Abstract
Coronary microvascular disease (CMD) causes myocardial ischemia in a variety of clinical scenarios. Clinical practice guidelines support routine testing for CMD in patients with ischemia with non-obstructive coronary artery disease (INOCA). Invasive testing to identify CMD requires Doppler or thermodilution measures of flow to determine the coronary flow reserve and measures of microvascular resistance. Acetylcholine coronary reactivity testing identifies concomitant endothelial dysfunction, microvascular spasm, or epicardial coronary spasm. Comprehensive testing may improve symptoms, quality of life, and patient satisfaction by establishing a diagnosis and guiding targeted medical therapy and lifestyle measures. Beyond INOCA, testing for CMD may play a role in patients with acute myocardial infarction, angina following coronary revascularization, heart failure with preserved ejection fraction, Takotsubo syndrome, and after heart transplantation. Additional education and provider awareness of CMD and its role in cardiovascular disease is needed to improve patient-centered outcomes of ischemic heart disease.
Keywords: angina pectoris, Microcirculation, Microvascular Angina, Acetylcholine, Hyperemia
Keywords: ANOCA, acetylcholine, adenosine, INOCA, angina, coronary flow reserve, coronary microvascular dysfunction, coronary spasm, index of microcirculatory resistance, ischemia, ischemic heart disease, microcirculation, microvascular angina, provocative testing
Background
Although the focus over the past century has been on the diagnosis and treatment of epicardial coronary artery disease (CAD), coronary microvascular disease (CMD) is an important cause of ischemic symptoms and contributor to adverse outcomes.1–3 Increasing awareness of CMD has led to renewed interest in the diagnosis. This review describes the clinical implications of CMD and relevant invasive methods for CMD assessment in the cardiac catheterization laboratory.
Anatomy and Physiology of the Coronary Microcirculation
The coronary vasculature is divided into three compartments (Figure 1): large epicardial conductive vessels, intermediate size pre-arterioles, and small intramyocardial arterioles and capillaries that are too small to image in vivo.4 Large conductive epicardial vessels (caliber >500 μm) account for only ~5% of coronary resistance to blood flow. Coronary pre-arterioles (100–500 μm) maintain stable pressures in the setting of variability in coronary perfusion pressures and account for 20% of the resistance to coronary flow. Small caliber intramyocardial coronary arterioles are responsible for 60% of coronary resistance to flow, and capillaries account for the remaining 15% of coronary resistance.4 At rest, coronary arterioles have a high resting tone, but they can vasodilate via endothelium-independent pathways in response to metabolic signaling from the myocardium in the setting of increased oxygen demand. As a result of arteriolar vasodilation and decreased coronary resistance, the healthy coronary circulation can increase blood flow and oxygen delivery >5-fold above baseline under physiologic conditions in the setting of maximal hyperemia.
Figure 1.
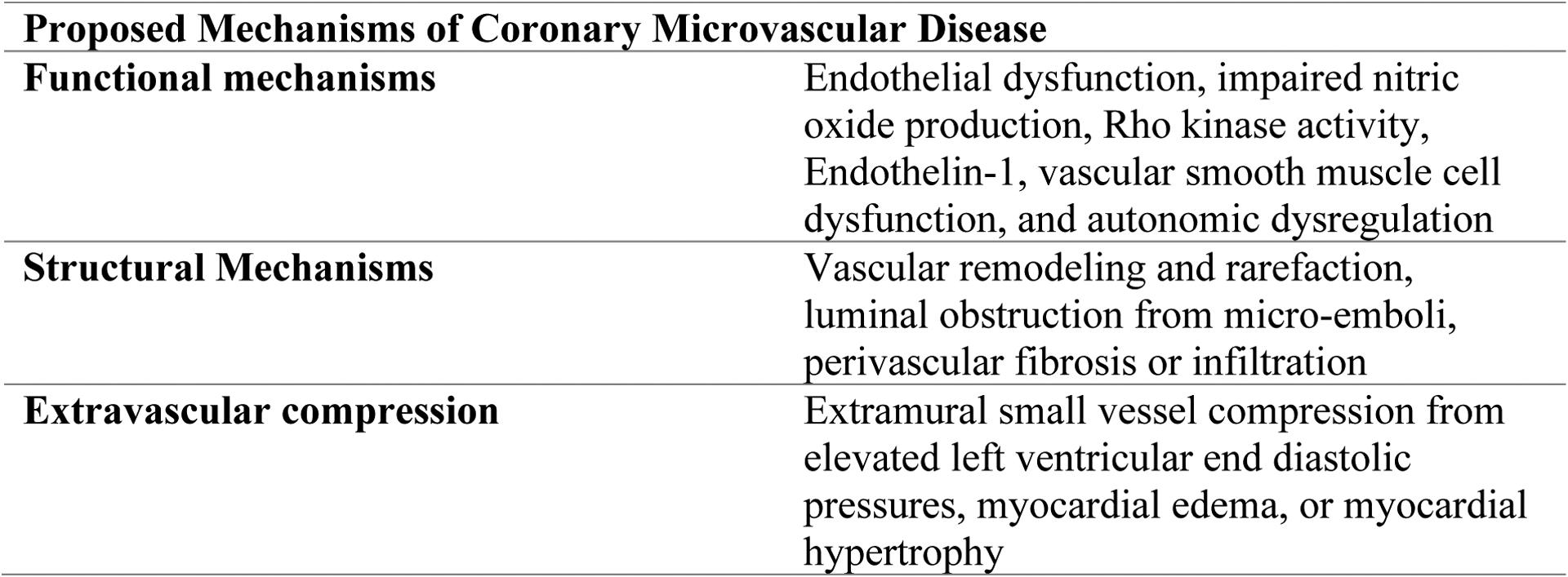
Anatomy of the Coronary Microcirculation and Potential Mechanisms of CMD
Pathobiology of Coronary Microvascular Disease
CMD represents a spectrum of functional and structural abnormalities that may be isolated or present in combination with abnormalities in the epicardial vessels or myocardial disorders.4 Examples of epicardial disorders include atherosclerotic CAD, muscle bridges, or coronary spasm. Thus, a proposed CMD classification scheme includes the following subtypes: (1) primary CMD in the absence of myocardial disease or obstructive CAD, (2) CMD in primary myocardial diseases, (3) CMD in obstructive CAD post myocardial infarction (MI), and (4) iatrogenic CMD associated with reperfusion injury and microvascular distal embolization following coronary revascularization.4
Mechanisms of CMD may be multifactorial. Functional etiologies of CMD include impaired relaxation, or in the case of microvascular spasm, transient vasoconstriction of the microcirculatory arterioles. This may be caused by endothelial dysfunction with insufficient nitric oxide (NO) production, or due to direct endothelin- or rho-kinase mediated constriction of microcirculatory vascular smooth muscle.4–7 In other cases, CMD may be characterized by an abnormal loss of microcirculatory tone in resting conditions, with little capacity for further vasodilation. Structural causes of CMD may include microvascular remodeling with narrowing of the lumen due to intimal and medial smooth muscle cell hypertrophy, fibrosis of the intramyocardial arterioles and capillaries, perivascular fibrosis (from inflammation or injury), intravascular platelet plugging, or capillary rarefaction, all of which may be associated with traditional risk factors for atherosclerosis. Inflammation, platelet dysfunction, hormonal imbalances and autonomic dysfunction have also been proposed as contributory factors. Extrinsic (extraluminal) microcirculatory compression from myocyte hypertrophy, infiltrative cardiomyopathies, or elevated left ventricular end diastolic pressures, may also effectively cause resistance to microcirculatory flow that may manifest as CMD. Potential mechanisms of CMD are depicted in Figure 1.
Invasive Approaches to Assess the Microcirculation
Coronary Flow Reserve
Since the coronary microcirculation cannot be visualized by angiography in vivo, invasive assessments are based on coronary flow (Table 1). The dynamic capacity of the coronary circulation to augment blood flow in response to maximal demand is expressed as the coronary flow reserve (CFR), defined as the ratio of maximal achievable coronary blood flow during hyperemia to that at rest. As CFR represents an integrated measure of coronary epicardial and microvascular flow, CFR is not specific to microcirculation and can be significantly affected by epicardial coronary stenoses, resting hemodynamics, and baseline coronary blood flow.8 In the absence of epicardial stenosis, impaired CFR <2 to 2.5 is a diagnostic hallmark of CMD and a well-established prognostic indicator of adverse long-term outcomes.2,3,9–11
Table 1:
Invasively derived measures of coronary microvascular function
| Measure | Technique | Abnormal Threshold Value | Formula |
|---|---|---|---|
| Coronary Flow Reserve (CFR) | Intracoronary Doppler or Thermodilution | < 2.0 – 2.5 | Doppler: APV hyperemia / APV rest Bolus Thermodilution: Tmn rest / Tmn hyperemia Continuous Thermodilution: Absolute Coronary flow hyperemia / Absolute Coronary flow rest |
| Index of Microcirculatory Resistance (IMR) | Intracoronary Thermodilution (Bolus dose) | ≥ 25 | Pd x Tmn during hyperemia |
| Hyperemic microvascular resistance (hMR) | Intracoronary Doppler | ≥ 2 – 2.5 | Pd / APV at hyperemia |
| Minimal microvascular resistance (mMR) | Intracoronary Doppler | Not Defined | Pd / APV at hyperemia during wave free period of diastole |
| Resistive reserve ratio (RRR) | Intracoronary Doppler or Thermodilution | ≥ 1.7–3.5 | Baseline microvascular resistance (BMR) / hyperemic microvascular resistance (HMR) |
| R micro | Intracoronary Thermodilution (Continuous Infusion) | ≥ 500 Woods units | Rmicro = Pd/absolute coronary flow |
| Microvascular Resistance Reserve (MRR) | Intracoronary Thermodilution (Continuous Infusion) | Not Defined | MRR = (CFR / FFR) x (Pa at rest / Pa at hyperemia) |
APV: Average peak velocity.
Pd: Distal coronary pressure
Invasive evaluation of CFR requires coronary thermodilution or Doppler-based assessments of coronary blood flow before and after a hyperemic stimulus. Hyperemia is typically induced in the catheterization laboratory by administering an endothelium independent (smooth muscle relaxing) agent such as Adenosine or Papaverine (Table 2). Intracoronary Doppler, the most established method of assessing coronary flow, uses a piezoelectric ultrasound transducer mounted at the tip of a coronary guidewire to measure average peak velocity (APV), the time-averaged peak over several cardiac cycles (Figure 2A). Coronary guidewires with a Doppler transducer alone (Doppler wire, Philips) or mounted alongside a pressure transducer (Combowire, Philips) can be used. Careful wire positioning should be guided by the auditory quality and intensity of the Doppler signal, visual appraisal of the Doppler envelope, and modification of the signal to noise ratio to optimize the Doppler signal. CFR is calculated as APV (during hyperemia)/APV (at rest), without the need to measure cross sectional area, as the latter remains relatively unchanged between conditions. Given the learning curve required for optimal Doppler signal acquisition, approximately 10% of studies yield poor quality data,9 and this method of assessment is best used at centers with substantial experience.
Table 2:
Hyperemic stimuli in the cardiac catheterization laboratory
| Drug | Dose | |
|---|---|---|
| Adenosine | IV Infusion | 140 μg/kg/min IV |
| IC Bolus | 100 μg in RCA or 200 μg in LCA | |
| IC Infusion Saline | IC Infusion * | 20cc/min infusion |
| IC Papaverine | IC Bolus | 8 mg in RCA or 12 mg in LCA |
| Regadenoson | IV Bolus | 400 μg IV over 10 seconds |
| Nitroprusside | IC Bolus | 0.6 μg/kg |
Via a dedicated infusion microcatheter.
Figure 2.
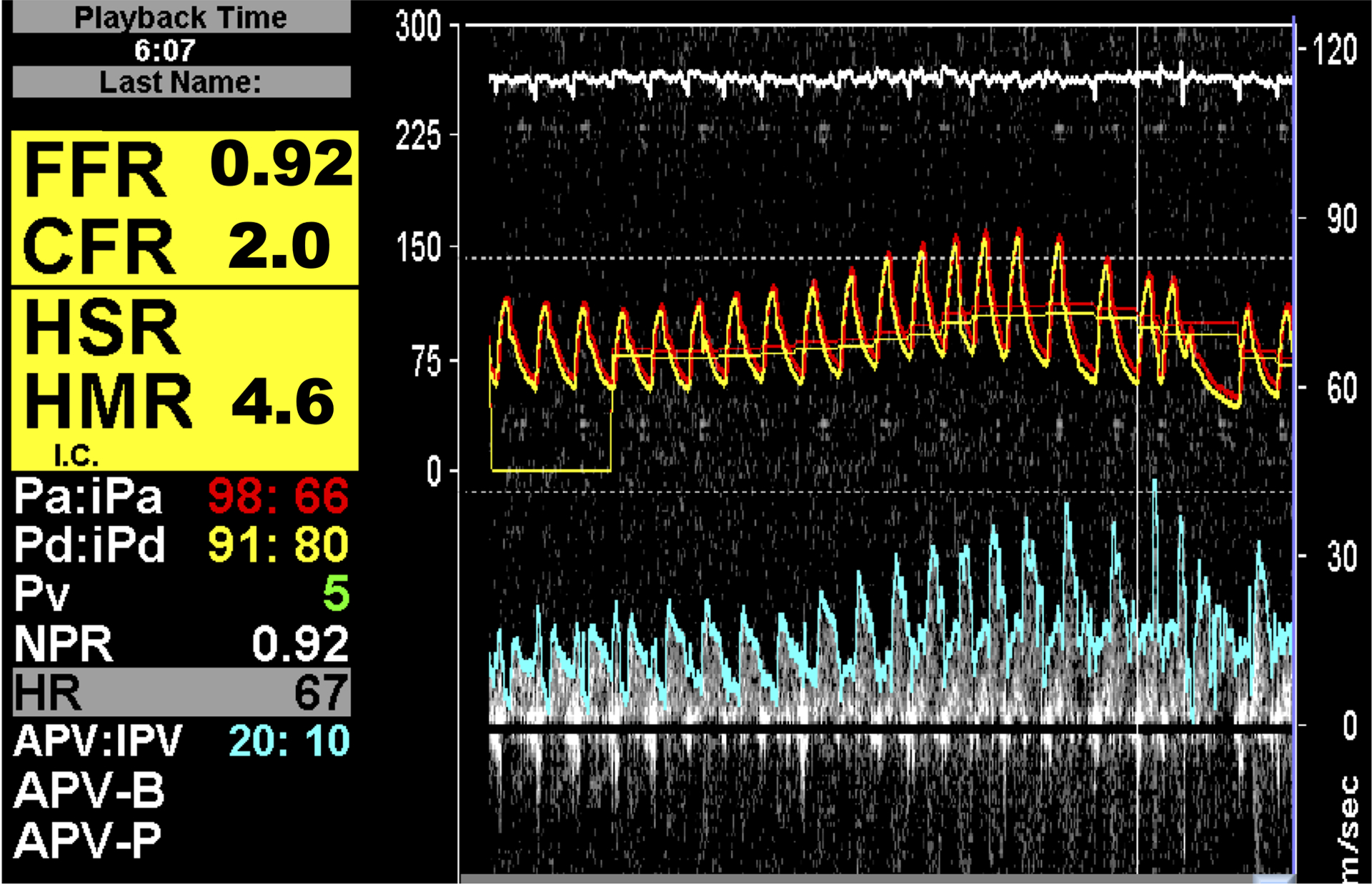
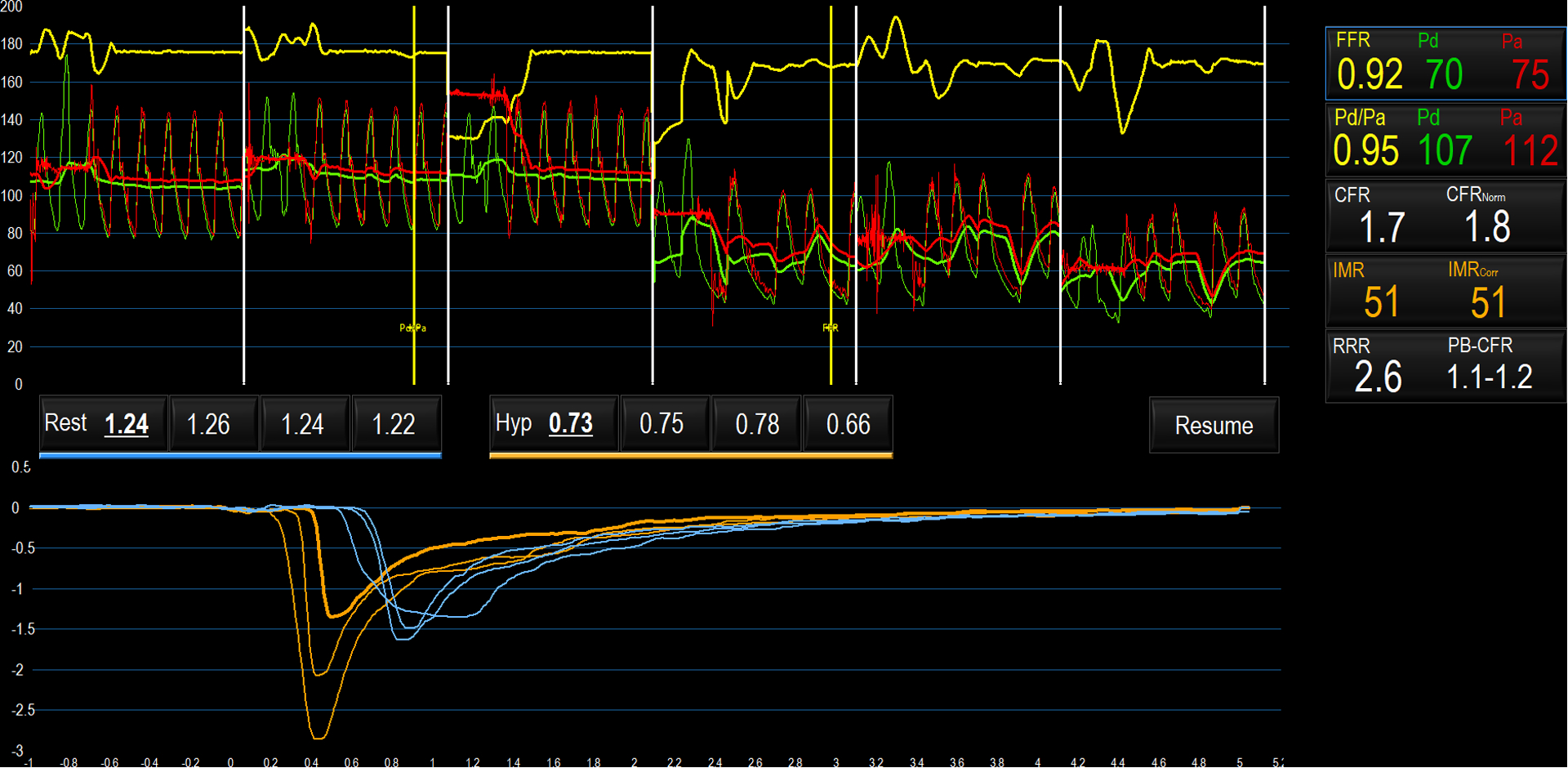
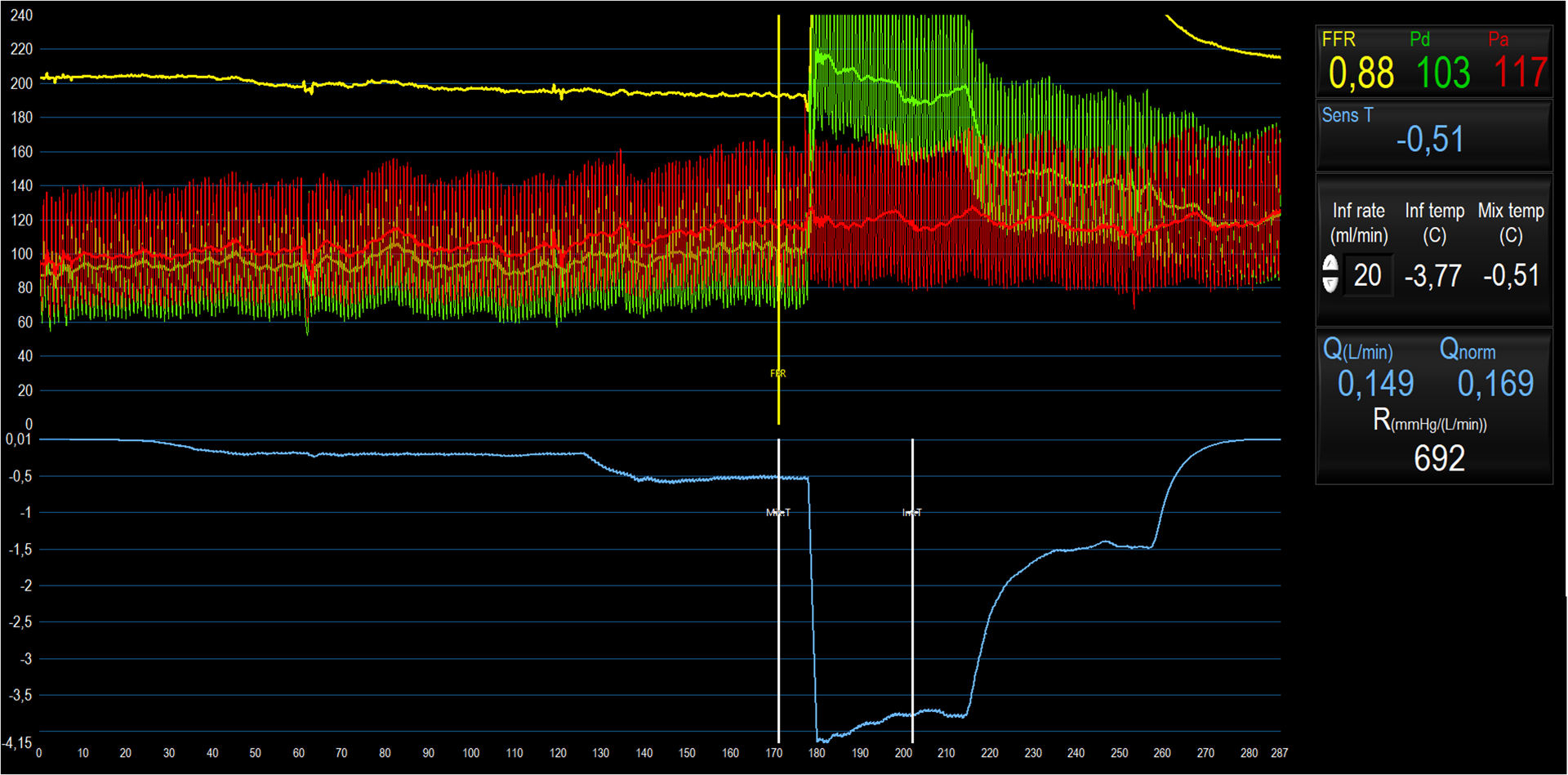
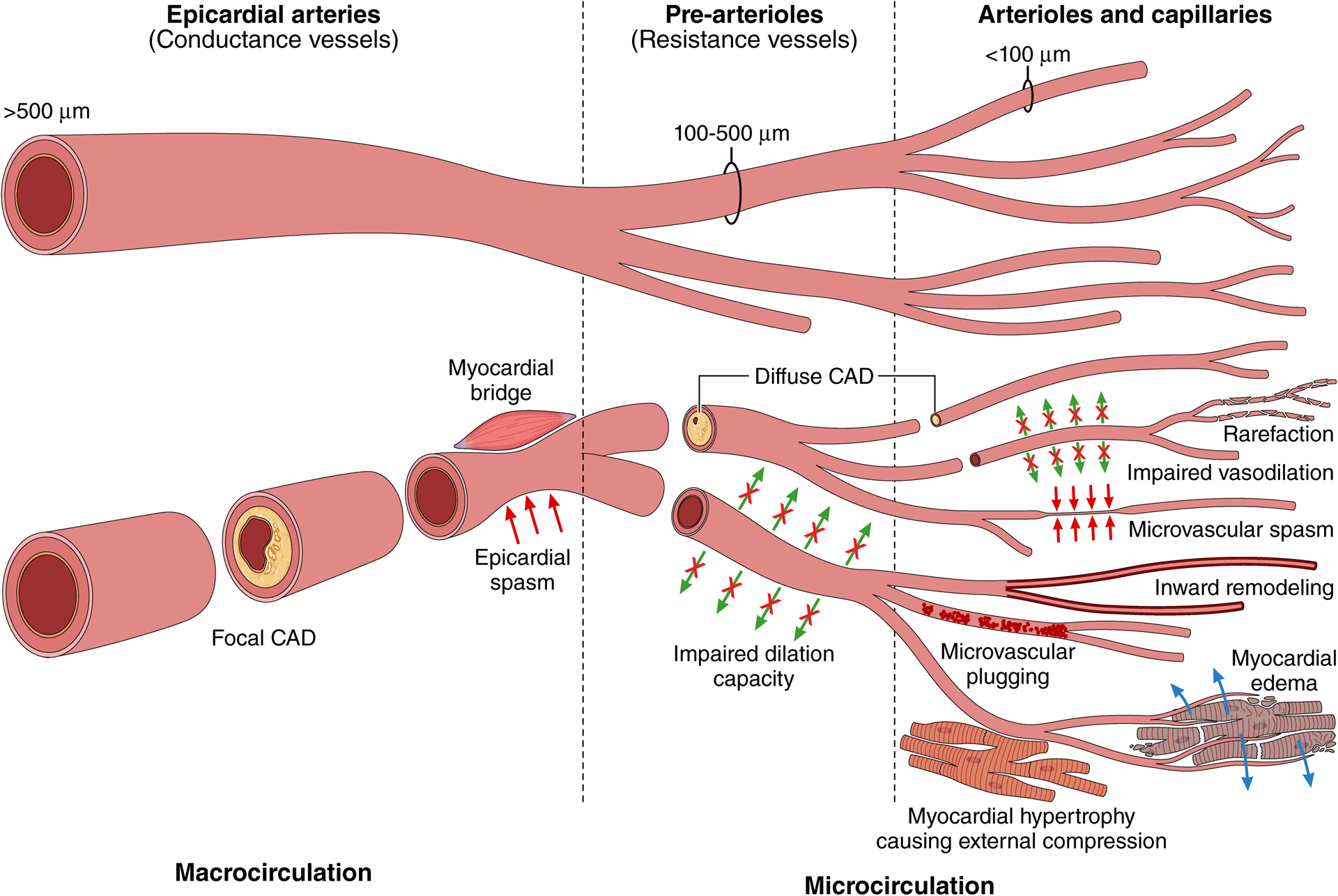
Coronary flow measurements by Doppler (Panel A), Bolus Thermodilution (Panel B), and Continuous Thermodilution (Panel C)
A: Average peak velocity (APV) is shown at rest and at peak hyperemia.
B: Pressures are shown on the y-axis in the upper panel. Green tracings reflect the distal coronary pressure, red tracings indicate aortic pressure. In the lower panel, temperature is shown on the y-axis. Blue thermodilution curves were assessed at baseline; yellow curves were measured at peak hyperemia. The x-axis indicates time. CFR and IMR are calculated.
C: Pressures are shown on the y-axis in the upper panel. Green tracings reflect the distal coronary pressure, red tracings indicate aortic pressure. Blue tracings indicate temperature recorded by the distal thermistor. The y-axis is temperature; x-axis is time. At 10–130 seconds, the thermistor is positioned in the distal coronary artery with saline infusion Qi=10ml/min (T at rest); 130–180 seconds: Qi=20ml/min (T at hyperemia); 180 seconds: rapid withdrawal of thermistor to Rayflow catheter tip; 190–220 seconds: Qi= 20ml/min (Ti at hyperemia); 220–250 seconds: Qi=10ml/min (Ti at rest); 250–280 seconds: infusion discontinued. Q at rest and hyperemia are calculated, from which CFR, MR at rest and MR at hyperemia can be derived.
Coronary flow can also be estimated using thermodilution techniques based on the indicator-dilution theory, where the indicator is the temperature difference between room temperature saline and blood (Figure 2B). Temperatures are measured using a coronary guidewire with two thermistors at a fixed distance apart, with the shaft of the wire itself acting as the proximal thermistor (PressureWire X, Abbott Vascular). To allow adequate mixing of saline with blood, the distal thermistor should be positioned >60mm from the tip of the guiding catheter during thermodilution measurements.12 Thermodilution can be measured with bolus or continuous saline infusions.13 For bolus thermodilution, 3 mL of room-temperature saline are rapidly injected through the guiding catheter to determine the mean transit time (Tmn). Coronary flow can be estimated by dividing the volume of saline by Tmn and, if the volumes are assumed to be constant, flow is inversely proportional to Tmn. CFR is calculated as Tmn (at rest) / Tmn (during hyperemia). Typically, serial Tmn measurements are made during each condition and the mean of 3 recordings (ideally with <10% variability) is used. CFR derived from bolus thermodilution correlates moderately well with Doppler-derived CFR, although the former overestimates the latter with the progressive discrepancy with increasing CFR; a CFR of ~2.5 by coronary thermodilution is likely equivalent to a Doppler CFR measurement of ~2.0.9
During continuous thermodilution (Figure 2C), a saline infusion mixes homogenously with blood in the coronary segment between the point of infusion and the distal thermistor, and volumetric coronary flow can be calculated as Ti/T x Qi x 1.08 (where Ti and T are the temperatures of the saline infusate and of the blood mixed with saline (at the distal thermistor) respectively, Qi is the rate of infusion of saline and 1.08 is a constant that accounts for the density and specific heat of blood and saline). Absolute coronary flow measured in this way requires saline infusion through a dedicated monorail microcatheter with side-holes (Rayflow, Hexacath). As there are fewer assumptions inherent in this technique, absolute flow may be a more accurate estimation of coronary flow than bolus thermodilution techniques. When calculating CFR, hyperemia can either be induced with adenosine, or by infusing saline at a higher rate, which has been shown to achieve comparable hyperemia, although the mechanisms remain unclear (Table 2). Typically, saline infusion rates of 10ml/min (for baseline flow) and 20ml/min (for hyperemic flow) are used.14
Microvascular Resistance
If distal coronary pressure (Pd) is measured simultaneously with flow, it is also possible to measure microvascular resistance (MR), which can be expressed as MR= Pd / flow, by analogy to Ohm’s Law. The specific technique used to measure flow determines how MR is calculated, namely hyperemic microvascular resistance using Doppler (hMR = Pd/ APV at hyperemia), index of microvascular resistance using bolus thermodilution (IMR= Pd x Tmn during hyperemia), and absolute resistance during continuous thermodilution (Rmicro = Pd/absolute flow). The microvascular resistance reserve ratio (RRR) is the basal resistance divided by hyperemic resistance. An RRR>2.0 is taken as normal.15 Other MR measurements are shown in Table 1. Microvascular resistance measurements have the theoretical advantage that they are specific to the microcirculation. Elevated MR may indicate structural mechanisms of CMD and provides an explanation for ischemic symptoms in patients with INOCA.16 However, the independent prognostic utility of resistance measures to predict major adverse cardiovascular events in patients with INOCA is unclear, whereas the prognostic utility of CFR is well established.17 In contrast, microvascular resistance measures are associated with prognosis in patients with microcirculatory dysfunction post-MI.
Wire-free measures of MR have also been developed to assess the microcirculation. Computational fluid dynamics models that estimate distal coronary pressure gradients can be combined with estimates of coronary flow using frame counts and vessel lengths measured from invasive coronary angiography to calculate an angiographic IMR (IMRangio).18 In studies of patients with STEMI, both hyperemic and non-hyperemic IMRangio demonstrated good agreement with conventional invasive IMR.19 Further research is necessary to validate angiography-based IMR in larger patient cohorts and determine best practices using this novel technology.
Integration and Interpretation of Flow Reserves and Microvascular Resistance
CMD, which is characterized by reduced CFR or elevated MR, has recently been divided into two distinct subtypes: structural CMD, in which hyperemic coronary flow is reduced due to pathologically increased minimal microvascular resistance (IMR>25 or hMR>2.5), and functional CMD, in which baseline coronary flow is high due decreased resting microvascular tone.11,16,20,21 The former has been ascribed to architectural changes leading to microcirculatory impairment, the later may be due to an abnormal metabolic state at rest or perhaps increased oxygen demand leading to high resting flow.11,17,21,22 Patients with functional and structural CMD have similar clinical presentations, characterized by exercise maladaptation, inducible ischemia, and comparable adverse prognosis, although the underlying pathophysiological mechanisms may be distinct. Among patients with INOCA, abnormal functional CMD appears to be more common in women, younger patients, and patients with fewer cardiovascular risk factors.21 Ultimately, both CFR and MR measures (e.g. IMR or hMR) are necessary to discriminate between these two subtypes; CFR is abnormally low in both cases, whereas MR is normal in functional CMD, while it is pathologically increased (IMR>25 or HMR>2.5) in structural CMD.17 Measurement of CFR alone is insufficient to identify mechanisms of CMD.
The prognostic impact of CFR and MR measurements have been investigated in patients with INOCA.17,20,23 In patients with abnormal CFR, long term outcomes such as major adverse cardiac events (MACE) and target vessel failure (TVF) are increased when compared to patients with normal CFR, irrespective of MR. Conversely, in patients with normal CFR, high MR was not associated with increased cardiovascular events.17 The clinical impact of elevated MR measurements in patients with INOCA requires further study.
Intracoronary Acetylcholine Reactivity Testing
Coronary infusion of acetylcholine (ACh) explores endothelium-dependent mechanisms of CMD and epicardial vasomotor disorders and is required for the definitive endotype stratification of patients with INOCA.3,24–27 Acetylcholine binds muscarinic receptors on endothelial and vascular smooth muscle cells. In normal coronaries, ACh likely stimulates endothelial calcium release and enhances nitric oxide production, which dilates the epicardial coronary diameter by >20% and augments microcirculatory coronary blood flow.28 Acetylcholine also acts at smooth muscle cells to cause vasoconstriction, and in vessels with endothelial dysfunction, vasoconstriction becomes the dominant response.29 ACh dosing, including manual or automated infusion, and the timing with respect to CMD testing, varies according to local preferences. A typical approach to assess for endothelial dysfunction involves sequential intracoronary infusion of escalating doses of acetylcholine at concentrations approximating 0.182, 1.82, and 18.2 mcg/ml (10−6, 10−5, and 10−4 mol/l, respectively) at 2 ml/min for 2 min using a mechanical pump.25 To evaluate for coronary spasm, ACh can be administered as an intracoronary bolus at doses of 20, 50, 100, and 200 mcg over 20 seconds to 3 minutes. Given the risk of acetylcholine-induced bradycardia, it is considered safer to administer a half-dose bolus in cases of left coronary dominance, or when testing the right coronary artery. Intracoronary ACh reactivity testing is generally safe, with major complications reported in 0.5% of cases.27 The most robust safety data is derived from protocols that use 3-minute infusions of ACh.27
Assessment of Endothelium-Dependent Epicardial Function
Epicardial coronary endothelial dysfunction is characterized by impairment of vasodilatation or mild vasoconstriction in response to intracoronary infusion of low doses (1–40 μg) of ACh. Endothelial dysfunction severity exists on a continuum, with more significant dysfunction reflected by greater degrees of narrowing in response to lower doses of intracoronary ACh.30,31 Epicardial coronary spasm, the pathophysiological mechanism underlying vasospastic angina (VSA), is a transient, severe epicardial coronary narrowing that abruptly reduces coronary flow. Epicardial spasm is defined by a 90% reduction in coronary diameter associated with angina and ischemic ECG changes in response to intracoronary ACh.2,25,32
Assessment of Endothelium-Dependent Microvascular Function
Responses to ACh provide important insights into endothelial-dependent microvascular function. In the absence of epicardial coronary spasm, a <50% augmentation of coronary blood flow in response to ACh (ACh flow reserve ≤ 1.5) indicates endothelial-dependent CMD is present.10,33–35 In this setting, volumetric coronary flow is frequently calculated from Doppler velocity measurements and quantitative coronary angiography using the formula: flow= ½ APV x cross sectional area, where area=π x diameter2/4). Microvascular coronary responses to ACh can also be evaluated by coronary thermodilution techniques to determine ACh flow reserve and MR.36
Microvascular spasm plays a role in the pathogenesis of microvascular angina (MVA) in selected cases.2,32 Microvascular spasm is diagnosed when infusion of ACh causes a transient reduction or cessation of coronary flow, or based on clinical criteria, when angina and ischemic ECG changes develop after ACh administration in the absence of epicardial coronary spasm.25 Microvascular spasm remains a poorly defined entity, and recent data indicate that not all patients with microvascular spasm diagnosed by clinical criteria have objective decreases in microvascular flow.37 Additionally, VSA and MVA may coexist, however, in presence of severe epicardial spasm, it may not be possible to detect the presence of microvascular spasm with ACh reactivity testing.2,29,38
Alternative approaches to test the integrity of endothelial-dependent microvascular function have also been studied. The endothelial agonist substance-P,39 dobutamine infusion,40 and supine bicycle exercise are vasodilatory stimuli that may identify inappropriate augmentation (or reduction) of coronary blood flow suggestive of endothelial-dependent microcirculatory dysfunction.20,39,40 Mental stress and anxiety can also provoke endothelial dysfunction, providing a potential explanation for anxiety-induced ischemic symptoms that might otherwise be classified as non-cardiac.41,42 However, accepted protocols and thresholds indicative of an abnormal response to these vasodilatory stimuli have not yet been established and require further study.
Clinical Manifestations of CMD
CMD plays a role in ischemia and non-obstructive coronary arteries (INOCA) and acute MI, and may contribute to angina following percutaneous coronary intervention (PCI), heart failure with preserved ejection fraction, Takotsubo syndrome, and complications after heart transplantation. The most common clinical scenarios are described herein.
Ischemia with non-obstructive coronary arteries (INOCA)
Nearly half of patients undergoing coronary angiography for the evaluation of stable angina do not have obstructive CAD.43 INOCA is a clinical syndrome characterized by ischemic symptoms in the absence of a major epicardial coronary artery diameter stenosis ≥50%.1–3 In a majority of cases, INOCA is caused by CMD (including microvascular spasm), epicardial coronary spasm, or a combination of pathologies, with or without traditional coronary risk factors and non-obstructive atherosclerosis. Patients with INOCA have impaired quality of life and are at increased risk of long-term cardiac events, recurrent hospital admissions, and increased health care costs.44 In CorMicA, patients randomly assigned to medical therapy guided by invasive physiology (CMD and ACh coronary reactivity testing) had sustained improvement in angina and better quality of life at 1 year compared to management per usual care.3 Despite the prevalence and clinical impact of INOCA, substantial gaps in knowledge remain due to heterogenous causes of this clinical syndrome and a dearth of large randomized controlled trials on therapies and outcomes.
Microvascular Obstruction after ST-segment Elevation MI
Despite advances in treatment for ST-segment elevation MI (STEMI) with PCI, microvascular obstruction (MVO) remains a prognostically significant complication without evidence-based therapies.45 MVO occurs in half of patients who present with STEMI and is associated with angiographic no-reflow or slow-flow despite revascularization.46 MVO can be measured invasively after primary PCI using IMR,47 and thermodilution-derived temperature recovery time.48 IMR thresholds >28 and >40 are independently predictive of the occurrence of intramyocardial hemorrhage, and all-cause death or heart failure.47
Heart Failure with Preserved Ejection Fraction
Myocardial ischemia due to epicardial CAD or CMD, or both, are implicated in the pathophysiology of heart failure with preserved ejection fraction (HFpEF).49,50 In a prospective cohort study of 75 patients with HFpEF, 66% had endothelium-independent CMD (defined as CFR <2.0 and/or IMR ≥25), and 24% had endothelium-dependent microvascular spasm.50 Thus, CMD is a common finding in HFpEF and may be an important therapeutic target.
Contemporary Guidelines
Based on cohort studies and the CorMicA trial,3 clinical practice guidelines now recommend evaluation of the coronary microcirculation in patients with suspected INOCA to determine mechanisms of ischemia and to guide medical therapy.24–26 European Society of Cardiology guidelines provide a Class IIa recommendation for guidewire-based measurement of CFR and/or microcirculatory resistance measurements in patients with INOCA, and a IIb recommendation for intracoronary ACh testing, although when VSA is clinically suspected, the recommendation is escalated to Class IIa.24 American clinical practice guidelines also provide a Class IIa recommendation for invasive coronary functional testing in INOCA (Figure 3).26 Non-invasive modalities, including stress CMR and PET imaging (Class IIa) and stress echocardiography with coronary flow velocity reserve measurement (Class IIb) are also recommended.26
Figure 3.
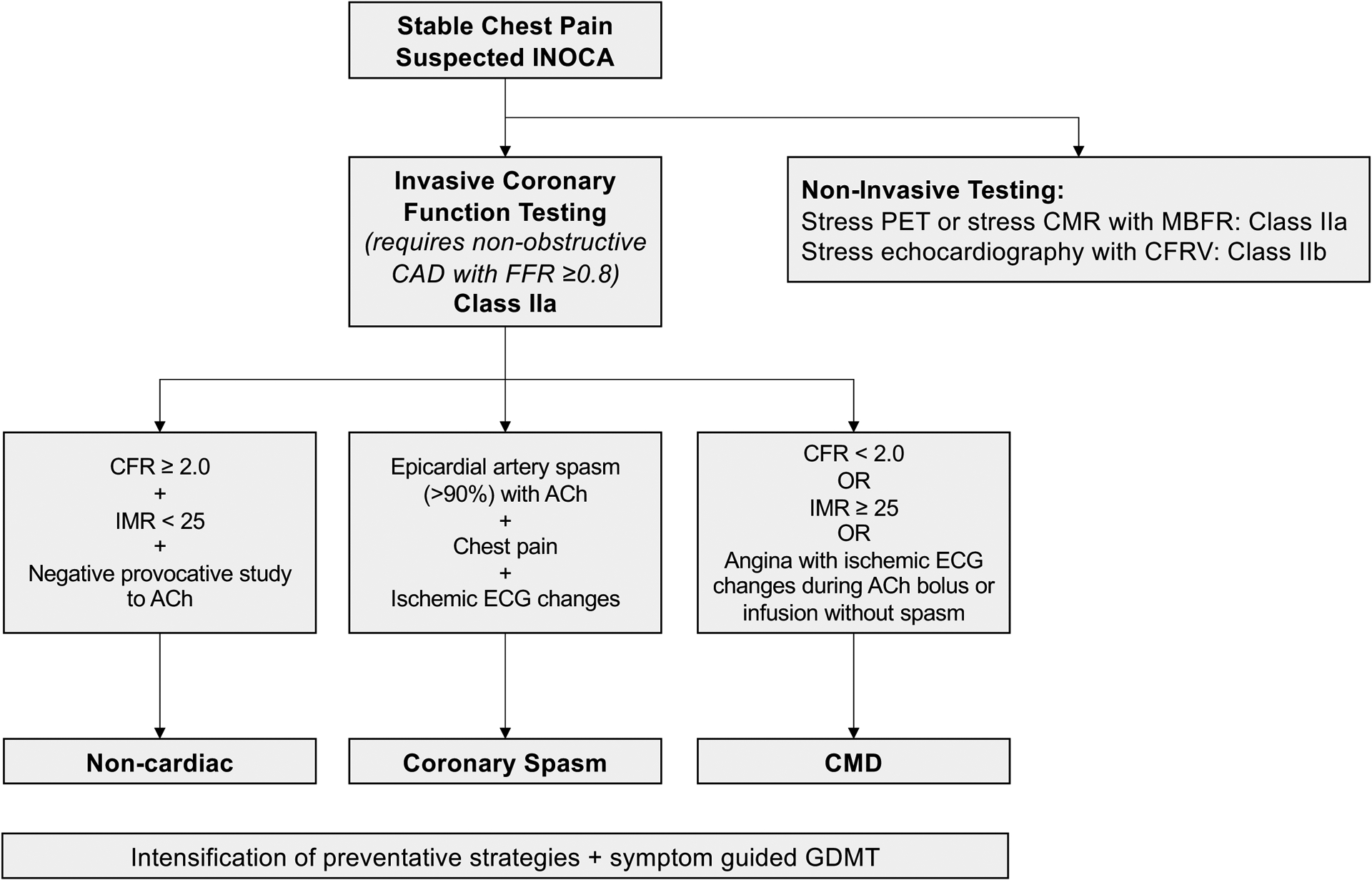
Contemporary paradigm to assess endotypes of INOCA.
Abbreviations: ACh, acetylcholine; CAD, coronary artery disease; CFR, coronary flow reserve; CFVR, coronary flow velocity reserve; CMD, coronary microvascular dysfunction; CMR, cardiac magnetic resonance imaging; ECG, electrocardiogram; FFR, fractional flow reserve; GDMT, guideline-directed medical therapy; IMR, index of microcirculatory restriction; INOCA, ischemia and no obstructive CAD; MBFR, myocardial blood flow reserve; PET, positron emission tomography
Future Directions & Research Priorities
Despite this clinical evidence, coronary microvascular function is not routinely measured in the catheterization laboratory. Inadequate physician education and training, patient involvement, and recognition by insurers and hospital providers are key barriers to CMD testing in daily clinical practice. A variety of invasive methods for CMD assessment, without a clear gold standard, may reduce confidence and hampers development in the field. Although absolute MR by continuous thermodilution has theoretical advantages, its clinical utility is yet to be systematically explored. Research priorities should address evidence gaps for mechanistically targeted medical therapy in CMD. The potential of endothelin receptor antagonists as a disease-modifying therapy in CMD is being assessed [ClinicalTrials.gov: NCT04097314]. Currently, there are no evidence-based medicines for CMD following acute MI. The ongoing Stratified Medicine of Eplerenone in Acute MI/Injury trial [ClinicalTrials.gov: NCT05198791] seeks to address this gap.
Conclusions
In conclusion, CMD is a widely prevalent disorder that plays a role in a variety of acute and chronic cardiovascular disease states. Guidelines support routine testing for CMD in patients with INOCA, and testing has potential to improve symptoms, quality of life, and patient satisfaction by establishing a diagnosis, mechanistically targeted medical therapy, and lifestyle measures.3 Going forward, education and provider awareness of CMD and its role in cardiovascular disease are needed.
Acknowledgements
We acknowledge Dr. Giulia Botti MD, Dr. Aish Sinha MRCP, and Dr. Stéphane Rinfret, MD, SM for their contributions in manuscript and figure preparation.
Sources of Funding:
Dr. Smilowitz is supported, in part, by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL150315. Dr. Perera receives research funding from National Institute for Health Research (19/109 - NIHR130593 and others), British Heart Foundation (RE/18/2/34213 and others), Medical Research Council (MR/S005714/1) and National Institutes for Health (R01HL159089–01). Colin Berry receives research funding from the British Heart Foundation grant (RE/18/6134217), Chief Scientist Office, EPSRC (EP/R511705/1, EP/S030875/1), European Union (754946–2), Medical Research Council (MR/S018905/1) and UK Research and Innovation (MC/PC/20014).
Footnotes
Disclosures:
Dr. Smilowitz serves on an advisory board for Abbott Vascular.
Dr. Toleva – Nothing to disclose.
Dr. Chieffo - Speaker/Consultant fees from Abbott, Abiomed, Boston Scientific, Bionsensor, Medtronc, Menarini, Shock Wave Medical
Dr. Perera receives speaker fees/research support from Abbott, Abiomed, Menarini, Philips, Shockwave
Dr. Berry - Colin Berry is employed by the University of Glasgow which holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Auxilius Pharma, Boehringer Ingelheim, Causeway Therapeutics, Coroventis, Genentech, GSK, HeartFlow, Menarini, Neovasc, Novartis, Siemens Healthcare, and Valo Health.
References
- 1.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN, Coronary Vasomotion Disorders International Study G. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068 [DOI] [PubMed] [Google Scholar]
- 3.Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 4.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889 [DOI] [PubMed] [Google Scholar]
- 5.Ford TJ, Corcoran D, Padmanabhan S, Aman A, Rocchiccioli P, Good R, McEntegart M, Maguire JJ, Watkins S, Eteiba H, et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J. 2020;41:3239–3252. doi: 10.1093/eurheartj/ehz915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford TJ, Rocchiccioli P, Good R, McEntegart M, Eteiba H, Watkins S, Shaukat A, Lindsay M, Robertson K, Hood S, et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J. 2018;39:4086–4097. doi: 10.1093/eurheartj/ehy529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, Sato K, Sugisawa J, Matsumoto Y, Miyata S, et al. Coronary Functional Abnormalities in Patients With Angina and Nonobstructive Coronary Artery Disease. J Am Coll Cardiol. 2019;74:2350–2360. doi: 10.1016/j.jacc.2019.08.1056 [DOI] [PubMed] [Google Scholar]
- 8.Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–2061. doi: 10.1161/circulationaha.105.603522 [DOI] [PubMed] [Google Scholar]
- 9.Demir OM, Boerhout CKM, de Waard GA, van de Hoef TP, Patel N, Beijk MAM, Williams R, Rahman H, Everaars H, Oxford Acute Myocardial Infarction S, et al. Comparison of Doppler Flow Velocity and Thermodilution Derived Indexes of Coronary Physiology. JACC Cardiovasc Interv. 2022;15:1060–1070. doi: 10.1016/j.jcin.2022.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol. 2019;73:684–693. doi: 10.1016/j.jacc.2018.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, Scannell C, Clapp B, Marber M, Webb A, et al. Coronary Microvascular Dysfunction Is Associated With Myocardial Ischemia and Abnormal Coronary Perfusion During Exercise. Circulation. 2019;140:1805–1816. doi: 10.1161/CIRCULATIONAHA.119.041595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–2006. [DOI] [PubMed] [Google Scholar]
- 13.Candreva A, Gallinoro E, van ‘t Veer M, Sonck J, Collet C, Di Gioia G, Kodeboina M, Mizukami T, Nagumo S, Keulards D, et al. Basics of Coronary Thermodilution. JACC Cardiovasc Interv. 2021;14:595–605. doi: 10.1016/j.jcin.2020.12.037 [DOI] [PubMed] [Google Scholar]
- 14.De Bruyne B, Adjedj J, Xaplanteris P, Ferrara A, Mo Y, Penicka M, Flore V, Pellicano M, Toth G, Barbato E, et al. Saline-Induced Coronary Hyperemia: Mechanisms and Effects on Left Ventricular Function. Circ Cardiovasc Interv. 2017;10:e004719. doi: 10.1161/CIRCINTERVENTIONS.116.004719 [DOI] [PubMed] [Google Scholar]
- 15.Layland J, Carrick D, McEntegart M, Ahmed N, Payne A, McClure J, Sood A, McGeoch R, MacIsaac A, Whitbourn R, et al. Vasodilatory capacity of the coronary microcirculation is preserved in selected patients with non-ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6:231–236. doi: 10.1161/CIRCINTERVENTIONS.112.000180 [DOI] [PubMed] [Google Scholar]
- 16.Ford TJ, Yii E, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, et al. Ischemia and No Obstructive Coronary Artery Disease: Prevalence and Correlates of Coronary Vasomotion Disorders. Circ Cardiovasc Interv. 2019;12:e008126. doi: 10.1161/CIRCINTERVENTIONS.119.008126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerhout CKM, de Waard GA, Lee JM, Mejia-Renteria H, Lee SH, Jung JH, Hoshino M, Echavarria-Pinto M, Meuwissen M, Matsuo H, et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease; from the multicentre international ILIAS registry. EuroIntervention. 2022;18:719–728. doi: 10.4244/EIJ-D-22-00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mejia-Renteria H, Lee JM, Choi KH, Lee SH, Wang L, Kakuta T, Koo BK, Escaned J. Coronary microcirculation assessment using functional angiography: Development of a wire-free method applicable to conventional coronary angiograms. Catheter Cardiovasc Interv. 2021;98:1027–1037. doi: 10.1002/ccd.29863 [DOI] [PubMed] [Google Scholar]
- 19.Scarsini R, Shanmuganathan M, Kotronias RA, Terentes-Printzios D, Borlotti A, Langrish JP, Lucking AJ, Ox AMISI, Ribichini F, Ferreira VM, et al. Angiography-derived index of microcirculatory resistance (IMR(angio)) as a novel pressure-wire-free tool to assess coronary microvascular dysfunction in acute coronary syndromes and stable coronary artery disease. Int J Cardiovasc Imaging. 2021;37:1801–1813. doi: 10.1007/s10554-021-02254-8 [DOI] [PubMed] [Google Scholar]
- 20.Rahman H, Demir OM, Khan F, Ryan M, Ellis H, Mills MT, Chiribiri A, Webb A, Perera D. Physiological Stratification of Patients With Angina Due to Coronary Microvascular Dysfunction. J Am Coll Cardiol. 2020;75:2538–2549. doi: 10.1016/j.jacc.2020.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nardone M, McCarthy M, Ardern CI, Nield LE, Toleva O, Cantor WJ, Miner SES. Concurrently Low Coronary Flow Reserve and Low Index of Microvascular Resistance Are Associated With Elevated Resting Coronary Flow in Patients With Chest Pain and Nonobstructive Coronary Arteries. Circ Cardiovasc Interv. 2022;15:e011323. doi: 10.1161/CIRCINTERVENTIONS.121.011323 [DOI] [PubMed] [Google Scholar]
- 22.Mejia-Renteria H, van der Hoeven N, van de Hoef TP, Heemelaar J, Ryan N, Lerman A, van Royen N, Escaned J. Targeting the dominant mechanism of coronary microvascular dysfunction with intracoronary physiology tests. Int J Cardiovasc Imaging. 2017;33:1041–1059. doi: 10.1007/s10554-017-1136-9 [DOI] [PubMed] [Google Scholar]
- 23.Toya T, Ahmad A, Corban MT, zcan I, Sara JD, Sebaali F, Escaned J, Lerman LO, Lerman A. Risk Stratification of Patients With NonObstructive Coronary Artery Disease Using Resistive Reserve Ratio. J Am Heart Assoc. 2021;10:e020464. doi: 10.1161/JAHA.120.020464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 25.Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, Prescott E, Karam N, Appelman Y, Fraccaro C, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504–3520. doi: 10.1093/eurheartj/ehaa503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001029 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Samuels BA, Li W, Parikh MA, Wei J, Moses JW, Fearon WF, Henry TD, Tremmel JA, Kobayashi Y, et al. Safety of Provocative Testing With Intracoronary Acetylcholine and Implications for Standard Protocols. J Am Coll Cardiol. 2022;79:2367–2378. doi: 10.1016/j.jacc.2022.03.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson C, Lee MD, McCarron JG. Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J Physiol. 2016;594:7267–7307. doi: 10.1113/JP272927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong P, Athanasiadis A, Sechtem U. Patterns of coronary vasomotor responses to intracoronary acetylcholine provocation. Heart. 2013;99:1288–1295. doi: 10.1136/heartjnl-2012-302042 [DOI] [PubMed] [Google Scholar]
- 30.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr., Lerman A Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948 [DOI] [PubMed] [Google Scholar]
- 31.Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/circulationaha.114.012636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN, Coronary Vasomotion Disorders International Study G. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. doi: 10.1093/eurheartj/ehv351 [DOI] [PubMed] [Google Scholar]
- 33.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1445–1453. doi: 10.1016/j.jcin.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 34.Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. doi: 10.1161/01.cir.85.5.1899 [DOI] [PubMed] [Google Scholar]
- 35.Perera D, Berry C, Hoole SP, Sinha A, Rahman H, Morris PD, Kharbanda RK, Petraco R, Channon K, Group UKCMDW. Invasive coronary physiology in patients with angina and non-obstructive coronary artery disease: a consensus document from the coronary microvascular dysfunction workstream of the British Heart Foundation/National Institute for Health Research Partnership. Heart. 2022;109:88–95. doi: 10.1136/heartjnl-2021-320718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nardone M, McCarthy M, Ardern CI, Edgell H, Toleva O, Nield LE, Miner SES. Characterization of the Human Coronary Microvascular Response to Multiple Hyperaemic Agents. CJC Open. 2021;3:133–141. doi: 10.1016/j.cjco.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohba K, Sugiyama S, Sumida H, Nozaki T, Matsubara J, Matsuzawa Y, Konishi M, Akiyama E, Kurokawa H, Maeda H, et al. Microvascular coronary artery spasm presents distinctive clinical features with endothelial dysfunction as nonobstructive coronary artery disease. J Am Heart Assoc. 2012;1:e002485. doi: 10.1161/JAHA.112.002485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seitz A, Feenstra R, Konst RE, Martinez Pereyra V, Beck S, Beijk M, van de Hoef T, van Royen N, Bekeredjian R, Sechtem U, et al. Acetylcholine Rechallenge: A First Step Toward Tailored Treatment in Patients With Coronary Artery Spasm. JACC Cardiovasc Interv. 2022;15:65–75. doi: 10.1016/j.jcin.2021.10.003 [DOI] [PubMed] [Google Scholar]
- 39.Melikian N, Kearney MT, Thomas MR, De Bruyne B, Shah AM, MacCarthy PA. A simple thermodilution technique to assess coronary endothelium-dependent microvascular function in humans: validation and comparison with coronary flow reserve. Eur Heart J. 2007;28:2188–2194. doi: 10.1093/eurheartj/ehm269 [DOI] [PubMed] [Google Scholar]
- 40.Collste O, Tornvall P, Alam M, Frick M. Coronary flow reserve during dobutamine stress in Takotsubo stress cardiomyopathy. BMJ Open. 2015;5:e007671. doi: 10.1136/bmjopen-2015-007671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O’Connor G, Betteridge J, Klein N, Steptoe A, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–2478. doi: 10.1161/01.cir.102.20.2473 [DOI] [PubMed] [Google Scholar]
- 42.Irfan S, McCarthy M, Miner S. Association Between Panic Attack and Coronary Ischemia Due to Reduction in Coronary Blood Flow. Can J Cardiol. 2023;39:71–72. doi: 10.1016/j.cjca.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 43.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990 [DOI] [PubMed] [Google Scholar]
- 45.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765 [DOI] [PubMed] [Google Scholar]
- 46.Carrick D, Haig C, Ahmed N, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay MM, Davie A, et al. Myocardial Hemorrhage After Acute Reperfused ST-Segment-Elevation Myocardial Infarction: Relation to Microvascular Obstruction and Prognostic Significance. Circ Cardiovasc Imaging. 2016;9:e004148. doi: 10.1161/CIRCIMAGING.115.004148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrick D, Haig C, Ahmed N, Carberry J, Yue May VT, McEntegart M, Petrie MC, Eteiba H, Lindsay M, Hood S, et al. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients With an Acute ST-Segment-Elevation Myocardial Infarction. Circulation. 2016;134:1833–1847. doi: 10.1161/CIRCULATIONAHA.116.022603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maznyczka AM, Carrick D, Oldroyd KG, James-Rae G, McCartney P, Greenwood JP, Good R, McEntegart M, Eteiba H, Lindsay MM, et al. Thermodilution-derived temperature recovery time: a novel predictor of microvascular reperfusion and prognosis after myocardial infarction. EuroIntervention. 2021;17:220–228. doi: 10.4244/EIJ-D-19-00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Ljung Faxen U, Fermer ML, Broberg MA, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39:3439–3450. doi: 10.1093/eurheartj/ehy531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rush CJ, Berry C, Oldroyd KG, Rocchiccioli JP, Lindsay MM, Touyz RM, Murphy CL, Ford TJ, Sidik N, McEntegart MB, et al. Prevalence of Coronary Artery Disease and Coronary Microvascular Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2021;6:1130–1143. doi: 10.1001/jamacardio.2021.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]


