Abstract
Background:
Cognitive impairment is common in Parkinson’s disease (PD) and has a substantial impact on quality of life. Despite numerous trials targeting various PD features, we still lack effective treatments for cognition beyond cholinesterase inhibitors.
Objective:
To identify the gaps in recent clinical trials with cognitive outcomes in PD and consider areas for improvement.
Methods:
We examined recent clinical trials with cognitive outcomes in PD registered on ClinicalTrials.gov, excluding trials without cognitive outcomes, non-interventional studies, and in atypical Parkinsonian disorders. Included trials were categorized by treatment approach (investigational medicinal product, behavioral, physical activity, device-based). Details of trial design and outcomes were collected.
Results:
178 trials at different stages of trial completion were considered. 46 trials were completed, 25 had available results. Mean follow-up duration was 29.9 weeks. Most common cognitive measure was Montreal Cognitive Assessment. Most were performed in North America or Europe. Majority of the participants identified as non-Hispanic and White. Only eight trials showed improvement in cognition, none showed improvement beyond four months. These included trials of international medicinal products, cognitive and physical interventions and devices. GRADE certainty levels ranged from Moderate to Very Low. Only mevidalen had a Moderate certainty for potential clinical effectiveness.
Conclusions:
Amongst a large number of trials for cognition in PD, only a small proportion were completed. Few showed significant improvement, with no proven long-lasting effects. Trial design, lack of enrichment for at-risk groups, short follow-up duration, insensitive outcome measures likely contribute to lack of detectable benefit and should be considered in future trials.
Keywords: Parkinson’s disease, Cognitive impairment, Clinical trials
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative condition, with a fast-growing prevalence [1]. Although PD is characterized by motor features, cognitive impairment is a common and feared symptom, with dementia six times more common in PD than in the general population [2] and affecting around 50% of people with PD within 10 years of diagnosis [3]. Parkinson’s disease dementia (PDD) is linked with other distressing symptoms of visual hallucinations, psychosis and depression [4] and is a cause of significant economic burden on the population and the healthcare system [5]. Parkinson’s disease with mild cognitive impairment (PD-MCI) can be determined based on gradual decline in cognitive ability, and cognitive deficits on either a global cognitive scale or on at least two tests in neuropsychological testing, without significant impact on functional independence [6]. In people with PD, MCI can predict the development of PDD, although reversion to normal cognition can also occur [7].
Currently, the mainstay of treatment for cognitive impairment in PD (for both PD-MCI and PDD) is the use of cholinesterase inhibitors, with level 1 evidence for rivastigmine [8,9], and similar efficacy for donepezil in PDD [10,11]. The use of galantamine, which is also a cholinesterase inhibitor, is only supported by open-label trials [11]. The NMDA receptor antagonist memantine may have some effects on cognition in PDD, but results have been mixed [12–14]. There has previously been interest in drugs active at noradrenaline receptors, such as atomoxetine and guanfacine [15], with a recent promising meta-analysis in Alzheimer’s disease [16]. However, most trials have shown minimal effects in improving cognition in PD, especially in people at earlier disease stages with no cognitive involvement [17,18]. More recently, other approaches, especially non-pharmacological interventions, have begun to be investigated for their potential effects on improving cognition in PD. These include physical exercise, non-invasive brain stimulation (including transcranial direct current stimulation or repetitive transcranial magnetic stimulation [rTMS]), and invasive brain stimulation (DBS), as well as combinations of these.
Here, we reviewed recent clinical trials that examine cognitive outcomes in PD from the previous five years, across the range of pharmacological and non-pharmacological interventions. Our aim was to identify emerging promising treatments, and to examine trial methodology, in order to determine whether there might be learnings to inform and improve future clinical trials for cognitive impairment in PD.
2. Methods
We searched ClinicalTrials.gov for clinical trials in the last five years that included cognitive outcomes in PD using the following search criteria: Condition: “Parkinson Disease”; Study type: “Interventional”; Phase: “Early Phase 1”, “Phase 1”, “Phase 2”, “Phase 3”, “Not Applicable”; Status: “Not yet recruiting”, “Recruiting”, “Enrolling by invitation”, “Active, not recruiting”, “Suspended”, “Terminated”, “Completed” or “Withdrawn”; Study start date: 01/01/2016. The search was performed on 24th February 2021. We deliberately used broad search criteria to include suspended, terminated or withdrawn trials in order to capture the full range of clinical trials attempted for cognition in PD.
We recorded trial title; trial number in ClinicalTrials.gov; start date; projected end date; primary completion date; actual end date; intervention name; age range of participants; number of participants planned for enrolment; number of study arms; outcome measures; availability of study results; type of funding source; sponsor/collaborators; and trial location. We then excluded from this list any trials that 1) did not include cognitive measures as either primary or secondary cognitive outcomes; 2) were non-interventional studies; 3) addressed only non-cognitive symptoms; or 4) assessed atypical Parkinsonian disorders (progressive supranuclear palsy, corticobasal degeneration, multiple system atrophy, vascular parkinsonism), including when these cases were tested alongside PD cases. As Dementia with Lewy bodies is widely considered to be on a spectrum with PD and PDD [19,20], where these cases were part of an analysis, the trial was not excluded. Next, we categorized trials according to treatment approach (investigational medicinal product [IMP], behavioral, physical activity, device-based, and other). Filtering and categorization were performed by three authors independently (EB, LB for the initial run, checked by BT). In cases of disagreement, the authors discussed in detail and came to a mutual conclusion for the trials they initially disagreed upon. Further information for each trial was gathered regarding cognitive status of recruited participants; type of cognitive outcome measure; duration of follow-up; geographical location of the trial; ethnicity and race of participants; serious adverse effects and availability of results and/or publications. This information was collected independently by teams cross-checking reports for consistency (PJ, RB, MG, TP, BT, RG).
We considered Michael J. Fox Foundation guidelines on goals for clinical trials including assessing whether people with PD populations, outcome measures, procedures and doses were appropriate for the trial aims and whether diversity had been considered for inclusion in the trial (www.michaeljfox.org). Once we had collated the trials, we examined trial outcomes for those trials where results were available either as peer-reviewed publications or directly from ClinicalTrials.gov. All trials were examined for risk of bias using Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) [21]. This tool assigns a semi-quantitative risk-of-bias assessment (i.e. high, medium, low) based on characteristics of the trial and published data. Phase 2 and 3 trials with positive results were then assessed using the Grades of Recommendation Assessment, Development and Evaluation (GRADE) approach [22].
Baseline demographic and disease-related data from trials with published results were reported as mean (standard deviation) or percentage. We accepted a threshold of p < .05 as significant when considering outcomes of clinical trials, and also considered whether correcting for multiple comparisons was performed.
3. Results
3.1. Study selection and characteristics
Our initial search identified 858 clinical trials examining cognitive outcomes in PD from the previous 5 years. Based on our exclusion criteria, 680 trials were excluded, bringing the total number of included trials to 178. These included trials at varying stages: 16 trials were registered but not yet recruiting, 85 were recruiting, 23 were active, 46 were completed and 8 were terminated or withdrawn. The total number of trials with published results was 25 (see below for detailed considerations of their findings). Trials included interventions ranging from IMP, cognitive training, physical training and devices (see Fig. 1 for a Prisma chart of trial selection).
Fig. 1. Prisma flow chart of trials identified in the search.
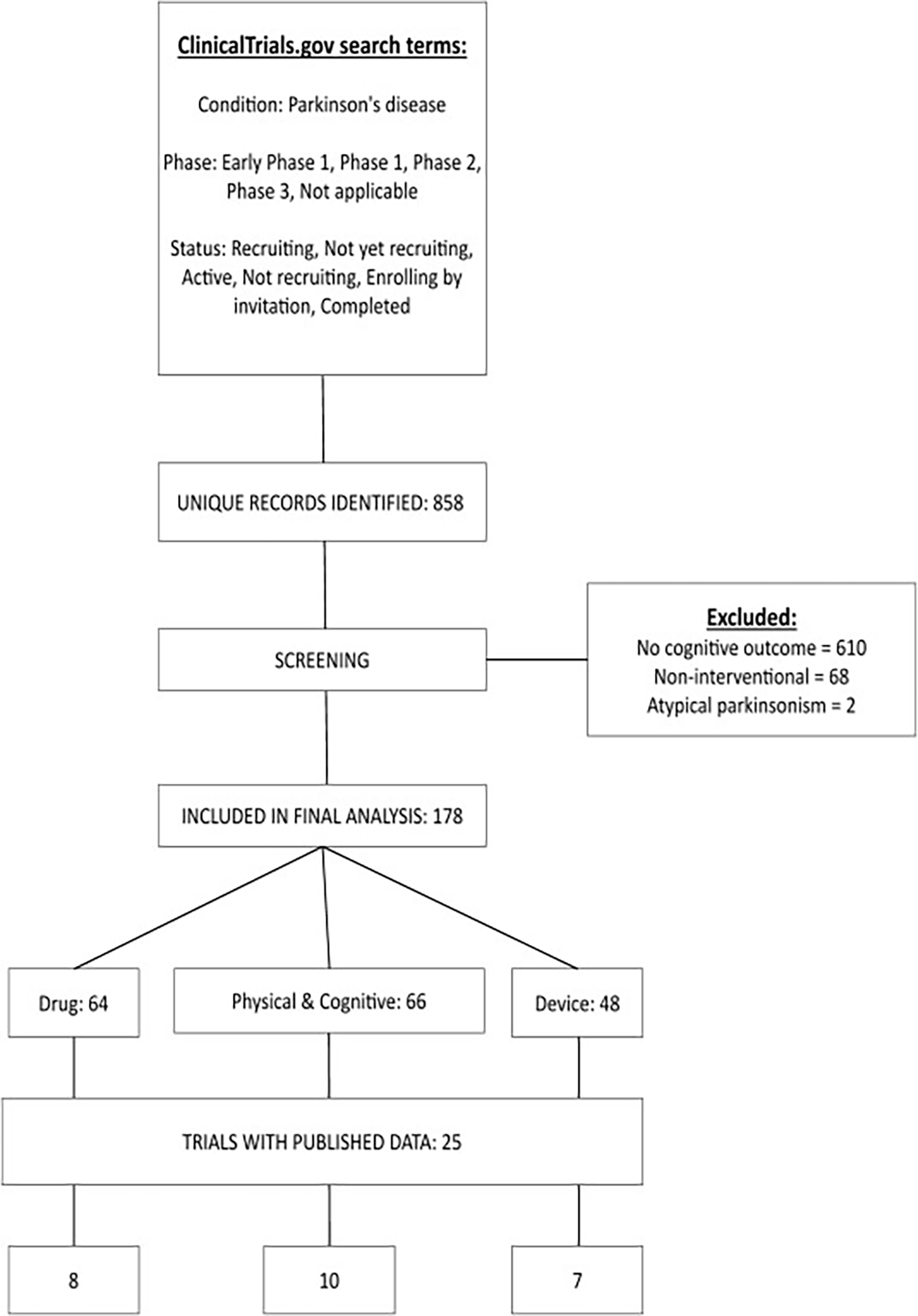
Trials were classified by intervention type. IMP: investigational medicinal product.
The 178 trials included a total of 15,657 participants, with a mean number of 88 people with PD across the trials. Most studies (n = 141) recruited cognitively normal people with PD; 18 trials included people with PD-MCI; 15 included people with PDD and four recruited people with PD across a range of cognitive states. Geographically, there were only 12 trials conducted across multiple continents with most trial sites located in North America (n = 85) and Europe (n = 63), 33 in Asia-Oceania, only five in South America and five in the Middle East and Africa.
3.2. Trials with available results
Of the 46 completed trials, 25 had data available for review in either peer-reviewed publications or as preprints, of which six were Phase 2 or Phase 3 (Table 1). A total number of 1,710 participants (mean number per trial = 68.4) with mean age of 66.3 (standard deviation [SD] = 3.19) and mean disease duration of 7.31 (SD = 4.36) years completed these studies. Overall, 33.2% (total n = 567) of participants were female. Ten of these studies reported information on ethnicity and race of participants (total n = 1,157); only 5.3% of the participants (total n = 61) identified as Hispanic. In terms of race, 0.2% (total n = 2) identified as American Indian or Alaska Native, 1.3% (total n = 15) as Asian, 1.7% (total n = 20) as Black, and 91.5% (n = 1,059) as White. There were minimal differences in geographic location with 14 studies undertaken in North America, one in North America and Europe, ten in Europe and one in the Middle East and Africa.
Table 1.
Phase 2/3 trials with published results
| Trial number | Source | Intervention | Control | N | Phase | Follow-up intervals | Cognitive measures | Cognitive result | Level of Evidence | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|
| IMP-based interventions | ||||||||||
| NCT03100149 | Pagano et al., 2022 | Prasinezumab | Placebo | 316 | 2 | 52 weeks | MoCA | No significant differences in MoCA at high or low dose | 2 | |
| NCT03769896 | Peball et al., 2020 | Nabilone | Placebo | 48 | 2 | 4 weeks | MoCA | No significant change in MoCA | 2 | |
| NCT04403399 | Albin et al., 2021 | Varenicline | Placebo | 34 | 2 | 2 × 3-week treatment & washout periods | MoCA, SAT | Significant improvement on SAT (without distractor, treatment difference 0.076, 95% CI 0.0073–0.14, p=.03; non-significant after correction for multiple comparisons); No significant change in other cognitive tests | 2 | Low |
| NCT02642393 | Mestre et al., 2021 | Inosine | Placebo | 298 | 3 | 2 years | MoCA | No significant change in MoCA | 2 | |
| NCT03305809 | Biglan et al., 2022 | Mevidalen | Placebo | 344 | 2 | 12 weeks | ADAScog, MoCA, ADCS-CGIC | No significant change in ADAScog or MoCA; Dose-dependent significant improvement in ADCS-CGIC in intervention group (minimal or better improvement: 32.9% of subjects in placebo vs 58.0% for 30 mg, 58.2% for 75 mg [p<.001 for both]; moderate or better improvement: 6.8% of subjects in placebo vs 21.7% for 30 mg, 30.9% for 75 mg [30mg p<.05; 75mg p<.001]) | 2 | Moderate |
| Device-based interventions | ||||||||||
| NCT03417271 | Dayal et al., 2020 | STN DBS | OFF | 16 | 2 | 4 weeks | Verbal fluency | No significant difference in verbal fluency | 2 | |
Abbreviations: GRADE = Grades of Recommendation Assessment, Development and Evaluation, IMP = Investigational Medicinal Product, MoCA = Montreal Cognitive Assessment, ADAScog = Alzheimer’s Disease Assessment Scale–Cognitive Subscale, ADCS-CGlC = Alzheimer’s Disease Cooperative Study - Clinical Global Impression of Change, SAT = Sustained Attention Test, STN DBS= Subthalamic nucleus deep brain stimulation, CI = Confidence interval
Only 15 studies were formal double-blind, placebo controlled randomized controlled trials, the gold standard for clinical trials. Follow-up duration in these trials ranged from 1 day to 104 weeks (mean = 16.9 weeks). Majority of the trials (n = 21) recruited people with PD with no cognitive impairment; three recruited people with PD-MCI and one recruited people with Lewy body dementia. The most commonly used cognitive measure was the Montreal Cognitive Assessment (MoCA) (11/25 studies), with baseline scores ranging from 15.7 to 28.1 (mean across trials = 25.44, SD = 2.93). Levodopa equivalent daily dose (LEDD) varied from 418 mg to 889 mg (mean across trials = 642.8 mg, SD = 239.9).
Only three trials reported study-related serious adverse events and notably, all of these were trials involving IMPs. In the allogeneic human mesenchymal stem cell infusion study (total n = 20), one participant with a prior history of lymphocytosis was diagnosed with asymptomatic chronic lymphocytic leukemia eight months after infusion [23]. In the prasinezumab trial (total n = 316), two participants had infusion reactions, one participant had influenza-like illness and one participant experienced worsening of PD [24]. In the mevidalen trial (total n = 344), one participant died from an unknown cause deemed to be related to treatment and 21 participants experienced at least one serious adverse event, most pronounced with the 75 mg dose [25]. Four participants experienced cardiovascular serious adverse events including congestive heart failure, hypertension, stroke, and hypertensive encephalopathy, leading to the discontinuation of the study drug.
3.2.1. IMP-based trials
Of the eight IMP-based trials, three demonstrated significant cognitive benefits. The first was four weeks of twice-weekly infusion of young plasma, in which baseline phonemic fluency (mean 41.14 (95% CI 34.8–47.5) improved to 44.73 (95% CI 38.4–51.1), p = .002) as one of two significant differences amongst 28 exploratory outcome measures [26]. However, this was primarily a safety and tolerability study that included 15 people with PD who all received the treatment and compared cognitive and other scores before and after treatment. Therefore, there was no placebo arm and participants were not blinded to treatment. The primary outcome was safety, rather than cognitive. Latest follow-up testing was one month after the infusion, so it is not known if effects were sustained.
A double-blind randomized placebo-controlled cross-over trial examined the effects of varenicline in 34 people with PD [27]. The trial reported improved attention as measured by the Sustained Attention Test (without distractor, treatment difference 0.076, 95% CI 0.0073–0.14, p = .03). However, the certainty for this trial is downgraded due to a moderate risk of bias; and further downgraded due to imprecision of results, as we note that only one measure of cognition out of 14 cognitive tests showed improvement and this did not survive correction for multiple comparisons. Assessments were made three weeks after the treatment period and longer-term effects were not assessed, further downgrading the certainty due to imprecision. This gives a GRADE score of a Low certainty rating.
Another positive trial was a 12-week placebo-controlled double-blind RCT investigating the effect of LY3154207 (Mevidalen; a centrally-acting dopamine D1-receptor positive allosteric modulator) on cognition in 344 people with Lewy body dementia [25]. Three different doses of mevidalen were trialled. Initial trial data on ClinicalTrials.gov revealed a dose-dependent improvement in Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change scale (moderate or better improvement: 30 mg p < .05; 75 mg p < .001) but not in Alzheimer’s Disease Assessment Scale-Cognitive Subscale or MoCA. However, the number of subjects who dropped out increased with higher dose, due to adverse events and physician decisions to withdraw. Using a GRADE approach, this would be upgraded due to a dose effect, but lowered due to inconsistency of effect, as no improvement was seen for cognitive scales such as the MoCA. Furthermore, higher drop-out with increasing effect would reduce the likelihood of this being tolerated for clinical use. This would give a Moderate certainty rating.
Other IMP-based trials identified in our search did not show significant improvements in cognitive outcomes. These had investigated prasinezumab, an anti-α-synuclein monoclonal antibody [24], nabilone, a synthetic cannabinoid [28], mesenchymal stem cells [23], niacin [29] and inosine, which increases serum urate [30].
Considering IMP trials, as a group, there is only Moderate to Low certainty for potential clinical effectiveness in two Phase 2 trials, downgraded due to imprecision and inconsistency of effects.
3.2.2. Cognitive/physical intervention trials
Of the ten cognitive/physical intervention trials with available results, two showed positive or potentially positive results. In a small (n = 18) single-blinded, randomized-controlled pilot study in early-mid stage PD of gait-cognitive training (which involved playing immersive visuospatial and working memory games while exercising on a treadmill), intervention group participants showed significant improvements in MoCA (p = .007) and symbol digit modalities test (p = .01, mean and SD values were not reported) [31]. The effect held after correction for comparisons of cognitive tests, although sustained effects beyond the period of testing were not examined. Participants were also not blinded to intervention, and the control group had no intervention. Using a GRADE approach, this would start as moderate certainty due to the issue of lack of blinding in participants, and further downgrading for imprecision due to the small sample size. Further downgrading would be supported by the lack of demonstration of sustained effects. This would produce a Very Low certainty rating.
Another small single-blinded, block-randomized, parallel-intervention group study of 11 people with PD-MCI investigated goal management training adapted for MCI, compared with psychoeducation with mindfulness [32]. Both groups showed improvement in subjective and objective measures of executive function (p = .033, 95% CI 10.75–15.23), but with no controlled intervention. The lack of controlled intervention and proper blinding would place this in a Low GRADE rating, with further downgrading due to the small sample size to Very Low certainty of effectiveness.
The other eight studies yielded no detectable benefit. These included an open label study of twice weekly 90-min art sessions for ten weeks [33]; WebFitForAll, a ten-week program of computerized physical training [34]; Qigong [35]; a balance training program [36,37]; high cadence cycling [38]; a music-based physical and cognitive multitasking activity, called the Ronnie Gardiner Method [39]; an eight-week program of online cognitive training [40]; and an eight-week program of computer-based cognitive training [41]. Notably, follow-up duration was short in most of these studies (mean across these trials 9.3 weeks, SD = 4.7).
Considering cognitive and physical intervention trials there is Very Low certainty for efficacy from the trials recently undertaken, due to design of trials (lack of blinding, small numbers and limited follow-up duration).
3.2.3. Device-based trials
Of the seven device-based intervention trials with available results, four showed potential effects on cognition in PD. An rTMS trial targeting the M1 motor cortical region showed a significant improvement on the MoCA by 3.44 points (19.11 [SD = 4.3] in the active group, compared with 15.67 [SD 5.16] in the sham group, p = .04) and Mini Mental State Examination (MMSE) by 3.21 points (24.64 [SD = 2.5] in the active group, compared with 21.43 [SD = 2.44] in the sham group, p = .002) immediately post stimulation [42]. However, benefits were not detectable at the one, two, or three month follow up interval. Although participants were blinded to treatment arm, outcome assessors were not blinded. Using a GRADE approach, this would be considered moderate evidence due to lack of blinding, with further downgrading due to moderate risk of bias. Lack of benefit at 3 months could further downgrade the certainty to Very Low.
Caloric vestibular stimulation (n = 33, double-blind, placebo controlled) also led to significant improvement in MoCA scores compared with sham stimulation at 17 weeks (improvement of 3, 95% CI 1.5–4.5) [43]. However, the effect dissipated at 32 weeks and control cases had a shorter disease duration than those in the active group. This well-designed trial would therefore be downgraded due to a moderate risk of bias to Moderate certainty, with further downgrading to Low certainty considering the lack of detectable effect after follow-up.
In a small trial including 23 participants to compare four weeks of virtual reality exergaming with more standard exercise therapy, participants in both the exergaming and exercise therapy groups showed improvement in cognitive outcome measures two weeks after the end of the sessions [44]. Participants in the exergaming group showed an interaction between follow-up time and group, with greater improvement in MoCA of 0.52 points (95% CI −2.2–3.2, p =.035), over and above exercise therapy. However, as follow-up extended to only two weeks, it is unclear whether the intervention has long term effects on cognition. The trial was also not fully blinded: participants were aware of their own intervention, but not aware of other groups, and the neurologists and evaluators were blinded, but the physical therapists conducting the physical interventions were not blinded. The lack of full blinding (design) and the small sample size (imprecision) would downgrade the certainty of effectiveness to Low.
Another trial examined delivery of theta rather than gamma stimulation during subthalamic nucleus DBS (STN-DBS) [45]. The trial involved 12 participants in a double-blinded cross-over randomized controlled design and showed improved category verbal fluency (18.0 (SEM 1.47) for theta stimulation versus 15.5 (SEM 1.47) for gamma stimulation, F (2,22) = 3.69, p = .04), but not for other cognitive tests, and without any corrections for multiple comparisons. The long-term effects of stimulating at this frequency were not examined. The small sample size (imprecision) and lack of long-term follow-up (consistency) would downgrade the certainty of this intervention to Low.
Three device-based intervention trials showed no significant effects on cognition. A six-session TMS trial using theta-burst stimulation over the course of a week targeted at the left dorsolateral prefrontal cortex showed a trend toward improving executive function at one month [46]. Two STN-DBS studies also showed no significant improvement on cognition. One used short compared to conventional pulse-width stimulation in a crossover trial over four weeks with STN-DBS devices in situ and failed to improve verbal fluency [47]; the other used asymmetric STN-DBS for treatment-resistant axial motor dysfunction but failed to show improvements in cognitive measures [48].
In summary, trials of devices for improving cognitive outcomes in PD show Low certainty for likely clinical effectiveness due to lack of blinding, small sample sizes and lack of follow-up.
3.2.4. Risk of bias
Risk of bias assessment showed that 15 of the 25 trials with available results showed low risk of bias; six showed some cause for concern and two had high overall risk of bias (Supplementary Figure). Sources of bias included lack of masking (n = 7), lack of control comparisons (n = 4) and missing data for uncertain reasons (n = 1).
3.3. Trials without available results
Amongst 153 trials without available outcome data: 16 were not yet recruiting (2 IMP-based, 8 physical, 6 device-based interventions); 81 were recruiting (27 IMP-based, 8 cognitive, 20 physical, 26 device-based interventions); 20 were active (10 IMP-based, 5 physical, 4 device-based interventions, 1 other); 29 were completed but had not published results (13 IMP-based, 4 cognitive, 8 physical, 4 device-based interventions) and 7 were withdrawn or terminated. For these trials, mean number of targeted participants per trial is 89 (SD = 129) and mean follow-up duration is 32 weeks (SD = 44).
4. Discussion
In this paper, we examined recent clinical trials for cognitive impairment in PD performed between 2016 and 2021. We found that trials for IMPs, cognitive and physical training and devices have been performed. The highest level of certainty for clinical effectiveness was for trials of IMPs, which had Moderate to Low certainty of evidence. From the IMPs, mevidalen had Moderate certainty of evidence. Higher levels of certainty in IMP trials were more likely to be fully double blinded and had higher numbers of participants, giving higher precision, than trials of cognitive and physical training and devices. Overall, the lowest level of evidence was for trials of cognitive and physical training, mostly due to lack of blinding in the trial designs.
We also noted several reports where the interventional arms were not fully balanced in demographic or disease-related variables. Follow-up duration was uniformly short, with most studies reporting outcomes at less than one month. To detect any effects on long-term cognition, trials will need to be designed with longer duration, ideally over 18 months, as is the case for trials of disease modifying treatments in dementia [49–51]. Other shortcomings in trials described here included missing outcome data, and selective reporting of results, both of which are likely to overestimate the beneficial effects of an intervention. The most common concern regarding statistical analyses was lack of correction for multiple comparisons; and we also noted several trials that described trend results as positive outcomes in their publications. Interestingly, despite publication bias generally favoring positive results, most of the trials we identified showed negative findings.
A more global concern that emerges from our review is the strong bias for interventional trials to be carried out in North America and Europe. This limited geographical diversity will impact the diversity of participants likely to be recruited to clinical trials. Lack of diversity and inclusion of minority ethnic and racial subjects in trials has recently been cited as an unmet need in terms of external validity of outcomes from trials [52]. Our review underscores this urgent need, as we observed that in completed trials with available ethnicity and race data, more than 90% of the participants self-identified as non-Hispanic and White. For potential treatments to have validity across the range of people with PD, it is vital to include diverse nationalities and ethnicities in clinical trials. This is reflected in directives from groups such as Michael J. Fox Foundation and Aligning Science Across Parkinson’s that have taken a more global approach with specific aims to recruit ethnically diverse people with PD and involve researchers globally [53]. Adequate representation of sexes and genders, which may be affected differently by PD [54,55], is also required to allow the applicability of results from clinical trials across all people with PD.
As a field, how can we do better and optimize the design of clinical trials for cognitive outcomes in PD, to maximize our chances of success and bring effective treatments into clinical practice?
Clinical trials are costly and require huge effort from both researchers and participants. It is imperative that they are performed in the best possible way, so that funds and energy are directed most effectively. Disease modification remains a focus as we aim to eventually prevent and cure cognitive decline in PD. However, the substantial burden associated with this debilitating symptom also supports the study of symptomatic treatments. Translation of preclinical trials with promising results to the clinical cohorts are challenging [56]. Thus, in the process of developing disease-modifying approaches, research focusing on symptom alleviation to maintain quality of life and reduce the burden remains impactful for people with PD as well as their caregivers and relatives.
We have identified three key areas that should be considered for any interventional trial for cognitive outcomes in PD. These are: 1) optimizing the target population; 2) optimizing trial design; and 3) optimizing outcome measures. We argue that by optimizing each of these areas, as new treatments for cognitive change in PD emerge, we will have the best possible chance of identifying the interventions with the greatest impact for lives of people with PD.
4.1. Optimizing the target population
A key question is which disease stage is the best time to intervene to improve cognitive outcomes in PD. Current treatment approaches in the clinic are targeted at people with established PDD and PD-MCI (especially the use of cholinesterase inhibitors) [9]. However, across the field of neurodegeneration, there is a move to treat people earlier in the disease course, before changes become irreversible [57,58]. This is reflected in the trials identified in our review, where the majority involved people with PD without cognitive impairment, followed by trials targeting PD-MCI, PDD and a mixed of cognitive stages.
A challenge in PD is that cognitive involvement is heterogeneous in timing and presentation [3]. Therefore, when designing trials aimed at slowing cognitive impairment in PD, cohorts can be enriched for people with PD most likely to develop significant cognitive decline. This will improve trial efficiency, as benefits are more likely to be seen in an at-risk group for dementia within the trial period. In recent years, clinical algorithms have emerged to identify at-risk groups for PDD [59–61]. Key predictors for dementia in PD include older age at onset, male sex, depression, REM sleep behavior disorder, axial motor features and visual dysfunction in PD [3,62–65]. We therefore recommend that clinical trials aimed at improving cognition in PD could be enriched for high-risk groups. A related consideration is to include homogenous PD subgroups to improve accuracy of cognitive outcomes prediction. Stratifying based on cognitive profile, genetic (presence of APOE4), neuroimaging and neuropathological (presence of AD hallmarks) biomarkers could reduce misinterpretation of findings due to baseline heterogeneity [66].
To ensure applicability of findings across all people with PD, we also recommend that trials specifically target a wider global and ethnic reach [52] and put more effort into recruiting ethnoracial minorities as well as sexual and gender minorities. This could be achieved, for example, by partnering with centres in wider geographical locations, greater use of multi-site studies, and enhancing outreach and educational activities to overcome barriers in recruitment of minorities in research.
4.2. Optimizing trial design
Sample sizes in trials in our review ranged between 13 and 298, with a mean of 68.4 across trials. Whilst some of these were pilot studies of safety, rather than a Phase 3 or 4 trials, there is clearly a need for interventional trials to be properly powered to detect potential outcomes.
A further important consideration is follow-up duration, as highlighted by Guimaraes and colleagues when considering trial design for motor outcomes in PD [67]. They have shown that longer follow-up duration produces improved power to detect change for a given sample sizes. Follow-up duration in trials in our review ranged between 1 day and 104 weeks, with a mean of 16.9 weeks. In PDD, cognitive decline is approximately 1.75 points per year on the MMSE [68] and this may be even smaller per year at less severe stages. Therefore, trials examining cognitive changes using global measures such as the MMSE with durations shorter than one year, are highly unlikely to detect any significant differences between intervention arms.
Another area of concern is the placebo effect in PD. Placebos stimulate the release of striatal dopamine and can alter the activity of dopaminergic neurons in people with PD [69]. This effect is common and can be very strong in PD trials. Although less studied then the placebo effect in PD, Hawthorne effect can also occur and cause a pre-placebo effect in PD trials [70]. Participating in research or awareness of being observed can alter the behavior of the participant [71–73]. We therefore recommend careful powering of trial design based on likely effect sizes, with samples of sufficient size to detect differences; follow-up duration of at least one year and a placebo arm.
4.3. Optimizing outcome measures
The most frequently used outcome measures used in the clinical trials assessed were global measures of cognition, specifically, the MoCA [74] and also the MMSE [75]. Whilst both can be useful, they have relatively poor sensitivity to detect decline in non-demented people with PD over a 1-year interval [76,77]. The MoCA may be preferable as it includes a measure of executive function and has been shown to have greater sensitivity than the MMSE [76]. More detailed neuropsychological testing including relevant cognitive domains would be likely to show greater sensitivity to change in PD, especially in earlier stages [66]. There is still a lack of consensus about the best way to measure cognitive change in PD. However, a detailed battery of tests across the five cognitive domains (especially executive functions, memory and visuospatial domains) will provide more insight to the cognitive status [6,78]. Where multiple tests are used, appropriate statistical corrections are required.
The selection of appropriate quantitative, sensitive outcome measures in PD can often be the most challenging of all when considering trial design and is an active area of research and debate across the spectrum of Lewy body disease [79]. An area of increasing interest is the use of biomarkers, including plasma measures, to track disease progression. A range of plasma measures are emerging that are beginning to show some potential in PD, such as neurofilament light, and plasma phosphorylated tau [80]. So far, imaging markers in PD have been less successful, but newer techniques are emerging with potential, including measures of brain iron [81]. In future, plasma or imaging biomarkers may be used alongside cognitive measures to assess the effects of novel interventions on PD. These could also have the beneficial effect of improving trial efficiency, allowing smaller sample sizes or even shorter follow-up duration, if they reveal effects before cognitive improvements are seen.
5. Conclusion
In conclusion, despite a high number of clinical trials for cognitive involvement in PD, the level of certainty for clinical effectiveness is Low for almost all trials, and none have shown sustained improvement beyond three months. Trials of cognitive and physical interventions and devices are hampered by small sample sizes, and the former also by the intrinsic limitations that these are challenging to fully blind. We propose that future trials for cognitive impairment in PD should enrich for people with PD at risk of cognitive impairment, ensure sufficient follow-up duration, and include sensitive outcome measures. We also highlight the importance of greater geographical and ethnic diversity for clinical trials to ensure their validity for people with PD across the world.
Supplementary Material
Acknowledgment
The authors wish to thank all the research participants and research teams that have contributed to the discussed trials to help advance the knowledge in this field and guide future research approaches. EB is supported by the National Institute on Aging (K99AG073453); KRC is supported by the Wellcome Trust and is in the advisory board of Roche and Scion; RSW is supported by the Wellcome Trust (205167/Z/16/Z).
Financial disclosures of all authors (for the preceding 12 months)
EB: Funding from the National Institutes of Health (K99AG073453, U01NS119562).
LB: Funding from Horizon 2020 (848002), GlaxoSmithKline (MA-RxRD-0000003242–2018). Received honoraria from Zambon.
PJ: Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University.
RB: Supported by the Italian Ministry of Health under Grant Number GR-2016–02361986. Received honoraria from Bial.
KRC: In the advisory board of AbbVie, UCB, GKC, Bial, Cynapsus, Lobsor, Stada, Medtronic, Zambon, Profile, Sunovion, Roche, Theravance, Scion, Britannia, Acadia, 4D; honoraria for lectures from AbbVie, Britannia, UCB, Zambon, Novartis, Boeringer Ingelheim, Bial and reports grants for investigator initiated studies from Britannia Pharmaceuticals, AbbVie, UCB, GKC, Bial; academic grants from EU, IMI EU, Horizon 2020, Parkinson’s UK, NIHR, PDNMG, Kirby Laing Foundation, NPF, MRC, Wellcome Trust.
RSW: Supported by the Wellcome Trust (205167/Z/16/Z) and received honoraria from GE Healthcare and Britannia.
Funding sources for the study
EB receives research funding from National Institute on Aging (K99AG073453), which in part supported this work.
Footnotes
Declaration of competing interest
KRC is supported by the Wellcome Trust and is in the advisory board of Roche and Scion; RSW is supported by the Wellcome Trust (205167/Z/16/Z).
Authors’ roles
1. Research Project: A Conception, B Organization, C Execution.
2. Statistical Analysis: A Design, B Execution, C Review and Critique.
3. Manuscript Preparation: A Writing of the first draft, B Review and Critique.
EB: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
LB: 1A, 1B, 1C, 2A, 2C, 3A, 3B
BT: 1C, 2A, 2B, 2C, 3A, 3B
RG: 1C, 3B
PJ: 1A, 1C, 2C, 3B
RB: 1A, 1C, 2C, 3B
MG: 1C, 3B
TP: 1C, 3B
CLO: 1A, 1C, 2C, 3A, 3B
KRC: 1A, 3B
RSW: 1A, 1C, 2A, 2C, 3A, 3B
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parkreldis.2023.105385.
References
- [1].Bloem BR, Okun MS, Klein C, Parkinson’s disease , Lancet 397 (2021) 2284–2303, 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- [2].Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P, Risk of dementia in Parkinson’s disease: a community-based, prospective study, Neurology 56 (2001) 730–736, 10.1212/WNL.56.6.730. [DOI] [PubMed] [Google Scholar]
- [3].Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, Barker RA, The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort, J. Neurol. Neurosurg. Psychiatry 84 (2013) 1258–1264, 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- [4].Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, Ballard C, Cognitive decline in Parkinson disease, Nat. Rev. Neurol. 13 (2017) 217, 10.1038/NRNEUROL.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Leroi I, McDonald K, Pantula H, Harbishettar V, Cognitive impairment in Parkinson disease, J. Geriatr. Psychiatr. Neurol. 25 (2012) 208–214, 10.1177/0891988712464823. [DOI] [PubMed] [Google Scholar]
- [6].Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M, Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement Disorder Society Task Force guidelines, Mov. Disord. 27 (2012) 349–356, 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pedersen KF, Larsen JP, Tysnes O-B, Alves G, Natural course of mild cognitive impairment in Parkinson disease, Neurology 88 (2017) 767–774, 10.1212/WNL.0000000000003634. [DOI] [PubMed] [Google Scholar]
- [8].Burn D, Emre M, McKeith I, De Deyn PP, Aarsland D, Hsu C, Lane R, Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease, Mov. Disord. 21 (2006) 1899, 10.1002/mds.21077.-1907. [DOI] [PubMed] [Google Scholar]
- [9].Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, Durif F, Kulisevsky J, van Laar T, Lees A, Poewe W, Robillard A, Rosa MM, Wolters E, Quarg P, Tekin S, Lane R, Rivastigmine for dementia associated with Parkinson’s disease, N. Engl. J. Med. 351 (2004) 2509–2518, 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- [10].Stinton C, McKeith I, Taylor JP, Lafortune L, Mioshi E, Mak E, Cambridge V, Mason J, Thomas A, O’Brien JT, Pharmacological management of Lewy body dementia: a systematic review and meta-analysis, Am. J. Psychiatr. 172 (2015) 731–742, 10.1176/APPI.AJP.2015.14121582. [DOI] [PubMed] [Google Scholar]
- [11].Wang H-F, Yu J-T, Tang S-W, Jiang T, Tan C-C, Meng X-F, Wang C, Tan MS, Tan L, Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis, J. Neurol. Neurosurg. Psychiatry 86 (2015) 135–143, 10.1136/jnnp-2014-307659. [DOI] [PubMed] [Google Scholar]
- [12].Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, Leroi I, Pozo-Rodriguez F, Minthon L, Londos E, Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial, Lancet Neurol. 8 (2009) 613–618, 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- [13].Emre M, Tsolaki M, Bonuccelli U, Destée A, Tolosa E, Kutzelnigg A, Ceballos-Baumann A, Zdravkovic S, Bladström A, Jones R, Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial, Lancet Neurol. 9 (2010) 969–977, 10.1016/S1474-4422(10)70194-0. [DOI] [PubMed] [Google Scholar]
- [14].Wesnes KA, Aarsland D, Ballard C, Londos E, Memantine improves attention and episodic memory in Parkinson’s disease dementia and dementia with Lewy bodies, Int. J. Geriatr. Psychiatr. 30 (2015) 46–54, 10.1002/gps.4109. [DOI] [PubMed] [Google Scholar]
- [15].Weintraub D, Mavandadi S, Mamikonyan E, Siderowf AD, Duda JE, Hurtig HI, Colcher A, Horn SS, Nazem S, Ten Have TR, Stern MB, Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease, Neurology 75 (2010) 448–455, 10.1212/WNL.0b013e3181ebdd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].David MCB, Del Giovane M, Liu KY, Gostick B, Rowe JB, Oboh I, Howard R, Malhotra PA, Cognitive and neuropsychiatric effects of noradrenergic treatment in Alzheimer’s disease: systematic review and meta-analysis, J. Neurol. Neurosurg. Psychiatry (2022), 10.1136/jnnp-2022-329136jnnp-2022-329136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Marsh L, Biglan K, Gerstenhaber M, Williams JR, Atomoxetine for the treatment of executive dysfunction in Parkinson’s disease: a pilot open-label study, Mov. Disord. 24 (2009) 277–282, 10.1002/mds.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hinson VK, Delambo A, Elm J, Turner T, A randomized clinical trial of atomoxetine for mild cognitive impairment in Parkinson’s disease, Mov. Disord. Clin. Pract. 4 (2017) 416–423, 10.1002/mdc3.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Goldman JG, Williams-Gray C, Barker RA, Duda JE, Galvin JE, The spectrum of cognitive impairment in Lewy body diseases, Mov. Disord. 29 (2014) 608–621, 10.1002/mds.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jellinger KA, Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies, J. Neural. Transm. 125 (2018) 615–650, 10.1007/s00702-017-1821-9. [DOI] [PubMed] [Google Scholar]
- [21].Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT, RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ (2019) l4898, 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- [22].Kavanagh BP, The GRADE system for rating clinical guidelines, PLoS Med. 6 (2009), e1000094, 10.1371/JOURNAL.PMED.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schiess M, Suescun J, Doursout M, Adams C, Green C, Saltarrelli JG, Savitz S, Ellmore TM, Allogeneic bone marrow–derived mesenchymal stem cell safety in idiopathic Parkinson’s disease, Mov. Disord. 36 (2021) 1825–1834, 10.1002/mds.28582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pagano G, Taylor KI, Anzures-Cabrera J, Marchesi M, Simuni T, Marek K, Postuma RB, Pavese N, Stocchi F, Azulay J-P, Mollenhauer B, López-Manzanares L, Russell DS, Boyd JT, Nicholas AP, Luquin MR, Hauser RA, Gasser T, Poewe W, Ricci B, Boulay A, Vogt A, Boess FG, Dukart J, D’Urso G, Finch R, Zanigni S, Monnet A, Pross N, Hahn A, Svoboda H, Britschgi M, Lipsmeier F, Volkova-Volkmar E, Lindemann M, Dziadek S, Holiga Š, Rukina D, Kustermann T, Kerchner GA, Fontoura P, Umbricht D, Doody R, Nikolcheva T, Bonni A, Trial of prasinezumab in early-stage Parkinson’s disease, N. Engl. J. Med. 387 (2022) 421–432, 10.1056/NEJMoa2202867. [DOI] [PubMed] [Google Scholar]
- [25].Biglan K, Munsie L, Svensson KA, Ardayfio P, Pugh M, Sims J, Brys M, Safety and efficacy of mevidalen in Lewy body dementia: a Phase 2, randomized, placebo-controlled trial, Mov. Disord. 37 (2022) 513–524, 10.1002/MDS.28879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Parker JE, Martinez A, Deutsch GK, Prabhakar V, Lising M, Kapphahn KI, Anidi CM, Neuville R, Coburn M, Shah N, Bronte-Stewart HM, Safety of plasma infusions in Parkinson’s disease, Mov. Disord. 35 (2020) 1905, 10.1002/mds.28198.-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Albin RL, Müller MLTM, Bohnen NI, Spino C, Sarter M, Koeppe RA, Szpara A, Kim K, Lustig C, Dauer WT, α4β2 * nicotinic cholinergic receptor target engagement in Parkinson disease gait–balance disorders, Ann. Neurol. 90 (2021) 130–142, 10.1002/ana.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peball M, Krismer F, Knaus H, Djamshidian A, Werkmann M, Carbone F, Ellmerer P, Heim B, Marini K, Valent D, Goebel G, Ulmer H, Stockner H, Wenning GK, Stolz R, Krejcy K, Poewe W, Seppi K, Non-motor symptoms in Parkinson’s disease are reduced by nabilone, Ann. Neurol. 88 (2020) 712–722, 10.1002/ana.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wakade C, Chong R, Seamon M, Purohit S, Giri B, Morgan JC, Low-dose niacin supplementation improves motor function in US veterans with Parkinson’s disease: a single-center, randomized, placebo-controlled trial, Biomedicines 9 (2021) 1881, 10.3390/biomedicines9121881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mestre TA, Macklin EA, Ascherio A, Ferreira JJ, Lang AE, Schwarzschild MA, Expectations of benefit in a trial of a candidate disease-modifying treatment for Parkinson disease, Mov. Disord. 36 (2021) 1964–1967, 10.1002/mds.28630. [DOI] [PubMed] [Google Scholar]
- [31].Lau J, Regis C, Burke C, Kaleda M, McKenna R, Muratori LM, Immersive technology for cognitive-motor training in Parkinson’s disease, Front. Hum. Neurosci. 16 (2022), 10.3389/fnhum.2022.863930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Giguère-Rancourt A, Plourde M, Racine E, Couture M, Langlois M, Dupré N, Simard M, Goal management training and psychoeducation/mindfulness for treatment of executive dysfunction in Parkinson’s disease: a feasibility pilot trial, PLoS One 17 (2022), e0263108, 10.1371/journal.pone.0263108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cucca A, Di Rocco A, Acosta I, Beheshti M, Berberian M, Bertisch HC, Droby A, Ettinger T, Hudson TE, Inglese M, Jung YJ, Mania DF, Quartarone A, Rizzo J-R, Sharma K, Feigin A, Biagioni MC, Ghilardi MF, Art therapy for Parkinson’s disease, Park. Relat. Disord. 84 (2021) 148–154, 10.1016/j.parkreldis.2021.01.013. [DOI] [PubMed] [Google Scholar]
- [34].Xefteris VR, Styliadis C, Anagnostopoulou A, Kartsidis P, Paraskevopoulos E, Klados M, Zilidou V, Karagianni M, Bamidis PD, Computerized physical exercise improves the functional architecture of the brain in patients with Parkinson’s Disease: a network science resting-state EEG study, medRxiv (2020), 10.1101/2020.10.21.20209502,2020.10.21.20209502. [DOI] [Google Scholar]
- [35].Moon S, Sarmento CVM, Steinbacher M, Smirnova IV, Colgrove Y, Lai S-M, Lyons KE, Liu W, Can Qigong improve non-motor symptoms in people with Parkinson’s disease - a pilot randomized controlled trial? Compl. Ther. Clin. Pract. 39 (2020), 101169 10.1016/j.ctcp.2020.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Joseph C, Leavy B, Mattsson S, Falk L, Franzén E, Implementation of the HiBalance training program for Parkinson’s disease in clinical settings: a feasibility study, Brain Behav. 8 (2018), e01021, 10.1002/brb3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].von Rosen P, Hagströmer M, Franzén E, Leavy B, Physical activity profiles in Parkinson’s disease, BMC Neurol. 21 (2021) 71, 10.1186/s12883-021-02101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McKee KE, Johnson RK, Chan J, Wills A, Implementation of high-cadence cycling for Parkinson’s disease in the community setting: a pragmatic feasibility study, Brain Behav. 11 (2021), 10.1002/brb3.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pohl P, Wressle E, Lundin F, Enthoven P, Dizdar N, Group-based music intervention in Parkinson’s disease – findings from a mixed-methods study, Clin. Rehabil. 34 (2020) 533–544, 10.1177/0269215520907669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].van Balkom TD, Berendse HW, van der Werf YD, Twisk JWR, Peeters CFW, Hoogendoorn AW, Hagen RH, Berk T, van den Heuvel OA, Vriend C, Effect of eight-week online cognitive training in Parkinson’s disease: a double-blind, randomized, controlled trial, Park. Relat. Disord. 96 (2022) 80–87, 10.1016/j.parkreldis.2022.02.018. [DOI] [PubMed] [Google Scholar]
- [41].Svaerke K, Faerk AK, Riis A, Stiegnitz von Ehrenfels SEM, Mogensen J, Lokkegaard A, Effects of computer-based cognitive rehabilitation on attention, executive functions, and quality of life in patients with Parkinson’s disease: a randomized, controlled, single-blinded pilot study, Dement. Geriatr. Cogn. Disord 50 (2021) 519–528, 10.1159/000520591. [DOI] [PubMed] [Google Scholar]
- [42].Khedr EM, Mohamed KO, Ali AM, Hasan AM, The effect of repetitive transcranial magnetic stimulation on cognitive impairment in Parkinson’s disease with dementia: pilot study, Restor. Neurol. Neurosci. 38 (2020) 55–66, 10.3233/RNN-190956. [DOI] [PubMed] [Google Scholar]
- [43].Wilkinson D, Podlewska A, Banducci SE, Pellat-Higgins T, Slade M, Bodani M, Sakel M, Smith L, LeWitt P, Ade KK, Caloric vestibular stimulation for the management of motor and non-motor symptoms in Parkinson’s disease, Park. Relat. Disord. 65 (2019) 261–266, 10.1016/j.parkreldis.2019.05.031. [DOI] [PubMed] [Google Scholar]
- [44].Hajebrahimi F, Velioglu HA, Bayraktaroglu Z, Helvaci Yilmaz N, Hanoglu L, Clinical evaluation and resting state fMRI analysis of virtual reality based training in Parkinson’s disease through a randomized controlled trial, Sci. Rep. 12 (2022) 8024, 10.1038/s41598-022-12061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lam J, Lee J, Williams M, Cohn M, Wilson M, Mark C, Esnaashari N, Petkus A, Hui J, Feigenbaum D, Liker M, Liu CY, Lee B, Lee DJ, Cognitive effects of theta frequency bilateral subthalamic nucleus stimulation in Parkinson’s disease: a pilot study, Brain Stimul. 14 (2021) 230–240, 10.1016/j.brs.2020.12.014. [DOI] [PubMed] [Google Scholar]
- [46].Lang S, Gan LS, Yoon EJ, Hanganu A, Kibreab M, Cheetham J, Hammer T, Kathol I, Sarna J, Martino D, Monchi O, Theta-burst stimulation for cognitive enhancement in Parkinson’s disease with mild cognitive impairment: a randomized, double-blind, sham-controlled trial, Front. Neurol. 11 (2020), 10.3389/fneur.2020.584374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dayal V, Grover T, Tripoliti E, Milabo C, Salazar M, Candelario-McKeown J, Athauda D, Zrinzo L, Akram H, Hariz M, Limousin P, Foltynie T, Short versus conventional pulse-width deep brain stimulation in Parkinson’s disease: a randomized crossover comparison, Mov. Disord. 35 (2020) 101–108, 10.1002/mds.27863. [DOI] [PubMed] [Google Scholar]
- [48].Lizárraga KJ, Gnanamanogaran B, Al-Ozzi TM, Cohn M, Tomlinson G, Boutet A, Elias GJB, Germann J, Soh D, Kalia SK, Hodaie M, Munhoz RP, Marras C, Hutchison WD, Lozano AM, Lang AE, Fasano A, Lateralized subthalamic stimulation for axial dysfunction in Parkinson’s disease: a randomized trial, Mov. Disord. 37 (2022) 1079–1087, 10.1002/mds.28953. [DOI] [PubMed] [Google Scholar]
- [49].Cummings J, Ritter A, Zhong K, Clinical trials for disease-modifying therapies in Alzheimer’s disease: a primer, lessons learned, and a blueprint for the future, J. Alzheim. Dis. 64 (2018), 10.3233/JAD-179901.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T, Lecanemab in early Alzheimer’s disease, N. Engl. J. Med. 388 (2023), 10.1056/NEJMoa2212948,9–21. [DOI] [PubMed] [Google Scholar]
- [51].Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM, Donanemab in early Alzheimer’s disease, N. Engl. J. Med. 384 (2021) 1691–1704, 10.1056/NEJMoa2100708. [DOI] [PubMed] [Google Scholar]
- [52].Lau YH, Podlewska A, Ocloo J, Gupta A, Gonde C, Bloem BR, Chaudhuri KR, Does ethnicity influence recruitment into clinical trials of Parkinson’s disease? J. Parkinsons Dis. 12 (2022) 975–981, 10.3233/JPD-213113. [DOI] [PubMed] [Google Scholar]
- [53].Riley EA, Schekman R, Open science takes on Parkinson’s disease, Elife 10 (2021), 10.7554/eLife.66546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Subramanian I, Mathur S, Oosterbaan A, Flanagan R, Keener AM, Moro E, Unmet needs of women living with Parkinson’s disease: gaps and controversies, Mov. Disord. 37 (2022) 444–455, 10.1002/mds.28921. [DOI] [PubMed] [Google Scholar]
- [55].Roy Lin CY, Rosendale N, Deeb W, Expanding sexual and gender minority research in movement disorders: more than awareness and acceptance, Park. Relat. Disord. 87 (2021) 162–165, 10.1016/J.PARKRELDIS.2021.05.019. [DOI] [PubMed] [Google Scholar]
- [56].Seyhan AA, Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles, Transl. Med. Commun. 4 (2019) 18, 10.1186/s41231-019-0050-7. [DOI] [Google Scholar]
- [57].Robinson L, Tang E, Taylor J-P, Dementia: timely diagnosis and early intervention, BMJ 350 (2015), 10.1136/bmj.h3029h3029-h3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fan D-Y, Wang Y-J, Early intervention in Alzheimer’s disease: how early is early enough? Neurosci. Bull. 36 (2020) 195–197, 10.1007/s12264-019-00429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM, Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: a cohort study, Lancet Neurol. 16 (2017) 66–75, 10.1016/S1474-4422(16)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liu G, Locascio JJ, Corvol J-C, Boot B, Liao Z, Page K, Franco D, Burke K, Jansen IE, Trisini-Lipsanopoulos A, Winder-Rhodes S, Tanner CM, Lang AE, Eberly S, Elbaz A, Brice A, Mangone G, Ravina B, Shoulson I, Cormier-Dequaire F, Heutink P, van Hilten JJ, Barker RA, Williams-Gray CH, Marinus J, Scherzer CR, Scherzer CR, Hyman BT, Ivinson AJ, Trisini-Lipsanopoulos A, Franco D, Burke K, Sudarsky LR, Hayes MT, Umeh CC, Sperling R, Growdon JH, Schwarzschild MA, Hung AY, Flaherty AW, Blacker D, Wills A-M, Sohur US, Mejia NI, Viswanathan A, Gomperts SN, Khurana V, Albers MW, Alora-Palli M, McGinnis S, Sharma N, Dickerson B, Frosch M, Gomez-Isla T, Greenberg S, Gusella J, Hedden T, Hedley-Whyte ET, Koenig A, Marquis-Sayagues M, Marshall G, Okereke O, Stemmer-Rachaminov A, Kloppenburg J, Schlossmacher MG, Growdon JH, Selkoe DJ, Sperling R, Yi T, Locascio JJ, Li H, Stalberg G, Liao Z, Barker R, Foltynie T, Williams-Gray C, Robbins T, Brayne C, Mason S, Winder-Rhodes S, Barker R, Williams-Gray C, Breen DP, Cummins G, Evans J, Winder-Rhodes S, van Hilten JJ, Marinus J, Corvol J-C, Brice A, Corvol J-C, Elbaz A, Mallet A, Vidailhet M, Bonnet A-M, Bonnet C, Corvol J-C, Elbaz A, Grabli D, Hartmann A, Klebe S, Lacomblez L, Mangone G, Vidailhet M, Bourdain F, Brandel J-P, Derkinderen P, Durif F, Mesnage V, Pico F, Rascol O, Brefel-Courbon C, Ory-Magne F, Forlani S, Lesage S, Mangone G, Tahiri K, Albin R, Alcalay R, Ascherio A, Bowman D, Chen-Plotkin A, Dawson T, Dewey R, German D, Saunders-Pullman R, Scherzer C, Vaillancourt D, Petyuk V, West A, Zhang J, Prediction of cognition in Parkinson’s disease with a clinical–genetic score: a longitudinal analysis of nine cohorts, Lancet Neurol. 16 (2017) 620–629, 10.1016/S1474-4422(17)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Velseboer DC, de Bie RMA, Wieske L, Evans JR, Mason SL, Foltynie T, Schmand B, de Haan RJ, Post B, Barker RA, Williams-Gray CH, Development and external validation of a prognostic model in newly diagnosed Parkinson disease, Neurology 86 (2016) 986–993, 10.1212/WNL.0000000000002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Anang JBM, Gagnon J-F, Bertrand J-A, Romenets SR, Latreille V, Panisset M, Montplaisir J, Postuma RB, Predictors of dementia in Parkinson disease: a prospective cohort study, Neurology 83 (2014) 1253–1260, 10.1212/WNL.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zarkali A, McColgan P, Leyland L, Lees AJ, Weil RS, Visual dysfunction predicts cognitive impairment and white matter degeneration in Parkinson’s disease, Mov. Disord. 36 (2021) 1191–1202, 10.1002/mds.28477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hamedani AG, Abraham DS, Maguire MG, Willis AW, Visual impairment is more common in Parkinson’s disease and is a risk factor for poor Health outcomes, Mov. Disord. 35 (2020) 1542–1549, 10.1002/mds.28182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Han G, Han J, Han K, Youn J, Chung T, Lim DH, Visual acuity and development of Parkinson’s disease: a nationwide cohort study, Mov. Disord. 35 (2020) 1532–1541, 10.1002/mds.28184. [DOI] [PubMed] [Google Scholar]
- [66].Biundo R, Weis L, Fiorenzato E, Antonini A, Cognitive rehabilitation in Parkinson’s disease: is it feasible? Arch. Clin. Neuropsychol. 32 (2017) 840–860, 10.1093/arclin/acx092. [DOI] [PubMed] [Google Scholar]
- [67].Guimaraes P, Kieburtz K, Goetz CG, Elm JJ, Palesch YY, Huang P, Ravina B, Tanner CM, Tilley BC, Non-linearity of Parkinson’s disease progression: implications for sample size calculations in clinical trials, Clin. Trials 2 (2005) 509–518, 10.1191/1740774505cn125oa. [DOI] [PubMed] [Google Scholar]
- [68].Kramberger MG, Auestad B, Garcia-Ptacek S, Abdelnour C, Olmo JG, Walker Z, Lemstra AW, Londos E, Blanc F, Bonanni L, McKeith I, Winblad B, de Jong FJ, Nobili F, Stefanova E, Petrova M, Falup-Pecurariu C, Rektorova I, Bostantjopoulou S, Biundo R, Weintraub D, Aarsland D, Long-term cognitive decline in dementia with Lewy bodies in a large multicenter, international cohort, J. Alzheim. Dis. 57 (2017) 787–795, 10.3233/JAD-161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lidstone SC, Great expectations: the placebo effect in Parkinson’s disease, Placebo (2014) 139–147, 10.1007/978-3-662-44519-8_8. [DOI] [PubMed] [Google Scholar]
- [70].Morberg BM, Malling AS, Jensen BR, Gredal O, Wermuth L, Bech P, The Hawthorne effect as a pre-placebo expectation in Parkinsons disease patients participating in a randomized placebo-controlled clinical study, Nord. J. Psychiatr. 72 (2018) 442–446, 10.1080/08039488.2018.1468480. [DOI] [PubMed] [Google Scholar]
- [71].Benedetti F, Carlino E, Piedimonte A, Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects, Lancet Neurol. 15 (2016) 736–747, 10.1016/S1474-4422(16)00066-1. [DOI] [PubMed] [Google Scholar]
- [72].Robles-García V, Corral-Bergantiños Y, Espinosa N, Jácome MA, García-Sancho C, Cudeiro J, Arias P, Spatiotemporal gait patterns during overt and covert evaluation in patients with Parkinson’s disease and healthy subjects: is there a Hawthorne effect? J. Appl. Biomech. 31 (2015) 189–194, 10.1123/jab.2013-0319. [DOI] [PubMed] [Google Scholar]
- [73].Choi W, Jung J, Grantcharov T, Impact of Hawthorne effect on healthcare professionals: a systematic review, Univ. Toronto Med. J. 96 (2019) 21–32. [Google Scholar]
- [74].Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment, J. Am. Geriatr. Soc. 53 (2005) 695–699, 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- [75].Folstein MF, Folstein SE, McHugh PR, Mini-mental state J Psychiatr. Res. 12 (1975) 189–198, 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [76].Biundo R, Weis L, Bostantjopoulou S, Stefanova E, Falup-Pecurariu C, Kramberger MG, Geurtsen GJ, Antonini A, Weintraub D, Aarsland D, MMSE and MoCA in Parkinson’s disease and dementia with Lewy bodies: a multicenter 1-year follow-up study, J. Neural. Transm. 123 (2016) 431–438, 10.1007/s00702-016-1517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Faust-Socher A, Duff-Canning S, Grabovsky A, Armstrong MJ, Rothberg B, Eslinger PJ, Meaney CA, Schneider RB, Tang-Wai DF, Fox SH, Zadikoff C, Kennedy N, Chou KL, Persad C, Litvan I, Mast BT, Gerstenecker AT, Weintraub S, Reginold W, Marras C, Responsiveness to change of the montreal cognitive assessment, mini-mental state examination, and SCOPA-cog in non-demented patients with Parkinson’s disease, Dement. Geriatr. Cogn. Disord 47 (2019) 187–197, 10.1159/000496454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hoogland J, van Wanrooij LL, Boel JA, Goldman JG, Stebbins GT, Dalrymple-Alford JC, Marras C, Adler CH, Junque C, Pedersen KF, Mollenhauer B, Zabetian CP, Eslinger PJ, Lewis SJG, Wu RM, Klein M, Rodriguez-Oroz MC, Cammisuli DM, Barone P, Biundo R, de Bie RMA, Schmand BA, Tröster AI, Burn DJ, Litvan I, Filoteo JV, Geurtsen GJ, Weintraub D, Detecting mild cognitive deficits in Parkinson’s disease: comparison of neuropsychological tests, Mov. Disord. 33 (2018) 1750–1759, 10.1002/mds.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rodriguez-Porcel F, Wyman-Chick KA, Abdelnour Ruiz C, Toledo JB, Ferreira D, Urwyler P, Weil RS, Kane J, Pilotto A, Rongve A, Boeve B, Taylor J-P, McKeith I, Aarsland D, Lewis SJG, Clinical outcome measures in dementia with Lewy bodies trials: critique and recommendations, Transl. Neurodegener. 11 (2022) 24, 10.1186/s40035-022-00299-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Simrén J, Ashton NJ, Blennow K, Zetterberg H, An update on fluid biomarkers for neurodegenerative diseases: recent success and challenges ahead, Curr. Opin. Neurobiol. 61 (2020) 29–39, 10.1016/j.conb.2019.11.019. [DOI] [PubMed] [Google Scholar]
- [81].Lanskey JH, McColgan P, Schrag AE, Acosta-Cabronero J, Rees G, Morris HR, Weil RS, Can neuroimaging predict dementia in Parkinson’s disease? Brain 141 (2018) 2545–2560, 10.1093/brain/awy211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


