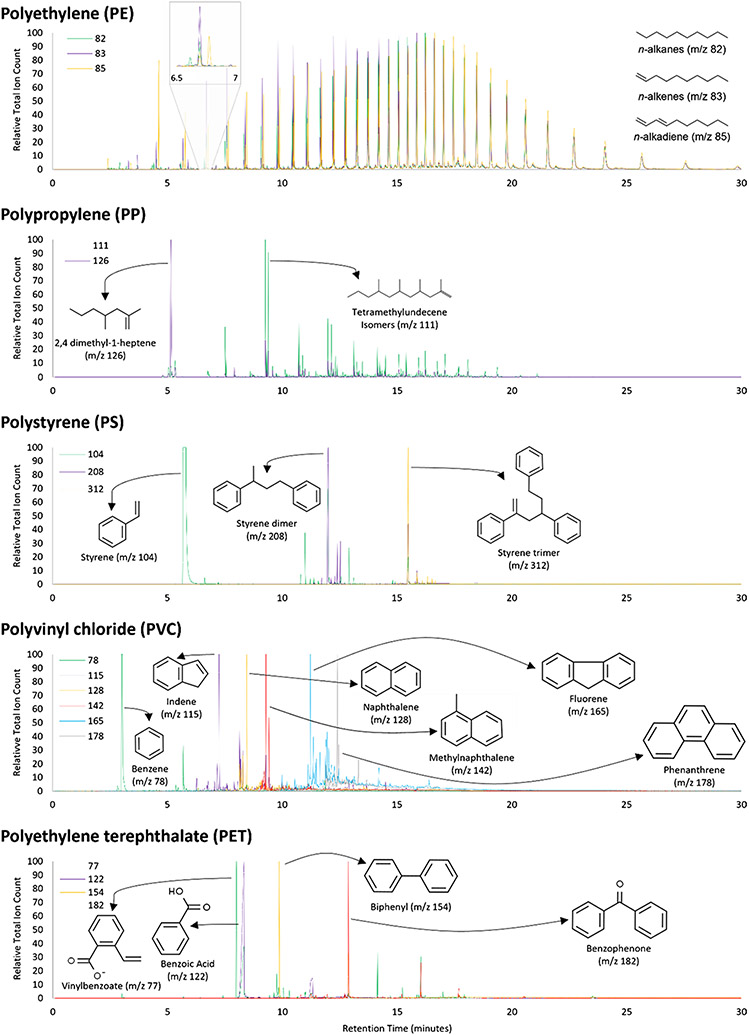
The pyrolysis behavior of most polymers is predictable, leading to the creation of specific characteristic pyrolyzate(s) that are identifiable by their mass spectra. These pyrolyzates are termed “marker compound(s)” when used to identify the polymer type of unknown plastics. In many cases, marker compounds are mono- or oligomeric components of the polymer. For example, polystyrene (PS) is identifiable by styrene monomer, dimer, and trimer. The PA marker compound is the monomer (N-methyl)-E-caprolactam for polyamide-6 and 1,8-diazacyclotetradecane-2,7-dione for polyamide-6,6. Common long-chain thermoplastics, polyethylene (PE) and polypropylene (PP), are characterized by a series of hydrocarbons from polymer decomposition. For PE, pyrolysis yields a series of n-alkane, alkene, and alkadiene triplets; for PP, 2,4-dimethyl hept-1-ene and a series of tetramethylundecene isomers. There is a growing interest in using Py–GC/MS to identify tire and road wear particles, as spectroscopic techniques are not robust enough for rubber analysis [60, 61]. Markers of tire-derived rubbers, including styrene butadiene rubber (SBR) and butadiene rubber (BR), are often small aromatic hydrocarbons such as benzene, ethylstyrene, styrene, butadiene, and vinylcyclohexene [17, 60, 61]. Natural rubber (NR) may also be distinguished via dipentene and isoprene markers [17]. Often, but not always, the best marker compound is the most abundant pyrolyzate of a polymer (see “Matrix interferences”).The extracted ion chromatograms of common marker compounds for five polymers are provided in Fig. 4. Details of pyrograms with marker compounds from a variety of additional polymers can be found in at least one reference textbook [37].
Fig. 4.
Extracted ion chromatograms of marker pyrolyzates for five common plastic polymers from HPU CMDR Polymer Kit 1.0. A single chromatogram is present for each marker and compound peak labeled with molecular structure. Pyrolysis conducted at 650 °C (analysis parameters detailed in Table S2)
Occasionally, the mass spectra of an entire thermogram or pyrogram (or an area of interest within a pyrogram) can be compared to reference polymers for identification. This is an option provided by the F-Search library (Frontier Labs, Koriyama, Fukushima, Japan). This approach, however, is not possible for complex mixtures and can be complicated by varying additive components between reference and sample polymers.
Polymer identification is simplest for a discrete piece of plastic but increases in complexity for mixtures. For individual particles, an advantage over spectroscopic methods is that copolymers can be identified more easily. However, the small sample size for pyrolysis could lead to only characterizing one component of a multilayer composite (a similar pitfall of surface-only spectroscopy). Taking multiple samples within a plastic item (e.g., outer and inner core) or carefully sampling across the entire composite can remedy this. In environmental samples with a mixture of polymers, manual inspection of the total pyrogram for a set of pyrolyzates is necessary to confirm polymer presence, even though only one marker compound may be used for quantification. For example, the presence of PE should be validated by confirming the presence of at least five of these homologous series of triplets in the C7–C41 range, even though one or two compounds may be selected for quantification [29].
Quantifying polymer mass–calibrant preparation
A unique capability of Py–GC/MS, in comparison to spectroscopic techniques, is quantifying the mass of a polymer. The quantification approach is based on external calibration curves of reference polymers using pyrolysis indicator compound(s). An indicator ion of the marker compound is extracted, and the peak area of that ion is used for calibration. This approach is akin to calibration curves for GC/MS quantification of organic compounds, including environmental pollutants. Unlike a single-compound analyte, however, it is best practice to confirm the presence of a polymer in a sample by identifying multiple marker compounds of that polymer. For example, the presence of PE should be validated by confirming the presence of at least five of these homologous series of triplets in the C7–C41 range, even though one or two compounds may be selected for quantification [29].
An important assumption behind quantifying polymer content with Py–GC/MS is that the marker compound yield is consistent between plastic varieties of the same bulk polymer type. While this is typically an acceptable assumption, recent findings have illustrated that tires are highly variable in SBR and BR content, meaning quantification of tire wear particle concentrations using a marker of SBR or BR may be inconsistent [61]. In some cases, multiple marker compound peak(s) may be integrated for improved tire wear quantification [60], a concept that can be applied to other plastics/markers.
External calibration curves can be created by weighing particles of a reference polymer for pyrolysis [16, 19-22, 24, 26, 30, 36]. Using this approach, linear calibration curves with coefficients of determination (i.e., R2) generally > 0.9, and limits of quantification as low as 0.3 μg per injection, have been obtained [16, 19]. This is constrained by the minimum weight limit and errors of the analytical balance, which often does not reach the limit of detection (based on peak signal-to-noise ratio parameters) and is a time-consuming process [19]. The upper calibration limit is constrained by the mass limit for the instrument (i.e., avoiding overloading the column or detector). To overcome these constraints, some have weighed polymers in an inert solid matrix and subsampled, achieving lower limits of detection [17, 32, 34, 50]. However, the heterogeneity of microplastics within a matrix may cause inaccuracies [19]. Similarly, Funck et al. [14] dispersed PS and PE microplastic standards in ethanol, achieving lower limits of quantification and detection for PS, which is soluble therein.
Indeed, dissolving calibrant standards in solvent is also widely used. Fisher and Scholz-Böttcher [16, 19] dissolved PS in dichloromethane (DCM) to lower the LOD an order of magnitude, from 0.3 to 0.03 μg, while continuing to weigh other calibrant polymers. Other common polymers are poorly soluble at room temperature. Accordingly, pressurized liquid extraction may be used to increase solubility. Okoffo et al. [29], Ribeiro et al. [27], and Leslie et al. [28] used pressurized fluid extraction (PFE) to dissolve polymer calibrants in DCM. This facilitated calibration curves for PE, PMMA, PS, PET, PC, polypropylene (PP), and polyvinyl chloride (PVC) by Okoffo et al.; Leslie et al. did not report using PC or PVC; PC was not tested by Ribeiro, and PET was not used as recoveries were too low (mean mass recovery: 32%). Hermabessiere and Rochman [25] reported that microwave-assisted extraction (MAE) in DCM facilitated extraction of PE, PP, PS, PMMA, PVC, and PC with gravimetric calibrant recoveries ranging from 93 to 120%, while PET was insoluble. Indeed, reporting calibrant recovery (i.e., weight of solid polymer retained in final solution) is recommended, as this varies between solvents. Krauskopf et al. [36] used tetrahydrofuran to dissolve PP, PS, and PVC for analysis, but weighed PE and PET calibrants citing these were insoluble at room temperature. However, they do not report the degree of solubility (i.e., a polymer calibrant percent recovery in solution). Steinmetz et al. [15] dissolved PE, PP, and PS in 1,2,4-trichlorobenzene (TCB), heated to 120 °C to facilitate dissolution. They reported that the plastics formed a solution phase that could be dispersed upon mixing. Efforts to use tailored solvents for different polymers for calibration have been carried out [32, 34]. For example, Matsueda et al. [34] used a 1:1 DCM:tetrahydrofuran (THF) mixture for PS, PVC, PMMA, acrylonitrile butadiene styrene (ABS), PC, and PUR, but hexafluoroisopropanol (HFIP) for nylon-6, nylon-6,6, and PET, while PE and PP were retained in solid suspension with deactivated silica. Once a polymer calibrant or standard has been brought into solution, in most cases, the extracted calibrants are diluted to create calibrant curves [15, 27-29, 34, 36]. In other cases, the calibrants may be concentrated, for example, under an inert gas stream such as N2 [25].
When using solvent-suspended polymer standards, solution stability is an important consideration. Some authors note that analysis should take place within a 3-h window post-extraction, so that the polymer does not precipitate [27, 29]. Alternatively, inter- and intra-day variability can be tested. Hermabessiere and Rochman [25] documented these values for PE, PP, PS, PMMA, PVC and PC following microwave extraction, reporting relative standard deviations of marker peak areas among sample runs (five replicates in one day, or runs over five consecutive days). The inter-day and intra-day relative standard deviations of selected markers ranged from 9.5% to 23.6% and 12.4% to 21.1%, respectively [25]. Other groups reported similar inter- and intra-day variabilities, generally under 20% and higher for inter-day than intra-day, when both are reported [14, 15, 23, 27, 29, 55]. These variabilities should be reported and used to validate marker choice when calibrants are generated in solution. For example, Hermabessiere and Rochman [25] found that the inter and intra-day variability for bisphenol A as a marker for PC were 42% and 81.7%, respectively, leading these authors to use a different marker pyrolyzate for quantification. This variability could be attributed to the precarious suspension of polymers in solvent, meaning calibrants of solid polymers would produce more reliable calibrations. In general, the calibrant preparation approach should be decided based on the sample type, recognizing the tradeoffs between variability involved in solvent dissolution compared to sensitivity. A summary of the limits of quantification reported in literature for different polymers/marker compounds is provided (Table 1).
Table 1.
Indicator compound and ions used for the analysis of different polymers with Py–GC/MS. The limits of quantitation (LOQ) are provided for each reference, categorized by calibrant preparation as a solid or in solution
| Polymer | Indicator compound | Ion(s) | LOQ solid (μg) | LOQ in solution (μg) |
|---|---|---|---|---|
| PE | Alkane(s) | 57, 71, 85, 99 | 1.0 [18], 4.0 [16], 2.3 [25] | 0.02 [27]a, 12.0 [36] |
| Alkene(s) | 56, 70, 83, 97 | 0.7 [24]a | 1.0 [18], 3.2 [25], 1.8 [26], 0.02 [27]a, 9.8 [36], 0.02 [28], 0.03 [29], 0.02 [27]a | |
| Alkadiene(s) | 55, 67, 81, 82, 95 | 0.44 [55], 0.5 [19], 0.5 [20], 0.7 [22], 9.2 [21], 4.0 [16], 3.6 [32]c, 32.0 [34]c, 0.13 [39] | 1.0–1.2 [14], 62.0 [36] | |
| PP | 2,4 Dimethyl-1-heptene | 70, 83, 126 | 0.03 [39]c, 0.85 [55], 8.0 [34]c, 0.77 [32]c, 0.3 [19, 20], 0.6 [20], 0.8 [21, 22], 0.9 [18] | 2.3 [28], 0.02 [27]a, 0.03 [29], 1.2 [25] |
| Tetramethylundecene isomers | 69 | 0.5 [24]a, 1.9 [26] | 1.4 [36], 3.0 [36], 1.7 [36] | |
| PS | Styrene | 104, 78 | 1.2 [26], 1.5 [35], 0.005 [50] | 0.001 [14], 1.1 [28] |
| Styrene dimer | 91, 130, 208 | 0.1 [24]a, 1.2 [26] | 0.02 [27]a, 0.03 [14], 0.35 [55], 0.9 [36] | |
| Styrene trimer | 91, 207, 312 | 0.005 [50], 0.2 [55], 0.8 [19, 20, 34], 0.9 [21, 22], 1.2 [16], 0.385 [39]c | 0.01 [29], 0.03 [14], 0.282 [19], 0.53 [32], 1.2 [25], 2.4 [36] | |
| PVC | Methylnaphthalene | 142 | 0.5 [24]a, 2.4 [26] | 5.7–6.2 [36] |
| Benzene | 178 | 0.3 [19], 0.7 [21], 0.8 [22], 2.9 [18], 3.0 [16] | 0.02 [27]a, 0.03 [29], 0.3 [20] | |
| Fluorene | 165 | 13.0 [36] | ||
| Indene | 115 | 2.3 [25] | ||
| Naphthalene | 128 | 0.54 [32], 1.1 [36], 4.0 [34] | ||
| Phenanthrene | 178 | 5.5 [36] | ||
| PET | Dimethyl terephthalateb | 163, 194 | 0.8 [18], 3.5 [26], 0.5 [24]a, 1.3 [36], 0.6 [19, 20], 0.7 [22], 0.9 [21], 5.0 [16], 0.025 [39]c | 0.43 [28] |
| Benzoic acid | 122 | 1.6 [34] | ||
| Benzophenone | 182 | 1.1 [32] | ||
| Ethyl or vinyl benzoateb | 105 | 25.0 [36] | 0.03 [29] | |
| PC | Dimethyl bisphenol A | 241 | 0.5 [16], 0.9 [19-22] | |
| 4-Isopropenylphenol | 134 | 0.1 [32] | 5.8 [25] | |
| Bisphenol A | 213, 288 | 0.2 [34] | 0.03 [29] | |
| p-Methoxy-tert-butylbenzeneb | 149 | 3.2 [26] | ||
| 2,2-Bis(4’methoxyphenyl) propaneb | 0.027 [39]c | |||
| PA | ε-Caprolactam | 113, 84/85 | 0.5 [19, 20], 1.0 [21, 22], 9.0 [16] | 0.1 [32], 0.28 [34] |
| N-Methyl caprolactumb | 127, 70 | 0.5 [19, 20], 1 [21, 22], 9.0 [16] | ||
| 1,8-Diazacyclotetradecane-2,7-dione | 226 | 1.2 [21] | ||
| Cyclopentanone | 84 | 1.3 [26] | 0.57 [32], 1.8 [34] | |
| Hexane | 84 | 0.5 [24]a | ||
| PU | 4,4’-Diphenylmethane diisocyanate (MDI) | 250 | 3.0 [34] | |
| 4,4’-Methylenbis(N,N-dimethylaniline) | 254 | 0.9 [22], 1.2 [21], 1.4 [19, 20] | ||
| 4,4’-Methylenedianiline | 198 | 1.1 [32] | ||
| SBR & BR | Vinylcyclohexene (butadiene dimer) | 54, 108 | 0.1 [17]a | 0.5 [32] |
| Styrene | 103, 78 | 0.13 [17]a | ||
| Butadiene | 39, 54 | 0.65 [17]a | ||
| Benzene | 78 | 1.0 [60] | ||
| α-Methylstyrene | 118 | 5.0 [60] | ||
| Ethylstyrene | 117 | 5.0 [60] | ||
| Butadiene trimer | 91 | 5.0 [60] | ||
| NR | Dipentene | 68, 136 | 0.03 [17]a | |
| Isoprene | 39, 68 | 0.04 [17]a | ||
| PMMA | Methyl methacrylate | 69, 100 | 0.4 [16], 0.5 [24]a, 0.8 [19-22], 3.3 [26], 0.035 [39]c | 0.02 [27]a, 0.09 [29], 0.26 [32], 0.33 [28], 0.8 [34], 1.6 [25] |
| ABS | 2-Phenethyl-4-phenylpent | 170 | 0.42 [32], 16 [34] |
Limit of detection; limit of quantitation not reported
Improved with thermochemolysis
Polymer diluted in solid matrix (e.g., glass fiber, deactivated silica, etc.)
An additional calibrant preparation consideration is whether to run polymer standards individually or in combination. When calibrants are weighed, the typical approach is to run calibrants individually, to obtain the lower and higher limits of calibration for each polymer while staying within instrumental loading recommendations [19]. This approach is also common for solvent-dissolved polymer standards, as the volume of the sample container (e.g., 80 μL) can be limiting. (Note: solvents are generally evaporated in a controlled manner prior to loading.) While individual calibration curves expand the calibration range, a drawback is that changes in relative signal intensity resulting from polymer interactions are not captured. Matsueda et al. [34] explored this with their solvent and inert solid matrix of 11 mixed polymer standards. They hypothesized that polymer interactions caused PE and PP to fit a quadradic calibration curve (as opposed to linear). In addition, they suggest that pyrolyzates of PUR and PET interacted, reducing the calibration quality (partially a caveat of secondary reactions between the PUR pyrolyzate, 4,4′-diphenylmethane diisocyanate, with the deactivated silica matrix used to dilute PE and PP, an issue that is unique to their sample preparations). Similarly, Steinmetz et al. [15] investigated the suitability of marker compounds based on potential interferences and found that PP may be overestimated (using 2,4-dimethyl-1-heptene as marker) when PE is present, but that interference among all polymers tested (PE, PP, PS) was generally under 10%. As such, individual calibration curves for polymers may result in inaccuracies in final calibration of samples with complex combinations of polymers. In reality, however, not all polymer interactions would be relevant for any given sample.
Regardless of sample preparation approach, careful consideration should be made on how often calibration is essential. A combination of charring, secondary reactions, and condensation of pyrolyzates can lead to residual organic material within the pyrolysis chamber [19]. Ghost peaks confounding subsequent analyses may be observed as a consequence [33]. In this case, running calibrant curves for each sample batch (e.g., group of < 20 samples) can improve calibration [19], as can running blanks (instrumental: no sample container or cup; procedural: empty sample cup) periodically [29, 32]. Notably, routine maintenance operations and reactive internal surfaces can also cause secondary reactions, resulting in calibrant variability [19].
Quantifying polymer type—internal standards
The use of internal standards can help to overcome many of the challenges associated with calibrant/sample preparation and instrument variability. Fischer and Scholz-Böttcher [19] reported improved calibration using a combination of internal standards: of 9-dodecyl-1,2,3,4,5,6,7,8-octahydro anthracene, anthracene-d10, androstane, and cholanic acid methyl ester (each 0.02 mg mL−1 in n-hexane), collectively termed ISTDPY. These represent aliphatic, planar aromatic, and non-polar aromatic compounds, respectively, as analogs of the marker compounds and not parent polymers. In some cases, 9-tetradecyl-1,2,3,4,5,6,7,8-octahydro anthracene was added to mimic methylation of acid groups during thermochemolysis. These authors determined the best internal standard for each polymer based on calibration curve fit, finding that standard deviation reduced from 100 to 6 μg with internal standards. Others have successfully used this ISTDPY solution [20-22], or just anthracene [50]. Deuterated PS (dPS; d5 or d8) can also be used as an internal standard, often in solution [14, 25], but solid powder is also available [23]. The importance of internal standards was highlighted in a 2013 study of tire wear particles, in which deuterated polyisoprene (d8), polybutadiene (d6), and dPS (d8) were used [17]. Nonetheless, not all Py–GC/MS studies use internal standards.
Advancing internal standards for plastic analysis is limited by the commercial availability of isotopically labeled plastics. While dPS is widely available, it is often in suspension and thus not analogous to microplastics. In addition, there is increased potential for hydrogen–deuterium ion exchange during pyrolysis, meaning the marker ions will not reliably identify the portion of dPS [62]. Lauschke et al. [62] illustrated that this H–D exchange reaction is heavily dependent upon the inorganic matrix and is less reliable when substrates such as aluminum oxide filters are used. Unfortunately, 13C-labeled polymers that would be better suited for these analyses have limited availability and are costly. To overcome this, Lauschke et al. [62] demonstrated that poly(4-fluorostyrene) (PRS) may be a better internal standard than dPS for PS, PP, and PE. Further work is needed to determine the best internal standards for plastic Py–GC/MS analysis. Ideally, however, these should be particles of similar size as calibration standards or plastics expected in the sample, and samples should be processed through all extraction/purification procedures with the internal standard present. This can help capture variability created by sample processing and matrix interferences. Additionally, multiple internal standards of different plastics (e.g., amorphous and semi-crystalline polymers) are necessary.
Sample preparation
Py–GC/MS is often known for requiring minimal sample preparation. While this is true for discrete pieces of plastics, from which an aliquot can be sliced and directly pyrolyzed [42, 43, 52, 53], most environmental samples require preparation. Sample preparation for Py–GC/MS is necessary to (1) concentrate the plastics within a sample for analysis and (2) minimize interference of the sample matrix.
Isolation of microplastics
Often, sample preparation for Py–GC/MS is similar to preparation for spectroscopic techniques. This includes physical separation of plastics from the matrix (e.g., density separation) and isolation from the matrix (e.g., chemical or enzymatic digestion; Fig. 1) [6]. In many workflows, samples will be concentrated on a filter which may be folded, cut, or crushed to fit into the pyrolysis chamber [15, 17, 20-22, 24, 26, 28, 39]. For example, Albignac et al. [39] digested marine benthic organisms in a potassium hydroxide solution, coarse filtered to remove any undigested material (> 500 μm), and concentrated the microplastics on a 20-μm filter, which was cryoground and aliquoted for Py–GC/MS. This split ratio can be lowered to overcome low analyte concentration.
Due to the minimal sample-processing requirements, unique approaches to Py–GC/MS analysis are also possible. Nakano et al. [50] quantified the PS concentration in individual daphnia fed PS microplastics in experimental conditions, demonstrating the possibility for Py–GC/MS to analyze discrete organismal samples [50]. In general, this split ratio can often be lowered to overcome low analyte concentration, but in the case of environmental or animal samples, this may overload the detector with matrix material.
Isolation of nanoplastics
Unlike spectroscopic approaches, there is no lower size limit of detection for Py–GC/MS, making it well poised to quantify nanoplastics [63-65]. For nanoplastics, the limiting factor is sample preparation to extract and concentrate nanoplastics above the instrument LOD. In one of the first publications of nanoplastics in oceanic waters, ter Halle et al. [66] concentrated the samples with ultrafiltration and analyzed this colloidal fraction with Py–GC/MS. In this study, the authors determined the relative abundance of the three most abundant polymers using chemometric principal component analysis of aromatic pyrolyzates but did not quantify polymer content by weight. They also used dynamic light scattering to confirm the presence of nanoparticulate prior to Py–GC/MS analysis [66]. Similarly, Mintenig et al. [64] proposed using crossflow-ultrafiltration to concentrate small micro- and nanoplastics, followed by asymmetrical field-flow fractionation (AF4) to size the particles. They demonstrated that Py–GC/MS is a viable technique for analysis of polymer type and concentration, with a lower limit of detection of 100 ng for PS nanoplastics [64]. This AF4 fractionation approach has been used to isolate nanoplastics in soil and identify them with Py–GC/MS [65].
Other mechanisms to concentrate nanoplastics for Py–GC/MS have been proposed. Zhou et al. [67] used cloud point extraction with a TritonX-45 (TX-45) surfactant to concentrate nanoplastics in water. They demonstrate a workflow in which micelles surrounding the nanoplastics are created and concentrated via centrifugation. To reduce the TX-45 interference, the surfactant was thermally desorbed at 190 °C prior to Py–GC/MS analysis. Theoretically, this could be accomplished with a double-shot Py–GC/MS. These authors successfully quantified PMMA- and PS-spiked nanoplastics in riverine water samples using this technique, but the nanoplastics in the environmental water samples were below the LOD. Zhou et al. [67] adapted a similar centrifugation approach to analyze nanoplastics in biota. Animal tissue was digested with TMAH and ethanol, filtered to exclude microplastics (> 1 μm) and centrifuged to create a pellet of nanoplastics and residual protein for Py–GC/MS analysis. This resulted in recoveries of ~ 80–90% of spiked PS and PMMA nanoplastics; PS nanoplastics were detected in tissue samples ranging from 0.8 to 2.7 μg g−1, but no PMMA was found. Sullivan et al. [68] proposed a technique to semi-quantify nanoplastics that were retained on 0.45-μm and 0.1-μm polytetrafluoroethylene (PTFE) filters. These authors used a slow temperature ramp up to 500 °C, followed by GC–TOF MS to increase detection capabilities. A laser cutter was used to subsample portions of the PTFE filter with cryomilled polymer standards or environmental samples. They demonstrated that the PS and PVC signals were above filter background, with relative standard deviation below 20% when using an internal standard. They provide an example of a river sample containing 241.8 mg L−1 PS nanoplastics [68].
A limitation on nanoplastics analysis is the availability of reference materials. While PS, PET, and PMMA spheres of nominal nanoplastic sizes are commercially available, this does not represent the variety of polymers or complexity of shapes/sizes in the environment [63]. As such, some laboratories have generated nanoplastics for experiments in the laboratory [69-71]. Although researchers may reach limits of detection low enough for nanoplastics by diluting standards of larger plastics (e.g., solvent-extracted microplastics), sample processing should be consistent to avoid dissimilarities in pyrolysis behavior. Moreover, methods to extract and concentrate nanoplastics require standards to test recovery; consequently, methodologies may be biased toward the polymers/shapes of nanoplastic standards and against environmentally relevant nanoplastics. Moving forward, the availability of nanoplastic standards of different polymers, shapes, and sizes, and/or techniques to generate nanoplastic reference materials (e.g., cryomilling) would improve measurements.
Solvent extraction
The same solvent extraction approaches used for calibrants can be used for environmental samples, but their analysis approach varies [15, 25, 27, 29, 36]. Okoffo et al. [29] directly aliquoted solvent-extracted biosolid samples and evaporated the solvent in the sample container, without pre-concentrating the extracted samples (biosolids were presumed homogeneous via pre-extraction freeze-drying, milling, and shaking). Ribeiro et al. [27] used the same pressurized extraction approach as Okoffo et al. [29], but pre-concentrated microplastics from seafood samples with matrix digestion before solvent extraction. Steinmetz et al. [15] applied an aliquot of solvent-extracted soil samples to a small filter for pyrolysis, without evaporating the solvent. Hermabessiere and Rochman [25] concentrated both sample and standard solvent extracts under N2. In some cases, only calibrants or samples, not both, have been prepared with solvent extraction. For example, Leslie et al. [28] concentrated the plastics on a filter for pyrolysis but extracted calibrants in DCM. Dierkes et al. [23] diluted calibrants for analysis in a solid matrix (silica), but solvent-extracted samples which were subsequently concentrated to dryness on silica, ground for homogenization, and subsampled for pyrolysis. Importantly, these authors noted that during concentration any polymers that suspended on vial walls were rinsed with DCM onto the silica gel [23]. As discussed with calibrants, the propensity for polymers to be resuspended in solvents is an important consideration, especially if samples are concentrated post-extraction.
Matrix interferences
A major consideration in the selection of indicator compounds is specificity to the polymer of interest. While some pyrolyzates are highly specific to a polymer type, others are common pyrolyzates of natural organic matter. For example, alkanes are a common pyrolyzate of fats and petroleum hydrocarbons, among others. Likewise, styrene (a PS marker) and benzene (a PVC, BR, and SBR marker) may condense during pyrolysis of aliphatic hydrocarbons (e.g., Diels–Alder reaction) [68]. This is why a styrene trimer is recommended over styrene as a PS marker, despite the relatively higher signal of styrene. Alternatively, the ratio of styrene to toluene can be used to confirm the presence of PS versus styrene monomer from natural organic matter [72].
It is recommended to test the interference of relevant organic matrices for proposed indicator compounds [15, 23, 29]. Dierkes et al. [23] found little interference from wood, leaf litter, humic acids, fir needles, fish filet, crayfish, engine oil, or filter paper for PS and PP, but that several matrix materials interfered with detection of PE (most specifically engine oil); however, sample pretreatment (methanol preextraction, THF solvent extraction) adequately reduced interference [23]. Likewise, Steinmetz et al. [15] tested their extraction procedure for removing interferences from soils. Okoffo et al. [29] tested the interference of fish filet, fir needles, humic acids, prawns, wood, engine oil, leaf litter, filter paper, and rice, and found that most polymers of interest (PE, PP, PS, PVC, PET, PC, PMMA) were not confounded by the matrix presence, notably due to the double-shot pyrolysis approach which could presumably devolve more volatile natural organic material in the thermal desorption step. They still reported interference from several materials for PE, however [29].
It is important to note that matrices can also interfere with the pyrolysis behavior of analytes. An extensive organic matrix can lead to different pyrolysis rates/products, cause ghost peaks, or create a variety of non-volatile products confounding polymer identification. Inclusion of an internal standard to mimic the pyrolysis reactions of the target analyte can directly help with this issue. Further, the composition of some non-petroleum-based plastics may generate pyrolyzates similar to natural organic matter. For example, Käppler et al. [18] found it difficult to identify cellulose-based fibers using Py–GC/MS, as the signals were similar to those of natural plant matter. With these considerations in mind, reducing matrix is recommended wherever possible. Further, even when an inert matrix (e.g., glass fiber filter) is used for samples, the same material should be included for calibration standards so that any potential differences in pyrolysis behaviors with/without matrix are captured.
Py–GC/MS vs. other techniques
Other thermoanalytical approaches
Py–GC/MS is just one of several thermoanalytical techniques used for the characterization of organic matter. The simplest of these is thermogravimetry (TGA), which tracks the weight differential of a sample over a heating program. TGA alone provides no chemical information; therefore, it is often combined with chemical analysis via methods such as MS, GC/MS, or FT-IR [8]. TGA on solid-phase adsorbers followed by (TD)–GC/MS combined is TED–GC/MS. This has been used for the analysis of plastics in environmental matrices, including tire wear particles [73, 74]. Another thermoanalytical method that is used for the analysis of plastics is DSC, which measures phase transitions [44, 46, 75]. In TED–GC/MS and DSC, the sample size can be one to two orders of magnitude greater than that in Py–GC/MS (1 to 10 mg versus 0.1 mg). However, condensation of the evolved gas in transfer lines may reduce transfer efficiencies to detectors [8, 44].
Analytical spectroscopy
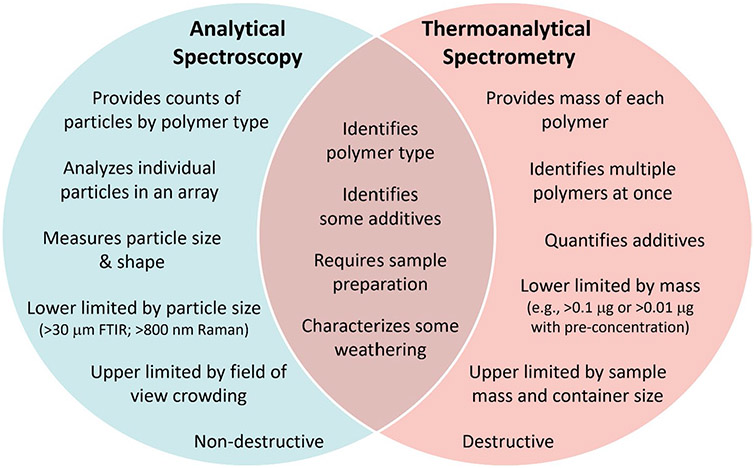
Spectroscopic techniques such as FT-IR and Raman are less complimentary to Py–GC/MS, but widely used for the analysis of plastics. While Py–GC/MS measures the mass, FT-IR and Raman use vibrational chemistry to identify the polymeric composition of individual particles, which may be counted. A variety of the differences and similarities between these approaches are summarized in Fig. 5.
Fig. 5.
Similarities and differences between common analytical spectroscopy techniques (i.e., FT-IR and Raman) and thermoanalytical spectrometry (e.g., Py–GC/MS) for the analysis of plastic in the environment
Three studies have compared datasets provided on the same sample from spectroscopic approaches and Py–GC/MS. In 2018, Käppler et al. provided a comparison between μ-attenuated total reflectance (ATR)-FT-IR and Py–GC/MS of 27 individual particles [18]. They found that both approaches were able to distinguish between plastic and non-plastic particles and identify polyolefins correctly. They highlighted the ability of Py–GC/MS to correctly identify additives in a PVC polymer, for which the polymer identification via FT-IR was confounded by additives. They also show that natural polymers as well as paint particles were more easily identified by FT-IR than Py–GC/MS; however, thermochemolysis with TMAH improved paint particle identification using Py–GC/MS. In addition, a sample was identified as ethylene vinyl–acetate by FT-IR but as PE by Py–GC/MS. The reference libraries available for spectroscopic data of environmental plastics are larger and more widely accessible [76] than libraries for Py–GC/MS, possibly biasing this result. Primpke et al. [22] undertook a similar comparison for a complex sample containing multiple particles on a filter, finding relatively similar polymer compositions between techniques. Firstly, the authors recognized that the PS pyrolyzate styrene could also derive from styrene acrylate used in paints, which would be considered a paint particle via FT-IR. Likewise, PS is likely a common co-polymer in samples, leading to its underestimation in FT-IR. These authors also used polymer density and particle size to estimate the mass of plastics identified by FT-IR. While these data were similar to mass estimates derived from Py–GC/MS, they were biased from the presence of a small number of large particles [22]. In an interlaboratory study, authors report that μRaman and Py–GC/MS were most accurate for polymer identification, but they did not attempt to normalize quantification (count vs. mass) for comparison [77].
Interestingly, several Py–GC/MS studies report larger estimates of PVC content than other techniques [22, 43, 78]. Primpke et al. [22] suggested that matrix material such as plant matter may also contribute benzene pyrolyzates, leading to the overestimation. However, in studies that investigate matrix interference in a variety of natural matrices, including plant matter, there were no observed interferences for PVC quantification. In fact, one group observed matrix interference may lower the PVC pyrolyzate signal [72]. It is possible that secondary reactions of multiple polymers in a sample can lead to benzene, or perhaps PVC is underestimated spectroscopically due to high additive (i.e., phthalate) content. Alternatively, Hendrickson et al. [43] found that chlorinated PE was identified as PVC via Py–GC/MS and PE via FT-IR. This bias of Py–GC/MS toward PVC has otherwise not been addressed in the literature. As such, the question remains—is the environment truly more polluted with PVC micro/nanoplastics than we realize, or is there an analytical bias created by Py–GC/MS?
Conclusions
In the context of this review, future opportunities to improve or expand upon Py–GC/MS analyses of plastics emerge. Suggested best practices include:
Sample and calibrant preparation procedures should be identical. Due to the complex pyrolytic nature of plastics and organic matter, secondary reactions that can magnify or reduce the production of a given pyrolyzate are possible. As such, samples and calibrants should be prepared similarly. If a sample is suspected to contain multiple polymers, calibrants of those polymers should be mixed. Similarly, if a sample is collected and analyzed on a filter, the same filter type should be used for calibrants.
Size-sort samples prior to analysis. A limitation of Py–GC/MS is the inability to quantify particle size. Toward rectifying this gap, sequential sieving or filtering of samples prior to Py–GC/MS could help determine the mass of polymers in a given particle size range. This, however, would not inform on particle form (i.e., fragment, fiber, etc.).
Use internal standards, preferably solid, carbon-labeled polymers, for quantification. Studies have demonstrated that internal standards improve Py–GC/MS polymer quantification. The most common approaches are a mix of deuterated organic compounds, anthracene [19-22, 50], or solvent-suspended dPS [14, 17, 23, 25]. While these improve quantification, Lauschke et al. [62] demonstrated that deuterated standards are subject to hydrogen–deuterium ion exchange in pyrolysis when some matrices are present [62]. Although expensive, carbon-labeled polymers of a similar state, size, and shape as calibrants/samples are ideal.
Use thermal desorption to identify or quantify additives. While some of the earliest work with Py–GC/MS demonstrated additive identification [52], little work since has been done to simultaneously quantify additives and polymers [55], or even identify additives within a sample. As mass spectral libraries of plastic additives expand, further work should use Py–GC/MS to better characterize additives in plastics and environmental samples, especially considering that additives may be an important component of toxicity [5].
Improve our understanding of the sensitivity of Py–GC/MS in scan versus selected ion monitoring (SIM) modes. While Py–GC/MS is destructive, efforts to preserve the data for later analyses are possible when samples are processed in full-scan mode with a wide ion range. This can facilitate future data mining as GC/MS reference libraries expand. The benefits of selected ion monitoring cannot be ignored, however; these include (a) improving calibration and lowering limits of detection, particularly for nanoplastics, and (b) increasing detection of low-concentration plastic additives [79]. When possible, duplicating sample analysis in scan and SIM modes could be considered a cautionary approach, as our understanding of pyrolyzate compound sensitivity increases.
Facilitate Py–GC/MS data sharing between users to improve sample characterization. By comparing data between labs working with Py–GC/MS, as has been developed for spectroscopic techniques [76], identification of both complex polymers and additives, as well as weathering patterns, could be improved.
Characterize extent of plastic weathering. A major data gap in plastics fate models is environmental residence time, which may be demystified by quantifying weathering severity. Research has illustrated that Py–GC/MS can detect polymeric changes due to photooxidation [46]. Likewise, the relative photooxidation of polluted petroleum has been characterized with Py–GC/MS [58]. Future investigations may expand this realm of research to help fill the “age” gap of polluted plastics.
Continue and improve quality assurance and quality control (QA/QC) measures. Recently, the plastic pollution field has made great strides in refining QA/QC protocols [80, 81]. Best practices include limiting contamination by plastics (particularly from clothing, supplies/instrumentation, and dust) in field and laboratory processes, as well as conducting field and laboratory blanks to characterize unavoidable contamination. Consistent with previous discussion, additional QA/QC requirements for Py–GC/MS should include furnace and inlet cleaning, as well as running calibrants for each sample batch. In addition, spike recovery experiments are recommended for all environmental matrices analyzed. This is particularly important as calibrant and sample preparation techniques evolve for Py–GC/MS. Strategic use of existing reference materials (such as NIST polystyrene nanosphere Standard Reference Materials (SRMs 1691 and 1964)) are invaluable in QA/QC, particularly for nanoplastics [67]. The field would benefit from development of environmental matrix reference materials that are certified for masses of micro- and nanoplastics, and additives therein.
In conclusion, Py–GC/MS is an advantageous technique for the analysis of plastics. This review highlights the approaches, benefits, and caveats of Py–GC/MS for the analysis of plastics, pointing toward the potential benefits of Py–GC/MS for future use.
Supplementary Material
Acknowledgements
The authors appreciate manuscript revision provided by Drs. John Kucklick, Katherine Shaw, Robert C. Hale, and Carlos Gonzalez. Graphical abstract created with biorender.com.
Funding
Funding for Dr. Seeley is provided by the National Research Council through the Research Associateship Program.
Footnotes
Competing interests The authors declare no competing interests.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00216-023-04671-1.
NIST disclaimer Certain commercial equipment, instruments, or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified is necessarily the best available for the purpose.
References
- 1.Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, Rist S, Karlsson T, Brennholt N, Cole M, Herrling MP, Hess MC, Ivleva NP, Lusher AL, Wagner M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ Sci Technol. 2019;53:1039–47. 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- 2.Arthur C, Baker J, Bamford H (2008) Proceedings of the international research workshop on the occurrence, effects and fate of microplastic marine debris. NOAA Technical Memorandum NOS-OR&R-30. https://repository.library.noaa.gov/view/noaa/2509/noaa_2509_DS1.pdf [Google Scholar]
- 3.Rochman CM, Brookson C, Bikker J, Djuric N, Earn A, Bucci K, Athey S, Huntington A, McIlwraith H, Munno K, De Frond H, Kolomijeca A, Erdle L, Grbic J, Bayoumi M, Borrelle SB, Wu T, Santoro S, Werbowski LM, Zhu X, Giles RK, Hamilton BM, Thaysen C, Kaura A, Klasios N, Ead L, Kim J, Sherlock C, Ho A, Hung C. Rethinking microplastics as a diverse contaminant suite. Environ Toxicol Chem. 2019;38:703–11. 10.1002/etc.4371. [DOI] [PubMed] [Google Scholar]
- 4.Bucci K, Rochman CM. Microplastics: a multidimensional contaminant requires a multidimensional framework for assessing risk. Micropl&Nanopl. 2022;2:7. 10.1186/s43591-022-00028-0. [DOI] [Google Scholar]
- 5.Hale RC, Seeley ME, La Guardia MJ, Mai L, Zeng EY. A global perspective on microplastics. J Geophys Res Oceans. 2020;125:e2018JC014719. 10.1029/2018JC014719. [DOI] [Google Scholar]
- 6.Lusher AL, Munno K, Hermabessiere L, Carr S. Isolation and extraction of microplastics from environmental samples: an evaluation of practical approaches and recommendations for further harmonization. Appl Spectrosc. 2020;74:1049–65. 10.1177/0003702820938993. [DOI] [PubMed] [Google Scholar]
- 7.Gouin T, Becker RA, Collot A, Davis JW, Howard B, Inawaka K, Lampi M, Ramon BS, Shi J, Hopp PW. Toward the development and application of an environmental risk assessment framework for microplastic. Environ Toxic Chem. 2019;38:2087–100. 10.1002/etc.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peñalver R, Arroyo-Manzanares N, López-García I, Hernández-Córdoba M. An overview of microplastics characterization by thermal analysis. Chemosphere. 2020;242:125170. 10.1016/j.chemosphere.2019.125170. [DOI] [PubMed] [Google Scholar]
- 9.Primpke S, Christiansen SH, Cowger W, De Frond H, Deshpande A, Fischer M, Holland EB, Meyns M, O’Donnell BA, Ossmann BE, Pittroff M, Sarau G, Scholz-Böttcher BM, Wiggin KJ. Critical assessment of analytical methods for the harmonized and cost-efficient analysis of microplastics. Appl Spectrosc. 2020;74:1012–47. 10.1177/0003702820921465. [DOI] [PubMed] [Google Scholar]
- 10.Rochman CM, Regan F, Thompson RC. On the harmonization of methods for measuring the occurrence, fate and effects of microplastics. Anal Methods. 2017;9:1324–5. 10.1039/C7AY90014G. [DOI] [Google Scholar]
- 11.Provencher JF, Covernton GA, Moore RC, Horn DA, Conkle JL, Lusher AL. Proceed with caution: the need to raise the publication bar for microplastics research. Sci Total Environ. 2020;748:141426. 10.1016/j.scitotenv.2020.141426. [DOI] [PubMed] [Google Scholar]
- 12.Dehaut A, Hermabessiere L, Duflos G. Microplastics Detection using pyrolysis-GC/MS-based methods. In: Rocha-Santos T, Costa M, Mouneyrac C, editors. Handbook of microplastics in the environment. Cham: Springer International Publishing; 2020. p. 1–35. [Google Scholar]
- 13.Picó Y, Barceló D. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: focus on organic matter and microplastics. TrAC Trends Anal Chem. 2020;130:115964. 10.1016/j.trac.2020.115964. [DOI] [Google Scholar]
- 14.Funck M, Yildirim A, Nickel C, Schram J, Schmidt TC, Tuerk J. Identification of microplastics in wastewater after cascade filtration using pyrolysis-GC–MS. MethodsX. 2020;7:100778. 10.1016/j.mex.2019.100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinmetz Z, Kintzi A, Muñoz K, Schaumann GE. A simple method for the selective quantification of polyethylene, polypropylene, and polystyrene plastic debris in soil by pyrolysis-gas chromatography/mass spectrometry. J Anal Appl Pyrolysis. 2020;147:104803. 10.1016/j.jaap.2020.104803. [DOI] [Google Scholar]
- 16.Fischer M, Scholz-Böttcher BM. Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography–mass spectrometry. Environ Sci Technol. 2017;51:5052–60. 10.1021/acs.est.6b06362. [DOI] [PubMed] [Google Scholar]
- 17.Unice KM, Kreider ML, Panko JM. Use of a deuterated internal standard with pyrolysis-GC/MS dimeric marker analysis to quantify tire tread particles in the environment. IJERPH. 2012;9:4033–55. 10.3390/ijerph9114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Käppler A, Fischer M, Scholz-Böttcher BM, Oberbeckmann S, Labrenz M, Fischer D, Eichhorn K-J, Voit B. Comparison of μ-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal Bioanal Chem. 2018;410:5313–27. 10.1007/s00216-018-1185-5. [DOI] [PubMed] [Google Scholar]
- 19.Fischer M, Scholz-Böttcher BM. Microplastics analysis in environmental samples – recent pyrolysis-gas chromatography-mass spectrometry method improvements to increase the reliability of mass-related data. Anal Methods. 2019;11:2489–97. 10.1039/C9AY00600A. [DOI] [Google Scholar]
- 20.Fischer M, Goßmann I, Scholz-Böttcher BM. Fleur de Sel—an interregional monitor for microplastics mass load and composition in European coastal waters? J Anal Appl Pyrolysis. 2019;144:104711. 10.1016/j.jaap.2019.104711. [DOI] [Google Scholar]
- 21.Dibke C, Fischer M, Scholz-Böttcher BM. Microplastic mass concentrations and distribution in German bight waters by pyrolysis–gas chromatography–mass spectrometry/thermochemolysis reveal potential impact of marine coatings: do ships leave skid marks? Environ Sci Technol. 2021;55:2285–95. 10.1021/acs.est.0c04522. [DOI] [PubMed] [Google Scholar]
- 22.Primpke S, Fischer M, Lorenz C, Gerdts G, Scholz-Böttcher BM. Comparison of pyrolysis gas chromatography/mass spectrometry and hyperspectral FTIR imaging spectroscopy for the analysis of microplastics. Anal Bioanal Chem. 2020;412:8283–98. 10.1007/s00216-020-02979-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dierkes G, Lauschke T, Becher S, Schumacher H, Földi C, Ternes T. Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal Bioanal Chem. 2019;411:6959–68. 10.1007/s00216-019-02066-9. [DOI] [PubMed] [Google Scholar]
- 24.Gomiero A, Øysæd KB, Agustsson T, van Hoytema N, van Thiel T, Grati F. First record of characterization, concentration and distribution of microplastics in coastal sediments of an urban fjord in south west Norway using a thermal degradation method. Chemosphere. 2019;227:705–14. 10.1016/j.chemosphere.2019.04.096. [DOI] [PubMed] [Google Scholar]
- 25.Hermabessiere L, Rochman CM. Microwave-assisted extraction for quantification of microplastics using pyrolysis–gas chromatography/mass spectrometry. Environ Toxicol Chem. 2021;40:2733–41. 10.1002/etc.5179. [DOI] [PubMed] [Google Scholar]
- 26.Kirstein IV, Hensel F, Gomiero A, Iordachescu L, Vianello A, Wittgren HB, Vollertsen J. Drinking plastics? – Quantification and qualification of microplastics in drinking water distribution systems by μFTIR and Py-GCMS. Water Res. 2021;188:116519. 10.1016/j.watres.2020.116519. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro F, Okoffo ED, O’Brien JW, Fraissinet-Tachet S, O’Brien S, Gallen M, Samanipour S, Kaserzon S, Mueller JF, Galloway T, Thomas KV. Quantitative analysis of selected plastics in high-commercial-value Australian seafood by pyrolysis gas chromatography mass spectrometry. Environ Sci Technol. 2020;54:9408–17. 10.1021/acs.est.0c02337. [DOI] [PubMed] [Google Scholar]
- 28.Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- 29.Okoffo ED, Ribeiro F, O’Brien JW, O’Brien S, Tscharke BJ, Gallen M, Samanipour S, Mueller JF, Thomas KV. Identification and quantification of selected plastics in biosolids by pressurized liquid extraction combined with double-shot pyrolysis gas chromatography–mass spectrometry. Sci Total Environ. 2020;715:136924. 10.1016/j.scitotenv.2020.136924. [DOI] [PubMed] [Google Scholar]
- 30.Coralli I, Giorgi V, Vassura I, Rombolà AG, Fabbri D. Secondary reactions in the analysis of microplastics by analytical pyrolysis. J Anal Appl Pyrolysis. 2022;161:105377. 10.1016/j.jaap.2021.105377. [DOI] [Google Scholar]
- 31.Hermabessiere L, Himber C, Boricaud B, Kazour M, Amara R, Cassone A-L, Laurentie M, Paul-Pont I, Soudant P, Dehaut A, Duflos G. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal Bioanal Chem. 2018;410:6663–76. 10.1007/s00216-018-1279-0. [DOI] [PubMed] [Google Scholar]
- 32.Ishimura T, Iwai I, Matsui K, Mattonai M, Watanabe A, Robberson W, Cook A-M, Allen HL, Pipkin W, Teramae N, Ohtani H, Watanabe C. Qualitative and quantitative analysis of mixtures of microplastics in the presence of calcium carbonate by pyrolysis-GC/MS. J Anal Appl Pyrolysis. 2021;157:105188. 10.1016/j.jaap.2021.105188. [DOI] [Google Scholar]
- 33.Matsui K, Ishimura T, Mattonai M, Iwai I, Watanabe A, Teramae N, Ohtani H, Watanabe C. Identification algorithm for polymer mixtures based on Py-GC/MS and its application for microplastic analysis in environmental samples. J Anal Appl Pyrolysis. 2020;149:104834. 10.1016/j.jaap.2020.104834. [DOI] [Google Scholar]
- 34.Matsueda M, Mattonai M, Iwai I, Watanabe A, Teramae N, Robberson W, Ohtani H, Kim Y-M, Watanabe C. Preparation and test of a reference mixture of eleven polymers with deactivated inorganic diluent for microplastics analysis by pyrolysis-GC–MS. J Anal Appl Pyrolysis. 2021;154:104993. 10.1016/j.jaap.2020.104993. [DOI] [Google Scholar]
- 35.Fabbri D, Rombolà AG, Vassura I, Torri C, Franzellitti S, Capolupo M, Fabbri E. Off-line analytical pyrolysis GC–MS to study the accumulation of polystyrene microparticles in exposed mussels. J Anal Appl Pyrolysis. 2020;149:104836. 10.1016/j.jaap.2020.104836. [DOI] [Google Scholar]
- 36.Krauskopf L-M, Hemmerich H, Dsikowitzky L, Schwarzbauer J. Critical aspects on off-line pyrolysis-based quantification of microplastic in environmental samples. J Anal Appl Pyrolysis. 2020;152:104830. 10.1016/j.jaap.2020.104830. [DOI] [Google Scholar]
- 37.Shin T, Hajime O, Chuichi W. Pyrolysis-GC/MS data book of synthetic polymers: pyrograms, thermograms and MS of pyrolyzates. 1st ed. Elsevier; 2011. [Google Scholar]
- 38.Harata K, Kitagawa S, Iiguni Y, Ohtani H. Identification of polymer species in a complex mixture by pyrolysis-gas chromatography-atmospheric pressure chemical ionization-high resolution time-of-flight mass spectrometry as a basis for environmental microplastic analysis. J Anal Appl Pyrolysis. 2020;148:104828. 10.1016/j.jaap.2020.104828. [DOI] [Google Scholar]
- 39.Albignac M, Ghiglione JF, Labrune C, ter Halle A. Determination of the microplastic content in Mediterranean benthic macrofauna by pyrolysis-gas chromatography-tandem mass spectrometry. Mar Pollut Bull. 2022;181:113882. 10.1016/j.marpolbul.2022.113882. [DOI] [PubMed] [Google Scholar]
- 40.The Center for Marine Debris Research (2020) Polymer Kit 1.0. https://www.hpu.edu/cncs/cmdr/img/polymerkit1.0_marketingbrochure.pdf
- 41.Chouchene K, Nacci T, Modugno F, Castelvetro V, Ksibi M. Soil contamination by microplastics in relation to local agricultural development as revealed by FTIR, ICP-MS and pyrolysis-GC/MS. Environ Pollut. 2022;303:119016. 10.1016/j.envpol.2022.119016. [DOI] [PubMed] [Google Scholar]
- 42.Doyen P, Hermabessiere L, Dehaut A, Himber C, Decodts M, Degraeve T, Delord L, Gaboriaud M, Moné P, Sacco J, Tavernier E, Grard T, Duflos G. Occurrence and identification of microplastics in beach sediments from the Hauts-de-France region. Environ Sci Pollut Res. 2019;26:28010–21. 10.1007/s11356-019-06027-8. [DOI] [PubMed] [Google Scholar]
- 43.Hendrickson E, Minor EC, Schreiner K. Microplastic abundance and composition in Western Lake Superior as determined via microscopy, Pyr-GC/MS, and FTIR. Environ Sci Technol. 2018;52:1787–96. 10.1021/acs.est.7b05829. [DOI] [PubMed] [Google Scholar]
- 44.Becker R, Altmann K, Sommerfeld T, Braun U. Quantification of microplastics in a freshwater suspended organic matter using different thermoanalytical methods – outcome of an interlaboratory comparison. J Anal Appl Pyrolysis. 2020;148:104829. 10.1016/j.jaap.2020.104829. [DOI] [Google Scholar]
- 45.Watteau F, Dignac M-F, Bouchard A, Revallier A, Houot S. Microplastic detection in soil amended with municipal solid waste composts as revealed by transmission electronic microscopy and pyrolysis/GC/MS. Front Sustain Food Syst. 2018;2:81. 10.3389/fsufs.2018.00081. [DOI] [Google Scholar]
- 46.Ainali NM, Bikiaris DN, Lambropoulou DA. Aging effects on low- and high-density polyethylene, polypropylene and polystyrene under UV irradiation: an insight into decomposition mechanism by Py-GC/MS for microplastic analysis. J Anal Appl Pyrolysis. 2021;158:105207. 10.1016/j.jaap.2021.105207. [DOI] [Google Scholar]
- 47.Herrera M, Matuschek G, Kettrup A. Fast identification of polymer additives by pyrolysis-gas chromatography/mass spectrometry. J Anal Appl Pyrol. 2003;70:35–42. 10.1016/S0165-2370(02)00078-5. [DOI] [Google Scholar]
- 48.Ni B-J, Zhu Z-R, Li W-H, Yan X, Wei W, Xu Q, Xia Z, Dai X, Sun J. Microplastics mitigation in sewage sludge through pyrolysis: the role of pyrolysis temperature. Environ Sci Technol Lett. 2020;7:961–7. 10.1021/acs.estlett.0c00740. [DOI] [Google Scholar]
- 49.Nardella F, Bellavia S, Mattonai M, Ribechini E. Co-pyrolysis of biomass and plastic: synergistic effects and estimation of elemental composition of pyrolysis oil by analytical pyrolysis–gas chromatography/mass spectrometry. Bioresource Technol. 2022;354:127170. 10.1016/j.biortech.2022.127170. [DOI] [PubMed] [Google Scholar]
- 50.Nakano R, Gürses RK, Tanaka Y, Ishida Y, Kimoto T, Kitagawa S, Iiguni Y, Ohtani H. Pyrolysis-GC–MS analysis of ingested polystyrene microsphere content in individual Daphnia magna. Sci Total Environ. 2022;817:152981. 10.1016/j.scitotenv.2022.152981. [DOI] [PubMed] [Google Scholar]
- 51.El Hayany B, El Fels L, Quénéa K, Dignac M-F, Rumpel C, Gupta VK, Hafidi M. Microplastics from lagooning sludge to composts as revealed by fluorescent staining- image analysis, Raman spectroscopy and pyrolysis-GC/MS. J Environ Manag. 2020;275:111249. 10.1016/j.jenvman.2020.111249. [DOI] [PubMed] [Google Scholar]
- 52.Fries E, Dekiff JH, Willmeyer J, Nuelle M-T, Ebert M, Remy D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci: Processes Impacts. 2013;15:1949. 10.1039/c3em00214d. [DOI] [PubMed] [Google Scholar]
- 53.Vilakati B, Sivasankar V, Mamba BB, Omine K, Msagati TAM. Characterization of plastic micro particles in the Atlantic Ocean seashore of Cape Town, South Africa and mass spectrometry analysis of pyrolyzate products. Environ Pollut. 2020;265:114859. 10.1016/j.envpol.2020.114859. [DOI] [PubMed] [Google Scholar]
- 54.Dekiff JH, Remy D, Klasmeier J, Fries E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ Pollut. 2014;186:248–56. 10.1016/j.envpol.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 55.La Nasa J, Biale G, Mattonai M, Modugno F. Microwave-assisted solvent extraction and double-shot analytical pyrolysis for the quali-quantitation of plasticizers and microplastics in beach sand samples. J Hazard Mater. 2021;401:123287. 10.1016/j.jhazmat.2020.123287. [DOI] [PubMed] [Google Scholar]
- 56.Lynch JM, Knauer K, Shaw KR. Plastic additives in the ocean. In: Andrady AL, editor. Plastics and the ocean. 1st ed. Wiley; 2022. p. 43–76. [Google Scholar]
- 57.Takahahashi T Operating principle of selective sampler. Frontier Lab. Technical report PYT-036E. https://www.frontier-lab.com/assets/file/technical-note/PYT-036E.pdf [Google Scholar]
- 58.Seeley ME, Wang Q, Bacosa H, Rosenheim BE, Liu Z. Environmental petroleum pollution analysis using ramped pyrolysis-gas chromatography–mass spectrometry. Org Geochem. 2018;124:180–9. 10.1016/j.orggeochem.2018.07.012. [DOI] [Google Scholar]
- 59.Lin C-C, Yang C-C, Ger J, Deng J-F, Hung D-Z. Tetramethylammonium hydroxide poisoning. Clin Toxicol. 2010;48:213–7. 10.3109/15563651003627777. [DOI] [PubMed] [Google Scholar]
- 60.Rødland ES, Samanipour S, Rauert C, Okoffo ED, Reid MJ, Heier LS, Lind OC, Thomas KV, Meland S. A novel method for the quantification of tire and polymer-modified bitumen particles in environmental samples by pyrolysis gas chromatography mass spectroscopy. J Hazard Mater. 2022;423:127092. 10.1016/j.jhazmat.2021.127092. [DOI] [PubMed] [Google Scholar]
- 61.Rauert C, Rødland ES, Okoffo ED, Reid MJ, Meland S, Thomas KV. Challenges with quantifying tire road wear particles: recognizing the need for further refinement of the ISO technical specification. Environ Sci Technol Lett. 2021;8:231–6. 10.1021/acs.estlett.0c00949. [DOI] [Google Scholar]
- 62.Lauschke T, Dierkes G, Schweyen P, Ternes TA. Evaluation of poly(styrene-d5) and poly(4-fluorostyrene) as internal standards for microplastics quantification by thermoanalytical methods. J Anal Appl Pyrolysis. 2021;159:105310. 10.1016/j.jaap.2021.105310. [DOI] [Google Scholar]
- 63.Cai H, Xu EG, Du F, Li R, Liu J, Shi H. Analysis of environmental nanoplastics: progress and challenges. Chem Eng J. 2021;410:128208. 10.1016/j.cej.2020.128208. [DOI] [Google Scholar]
- 64.Mintenig SM, Int-Veen I, Löder MGJ, Primpke S, Gerdts G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017;108:365–72. 10.1016/j.watres.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 65.Wahl A, Le Juge C, Davranche M, El Hadri H, Grassl B, Reynaud S, Gigault J. Nanoplastic occurrence in a soil amended with plastic debris. Chemosphere. 2021;262:127784. 10.1016/j.chemosphere.2020.127784. [DOI] [PubMed] [Google Scholar]
- 66.Ter Halle A, Jeanneau L, Martignac M, Jardé E, Pedrono B, Brach L, Gigault J. Nanoplastic in the North Atlantic subtropical gyre. Environ Sci Technol. 2017;51:13689–97. 10.1021/acs.est.7b03667. [DOI] [PubMed] [Google Scholar]
- 67.Zhou X, Hao L, Wang H, Li Y, Liu J. Cloud-point extraction combined with thermal degradation for nanoplastic analysis using pyrolysis gas chromatography–mass spectrometry. Anal Chem. 2019;91:1785–90. 10.1021/acs.analchem.8b04729. [DOI] [PubMed] [Google Scholar]
- 68.Sullivan GL, Gallardo JD, Jones EW, Hollliman PJ, Watson TM, Sarp S. Detection of trace sub-micron (nano) plastics in water samples using pyrolysis-gas chromatography time of flight mass spectrometry (PY-GCToF). Chemosphere. 2020;249:126179. 10.1016/j.chemosphere.2020.126179. [DOI] [PubMed] [Google Scholar]
- 69.Blancho F, Davranche M, Fumagalli F, Ceccone G, Gigault J. A reliable procedure to obtain environmentally relevant nanoplastic proxies. Environ Sci: Nano. 2021;8:3211–9. 10.1039/D1EN00395J. [DOI] [Google Scholar]
- 70.El Hadri H, Gigault J, Maxit B, Grassl B, Reynaud S. Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. NanoImpact. 2020;17:100206. 10.1016/j.impact.2019.100206. [DOI] [Google Scholar]
- 71.Mitrano DM, Beltzung A, Frehland S, Schmiedgruber M, Cingolani A, Schmidt F. Synthesis of metal-doped nanoplastics and their utility to investigate fate and behaviour in complex environmental systems. Nat Nanotechnol. 2019;14:362–8. 10.1038/s41565-018-0360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bouzid N, Anquetil C, Dris R, Gasperi J, Tassin B, Derenne S. Quantification of microplastics by pyrolysis coupled with gas chromatography and mass spectrometry in sediments: challenges and implications. Microplastics. 2022;1:229–39. 10.3390/microplastics1020016. [DOI] [Google Scholar]
- 73.Dümichen E, Eisentraut P, Bannick CG, Barthel A-K, Senz R, Braun U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere. 2017;174:572–84. 10.1016/j.chemosphere.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 74.Eisentraut P, Dümichen E, Ruhl AS, Jekel M, Albrecht M, Gehde M, Braun U. Two birds with one stone—fast and simultaneous analysis of microplastics: microparticles derived from thermoplastics and tire wear. Environ Sci Technol Lett. 2018;5:608–13. 10.1021/acs.estlett.8b00446. [DOI] [Google Scholar]
- 75.Kühn S, van Oyen A, Booth AM, Meijboom A, van Franeker JA. Marine microplastic: preparation of relevant test materials for laboratory assessment of ecosystem impacts. Chemosphere. 2018;213:103–13. 10.1016/j.chemosphere.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 76.Cowger W, Steinmetz Z, Gray A, Munno K, Lynch J, Hapich H, Primpke S, De Frond H, Rochman C, Herodotou O. Microplastic spectral classification needs an open source community: open specy to the rescue! Anal Chem. 2021;93:7543–8. 10.1021/acs.analchem.1c00123. [DOI] [PubMed] [Google Scholar]
- 77.Müller YK, Wernicke T, Pittroff M, Witzig CS, Storck FR, Klinger J, Zumbülte N. Microplastic analysis—are we measuring the same? Results on the first global comparative study for microplastic analysis in a water sample. Anal Bioanal Chem. 2020;412:555–60. 10.1007/s00216-019-02311-1. [DOI] [PubMed] [Google Scholar]
- 78.Fabbri D. Use of pyrolysis-gas chromatography/mass spectrometry to study environmental pollution caused by synthetic polymers: a case study: the Ravenna Lagoon. J Anal Appl Pyrol. 2001;58–59:361–70. 10.1016/S0165-2370(00)00170-4. [DOI] [Google Scholar]
- 79.Hermabessiere L, Dehaut A, Paul-Pont I, Lacroix C, Jezequel R, Soudant P, Duflos G. Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere. 2017;182:781–93. 10.1016/j.chemosphere.2017.05.096. [DOI] [PubMed] [Google Scholar]
- 80.Brander SM, Renick VC, Foley MM, Steele C, Woo M, Lusher A, Carr S, Helm P, Box C, Cherniak S, Andrews RC, Rochman CM. Sampling and quality assurance and quality control: a guide for scientists investigating the occurrence of microplastics across matrices. Appl Spectrosc. 2020;74:1099–125. 10.1177/0003702820945713. [DOI] [PubMed] [Google Scholar]
- 81.Cowger W, Booth AM, Hamilton BM, Thaysen C, Primpke S, Munno K, Lusher AL, Dehaut A, Vaz VP, Liboiron M, Devriese LI, Hermabessiere L, Rochman C, Athey SN, Lynch JM, De Frond H, Gray A, Jones OAH, Brander S, Steele C, Moore S, Sanchez A, Nel H. Reporting guidelines to increase the reproducibility and comparability of research on microplastics. Appl Spectrosc. 2020;74:1066–77. 10.1177/0003702820930292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.