Abstract
Purpose:
This global phase 1 trial investigated the safety, efficacy, pharmacokinetics, and pharmacodynamics of lisaftoclax (APG-2575), a novel, orally active, potent selective BCL-2 inhibitor, in patients with relapsed or refractory chronic or small lymphocytic leukemia (R/R CLL/SLL) and other hematologic malignancies (HMs).
Materials and methods:
Maximum tolerated dose (MTD) and recommended phase 2 dose were evaluated. Outcome measures were safety and tolerability (primary) and pharmacokinetic variables and antitumor effects (secondary). Pharmacodynamics in patient tumor cells were explored.
Results:
Among 52 patients receiving lisaftoclax, MTD was not reached. Treatment-emergent adverse events (TEAEs) included diarrhea (48.1%); fatigue (34.6%); nausea (30.8%); anemia and thrombocytopenia (28.8% each); neutropenia (26.9%); constipation (25.0%); vomiting (23.1%); headache (21.2%); peripheral edema and hypokalemia (17.3% each); and arthralgia (15.4%). Grade ≥ 3 hematologic TEAEs included neutropenia (21.2%); thrombocytopenia (13.5%); and anemia (9.6%), none resulting in treatment discontinuation. Clinical pharmacokinetic and pharmacodynamic results demonstrated that lisaftoclax had a limited plasma residence and systemic exposure and elicited rapid clearance of malignant cells. With a median treatment of 15 (range, 6-43) cycles, 14 of 22 efficacy-evaluable patients with R/R CLL/SLL experienced partial responses, for an objective response of 63.6% and median time to response of 2 (range, 2-8) cycles.
Conclusions:
Lisaftoclax was well tolerated, with no evidence of tumor lysis syndrome. Dose-limiting toxicity was not reached at the highest dose level. Lisaftoclax has a unique pharmacokinetic profile compatible with a potentially more convenient daily (vs. weekly) dose ramp-up schedule and induced rapid clinical responses in patients with CLL/SLL, warranting continued clinical investigation.
Keywords: apoptosis, BCL-2, chronic lymphocytic leukemia, hematologic malignancies, small lymphocytic lymphoma
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in western societies, accounting for approximately 7% of non-Hodgkin lymphoma (NHL) cases, 15,000 deaths, and 5,000 newly diagnosed US cases annually.1,2 Despite recent treatment advances, many patients experience relapse on chemoimmunotherapy.
B-cell lymphoma 2 (BCL-2) is overexpressed in most cases of CLL and plays an important role in its pathogenesis, tumorigenicity, and aggressiveness. The BCL-2 protein (one of the antiapoptotic members of the BCL-2 family) controls apoptosis, and its dysregulation promotes tumor cell survival. Small-molecular inhibitors of BCL-2 can enhance cancer cell apoptosis across many hematologic malignancies (HMs).3-8
Structural insights into interactions between proapoptotic and antiapoptotic proteins culminated in the discovery and clinical development of the first BCL-2 homology domain 3 (BH3) mimetic: navitoclax (ABT-263).9 Despite clinical efficacy in advanced cancer, high-grade thrombocytopenia driven by on-target BCL-extra-large (BCL-xL)–mediated platelet inhibition impeded its development.10,11 Subsequently, venetoclax (BH3-mimetic ABT-199)12,13 became the first FDA–approved BCL-2–selective small-molecule inhibitor,12-16 although hematologic toxicities and tumor lysis syndrome (TLS) remain its clinical challenge.
To address these issues, we have designed lisaftoclax as a novel molecule, with a potent BCL-2 inhibitory and unique pharmacokinetic profile, to enhance efficacy and minimize toxicities. The aim of this first-in-human phase 1 study was to investigate lisaftoclax in patients with relapsed or refractory HMs.
Materials and Methods
Study design
This phase 1 multicenter open-label, nonrandomized study investigated the safety, efficacy, and pharmacologic (pharmacokinetic and pharmacodynamic) profiles of lisaftoclax in patients with HMs that are refractory, relapsed, or intolerant or ineligible to therapies with known clinical benefits. Conduct of the trial was consistent with the Declaration of Helsinki, including patient informed written consent and institutional/ethical reviews.
Study population
Eligible patients were ≥ 18 years of age and had a histologically confirmed diagnosis of a B-cell HM, including CLL, NHL, small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), Waldenström macroglobulinemia (WM), and multiple myeloma (MM). The malignancies were relapsed, refractory or intolerant to, or considered ineligible for therapies that are known to provide clinical benefit, and the patients were required to have adequate function of major organs. Exclusion criteria included previous allogeneic stem-cell transplantation within 1 year, active infection, active central nervous system involvement, and gastrointestinal conditions that could affect the absorption of the study drug. A summary of the representativeness of study participants is outlined in Supplementary Table S1.
Protocol
For each treatment cycle, lisaftoclax was administered orally once daily for 28 consecutive days. Dose escalation commenced in single-patient cohorts to minimize exposure to subtherapeutic doses and allow for intrapatient dose escalation. Blinding was not used in the study. Disease assessments were performed at baseline, then every 2 cycles during the first year, followed by every 3 cycles thereafter.
In TLS intermediate/high-risk groups, defined according to criteria outlined by an expert TLS panel,17 the daily dose ramp-up was initiated at 20 mg on Day 1 (D1), followed by 50 mg on D2, 100 mg on D3, 200 mg on D4, and 400 mg on D5 (for the target dose of 400 mg, D5 was also Cycle 1 Day 1 [C1D1]). All patients were hospitalized and monitored closely for TLS during the dose ramp-up period. To mitigate the risk of TLS, standard prophylaxis with oral antihyperuricemic agents (rasburicase recommended) and oral hydration was instituted ≥ 72 hours before the first dose, along with intravenous hydration during hospitalization.
For pharmacokinetic analyses, blood samples were collected before lisaftoclax dosing and at 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 hours on C1D1 and C1D28. For pharmacodynamic analyses, specimens from patients with CLL/SLL were collected and analyzed and subjected to BH3 profiling as described previously.18-22
Outcome objectives
The primary objective was safety assessments including maximum tolerated dose (MTD) and dose limiting toxicity (DLT). Secondary objectives included pharmacokinetic characterizations, such as maximum concentration (Cmax), systemic exposure (area under the concentration-time curve [AUC]) over 28 days, and antitumor effects.
Objective disease assessments, including disease-specific scans, bone marrow biopsy/aspirate, and other appropriate hematologic and biochemical laboratory results, were performed at baseline, and then every 8 weeks beginning at odd cycles (Cycles 3, 5, 7, etc.). Minimal residual disease (MRD) was evaluated in patients achieving CR (≥ 6 weeks after first achievement of MRD), then MRD was also be assessed every 12 weeks in peripheral blood until achievement of MRD negativity; once MRD negativity was achieved in peripheral blood, MRD assessment was conducted in bone marrow.
Statistical analyses
This is an open-label, non-randomized study. Statistical analyses were descriptive, including means, standard deviations, medians, ranges for continuous variables and frequency counts, and percentages for categorical variables. Sex was not included as a biological variable and attrition rates were not analyzed. Objective response rates (ORR) with 95% confidence interval (CI) values were also calculated. Sample size is not based on formal statistical calculation as there is no formal hypothesis testing. Missing data were not imputed, and all safety analyses were based on the safety population, which was defined as all patients who received ≥ 1 dose of lisaftoclax.
Data availability
All data associated with this study, including the trial protocol, are present in the paper or Supplementary Materials. The authors agree to make additional data supporting the results or analyses presented in their paper available from the corresponding authors (YZ and ACK) upon reasonable request.
Results
Patient disposition and baseline characteristics
As of this study data cutoff date (October 22, 2022), a total of 52 patients were enrolled, the dose escalation phase is complete. Lisaftoclax was administered orally once a day at doses ranging from 20 mg to 1,200 mg. The median age of the 52 patients enrolled was 68 (range, 39-89) years, and 36 (69.2%) of these individuals were male. Of the 52 patients, 17 (32.7%) remained on treatment and 35 discontinued (n = 23, because of progressive disease; n = 5 each because of physician decision or withdrawal by patient, mainly due to lack of response and switched to other therapies; Supplementary Fig. S1). Patients received a median 7 (range, 1-43) cycles, including 23 (44.2%) with CLL/SLL and 11 (21.2%) with MM (Table 1).
Table 1.
Baseline characteristics (N = 52).
| Characteristic | |
|---|---|
| Median (range) age, y | 68 (39-89) |
| Sex, no. (%) | |
| Male | 36 (69) |
| Female | 16 (31) |
| Type of cancera, no. (%) | |
| CLL/SLL | 23 (44) |
| NHL | 14 (27) |
| MM | 11 (21) |
| AML | 1 (2) |
| MDS | 1 (2) |
| Hairy cell leukemia | 1 (2) |
| Castleman disease | 1 (2) |
| No. of prior therapies, median (range) | 2 (1-13) |
Certain percentages do not sum to 100 because of rounding. AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; SLL, small lymphocytic leukemia.
Among the 23 patients with CLL/SLL who met the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria for treatment at entry, the mean age was 67 (range, 48-83) and median number of previous treatments 2 (range, 1-3; Table 2). Most patients (n = 17; 73.9%) had intermediate, high, or very high-risk disease per the International Prognostic Index and/or adverse prognostic features (n = 21; 91.3%), including unmutated IgVH (n = 10; 43.5%). Prior treatments included anti-CD20 antibody, fludarabine/chemotherapy, and Bruton tyrosine kinase (BTK) inhibitors (Table 2). All patients had experienced disease relapse after 1 or 2 standard-of-care regimens.
Table 2.
Baseline characteristics of patients with CLL or SLL (N = 23).
| Characteristic | |
|---|---|
| Median (range) age, y | 67 (48-83) |
| Diagnosis, no. (%) | |
| CLL | 22 (96) |
| SLL | 1 (4) |
| Rai stage, no. (%) | |
| I-II | 12 (52) |
| III-IV | 11 (48) |
| IPI risk group, no. (%) | |
| Low | 6 (26) |
| Intermediate | 8 (35) |
| High | 8 (35) |
| Very high | 1 (4) |
| Prognostic features, no. (%) | |
| Del(17p)/TP53 mutation | 3 (13) |
| Del(11q) | 2 (9) |
| Del(13q) | 9 (39) |
| Trisomy 12 | 2 (9) |
| CD38 + | 3 (13) |
| Unmutated IgVH | 10 (43) |
| Complex cytogeneticsa | 3 (13) |
| No. prior therapies, median (range) | 2 (1-3) |
| Prior therapies, no. (%) | |
| Fludarabine/chemo-based | 9 (39) |
| CD20 antibody-based | 22 (96) |
| BTKi | 10 (43) |
| Bulky adenopathyb, no. (%) | 6 (26) |
Complex cytogenetics is defined as ≥ 3 unrelated chromosome abnormalities.
Bulky adenopathy is defined as any single lymph node mass measuring > 5 cm in any single dimension. BTKi, Bruton tyrosine kinase inhibitor; CLL, chronic lymphocytic leukemia; IPI, International Prognostic Index; SLL, small lymphocytic leukemia.
Safety and tolerability measures
Lisaftoclax was well tolerated, with no dose-limiting toxicities at doses of up to 1,200 mg. MTD was not reached. Among lisaftoclax recipients (n = 52) at the time of data cutoff, treatment-emergent neutropenia was observed in 14 (26.9%) with grade ≥ 3 neutropenia in 11 (21.2%); diarrhea, 25 (48.1%); fatigue, 18 (34.6%); nausea, 16 (30.8%); anemia or thrombocytopenia, 15 (28.8% each); constipation, 13 (25.0%); vomiting, 12 (23.1%); headache, 11 (21.2%); peripheral edema or hypokalemia, 9 (17.3% each); and arthralgia, 8 (15.4%) (Table 3). None of these adverse events led to treatment discontinuation. Among the 23 patients with CLL/SLL in the safety population, 6 (26.1%) had grade 3 or 4 treatment-emergent neutropenia; 3 (13.0%) increased lipase; and 2 (8.7% each) thrombocytopenia, pneumonia, and colitis.
Table 3.
Selected treatment-emergent adverse events a (N = 52).
| Any gradeb (≥ 15%) |
Grade 3/4 | |
|---|---|---|
| Adverse event, no. (%) | ||
| Diarrhea | 25 (48.1) | 1 (1.9) |
| Fatigue | 18 (34.6) | 1 (1.9) |
| Nausea | 16 (30.8) | 2 (3.8) |
| Anemia | 15 (28.8) | 5 (9.6) |
| Thrombocytopenia | 15 (28.8) | 7 (13.5) |
| Neutropenia | 14 (26.9) | 11 (21.2) |
| Constipation | 13 (25.0) | 1 (1.9) |
| Vomiting | 12 (23.1) | |
| Headache | 11 (21.2) | 2 (3.8) |
| Peripheral edema | 9 (17.3) | 2 (3.8) |
| Hypokalemia | 9 (17.3) | 1 (1.9) |
| Arthralgia | 8 (15.4) |
Highest-frequency adverse events.
A patient with > 1 adverse event is counted once.
Three patients experienced grade 3 or 4 treatment-related hematologic toxicities. One patient receiving lisaftoclax 400 mg experienced grade 3 neutropenia in C1, which led to dose interruption. The absolute neutrophil count recovered to 1.15 × 109/L (103/μL) after the lisaftoclax dose was held for 8 days (without other interventions), and subsequent treatment (C2 onward) with lisaftoclax was well tolerated. The other patient with CLL who received lisaftoclax 1,200 mg experienced grade 3 neutropenia without dose interruption or growth factor support. Neutrophil count remained stable, and the patient continued through C10. One patient treated with lisaftoclax 1,200 mg had grade 4 treatment-related thrombocytopenia, which led to dose interruption in C6. After the dose was held for 14 days during a splenectomy procedure, the platelet count recovered to 59 × 109/L, and lisaftoclax 1,200 mg was restarted. One week later, treatment-related grade 4 thrombocytopenia recurred and led to treatment discontinuation during C7. The recommended phase 2 dose of lisaftoclax monotherapy for patients with CLL/SLL is 600 mg.
Tumor lysis syndrome
There was no evidence of clinical or laboratory TLS with lisaftoclax, even though 76% of the patients were in the TLS intermediate, high, or very high-risk categories and 85% of the patients were treated at dose levels ranging from 600 to 1,200 mg. One case of grade 1 hyperuricemia was reported in a patient with CLL but recovered with standard TLS prophylaxis. To assess the effects of lisaftoclax on patients at elevated risk of TLS, we evaluated 5 individuals with CLL (CLL-007, CLL-008, CLL-017, CLL-026, and CLL-036) who were treated at respective lisaftoclax doses of 400, 400, 800, 1,000, and 600 mg, and whose baseline mean absolute lymphocyte count (ALC) was > 25 × 103/μL. The highest ALC was 104.53 × 109/L (in subject CLL-008). Biochemical changes occurred in certain TLS markers after the first dose of lisaftoclax (as low as 20 mg), but they resolved within 24 hours. Based on prespecified criteria, none of these isolated biochemical changes led to a diagnosis of TLS. Detailed TLS marker curves of these 5 patients are shown in Supplementary Fig. S2.
Efficacy
A total of 14 of 22 evaluable patients with CLL/SLL had a partial response (PR) per 2008 International Workshop on CLL criteria23 over a median of 15 (range, 6-43) treatment cycles, for an ORR of 63.6% (95% CI = 41%-83%; Fig. 1). The median time to response was 2 (range, 2-8) cycles. Patients with adverse prognostic features (as noted below) experienced PRs: 66.7% (2/3) with del(17p)/TP53 mutation; 55.6% (5/9) with del(13q); and 80.0% (8/10) with IGHV unmutated. No patients had prior venetoclax treatment, and 10 patients were previously treated with BTKi. Among 4 patients who relapsed to prior BTKi, 1 patient achieved PR after 2 cycles of treatment of lisaftoclax. In patients with BTKi-intolerant disease, the ORR was 80.0% (4 out of 5 evaluable patients). In the responders, only one patient who achieved PR in Cycle 9 progressed in Cycle 31, while others maintained their responses by the cutoff date. Among 28 evaluable patients without CLL/SLL, clinical benefit was observed in 14 (50.0%). One patient with MM achieved PR, and another carrying t(11;14) exhibited minimal response, after 2 cycles of treatment. One patient with WM exhibited minimal response after 18 cycles. Nineteen patients (8 CLL, 3 each WM and FL, 2 MM, and 1 each hairy cell leukemia [HCL], Castleman disease, and DLBCL) had stable disease as the best response (Table 4; Fig. 1). No patient experienced a CR.
Figure 1.
Lisaftoclax response assessments in patients with hematologic malignancies. Swimmer’s plot of all patients treated with lisaftoclax. Patients are grouped according to the hematologic malignancy and the colors of the bars identifies each disease type. For patients achieving a partial response (PR), the time on study to reach PR is indicated by an orange star. When a patient discontinued the study, the reason is indicated on the right end of each colored bar, and for patients still receiving lisaftoclax, their duration on the study according to the number of cycles is indicated. AE, adverse event; AML, acute myeloid leukemia; CLL, chronic lymphocytic lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HCL, hairy cell leukemia; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; MR, minor response; *SD, stable disease; SLL, small lymphocytic lymphoma.
Table 4.
Treatment responses in malignancy subgroups.
| Non-CLL/SLL | |||||||
|---|---|---|---|---|---|---|---|
| CLL/SLL | NHLa | MM | Myeloid malignancyb |
HCL | Castleman disease |
Overall response |
|
| Population, no. | 23 | 14 | 11 | 2 | 1 | 1 | 29 |
| Evaluable population, no. | 22 | 13 | 11 | 2 | 1 | 1 | 28 |
| Median (range) duration of treatment, cycles | 15 (6-43) | 4 (1-40) | 3 (1-31) | 2 (2-2) | 4 (4-4) | 11 (11-11) | 7 (1-43) |
| Best responsec, no. (%) | |||||||
| PR | 14 (63.6) | 0 (0) | 1e (9.1) | 0 (0) | 0 (0) | 0 (0) | 1 (3.6) |
| MR | 0 (0) | 1d (7.7) | 1f (9.1) | 0 (0) | 0 (0) | 0 (0) | 2 (7.1) |
| SD | 8 (36.4) | 7 (53.8) | 2 (18.2) | 0 (0) | 1 (100.0) | 1 (100.0) | 11 (39.3) |
| PD | 0 (0) | 5 (38.5) | 7 (63.6) | 2 (100.0) | 0 (0) | 0 (0) | 14 (50.0) |
| ORR | 14 (63.6) | 0 (0) | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) | 1 (3.6) |
| Clinical benefit rate, no. (%) | 14 (63.6) | 8 (61.5) | 4 (36.4) | 0 (0) | 1 (100.0) | 1 (100.0) | 14 (50.0) |
Median prior regimens in patients without CLL/SLL: 3 (range 1-13).
NHL includes WM/LPL (n = 5), FL (n = 5), MCL (n = 1), and DLBCL (n = 3; including one patient who was not evaluable for efficacy).
Myeloid malignancy includes AML (n = 1) and MDS (n = 1).
Certain percentages do not sum to 100 because of rounding.
One patient with WM achieved MR after 18 cycles of treatment and maintains MR.
One patient with MM achieved PR after 2 cycles of treatment and discontinued due to disease progression at the end of Cycle 6.
One patient with MM carrying t(11;14) achieved MR after 2 cycles of treatment and maintained MR at the end of treatment (Cycle 9). AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; HCL, hairy cell leukemia; LPL, lymphoplasmacytic lymphoma; MM, multiple myeloma; MR, minor response; NHL, non-Hodgkin lymphoma; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; SLL, small lymphocytic lymphoma; WM, Waldenström macroglobulinemia.
Rapid reductions in nodal and/or splenic size after lisaftoclax treatment were observed in patients with CLL/SLL (Supplementary Fig. S3). A total of 9 (42.9%) of 21 patients experienced ≥ 70% reductions. A total of 12 patients experienced lymph node shrinkage and a PR after 2 to 8 treatment cycles (Supplementary Fig. S3).
Among 23 patients with CLL/SLL, ALC values decreased markedly after initiation of lisaftoclax treatment. This decrease was noted as early as during the dose ramp-up period (mean decrease 69.4% from ramp-up D1 to C1D1) and was 92.8% at completion of the first cycle (from ramp-up Day 1 to C2D1; Supplementary Fig. S4A). Notably, 5 patients at respective doses of 400, 400, 800, 1,000, and 600 mg had a higher risk of TLS at screening (with ALC > 25 × 103/μL) and demonstrated rapid decreases in ALC of 89%, 99%, 97%, 96%, and 99%, respectively, without evidence of TLS. The median time to 50% ALC reduction was 3 (range, 2-4) days (Supplementary Fig. S4B-C). These data show leukemic cell susceptibility to lisaftoclax not only as early as the first dose but also with the lowest starting dose (20 mg). Representative patient data after treatment with different doses of lisaftoclax are presented in Supplementary Fig. S2 and Supplementary Fig. S5A-D.
Pharmacokinetics
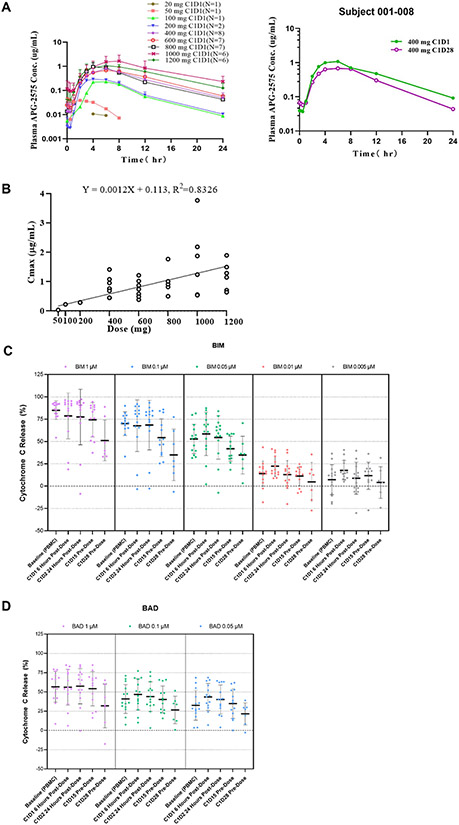
The pharmacokinetic (PK) profile of lisaftoclax and mean concentration versus time profiles from different dose cohorts are displayed alongside a representative profile of patient CLL-008 (Fig. 2A). The PK parameters are summarized in Table 5. After a single or multiple doses of orally administered lisaftoclax, the median time to reach maximum plasma concentration ranged from 3 to 8 hours across the dose range. A preliminary dose proportionality assessment showed that plasma exposure (Cmax and AUC0-24) generally increased with ascending doses (Fig. 2B), although there was a high inter-patient variability (CV approximately 72%). Lisaftoclax had a plasma elimination half-life (t1/2) of approximately 3 to 5 hours and a median time to maximum concentration (Tmax) of 3 to 6 hours. No significant accumulation was observed after multiple oral daily dosing, with an accumulation ratio of approximately 1. At 400 mg, the steady-state mean (coefficient of variation [CV%]) Cmax of lisaftoclax was 981 (43%) ng/mL and the AUC0-24h 10,130 (40%) ng•h/mL.
Figure 2.
Pharmacokinetic (PK) and BH3 profile of lisaftoclax. (A), Mean concentration vs time profiles performed using different dose cohorts are shown (left panel) with a representative profile from patient CLL-008 (right panel). Plasma lisaftoclax concentrations were determined by LC-MS/MS, with a lower limit of quantitation of 5 ng/mL. Noncompartmental PK analysis of lisaftoclax was performed using validated Phoenix WinNonlin software (version 8.1; Certara). (B) Correlation between lisaftoclax dose and maximum plasma concentration (Cmax) following a single oral administration across dose range studied. The circles represent individual data. (C-D) Figure 2C represents individual BH3 profiling data on PBMC samples collected from patients with CLL/SLL at baseline and after lisaftoclax treatments. There were 14 PBMC samples available at the baseline for the assay. The profile of mitochondrial cytochrome c release triggered by various concentrations of BIM (C) and BAD (D) peptides are shown with the median percentage of cytochrome c release. ALC, Absolute lymphocyte count; CLL, chronic lymphocytic lymphoma; Cyto, cytochrome; PBMC, peripheral blood mononuclear cells; SLL, small lymphocytic lymphoma. There was no statistically significantly difference in mitochondrial priming over time.
Table 5.
Summary of lisaftoclax PK parameters following a single oral administration.
| Analyte | Dose (mg) |
Subject Number |
T1/2 (h) |
Tmax (h) |
Cmax (μg/mL) |
AUC0-24 (h*μg/mL) |
|---|---|---|---|---|---|---|
| Lisaftoclax (APG-2575) | 50 | 1 | 2.84 | 3 | 0.0391 | 0.261 |
| 100 | 1 | 3.85 | 6 | 0.229 | 1.80 | |
| 200 | 2 | 3.84 ± 0.16 | 4 (4~4) | 0.294 ± 0.004 | 2.41 ± 0.37 | |
| 400 | 8 | 4.43 ± 0.57 | 6 (4~8) | 0.822 ± 0.334 | 6.85 ± 2.91 | |
| 600 | 7 | 4.68 ± 1.03 | 6 (4~8) | 0.761 ± 0.298 | 7.81 ± 2.84 | |
| 800 | 7 | 4.34 ± 0.53 | 4 (4~6) | 0.982 ± 0.384 | 7.25 ± 2.24 | |
| 1,000 | 6 | 6.10 ± 1.52 | 8 (6~8) | 1.69 ± 1.22 | 18.3 ± 13.1 | |
| 1,200 | 6 | 4.86 ± 1.01 | 6 (3~8) | 1.20 ± 0.48 | 12.3 ± 4.82 |
Data was represented as Mean ± SD; Tmax was represented as Median (range).
Pharmacodynamics
On-target pharmacodynamic analysis using BH3 profiling was conducted on bone marrow mononuclear cell (BMMC), or peripheral blood mononuclear cell (PBMC) samples collected from patients with CLL (n = 13) or SLL (n = 1) after lisaftoclax treatment. As an indicator of overall mitochondrial priming (the threshold for a cell to commit to apoptosis),24 BCL-2-like protein 11 (BIM) peptide triggered cytochrome c release in a dose-dependent manner (Fig. 2C), and this phenomenon was greater in PBMCs than BMMCs (Supplementary Fig. S6A-B). There was a transient increase in cytochrome c release in PBMCs collected from C1D1 6 hours after lisaftoclax treatment. However, cytochrome c release gradually decreased in samples collected from C1D2 (24 hours) and C1D15 (predosing) and was markedly decreased after the first cycle (C1D28).
Peptides of BCL-2-associated agonist of cell death (BAD), including MS1, HRK-y, and FS1, are specific interaction partners for BCL-2, myeloid-cell leukemia 1 (MCL-1), BCL-xL, and Bfl-1, respectively. The general pattern of cytochrome c loss triggered by these peptides was similar to that observed with BIM peptide (Fig. 2C and Supplementary Fig. S7A). As expected, BCL-2 was the dominant antideath protein in samples from patients with CLL, in which BAD peptide at 0.05 μM triggered 25% to 50% mitochondrial cytochrome c release compared to other peptides at 5 μM (Fig. 2D and Supplementary Fig. S7A).
Further analysis of samples collected across lisaftoclax dose cohorts showed that cytochrome c release induced by BAD (0.1 μM) and MS1 (10 μM) peptides in PBMC samples collected at baseline and C1D15 (predose) were inversely correlated with time to ALC normalization (Supplementary Fig. S7B-D). Cytochrome c release by BIM (0.05 μM) peptide treatment in either baseline or post-lisaftoclax treatment PBMCs did not show such a correlation (Supplementary Fig. S7B-D). Key peptide responses in patient samples, together with treatment dose cohorts and best clinical responses for patients, are summarized in Supplementary Table S2.
Discussion
Chronic lymphocytic leukemia is recognized to be one of the most BCL-2–dependent HMs. Our clinical study suggests that lisaftoclax (administered via a daily dose ramp-up schedule) is a novel BH3 mimetic. Clinically, the ability to have an abbreviated ramp-up with lisaftoclax can be a significant advantage in expeditiously achieving therapeutic doses, especially by minimizing and averting the risk of TLS and other dose-limiting adverse drug reactions (e.g., thrombocytopenia, neutropenia). The daily (vs weekly) ramp-up period with lisaftoclax may also be more convenient (“user friendly”) for patients and potentially reduce burdens on the healthcare system. Among individuals with R/R CLL or SLL, lisaftoclax treatment was associated with an ORR of 63.6% and rapid reduction in ALC even at the lowest dose evaluated (entry level 20 mg) and as early as the first day of dosing. In patients with CLL/SLL and del(17p), preliminary data showed an ORR of 66.7%
Mechanistically, BCL-2 inhibitors rapidly achieve a mitochondrial threshold that triggers apoptosis; thus, their effects are driven by Cmax.5 Lisaftoclax was designed as a potent and efficacious BCL-2 inhibitor with a unique pharmacokinetic profile, including a high Cmax and a shorter t1/2 (3-5 hours) compared to venetoclax (26 hours),25 resulting in a high Cmax but relatively low systemic exposure. Although preliminary, our clinical observations evidently support the intended pharmacologic properties of lisaftoclax, with a limited plasma residence and exposure that are likely responsible for its encouraging safety profile. This includes minimal effects of the BCL-2 inhibitor on the risk of TLS (despite an abbreviated dose ramp-up schedule) and a relatively low overall incidence (as well as rapid recovery) of grade 3 or 4 neutropenia (26.1%; 6/23 evaluable patients with CLL/SLL). Furthermore, in contrast to the dual BCL-2/BCL-xL inhibitor navitoclax, lisaftoclax also did not elicit clinically significant changes in platelet counts. These results support the feasibility and overall safety of implementing a more rapid, daily dose ramp-up protocol currently under evaluation with lisaftoclax.
Compared with a weekly ramp-up schedule of venetoclax, the potential 1-month reduction resulting from the shorter lisaftoclax ramp-up period may minimize potentially treatment-limiting adverse drug reactions, including thrombocytopenia, neutropenia, and TLS. While changes in some isolated TLS biochemical markers were observed, they tended to recover within 24 hours, and none constituted a full TLS. At this writing, no patient with CLL/SLL in this study has experienced TLS, even in the TLS high-risk group and in patients receiving high lisaftoclax doses (e.g., 1,200 mg). This minimal risk of TLS with lisaftoclax may differentiate it from other BCL-2 inhibitors and potentially warrants assessments of further reductions in lisaftoclax ramp-up intervals. Additional research using lisaftoclax in patients with different levels of pretreatment tumor burden and kinetics of ALC reductions are warranted to confirm our findings, as these are pivotal risk factors for TLS.16,26 By one estimate, the risk of developing TLS increases by 32% (P = .02) for each decrease of 10 × 103/μL in ALC.26
These data are consistent with the clinical observation of rapid reductions in ALC among most patients with CLL/SLL treated with lisaftoclax, indicating that the antileukemic activity of lisaftoclax depends on BCL-2 priming and on-target disruption of BCL-2 complex, a Cmax-dependent phenomenon. In lisaftoclax-treated PBMCs, the correlation of BCL-2 complex changes (indicated by BAD peptide response) with the time to ALC normalization seems to be confounded by the ramp-up duration and variation among different lisaftoclax doses (e.g., 4 to 8 days for the 400 to 1,200 mg cohort). At baseline, both BIM and BAD peptide responses were lower in BMMCs (Supplementary Fig. S6A-B) than PBMCs, indicating that BMMCs have a higher mitochondrial apoptosis threshold (probably because of the protective microenvironment in bone marrow), clinically correlating with peripheral blood clearing of leukemic cells before those in bone marrow. Cytochrome c release, however, did not show a relationship with lisaftoclax dose. These findings extend and reinforce preclinical observations, including rapid entry into tumor cells, on-target engagement, disruption of BCL-2 complexes, and BAX/BAK-dependent caspase-mediated apoptosis.27
While recognizing the preliminary nature of our observations, we report an overall encouraging safety profile of lisaftoclax, which may be relevant to the care of patients with CLL who are often elderly and thus more susceptible to cytopenias and effects of TLS than their younger counterparts.28
Treatment-emergent adverse events occurring in > 15% patients included neutropenia, thrombocytopenia and anemia (hematologic; Table 3), as well as diarrhea, fatigue, nausea, constipation, vomiting, headache, peripheral edema, hypokalemia, and arthralgia (nonhematologic; Table 3). Of 23 patients with CLL/SLL, only 6 (26.1%) had treatment-related neutropenia. The neutrophil count recovered rapidly and was stably maintained without granulocyte colony-stimulating factor (G-CSF) support. In contrast, 36% to 41% of patients (~75% of patients with grade 3/4 neutropenia) treated with venetoclax may require G-CSF support or dose reductions.16,29,30 In addition, neutropenic sequelae, such as fever and infection, were not observed with lisaftoclax, as compared to 72% of venetoclax recipients experiencing infection.29 In contrast, neutropenia was the leading adverse event leading to venetoclax dose adjustments in early-phase trials.28 Recent real-world-evidence studies offer further support for the healthcare burden of early, potentially treatment-limiting adverse events with venetoclax.31,32 These include grade ≥ 3 neutropenia in up to 47% of venetoclax recipients; grade ≥ 3 thrombocytopenia in up to 36%; grade ≥ 2 diarrhea in up to 18%; grade ≥ 3 neutropenic fever in up to 13%; and TLS in up to 13.4%.31,32
Our observations on lisaftoclax have been further validated in studies involving larger and more diverse patient populations with longer follow-up periods. Encouraging results in CLL patients have prompted us to initiate focused clinical trials in which we are evaluating the combination of lisaftoclax with the BTK-inhibitor acalabrutinib or the anti-CD20 monoclonal antibody rituximab in patients with R/R CLL/SLL (NCT04494503). Multiple ongoing studies are assessing additional efficacy endpoints such as CR rate, PFS and minimal residual disease, and safety profiles in other hematologic malignancies.
In conclusion, BCL-2 inhibitor lisaftoclax is a potentially safe, well-tolerated, and efficacious treatment for patients with R/R CLL/SLL and other HMs, supporting its further clinical development.
Supplementary Material
Translational relevance.
Management of chronic lymphocytic leukemia (CLL) and other B-cell hematologic malignancies (HMs) frequently results in treatment relapse and unfavorable outcomes. Although these malignancies are highly dependent on antiapoptotic BCL-2 protein for their survival, only one BCL-2-selective inhibitor has been approved. Despite its clinical efficacy, specific toxicity profiles, including tumor lysis syndrome (TLS), neutropenia, and thrombocytopenia, continue to pose a clinical challenge. This phase 1 study showed that, among patients with HMs including CLL, treatment with oral, small-molecule BCL-2 inhibitor lisaftoclax (APG-2575) was efficacious, safe, and well tolerated, including low frequencies of thrombocytopenia and neutropenia, with no clinical or laboratory evidence of TLS despite an abbreviated (5-day) dose ramp-up. These results are broadly consistent with the pharmacokinetic profile of lisaftoclax, including high maximum concentration, short-lived plasma residence, and low systemic exposure.
Acknowledgments
We wish to thank the patients and families for their participation in this trial. We thank Rachel Pop for her contributions in acquiring, managing, and analyzing patient data in the Mayo Clinic. We also recognize the Ascentage CMC and Analytic Center for providing lisaftoclax. Ashutosh K. Pathak, MD, PhD, MBA, FRCP (Edin.), Stephen W. Gutkin, Ndiya Ogba, PhD, and Paul O. Fletcher, PhD with Ascentage Pharma as well as Jing Deng, PhD (formerly with Ascentage Pharma), provided further input in manuscript research and preparation.
Financial support:
This study and its report were supported by Ascentage Pharma Group Corp Ltd (Hong Kong) as well as grants from the Major Innovation Team of Soochow (BZ2017035) and International Science & Technology Cooperation Program of Jiangsu Province (BZ2017035), China. Experiments and analyses conducted in the Mayo Clinic were supported in part by the Daniel Foundation of Alabama, the Predolin Foundation, the Mayo Clinic Cancer Center, and the Mayo Clinic Multiple Myeloma Specialized Program of Research Excellence (SPORE P50).
Footnotes
Prior presentation
Presented at the 2021 American Society of Clinical Oncology Virtual Congress, June 4-8, 2021.
Authors’ disclosures
Asher Chanan-Khan, Sikander Ailawadhi, and Aneel Paulus serve on the advisory board for Ascentage Pharma. Dr. Paulus also reports that he is the recipient of grants (funding for research purposes) from Ascentage Pharma. Matthew Davids reports grants and personal fees from AbbVie, personal fees from Adaptive Biotechnologies, grants and personal fees from Ascentage Pharma, grants and personal fees from AstraZeneca, personal fees from BeiGene, personal fees from Celgene, grants and personal fees from Genentech, personal fees from Gilead Sciences, personal fees from Janssen, grants and personal fees from MEI Pharma, grants and personal fees from Pharmacyclics, personal fees from Research to Practice, grants from Surface Oncology, personal fees from Syros Pharmaceuticals, grants and personal fees from TG Therapeutics, grants and personal fees from Verastem, and personal fees from Zentalis outside the submitted work. Zi Chen, Bo Huang, Mingyu Li, Lei (Tommy) Fu, Hengbang Wang, Lichuang Men, Mohammad Ahmad, Eric Liang, Divya J. Mekala, Zhicong He, Laura Glass, Dajun Yang, and Yifan Zhai are full-time employees of and/or equity shareholders in Ascentage Pharma. Drs Yang and Zhai also occupy leadership positions within the company. All other authors declare that they have no competing interests.
References
- 1.Falchi L, Vitale C, Keating MJ, Lerner S, Wang X, Elhor Gbito KY, et al. Incidence and prognostic impact of other cancers in a population of long-term survivors of chronic lymphocytic leukemia. Ann Oncol 2016;27:1100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma, version 1.2015. J Natl Compr Canc Netw 2015;13:326–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhola PD, Letai A. Mitochondria-judges and executioners of cell death sentences. Mol Cell 2016;61:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ 2015;22:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalkavan H, Green DR. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ 2018;25:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulus A, Akhtar S, Yousaf H, Manna A, Paulus SM, Bashir Y, et al. Waldenstrom macroglobulinemia cells devoid of BTK(C481S) or CXCR4(WHIM-like) mutations acquire resistance to ibrutinib through upregulation of Bcl-2 and AKT resulting in vulnerability towards venetoclax or MK2206 treatment. Blood Cancer J 2017;7:e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roufayel R. Regulation of stressed-induced cell death by the Bcl-2 family of apoptotic proteins. Mol Membr Biol 2016;33:89–99. [DOI] [PubMed] [Google Scholar]
- 8.Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia 2014;28:210–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005;435:677–81. [DOI] [PubMed] [Google Scholar]
- 10.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008;68:3421–8. [DOI] [PubMed] [Google Scholar]
- 11.Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 2010;11:1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013;19:202–8. [DOI] [PubMed] [Google Scholar]
- 14.Davids MS, von Keudell G, Portell CA, Cohen JB, Fisher DC, Foss F, et al. Revised dose ramp-up to mitigate the risk of tumor lysis syndrome when initiating venetoclax in patients with mantle cell lymphoma. J Clin Oncol 2018;36:JCO1800359. [DOI] [PubMed] [Google Scholar]
- 15.Roeker LE, Fox CP, Eyre TA, Brander DM, Allan JN, Schuster SJ, et al. Tumor lysis, adverse events, and dose adjustments in 297 venetoclax-treated CLL patients in routine clinical practice. Clin Cancer Res 2019;25:4264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol 2017;18:230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairo MS, Coiffier B, Reiter A, Younes A, Panel TLSE. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol 2010;149:578–86. [DOI] [PubMed] [Google Scholar]
- 18.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 2006;9:351–65. [DOI] [PubMed] [Google Scholar]
- 19.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 2007;12:171–85. [DOI] [PubMed] [Google Scholar]
- 20.Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS. Bruton's tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia 2017;31:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan J, Montero J, Rocco J, Letai A. iBH3: simple, fixable BH3 profiling to determine apoptotic priming in primary tissue by flow cytometry. Biol Chem 2016;397:671–8. [DOI] [PubMed] [Google Scholar]
- 22.Villalobos-Ortiz M, Ryan J, Mashaka TN, Opferman JT, Letai A. BH3 profiling discriminates on-target small molecule BH3 mimetics from putative mimetics. Cell Death Differ 2020;27:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koss B, Ryan J, Budhraja A, Szarama K, Yang X, Bathina M, et al. Defining specificity and on-target activity of BH3-mimetics using engineered B-ALL cell lines. Oncotarget 2016;7:11500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones AK, Freise KJ, Agarwal SK, Humerickhouse RA, Wong SL, Salem AH. Clinical predictors of venetoclax pharmacokinetics in chronic lymphocytic leukemia and non-Hodgkin's lymphoma patients: a pooled population pharmacokinetic analysis. AAPS J 2016;18:1192–202. [DOI] [PubMed] [Google Scholar]
- 26.Koenig KL, Huang Y, Dotson EK, Sheredy S, Bhat SA, Byrd JC, et al. Safety of venetoclax rapid dose escalation in CLL patients previously treated with B-cell receptor signaling antagonists. Blood Adv 2020;4:4860–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng J, Paulus A, Fang DD, Manna A, Wang G, Wang H, et al. Lisaftoclax (APG-2575) is a novel BCL-2 inhibitor with robust antitumor activity in preclinical models of hematologic malignancy. Clin Cancer Res 2022:OF1–OF14. [DOI] [PubMed] [Google Scholar]
- 28.Jain N, Keating M, Thompson P, Ferrajoli A, Burger J, Borthakur G, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med 2019;380:2095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davids MS, Hallek M, Wierda W, Roberts AW, Stilgenbauer S, Jones JA, et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res 2018;24:4371–9. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Cheng L, Shen K, Jin H, Li H, Cheng Y, et al. Efficacy and safety of Bcl-2 inhibitor venetoclax in hematological malignancy: a systematic review and meta-analysis of clinical trials. Front Pharmacol 2019;10:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cozad M, Stump SE, Buhlinger K, Collins Jt, Muir M, Coombs CC, et al. Evaluation of an interdisciplinary venetoclax initiation process in minimizing risk of tumor lysis syndrome. Leuk Lymphoma 2022:1–8. [DOI] [PubMed] [Google Scholar]
- 32.Mato AR, Thompson M, Allan JN, Brander DM, Pagel JM, Ujjani CS, et al. Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States. Haematologica 2018;103:1511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study, including the trial protocol, are present in the paper or Supplementary Materials. The authors agree to make additional data supporting the results or analyses presented in their paper available from the corresponding authors (YZ and ACK) upon reasonable request.