Abstract
Objectives:
People with HIV (PWH) have a higher risk of myocardial infarction (MI) than the general population, with a greater proportion of type 2 MI (T2MI), due to oxygen demand-supply mismatch, compared to type 1 (T1MI) resulting from atherothrombotic plaque disruption. PWH report greater prevalence of smoking and alcohol use than the general population. Alcohol use and smoking as risk factors for MI by type are not well studied among PWH; herein we examine longitudinal associations between smoking and alcohol use patterns and MI by type among PWH.
Design and Methods:
Using longitudinal data from the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) cohort, we conducted time-updated Cox proportional hazards models to determine the impact of smoking and alcohol consumption on adjudicated T1MI and T2MI.
Results:
Among 13,506 PWH, median 4 years follow-up, we observed 177 T1MI and 141 T2MI. Current smoking was associated with a 60% increase in risk of both T1MI and T2MI. In addition, every cigarette smoked/day was associated with a 4% increase in risk of T1MI, with suggestive, but not significant, 2% increase for T2MI. Cigarette use had a greater impact on T1MI for men than women and T2MI for women than men. Increasing alcohol use was associated with a lower risk of T1MI, but not T2MI. Frequency of heavy-episodic alcohol use was not associated with MI.
Conclusions:
Our findings reinforce prioritizing smoking reduction, even without cessation, and cessation among PWH for MI prevention and highlight different impacts on MI type by gender.
Keywords: type 1 myocardial infarction, type 2 myocardial infarction, smoking, alcohol use, HIV, people with HIV, binge drinking
Background
People with HIV (PWH) have a higher risk of myocardial infarction (MI) and other cardiovascular disease (CVD) compared to those without HIV [1–8]. According to the Universal Definition of MI, there are five types based on underlying mechanisms of myocardial ischemia [9]. Type I MI (T1MI) is attributable to disruption of atherothrombotic plaques [9]. Type 2 MI (T2MI) results from an acute imbalance in myocardial oxygen (i.e., increased demand or decreased supply), such as occurs with hypotension or vasospasm [9]. Type 3 MIs, defined by MI-related death without cardiac biomarkers, and type 4 and 5 MIs that occur in coronary revascularization, are rare. In the general population, T1MI is 5–10 times more common than T2MI [10–14]. In contrast, we have demonstrated that the incidence of T2MI is almost as frequent as T1MI among PWH receiving care across the US [15].
Associations between MI and both tobacco cigarette smoking and alcohol consumption have been widely studied in the general population, but to a lesser extent among PWH, and more importantly not by MI type. Cigarette smoking is considered one of the leading risk factors for CVD events [16]. In large cohort studies of the general population, people who smoke tend to have ≥2 times the risk of MI than those who never smoked [17–22], with hazard ratios consistently higher for women than men [18–22]. Conversely, alcohol consumption has been reported to be protective against MI in most studies of the general population [23–28]. Several large cohort and case-control studies have demonstrated that increased alcohol intake [25, 27, 29, 30], even above recommended limits [24, 27], had a greater protective effect than no or light alcohol consumption. PWH report a greater prevalence of smoking [31, 32] than the general population and meta-analysis suggests 24% prevalence of alcohol use disorder among PWH compared with 5–15% in the general population [33]. In addition, both smoking and alcohol use have been associated with lower likelihood of HIV viral suppression in large diverse samples [34–38].
Given different epidemiological presentation of MI among PWH and the high prevalence of smoking and alcohol use, it is important to assess risk factors for MI by type among PWH to determine if the dynamics of MI are the same as in the general population. Using a large, well-characterized cohort of PWH with comprehensive clinical data, including alcohol use and smoking, as well as clinical MI adjudication by type, we examined the associations of alcohol use and smoking with T1MI and T2MI.
Methods
Population, Setting and Data Sources
Data from Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) cohort, comprising >37,000 PWH in care at eight United States (US) clinical sites across the United States (http://www.uab.edu/cnics/) were included [39]. CNICS sites received Institutional Review Board approval for use of data collected on participants. The CNICS data repository integrates comprehensive longitudinal data from outpatient and inpatient encounters including demographic, clinical, medication, and laboratory data from each site’s electronic health record and other data sources [39]. Data from CNICS clinical assessments of patient-reported outcomes and measures (PRO) are also integrated into the data repository [40].
We included data from six sites (University of Alabama at Birmingham, University of Washington, University of California at San Diego, University of California at San Francisco, University of North Carolina, and Johns Hopkins University) where sufficient MI adjudication was completed. Last adjudication date varied by site with on-going assessments. PWH who completed at least one PRO were included in the analysis. Baseline date was defined as the initial CNICS visit date plus 6 months or first completed PRO, whichever was later and within the MI adjudication period for that site, resulting in a study time-period from September 2005 to December 2017. Participants who had a potential MI event before baseline (n=295) were excluded, as were those with incomplete data (n=139). Cohort exit, i.e., when data collection for an individual stopped, occurred on the earliest of the following: (a) date of first MI, (b) nine months after last CNICS visit or lab test, (c) death, or (d) end of the site-specific MI adjudication period.
Predictors and Covariates
Data on cigarette smoking and alcohol consumption were collected via PRO at routine care appointments every ~4–6 months. Participants were asked if they have ever smoked or currently smoke tobacco cigarettes, including the current number of cigarettes smoked per day. Current alcohol consumption was assessed using the Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) [41, 42], and modelled as (1) continuous AUDIT-C scores (0–12) [42] and (2) AUDIT-C derived categories; (3) AUDIT-C use frequency and (4) heavy-episodic use, defined as ≥6 drinks in one sitting. We categorized AUDIT-C scores into no current use, no current use with a prior alcohol use disorder (AUD), non-hazardous alcohol use, and hazardous alcohol use defined as ≥5 drinks for men and ≥4 for women per day [43]. Prior AUD was defined by either clinical diagnoses of alcohol abuse/dependency in the participants’ medical records or ever reporting treatment for an AUD on the CNICS PROs. Both alcohol use and smoking were time updated for every PRO completed and carried forward until the next measurement.
CNICS has standard operational definitions for other key covariates at baseline. Diabetes was defined as a prior hemoglobin A1c level of ≥6.5, or use of a diabetes-specific or diabetes-associated medication in the setting of also having a diabetes diagnosis [44]. Hypertension was defined as a recorded diagnosis of hypertension and receiving an antihypertensive medication prescription. Dyslipidemia was identified by receipt of lipid-lowering medications, such as statins. Estimated glomerular filtration rate (eGFR) was calculated [45] based on baseline serum creatinine, age, sex and race/ethnicity, with an eGFR<30 defined as severe kidney disease [46]. HIV viral load (VL) and CD4 counts assessed as part of clinical care were time updated for each new result from baseline until cohort exit.
Outcomes
All MI events were adjudicated as previously described [15, 47]. Ascertainment for potential MIs includes MI diagnoses, elevated cardiac biomarkers (e.g., troponin I or T), or documentation of coronary interventions (e.g., coronary artery bypass). For each potential event, sites assemble and upload de-identified packets of primary data to a secure, central review site. Event packets including medical notes, laboratory tests, imaging results, and electrocardiograms, are reviewed independently by two expert physicians, who categorize potential events as no, probable, or definite MI, with further differentiation into MI types. Discordance between reviewers’ findings results in a third physician review, and the three resolve any discrepancies. MIs for this study include all events adjudicated as definite or probable MI, and only the first MI a participant has had.
Statistical Analyses
Unadjusted summary statistics, including comparisons of central tendency and frequencies, were applied to baseline measures collected from clinical assessments to describe the cohort by type of MI event. As other MI types were rare (e.g., <10 type 4 or 5 MIs in CNICS to date), they are not discussed further.
We used time-updated Cox proportional hazards models to determine associations between cigarette smoking or alcohol consumption and MI by type, adjusted for known MI risk factors, in which smoking status, alcohol consumption, VL and CD4 count were updated as new data became available. Alcohol use was based on each of the four models described above and shown in Table 1. Smoking status (never, former, current) and number of cigarettes smoked per day among current smokers, centered on the median number of cigarettes, was modelled the same way in all models. Given associations of current smoking with both T1MI and T2MI and potentially differential associations by age and sex by type of MI, we graphed type-specific MI-free survival among smokers and non-smokers by age and cigarettes per day (pack equivalent) among males and females in models that included smoking, but not alcohol. All models were adjusted for age, birth sex, race/ethnicity, hepatitis C virus (HCV) infection, hepatitis B virus (HBV) infection, dyslipidemia, hypertension, diabetes, and time updated VL and CD4 count. The assumption of proportional hazards was assessed by Schoenfeld residuals. Analyses were conducted using STATA v17.0 (College Station, TX).
Table 1:
Baseline demographic, behavioral and clinical characteristics of participants by occurrence of MI and type of MI during the study period (N=13,506)
| Characteristics | Total (n=13,506) |
No MI (n=13,188) |
Type 1 MI (N=177) |
Type 2 MI (n=141) |
|---|---|---|---|---|
| N (%) * | N (%) * | N (%) * | N (%) * | |
| Age mean (SD) | 44 (10.9) | 43 (10.9) | 51 (8.5) | 49 (10.5) |
| Female | 2491 (18.4) | 2440 (18.5) | 16 (9.0) | 35 (24.8) |
| Race/Ethnicity | ||||
| White | 5819 (43.1) | 5679 (43.1) | 93 (52.5) | 47 (33.3) |
| Black | 5155 (38.2) | 5021 (38.1) | 54 (30.5) | 80 (56.7) |
| Hispanic | 1903 (14.1) | 1866 (14.2) | 25 (14.1) | 12 (8.5) |
| Other | 629 (4.7) | 622 (4.7) | 5 (2.8) | 2 (1.4) |
| Smoking status | ||||
| Never | 4948 (36.6) | 4853 (36.8) | 55 (31.1) | 40 (28.4) |
| Former | 3109 (23.0) | 3047 (23.1) | 37 (20.9) | 25 (17.7) |
| Current | 5449 (40.4) | 5288 (40.1) | 85 (48.0) | 76 (53.9) |
| cigarettes/ day among those ever smoking (n=8,558) Median (IRQ) | 10 (5–15) | 10 (5–15) | 12.5 (10–15) | 10.0 (5–15) |
| AUDIT-C score mean (SD) | 2.1 (2.5) | 2.1 (2.5) | 1.6 (2.1) | 1.7 (2.8) |
| Hazardous alcohol consumption | ||||
| No consumption | 4948 (36.6) | 4782 (36.3) | 89 (50.3) | 79 (56.0) |
| Non-hazardous drinking | 6263 (46.4) | 6155 (46.7) | 69 (39.0) | 39 (27.7) |
| Hazardous drinking | 2295 (17.0) | 2251 (17.1) | 19 (10.7) | 23 (16.3) |
| Current alcohol use | 8558 (63.4) | 8408 (63.8) | 88 (49.7) | 62 (44.0) |
| Frequency use (in prior 30 days) among users (n=8558), days, mean (SD) | 5.3 (6.1) | 5.3 (6.1) | 4.5 (5.4) | 6.5 (7.1) |
| Binge alcohol use | 4410 (32.7) | 4336 (32.9) | 45 (25.4) | 29 (20.6) |
| Frequency (prior 30 days) in binge users (n=4410), days, mean (SD) | 2.5 (6.3) | 2.5 (6.2) | 2.6 (7.4) | 8.1 (12.6) |
| Hepatitis C | 2373 (17.6) | 2293 (17.4) | 34 (19.2) | 46 (32.6) |
| Hepatitis B | 750 (5.6) | 728 (5.2) | 14 (7.9) | 8 (5.7) |
| Diabetes | 1105 (8.2) | 1037 (7.9) | 36 (20.3) | 32 (22.7) |
| Hypertension | 3257 (24.1) | 3104 (23.5) | 89 (50.3) | 64 (45.4) |
| Dyslipidemia | 2113 (15.6) | 2004 (15.2) | 75 (42.4) | 34 (24.1) |
| Severe kidney disease (eGFR <30) | 176 (1.3) | 150 (1.1) | 10 (5.7) | 16 (11.4) |
| Detectable HIV viral load | 2879 (21.32) | 2793 (21.2) | 41 (23.2) | 45 (31.9) |
| CD4 count cells/mm3 Median | 484 | 486 | 455 | 373 |
| (IQR) | (301–691) | (303–692) | (278–688) | (161–572) |
unless otherwise specified
Results
13,506 PWH were included, with a median follow up time of 4.04 years (IQR: 1.8–12.3 years), median of 8.1 PROs per person (IQR: 3.6–24.6), and a mean age 44 years (median=44 years, range 19–87 years) at baseline; 18% of participants were female (n=2491); 43% reported White, 38% reported African American/Black, and 14% reported Hispanic/Latinx race/ethnicity (Table 1). T1MI occurred in 177 participants and 141 had a T2MI during the study period. In univariate analyses, participants who had either T1MI or T2MI were significantly older and were more likely to report current smoking and smoking more cigarettes per day at baseline than those who did not experience an MI during the study period (Table 1). Current alcohol use, frequency of use, heavy-episodic use, frequency of heavy-episodic consumption, and AUDIT-C scores were lower among those who experienced a T1MI compared to those with no MI; those who had a T1MI or T2MI were also more likely to report not consuming alcohol than those who did not have MI. Female participants were significantly less likely than males to have a T1MI, but more likely to have a T2MI.
Adjusting for potential confounders, including time-updated VL and CD4 count, those reporting current cigarette use, compared to never smoking, had a consistently increased risk of T1MI regardless of how alcohol was modelled (Hazard ratio (HR) range 1.61–1.67) (Table 2). Furthermore, the risk of T1MI increased by 4% for every cigarette currently smoked per day in all models. Current cigarette use was associated with a similar significant increase in risk for T2MI across all four models of alcohol use (HR range 1.57–1.64 (Table 2)). A similar pattern to that of T1MI was observed in T2MI for impact of cigarettes smoked per day, including similar stability in the point estimate and confidence intervals, but did not achieve significance in T2MI models. Those reporting former smoking did not have an increased risk of either MI type compared to those who had never smoked. Consistent results were observed in sensitivity analyses including time updated body mass index (BMI) as a confounder in the models (Supplemental Table 1). Similarly, in sensitivity analyses including polynomial terms for smoking to examine linearity of association and separate models to examine the impact of cigarettes per day on only PWH who reported ever smoking, we observed consistent associations for dose-dependent effects (Supplemental Table 2). Generalized additive model (GAM) plots provide further visualization of the impact of cigarettes per day on risk of T1MI (Supplemental Figure 1) and T2MI (Supplemental Figure 2).
Table 2:
Risk for myocardial infraction (MI), by type and alcohol use and tobacco cigarette smoking, in time updated adjusted analyses among PWH* (n=13,506)
| Model | Type 1 MI | Type 2 MI | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| 1 | AUDIT-C score | 0.91 | 0.84, 0.98 | 0.014 | 0.97 | 0.90, 1.04 | 0.385 |
| Cigarette use: Never | REF | REF | |||||
| Former | 1.02 | 0.68, 1.53 | 0.931 | 1.28 | 0.79, 2.07 | 0.303 | |
| Current | 1.67 | 1.14, 2.45 | 0.009 | 1.64 | 1.08, 2.49 | 0.021 | |
| Current cigarettes per day | 1.04 | 1.01, 1.06 | 0.003 | 1.02 | 0.99, 1.05 | 0.148 | |
| 2 | Hazardous alcohol | ||||||
| No consumption, no AUD | REF | REF | |||||
| No consumption, former AUD | 1.02 | 0.64, 1.63 | 0.940 | 1.20 | 0.74, 1.95 | 0.460 | |
| Non-hazardous drinking | 0.64 | 0.45, 0.90 | 0.011 | 0.61 | 0.40, 0.93 | 0.020 | |
| Hazardous drinking | 0.51 | 0.29, 0.80 | 0.017 | 0.87 | 0.51, 1.46 | 0.595 | |
| Cigarette use: Never | REF | REF | |||||
| Former | 1.02 | 0.68, 1.54 | 0.916 | 1.29 | 0.80, 2.08 | 0.293 | |
| Current | 1.66 | 1.13, 2.44 | 0.009 | 1.60 | 1.05, 2.44 | 0.028 | |
| Current cigarettes per day | 1.03 | 1.01, 1.06 | 0.004 | 1.02 | 0.74, 1.95 | 0.460 | |
| 3 | Alcohol frequency (days/month) | 0.98 | 0.95,1.01 | 0.117 | 1.00 | 0.96, 1.03 | 0.744 |
| Cigarette use: Never | REF | REF | |||||
| Former | 1.00 | 0.66, 1.50 | 0.991 | 1.27 | 0.79, 2.05 | 0.321 | |
| Current | 1.61 | 1.10, 2.36 | 0.014 | 1.61 | 1.06, 2.45 | 0.025 | |
| Current cigarettes per day | 1.04 | 1.01,1.06 | 0.003 | 1.02 | 0.99, 1.05 | 0.154 | |
| 4 | Binge frequency (days/month) | 0.94 | 0.85 ,1.03 | 0.2 | 1.03 | 0.99, 1.06 | 0.122 |
| Cigarette use: Never | REF | REF | |||||
| Former | 0.99 | 0.66, 1.49 | 0.961 | 1.26 | 0.78, 2.02 | 0.344 | |
| Current | 1.62 | 1.11, 2.37 | 0.013 | 1.57 | 1.03, 2.39 | 0.034 | |
| Current cigarettes per day | 1.04 | 1.01, 1.06 | 0.002 | 1.02 | 0.99, 1.04 | 0.183 | |
Cox Proportional Hazards models adjusted for age, birth sex, race/ethnicity, hepatitis C infection, hepatitis B infection, dyslipidemia, hypertension, diabetes, and time updated viral load and CD4 count.
PWH who had a higher AUDIT-C score by continuous measure were significantly less likely to experience a T1MI (HR: 0.91, 95% CI: 0.84–0.98), but not a T2MI (Table 2). Additionally, in models where alcohol use was categorized into current non-use without AUD, non-use with AUD, non-hazardous drinking, and hazardous drinking, the risk of T1MI was reduced among those reporting alcohol consumption, regardless of category. In contrast, the risk of T2MI was reduced for those reporting non-hazardous alcohol consumption, but not hazardous consumption. Neither frequency of alcohol use nor heavy-episodic consumption in the past 30 days was associated with either MI type.
Given that aging is associated with increased risk of MI in the general population, and among PWH [48], as well as in this study (data not shown), we examined the impact of aging and smoking within our models of T1MI and T2MI by plotting survival curves for decade of age and smoking status (Figure 1). For every decade of age (from 30–60 years), current smoking increased our participants’ risk of T1MI by approximately one age decade. However, for T2MI the increased risk from smoking was greater than a single decade of age. Similarly, when we plotted amount smoked by sex, we saw different patterns for the types of MI (Figure 2). The impact of increased number of cigarettes per day (assessed by intervals of half packs) on T1MI was greater for men than women. However, the reverse pattern was seen for T2MI
Figure 1: A: T1MI and B: T2MI free survival by daily, current cigarette use and age.
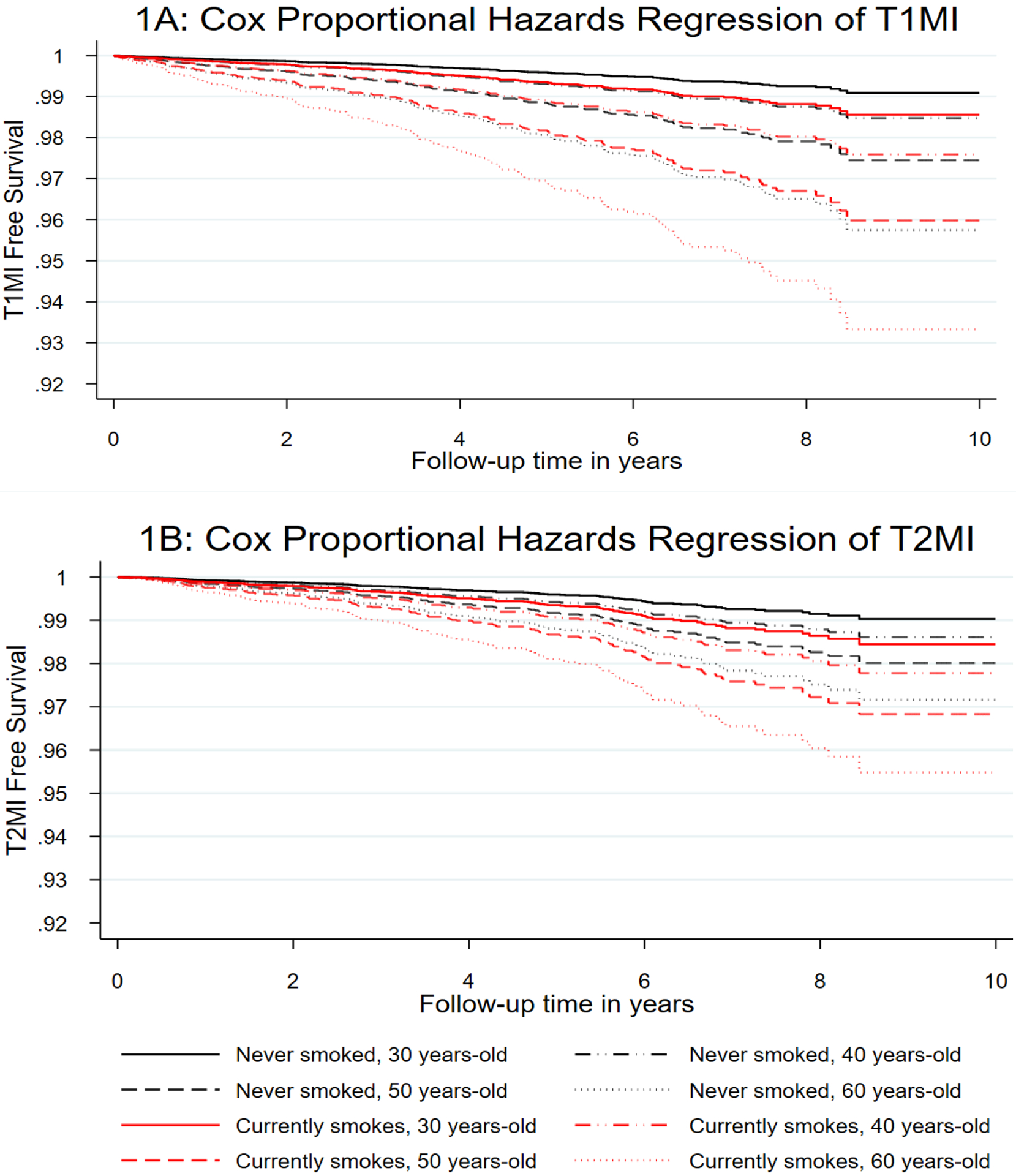
adjusted for number of cigarettes smoked per day for current smokers, sex, race/ethnicity, HCV infection, HBV infection, dyslipidemia, treated hypertension, diabetes, severe chronic kidney disease and time updated viral load and CD4 count. Panel 1A shows that current smokers have a T1MI risk similar to that of a PWH a decade older, whereas the risk of T2MI is similar to that of someone even more than a decade older.
Figure 2: A: T1MI and B :T2MI free survival by sex and current daily cigarette use.
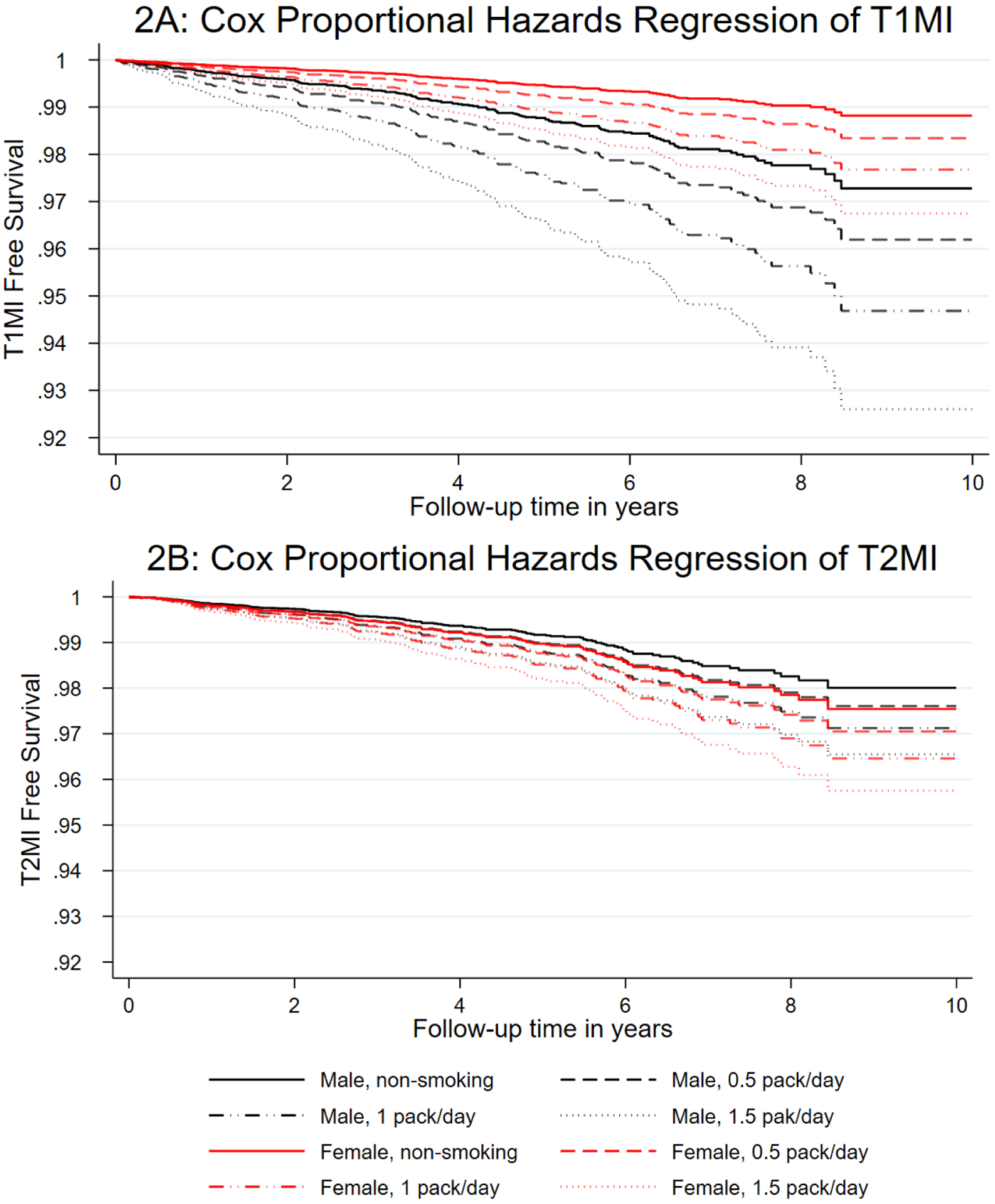
adjusted for age, race/ethnicity, HCV infection, HBV infection, dyslipidemia, treated hypertension, diabetes, severe chronic kidney disease and time updated viral load and CD4 count. Panel 2A highlights the impact of sex on T1MI, where male non-smokers had a similar risk to females who smoked more than a pack/ day, however, this observation was reversed for T2MI (panel 2B), where women had a significantly greater risk of T2MI than men.
Discussion
Among PWH who completed detailed, longitudinal assessments on cigarette/alcohol use with a median follow-up of 4 years, there was an 60% higher risk of either T1MI or T2MI among those who currently smoked compared to those who never smoked. Furthermore, we observed that current smoking increased the risk of MI by the equivalent of one or more decade of age, and that smoking appeared to have a greater association with T1MI risk in men and T2MI risk in women. There was no difference in risk for either type of MI for those who formerly smoked compared to those who never smoked. These findings taken together with the observed increasing risk per cigarette smoked daily for T1MI suggests that both smoking cessation and reduction among PWH are important for mitigating their already increased risk of MI. The association of alcohol consumption with MI among PWH was similar to that in the general population, where alcohol use was associated with lower MI risk. However, this association was only observed for AUDIT-C score and hazardous/non-hazardous drinking for T1MI and non-hazardous alcohol consumption for T2MI. Our study is also one of the few to examine T1MI and T2MI separately regardless of population.
We found that current, but not former, smoking among PWH was associated with an increase in MI risk with a similar or slightly smaller effect size compared to most general population studies [17–22]. In contrast to our study, most prior studies did not differentiate between T1MI and T2M. Our study also collected smoking status at more frequent intervals than other studies, and these were time updated in our models. We adjusted for a number of factors known to be associated with both MI and smoking, including HCV infection, diabetes and hypertension, while prior studies rarely adjusted for these [21]. Our study had greater racial/ethnic diversity and a higher prevalence of smoking than many of the general population studies, as representative of PWH.
Similarities in MI risk in our cohort and the general population were also observed for the impact of number of cigarettes smoked per day, where greater numbers of cigarettes smoked per day was associated with increased MI risk [18, 19, 30, 49]. While general population studies tended to categorize cigarettes used per day, we examined this association continuously, demonstrating an 4% increase in T1MI risk per cigarette per day. While this association did not reach significance in our T2MI analyses, Figure 2 demonstrates the impact that each half pack (i.e., 10 cigarettes/day) has on increasing risk of T1MI and T2MI. This could be explained by reduced power for T2MI, due to fewer events, or differences in mechanism of how cigarette smoking results in T1MI and T2MI. Non-nicotine components of cigarettes have been shown to result in platelet activation, which has been associated with atherosclerotic plaque formation [50], increasing the risk for T1MI, which would be consistent with a dose-response dynamic. A proposed mechanism for smoking resulting in T2MI susceptibility suggests that an oxygen supply versus demand imbalance is created when high blood carboxyhemoglobin is present, reducing oxygen levels in the blood, while nicotine stimulates the sympathetic nervous system resulting in elevated heart rate and blood pressure as well as coronary vasoconstriction [16, 51]. This could be dose-response related (i.e., the longer one smokes at any one time the higher the risk), but not necessarily over the long-term.
Interestingly, our data demonstrated differential risk for men and women by MI type with respect to smoking. Most studies of MI, regardless of population, combine all types together, therefore less is known about sex differences with respect to MI type. Recent studies examining sex differences in the general population highlight that while men tend to be more likely to experience T1MI than women, men and women experience T2MI at similar rates[52,53]. This is consistent with our T1MI findings, reinforcing the idea that T1MI is also likely to be higher among men in PWH. With respect to T2MI, our previous work demonstrated that the top three causes in our cohort were sepsis/bacteremia, illicit drug use, and hypertensive urgency/emergency. While information on sepsis/bacteremia was not available for PWH in the analysis cohort, a greater proportion of women in our cohort report injection drug use as an HIV risk factor than men, suggesting they may have greater risk of sepsis/bacteremia. Additionally, women were more likely than men to have hypertension and report regular use of opioids and cocaine, suggesting that these could contribute to the higher risk of T2MI with smoking observed in women; however, we suggest the need for further investigations of mechanisms that may result in differential impact of smoking on sex and MI type. This study, taken with evidence from the general population, suggests that both smoking cessation and reduction are critically important for preventing MI risk among PWH. In addition to assessing and preventing atherosclerosis for both men and women with HIV who smoke, careful attention to factors associated with T2MI, such as infection, low CD4 cell count, higher viral loads, and stimulant use, is also important for preventing and managing MI in PWH, particularly among women with HIV who smoke.
We observed an inverse association between continuous AUDIT-C score and both hazardous and non-hazardous categories of AUDIT-C compared to no alcohol use with T1MI and non-hazardous AUDIT-C category compared to no alcohol use with T2MI. These associations were not observed for frequency of alcohol or heavy-episodic use over a 30-day period. Most general population studies demonstrated more consistent inverse associations with measures of alcohol use, however, they used different categories of alcohol consumption than we did, such as moderate and heavy [24, 26], sometimes and regularly [23], any consumption in the past 12 months [54], timing of consumption prior to MI (e.g., hours, weeks, days) [55, 56], and grams/day or week [27, 29], which might explain why we saw an inverse association with MI on some measures and not others. Indeed, when we examined associations between T1MI/T2MI and categories of numbers of drinks per month (i.e., 0, 1–4, ≥5), we observed a similar association to studies in the general population categorizing frequency of consumption [25, 30]. Our study was consistent with results regarding no association between heavy episodic/binge alcohol consumption and MI [26]. It is also important to note that the inverse association between alcohol use on MI is not observed in all populations [54], and our sample is racially/ethnically diverse. Furthermore, epidemiological studies suggest that the mechanisms by which alcohol may lower the risk of MI include lowering blood lipids [23], increasing HDL cholesterol [29], and improving insulin sensitivity [29]. Experimental studies demonstrate that feeding participants ≥30 g of alcohol/day increased HDL cholesterol [57] and insulin sensitivity [58, 59]. However, HIV infection results in increased dyslipidemia and insulin resistance [59, 60], which may attenuate the positive effects of moderate alcohol consumption observed in the general population, and might explain the moderate and inconsistent association between alcohol consumption and MI in our study.
While this study, and most other studies among the general population, identify a potentially moderate protective effect of alcohol consumption on the risk of T1MI, these findings should be tempered with regard to health more generally. Studies examining the effects of alcohol in the general population on all types of CVD demonstrate heterogeneous effects,[24, 27] where the risk of stroke and other cardiovascular events are increased by alcohol consumption, even though alcohol consumption appears protective for MI. When comparing heavy alcohol consumption (>167g/week) to more moderate consumption (12–83g/week), an increased risk of MI was observed in a moderately sized cohort from Northern Europe [61]. Furthermore, while alcohol consumption may be associated with reduced risk of MI, it has also been associated with increased risk of cancer and all-cause mortality within the same study [26]. Other studies have demonstrated associations with increased risk of renal damage [62], cirrhosis [63], cancer [64], and all-cause mortality [64, 65]. Furthermore, alcohol consumption is the seventh leading cause of death worldwide [66]. While complete alcohol cessation has been recommended for PWH [67], this study suggests that smoking cessation and/or reduction might be a more important priority for long-term health outcomes over cessation of light/moderate alcohol use among PWH who do not have HCV infection or liver disease.
Our study had several limitations with respect to measurement of primary exposures and follow-up. While we collected robust measures on participant smoking behavior, passive exposure to cigarette smoke, which has been associated with increased risk for MI [18], was not measured in our study. If non-smoking participants had significant exposure to second-hand cigarette smoke, this could reduce the strength of the association between smoking and MI observed in our study. Additionally, we did not have the opportunity to include vaping/ e-cigarette use in these analyses, as data collection on this measure started in the final year of cohort inclusion resulting in only 44% answering this question, among whom 10% reported ever vaping/using e-cigarettes. Associations between MI by type and vaping/e-cigarette use is of interest and will be examined in future analyses. While we used AUDIT-C as a robust measure of alcohol consumption, we did not measure whether alcohol was paired with food, which has been shown support insulin sensitivity [68], a mechanism by which alcohol is thought to be protective against MI [29]. Additionally, we measured alcohol consumption over discrete time-periods rather than by daily journaling. While this has advantages for understanding overall impact, we could not examine the effect of alcohol consumption on the day of, or just before, an MI. Previous studies have shown that alcohol consumption has been associated with an acute increased risk of MI within a few hours after consumption [54–56]. Additionally, we did not examine modifications in associations between smoking and MI with respect to illicit substance use, as illicit substance use was not measured on the entire cohort. While there is significant interest regarding the impact of illicit substance use on MI by type, the focus of this study was to determine if the effects of alcohol and smoking on MI among PWH differed from the general population, where illicit substance use is not normally studied. While we had a sufficiently long median follow-up of 4 years, events and longitudinal follow-up continue to accrue, allowing greater opportunity to examine changes in alcohol use and smoking patterns in the future, as well as effects of illicit substance use.
Our study has several strengths. CNICS has a large population with demographic, clinical, and geographic diversity. CNICS collects consistent and robust repeated measures on alcohol use, smoking, clinical laboratory measures, and other health measures, which allowed for time varying assessment of important factors within this study. The assessment of MI through our clinical adjudication process reduces the risk of misclassification of the outcome and allows for examination of risk by MI type. Our data collection and assessment processes also enhance completeness of data on all participants.
In this study of 13,506 PWH with repeated measures on smoking/alcohol consumption over a median of 4 years follow-up, we demonstrate that current cigarette smoking is associated with a 1.6-fold increased risk of both T1MI and T2MI compared with non-smoking. Furthermore, 4% of this risk could be decreased for every cigarette per day reduction, and this had a greater impact on T1MI in men and T2MI in women. While alcohol consumption was associated with moderately lower risk of T1MI compared to no consumption, we would not recommend an increase in alcohol consumption among PWH due to other serious consequences of alcohol. This study highlights the potential benefits of not only cessation, but also reducing number of cigarettes per day even without achieving cessation, on CVD health among PWH, with potentially different impact on MI type between men and women.
Supplementary Material
Acknowledgements:
We would like to thank our participants for their generosity in sharing their de-identified information to support research that can lead to better health outcomes for all people living with HIV. We would also like to thank all care providers, staff and researchers across CNICS for time and effort in supporting all CNICS activities, making this research possible.
Funding:
This study was funded by the National Heart, Lung, and Blood Institute (NHLBI) R01 HL126538; the National Institute of Alcohol Abuse and Alcoholism (NIAAA) [U24AA020801, U01AA020793 and U01AA020802], the National Institute of Allergy and Infectious Diseases (NIAID) [CNICS R24 AI067039; UW CFAR NIAID Grant P30 AI027757; UAB CFAR grant P30 AI027767; UNC CFAR grant P30 AI050410; and JHU CFAR grant P30 AI094189], the National Institute on Drug Abuse (NIDA) [R01DA047045 and R01DA044112], and the National Institute on Aging (NIA) [R33AG067069].
Footnotes
Conflict of Interest: No conflict of interest has been declared for any of the authors.
References
- 1.Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV infection and incidence of cardiovascular diseases: an analysis of a large healthcare database. Journal of the American Heart Association 2019; 8(14):e012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drozd DR, Kitahata MM, Althoff KN, Zhang J, Gange SJ, Napravnik S, et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared to the general population. Journal of acquired immune deficiency syndromes (1999) 2017; 75(5):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durand M, Sheehy O, Baril J-G, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case–control study using Quebec’s public health insurance database. JAIDS Journal of Acquired Immune Deficiency Syndromes 2011; 57(3):245–253. [DOI] [PubMed] [Google Scholar]
- 4.Eyawo O, Brockman G, Goldsmith CH, Hull MW, Lear SA, Bennett M, et al. Risk of myocardial infarction among people living with HIV: an updated systematic review and meta-analysis. BMJ open 2019; 9(9):e025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang C-CH, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine 2013; 173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PloS one 2017; 12(5):e0176686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SG, Galaviz KI, Gay HC, Wei J, Armstrong WS, Del Rio C, et al. Factors associated with excess myocardial infarction risk in HIV-infected adults: a systematic review and meta-analysis. Journal of acquired immune deficiency syndromes (1999) 2019; 81(2):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenson RS, Hubbard D, Monda KL, Reading SR, Chen L, Dluzniewski PJ, et al. Excess risk for atherosclerotic cardiovascular outcomes among US adults with HIV in the current era. Journal of the American Heart Association 2020; 9(1):e013744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). European heart journal 2019; 40(3):237–269. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Vaidya SR, Arora S, Bahekar A, Devarapally SR. Type 2 versus type 1 myocardial infarction: a comparison of clinical characteristics and outcomes with a meta-analysis of observational studies. Cardiovascular diagnosis and therapy 2017; 7(4):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Cuenca A, Gómez-Molina M, Flores-Blanco PJ, Sánchez-Martínez M, García-Narbon A, De Las Heras-Gómez I, et al. Comparison between type-2 and type-1 myocardial infarction: clinical features, treatment strategies and outcomes. Journal of geriatric cardiology: JGC 2016; 13(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy CP, Kolte D, Kennedy KF, Vaduganathan M, Wasfy JH, Januzzi JL Jr. Patient characteristics and clinical outcomes of type 1 versus type 2 myocardial infarction. Journal of the American College of Cardiology 2021; 77(7):848–857. [DOI] [PubMed] [Google Scholar]
- 13.Putot A, Derrida SB, Zeller M, Avondo A, Ray P, Manckoundia P, et al. Short-term prognosis of myocardial injury, type 1, and type 2 myocardial infarction in the emergency unit. The American journal of medicine 2018; 131(10):1209–1219. [DOI] [PubMed] [Google Scholar]
- 14.Sandoval Y, Jaffe AS. Type 2 myocardial infarction: JACC review topic of the week. Journal of the American College of Cardiology 2019; 73(14):1846–1860. [DOI] [PubMed] [Google Scholar]
- 15.Crane HM, Paramsothy P, Drozd DR, Nance RM, Delaney JC, Heckbert SR, et al. Types of Myocardial Infarction Among Human Immunodeficiency Virus–Infected Individuals in the United States. JAMA cardiology 2017; 2(3):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Services UDoHaH. The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. In. Atlanta, GA, USA; 2010. [PubMed] [Google Scholar]
- 17.Banks E, Joshy G, Korda RJ, Stavreski B, Soga K, Egger S, et al. Tobacco smoking and risk of 36 cardiovascular disease subtypes: fatal and non-fatal outcomes in a large prospective Australian study. BMC medicine 2019; 17(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iversen B, Jacobsen BK, Løchen M-L. Active and passive smoking and the risk of myocardial infarction in 24,968 men and women during 11 year of follow-up: the Tromsø Study. European journal of epidemiology 2013; 28(8):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonsdottir LS, Sigfússon N, Gunason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study. Journal of cardiovascular risk 2002; 9(2):67–76. [PubMed] [Google Scholar]
- 20.Nyboe J, Jensen G, Appleyard M, Schnohr P. Smoking and the risk of first acute myocardial infarction. American heart journal 1991; 122(2):438–447. [DOI] [PubMed] [Google Scholar]
- 21.Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. Bmj 1998; 316(7137):1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mähönen M, McElduff P, Dobson A, Kuulasmaa K, Evans A. Current smoking and the risk of non-fatal myocardial infarction in the WHO MONICA Project populations. Heart 2004; 90(12):1416–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hange D, Sigurdsson JA, Björkelund C, Sundh V, Bengtsson C. A 32-year longitudinal study of alcohol consumption in Swedish women: Reduced risk of myocardial infarction but increased risk of cancer. Scandinavian journal of primary health care 2015; 33(3):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. bmj 2017; 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gémes K, Janszky I, Laugsand L, Laszlo K, Ahnve S, Vatten L, et al. Alcohol consumption is associated with a lower incidence of acute myocardial infarction: results from a large prospective population‐based study in Norway. Journal of internal medicine 2016; 279(4):365–375. [DOI] [PubMed] [Google Scholar]
- 26.Smyth A, Teo KK, Rangarajan S, O’Donnell M, Zhang X, Rana P, et al. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. The Lancet 2015; 386(10007):1945–1954. [DOI] [PubMed] [Google Scholar]
- 27.Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. The Lancet 2018; 391(10129):1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wine Cleophas T., beer and spirits and the risk of myocardial infarction: a systematic review. Biomedicine & pharmacotherapy 1999; 53(9):417–423. [DOI] [PubMed] [Google Scholar]
- 29.Mukamal KJ, Jensen MK, Grønbæk M, Stampfer MJ, Manson JE, Pischon T, et al. Drinking frequency, mediating biomarkers, and risk of myocardial infarction in women and men. Circulation 2005; 112(10):1406–1413. [DOI] [PubMed] [Google Scholar]
- 30.Tavani A, Bertuzzi M, Negri E, Sorbara L, La Vecchia C. Alcohol, smoking, coffee and risk of non-fatal acute myocardial infarction in Italy. European journal of epidemiology 2001; 17(12):1131–1137. [DOI] [PubMed] [Google Scholar]
- 31.Frazier EL, Sutton MY, Brooks JT, Shouse RL, Weiser J. Trends in cigarette smoking among adults with HIV compared with the general adult population, United States-2009–2014. Preventive medicine 2018; 111:231–234. [DOI] [PubMed] [Google Scholar]
- 32.Johnston PI, Wright SW, Orr M, Pearce FA, Stevens JW, Hubbard RB, et al. Worldwide relative smoking prevalence among people living with and without HIV. AIDS 2021; 35(6):957–970. [DOI] [PubMed] [Google Scholar]
- 33.Duko B, Ayalew M, Ayano G. The prevalence of alcohol use disorders among people living with HIV/AIDS: a systematic review and meta-analysis. Substance abuse treatment, prevention, and policy 2019; 14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King D, Grasso C, Dant L, Elsesser SA, Crane HM, Cropsey KL, et al. Treatment outcomes associated with quitting cigarettes among sexual minority men living with HIV: antiretroviral adherence, engagement in care, and sustained HIV RNA suppression. AIDS and Behavior 2018; 22(9):2868–2876. [DOI] [PubMed] [Google Scholar]
- 35.O’Cleirigh C, Valentine SE, Pinkston M, Herman D, Bedoya CA, Gordon JR, et al. The unique challenges facing HIV-positive patients who smoke cigarettes: HIV viremia, ART adherence, engagement in HIV care, and concurrent substance use. AIDS and Behavior 2015; 19(1):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cropsey KL, Willig JH, Mugavero MJ, Crane HM, McCullumsmith C, Lawrence S, et al. Cigarette smokers are less likely to have undetectable viral loads: results from four HIV clinics. Journal of addiction medicine 2016; 10(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The impact of alcohol use and related disorders on the HIV continuum of care: a systematic review. Current HIV/AIDS Reports 2015; 12(4):421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams EC, McGinnis KA, Edelman EJ, Matson TE, Gordon AJ, Marshall BD, et al. Level of alcohol use associated with HIV care continuum targets in a national US sample of persons living with HIV receiving healthcare. AIDS and Behavior 2019; 23(1):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, et al. Cohort profile: the centers for AIDS research network of integrated clinical systems. International journal of epidemiology 2008; 37(5):948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crane HM, Lober W, Webster E, Harrington RD, Crane PK, Davis TE, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Current HIV research 2007; 5(1):109–118. [DOI] [PubMed] [Google Scholar]
- 41.Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT‐C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism: Clinical and Experimental Research 2005; 29(5):844–854. [DOI] [PubMed] [Google Scholar]
- 42.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Archives of internal medicine 1998; 158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 43.Gual A, Segura L, Contel M, Heather N, Colom J. AUDIT-3 and AUDIT-4: effectiveness of two short forms of the alcohol use disorders identification test. Alcohol 2002; 37(6):591–596. [DOI] [PubMed] [Google Scholar]
- 44.Crane HM, Kadane JB, Crane PK, Kitahata MM. Diabetes case identification methods applied to electronic medical record systems: their use in HIV-infected patients. Curr HIV Res 2006; 4(1):97–106. [DOI] [PubMed] [Google Scholar]
- 45.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Annals of internal medicine 1999; 130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 46.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. New England Journal of Medicine 2006; 354(23):2473–2483. [DOI] [PubMed] [Google Scholar]
- 47.Crane H, Heckbert S, Drozd D, Budoff M, Delaney J, Rodriguez C, et al. Lessons learned from the design and implementation of myocardial infarction adjudication tailored for HIV clinical cohorts. American journal of epidemiology 2014; 179(8):996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crane HM, Nance RM, Whitney BM, Heckbert SR, Budoff M, High K, et al. Brief Report: Differences in Types of Myocardial Infarctions Among People Aging With HIV. JAIDS Journal of Acquired Immune Deficiency Syndromes 2021; 86(2):208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rastogi T, Jha P, Reddy K, Prabhakaran D, Spiegelman D, Stampfer M, et al. Bidi and cigarette smoking and risk of acute myocardial infarction among males in urban India. Tobacco Control 2005; 14(5):356–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armani C, Landini L Jr, Leone A. Molecular and biochemical changes of the cardiovascular system due to smoking exposure. Current pharmaceutical design 2009; 15(10):1038–1053. [DOI] [PubMed] [Google Scholar]
- 51.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Progress in cardiovascular diseases 2003; 46(1):91–111. [DOI] [PubMed] [Google Scholar]
- 52.Kimenai DM, Lindahl B, Chapman AR, Baron T, Gard A, Wereski R, et al. Sex differences in investigations and outcomes among patients with type 2 myocardial infarction. Heart 2021; 107(18):1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phreaner N, Daniels LB. Sex differences in type 2 myocardial infarction: learning that we still have a lot to learn. In: BMJ Publishing Group Ltd and British Cardiovascular Society; 2021. pp. 1444–1445. [DOI] [PubMed] [Google Scholar]
- 54.Leong DP, Smyth A, Teo KK, McKee M, Rangarajan S, Pais P, et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case–control study. Circulation 2014; 130(5):390–398. [DOI] [PubMed] [Google Scholar]
- 55.Mostofsky E, Chahal HS, Mukamal KJ, Rimm EB, Mittleman MA. Alcohol and immediate risk of cardiovascular events: a systematic review and dose–response meta-analysis. Circulation 2016; 133(10):979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mostofsky E, van der Bom JG, Mukamal KJ, Maclure M, Tofler GH, Muller JE, et al. Risk of myocardial infarction immediately after alcohol consumption. Epidemiology (Cambridge, Mass) 2015; 26(2):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. Bmj 1999; 319(7224):1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sierksma A, Patel H, Ouchi N, Kihara S, Funahashi T, Heine RJ, et al. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-α, and insulin sensitivity. Diabetes care 2004; 27(1):184–189. [DOI] [PubMed] [Google Scholar]
- 59.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. Jama 2002; 287(19):2559–2562. [DOI] [PubMed] [Google Scholar]
- 60.Molina PE, Simon L, Amedee AM, Welsh DA, Ferguson TF. Impact of alcohol on HIV disease pathogenesis, comorbidities and aging: Integrating preclinical and clinical findings. Alcohol and Alcoholism 2018; 53(4):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ilomäki J, Hajat A, Kauhanen J, Kurl S, Kaufman JS, Tuomainen T-P, et al. Relationship between alcohol consumption and myocardial infarction among ageing men using a marginal structural model. The European Journal of Public Health 2012; 22(6):825–830. [DOI] [PubMed] [Google Scholar]
- 62.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Brabec BA, O’Corragain O, Edmonds P, et al. High alcohol consumption and the risk of renal damage: a systematic review and meta-analysis. QJM: An International Journal of Medicine 2015; 108(7):539–548. [DOI] [PubMed] [Google Scholar]
- 63.Jani BD, McQueenie R, Nicholl BI, Field R, Hanlon P, Gallacher KI, et al. Association between patterns of alcohol consumption (beverage type, frequency and consumption with food) and risk of adverse health outcomes: a prospective cohort study. BMC medicine 2021; 19(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in US adults. Journal of the American College of Cardiology 2017; 70(8):913–922. [DOI] [PubMed] [Google Scholar]
- 65.Rehm J, Gmel GE Sr, Gmel G, Hasan OS, Imtiaz S, Popova S, et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction 2017; 112(6):968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 2018; 392(10152):1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bryant KJ. Expanding research on the role of alcohol consumption and related risks in the prevention and treatment of HIV_AIDS. Substance use & misuse 2006; 41(10–12):1465–1507. [DOI] [PubMed] [Google Scholar]
- 68.ALGSTABCNEV C. Alcohol consumption and acute myocardial infraction: a benefit of alcohol consumed with meals? Epidemiology 2004; 15(6):767–769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


