Abstract
Purpose:
Devimistat (CPI-613®) is a novel inhibitor of tumoral mitochondrial metabolism. We investigated effect of devimistat in vitro and in a phase 1b clinical trial in patients with advanced biliary tract cancer (BTC).
Patients and Methods:
Cell viability assays of devimistat +/− GC were performed and effect of devimistat on mitochondrial respiration via oxygen consumption rate (OCR) was evaluated. A phase 1b/2 trial was initiated in patients with untreated advanced BTC. In phase 1b, devimistat was infused over 2 hours in combination with GC on days 1 and 8 every 21 days with a primary objective to determine recommended phase 2 dose (RP2D). Secondary objectives included safety, overall response rate (ORR), progression-free survival (PFS) and overall survival (OS).
Results:
In vitro, devimistat with GC had synergistic effect on two cell lines. Devimistat significantly decreased OCR at higher doses and in arms with divided dosing. In the phase 1b trial, 20 patients received a median of 9 cycles (range 3–19). One DLT was observed and the RP2D of devimistat was determined to be 2000 mg/m2 in combination with GC. Most common grade 3 toxicities included neutropenia (n=11, 55%), anemia (n=4, 20%), and infection (n=3, 15%); with no grade 4 toxicities. After a median follow-up of 15.6 months, ORR is 45% and median PFS is 10 months (95% CI, 7.1 – 14.9). Median OS is not yet estimable.
Conclusion:
Devimistat in combination with GC is well tolerated and has an acceptable safety profile in patients with untreated advanced BTC.
Keywords: Biliary cancer, cholangiocarcinoma, CPI-613, gemcitabine, cisplatin
Introduction
Biliary tract cancers (BTC) are comprised of intra- and extra-hepatic (perihilar and distal) cholangiocarcinoma and gallbladder cancer. Advanced BTCs are aggressive malignancies with median survival times of less than 12 months, and a five-year overall survival (OS) of less than 5% despite treatment 1,2. Systemic chemotherapy options remain limited with few meaningful improvements over the past two decades.
In the phase 3 ABC-02 trial, 410 patients with locally advanced or metastatic BTC were randomly assigned to receive gemcitabine +/− cisplatin 2. Patients on the combination arm demonstrated an objective response rate (ORR) of 26.1% and an improvement in OS (11.7 vs 8.1 months; hazard ratio (HR), 0.64; 95% CI 0.52–0.80; p<0.001) as compared to gemcitabine alone. The ABC-02 trial established gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2, days 1 and 8 every 21 days, as a standard first line combination chemotherapy regimen for patients with advanced BTC.
Devimistat (CPI-613®) is a stable analog of normally transient, acylated catalytic intermediates of lipoic acid (lipoate), an essential co-factor for mitochondrial enzyme complexes, including pyruvate dehydrogenase (PDH), -ketoglutarate dehydrogenase (KGDH), both central to the tricarboxylic acid cycle, as well as the branched chain keto acid dehydrogenase 3–5. Lipoic intermediates and their flux through these enzymes are monitored by regulatory systems, which are significantly modified in cancer, making these intratumoral enzymes more susceptible to devimistat. Devimistat selectively inactivates PDH and KGDH, blocking entry of glucose and glutamine derived carbons into the TCA cycle. Inactivation of PDH and KGDH collapses mitochondrial metabolism in tumor cells and leads to redundant activation of apoptotic and necrotic cell death pathways 4. Given these specific intratumoral metabolic targets, a phase 1 dose-escalation trial of devimistat as a single agent in 26 patients with advanced hematologic malignancies was conducted to a dose 2,940 mg/m2 infused over two hours with no dose limiting toxicities (DLTs) observed. The trial established a maximum tolerated dose of 2,940 mg/m2 infused over 0.5–2 hours and pharmacokinetic profile defined a devimistat half-life of 1.34 hours 6.
Based on the encouraging results from a phase 1 trial of devimistat with modified FOLFIRINOX in metastatic pancreatic cancer 7 as well as clinical similarities between pancreas cancer and BTC, we investigated the role of devimistat in combination with gemcitabine and cisplatin (GC) in BTC. Here we report in vitro studies and results of a phase 1b clinical trial of devimistat in combination with gemcitabine and cisplatin in patients with advanced BTC.
Methods
2.1. Cell Culture
TFK-1 (gift from N. Neamati; June 2017), RCB1292 (Riken BRC; March 2021) and RCB1293 (Riken BRC; March 2021) human BTC cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator. All cells were mycoplasma-free with latest testing completed August 26th, 2022. The approximate passage number between thawing and usage in experiments for cell lines is 25.
2.2. Viability Assay
Cells (n=3,000) were seeded to each well in 96-well plates, allowed to adhere overnight, and then treated with devimistat, gemcitabine and cisplatin once. After three days, viability was quantified by crystal violet assay. Briefly, cells were fixed and then stained with 0.5% crystal violet staining solution on bench rocker for 30 minutes. The plates were washed with deionized water and left to dry. 150 μM methanol was added to each well and absorbance was measured with a spectrophotometer at 570 nm. Absorbance of blank wells was subtracted during calculation.
2.3. Oxygen Consumption Rate (OCR) Analysis
15,000 cells were seeded to 96-well Agilent Seahorse cell culture plates and allowed to attach overnight. On the day of the assay, medium was replaced with 180μL of Seahorse XF medium and the plate was incubated in a CO2-free incubator at 37°C. OCR measurements were performed by Seahorse XFe96 analyzer at baseline level and after each injection of devimistat of indicated concentration. In addition, 15,000 cells were seeded to each well in 96-well plates (Falcon, no. 353072) and allowed to attach overnight for real time OCR via Resipher. Indicated treatments were applied and Resipher oxygen-sensing lid (Lucid Scientific) was attached. OCR was measured for 48 hours.
2.4. Clinical Trial
The clinical trial was designed as a single-arm, open-label, multicenter, phase 1b dose escalation study in previously untreated patients with advanced BTC to assess safety and determine the recommended phase 2 dose (RP2D) of devimistat (dose levels 500, 1000, 1500 and 2000 mg/m2) infusion over 2 hours in combination with gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2 given intravenously on days 1 and 8 of a 21-day treatment cycle; Supplementary Figure 1. All patients provided written informed consent approved by the Institutional Review Board prior to any study specific participation. The study was performed in accordance with Good Clinical Practice, as defined by the International Conference on Harmonization, WHO, and any local derivatives.
The phase 1b primary endpoint was the presence or absence of dose-limiting toxicity up to and including cycle 2 day 1, attributed as possibly, probably, or definitely related to the drug combination. The secondary endpoints included safety and tolerability, ORR, progression-free survival (PFS) and OS.
2.5. Patient Eligibility
Patients aged 18 years with pathologically or cytologically confirmed BTC not eligible for curative resection, transplantation, or ablative therapies, and receiving no previous systemic treatment for advanced BTC were eligible for inclusion in this study. Patients with prior perioperative chemotherapy for localized disease were permitted provided treatment was completed more than 6 months from study enrollment. Patients might also have received prior radiation, embolization, ablation, or hepatic resection, if completed more than 4 weeks prior to enrollment. Extrahepatic palliative radiation was permitted if completed more than 2 weeks prior to enrollment. Other inclusion criteria included Eastern Cooperative Oncology Group ECOG) performance status of 0–1, radiographically measurable disease per RECIST v1.1 in at least one site not previously treated, adequate organ function, no evidence of ongoing active infection, no active cardiopulmonary disease (NYHA class III or IV heart failure, unstable angina, uncontrolled arrhythmia, or interstitial lung disease), and QTcF interval < 480 msec.
2.6. Statistical Design
Given the poor prognosis for patients with advanced BTC and potential for only additive toxicity from the combination therapy, DLTs up to but not exceeding a 35% DLT rate during the first 22 days of therapy was considered acceptable. A dose level with estimated probability closest to, but not exceeding the DLT target rate was considered the RP2D. Up to 20 patients were expected to be enrolled with dose allocation using the Time-to-Event (TiTE) method 8,9. This method assumes a model for the time to occurrence of toxic responses as a function of the dose and allows information from all patients treated, even those with only partial observation, to contribute to dose-toxicity calculation. This method allows for continuous recruitment and flexibility regarding the number of patients treated at each dose level. Every patient enrolled and receiving at least one dose of devimistat was considered evaluable for estimation of the probability of DLT. If a patient withdrew for any reason during the first cycle of therapy, not primarily or secondarily associated with toxicity, that patient’s follow-up was used in the ongoing estimation of the probability of toxicity. However, such a patient would have been replaced when calculating the maximum trial size. Patients stopping treatment early due in part or secondary to toxicity were considered to have had a DLT.
2.7. Safety and Toxicity
Any patient who received treatment on trial was evaluated for toxicity. Toxicity was assessed according to the NCI Common Terminology Criteria for Adverse Events version 5.0. Dose adjustments were made according to the adverse event with the greatest degree of toxicity. Treatment delays of more than 28 days from last intended therapy resulted in treatment discontinuation. If both cisplatin and gemcitabine were held or discontinued on a treatment day, devimistat was also held or discontinued. If one of the study drugs was discontinued due to toxicity attributed to that agent, patients were allowed to continue a modified regimen within the study arm. However, monotherapy with cisplatin was not permitted.
2.8. Data Availability
The data generated in this study are available within the article, its supplementary data files and available upon request from the corresponding author.
Results
3.1. In vitro Studies - Drug Synergism and Duration of Infusion
To characterize the response of BTC to devimistat and demonstrate the combinational effects of standard of care chemotherapy and devimistat in vitro, we used TFK-1 (extrahepatic; RRID: CVCL_2214) and RCB1292 (intrahepatic; RBE, RRID: CVCL_4896) and RCB1293 (intrahepatic; RRID: CVCL_4902) human BTC cell lines. The genomic profile of the cell lines is characterized in a Circos plot10; Supplementary Figure 2. The three cell lines responded to devimistat similarly, with IC50 ranging from 139μM to 200μM; Supplementary Figure 3. We also treated the cells with gemcitabine and cisplatin alone, devimistat alone or the combination and then examined viability and calculated synergy score. The combination of devimistat, gemcitabine and cisplatin had synergistic effect on RCB1292 and RCB1293 (synergy scores 11.24 and 10.85, respectively), and additive effects on TFK-1 (synergy score 5.47; Figure 1A) using SynergyFinderPlus11.
Figure 1. Synergism of devimistat with chemotherapy and effect on mitochondrial respiration in biliary cancer cell lines.
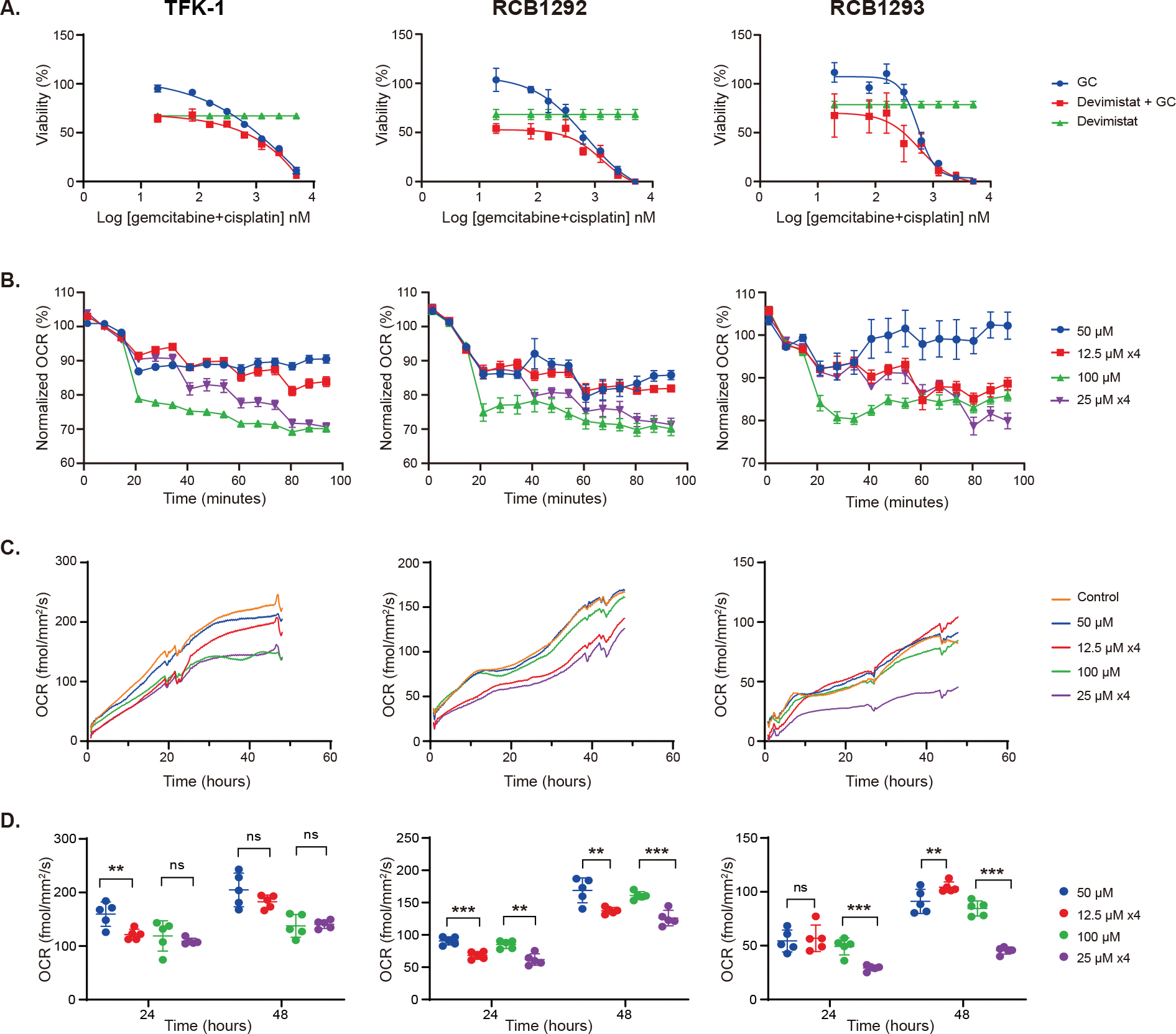
(A) Viability of the biliary cancer cell lines (TFK-1, RCB1292 and RCB1293) in response to different concentrations of gemcitabine and cisplatin (GC) alone, devimistat alone (dose less than IC50 for each cell line; 100μM for TFK-1 and RCB1292, 150μM for RCB1293), or the combination. Data presented as mean ± SD (n=4). Data were fitted into a four-parameter dose-response curve. Synergy scores for GC + devimistat showed synergism for RCB1292 (11.24; p<0.001) and RCB1293 (10.85; p<0.001), and additive effect for TFK-1 (5.47; p<0.001) per HSA model by SynergyFinderPlus; (B) Oxygen consumption rate (OCR) with devimistat via Agilent Seahorse assay using four arms: 50μM once, 100μM once, 12.5μM every 18 minutes x 4 doses, and 25μM every 18 minutes x 4 doses. No difference between divided dosing versus one-time dosing at 90-minutes. Data presented as mean ± SEM (n=5); (C) OCR with devimistat via Resipher assay using 4 arms in (B) along with control (DMSO) (n=5). (D) Quantitation of OCR change via Resipher assay at 24 hours and 48 hours (mean ± SD; n=5) using non-paired two-tail t-test. ns, not significant, **: p<0.01, ***: p<0.001.
Due to the short half-life of devimistat, we hypothesized a longer infusion time may improve drug availability and efficacy 6. To explore this potential benefit, we treated the cells with either 12.5μM or 25μM devimistat every 18 minutes for 4 times (accumulating to 50μM or 100μM, respectively), or with 50μM or 100μM once. The 100μM dose led to significantly lower OCR compared to 50μM across all three cell lines and both Agilent Seahorse and Resipher assays; Figure 1B and 1C, respectively, and Supplementary Table 1. OCR, as measured by Agilent Seahorse, dropped immediately after each addition, demonstrating the minimizing effect of devimistat on the mitochondrial respiration. At 90 minutes, cells that received 4 divided treatments had similar OCR compared respectively to arms treated once at full dose, indicating no difference between the arms; Figure 1B. A lack of benefit from divided versus one-time dosing was expected at the 90-minute analysis due to 1.34 hour half-life of devimistat. However, at 24- and 48-hour evaluations, the divided dosing led to significantly lower OCR (RCB1292 and RCB1293) when compared to one-time dose per the Resipher assay; Figure 1D.
3.2. Phase 1b Trial
A total of 20 patients with advanced BTC were enrolled onto phase 1b of the study across three institutions and received study treatment; Table 1 along with study participant representativeness in Supplementary Table 2. The median age was 65 years (range 43–75), there were 9 women (45%) and ECOG performance status was 0 or 1 in 11 and 9 patients, respectively. Nine patients (45%) had an intrahepatic cholangiocarcinoma, 8 patients (40%) had an extrahepatic cholangiocarcinoma, 3 patients (15%) had gallbladder carcinoma. Majority of patients (75%) had metastatic disease.
Table 1.
Baseline Characteristics of Patient Population
|
| ||
| Patients, N (%) | 20 (100) | |
|
| ||
| Sex, N (%) | Female | 9 (45) |
|
| ||
| ECOG performance status, N (%) | 0 | 11 (55) |
| 1 | 9 (45) | |
|
| ||
| Age, years | Median (range) | 65 (43–75) |
|
| ||
| Race/Ethnicity, N (%) | White, Non-Hispanic | 15 (75) |
| White, Hispanic | 2 (10) | |
| Asian | 1 (5) | |
| Not reported | 2 (10) | |
|
| ||
| Anatomic subtype, N (%) | Extrahepatic, Distal | 2 (10) |
| Extrahepatic, Hilar | 6 (30) | |
| Gallbladder | 3 (15) | |
| Intrahepatic | 9 (45) | |
|
| ||
| Disease extent, N (%) | Locally Advanced | 5 (25) |
| Metastatic | 15 (75) | |
|
| ||
| Prior therapy, N (%) | Radiotherapy | 3 (15) |
| Surgery | 7 (35) | |
|
| ||
| CA 19–9 at baseline, U/mL | Median (range) | 170.5 (58.5 – 1357) |
CA 19–9, carbohydrate antigen 19–9; ECOG, Eastern Cooperative Oncology Group
No DLT was observed at devimistat dose levels of 500 mg/m2 (n=1), 1000 mg/m2 (n = 1), or 1500 mg/m2 (n = 2). At 2000 mg/m2 (n = 16), one patient had DLT with grade 2 renal dysfunction (creatinine 0.91 mg/dL at baseline to 2.26 mg/dl on cycle 1 day 8). The RP2D was determined to be devimistat 2000 mg/m2, gemcitabine 1000 mg/m2, and cisplatin 25 mg/m2, based upon the posterior probability of toxicity as estimated by the TiTE continual reassessment methodology; Supplementary Table 3.
Patients in the phase 1b portion of this study received a median of 9 treatment cycles (range 3 – 19). Treatment-related adverse events in all 20 subjects are listed in Table 2. There were no grade 4 toxicities. The most common grade 3 hematologic toxicities included neutropenia (n=11, 55%) and anemia (n=4, 20%). Grade 3 non-hematologic toxicities included infection (n=3, 15%) and febrile neutropenia (n=2, 10%). Dose reductions were required in 15 (75%) patients and white blood cell growth factors were used in 4 (20%) patients. Only one patient discontinued study therapy due to toxicity (DLT in cycle 1).
Table 2.
Treatment-Related Adverse Events per Patient at Grade 2 or Higher
| Event† | Grade 2 | Grade 3 |
|---|---|---|
| Hematologic Events | ||
| Neutropenia | 1 (5%) | 11 (55%) |
| Leukopenia | 1 (5%) | 2 (10%) |
| Anemia | 3 (15%) | 4 (20%) |
| Thrombocytopenia | 3 (15%) | 2 (10%) |
| Non-Hematologic Events | ||
| Fatigue | 7 (35%) | 0 |
| Anorexia | 2 (10%) | 0 |
| Nausea/vomiting | 5 (25%) | 0 |
| Constipation | 4 (20%) | 0 |
| Diarrhea | 1 (5%) | 1 (5%) |
| Gastroesophageal reflux | 1 (5%) | 0 |
| Infection | 2 (10%) | 3 (15%) |
| Febrile neutropenia | 0 | 2 (10%) |
| Creatinine elevation | 2 (10%) | 0 |
| Thromboembolic event | 0 | 1 (5%) |
| Hyponatremia | 0 | 1 (5%) |
| Infusion related reaction | 3 (15%) | 0 |
| Hypertension | 2 (10%) | 0 |
| Alopecia | 1 (5%) | 0 |
| Hot flashes | 1 (5%) | 0 |
Only the laboratory abnormalities which required protocol treatment to be modified or treatment to be rendered were required to be reported.
In 20 patients treated on phase 1b, ORR was 45% measured by site radiologist(s), including one patient with complete response and 8 patients (40%) with partial responses; Figure 2. Median PFS is estimated to be 10 months (95% CI, 7.1 – 14.9) with two patients still on treatment; Figure 3. Median OS is not yet estimable with 14 (70%) patients still alive. The median follow-up time is 15.6 months using the reverse censoring method. The genomic data of these 20 patients is included in Supplementary Table 4.
Figure 2. Swimmer’s plot for patients treated on the Phase 1b trial.
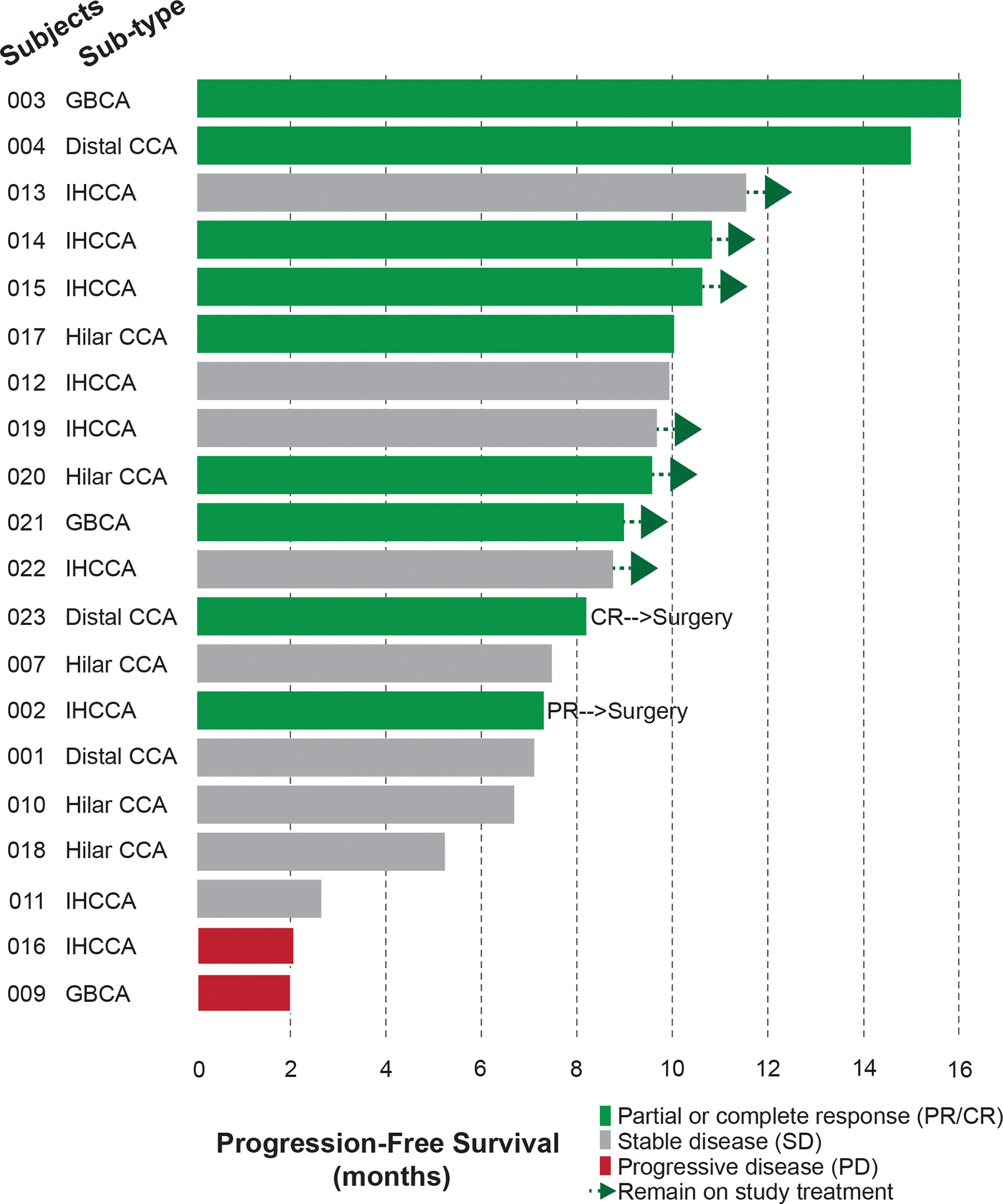
Median PFS is estimated to be 10 months (95% CI, 7.1 – 14.9) with two patients still on treatment. Two patients underwent surgical resection. CCA, cholangiocarcinoma; GBCA, gallbladder carcinoma; IHCCA, intrahepatic cholangiocarcinoma.
Figure 3. Kaplan-Meier analyses of progression-free survival and overall survival.
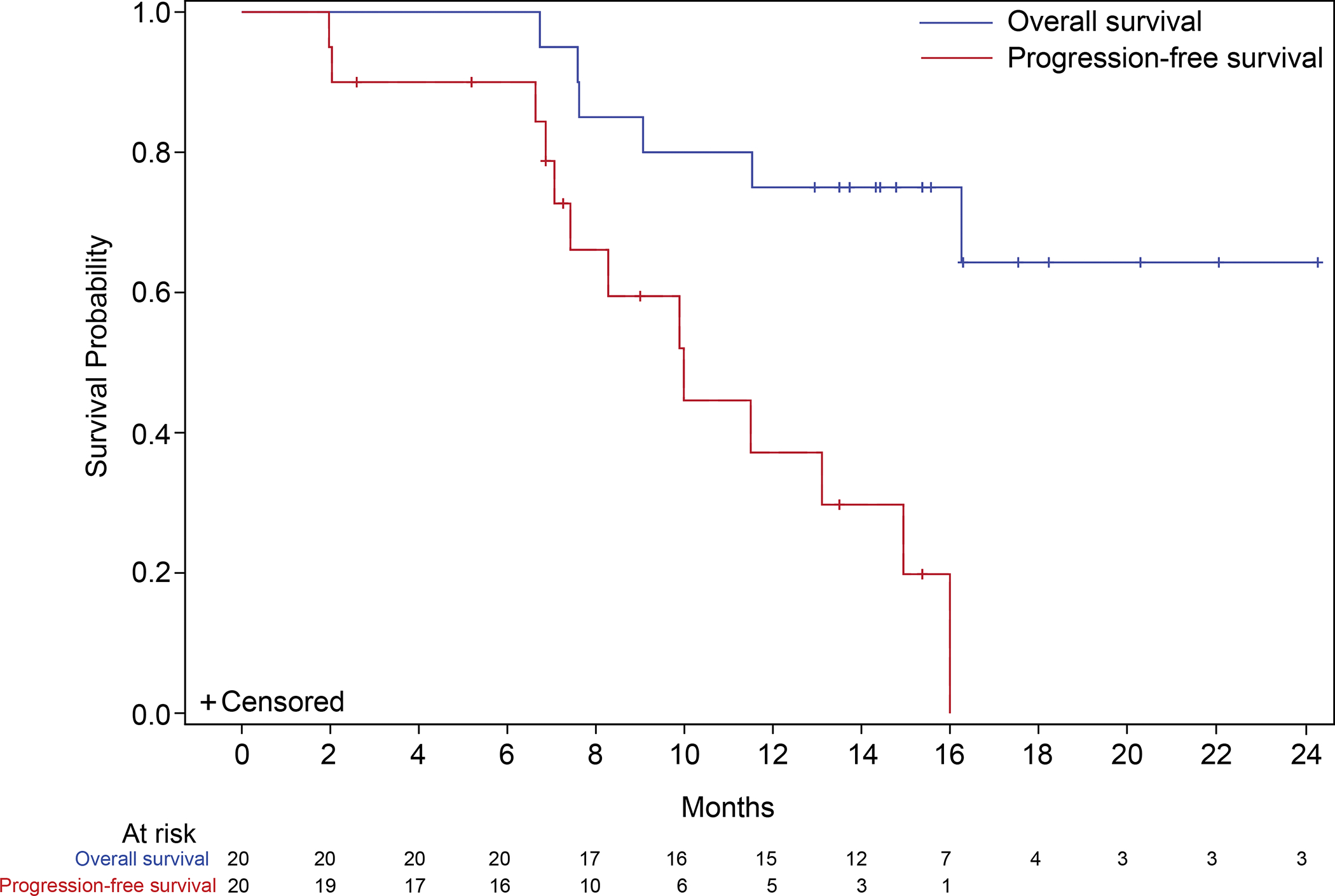
The median progression-free survival (red line) was 10.0 (95% CI, 7.1 – 14.9) months. The median overall survival (blue line) is not estimable after a median follow-up of 15.6 months.
Two patients proceeded to surgical therapy following study treatment. The first patient presented with a 16.4 cm intrahepatic cholangiocarcinoma and received 9 cycles of study therapy with interval reduction in primary mass by 63.2% to 6.0 cm; Figure 4. The patient underwent an open central hepatectomy with resection of segments I-IV, portal lymphadenectomy and cholecystectomy. Pathologic review noted complete pathologic response with extensive treatment effect and fibrosis; 3 of 3 lymph nodes were negative for carcinoma. A second patient with history of pancreaticoduodenectomy for a T3N0 distal extrahepatic cholangiocarcinoma in November 2018 was enrolled on the trial with limited intraperitoneal recurrence in July 2021, confirmed via biopsy of a peritoneal nodule measuring 1.7 cm. The patient subsequently received 8 cycles of study therapy with complete radiologic response following which the patient underwent a peritoneal metastasectomy and then heated intraperitoneal chemotherapy. On pathologic review of the two resected peritoneal lesions, only scattered cancer cells were seen with a significant treatment effect. Following surgical treatment, both patients remain free of recurrent disease at 19.0 and 8.8 months, respectively.
Figure 4. Patient with complete pathological response.
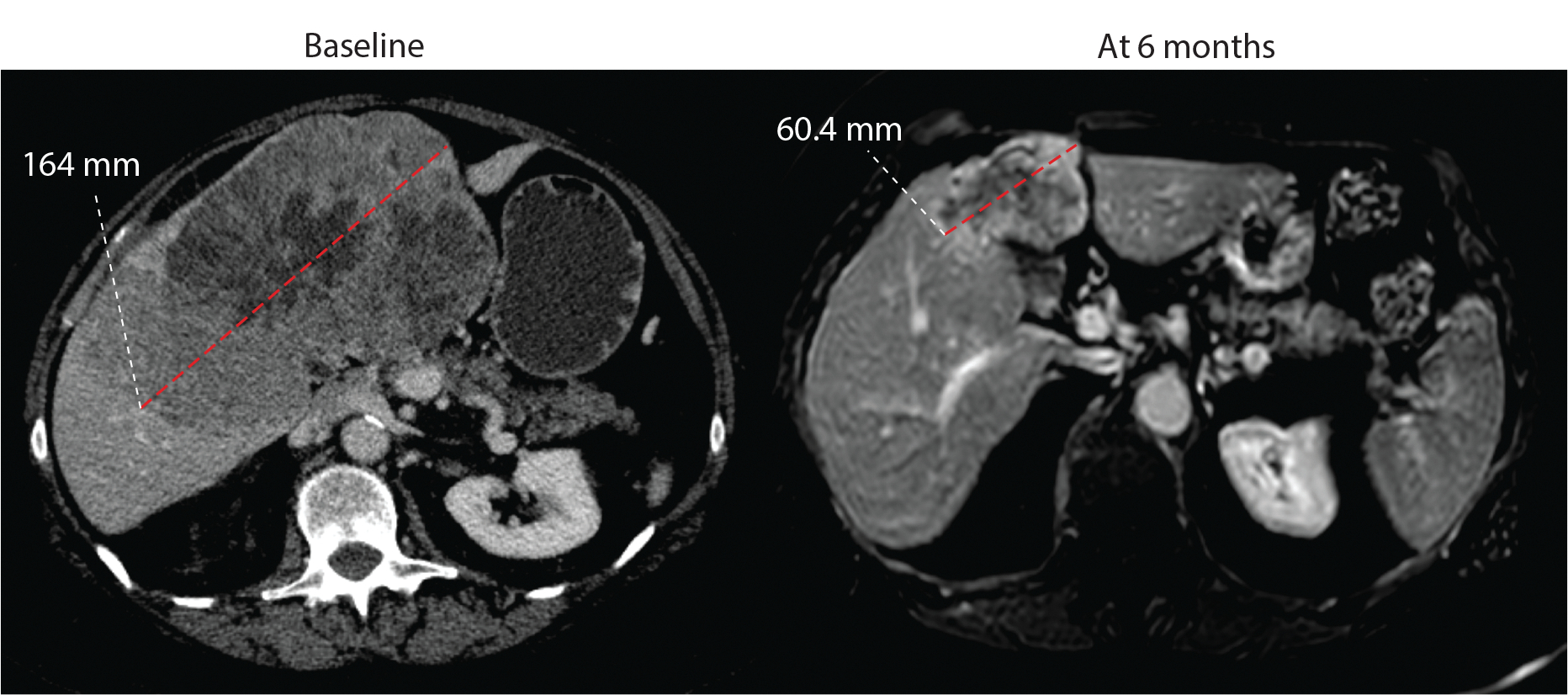
Patient 002 had a partial response with −63.2% response at 6 months following which underwent surgical resection. Pathology report noted no viable tissue with complete pathologic response.
Discussion
This phase 1b study of devimistat in combination with gemcitabine and cisplatin in patients with previously untreated advanced stage BTC successfully identified a RP2D and demonstrated a favorable safety profile.
The type and frequency of treatment-related adverse events are similar to those reported for patients treated on the ABC-02 and TOPAZ-1 trials and no unexpected toxicities were observed 2,12. The ORR, PFS, and OS appear promising; however, these initial measures of efficacy are not powered for comparison to historical data due to limited sample size. Final efficacy data will need to be evaluated in the randomized phase 2 portion of this study. Furthermore, if the randomized portion shows a significant improvement with addition of devimistat, a follow-up study investigating use of devimistat in combination with gemcitabine, cisplatin and durvalumab will be an additional area of investigation given recent advances in front line therapy options for BTC12.
Recently, a phase 3 clinical trial in previously untreated, metastatic pancreas cancer comparing devimistat plus modified FOLFIRINOX to FOLFIRINOX failed to demonstrate a significant improvement in survival (AVENGER 500). In this trial, devimistat was dosed at 500 mg/m2 with an average infusion time of 21.5 minutes (Cornerstone Pharmaceuticals, personal communication, October 19, 2022). Due to the short half-life of devimistat, we hypothesized that a longer infusion time of devimistat may improve drug availability and thus efficacy of the drug in combination with chemotherapy 6. Pre-clinical studies reported here demonstrate synergism in combination with gemcitabine and cisplatin, and significant decrease in mitochondrial respiration at higher doses (10μM versus 50μM) as well as in arms with divided dosing over an hour compared to one-time dosing. These data support the study design in this BTC trial with higher dose levels (RP2D for devimistat was 2000 mg/m2) and a prolonged infusion duration of 2 hours. Furthermore, the evaluation of devimistat with gemcitabine and cisplatin in BTC, a different malignancy with molecular profile distinct from pancreatic cancer, may render this disease to a different metabolic susceptibility to devimistat 13,14.
Following the determination of the RP2D of devimistat and the chemotherapy combination from phase 1b, additional patients are enrolling in a randomized phase 2 portion of the study with 2:1 randomization to arms A (combination regimen at RP2D) or B (gemcitabine and cisplatin alone), respectively. Randomization is stratified by disease extent - locally advanced versus metastatic cancer. The primary endpoint for the phase 2 study is the best ORR per RECIST v1.1 criteria during active study treatment. Secondary endpoints include median PFS and OS. The statistical comparison of the phase 2 portion will be between experimental treatment arm and historical ORR. The estimated ORR from the limited-sized control arm will be used to confirm the control arm has a similar response rate as the historical ORR in our patient population.
Recruitment to the randomized portion of this phase 1b/2 study completed accrual in March 2023. The phase 1b/2 results will include final description of ORR, PFS, and OS analysis in comparison to the historical data and will provide valuable data into further understanding of the role of devimistat in combination with chemotherapy as a first-line treatment option for patients with advanced BTC. In addition, exploratory analysis including genomic, transcriptomic and metabolomic analyses along with efficacy association will be reported.
Conclusions
This study found that devimistat targets mitochondrial metabolism in BTC in vitro and phase 1b results reported here indicate the combination of devimistat at 2000 mg/m2 given over 2 hours with gemcitabine and cisplatin is well tolerated with an acceptable safety profile and promising initial efficacy in this patient population.
Supplementary Material
Statement of Translational Relevance.
In this report, we describe in vitro data and results of a multicenter phase 1b clinical trial of devimistat (CPI-613) in combination with gemcitabine and cisplatin in patients with advanced biliary tract cancer. Devimistat is a novel inhibitor of key mitochondrial enzymes in the tricarboxylic acid cycle. Pre-clinical studies demonstrate synergism of devimistat in combination with gemcitabine and cisplatin, and at higher doses or prolonged exposures to devimistat, a significant decrease in mitochondrial respiration. These data inform and support the design of this phase 1b/2 trial which investigates higher doses and longer infusion times of devimistat as compared to prior investigations in pancreatic cancer. Results of the phase 1b portion of the study demonstrate safety with promising initial measures of efficacy. The recommended dose and schedule identified in phase 1b is under evaluation in a larger phase 2 trial and, if improved efficacy confirmed, would suggest further investigation of devimistat in biliary cancer and other malignancies.
Acknowledgements
The investigational study drug devimistat (CPI-613®) was supplied by Cornerstone Pharmaceuticals. The pre-clinical work was supported by Cornerstone Pharmaceuticals (PI: V. Sahai; supported F. Wuchu, D. Nagrath), Cholangiocarcinoma Foundation (PI: S. Choppara). The clinical trial was supported by Cornerstone Pharmaceuticals (PI: V. Sahai; supported D. Dippman, K. Griffith, C. Kumar-Sinha) and University of Michigan Rogel Cancer Center (P30CA046592; PI: E Fearon; supported V. Sahai, D. Dippman). D. Nagrath is supported by grants from NCI R01CA227622 and R01CA204969, R01CA222251. The authors are immensely grateful to the patients and their families for their participation, and all the investigators and research staff for enrolling patients.
Footnotes
Conflict of Interest
AM – None. KAG – None. FW – None. DBZ – Institutional grant funding from AstraZeneca, Bristol Meyers Squibb, Cornerstone, Daiichi-Sankyo, Eli Lilly, Roche/Genentech, Legend Biotech, Merck, Noxxon, SeaGen; and consultant fees from Ipsen and QED Therapeutics. CK – None. OC – Institutional grant funding from Surface Oncology and Genentech. DH – Institutional grant funding from Pfizer. TE – Institutional grant funding from Amgen, AstraZeneca, CanBas, Exelixis, GlaxoSmithKline, NeoImmuneTech. DD – None. VG – None. AA – None. OA – None. SC – None. AMC – None. DN – None. MMZ – Institutional grant funding from AstraZeneca, MedImmune and Seattle Genetics. VS – Institutional grant funding from Agios, Bristol-Myers Squibb, Celgene, Clovis, Cornerstone, Exelixis, Fibrogen, Incyte, Ipsen, Medimmune, Merck, NCI, Rogel Cancer Center, Repare, Relay, Servier, Syros and Transthera; and consultant fees from AstraZeneca, Autem, Cornerstone, Delcath Systems, GlaxoSmithKline, Helsinn, Histosonics, Incyte, Ipsen, Kinnate, Lynx Group, QED, Servier and Taiho.
Trial Registration: NCT04203160
References
- 1.Nathan H, Pawlik TM, Wolfgang CL, et al. : Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 11:1488–96; discussion 1496–7, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Valle J, Wasan H, Palmer DH, et al. : Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–81, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Stuart SD, Schauble A, Gupta S, et al. : A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab 2:4, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zachar Z, Marecek J, Maturo C, et al. : Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med (Berl) 89:1137–48, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Reid MA, Bose S, Pladna KM, et al. : Predictive targeting of mitochondrial metabolism in Acute Myeloid Leukemia patients with a lipoic acid analog. medRxiv:2021.06.03.21257935, 2021 [Google Scholar]
- 6.Pardee TS, Lee K, Luddy J, et al. : A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies. Clin Cancer Res 20:5255–64, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alistar A, Morris BB, Desnoyer R, et al. : Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol 18:770–778, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung YK, Chappell R: Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics 56:1177–82, 2000 [DOI] [PubMed] [Google Scholar]
- 9.O’Quigley J, Pepe M, Fisher L: Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics 46:33–48, 1990 [PubMed] [Google Scholar]
- 10.Krzywinski M, Schein J, Birol I, et al. : Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng S, Wang W, Aldahdooh J, et al. : SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genomics Proteomics Bioinformatics 20:587–596, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh D-Y, He AR, Qin S, et al. : Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evidence 1:EVIDoa2200015, 2022 [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Zheng Y, Yang F, et al. : The molecular biology of pancreatic adenocarcinoma: translational challenges and clinical perspectives. Signal Transduct Target Ther 6:249, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain A, Javle M: Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol 7:797–803, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available within the article, its supplementary data files and available upon request from the corresponding author.


