Abstract
Introduction:
Regenerative medicine involves the replacement of damaged cells, tissues, or organs to restore normal function. Mesenchymal stem cells (MSCs) and exosomes secreted by MSCs have unique advantages that make them a suitable candidate in the field of regenerative medicine.
Areas covered:
This article provides a comprehensive overview of regenerative medicine, focusing on the use of MSCs and their exosomes as potential therapies for replacing damaged cells, tissues, or organs. This article discusses the distinct advantages of both MSCs and their secreted exosomes, including their immunomodulatory effects, lack of immunogenicity, and recruitment to damaged areas. While both MSCs and exosomes have these advantages, MSCs also have the unique ability to self-renew and differentiate. This article also assesses the current challenges associated with the application of MSCs and their secreted exosomes in therapy. We have reviewed proposed solutions for improving MSC or exosome therapy, including ex-vivo preconditioning strategies, genetic modification, and encapsulation. Literature search was conducted using Google Scholar and PubMed databases.
Expert opinion:
Providing insight into the future development of MSC and exosome-based therapies and to encourage the scientific community to focus on the identified gaps, develop appropriate guidelines, and enhance the clinical application of these therapies.
Keywords: mesenchymal stem cells, exosomes, extracellular vesicles, regenerative medicine
Graphical Abstract:
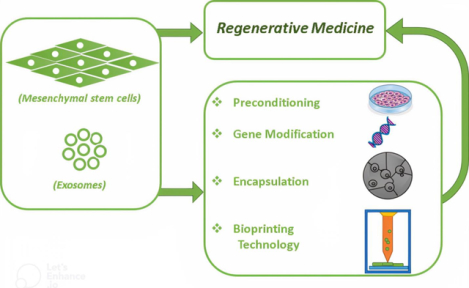
This graphical abstract illustrates the potential for direct use of MSCs or their secreted exosomes in regenerative medicine, as well as various strategies to enhance their efficacy.
1. Introduction
The term ‘regenerative medicine’ refers to the ability to restore or replace tissues and organs that have been injured or lost by illness, trauma, aging, or other factors. Regenerative medicine is a promising alternative therapeutic option to address problems in tissue or cell transplantation, such as donor supply shortages or immune complications [1,2].
Since both synthetic and biomaterials are extensively used in tissue repair and therapeutic procedures, material development is a high priority in regenerative medicine. Although some synthetic materials, such as silicone and tetrafluoroethylene, were developed to repair damaged tissues, their tissue-specific functional characteristics were not preserved [3]. Therefore, in recent decades, living materials that are biologically generated by living cells have been of great interest due to their ability to be well-tolerated by the body and to promote biological activity [1]. To develop living materials, human cells, especially those with the potential for self-renewal, expansion, and differentiation into other types of cells, have been a crucial focus in the field of regenerative medicine [4].
Stem cells are known to have the abilities of proliferation and the generation of identical daughter cells, as well as the capability of differentiation into other types of cells. According to their sources, there are four types of stem cells: embryonic stem cells, placental and umbilical cord stem cells, adult stem cells, and induced pluripotent stem cells (iPSCs) [5,6]. Even without considering the moral and ethical implications, human embryos are not the ideal source from a technical perspective. Other sources of stem cells, such as the placenta, umbilical cord, and many adult tissues, possess MSCs, which are multipotent cells that can differentiate into several types of cells [4,7,8]. Especially, extracellular vesicles (EVs), mainly exosomes, secreted by MSCs have emerged as a promising therapeutic strategy to treat a variety of diseases [9,10]. In this study, we conducted a literature search using databases such as Google Scholar and PubMed to identify relevant articles on the therapeutic applications of mesenchymal stem cells (MSCs) and their exosomes in the context of degenerative medicine. The search was performed with the following keywords: ‘MSC,’ ‘exosome and MSC,’ ‘degenerative medicine,’ ‘encapsulation,’ ‘preconditioning,’ ‘gene modification,’ ‘bioprinting,’ and ‘clinical trials.’ We searched for articles published between 2017 and 2022. The aim of this review is to provide an overview of the applications of MSCs and their secreted exosomes in regenerative medicine.
2. Mesenchymal stem cells (MSCs)
2.1. Sources, characterization, and properties
MSCs, as adult stem cells, can be isolated from different types of human tissues, including bone marrow, adipose tissue, Wharton’s jelly, the umbilical cord, the chorionic villi of the placenta, and fetal or neonatal tissue [11]. In addition, other uncommon sources have been introduced for MSC isolation, such as amniotic fluid [12], dental pulp [13], endometrium [14], tonsils [15], salivary gland [16], urine [17], menstrual blood [18], peripheral blood [19], synovial fluid [20], and most of human tissues, such as kidney, liver, and pancreas [21]. The IPSCs derived from somatic cells can also be differentiated into MSCs [22]. Regardless of their sources, MSCs possess two important intrinsic characteristics: the abilities to self-renew and the ability to differentiate into other cell lineages [23]. It has been reported that MSCs can express nestin and NANOG genes, which are crucial markers for maintaining pluripotency and self-renewal [23]. In addition, MSCs, as multipotent cells, can produce not only mesodermal lineage cells but also non-mesodermal cells like neuroblasts. Although the mechanism behind the differentiation of MSCs into non-mesodermal cells is not completely clear, some studies have reported the transdifferentiation potential of MSCs, meaning that MSCs can dedifferentiate to the primitive stem cell stage and subsequently differentiate to other lineages [24–26].
As there are no specific criteria for the characterization of MSCs, to classify human MSCs according to the 2006 declaration of the International Society for Cellular Therapy (ISCT), (a) MSCs must be plastic-adherent and have fibroblastic MSC-like morphology under standard culture conditions; (b) they must express some specific surface antigens such as CD105, CD90, CD73, CD44, etc. but lack the expression of CD34, CD45, CD11b, and CD31 to exclude hematopoietic, myeloid, and endothelial cell contamination; (c) they must be able to differentiate into some specific mesenchymal lineages such as chondrocytes, osteoblasts, and adipocytes [27]. However, in recent years, the expression of the highly procoagulant tissue factor (TF/CD142), which initiates coagulation, has been thought to reduce the risk of a thromboembolic adverse effect when using MSCs in clinic [28].
The advantages in the clinical application of MSCs over other types of stem cells can be attributed to their low immunogenicity and unique immunomodulatory properties [29–31]. The risk of immune rejection is the most imminent concern regarding the use of stem cells in cell therapy. Although all types of stem cells have low expression of major histocompatibility complex (MHC) classes I and II, the differentiation of stem cells into more differentiated cells may cause them to lose their low immunogenicity [32]. Therefore, the immunomodulatory properties of MSCs have emerged as an important factor in cell therapy. It has been reported that MSCs can affect both innate and adaptive immunity and inhibit immune responses [33]. MSCs can exert immunomodulatory paracrine factors and direct cell-cell contact between MSCs and immune cells such as T cells, B cells, natural killer cells, macrophages, monocytes, dendritic cells, and neutrophils. MSCs can secrete some cytokines, chemokines, and growth factors such as transforming growth factor-1 (TGF-β1), interferon-γ (IFN-γ), tumor necrosis factor- (TNF-α), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), indoleamine-pyrrole 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), and nitric oxide (NO), which are responsible for the immunosuppressive effects of MSCs via interaction with the innate and adaptive immune systems [33].
Recently, enhancing the immunomodulatory properties of MSCs via either preconditioning or engineering of MSCs has attracted much attention. For example, Zimmermann et al. showed that preconditioning of MSC spheroids cultured in agarose micro-wells with IFN-γ and TNF-α resulted in the increased secretion of immunomodulatory factors PGE2, IDO, and interleukin-6 as well as the inhibition of secretion of TNF-α from macrophage under trans-well co-culture conditions, in comparison with untreated spheroids [34]. Moreover, Garcia et al. encapsulated MSCs with IFN-γ-tethered hydrogels to enhance their immunomodulatory activity and observed an increase in secretion of IDO and programmed death ligand-1 (PD-L1) levels, as well as the capacity of MSCs to suppress proliferation of activated T-cell and finally an acceleration in wound healing in mice treated with MSCs encapsulated with IFN-γ-tethered hydrogels in comparison with MSCs pre-treated with IFN-γ and untreated MSCs [35]. Filho et al. proposed that MSC engineering using the CRISPR-Cas system can enhance their secretum, survival, and migration abilities, which may improve the therapeutic efficiency of MSCs [36]. Another advantage of MSCs that makes them a considerable candidate for cell therapy is their ability to migrate to injured sites. The most important mechanism involved in the migration of MSCs to injured tissues is the stromal cell-derived factor 1 (SDF-1) (CXCL-12)/C-X-C chemokine receptor type 4 (CXCR4) axis. Binding of SDF-1 to CXCR4 expressed on the surface of MSCs can trigger several signaling cascades in MSCs, including p44/p42 extracellular signal-regulated kinases (ERK1/2) and phosphatidylinositol-3-kinase (PI3K)/Akt, which result in the mobilization of stem cells [37,38]. It was reported that overexpression of CXCR4 could lead to increased homing to intestine tumors in the mice which enhanced the anti-tumor function of MSCs [39].
2.2. Mechanisms underlying MSC-based therapy
Several mechanisms have been presented to describe the mechanisms how MSCs can influence target cells (Figure 1). The functions of MSCs in a specific injured tissue include differentiation into specific tissue cells and effect on both specific tissue cells and immune cells. The effect of MSCs on target cells can be mediated through direct cell-cell contact or the release of biomolecules. Wang et al. demonstrated that MSCs can differentiate into cardiomyocytes and smooth muscle cells only through direct co-culture with cardiomyocytes and smooth muscle cells only through direct co-culture with these cells, but not through indirect co-culture or conditioned culture [40]. The effect of MSCs on target cells through direct cell-cell interactions was further supported by another study conducted by Duffy et al, where it was reported that MSCs can inhibit the differentiation of CD4+ T cells into T-helper 17 cells under Th17-inducing conditions by cell-cell contact via the PGE2/EP4 receptor signaling pathway; either PGE2 or EP4 receptor antagonists reversed T-helper 17 cell differentiation inhibition [41]. Moreover, Li et al. showed an increased immunomodulatory effect of MSCs through cell-cell interactions in certain mouse models of abortion. They observed that direct interaction between MSCs and proinflammatory macrophages can increase in CD200 expression on the surface of MSCs, leading to interactions with CD200R on the surface of proinflammatory macrophages and the shift of these cells to an anti-inflammatory phenotype [42].
Figure 1. The mechanisms of mesenchymal stem cells’ (MSCs) actions.
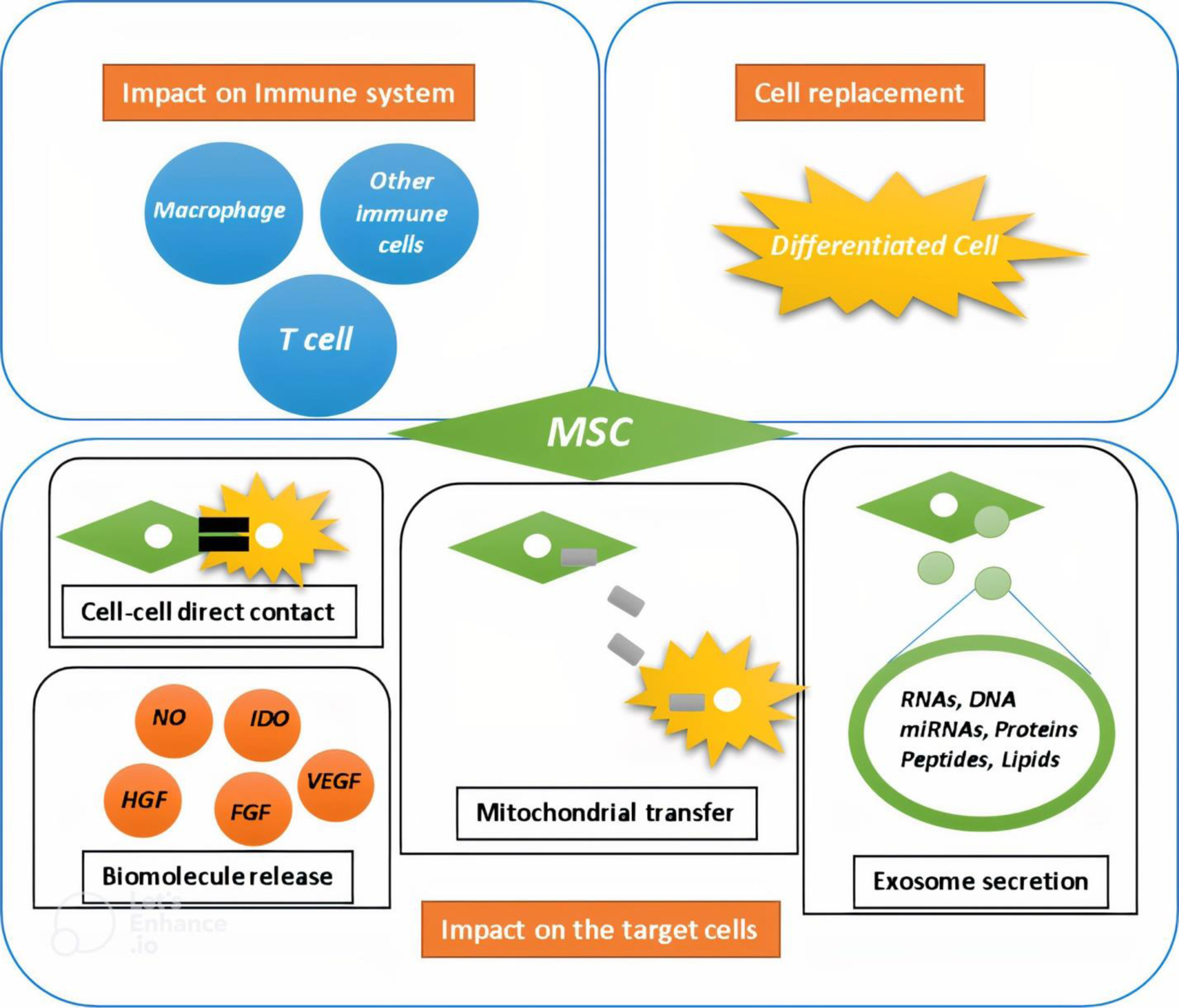
(a) MSCs can differentiate into desired cell types, allowing them to replace injured cells. (b) MSCs possess immunomodulatory properties that enable them to impact immune cells and inhibit inflammation. (c) MSCs can directly contact target cells and affect intracellular cascades to correct abnormalities. Additionally, MSCs may function through mitochondrial transfer, secretion of exosomes, and release of other biomolecules. Examples of these biomolecules include nitric oxide (NO), indoleamine-pyrrole 2,3-dioxygenase (IDO), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and hepatocyte growth factor (HGF).
Another proposed mechanism by which MSCs influence their target cells is through mitochondrial transfer. Several mechanisms have been suggested for the transfer of mitochondria from MSCs to injured cells, including direct cell-to-cell contact, cell fusion, the formation of microvesicles containing mitochondria, and transportation via tunneling nanotubes [43]. Mori et al. confirmed the transfer of human MSC mitochondria to cardiomyocytes in a rat model of myocardial infarction by detecting human MSC mitochondrial DNA in the rat myocardium [43]. Jiang’s study showed that MSCs could transfer their mitochondria to corneal epithelial cells, protecting the cells from mitochondrial dysfunction induced by oxidative stress and that tunneling nanotubes between MSCs and epithelial cells contribute to mitochondrial transfer [44]. Additionally, Rackham et al. demonstrated that MSCs can donate mitochondria to human islet β-cells under coculture conditions, and tunneling nanotube-like structures were also shown to play a role in mitochondrial transfer [45]. Morrison et al. observed mitochondrial transfer from MSCs to murine alveolar macrophages via EVs. By staining EVs isolated from MSCs with a MitoTracker dye and using them to treat macrophages, they found that the treated macrophages had an anti-inflammatory phenotype and were able to improve a mouse lung injury model, likely due to increased oxidative phosphorylation-related metabolism [46].
In comparison, the paracrine function of MSCs is a widely accepted mechanism by which MSCs can exert their effects on target cells. In addition to immunomodulatory factors, certain factors secreted by MSCs, such as vascular endothelial growth factor (VEGF), monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8), and angiogenin, have been suggested to play a key role in vascular regeneration [47]. For instance, MSCs secrete various factors and cytokines that have immunomodulatory, anti-apoptotic, and anti-inflammatory effects, and can modulate the reactivity of astrocytes and microglia to promote neurodegeneration. The therapeutic effect of MSCs is due to a paracrine mechanism of action, which highlights the importance of MSC survival and their secretory phenotype [48]. However, the secretory phenotype of MSCs can vary depending on the donor, indicating that there are differences in their biological properties and therapeutic potential [48–50]. Recently, scientists have focused on improving MSC secretome production. For example, Su et al. used electrospun fibrous scaffolds made of polycaprolactone to enhance the production of pro-angiogenic and anti-inflammatory factors by MSCs, and found that culturing MSCs in these scaffolds increased their paracrine function compared to 2D culture [51]. Drzeniek et al. showed that encapsulating of MSCs with a hydrogel made of collagen I-hyaluronic acid led to a significant increase of their secretory factors [52]. Moreover, the effect of the stiffness of alginate hydrogels as a matrix for MSCs on their paracrine function was evaluated by Lin et al, who found that a stiffer matrix could promote the secretion of paracrine factors from MSCs compared to a soft matrix, due to the polymerization of F-actin and subsequent activation of Yes-associated protein (YAP) [53]. In another study, the effect of pulsed electromagnetic fields on the paracrine functions of MSCs was assessed by Parate et al, who found that the medium after exposure of MSCs to pulsed electromagnetic fields exhibited the potential to induce the differentiation of MSCs into chondrocytes [54].
Some effects of MSCs on their target cells can also be attributed to exosomes secreted by MSCs. Exosomes are small EVs that are produced through the endocytic process and contain active signaling components. These components are surrounded and protected by the two layers of lipid molecules that make up the exosome [55]. Based on available evidence, exosomes derived from MSCs include a variety of proteins, coding and non-coding RNAs [56–58]. Recently, exosomes have been suggested as a nanodelivery system due to their low immunogenicity, long half-life, and ability to cross the blood-brain barrier. They are considered an alternative to MSC-based therapy in regenerative medicine and are being used in a number of studies [55,59].
2.3. Application of MSCs in regenerative medicine and challenges
Regenerative medicine utilizes a combination of synthetic or natural materials to treat a variety of diseases and to regenerate damaged tissue. Over the last few decades, MSCs have become increasingly popular in regenerative medicine due to their unique advantageous properties, including the potential for self-renewal, capacity for differentiation into other cells, ability to target injured tissues, low immunogenicity, and immunomodulatory functions [7,60,61].
Despite the numerous advantages of MSCs, their clinical application still presents challenges in terms of preparation and use for disease treatment. The most important challenge related to preparing MSCs is their inherent heterogeneity, which can be attributed to their isolation from various sources and donors, resulting in different differentiation capacities, stemness stability, and expansion capacities. Additionally, challenges still exist during the application of MSCs to treat diseases, due to factors such as the impact of administration conditions on homing, the effect of the host microenvironment on the MSC secretion, and the influence of immune compatibility between donors and patients on the risk of rejection [62]. Therefore, the optimization of MSC-based therapy has been considered to address these challenges. To this end, a wide range of strategies have been applied to enhance the survival, stability, and secretory capacity of MSCs. MSCs derived from different sources have been used in vitro, in experimental animal models, and in clinical trials as non-engineered MSCs, pre-conditioned MSCs, engineered MSCs, and encapsulated MSCs to alleviate various types of degenerative or non-degenerative diseases (Figure 2).
Figure 2. Application of mesenchymal stem cells (MSCs) in regenerative medicine.
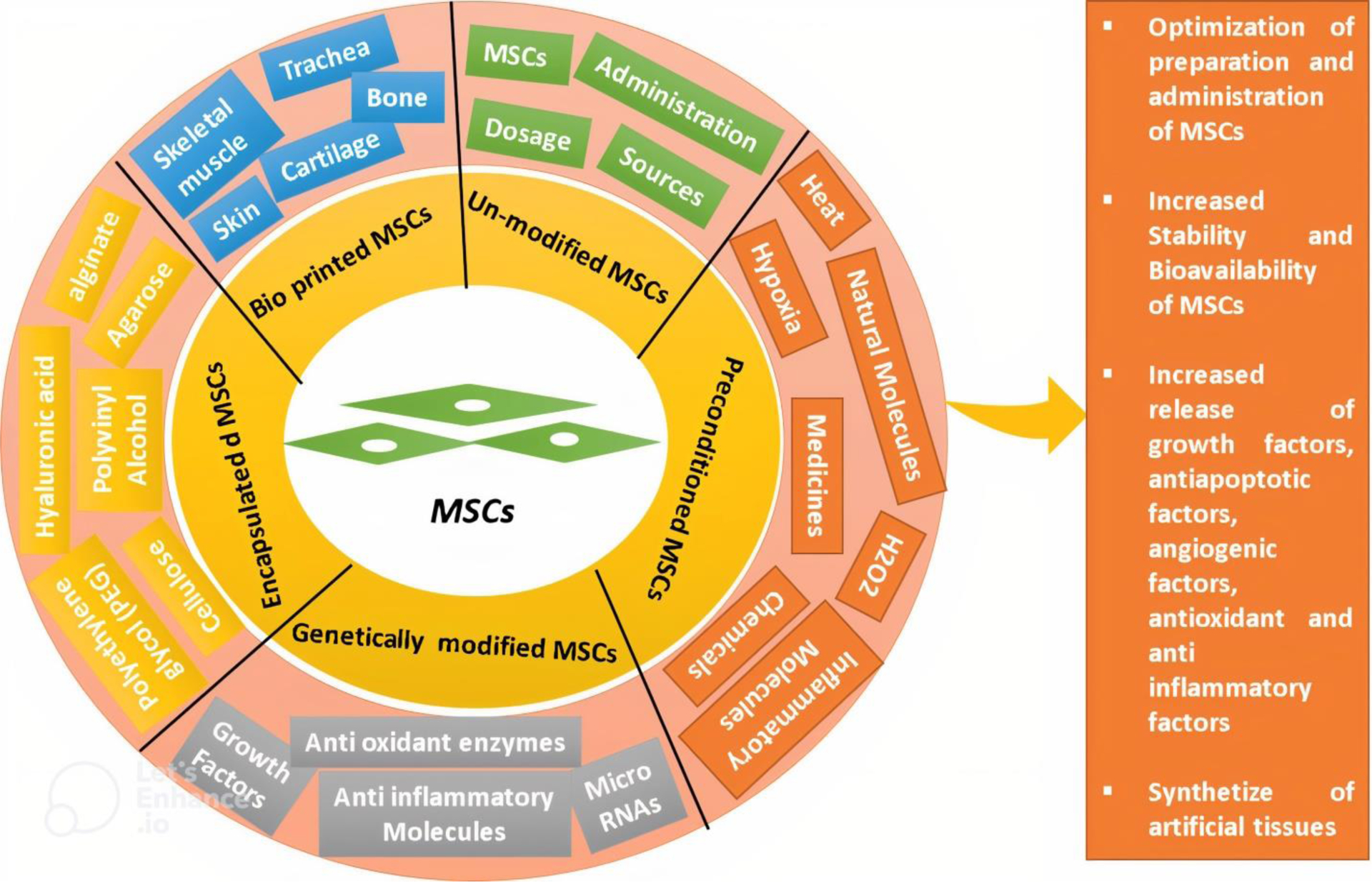
MSCs can be used as unmodified cells due to their therapeutic effects in treating a wide range of diseases. Preconditioning of MSCs with heat, hypoxia, chemicals, natural molecules, and biomolecules has been shown to improve their efficiency. Genetically modified MSCs, which overexpress certain biomolecules, may increase the therapeutic effect of MSCs. Encapsulating MSCs improves their stability and survival and allows for controlled release of MSCs to target tissues. Additionally, MSCs can be applied to scaffolds to create artificial tissues using 3D bioprinting technology.
2.3.1. Strategies to improve MSC-based therapy
The protective effects of MSCs have been reported in various disease models, including diabetic retinopathy [63,64], meniscus repair [65], lumbar spinal degeneration [66], spinal cord injury [48], cardiac degeneration [67], reproductive diseases [68], osteoporosis [69], inflammatory diseases [70], and autoimmune diseases [71]. Furthermore, various preconditioning strategies have been introduced to enhance the therapeutic potential of MSCs, such as incubation with cytokines and chemicals, as well as exposure to hypoxia and heat shock prior to transplantation into animal models. For instance, Ishiuchi et al. demonstrated that preconditioning MSCs in hypoxic conditions can increase their efficiency in preventing inflammation and fibrosis induced by ischemic/reperfusion injury of the kidney in mice. They found that hypoxic preconditioning increased the production of VEGF and HGF, which are responsible for suppressing fibrosis [72]. In addition, preconditioning MSCs at 42 °C for 1 h as heat shock showed an increase in the heat shock protein 70 (HSP70) level, which can suppress macrophage activation and improve acute lung injury in a mouse model [73]. Moreover, preconditioning MSCs with an optimal concentration of H2O2 has been shown to improve MSCs and increase their therapeutic activity for wound healing [74]. Furthermore, there are a large number of studies showing the use of chemicals, cytokines, and growth factors for preconditioning MSCs and thereby optimizing cell therapy. For example, Zhao et al. found that antioxidative enzymes in conditioned medium from MSCs treated with melatonin could protect human kidney cells against cisplatin toxicity [75]. Also, preconditioning of MSCs with resveratrol [76], thrombin [77], dimethyloxalylglycine [78], rapamycin [79], sevoflurane [80], and lithium chloride [81] were reported to enhance the protective effects of MSCs against diabetic cardiomyopathy, severe hypoxic ischemic encephalopathy, Alzheimer disease, liver ischemia/reperfusion injury, myocardial ischemia/reperfusion injury, and degenerated intervertebral disc in animal models, respectively.
Genetically engineered MSCs have been used in successful MSC-based therapy [82]. For example, VEGF-expressing MSCs were used to evaluate their effect on pain in a mouse model of Parkinson’s disease, where the cell therapy with VEGF-expressing MSCs was shown to reduce pain behaviors in mice, and inhibition of the expression of transient receptor potential vanilloid 1 (TRPV1) was shown to contribute to analgesia [83]. Rostami et al. used interleukin 23 receptor (RIL-23R)-expressed MSCs to treat a mouse model of autoimmune encephalomyelitis, which resulted in enhanced myelination and decreased inflammation in the white matter [84]. Hombach et al. used engineered MSCs expressing IL7-IL12 to activate chimeric antigen receptor (CAR) T cells and improve their efficiency in treating colorectal cancer cells. Genetically altered MSCs were used to transport immuno-modulatory proteins to tumor tissue, enhancing the effectiveness of CAR T cells in treating solid tumors [85].
Encapsulation has been considered as a means of increasing the survival of MSCs. For example, Sahu et al. prepared microbeads by encapsulating MSCs with alginate, and showed that microencapsulation of MSCs can increase their secretion of cytokines such as IL-10, HGF, and sFAS. Co-culture of patient-derived osteoarthritis tissue explants with MSC microbeads increased the DNA content and number of Ki67+ cells, indicating proliferation and regeneration [86]. Similarly, Kim et al. showed that gelatin−hydroxyphenyl propionic acid (GH) hydrogel can be serve as a delivery platform for MSCs to be injected into the myocardium. Encapsulation of MSCs using GH hydrogel improved cell retention and survival both in vitro and in vivo. In a mouse myocardial infarction model, the use of MSC-encapsulating GH hydrogels resulted in improved cardiac functional parameters, reduced fibrosis, and thicker infarcted walls [87]. Furthermore, Wang et al. demonstrated that an injectable hydrogel can be a suitable carrier for MSCs derived from the nucleus pulposus to treat degenerative intervertebral discs in rats. Their results showed that a combination of MSCs and a 3D-RGD peptide-modified polysaccharide hydrogel can promote the efficiency of MSCs in repairing degenerative intervertebral discs in rats [88].
MSCs have been explored for their potential use in constructing artificial tissues or organoids. For example, Pitacco et al. created a cartilaginous graft using MSCs and fibrinogen to repair bone defects. They used a 3D bioprinting strategy to create a fibrin-based scaffold and seeded it with MSCs. The scaffold was then cultured under chondrogenesis conditions to form a cartilaginous structure, which was then implanted into a rat femoral bone defect model. The results showed that the cartilaginous structure was remodeled into bone with a high level of vascularization [89]. In addition, Lin et al. developed scaffolds made of a gelatin-methacryloyl matrix and various concentrations of calcium silicate, which were loaded with human dental pulp stem cells for odontogenic regeneration. They demonstrated that the stem cells can differentiate into odontocyte-like cells in the presence of calcium silicate, which suggests a possible approach for dental tissue engineering [90]. Furthermore, Ke et al. used a bioprinting strategy to create a trachea construct made of polycaprolactone and MSC-laden hydrogels, where the bioprinted trachea had both smooth muscle and cartilage structures expressing the related biomarkers [91].
2.4. Application of MSC-based therapy in clinical trials
Various clinical trials have utilized MSCs derived from different sources for treating different diseases, as shown in Table 1. Most of these trials have demonstrated the safety of MSCs without any serious side effects. However, one study evaluated the safety and efficacy of using autologous MSCs in combination with standard therapy for treating kidney transplant recipients with biopsy-proven chronic active antibody-mediated rejection (AMR). The study, which enrolled three patients, was terminated early due to adverse events. The results indicated that the treatment did not improve AMR in any of the patients, and serious adverse events occurred in one patient when the therapy was administered in the late phase after kidney transplantation [92]. The effectiveness of MSCs has been demonstrated in clinical trials for treating ischemic stroke, newly diagnosed type-1 diabetes patients, type 2 diabetes, diabetic foot ulcers, knee osteoarthritis, and detrusor underactivity [93–98]. However, the effectiveness of MSCs in treating COVID-19 remains inconclusive, with some studies showing significant effects on disease response while others have not demonstrated any significant effects [99–102].
Table 1.
Examples of clinical trials using MSCs for various diseases
| Type of cell/Autologous or Allogenic | Dosage | Number of treatments | Delivery method | Type of disease | Type of study | Country | Time after cell therapy | Result | References |
|---|---|---|---|---|---|---|---|---|---|
| Bone marrow-derived MSCs/Autologous | Ranged from 1.2×107 to 6.5×107 cells | 1 | Intracardiac catheter-based injection | Cardiomyopathy | Long-term results of a previous clinical trial | Japan | 10 years | Medical history of 8 patients: 3 died (pneumonia, liver cancer, heart failure), 5 had heart-related issues, 1 needed a heart transplant, 2 survived without worsening heart condition for 10 years | [133] |
| Bone marrow-derived MSCs/Autologous | Not reported | Not reported | Not reported | Ischemic stroke | An investigator-initiated, prospective, randomized, open-label, controlled trial with blinded outcome evaluation | South Korea | 90 days | Increase in the improvement ratio of the Fugl-Meyer assessment score, a decrease in corticospinal tract and posterior limb of the internal capsule fractional anisotropy, an increase in Interhemispheric connectivity and ipsilesional connectivity | [95] |
| Adipose-derived MSCs/Allogenic | 1 million cells per kilogram | 1 | IV | Acute ischemic stroke | A Phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial | Spain | 24 months | No difference in adverse effect between treated and placebo group, no difference in mRS | [134] |
| Bone marrow derived-MSC/Autologous | 1 × 106 per kilogram | 2 | IV | Newly diagnosed type-1 diabetes patients | Phase I/II randomized placebo-controlled clinical trial | Iran | 1 year | Improvement of glycated hemoglobin (HbA1c), changing serum cytokine patterns from pro-inflammatory to anti-inflammatory, an increase in the number of regulatory T-cells in the peripheral blood | [94] |
| Umbilical cord-derived MSCs/Allogenic | 1 × 106 per kilogram | 3 | IV | Type 2 diabetes | A single-center, double-blinded, randomized, placebo-controlled phase II trial | China | 48 weeks | Insulin reduction, a decrease in HbA1c/an increase in GIR, no improvement in islet β-cell function | [96] |
| Umbilical cord-derived MSCs/Allogenic | 2 × 105 cells per kilogram with an upper limit of 1 × 107 cells | 3 | IV and topical | Diabetic foot ulcer and peripheral arterial disease | A phase I pilot study with a 3-year follow-up | China | 3 years | Ulcer disclosure within 1.5 month after treatment, an alleviation in symptoms of chronic limb ischemia, 3-year amputation-free survival rate | [97] |
| Bone marrow-derived MSCs/Allogeneic | 45–50 × 106 cells | 1 | Intranasally | Perinatal arterial ischemic stroke | A first-in-human, open-label intervention study | Netherlands | 3 months | No serious adverse events, increase in thrombocytes after MSC administration but were within physiological ranges for all patients, No signs of infection or inflammatory reactions to MSC administration | [135] |
| Bone marrow-derived MSCs/Autologous | 1 × 106 cells | 1 | intra-articular injection | Knee osteoarthritis | Randomized control trial of mesenchymal stem cells versus hyaluronic acid | China | 1 year | Improvement in quality of life and reduction in pain, a decrease in T2-relaxation time | [93] |
| Adipose-derived MSCs/Autologous | 2 × 106 cells | 2 | intravesical injection | Detrusor underactivity | Open clinical trial | Brazil | 60 days | An increase in maximum flow, mean flow, urinated volume and a decrease in residual volume in the uroflowmetry exam, an improvement in quality of life and a return to daily activities. No complications in the 6-month follow-up after cell therapy | [98] |
| Bone marrow-derived MSCs/Autologous | 1 × 106 cells per kilogram | 3 | IV | Chronic active antibody-mediated kidney graft rejection | Single-center clinical study | Slovenia | 12 months | No improvement in kidney graft function and no protective effect on histological and molecular indicators of active antibody rejection activity, activation of the T-lymphocyte response as a serious adverse effect in 1 out of 3 patient results in prematurely termination of study | [92] |
| Bone marrow-derived MSCs/Haploidentical Allogeneic | 2.0×106 cells per kilogram (First dose) 3.0×106 cells per kilogram (second dose) | 2 | IV | Systemic lupus erythematosus | A nonrandomized, single center, open-label, single-arm, phase I trial | South Korea | 28 days | No dose-limiting toxicity, three adverse events (one diarrhea, one toothache, and one arthralgia), safe | [103] |
| Wharton’s jelly-Mesenchymal stem cells/Allogenic | 150 × 106 cells | 2 | IV | Severe COVID-19 | Pilot study | Iran | 14 days | A decrease in neutrophil, ESR, ferritin, and CRP, an increase in lymphocyte, no change in LDH, no abnormal tumor markers and CT after 1 year | [102] |
| Placenta-derived MSC/Allogenic | 1 × 106 cells per kilogram | 1 | IV | Acute respiratory distress syndrome (ARDS) caused by COVID-19 | Phase I clinical trial | Iran | 28 days | No difference in mean length of hospitalization, serum oxygen saturation, and other clinical and laboratory parameters/no adverse events | [99] |
| Umbilical cord-derived MSCs/Allogenic | 1 × 106 cells | 3 | IV | COVID-19- induced mild to moderate acute respiratory distress syndrome | Phase 1, control-placebo group, clinical trial | Iran | 17 days | Significant improvement in SPO2/FIO2 ratio and serum CRP levels, a significant decrease (P < 0.05) in serum cytokine levels of IL-6, IFN-g, TNF-α, IL-17 A and a significant increase in serum cytokine levels of TGF-B, IL-1B and IL-10/no serious adverse effects | [101] |
| Bone marrow derived-MSC/Allogenic | 1.5–3×106 cells per kilogram | 3 | IV | Severe COVID-19 | Phase I/II Clinical Trial | Belgium | 28 and 60 days | Higher survival, no significant difference in the need for mechanical ventilation nor in the number of invasive ventilation-free days, high flow nasal oxygenation-free days, oxygen support-free days and ICU-free days, decrease in day-7 D-dimer value | [100] |
MSC: mesenchymal stem cells; IV: intravenously; CRP: C-reactive protein; ICU: intensive care unit; LDH: lactate dehydrogenase; GIR: glucose infusion rate; mRS: modified Rankin Scale
In regard to the use of autologous or allogenic MSCs, the evidence has shown that autologous MSCs have a positive effect on ischemic stroke [95], type 1 diabetes [94], knee osteoarthritis [93], and detrusor underactivity [98], while they have not shown a positive effect on cardiomyopathy and chronic active antibody-mediated kidney graft rejection. It appears that most studies using autologous MSCs have reported positive outcomes. However, the application of allogenic MSCs to treat certain diseases, such as type 2 diabetes [96], diabetic foot ulcer [97], systemic lupus erythematosus [103], and COVID-19 [101,102], has been reported to be safe and effective.
3. Exosome secreted by MSCs
3.1. Isolation and characterization
Extracellular vesicles (EVs) include a variety of vesicles that differ in size, content, and biogenesis. The three main types of extracellular vesicles are exosomes, microvesicles, and apoptotic bodies. Microvesicles and apoptotic bodies are released from living or dying cells, respectively, by outward budding of the plasma membrane. Exosomes are typically smaller than microvesicles and apoptotic bodies and are formed through the endocytic pathway [104]. Exosomes released from MSCs are being discovered as mediators for cell-free regenerative medicine. These small EVs, with sizes less than 150 nm, are produced from endosomes, created by the invagination of the plasma membrane, and released through membrane fusion. Exosomes contain two layers of phospholipids enriched in ceramide, sphingomyelin, and cholesterol. Exosomes also contain various biomolecules, including transmembrane proteins (CD63, CD9, and CD81), mRNA, microRNA, and DNA, which make them potential therapeutic agents [105,106].
There are several methods available for isolating and enriching exosomes secreted from cells, including ultracentrifugation, size exclusion chromatography, polymer-based precipitation, immunoaffinity capture, and ultrafiltration. Each method has its own advantages and limitations, and the choice of isolation method should be based on the specific research question and characteristics of the exosome sample being studied [107].
To characterize exosomes, several criteria are typically used. According to the MISEV2018 guidelines, in order to demonstrate the extracellular vesicle nature of exosomes, the presence of certain proteins, such as transmembrane or GPI-anchored proteins (e.g., CD63), cytosolic proteins (e.g., heat shock protein HSP70), and the absence of major components of non-extracellular vesicle co-isolated structures (e.g., apolipoproteins A1/2 for lipoproteins or Tamm-Horsfall protein for protein and protein/nucleic acid aggregates) must be demonstrated. To validate small EVs, it is necessary to evaluate transmembrane, lipid-bound, and soluble proteins associated with other intracellular compartments other than the plasma membrane or endosomes (e.g., histones for the nucleus or cytochrome C for mitochondria). Additionally, to confirm the functional activities of EVs, it is necessary to analyze secreted proteins recovered with EVs such as cytokines and growth factors. In addition to measuring protein content, it is necessary to characterize EVs using two distinct methods of single vesicle analysis. Various methods are available for evaluating single vesicles, including imaging techniques such as electron or atomic force microscopy, which can reveal the bilayer lipid membrane of EVs, and single-particle analyzers such as nanoparticle tracking analysis (NTA), which can determine the size of EVs (Figure 3) [108].
Figure 3. The methods for exosome characterization.
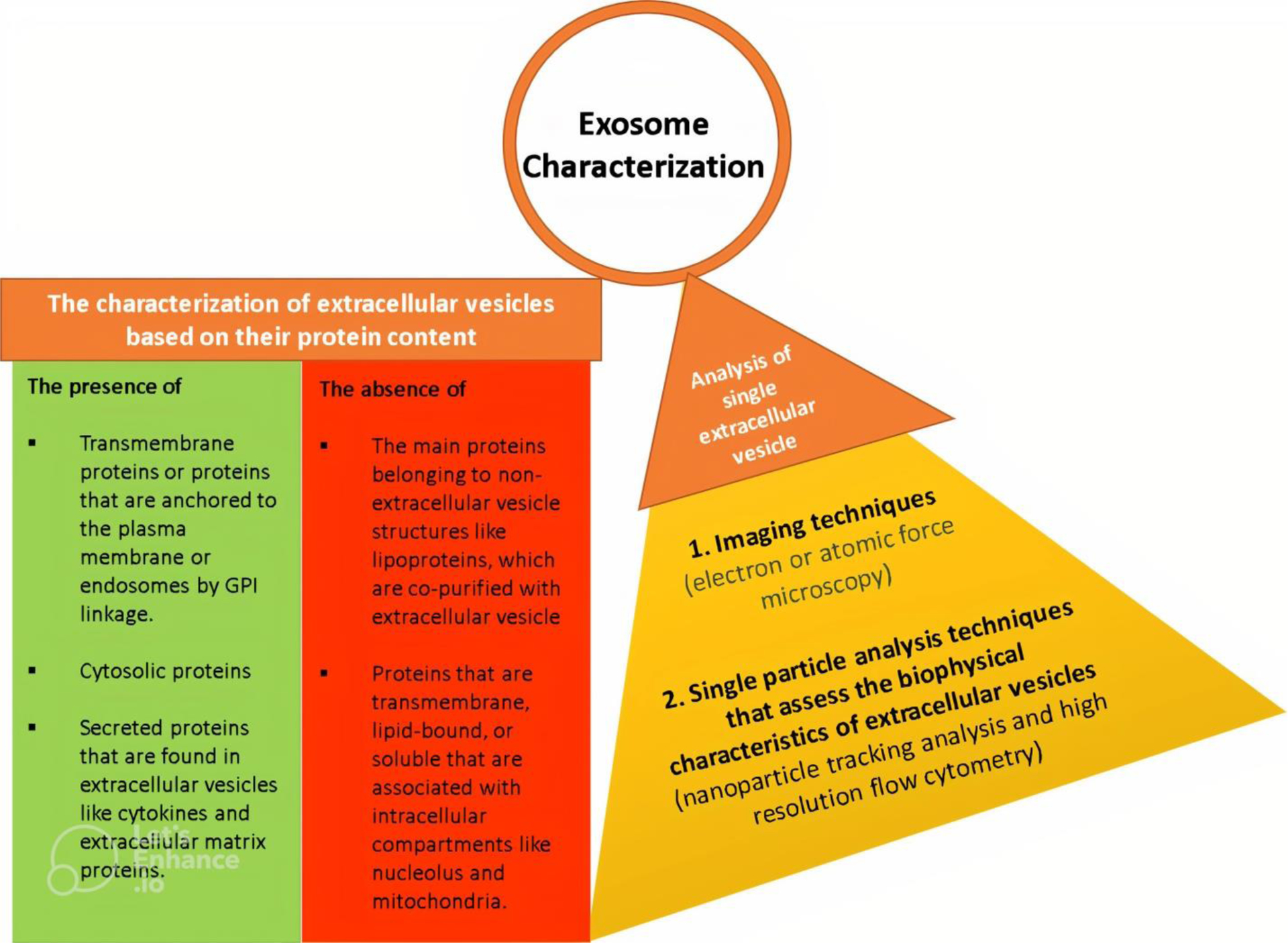
To characterize exosomes, their protein contents are examined by checking for the presence of positive protein markers and the absence of negative markers. Additionally, it is necessary to use two different techniques to assess exosomes at the single extracellular vesicle level, namely imaging methods and single particle analysis techniques which analyze the biophysical properties of the vesicles.
Mass spectrometry, which ionizes molecules and separates them based on their mass-to-charge ratio, is also a valuable analytical technique for identifying and characterizing exosomes. Mass spectrometry can detect the proteins, lipids and other molecules present in exosomes, providing a comprehensive overview of their content [107]. Due to its ability to identify, mass spectrometry is a powerful tool for characterizing exosomes.
3.2. Application of MSCs-derived exosomes in regenerative medicine and challenges
Exosomes derived from MSCs possess unique properties that make them a suitable candidate to be used for the treatment of certain diseases. These properties include the capacity for regeneration, immunomodulation, and even anti-tumor effects. They can migrate to injured tissues such as MSCs and penetrate tight tissues like the blood-brain barrier. Using exosomes as an alternative to MSC-based therapy appears to have some advantages, such as avoiding harmful effects like malignant transformation, immune rejection, and the risk of infusion toxicity. In recent years, a wide range of studies have focused on the use of exosomes derived from MSCs to improve diseases in vitro, in vivo, and in clinical trials [109]. In the line of research, Rong et al. used exosomes released from bone marrow-derived MSCs to improve fibrosis in a rat model of liver fibrosis. Their findings demonstrated that exosomes can contribute to the inhibition of hepatic stellate cell activation through the Wnt/β-catenin pathway, which is responsible for liver fibrosis [110]. In another study, exosomes derived from MSCs were found to promote neurogenesis and improve cognitive function in an Alzheimer’s disease mouse model. This was indicated by the increased levels of doublecortin and polysialylated neuronal cell adhesion molecules in the subventricular zones of the brains of mice, indicating neurogenesis and the presence of neuroblastoma in these areas [111]. In addition, Zhang et al. applied exosomes derived from MSCs to improve diabetic osteoporosis in rats, and showed that exosome therapy suppressed NLRP3 inflammasome activation in osteoclasts, resulting in a decrease in bone resorption [112]. It has been reported that ultrasonication for one minute can be used to shear intact human umbilical cord MSCs and extract a higher yield of EVs. The EVs extracted using ultrasonication demonstrate similar properties to those extracted without the ultrasonication step. Furthermore, EVs derived from MSCs have been shown to increase the proliferation of dermal fibroblasts, upregulate the expression of elastin, collagen, and fibronectin proteins, and downregulate matrix metalloproteinases-1 (MMP-1) and MMP-3 in vitro. An in vivo study has also demonstrated wound healing in mice after treatment with EVs [113].
Exosomes have recently been considered as nanoparticle carriers for drug and biomolecule delivery in biological systems. There are some advantages of using exosomes over synthetic nanoparticles in drug delivery such as biocompatibility and durability, as well as capability to communicate with distant cells via their intrinsic delivery mechanism, fusion, and intercellular communication capacities [109].
3.3. Strategies to improve MSC-secreted exosomes therapy
Table 2 lists several strategies that have been used to engineer exosomes to be more potent in the treatment of a wide range of diseases. Several methods have been used to load cargo into exosomes, including incubation of exosomes or exosome-derived MSCs with cargo, transduction of the desired cargos into exosomes or exosome-secreting MSCs using a transfection reagent, and physical treatment of exosomes to create some micropores on the surface of the exosomes, allowing cargo to enter. Some methods such as electroporation, freeze-thaw, dialysis, surfactant treatment, and sonication are among the physical techniques used to load exosomes and extracellular vesicle with cargo [114,115].
Table 2.
Application of MSCs-derived exosomes with different strategies to treat diseases
| Strategy of optimization | Type of study | Delivery method | Disorder | Result | References |
|---|---|---|---|---|---|
| Preconditioning | |||||
| Exosome derived from MSCs preconditioned with hypoxia | In vitro (BV2 microglia) In vivo (Mouse) |
Tail vein injection | Spinal cord injury | Increased the level of miR-216a-5p in exosomes (in vitro), polarization of microglia from M1 to M2 (both in vivo and in vitro), and injury repair (in vivo) | [136] |
| Exosomes derived from MSCs pretreated with IFN-γ | In vivo (Mouse) | Intra vascular injection | Colitis | Increased levels of miR-125a and miR-125b in exosomes which lead to inhibition of Th17 cell differentiation and suppression of inflammation | [117] |
| Exosome incubated with doxorubicin | In vitro (MG63 cells) | Osteosarcoma | Increase in cellular uptake and anti-tumor effect | [137] | |
| Transduction | |||||
| Exosome derived from MSCs transduced with MIF | In vivo (Rat) | Intramuscularly injection into the peri-infarct region | Myocardial Infarction | Improved heart function; reduction of heart remodeling; decreased fragmentation of cardiomyocyte mitochondria and apoptosis | [138] |
| Exosome derived from MSCs transduced with miR-145 | In vitro (T47D cells) | Breast cancer | Inhibition of apoptosis and metastasis | [139] | |
| Exosome derived from MSCs transduced with miR-125b | In vivo (Rat) | Intra vascular injection | Neointimal hyperplasia | Suppression of vascular smooth muscle cell proliferation and migration | [140] |
| Exosome derived from MSCs transduced with hypoxia-inducible factor 1-alpha | In vivo (Rat) | Tail vein injection | Myocardial infarction | Increased angiogenesis, fibrosis inhibition, and cardio protection | [119] |
| Encapsulation | |||||
| Exosomes derived from MSCs encapsulated with PF-127 hydrogel | In vivo (Rat) | Topical application | Diabetic wound | Increased angiogenesis and proliferation in the wound area and wound healing | [121] |
| Exosomes immobilized in a peptide-modified adhesive hydrogel | In vivo (Mouse) | Topical application | Spinal cord injury | Inhibition of inflammation and oxidation, nerve recovery | [141] |
| Exosomes derived from MSCs encapsulated in functional peptide hydrogel | In vivo (Rat) | Injection into the infarcted border zone of rat hearts | Cardiac infarction | Improved the myocardial function, reduction in fibrosis, inflammation and apoptosis | [142] |
| 3D bioprinting | |||||
| 3D printed cartilage extracellular matrix/gelatin methacrylate/exosome scaffold | In vivo (Rabbit) | Implantation | Osteochondral defect | Restoration of chondrocyte mitochondria, polarization of macrophages from M1 to M2, and cartilage regeneration | [125] |
| 3D-printed SF/COL-I/nHA/exosomes scaffolds | In vivo (Rat) | Implantation | Alveolar bone defects | Increased angiogenesis and osteogenesis | [127] |
| 3D-printed BDNF-stimulated MSCs-derived exosomes/collagen/chitosan scaffold | In vivo (Rat) | Implantation | Traumatic brain injury | Recovery of neuromotor function and cognitive function improvement | [126] |
BDNF= Brain derived neurotrophic factor; PF-127= Pluronic F127; SF/COL-I/nHA= Silk fibroin/collagen I/nano-hydroxyapatite; MIF: macrophage migration inhibitory factor
Wei et al. prepared doxorubicin-loaded exosomes by incubating exosomes isolated from MSCs with doxorubicin and demonstrated that the doxorubicin-loaded exosomes could be more efficiently entered into the osteosarcoma MG63 cell line than free doxorubicin and exerted significantly improved anti-tumor effects [116]. Yang et al. demonstrated a higher content of miR-125a and miR-125b in exosomes derived from IFN-γ-pretreated MSCs. The targets of miR-125a and miR-125b are the 3′-UTR of Stat3, which can suppress Th17 cell differentiation upon binding with miR-125a and miR-125b. The researchers treated a mouse model of colitis with these engineered exosomes and observed a suppression of inflammation via inhibition of Th17 cell differentiation and an increased ratio of Treg cells. This, in turn, resulted in a significant improvement in the disease activity index and histological score of colitis [117].
Furthermore, Chen et al, generated exosomes enriched in miR-375 by using lentiviral transfection to load MSC-derived exosomes with miR-375, and demonstrated that the exosomes enriched in miR-375 improved the differentiation of MSCs into osteoblasts and increased bone repair in a calvarial defect model of the rat. They also showed that the combination of exosomes with hydrogel can regulate their release and enhance their efficiency [118]. In a separate study, exosomes were extracted from MSCs that had been transduced with lentivirus carrying HIF-1α and applied to improve a model of myocardial infarction in vitro. For this purpose, human umbilical vein endothelial cells (HUVECs) were cultured under hypoxic condition in the presence or absence of exosome derived from HIF-1α-overexpressed MSCs. The results showed that HIF-1α-overexpressed exosomes can promote the proliferation, migration, and vessel formation ability of HUVECs. Furthermore, administration of HIF-1α-overexpressed exosomes in a rat model of myocardial infarction resulted in improved heart function by inducing the overexpression of proangiogenic factors and enhancing angiogenesis [119].
Hydrogel encapsulation was used to improve the efficiency of exosomes derived from MSCs in other studies. In this regard, Zhang et al. used hyaluronic acid hydrogel to encapsulate MSC-derived exosomes and combined them with a scaffold made of nanohydroxyapatite/poly-ε-caprolactone to accelerate bone repair in a rat model of cranial defect. Their results showed an acceleration in bone repair that can be attributed to the angiogenic action of exosomal miR-21 [120]. Furthermore, Yang et al. utilized F127 hydrogel to encapsulate exosomes derived from MSCs for the treatment of full-thickness cutaneous wound in a diabetic rat model. Their findings demonstrated that the combination of F127 hydrogel with exosomes from MSCs can enhance the efficiency of exosomes. Encapsulated exosomes promoted angiogenesis in granulation tissues in a chronic diabetic wound, thereby accelerating wound healing and skin regeneration [121]. However, Kostennikov et al. compared the efficacy of local (encapsulated in fibrin matrix) and systemic transplantation of MSC-EVs in promoting spinal cord regeneration in rats after injury. The study showed that intravenous transplantation of MSC-EVs showed more significant therapeutic effects compared to the treatment of fibrin matrix-encapsulated MSC-EVs in the spinal cord injury area [122].
Surface modification of exosomes is reported as a strategy to increase the therapeutic potential of exosomes. Huang et al. modified the surface of exosomes using a cell-penetrating peptide-linked phospholipid to enhance the penetration of exosomes to endothelial cells. For this purpose, cell-penetrating peptide-linked phospholipids were synthesized and mixed with exosomes to incorporate into their surfaces. They showed that following incubation of endothelial cells with exosomes, the modified exosomes can be highly detected in the endothelial cells compared to the unmodified exosome [123]. Also, Wu et al. modified the surface of exosomes derived from MSCs with the CREKA peptide, which is able to target fibrin. They showed that exosomes containing the CREKA peptide can accumulate in the bones of rats with a femoral defect model and increase bone repair [124].
Recently, bioprinting technology has been used to create scaffolds containing exosomes derived from MSCs for use in regenerative medicine [125–127]. For instance, Chen et al. developed a scaffold made of cartilage extracellular matrix, gelatin methacrylate, and exosomes from MSCs to regenerate osteochondral defects in rabbits. The scaffold was printed using desktop-stereolithography technology, and its effectiveness was evaluated in a rabbit model of osteochondral defects. The results showed that the scaffold led to the restoration of chondrocyte mitochondria, polarization of macrophages from M1 to M2, and regeneration of cartilage in the osteochondral defect model [125]. In a separate study, Sun et al. integrated exosomes derived from MSCs into a scaffold made of bioprinted silk fibroin, collagen I, and nano-hydroxyapatite. They then implanted this scaffold into a rat model of alveolar bone defects. Their findings showed that the scaffold containing exosomes promoted the formation of blood vessels and bone tissue, effectively repairing the defects in the alveolar bone [127].
There are, however, several limitations in the clinical application of exosomes. Exosome production by MSCs is associated with the sources, activities, and microenvironment of MSCs, and there is no specific criteria for MSC-derived exosome identification. Another important problem in exosome preparation is the heterogeneity of the extracellular vesicle population. Some other problems, like low yield, various methods of exosome extraction, and a lack of quality assurance assays, remain unsolved [109,128]. However, several methods have been developed to increase the production of EVs. These methods can be classified into three main categories. Firstly, one approach involves creating high-capacity “cell factories” that can naturally produce more EVs. This can be achieved through the use of bioreactor systems and by modulating culture conditions to optimize EV production. Secondly, researchers induce EV secretion by exposing cells to various stressors such as physical and chemical stimulation. This approach has been shown to increase the yield of EVs, although further optimization is necessary. A third approach involves fragmenting cells to create biomimetic vesicles that closely mimic the properties of natural EVs. This can be achieved using techniques such as low-frequency ultrasound and nitrogen cavitation [129].
3.4. Application of exosomes secreted by MSCs in clinical trials
Exosomes, which are secreted by MSCs, have been used to treat certain illnesses such as COVID-19 in recent years. Sengupta et al. administered a single dose of ExoFlo, a bone marrow MSC-derived exosome agent, an intravenous treatment, to 24 critically ill COVID-19 patients suffering from acute respiratory distress syndrome. The treatment was well-tolerated, with no negative effects observed within 72 hours of administration, and 83% of patients survived. Among the survivors, 71% recovered, 13% remained in critical condition but stable, and 16% died from causes unrelated to the treatment. After receiving the treatment, the patients’ clinical condition and oxygenation improved substantially, as evidenced by improvements in immune cell counts and acute phase reactants in laboratory values. ExoFlo appears to be a promising candidate for treating severe COVID-19 due to its safety profile and its ability to improve oxygenation, regulate cytokine storm, and restore immunity [130]. Also, exosomes derived from human adipose-derived MSCs were administered via nebulizer as a method of treating COVID-19 in a phase 2a single-arm trial called MEXCOVID. The trial aimed to explore the safety and efficacy of aerosol inhalation of exosomes in seven COVID-19 patients. The patients were given daily doses of haMSC-Exos for five days, and no adverse events or clinical instability occurred during or after the treatment. Additionally, patients experienced a slight increase in serum lymphocyte counts and varying degrees of improvement in pulmonary lesions [131]. Moreover, a pilot trial was conducted to investigate the safety and efficacy of nebulization therapy with exosomes of mesenchymal stem cells (MSCs) for treating COVID-19 pneumonia by Chu et al. The trial involved seven patients who were treated with nebulization of MSC-derived exosomes. The study found that nebulization of MSC-derived exosomes was a safe and effective method that improved patient outcomes. Patients showed improved absorption of pulmonary lesions and reduced hospitalization duration for mild cases of COVID-19 pneumonia [132]. The mentioned studies on exosome therapy for COVID-19 demonstrated no adverse effects following administration through both intravenous and inhalational routes. Furthermore, the positive effects of exosomes derived from MSCs were observed in patients with COVID-19, including improvements in immune cell counts, acute phase reactants, pulmonary lesions, and oxygenation levels.
4. Expert opinion
In recent years, the use of MSCs and their secreted exosomes has shown unique advantages in regenerative medicine. Several mechanisms have been proposed to explain how MSCs can influence their target cells or tissues. Among them, exosomes secreted by MSCs have attracted much attention for their key role in the therapeutic effects of MSCs on their targets. In preclinical studies, both MSCs and their secreted exosomes have demonstrated significant potential in the treatment of various diseases. Promising results in clinical trials have also supported the safety of MSC and exosome therapy, underscoring the need for further exploration in this field. However, the efficacy of MSCs in treating some diseases remains uncertain, necessitating the implementation of strategies aimed at enhancing their therapeutic benefits. In certain situations, exosomes may serve as a viable alternative to MSCs, particularly in the context of lung disease where their small size enables inhalation therapy.
Regarding economics, MSC-based therapies can indeed be costly; however, they have the potential to be cost-effective, particularly in cases of chronic diseases that require prolonged treatment. Additionally, MSC-based therapies may decrease the need for conventional drugs and improve patient outcomes. Nonetheless, the absence of established guidelines for the application of MSCs or their secreted exosomes underscores the need for continued patient follow-up in clinical trials, as this will help standardize methods and facilitate the development of more efficient treatments using MSCs or their secreted exosomes.
Despite the promising potential of MSCs and their exosomes, there are several unsolved challenges and limitations that need to be addressed. In regenerative medicine, the lack of reproducibility and comparability of results between studies poses a challenge for the use of MSCs or their exosomes. Variations in the preparation of MSCs from different tissue and different donors, variation in the routes of administration, and individual host responses make it difficult to analyze results from different studies. To overcome these challenges, it is necessary to determine the optimal stage of each degenerative disease for MSC-based therapy, identify the most suitable MSC source for each specific disease, establish the best administration method for each degenerative condition, and consider personalized medicine. By conducting a series of well-organized studies for each disease, it may be possible to develop separate guidelines for MSC-based or exosome therapy, offering a promising avenue for efficient treatment and improving the quality of life for patients suffering from degenerative diseases.
In the context of exosomes, while they appear to be safe, the yield and purity of exosomes derived from MSCs are often low. To address this issue, several methods have been developed to increase exosome production, including bioreactor systems, culture condition modulation, induction of various stressors, and cell fragmentation. While these methods show promise for increasing exosome production, further research is needed to optimize them and improve the yield and quality of exosomes. Achieving this may enable the enhancement of the therapeutic potential of exosomes and advance their use in clinical applications. Currently, several methods have been developed to improve the stability and efficiency of exosomes, including genetic engineering of exosome content, encapsulation, and bioprinting technology. However, the development of novel strategies for modifying MSCs and their exosomes holds promise for further enhancing their efficiency.
On the other hand, since MSC-based therapies are classified as biological products, regulatory agencies have strict guidelines for their use in clinical practice. These guidelines need to be standardized, streamlined, and harmonized across different countries and regions to facilitate widespread adoption.
The ultimate goal in the field of MSCs or their exosomes-based therapy is to develop safe and effective therapies for a wide range of degenerative diseases, which includes optimizing cell-based therapies by using novel strategies to increase their efficiency. Further research can lead to the development of new combination therapies that utilize MSCs and their products to improve the outcomes of existing treatments, as well as provide new insights into disease pathology and development.
While the study of MSCs and their exosomes is a promising area of research, it is important to focus on novel strategies to enhance the efficiency of MSCs or their exosomes for application in regenerative medicine. Additionally, it is crucial to develop proper guidelines for using specific MSCs or their exosomes to treat specific stages of particular degenerative conditions. Moreover, the combination of MSC (exosome)-based therapy with other treatments may offer potential benefits. Finally, the field of MSCs and their secreted exosomes is expected to continue evolving and growing in the coming years, with ongoing research aimed at improving the understanding of MSCs and exosome biology and developing more effective therapies for a range of diseases and conditions.
Article highlights.
MSCs and their secreted exosomes have emerged as a promising therapeutic strategy to treat a variety of diseases.
MSCs can influence their target cells through direct contact, mitochondrial transfer, paracrine function, and exosomes, each of which plays a unique role in regenerative medicine.
Exosomes derived from MSCs are useful for cell-free regenerative medicine due to their capacity for regeneration, immunomodulation, and anti-tumor effects, among other properties.
Despite the advantages of MSCs, challenges in their preparation and application still exist, leading to the development of various strategies to optimize MSC-based therapy.
To optimize MSC or their secreted exosome-based therapy, a variety of strategies have been applied to enhance their survival, stability, and secretory capacity. These strategies include ex-vivo preconditioning strategies, genetic modification, encapsulation and the application of bioprinting technology.
Funding
This paper was funded by the Brain Pool Program funded by the Ministry of Science and ICT through the National Research Foundation of Korea (grant 2022H1D3A2A01063847), the Basic Science Research Program funded by the Ministry of Education through the National Research Foundation of Korea (grant 2016R1D1A1B02008854), the Basic Science Research Capacity Enhancement Project through the Korea Basic Science Institute (National Research Facilities and Equipment Center) funded by the Ministry of Education (grant 2019R1A6C1010030), and the National Cancer Institute (grant 1R01 CA258240 to DM Lubman).
Abbreviations
- MSC
Mesenchymal stem cell
- iPSCs
induced Pluripotent stem cells
- ISCT
International Society for Cellular Therapy
- TF
Tissue factor
- MHC
Major histocompatibility complex
- TGF-β1
Transforming growth factor-1
- IFN-γ
Interferon-γ
- TNF-α
Tumor necrosis factor- α
- HGF
Hepatocyte growth factor
- FGF
Fibroblast growth factor
- IDO
Indoleamine-pyrrole 2,3-dioxygenase
- PGE2
Prostaglandin E2
- NO
Nitric oxide
- PD-L1
Programmed death ligand-1
- SDF-1
Stromal cell-derived factor 1
- CXCR4
Chemokine receptor type 4
- ERK1/2
Extracellular signal-regulated kinases
- EVs
Extracellular Vesicle
- PI3K
Phosphatidylinositol-3-kinase
- VEGF
Vascular endothelial growth factor
- MCP-1
Monocyte chemoattractant protein-1
- IL-8
interleukin-8
- YAP
Yes-associated protein
- HSP70
Heat shock protein 70
- TRPV1
Transient receptor potential vanilloid 1
- RIL-23R
Interleukin 23 receptor
- CAR
Activate chimeric antigen receptor
- GH
Gelatin−hydroxyphenyl propionic acid
- NTA
Nanoparticle tracking analysis
- MMP-1
Matrix metalloproteinases-1
- HUVECs
Human umbilical vein endothelial cells
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Yu Y, Wang Q, Wang C, et al. Living materials for regenerative medicine. Engineered Regeneration. 2021;2:96–104. [Google Scholar]
- 2.Mao AS, Mooney DJ. Regenerative medicine: current therapies and future directions. Proceedings of the National Academy of Sciences. 2015;112(47):14452–14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atala A Regenerative medicine strategies. Journal of pediatric surgery. 2012;47(1):17–28. [DOI] [PubMed] [Google Scholar]
- 4.Mason C, Dunnill P. A brief definition of regenerative medicine. 2008. [DOI] [PubMed]
- 5.Escacena-Acosta N, Lopez-Beas J, Lachaud CC, et al. Stem Cells: Concept, Properties, and Characterization. Corneal Regeneration: Springer; 2019. p. 41–55. [Google Scholar]
- 6.Adelipour M, Saleth LR, Ghavami S, et al. The role of autophagy in the metabolism and differentiation of stem cells. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2022;1868(8):166412. [DOI] [PubMed] [Google Scholar]
- 7.Adelipour M, Allameh A, Tavangar S, et al. Inhibition of breast tumor growth and abnormal angiogenesis in mice treated with endothelial cells and their progenitor mesenchymal stem cells derived from bone marrow. Neoplasma. 2016;63(6):911–924. [DOI] [PubMed] [Google Scholar]
- 8.Allameh A, Jazayeri M, Adelipour M. In vivo vascularization of endothelial cells derived from bone marrow mesenchymal stem cells in SCID mouse model. Cell Journal (Yakhteh). 2016;18(2):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10(8):1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou Y, Liao L, Dai J, et al. Mesenchymal stem cell-derived extracellular vesicles/exosome: A promising therapeutic strategy for intracerebral hemorrhage. Regenerative Therapy. 2023;22:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S, Jung S-C. New Sources, Differentiation, and Therapeutic Uses of Mesenchymal Stem Cells. MDPI; 2021. p. 5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roubelakis MG, Pappa KI, Bitsika V, et al. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem cells and development. 2007;16(6):931–952. [DOI] [PubMed] [Google Scholar]
- 13.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. Journal of bone and mineral research. 2003;18(4):696–704. [DOI] [PubMed] [Google Scholar]
- 14.Gargett CE, Schwab KE, Zillwood RM, et al. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biology of reproduction. 2009;80(6):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J-S, Lee B-J, Park H-Y, et al. Effects of donor age, long-term passage culture, and cryopreservation on tonsil-derived mesenchymal stem cells. Cellular Physiology and Biochemistry. 2015;36(1):85–99. [DOI] [PubMed] [Google Scholar]
- 16.Rotter N, Oder J, Schlenke P, et al. Isolation and characterization of adult stem cells from human salivary glands. Stem cells and development. 2008;17(3):509–518. [DOI] [PubMed] [Google Scholar]
- 17.Schosserer M, Reynoso R, Wally V, et al. Urine is a novel source of autologous mesenchymal stem cells for patients with epidermolysis bullosa. BMC research notes. 2015;8(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musina R, Belyavski A, Tarusova O, et al. Endometrial mesenchymal stem cells isolated from the menstrual blood. Bulletin of experimental biology and medicine. 2008;145(4):539–543. [DOI] [PubMed] [Google Scholar]
- 19.Kassis I, Zangi L, Rivkin R, et al. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone marrow transplantation. 2006;37(10):967–976. [DOI] [PubMed] [Google Scholar]
- 20.Jia Z, Liang Y, Xu X, et al. Isolation and characterization of human mesenchymal stem cells derived from synovial fluid by magnetic-activated cell sorting (MACS). Cell biology international. 2018;42(3):262–271. [DOI] [PubMed] [Google Scholar]
- 21.Machado CdV, Telles PDdS, Nascimento ILO. Immunological characteristics of mesenchymal stem cells. Revista brasileira de hematologia e hemoterapia. 2013;35:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hynes K, Menicanin D, Mrozik K, et al. Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem cells and development. 2014;23(10):1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Xu SQ, Zhao YM, et al. Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Molecular medicine reports. 2018;18(6):4969–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. The FASEB Journal. 2004;18(9):980–982. [DOI] [PubMed] [Google Scholar]
- 25.Naji A, Eitoku M, Favier B, et al. Biological functions of mesenchymal stem cells and clinical implications. Cellular and Molecular Life Sciences. 2019;76(17):3323–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding D-C, Shyu W-C, Lin S-Z. Mesenchymal stem cells. Cell transplantation. 2011;20(1):5–14. [DOI] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 28.Moll G, Ankrum JA, Olson SD, et al. Improved MSC minimal criteria to maximize patient safety: a call to embrace tissue factor and hemocompatibility assessment of MSC products. Stem cells translational medicine. 2022;11(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Z, Liang J, Huang W, et al. Immunomodulatory effects of mesenchymal stem cells for the treatment of cardiac allograft rejection. Experimental Biology and Medicine. 2021;246(7):851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon DG, Kim MK, Jeon YS, et al. State of the art: the immunomodulatory role of MSCs for osteoarthritis. International Journal of Molecular Sciences. 2022;23(3):1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Shen S, Fu H, et al. Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells International. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otsuka R, Wada H, Murata T, et al. Immune reaction and regulation in transplantation based on pluripotent stem cell technology. Inflammation and Regeneration. 2020;40(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends in Pharmacological Sciences. 2020;41(9):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmermann JA, Mcdevitt TC. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy. 2014;16(3):331–345. [DOI] [PubMed] [Google Scholar]
- 35.García JR, Quirós M, Han WM, et al. IFN-γ-tethered hydrogels enhance mesenchymal stem cell-based immunomodulation and promote tissue repair. Biomaterials. 2019;220:119403. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Use of encapsulation strategy to enhance MSC-based therapy efficienc
- 36.de Carvalho Ribeiro P, Oliveira LF, dos Santos ALRT, et al. Enhancing the therapeutic potential of mesenchymal stem cells with the CRISPR-Cas system. Stem Cell Reviews and Reports. 2019;15(4):463–473. [DOI] [PubMed] [Google Scholar]
- 37.Ling L, Hou J, Liu D, et al. Important role of the SDF-1/CXCR4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (hAD-MSCs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (POI). Stem Cell Research & Therapy. 2022;13(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin W, Liang X, Brooks A, et al. Modelling of the SDF-1/CXCR4 regulated in vivo homing of therapeutic mesenchymal stem/stromal cells in mice. PeerJ. 2018;6:e6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng X-B, He X-W, Zhang L-J, et al. Bone marrow-derived CXCR4-overexpressing MSCs display increased homing to intestine and ameliorate colitis-associated tumorigenesis in mice. Gastroenterology report. 2019;7(2):127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Xu Z, Jiang W, et al. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. International journal of cardiology. 2006;109(1):74–81. [DOI] [PubMed] [Google Scholar]
- 41.Duffy MM, Pindjakova J, Hanley SA, et al. Mesenchymal stem cell inhibition of T-helper 17 cell-differentiation is triggered by cell–cell contact and mediated by prostaglandin E2 via the EP4 receptor. European journal of immunology. 2011;41(10):2840–2851. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Zhang D, Xu L, et al. Cell–cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cellular & molecular immunology. 2019;16(12):908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori D, Miyagawa S, Kawamura T, et al. Mitochondrial Transfer Induced by Adipose-Derived Mesenchymal Stem Cell Transplantation Improves Cardiac Function in Rat Models of Ischemic Cardiomyopathy. Cell Transplantation. 2023;32:09636897221148457. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Discovery of one of mechanises by wich MSCs can affect on their target cells.
- 44.Jiang D, Gao F, Zhang Y, et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell death & disease. 2016;7(11):e2467–e2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rackham CL, Hubber EL, Czajka A, et al. Optimizing beta cell function through mesenchymal stromal cell-mediated mitochondria transfer. Stem Cells. 2020;38(4):574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. American journal of respiratory and critical care medicine. 2017;196(10):1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H-K, Lee S-G, Lee S-W, et al. A subset of paracrine factors as efficient biomarkers for predicting vascular regenerative efficacy of mesenchymal stromal/stem cells. Stem Cells. 2019;37(1):77–88. [DOI] [PubMed] [Google Scholar]
- 48.Mukhamedshina Y, Shulman I, Ogurcov S, et al. Mesenchymal stem cell therapy for spinal cord contusion: a comparative study on small and large animal models. Biomolecules. 2019;9(12):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alessio N, Özcan S, Tatsumi K, et al. The secretome of MUSE cells contains factors that may play a role in regulation of stemness, apoptosis and immunomodulation. Cell Cycle. 2017;16(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Assoni A, Coatti G, Valadares MC, et al. Different donors mesenchymal stromal cells secretomes reveal heterogeneous profile of relevance for therapeutic use. Stem cells and development. 2017;26(3):206–214. [DOI] [PubMed] [Google Scholar]
- 51.Su N, Gao P-L, Wang K, et al. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials. 2017;141:74–85. [DOI] [PubMed] [Google Scholar]
- 52.Drzeniek NM, Mazzocchi A, Schlickeiser S, et al. Bio-instructive hydrogel expands the paracrine potency of mesenchymal stem cells. Biofabrication. 2021;13(4):045002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin C, Xu K, He Y, et al. A dynamic matrix potentiates mesenchymal stromal cell paracrine function via an effective mechanical dose. Biomaterials Science. 2020;8(17):4779–4791. [DOI] [PubMed] [Google Scholar]
- 54.Parate D, Kadir ND, Celik C, et al. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Stem cell research & therapy. 2020;11(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marote A, Teixeira FG, Mendes-Pinheiro B, et al. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Frontiers in pharmacology. 2016;7:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z-g, He Z-y, Liang S, et al. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Research & Therapy. 2020;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Capomaccio S, Cappelli K, Bazzucchi C, et al. Equine adipose-derived mesenchymal stromal cells release extracellular vesicles enclosing different subsets of small RNAs. Stem cells international. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bi Y, Qiao X, Liu Q, et al. Systemic proteomics and miRNA profile analysis of exosomes derived from human pluripotent stem cells. Stem Cell Research & Therapy. 2022;13(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janockova J, Slovinska L, Harvanova D, et al. New therapeutic approaches of mesenchymal stem cells-derived exosomes. Journal of Biomedical Science. 2021;28(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Margiana R, Markov A, Zekiy AO, et al. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Research & Therapy. 2022;13(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sel FA, Oguz FS. Regenerative Medicine Application of Mesenchymal Stem Cells. Springer; 2022. [DOI] [PubMed] [Google Scholar]
- 62.Zhou T, Yuan Z, Weng J, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. Journal of Hematology & Oncology. 2021;14(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Araujo RS, Silva MS, Santos DF, et al. Dysregulation of trophic factors contributes to diabetic retinopathy in the Ins2Akita mouse. Experimental Eye Research. 2020;194:108027. [DOI] [PubMed] [Google Scholar]
- 64.Elshaer SL, Evans W, Pentecost M, et al. Adipose stem cells and their paracrine factors are therapeutic for early retinal complications of diabetes in the Ins2Akita mouse. Stem Cell Research & Therapy. 2018;9(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sekiya I, Koga H, Katano H, et al. Second-look arthroscopy after meniscus repair and synovial mesenchymal stem cell transplantation to treat degenerative flaps and radial tears of the medial meniscus: a case report. Journal of Orthopaedic Science. 2022;27(4):821–834. [DOI] [PubMed] [Google Scholar]
- 66.Atluri S, Muy MB, Dragella R, et al. Evaluation of the effectiveness of autologous bone marrow mesenchymal stem cells in the treatment of chronic low back pain due to severe lumbar spinal degeneration: a 12-month, open-label, prospective controlled trial. Pain Physician. 2022;25(2):193. [PubMed] [Google Scholar]
- 67.Meligy FY, El-Deen Mohammed HS, Mostafa TM, et al. Therapeutic Potential of Mesenchymal Stem Cells versus Omega n− 3 Polyunsaturated Fatty Acids on Gentamicin-Induced Cardiac Degeneration. Pharmaceutics. 2022;14(7):1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y-x, Chen S-r, Su P-p, et al. Using mesenchymal stem cells to treat female infertility: an update on female reproductive diseases. Stem cells international. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang Y, Zhang P, Zhang X, et al. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell proliferation. 2021;54(1):e12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Regmi S, Pathak S, Kim JO, et al. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. European journal of cell biology. 2019;98(5–8):151041. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Yu Q, Hu Y, et al. Current research and use of mesenchymal stem cells in the therapy of autoimmune diseases. Current Stem Cell Research & Therapy. 2019;14(7):579–582. [DOI] [PubMed] [Google Scholar]
- 72.Ishiuchi N, Nakashima A, Yoshida K, et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem cell research & therapy. 2020;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lv H, Yuan X, Zhang J, et al. Heat shock preconditioning mesenchymal stem cells attenuate acute lung injury via reducing NLRP3 inflammasome activation in macrophages. Stem Cell Research & Therapy. 2021;12(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo L, Du J, Yuan D-f, et al. Optimal H2O2 preconditioning to improve bone marrow mesenchymal stem cells’ engraftment in wound healing. Stem cell research & therapy. 2020;11(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao J, Young YK, Fradette J, et al. Melatonin pretreatment of human adipose tissue-derived mesenchymal stromal cells enhances their prosurvival and protective effects on human kidney cells. American Journal of Physiology-Renal Physiology. 2015;308(12):F1474–F1483. [DOI] [PubMed] [Google Scholar]
- 76.ShamsEldeen AM, Ashour H, Shoukry HS, et al. Combined treatment with systemic resveratrol and resveratrol preconditioned mesenchymal stem cells, maximizes antifibrotic action in diabetic cardiomyopathy. Journal of Cellular Physiology. 2019;234(7):10942–10963. [DOI] [PubMed] [Google Scholar]
- 77.Kim YE, Sung SI, Chang YS, et al. Thrombin Preconditioning Enhances Therapeutic Efficacy of Human Wharton’s Jelly–Derived Mesenchymal Stem Cells in Severe Neonatal Hypoxic Ischemic Encephalopathy. International journal of molecular sciences. 2019;20(10):2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Esmaeilzade B, Artimani T, Amiri I, et al. Dimethyloxalylglycine preconditioning enhances protective effects of bone marrow-derived mesenchymal stem cells in Aβ-induced Alzheimer disease. Physiology & behavior. 2019;199:265–272. [DOI] [PubMed] [Google Scholar]
- 79.Zheng J, Li H, He L, et al. Preconditioning of umbilical cord-derived mesenchymal stem cells by rapamycin increases cell migration and ameliorates liver ischaemia/reperfusion injury in mice via the CXCR4/CXCL12 axis. Cell Proliferation. 2019;52(2):e12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang J, Tang L, Zhang F, et al. Sevoflurane preconditioning promotes mesenchymal stem cells to relieve myocardial ischemia/reperfusion injury via TRPC6-induced angiogenesis. Stem cell research & therapy. 2021;12(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu Z, Xing H, Tang R, et al. The preconditioning of lithium promotes mesenchymal stem cell-based therapy for the degenerated intervertebral disc via upregulating cellular ROS. Stem Cell Research & Therapy. 2021;12(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ebrahim N, James V, Rizvanov AA, et al. Genetic Modification of Mesenchymal Stem Cells for Neurological Disease Therapy: What Effects Does it Have on Phenotype/Cell Behavior, Determining Their Effectiveness? Molecular Diagnosis & Therapy. 2020;24(6):683–702. [DOI] [PubMed] [Google Scholar]
- 83.Li M, Li J, Chen H, et al. VEGF-Expressing Mesenchymal Stem Cell Therapy for Safe and Effective Treatment of Pain in Parkinson’s Disease. Cell Transplantation. 2023;32:09636897221149130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rostami M, Haidari K, Amini H, et al. Genetically Engineered Mesenchymal Stem Cell Therapy Against Murine Experimental Autoimmune Encephalomyelitis. Molecular Neurobiology. 2022:1–9. [DOI] [PubMed] [Google Scholar]
- 85.Hombach AA, Geumann U, Günther C, et al. IL7-IL12 engineered mesenchymal stem cells (MSCs) improve a CAR T cell attack against colorectal cancer cells. Cells. 2020;9(4):873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sahu N, Agarwal P, Grandi F, et al. Encapsulated mesenchymal stromal cell microbeads promote endogenous regeneration of osteoarthritic cartilage ex vivo. Advanced Healthcare Materials. 2021;10(8):2002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim CW, Kim CJ, Park E-H, et al. MSC-encapsulating in situ cross-linkable gelatin hydrogels to promote myocardial repair. ACS Applied Bio Materials. 2020;3(3):1646–1655. [DOI] [PubMed] [Google Scholar]
- 88.Wang F, Nan L-p, Zhou S-f, et al. Injectable hydrogel combined with nucleus pulposus-derived mesenchymal stem cells for the treatment of degenerative intervertebral disc in rats. Stem cells international. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pitacco P, Sadowska JM, O’Brien FJ, et al. 3D bioprinting of cartilaginous templates for large bone defect healing. Acta Biomaterialia. 2022. [DOI] [PubMed] [Google Scholar]
- 90.Lin Y-T, Hsu T-T, Liu Y-W, et al. Bidirectional Differentiation of Human-Derived Stem Cells Induced by Biomimetic Calcium Silicate-Reinforced Gelatin Methacrylate Bioink for Odontogenic Regeneration. Biomedicines. 2021;9(8):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ke D, Yi H, Est-Witte S, et al. Bioprinted trachea constructs with patient-matched design, mechanical and biological properties. Biofabrication. 2019;12(1):015022. [DOI] [PubMed] [Google Scholar]
- 92.Večerić-Haler Ž, Sever M, Kojc N, et al. Autologous Mesenchymal Stem Cells for Treatment of Chronic Active Antibody-Mediated Kidney Graft Rejection: Report of the Phase I/II Clinical Trial Case Series. Transplant International. 2022:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ho KK-w, Lee WY-w, Griffith JF, et al. Randomized control trial of mesenchymal stem cells versus hyaluronic acid in patients with knee osteoarthritis–A Hong Kong pilot study. Journal of Orthopaedic Translation. 2022;37:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The use of MSCs in clinical traials in comparision with current treatment.
- 94.Izadi M, Sadr Hashemi Nejad A, Moazenchi M, et al. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Research & Therapy. 2022;13(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee J, Chang WH, Chung J-W, et al. Efficacy of intravenous mesenchymal stem cells for motor recovery after ischemic stroke: a neuroimaging study. Stroke. 2022;53(1):20–28. [DOI] [PubMed] [Google Scholar]
- 96.Zang L, Li Y, Hao H, et al. Efficacy and safety of umbilical cord-derived mesenchymal stem cells in Chinese adults with type 2 diabetes: a single-center, double-blinded, randomized, placebo-controlled phase II trial. Stem Cell Research & Therapy. 2022;13(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang C, Huang L, Wang X, et al. Topical and intravenous administration of human umbilical cord mesenchymal stem cells in patients with diabetic foot ulcer and peripheral arterial disease: a phase I pilot study with a 3-year follow-up. Stem Cell Research & Therapy. 2022;13(1):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coelho HRS, Neves SCd, da Silva Menezes JN, et al. Autologous adipose-derived mesenchymal stem cell therapy reverses detrusor underactivity: open clinical trial. Stem Cell Research & Therapy. 2023;14(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aghayan HR, Salimian F, Abedini A, et al. Human placenta-derived mesenchymal stem cells transplantation in patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 (phase I clinical trial): safety profile assessment. Stem Cell Research & Therapy. 2022;13(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grégoire C, Layios N, Lambermont B, et al. Bone Marrow-Derived Mesenchymal Stromal Cell Therapy in Severe COVID-19: Preliminary Results of a Phase I/II Clinical Trial. Frontiers in immunology. 2022:3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaffash Farkhad N, Sedaghat A, Reihani H, et al. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial. Stem Cell Research & Therapy. 2022;13(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saleh M, Vaezi AA, Sohrabpour AA, et al. Wharton’s jelly-mesenchymal stem cells treatment for severe COVID 19 patients: 1-year follow-up. Gene Reports. 2022;29:101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chun S, Choi C-B, Kim MS, et al. Safety and tolerability of bone marrow–derived mesenchymal stem cells in lupus animal models and a phase I clinical trial in humans. Lupus. 2022;31(10):1245–1253. [DOI] [PubMed] [Google Scholar]
- 104.Davidson SM, Boulanger CM, Aikawa E, et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: From exosomes to microvesicles. Cardiovascular research. 2023;119(1):45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jing H, He X, Zheng J. Exosomes and regenerative medicine: state of the art and perspectives. Translational Research. 2018;196:1–16. [DOI] [PubMed] [Google Scholar]
- 106.Jalaludin I, Lubman DM, Kim J. A guide to mass spectrometric analysis of extracellular vesicle proteins for biomarker discovery. Mass Spectrometry Reviews. 2021:e21749. [DOI] [PubMed] [Google Scholar]
- 107.Burton JB, Carruthers NJ, Stemmer PM. Enriching extracellular vesicles for mass spectrometry. Mass Spectrometry Reviews. 2023;42(2):779–795. [DOI] [PubMed] [Google Scholar]
- 108.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of extracellular vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei W, Ao Q, Wang X, et al. Mesenchymal stem cell–derived exosomes: a promising biological tool in nanomedicine. Frontiers in pharmacology. 2021;11:590470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rong X, Liu J, Yao X, et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem cell research & therapy. 2019;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, et al. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regeneration Research. 2019;14(9):1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang L, He F, Gao L, et al. Engineering exosome-like nanovesicles derived from Asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. International Journal of Nanomedicine. 2021;16:1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang L, Abhange KK, Wen Y, et al. Preparation of engineered extracellular vesicles derived from human umbilical cord mesenchymal stem cells with ultrasonication for skin rejuvenation. ACS omega. 2019;4(27):22638–22645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fu S, Wang Y, Xia X, et al. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. [Google Scholar]
- 115.Kwon S, Shin S, Do M, et al. Engineering approaches for effective therapeutic applications based on extracellular vesicles. Journal of Controlled Release. 2021;330:15–30. [DOI] [PubMed] [Google Scholar]
- 116.Wei H, Chen J, Wang S, et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. International journal of nanomedicine. 2019;14:8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang R, Huang H, Cui S, et al. IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death & Disease. 2020;11(7):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen S, Tang Y, Liu Y, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Proliferation. 2019;52(5):e12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun J, Shen H, Shao L, et al. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Research & Therapy. 2020;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y, Xie Y, Hao Z, et al. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Applied Materials & Interfaces. 2021;13(16):18472–18487. [DOI] [PubMed] [Google Scholar]
- 121.Yang J, Chen Z, Pan D, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. International Journal of Nanomedicine. 2020:5911–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kostennikov A, Kabdesh I, Sabirov D, et al. A Comparative Study of Mesenchymal Stem Cell-Derived Extracellular Vesicles’ Local and Systemic Dose-Dependent Administration in Rat Spinal Cord Injury. Biology. 2022;11(12):1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang T, Sato Y, Kuramochi A, et al. Surface modulation of extracellular vesicles with cell-penetrating peptide-conjugated lipids for improvement of intracellular delivery to endothelial cells. Regenerative Therapy. 2023;22:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu Q, Fu X, Li X, et al. Modification of adipose mesenchymal stem cells-derived small extracellular vesicles with fibrin-targeting peptide CREKA for enhanced bone repair. Bioactive Materials. 2023;20:208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen P, Zheng L, Wang Y, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The use of 3-D bioprinting technique for exosome therapy
- 126.Liu X, Zhang J, Cheng X, et al. Integrated printed BDNF-stimulated HUCMSCs-derived exosomes/collagen/chitosan biological scaffolds with 3D printing technology promoted the remodelling of neural networks after traumatic brain injury. Regenerative Biomaterials. 2023;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun X, Mao Y, Liu B, et al. Mesenchymal Stem Cell-Derived Exosomes Enhance 3D-Printed Scaffold Functions and Promote Alveolar Bone Defect Repair by Enhancing Angiogenesis. Journal of Personalized Medicine. 2023;13(2):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jalaludin I, Lubman DM, Kim J. MALDI-MS: A Powerful but Underutilized Mass Spectrometric Technique for Exosome Research. Mass Spectrometry Letters. 2021;12(3):93–105. [Google Scholar]
- 129.Syromiatnikova V, Prokopeva A, Gomzikova M. Methods of the Large-Scale Production of Extracellular Vesicles. International Journal of Molecular Sciences. 2022;23(18):10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sengupta V, Sengupta S, Lazo A, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem cells and development. 2020;29(12):747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu Y-G, Shi M-m, Monsel A, et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study. Stem Cell Research & Therapy. 2022;13(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The use of nebulizer of exosomes to treat COVID-19 in clinic
- 132.Chu M, Wang H, Bian L, et al. Nebulization therapy with umbilical cord mesenchymal stem cell-derived exosomes for COVID-19 pneumonia. Stem Cell Reviews and Reports. 2022;18(6):2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yagyu T, Yasuda S, Nagaya N, et al. Long-term results of intracardiac mesenchymal stem cell transplantation in patients with cardiomyopathy. Circulation Journal. 2019;83(7):1590–1599. [DOI] [PubMed] [Google Scholar]
- 134.de Celis-Ruiz E, Fuentes B, Alonso de Leciñana M, et al. Final results of allogeneic adipose tissue–derived mesenchymal stem cells in acute ischemic stroke (AMASCIS): A phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Cell Transplantation. 2022;31:09636897221083863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baak LM, Wagenaar N, van der Aa NE, et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): a first-in-human, open-label intervention study. The Lancet Neurology. 2022;21(6):528–536. [DOI] [PubMed] [Google Scholar]
- 136.Liu W, Rong Y, Wang J, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. Journal of neuroinflammation. 2020;17(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wei H, Chen J, Wang S, et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. International journal of nanomedicine. 2019:8603–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu X, Li X, Zhu W, et al. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. Journal of cellular physiology. 2020;235(11):8010–8022. [DOI] [PubMed] [Google Scholar]
- 139.Sheykhhasan M, Kalhor N, Sheikholeslami A, et al. Exosomes of mesenchymal stem cells as a proper vehicle for transfecting miR-145 into the breast cancer cell line and its effect on metastasis. BioMed Research International. 2021;2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang D, Gao B, Yue J, et al. Exosomes from mesenchymal stem cells expressing miR-125b inhibit neointimal hyperplasia via myosin IE. Journal of Cellular and Molecular Medicine. 2019;23(2):1528–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li L, Zhang Y, Mu J, et al. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano letters. 2020;20(6):4298–4305. [DOI] [PubMed] [Google Scholar]
- 142.Han C, Zhou J, Liang C, et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomaterials science. 2019;7(7):2920–2933. [DOI] [PubMed] [Google Scholar]


