Abstract
To coordinate, adapt and respond to biological signals, cells convey specific messages to other cells. An important aspect of cell–cell communication involves secretion of molecules into the extracellular space. How these molecules are selected for secretion has been a fundamental question in the membrane trafficking field for decades. Recently, extracellular vesicles (EVs) have been recognized as key players in intercellular communication, carrying not only membrane proteins and lipids but also RNAs, cytosolic proteins, and other signaling molecules to recipient cells. To communicate the right message, it is essential to sort cargoes into EVs in a regulated and context-specific manner. In recent years, a deluge of lipidomic, proteomic, and RNA sequencing studies have revealed that EV cargo composition differs depending upon the donor cell type, metabolic cues, and disease states. In many cases, analyses of these distinct cargo “fingerprints” have uncovered mechanistic linkages between the activation of specific molecular pathways and cargo sorting. In addition, cell biological studies are beginning to reveal novel biogenesis mechanisms regulated by cellular context. Here, we review context-specific mechanisms of EV cargo sorting, focusing on how cell signaling and cell state influence which cellular components are ultimately targeted to EVs.
II. Introduction
Once regarded as an essential waste disposal pathway1 or a process that only takes place under specialized circumstances2,3, secretion of extracellular vesicles (EVs) is now recognized as a bona fide mechanism to exchange molecules and convey signals between cells. EVs can circulate through the blood to affect distant tissues or remain near the site of secretion to promote autocrine or paracrine signaling4. Two major subtypes of EVs – exosomes and ectosomes – have been categorized based on their origin in the cell5. Exosomes are small (~50–150 nm diameter) EVs that form when the endosomal membrane buds inwardly to create intralumenal vesicles (ILVs). These ILVs are secreted as exosomes when the endosome, now called a multivesicular body (MVB), fuses with the plasma membrane (Fig. 1a,5–7). On the other hand, ectosomes arise from outward protrusions of plasma membrane that are excised and shed into the extracellular space (Fig. 1b,5–7). Ectosomes range in size from less than 100 nanometers to several micrometers in diameter and include a variety of vesicle types, including large oncosomes. Recently, additional EV subtypes have been described, such as migrasomes (Fig. 1c) that fit less well into these two main categories and are still being characterized8. Apoptotic bodies that form when cells fragment during programmed cell death are also categorized as EVs, in some cases forming from protrusions called apoptopodia (Fig. 1d)9. While EV subtypes are defined by biogenesis mechanism, they can be difficult to distinguish from each other in typical biochemical preparations, leading to recent proposed nomenclature standards in which purified EVs are described as small and large EVs (Box 1)10. In this review, we will use these nomenclature standards, except in cases where the biogenesis mechanism has been defined by other methods, such as imaging.
Figure 1: A road map of EV biogenesis.

a | After endocytosis, early endosomes undergo maturation into multivesicular bodies (MVBs) containing intraluminal vesicles formed through inward membrane budding into the endosome lumen. Intraluminal vesicles can be directed towards lysosomal degradation by MVB-lysosome fusion or towards secretion into the extracellular space through MVB-plasma membrane fusion. The immunoelectron micrograph (i) shows gold-labelled transferrin receptor on the surface of exosomes2. b | A variety of biogenesis pathways give rise to ectosomes, but in all cases stem from surface blebs or protrusions that are cut off from the plasma membrane. Bladder cancer cells release large oncosomes (ii), shown by staining with cholera toxin B and visualization by confocal microscopy323. Smaller “classical” ectosomes immunostained for annexin A1 are visible by structured illumination microscopy budding off of the plasma membrane of colorectal cancer cells (iii)178. Much smaller ectosomes can also bud off of the plasma membrane through a pathway depending on arrestin domain-containing protein 1 (ARRDC) (iv). These ARMMs (ARRDC1-mediated microvesicles), are detected on the surface of cells expressing ARRDC1-mCherry by immunoelectron microscopy with gold-labelled anti-mCherry93. Small ectosomes may also bud off of the tips and sides of surface protrusions (v). Inset (v) exemplifies ectosome budding off the tips of microvilli in the brush border of an ATP-treated rat small intestine324. c | Other types of extracellular vesicles include migrasomes, EVs containing internal vesicles that arise from retraction fibers (transmission electron micrograph (vi))8, and vesicles originating from amphisomes that are formed when when the outer autophagosome membrane fuses with a late endosome. d | Apoptotic bodies arise from orderly fragmentation of apoptotic cells. Caspase-3 substrates include key players in apoptotic body formation, including ROCK1 and other regulators such as Pannexin-1 and Plexin-B29,172,173. ROCK1 activates actomyosin contractility, resulting in blebbing from the plasma membrane itself or from the tips of surface protrusions termed apoptopodia9,173. Apoptopodia can undergo beading along their length, visualized by DIC microscopy of apoptotic THP-1 monocytes in (vii)9.
Box 1: EV nomenclature describing EV size and origin.
Historically, EVs have been described by a variety of names, including exosomes, microvesicles, microparticles, and ectosomes. Most typically, the term exosomes has been used to describe small EVs that are formed within MVBs in cells, whereas microvesicles and ectosomes have referred to larger EVs that bud from the cell surface. However, many early papers used these terms interchangeably, making it difficult to identify what type of EV was being studied without digging deeply into the methods. Moreover, EVs are often studied after isolation from biofluids or cell culture conditioned media, which contain mixtures of EVs from a variety of membrane or organelle sources. Finally, it has become clear that not all small EVs are exosomes, since EVs less than 150 nm in diameter have been shown to both bud from the plasma membrane and be formed within endosomes as ILVs. Due to these issues, the EV field has recently sought to define vesicles based on physical characteristics rather than the mode of biogenesis10, often to the confusion of many readers. Generally, material obtained by lower-speed ultracentrifugation (10,000–16,000xg) contains “large EVs,” while material obtained by high-speed ultracentrifugation (≥100,000xg) contains “small EVs”. However, this method cannot be used to separate ectosomes from exosomes because some ectosome biogenesis pathways produce small EVs of equivalent size and density to exosomes93.
Although it is experimentally practical to categorize EVs by size, it is important to bear in mind that EV subtypes (e.g., exosomes, ectosomes, apoptotic bodies) can also be useful terms when there is evidence to support their use. These biological subtypes are defined by where they form in the cell and further classified by their biogenesis mechanism (e.g., ectosome blebbing vs. virus-like budding; and ESCRT-independent exosomes vs. ESCRT-dependent exosomes). EV subtypes can share some similarities with one another – for instance, ESCRT subunits mediate both exosome budding in endosomes and ARMM-type ectosome budding on the plasma membrane21,27,93. This biological terminology is necessary for a field-wide discussion of EV biogenesis and cargo sorting from a molecular, mechanistic and cell biological point of view. Attributing changes in EV composition or function to specific EV subtypes will help identify key regulatory pathways that control cellular communication. As an analogy, if cell biologists decided to define cell types (neurons, immune cells, red blood cells) based solely on their size, it would be difficult to discern anything about the logic of transcriptional programs and differentiation underlying cellular heterogeneity. Thus, there is a need for the EV field to move beyond a purely size-based nomenclature.
Microscopy and genetic perturbation studies have already brought progress in defining EV subtypes based on the subcellular location from which they originate17,87,88,327. New technologies for single-EV molecular analysis (e.g., EV flow cytometry or super-resolution microscopy328,329) may facilitate the identification of EV subtypes within complex mixtures of EVs, with the caveat that there is no current consensus on how molecular markers currently correspond to EV membrane of origin. For example, CD63 is typically a good exosome marker in most (but not all) cell types87,89,327, but it is probably not present in all exosomes; therefore, its absence is not evidence for plasma membrane origin of a small EV. Thus, subtypes would need to be distinguishable based on multiple characteristics – perhaps by multiple surface markers in combination with size – much as how single-cell technologies are used to distinguish cell types. If successfully employed, single-EV methods in combination with cell biology investigations could facilitate identification of EV subtypes within purified EV preparations. For instance, one could use microscopy to assess how a genetic perturbation affects EV cargo sorting or biogenesis at the relevant subcellular compartments, and then use single-vesicle techniques to identify the affected EV subpopulations out of a complex mixture and characterize how their cargo compositions change.
The first EV cargo to be discovered was the transferrin receptor, which reticulocytes[G] expel on exosomes as they mature into red blood cells2,3. Since then, the list of EV cargoes has expanded to include a complex assortment of proteins, RNAs, and signaling lipids that differ across cell types, cell signaling states, and disease conditions. Autocrine responses to EVs modulate many cellular processes, such as cell migration and serum- and anchorage-independent growth11–15. In other cases, EVs secreted by a “donor” cell induce phenotypic changes in a distinct type of “recipient” cell. To effect such changes, EV cargoes on the external face of EVs may be presented to recipient cell surface receptors as ligands, and induce cytoplasmic signaling events either at the plasma membrane or following endocytosis16–18. By contrast, internal EV cargoes require delivery to the cytoplasm through fusion with cellular membranes, including the plasma membrane and endosomal membranes. Endocytosed EVs may also undergo degradation in the lysosomes without delivering cargoes to the cytoplasm.
Central to the EV communication hypothesis is the ability of cells to control EV cargo selection and thereby convey specific messages in a regulated and selective manner. In this review, we discuss the existing evidence for different pathways of EV biogenesis, with a focus on cargo sorting. We will also review how cell signaling, metabolic and disease state regulate EV cargo sorting, in many cases through post-translational modification of the cargo itself.
III. A cargo-centered view of EV biogenesis
In conventional membrane trafficking[G] pathways, cargoes destined for a given organelle recruit the machinery required for their own sorting and trafficking19. As a general outline, “trafficking machineries” sequester cargoes onto patches of membrane, remodel the surrounding membrane into the shape of a vesicle, and ultimately sever the vesicle from the membrane source6. EV cargoes appear to follow the same basic itinerary, binding trafficking effectors (Table I) that enrich cargoes in endosomal and plasma membrane patches and recruiting membrane bending and scission machineries to generate an EV. One outcome of having cargo-specific biogenesis pathways could be to produce multiple subpopulations of EVs, each of which is regulated independently. Cargo-initiated biogenesis is thus one potential mechanism to explain the increasingly appreciated heterogeneity of EVs (Box 2).
Table I.
Effectors of EV cargo loading*
| Sorting Effector(s) | Cargo | Ref. |
|---|---|---|
| Hrs | Ub-modifications | 22 |
| PD-L1 | 270 | |
| TSG101 | CD63 | 27 |
| MHC-II | ||
| ARRDC1 | TSG101 | 93,94 |
| NOTCH2 | ||
| CD63 | Pmel17 | 79 |
| LMP1 | 47 | |
| CD9 | CD10 | 312 |
| β-catenin | 81 | |
| CD82 | β-catenin | 81 |
| Ezrin | 97 | |
| CD81 | Rac | 75 |
| Syntenin | CD63 | 39 |
| Syndecan-1 | ||
| lysyl-tRNA synthetase | 313 | |
| Syndecan-1 | β-integrin | 43 |
| Fibronectin | ||
| ALIX | Syntenin | 39 |
| PAR1 | 314 | |
| PD-L1 | 271 | |
| Arf6 | MHC-I | 45,136 |
| pre-miRNA | ||
| Exportin-5 | ||
| Dicer | ||
| Ago2 | ||
| VPS4 CHMP4 |
β-catenin | 315 |
| Caveolin-1 | sortillin | 301 |
| hnRNPA1 Caveolin-1 |
miR-27a-3p miR-27b-3p |
162 |
| miR-92a-3p | ||
| miR-221–3p | ||
| miR-21 | ||
| hnRNPA2B1 |
miR-198 | 148 |
| miR-601 | ||
| miR-451 | ||
| miR-575 | ||
| miR-125a-3p | ||
| miR-887 | ||
| miR-17 | 137 | |
| miR-93 | ||
| miR-122–5p | 149 | |
| H19 lncRNA | 165 | |
| AGAP2-AS1 lncRNA | 167 | |
| AFAP1-AS1 lncRNA | 166 | |
| YBX1 | miR-133 | 138 |
| FMRP | miR-155 | 153 |
| SYNCRIP | miR-3470a | 151 |
| miR-194–2-3p | ||
| hnRNPU | miR-30c-5p | 316 |
| MEX3C/AP-2 | miR-451a | 148 |
| La protein | miR-126 | 257 |
| miR-145 | ||
| miR-486 | ||
| miR-122 | ||
| miR-142 |
Accompaniment of cargo by the listed effector to sites of EV loading is not inherently implied. Although direct binding between effector and cargo to mediate secretion in EVs have been demonstrated in some cases, cargo loading regulation may also represent changes in intracellular trafficking or other upstream processes.
Box 2: Identifying selective EV cargo sorting: Active vs. passive processes.
The EV field has long contemplated whether cargoes are selectively sorted into EVs, or whether EV cargoes represent nonselective samples of cytoplasm and source membranes330. EV cargo “selection” and “sorting” are defined as a process that drives the local increase in cargo concentration within a nascent EV compared to the surrounding concentration. This process is driven through recognition by the biogenesis machinery and by cargo-specific interactions. Cargoes increase in local concentration because they are captured and clustered by EV biogenesis machineries. For instance, the ESCRT machinery binds specifically to ubiquitinated transmembrane proteins (e.g., integrin α5331, EGFR23), on the endosome membrane, creating patches of membrane with higher cargo concentrations that are then incorporated into budding EVs. Similarly, cytosolic cargoes may also be captured and locally concentrated by adaptor proteins, although in many cases the adaptors are less defined. In one proposed mechanism, FMRP acts as an adaptor between the ESCRT machinery and cytosolic microRNA cargoes153. Several studies have shown that post-translational modifications provide a mechanism for regulating the selective loading of certain EV cargoes. For example, O-GlcNAcylation of hnRNPA2B1 under oxidative stress enhances its interactions with microRNAs secreted in EVs137.
In contrast to selected cargoes, “passively” or “randomly” loaded EV cargoes are defined as those that do not undergo local increases in concentration by clustering at EV biogenesis sites. The extent to which passive loading occurs is unknown and would presumably occur upon nascent EVs randomly sampling the cytoplasm and source membrane. In principle, levels of passively loaded cargoes in EVs could increase or decrease depending on cellular expression or the rate of EV production; which could also impact the loading of selectively sorted cargoes. Therefore, changes in EV composition do not necessarily prove selective sorting at the site of biogenesis in the absence of other information. As a hypothetical example, manipulation of factors involved in nuclear import or export of RNAs could conceivably affect EV RNA levels indirectly by changing cytoplasmic RNA levels, without a specific capture step by the EV biogenesis machinery or associated adaptors. For this reason, it is important to determine at what point in a cargo’s trafficking itinerary (from synthesis to secretion) a proposed selection event occurs.
This section will describe the field’s current understanding of basic EV cargo sorting mechanisms. Section IV will then discuss how cell, tissue and disease context regulates those sorting mechanisms. As most EV cargo sorting mechanisms have been described in exosomes, we will begin by describing those, noting that many of the same principles apply to cargo sorting into certain types of ectosomes. In fact, inward budding of the endosome is topologically equivalent to outward budding of the plasma membrane, so in principle similar biogenesis machineries could be activated at different places in the cell to generate exosomes and ectosomes20. For instance, the ESCRT machinery[G] is involved in both exosome and ectosome biogenesis20. We will review situations in which sorting mechanisms appear similar for ectosomes and exosomes, and other situations in which sorting mechanisms appear unique.
IIIa. Biogenesis of exosomes
Many EV cargo trafficking effectors have been identified (Table I) that are involved in binding cargo at the early endosomal membrane. The endosomal membrane then buds into the lumen of the endosome, and an intraluminal vesicle (ILV) is freed by scission of the neck of the bud. The process of ILV formation, combined with removal of other cargoes to vesicles destined for other cellular locations, leads to endosome maturation into late endosomal multivesicular bodies (MVBs)6. Thus, biogenesis of exosomes is typically defined as ILV formation, and exosomes are by definition the subset of ILVs that undergo secretion upon MVB fusion with the plasma membrane.
IIIa1. ESCRT-mediated exosome biogenesis
The first exosome biogenesis pathway discovered was the ESCRT pathway, involving the action of all four ESCRT complexes (ESCRT-0, -I, -II, and -III) along with disassembly and deubiquitinating enzymes on the endosome membrane (Fig. 2a). In mammalian cells, ESCRT-0, -I, -II, -III are sequentially recruited to maturing endosomes. The “early” ESCRT machinery, comprised of ESCRT-0, -I, and -II, recruit and sequester transmembrane proteins such as epidermal growth factor receptor (EGFR) to ILVs by binding multivalently to lysine-63-linked polyubiquitinated residues or multiple monoubiquitinated residues (Table I)21,22. Since transmembrane receptors are frequently ubiquitinated and endocytosed upon ligand-induced signaling23, this mechanism of ESCRT recruitment specifically leads to capture of ligand-receptor signaling complexes and their incorporation into ILVs. Specifically, the Hrs subunit of the ESCRT-0 dimer (Hrs and STAM1/2) coordinates early steps in ILV biogenesis by binding to ubiquitinated cargoes and recruiting clathrin to the early endosome24. This interaction between Hrs and clathrin is essential for clustering Hrs into a distinct type of endosomal microdomain and for sorting cargoes into ILVs22,25. Roles for ESCRT proteins in ILV biogenesis are conserved, and deletion of ESCRT proteins in budding yeast results in prevacuolar, endosomal compartments that lack ILVs21,26. An shRNA screen of ESCRT components in HeLa cells showed that depletion of ESCRT-0 proteins Hrs or STAM1, or the ESCRT-I subunit TSG101 inhibits small EV secretion27. By contrast, depletion of ESCRT-II and -III proteins had little effect, suggesting that human cells express redundant isoforms of ESCRT-II and -III components.
Figure 2: Mechanisms of EV biogenesis and cargo sorting.

a | In the ESCRT pathway, ESCRT-0 (Hrs:STAM dimers) and other ESCRT components underly flat clathrin domains on the endosome and cluster ubiquitinated cargoes for incorporation into ILVs. Current models suggest that ESCRT-I and -II mediate membrane bending, and ESCRT-III filaments promote membrane scission in concert with VPS4. ESCRT also recruits deubiquitinating enzymes (DUbs) that remove ubiquitin from cargoes. b | An ALIX-syntenin pathway of ILV formation bypasses early ESCRT machinery. Acting as a scaffolding protein through its Bro1 domain and PRR33,39,42, ALIX interacts with LBPA, phosphatidic acid (PA), other phospholipids (PLs), CHMP4B (ESCRT-III) and TSG101 (ESCRT-I). The V-domain of ALIX also engages short LYPX(n)L motifs on syntenin, which serves as a cargo adaptor for exosome cargoes like CD63 and syndecan39. Syntenin captures cargo through its tandem PDZ domains, which can engage syndecan325. c | Lipids and membrane proteins may promote ILV generation by acting as cone-shaped wedges that bend the endosome membrane. nSMase2 removes the head group of sphingomyelin (SM) to produce ceramide (Cer), a cone-shaped lipid sufficient for in vitro ILV formation. nSMase2 is activated by factor associated with nSMase (FAN) and is pharmacologically inhibited by the small molecule GW486953. Cargoes sorted through this pathway localize to lipid rafts enriched in flotillin and cholesterol. Tetraspanins are also cone-shaped and can promote membrane bending. d | Large ectosomes originate as plasma membrane blebs that decouple from the cortical actin cytoskeleton (1). Myosin-II sliding promotes drawstring-like closing of the bleb (2). The bud is cut off from the plasma membrane (3), releasing an ectosome into the extracellular space (4).
In addition to their cargo-sequestering activities, the ESCRT-I and -II complexes play a role in bending the endosomal membrane into an ILV19,28. In reconstituted systems, ESCRT-II association with cholesterol-rich membranes promotes the formation of liquid-ordered domains (Lo domains)29 and ILVs enriched with Lo lipids30, suggesting that the ESCRT pathway may depend on the Lo domain microenvironment, and potentially specific lipid interactions therein, to mediate EV biogenesis. It is possible that Lo domains themselves contribute to membrane bending when bound by ESCRT-II. Lo domains may also contribute to cargo selection due to enrichment with certain membrane proteins31. If Lo domain formation does underlie the ESCRT pathway generally, it would help explain why small EVs are often enriched in Lo domain lipids like sphingomyelin and cholesterol compared to cells32. Advances in the lipid imaging field have now enabled Lo domain visualization in a close-to-native context31, making this longstanding hypothesis more accessible to experimentation in the future. Capping off the process of exosome biogenesis, ESCRT-II induces the formation of ESCRT-III filaments that sever the neck of the nascent exosome from the endosome membrane19,28. The ESCRT-III complex can also be recruited for this process by ALIX, an ESCRT “accessory” protein that binds lysobisphosphatidic acid (LBPA) on the MVB membrane33. It has been suggested that ESCRT-III is directed to the vesicle bud neck by sensing negative membrane curvature34 or promotes membrane curvature to drive the fission event35; however, these roles are still being investigated36. The AAA ATPase Vps4 then removes ESCRT-III filaments from the membrane to reset the ESCRT system and possibly facilitate scission37,38.
IIIa2. Variations on the ESCRT pathway
Exosomes carrying different cargoes exhibit discrete requirements for ESCRT proteins during biogenesis, presumably reflecting differences in how the cargo is recruited into the ESCRT pathway. These requirements can be difficult to delineate in mammalian cells due to the expression of multiple ESCRT protein isoforms with partially redundant functions and the pleotropic effects of ESCRT depletion27. However, studies have identified one variation on the ESCRT pathway – the syndecan-syntenin-ALIX pathway – that is specifically compromised by knockdown of the ESCRT-I protein TSG101, the ESCRT-II subunit VPS22, or the ESCRT-III filament protein CHMP4 (Fig. 2b)39. Syndecan-1 is a transmembrane heparan sulfate proteoglycan that oligomerizes and promotes signaling through multivalent binding by growth factors and chemokines. On endosomes, the scaffold protein syntenin interacts with the syndecan-1 intracellular domain, linking it to CD63, and the ESCRT accessory protein Alix39,40. Likewise, syndecans 2–4 can control EV biogenesis39,41. ALIX serves as a second organizing protein at the MVB surface, binding to the lipid LBPA and the ESCRT proteins TSG101 and CHMP4B (Fig. 2b)33,42. Syntenin, syndecan-1 and ALIX all appear to play a role in sorting cargo during this process (Table I).
Syndecan-syntenin-ALIX-mediated exosome biogenesis is regulated by activation of the oncogenic tyrosine kinase Src[G]. Src phosphorylates syndecan-1 on its intracellular domain, inducing syndecan-1 endocytosis. Src also phosphorylates syntenin and Alix, which together with syndecan-1 phosphorylation stimulate exosome biogenesis43. Syntenin-mediated exosome biogenesis is also dependent on the GTPase ADP ribosylation factor-6 (Arf6), a regulator of endocytosis and vesicle trafficking, and phospholipase D2 (PLD2)44. While their molecular contributions have not yet been defined in this context, Arf6 and PLD2 act downstream of Src43 and could potentially impinge on ILV budding by producing phosphatidic acid to bind syntenin44,45. The syndecan-syntenin-ALIX pathway is also hijacked by Epstein-Barr virus to load exosomes with latent membrane protein 1 (LMP1), the major EBV oncogene46,47.
Another variation of the ESCRT pathway involves accessory ESCRT III proteins CHMP1, CHMP5 and IST1 that that are important for formation of a unique class of exosomes in cells subjected to glutamine deprivation and/or mTOR/Akt inhibition48,49. Surprisingly, these exosomes are formed within Rab11-positive recycling endosomes as opposed to the classical Rab5/Rab7-positive endosomes. These interesting studies reveal functions for the poorly studied accessory ESCRT III proteins as well as their role in stress-induced EV biogenesis.
IIIa3. Lipids in exosome biogenesis
EVs are rich in cholesterol, phosphatidylcholine, phosphatidylserine, sphingomyelin and ceramide, which play diverse roles in EV biogenesis, uptake and functional outcomes in recipient cells50. Lipids control many aspects of endosome biology, including cholesterol-regulated endosome positioning51 and size52 and ILV formation through ceramide53. There are reports that certain cargoes sort to ILVs regardless of ESCRT expression, and that ILVs containing such cargoes are formed through a lipid-dependent pathway (Fig. 2c). For instance, proteolipid protein (PLP), an abundant membrane protein in the central nervous system54, is still able to sort into endosomes in oligodendrocytes even when Tsg101, ALIX, or Hrs is siRNA-depleted or a dominant-negative VPS4 is expressed53. Endosomal PLP localizes not to Hrs-containing membrane domains but rather to domains containing flotillin-2, an Lo domain marker, raising the hypothesis that unique raft-associated lipids might facilitate the formation of ILVs containing PLP53,55. Indeed, the cone-shaped structure of certain lipids such as ceramide and phosphatidic acid can induce spontaneous negative membrane curvature, leading to invagination into the endosome membrane if cone-shaped lipids are produced on the outer leaflet53,56. Supporting this idea, inhibition of cellular neutral sphingomyelinase-2 (nSMase2), a ceramide-producing enzyme, prevents PLP from entering ILVs53. In addition to PLP, nSMase2 controls the EV-mediated release of other proteins (including TDP-4357, interleukin-3357,58, vacuolar H+-ATPase59, prion proteins60) and miRNAs61–66, although it is not always clear that the affected EVs are exosomes. This distinction is important because nSMase2 inhibition could also affect ectosome release67 or apoptotic body formation (rev. in68). nSMase2-dependent and ALIX-dependent pathways can act concurrently to generate unique subsets of EVs from the same cell69. For instance, a polarized epithelial cell line was found to release nSMase-2 dependent EVs from the basolateral surface along with ALIX-dependent EVs from the apical surface69. These EV subpopulations contained unique molecular markers, supporting the premise that different EV cargoes follow different biogenesis pathways, resulting in EV heterogeneity69; however some of those cargoes could conceivably follow those pathways simply due to their enrichment in apical or basal membranes (Box 2). In addition to nSMase2, exosome biogenesis is also impacted by ceramide through trafficking of existing ceramide from the endoplasmic reticulum (ER) to the endosome membrane by the ceramide transporter (CERT)70–72 and receptor-mediated signaling at MVBs by the ceramide metabolite S1P73. CERT-mediated transfer from the ER is also likely to affect ectosome biogenesis at ER-plasma membrane sites71.
IIIa4. Tetraspanins in exosome biogenesis
Tetraspanins[G] are integral membrane surface proteins that are frequently enriched in small EVs. At the cell membrane, tetraspanins interact with integrins and other associated proteins to form highly ordered tetraspanin enriched microdomains (TEMs)74. As such, classical tetraspanins that are present as EV cargoes, including CD63, CD81, and CD9, can themselves promote EV biogenesis, incorporating and directing the sorting of other TEM-associated proteins into EVs (Table I). In fact, one study using tetraspanin pulldowns determined that as much as 45% of the EV proteome from lymphoblasts interacts with TEMs75.
Reflecting the role of tetraspanins in membrane bending and vesicle formation76, crystal structures show that the four transmembrane helices common to all tetraspanins form an inverted conical wedge with a pocket that binds lipids77,78. Given the structural similarity between tetraspanins and ceramide, it is intriguing to speculate that both molecular classes utilize a common biophysical mechanism to promote membrane curvature. However, efforts to define the specific process by which tetraspanins contribute to exosome or ectosome biogenesis have been confounded by conflicting knockdown effects across studies. For instance, while knockdown of CD63 in melanoma cells decreases the number of ILVs per endosome79, opposing effects on total secreted small EVs are seen in other cell types80. Similar discrepancies have been reported for CD9 depletion and knockout studies81,82. One possible reason for such discrepancies may be that multiple tetraspanins can be present in the same tetraspanin-enriched membrane domains83,84, and may act upon exosome biogenesis within the same pathway. For example, syntenin-1 directly interacts with both CD63 and tetraspanin-6 during exosome biogenesis39,41,85,86. It is also possible that downregulation of exosome biogenesis could lead to upregulation of ectosome biogenesis, leading to an overall increase in EV number.
The tetraspanin CD63 is considered a classical marker of small EVs arising from exosome biogenesis, localizing primarily to intracellular endolysosomal compartments and incorporating into exosome-destined ILVs87,88. However, plasma membrane localization of CD63 has been observed89. Conversely, CD9 and CD81, which reside mostly at the plasma membrane, also associate with late endosomes and are often identified with CD63 in MVB-derived EVs88,90. Thus, the presence or absence of any given tetraspanin in a mixture of small EVs cannot be used to classify the EVs as exosomes or ectosomes.
IIIb. Biogenesis of Ectosomes
Unlike exosomes, ectosomes originate as outward buds of the plasma membrane that undergo fission to release the vesicles91,92. Compared to exosomes, a larger variety in size has been observed with ectosomes, which may also reflect differences in biogenesis mechanisms (Fig. 1b).
IIIb1. Small ectosome formation
Formation of small ectosomes has many similarities to exosome biogenesis, utilizing much of the same machinery and forming vesicles of a similar size as exosomes (~100 nm diameter). These machineries include both ESCRT proteins and tetraspanins. For instance, the ESCRT-I protein TSG101 is recruited to the plasma membrane through interaction with the Hrs-mimicking PSAP motif of arrestin domain-containing protein 1 (ARRDC1), where it participates in VPS4-dependent small ectosome production (Fig. 1b)93. Ectosomes formed through this pathway are called “ARMMs” (arrestin domain-containing protein 1-mediated microvesicles)93. Overexpression of ARRDC1 increases levels of ESCRT and Notch signaling proteins in EVs, suggesting that these are cargoes of ARMMs (Table I)94. Entry of ARRDC1 and Notch2 into EVs depends on expression of their respective E3 ubiquitin ligases93,94, similar to the ubiquitin-mediated ESCRT sorting that occurs on the endosome. Tetraspanins also play roles in ectosome budding and cargo sorting. CD9 and CD81 link to the actin cytoskeleton through interacting ERM[G] and EWI proteins, and thereby participate in plasma membrane organization. This interactome is thought to influence signaling, cargo sorting, and vesicle budding95,96, as exemplified by CD82’s recruitment of the ERM protein ezrin to membrane blebs[G] for release in ectosomes97.
Various types of membrane protrusions have now been shown to shed small ectosomes, including filopodia98–103, cilia104–107, and microvilli108–111 (Fig. 1b). For example, filopodia-like protrusions at sites of plasma membrane damage undergo Vps4B-dependent severing to produce ectosomes (Fig. 1b)112. The ESCRT-III component CHMP4B localizes to damaged sites and may play a role on the plasma membrane similar to its role in exosome formation, at least when overexpressed112. Crowding of glycocalyx[G] complexes on the outer plasma membrane of cells also promotes ectosome shedding from filopodia in response to DNA damage, producing semi-attached chains of “pearled” small EVs113. Small ectosome biogenesis from non-damaged cellular protrusions has also been described, through mechanisms involving hyaluronan[G] production, the actin cytoskeleton, inverse BAR domain[G]-containing proteins and cholesterol99,102,108,109,114. HIV-1 particles can also assemble at the tips of filopodia, suggesting that filopodia formation may contribute to ectosome/retrovirion biogenesis in ways that are not yet fully understood (Fig. 1b, Supplementary box 1)115,116.
Like filopodia, cilia also shed small ectosomes104–107. In many cases these cilia-generated EVs carry cargoes related to diseases known as “ciliopathies”, including polycystic kidney disease and Bardet-Biedl syndrome117. In C. elegans, release of ectosomes containing GFP-tagged polycystins (associated with polycystic kidney disease) has been observed at the base of cilia sensory neurons and those ectosomes regulate worm mating behavior118. Ciliary release of EVs is also supported by studies that impair ciliogenesis by knocking out cilia-related genes, which reduces EV-associated secretion of specific cargoes, including hedgehog and Wnt signaling proteins119–121 . Altered ectosome release is also associated with the pathogenesis of retinal ciliopathies. The specialized cilia of rod photoreceptor cells are capable of producing vast quantities of ectosomes, but are inhibited from doing so by the tetraspanin peripherin-2122. This suppression enables development of the ciliary outer segments; consequently, peripherin-2 gene knockout leads to retinal degeneration122.
IIIb2. Large ectosome formation
The formation of large ectosomes is less well understood than that of exosomes and small ectosomes. It is unclear whether the process involves early ESCRT machineries or tetraspanins, or any other features in common with exosome or small ectosome biogenesis. Instead, actin cytoskeletal rearrangements have been shown to underlie the process of plasma membrane blebbing and scission to release large EVs (Fig. 2d)123. Molecular reorganizations within the plasma membrane are also implicated in the process, including alterations in proteins, lipids and electrolyte levels. For example, altered levels of Ca2+ activate a family of lipid scramblases that disrupt membrane lipid asymmetry124,125. This process is thought to yield greater exposure of phosphatidylserine on the outer leaflet, one of the main features of ectosomes45,126. Phosphatidylethanolamine exposure on the outer leaflet may also promote ectosome formation since in early C. elegans embryos, formation of ~200 nm sized ectosomes is inhibited by the phosphatidylethanolamine flippase TAT-5127. Loss of TAT-5 activity on the plasma membrane leads to the accumulation of large ectosomes in spaces between cells, and loss of TAT-5 disrupts gastrulation127,128.
Both large and small EVs contain sphingomyelin and ceramide32,129 and the lipid raft marker caveolin-1130, suggesting that EVs either contain or are derived from lipid raft-associated membrane domains. Caveolin is also known to regulate small EV biogenesis through binding cholesterol131; that principle may also apply to ectosomes. However, studies to systematically address the role of cholesterol and lipid rafts in EV biogenesis are lacking, and it seems possible that the ectosomal membrane is simply derived from the plasma membrane and associated lipid rafts.
Beside alterations in membrane composition, local disassembly of the cortical actin cytoskeleton combined with actomyosin contractility can promote plasma membrane blebbing and subsequent formation of large ectosomes (Fig. 1b, Fig. 2d)45,132–134. This mechanism is used by abnormally large blebs on the plasma membranes of non-apoptotic cells shifting from a highly metastatic, mesenchymal state to the even more migratory and metastatic “amoeboid” phenotype. In prostate and breast cancer cells, loss of the actin nucleator Diaphanous-Related-Formin 3 (DIAPH3) induces the formation of large oncosomes (LO)123. The small GTPase RhoA, together with its downstream targets ROCK[G] and the LIM kinase (LIMK), also regulates release of large ectosomes from breast cancer cells by respectively enhancing contractility and downregulating the activity of the actin severing protein cofilin135.
In addition to their role in the syndecan-syntenin-ALIX pathway of exosome biogenesis (section IIIa2), Arf6 and PLD2 also regulate ectosome formation. At the plasma membrane, ARF6 activates phospholipase D, which in turn leads to recruitment of Erk[G] and downstream phosphorylation of myosin light chain kinase and myosin light chain, increasing actomyosin contractility and fission of the membrane bleb45. Arf6 and PLD2 also facilitate the selective incorporation of pre-miRNAs into ectosomes. Following pre-miRNA nuclear export by exportin-5, Arf6 in conjunction with GRP1 mediates transport of the pre-miRNAs and exportin-5 to the cell periphery and into ectosomes136. Overall, it seems that the biogenesis of ectosomes is highly dependent on a combination of plasma membrane phospholipid redistribution and activity of the actomyosin contractile machinery
IIIc. RNA recruitment to EVs
While cargo selection of transmembrane receptors via ubiquitination and recognition by ESCRT components has been well established in both exosome and ectosome biogenesis21–23,93, the mechanisms by which cytoplasmic cargoes such as RNAs and RNA-binding proteins (RBPs) are incorporated into EVs are much less clear. Many reports have found that EVs – especially small EVs – are enriched in specific RNAs relative to cellular levels, suggesting that selective sorting mechanisms exist137–144. In addition, the RNA composition of small EVs is distinct from large EVs, with enrichment in select subtypes of small RNAs, including miRNAs, snoRNAs[G], tRNA fragments, and mRNA fragments, and a nominal presence of short full-length mRNA transcripts (≤1 kb in length)90,145–147. In sections IV and V, we will describe how cell signaling state and cell type can regulate the RNA content of EVs. However, for both small and large EVs, the prevailing model is that RNA-binding proteins (RBPs) localize to EV biogenesis sites and act as adaptors between the RNA cargo and EV biogenesis machineries (Table I)148–153. Confocal fluorescence microscopy supports this model, revealing the localization of RBPs such as YBX1139, hnRNPK154, hnRNPQ71, SafB154, and Ago2[G]71 in the lumen of enlarged Rab5Q79L+ multivesicular endosomes.
miRNAs in small EVs are the most thoroughly studied RNA cargo in terms of both biogenesis and function. A number of studies have described selective sorting of miRNAs into small EVs148,155,156, based on recognition of cell type-specific but mostly GC-rich specific sequence elements by RBPs144,157 . Such sequence preferences have been defined for hnRNPA2B1 (GGAG/UGCA, AGG/UAG)148,158, SYNCRIP (GGCU)151,152, and FMRP (AAUGC)153. Among these, the most extensively reported effector is hnRNPA2B1, which has been linked to the regulated loading of numerous miRNAs (Table I)137,148,149. While the short miRNA length lends itself more to recognition by sequence motifs than structural elements, one notable exception is a noncanonical upstream structural motif that contributes to SYNCRIP miRNA recognition152. In the case of FMRP, its loading of AXXGC-containing miRNAs into exosome-destined ILVs is triggered by inflammasome[G] activation153. hnRNPA1 also interacts with a large number of miRNAs in various cancer cell types through an as yet unknown targeting mechanism and regulates their sorting to EVs (Table 1)159–162. It should be noted that interactions with specific RBPs could also sequester miRNA and inhibit their loading into small EVs, as has been described in endothelial cells for miR-503 regulation by hnRNPA2B1 and Annexin A2163.
Long noncoding RNA, short mRNAs, and mRNA fragments are also sorted to small EVs through interaction with RBPs147. The lncRNAs AFAP1-AS1, AGAP2-AS1, H19 and LNMAT2 are targeted to small EVs by hnRNPA2B1 (Tables I and II), with its canonical GGAG binding motif validated in the latter two transcripts164–167. One study in HEK293 cells identified sequence elements that direct mRNA to EVs. EV secretion of mRNA fragments is linked to the presence of three sequence motifs that are bound by SYNCRIP and NSun2. Interestingly, the location of those sequence motifs within the mRNA transcript dictates the specificity of binding by the two RBPs and the efficacy of mRNA targeting to small EVs168. Unlike small EVs, large EVs can also contain full-length mRNAs >1 kb, in addition to mRNA fragments and other RNA subtypes147.
Table II.
Context-dependent regulation of cargo sorting*
| Cargo | Context/Disease Association | Ref. |
|---|---|---|
| miRNAs | ||
| miR-17 | Hyperoxia-stressed lung epithelial cells; regulated by caveolin-1 and hnRNPA2B1 | 137 |
| miR-93 | ||
| miR-221 | Hyperoxia-stressed lung epithelial cells | 229 |
| miR-223 | Irradiation-induced senescence in human dermal fibroblasts | 232 |
| miR-15b-5p | Hydrogen peroxide-induced senescence in human dermal fibroblasts Hydrogen peroxide-induced senescence in human dermal fibroblasts |
233
233 |
| miR-30a-3p | ||
| miR-23a-3p | Hydrogen peroxide-induced senescence in human dermal fibroblasts | 317 |
| miR-155 | Inflammasome activated THP-1-differentiated macrophages; regulated by FMRP | 153 |
| miR-100 | DKO-1 mutant KRAS colorectal cancer cells expressing Ago2-S387A | 63,196 |
| miR-320a | DKO-1 mutant KRAS colorectal cancer cells expressing Ago2-S387A, hyperoxia-stressed lung epithelial cells | 196,229 |
| let-7a | DKO-1 mutant KRAS colorectal cancer cells expressing Ago2-S387A | 196 |
| miR-122 | Metabolically stressed Huh7 cells; regulated by HuR ubiquitination | 318 |
| miR-193a | Liver metastasis of mouse colon cancer; regulated by major vault protin | 260 |
| miR-503 | VEGF, bFGF-treated HUVEC cells; regulated by hnRNPA2B1 | 163 |
| Other RNA | ||
| LNMAT2 lncRNA | Bladder cancer 5637 and UM-UC-3 cells; regulated by hnRNPA2B1 |
164 |
| NFAT mRNA | TGF-β2 stimulation of cardiomyocytes | 187 |
| HDAC5 mRNA | ||
| Proteins | ||
| Ago2 | DKO-1 mutant KRAS colorectal cancer cells; regulated by Ago2 S387 phosphorylation | 196 |
| ApoE | Amyloid-β protofibrils exposure of primary differentiated cerebral cortical cells | 319 |
| bHLHE40 | TGF-β1 stimulation of pulmonary artery smooth muscle cells |
185 |
| Palladin | ||
| β-integrin-1 | Lysophosphatidylcholine-induced inflammation in hepatocytes | 197 |
| Metastasis of breast cancer and melanoma | 238 | |
| Caspase-3 | Hyperoxia-stressed lung epithelial cells | 230 |
| Caveolin-1 | Hyperoxia-stressed endothelial progenitor cells; regulated by caveolin-1 Y14 phosphorylation | 137 |
| c-Src | Onco-Dbl expressing mouse embryonic fibroblasts (MEFs) | 191 |
| FAK | MDAMB231, BT-549, Hs-578T breast cancer cells, onco-Dbl expressing MEFs | 191 |
| Fibronectin | UVB irradiation of melanocytes | 320 |
| Migratory fibrosarcoma cells; dependent on binding to integrin receptors | 11 | |
| TGF-β2 stimulation of HCT116 colorectal cancer cells | 186 | |
| HMGB1 | LPS and hydrogen peroxide-induced activation of MEFs, THP-1 and RAW264.7 macrophages; LC3- and RAGE receptor-dependent | 201,321 |
| lysyl-tRNA synthetase | Serum starvation-induced release by HCT116 colorectal cancer cells; regulated by caspase-8 | 313 |
| MHC-I | LOX melanoma cells, regulated by Arf6 | 45 |
| MHC-II | T-cell activated dendritic cells | 267 |
| Survivin | Paclitaxel-induced in MDAMB231 breast cancer cells | 322 |
| TDP-43 | ALS temporal cortex postmortem tissue | 57 |
Cargos listed represent selected examples of RNA and proteins that are enriched in EVs in a context-regulated manner, not solely due to altered cellular expression.
While the importance of RBPs in mediating RNA sorting to EVs is clear, how those RBPs connect to the EV biogenesis machineries is largely unclear. One exception is FMRP, which is recruited to MVBs through its binding to Rab interacting lysosomal protein (RILP) and Hrs, thus linking FMRP-mediated miRNA sorting with the ESCRT-mediated process of exosome biogenesis153. Another mechanism for RBP sorting to exosomes involves recognition of LC3-interacting regions in cargo RBPs by the autophagy protein LC3B154. RBPs might not be captured as individual RBP-RNA complexes but rather as larger assemblies; for instance, one recent study identified a possible role for RNA-containing membraneless granules in mediating sorting of miR-233 to MVBs139. Another study identified membrane contact sites[G] between the ER and MVBs as a key event in the formation of RNA-containing EVs. This work identified roles for the integral membrane ER tether protein VAP-A and ceramide transport protein CERT in the biogenesis of both large and small EVs enriched in RNA and RBPs71. As the ER serves as a scaffold for many types of RNA granules, it seems likely that regulation of ER-localized RNA-RBP complexes is a key component to cargo-driven biogenesis of RNA-containing EVs.
IIId. DNA in EVs
DNA can be detected in the extracellular space and in cell culture supernatants in both non-EV and EV-associated forms. In the circulation, much of the EV-contained DNA has been assumed to come from apoptotic bodies, but this is an active area of investigation as increasingly non-apoptotic EVs have been described to contain DNA169. A comparative study on large and small EVs purified from prostate cancer cells and patient plasma revealed that most of the DNA is contained in the large EVs170. Another study demonstrated that the DNA abundance in non-apoptotic large EVs increases in cancer cells undergoing nuclear shape instability171, suggesting that ectosomes may enclose and export free cytosolic DNA generated through genomic instability in rapidly dividing cancer cells.
For apoptotic bodies, disassembly of the cell and formation of apoptotic bodies is an orderly, regulated process172. In apoptotic cells, caspase-3 cleaves an autoinhibitory domain of ROCK1 to increase actomyosin contractility, resulting in blebbing from the plasma membrane directly or from the tips of surface protrusions termed apoptopodia9,172,173. Apoptopodia can also form beads of apoptotic bodies along their length, which is regulated by actomyosin contractility and the caspase-3 targets Plexin-B2 and Pannexin-1 (Fig. 1d)9. It is unknown whether apoptotic bodies are released by shear force or in a regulated manner172. Pannexin-1 also regulates the incorporation of nuclear material, including DNA, into apoptotic bodies9,174.
Exosomes and other small EVs may also contain fragmented nuclear DNA175,176, although in many cases the DNA may simply co-isolate with EVs, potentially adhering to the EV surface177,178. The mechanisms regulating DNA recruitment to exosomes and other small EVs are largely unknown, and it has been difficult to fathom how DNA might enter MVBs, although immunoelectron microscopy has localized DNA to the interior of ILVs within MVBs179. A few reports have successfully demonstrated the presence of DNA in small EVs175,176,180; this may reflect the context-specific nature of DNA sorting to exosomes/small EVs. Indeed, senescence (including oncogene-induced senescence) and DNA damage upregulate the levels of DNA found in MVBs and small EVs179,181,182, which may explain why the presence of DNA in small EVs has been primarily reported for cancer cell-derived EVs. Furthermore, DNA-containing small EVs appear to be a subpopulation of the small EVs released from cells177.
IV. Cargo regulation based on cell state
As the EV field has evolved, it has become clear that there are no absolute rules with respect to what is or is not an EV cargo. In many cases, it is studies on the regulation of EV cargoes by cell signaling or other cell state changes (Table II) that have led to this realization, identifying cases in which cargoes found infrequently in EVs are upregulated in a specific context. Some common themes are highlighted here.
IVa. Growth factor and oncogene signaling
A consequence of growth factor signaling, which may propagate its effects to recipient cells, is modulation of EV cargo loading. For example, fibroblast growth factor signaling in cultured hippocampal neurons, which regulates neuronal plasticity and wound repair in vivo, greatly alters the cargo of secreted EVs and stimulates MVB-plasma membrane fusion events183,184. EV cargoes are also altered by growth factor signaling in lung tissue, where EVs mediate cross talk between endothelial and smooth muscle cells. Excessive stimulation of pulmonary artery smooth muscle cells with transforming growth factor-β (TGF-β1) both induces secretion and enhances uptake of EVs enriched in RNAs encoding cytoskeletal factors and the transcription regulator bHLHE40185. Similarly, TGF-β2 signaling influences the levels of proteasome subunits, fibronectin, and histones carried by EVs from cultured colorectal cancer cell lines186, and induces the loading of mRNAs encoding nuclear factor of activated T-cells 5 (NFAT5) and histone deacetylase 5 (HDAC5) into cardiomyocyte EVs187 (Table II).
The transforming capacity of cancer cells is greatly expanded by the effect that oncogenic signaling has on redefining EV cargo content. A prime example is in glioblastoma tumors, in which tumor heterogeneity is maintained by different oncogenic cell subtypes through the exchange of EVs with distinct pro-tumorigenic protein cargos188. One of these subtypes is driven by expression of the EGFRvIII oncogene, whose signaling profoundly alters both the expression of EV biogenesis components and sorting of EV cargoes, including invasion-promoting factors and adhesion molecules143,189,190. Similarly, the oncogenic form of the guanine nucleotide exchange factor Dbl drives production of ectosomes that transfer focal adhesion kinase[G] (FAK) to recipient cells, promoting apoptotic evasion and anchorage independent growth191. In breast cancer cells, signaling by the oncogenic nonreceptor protein kinase Src enhances loading of the syndecan binding partners β1 integrin and fibronectin into small EVs that stimulate migration of recipient cells43. At the molecular level, phosphorylation of both syndecan and syntenin by Src promotes endocytosis and exosomal sorting of these cargoes through the ARF6-PLD2 pathway (Fig. 3a)43.
Figure 3: Examples of context-specific regulation of cargo sorting.
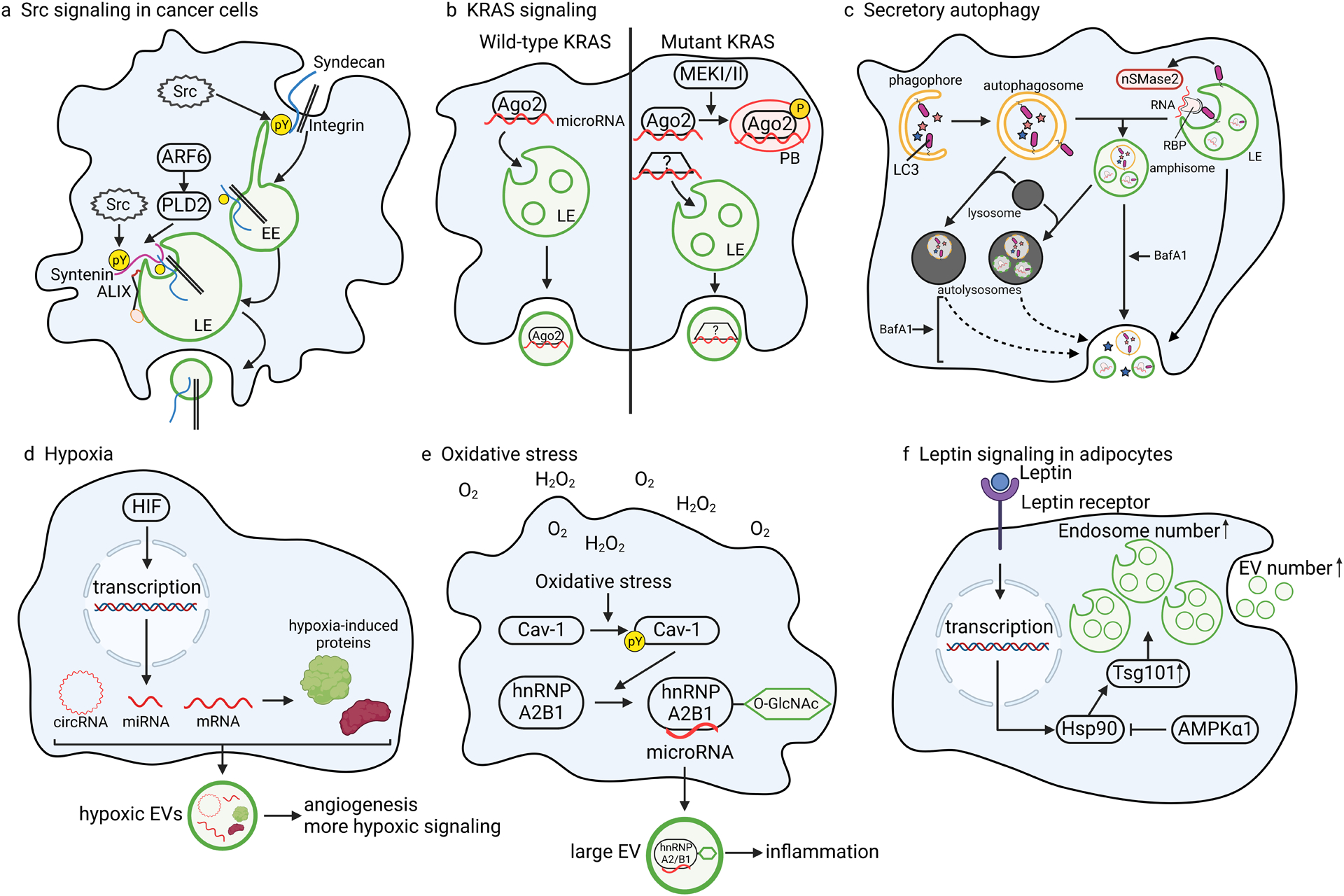
a | Src directly phosphorylates syndecan and syntenin to regulate integrin cargo sorting through ARF6-PLD243,44. b | Oncogenic mutant KRAS signaling in colon cancer cells inhibits Ago2 entry into small EVs by promoting MEKI/II-dependent phosphorylation of Ago2. Phosphorylation of Ago2 inhibits its incorporation into ILVs and increases its association with processing bodies (PBs), resulting in altered RNA and protein content in small EVs compared to small EVs secreted from isogenic colon cancer cells with wild-type KRAS196. Nonetheless, certain miRNAs are secreted at enhanced levels from mutant KRAS-expressing cells63, and are presumably bound by other yet to be identified RNA-binding proteins. c | Lipidated LC3 assists in nonselective engulfment of autophagy cargoes (denoted as stars) by the phagophore, a double-membrane organelle that fuses to itself to create an autophagosome. In an independent process termed LC3-dependent EV loading and secretion (LDELS), lipidated LC3 captures RNA-binding proteins (via LC3 interaction motifs) and associated RNAs onto the endosome membrane to promote nSMase2-dependent exosome biogenesis154. Secretion of LC3-induced exosomes may occur via normal fusion of MVBs with the plasma membrane or after fusion of MVBs and autophagosomes to form amphisomes. Under conditions of lysosomal dysfunction, such as with inhibition of acidification by Bafilomycin A1 (BafA1), there is enhanced secretion of autophagic proteins (blue and pink stars), both inside and outside of EVs, presumably due to fusion of both amphisomes and autolysosomes with the plasma membrane326. Arrows represent membrane fusion events. d | Hypoxia activates HIF-mediated transcriptional upregulation of targeted circRNAs, miRNAs, and mRNAs, which enter EVs along with the protein products of the affected mRNAs. e | Oxidative stress from reactive oxygen species leads to caveolin-1 (Cav-1) phosphorylation, stabilizing the RNA-binding protein hnRNPA2B1 and promoting its O-GlcNAcylation. This post-translational modification enhances hnRNPA2B1 binding to microRNAs that are trafficked to large EVs137. f | Leptin signaling activates transcription of Hsp90, which stabilizes Tsg101 to increase the number of endosomes and EVs. However, Hsp90 also antagonizes this process through its interaction with AMPKα1308.
Oncogenic signaling can also affect sorting of RNA cargoes into EVs. Oncogenic KRAS mutations are present in many cancers, including more than 30% of colorectal and lung adenocarcinomas and more than 80% of pancreatic cancers192. Isogenic colorectal cancer cell lines expressing wild type or mutant KRAS produce small EVs with vastly different cargo (Table II). Cells expressing mutant KRAS generate small EVs enriched in cell signaling proteins and metabolic enzymes, whereas cells expressing wildtype KRAS produce small EVs laden with RBPs193. Similar effects of mutant KRAS on EV sorting have been reported in non-small cell lung cancer cells194. Certain miRNAs such as miR-100 are also enriched in small EVs from mutant KRAS-expressing cells and can be functionally transferred to recipient cells63. Moreover, mutant KRAS-derived EVs promote growth of tumor cells in soft agar; however, the specific cargoes responsible for this pro-tumorigenic behavior remain unconfirmed195. Finally, RBP and RNA sorting in mutant KRAS-expressing cells are modulated by downstream MEK/Erk signaling, which inhibits Ago2 sorting into EVs by shifting its subcellular distribution away from endosomes and into RNA processing bodies (Fig. 3b, Table II)196. The functional consequence of these changes in RNA-RBP trafficking remain to be defined but likely include altering the RNA expression profile of both donor and recipient cells.
IVb. Metabolic regulation of cargo sorting
IVbi. Fatty acid metabolism
As discussed in the EV biogenesis section, lipids regulate EV formation by facilitating membrane curvature and forming lipid ordered domains that provide a platform for sorting proteins. Excess fatty acid metabolism, such as occurs in non-alcoholic steatotic hepatitis[G] (NASH), also imposes dramatic changes in EV sorting, provoking the selective release of integrin β1 as EV cargo from hepatocytes197. Similar alterations in EV cargoes are brought on by fatty acid treatment of hepatic and hepatic carcinoma cells, including by myristic acid[G]198 and palmitic acid[G]70, the latter of which was shown to stimulate EV biogenesis at ER membrane contact sites. Palmitic acid may also promote EV biogenesis and cargo loading via palmitoylation of EV cargoes, potentially targeting them to the membrane at EV biogenesis sites130.
IVbii. Autophagy
Autophagy is the process whereby cells sequester cytoplasmic components and deliver them to lysosomes for metabolic recycling. Autophagy encompasses both macroautophagy, in which cargoes are delivered to lysosomes via an intermediate organelle termed the autophagosome, and microautophagy, in which cargoes are directly internalized by endosomes or lysosomes.199 It is now understood that some autophagy machinery also functions in secretory pathways, including EV biogenesis (Fig. 3c)154,200. A recent breakthrough study found that RNA sorting to exosomes is closely linked with secretory autophagy154. In this proposed mechanism, the autophagy adaptor protein LC3B and related proteins associate with the endosome membrane and recognize RBPs containing a four amino acid-long LC3-interacting region (LIR)154. To couple ILV budding with cargo sorting, LC3B also recruits FAN (factor associated with nSMase) through its LIR to activate localized ceramide production154. LC3-dependent sorting into EVs has also been shown for the nuclear protein HMGB1 (Table II)201. This new role of LC3 is distinct from its classical role in capturing substrates into the autophagosome, a double-membrane organelle that directly fuses with the lysosome, since knockout of genes important for autophagosome formation downstream of LC3B lipidation do not inhibit LC3-induced exosome biogenesis154. Nonetheless, the two roles of LC3 may interconnect in cases where the outer autophagosome membrane fuses with MVBs to create an “amphisome,” which itself can fuse with the plasma membrane to release conventional exosomes along with inner autophagosome contents202,203 (Fig 3c). In another variety of endosomal microautophagy, the chaperone HSC70 captures protein cargoes containing a KFERQ peptide and not only delivers them to lysosomes but also to endosomes for secretion in exosomes204,205.
IVbiii. Oxidative Stress
In response to environmental stress like hypoxia, hyperoxia, and chemotherapeutic agents, the production of excess reactive oxygen species (ROS) triggers an oxidative stress response that alters metabolic signaling and EV content. For example, chemotherapy results in the massive accumulation of oxidized and ubiquitylated proteins that are cleared as EV cargo206. ROS generated as metabolic byproducts or during immune defense also alter EV content and convey either cytoprotective or injurious effects upon recipient cells.
Under conditions of hypoxia such as in the tumor microenvironment, fibrotic lung, or ischemic myocardial tissue, the protein composition of EV cargo is dramatically altered (Fig 3d). For example, glioblastoma cells exposed to hypoxic conditions release EVs enriched in anti-apoptotic and pro-metastatic proteins207. In other hypoxic cancer models, EVs are differentially laden with adhesion proteins and factors involved in epithelial mesenchymal transition208,209. Hypoxia likely exploits secretory autophagy pathways to regulate EV content, since hypoxia-induced increases in the loading of EVs with pro-angiogenic factors depends on the LC3-like autophagy factor GABARAPL1210.
Hypoxia also alters the nucleic acid content of EVs (Fig 3d). Expression of many miRNAs are transcriptionally upregulated by hypoxia inducible factor 1a[G] (HIF-1a) and therefore more highly represented in hypoxic EVs under hypoxic conditions. These include miR-210 and miR-2162,211–215; in models of myocardial hypoxia, both confer anti-apoptotic cardioprotective effects216,217. In numerous cancer and cardiac disease models, hypoxia-induced EVs contain increased levels of pro-angiogenic miRNAs into EVs and promote angiogenesis in vivo216,218–220. Interestingly, these types of miRNA functions can be abrogated by circular RNAs[G], and hypoxic signaling appears to fine-tune the downstream effects of EVs by modulating both miRNA and circular RNA cargos. For instance, in hypoxic pancreatic cancer cells, the circular RNA circZNF91 is upregulated by HIF-1a and transferred as EV cargo to recipient cells, where it acts as sponge to inhibit miR-23b-3p and further propagate HIF-1a-mediated transcriptional reprogramming221. Hypoxic upregulation of circular RNA cargo in EVs is associated with ischemic cardiac disease, colorectal cancer and diabetic retinopathy222–226. Hypoxic conditions also induce HIF1-mediated upregulation of mRNAs (and their cognate proteins) in glioblastoma EVs (Fig 3d)227 and altered levels of cardiac fibrosis-promoting long noncoding RNAs in cardiomyocyte EVs228. Importantly, HIF-1a also amplifies nSMase2 expression, effectively exerting its own selective process to drive the production of EVs laden with hypoxia-induced cargoes62.
Hyperoxia is an iatrogenic form of oxidative stress that lung cells may experience due to the formation of oxygen free radicals following administration of excess supplementary oxygen. In models of hyperoxia-induced acute lung injury, robust secretion of EVs enriched in caspase-3, miR-221 and miR-320a activates macrophage pro-inflammatory responses that stimulate tissue repair (Table II)229,230. Conversely, hyperoxia stress in a rat model of bronchopulmonary dysplasia releases EVs containing the pyroptotic p30 fragment of gasdermin D[G], inciting widespread inflammatory, lung and brain injury231. Studies of hyperoxia have also revealed important mechanistic insights into the recruitment of RNA to EVs. Specifically, hyperoxia or H2O2 exposure trigger phosphorylation of the lipid raft protein caveolin-1 at tyrosine-14, which then binds hnRNPA2B1. This interaction protects hnRNPA2B1 from degradation and increases its O-GlcNAcylation in the RRM1 domain (Fig. 3e)137. hnRNPA2B1 is also sumoylated in its RRM2 domain. Both sumoylation and O-GlcNAcylation enhance the interaction of hnRNPA2B1 with specific miRNAs, allowing hnRNPA2B1 to sort miRNAs into EVs (Table II)137,148.
IVbiv. Senescence:
Unchecked ROS can also lead to DNA damage, leakage of DNA into the cytoplasm, and induction of cell senescence or apoptosis. EV-mediated clearance of the offending cytoplasmic DNA provides a way to evade this potential cell fate179,182. Senescent macrophages and fibroblasts secrete more, small EVs and different microRNAs compared to non-senescent counterparts, with macrophages downregulating miR-223 sorting and fibroblasts upregulating miR-15b-5p and miR-30a-3p sorting to EVs (Table II)232,233. Senescence in primary fibroblasts also increases release of EVs carrying interferon-induced transmembrane protein 3, which leads to a more senescent phenotype in recipient cells234.
V. Consequences of disease on EV cargoes
Since EVs were discovered, the idea that their cargo could serve as disease biomarkers has excited many fields. In some cases, the cargoes are unique to specific cell types and thus serve as a marker of altered activity in a specific tissue. Stage-dependent changes in cargo content have also been documented in many diseases, demonstrating the value of EV cargo profiling for monitoring disease progression235. In many cases, these changes arise simply as a consequence of altered transcriptional activity driven by the disease state. However, in some instances, bonafide differences in the EV content of certain cargos as compared to cellular levels have been carefully documented. These disease-specific influences on EV cargo sorting warrant underscoring as they represent fascinating examples of how EV biogenesis and secretion may be targeted to modulate cell biology. Here, we highlight the effect of certain disease pathologies on cargo sorting in the associated cell types.
Va. Cancer
Perhaps the best characterized examples of context-specific regulation of EV cargo sorting come from cancer biology. Across model systems, EVs released by tumor cells and their neighboring untransformed cells have been shown to both support and inhibit cancer progression and metastasis236. Tumor-derived EVs contribute to formation of premetastatic niches by delivering proteins and miRNAs that reprogram target cells toward a pro-metastatic phenotype237–243. Accordingly, proteomics and transcriptomics have shown major differences in the EV cargo of untransformed and cancer cells244
As noted in earlier sections, upstream oncogenic signaling and alterations in the tumor microenvironment can potentially act in concert to regulate EV biogenesis in cancer cells. This paradigm is illustrated well in the findings of a recent study of glutamine deprivation in colorectal, prostate and cervical cancer cell lines. Glutamine deprivation leads to an altered metabolic state and reduced Akt/mTOR signaling that promotes biogenesis of a unique class of exosomes from Rab11-positive recycling endosomal compartments49. These exosomes have growth promoting activities and unique cargoes. Intrinsic effects of tumor metabolism may also influence EV cargo. Thus, the Warburg effect[G] on cancer cell metabolism is reflected in the cargo of cancer cell EVs and passed on to recipient cells. Small EVs released from colorectal cancer cells expressing mutant KRAS are enriched in a subset of metabolic enzymes and metabolites, including glucose transporter 1 (GLUT1)193,245,246; transfer of these EVs to recipient cells promotes a metabolic shift toward aerobic glycolysis245. Similarly, free amino acids and other metabolites present in prostate cancer cell small EVs confer metabolic reprogramming in recipient cells247. Similar findings have been reported across diverse cancer cell types for other glycolytic enzymes, including pyruvate kinase-2, glucose-6-phosphate dehydrogenase, transketolase and transaldolase 1248–250.
Large EVs from tumor cells are also enriched in metabolic enzymes and can drive glutamine metabolism in recipient cells130,251. In some cases, this may reflect the presence of mitochondrial enzymes, as quantitative proteomics has shown enrichment of mitochondrial proteins in large EVs252,253. Consistent with that idea, electron microscopy imaging has revealed whole mitochondria and mitochondrial fragments inside various types of large EVs254–256 .Whether this represents specific recruitment of mitochondria or the bulk engulfment of cytoplasm, including whole or fragmented organelles, into ectosomes is currently unknown.
Particular attention has been paid in cancer cell biology to the transformative potential of miRNAs released in EVs. It has been broadly demonstrated that tumor cells release EVs containing altered levels of specific miRNAs relative to cellular levels, whose uptake impart significant downstream effects on tumorigenesis257–260. In colorectal cancer (CRC), miR-10063 and miR-410–3p261 are selectively sorted in EVs. Both miRNAs have been shown to transform cells through EV uptake, with transfer of miR-410–3p by colorectal cancer cell EVs exerting PI3K-driven tumorigenic effects on recipient cells261 and miR-100 transfer in lung cancer cell EVs imparting recipient cells with cisplatin resistance262. EV loading with miR-100 in CRC is enhanced by constitutively activated KRAS63 and downregulated by Ago2 phosphorylation (Table II)196. Additionally, hnRNPK has been shown to load miRNAs containing the AsUGnA motif into exosomes, with trafficking of hnRNPK to MVBs occurring through a process dependent on caveolin-1 and ceramide150.
Vb. Immune cells
One of the earliest functions identified for EVs is in facilitating antigen presentation. Antigen-presenting cells such as dendritic cells and B lymphocytes load antigen peptides onto MHC-II[G] inside specialized endosomal structures called antigen-processing compartments263. Fusion of these specialized MVBs with the plasma membrane releases MHC-II-containing ILVs as exosomes and also places MHC-II on the plasma membrane where it can be released in ectosomes264,265. One study found that EVs of all sizes from unstimulated dendritic cells contain MHC-II and can activate CD4+ T cells, with small and medium EVs evoking more of a Th1 phenotype and larger EVs evoking more of a Th2 phenotype266. Loading of MHC-II into EVs released by dendritic cells is upregulated in response to antigen stimulation, a process that is dependent on their cognate interaction with T cells267. Uptake of the EVs carrying antigen-bound MHC-II by recipient dendritic cells leads to further activation of T cells and powerful modulation of the immune response268,269. Immunoelectron microscopy studies have also shown clusters of exosomes carrying MHC-II at the T cell plasma membrane267.
Since interactions between tumor cells and immune cells play a central role in cancer, immune cell EVs have been studied in the context of tumorigenesis. One mechanism by which transformed cells navigate immune escape is through exosomal secretion of the transmembrane protein programmed cell death ligand 1[G] (PD-L1). In melanoma cells, stimulation with interferon-gamma triggers the loading of PD-L1 into exosomes, dependent on the ESCRT-0 protein Hrs270. In breast cancer cells, the ESCRT accessory protein ALIX is necessary for loading of PD-L1 into exosomes (Table I)271. Binding of exosomal PD-L1 to its receptor PD-1 inactivates T lymphocytes by concurrently blocking MHC-II/T cell receptor signaling and costimulatory receptors required for full T cell activation272,273. Similarly, downregulatory effects on natural killer cells and CD8+ cytotoxic T lymphocytes are conferred by exosomes presenting the NKG2D ligand to its lectin-like activating receptor274,275. Other exosomal cargoes can have the opposing effect, activating immune defense mechanisms, such as exosomes carrying pigment epithelium derived factor (PEDF) that activate patrolling monocytes at metastatic sites276.
EVs selectively loaded with non-coding (nc)RNAs also mediate tumor immune surveillance. tRNA and tRNA fragments are sorted preferentially into cancer cell- and immune cell-derived EVs257,277. Similar in length to miRNAs, tRNA fragments can regulate gene expression278–280, apoptosis281, and the immune response. In activated T cells, tRNA fragments are the most abundant form of EV RNA cargo. EV-mediated secretion of tRNA fragments and other ncRNAs generated during the immune response has been shown to thwart the development of chronic inflammation277,282. Intriguingly, immunosuppressive vitamin D3 and immunostimulatory lipopolysaccharide [G] (LPS) treatments of primary dendritic cells induce opposing changes in Y-RNA[G], miRNA, and small nucleolar RNA levels in EVs283.
Vc. Neurons and glial cells
Synaptic plasticity in the central nervous system is regulated by context-specific EV loading in both normal physiology and disease pathogenesis. For instance, oligodendrocyte-derived EVs contain myelination factors, astrocyte-derived EVs contain protein cargoes that function in neurite outgrowth, neuroprotection, and synapse formation, and neuronal EVs contain proteins related to synaptic function284–287. In Drosophila, neuronal EVs transfer Evenless Interrupted – a transmembrane protein that binds to Wingless (Wnt in mammals) – across neuromuscular junctions, facilitating synapse development288. External stimulation can affect the number and cargo content of the released EVs. Thus, neurotransmitters promote secretion of small EVs by oligodendrocytes, whereas glutaminergic activity induces small EV secretion by neurons289,290. In astrocytes, ethanol-induced activation of the inflammatory response produces EVs enriched in neuroinflammatory proteins and miRNA291. Furthermore, when exposed to trophic, inflammatory, or anti-inflammatory stimuli, astrocytes produce EVs loaded with functionally distinct cargoes292.
EVs are also vectors for transmission of pathological proteins, which is thought to mediate spreading of neurogenerative disease pathology through tissues in diseases such as Parkinson’s, prion-related diseases, and Alzheimer’s293. In Alzheimer’s disease, EV-associated Tau and amyloid-β may play a central role in this process as exosome-associated Tau released from synapses can induce accumulation of additional Tau in an amyloid-β-dependent manner294. Tau has also been reported to be secreted in neuronal large EVs295. Tau expression in iPSC cells dramatically alters cargo loading to small EVs, raising the possibility that it might also have a role in EV biogenesis in neurons296. Likewise, the prefrontal cortex of AD patients exhibits robust upregulation of tetraspanin-6, which stimulates secretion of amyloid-β-loaded exosomes by multiple mechanisms, including altering the turnover of Alzheimer’s precursor protein and recruitment of syntenin to MVBs85.
Vd. Cardiac cells
Several highly discriminant alterations in the cargo of serum EVs have been defined in patient sera following specific types of cardiac events. In addition to the aforementioned hypoxia-triggered alterations in long noncoding RNA cargo of cardiomyocyte EVs, CD31/annexin V double positive large EVs297 and VE-cadherin positive large EVs298 are released by injured endothelial cells and correlate with poor prognosis in coronary artery disease. A similar study of disease-specific cargo in circulating EVs following an acute coronary syndrome identified factors with known cardiac disease connections as well as a previously unknown association for polymeric immunoglobulin receptor (pIgR)299. After myocardial infarction in mice, large EVs carrying cardiac muscle markers transiently increase in abundance in the left ventricle300. While many such studies have uncovered valuable disease biomarker candidates with the potential to avert recurrent cardiac events, few have provided mechanistic insight to the processes that translate disease-specific signaling cues into altered EV sorting. One noteworthy exception is an elegant analysis of vascular calcification factors that revealed an active role for EVs in directing vascular calcification301. The primary effector of calcification is the ubiquitous enzyme tissue nonspecific alkaline phosphatase (TNAP). Remarkably, TNAP-mediated calcification of smooth muscle depends on the deposition of EVs carrying both TNAP and the transmembrane glycoprotein sortillin. TNAP and sortillin are trafficked to lipid rafts and are dependent on Rab11, Cav-1 and phosphorylation of sortillin’s C-terminal tail by FAM20 and CK2 for loading into EVs301.
Ve. Adipocytes and obesity
Adipocytes regulate metabolism in other tissues directly through lipogenesis, lipolysis, and fatty acid oxidation and indirectly through the immune response via release of cytokines and adipokines. In addition to these conventional secreted factors, white and brown adipocyte cells also secrete small EVs containing enzymes involved in glycolysis and fatty acid production302. The small EVs secreted from white adipocytes are notably enriched in cholesterol compared to large EVs, while large EVs are marked by phosphoserine on the outer membrane leaflet303. Within white adipose tissue, adipocytes intermingle with adipose stromal cells, epithelial cells, and immune cells (macrophages, mast cells and eosinophils), which together make up the stromal vascular fraction (SVF). The EVs released from this tissue impact metabolic homeostasis in both an autocrine and paracrine fashion, most notably exerting powerful influences on tumor cell growth and pancreatic β-cell function.
Adipose dysfunction is primarily associated with obesity, as hypoxia within the expanding fat pads induces increased EV secretion of fatty acid synthesis factors that trigger adipocyte hypertrophy304. In obese patients, adipose-derived EV size, number and cargo composition are all altered (rev. in305), in part mediated through metabolic increases in the cellular levels of palmitic acid and ceramide306,307, which escalate EV biogenesis and secretion of obesity-associated cargo. Obesity also is associated with increased levels of the fat-regulatory hormone leptin[G], which among its many effects includes stimulation of EV release from adipocytes. Mechanistically, leptin signaling through its receptor drives increased exosome biogenesis through a process dependent on Hsp90 interaction with Tsg101 (Fig. 3f). The secreted EVs are enriched in Hsp90 and other leptin signaling factors, further propagating the activation of leptin signaling in recipient cells (Fig. 3f)308. This pathway may be counteracted by metformin, which acts to mitigate non-alcoholic fatty liver disease by activating the metabolic sensor kinase AMPKα1, which then interacts with Hsp90 and inhibits Tsg101-mediated exosome biogenesis in adipocytes309. Higher circulating leptin levels are also associated with increased breast cancer risk, with leptin signaling stimulating EV release from breast cancer cells and promoting angiogenesis, invasiveness, and migration308. Indeed, recent studies have uncovered extensive cross talk via EV transfer between adipocytes, cardiac cells, β cells and cancer cells (rev. in310), suggesting that EV dysregulation may underlie the development of metabolic comorbidities, e.g., obesity, cardiovascular disease, diabetes, and cancer (rev. in311).
VI. Conclusions and Perspectives
While EVs were once thought to be fairly uniform, we now know that cells from any organism release EV populations that are highly heterogeneous, with diverse cargoes and multiple biogenesis mechanisms. Indeed, EVs do not just differ because of their origin as ILVs within MVBs or plasma membrane blebs, although this is a convenient way to classify many types of EVs. The central role of cargo in recruiting various machineries illustrates how such heterogeneity can be generated, even within EV subtypes. The example of specific recruitment mechanisms through posttranslational modifications of cargo speaks to the selectivity of the process. This is particularly clear for such cargos as transmembrane cargoes that are ubiquitinated, recruiting the ESCRT machinery; however, the more recently described alternate mechanisms by which cargo engage the vesicle formation machinery (e.g., via syntenin-Alix, tetraspanin, and ceramide formation pathways) appear nonetheless specific. As another example, RNAs are selectively sorted into EVs in that the levels in EVs is not simply reflective of their cellular levels63 and may depend on their location at ER membrane contact sites with EV biogenesis membranes71. However, the principal architects, conductors, and/or couriers that steer RNA-RBP complexes to these sites and into the vesicular lumen remain largely unknown. The location of cargo on the plasma membrane versus the endosome is also a likely determining factor in whether a certain cargo is incorporated into an ectosome versus an endosome.
A deeper understanding of the molecular mechanisms that drive biogenesis of distinct EV populations carrying diverse cargoes is important to drive understanding of how EVs drive cell-cell communication, as well as for biomarker and therapeutic vesicle development. In addition, a greater appreciation for the role of cell and tissue context in generating EV cargo diversity is needed. For example, to what extent are the EV cargoes of different cell types driven by the levels of expression of certain cargoes and stimulation of overall EV biogenesis pathways? And are certain cargoes more tightly controlled than others, for example by signaling and/or metabolic state (Table II)? Future studies taking in account the role of cellular context and regulation in the generation of EV diversity will be important to address such questions and to enable better engineering of therapeutic EVs loaded with specific cargoes (Box 3). It will also be important to perform comparative studies with appropriate models that allow tracking of EV cargo sorting in donor cells and EV cargo delivery to target cells.
Box 3: Engineering EVs with specific cargoes.
Although the rules for driving cargo selection and EV biogenesis are not yet entirely understood, some efforts have already been made to engineer EVs with specific cargoes. One example is the use of the C1C2 peptide of lactadherin/MFG-E8, which binds to phosphatidylserine and has been used to display a variety of proteins on the surface of phosphatidylserine-containing EVs332–334. Other investigators have fused surface cargoes to EV resident proteins, such as CD63, to facilitate targeting to specific cells335. For targeting of luminal cargoes, several approaches have been utilized. Fusion of a palmitoylation motif to fluorescent proteins was used as a method of labeling EVs for in vivo tracking; this approach links proteins to the inner leaflet of the EV bilayer membrane336. Fusion of optogenetically controlled plant protein module CIBN to the cytoplasmic tail of the tetraspanin CD9 allowed light-dependent recruitment of CRY2-fusion proteins and EV targeting337. While lipid or tetraspanin targeting has been successful, it is clear that only a subset of vesicles is targeted. Most recently, enriched proteins identified via a proteomics screen were used to engineer fusion proteins targeting EV surfaces (PTGFRN) or lumens (MARCKS family proteins)338. While this approach seems to target a larger fraction of vesicles, more validating studies are required to determine if this holds across a variety of cell systems.
For packaging of RNAs into EVs, both brute force overexpression and engineered targeting have been employed. Cre mRNA can be transmitted into EVs via overexpression in donor cells, but the efficiency is generally low339–341. Small noncoding RNAs are often enriched in EVs and one group recently leveraged the packaging selectivity of miR-451 to drive packaging of miRNAs and siRNAs into EVs. Reshke et al.342 identified miR-451 to be a highly enriched miRNA in EVs and utilized the structural backbone of pre-miR-451 to incorporate a variety of siRNAs, including siRNAs targeting GFP and the disease genes superoxide dismutatase or transthyretin. This approach for identifying naturally selected cargoes to use as a platform for engineering may turn out to be fruitful for additional RNA cargoes, despite the paucity of knowledge about their trafficking mechanisms.
Supplementary Material
Acknowledgements:
We thank the members of the Weaver and Di Vizio groups for many helpful discussions. Funding support came from NIH/NSF grants R01CA206458, R01CA249684, P01CA229123, R01CA249424, U54 CA217450, U01CA224276, NSF-2036809 to AMW; from NIH/NCI (R01 CA234557 and R01CA218526) to DDV. ACD was also supported by a NIH T32 training grant (1T32GM137793-01) and NSF GRFP # 1937963. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the National Institutes of Health.
Glossary:
- Reticulocyte
Nucleated precursor cell type to red blood cells.
- Membrane trafficking
the process of sorting molecular cargoes, especially lipids and proteins, into different compartments within the cell
- ESCRT
endosomal sorting complex required for transport, comprising five protein complexes (ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, and Vps4) that function in cargo sorting, membrane remodeling and scission
- Src
membrane-associated non-receptor tyrosine kinase that localizes to endosomes and regulates exosome biogenesis and cargo sorting
- Tetraspanins
a conserved family of four-pass transmembrane proteins that organize lipid and signaling domains in membranes. Tetraspanins are enriched in many EVs and also in some cases regulate EV biogenesis.
- Glycocalyx
from Greek, sweet husk; a tough casing consisting of glycoproteins, proteoglycans, and glycolipids that covers a cell
- Hyaluronan
a long, linear polymer of disaccharides that forms a part of the extracellular matrix and resists compression
- BAR domain
BIN, amphiphysin, and Rvs161 and Rvs167 domain; a conserved protein domain that promotes and/or senses positive membrane curvature; conversely, inverse BAR domains associate with negatively curved membranes.
- ERM proteins
ezrin, radixin, and moesin proteins; proteins that link the actin cytoskeleton to transmembrane proteins in the plasma membrane, playing roles in cytoskeleton organization at the cell cortex and signal transduction
- Blebs
Bulbous protrusions of plasma membrane that form when the cortical cytoskeleton decouples from the plasma membrane
- ROCK
Rho-associated coiled coil containing serine/threonine kinase that acts downstream of RhoA small GTPase in cytoskeletal remodeling and membrane blebbing
- Erk
extracellular signal-regulated kinase, also known as MAPK (mitogen-activated protein kinase); a protooncogene and serine/threonine protein kinase activated by EGFR and RAS-RAF-MEK signaling
- snoRNAs
small nucleolar RNAs; function to perform direct covalent modification of ribosomal RNAs, snRNAs, and other RNAs. Two classes of snoRNAs include C/D box snoRNAs, which direct 2’-O-ribose methylation, and H/ACA box snoRNAS, which direct pseudouridylation.
- inflammasome
a large molecular weight protein complex that activates pyroptosis, a pro-inflammatory and lytic type of programmed cell death, especially as a response to infection
- Membrane contact sites
regions where two organelle membranes are tethered together (typically at a distance of ~10–40 nm) to perform a function other than fusion.
- Focal adhesion kinase
a tyrosine kinase activated by integrin and growth factor signaling that controls the actin cytoskeleton to influence cell shape, focal adhesion assembly and disassembly, and cell migration.
- Ago2
Argonaute 2; the central component of the RNA-induced silencing complex. Ago2 is guided by a microRNA to the open reading frame or 3’ untranslated region of a target mRNA, where it recruits other factors for translational silencing, depolyadenylation, and/or degradation of the target mRNA. Alternatively, Ago2 activates its “slicer” (endonuclease) activity when directed to an mRNA target by an exogenous small interfering RNA (siRNA) that is perfectly complementary to the target mRNA.
- Non-alcoholic steatotic hepatitis
a chronic, severe inflammatory liver disease caused by metabolic alterations associated with obesity and typified by fat accumulation in the liver.
- Myristic acid
a saturated fatty acid with fourteen carbons (14:0)
- Palmitic acid
a saturated fatty acid with sixteen carbons (16:0)
- HIF-1a
hypoxia-inducible factor 1a; a transcription factor activated under low oxygen conditions that regulates angiogenesis, apoptosis, and many other cellular processes
- Circular RNAs
single-stranded, circularized RNAs arising when the 3’ end of one exon back-splices to its own 5’ end or the 5’ end of another exon.
- Gasdermin D
effector of pyroptosis activated by caspase-1-mediated proteolytic cleavage downstream of the inflammasome. Cleaved gasdermin D forms pores in the plasma membrane and organelle membranes, resulting in cell death.
- Warburg effect
the tendency of cancer cells to upregulate glucose uptake and aerobic glycolysis
- MHC-II
Major Histocompatibility Complex II, a transmembrane protein complex found on professional antigen presenting cells that binds to exogenous antigen peptides and presents these peptides to immune cells
- Programmed cell death ligand 1
a ligand that activates its receptor (PD-1) on T cells to inhibit T-cell activation.
- LPS
lipopolysaccharide; a potent immunostimulatory glycolipid present on the outer membrane of Gram-negative bacteria
- Y-RNA
A subtype of RNA transcribed by RNA polymerase III that is conserved among vertebrates but has poorly understood functions. Four Y-RNAs are present in humans.
- Leptin
a hormone secreted by fat cells that regulates hunger and body mass
Footnotes
Declaration of Interests: There are no declarations of interests.
References
- 1.Johnstone RM, Mathew A, Mason AB & Teng K Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol 147, 27–36, doi: 10.1002/jcp.1041470105 (1991). [DOI] [PubMed] [Google Scholar]
- 2.Pan BT, Teng K, Wu C, Adam M & Johnstone RM Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101, 942–948, doi: 10.1083/jcb.101.3.942 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding C, Heuser J & Stahl P Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol 35, 256–263 (1984). [PubMed] [Google Scholar]
- 4.van Niel G et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol, doi: 10.1038/s41580-022-00460-3 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Maas SLN, Breakefield XO & Weaver AM Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol 27, 172–188, doi: 10.1016/j.tcb.2016.11.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Niel G, D’Angelo G & Raposo G Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19, 213–228, doi: 10.1038/nrm.2017.125 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Raposo G & Stoorvogel W Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200, 373–383, doi: 10.1083/jcb.201211138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res 25, 24–38, doi: 10.1038/cr.2014.135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkin-Smith GK et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun 6, 7439, doi: 10.1038/ncomms8439 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thery C et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7, 1535750, doi: 10.1080/20013078.2018.1535750 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sung BH, Ketova T, Hoshino D, Zijlstra A & Weaver AM Directional cell movement through tissues is controlled by exosome secretion. Nat Commun 6, 7164, doi: 10.1038/ncomms8164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha S et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol 214, 197–213, doi: 10.1083/jcb.201601025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeRita RM et al. Tumor-Derived Extracellular Vesicles Require beta1 Integrins to Promote Anchorage-Independent Growth. iScience 14, 199–209, doi: 10.1016/j.isci.2019.03.022 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kia V, Mortazavi Y, Paryan M, Biglari A & Mohammadi-Yeganeh S Exosomal miRNAs from highly metastatic cells can induce metastasis in non-metastatic cells. Life Sci 220, 162–168, doi: 10.1016/j.lfs.2019.01.057 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Kriebel PW et al. Extracellular vesicles direct migration by synthesizing and releasing chemotactic signals. J Cell Biol 217, 2891–2910, doi: 10.1083/jcb.201710170 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French KC, Antonyak MA & Cerione RA Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin Cell Dev Biol 67, 48–55, doi: 10.1016/j.semcdb.2017.01.002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathieu M, Martin-Jaular L, Lavieu G & Thery C Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21, 9–17, doi: 10.1038/s41556-018-0250-9 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Schwager SC & Reinhart-King CA Mechanobiology of microvesicle release, uptake, and microvesicle-mediated activation. Curr Top Membr 86, 255–278, doi: 10.1016/bs.ctm.2020.08.004 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Henne WM, Buchkovich NJ & Emr SD The ESCRT pathway. Dev Cell 21, 77–91, doi: 10.1016/j.devcel.2011.05.015 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Jackson CE, Scruggs BS, Schaffer JE & Hanson PI Effects of Inhibiting VPS4 Support a General Role for ESCRTs in Extracellular Vesicle Biogenesis. Biophys J 113, 1342–1352, doi: 10.1016/j.bpj.2017.05.032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raiborg C & Stenmark H The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452, doi: 10.1038/nature07961 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Raiborg C et al. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol 4, 394–398, doi: 10.1038/ncb791 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Katzmann DJ, Odorizzi G & Emr SD Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol 3, 893–905, doi: 10.1038/nrm973 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Raiborg C, Bache KG, Mehlum A, Stang E & Stenmark H Hrs recruits clathrin to early endosomes. EMBO J 20, 5008–5021, doi: 10.1093/emboj/20.17.5008 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raiborg C, Wesche J, Malerod L & Stenmark H Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J Cell Sci 119, 2414–2424, doi: 10.1242/jcs.02978 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Coonrod EM & Stevens TH The yeast vps class E mutants: the beginning of the molecular genetic analysis of multivesicular body biogenesis. Mol Biol Cell 21, 4057–4060, doi: 10.1091/mbc.E09-07-0603 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colombo M et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126, 5553–5565, doi: 10.1242/jcs.128868 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Hurley JH & Hanson PI Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Biol 11, 556–566, doi: 10.1038/nrm2937 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boura E, Ivanov V, Carlson LA, Mizuuchi K & Hurley JH Endosomal sorting complex required for transport (ESCRT) complexes induce phase-separated microdomains in supported lipid bilayers. J Biol Chem 287, 28144–28151, doi: 10.1074/jbc.M112.378646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booth A, Marklew CJ, Ciani B & Beales PA The influence of phosphatidylserine localisation and lipid phase on membrane remodelling by the ESCRT-II/ESCRT-III complex. Faraday Discuss 232, 188–202, doi: 10.1039/d0fd00042f (2021). [DOI] [PubMed] [Google Scholar]
- 31.Levental I, Levental KR & Heberle FA Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol 30, 341–353, doi: 10.1016/j.tcb.2020.01.009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llorente A et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta 1831, 1302–1309, doi: 10.1016/j.bbalip.2013.04.011 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Larios J, Mercier V, Roux A & Gruenberg J ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol 219, doi: 10.1083/jcb.201904113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee IH, Kai H, Carlson LA, Groves JT & Hurley JH Negative membrane curvature catalyzes nucleation of endosomal sorting complex required for transport (ESCRT)-III assembly. Proc Natl Acad Sci U S A 112, 15892–15897, doi: 10.1073/pnas.1518765113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiaruttini N et al. Relaxation of Loaded ESCRT-III Spiral Springs Drives Membrane Deformation. Cell 163, 866–879, doi: 10.1016/j.cell.2015.10.017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertin A et al. Human ESCRT-III polymers assemble on positively curved membranes and induce helical membrane tube formation. Nat Commun 11, 2663, doi: 10.1038/s41467-020-16368-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adell MAY et al. Recruitment dynamics of ESCRT-III and Vps4 to endosomes and implications for reverse membrane budding. Elife 6, doi: 10.7554/eLife.31652 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgar JR, Eden ER & Futter CE Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic 15, 197–211, doi: 10.1111/tra.12139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baietti MF et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14, 677–685, doi: 10.1038/ncb2502 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Stepp MA, Pal-Ghosh S, Tadvalkar G & Pajoohesh-Ganji A Syndecan-1 and Its Expanding List of Contacts. Adv Wound Care (New Rochelle) 4, 235–249, doi: 10.1089/wound.2014.0555 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghossoub R et al. Tetraspanin-6 negatively regulates exosome production. Proc Natl Acad Sci U S A 117, 5913–5922, doi: 10.1073/pnas.1922447117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elias RD et al. Proline-rich domain of human ALIX contains multiple TSG101-UEV interaction sites and forms phosphorylation-mediated reversible amyloids. Proc Natl Acad Sci U S A 117, 24274–24284, doi: 10.1073/pnas.2010635117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imjeti NS et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc Natl Acad Sci U S A 114, 12495–12500, doi: 10.1073/pnas.1713433114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghossoub R et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun 5, 3477, doi: 10.1038/ncomms4477 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Muralidharan-Chari V et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 19, 1875–1885, doi: 10.1016/j.cub.2009.09.059 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rider MA et al. The interactome of EBV LMP1 evaluated by proximity-based BioID approach. Virology 516, 55–70, doi: 10.1016/j.virol.2017.12.033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nkosi D et al. Epstein-Barr Virus LMP1 Promotes Syntenin-1- and Hrs-Induced Extracellular Vesicle Formation for Its Own Secretion To Increase Cell Proliferation and Migration. mBio 11, doi: 10.1128/mBio.00589-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marie PP et al. Accessory ESCRT-III proteins selectively regulate Rab11-exosome biogenesis in Drosophila secondary cells. bioRxiv, 2020.2006.2018.158725, doi: 10.1101/2020.06.18.158725 (2020). [DOI] [Google Scholar]
- 49.Fan SJ et al. Glutamine deprivation alters the origin and function of cancer cell exosomes. EMBO J 39, e103009, doi: 10.15252/embj.2019103009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donoso-Quezada J, Ayala-Mar S & Gonzalez-Valdez J The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic 22, 204–220, doi: 10.1111/tra.12803 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rocha N et al. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol 185, 1209–1225, doi: 10.1083/jcb.200811005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobo K et al. Late endosomal cholesterol accumulation leads to impaired intra-endosomal trafficking. PLoS One 2, e851, doi: 10.1371/journal.pone.0000851 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trajkovic K et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247, doi: 10.1126/science.1153124 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Knapp PE Proteolipid protein: is it more than just a structural component of myelin? Dev Neurosci 18, 297–308, doi: 10.1159/000111420 (1996). [DOI] [PubMed] [Google Scholar]
- 55.Otto GP & Nichols BJ The roles of flotillin microdomains--endocytosis and beyond. J Cell Sci 124, 3933–3940, doi: 10.1242/jcs.092015 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Matsuo H et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303, 531–534, doi: 10.1126/science.1092425 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Iguchi Y et al. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain 139, 3187–3201, doi: 10.1093/brain/aww237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katz-Kiriakos E et al. Epithelial IL-33 appropriates exosome trafficking for secretion in chronic airway disease. JCI Insight 6, doi: 10.1172/jci.insight.136166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choezom D & Gross JC Neutral sphingomyelinase 2 controls exosome secretion by counteracting V-ATPase-mediated endosome acidification. J Cell Sci 135, doi: 10.1242/jcs.259324 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo BB, Bellingham SA & Hill AF The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem 290, 3455–3467, doi: 10.1074/jbc.M114.605253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kosaka N et al. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem 288, 10849–10859, doi: 10.1074/jbc.M112.446831 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu J et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells Nanomed Biotechnol 46, 1659–1670, doi: 10.1080/21691401.2017.1388249 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cha DJ et al. KRAS-dependent sorting of miRNA to exosomes. Elife 4, e07197, doi: 10.7554/eLife.07197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tahara H, Kay MA, Yasui W & Tahara E MicroRNAs in Cancer: the 22nd Hiroshima Cancer Seminar/the 4th Japanese Association for RNA Interference Joint International Symposium, 30 August 2012, Grand Prince Hotel Hiroshima. Jpn J Clin Oncol 43, 579–582, doi: 10.1093/jjco/hyt037 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Mittelbrunn M et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2, 282, doi: 10.1038/ncomms1285 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kosaka N et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285, 17442–17452, doi: 10.1074/jbc.M110.107821 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menck K et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles 6, 1378056, doi: 10.1080/20013078.2017.1378056 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shamseddine AA, Airola MV & Hannun YA Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Adv Biol Regul 57, 24–41, doi: 10.1016/j.jbior.2014.10.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsui T, Osaki F, Hiragi S, Sakamaki Y & Fukuda M ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. EMBO Rep 22, e51475, doi: 10.15252/embr.202051475 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukushima M et al. StAR-related lipid transfer domain 11 (STARD11)-mediated ceramide transport mediates extracellular vesicle biogenesis. J Biol Chem 293, 15277–15289, doi: 10.1074/jbc.RA118.002587 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barman B et al. VAP-A and its binding partner CERT drive biogenesis of RNA-containing extracellular vesicles at ER membrane contact sites. Dev Cell 57, 974–994 e978, doi: 10.1016/j.devcel.2022.03.012 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crivelli SM et al. Function of ceramide transfer protein for biogenesis and sphingolipid composition of extracellular vesicles. J Extracell Vesicles 11, e12233, doi: 10.1002/jev2.12233 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kajimoto T et al. Involvement of Gbetagamma subunits of Gi protein coupled with S1P receptor on multivesicular endosomes in F-actin formation and cargo sorting into exosomes. J Biol Chem 293, 245–253, doi: 10.1074/jbc.M117.808733 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M & Sanchez-Madrid F Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 19, 434–446, doi: 10.1016/j.tcb.2009.06.004 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Perez-Hernandez D et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem 288, 11649–11661, doi: 10.1074/jbc.M112.445304 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McMahon HT & Boucrot E Membrane curvature at a glance. J Cell Sci 128, 1065–1070, doi: 10.1242/jcs.114454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zimmerman B et al. Crystal Structure of a Full-Length Human Tetraspanin Reveals a Cholesterol-Binding Pocket. Cell 167, 1041–1051 e1011, doi: 10.1016/j.cell.2016.09.056 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Umeda R, Nishizawa T & Nureki O Crystallization of the human tetraspanin protein CD9. Acta Crystallogr F Struct Biol Commun 75, 254–259, doi: 10.1107/S2053230X1801840X (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Niel G et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 21, 708–721, doi: 10.1016/j.devcel.2011.08.019 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petersen SH et al. The role of tetraspanin CD63 in antigen presentation via MHC class II. Eur J Immunol 41, 2556–2561, doi: 10.1002/eji.201141438 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Chairoungdua A, Smith DL, Pochard P, Hull M & Caplan MJ Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol 190, 1079–1091, doi: 10.1083/jcb.201002049 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brzozowski JS et al. Extracellular vesicles with altered tetraspanin CD9 and CD151 levels confer increased prostate cell motility and invasion. Sci Rep 8, 8822, doi: 10.1038/s41598-018-27180-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andreu Z & Yanez-Mo M Tetraspanins in extracellular vesicle formation and function. Front Immunol 5, 442, doi: 10.3389/fimmu.2014.00442 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gurung S, Perocheau D, Touramanidou L & Baruteau J The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 19, 47, doi: 10.1186/s12964-021-00730-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guix FX et al. Tetraspanin 6: a pivotal protein of the multiple vesicular body determining exosome release and lysosomal degradation of amyloid precursor protein fragments. Mol Neurodegener 12, 25, doi: 10.1186/s13024-017-0165-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Latysheva N et al. Syntenin-1 is a new component of tetraspanin-enriched microdomains: mechanisms and consequences of the interaction of syntenin-1 with CD63. Mol Cell Biol 26, 7707–7718, doi: 10.1128/MCB.00849-06 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sung BH et al. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat Commun 11, 2092, doi: 10.1038/s41467-020-15747-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mathieu M et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun 12, 4389, doi: 10.1038/s41467-021-24384-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Booth AM et al. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol 172, 923–935, doi: 10.1083/jcb.200508014 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crescitelli R et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles 2, doi: 10.3402/jev.v2i0.20677 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antonyak MA & Cerione RA Microvesicles as mediators of intercellular communication in cancer. Methods Mol Biol 1165, 147–173, doi: 10.1007/978-1-4939-0856-1_11 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A & Ratajczak MZ Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495, doi: 10.1038/sj.leu.2404296 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Nabhan JF, Hu R, Oh RS, Cohen SN & Lu Q Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A 109, 4146–4151, doi: 10.1073/pnas.1200448109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Q & Lu Q Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat Commun 8, 709, doi: 10.1038/s41467-017-00767-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Umeda R et al. Structural insights into tetraspanin CD9 function. Nat Commun 11, 1606, doi: 10.1038/s41467-020-15459-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sala-Valdes M et al. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J Biol Chem 281, 19665–19675, doi: 10.1074/jbc.M602116200 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Huang C, Hays FA, Tomasek JJ, Benyajati S & Zhang XA Tetraspanin CD82 interaction with cholesterol promotes extracellular vesicle-mediated release of ezrin to inhibit tumour cell movement. J Extracell Vesicles 9, 1692417, doi: 10.1080/20013078.2019.1692417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott G Demonstration of melanosome transfer by a shedding microvesicle mechanism. J Invest Dermatol 132, 1073–1074, doi: 10.1038/jid.2012.20 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Rilla K et al. Hyaluronan production enhances shedding of plasma membrane-derived microvesicles. Exp Cell Res 319, 2006–2018, doi: 10.1016/j.yexcr.2013.05.021 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Deen AJ et al. UDP-sugar substrates of HAS3 regulate its O-GlcNAcylation, intracellular traffic, extracellular shedding and correlate with melanoma progression. Cell Mol Life Sci 73, 3183–3204, doi: 10.1007/s00018-016-2158-5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noble JM et al. Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J Struct Biol 210, 107474, doi: 10.1016/j.jsb.2020.107474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nishimura T et al. Filopodium-derived vesicles produced by MIM enhance the migration of recipient cells. Dev Cell 56, 842–859 e848, doi: 10.1016/j.devcel.2021.02.029 (2021). [DOI] [PubMed] [Google Scholar]
- 103.Ando H et al. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol 132, 1222–1229, doi: 10.1038/jid.2011.413 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Dubreuil V, Marzesco AM, Corbeil D, Huttner WB & Wilsch-Brauninger M Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol 176, 483–495, doi: 10.1083/jcb.200608137 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kesimer M et al. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J 23, 1858–1868, doi: 10.1096/fj.08-119131 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J et al. Sensory cilia act as a specialized venue for regulated extracellular vesicle biogenesis and signaling. Curr Biol 31, 3943–3951 e3943, doi: 10.1016/j.cub.2021.06.040 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cao M et al. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. Elife 4, doi: 10.7554/eLife.05242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marzesco AM et al. Release of extracellular membrane vesicles from microvilli of epithelial cells is enhanced by depleting membrane cholesterol. FEBS Lett 583, 897–902, doi: 10.1016/j.febslet.2009.01.048 (2009). [DOI] [PubMed] [Google Scholar]
- 109.Marzesco AM et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 118, 2849–2858, doi: 10.1242/jcs.02439 (2005). [DOI] [PubMed] [Google Scholar]
- 110.McConnell RE et al. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol 185, 1285–1298, doi: 10.1083/jcb.200902147 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hara M et al. Podocyte membrane vesicles in urine originate from tip vesiculation of podocyte microvilli. Hum Pathol 41, 1265–1275, doi: 10.1016/j.humpath.2010.02.004 (2010). [DOI] [PubMed] [Google Scholar]
- 112.Mageswaran SK, Yang WY, Chakrabarty Y, Oikonomou CM & Jensen GJ A cryo-electron tomography workflow reveals protrusion-mediated shedding on injured plasma membrane. Sci Adv 7, doi: 10.1126/sciadv.abc6345 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shurer CR et al. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell 177, 1757–1770 e1721, doi: 10.1016/j.cell.2019.04.017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han J et al. RhoB/ROCK mediates oxygen-glucose deprivation-stimulated syncytiotrophoblast microparticle shedding in preeclampsia. Cell Tissue Res 366, 411–425, doi: 10.1007/s00441-016-2436-4 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Aggarwal A et al. Mobilization of HIV spread by diaphanous 2 dependent filopodia in infected dendritic cells. PLoS Pathog 8, e1002762, doi: 10.1371/journal.ppat.1002762 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bracq L, Xie M, Benichou S & Bouchet J Mechanisms for Cell-to-Cell Transmission of HIV-1. Front Immunol 9, 260, doi: 10.3389/fimmu.2018.00260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reiter JF & Leroux MR Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol 18, 533–547, doi: 10.1038/nrm.2017.60 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang J et al. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol 24, 519–525, doi: 10.1016/j.cub.2014.01.002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cruz NM et al. Modelling ciliopathy phenotypes in human tissues derived from pluripotent stem cells with genetically ablated cilia. Nat Biomed Eng 6, 463–475, doi: 10.1038/s41551-022-00880-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Volz AK et al. Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles. Nat Commun 12, 5671, doi: 10.1038/s41467-021-25929-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mohieldin AM et al. Ciliary extracellular vesicles are distinct from the cytosolic extracellular vesicles. J Extracell Vesicles 10, e12086, doi: 10.1002/jev2.12086 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salinas RY et al. Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J Cell Biol 216, 1489–1499, doi: 10.1083/jcb.201608081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di Vizio D et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res 69, 5601–5609, doi: 10.1158/0008-5472.CAN-08-3860 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suzuki J, Umeda M, Sims PJ & Nagata S Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468, 834–838, doi: 10.1038/nature09583 (2010). [DOI] [PubMed] [Google Scholar]
- 125.Bricogne C et al. TMEM16F activation by Ca(2+) triggers plasma membrane expansion and directs PD-1 trafficking. Sci Rep 9, 619, doi: 10.1038/s41598-018-37056-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lima LG, Chammas R, Monteiro RQ, Moreira ME & Barcinski MA Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett 283, 168–175, doi: 10.1016/j.canlet.2009.03.041 (2009). [DOI] [PubMed] [Google Scholar]
- 127.Beer KB et al. Extracellular vesicle budding is inhibited by redundant regulators of TAT-5 flippase localization and phospholipid asymmetry. Proc Natl Acad Sci U S A 115, E1127–E1136, doi: 10.1073/pnas.1714085115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wehman AM, Poggioli C, Schweinsberg P, Grant BD & Nance J The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr Biol 21, 1951–1959, doi: 10.1016/j.cub.2011.10.040 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haraszti RA et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles 5, 32570, doi: 10.3402/jev.v5.32570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mariscal J et al. Comprehensive palmitoyl-proteomic analysis identifies distinct protein signatures for large and small cancer-derived extracellular vesicles. J Extracell Vesicles 9, 1764192, doi: 10.1080/20013078.2020.1764192 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Albacete-Albacete L et al. ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting. J Cell Biol 219, doi: 10.1083/jcb.202006178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crespin M, Vidal C, Picard F, Lacombe C & Fontenay M Activation of PAK1/2 during the shedding of platelet microvesicles. Blood Coagul Fibrinolysis 20, 63–70, doi: 10.1097/MBC.0b013e32831bc310 (2009). [DOI] [PubMed] [Google Scholar]
- 133.Ciardiello C et al. Large oncosomes overexpressing integrin alpha-V promote prostate cancer adhesion and invasion via AKT activation. J Exp Clin Cancer Res 38, 317, doi: 10.1186/s13046-019-1317-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bergert M, Chandradoss SD, Desai RA & Paluch E Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc Natl Acad Sci U S A 109, 14434–14439, doi: 10.1073/pnas.1207968109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li B, Antonyak MA, Zhang J & Cerione RA RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31, 4740–4749, doi: 10.1038/onc.2011.636 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clancy JW, Zhang Y, Sheehan C & D’Souza-Schorey C An ARF6-Exportin-5 axis delivers pre-miRNA cargo to tumour microvesicles. Nat Cell Biol 21, 856–866, doi: 10.1038/s41556-019-0345-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee H et al. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J Exp Med 216, 2202–2220, doi: 10.1084/jem.20182313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin F et al. YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT. Stem Cell Res Ther 10, 263, doi: 10.1186/s13287-019-1377-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu XM, Ma L & Schekman R Selective sorting of microRNAs into exosomes by phase-separated YBX1 condensates. Elife 10, doi: 10.7554/eLife.71982 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lu P et al. MEX3C interacts with adaptor-related protein complex 2 and involves in miR-451a exosomal sorting. PLoS One 12, e0185992, doi: 10.1371/journal.pone.0185992 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hinger SA et al. Diverse Long RNAs Are Differentially Sorted into Extracellular Vesicles Secreted by Colorectal Cancer Cells. Cell Rep 25, 715–725 e714, doi: 10.1016/j.celrep.2018.09.054 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Valadi H et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9, 654–659, doi: 10.1038/ncb1596 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Skog J et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10, 1470–1476, doi: 10.1038/ncb1800 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garcia-Martin R et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 601, 446–451, doi: 10.1038/s41586-021-04234-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lunavat TR et al. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells--Evidence of unique microRNA cargos. RNA Biol 12, 810–823, doi: 10.1080/15476286.2015.1056975 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mateescu B et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J Extracell Vesicles 6, 1286095, doi: 10.1080/20013078.2017.1286095 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wei Z et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun 8, 1145, doi: 10.1038/s41467-017-01196-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Villarroya-Beltri C et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 4, 2980, doi: 10.1038/ncomms3980 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li C et al. hnRNPA2B1-Mediated Extracellular Vesicles Sorting of miR-122–5p Potentially Promotes Lung Cancer Progression. Int J Mol Sci 22, doi: 10.3390/ijms222312866 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Robinson H et al. Caveolin-1-driven membrane remodelling regulates hnRNPK-mediated exosomal microRNA sorting in cancer. Clin Transl Med 11, e381, doi: 10.1002/ctm2.381 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Santangelo L et al. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep 17, 799–808, doi: 10.1016/j.celrep.2016.09.031 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Hobor F et al. A cryptic RNA-binding domain mediates Syncrip recognition and exosomal partitioning of miRNA targets. Nat Commun 9, 831, doi: 10.1038/s41467-018-03182-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wozniak AL et al. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J Cell Biol 219, doi: 10.1083/jcb.201912074 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leidal AM et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Biol 22, 187–199, doi: 10.1038/s41556-019-0450-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nolte-’t Hoen EN et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res 40, 9272–9285, doi: 10.1093/nar/gks658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xiao D et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One 7, e46874, doi: 10.1371/journal.pone.0046874 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bolukbasi MF et al. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids 1, e10, doi: 10.1038/mtna.2011.2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu B et al. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun 9, 420, doi: 10.1038/s41467-017-02770-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.D’Souza A et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J Control Release 338, 505–526, doi: 10.1016/j.jconrel.2021.08.038 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang H et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemoresistance in gastric cancer. Mol Cancer 19, 43, doi: 10.1186/s12943-020-01168-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao X et al. Chronic myelogenous leukemia cells remodel the bone marrow niche via exosome-mediated transfer of miR-320. Theranostics 9, 5642–5656, doi: 10.7150/thno.34813 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li Y et al. Heterogeneous Nuclear Ribonucleoprotein A1 Loads Batched Tumor-Promoting MicroRNAs Into Small Extracellular Vesicles With the Assist of Caveolin-1 in A549 Cells. Front Cell Dev Biol 9, 687912, doi: 10.3389/fcell.2021.687912 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Perez-Boza J, Boeckx A, Lion M, Dequiedt F & Struman I hnRNPA2B1 inhibits the exosomal export of miR-503 in endothelial cells. Cell Mol Life Sci 77, 4413–4428, doi: 10.1007/s00018-019-03425-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen C et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin Invest 130, 404–421, doi: 10.1172/JCI130892 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lei Y et al. Tumorreleased lncRNA H19 promotes gefitinib resistance via packaging into exosomes in nonsmall cell lung cancer. Oncol Rep 40, 3438–3446, doi: 10.3892/or.2018.6762 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Han M et al. Exosome-mediated lncRNA AFAP1-AS1 promotes trastuzumab resistance through binding with AUF1 and activating ERBB2 translation. Mol Cancer 19, 26, doi: 10.1186/s12943-020-1145-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Zheng Z, Chen M, Xing P, Yan X & Xie B Increased Expression of Exosomal AGAP2-AS1 (AGAP2 Antisense RNA 1) In Breast Cancer Cells Inhibits Trastuzumab-Induced Cell Cytotoxicity. Med Sci Monit 25, 2211–2220, doi: 10.12659/MSM.915419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kossinova OA et al. Cytosolic YB-1 and NSUN2 are the only proteins recognizing specific motifs present in mRNAs enriched in exosomes. Biochim Biophys Acta Proteins Proteom 1865, 664–673, doi: 10.1016/j.bbapap.2017.03.010 (2017). [DOI] [PubMed] [Google Scholar]
- 169.Grabuschnig S et al. Putative Origins of Cell-Free DNA in Humans: A Review of Active and Passive Nucleic Acid Release Mechanisms. Int J Mol Sci 21, doi: 10.3390/ijms21218062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vagner T et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J Extracell Vesicles 7, 1505403, doi: 10.1080/20013078.2018.1505403 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reis-Sobreiro M et al. Emerin Deregulation Links Nuclear Shape Instability to Metastatic Potential. Cancer Res 78, 6086–6097, doi: 10.1158/0008-5472.CAN-18-0608 (2018). [DOI] [PubMed] [Google Scholar]
- 172.Santavanond JP, Rutter SF, Atkin-Smith GK & Poon IKH Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Subcell Biochem 97, 61–88, doi: 10.1007/978-3-030-67171-6_4 (2021). [DOI] [PubMed] [Google Scholar]
- 173.Poon IK et al. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507, 329–334, doi: 10.1038/nature13147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Phan TK et al. Pannexin-1 channel regulates nuclear content packaging into apoptotic bodies and their size. Proteomics 21, e2000097, doi: 10.1002/pmic.202000097 (2021). [DOI] [PubMed] [Google Scholar]
- 175.Thakur BK et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 24, 766–769, doi: 10.1038/cr.2014.44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kahlert C et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 289, 3869–3875, doi: 10.1074/jbc.C113.532267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lazaro-Ibanez E et al. DNA analysis of low- and high-density fractions defines heterogeneous subpopulations of small extracellular vesicles based on their DNA cargo and topology. J Extracell Vesicles 8, 1656993, doi: 10.1080/20013078.2019.1656993 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jeppesen DK et al. Reassessment of Exosome Composition. Cell 177, 428–445 e418, doi: 10.1016/j.cell.2019.02.029 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Takahashi A et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 8, 15287, doi: 10.1038/ncomms15287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Balaj L et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2, 180, doi: 10.1038/ncomms1180 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yokoi A et al. Mechanisms of nuclear content loading to exosomes. Sci Adv 5, eaax8849, doi: 10.1126/sciadv.aax8849 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hitomi K et al. DNA Damage Regulates Senescence-Associated Extracellular Vesicle Release via the Ceramide Pathway to Prevent Excessive Inflammatory Responses. Int J Mol Sci 21, doi: 10.3390/ijms21103720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kumar R et al. Basic Fibroblast Growth Factor 2-Induced Proteome Changes Endorse Lewy Body Pathology in Hippocampal Neurons. iScience 23, 101349, doi: 10.1016/j.isci.2020.101349 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kumar R et al. Fibroblast Growth Factor 2-Mediated Regulation of Neuronal Exosome Release Depends on VAMP3/Cellubrevin in Hippocampal Neurons. Adv Sci (Weinh) 7, 1902372, doi: 10.1002/advs.201902372 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.de la Cuesta F et al. Extracellular vesicle cross-talk between pulmonary artery smooth muscle cells and endothelium during excessive TGF-beta signalling: implications for PAH vascular remodelling. Cell Commun Signal 17, 143, doi: 10.1186/s12964-019-0449-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fricke F et al. SILAC-Based Quantification of TGFBR2-Regulated Protein Expression in Extracellular Vesicles of Microsatellite Unstable Colorectal Cancers. Int J Mol Sci 20, doi: 10.3390/ijms20174162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Genneback N et al. Growth factor stimulation of cardiomyocytes induces changes in the transcriptional contents of secreted exosomes. J Extracell Vesicles 2, doi: 10.3402/jev.v2i0.20167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ricklefs F et al. Extracellular Vesicles from High-Grade Glioma Exchange Diverse Pro-oncogenic Signals That Maintain Intratumoral Heterogeneity. Cancer Res 76, 2876–2881, doi: 10.1158/0008-5472.CAN-15-3432 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Choi D et al. The Impact of Oncogenic EGFRvIII on the Proteome of Extracellular Vesicles Released from Glioblastoma Cells. Mol Cell Proteomics 17, 1948–1964, doi: 10.1074/mcp.RA118.000644 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Al-Nedawi K et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10, 619–624, doi: 10.1038/ncb1725 (2008). [DOI] [PubMed] [Google Scholar]
- 191.Kreger BT, Dougherty AL, Greene KS, Cerione RA & Antonyak MA Microvesicle Cargo and Function Changes upon Induction of Cellular Transformation. J Biol Chem 291, 19774–19785, doi: 10.1074/jbc.M116.725705 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Timar J & Kashofer K Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev 39, 1029–1038, doi: 10.1007/s10555-020-09915-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Demory Beckler M et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics 12, 343–355, doi: 10.1074/mcp.M112.022806 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Clark DJ, Fondrie WE, Yang A & Mao L Triple SILAC quantitative proteomic analysis reveals differential abundance of cell signaling proteins between normal and lung cancer-derived exosomes. J Proteomics 133, 161–169, doi: 10.1016/j.jprot.2015.12.023 (2016). [DOI] [PubMed] [Google Scholar]
- 195.Hinger SA et al. Rab13 regulates sEV secretion in mutant KRAS colorectal cancer cells. Sci Rep 10, 15804, doi: 10.1038/s41598-020-72503-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McKenzie AJ et al. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep 15, 978–987, doi: 10.1016/j.celrep.2016.03.085 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Guo Q et al. Integrin beta1-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol 71, 1193–1205, doi: 10.1016/j.jhep.2019.07.019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Speziali G et al. Myristic acid induces proteomic and secretomic changes associated with steatosis, cytoskeleton remodeling, endoplasmic reticulum stress, protein turnover and exosome release in HepG2 cells. J Proteomics 181, 118–130, doi: 10.1016/j.jprot.2018.04.008 (2018). [DOI] [PubMed] [Google Scholar]
- 199.Schuck S Microautophagy - distinct molecular mechanisms handle cargoes of many sizes. J Cell Sci 133, doi: 10.1242/jcs.246322 (2020). [DOI] [PubMed] [Google Scholar]
- 200.Murrow L, Malhotra R & Debnath J ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol 17, 300–310, doi: 10.1038/ncb3112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kim YH et al. Secretory autophagy machinery and vesicular trafficking are involved in HMGB1 secretion. Autophagy 17, 2345–2362, doi: 10.1080/15548627.2020.1826690 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fader CM, Sanchez D, Furlan M & Colombo MI Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic 9, 230–250, doi: 10.1111/j.1600-0854.2007.00677.x (2008). [DOI] [PubMed] [Google Scholar]
- 203.Hessvik NP et al. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell Mol Life Sci 73, 4717–4737, doi: 10.1007/s00018-016-2309-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ferreira JV et al. LAMP2A regulates the loading of proteins into exosomes. Sci Adv 8, eabm1140, doi: 10.1126/sciadv.abm1140 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sahu R et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20, 131–139, doi: 10.1016/j.devcel.2010.12.003 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yarana C & St Clair DK Chemotherapy-Induced Tissue Injury: An Insight into the Role of Extracellular Vesicles-Mediated Oxidative Stress Responses. Antioxidants (Basel) 6, doi: 10.3390/antiox6040075 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kore RA et al. Hypoxia-derived exosomes induce putative altered pathways in biosynthesis and ion regulatory channels in glioblastoma cells. Biochem Biophys Rep 14, 104–113, doi: 10.1016/j.bbrep.2018.03.008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ramteke A et al. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog 54, 554–565, doi: 10.1002/mc.22124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Samoylenko A et al. Time-gated Raman spectroscopy and proteomics analyses of hypoxic and normoxic renal carcinoma extracellular vesicles. Sci Rep 11, 19594, doi: 10.1038/s41598-021-99004-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Keulers TG et al. Secretion of pro-angiogenic extracellular vesicles during hypoxia is dependent on the autophagy-related protein GABARAPL1. J Extracell Vesicles 10, e12166, doi: 10.1002/jev2.12166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen X et al. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett 435, 80–91, doi: 10.1016/j.canlet.2018.08.001 (2018). [DOI] [PubMed] [Google Scholar]
- 212.Makiguchi T et al. Serum extracellular vesicular miR-21–5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir Res 17, 110, doi: 10.1186/s12931-016-0427-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bang C et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124, 2136–2146, doi: 10.1172/JCI70577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.King HW, Michael MZ & Gleadle JM Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12, 421, doi: 10.1186/1471-2407-12-421 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tadokoro H, Umezu T, Ohyashiki K, Hirano T & Ohyashiki JH Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem 288, 34343–34351, doi: 10.1074/jbc.M113.480822 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Namazi H et al. Exosomes secreted by hypoxic cardiosphere-derived cells enhance tube formation and increase pro-angiogenic miRNA. J Cell Biochem 119, 4150–4160, doi: 10.1002/jcb.26621 (2018). [DOI] [PubMed] [Google Scholar]
- 217.Zhang J et al. Overexpression of Exosomal Cardioprotective miRNAs Mitigates Hypoxia-Induced H9c2 Cells Apoptosis. Int J Mol Sci 18, doi: 10.3390/ijms18040711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hsu YL et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 36, 4929–4942, doi: 10.1038/onc.2017.105 (2017). [DOI] [PubMed] [Google Scholar]
- 219.Umezu T et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 124, 3748–3757, doi: 10.1182/blood-2014-05-576116 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bister N et al. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. J Extracell Vesicles 10, e12002, doi: 10.1002/jev2.12002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zeng Z et al. Hypoxic exosomal HIF-1alpha-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene 40, 5505–5517, doi: 10.1038/s41388-021-01960-w (2021). [DOI] [PubMed] [Google Scholar]
- 222.Yang K, Zhang J & Bao C Exosomal circEIF3K from cancer-associated fibroblast promotes colorectal cancer (CRC) progression via miR-214/PD-L1 axis. BMC Cancer 21, 933, doi: 10.1186/s12885-021-08669-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang C, Wang H, Li J & Ma L Circular RNA Involvement in the Protective Effect of Human Umbilical Cord Mesenchymal Stromal Cell-Derived Extracellular Vesicles Against Hypoxia/Reoxygenation Injury in Cardiac Cells. Front Cardiovasc Med 8, 626878, doi: 10.3389/fcvm.2021.626878 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ye L et al. Exosomal circEhmt1 Released from Hypoxia-Pretreated Pericytes Regulates High Glucose-Induced Microvascular Dysfunction via the NFIA/NLRP3 Pathway. Oxid Med Cell Longev 2021, 8833098, doi: 10.1155/2021/8833098 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yang H et al. Hypoxia induced exosomal circRNA promotes metastasis of Colorectal Cancer via targeting GEF-H1/RhoA axis. Theranostics 10, 8211–8226, doi: 10.7150/thno.44419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang Y et al. Exosomal circHIPK3 Released from Hypoxia-Pretreated Cardiomyocytes Regulates Oxidative Damage in Cardiac Microvascular Endothelial Cells via the miR-29a/IGF-1 Pathway. Oxid Med Cell Longev 2019, 7954657, doi: 10.1155/2019/7954657 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kucharzewska P et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A 110, 7312–7317, doi: 10.1073/pnas.1220998110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kenneweg F et al. Long Noncoding RNA-Enriched Vesicles Secreted by Hypoxic Cardiomyocytes Drive Cardiac Fibrosis. Mol Ther Nucleic Acids 18, 363–374, doi: 10.1016/j.omtn.2019.09.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee H, Zhang D, Zhu Z, Dela Cruz CS & Jin Y Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep 6, 35250, doi: 10.1038/srep35250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Moon HG et al. Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway. Cell Death Dis 6, e2016, doi: 10.1038/cddis.2015.282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ali A et al. Hyperoxia-activated circulating extracellular vesicles induce lung and brain injury in neonatal rats. Sci Rep 11, 8791, doi: 10.1038/s41598-021-87706-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Alibhai FJ et al. Cellular senescence contributes to age-dependent changes in circulating extracellular vesicle cargo and function. Aging Cell 19, e13103, doi: 10.1111/acel.13103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Terlecki-Zaniewicz L et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging (Albany NY) 10, 1103–1132, doi: 10.18632/aging.101452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Borghesan M et al. Small Extracellular Vesicles Are Key Regulators of Non-cell Autonomous Intercellular Communication in Senescence via the Interferon Protein IFITM3. Cell Rep 27, 3956–3971 e3956, doi: 10.1016/j.celrep.2019.05.095 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kosaka N et al. Exploiting the message from cancer: the diagnostic value of extracellular vesicles for clinical applications. Exp Mol Med 51, 1–9, doi: 10.1038/s12276-019-0219-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wortzel I, Dror S, Kenific CM & Lyden D Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell 49, 347–360, doi: 10.1016/j.devcel.2019.04.011 (2019). [DOI] [PubMed] [Google Scholar]
- 237.Costa-Silva B et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17, 816–826, doi: 10.1038/ncb3169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hoshino A et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335, doi: 10.1038/nature15756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Garcia-Silva S et al. Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism. Nat Cancer 2, 1387–1405, doi: 10.1038/s43018-021-00272-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li XQ, Zhang R, Lu H, Yue XM & Huang YF Extracellular vesicle-packaged CDH11 and ITGA5 induce the premetastatic niche for bone colonization of breast cancer cells. Cancer Res, doi: 10.1158/0008-5472.CAN-21-1331 (2022). [DOI] [PubMed] [Google Scholar]
- 241.Hsu YL et al. Bone-marrow-derived cell-released extracellular vesicle miR-92a regulates hepatic pre-metastatic niche in lung cancer. Oncogene 39, 739–753, doi: 10.1038/s41388-019-1024-y (2020). [DOI] [PubMed] [Google Scholar]
- 242.Wills CA et al. Chemotherapy-Induced Upregulation of Small Extracellular Vesicle-Associated PTX3 Accelerates Breast Cancer Metastasis. Cancer Res 81, 452–463, doi: 10.1158/0008-5472.CAN-20-1976 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Peinado H et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18, 883–891, doi: 10.1038/nm.2753 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hosseini-Beheshti E, Pham S, Adomat H, Li N & Tomlinson Guns ES Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol Cell Proteomics 11, 863–885, doi: 10.1074/mcp.M111.014845 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang Q et al. Mutant KRAS Exosomes Alter the Metabolic State of Recipient Colonic Epithelial Cells. Cell Mol Gastroenterol Hepatol 5, 627–629 e626, doi: 10.1016/j.jcmgh.2018.01.013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Preet R & Dixon DA Mutant KRAS Exosomes Influence the Metabolic State of the Colon Microenvironment. Cell Mol Gastroenterol Hepatol 5, 647–648, doi: 10.1016/j.jcmgh.2018.01.021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhao H et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife 5, e10250, doi: 10.7554/eLife.10250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yi H et al. Exosomes mediated pentose phosphate pathway in ovarian cancer metastasis: a proteomics analysis. Int J Clin Exp Pathol 8, 15719–15728 (2015). [PMC free article] [PubMed] [Google Scholar]
- 249.Wan L et al. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: a role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J 33, 8530–8542, doi: 10.1096/fj.201802675R (2019). [DOI] [PubMed] [Google Scholar]
- 250.Lim AR, Vincent BG, Weaver AM & Rathmell WK Sunitinib and Axitinib increase secretion and glycolytic activity of small extracellular vesicles in renal cell carcinoma. Cancer Gene Ther, doi: 10.1038/s41417-021-00345-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Minciacchi VR et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 6, 11327–11341, doi: 10.18632/oncotarget.3598 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Crescitelli R et al. Subpopulations of extracellular vesicles from human metastatic melanoma tissue identified by quantitative proteomics after optimized isolation. J Extracell Vesicles 9, 1722433, doi: 10.1080/20013078.2020.1722433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lischnig A, Bergqvist M, Ochiya T & Lasser C Quantitative Proteomics Identifies Proteins Enriched in Large and Small Extracellular Vesicles. Mol Cell Proteomics 21, 100273, doi: 10.1016/j.mcpro.2022.100273 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Phinney DG et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 6, 8472, doi: 10.1038/ncomms9472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jiao H et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell 184, 2896–2910 e2813, doi: 10.1016/j.cell.2021.04.027 (2021). [DOI] [PubMed] [Google Scholar]
- 256.D’Acunzo P et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci Adv 7, doi: 10.1126/sciadv.abe5085 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Temoche-Diaz MM et al. Distinct mechanisms of microRNA sorting into cancer cell-derived extracellular vesicle subtypes. Elife 8, doi: 10.7554/eLife.47544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tuzesi A et al. Pediatric brain tumor cells release exosomes with a miRNA repertoire that differs from exosomes secreted by normal cells. Oncotarget 8, 90164–90175, doi: 10.18632/oncotarget.21621 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ye SB et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 5, 5439–5452, doi: 10.18632/oncotarget.2118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Teng Y et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun 8, 14448, doi: 10.1038/ncomms14448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hu X et al. Exosomes Derived from Hypoxic Colorectal Cancer Cells Transfer miR-410–3p to Regulate Tumor Progression. J Cancer 11, 4724–4735, doi: 10.7150/jca.33232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Qin X et al. Cisplatin-resistant lung cancer cell-derived exosomes increase cisplatin resistance of recipient cells in exosomal miR-100–5p-dependent manner. Int J Nanomedicine 12, 3721–3733, doi: 10.2147/IJN.S131516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Roche PA & Furuta K The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 15, 203–216, doi: 10.1038/nri3818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Raposo G et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183, 1161–1172, doi: 10.1084/jem.183.3.1161 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wheway J, Latham SL, Combes V & Grau GE Endothelial microparticles interact with and support the proliferation of T cells. J Immunol 193, 3378–3387, doi: 10.4049/jimmunol.1303431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tkach M et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J 36, 3012–3028, doi: 10.15252/embj.201696003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Buschow SI et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 10, 1528–1542, doi: 10.1111/j.1600-0854.2009.00963.x (2009). [DOI] [PubMed] [Google Scholar]
- 268.Thery C et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3, 1156–1162, doi: 10.1038/ni854 (2002). [DOI] [PubMed] [Google Scholar]
- 269.Muntasell A, Berger AC & Roche PA T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J 26, 4263–4272, doi: 10.1038/sj.emboj.7601842 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chen G et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386, doi: 10.1038/s41586-018-0392-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Monypenny J et al. ALIX Regulates Tumor-Mediated Immunosuppression by Controlling EGFR Activity and PD-L1 Presentation. Cell Rep 24, 630–641, doi: 10.1016/j.celrep.2018.06.066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yang Y et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res 28, 862–864, doi: 10.1038/s41422-018-0060-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ricklefs FL et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv 4, eaar2766, doi: 10.1126/sciadv.aar2766 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lundholm M et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One 9, e108925, doi: 10.1371/journal.pone.0108925 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ashiru O et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res 70, 481–489, doi: 10.1158/0008-5472.CAN-09-1688 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Plebanek MP et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun 8, 1319, doi: 10.1038/s41467-017-01433-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Chiou NT, Kageyama R & Ansel KM Selective Export into Extracellular Vesicles and Function of tRNA Fragments during T Cell Activation. Cell Rep 25, 3356–3370 e3354, doi: 10.1016/j.celrep.2018.11.073 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Keam SP, Sobala A, Ten Have S & Hutvagner G tRNA-Derived RNA Fragments Associate with Human Multisynthetase Complex (MSC) and Modulate Ribosomal Protein Translation. J Proteome Res 16, 413–420, doi: 10.1021/acs.jproteome.6b00267 (2017). [DOI] [PubMed] [Google Scholar]
- 279.Kim HK et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature 552, 57–62, doi: 10.1038/nature25005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Goodarzi H et al. Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell 161, 790–802, doi: 10.1016/j.cell.2015.02.053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Saikia M et al. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol Cell Biol 34, 2450–2463, doi: 10.1128/MCB.00136-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Alexander M et al. Rab27-Dependent Exosome Production Inhibits Chronic Inflammation and Enables Acute Responses to Inflammatory Stimuli. J Immunol 199, 3559–3570, doi: 10.4049/jimmunol.1700904 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Driedonks TAP et al. Immune stimuli shape the small non-coding transcriptome of extracellular vesicles released by dendritic cells. Cell Mol Life Sci 75, 3857–3875, doi: 10.1007/s00018-018-2842-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Patel MR & Weaver AM Astrocyte-derived small extracellular vesicles promote synapse formation via fibulin-2-mediated TGF-beta signaling. Cell Rep 34, 108829, doi: 10.1016/j.celrep.2021.108829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Vilcaes AA, Chanaday NL & Kavalali ET Interneuronal exchange and functional integration of synaptobrevin via extracellular vesicles. Neuron 109, 971–983 e975, doi: 10.1016/j.neuron.2021.01.007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Silverman JM et al. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem 294, 3744–3759, doi: 10.1074/jbc.RA118.004825 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kramer-Albers EM et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl 1, 1446–1461, doi: 10.1002/prca.200700522 (2007). [DOI] [PubMed] [Google Scholar]
- 288.Korkut C et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139, 393–404, doi: 10.1016/j.cell.2009.07.051 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Fruhbeis C et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11, e1001604, doi: 10.1371/journal.pbio.1001604 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lachenal G et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci 46, 409–418, doi: 10.1016/j.mcn.2010.11.004 (2011). [DOI] [PubMed] [Google Scholar]
- 291.Ibanez F, Montesinos J, Urena-Peralta JR, Guerri C & Pascual M TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J Neuroinflammation 16, 136, doi: 10.1186/s12974-019-1529-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Datta Chaudhuri A et al. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia 68, 128–144, doi: 10.1002/glia.23708 (2019). [DOI] [PubMed] [Google Scholar]
- 293.Hill AF Extracellular Vesicles and Neurodegenerative Diseases. J Neurosci 39, 9269–9273, doi: 10.1523/JNEUROSCI.0147-18.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Miyoshi E et al. Exosomal tau with seeding activity is released from Alzheimer’s disease synapses, and seeding potential is associated with amyloid beta. Lab Invest 101, 1605–1617, doi: 10.1038/s41374-021-00644-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Dujardin S et al. Ectosomes: a new mechanism for non-exosomal secretion of tau protein. PLoS One 9, e100760, doi: 10.1371/journal.pone.0100760 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Podvin S et al. Dysregulation of Exosome Cargo by Mutant Tau Expressed in Human-induced Pluripotent Stem Cell (iPSC) Neurons Revealed by Proteomics Analyses. Mol Cell Proteomics 19, 1017–1034, doi: 10.1074/mcp.RA120.002079 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sinning JM et al. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J 32, 2034–2041, doi: 10.1093/eurheartj/ehq478 (2011). [DOI] [PubMed] [Google Scholar]
- 298.Gidlof O et al. Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone. Sci Rep 9, 8991, doi: 10.1038/s41598-019-45473-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.de Hoog VC et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2, 53–60, doi: 10.1177/2048872612471212 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Loyer X et al. Intra-Cardiac Release of Extracellular Vesicles Shapes Inflammation Following Myocardial Infarction. Circ Res 123, 100–106, doi: 10.1161/CIRCRESAHA.117.311326 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Goettsch C et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest 126, 1323–1336, doi: 10.1172/JCI80851 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Garcia-Martin R, Brandao BB, Thomou T, Altindis E & Kahn CR Tissue differences in the exosomal/small extracellular vesicle proteome and their potential as indicators of altered tissue metabolism. Cell Rep 38, 110277, doi: 10.1016/j.celrep.2021.110277 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Durcin M et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles 6, 1305677, doi: 10.1080/20013078.2017.1305677 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sano S et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun 445, 327–333, doi: 10.1016/j.bbrc.2014.01.183 (2014). [DOI] [PubMed] [Google Scholar]
- 305.Kwan HY, Chen M, Xu K & Chen B The impact of obesity on adipocyte-derived extracellular vesicles. Cell Mol Life Sci 78, 7275–7288, doi: 10.1007/s00018-021-03973-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kim JI et al. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol 35, 1686–1699, doi: 10.1128/MCB.01321-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Li Y, Talbot CL & Chaurasia B Ceramides in Adipose Tissue. Front Endocrinol (Lausanne) 11, 407, doi: 10.3389/fendo.2020.00407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Giordano C et al. Leptin Modulates Exosome Biogenesis in Breast Cancer Cells: An Additional Mechanism in Cell-to-Cell Communication. J Clin Med 8, doi: 10.3390/jcm8071027 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Yan C et al. A High-Fat Diet Attenuates AMPK alpha1 in Adipocytes to Induce Exosome Shedding and Nonalcoholic Fatty Liver Development In Vivo. Diabetes 70, 577–588, doi: 10.2337/db20-0146 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Guay C & Regazzi R Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab 19 Suppl 1, 137–146, doi: 10.1111/dom.13027 (2017). [DOI] [PubMed] [Google Scholar]
- 311.Rome S, Blandin A & Le Lay S Adipocyte-Derived Extracellular Vesicles: State of the Art. Int J Mol Sci 22, doi: 10.3390/ijms22041788 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mazurov D, Barbashova L & Filatov A Tetraspanin protein CD9 interacts with metalloprotease CD10 and enhances its release via exosomes. FEBS J 280, 1200–1213, doi: 10.1111/febs.12110 (2013). [DOI] [PubMed] [Google Scholar]
- 313.Kim SB et al. Caspase-8 controls the secretion of inflammatory lysyl-tRNA synthetase in exosomes from cancer cells. J Cell Biol 216, 2201–2216, doi: 10.1083/jcb.201605118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Dores MR et al. ALIX binds a YPX(3)L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J Cell Biol 197, 407–419, doi: 10.1083/jcb.201110031 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Han Q et al. Vps4A mediates the localization and exosome release of beta-catenin to inhibit epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett 457, 47–59, doi: 10.1016/j.canlet.2019.04.035 (2019). [DOI] [PubMed] [Google Scholar]
- 316.Zietzer A et al. The RNA-binding protein hnRNPU regulates the sorting of microRNA-30c-5p into large extracellular vesicles. J Extracell Vesicles 9, 1786967, doi: 10.1080/20013078.2020.1786967 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Terlecki-Zaniewicz L et al. Extracellular Vesicles in Human Skin: Cross-Talk from Senescent Fibroblasts to Keratinocytes by miRNAs. J Invest Dermatol 139, 2425–2436 e2425, doi: 10.1016/j.jid.2019.05.015 (2019). [DOI] [PubMed] [Google Scholar]
- 318.Mukherjee K et al. Reversible HuR-microRNA binding controls extracellular export of miR-122 and augments stress response. EMBO Rep 17, 1184–1203, doi: 10.15252/embr.201541930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Nikitidou E et al. Increased Release of Apolipoprotein E in Extracellular Vesicles Following Amyloid-beta Protofibril Exposure of Neuroglial Co-Cultures. J Alzheimers Dis 60, 305–321, doi: 10.3233/JAD-170278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bin BH et al. Fibronectin-Containing Extracellular Vesicles Protect Melanocytes against Ultraviolet Radiation-Induced Cytotoxicity. J Invest Dermatol 136, 957–966, doi: 10.1016/j.jid.2015.08.001 (2016). [DOI] [PubMed] [Google Scholar]
- 321.Wang G et al. LPS-induced macrophage HMGB1-loaded extracellular vesicles trigger hepatocyte pyroptosis by activating the NLRP3 inflammasome. Cell Death Discov 7, 337, doi: 10.1038/s41420-021-00729-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kreger BT, Johansen ER, Cerione RA & Antonyak MA The Enrichment of Survivin in Exosomes from Breast Cancer Cells Treated with Paclitaxel Promotes Cell Survival and Chemoresistance. Cancers (Basel) 8, doi: 10.3390/cancers8120111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Di Vizio D et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol 181, 1573–1584, doi: 10.1016/j.ajpath.2012.07.030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.McConnell RE & Tyska MJ Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J Cell Biol 177, 671–681, doi: 10.1083/jcb.200701144 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Grootjans JJ et al. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci U S A 94, 13683–13688, doi: 10.1073/pnas.94.25.13683 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Solvik TA et al. Secretory autophagy maintains proteostasis upon lysosome inhibition. J Cell Biol 221, doi: 10.1083/jcb.202110151 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Verweij FJ et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J Cell Biol 217, 1129–1142, doi: 10.1083/jcb.201703206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.McNamara RP et al. Imaging of surface microdomains on individual extracellular vesicles in 3-D. J Extracell Vesicles 11, e12191, doi: 10.1002/jev2.12191 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hilton SH & White IM Advances in the analysis of single extracellular vesicles: A critical review. Sens Actuators Rep 3, doi: 10.1016/j.snr.2021.100052 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Tosar JP, Witwer K & Cayota A Revisiting Extracellular RNA Release, Processing, and Function. Trends Biochem Sci 46, 438–445, doi: 10.1016/j.tibs.2020.12.008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Lobert VH et al. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell 19, 148–159, doi: 10.1016/j.devcel.2010.06.010 (2010). [DOI] [PubMed] [Google Scholar]
- 332.Wang JH et al. Anti-HER2 scFv-Directed Extracellular Vesicle-Mediated mRNA-Based Gene Delivery Inhibits Growth of HER2-Positive Human Breast Tumor Xenografts by Prodrug Activation. Mol Cancer Ther 17, 1133–1142, doi: 10.1158/1535-7163.MCT-17-0827 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Komuro H et al. Engineering Extracellular Vesicles to Target Pancreatic Tissue In Vivo. Nanotheranostics 5, 378–390, doi: 10.7150/ntno.54879 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Delcayre A et al. Exosome Display technology: applications to the development of new diagnostics and therapeutics. Blood Cells Mol Dis 35, 158–168, doi: 10.1016/j.bcmd.2005.07.003 (2005). [DOI] [PubMed] [Google Scholar]
- 335.Liang G et al. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomedicine 13, 585–599, doi: 10.2147/IJN.S154458 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lai CP et al. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun 6, 7029, doi: 10.1038/ncomms8029 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Yim N et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun 7, 12277, doi: 10.1038/ncomms12277 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Dooley K et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol Ther 29, 1729–1743, doi: 10.1016/j.ymthe.2021.01.020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zomer A et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057, doi: 10.1016/j.cell.2015.04.042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ridder K et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology 4, e1008371, doi: 10.1080/2162402X.2015.1008371 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Schneider J et al. Cre mRNA Is Not Transferred by EVs from Endothelial and Adipose-Derived Stromal/Stem Cells during Vascular Network Formation. Int J Mol Sci 22, doi: 10.3390/ijms22084050 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Reshke R et al. Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat Biomed Eng 4, 52–68, doi: 10.1038/s41551-019-0502-4 (2020). [DOI] [PubMed] [Google Scholar]
- 343.Nolte-’t Hoen E, Cremer T, Gallo RC & Margolis LB Extracellular vesicles and viruses: Are they close relatives? Proc Natl Acad Sci U S A 113, 9155–9161, doi: 10.1073/pnas.1605146113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rauch S & Martin-Serrano J Multiple interactions between the ESCRT machinery and arrestin-related proteins: implications for PPXY-dependent budding. J Virol 85, 3546–3556, doi: 10.1128/JVI.02045-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Jolly C & Sattentau QJ Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J Virol 81, 7873–7884, doi: 10.1128/JVI.01845-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Dahmane S et al. Nanoscale organization of tetraspanins during HIV-1 budding by correlative dSTORM/AFM. Nanoscale 11, 6036–6044, doi: 10.1039/c8nr07269h (2019). [DOI] [PubMed] [Google Scholar]
- 347.Gerber PP et al. Rab27a controls HIV-1 assembly by regulating plasma membrane levels of phosphatidylinositol 4,5-bisphosphate. J Cell Biol 209, 435–452, doi: 10.1083/jcb.201409082 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Kerviel A, Zhang M & Altan-Bonnet N A New Infectious Unit: Extracellular Vesicles Carrying Virus Populations. Annu Rev Cell Dev Biol 37, 171–197, doi: 10.1146/annurev-cellbio-040621-032416 (2021). [DOI] [PubMed] [Google Scholar]
- 349.Chen YH et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 160, 619–630, doi: 10.1016/j.cell.2015.01.032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Santiana M et al. Vesicle-Cloaked Virus Clusters Are Optimal Units for Inter-organismal Viral Transmission. Cell Host Microbe 24, 208–220 e208, doi: 10.1016/j.chom.2018.07.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Giannessi F, Aiello A, Franchi F, Percario ZA & Affabris E The Role of Extracellular Vesicles as Allies of HIV, HCV and SARS Viruses. Viruses 12, doi: 10.3390/v12050571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


