Abstract
Objective:
Limited pharmacokinetic (PK)/pharmacodynamic (PD) data is a barrier to the safe scale-up of dolutegravir-based antiretroviral therapy (ART) in children. We examined the PK/PD of the adult film-coated dolutegravir 50 mg tablets in children with human immunodeficiency virus (HIV) infection weighing at least 20 kg.
Design:
A prospective, observational pharmacokinetic and safety study
Methods:
Treatment-experienced children with HIV weighing ≥20 kg and evidence of viral load suppression on ART were enrolled and switched to dolutegravir-based therapy. After at least 4 weeks and 7 months on dolutegravir-based therapy, blood samples were collected at 0, 1, 4, 8, 12 and 24-hour post-dose. Dolutegravir concentrations were measured using validated LCMS/MS and PK parameters calculated by noncompartmental analysis. Descriptive statistics were used to summarize PK parameters and comparisons with published reference values.
Results:
Of 25 participants, 92% were on efavirenz-based ART and 60.0% were male. Dolutegravir mean exposure, peak and trough concentrations at both PK visits were higher than the mean reference values in adults and children weighing 20 kg to less than 40 kg treated with 50 mg once daily, but were closer to the mean values in adults given 50 mg twice a day. Children weighing 20 kg to less than 40 kg had even higher dolutegravir exposures. The regimens were well tolerated with good virologic efficacy through week 48.
Conclusions:
The higher dolutegravir exposure in our study population suggest that further studies and close monitoring should investigate the adverse effects of dolutegravir in more children and in the long term.
Keywords: Dolutegravir, adult tablets, treatment-experienced, pharmacokinetics, pharmacodynamics, children
Introduction
The global response to human immunodeficiency virus (HIV) epidemic is failing to meet the needs of children. According to UNAIDS, 52% of children living with HIV (CLWH) globally received antiretroviral therapy (ART) compared to 76% of adults with HIV in 2021, well short of the global target of 95% treatment coverage [1]. Children living with HIV have not fully benefited from the recent advances in ART because of the delays in developing age-appropriate formulations or establishing pharmacokinetic (PK)/pharmacodynamic (PD) data that will inform optimal dosing recommendations [2]. The highly potent and safer second-generation integrase strand transfer inhibitors (INSTIs) with higher barrier to emergence of drug resistance such as dolutegravir and bictegravir are the cornerstone of preferred ART regimens in adults in the United States (US) [3]. In 2018, World Health Organization (WHO) interim HIV treatment guidelines recommended the fixed-dose combination of tenofovir disoproxil fumarate/lamivudine/dolutegravir 300mg /300mg /50 mg (TLD) as the preferred first-line ART globally [4]. A subsequent systematic review reaffirmed that dolutegravir-based regimens led to higher viral load suppression, lower risk of treatment discontinuations and developing drug resistance mutations compared to efavirenz-based regimens in ART-naïve patients [5]. Thus, in July 2019, WHO updated the guidelines to recommend dolutegravir-containing regimens as the preferred first- and second-line ART for adults and adolescent living with HIV globally [5]. In addition, without supportive PK and safety data, the updated guidelines recommended the investigational adult dolutegravir dose of 50 mg/day for children weighing ≥20 kg [5].
Recently, data from the nested PK and safety sub-study of ODYSSEY trial that enrolled children at the Uganda and Zimbabwe sites showed that the adult film-coated dolutegravir 50 mg tablets given once daily achieved appropriate PK profiles in children weighing 20 kg to less than 40 kg with no safety signals [6]. The mean trough concentration (Ctrough) of 0.63 to 0.77 mg/L in the children (depending on weight-band) were comparable to the adult Ctrough reference value of 0.83 mg/L, and no participant had Ctrough below dolutegravir EC90 of 0.32 mg/L[6]. These findings allowed for the US Food and Drug Administration (FDA) and European Medicines Agency to approve the adult dolutegravir tablets for children weighing 20 kg or more. Current WHO consolidated guidelines now recommend dolutegravir-based regimens using existing adult formulations as first-line and second-line preferred ART for adults, adolescents and children with HIV weighing at least 20 kg [7]. While harmonization with adult treatment has allowed for rapid scale-up of dolutegravir-based regimens in a large population of children, more PK and safety data are needed in pediatric populations not studied to date. While the film-coated adults dolutegravir formulations are expected to be efficacious in most children, verification that PK profiles are comparable to references in adults is necessary. The current study sought to investigate the PK/PD of dolutegravir in Ghanaian children who switched from efavirenz- or lopinavir-ritonavir-based ART to TLD or dolutegravir 50 mg once daily plus 2 nucleoside reverse transcriptase inhibitors (NRTIs).
Methods:
Study population
A prospective observational PK and safety study was performed at Komfo Anokye Teaching Hospital in Ghana. Children and adolescents with HIV aged 6 to 17 years old and weighing at least 20 kg who were stable on ART with evidence of viral load suppression in the past 6 months were enrolled. All participants were naïve to dolutegravir or raltegravir. At the time of the study, Ghana National AIDS Control required ART-experienced patients to have HIV RNA < 1000 copies/mL on their current regimen to be eligible to switch to dolutegravir-based as pre-switch HIV drug resistance testing was not available to optimize the NRTI backbone. Exclusion criteria included body weight <20 kg at study entry, active opportunistic infection or pregnancy at screening visit. The Institutional Review Boards of Kwame Nkrumah University of Science and Technology (Ref # CHRPE/AP/478/21) and University of Florida (Ref # IRB202002216) reviewed and approved the study. All parents and guardians signed an informed consent. Signed assent was obtained from children older than 8 years old.
Antiretroviral regimen and study procedures
At study entry, a complete medical history, physical examination was performed and relevant data collected using standardized forms. Baseline tests included complete blood count (CBC), basic metabolic panel (BMP), liver function tests (LFTs), and HIV-1 RNA level at study entry. The ART regimen was TLD FDC tablet for children weighing at least 30 kg and the film-coated dolutegravir 50 mg daily plus either zidovudine plus lamivudine or abacavir plus lamivudine for those weighing < 30 kg. Participants were followed up at specified times to assess for ART side effects using a questionnaires and clinical response to ART.
Pharmacokinetic testing and analysis
Intensive PK sampling was performed after at least 4 weeks of starting dolutegravir-based ART and repeated after 7 months on therapy. A week prior to sampling, caregivers or participants were called daily on the phone to verify that medications were ingested. A day prior to sampling, participants were admitted to the hospital for the sampling. On the day of sampling, ART was ingested after an overnight fast. A standard breakfast was provided 30 minutes after dosing. Blood samples were collected at 0, 1, 4, 8, 12 and 24-hour post-dose. In addition, a timed 24-hour sample was collected at 2 weeks of ART and a random sample at 3- and 6-months visit for dolutegravir concentrations. The samples were processed and plasma stored at −80°C until shipment on dry ice to Infectious Disease Pharmacokinetics Lab at University of Florida. Drug concentrations were determined using validated liquid chromatography tandem with mass spectrometry (LC/MS/MS) methods. The detection range was 0.10 to 20 mg/L. Inter- and intra-batch precision percent coefficient of variation (%CV) was <11% and accuracy 96-103%. The maximum concentration (Cmax) and time to Cmax (Tmax) were determined by inspection of the serum concentration-time graphs. The calculation of AUC from time 0 to 24 hours (AUC0-24h), apparent oral clearance (CL/F) and volume of distribution (Vd/F) was performed using noncompartmental analysis on Phoenix WinNonlin (Certara, v8.3).
Statistical analysis
Statistical analyses were performed using SAS 9.4 software (SAS Institute Inc, Cary, NC) and SigmaPlot 14.0 (Systat Software, San Jose, CA). Continuous variables were summarized by geometric means with coefficient of variation or median with interquartile range (IQR) and compared by Wilcoxon Rank Sum test, and categorical variables are summarized by count and percentage and compared by Fisher exact test. The proportion with trough concentration < 0.32 mg/L, frequency of side effects as well as virologic suppression were also reported. Wilcoxon Rank Sum test was used to compare PK parameter between weight < 40 and ≥ 40 kg. For all analyses, a P value of <0.05 was considered significant. For comparison of actual weight gain over 8 months of observation to expected weight change based on WHO standard growth curves, we first found the centile for weight-age-sex at study entry and then projected the expected weight change on that centile after 8 months. For example for DTG-A009 who was 9 years old male with baseline weight 20kg was on the 1st centile. The projected weight gain over 8 months on the 1st centile 1.6 kg but the observed weight gain was 4.0 kg. Thus, excess weight gain was 2.4 kg. Similar process was performed for comparisons of actual to expected BMI change using WHO BMI-for-age for boys and girls to determine the expected change.
Results
Participants characteristics
Between February 2021 and June 2021, 25 CLWH weighing 20 kg or more who were stable on ART and eligible to switch to dolutegravir-based therapy were screened and enrolled. There were no screen failures. Of the 25 participants, 23 (92%) were on efavirenz-based ART, 21 (84%) had HIV RNA < 20 copies/mL or target not detected (TND) at entry and 15 (60.0%) were male (Table 1). The median (IQR) age was 10.5 (8.8 – 13.9) years and weight 29.3 (24.4 – 38.8) kg (Table 1). The median time on prior ART was 6.3 (1.9 −10.8) years. Overall, 16 (64%) were started on TLD and 9 (36%) were given dolutegravir plus two NRTIs (Table 1).
Table 1.
Baseline demographic and clinical characteristics
| Variable | n (%) or median (IQR)* |
|---|---|
| Median (IQR) age (years) | 11 (9.0 – 14.0) |
| Age (years) | |
| 6 – 9 | 7 (28%) |
| 10 – 14 | 13 (52%) |
| 15 – 17 | 5 (20%) |
| Sex | |
| Male | 15 (60%) |
| Female | 10 (40%) |
| Body weight (kg) | 30.0 (24.5 – 40.5) |
| Height (cm) | 132.0 (128.2 – 152.7) |
| Body mass index (kg/m2) | 16.7 (15.6 – 17.6) |
| Weight band | |
| 20 to < 25 kg | 6 (24%) |
| 25 to < 30 kg | 4 (16%) |
| 30 to < 40 kg | 9 (36%) |
| ≥ 40 kg | 6 (24%) |
| Previous ART | |
| Tenofovir disoproxil/ lamivudine/ efavirenz | 6 (24%) |
| Zidovudine/lamivudine/efavirenz | 17 (68%) |
| Tenofovir disoproxil/ lamivudine/lopinavir/ritonavir | 2 (8%) |
| Study regimen | |
| Tenofovir disoproxil/lamivudine/dolutegravir | 16 (64%) |
| Zidovudine/lamivudine/dolutegravir | 7 (28%) |
| Abacavir/lamivudine/dolutegravir | 2 (8%) |
| Entry HIV RNA | |
| < 20 copies/mL or target not detected | 21 (84%) |
| 20 - < 200 copies/mL | 2 (8%) |
| 200 - < 1000 copies/mL | 0 (0%) |
| > 1000 copies/mL | 2 (8%) |
| Time since HIV diagnosis (years) | 6.3 (1.8 – 10.8) |
| Time on prior ART (years) | 6.3 (1.9 – 10.8) |
| White blood cell count (x 109/L) | 4.4 (4.1 – 5.4) |
| Absolute neutrophil count (x 109/L) | 1.1 (0.9 – 1.9) |
| Hemoglobin (g/dL) | 11.6 (10.4 – 12.1) |
| Platelets (x 109/L) | 312 (264 – 365) |
| Blood urea nitrogen (mmol/L) | 2.5 (2.0 – 3.20) |
| Serum creatinine (μmol/L) | 39.0 (31.4 – 46.6) |
| Aspartate transaminase (IU/L) | 27.5 (22.3 – 40.8) |
| Alanine transaminase (IU/L) | 17.8 (12.3 – 29.3) |
| Alkaline phosphatase (IU/L) | 249 (151 – 279) |
| Total bilirubin (μmol/L) | 4.58 (3.7 – 6.4) |
| Direct bilirubin (μmol/L) | 1.0 (1.0 – 1.0) |
| Albumin (g/L) | 39.5 (35.3 – 41.9) |
number (percent) or median (interquartile range)
Pharmacokinetics
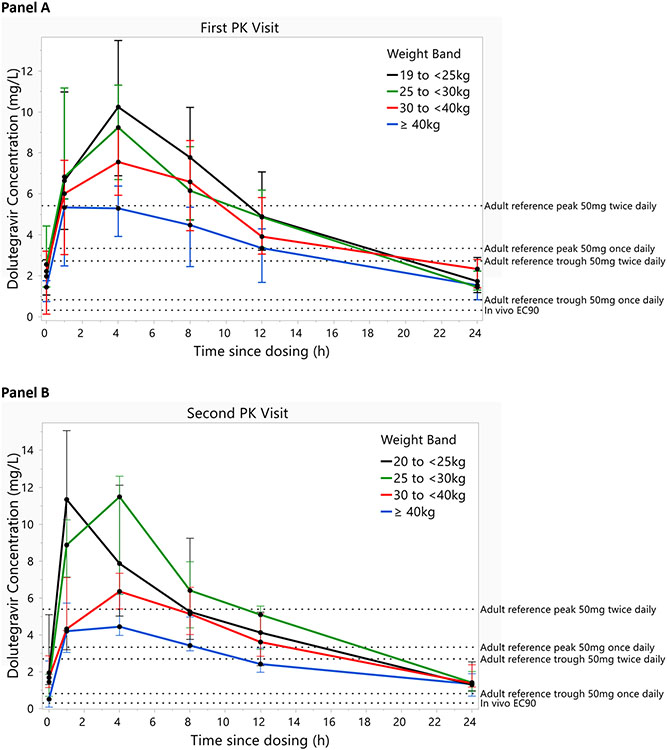
The median (range) time to first and second PK sampling were 4.5 (4.0 to 5.6) weeks and 31.8 (29.6 to 34.1) weeks after starting dolutegravir-based therapy, respectively. The median concentration-time profiles by weight bands for the first and second PK visits are shown in Fig. 1A & B. At both visits, the patterns were similar with lowest dolutegravir concentrations observed in the children weighing ≥40 kg. Also, the median dolutegravir concentrations and exposure were numerically higher at the first than the second PK visit (Fig. S1).
Fig 1.
Dolutegravir median concentration versus time profiles by weight band and by pharmacokinetic sampling visit. First PK visit at a median (range) of 4.5 (4.0 to 5.6) weeks of dolutegravir based therapy, Second PK visit at a median (range) of 31.8 (29.6 to 34.1) weeks after starting dolutegravir-based therapy. The error bars represent interquartile ranges. Labeled dashed lines indicate adult reference geometric mean trough and peak concentration values on dolutegravir 50 mg once daily and 50 mg twice daily. Dashed line for the in vivo EC90 (0.32 mg/L) is also shown.
A summary of dolutegravir PK parameters are shown in Table 2. The median (IQR) of AUC0-24h, Cmax and C24h at the first PK visit were 98.8 (86.8 – 138.2) mg*h/L, 7.3 (5.8 – 9.7) mg/L and 1.5 (1.3 – 2.3) mg/L, respectively. At the second visit, median (IQR) of AUC0-24h, Cmax and C24h were 86.8 (69.2 – 120.0) mg*h/L, 7.0 (4.6 – 8.7) mg/L and 1.4 (1.1 – 2.1) mg/L, respectively. All participants except one (at the second PK visit) had dolutegravir C24h greater than dolutegravir in vivo EC90 of 0.32 mg/L [8]. Dolutegravir median AUC0-24h at both PK visits in our study were at least 2 times higher than the reference AUC0-24h of 43.4 mg*h/L in adults given 50 mg once daily [8], but were similar to the mean value of 93.4 mg*h/L in adults given 50 mg twice a day [9, 10]. Mean C24h in our study population at both visits were also higher than the reference of 0.83 mg/L in adults given 50 mg once daily [8]. Children weighing 20 kg to < 40 kg compared to those weighing ≥ 40 kg had significantly higher dolutegravir AUC0-24h and Cmax and lower CL/F and Vd/F at both PK visits (Table 3). At both PK visits, geometric mean of dolutegravir AUC0-24h, Cmax and C24h for each weight band in our study were higher than values reported for corresponding weight bands in the ODYSSEY PK sub-study[6] (Supplement Table 1). We also collected spot sample Ctrough at 2 weeks and random concentrations at 3- and 6-months visits (Table 4). The median (range) dolutegravir trough concentration at 2 weeks after ART switch was 0.88 (0 – 7.0) mg/L, with 2 participants having concentrations reported as trace or below the limit of quantitation.
Table 2.
Dolutegravir steady-state pharmacokinetic parameters and published adult reference pharmacokinetic parameters
| Pharmacokinetic visit at a median of 4.5 weeks on ART |
Pharmacokinetic visit at a median of 32 weeks on ART |
Adults ≥ 40 kg |
||||
|---|---|---|---|---|---|---|
| 50 mg QD | 50 mg BID | |||||
| (N=25) | (N=25) | (N=10)[1] | (N=23)[2, 3] | |||
| Parameter | GM (CV%) | Median (IQR) | GM (CV%) | Median (IQR) | GM (CV%)$ | GM (CV%)$ |
| T1/2 | 9.7 (29.6%) | 9.4 (8.3 – 11.2) | 9.5 (40.8) | 8.5 (7.4 – 11.0) | 12.0 (22%)a | |
| Tmax (h) | 3.2 (52.4%) | 4.0 (1.8 – 4.2) | 2.8 (62.8%) | 3.9 (1.0 – 4.1) | 2.0 (0.97-4.0)b | 2.0 (0-7.9)b |
| Cmax (mg/L) | 7.6 (40.1%) | 7.3 (5.8 – 9.7) | 7.0 (46.0%) | 7.0 (4.6 – 8.7) | 3.34 (16%)a | 5.41 (40%)a |
| C24h (mg/L) | 1.6 (52.7%) | 1.5 (1.3 – 2.3) | 1.4 (47.1%) | 1.4 (1.13 – 2.10) | 0.83 (26%) | 2.72 (70%)a |
| AUC0-24h (mg *h/mL) | 101.8 (37.7%) | 98.8 (86.8 – 138.2) | 88.6 (40.5%) | 86.6 (69.15 – 119.97) | 43.4 (20%) | 93.36 (50%)a |
| Vd/F (L) | 6.9 (52.3%) | 6.8 (4.0 – 9.3) | 7.8 (75.8%) | 7.0 (4.19 – 11.49) | ||
| CL/F (L/h) | 0.49 (46.6%) | 0.47 (0.35 – 0.57) | 0.56 (74.6%) | 0.51 (0.39 – 0.72) | 1.15 (20%)c | 0.54 (70%)c |
GM, geometric mean, CV, coefficient of variation, QD, once daily, BID, twice daily
GM (CV%) or otherwise specified
geometric mean (coefficient of variation)
median (range), T1/2, half-life, Tmax, time to peak concentration; Cmax, peak concentration; C24h, concentration at 24 hours post-dose, AUC0-24h, total area under the curve from time 0 hours to 24hours; Vd/F, apparent volume of distribution, CL/F, apparent oral clearance.
Table 3.
Comparison of dolutegravir pharmacokinetic (PK) parameters by body weight at each intensive PK sampling visit
| Pharmacokinetic visit at a median of 4.5 weeks on dolutegravir |
Pharmacokinetic visit at a median of 32 weeks on dolutegravir |
|||||
|---|---|---|---|---|---|---|
| Parameter | 20 to <40 kg (N = 18) | ≥40 kg (N = 7) | P value | 20 to <40 kg (N = 17) | ≥40 Kg (N = 8) | P value |
| T1/2 | 9.1 (8.1 – 10.7) | 11.4 (8.3 – 15.1) | 0.215 | 8..5 (7.6 – 10.3) | 12.0 (6.3 – 17.4) | 0.503 |
| Tmax (h) | 4.1 (2.7 – 4.2) | 3.9 (1.2 – 4.2) | 0.431 | 4.0 (1.1 – 4.1) | 4.0 (1.0 – 4.3) | 1.000 |
| Cmax (mg/L) | 8.9 (7.1 – 11.7) | 5.6 (4.1 – 6.4) | 0.002 | 7.7 (6.7 – 12.0) | 4.6 (4.2 – 6.2) | 0.006 |
| C24h (mg/L) | 1.8 (1.3 – 2.6) | 1.5 (0.8 – 2.2) | 0.289 | 1.4 (1.2 – 2.3) | 1.3 (0.7 – 1.9) | 0.503 |
| AUC0-24h (mg *h/mL) | 125.1 (88.9 – 148.6) | 86.8 (46.8 – 101.8) | 0.032 | 114.9 (74.7 – 140.9) | 66.3 (53.2 – 98.8) | 0.016 |
| Vd/F (L) | 6.5 (3.9 – 7.6) | 12.7 (9.0 – 17.2) | 0.008 | 5.8 (4.0 – 7.4) | 13.0 (9.5 – 18.1) | 0.007 |
| CL/F (L/h) | 0.41 (0.34 – 0.56) | 0.58 (0.49 – 1.1) | 0.032 | 0.44 (0.36 – 0.67) | 0.76 (0.52 – 0.95) | 0.013 |
T1/2, half-life, Tmax, time to peak concentration; Cmax, peak concentration; C24h, concentration at 24 hours post-dose, AUC0-24h, total area under the curve from time 0 hours to 24hours; Vd/F, apparent volume of distribution, CL/F, apparent oral clearance.
Table 4.
Clinical outcome
| Entry | 2 weeks (N=25) |
4 weeks (N=25) |
3 months (n=24) |
6 months (N=25) |
8 months (N=25 |
12 months (N=25) |
|
|---|---|---|---|---|---|---|---|
| HIV RNA levels | |||||||
| < 20 copies/mL or TND | 21 (84%) | 22 (88%) | 23 (92%) | 22 (88%) | 22 (88%) | 22 (88%) | |
| 20 to < 200 copies/mL | 2 (8%) | 3 (12%) | 1 (4%) | 1 (4%) | 2 (8%) | 0 (0%) | |
| 200 to 1000 copies/mL | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) | 2 (8%) | |
| > 1000 copies/mL | 2 (8%) | 0 (0%) | 1 (4%) | 1 (4%) | 1 (4%) | 1 (4%) | |
| Dolutegravir concentrations | |||||||
| Median (range) C24h (mg/L) | 0.86 (0 -7.0)$ | 1.5 (0.5-5.0) | 1.4 (0.1-3.3) | ||||
| Median (range) Crandom (mg/L) | 6.0 (0 - 9.5) | 2.2 (0 -9.6) | |||||
| Side effects | |||||||
| Headache | 6 (24%) | 7 (28%) | 7 (29%) | 5 (20%) | 2 (8%) | ||
| Dizziness | 1 (4%) | 0 (0%) | 3 (13%) | 2 (8%) | 0 (0%) | ||
| Insomnia | 1 (4%) | 3 (12%) | 2 (8%) | 2 (8%) | 0 (0%) | ||
| Anxiety | 1 (4%) | 1 (4%) | 1 (4%) | 1 (4%) | 1 (4%) | ||
| Depression | 2 (8%) | 1 (4%) | 1 (4%) | 2 (8%) | 0 (0%) | ||
| Nausea | 2 (8%) | 2 (8%) | 2 (8%) | 1 (4%) | 2 (8%) | ||
| Vomiting | 2 (8%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Diarrhea | 3 (12%) | 2 (8%) | 3 (13%) | 2 (8%) | 1 (4%) | ||
| Abdominal pain | 8 (32%) | 5 (20%) | 2 (8%) | 1 (4%) | 3 (12%) | ||
| Rash | 2 (8%) | 3 (12%) | 1 (4%) | 0 (0%) | 1 (4%) | ||
| Fatigue | 2 (8%) | 3 (12%) | 2 (8%) | 2 (8%) | 0 (0%) | ||
| Other | 2 (8%) | 0 (0%) | 1 (4%) | 0 (0%) | 0 (0%) | ||
| Grade 3 or 4 lab events | |||||||
| Anemia | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Neutropenia | 0 (0%) | 2 (8%) | 1 (4%) | ||||
| Thrombocytopenia | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Elevated creatinine | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Total bilirubin | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Elevated transaminase | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Alkaline phosphatase | 0 (0%) | 0 (0%) | 0 (0%) |
TND, target not detected, C24h, concentration at 12 hours post-dose, Crandom, random concentration at study visit
timed C24h sample, but dose before sample was not observed.
Safety and efficacy
The tolerability and HIV RNA suppression data are shown in Table 4. Two participants who were previously suppressed on ART had entry HIV RNA of 1544 and 5892 copies/mL. At 2 weeks of dolutegravir-based therapy, 22 (88%) maintained or achieved HIV RNA levels < 20 copies/mL or target not detected(TND), two participants with low viremia (33 copies/mL and 58 copies/mL) at study entry had unchanged viral load and one with viral load of TND at entry had a blip to 31 copies/mL. The two participants with entry HIV RNA > 1000 copies/mL achieved HIV RNA < 20 at 2 weeks of dolutegravir-based therapy. After achieving HIV RNA < 20 c/mL or TND in all participants, there were three episodes of viral load blips between 20 and < 200 c/mL with full suppression at a subsequent testing. There were six episodes of virological rebound ranging from 561 to 35,493 copies/mL. At the end of 12 months follow up, 22/25 (88%) had RNA levels < 20 copies/mL or TND. One participant had HIV RNA > 1000 copies/mL on two consecutive measures at 6 and 12 months; this participant was on TLD, had dolutegravir trough concentrations of 2.8 and 1.4 mg/L at the two intensive PK visits and a random concentration of 0.4 mg/L at 6 months visit, which may suggest poor medication adherence around the 6 months visit. Overall, 21 (84%) participants reported perfect ART adherence at all visit, 3 reported missing 2 doses in 7-day recall at one visit and 1 reported missing 2 doses in 7-day recall at two visits. We did not find a clear relationship between dolutegravir trough or random concentrations with viral load rebound. There were 6 episodes of spot dolutegravir concentrations below the limit of quantitation or trace, but only 1 such episode coincided with a viral load blip. Headache and abdominal pain were the most common symptoms during follow-up but neuropsychiatric symptoms (such as dizziness, anxiety, insomnia, depression) were infrequent (Table 4). Grade 3 neutropenia was seen in 2 participants at 2 weeks and persisted in 1 of them at 4 weeks of dolutegravir-based ART. There were no ART discontinuations or changes because of medication side effects. The median weight change (range) over 8 months of observation was 2.0 (−2 to 7) kg. Of the 7 participants aged less than 10 years for whom there are WHO standardized growth curves, 2 (28.6%) had excess weight gain of 2.4 kg and 2.5 kg. The median change (range) in BMI over 8 months was 0.5 (−4.3 to 2) kg/m2. The expected median (range) change in BMI based on age and sex of the study population using WHO reference BMI-for-age for girls and boys 5 to 19 years was 0.3 (0.1 to 0.6) kg/m2. Overall, 13 (52%) of 25 participants had an increase in BMI greater than expected; the increased ranged from 0.2 – 1.8 kg/m2. Table S2 also show the median values of weight, height and BMI at each study visit.
Discussion
In this study, we found that the film-coated adult dolutegravir 50mg (TLD or standalone) tablets achieved high plasma exposure and trough concentrations in ART-experienced Ghanaian children with HIV weighing at least 20 kg. Unlike the recent report of appropriate dolutegravir PK profiles in children with HIV weighing 20 kg to less than 40 kg treated with the adult dolutegravir 50 mg film-coated tablets [6], we found mean dolutegravir AUC0-24h, Cmax and C24h to be higher than reference values in adults treated with dolutegravir 50 mg once daily [8]. The C24h at about 1 and 8 months on dolutegravir-based ART in our study population was nearly twice the reference value of in adults treated with dolutegravir 50 mg once daily [8]. Surprisingly, the AUC0-24h and Cmax at the first PK visit and second PK visits in our study were comparable or even higher than the adult reference values reported in HIV-infected adults with genotypic evidence of raltegravir resistance who were treated with dolutegravir 50 mg twice daily [9, 10]. Our trough concentrations were lower than the mean Ctrough in the adults given dolutegravir 50 mg twice daily [9, 10], but the dosing interval in our study was once daily. Despite the higher dolutegravir plasma exposures or concentrations in our study population, the adult film-coated 50 mg tablets were well tolerated with no treatment-limiting toxicities and the regimens were effective in maintaining viral suppression through 48 weeks of follow up.
It is reassuring that dolutegravir exposures in our study were consistent with exposures that led to more durable viral suppression in ART-experienced adults with high level drug resistance treated with dolutegravir 50 mg twice a day [10]. It is also reassuring that dolutegravir Ctrough at 2 weeks and 4 weeks after switching from predominantly efavirenz-based ART were adequate and effective in nearly all patients. In healthy volunteers, efavirenz reduced dolutegravir Ctrough by 75%, with coadministration leading to a geometric mean Ctrough of 0.22 mg/L, well below the clinical target of 0.32 mg/L [11]. While there is no consensus on dolutegravir PK target(s) for efficacy and safety, dolutegravir Ctrough was associated with viral load reduction in a 10-day dolutegravir monotherapy study, with a Ctrough above the calculated EC90 of 0.32 mg/L generally accepted as the cutoff for efficacy [8]. Our study enrolled children with evidence of viral suppression on efavirenz-based or lopinavir/ritonavir-based ART prior to switch to dolutegravir. However, current WHO and updated Ghana National HIV treatment guidelines recommend transition to dolutegravir regimens for children established on first-line ART [7, 12]. Acquired or pretreatment HIV drug resistance is common in children and adolescents in resource-limited settings [13-17]. A large number of treatment-experienced CLWH weighing 20 kg or more in our settings will be switched to adult dolutegravir 50 mg tablets without the benefit of prior viral load or HIV drug resistance testing and could be on functional monotherapy [16, 17]. The significantly higher dolutegravir plasma exposures may provide durable viral load suppression but further evaluation of the 50 mg adult tablets in other pediatric populations and closer monitoring for adverse events are needed as the rollout of the regimen is scaled up globally.
Our study enrolled children aged 6 to 17 years old with body weight below and above 40 kg, which enable us compare PK parameters in 4 weight bands (Fig. 1). We found higher dolutegravir C24h in the three weight bands in the children weighing 20 kg to < 40 kg to be higher than the mean Ctrough concentrations of 0.63 to 0.77 mg/L in the ODYSSEY PK sub-study [6]. Similarly, dolutegravir AUC0-24h in the three weight bands in the children weighing 20 kg to <40 kg was also much higher than corresponding weight bands in the ODYSSEY PK sub-study (Supplement Table 1). In the children weighing ≥40 kg, mean dolutegravir AUC0-24h and C24h were also higher than the corresponding values among adolescents in the IMPAACT P1093 PK study [18]. In contrast, the median C24h of 1.8 mg/L among our study participants weighing 20 to <40 kg is similar to the mean Ctrough of 1.93 mg/L in a multicenter retrospective study of therapeutic drug monitoring of children weighing 20 to 40 kg living in the Netherlands who were treated with 50 mg dolutegravir dose [19]. It is unclear why we found a significantly higher exposure in our study population compared to the children in the ODYSEEY or IMPAACT P1093 studies mentioned above. Coadministration of dolutegravir with food is known to increase exposure[20], but it was administered after an overnight fast in in our study as in the ODYSSEY PK study. Differences in genetic factors and/or adherence could explain the differences in dolutegravir PK between our study and other population.
All participants in our study rapidly achieved or maintained viral suppression within 2 weeks of switch to dolutegravir-based regimens. Overall, 88% of the children in our study achieved HIV RNA < 20 c/mL at end of follow-up at week 48, which is higher than the 61% who had HIV RNA < 50 copies/mL in the IMPACT P1093 study [18]. Whether the difference is related to the higher dolutegravir exposure achieved in our study population, it is not clear but dolutegravir-based ART in children is highly efficacious. In the large ODYSSEY trial with a longer duration of follow up, 81% of ART-naïve and ART-experienced children in the dolutegravir arm achieved viral load < 50 c/mL at 96 weeks [21]. Three participants in our study had virologic rebound at 48 weeks but only one had viral load greater than 1000 copies/mL on two consecutive testing. We had no access to resistance testing, so it is unclear if that participant had emergent dolutegravir resistance mutations or not. We found no clear relationship with dolutegravir PK and viral suppression or rebound.
Despite high dolutegravir plasma exposure in our study population, the film-coated adult dolutegravir 50 mg tablets were well tolerated, with no changes or discontinuation in ART. Except for headache and transient abdominal pain, side effects were infrequent. We were not able to establish any relationship between reported side effect and dolutegravir PK given the small sample size. In addition, we did not complete the side effects questionnaires at study entry, which make it difficult to attribute the reported frequent symptoms (especially headache and abdominal pain) to dolutegravir. Among Japanese PLWH, dolutegravir Ctrough were higher in patients with neuropsychiatric adverse events (NP AEs) than those without NP AEs [22]. In that study, a C24h ≥1.06 mg/L was associated with NP AEs [22]. We found mean C24h of 1.6 and 1.4 at the 2 PK visit but NP AEs were infrequent and not consistent in most patients. Our sample size was too small to adequate describe changes in anthropometric characteristics. Weight gain through 8 months was variable. Two children younger than 10 years old had excess weight gain on dolutegravir-based therapy. Similarly, changes in BMI were variable but over 50% of our population had greater than expected changes based on WHO references for boys and girls. Future studies should carefully monitor changes in weight and effects on child health. The low frequency of side effects or treatment-limiting toxicities in our study is similar to other studies that have evaluated PK and safety of adult tablets in children weighing 20 kg or more [6, 19].
In summary, our results provide additional supportive data for current WHO [23] and Ghana NACP [12] consolidated guidelines that recommend a transition to the simplified dolutegravir 50mg mg once daily in ART-experienced children with HIV weighing at least 20 kg as in adolescents and adults [7, 12]. We also found higher median dolutegravir AUC0-24h and lower CL/F in children weighing 20 to < 40 kg compared to those weighing ≥40 kg, suggesting that children weighting 20 to < 40 kg would have prolonged exposure to higher dolutegravir exposures. While harmonization of dolutegravir-based ART with adult treatment programs has several advantages including minimizing effects of stock out of pediatric formulations. However, further studies and routine monitoring should investigate long-term adverse effects of dolutegravir in more children weighing 20 to less than 40 kg given the higher dolutegravir plasma exposures.
Supplementary Material
Acknowledgment
We thank the study participants, the supportive staff of the TB and HIV clinic, the Pharmacy and the malnutrition ward at Komfo Anokye Teaching Hospital (KATH) who helped with patient enrolment and follow up. We thank Joyce Manu of KATH for phlebotomy services. We also thank Benjamin Osei Kuffour and Richard Morgan of the TB and HIV clinic pharmacy for adherence counselling/monitoring. The study was funded by grant support from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) [grant number R21 AI158263]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest and Source of Funding:
All authors declare no conflict of interest. This work was primarily supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) [grant number R21 AI158263].
References
- 1.UNAIDS. IN DANGER: UNAIDS Global AIDS Update 2022. Geneva: Joint United Nations Programme on HIV/AIDS; 2022. Available at: https://www.unaids.org/sites/default/files/media_asset/2022-global-aids-update_en.pdf. Accesed on Februry 9, 2023. 2022. [Google Scholar]
- 2.Penazzato M, Palladino C, Sugandhi N, Paediatric ARVDOMp. Prioritizing the most needed formulations to accelerate paediatric antiretroviral therapy scale-up. Curr Opin HIV AIDS 2017; 12(4):369–376. [DOI] [PubMed] [Google Scholar]
- 3.Adolescents. PoAGfAa. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed on April 28, 2020. 2020. [Google Scholar]
- 4.WHO. Dolutegravir (DTG) and the fixed dose combination (FDC) of tenofovir/lamivudine/dolutegravir (TLD. april 30 2018 briefing note. Available at: https://www.who.int/hiv/pub/arv/DTG-TLD-arv_briefing_2018.pdf. Accessed on February 28, 2020. 2018. [Google Scholar]
- 5.WHO. Update of recommendations on first- and second-line antiretroviral regimens. July 2019. Geneva, Switzerland: World Health Organization; 2019 (WHO/CDS/HIV/19.15). Avialable at: https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf. Accessed on January 13 2020. [Google Scholar]
- 6.Bollen PDJ, Moore CL, Mujuru HA, Makumbi S, Kekitiinwa AR, Kaudha E, et al. Simplified dolutegravir dosing for children with HIV weighing 20 kg or more: pharmacokinetic and safety substudies of the multicentre, randomised ODYSSEY trial. Lancet HIV 2020; 7(8):e533–e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. Available at: https://www.who.int/publications/i/item/9789240031593. Accessed (August 7, 2021). 2021. [PubMed] [Google Scholar]
- 8.Min S, Sloan L, DeJesus E, Hawkins T, McCurdy L, Song I, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS 2011; 25(14):1737–1745. [DOI] [PubMed] [Google Scholar]
- 9.ClinicalTrials.gov. A pilot study assessing the integrrase inhibitor GSK1349572 in HIV-infected persons with virus resistant to raltegravir. Study results. aavilable at: https://clinicaltrials.gov/ct2/show/results/NCT00950859 (accessed January 27, 2023). 2013. [Google Scholar]
- 10.Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis 2013; 207(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song I, Borland J, Chen S, Guta P, Lou Y, Wilfret D, et al. Effects of enzyme inducers efavirenz and tipranavir/ritonavir on the pharmacokinetics of the HIV integrase inhibitor dolutegravir. Eur J Clin Pharmacol 2014; 70(10):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NACP. Consolidated guidelines for HIV care in Ghana. Test, treat & track. 2022. [Google Scholar]
- 13.Jordan MR, Penazzato M, Cournil A, Vubil A, Jani I, Hunt G, et al. Human Immunodeficiency Virus (HIV) Drug Resistance in African Infants and Young Children Newly Diagnosed With HIV: A Multicountry Analysis. Clin Infect Dis 2017; 65(12):2018–2025. [DOI] [PubMed] [Google Scholar]
- 14.Nyandiko W, Holland S, Vreeman R, DeLong AK, Manne A, Novitsky V, et al. HIV-1 Treatment Failure, Drug Resistance, and Clinical Outcomes in Perinatally Infected Children and Adolescents Failing First-Line Antiretroviral Therapy in Western Kenya. J Acquir Immune Defic Syndr 2022; 89(2):231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigaloff KC, Calis JC, Geelen SP, van Vugt M, de Wit TF. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect Dis 2011; 11(10):769–779. [DOI] [PubMed] [Google Scholar]
- 16.Kamori D, Barabona G, Rugemalila J, Maokola W, Masoud SS, Mizinduko M, et al. Emerging integrase strand transfer inhibitor drug resistance mutations among children and adults on ART in Tanzania: findings from a national representative HIV drug resistance survey. J Antimicrob Chemother 2023. [DOI] [PubMed] [Google Scholar]
- 17.Salou M, Butel C, Comlan AS, Konou AA, Tegueni K, Ehlan A, et al. Challenges of scale-up to dolutegravir-based regimens in sub-Saharan Africa. AIDS 2020; 34(5):783–787. [DOI] [PubMed] [Google Scholar]
- 18.Viani RM, Alvero C, Fenton T, Acosta EP, Hazra R, Townley E, et al. Safety, Pharmacokinetics and Efficacy of Dolutegravir in Treatment-experienced HIV-1 Infected Adolescents: Forty-eight-week Results from IMPAACT P1093. Pediatr Infect Dis J 2015; 34(11):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waalewijn H, Stol K, van der Knaap L, Fraaij PLA, Vermont C, van Rossum AMC, et al. Adequate exposure of 50 mg dolutegravir in children weighing 20 to 40 kg outside of sub-Sahara Africa. AIDS 2022; 36(14):2077–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song I, Borland J, Chen S, Patel P, Wajima T, Peppercorn A, et al. Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother 2012; 56(3):1627–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turkova A, White E, Mujuru HA, Kekitiinwa AR, Kityo CM, Violari A, et al. Dolutegravir as First- or Second-Line Treatment for HIV-1 Infection in Children. N Engl J Med 2021; 385(27):2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yagura H, Watanabe D, Kushida H, Tomishima K, Togami H, Hirano A, et al. Impact of UGT1A1 gene polymorphisms on plasma dolutegravir trough concentrations and neuropsychiatric adverse events in Japanese individuals infected with HIV-1. BMC Infect Dis 2017; 17(1):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. Avialable at https://www.who.int/publications/i/item/9789240046764. Accesssed Octocber 27, 2022. 2022. [PubMed] [Google Scholar]
- 1.Min S, Sloan L, DeJesus E, Hawkins T, McCurdy L, Song I, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS 2011; 25(14):1737–1745. [DOI] [PubMed] [Google Scholar]
- 2.ClinicalTrials.gov. A pilot study assessing the integrrase inhibitor GSK1349572 in HIV-infected persons with virus resistant to raltegravir. Study results. aavilable at: https://clinicaltrials.gov/ct2/show/results/NCT00950859 (accessed January 27, 2023). 2013. [Google Scholar]
- 3.Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis 2013; 207(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.