Abstract
Background:
Animal models are vital for the development of radiation medical countermeasures for the prophylaxis or treatment of acute radiation syndrome and for the delayed effects of acute radiation exposure. Nonhuman primates (NHPs) play an important role in the regulatory approval of such agents by the United States Food and Drug Administration following the Animal Rule. Reliance on such animal models requires that such models are well characterized.
Methods:
Data gathered from both male and female animals under the same conditions and gathered concurrently are limited; therefore, the authors compared and contrasted here the radiosensitivity of both male and female NHPs provided different levels of clinical support over a range of acute, total-body gamma irradiation, as well as the influence of age and body weight.
Results:
Under matched experimental conditions, the authors observed only marginal, but clearly evident differences between acutely irradiated male and female NHPs relative to the measured response endpoints (rates of survival, blood cell changes and cytokine fluctuations). These differences appeared to be accentuated by level of exposure as well as by nature of clinical support.
Conclusion:
Additional studies with both sexes under various experimental conditions and different radiation qualities run concurrently are needed.
Keywords: Acute radiation syndrome, females, males, nonhuman primates, weights, age, radiation dose, survival, blood cell counts
1. Introduction
The development of effective radiation medical countermeasures (MCMs) for radiation injury depends on a number of factors. Importantly, having a full understanding of the scope and nature of ionizing radiation (IR) injuries incurring in both male and females, but also having the full knowledge of the strengths and weaknesses of given experimental model systems are essential for the experimental documentation that relates to MCM research [1–3]. As it is not always possible to glean from clinical human studies the essential details required for such documentation, especially in terms of measures of effectiveness for given MCMs under test; therefore, the use of appropriate animal models for testing are considered essential (due largely to the ethical constrains of irradiating and MCM testing in healthy subjects). Accordingly, the United States Food and Drug Administration (US FDA) has put into place guidance, commonly referred to as the Animal Rule that circumvents this regulatory stumbling block [4–6].
Development and regulatory approval of such MCMs are accomplished following the Animal Rule [4,6]. For approval under the Animal Rule, safety and efficacy must be demonstrated in either two different animal species, or in a single ‘sufficiently well characterized’ species. Rodents are a good choice for early efficacy screening due to low housing and care costs compared to large animal species. Despite this, the data generated by rodents is particularly limited in reference to its transferability to the clinic. Nonhuman primate (NHPs), such as the rhesus macaque, remain the “gold standard” animal model for the development and eventual regulatory approval of MCMs. This status can be attributed to their genetic and physiological similarities to humans, in addition to the well-understood pathophysiology of the progression of radiation injury in these animals [7]. Both total-body as well as partial-body irradiation (TBI and PBI) NHP models are capable of generating data that is satisfactory for comparison to radiation accident victims. Additionally, irradiated NHPs that are administered supportive care are capable of closely reflecting the course of treatment that is provided clinically to humans exposed to radiation [8–12]. For these reasons, NHP models of TBI and PBI have been developed, and these models have been and continue to be instrumental in the research and development of FDA approved MCMs [13–22]. Nevertheless and despite the above rationale for using NHPs, the current reality of having full and proper health risk assessments of humans subjected to excessive and unwanted radiation exposures strongly suggests that these experimentally-based assessments need to be carried out using both sexes; these assessments include not only overall evaluation of health risks, but also for the development of safe and effective MCMs [1]. Unfortunately, only a single sex, namely males, have been used in a majority of experiments using NHPs for MCM development.
2. Materials and Methods
2.1. General experimental design
Sex-based comparisons of acutely irradiated NHPs were retrospectively accomplished using both male and female rhesus NHPs (Macaca mulatta). These animals were exposed (or not exposed as in the case of ‘sham-irradiated’ control groups) under defined, previously reported, conditions of TBI using cobalt-60 gamma-radiation in order to induce varying degrees of bodily injury to vital organ systems (i.e., hematopoietic and gastrointestinal type injuries) [23,24]. Animals were irradiated with different doses of radiation inducing hematopoietic ARS (H-ARS) and were treated post-irradiation with different levels of supportive care, including the use of blood products in selected studies. Survival and hematopoietic recovery based on complete blood count (CBC) analysis were used for comparison purposes.
2.2. Animals
A total of 116 naïve rhesus macaques (Macaca mulatta, 60 males and 56 females) were used for the work presented here. The animals’ ages ranged between 3 – 8 years, weighing 3 – 9 kg. All animals were maintained in a facility accredited by AAALAC-International. Prior to the initiation of each experiment, all animals were quarantined for six weeks. All details of animal housing, health monitoring, care, and enrichment during the experimental period have been described in detail earlier [23,24]. The recommendations made in the Guide for the Care and Use of Laboratory Animals were strictly adhered to throughout the course of all studies [25].
2.3. Total-body irradiation
All irradiation procedures and dosimetry were reported previously in detail [10]. In brief, the salient features of those procedures are as follows: food was withheld for 12 – 18 h prior to the exposures; animals were sedated 30 – 45 min prior to exposure with intramuscular (im) ketamine (10 – 15 mg/kg) injections and then placed in separate Plexiglas irradiation boxes; prior to these placements, the animals were paired based on the similarity (+/− 1 cm) of their girth dimensions. Abdominal widths were measured with digital calipers a few days prior to scheduled irradiation. Animals that were not within one cm of another animal were irradiated separately. This strategy was used to make sure each animal received the desired radiation dose to the core of the body, irrespective of body weight. Two NHPs were placed on the irradiation platform and secured in seated, back-to-back positions and exposed to a specific radiation dose of 60Co γ-radiation at a dose rate of 0.6 Gy/min from both sides to the core of the abdomen (bilateral, simultaneous exposure). All irradiation procedures and dosimetry are reported and discussed in detail earlier [10]. The radiation field in the area of the NHP location was uniform within ± 1.5%. The dosimetry for photons was based on the alanine/EPR (electron paramagnetic resonance) dosimetry system [26]. The dosimetry system was calibrated in terms of absorbed dose to water using the US National Standard Radiation Sources. The dose rate for irradiation was 0.6 Gy/min across all experiments reported here [27].
2.4. Cage-side animal observations
All NHPs were observed pre-irradiation and for 60 d post-irradiation with survival being the primary measured endpoint. Daily observations for signs of pain and distress were made no less than twice a day by husbandry or research staff members. All procedures and routine protocols on ‘animal observations’ have been previously described in detail and reported [9,10].
There were three major categories or levels of supportive care provided to the animals under test: 1) no supportive care; 2) minimal supportive care, encompassing the clinical use of antibiotics, parenteral fluids, anti-diarrheal, anti-ulcer, anti-emetics and analgesics, along with nutritional support that included re-hydration fluids and extra enrichment items. Antibiotics (e.g., Baytril) were administered only under select clinical conditions, namely when absolute neutrophil counts fell below 500 cells/μl and discontinued when neutrophil counts recovered to 500 cells/μl or greater; and 3) full supportive care that entailed not only the above mentioned treatments (under minimal care), but the administration of whole blood and blood products as clinically required.
2.5. Blood collection
Blood draws were accomplished by venipuncture from the saphenous or brachial veins approximately 1 – 3 h after animals were fed, and were subsequently analyzed for CBCs. Detailed methods for such blood collection are described earlier [28]. Furthermore, the methodology for serum collection for cytokine analysis is described elsewhere [29]. In brief, the blood was placed in serum separating tubes and allowed to clot for at least 30 minutes prior to being centrifuged (10 min, 400 x g). The serum was aliquoted into empty specimen tubes which were then stored at −70 °C until use.
2.6. CBC analysis
Whole blood cells were counted using a hematology analyzer [9]. Twenty blood parameters were analyzed: white blood cells (WBC), red blood cells (RBC), hematocrit (HCT), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets, and absolute counts and percentages for neutrophils, lymphocytes, monocytes, eosinophils, basophils, and reticulocytes [11].
2.7. Multiplex analysis of cytokines
A Luminex 200 analyzer (Luminex Corp, Austin, TX, USA) was used to detect the cytokines, chemokines, and growth factors in the serum using custom-made multiplex kits (up to 48 plex) (Bio-Rad Laboratories, Hercules, CA, USA). A list of these 48 cytokines has been provided earlier [23]. Standard curves for each cytokine were prepared by serial dilution and run in duplicates. Cytokine concentration (pg/ml) was determined by fluorescence intensity and its quantification was performed using Bio-Plex Manager software, version 6.1 (Bio-Rad Inc.) [30]. The lower limit of detection for the selected cytokines were as follows: IL-6: 1.47 pg/ml, IL-8: 2.64 pg/ml, IL-10: 4.29 pg/ml, IL-1β: 1.06 pg/ml, G-CSF: 25.49 pg/ml, and GM-CSF: 2.22 pg/ml.
2.8. Medical management/symptomatic palliative care
After any specialized procedures, NHPs were monitored at least twice daily for signs of complications. The type of supportive care provided was based on CBC analysis and cage side observations [23,24]. Antibiotics were initiated when the absolute neutrophil count was <500 cells/μl and continued until the count reached >500 cells/μl. The primary antibiotic used was enrofloxacin (Baytril Bayer HealthCare LLC, Shawnee Mission, KS) and the administered dose was 5 mg/kg im or subcutaneously (sc) twice a day (BID), or 10 mg/kg administered im or intravenous (iv) once daily (QD). If the body temperature was >39.4 °C (in addition to neutrophil count <500 cells/μl), ceftiofur (Zoetis Inc.) was administered at 5 mg/kg, sc for 2 days or 20 mg/kg for 7 days. Alternatively, gentamicin sulfate (GentaMax, Phoenix Scientific, Inc.) (5 mg/kg, im or iv, QD) was administered in combination with Baytril. If high fever persisted, ceftriaxone (Rocephin, Roche Laboratories Inc., Nutley, NJ) (50 mg/kg, im, every 24 h) was administered after gentamicin sulfate was discontinued or if microbial resistance was demonstrated to enrofloxacin or gentamicin. When microbial resistance was demonstrated to enrofloxacin, gentamicin and ceftriaxone, an alternative antibiotic, at the discretion of the study’s veterinarian, was used under such situation. Additional supportive care measures included rehydration fluids, alternate antibiotics, antipyretics, antidiarrheal agents, analgesics, antiemetics, treatment for mucosal ulcers, nutritional support, and blood transfusions. These treatments have been described and reported in detail previously [23]. Dehydrated animals in the study with supportive care were provided fluids intraparenterally, as needed.
2.9. Euthanasia
Euthanasia was conducted in accordance with the most recently approved versions of the IACUC protocol, the Guide, and the American Veterinary Medical Association (AVMA) guidelines [31]. When an animal reached a state of moribundity, the animal was euthanized. Moribundity was used as a surrogate for mortality, and animals were euthanized in order to minimize pain and distress [32]. The following parameters were used as guidelines for moribundity: significant weight loss (10%) from baseline; inappetence (complete anorexia for 2 days and deteriorating conditions); minimal or absence of response to stimuli, severe anemia (<13% hematocrit due to acute blood loss or <40 g/dL hemoglobin) and core body temperature below 96.6 °F following a period of febrile neutropenia (such as >103 °F and <500 neutrophils/μl); weakened/inability to obtain feed or water; severe thrombocytopenia (<10,000 platelets/μl) or other signs of severe organ dysfunction with poor prognosis as determined by the veterinarian such as dyspnea or severe cyanosis; sustained vomiting or diarrhea, obstruction, intussusception and peritonitis; renal failure as determined by clinical chemistry and urinalysis; sustained CNS depression, seizures, or paralysis of one or more extremities; non-healing wounds, repeated self-trauma, and severe skin infections; and severe organ system dysfunction with poor prognosis. Any single parameter from the above-listed guidelines did not lead to euthanasia of any animal. Moribundity status of the animal was determined by a joint effort between the institutional veterinarian, principal investigator, research staff, veterinary technicians, and husbandry staff based on the combination of criteria described above. The moribund animals were given pentobarbital sodium iv (Virbac AH Inc., Fort Worth, TX) using either saphenous or cephalic veins, needle size 20 – 25 gauge (100 mg/kg, 1 – 5 ml). Prior to pentobarbital sodium administration, animals were sedated using ketamine hydrochloride injection (Mylan Institutional LLC, Rockford, IL) (5 – 15 mg/kg, im). Intra-cardiac administration was performed if unable to administer pentobarbital sodium through peripheral veins. The animals were deeply anesthetized by Isoflurane (Baxter Healthcare Corporation, Deerfield, IL) (1 – 5%) with oxygen at 1 – 4 liters per minute via mask before administering the intra-cardiac injection. The animals were euthanized only under the guidance of a staff veterinarian or a trained technician in consultation with the veterinarian. After pentobarbital sodium administration, the animals were examined by assessing the heart auscultation and pulse to confirm death.
2.10. Data analysis
Statistical software SPSS v.28 was used for all statistical analyses. For both CBC and survival data each group was required to have a minimum of three animals in order to determine significance at any given time point. All error bars represent standard deviations. Analysis of variance (ANOVA) was used to detect significant differences between male and female CBC data for each exposure group. The Tukey-Kramer test was used to determine which pairwise comparisons were significant, with the p value set at 0.05. For survival data, chi-square tests were performed to compare survival rates of males and females within each exposure group; however, statistical significance was not evaluated as the sample size in each comparison was deemed insufficient. For the analyses of body weights and age relative to survival outcomes, Student t-test was used to assess levels of statistical significance (as defined by p values of < 0.05) between various groups (e.g., surviving vs non-surviving animals within 60 d post-exposure period) of male and female animals.
3. Results
3.1. Survival patterns
The overall cumulative survival rates of NHPs that lacked the benefit of clinical support following lethal exposures (ranging from 5.8 to 7.2 Gy) were ~72% and ~65% survival for male and female animals, respectively (Table 1). Survival rates of these animals at specific levels of exposure were as follows: for males exposed to 5.8, 6.5 or 7.2 Gy, survival rates were 73.3, 70.0 and 60.0%, respectively; while for females, survival rates were found to be 55.6%, 64.3%, and 33.3%, respectively (Table 2).
Table 1.
Survival outcome of male and female NHPs exposed to ionizing radiation in relation to age and body weight.
| Gender | Survivors | N | Body Weight (kg) | Age (months) | Survival (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Average | SD | p value1 | Range | Average | SD | p value1 | ||||
| Without supportive care | |||||||||||
| Females | Total | 23 | 3.8 – 6.6 | 5.19 | 0.772 | 0.105 | 45 – 72 | 61.2 | 8.87 | 0.145 | 65.2 |
| Survival | 15 | 5.05 | 0.820 | 63.4 | 8.11 | ||||||
| Deaths | 8 | 5.45 | 0.639 | 58.3 | 10.01 | ||||||
| Males | Total | 25 | 3.6 – 8.3 | 5.85 | 1.59 | 0.173 | 34 – 70 | 56.7 | 8.50 | 0.422 | 72 |
| Survival | 19 | 6.01 | 1.70 | 56.9 | 8.85 | ||||||
| Deaths | 6 | 5.44 | 1.11 | 56.1 | 8.15 | ||||||
| With full supportive care | |||||||||||
| Females | Total | 17 | 4.7 – 7.4 | 5.37 | 0.708 | 0.287 | 51 – 88 | 63.71 | 10.98 | 0.077 | 35.3 |
| Survival | 6 | 5.53 | 0.958 | 59.86 | 4.06 | ||||||
| Deaths | 11 | 5.28 | 0.564 | 66.7 | 13.28 | ||||||
| Males | Total | 19 | 3.6 – 6.8 | 4.85 | 0.939 | 0.094 | 35 – 80 | 52.5 | 13.14 | 0.428 | 47.4 |
| Survival | 9 | 5.17 | 1.019 | 52.0 | 16.38 | ||||||
| Deaths | 10 | 4.58 | 0.813 | 53.1 | 9.27 | ||||||
P values determined by Student’s t-test: single tailed distribution, two samples, unequal variance.
Percent survival in studies without supportive care are higher than studies with supportive care in this table. This is because we have pooled animals with different radiation doses used in experiments with as well as without supportive care. In studies with supportive care, we have used higher radiation doses (6.0 Gy, 6.5 Gy, 7.0 Gy, 7.5 Gy, 8.0 Gy, and 8.5 Gy) compared to studies without supportive care (5.8 Gy, 6.5 Gy, and 7.2 Gy).
Table 2.
Survival outcome of male and female NHPs exposed to different doses of radiation.
| Supportive Care | Radiation Dose (Gy) | Percent Survival | |
|---|---|---|---|
| Female | Male | ||
| With | 5.8 | 55.6 | 73.3 |
| 6.5 | 64.3 | 70.0 | |
| 7.2 | 33.3 | 60.0 | |
|
| |||
| Without | 6.0 | 50.0 | 100.0 |
| 6.5 | 33.3 | 66.7 | |
| 7.0 | 40.0 | 0.0 | |
| 7.5 | 0.0 | 80.0 | |
| 8.0 | 0.0 | 20.0 | |
| 8.5 | 33.3 | 0.0 | |
By comparison, the overall cumulative survival rates of male and female animals receiving benefit of clinical support following a range of specific and acute exposures (i.e., 6.0 to 8.5 Gy) were ~47% and ~35%, respectively. Survival rates of these animals at specific levels of exposure were as follows: for males exposed to 6.0, 6.5, 7.0, 7.5, 8.0 and 8.5 Gy, fractional survival rates were 100.0, 66.7, 0.0, 80.0, 20.0, and 0%, respectively; while for equivalently exposed females, the survival rates were 50.0, 33.3, 40.0, 0.0, 0.0, 33.3%, respectively (Table 2).
Comparison of the estimated probits suggested that females had higher mortality than males at the same radiation doses, and supportive care with blood transfusions increased survival of males at lower doses but not at high doses of radiation exposure. It is important to note that all experiments were not conducted under exactly the same experimental conditions concurrently for intended comparisons. However, the limitation of these analyses are due to the relatively small sample size of the male and female animals in various experiments.
3.2. Potential influence of body weight and age at time of radiation exposure
The influence of body weight at the time of irradiation relative to survival outcomes of both sexes within both experimental groups (group lacking clinical support versus group receiving clinical support) were found to be largely insignificant (as defined statistically by p value >0.05) (Table 1). However, in the case of the male group that received clinical support, body weight and survival outcome approached statistical significance (p value = 0.094).
The influence of age at the time of irradiation relative to survival outcome was also examined and again found to be largely insignificant for both sexes and in both experimental groups (with/without clinical support) (Table 1). However, in the case of the female group that had received clinical support, age and survival approached statistical significance (p value = 0.077).
3.3. Blood response patterns
Temporal blood response patterns of male and female NHPs acutely irradiated at potentially lethal exposure levels (i.e., 5.8 and 6.5 Gy TBI) and that had not been provided clinical support are shown in Figure 1 A, B. In general, the patterns are remarkably similar, apart from a few exceptions. However, blood counts of most of the assayed blood elements appeared to be slightly more depressed in females than those of males at or near response nadirs, including the marginal differences between males and females in blood neutrophil and platelet values. By contrast, response patterns between sexes relative to hemoglobin levels and hematocrit values appeared different (did not reach significance) at the lower exposure level of 5.8 Gy, but at the high exposure level of 6.5 Gy there was no apparent difference (e.g., Figure 1 A, B).
Figure 1.
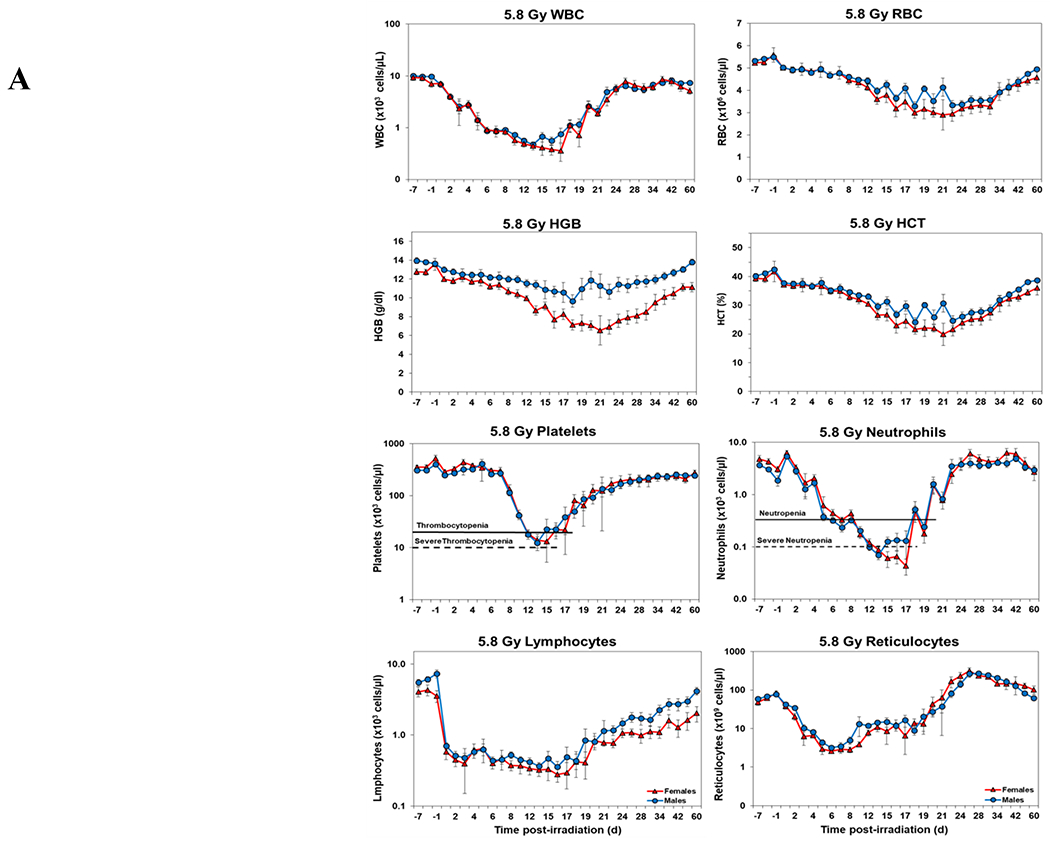
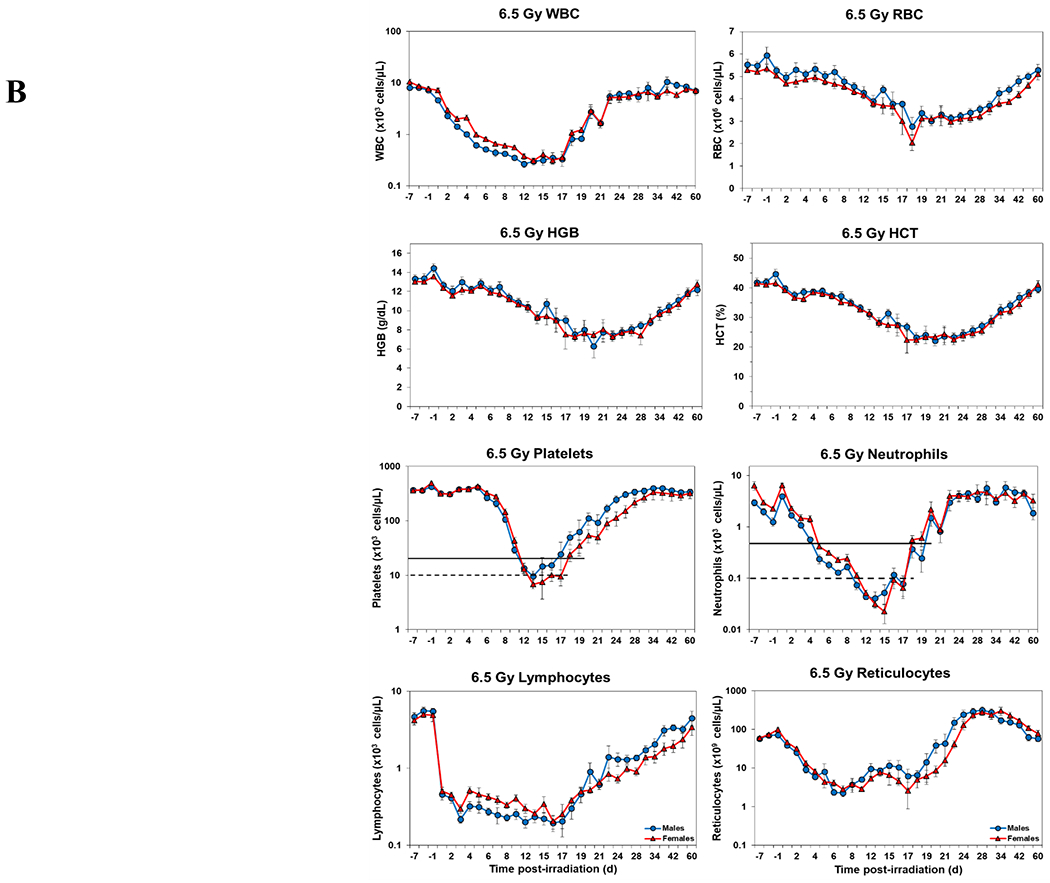
Complete blood counts of acutely irradiated NHPs not provided clinical support. The data for each time point is presented as the mean for each group. A. Represents CBC responses of animals exposed at 5.8 Gy (24 total animals, with 15 males and 9 females). B. Represents responses of animals exposed at 6.5 Gy (24 total animals, with 10 males and 14 females).
Blood responses of male and female NHPs acutely irradiated at lethal exposure levels (i.e., 6.0 – 8.5 TBI) and provided the benefit of clinical support are shown in Figure 2 A – F. At the lower exposure levels (i.e., 6.0 & 6.5 Gy) the temporal blood response patterns between sexes were similar, but not identical: e.g., the level of suppression of both platelet and neutrophil levels appeared greater in females than in males at the lower exposure level of 6.0 Gy, but not at the slightly higher exposure level of 6.5 Gy (Figure 2 A vs B).
Figure 2.
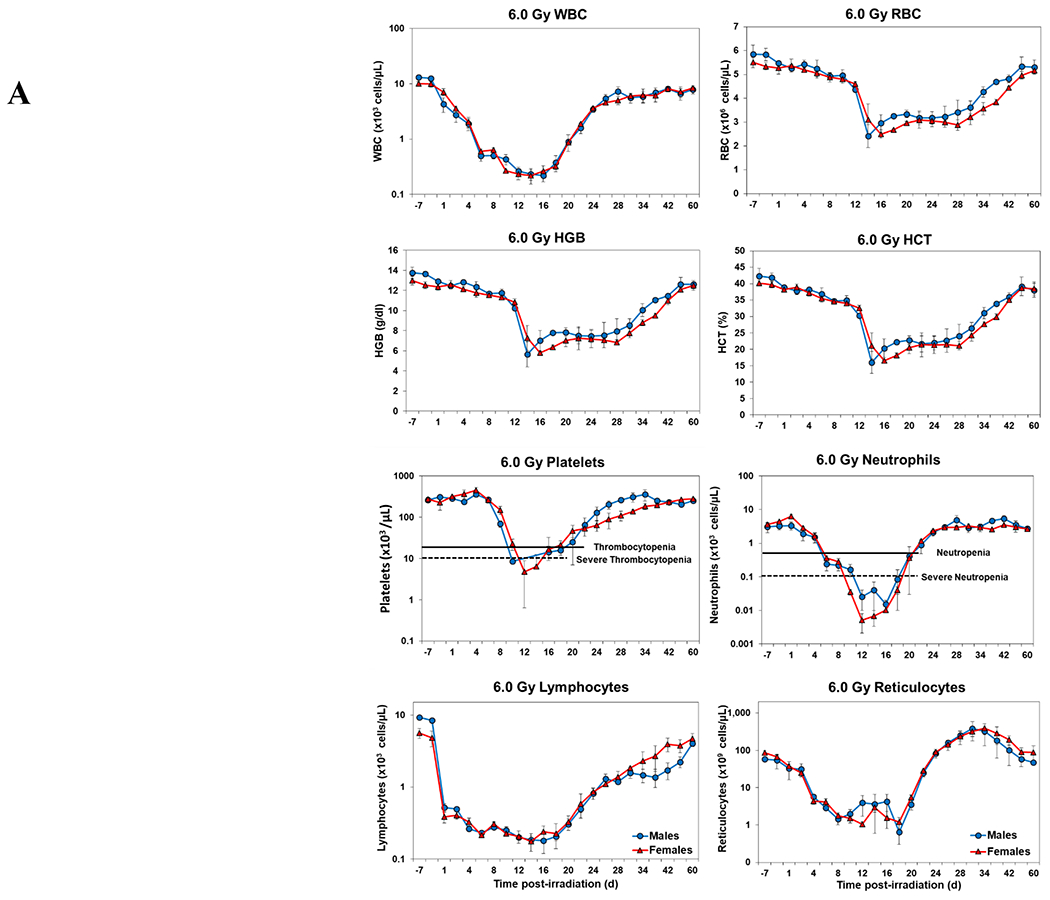
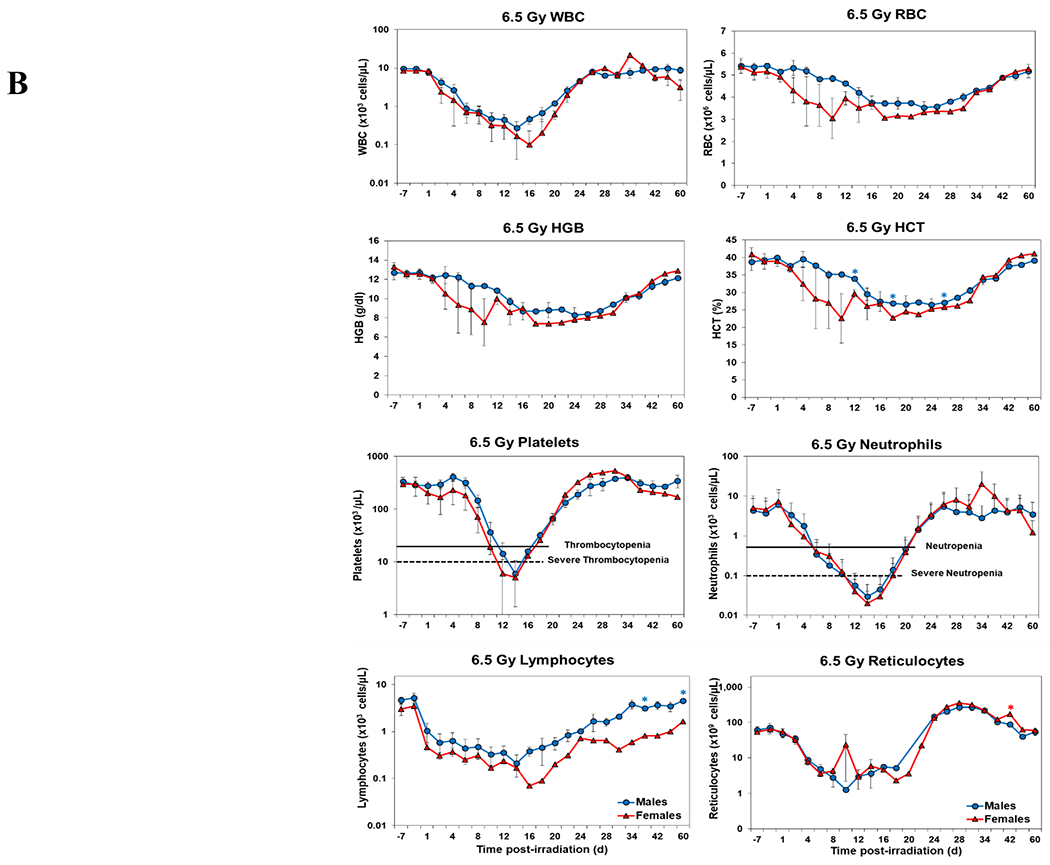
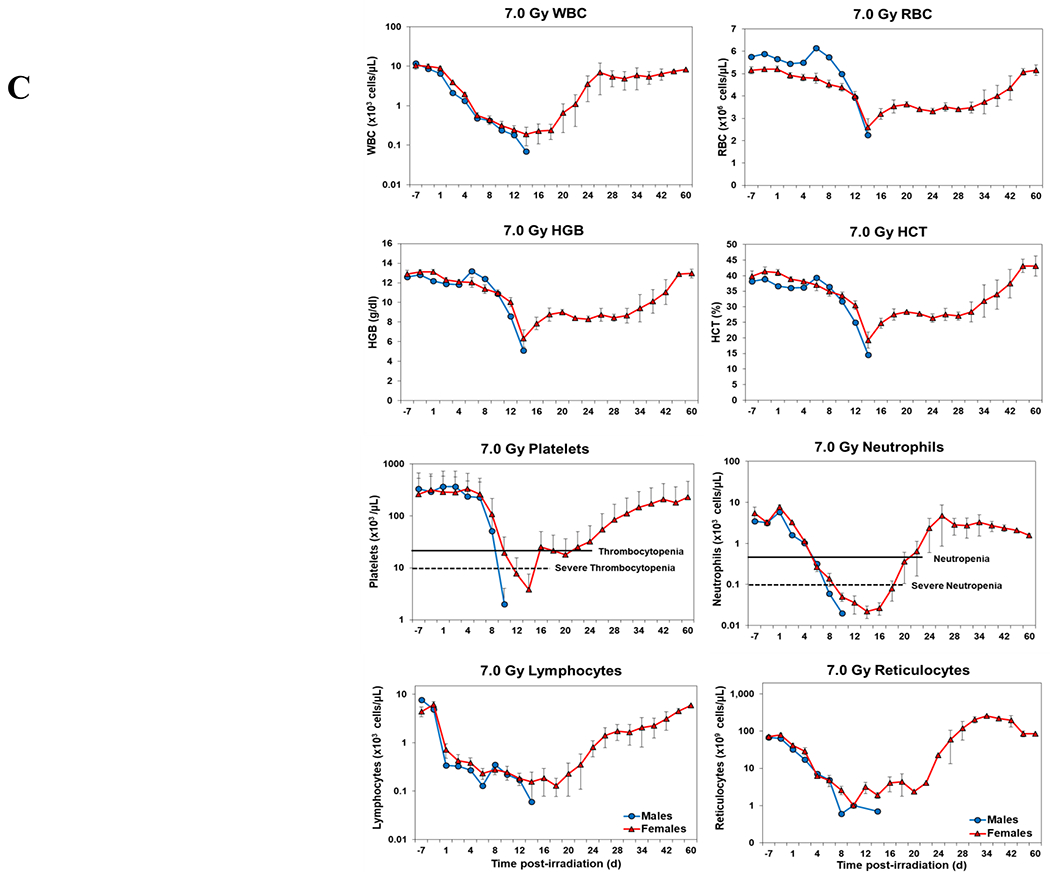
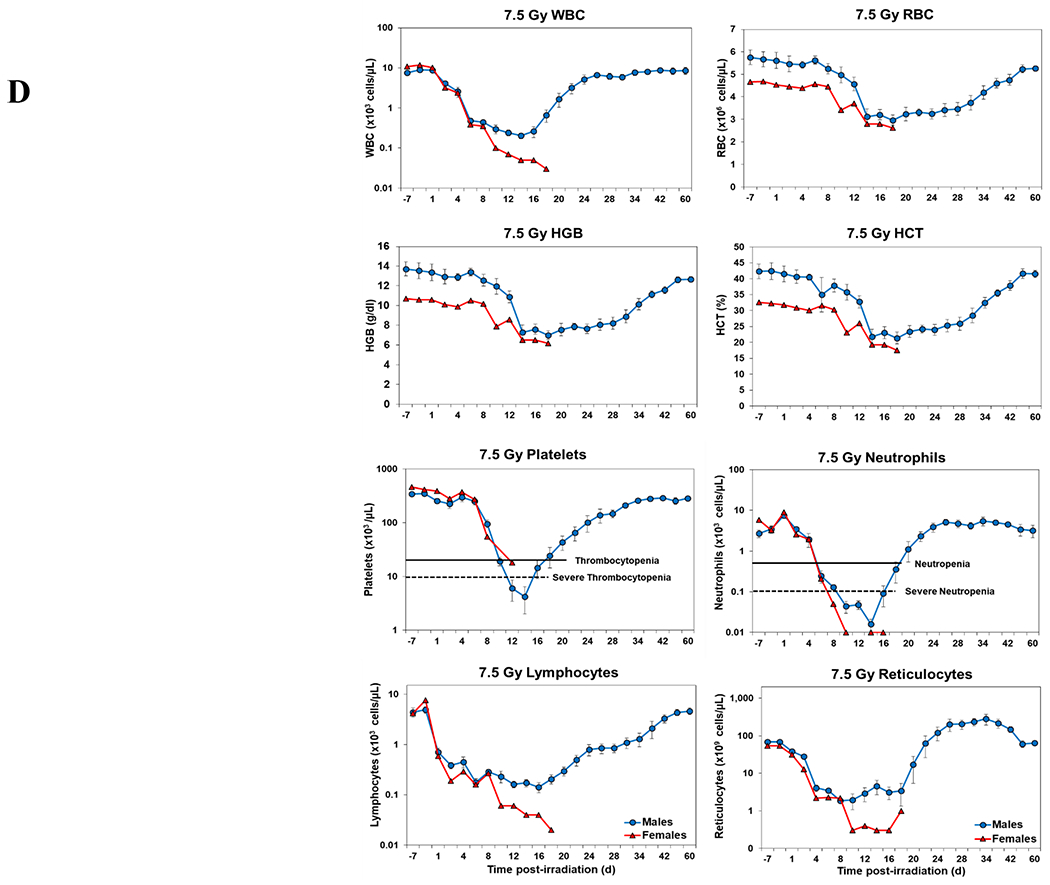
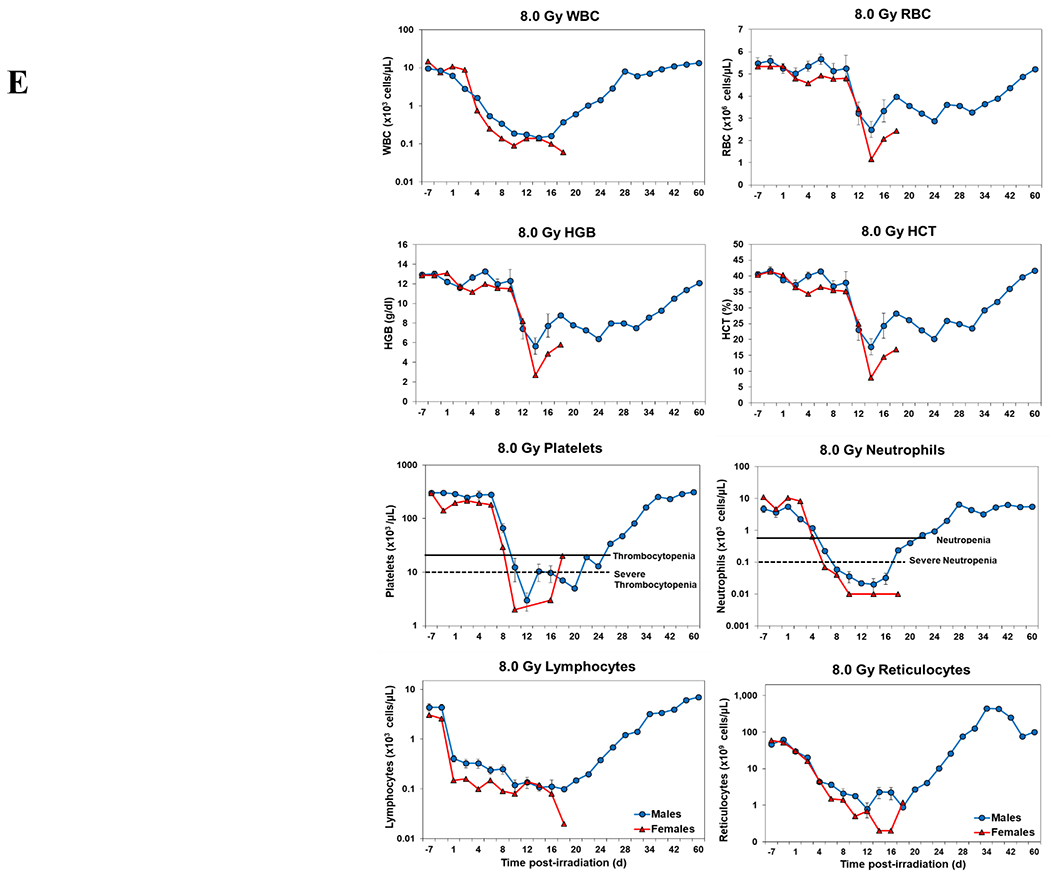
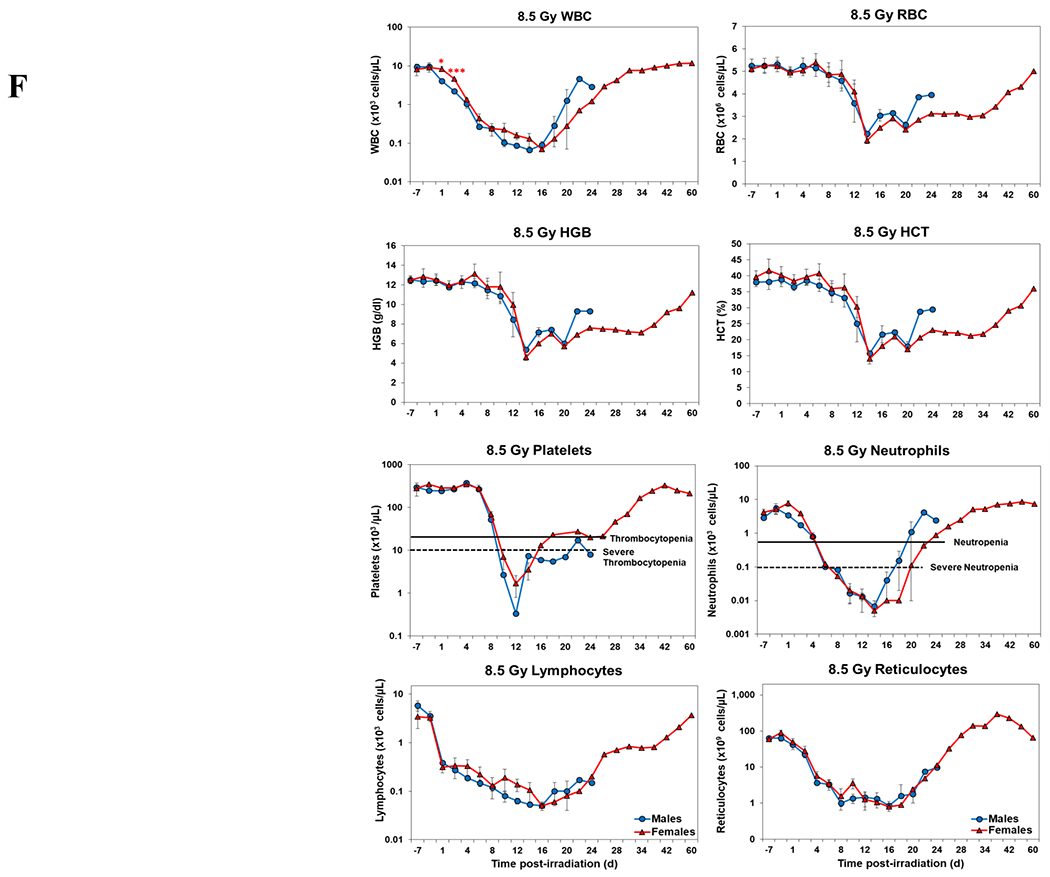
Complete blood counts of acutely irradiated NHPs provided clinical support. The data for each time point is presented as the mean for each group. Red asterisks indicate a significantly higher difference in females, while blue asterisks denote a significantly higher difference in males. The number of asterisks corresponds to the level of statistical significance as follows: *p<0.05, ***p<0.001. A. CBC responses of animals exposed at 6.0 Gy (6 total animals, with 2 males and 4 females). B. CBC responses of animals exposed at 6.5 Gy (6 total animals, with 3 males and 3 females). C. CBC responses of animals exposed at 7.0 Gy (6 total animals, with 1 male and 5 females). D. Represents responses of animals exposed at 7.5 Gy (6 total animals, with 5 males and 1 female). E. CBC responses of animals exposed at 8.0 Gy (6 total animals, with 5 males and 1 female). F. Represents responses of animals exposed at 8.5 Gy (6 total animals, with 3 males and 3 females).
At still higher, more lethal exposure levels at or greater than 7.0 Gy, distinct, sex-specific patterns were difficult to discern due to either the limited number of animals of a given sex or due to early deaths. In general, however, at the higher exposure levels (> 7.5 Gy) tested, females appeared to respond more poorly over the sixty day test period.
3.4. Cytokine response patterns
Temporal patterns of blood serum cytokine responses of acutely irradiated NHPs not receiving clinical support were similar in general. We have presented the data of selected cytokines which are usually upregulated in response to irradiation. With the exception of IL-8 and IL-1β serum levels, cytokine levels tended to rise from relatively low, pre-exposure baseline values to peak values at 4 h and declining thereafter (Figure 3 A, B). Further, comparing the temporal patterns of these cytokine responses of the two sexes, it appeared that peak values were higher generally in males than in females exposed at 5.8 Gy (Figure 3 A). These response patterns were noted for the majority of the cytokines, but not all (e.g., except IL-10 and G-CSF) (Figure 3 A). By contrast, at the higher exposure level of 6.5 Gy, only IL-6 and IL-8 levels were comparably elevated in males relative to females and peak levels of GM-CSF levels were lower in males than in females (Figure 3 B). By comparison, the temporal response patterns of serum cytokines of acutely irradiated NHPs that had received post-exposure clinical support were distinct from those patterns found in animals not given clinical support (Figure 4 A, B). Relative to specific cytokines and to the level of exposure and sex, the noted response patterns varied considerably and were less consistent relative to the responses noted earlier with animals not given clinical support. For example, at the lower levels of exposure (6.0, 6.5, and 7.0 Gy) select cytokines (e.g., G-CSF) appeared unresponsive to the stress of exposure over the initial 48 h post-exposure; the characteristic, early rise and ‘peaking’ of serum cytokine levels at ~1 h appeared absent (Figure 4 A). At the higher exposure levels (7.5, 8.0, and 8.5 Gy) and for most of the assayed cytokines, the general features of the time-dependent cytokine responses were retained, including flattening and/or declining of the responses over the initial post-exposure period (Figure 4 B). Of particular note was the late and continued rise (up to 96 h) in blood serum levels of G-CSF of females that contrasted to the reduced serum levels of G-CSF in males at the 96 h sampling point (Figure 4 B). Blood transfusion occurred only after a week post-irradiation in study with supportive care. Thus, during first week of study, animals with or without supportive care are the same. All cytokine changes discussed above are related only to the initial day of irradiation and shortly thereafter.
Figure 3.
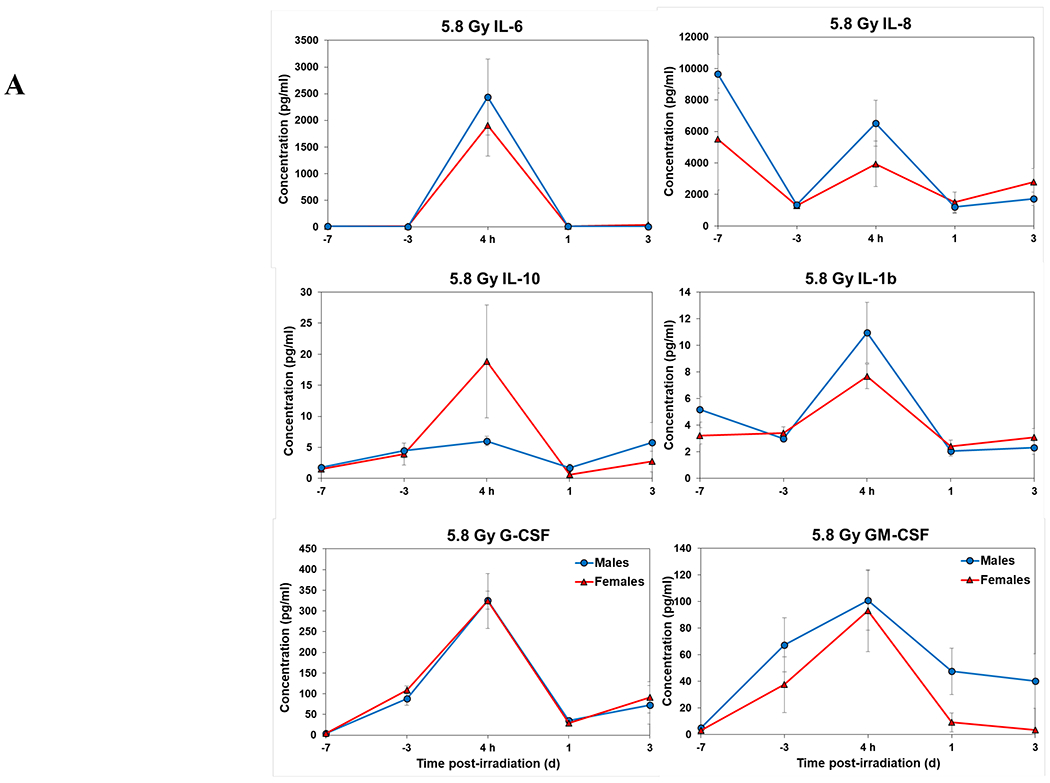
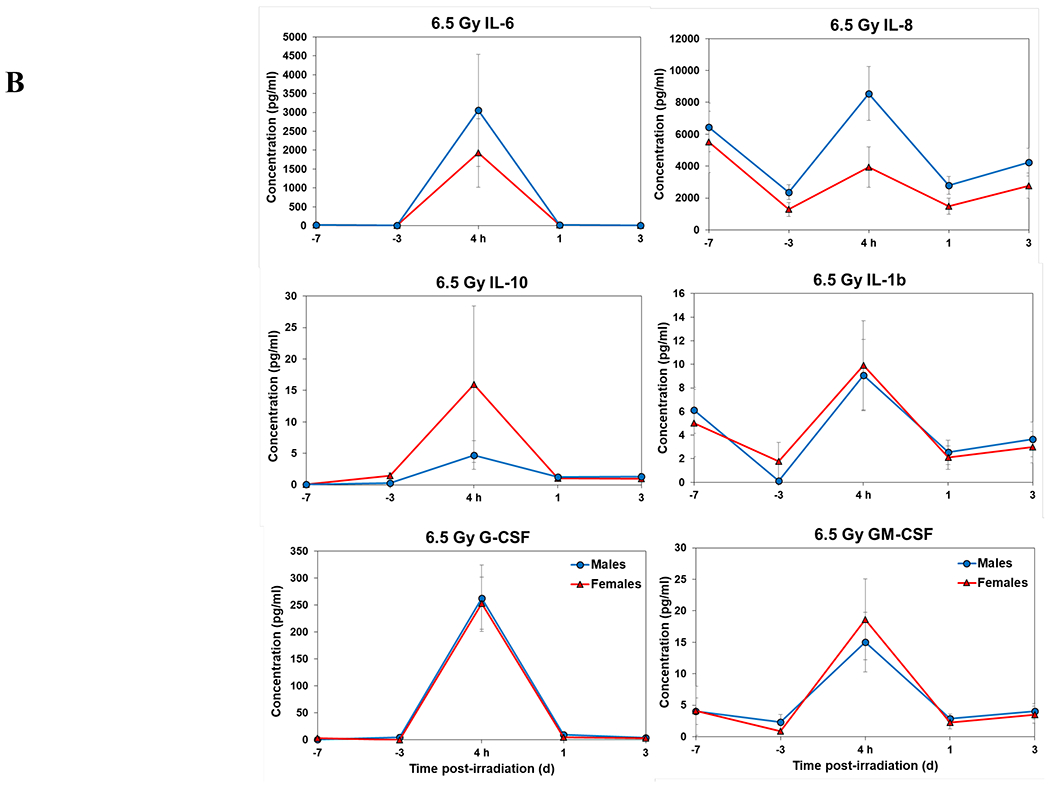
Cytokine responses in male and female NHPs not receiving clinical support following either 5.8 Gy or 6.5 Gy irradiation. A. 5.8 Gy, 24 NHPs in total, comprised of 15 males and 9 females. B. 6.5 Gy, 24 NHPs total, comprised of 10 males and 14 females.
Figure 4.
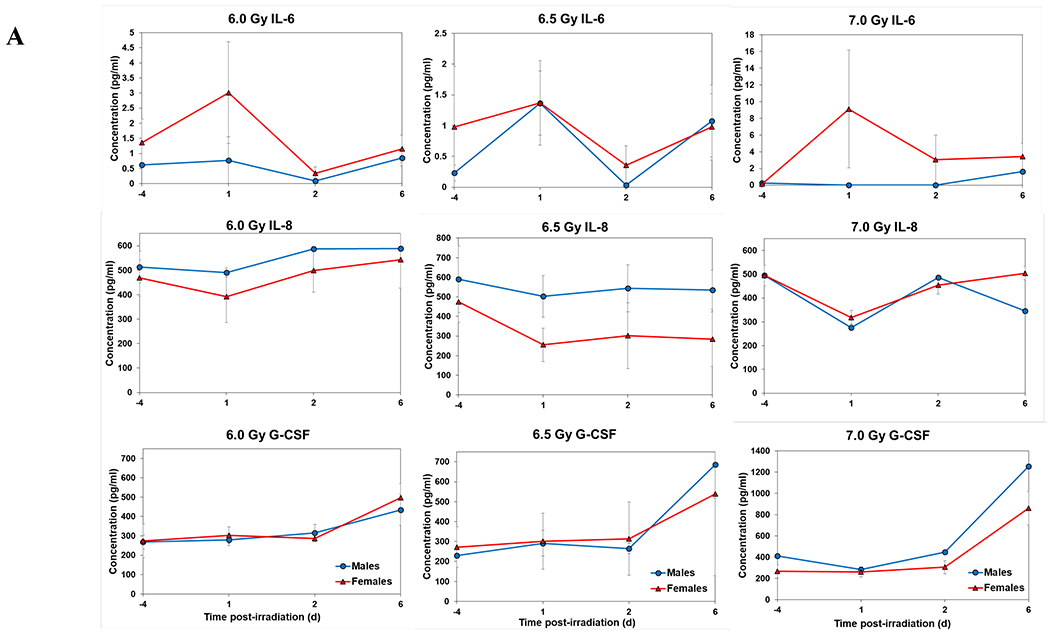
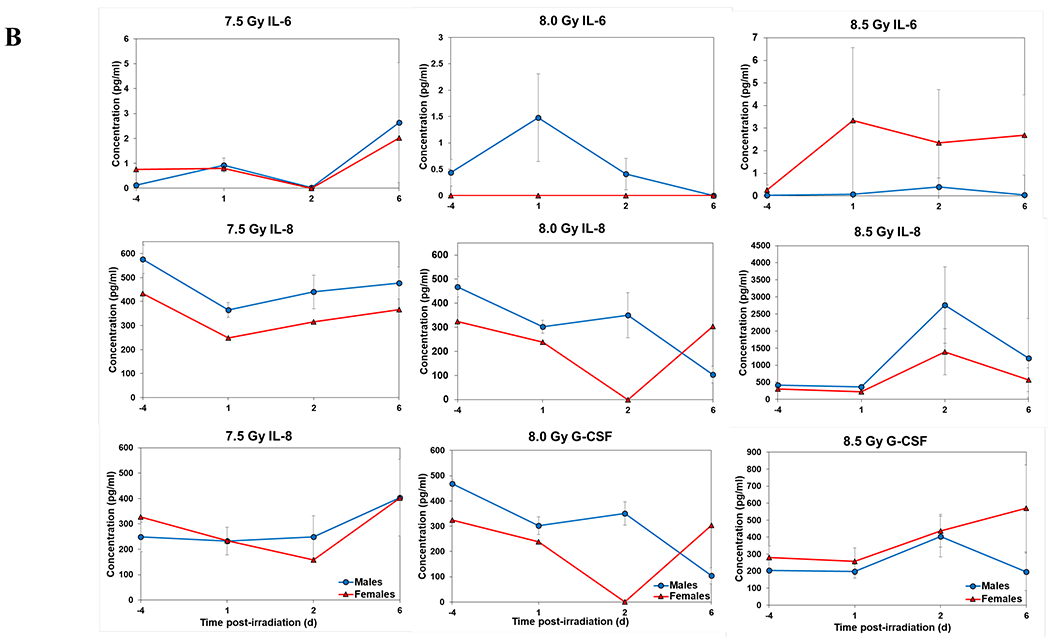
Cytokine responses in male and female NHPs receiving the benefit of full clinical support following either 6.0, 6.5, 7.0, 7.5, 8.0, or 8.5 Gy irradiation. A. For 6.0, 6.5, or 7.0, there were a total of 6 animals at each dose level, comprised of 2, 3, and 1 male, respectively; whereas there were 4, 3, and 5 females, respectively. B. For 7.5, 8.0, or 8.5 Gy, there were a total of 6 animals at each dose level, comprised of 5, 5, and 3 males, respectively; whereas there were 1, 1, and 3 females, respectively.
4. Discussion
For the research and development and eventual approval of new MCMs by the US FDA for human use to prevent, mitigate or to treat unwanted radiation exposure-associated injuries, these agents need to be thoroughly tested for efficacy and safety and approved by the regulatory authority. Because of the obvious ethical limitations of testing a given agent’s efficacy in countering the effects of ionizing radiation in normal, healthy individuals, the FDA developed and promulgated a new Animal Rule that allows new agents to be tested for efficacy using appropriate, well-developed and well-understood animal models that encompass both small (e.g., mice) and large (e.g., nonhuman primates) experimental animals [6,33–35]. Although this new Animal Rule and its various testing stipulations are readily understood and clearly serve the drug developer for efficacy documentation, the limitations of the agency’s recommendation (and the general consensus among researchers and funding agencies) is that not only should large animal models (to complement initial preclinical results using small animal models) be employed during advanced, often pivotal preclinical testing, but both sexes should also be incorporated into the testing schema. This inadvertently creates a series of rather significant logistical problems for researchers/drug developers in attempting to move a new medicinal through the various Research and Development steps required to gain final regulatory approval. The limited availability, high purchase cost and maintenance of a required number of large animals of both sexes for proper drug testing all accentuate the cost of doing business.
Clearly, there are response differences between the sexes, and these differences have been documented relative to the health consequences associated with varying regiments of IR exposures [1,36–38]. Relative to acute, potentially lethal IR exposures of large experimental animals, namely NHPs, we ask here the basic question of whether or not the health outcomes of male NHPs differ substantially from female NHPs. In general, the answer is ‘no’, but there are caveats/exceptions to this. For example, we note here that the survival rates of male and female NHPs exposed to a lower range of acute irradiation (i.e., near-lethal doses) differed, but only marginally. At the two low exposure levels of 5.8 and 6.5 Gy and in those animals lacking clinical support, survival rates were slightly greater in males than in females (~72% in males vs ~65%% in females). These rather slight differences between the sexes appeared greater when a baseline level of clinical support was provided: i.e., at the 6.0 and 6.5 Gy exposure levels, survival rates of males were 100% and ~67%, respectively; whereas for females, survival rates appeared much lower at 50% and 33%, respectively. By contrast, at the highest exposure level examined (8.5 Gy) and when full clinical support was provided, females seemed to fare better in terms of survival than did males. In general, our observations concerning the apparent differences in rates of survival between male and female NHPs following acute, potentially lethal radiation exposures are consistent with recent findings by Beach et al. [1]. Furthermore, comparable differences between the two sexes were noted, not only in terms of survival, but also in terms of blood response and induction patterns of select serum cytokines.
In this exercise here, we have compared and contrasted various response patterns (e.g., survival, blood, cytokine responses) of both male and female NHPs that were subjected to acute, intense and potentially lethal radiation exposures and have found that these ‘patterns’ for the two sexes to be quite similar, but certainly not identical. The question is whether these differences are sufficiently large to cause major concern when attempts to interpret efficacy and safety data generated from given ‘radiation countermeasure’ experiments that have used predominately one sex are used. The short answer here is that we do not believe so, but we still harbor a concern about attempting to generalize noted responses of a single sex to that of both sexes. Regardless, we do believe that differences, however minor, in the response patterns between the two sexes need to be ultimately accounted for; however, this should not be perceived as a major roadblock in pushing forward and developing new, promising countermeasures. Accordingly, we offer the following account of recent experiments related to ‘countermeasure research’ and our experience in noting sex-based response patterns.
Gamma-tocotrienol (GT3) is one such promising radiation countermeasure that is under advanced development using NHP models of total and partial-body irradiation [9–11,24,39–42]. In brief, we compared male and female irradiated animals pre-treated with GT3 and did not find any significant difference in respect of CBC recovery or cytokine induction (Supplementary Figures 1–3), although GT3 prophylaxis enhanced CBC recovery within drug-treated- versus vehicle-treated NHPs acutely exposed to 5.8 Gy as well as 6.5 Gy cobalt-60 TBI [24]. Furthermore, we observed (as previously noted) some difficulty in comparing sex-based responses (e.g., survival patterns), as the numbers of male or female animals employed were often limited at select exposure levels.
Other investigators have also noted similar issues when attempting to compare and contrast survival and blood response patterns, as the vast majority of these studies have used animals of only one sex. Furthermore, using different radiation sources, in turn, different radiation qualities, all served to complicate theses analyses (i.e., sex-based response analyses) even more, as does the use of various types and levels of supportive care provided to the acutely irradiated animals. As suggested earlier, there are simply too many variables that investigators need to contend with when attempting to incorporate both sexes into single experiments. This problem is a potential roadblock that might be alleviated by addressing sex-based issues via serial assessments. Regardless, the use of both sexes in any given experiment will serve no doubt to double the number of animals (i.e., in order to maintain sufficient levels of statistical power) and, in turn, double the cost of the research. The use of ‘historical data sets’ to establish essential response baselines for the sexes might be better and more extensively employed. To this point, a recent, well-developed and most welcomed report by Beach et al [1] examined survival patterns of acutely irradiated NHPs of both sexes under slightly different modes of post-exposure supportive care (i.e., standard regimen of clinical support alone versus a more complete regimen that included blood transfusions). The results appeared to highlight slight, but apparently significant differences in survival patterns between the two sexes. A comparison of estimated probits suggested that females have higher mortality than males at the same radiation doses, and supportive care with blood transfusions increased survival of males at lower doses but not at high doses of radiation exposure. It is important to note, however, that all experiments were not conducted under exactly the same experimental conditions or concurrently for intended comparisons; nevertheless, more observations were noted and useful insights were obtained.
Lastly, we suggest that the fundamental limitation in all of these studies relate to sample size of males and females in given experiments. Without question, we support the basic concept that both sexes of the primary large animal species should be utilized for advanced MCM R&D. This most certainly include the evaluation of both the efficacy and the safety of various ‘supportive care regimens’.
Supplementary Material
Acknowledgements
The authors would like to thank the staff of the Veterinary Science Department of AFRRI; the Comparative Medicine Department of the University of Maryland, Baltimore, BIOQUAL Inc., Rockville for animal care; and to the staff of the Radiation Science Department, AFRRI, for dosimetry and radiation exposure to the animals.
Funding:
The authors gratefully acknowledge the research support from the National Institute of Allergy and Infectious Diseases (AAI12044-001-07000, Work plan G), the Congressionally Directed Medical Research Programs (W81XWH-15-C-0117, JW140032), the Defense Threat Reduction Agency (CBM.RAD.01.10.AR.005), and the Uniformed Services University of the Health Sciences/Armed Forces Radiobiology Research Institute (grant # AFR-B4-10978 and 12080) to VK Singh.
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Ethics Statement
All procedures involving animals were approved by the Institutional Animal Care and Use Committee (AFRRI protocol #’s 2010-12-017, 2015-12-011; University of Maryland protocol # 0518005; and BIOQUAL Inc. protocol #18-060) and the Department of Defense Animal Care and Use Review Office (ACURO). This study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Data Availability:
All relevant data are within the manuscript.
References
Papers of special note have been highlighted as either of interest (*) or of high interest (**) to readers.
- 1.Beach T, Authier S, Javitz HS, et al. Total body irradiation models in NHPs - consideration of animal sex and provision of supportive care to advance model development. Int J Radiat Biol 2021;97:126–30 [DOI] [PubMed] [Google Scholar]; ** This study suggests that the use of single sex animal models may lead to an incomplete understanding of potential sex differences in the dose to mortality response of the total-body irradiation model.
- 2.Singh VK, Seed TM. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int J Radiat Biol 2017;93:851–69 [DOI] [PubMed] [Google Scholar]; ** This three part review article covers various subsyndromes of ARS and radiation countermeasures under development using various animal models.
- 3.Champlin RE, Kastenberg WE, Gale RP. Radiation accidents and nuclear energy: medical consequences and therapy. Ann Intern Med 1988;109:730–44 [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. Animal Rule information. 2022. Available at: http://www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/MCMRegulatoryScience/ucm391604.htm [Last accessed October 20, 2022]
- 5. U.S. Food and Drug Administration. Animal Rule approvals. 2022. Available at: https://www.fda.gov/drugs/nda-and-bla-approvals/animal-rule-approvals [Last accessed December 5, 2022] There is a list of all drugs approved by US FDA following Animal Rule.
- 6.U.S. Food and Drug Administration. Guidance document: Product development under the Animal Rule. 2015. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf [Last accessed October 20, 2022]; * This is the US FDA draft guidance document for drug development under the Animal Rule, and it demonstrates the relevance of biomarkers for the development of countermeasures.
- 7.Singh VK, Olabisi AO. Nonhuman primates as models for the discovery and development of radiation countermeasures. Expert Opin Drug Discov 2017;12:695–709 [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is an important article providing a wealth of information regarding the use of nonhuman primate models for studying radiation injury and developing radiation countermeasures for acute radiation syndrome following the US Food and Drug Administration Animal Rule.
- 8.Dorr H, Lamkowski A, Graessle DH, et al. Linking the human response to unplanned radiation and treatment to the nonhuman primate response to controlled radiation and treatment. Health Phys 2014;106:129–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg S, Garg TK, Miousse IR, et al. Effects of gamma-tocotrienol on partial-body irradiation-induced intestinal injury in a nonhuman primate model. Antioxidants (Basel) 2022;11:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg S, Garg TK, Wise SY, et al. Effects of gamma-tocotrienol on intestinal injury in a GI-specific acute radiation syndrome model in nonhuman primate. Int J Mol Sci 2022;23:4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg TK, Garg S, Miousse IR, et al. Gamma-tocotrienol modulates total-body irradiation-induced hematopoietic injury in a nonhuman primate model. Int J Mol Sci 2022;23: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res 2010;173:557–78 [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This article discusses various animal models for developing medical countermeasures.
- 13.Farese AM, Cohen MV, Katz BP, et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res 2013;179:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hankey KG, Farese AM, Blaauw EC, et al. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat Res 2015;183:643–55 [DOI] [PubMed] [Google Scholar]
- 15.Wong K, Bunin DI, Bujold K, et al. Romiplostim (Nplate) alone and in combination with pegfilgrastim (Neulasta) increased survival and reduces incidence, duration, and severity of thrombocytopenia post-iradiation in non-human primates. 66th Annual Conference of Radiation Research Society. Virtual https://www.radres.org/events/EventDetails.aspx?id=1336280 2020 [Google Scholar]
- 16.Wong K, Chang PY, Fielden M, et al. Pharmacodynamics of romiplostim alone and in combination with pegfilgrastim on acute radiation-induced thrombocytopenia and neutropenia in non-human primates. Int J Radiat Biol 2020;96:155–66 [DOI] [PubMed] [Google Scholar]
- 17.Clayton NP, Khan-Malek RC, Dangler CA, et al. Sargramostim (rhu GM-CSF) improves survival of non-human primates with severe bone marrow suppression after acute, high-dose, whole-body irradiation. Radiat Res 2021;195:191–99 [DOI] [PubMed] [Google Scholar]
- 18.Zhong Y, Pouliot M, Downey AM, et al. Efficacy of delayed administration of sargramostim up to 120 hours post exposure in a nonhuman primate total body radiation model. Int J Radiat Biol 2021;97:S100–S16 [DOI] [PubMed] [Google Scholar]
- 19.Singh VK, Seed TM. An update on sargramostim for treatment of acute radiation syndrome. Drugs Today (Barc) 2018;54:679–93 [DOI] [PubMed] [Google Scholar]
- 20.Singh VK, Seed TM. An update on romiplostim for treatment of acute radiation syndrome. Drugs Today (Barc) 2022;58:133–45 [DOI] [PubMed] [Google Scholar]
- 21.MacVittie TJ, Farese AM, Parker GA, et al. The time course of radiation-induced lung injury in a nonhuman primate model of partial-body irradiation with minimal bone marrow sparing: Clinical and radiographic evidence and the effect of neupogen administration. Health Phys 2019;116:366–82 [DOI] [PubMed] [Google Scholar]
- 22.Singh VK, Seed TM. Radiation countermeasures for hematopoietic acute radiation syndrome: growth factors, cytokines and beyond. Int J Radiat Biol 2021;97:1526–47 [DOI] [PubMed] [Google Scholar]; * This is a recent article discussing all radiation medical countermeasures approved by the US Food and Drug Administration following the Animal Rule.
- 23.Singh VK, Fatanmi OO, Wise SY, et al. Determination of lethality curve for cobalt-60 gamma-radiation source in rhesus macaques using subject-based supportive care. Radiat Res 2022;198:599–614 [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study has determined the lethality curve for cobalt-60 gamma-radiation source using both male and female rhesus nonhuman primates and subject-based supportive care.
- 24.Singh VK, Kulkarni S, Fatanmi OO, et al. Radioprotective efficacy of gamma-tocotrienol in nonhuman primates. Radiat Res 2016;185:285–98 [DOI] [PubMed] [Google Scholar]; ** This study demonstrates radioprotective efficacy of gamma-tocotrienol using both male and female nonhuman primates and total-body gamma-irradiation.
- 25.National Research Council of the National Academy of Sciences. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academies Press, 2011 [Google Scholar]
- 26.Vellichirammal NN, Sethi S, Pandey S, et al. Lung transcriptome of nonhuman primates exposed to total- and partial-body irradiation. Mol Ther Nucleic Acids 2022;29:584–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheema AK, Li Y, Moulton J, et al. Identification of novel biomarkers for acute radiation injury using multiomics approach and nonhuman primate model. Int J Radiat Oncol Biol Phys 2022;114:310–20 [DOI] [PubMed] [Google Scholar]; ** An elegant study using an NHP model and multi-omics approach to identify and validate proteomic/metabolomic biomarkers for radiation injury with six different doses of radiation.
- 28.Li Y, Singh J, Varghese R, et al. Transcriptome of rhesus macaque (Macaca mulatta) exposed to total-body irradiation. Sci Rep 2021;11:6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter AD, Li Y, Janocha BL, et al. Analysis of the proteomic profile in serum of irradiated nonhuman primates treated with Ex-Rad, a radiation medical countermeasure. J Proteome Res 2023;(in press): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni S, Singh PK, Ghosh SP, et al. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine 2013;62:278–85 [DOI] [PubMed] [Google Scholar]
- 31.American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. 2020. Available at: https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf [Last accessed December 29, 2022]
- 32.Phipps AJ, Bergmann JN, Albrecht MT, et al. Model for evaluating antimicrobial therapy to prevent life-threatening bacterial infections following exposure to a medically significant radiation dose. Antimicrob Agents Chemother 2022. e0054622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snoy PJ. Establishing efficacy of human products using animals: the US food and drug administration’s “animal rule”. Vet Pathol 2010;47:774–8 [DOI] [PubMed] [Google Scholar]
- 34.Aebersold P. FDA experience with medical countermeasures under the Animal Rule. Adv Prevent Med 2012;2012:507571. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This article highlights the FDA Animal Rule and use of biomarkers for drug development.
- 35.Park GD, Mitchel JT. Working with the U.S. Food and Drug Administration to obtain approval of products under the Animal Rule. Ann N Y Acad Sci 2016;1374:10–6 [DOI] [PubMed] [Google Scholar]
- 36.Orschell CM, Wu T, Patterson AM. Impact of age, sex, and genetic diversity in murine models of the hematopoietic acute radiation syndrome (H-ARS) and the delayed effects of acute radiation exposure (DEARE). Current Stem Cell Reports 2022;8:139–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson AM, Vemula S, Plett PA, et al. Age and sex divergence in hematopoietic radiosensitivity in aged mouse models of the hematopoietic acute radiation syndrome. Radiat Res 2022;198:221–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiang JG, Cannon G, Olson MG, et al. Female mice are more resistant to the mixed-field (67% neutron + 33% gamma) radiation-induced injury in bone marrow and small intestine than male mice due to sustained increases in G-CSF and the Bcl-2/Bax ratio and lower miR-34a and MAPK activation. Radiat Res 2022;198:120–33 [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This article demonstrates that the female mice are more resistant to the mixed-field radiation-induced injury in bone marrow and small intestine than male mice. This may be due to sustained increases in G-CSF and the Bcl-2/Bax ratio and lower miR-34a and MAPK activation.
- 39.Singh VK, Seed TM. Development of gamma-tocotrienol as a radiation medical countermeasure for the acute radiation syndrome: Current status and future perspectives. Expert Opin Investig Drugs 2023;32:25–35 [DOI] [PubMed] [Google Scholar]
- 40.Rosen E, Fatanmi OO, Wise SY, et al. Gamma-tocotrienol, a radiation countermeasure, reverses proteomic changes in serum following total-body gamma irradiation in mice. Sci Rep 2022;12:3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pannkuk EL, Laiakis EC, Fornace AJ Jr., et al. A metabolomic serum signature from nonhuman primates treated with a radiation countermeasure, gamma-tocotrienol, and exposed to ionizing radiation. Health Phys 2018;115:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheema AK, Mehta KY, Fatanmi OO, et al. A Metabolomic and lipidomic serum signature from nonhuman primates administered with a promising radiation countermeasure, gamma-tocotrienol. Int J Mol Sci 2017;19:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript.


