Abstract
The potent antitumor antibiotic pactamycin is an aminocyclopentitol-containing natural product produced by the soil bacterium Streptomyces pactum. Recent studies showed that the aminocyclopentitol unit is derived from N-acetyl-D-glucosamine, which is attached to an acyl carrier protein (ACP)-bound polyketide by a glycosyltransferase enzyme, PtmJ. Here, we report a series of post-glycosylation modifications of the sugar moiety of the glycosylated polyketide while it is still attached to the carrier protein. In vitro reconstitution of PtmS (an AMP-ligase), PtmI (an ACP), PtmJ, PtmN (an oxidoreductase), PtmA (an aminotransferase), and PtmB (a putative carbamoyltransferase) showed that the N-acetyl-D-glucosamine moiety of the glycosylated polyketide is first oxidized by PtmN and then transaminated by PtmA to give ACP-bound 3-amino-3-deoxy-N-acetyl-D-glucosaminyl polyketide. The amino group is then coupled with carbamoyl phosphate by PtmB to give a urea functionality. We also show that PtmG is a deacetylase that hydrolyses the C-2 N-acetyl group to give a free amine.
Keywords: pactamycin, post-glycosylation modification, protein-bound, carbamoylation, biosynthesis
Entry for the Table of Contents

A series of post-glycosylation modifications is observed on a glycosylated polyketide while it is still attached to the carrier protein. These unprecedented biotransformations represent some of the hallmarks of the highly unusual biosynthetic pathway to the potent antitumor antibiotic pactamycin. The enzymes, which show substrate promiscuity, may be developed as tools for sugar modifications on glycoproteins or other biomolecules.
Introduction
Aminocyclopentitol-containing natural products represent a family of microbial secondary metabolites with significant biological activities. Structurally, the five-membered aminocyclitol units in those metabolites bear a high resemblance to ribose (ribomimetics), and they can be attached to sugars, nucleobases, or can be decorated by various functionalities.[1] One of the prominent members of this family of natural products is pactamycin (1) (Scheme 1), a potent inhibitor of protein synthesis.[2] Pactamycin exhibits potent antibacterial, antitumor, antiviral, and antiprotozoal activities.[3] It contains a unique aminocyclitol pharmacophore which is decorated by 6-methylsalicylate (6MSA), 3-aminoacetophenone (3AAP), and a 1,1-dimethylurea.[4] X-ray crystallographic studies showed that pactamycin adopts a specific conformation within the ribosome that mimics an RNA dinucleotide. The cyclitol ring resembles the RNA sugar-phosphate backbone while the two aromatic rings appear like stacked RNA bases.[2c] Incorporation studies with isotopically labeled precursors showed that the aminocyclopentitol core is derived from glucose or N-acetylglucosamine (GlcNAc), whereas the 6MSA moiety is derived from acetate.[4] The 3AAP moiety is derived from 3-aminobenzoic acid (3ABA) (2), whose conversion is catalysed by a set of discrete polyketide synthase (PKS) proteins.[5] Interestingly, results from incorporation experiments suggest that free 3AAP is not an intermediate in the pactamycin pathway. Although 3AAP can be glycosylated by the glycosyltransferase PtmJ, the product N-acetyl-D-glucosaminyl-3AAP is not involved in pactamycin biosynthesis. A recent study showed that PtmJ is involved in pactamycin biosynthesis by glycosylating 3-aminophenyl-β-oxopropanoyl moiety while it is still attached to a carrier protein, PtmI (Scheme 1).[5a] However, since direct hydrolysis of the glycosylated polyketide intermediate produces N-acetyl-D-glucosaminyl-3AAP, which is not involved in pactamycin biosynthesis,[5] it is postulated that the glycosylated polyketide intermediate should undergo additional modifications before being released from the carrier protein.
Scheme 1.
Proposed biosynthetic pathway to pactamycin.
Within the pactamycin biosynthetic gene cluster (Figure S1), there are four genes, ptmN, ptmA, ptmB, and ptmG, that are likely to be involved in the post-glycosylation modifications of the sugar moiety of the glycosylated ACP-bound polyketide intermediate.[6] PtmN encodes a protein homologous to oxidoreductases, ptmA encodes a PLP-dependent aminotransferase, PtmB encodes a putative carbamoyltransferase, and ptmG encodes a putative deacetylase. On the basis of their putative functions, it may be proposed that the first step would be the oxidation of the sugar C-3 hydroxy group, catalysed by PtmN. The oxidized product then undergoes a transamination reaction, catalysed by PtmA. Next, coupling between the newly installed C-3 amino group and carbamoyl phosphate catalysed by PtmB would give a urea moiety. Finally, PtmG hydrolyses the C-2 N-acetyl group to give a free amine (Scheme 1). This biosynthetic logic has been observed in the lipid A biosynthetic pathway in Acidithiobacillus ferrooxidans with some minor differences (Figure S2). In the lipid A pathway, the UDP-GlcNAc is first converted to the keto-intermediate UDP-3´´-oxo-GlcNAc by an NAD+-dependent alcohol dehydrogenase, GnnA. The keto-intermediate subsequently undergoes a transamination reaction catalysed by the PLP-dependent aminotransferase GnnB. The newly introduced 3´´-amino group is then acylated by the acyltransferase LpxA, followed by N-deacetylation at C-2´´ of UDP-GlcNAc3N by the N-deacetylase LpxC.[7]
Previous studies on PctP (PtmN) and PctC (PtmA) from S. pactum NBRC13433 revealed their ability to convert GlcNAc-3AAP to its corresponding 3′-amino derivative. PctP can also oxidize GlcNAc-3ABA and GlcNAc-3-aminophenyl-β-oxopropanoic acid ethyl ester, but it does not accept UDP-GlcNAc, glucosaminyl-3AAP, and 4′-C-methyl-N-acetyl-D-galactosaminyl-3-aminoacetophenone as substrates.[8] These findings suggest that the oxidation and transamination reactions take place after glycosylation and before N-deacetylation and other downstream reactions in pactamycin biosynthesis.[8] However, it is unclear if PtmN and PtmA can catalyse the oxidation and transamination reactions on glycosylated polyketides that are attached to a carrier protein (PtmI). Moreover, the functions of PtmB as a putative carbamoyltransferase that is believed to form the urea moiety of pactamycin, and PtmG, a putative deacetylase, have not been characterized.
While O-carbamoylation has been observed in the biosynthesis of various secondary metabolites, e.g., novobiocin,[9] irumamycin,[10] nebramycin,[11] and maytansine,[12] very few examples were found for N-carbamoylation that leads to urea formation.[13] In fact, N-carbamoylation more prominently occurs in primary metabolism, involving enzymes such as ornithine carbamoyltransferases and aspartate carbamoyltransferases.[14] PtmB shows phylogenetic similarity to the NodU family of carbamoyltransferases. Interestingly, its amino acid sequence is more similar to carbamoyltransferases that catalyse O-carbamoylation than those that catalyse N-carbamoylation.
The putative deacetylase PtmG is similar to MitC (38% identity) from the mitomycin biosynthetic gene cluster. MitC has been proposed to play a role in the formation of the mitosane core structure during mitomycin biosynthesis.[15] In mitomycin biosynthesis, GlcNAc is assembled into the mitosane core structure via condensation with ACP-bound 3-amino-5-hydroxybenzoic acid by the glycosyltransferase MitB,[16] followed by subsequent biotransformations including deacetylation by MitC.
Here, we report the characterization of PtmN, PtmA, and PtmB which catalyse a series of post-glycosylation modifications of a glycosylated polyketide while it is still attached to a carrier protein. We also show that PtmG is a deacetylase that hydrolyses the C-2 N-acetyl group of the sugar moiety to give a free amine.
Results and Discussion
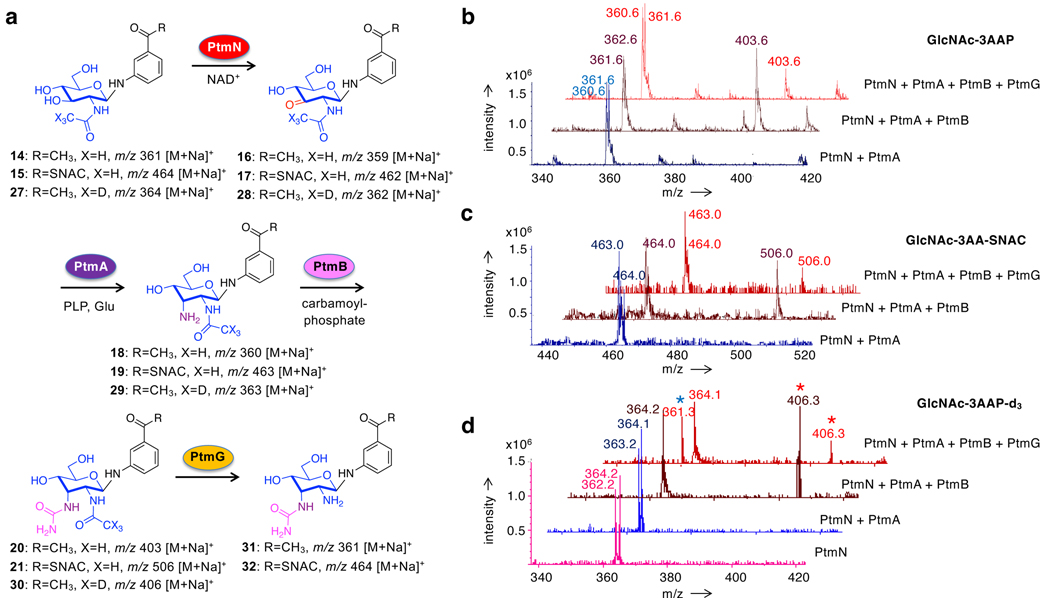
To characterize the function of PtmN, PtmA, PtmB, and PtmG, their genes were cloned and heterologously expressed in E. coli BL21 (DE3) pLysS and the recombinant enzymes were purified to homogeneity and used in in vitro experiments (Figure S3). First, GlcNAc-3AAP (14) was used as a substrate to confirm the function of PtmN and PtmA as well as to characterize the function of PtmB. GlcNAc-3AAP was incubated with PtmN, in the presence of the cofactor NAD+ as well as pyruvate and lactate dehydrogenase, which recycles NADH back to NAD+, for 10 h (Figure 1a). Mass spectrometry (MS) analysis of the reaction mixture showed the conversion of GlcNAc-3AAP (m/z 361.3 [M+Na]+) to the expected keto-intermediate 16 (m/z 359.3 [M+Na]+) (Figure 1b). In parallel, GlcNAc-3AAP was incubated with PtmN and PtmA, in the presence of required cofactors for both enzymes, NAD+, L-glutamate, and PLP, as well as pyruvate and lactate dehydrogenase. MS analysis of the reaction mixture showed two new molecular ion peaks at m/z 359.3 and m/z 360.3 that correspond to the oxidation and the transamination products of GlcNAc-3AAP, respectively (Figure 1b). Subsequently, the two-enzyme assay was repeated and PtmB was added to the mixture together with ATP, carbamoyl phosphate, and MgCl2. MS analysis of the reaction mixture showed the appearance of a new molecular ion peak at m/z 403.3 (Figure 1b), which is consistent with the formation of an N-carbamoylated (urea) product (20). The results established the catalytic functions of PtmN, PtmA, and PtmB as an oxidoreductase, an aminotransferase, and an N-carbamoyltransferase, respectively.
Figure 1.
Characterization of PtmN, PtmA, and PtmB. (a) reaction schemes PtmN, PtmA, and PtmB assays using GlcNAc-3AAP and GlcNAc-3ABA-SNAC as substrates; (b) mass spectrometry analysis of PtmN, PtmA, and PtmB reactions using GlcNAc-3AAP as a substrate; and (c) mass spectrometry analysis of PtmN, PtmA, and PtmB reactions using GlcNAc-3ABA-SNAC as a substrate. Red stars indicate the N-carbamoylated (urea) products.
Next, the N-acetylcysteamine thioester of 3-aminobenzoic acid (3ABA-SNAC) was used as a mimic of an ACP-bound substrate. The 3ABA-SNAC was first glycosylated with the glycosyltransferase PtmJ, and the product, GlcNAc-3ABA-SNAC (15), was incubated with PtmN, PtmA, and PtmB in the same manner as described for GlcNAc-3AAP. MS analysis of the reaction of GlcNAc-3ABA-SNAC with PtmN showed an oxidation product with an ion peak at m/z 462.2 [M+Na]+ (Figure 1c), whereas the reaction with PtmN and PtmA showed ion peaks at m/z 462.2 [M+Na]+ and m/z 463.3 [M+Na]+, which correspond to the oxidation and the transamination products of GlcNAc-3ABA-SNAC, respectively (Figure 1c). Subsequently, GlcNAc-3ABA-SNAC (15) was incubated with the three enzymes PtmN, PtmA, and PtmB, in the presence of their respective cofactors, for 13 h. MS analysis of the reaction mixture showed the presence of ion peaks at m/z 462.2 [M+Na]+ and m/z 463.3 [M+Na]+, and a new peak at m/z 506.4 [M+Na]+ which corresponds to the N-carbamoylated (urea) product 21 (Figures 1a and 1c), suggesting that PtmN, PtmA, and PtmB are able to process ACP-bound substrates.
While oxidation or transamination of an ACP-bound substrate in the context of PKS and/or non-ribosomal peptide synthases have been well established,[17] oxidation, transamination, and N-carbamoylation of a glycosylated polyketide that is still attached to a carrier protein have never been reported. To confirm whether these enzymes can indeed process ACP-bound substrates, we used GlcNAc-3ABA-PtmI as a model substrate. The compound was prepared by in vitro reconstitution of PtmI, PtmS (an AMP-ligase), and PtmJ. The product GlcNAc-3ABA-PtmI (23) was then incubated with PtmN and PtmA in the presence of their respective cofactors at 30 °C for 13 h (Figure 2a). Since the molecular mass of the expected product is only one atomic mass unit different from that of the substrate (12716.29 Da), the molecular ion peaks of the substrate and the product are indistinguishable in the protein mass spectrometry (Figure 2b). However, when PtmB was added to the reaction, a new peak at 12758.25, which corresponds to the molecular mass of an N-carbamoylated product 26, was observed (Figure 2c). These results indicate that PtmN, PtmA, and PtmB are responsible for the formation of the urea moiety in pactamycin by modifying the glycosylated polyketide intermediate while it is still attached to the ACP PtmI.
Figure 2.
Post-glycosylation modifications of sugars on acyl carrier protein-bound polyketides. (a) Enzymatic reaction scheme to produce glycosylated 3ABA-PtmI and modifications of the sugar moiety; (b) Deconvoluted mass spectrum of a reaction mixture containing PtmS, PtmI, PtmJ, PtmN, and PtmA; and (c) Deconvoluted mass spectrum of a reaction mixture containing PtmS, PtmI, PtmJ, PtmN, PtmA, and PtmB.
To confirm the function of PtmG as a deacetylase, GlcNAc-3AAP (14) was incubated with PtmN, PtmA, and PtmB in the presence of all necessary cofactors to form N-carbamoylated GlcNAc-3AAP (20) (Figure 3a). Recombinant PtmG was then added to the mixture, incubated for 10 h, and the product was analysed by MS. The results showed that after incubation with PtmG, the carbamoylated GlcNAc-3AAP was partially consumed, as reflected by its reduced sodiated molecular ion at m/z 403.6 (Figure 3b). The same results were also observed when GlcNAc-3ABA-SNAC (15) was used as a substrate (Figure 3c). However, the expected molecular ions of the deacetylated products (m/z 361.6 (Figure 3b) and 464.0 (Figure 3c)) overlap with those of the unreacted substrates, making it difficult to confirm the products. This analytical challenge dissuaded us from pursuing further assay using a carrier protein (PtmI)-bound substrate, which requires reconstitution of seven enzymes to make. In addition, the presence of a molecular ion at m/z 360.3 in the mixture (Figure 3b) has also raised the question of whether the PtmG product is a deacetylated or a decarbamoylated compound. To confirm PtmG activity, we synthesized isotopically labelled GlcN[2H3]Ac-3AAP (27) (m/z 364.2) (Figures 3a and S4) and incubated it with PtmN, PtmA, and PtmB to give N-carbamoyl-3´-amino-3´-deoxy-GlcN[2H3]Ac-3AAP (30)(m/z 406.3), in which only the acetyl group is isotopically labelled (Figure 3d (red stars)). Subsequently, the addition of PtmG to the mixture resulted in the consumption of N-carbamoyl-3´-amino-3´-deoxy-GlcN[2H3]Ac-3AAP (30) and the production of unlabelled N-carbamoyl-3´-amino-3´-deoxy-glucosamine-3AAP (31) (m/z 361.3) (Figure 3d (blue star)), confirming that PtmG is a deacetylase, not a decarbamoylase. On the other hand, incubations of GlcNAc-3AAP (14) with PtmG, PtmN/PtmG, or PtmN/PtmA/PtmG did not give any deacetylated product (Figure S5), suggesting that PtmG works last among these four-enzymes and only deacetylates carbamoylated substrates.
Figure 3.
Biotransformation of N-[2H3]acetyl-D-glucosaminyl-3-aminoacetophenone with PtmN, PtmA, PtmB, and PtmG. (a) Reaction scheme of the enzyme assays; (b) Mass spectrometry analysis of PtmN, PtmA, PtmB, and PtmG reactions using GlcNAc-3AAP as a substrate; (c) using GlcNAc-3AA-SNAC as a substrate; and (d) using GlcN[2H3]Ac-3AAP as a substrate. Red stars indicate the N-carbamoylated (urea) product. Blue star indicates the deacetylated product.
To further investigate the unusual capability of these enzymes to process both small molecules and ACP-bound substrates, we constructed modelled structures of PtmN, PtmA, PtmB, and PtmG in complex with PtmI using ColabFold.[18] The structures were uploaded to the Dali server to find similar structures. The structure of WlbA, an NAD+-dependent dehydrogenase involved in the biosynthesis of 2,3-diacetomido-2,3-dideoxy-D-mannuronic acid, was found to be most similar to PtmN.[19] WlbA catalysed the oxidation of the C-3′ hydroxyl group of the UDP-linked sugar to a keto moiety. Superimposition of the structures of WlbA containing NAD+ and the PtmN-PtmI complex showed relatively high overall structural similarity between WlbA and PtmN (Figures 4 and S6; RMSD: 2.55). However, NAD+ in WlbA is located close to the surface of the enzyme, whereas NAD+ in PtmN is located deep in the active site that is only accessible through a 20 Å tunnel (Figure 4a). This is consistent with our finding that PtmN can process a substrate that is attached to a phosphopantetheine arm (~14 Å) of PtmI (Figure S7). We also observed similar long active site pockets in PtmA (~20 Å) and PtmB (~22 Å), consistent with the fact that PtmA and PtmB can also process ACP-bound substrates (Figure 4b, 4c and S8, S9). However, comparisons of the modelled structure of PtmG with the crystal structure of its close homologue, teicoplanin deacetylase (Orf2), did not show any significant differences in their active sites (both have a ~10 Å active site cavity) (Figure 4d and S10), making it unclear at this point whether PtmG can process an ACP-bound substrate.
Figure 4.
Partial modelled structures of PtmN, PtmA, PtmB and PtmG in complex with PtmI (cyan). (a) putative active site pocket of PtmN, (b) putative active site pocket of PtmA, (c) putative active site pocket of PtmB, (d) putative active site pocket of PtmG. The Ser residue in PtmI is conserved for the phosphopantetheinyl moiety attachment. The location of cofactors was determined based on superimposition of the modelled structures with the X-ray crystal structures of similar enzymes. The black dashed lines show the tunnels for phosphopantetheinyl-bound substrates. The yellow dashed line shows the distance between the conserved Ser residue of PtmI and Zn2+ ion.
Conclusion
All together the results showed that modifications of the glycosylated polyketide intermediates in pactamycin biosynthesis by PtmN, PtmA, and PtmB take place when the compounds are still attached to the carrier protein. These unprecedented biotransformations represent some of the hallmarks of the highly unusual biosynthetic pathway to pactamycin.[5a, 20] In addition to modifying ACP-bound substrates, PtmN, PtmA, and PtmB can accept sugar-containing small molecules as substrates. While PtmG functions last among the four enzymes studied, the possibility that PtmG only recognizes free substrates cannot be ruled out. The promiscuous hydrolase PtmO,[5a] may hydrolyse the PtmB product first and the free product is then deacetylated by PtmG. A modelled structure of PtmO in complex with PtmI showed a tunnel where the distance between the Ser residue of PtmI and the putative catalytic residue His238 in PtmO is ~23 Å (Figure S11), confirming its function as a hydrolase that catalyses the release of an ACP-bound product.[5a]
Supplementary Material
Acknowledgements
The authors thank S. Tanoeyadi, N. Kamarudheen, L. Yang, and J. Morre for technical help. This work was supported by a grant AI129957 (to T.M.) from the National Institute of Allergy and Infectious Diseases. A. Samadi was supported by a T32 training grant AT010131 from the National Center for Complementary & Integrative Health. The content is solely the responsibility of the authors and does not represent the official views of the National Institute of Allergy and Infectious Diseases, the National Center for Complementary & Integrative Health, or the National Institutes of Health (NIH).
Dedicated to the memory of Professor Heinz G. Floss (1934–2022), a grandmaster in natural products biosynthesis and a highly regarded mentor
Footnotes
Supporting Information
The authors have cited additional references within the Supporting Information.[21–34]
References
- [1].Flatt PM, Mahmud T, Nat. Prod. Rep 2007, 24, 358–392. [DOI] [PubMed] [Google Scholar]
- [2] a).Felicetti L, Colombo B, Baglioni C, Biochim. Biophys. Acta 1966, 119, 120–129; [PubMed] [Google Scholar]; b) Bhuyan BK, Biochem. Pharmacol 1967, 16, 1411–1420; [DOI] [PubMed] [Google Scholar]; c) Brodersen DE, Clemons WM Jr., Carter AP, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V, Cell 2000, 103, 1143–1154. [DOI] [PubMed] [Google Scholar]
- [3].a) Bhuyan BK, Appl. Microbiol 1962, 10, 302–304; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Taber R, Rekosh D, Baltimore D, Virol J. 1971, 8, 395–401; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) White FR, Cancer Chemother. Rep 1962, 24, 75–78; [PubMed] [Google Scholar]; Otoguro dK, Iwatsuki M, Ishiyama A, Namatame M, Nishihara-Tukashima A, Shibahara S, Kondo S, Yamada H, Omura S, J. Antibiot 2010, 63, 381–384. [DOI] [PubMed] [Google Scholar]
- [4].Weller DD, Rinehart KL Jr., J. Am. Chem. Soc 1978, 100, 6757–6760. [Google Scholar]
- [5].a) Eida AA, Abugrain ME, Brumsted CJ, Mahmud T, Nat. Chem. Biol 2019, 15, 795–802; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kudo F, Zhang J, Sato S, Hirayama A, Eguchi T, ChemBioChem 2019, 20, 2458–2462. [DOI] [PubMed] [Google Scholar]
- [6].a) Ito T, Roongsawang N, Shirasaka N, Lu W, Flatt PM, Kasanah N, Miranda C, Mahmud T, ChemBioChem 2009, 10, 2253–2265; [DOI] [PubMed] [Google Scholar]; b) Kudo F, Kasama Y, Hirayama T, Eguchi T, J. Antibiot 2007, 60, 492–503. [DOI] [PubMed] [Google Scholar]
- [7].a) Manissorn J, Sitthiyotha T, Montalban JRE, Chunsrivirot S, Thongnuek P, Wangkanont K, ACS Chem. Biol 2020, 15, 3235–3243; [DOI] [PubMed] [Google Scholar]; b) Sweet CR, Ribeiro AA, Raetz CR, J. Biol. Chem 2004, 279, 25400–25410; [DOI] [PubMed] [Google Scholar]; c) Sweet CR, Williams AH, Karbarz MJ, Werts C, Kalb SR, Cotter RJ, Raetz CR, J. Biol. Chem 2004, 279, 25411–25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hirayama A, Chu J, Goto E, Kudo F, Eguchi T, ChemBioChem 2018, 19, 126–130. [DOI] [PubMed] [Google Scholar]
- [9].Xu H, Wang ZX, Schmidt J, Heide L, Li SM, Mol. Genet. Genomics 2002, 268, 387–396. [DOI] [PubMed] [Google Scholar]
- [10].Nakagawa A, Tanaka Y, Otoguro K, Omura S, Takiguchi T, Arai Y, J. Antibiot 1985, 38, 1266–1269. [DOI] [PubMed] [Google Scholar]
- [11].Kharel MK, Basnet DB, Lee HC, Liou K, Woo JS, Kim BG, Sohng JK, FEMS Microbiol. Lett 2004, 230, 185–190. [DOI] [PubMed] [Google Scholar]
- [12].Yu TW, Bai L, Clade D, Hoffmann D, Toelzer S, Trinh KQ, Xu J, Moss SJ, Leistner E, Floss HG, Proc. Natl. Acad. Sci. U.S.A 2002, 99, 7968–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kevany BM, Rasko DA, Thomas MG, Appl. Environ. Microbiol 2009, 75, 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) de Las Rivas B, Fox GC, Angulo I, Ripoll MM, Rodriguez H, Munoz R, Mancheno JM, J. Mol. Biol 2009, 393, 425–434; [DOI] [PubMed] [Google Scholar]; b) Rabinowitz JD, Hsiao JJ, Gryncel KR, Kantrowitz ER, Feng XJ, Li G, Rabitz H, Biochemistry 2008, 47, 5881–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15] a).Mao Y, Varoglu M, Sherman DH, Chem Biol 1999, 6, 251–263; [DOI] [PubMed] [Google Scholar]; b) Mao Y, Varoglu M, Sherman DH, Bacteriol J. 1999, 181, 2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Nguyen HP, Yokoyama K, Biochemistry 2019, 58, 2804–2808; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ogasawara Y, Nakagawa Y, Maruyama C, Hamano Y, Dairi T, Bioorg. Med. Chem. Lett 2019, 29, 2076–2078. [DOI] [PubMed] [Google Scholar]
- [17].a) Tillett D, Dittmann E, Erhard M, von Dohren H, Borner T, Neilan BA, Chem. Biol 2000, 7, 753–764; [DOI] [PubMed] [Google Scholar]; b) Fewer DP, Osterholm J, Rouhiainen L, Jokela J, Wahlsten M, Sivonen K, Appl. Environ. Microbiol 2011, 77, 8034–8040; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Holmes TC, May AE, Zaleta-Rivera K, Ruby JG, Skewes-Cox P, Fischbach MA, DeRisi JL, Iwatsuki M, Omura S, Khosla C, J. Am. Chem. Soc 2012, 134, 17797–17806; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Etzbach L, Plaza A, Garcia R, Baumann S, Muller R, Org. Lett 2014, 16, 2414–2417; [DOI] [PubMed] [Google Scholar]; e) Mares J, Hajek J, Urajova P, Kopecky J, Hrouzek P, PLoS One 2014, 9, e111904; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Masschelein J, Clauwers C, Awodi UR, Stalmans K, Vermaelen W, Lescrinier E, Aertsen A, Michiels C, Challis GL, Lavigne R, Chem. Sci 2015, 6, 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mirdita M, Schutze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M, Nat. Methods 2022, 19, 679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thoden JB, Holden HM, Biochemistry 2011, 50, 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].a) Abugrain ME, Brumsted CJ, Osborn AR, Philmus B, Mahmud T, ACS Chem. Biol 2017, 12, 362–366; [DOI] [PubMed] [Google Scholar]; b) Abugrain ME, Lu W, Li Y, Serrill JD, Brumsted CJ, Osborn AR, Alani A, Ishmael JE, Kelly JX, Mahmud T, ChemBioChem 2016, 17, 1585–1588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.