Abstract
Background:
T cell mediated hyperinflammatory responses such as cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS) are now well-established toxicities of chimeric antigen receptor (CAR) T cells. As the field of CAR T cells advances, however, there is increasing recognition that hemophagocytic lymphohistiocytosis (HLH)-like toxicities following CAR T cell infusions are occurring broadly across patient populations and CAR T cell constructs. Importantly, these HLH-like toxicities are often not as directly associated with CRS and/or its severity as initially described. This emergent toxicity, however ill-defined, is associated with life-threatening complications, creating an urgent need for improved identification and optimal management.
Objectives:
With the goal to improve patient outcomes and formulate a framework to characterize and study this HLH-like syndrome, we established an American Society for Transplantation and Cellular Therapy (ASTCT) panel comprised of experts in primary and secondary HLH, pediatric and adult HLH, infectious disease, rheumatology and hematology, oncology, and cellular therapy.
Results:
Through this effort, we provide an overview of the underlying biology of classical primary and secondary HLH, its relationship with similar manifestations following CAR T cell infusions and propose the term “immune effector cell (IEC) associated HLH-like syndrome (IEC-HS)” to describe this emergent toxicity. Further, we delineate a framework for identification of IEC-HS and put forward a grading schema which can be used to assess severity and facilitate cross-trial comparisons. Additionally, given the critical need to optimize outcomes for patients experiencing IEC-HS, we provide insights into potential treatment approaches, strategies to optimize supportive care and delineate alternate etiologies which should be considered in a patient presenting with IEC-HS.
Conclusion:
By collectively defining IEC-HS as a hyperinflammatory toxicity we can now embark on further study of the pathophysiology underlying this toxicity profile and make strides towards a more comprehensive assessment and treatment approach.
Keywords: Chimeric antigen receptor T cell (CAR T cell), hemophagocytic lymphohistiocytosis (HLH), macrophage activation syndrome (MAS)
Introduction
Hemophagocytic lymphohistiocytosis (HLH), as classically defined, is a severe hyperinflammatory syndrome characterized by hyperferritinemia, coagulopathy, hepatic dysfunction and cytopenias amongst a host of other manifestations.1,2 The unifying pathophysiology is hyperinflammation related to unmitigated T cell and macrophage activation, often with concurrent NK-cell dysfunction, which induces a cascade of cytokine-mediated systemic toxicities. With chimeric antigen receptor (CAR) T cells, the earliest reports of severe cytokine release syndrome (CRS) following CD19 CAR T cells described subsets of patients who presented with clinical manifestations mimicking HLH and with cytokine elevations mirroring those seen in HLH/macrophage activation syndrome (MAS).3–5 Similar toxicities have also been described in patients receiving blinatumomab, a bispecific T cell engager.6
As the field of CAR T cells advances, there is increasing recognition that HLH-like toxicities may not be as clearly superimposed with CRS or as directly associated with CRS severity as initially described.7–13 Indeed, reports of delayed manifestations are being seen more frequently,8,14,15 with a variable incidence across CAR T cell constructs and patient populations. Of particular concern, HLH-like toxicities can be life-threatening and associated with multiorgan dysfunction and coagulopathy. Accordingly, recent United States Food and Drug Administration (FDA) package inserts for both commercially-approved B-cell maturation antigen (BCMA)-targeted CAR T cell constructs incorporate the risk of “HLH/MAS” as a potentially fatal, life-threatening toxicity, of infusion.16,17 Given potentially dire outcomes of this complication for a potentially life-saving cell therapy, there is an urgent need to understand HLH-like toxicities following CAR T cells: specifically to facilitate recognition, establish common nomenclature and grading approaches, implement effective therapies, investigate in a coherent fashion, and improve patient outcomes.
To better define and understand HLH-like toxicities following CAR T cells, or broadly, after immune effector cell (IEC) based therapies, we developed a working group through the American Society of Transplantation and Cellular Therapy (ASTCT) Committee on Cellular Therapy to focus on this entity. Comprised of 30 experts in primary and secondary HLH, pediatric and adult HLH, infectious disease, rheumatology, and CAR T cell use across B-cell acute lymphoblastic leukemia, B-cell lymphoma, and multiple myeloma—we focused on 1) underlying biology of historically-defined HLH, 2) guidelines for identification, 3) grading, 4) potential treatment options and supportive care, and 5) alternate etiologies leading to HLH-like manifestations. Despite the limited data available on HLH-like toxicities, with the urgent need to improve outcomes, our manuscript provides a framework to foster recognition and further study of this important IEC-associated toxicity, now newly termed: IEC-associated HLH-like syndrome (IEC-HS).
1. Primary and Secondary HLH: Biology and Definitions
HLH, as classically defined, is not an individual disease, but a syndrome characterized by pathologic immune activation that results in fever, hepatosplenomegaly, organ failure, neurologic toxicities, coagulopathy, cytopenias, hyperferritinemia, and/or hypertriglyceridemia.1,18 HLH can be broadly categorized as primary (genetic/inherited) or secondary (acquired), although considerable overlap exists.
1.1. Primary HLH
Primary HLH (pHLH) encompasses inborn errors of immunity which heavily predisposes to HLH and often presents as systemic disease in infants and young children.18,19 Primary HLH is generally caused by pathogenic variants in genes affecting cytotoxic T cell and NK-cell function, lymphocyte survival, or inflammasome activation, and are most often autosomal recessive (Supplemental Table 1).1,19,20 Familial HLH is a specific type of pHLH in which CD8+ T-lymphocytes with defects in granule-mediated cytotoxicity are activated in an uncontrolled manner and release interferon-gamma (IFNγ) which stimulates macrophages that then release T cell activating cytokines in a positive feedback loop (Figure 1A). As a result, additional cytokines including IL-1β, IL-6, IL-10, IL-12, IL-18, and tumor necrosis factor-alpha (TNFα) are released in relative abundance.1,21,22 Other types of pHLH include Epstein-Barr Virus susceptibility disorders, lysosomal-related pigmentary disorders, and X-linked lymphoproliferative diseases, and inflammasopathies involving disrupted inflammasome regulation, increased macrophage activity, and highly elevated IL-18 levels.1,19,23 Overall, the number of identified genes associated with HLH has grown significantly since discovery of PRF1, LYST, and SH2D1A, with new HLH-associated genes identified annually.1,24–29
Figure 1.
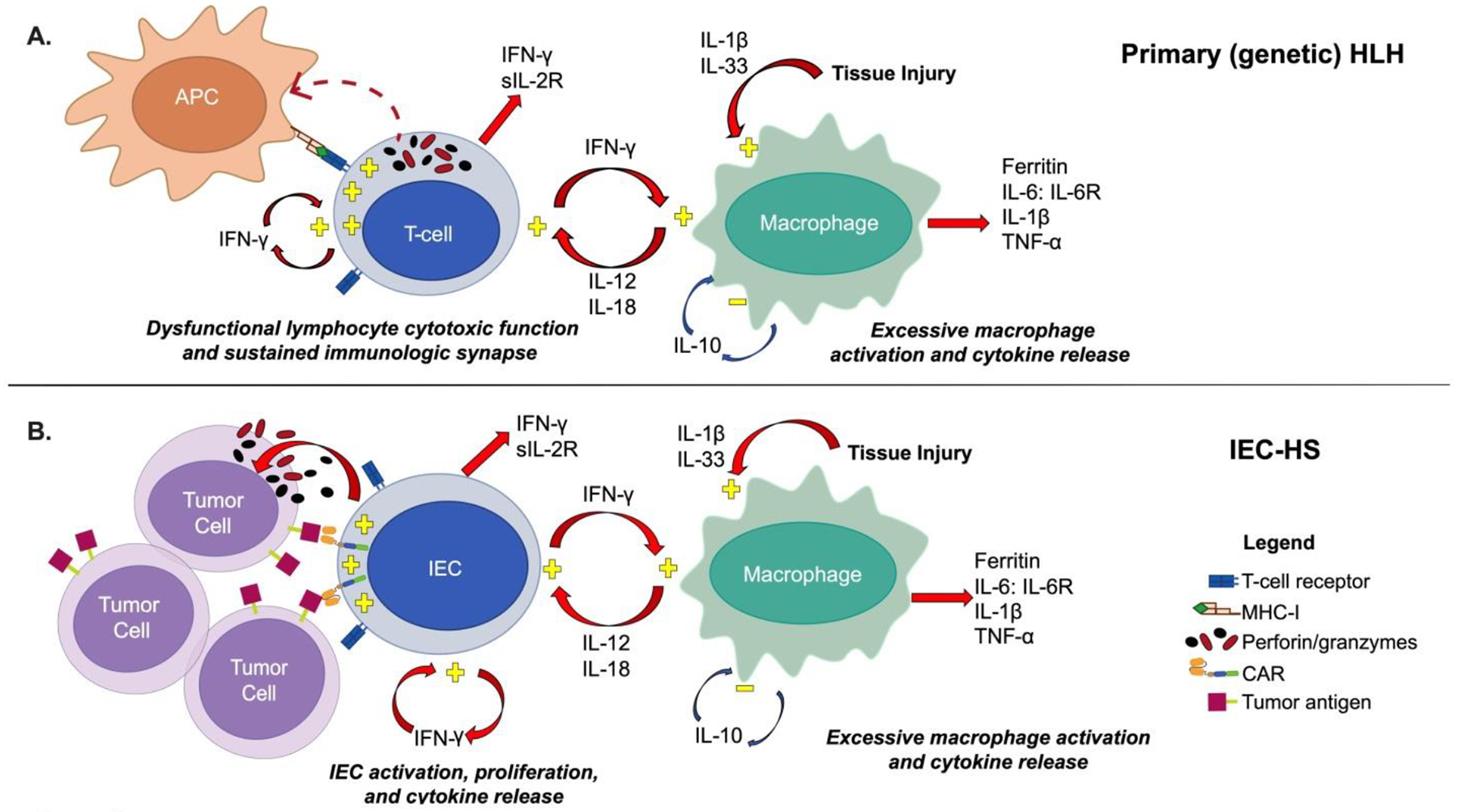
Primary HLH and IEC-Associated HLH-like syndrome (IEC-HS)
A. Top panel: In primary (genetic) HLH, cytotoxic T cells with insufficient cytotoxic function due to defects in cytotoxic granule release or perforin are activated, cannot terminate their immunologic synapse, and with insufficient downregulation promote macrophage activation and cytokine release, which further activates cytotoxic T cells. The resulting T cell and macrophage activation results in release of IFN-γ, sIL-2R, IL-6, IL-10, IL-12, IL-18, IL-1β, TNF-α, IL-33, and ferritin.
B. Bottom panel: In IEC-HS, CAR T cells are activated by CAR T cell recognition of tumor antigen which leads to cytotoxic granule release and tumor lysis. Sustained activation via engineered CAR recognition of tumor antigen results in T cell activation, proliferation, and cytokine release and resultant macrophage activation and release of soluble factors as seen with primary HLH.
1.2. Secondary HLH
Secondary HLH (sHLH) refers to the clinical syndrome of HLH occurring in the absence of an identifiable heritable defect in lymphocyte cytotoxicity, survival, or inflammasome activation.18 Secondary HLH has been categorized by underlying triggers, including: (1) infections, (2) malignancies, (3) metabolic disorders, (4) rheumatologic diseases (i.e. MAS), and (5) therapy-related (e.g., following CAR T cells and bispecific T cell engagers).1,5,18 sHLH may occur in patients heterozygous for variants in familial HLH genes following only a modest trigger,18 and can also occur in individuals with primary immune deficiencies that develop serious infections (e.g. chronic granulomatous disease and overwhelming bacterial or fungal infection).30
1.3. Role for Molecular Diagnostics
Molecular diagnoses are made more often in children than in adults presenting with HLH. In contrast, most adults with HLH do not have an identifiable genetic contributor,31,32 and sHLH is the predominant manifestation. However, as large-scale efforts that include whole genome sequencing and epigenetic profiling are more available, novel genetic causes of HLH, even in adults, may become more apparent.
1.4. Diagnostic criteria: pHLH and sHLH
Multiple diagnostic criteria have been developed for HLH, with HLH-2004 criteria established in pHLH, the most frequently utilized. The HLH-2004 criteria are comprised of a set of 8 criteria including fever, splenomegaly, cytopenias, hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis along with low/absent NK-cell activity, hyperferritinemia and high soluble IL2-receptor (sIL-2R) / soluble CD25 (sCD25) levels.33 Other diagnostic criteria for specific patient populations and based on unique triggers (e.g., HScore, derived in adults with sHLH,34 as well as MAS/HLH criteria specifically for systemic juvenile idiopathic arthritis (sJIA)35) have been developed by expert consensus and validated in select populations. While these criteria have considerable strengths and facilitate diagnosis of HLH, they are less-well suited for some forms of sHLH. Further, certain biomarkers may be more diagnostically useful in children than adults. For instance, ferritin values of 500 to 2,000 ng/mL may be clinically meaningful and reflect HLH in children with pHLH but lack specificity in adults.18,36 Malignancy-associated HLH is another variant where new diagnostic criteria were needed as neither HLH-2004 nor the HScore34 were optimally applicable due to overlapping manifestations of underlying disease processeses.37 Nevertheless, recent studies report that both the HLH-2004 criteria and HScore have high diagnostic reliability to identify sHLH in critically ill patients.38,39
2. CAR T cells and hyperinflammatory syndromes
2.1. Cytokine release syndrome and immune effector cell associated neurotoxicity syndrome
CAR T cells express proteins with antigen-binding, transmembrane, and co-stimulatory domains, allowing T cells to recognize extracellular tumor antigens, proliferate, and mediate tumor lysis, often resulting in ample cytokine release.40–42 Well-described toxicities of CAR T cell therapy include CRS and IEC-associated neurotoxicity syndrome (ICANS).5,43 CRS, like HLH, involves fever and, in severe forms, respiratory failure, and hypotension. Cytopenias, hyperferritinemia, coagulopathy, and hypertriglyceridemia are seen in both conditions. Overlapping cytokine (e.g., IFN-γ, IL-6, IL-10, IL-18, sIL-2R, CXCL9), and proteomic profiles are described in HLH and severe CRS.8,44–46
While some patients who receive CAR T cells experience severe CRS with abnormal immunopathologic sequelae (e.g., hepatopathy, hypofibrinogenemia, or hemophagocytosis), a subset of patients develop a different pathophysiologic entity with HLH-like features manifesting primarily after traditional CRS has resolved. While disease-specific factors (disease burden,9,47 proliferation dynamics, immune resistance mechanisms), IEC-associated factors (antigen target,8 CAR T cell construct,48 co-stimulatory domain, T cell selection,8 cell dose) and patient characteristics (baseline inflammation,9,49,50 immune suppression, cytopenias, genetic predisposition)47,51 likely influence development of IEC-HS, the underlying biology of IEC-HS requires further study.
2.2. Immune Effector Cell associated HLH-like Syndrome (IEC-HS)
Because of overlapping features of CRS and underlying hematologic malignancies,52 current diagnostic criteria for HLH are suboptimal for use following CAR T cells.53,54 While other terms (e.g., carHLH, MAS-L, HLH/MAS) to describe HLH-like toxicities following CAR T cells have been applied, in order to: 1) unify the field on HLH-like toxicities following IEC-based therapies—which aligns with ICANS nomenclature, and 2) acknowledge a pathophysiology and hyperinflammatory process distinct from pHLH, albeit with similar manifestations, we opted to identify this as an “HLH-like” syndrome. Accordingly, the term “IEC-associated HLH-like syndrome (IEC-HS)” was established.
This model suggests that IECs, such as CAR T cells, become activated by tumor antigens and transactivate macrophages leading to concurrent CAR T cell/macrophage activation and cytokine release creating a positive feedback loop. With limited ability to sufficiently down-regulate, the onset of inflammation-mediated organ injury that extends beyond recovery from CRS is delayed (Figure 1B).
2.3. IEC-HS Definition
The ASTCT CRS consensus grading scale encompasses clinical parameters of fever, hypotension and hypoxia to identify and rate severity.45 Importantly, however, many early grading systems for CRS incorporated elements of end-organ toxicities55 as patients with moderate to severe CRS could have clinical features and cytokine abnormalities mimicking HLH-like toxicities (e.g., elevated ferritin, transaminitis, hemophagocytosis and coagulopathy), which usually resolved without delayed sequelae or need for additional immunomodulatory therapy.43,45,55–61 While acknowledging the biochemical and pathologic similarities between CRS, ICANS and HLH (Figure 2A), a key factor in diagnosing IEC-HS, is its clinical independence from CRS.
Figure 2.
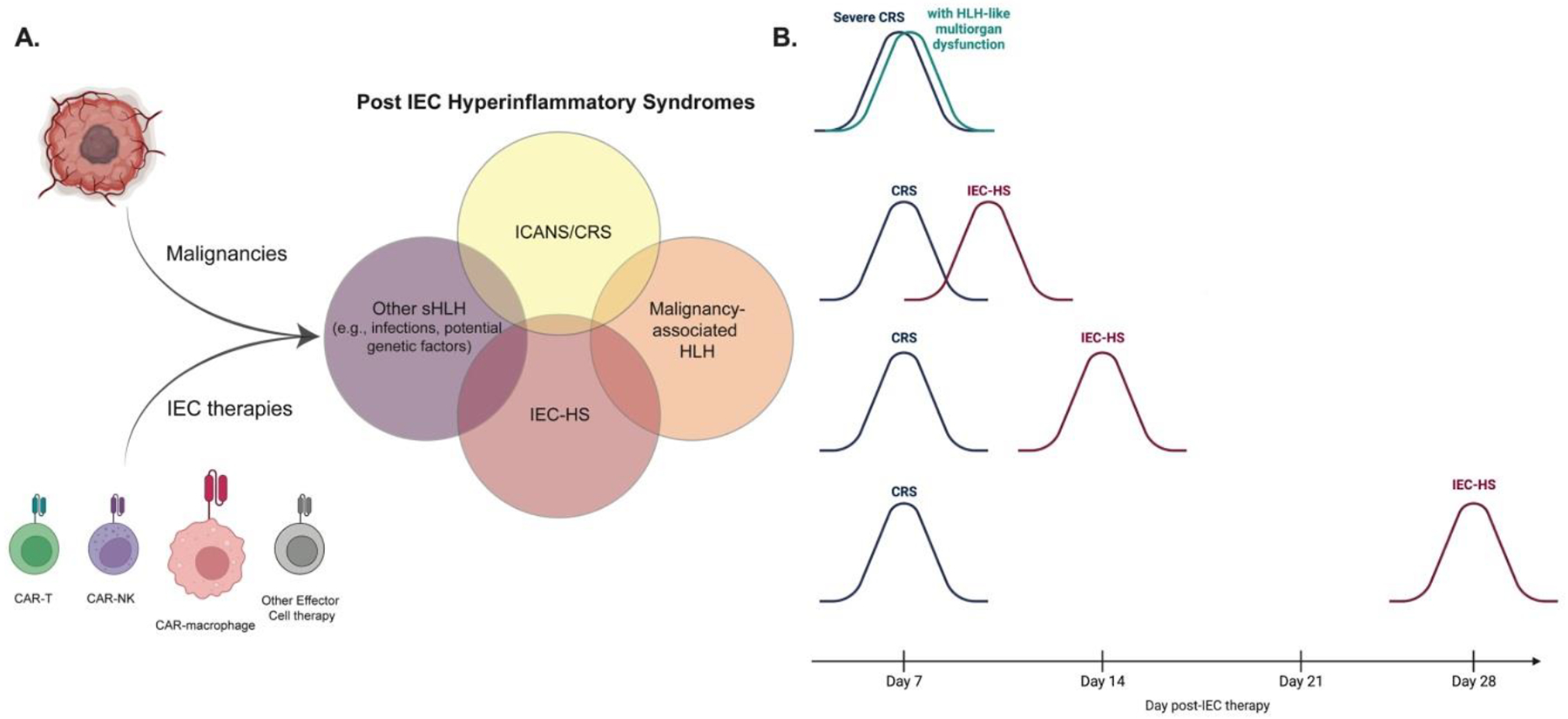
Intersection of IEC related toxicities and Timing of CRS and IEC-HS
A. Visual of how various IEC based therapies contribute to inducing a host of IEC-associated hyperinflammatory syndromes—highlighting both the inter-relatedness and yet unique presentations of the various toxicities.
B. Suggested paradigms for severe CRS with HLH-like multiorgan dysfunction and how this chronologically differs from IEC-HS presentations.
We ultimately define IEC-HS as the development of a pathological and biochemical hyperinflammatory syndrome that 1) manifests with features of macrophage activation/HLH, 2) is attributable to IEC therapy, and 3) is associated with progression or new onset of cytopenias, hyperferritinemia, coagulopathy with hypofibrinogenemia and/or transaminitis. While HLH-like manifestations are frequently seen in in patients with severe CRS, IEC-HS is often delayed in onset and manifests as CRS is resolved/resolving and should not be used to describe solely manifestations of severe CRS (Figure 2B).
We further delineate more specific criteria (of which hyperferritinemia (> 2 x upper limit of normal or baseline (at time of infusion)) and/or rapidly rising (per clinical assessment) is a pre-requisite) based upon HLH-2004 diagnostic criteria, analysis of published cases3,4,7–12,14,15,33,46,48,62–64 and experiences of our expert panel. (Table 1) Indeed, this analysis of data emerging from single or multi-case series, (Supplemental Figure 1) alongside unpublished experiences, was critical as we developed our definitions and approach. Accordingly, this effort builds upon these initial observations and serves to lay the foundation to collectively learn more about IEC-HS
Table 1.
Immune effector cell associated HLH-like syndrome (IEC-HS): Definition and Identification
| IEC-HS Definition | The development of a pathological and biochemical hyperinflammatory syndrome independent from CRS and ICANS that 1) manifests with features of macrophage activation/HLH, 2) is attributable to IEC therapy, and 3) is associated with progression or new onset of cytopenias, hyperferritinemia, coagulopathy with hypofibrinogenemia and/or transaminitis. |
| Criteria for identification of IEC-HS ^ | Clinical or laboratory manifestations |
| Most common manifestations $ | REQUIRED: Elevated ferritin (> 2 × ULN or baseline (at time of infusion)) and/or rapidly rising (per clinical assessment) |
| Onset with resolving/resolved CRS or worsening inflammatory response after initial improvement with CRS directed therapy* | |
| Hepatic transaminase elevation** (> 5 × ULN (if baseline was normal) or > 5 x baseline if baseline was abnormal) | |
| Hypofibrinogenemia (< 150 mg/dL or < LLN)^^ | |
| Hemophagocytosis in bone marrow or other tissue^^ | |
| Cytopenias (new onset, worsening, or refractory&) | |
| Other manifestations that may be present | Lactate dehydrogenase elevations (> ULN) |
| Other coagulation abnormalities (e.g., elevated PT/PTT) | |
| Direct hyperbilirubinemia | |
| New onset splenomegaly | |
| Fevers (new# or persistent)^^ | |
| Neurotoxicity | |
| Pulmonary manifestations (e.g., hypoxia, pulmonary infiltrates, pulmonary edema) | |
| Renal insufficiency (new onset) | |
| Hypertriglyceridemia (fasting level, >265 mg/dL^^) |
Diagnosis is made only when it is not attributable to alternative etiologies, including CRS, infection and/or disease progression;
Although most cases of IEC-HS have been seen with antecedent CRS, this may not always be the case and emerging experience will shed light on how IEC-HS may present;
Generally at least one lineage will be a grade 4 cytopenia (platelets, neutrophils, hemoglobin).
As distinguished from CRS onset or recrudescence of CRS;
Constellation of findings typically simultaneously (e.g., all within 72 hours);
consistent with Grade 3 hepatic transaminase elevations as per CTCAE v 5.0;
as per HLH-2004
2.4. IEC-HS Diagnosis
2.4.1. Clinical suspicion:
Clinical presentation of IEC-HS closely resembles the onset of an exaggerated inflammatory response following CRS or CAR T cell expansion. The timing of IEC-HS in relation to antecedent CRS is variable, but generally delayed. Importantly, all reported patients with HLH-like toxicities to date have had an antecedent or ongoing CRS.8,9
Despite potential overlap, we strongly caution against use of IEC-HS nomenclature to describe patients with severe CRS involving multi-organ dysfunction due to unique implications on treatment approach and to avoid designation of new nomenclature on a previously established process. Accordingly, while fever is an important criterion for pHLH/sHLH and may be seen with IEC-HS, fever was excluded from the proposed diagnosis of IEC-HS to avoid confusion as it represents the sole criterion for grade 1 CRS.45 Hence, it is imperative to distinguish suspected IEC-HS from CRS recurrence or a prolonged severe CRS, to pursue treatment options and monitoring accordingly.
2.4.2. Laboratory monitoring:
2.4.2.1. Ferritin
Published recommendations based primarily on single-center experiences have suggested use of elevated ferritin above 10,000 ng/mL with CD19 CAR T cells,65 above 100,000 ng/mL with CD22 CAR T cells,8 or rapid increases by ≥ 100 μg/L/hour within 24-hours with BCMA-targeted CAR T cells7 as a primary criterion in diagnosing HLH-like toxicities. However, with ongoing or impending multi-organ failure, waiting to reach an otherwise arbitrary ferritin elevation may result in unnecessary delay in diagnosis and potential intervention. Given limitations in proposing these cut-offs due to a lack of supporting evidence and vast heterogeneity in baseline ferritin values across patient and disease cohorts, we refrained from using specific ferritin values in the proposed criteria. Nonetheless, given the high negative predictive value of a normal ferritin,2 substantial elevations in ferritin are a prerequisite to a diagnosis of IEC-HS and normal ferritin values with ongoing inflammation should prompt considerations of alternative etiologies. In addition, as monitoring frequency of ferritin varies by center, standardization of the rate of rise was precluded. Alternatively, we included a “rapidly rising” level of ferritin as a subjective, investigator-determined criterion for IEC-HS, in context with other clinical and laboratory parameters as listed in Table 1.
2.4.2.2. Cytokine profiling
While cytokine profiling may shed insights into the pathophysiology and/or diagnosis of IEC-HS, results are rarely available in real-time, may delay diagnosis and may be confounded by prior CRS and/or cytokine directed treatments employed. Therefore, cytokine levels were not considered diagnostic at present. Nevertheless, as rapid turnaround becomes more commonplace, cytokine profiling may become more integrated into the diagnostic algorithms for IEC-HS and should be re-visited.
2.4.3. Overall diagnosis
Ultimately, we emphasize the holistic consideration of the clinical picture and bedside assessment for IEC-HS identification and to evaluate severity, rather than reliance on any one laboratory parameter. Our current IEC-HS definition serves as a foundation. We anticipate a more precise delineation of these diagnostic criteria and improved methods to distinguish IEC-HS from severe CRS, as additional data emerges.
3. Grading of IEC-HS
While IEC-HS can be associated with fatal and/or life-threatening complications, we have also observed patients who have clear evidence of a hyperinflammatory process but remain clinically well, and in whom laboratory abnormalities may resolve without intervention. Given variability in presentation, there is a need to assess severity to facilitate cross-trial comparisons of toxicity and identify IEC-HS before it leads to substantial complications. Thus, we propose a grading schema (Table 2) based largely off the NCI Common Terminology for Adverse Events (CTCAE) utilizing the “Immune system disorder, other” category. Importantly, grading is intended to be kept distinct from treatment approaches, primarily since we anticipate that pre-emptive and combinatorial agents may be used for IEC-HS and that titrating optimal dosing regimens should not influence the severity assessment of clinical and laboratory manifestations.
Table 2.
Immune effector cell associated HLH-like syndrome (IEC-HS): Grading
| Grade | |||||
|---|---|---|---|---|---|
| Adverse Event | 1 | 2 | 3 | 4 | 5 |
| IEC-HS * | Asymptomatic or mild symptoms; requires observation and/or clinical and diagnostic evaluations. Intervention not indicated. | Mild to moderate symptoms, with intervention indicated. (e.g., immunosuppressive agents directed at IEC-HS, transfusions for asymptomatic hypofibrinogenemia) | Severe or medically significant, but not immediately life threatening (e.g., coagulopathy with bleeding requiring transfusion support, or hospitalization required for new onset acute kidney injury, hypotension or respiratory distress) | Life-threatening consequences: urgent intervention indicated (e.g., life-threatening bleeding or hypotension, respiratory distress requiring intubation, dialysis indicated for acute kidney injury) | Death |
Not attributable to other causes; Defined by the development of pathological and biochemical features of macrophage activation/HLH that is attributable to IEC therapy and associated with progression or new onset of cytopenias, hyperferritinemia, coagulopathy with hypofibrinogenemia and hepatic transaminitis (>5 x ULN)). While HLH-like manifestations are frequently seen in in patients with severe CRS (as defined by ASTCT), IEC-HS is often delayed in onset and manifests as CRS is resolved/resolving
4. Treatment of IEC-HS and Supportive Care
4.4. Current Treatment Approaches in Primary and Secondary HLH
Due to overlap in underlying pathophysiology, analyzing therapies used for pHLH is valuable for multiple reasons: first, data are available from large, prospective, international clinical trials, and second, pHLH most often affects children, who generally have few confounding diagnoses and treatments. These factors facilitate comparison of various anti-hyperinflammatory treatments. We therefore summarize management strategies in genetically confirmed HLH, alongside the short-term outcomes, as relevant to IEC-HS (Supplemental Table 2).
4.4.1. Treatment of Primary HLH
The largest HLH studies are HLH-9466 and HLH-2004,33 both etoposide/dexamethasone-based and focused on “severe” and “life-threatening” disease, with the vast majority of children with verified familial HLH proceeding to hematopoietic stem cell transplantation (HSCT) (i.e., 133/167 (80%) in HLH-2004). The mechanism of action of etoposide in HLH involves targeting pathologically activated T cells via programmed cell death (e.g. apoptosis) rather than proinflammatory lytic cell death (e.g. pyroptosis), conceivably ameliorating subsequent systemic inflammation.67–69 Studies evaluating etoposide included both pHLH and sHLH but were limited to children. Recent information from the HLH Registry indicate that 74% of patients receiving first-line etoposide were alive after 1 year.70 Additionally, etoposide has shown efficacy in “low-grade” disease, by limiting disease progression in a cohort of asymptomatic children with genetically confirmed pHLH.71 Other approaches utilized in pHLH include alemtuzumab for frontline and salvage therapy, with good effect72,73 and antithymocyte globulin, with limited experience.74 Emapalumab, an IFNγ-targeted monoclonal antibody, has also been used for both frontline and salvage therapy in children. Among 34 children treated with emapalumab plus dexamethasone, 22/34 (65%) proceeded to HSCT; however, ~60% of this group did receive adjunctive etoposide. Of the seven previously untreated children, 0/7 achieved complete remission and 2/7 survived without adjunctive etoposide.75,76 There is limited or no data available for the use of ruxolitinib, tocilizumab or anakinra in pHLH.
4.4.2. Treatment of secondary HLH
Secondary HLH is more heterogeneous than pHLH, with a varied patient population, diverse triggering etiologies, and multiple treatment regimens. In most patients, systemic corticosteroids are both an initial therapy and a component of subsequent treatments.2,77,78 Beyond this however, there is a large degree of variation, and selection of initial/subsequent therapies is etiology-driven.2 Etoposide, with or without combination, has been utilized for sHLH, and reductions in both frequency and dosage (compared to induction therapy) have been suggested.2 Despite a strong theoretical rationale,67,68 real-world data for etoposide is mixed; most studies show improved outcomes,79–84 while others found no benefit.31,85
4.4.3. Drugs used in the treatment of sHLH beyond etoposide
4.1.3.1. Anakinra
Anakinra, an IL1-receptor antagonist, has the second largest amount of evidence in sHLH, after etoposide. It is a relatively low-toxicity agent, has a wide therapeutic dosing range, a short half-life allowing rapid titration to effect and, conversely, rapid clearance if necessary, and an acceptable side effect profile and pharmacokinetic data to support both intravenous and subcutaneous use.86 The strongest evidence supporting its use emerges from MAS.2 In this context, anakinra is often a first-line agent in conjunction with systemic corticosteroids,87–89 particularly in severe or progressive disease.77 Anakinra has also been effective in critically ill patients with sHLH triggered by non-rheumatologic etiologies.90–92
4.1.3.2. Ruxolitinib
Ruxolitinib has been used in a small number of studies as both front-line and as salvage for sHLH. Ruxolitinib inhibits Janus kinase 1 (JAK1) and JAK2, thereby blocking signal transduction via the JAK/STAT (signal transducer and activator of transcription) pathway, allowing it to inhibit multiple key proinflammatory cytokine activity, including IFNγ, IL-2, and IL-6.93,94 As front-line, a 69% (36/52) overall response rate to ruxolitinib monotherapy has been reported.94 In refractory disease, overall response rates of 74% (25/34)95 and 78% (32/41)96 have been seen – although these latter studies combine ruxolitinib with a variety of other agents, including corticosteroids.
4.1.3.3. Other cytokine directed therapies
Other cytokine-directed therapies have limited evidence in sHLH, including tocilizumab and emapalumab. Smaller studies of tocilizumab report variable outcomes, with one showing an increased risk of infectious complications and death (n=8),97 and another showing improved survival (n=9).98 Moreover, despite therapeutic efficacy in sJIA, the occasional simultaneous occurrence of MAS in patients receiving tocilizumab has led to suggestions that IL-6 may play a limited role in the development of MAS/sHLH.99 While published data on use of emapalumab in sHLH is limited, off-label use is increasing and may provide insights on its role in the future. There is currently an ongoing single-arm clinical trials evaluating emapalumab for MAS with preliminary data showing biologic efficacy in a sJIA and MAS cohort.100,101
4.5. Treatment Approaches for IEC-HS
Due to lack of prospective clinical trials for IEC-HS and limited literature on treatment approaches for HLH-like manifestations following CAR T cells—the following treatment recommendations are derived from expert opinion, rely on available evidence from CRS/IEC-HS cohorts and previous evidence with treatment of pHLH and sHLH, and warrant prospective studies. Therapy should generally be initiated with corticosteroids +/− anakinra prior to development of life-threatening complications and, if possible, a time interval to evaluate for efficacy (e.g., 48 hours) should elapse before additional agents are added to avoid cumulative toxicity of multiple immunosuppressive agents. The impact of any potential therapies on CAR T cell efficacy must also be considered, and first-and-early line therapies should be selected at least partially based on lower risk of impeding CAR T cell activity and persistence, with progressively less consideration given to this as the severity of IEC-HS increases. Initial recommendations for specific therapies, dosages, and escalation of care are presented in Figure 3 and Table 3.
Figure 3.
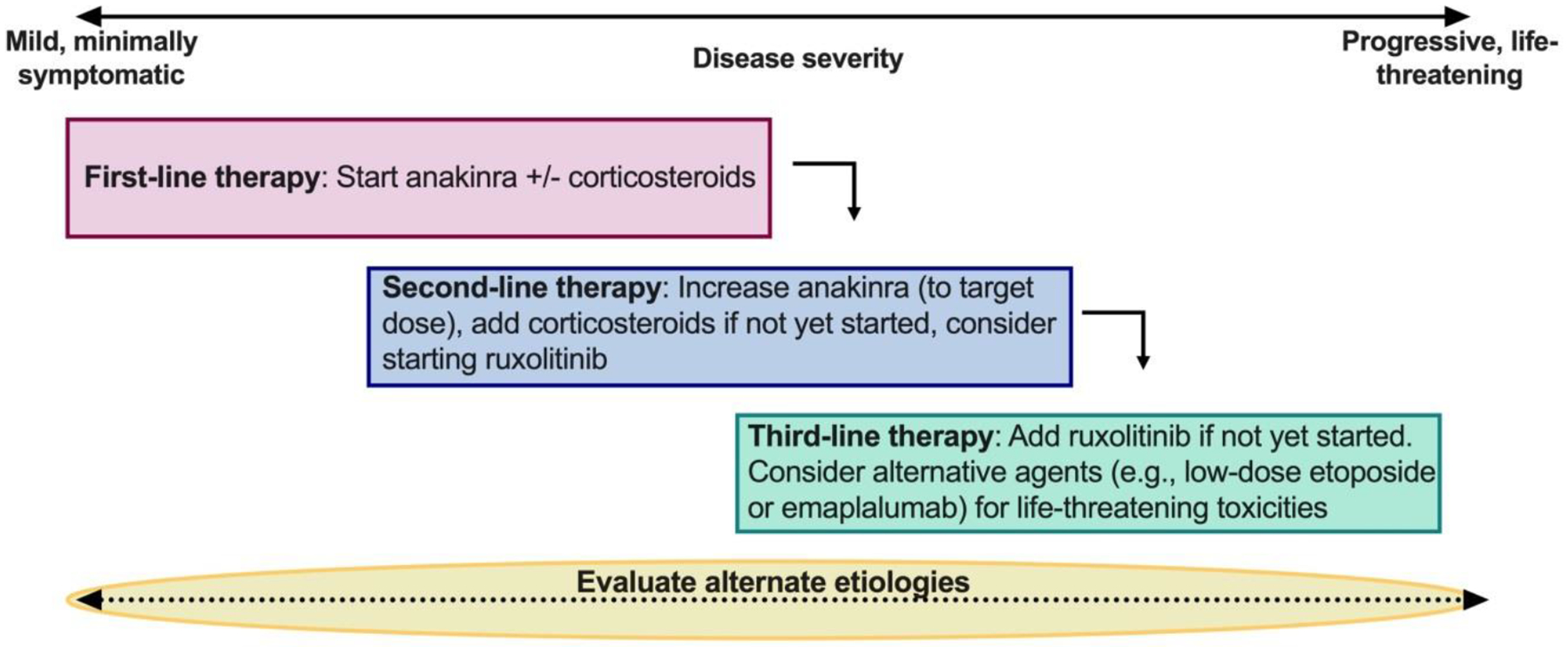
Stepwise approach to treatment of IEC-HS
Treatment recommendations are derived from expert opinion and warrant prospective study. Nonetheless, therapy should generally be initiated prior to development of life-threatening complications and, if possible, a time interval (e.g., 48 hours) should be allowed to observe for efficacy before additional agents are added to avoid cumulative toxicity of multiple immunosuppressive agents. Patients should be closely monitored for response to interventions, with potential for escalation in care and evaluation of alternative etiologies if there is worsening. (See table 3 for specific dosing recommendations)
First-line therapy: Can start with anakinra +/− corticosteroids. While a range of dosing is provided, use of one or both agents and/or low versus higher dose is dependent on the clinical presentation of the patient and how rapidly the inflammatory response is worsening. For patients with grade 2 IEC-HS, starting with the lower end of the range for dual therapy, or higher-dosing for single agent intervention could both be considered.
Second-line therapy: For patients with clearly worsening inflammatory parameters who are developing more severe manifestations of IEC-HS, recommendations are for dual-agent therapy at the higher-end of the range—with consideration for adding additional agents if the patient is rapidly worsening.
Third-line therapy: Typically, corticosteroids/anakinra would continue as additional therapies are added to achieve control of the inflammatory response. Choice of therapy beyond ruxolitinib can be informed by unique patient considerations (e.g., etoposide for CAR T-cell associated lymphocytosis or emapalumab for highly elevated IFN-γ).
An ongoing assessment for alternative etiologies should continue throughout the IEC-HS manifestations and treatment course. Consultation with infectious disease specialists and rheumatology should be considered to optimize supportive care for patients with sub-optimal response to initial therapeutic approaches or those with worsening manifestations. As clinical and laboratory parameters stabilize, gradually tapering immunosuppression is encouraged while monitoring for recrudescence of symptoms
Table 3.
Potential pharmacologic approaches for treatment of IEC-HS
| Agent | Mechanism and Rationale for Inclusion in IEC-HS Treatment Algorithm | Side Effect Profile and Potential Considerations | Starting dose in IEC-HS (General Considerations) |
|---|---|---|---|
| Anakinra | Recombinant IL-1 receptor antagonist. Elevated IL-1β present in MAS/sHLH; blockade with anakinra has documented efficacy in both rheumatologic and non-rheumatologic etiologies, including in severe disease.87,89–92 IL-1β upregulation has been documented specifically in the IEC-HS context.8,48 Evidence of efficacy in treatment/prevention of other CAR T-associated toxicities (refractory CRS, ICANS).10,104,105 Relatively high degree of familiarity with use in HLH context. A short half-life allowing rapid titration to effect and, conversely, rapid clearance if necessary. |
Well-studied, well-tolerated side-effect profile (in rheumatology context);144 less data regarding side-effect profile in CAR T-context but appears similar.105 Unknown effect on CAR T-efficacy but expected to be minimal. |
Adults: 100–200 mg SQ/IV q6-12 hours Peds: Can start at 5–7 mg/kg/day or use higher dose of 8–10 mg/kg/day divided BID or TID or 4 mg/kg IV q6 hours Continuous IV infusion regimens have been used for other indications. Discuss with rheumatology consultants. |
| Corticosteroids | Established use in conjunction with multiple other agents in pHLH113,114 and sHLH.2,77,78 Evidence of efficacy in treatment, prevention, and reduction of severity of other CAR T-associated toxicities (CRS, ICANS).102,103,145 |
Known but prominent side effect profile. Mixed data on impact of corticosteroids on long-term CAR T-efficacy and outcomes.106,108,146 |
Adults: Dexamethasone 10–40 mg daily (10 mg q6 hours most common) Peds: Dexamethasone 10 mg/m2/day or methylprednisol one 1 −2 mg/kg IV/PO q6-12 hours |
| Ruxolitinib | Inhibition of JAK1/JAK2, thereby blocking downstream transduction via the JAK/STAT pathway, attenuating the action of multiple key proinflammatory cytokines including IFN-γ, IL-2, and IL-6.93 Evidence of efficacy in sHLH in both frontline94 and refractory95,96 settings. Theoretical therapeutic rationale for use exists in IEC-HS, e.g., inhibition of multiple cytokines shown to be involved in IEC-HS pathogenesis.8 Relatively commonly used agent in HSCT/cell therapy setting; high degree of familiarity. A short half-life allowing rapid titration to effect and, conversely, rapid clearance if necessary. |
Risk of exacerbating post CAR-T cytopenias and increased risk of infection, especially viral re-activation.147 CYP3A4 inhibition: risk of drug interaction especially with azole prophylaxis.147 Unknown effect on CAR T-efficacy (? expected to be minimal) |
Adults (≥14 years): 10 mg BID (and can consider increasing to 20 mg BID) Peds (<14 years): >25kg: 5 mg BID; <25kg: 2.5 mg BID (can consider increasing) (*Dose-adjustment required with strong CYP450 inhibitors (e.g., azoles) |
| Etoposide | Inhibition of topoisomerase II. Robust evidence of efficacy in pHLH,113,114 and most studies show evidence of efficacy in sHLH.31,79–82,84,85 Strong theoretical mechanistic rationale for use in IEC-HS (e.g., targeted induction of T cell apoptosis and blunting of subsequent inflammatory response).67,68 May best be utilized as last-line therapy in or to prevent high-grade/life threatening IEC-HS. Impact on CAR T cells is unknown but given the direct impact on lymphocytes, may also eradicate/diminish CAR T cells) |
Significant side effect profile compared to other agents discussed. Importantly, however, use of etoposide for IEC-HS would be at a substantially lower dose than with induction dosing for pHLH. Evidence of extended use in adults as well as children.83 Risk of secondary malignancy – likely very low with the suggested dosing.113–116 Putative effect in IEC-HS is via direct ablation of activated T-lymphocytes;69 removal of CAR T cells therefore possible. |
50–100 mg/m2/dose (× 1 to initiate) |
| Emapalumab | Human IgG1 anti-IFNγ monoclonal antibody; binds both free and receptor-bound IFNγ. May be considered as a possible alternative agent in severe/life-threatening IEC-HS, especially in the setting of documented IFNγ elevation Elevated IFN-γ has been documented in both pHLH125–127 and in CAR T-associated toxicities.120,122,123 Evidence of clinical efficacy in pediatric pHLH in both frontline and salvage settings.76 (*notably several patients required additional etoposide) Directly measured cytokine levels support mechanism of action in pHLH, e.g., rapid decline in CXCL9 during emapalumab therapy76 (CXCL9 is induced by IFN-γ and may be used as a surrogate for activity). Theoretical rationale exists for use specifically in IEC-HS, e.g., elevated IFNγ shown to be involved in IEC-HS pathogenesis.8,48 |
Increased infection risk, including viral and fungal.76 Otherwise relatively low side effect profile, but (a) limited data available, and (b) no adult data reported. Minimal (but positive) data supporting use in pediatric sHLH.100,101 No data supporting use in adult patients. Potentially cost-prohibitive. |
Per standard package insert |
Due to the lack of prospective clinical trials for IEC-HS (as this entity is only now being formally defined and recognized) and the limited literature on treatment approaches for HLH-like manifestations following CAR T cells—which is based a small number of case-control and cohort studies, case series, and case reports, theserecommendations are not evidence-based, are derived from expert opinion, and warrant prospective study.
Therapy should generally be initiated prior to development of life-threatening complications and, if possible, a time interval (e.g., 48 hours) should be allowed to observe for efficacy before additional agents are added to avoid cumulative toxicity of multiple immunosuppressive agents. Patients should be closely monitored for response to interventions, with potential for escalation in care and evaluation of alternative etiologies if there is worsening.
Abbreviations: CXCl9: C-X-C motif chemokine ligand 9. MAS: Macrophage activation syndrome. ICANS: Immune effector cell associated neurotoxicity syndrome. IFN-γ: Interferon gamma. IL: Interleukin. JAK: janus kinase. pHLH: primary hemophagocytic lymphohistiocytosis. sHLH: secondary hemophagocytic lymphohistiocytosis. STAT: signal transducer and activator of transcription
The role of pre-emptive treatment to prevent severe IEC-HS is unknown but is reasonable to explore, particularly within a clinical study. Similar to use of tocilizumab and corticosteroids as prevention or with low-grade CRS/ICANS to mitigate development of severe CRS/ICANS,102,103 this may help prevent severe complications and decrease prolonged need for immunosuppression. We anticipate that with improved recognition of IEC-HS a role for pre-emptive approaches may evolve.
Similarly, the best approach to tapering and withdrawal of therapy remains unclear—particularly in patients with protracted IEC-HS. Nonetheless, given the deleterious impact of immunosuppression, including risk of infection—when clinical and laboratory parameters have stabilized, gradually tapering therapy is encouraged while monitoring for recrudescence of symptoms. Similar to sHLH, a more rapid cessation of IEC-HS directed therapy may be tolerated as compared to pHLH, but further studies are needed.
4.5.1. Agent-Specific Considerations in the Treatment of IEC-HS (Table 3)
4.5.1.1. Anakinra
Anakinra was recommended as a first-line agent due largely to its acceptable side effect profile, evidence of IL-1β upregulation in IEC-HS,8 use in sHLH, increasing use in CRS, and familiarity of the committee with its use in this context. Additionally, there is emerging evidence that anakinra may treat or prevent ICANS,10,104,105 for which prospective studies are ongoing. Compared to other therapeutic options, given its evolving role in treatment of CAR T cell associated toxicities in general, it was considered as a reasonable initial strategy although future prospective efforts will be needed to evaluate its role, optimal timing of initiation, dosing and duration.
4.5.1.2. Corticosteroids
Additionally, corticosteroids were also considered an acceptable first-line agent for use with or without anakinra. Notably, steroids have more known side effects, including metabolic derangements, hypertension, and increased risk of infection, particularly fungal infection. There are also concerns about potential adverse effects of corticosteroid therapy on CAR T cell function and persistence,106,107 though most data suggest that short-term corticosteroids can be utilized in the treatment of complications including CRS and ICANS without a definite increase in relapse rates.56,108 The dosing of corticosteroids was based on expert opinion and consensus, with the general approach that lower doses be used initially, with up-titration for increasing disease severity. In the absence of evidence, doses are suggested over a wide range, with increased dosage likely to be required in more severe cases.
4.5.1.3. Ruxolitinib
For patients in whom additional agents are needed (e.g., with worsening inflammatory parameters and/or with evidence of progressive end-organ involvement) to treat IEC-HS, second-line options included ruxolitinib based on use in HSCT and in the treatment for HLH. It was considered a reasonable therapeutic option following anakinra and corticosteroids, but not suggested as a first-line approach largely due to the lack of experience and potential risk of exacerbating cytopenias and infection (i.e., viral reactivation).109 As ruxolitinib levels increase with renal dysfunction and with concurrent CYP3A4 inhibitors, including antifungal agents (i.e., azoles), appropriate dose reductions must be made.
4.5.1.4. Additional considerations in the treatment of IEC-HS
Beyond these initial recommendations, there was either no consensus, or highly divergent opinions. Thereby, we present additional options as an important part of the discussion—but strongly encourage patient-specific considerations when IEC-HS is rapidly worsening and if alternate agents are needed—as a “best approach” is lacking. We also strongly encourage evaluation for alternative etiologies that may be contributing to the clinical presentation as this may also play a critical role in guiding management.
4.5.1.4.1. T cell targeted therapies
In rapidly progressive or life-threatening high-grade IEC-HS, T cell targeted therapies may be considered, after first- and second-line therapies have failed. In these cases, it is understood that the patient’s survival takes precedence over the longevity of CAR T cells. In patients with targetable safety switches (e.g., cetuximab for truncated-EGFR based constructs), turning “off” the CAR T cell with directed specificity should be considered. When this is not an option, due to the large amount of data available for etoposide in both primary and sHLH, etoposide is the preferred T cell depleting agent over alternate strategies (i.e., alemtuzumab) which may be more immunosuppressive or where clinical data (e.g., with dasatinib) is sparse.110,111 Importantly, we specifically advise against the use of intensive etoposide as per HLH-94 induction therapy (i.e., 150 mg/m2 twice weekly). Rather, etoposide may be best utilized as a one-time, moderate dose of 50–100mg/m2 with subsequent monitoring for response with consideration for re-dosing on a once weekly basis.84,112 With the limited use of low-dose etoposide used once or twice, we do not anticipate secondary malignancies—which with even extended and extensive use, the risk remains low (0.3%−3.8%).113–117 Given its high degree of efficacy in pHLH, rationale for use with IEC-HS, and a large evidence base compared to the other options presented here, etoposide may have a role as an alternate therapy in life-threatening, refractory IEC-HS—as has been recently reported on,118 but its optimal utilization was heavily debated without reaching a clear consensus. With concurrent leukemia as a contributing factor for HLH-like manifestations, low-dose etoposide may also provide benefit in simultaneously targeting underlying disease. Its impact on CAR T cells warrants further study.
4.5.1.4.2. Targeting of IFNγ
Due to the small but expanding body of literature showing the importance of IFNγ in CAR T cell-associated toxicities,8,119–122 and that blockade may not impede CAR T cell efficacy in a preclinical model,120 emapalumab can be considered as a possible agent for life-threatening IEC-HS. However, literature supporting use of emapalumab is limited to a small pediatric pHLH cohort and cost and procurement under urgent circumstances may be prohibitive. Use of emapalumab in adults with IEC-HS is extrapolative at best, leading to ongoing debate for its use. If elevations in IFNγ are confirmed in IE-CHS, emapalumab may present a rational choice, guided by patient-specific considerations.
4.5.1.4.3. Potential Alternative Agents for Treatment of IEC-HS
Based on growing understanding of cytokine profiles in CAR T cell-associated toxicities,120,122–124 HLH,125–127 and IEC-HS, theoretical mechanistic rationales exist for potential use of several additional agents—including those targeting T cells or a cytokine pathway. However, based on extremely limited evidence supporting use in IEC-HS, the working group could not recommend these agents, but instead point out their potential uses as salvage agents (Supplemental Table 3). In general, caution was advised with use of these agents, particularly long-acting drugs (e.g., alemtuzumab, basiliximab and antithymocyte globulin), based on elevated infectious risks, especially in patients who received other immunosuppressive drugs such as corticosteroids, ruxolitinib, etc. Additionally, despite established efficacy in CRS,128 use of tocilizumab or other IL-6 blocking agents (e.g., siltuximab) for IEC-HS, without CRS manifestations, was specifically discouraged due to lack of evidence for efficacy in any HLH-related context, and since most patients will have already received tocilizumab prior to the onset of IEC-HS. Additionally, plasma IL-6 levels can be hard to interpret with IL-6 pathway blockade.8 Individual patient considerations may make use of these alternative agents appropriate in specific circumstances, but general use cannot be recommended at this time. Treatment of alternative etiologies (as discussed below) is also an important consideration.
4.5.2. Recommendations for Monitoring and Supportive Care in Patients with Known or Suspected IEC-HS
4.5.2.1. Initial Work-Up
IEC-HS can mimic a number of inflammatory conditions and major categories of alternative causes include malignancy (including malignancy-HLH), infection (including infection-induced sHLH), autoimmune diseases, and drug reactions.37,129,130 Additionally, depending on timing from IEC therapy, new onset or recurrent CRS should be considered as a possible etiology.
Expediency should be given to excluding the most common and dangerous concurrent diagnoses, including infection and/or progressive malignancy. Infectious causes are the most eminently intervenable processes, and routine workup for infection should be performed in patients with hyperinflammation after IEC therapy.129 This should include assessment for bacterial, viral reactivation or new infection, and fungal disease, in blood, urine, and sputum cultures, +/− sampling of other possible infectious sources (e.g., bronchoscopy, cerebrospinal fluid), as clinically indicated. Additional efforts to exclude oncologic progression and/or malignancy associated-HLH are also prudent (e.g., bone marrow biopsy, flow cytometry, PET-CT).
Beyond ferritin, the effect of IEC therapy on classic HLH diagnostic parameters like soluble CD25, NK-cell function, triglycerides, IFNγ, CXCL9 ratio, CXCL10, IL-10, IL-18 remain to be seen. Current data is lacking on their utility in differentiating IEC-HS from alternative etiologies.8,37,76,131 Furthermore, inadequate lymphocyte recovery may impair utility of any lymphocyte-based assays and functional NK deficiency has not been reported in IEC-HS patients.8 Clearly further study is warranted.
4.5.2.2. Monitoring strategies (Table 4)
Table 4.
Supportive Care Considerations in Patients Who Develop IEC-HS.
Monitoring:
| |||
| 4a. Cytopenias and coagulopathy | |||
| Cytopenias |
|
||
| Coagulopathy |
|
||
| 4b. Infections | |||
| Infectious Disease^ Considerations | Immunosuppressive therapy | Associated infection risk | Prophylaxis/preemptive therapy*129,150 and monitoring |
| Steroids (e.g., dexamethasone, methylprednisolone) |
Fungal infection, viral reactivation, PJP | Mold active antifungal HSV prophylaxis (if seropositive) CMV pre-emptive therapy Consider weekly monitoring for viral reactivation (e.g., CMV) |
|
| IL-1 Receptor Antagonist (anakinra) | No specific infections described with single-agent use. In combination with other agents (e.g., tocilizumab, corticosteroids), risk of infection may be high.105,151,152 |
Recommend infectious disease consultation to guide optimal management Consider: Mold active antifungal HSV prophylaxis (if seropositive) CMV pre-emptive therapy Consider weekly monitoring for viral reactivation (e.g., CMV) Recommendations will depend upon the adjunctive agents used in combination with anakinra |
|
| IL-6 Receptor Antagonist (tocilizumab) | Tuberculosis, invasive fungal, bacterial, viral, protozoal | Mold active antifungal HSV prophylaxis (if seropositive) CMV pre-emptive therapy153 Consider bacterial prophylaxis during neutropenia |
|
| JAK 1 / 2 Inhibitors (ruxolitinib) | Tuberculosis, herpes zoster, esophageal candidiasis, PJP, CMV, cryptococcal infections | Fungal prophylaxis154 PJP prophylaxis VZV prophylaxis (if seropositive) CMV pre-emptive therapy |
|
| Chemotherapy (etoposide) | Bacterial infections with neutropenia | Consider bacterial prophylaxis during neutropenia | |
HSV: herpes simplex virus, CMV: cytomegalovirus, VZV: varicella zoster virus PJP: pneumocystis jiroveci pneumonia
Agents used for anti-infective prophylaxis: mold active antifungals include voriconazole, posaconazole, isavuconazole; antivirals for HSV and VZV prophylaxis include acyclovir and valacyclovir; antivirals for CMV pre-emptive therapy include ganciclovir, valganciclovir and foscarnet; agents for PJP prophylaxis include TMP-SMX, atovaquone, pentamidine; antibacterial prophylaxis with levofloxacin is recommended.
This is not intended to be an exhaustive list, and management decisions should be made in consultation with an Infectious Diseases specialist.
Daily monitoring of complete blood cell count with differential, coagulation parameters (PT/PTT) and fibrinogen are recommended for patients with IEC-HS. We also recommend frequent (e.g., daily) evaluation for renal and hepatic dysfunction, particularly since these patients can rapidly progress and investigation of alternative etiologies may be indicated (see below).
4.5.2.3. Cytopenias
Cytopenias are a common manifestation and part of the diagnostic criteria of IEC-HS.9,48 Supportive care with transfusions is often required. Recombinant thrombopoietin can be considered given some evidence for improved outcomes in patients with HLH.132 In cases of persistent cytopenias, particularly in cases where IEC-HS directed therapy has been instituted, bone marrow assessments for evaluation for alternative etiologies, including assessment for viral infections, should be considered,. The use of granulocyte-colony stimulating factor (G-CSF) to maintain an absolute neutrophil count (ANC) ≥ 500 can be considered to mitigate from infectious complications but should be approached with caution given rare reports of G-CSF/GM-CSF mediated worsening of pHLH or sHLH.133,134 While further study is warranted, and emerging experience with use in CAR T cell therapy generally supports its safe implementation, how that applies in the setting of active IEC-HS needs to be evaluated.135,136 Lastly, although stem-cell boosts are increasingly being explored for treatment of non IEC-HS associated delayed cytopenias,137–139 its utilization may be best considered for persistent cytopenias after acute inflammation has resolved.
4.5.2.4. Coagulopathy
Patients with IEC-HS may develop severe hypofibrinogenemia and variable PT/INR and PTT prolongation and are at high risk for delayed, severe, and protracted bleeding implications. Characterization and management of IEC-HS (and CRS) associated coagulopathy is similar to approaches in pHLH/sHLH with particular attention to fibrinogen replacement.140,141
4.5.2.5. Organ Dysfunction
Organ dysfunction should be managed per standard of care with incorporation of specific organ-specific consultants as dictated by severity. Cardiac dysfunction can also occur and appropriate evaluations (e.g., echocardiogram and ECG) should be performed as clinically indicated. Pulmonary failure in IEC-HS can have phenotypic overlap with CRS with fluid overload or pulmonary edema secondary to capillary leak and/or underlying infection—and a broad differential should be explored. Additionally, given the potential for overlap of ICANS with CNS manifestations of HLH,142,143 monitoring for neurotoxicity should continue; neurologic manifestations attributed IEC-HS are unknown at present.
4.2.2.3. Infection Prophylaxis and Management
As the risk of infection in patients with IEC-HS incrementally increases with additive use of immunosuppression, infectious disease consultation is strongly encouraged—particularly with clinical deterioration. Considerations should include 1) role for empiric management; 2) evaluation to rule out infectious causes of sHLH (discussed below); 3) prophylactic antimicrobials in those with IEC-HS and 4) pre-emptive monitoring for infections (Table 5b). Recommendations should incorporate considerations of prior infectious exposures, concomitant, or prior use of corticosteroids/tocilizumab for CRS/ICANS, results of pre-CAR T cell infectious disease evaluations, and risk of infection based on degree of immunosuppression in an immunocompromised host. Empiric management includes obtaining blood cultures with new fever, change or worsening clinical status, and with increasing immunosuppression, even with absence of fever particularly when patients are on immunosuppressive therapy. Empiric antibiotics for fever and neutropenia should be per institutional guidelines, with use of definitive treatment for identified infections. Prophylactic and pre-emptive antimicrobials should be selected based on underlying and/or additive risks of additional immunosuppression.
Table 5.
Diagnostic alternatives to IEC-HS
| Infectious | Neoplastic | Other |
|---|---|---|
Bacterial infections
|
Progressive underlying disease Second malignant neoplasm EBV-PTLD |
CRS Budd-Chiari Pulmonary Emboli Drug induced liver injury DRESS |
Viral Infections
| ||
Fungal
| ||
Vector borne
| ||
Parasites
| ||
Mycobacterial
|
Abbreviations: CMV: cytomegalovirus; COVID-19: coronavirus-disease of 2019; CRS: cytokine release syndrome; DRESS: drug rash with eosinophilia and systemic symptoms; EBV: Epstein-Barr virus; HHV-6: human herpes virus-type 6; HAV: hepatitis A virus; HBV: hepatitis B virus; HCV: hepatitis C virus; HEV: hepatitis E virus; HIV: human immunodeficiency virus; HSV: herpes simplex virus; PTLD: post-transplant lymphoproliferative disease; VZV: varicella zoster virus. BOLD font indicates those that are most likely of concern
5. Alternative Etiologies (Table 5)
Given the mortality associated with IEC-associated hyperinflammation, it is imperative to identify and exclude eminently treatable/reversible non-IEC-HS conditions. Frustratingly, many alternate diagnoses are also capable of independently driving HLH-like manifestations or heighten IEC-related toxicities, including progressive disease. Ascertaining the underlying etiology or etiologies is critical to intervening with appropriate medical remedies, especially among patients who may be receiving added immunosuppressive therapy to treat existing IEC toxicity.
5.4. Additional evaluations
Additional workup for less common etiologies of non-IEC-HS conditions beyond the initial evaluations should be driven by history, exam, laboratory data and known risks based on the level and duration of immunosuppression, alongside patient-specific risk factors.129 As the most common clinical presentations of IEC-HS consists of coagulopathy, liver injury, and/or cytopenias (though additional manifestations may include hypoxia and/or renal insufficiency), focused evaluation, in addition to standard workup, for these presentations may be pursued.
In our clinical experience, a liver injury pattern is present in an overwhelming majority of IEC-HS cases and the absence of this parameter should prompt consideration of alternate diagnoses. However, when a liver injury pattern is the overwhelmingly predominant feature, this should trigger evaluation for viral hepatitis, hepatic vascular and parenchymal assessment via imaging, and a re-evaluation of hepatotoxic medications and associated interactions for possible drug-induced liver injury (DILI) or drug reaction with eosinophilia and systemic symptoms (DRESS). Coagulopathy should trigger a full assessment of coagulation parameters, vitamin deficiency, possible infections/sepsis, and drug interactions. Additional studies for cytopenias beyond the initial workup may include assessments for parvovirus, vitamin deficiencies, a bone marrow biopsy, and consideration of expanded infectious etiologies or medication-induced cytopenias.
Furthermore, as infection remains a leading alternative diagnosis for non-IEC-HS, it is prudent to determine patient-specific risk factors for less common infectious diseases (e.g., risk based on travel history). Specific clinical historical parameters can direct testing and multidisciplinary consultation is recommended for appropriate diagnosis, testing, and treatment. Finally, while unmasking of pHLH is theoretically possible to coincide with the immunologic challenge mounted by IEC therapy, we do not feel that routine germline is warranted except in atypical cases with exceptionally high clinical suspicion.
Future Directions
With enhanced recognition of IEC-HS, critical next steps include both retrospectively and prospectively characterizing patient cohorts to distinguish those with severe CRS complicated by HLH-like manifestations from those with delayed IEC-HS and better understanding the natural history of both presentations. How these manifestations vary across different IEC-based therapies (i.e., bispecific T cell engagers versus CAR T cells) is also an area of future research. A deeper exploration into the biology will be critical—particularly as we try to understand the best treatment approaches. Lastly, understanding risk factors for IEC-HS—including analysis of baseline clinical characteristics and inflammatory profiles and/or genetic predisposition will help to elucidate potentially modifiable factors to improve outcomes.
Conclusion
IEC-HS is a life-threatening complication of IEC therapy. Current diagnostic approaches, understanding of the pathophysiology, grading schema and management represent areas of unmet need. With the overall goal to improve patient outcomes, we provide a uniform approach to identification of IEC-HS as an HLH-like syndrome, apply a grading schema that is guided by practical considerations and informed by patient acuity, and discuss underlying biology. We also delineate potential treatment approaches for IEC-HS and endorse evaluations for alternate etiologies, particularly in those whose course is rapidly declining. By collectively acknowledging IEC-HS as a hyperinflammatory toxicity we can embark on further study of the pathophysiology underlying this emergent toxicity and make strides towards a more comprehensive assessment and treatment approach.
Supplementary Material
Table 1. Primary (genetic) HLH and associated genes
Table 2. Treatment approaches for primary and secondary HLH
Table 3. Potential alternative/salvage therapies for IEC-HS
Figure 1. Reported incidence of specific HLH-like manifestations following CAR T cell therapy across 16 publications. 9 of the 16 publications classifying HLH-like toxicity following CAR T cell infusion reported individual patient manifestations. For Supplemental Figures 1A–H, the frequency of coagulopathy, cytopenia, hemophagocytosis, hyperferritinemia, hypertension, hypofibrinogenemia, hypotension, and infection post CAR T cell infusion are reported. Given that some manifestations were either not reported or not considered in a publication, the sample size for each manifestation is different. Furthermore, threshold values for each manifestation are respective to the criteria used in each publication.
Highlights.
Hemophagocytic lymphohistiocytosis (HLH)-like toxicities occur after CAR T cells
This is now termed immune effector cell (IEC) associated HLH-like syndrome (IEC-HS)
Independent of cytokine release syndrome (CRS) and neurotoxicity, IEC-HS can be fatal
Consensus for identification and grading of IEC-HS has been developed by experts
Treatment, supportive care, and future research are imperative to improving outcomes of IEC-HS
Acknowledgements
The authors would like to acknowledge Anna Hawkshead, ASTCT Education and Board Relations Senior Coordinator for her support of this effort through the ASTCT Committee on Cellular Therapy.
Disclosures/Conflicts of Interest
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and the Warren Grant Magnuson Clinical Center (ZIA BC 011823, N. Shah).
Dr. Nirali N Shah receives royalties from Cargo, Inc and has participated in Advisory Boards for Sobi and VOR.
Dr. Tania Jain receives institutional research support from CTI Biopharma, SyneosHealth, Incyte; Advisory board participation with Care Dx, Bristol Myers Squibb, Incyte, Abbvie, CTI, and Kite.
Dr. Perales reports honoraria from Adicet, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene, and Sellas Life Sciences, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune and Omeros. He has received institutional research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis.
Dr Henter serves as a consultant for Sobi.
Dr. Michael Jain reports honoraria from Kite/Gilead, BMS, Novartis, and Myeloid Therapeutics. He reports research funding from Kite/Gilead and Incyte.
Dr. JA Hill reports honoraria from Gilead Sciences, Pfizer/Amplyx, Allovir, Allogene therapeutics, CRISPR therapeutics, CSL Behring, OptumHealth, Octapharma, Karius, and Takeda and research funding from Takeda, Allovir, Karius, Merck, Deverra, and Gilead Sciences, all unrelated to this manuscript. He reports no other relevant COI
Dr. Maron reports research funding from Astellas Inc and Symbio Pharma Inc, both unrelated to this manuscript
Dr. Hines receives institutional funding from Incyte for a clinical trial utilizing ruxolitinib for HLH.
Dr. Neelapu received research support from Kite/Gilead, BMS, Cellectis, Poseida, Allogene, Unum Therapeutics, Precision Biosciences, and Adicet Bio; served as Advisory Board Member/Consultant for Kite/Gilead, Merck, Novartis, Sellas Life Sciences, Athenex, Allogene, Incyte, Adicet Bio, BMS, Legend Biotech, Bluebird Bio, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, Morphosys, Janssen, Chimagen, ImmunoACT, and Orna Therapeutics; has received royalty income from Takeda Pharmaceuticals; has stock options from Longbow Immunotherapy, Inc; and has intellectual property related to cell therapy.
Dr. Nirav Shah reports participation on advisory boards and/or consultancy for Kite Pharma, BMS, TG therapeutics, Miltenyi Biotec, Lilly Oncology, Epizyme, Incyte, Novartis, Seattle Genetics, and Umoja. He has research funding and honoraria from Lilly Oncology and Miltenyi Biotec. In addition, N.S. is on a scientific advisory board for Tundra Therapeutics. These relationships are all unrelated to this manuscript
Dr Sairah Ahmed has research support to institution for clinical trials from Seattle Genetics, Merck, Xencor, Chimagen and Tessa Therapeutics, has membership on Tessa Therapeutic’s and Chimagen scientific advisory committee, she serves on Data Safety Monitoring Board for Myeloid Therapeutics; she is a consultant for ADC therapeutics, KITE/Gilead
Dr. Matthew Frigault reports consulting/honoraria from Kite/Gilead, Novartis, BMS, JnJ/Legend, Incyte, and Arcellx.
Dr. Mahadeo has served as a consultant and has received research support from Atara Biotherapuetics and Jazz.
Dr. Komanduri has served as an ad hoc consultant for Kite/Gilead, BMS, Novartis, Adaptimmune, Autolus, Janssen, Genentech, Cargo Therapeutics, Takeda, CRISPR, Incyte, Optum Health and the Bill and Melinda Gates Foundation. He serves on scientific advisory boards for Avacta Therapeutics and Aegle Therapeutics and as a voluntary member of the Board of Directors of the National Marrow Donor Program.
Dr. Frank has participated in Advisory Boards for Kite/Gilead and Cargo Inc and received research support from Adaptive Biotechnology and Allogene Biotechnologies
Dr. Nikiforow reports participation in ad hoc advisory boards for A2 Bio, GlaxoSmithKline, Iovance, Kite/Gilead and Sobi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Canna SW, Marsh RA. Pediatric hemophagocytic lymphohistiocytosis. Blood. 2020;135(16):1332–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Rosee P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477. [DOI] [PubMed] [Google Scholar]
- 3.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Crit Care Med. 2017;45(2):e124–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teachey DT, Lacey SF, Shaw PA, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6(6):664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121(26):5154–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy VE, Wong C, Huang CY, et al. Macrophage activation syndrome-like (MAS-L) manifestations following BCMA-directed CAR T cells in multiple myeloma. Blood Adv. 2021;5(23):5344–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenstein DA, Schischlik F, Shao L, et al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells. Blood. 2021;138(24):2469–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hines MR, Keenan C, Maron Alfaro G, et al. Hemophagocytic lymphohistiocytosis-like toxicity (carHLH) after CD19-specific CAR T-cell therapy. Br J Haematol. 2021;194(4):701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreyzin A, Jacobsohn D, Angiolillo A, et al. Intravenous anakinra for tisagenlecleucel-related toxicities in children and young adults. Pediatr Hematol Oncol. 2022;39(4):370–378. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Rojas RM, Gomez-Centurion I, Bailen R, et al. Hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) following treatment with tisagenlecleucel. Clin Case Rep. 2022;10(1):e05209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter TJ, Lazarevic A, Ziggas JE, et al. Hyperinflammatory syndrome resembling haemophagocytic lymphohistiocytosis following axicabtagene ciloleucel and brexucabtagene autoleucel. Br J Haematol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Major A, Collins J, Craney C, et al. Management of hemophagocytic lymphohistiocytosis (HLH) associated with chimeric antigen receptor T-cell (CAR-T) therapy using anti-cytokine therapy: an illustrative case and review of the literature. Leuk Lymphoma. 2021;62(7):1765–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashmi H, Bachmeier C, Chavez JC, et al. Haemophagocytic lymphohistiocytosis has variable time to onset following CD19 chimeric antigen receptor T cell therapy. Br J Haematol. 2019;187(2):e35–e38. [DOI] [PubMed] [Google Scholar]
- 16.FDA US. ABECMA (idecabtagene vicleucel) package insert. 2021.
- 17.FDA US. CARVYKTI (Ciltacabtagene autoleucel) package insert. 2022.
- 18.Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125(19):2908–2914. [DOI] [PubMed] [Google Scholar]
- 19.Marsh RA, Haddad E. How i treat primary haemophagocytic lymphohistiocytosis. Br J Haematol. 2018;182(2):185–199. [DOI] [PubMed] [Google Scholar]
- 20.Sepulveda FE, de Saint Basile G. Hemophagocytic syndrome: primary forms and predisposing conditions. Curr Opin Immunol. 2017;49:20–26. [DOI] [PubMed] [Google Scholar]
- 21.Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66(11):e27929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canna SW, de Jesus AA, Gouni S, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46(10):1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbosa MD, Nguyen QA, Tchernev VT, et al. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature. 1996;382(6588):262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinn IK, Eckstein OS, Peckham-Gregory EC, et al. Genetic and mechanistic diversity in pediatric hemophagocytic lymphohistiocytosis. Blood. 2018;132(1):89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coffey AJ, Brooksbank RA, Brandau O, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20(2):129–135. [DOI] [PubMed] [Google Scholar]
- 27.Nichols KE, Harkin DP, Levitz S, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95(23):13765–13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayos J, Wu C, Morra M, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395(6701):462–469. [DOI] [PubMed] [Google Scholar]
- 29.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286(5446):1957–1959. [DOI] [PubMed] [Google Scholar]
- 30.Parekh C, Hofstra T, Church JA, Coates TD. Hemophagocytic lymphohistiocytosis in children with chronic granulomatous disease. Pediatr Blood Cancer. 2011;56(3):460–462. [DOI] [PubMed] [Google Scholar]
- 31.Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin Proc. 2014;89(4):484–492. [DOI] [PubMed] [Google Scholar]
- 32.Riviere S, Galicier L, Coppo P, et al. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med. 2014;127(11):1118–1125. [DOI] [PubMed] [Google Scholar]
- 33.Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. [DOI] [PubMed] [Google Scholar]
- 34.Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620. [DOI] [PubMed] [Google Scholar]
- 35.Canna SW, Behrens EM. Not all hemophagocytes are created equally: appreciating the heterogeneity of the hemophagocytic syndromes. Curr Opin Rheumatol. 2012;24(1):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schram AM, Campigotto F, Mullally A, et al. Marked hyperferritinemia does not predict for HLH in the adult population. Blood. 2015;125(10):1548–1552. [DOI] [PubMed] [Google Scholar]
- 37.Setiadi A, Zoref-Lorenz A, Lee CY, Jordan MB, Chen LYC. Malignancy-associated haemophagocytic lymphohistiocytosis. Lancet Haematol. 2022;9(3):e217–e227. [DOI] [PubMed] [Google Scholar]
- 38.Valade S, Monseau G, Mariotte E, Darmon M. Diagnostic Performance of Hemophagocytic Lymphohistiocytosis Criteria and HScore in Critically Ill Patients With Severe Hemophagocytic Syndrome. Crit Care Med. 2021;49(9):e874–e879. [DOI] [PubMed] [Google Scholar]
- 39.Knaak C, Nyvlt P, Schuster FS, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116(19):3875–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diorio C, Shraim R, Myers R, et al. Comprehensive Serum Proteome Profiling of Cytokine Release Syndrome and Immune Effector Cell-Associated Neurotoxicity Syndrome Patients with B-Cell ALL Receiving CAR T19. Clin Cancer Res. 2022;28(17):3804–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. [DOI] [PubMed] [Google Scholar]
- 46.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNerney KO LS, Ishikawa K, Dreyzin A, Vatsayan A, Chen JJ, Baggott C, Prabhu S, Pacenta H, Phillips CL, Rossoff J, Stefanski HE, Talano JA, Moskop A, Verneris M, Myers D, Karras NA, Brown P, Qayed M, Hermiston M, Satwani P, Krupski C, Keating AK, Baumeister S, Fabrizio VA, Chinnabhandar V, Egeler E, Mavroukakis S, Curran KJ, Mackall CL, Laetsch TW, Schultz LM. Chimeric Antigen Receptor T-cell-Associated Hemophagocytic Lymphohistiocytosis (carHLH) Predicts Poor Survival with Real-World Use of Tisagenlecleucel for B-ALL. Preprints with The Lancet: SSRN; 2022:32. [Google Scholar]
- 48.Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J Clin Oncol. 2020;38(17):1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faramand R, Jain M, Staedtke V, et al. Tumor Microenvironment Composition and Severe Cytokine Release Syndrome (CRS) Influence Toxicity in Patients with Large B-Cell Lymphoma Treated with Axicabtagene Ciloleucel. Clin Cancer Res. 2020;26(18):4823–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burleigh KKA, Long A, Jensen MC, Masters S, Vince JE, Webb-Robertson BJ, Gardner RA, Gustafson H. Poor Clinical Outcome in Pediatric Immunotherapy is Mediated by a Pre-Existing Overactive IL-18-IFNg Immune Phenotype. Blood. Vol. 138; 2021. [Google Scholar]
- 51.Ahmed SFF, Strati P, Westin J, Fayad L, Hagemeister FB, Lee HJ, Iyer SP, Nair R, Nastoupil LJ, Parmar S, Rodriguez MA, Samaniego F, Steiner R, Wang M, Pinnix CC, Flowers C, Horowitz SB, Hawkins M, Neelapu SS. Haemophagocytic lymphohistiocytosis (HLH) in patients with large B-cell lymphoma treated with standard of care (SOC) axicabtagene ciloleucel (Axi-cel). Journal of Clinical Oncology. Vol. 38; 2020. [Google Scholar]
- 52.Zoref-Lorenz A, Murakami J, Hofstetter L, et al. An improved index for diagnosis and mortality prediction in malignancy-associated hemophagocytic lymphohistiocytosis. Blood. 2022;139(7):1098–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandler RD, Tattersall RS, Schoemans H, et al. Diagnosis and Management of Secondary HLH/MAS Following HSCT and CAR-T Cell Therapy in Adults; A Review of the Literature and a Survey of Practice Within EBMT Centres on Behalf of the Autoimmune Diseases Working Party (ADWP) and Transplant Complications Working Party (TCWP). Front Immunol. 2020;11:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim DW, Bukhari A, Lutfi F, et al. Low utility of the H-Score and HLH-2004 criteria to identify patients with secondary hemophagocytic lymphohistiocytosis after CAR-T cell therapy for relapsed/refractory diffuse large B-Cell lymphoma. Leuk Lymphoma. 2022;63(6):1339–1347. [DOI] [PubMed] [Google Scholar]
- 55.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019;134(24):2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maron GM, Hijano DR, Epperly R, et al. Infectious Complications in Pediatric, Adolescent and Young Adult Patients Undergoing CD19-CAR T Cell Therapy. Front Oncol. 2022;12:845540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masih KE, Ligon JA, Yates B, et al. Consequences of hemophagocytic lymphohistiocytosis-like cytokine release syndrome toxicities and concurrent bacteremia. Pediatr Blood Cancer. 2021;68(10):e29247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishii K, Pouzolles M, Chien CD, et al. Perforin-deficient CAR T cells recapitulate late-onset inflammatory toxicities observed in patients. J Clin Invest. 2020;130(10):5425–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neelapu SS, Tummala S, Kebriaei P, et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL’. Nat Rev Clin Oncol. 2018;15(4):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henter JI, Samuelsson-Horne A, Arico M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367–2373. [DOI] [PubMed] [Google Scholar]
- 67.Palmblad K, Schierbeck H, Sundberg E, et al. Therapeutic administration of etoposide coincides with reduced systemic HMGB1 levels in macrophage activation syndrome. Mol Med. 2021;27(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersson U Hyperinflammation: On the pathogenesis and treatment of macrophage activation syndrome. Acta Paediatr. 2021;110(10):2717–2722. [DOI] [PubMed] [Google Scholar]
- 69.Johnson TS, Terrell CE, Millen SH, Katz JD, Hildeman DA, Jordan MB. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol. 2014;192(1):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehmberg K, Ledig S, Wustrau K, et al. Etoposide for Primary HLH- Better Than Its Reputation. Abstracts from the 38th Annual Meeting of the Histiocyte Society Held Virtually and In Person in Stockholm, Sweden from September 18–20, 2022. Pediatric Blood & Cancer. 2023;70(S1):e30097; 30528. [Google Scholar]
- 71.Lucchini G, Marsh R, Gilmour K, et al. Treatment dilemmas in asymptomatic children with primary hemophagocytic lymphohistiocytosis. Blood. 2018;132(19):2088–2096. [DOI] [PubMed] [Google Scholar]
- 72.Marsh RA, Allen CE, McClain KL, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013;60(1):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moshous D, Briand C, Castelle M, et al. Alemtuzumab as First Line Treatment in Children with Familial Lymphohistiocytosis. Blood. 2019;134(Supplement_1):80–80. [Google Scholar]
- 74.Mahlaoui N, Ouachee-Chardin M, de Saint Basile G, et al. Immunotherapy of familial hemophagocytic lymphohistiocytosis with antithymocyte globulins: a single-center retrospective report of 38 patients. Pediatrics. 2007;120(3):e622–628. [DOI] [PubMed] [Google Scholar]
- 75.Henter JI, von Bahr Greenwood T, Bergsten E. Emapalumab in Primary Hemophagocytic Lymphohistiocytosis. N Engl J Med. 2020;383(6):596–598. [DOI] [PubMed] [Google Scholar]
- 76.Locatelli F, Jordan MB, Allen C, et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N Engl J Med. 2020;382(19):1811–1822. [DOI] [PubMed] [Google Scholar]
- 77.Hines MR, von Bahr Greenwood T, Beutel G, et al. Consensus-Based Guidelines for the Recognition, Diagnosis, and Management of Hemophagocytic Lymphohistiocytosis in Critically Ill Children and Adults. Crit Care Med. 2022;50(5):860–872. [DOI] [PubMed] [Google Scholar]
- 78.Hayden A, Park S, Giustini D, Lee AY, Chen LY. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: A systematic scoping review. Blood Rev. 2016;30(6):411–420. [DOI] [PubMed] [Google Scholar]
- 79.Arca M, Fardet L, Galicier L, et al. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: impact of triggering disease and early treatment with etoposide. Br J Haematol. 2015;168(1):63–68. [DOI] [PubMed] [Google Scholar]
- 80.Imashuku S, Kuriyama K, Sakai R, et al. Treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis (EBV-HLH) in young adults: a report from the HLH study center. Med Pediatr Oncol. 2003;41(2):103–109. [DOI] [PubMed] [Google Scholar]
- 81.Song Y, Wang Y, Wang Z. Requirement for etoposide in the initial treatment of Epstein-Barr virus-associated haemophagocytic lymphohistiocytosis. Br J Haematol. 2019;186(5):717–723. [DOI] [PubMed] [Google Scholar]
- 82.Kogawa K, Sato H, Asano T, et al. Prognostic factors of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in children: report of the Japan Histiocytosis Study Group. Pediatr Blood Cancer. 2014;61(7):1257–1262. [DOI] [PubMed] [Google Scholar]
- 83.Song Y, Wang J, Wang Y, Wu L, Wang Z. Requirement for containing etoposide in the initial treatment of lymphoma associated hemophagocytic lymphohistiocytosis. Cancer Biol Ther. 2021;22(10–12):598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horne A, von Bahr Greenwood T, Chiang SCC, et al. Efficacy of Moderately Dosed Etoposide in Macrophage Activation Syndrome-Hemophagocytic Lymphohistiocytosis. J Rheumatol. 2021;48(10):1596–1602. [DOI] [PubMed] [Google Scholar]
- 85.Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93(2):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phadke O, Rouster-Stevens K, Giannopoulos H, Chandrakasan S, Prahalad S. Intravenous administration of anakinra in children with macrophage activation syndrome. Pediatr Rheumatol Online J. 2021;19(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ravelli A, Davi S, Minoia F, Martini A, Cron RQ. Macrophage Activation Syndrome. Hematol Oncol Clin North Am. 2015;29(5):927–941. [DOI] [PubMed] [Google Scholar]
- 89.Stoll ML, Cron RQ. Treatment of juvenile idiopathic arthritis: a revolution in care. Pediatr Rheumatol Online J. 2014;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gregory J, Greenberg J, Basu S. Outcomes Analysis of Children Diagnosed With Hemophagocytic Lymphohistiocytosis in the PICU. Pediatr Crit Care Med. 2019;20(4):e185–e190. [DOI] [PubMed] [Google Scholar]
- 91.Charlesworth JEG, Wilson S, Qureshi A, et al. Continuous intravenous anakinra for treating severe secondary haemophagocytic lymphohistiocytosis/macrophage activation syndrome in critically ill children. Pediatr Blood Cancer. 2021;68(9):e29102. [DOI] [PubMed] [Google Scholar]
- 92.Wohlfarth P, Agis H, Gualdoni GA, et al. Interleukin 1 Receptor Antagonist Anakinra, Intravenous Immunoglobulin, and Corticosteroids in the Management of Critically Ill Adult Patients With Hemophagocytic Lymphohistiocytosis. J Intensive Care Med. 2019;34(9):723–731. [DOI] [PubMed] [Google Scholar]
- 93.Henderson LA, Degar BA. HLH treatment: smarter, not harder. Blood. 2022;139(24):3453–3455. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Q, Zhao YZ, Ma HH, et al. A study of ruxolitinib response-based stratified treatment for pediatric hemophagocytic lymphohistiocytosis. Blood. 2022;139(24):3493–3504. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, Wang Y, Wu L, et al. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica. 2020;105(5):e210–e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J, Zhang R, Wu X, et al. Ruxolitinib-combined doxorubicin-etoposide-methylprednisolone regimen as a salvage therapy for refractory/relapsed haemophagocytic lymphohistiocytosis: a single-arm, multicentre, phase 2 trial. Br J Haematol. 2021;193(4):761–768. [DOI] [PubMed] [Google Scholar]
- 97.Kim JY, Kim M, Park JK, Lee EB, Park JW, Hong J. Limited efficacy of tocilizumab in adult patients with secondary hemophagocytic lymphohistiocytosis: a retrospective cohort study. Orphanet J Rare Dis. 2022;17(1):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dufranc E, Del Bello A, Belliere J, Kamar N, Faguer S, group Ts. IL6-R blocking with tocilizumab in critically ill patients with hemophagocytic syndrome. Crit Care. 2020;24(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12(5):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grom AA, Bachir J, Asnaghi V, De Benedetti F. Trials in Progress: A Two-Cohort, Open-Label, Single-Arm Study of Emapalumab, an Anti-Interferon Gamma (IFNγ) Monoclonal Antibody, in Patients with Macrophage Activation Syndrome (MAS) in Rheumatic Diseases. Blood. 2021;138(Supplement 1):4195–4195. [Google Scholar]
- 101.De Benedetti F, Grom A, Brogan P, et al. Emapalumab, an Anti-IFNγ Antibody in Patients with Macrophage Activation Syndrome (MAS) Complicating Systemic Juvenile Idiopathic Arthritis (sJIA) Who Had Failed High-Dose Glucocorticoids (GCs). Arthritis Rheumatol. 2021;73(American College of Rheumatology Meeting Abstracts). [Google Scholar]
- 102.Topp MS, van Meerten T, Houot R, et al. Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 2021;195(3):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oluwole OO, Bouabdallah K, Munoz J, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 2021;194(4):690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park JH, Sauter CS, Palomba ML, et al. A Phase II Study of Prophylactic Anakinra to Prevent CRS and Neurotoxicity in Patients Receiving CD19 CAR T Cell Therapy for Relapsed or Refractory Lymphoma. Blood. 2021;138(Supplement 1):96–96. [Google Scholar]
- 105.Wehrli M, Gallagher K, Chen YB, et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J Immunother Cancer. 2022;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strati P, Ahmed S, Furqan F, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137(23):3272–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu S, Deng B, Yin Z, et al. Corticosteroids do not influence the efficacy and kinetics of CAR-T cells for B-cell acute lymphoblastic leukemia. Blood Cancer J. 2020;10(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abedin S, McKenna E, Chhabra S, et al. Efficacy, Toxicity, and Infectious Complications in Ruxolitinib-Treated Patients with Corticosteroid-Refractory Graft-versus-Host Disease after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2019;25(8):1689–1694. [DOI] [PubMed] [Google Scholar]
- 110.Mestermann K, Giavridis T, Weber J, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med. 2019;11(499). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weber EW, Lynn RC, Sotillo E, Lattin J, Xu P, Mackall CL. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 2019;3(5):711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koyama M, Sawada A, Yasui M, Inoue M, Kawa K. Encouraging results of low-dose etoposide in the treatment of early-onset hemophagocytic syndrome following allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2007;86(5):466–467. [DOI] [PubMed] [Google Scholar]
- 113.Bergsten E, Horne A, Arico M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trottestam H, Horne A, Arico M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the Use of Etoposide-Based Therapy and Bone Marrow Transplantation for the Treatment of HLH: Consensus Statements by the HLH Steering Committee of the Histiocyte Society. J Allergy Clin Immunol Pract. 2018;6(5):1508–1517. [DOI] [PubMed] [Google Scholar]
- 116.Imashuku S, Teramura T, Kuriyama K, et al. Risk of etoposide-related acute myeloid leukemia in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Int J Hematol. 2002;75(2):174–177. [DOI] [PubMed] [Google Scholar]
- 117.Pui CH, Ribeiro RC, Hancock ML, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med. 1991;325(24):1682–1687. [DOI] [PubMed] [Google Scholar]
- 118.Peterlin P, Garnier A, Le Bourgeois A, et al. Dramatic Recovery after Etoposide Phosphate Infusion for Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome following Treatment with Tisagenlecleucel in a Young Patient with Relapsed Acute Lymphoblastic Leukemia: A Case Report. Acta Haematol. 2022;145(5):537–541. [DOI] [PubMed] [Google Scholar]
- 119.Srinagesh HK, Baird J, Patel S, et al. Proinflammatory Cytokines are Associated with CAR-22 Macrophage Activation Syndrome. Transplantation and Cellular Therapy, Official Publication of the American Society for Transplantation and Cellular Therapy. 2022;28(3):S162–S163. [Google Scholar]
- 120.Bailey SR, Vatsa S, Larson RC, et al. Blockade or Deletion of IFNgamma Reduces Macrophage Activation without Compromising CAR T-cell Function in Hematologic Malignancies. Blood Cancer Discov. 2022;3(2):136–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McNerney KO, DiNofia AM, Teachey DT, Grupp SA, Maude SL. Potential Role of IFNgamma Inhibition in Refractory Cytokine Release Syndrome Associated with CAR T-cell Therapy. Blood Cancer Discov. 2022;3(2):90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Larson RC, Kann MC, Bailey SR, et al. CAR T cell killing requires the IFNgammaR pathway in solid but not liquid tumours. Nature. 2022;604(7906):563–570. [DOI] [PubMed] [Google Scholar]
- 123.Hunter BD, Jacobson CA. CAR T-Cell Associated Neurotoxicity: Mechanisms, Clinicopathologic Correlates, and Future Directions. J Natl Cancer Inst. 2019;111(7):646–654. [DOI] [PubMed] [Google Scholar]
- 124.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bracaglia C, de Graaf K, Pires Marafon D, et al. Elevated circulating levels of interferon-gamma and interferon-gamma-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76(1):166–172. [DOI] [PubMed] [Google Scholar]
- 126.Xu XJ, Tang YM, Song H, et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012;160(6):984–990 e981. [DOI] [PubMed] [Google Scholar]
- 127.Henter JI, Elinder G, Soder O, Hansson M, Andersson B, Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78(11):2918–2922. [PubMed] [Google Scholar]
- 128.Le RQ, Li L, Yuan W, et al. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist. 2018;23(8):943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136(8):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7(12):814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maruoka H, Inoue D, Takiuchi Y, et al. IP-10/CXCL10 and MIG/CXCL9 as novel markers for the diagnosis of lymphoma-associated hemophagocytic syndrome. Ann Hematol. 2014;93(3):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y, Wang Z, Wu L, Zhang J, Wang J, Yan L. Recombinant human thrombopoietin is an effective treatment for thrombocytopenia in hemophagocytic lymphohistiocytosis. Ann Hematol. 2013;92(12):1695–1699. [DOI] [PubMed] [Google Scholar]
- 133.Padhi S, Varghese RG, Ramdas A, Phansalkar MD, Sarangi R. Hemophagocytic lymphohistiocytosis: critical reappraisal of a potentially under-recognized condition. Front Med. 2013;7(4):492–498. [DOI] [PubMed] [Google Scholar]
- 134.Wang S, Degar BA, Zieske A, Shafi NQ, Rose MG. Hemophagocytosis exacerbated by G-CSF/GM-CSF treatment in a patient with myelodysplasia. Am J Hematol. 2004;77(4):391–396. [DOI] [PubMed] [Google Scholar]
- 135.Miller KC, Johnson PC, Abramson JS, et al. Effect of granulocyte colony-stimulating factor on toxicities after CAR T cell therapy for lymphoma and myeloma. Blood Cancer J. 2022;12(10):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galli E, Allain V, Di Blasi R, et al. G-CSF does not worsen toxicities and efficacy of CAR-T cells in refractory/relapsed B-cell lymphoma. Bone Marrow Transplant. 2020;55(12):2347–2349. [DOI] [PubMed] [Google Scholar]
- 137.de Tena PS, Bailen R, Oarbeascoa G, et al. Allogeneic CD34-selected stem cell boost as salvage treatment of life-threatening infection and severe cytopenias after CAR-T cell therapy. Transfusion. 2022;62(10):2143–2147. [DOI] [PubMed] [Google Scholar]
- 138.Rejeski K, Burchert A, Iacoboni G, et al. Safety and feasibility of stem cell boost as a salvage therapy for severe hematotoxicity after CD19 CAR T-cell therapy. Blood Adv. 2022;6(16):4719–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mullanfiroze K, Lazareva A, Chu J, et al. CD34+-selected stem cell boost can safely improve cytopenias following CAR T-cell therapy. Blood Adv. 2022;6(16):4715–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Valade S, Joly BS, Veyradier A, et al. Coagulation disorders in patients with severe hemophagocytic lymphohistiocytosis. PLoS One. 2021;16(8):e0251216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Buechner J, Grupp SA, Hiramatsu H, et al. Practical guidelines for monitoring and management of coagulopathy following tisagenlecleucel CAR T-cell therapy. Blood Adv. 2021;5(2):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Debinski C, Goergen S, McLean C, et al. Exploring the Intersection of Isolated-CNS Hemophagocytic Lymphohistiocytosis and Pediatric Chronic Lymphocytic Inflammation With Pontine Perivascular Enhancement Responsive to Steroids. J Child Neurol. 2021;36(11):935–942. [DOI] [PubMed] [Google Scholar]
- 143.Blincoe A, Heeg M, Campbell PK, et al. Neuroinflammatory Disease as an Isolated Manifestation of Hemophagocytic Lymphohistiocytosis. J Clin Immunol. 2020;40(6):901–916. [DOI] [PubMed] [Google Scholar]
- 144.Fleischmann RM, Tesser J, Schiff MH, et al. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(8):1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22(2):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sanderson R, Kuhnl A, Tholouli E, et al. CAR-T Toxicity Management and Steroid Use in High-Grade B-Cell Lymphoma: Impact on Real-World Survival Outcomes in the UK. Blood. 2021;138(Supplement 1):531–531.33851211 [Google Scholar]
- 147.Haq M, Adnan G. Ruxolitinib. StatPearls. Treasure Island (FL); 2022. [Google Scholar]
- 148.Vlaar AP, Oczkowski S, de Bruin S, et al. Transfusion strategies in non-bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2020;46(4):673–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–1619. [DOI] [PubMed] [Google Scholar]
- 150.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Diorio C, Vatsayan A, Talleur AC, et al. Anakinra utilization in refractory pediatric CAR T-cell associated toxicities. Blood Adv. 2022;6(11):3398–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zurko JC, Johnson BD, Aschenbrenner E, et al. Use of Early Intrathecal Therapy to Manage High-Grade Immune Effector Cell-Associated Neurotoxicity Syndrome. JAMA Oncol. 2022;8(5):773–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hakki M, Aitken SL, Danziger-Isakov L, et al. American Society for Transplantation and Cellular Therapy Series: #3-Prevention of Cytomegalovirus Infection and Disease After Hematopoietic Cell Transplantation. Transplant Cell Ther. 2021;27(9):707–719. [DOI] [PubMed] [Google Scholar]
- 154.Dadwal SS, Hohl TM, Fisher CE, et al. American Society of Transplantation and Cellular Therapy Series, 2: Management and Prevention of Aspergillosis in Hematopoietic Cell Transplantation Recipients. Transplant Cell Ther. 2021;27(3):201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Primary (genetic) HLH and associated genes
Table 2. Treatment approaches for primary and secondary HLH
Table 3. Potential alternative/salvage therapies for IEC-HS
Figure 1. Reported incidence of specific HLH-like manifestations following CAR T cell therapy across 16 publications. 9 of the 16 publications classifying HLH-like toxicity following CAR T cell infusion reported individual patient manifestations. For Supplemental Figures 1A–H, the frequency of coagulopathy, cytopenia, hemophagocytosis, hyperferritinemia, hypertension, hypofibrinogenemia, hypotension, and infection post CAR T cell infusion are reported. Given that some manifestations were either not reported or not considered in a publication, the sample size for each manifestation is different. Furthermore, threshold values for each manifestation are respective to the criteria used in each publication.


