Abstract
A 12-year-old female presented with weight gain, edema, and shortness of breath. Lab and urine studies confirmed nephrotic syndrome and presence of a mediastinal mass, identified as a mature teratoma after resection. Nephrotic syndrome persisted despite resection and renal biopsy confirmed minimal change disease, which ultimately responded to steroid treatment. She had two relapses of nephrotic syndrome after vaccination administration, both of which occurred within eight months of tumor resection and were responsive to steroids. Autoimmune and infectious workup for other causes of nephrotic syndrome was negative. This is the first reported case of nephrotic syndrome associated with mediastinal teratoma.
Keywords: mature teratoma, nephrotic syndrome, pediatrics, minimal change disease, mediastinal mass
BACKGROUND
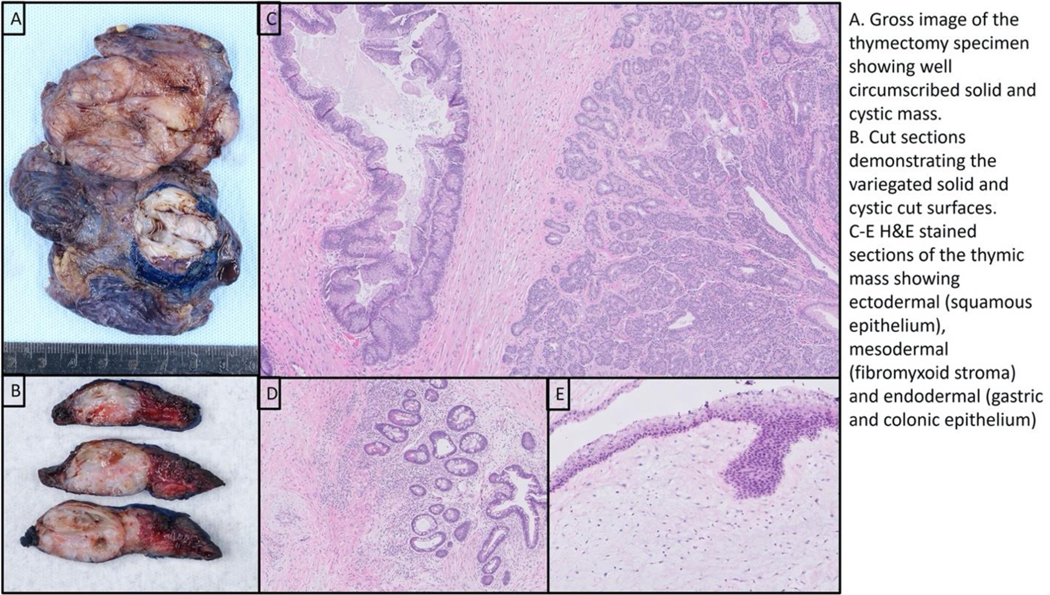
A 12-year-old previously healthy female presented to the Emergency Room with significant edema, five-pound weight gain in approximately a week, chest tightness, and shortness of breath. Her vital signs were normal for age. Her physical exam was notable for signs of fluid overload, including periorbital and lower extremity edema. Her exam was negative for splenomegaly and lymphadenopathy. Labs were notable for hypoalbuminemia with serum albumin of 1.8 g/dL, urinalysis with 3+ protein, urine protein >2,000mg/dL, urine creatinine 104.69mg/dL (incalculable protein/creatinine ratio), and hyperlipidemia consistent with nephrotic syndrome (NS). D-dimer was elevated at 1,084 ng/mL, prompting CT imaging given concern for possible pulmonary embolism in the setting of NS, chest tightness, and shortness of breath. A chest CT did not identify any pulmonary emboli, rather demonstrated enhancing nodularity within the anterior mediastinum, concerning for an underlying mass. Follow up chest MRI showed a heterogeneous thymic mass measuring 4.1 × 3.8 × 5.1 cm without mediastinal hilar adenopathy. She was admitted for treatment of NS and evaluation of the mediastinal mass. Flow cytometry of peripheral blood was negative and alpha fetoprotein and beta-human chorionic gonadotropin were both within normal range. On the sixth day of hospitalization, she underwent thoracoscopic resection of the mediastinal mass and a superior mediastinal lymph node. Pathology confirmed mature teratoma with negative margins. (Fig. 1)
Figure 1.
Mediastinal Mass
Work-up for secondary infectious and autoimmune causes of NS was negative. Infectious studies included EBV PCR, EBV Ab panel, Bartonella, Brucella, Histoplasma Ag, dimorphic fungi PCR, Cocci ID, HIV, and CMV. Autoimmune studies included ASO, ANA, anti-dsDNA, C3, and C4.
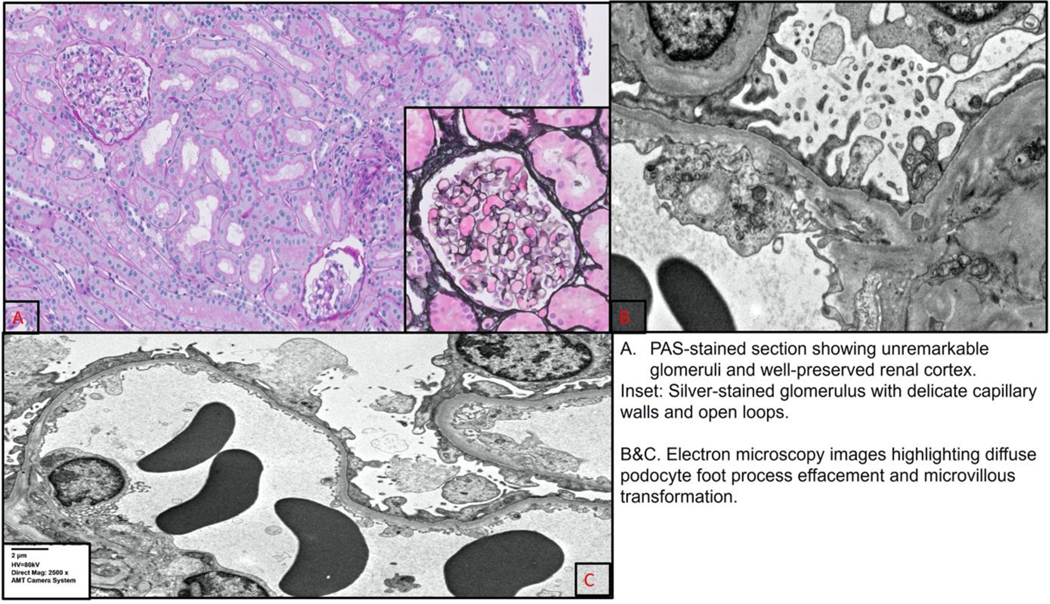
Her NS was managed initially with albumin and furosemide. She did not undergo a kidney biopsy and was not started on steroids as the NS was presumed to be likely secondary to the mediastinal mass. She continued to have nephrotic range proteinuria and generalized edema one-month post-surgical resection of her mediastinal mature teratoma. Thus, a kidney biopsy was performed at that time with pathology consistent with minimal change disease (MCD). The renal biopsy demonstrated unremarkable glomeruli with no chronic interstitial fibrosis and tubular atrophy by light microscopy. Immunofluorescence examination was negative for immune-complex mediated disease. Electron microscopic examination demonstrated extensive epithelial cell foot process effacement, diagnostic of podocytopathy. (Fig. 2)
Figure 2.
Kidney Biopsy
She was started on 60 mg/day of prednisone following the biopsy results and entered remission after five weeks of treatment. Fortunately, she did not develop serious complications of NS such as infection or blood clot prior to achieving remission. She was gradually weaned off of steroids and was doing well for several months but had a NS relapse following her second SARS-CoV2 vaccine requiring a second course of steroids. She remained steroid sensitive with resolution of this relapse. She relapsed a second time approximately 8 months after resection after receiving her annual influenza vaccine and, again, was responsive to steroids. She has remained in remission thereafter and did not experience relapse with subsequent SARS-CoV2 booster. At the time of this report, she has been in remission for over a year. She underwent surveillance MRI chest at 6 months post-surgical resection, which did not show any evidence of recurrent disease.
OBSERVATIONS
Mediastinal mature teratomas are extremely rare in children. Germ cell tumors (GCTs) as a group can occur anywhere in the body from the pineal to coccygeal region, though are most found in the coccygeal region, testes, ovaries, and brain. GCTs represent only 3–5% of all pediatric cancers and only 2–4% of all germ cell tumors are located in the mediastinum.1 Mature teratomas represent only a fraction of these GCTs and are more often found in the coccyx at birth, in testicles during the first 6 months of life, and in ovaries from ages 9–15.2
While data is limited due to the very rare occurrence of mediastinal mature teratomas, the prognosis is excellent with complete surgical resection alone. A publication by De Pasquale and colleagues included 7 patients with mature teratomas treated with primary resection all of whom had both event free survival and overall survival of 100% with median follow up of 89 months.1 Similarly Gobel and colleagues describe a cohort of 329 patients with teratomas in which 9 were mediastinal. None with mediastinal teratomas experienced death or relapse with the shortest observation period of 18 months in studied cases.2 A publication by Yalçın and colleagues followed 24 patients with mediastinal GCTs, 13 of whom had mediastinal mature teratomas who all underwent surgical resection alone and none of whom experienced relapse or death during the median follow up period of 207 months.3 The cornerstone of treatment is surgical resection and there is no documented experience in the literature demonstrating a need for additional treatment. Our patient is the first reported case of NS associated with a mediastinal teratoma. NS associated with malignancies is uncommon but well documented, with membranous nephropathy being the most common histologic finding seen in patients with solid tumors and MCD seen more commonly in association with Hodgkin Lymphoma.4 The literature documents four cases of mature teratomas associated with NS, and all four cases were associated with ovarian mature teratomas.4–7 The earliest case published in 1989 describes a 7 year-old female who presented with NS refractory to steroids. She was ultimately found to have an ovarian mass proven to be mature teratoma. NS remission was achieved 10 days after surgical resection of the teratoma.7 A second case reports a 36 year-old female with NS in the context of a mature ovarian teratoma who responded briskly to prednisone treatment and tumor resection.4 A third case reports a 55 year-old female with NS in the context of mature cystic teratoma of the ovary who achieved complete remission of the NS with steroids within 3 weeks of surgical removal of the tumor.6 Renal biopsies of the latter two cases confirmed MCD. The last case describes a 16-year-old female with NS and ovarian mature teratoma. Renal biopsy showed mesangial proliferative glomerulonephritis, which responded to tumor resection and cyclosporine.5
In general, pathophysiology of a paraneoplastic syndrome resulting in MCD is based on hypotheses that humoral tumor factors, for example cytokines, result in damage to podocyte integrity causing protein leakage. The mechanisms of proteinuria in MCD still remain incompletely understood, however it has been found that both cellular and humoral immune responses are altered in MCD. Cytokines and growth factors, especially vascular endothelial growth factor (VEGF), also seem to play a role in increasing glomerular basement membrane permeability. 15 In the wider literature of paraneoplastic glomerulonephritis, membranous nephropathy is the most common renal finding as a result of paraneoplastic glomerular nephropathy from solid tumors. Membranous nephropathy is hypothesized to be the result of tumor inducing target auto-antigens that result in deposition of immune complexes into the glomerular membrane, thus resulting in the characteristic membrane thickening seen histologically in membranous nephropathy. Groundbreaking discoveries of target antigens specifically implicated in glomerular diseases and malignancy include thrombospondin type q domain containing protein 7A (THSD7A) and neural epidermal growth factor like 1 protein (NELL-1), both of which are target podocyte antigens that have been stained positive on case reports of solid tumors and suggest a causal link between solid tumor and paraneoplastic glomerulonephropathy.8 To date, the mechanism of renal injury in paraneoplastic syndromes remains hypothetical rather than causal.
The diagnosis of a paraneoplastic process can be considered if there is a temporal relationship between NS and malignancy, if resolution of NS is observed with cure or remission of malignancy, if recurrence of NS accompanies recurrence of malignancy, or if no alternate explanation for the NS is identified.9 The ultimate proof of causality is the detection of tumor antigens in the glomerular basement membrane, however such cases are rare. Theoretically, the diagnosis should rely on the aforementioned criteria, however relatively few papers have reported NS remission following cure or remission of malignancy alone, likely due to the fact that majority of cancers are incurable at presentation.11
Our patient demonstrated a temporal relationship between the diagnosis of mediastinal teratoma and NS, though it remains unclear how long the teratoma was present before it was discovered by imaging. She did not have any previous chest imaging prior to her presentation with NS. At initial presentation of NS, secondary causes are always sought, especially in children who are older in age (>12 years). In our patient, workup for secondary infectious and autoimmune causes were negative. In the absence of other secondary causes and identifiable triggers for NS, we postulate that the mediastinal teratoma was the trigger for her initial NS presentation based on the patient’s clinical data we have to date. As mentioned above, the pathophysiology of MCD is thought to be due to immune dysregulation affecting the podocytes. Thus, it seems plausible that the presence of a teratoma, which can result in an immune and inflammatory response, may have been the NS trigger for our patient. It is also important to note that there is an identifiable trigger in many cases of nephrotic syndrome relapse, with the most common being acute respiratory illness. 13 In several studies examining relapses in children with nephrotic syndrome, around 60% of all relapses were associated with acute respiratory illnesses. 12–14 In one retrospective study consisting of 367 children followed for a mean of 3.1 years, there were 450 relapses. Of the relapses, 62.8% were associated with infections, 10.4% with poor compliance, and 26.6% had no identifiable trigger. 12 Other commonly identified triggers include other infections such as urinary tract infection, stress, and atopic disease. Although the precise mechanism underlying the pathogenesis of nephrotic syndrome has yet to be determined, the tendency of the disease to manifest and relapse with viral illnesses, atopic episodes, and lymphoma, as well as response to steroids lends support to the immune dysregulation hypothesis. 13
Our patient notably did not enter remission of her NS one month following tumor resection. Some cases report disappearance of proteinuria in a matter of weeks, 7 others report months.11 Our team decided to wait one-month post-surgery prior to starting her on steroid therapy after considering the risks of prolonged NS and its potential complications. Hence, it is unclear if the patient would have entered spontaneous NS remission if given more time.
Although her NS resolved after removal of the teratoma and steroid therapy, we believe that the lag in timing and brief but proximal relapses in relation to receiving her SARS-COV2 and then influenza vaccines were not evidence of a separate etiology. In particular, she had no further relapse with a SARS-COV2 booster received approximately 10 months after resection, suggesting recovery of podocyte injury by that time. While we believe the subsequent episodes of NS were still temporally related to the mediastinal teratoma, these vaccine related relapses point to either an immunologic mechanism or immunologic vulnerability of the underlying podocyte injury.
Limited guidance exists on surveillance of fully resected mediastinal teratomas. Therefore, a plan for surveillance was extrapolated from ovarian mature teratoma literature, acknowledging that rates of recurrence are higher in ovarian teratomas compared to mediastinal counterparts. Our patient had no elevation of her tumor markers at initial presentation and her teratoma was fully resected, therefore we do not plan to pursue ongoing monitoring of tumor markers.10 As previously discussed, a surveillance chest MRI at 6 months post resection was reassuring against recurrence of tumor. Based on a discussion amongst multidisciplinary members present at our tumor board, we will plan for an additional surveillance chest MRI at 18 months post resection, and if negative, we will not pursue further imaging surveillance unless clinically indicated.
CONCLUSIONS
To date, this is the first reported case of NS presenting in association with a mediastinal mature teratoma. Only four other cases of teratomas in association with NS have been reported in the literature, all of which were ovarian teratomas treated with tumor resection; three responded to short term steroids and the fourth responded to cyclosporine.
Despite the challenge of proving causal versus incidental correlation of the teratoma to NS in this case report, we believe this case highlights several important management considerations for providers. First, this case highlights the importance of chest imaging in evaluating patients with initial NS presentation, especially those with respiratory symptoms, as shortness of breath is not a classic feature of NS in the absence of significant pleural effusions. In addition, this case corroborates the need for steroid treatment in addition to tumor resection when NS is encountered in the setting of a teratoma, regardless of tumor location. The common approach to treatment of NS when a tumor is temporally identified is to permit for spontaneous resolution if tumor resection is feasible. Based on this experience we advocate for earlier initiation of steroids even if a teratoma is identified in order to avoid prolonged proteinuria and its potential complications.
Sources of Support:
Research reported in this publication was supported by both the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR003143 as well as the National Institute of Mental Health under Award Number 2T32MH01990828. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.De Pasquale MD ebor., Crocoli A, Conte M, et al. Mediastinal Germ Cell Tumors in Pediatric Patients: A Report From the Italian Association of Pediatric Hematology and Oncology. Pediatr Blood Cancer. 2016;63(5). doi: 10.1002/pbc.25895 [DOI] [PubMed] [Google Scholar]
- 2.Gobel U, Calaminus G, Engert J, et al. Teratomas in infancy and childhood. Med Pediatr Oncol. 1998;31(1). doi: [DOI] [PubMed] [Google Scholar]
- 3.Yalçin B, Demir HA, Tanyel FC, et al. Mediastinal germ cell tumors in childhood. Pediatr Hematol Oncol. 2012;29(7). doi: 10.3109/08880018.2012.713084 [DOI] [PubMed] [Google Scholar]
- 4.Jeroudi A, Kadikoy H, Gaber L, et al. Profound nephrotic syndrome in a patient with ovarian teratoma. Saudi J Kidney Dis Transpl. Published online 2013. doi: 10.4103/1319-2442.113883 [DOI] [PubMed] [Google Scholar]
- 5.Kilis-Pstrusinska K, Szajerka U, Zwolinska D. Unspecific increase of tumor markers in a girl with nephrotic syndrome and ovarian teratoma. Ren Fail. Published online 2013. doi: 10.3109/0886022X.2013.780614 [DOI] [PubMed] [Google Scholar]
- 6.Benabdellah N, Izzedine H, Bentata Y, Haddiya I. Ovarian tumor and glomerulopathies: Case report and review of the literature. Pan Afr Med J. Published online 2019. doi: 10.11604/pamj.2019.34.75.6008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauvais P, Vaudour G, Gibod LB, Levy M. Membranous nephropathy associated with ovarian tumour in a young girl: recovery after removal. Eur J Pediatr. 1989;148(7):624–625. doi: 10.1007/BF00441515 [DOI] [PubMed] [Google Scholar]
- 8.Jeyabalan A, Trivedi M. Paraneoplastic Glomerular Diseases. Adv Chronic Kidney Dis. 2022;29(2):116–126.e1. doi: 10.1053/j.ackd.2022.02.009 [DOI] [PubMed] [Google Scholar]
- 9.Bacchetta J, Juillard L, Cochat P, Droz J-P. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. 2009;70(1):39–58. doi: 10.1016/j.critrevonc.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 10.Łuczak J, Bagłaj M. Ovarian teratoma in children: a plea for collaborative clinical study. J Ovarian Res. 2018;11(1):75. doi: 10.1186/s13048-018-0448-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronco Pierre M. “Paraneoplastic glomerulopathies: new insights into an old entity.” Kidney International 56.1 1999. 355–377. [DOI] [PubMed] [Google Scholar]
- 12.Moorani KN Infections are common a cause of relapse in children with Nephrotic syndrome. Pak Paed J. 2011. 35(4), 213–9. [Google Scholar]
- 13.Uwaezuoke SN. Steroid-sensitive nephrotic syndrome in children: triggers of relapse and evolving hypotheses on pathogenesis. Ital J Pediatr. 2015;41:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald N, Wolfish N, McLaine P, Phipps P, Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. The Journal of Pediatrics. 1986; 108(3): 378–382 [DOI] [PubMed] [Google Scholar]
- 15.Bacchetta J, Juillard L, Cochat P, Droz JP. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. 2009;70(1):39–58. [DOI] [PubMed] [Google Scholar]