Abstract
Genome driven precision oncology has transformed the landscape of multiple cancers. However, access barriers exist. A recent study exemplified a direct-to-patient outreach program via social media through the implementation of a global program that offered free tumor genomic testing with a focus on rare cancers.
Keywords: precision medicine, targeted therapy, outreach, social media, NGS
In this issue of Clinical Cancer Research, Doe-Tetteh and colleagues,(1) sought to address the obstacles faced by patients with rare cancers in accessing genomic testing, by establishing a program that provided free clinical tumor genomic testing worldwide. Patients were recruited via social media outreach and disease-specific advocacy groups, with a particular rare cancer focus eg. histiocytosis, germ cell tumors, and pediatric cancers. The study utilized the MSK-IMPACT next-generation sequencing assay for tumor analysis, and the findings were shared with patients and their local healthcare providers.
Since the inception of the Human Genome Project, the cost of next-generation sequencing (NGS) technologies has decreased considerably enabling large scale genomic testing for cancer and other diseases. In parallel, there has been a notable shift in cancer treatment from broad-spectrum cytotoxic drugs to genomically targeted therapy and immunotherapy(2). These approaches now constitute essential pillars of cancer therapy. We are witnessing a surge in the incorporation of tumor genomic profiling into the care continuum for diverse malignancies. This is attributed to the remarkable headway in outcomes achieved with targeted therapy across a spectrum of tumor types.
Apart from a multitude of precision drugs being approved for tissue-specific indications, such as osimertinib for EGFR-driven lung cancer or pemigatinib for FGFR2-positive cholangiocarcinoma, the FDA has approved several drugs in a tissue-agnostic manner. Examples include pembrolizumab for MSI-H/d-MMR and high TMB cancers, larotrectinib and entrectinib for NTRK-positive cancers, selpercatinib for RET-positive cancers, and dabrafenib/trametinib for BRAF V600+ cancers (3). As these genomic alterations are present in a wide range of tumor histologies, this presents an opportunity to employ analogous therapeutic approaches for uncommon tumor histologies, where conducting large-scale studies may be challenging.(4) However, there may be significant barriers to genomic testing that prevent many patients from accessing these innovative treatments. These include lack of awareness among patients and physicians about the benefits and availability of genomic testing, high costs and insurance coverage issues, limited access to testing facilities and qualified personnel, variability in test quality and interpretation, ethical and legal challenges related to data sharing and privacy.(5)
The study by Doe-Tetteh and colleagues enrolled a total of 333 patients, from whom 288 tumor tissue samples were collected(1). Of these, 250 samples were of sufficient quality for MSK-IMPACT testing. Subsequently, 18 patients with histiocytosis were treated with genomically guided therapy, resulting in clinical benefit for 17 of them. The mean treatment duration was 21.7 months (range 6–40+).Furthermore, the whole exome sequencing of ovarian germ cell tumors uncovered a unique subset with haploid genotypes - a phenotype rarely observed in other cancer types. Although actionable genomic alterations were infrequent in ovarian germ cell tumors, two patients with ovarian germ cell tumors with squamous transformation exhibited a high tumor mutational burden. One patient had a complete response to pembrolizumab.
The study highlights the importance of direct-to-patient outreach in constructing sufficient rare cancer cohorts to define their genomic landscape and deliver precision medicine ( Figure 1). This can be further amplified with engagement of patient advocacy organizations, as shown by this study where 27% of referrals originated from a disease advocacy group. Tumor genotype driven patient advocacy groups (for example: KRAS Kickers, EGFR Resisters, ROS1ders, RET positives) are organizations that represent and support patients who have cancers with specific genetic alterations. These groups play a crucial role in oncology by providing education, resources, and guidance to patients and their families, as well as influencing research priorities, clinical trials design, and policy changes that affect their communities.(6). More and more, crowdsourcing initiatives are contributing to patient recruitment for clinical trials and research studies. They are also providing extensive and diverse patient data, creating funding opportunities for research that addresses patient-related inquiries, and establishing communication channels for patient-centered discussions.(7, 8)
Figure 1:
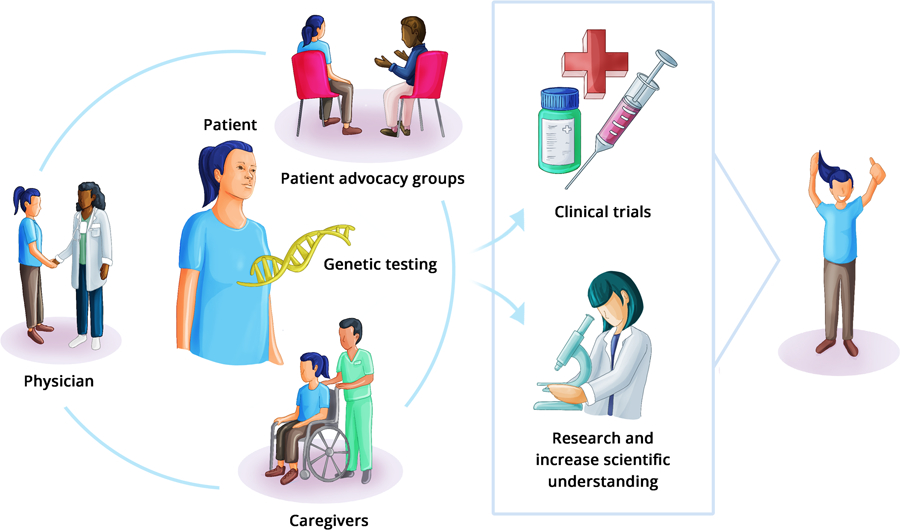
Patient driven genomic testing and rare disease patient advocacy groups. (Figure by DrawImpacts.)
This study highlights the crucial role of the treating oncologist in bridging the care gap through both genomic testing and targeted therapy, given that approximately 50% of genomic testing referrals were made by physicians. This is especially important for patients in lower middle income countries who may have limited clinical trial access and such efforts can align treatment access with cancer burden.(9–11) Notably, in this study 37% of patients were enrolled from sites outside the United States across 17 countries. Despite this, ethnic minorities including African American, and Hispanic patients were underrepresented in both the histiocytosis (13%) and ovarian tumor (19%) cohorts in the study. This is specifically important to study genetic, and ancestral differences in tumor makeup based on race and ethnicity, and improving generalization and applicability of results from clinical trials.(12)
Doe-Tetteh et al(1). also provided insights into application of novel approaches such as incorporation of tele health, virtual consent procedures, and stepwise use of whole exome sequencing when no oncogenic alterations were identified by usual next generation sequencing techniques. This approach facilitated the integration of specialized cancer centers’ expertise in treating rare cancers into community oncologic care thus ensuring that patients receive optimal genome informed therapies. Taking this proof of concept and leveraging advances in technology development during the pandemic can potentially alter the next generation of evidence based medicine.(13, 14)
One challenge with application of precision oncology is the availability of tumor DNA of sufficient quality. Out of the 288 patients for which tissue was received, only 250 (86.8%) could undergo NGS testing. The emergence of circulating tumor DNA technologies has facilitated the identification of genomic drivers in this context, thereby informing treatment selection.(15) Employing such minimally invasive techniques to inform treatment selection investigate resistance pathways and is crucial for patients experiencing resistance to standard targeted therapies and for the development of future targeted therapies. One such effort is the Studying Pathways of Resistance in KRAS-driven Cancers (SPARK) study (NCT05272423) which aims to study pathways of resistance to KRASG12C inhibitors (sotorasib and adagrasib) through a remote participation plasma NGS study. However, given the rapid pace of these significant developments, it is imperative that we do not fall behind in implementation and ensure global access and affordability to prevent further widening of cancer disparities.(11, 16)
In conclusion, tumor genomic profiling has the potential to revolutionize cancer diagnosis and treatment. However, barriers to access still exist. Direct-to-patient outreach programs can be instrumental in overcoming barriers to precision oncology. Such programs can increase awareness and scientific understanding of rare diseases, address concerns about cost, and promote equal access to this technology. By working collaboratively to overcome these obstacles, we can unlock the potential of precision oncology to improve patient outcomes. Taking small steps in this direction can result in giant leaps towards significant advances.
Acknowledgements:
Vivek Subbiah (VS) is an Andrew Sabin Family Foundation fellow at the University of Texas MD Anderson Cancer Center. VS acknowledges the support of the Jacquelyn A. Brady Fund. VS is supported by a US National Institutes of Health (NIH) grant (no. R01CA242845 and R01CA273168); MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (no. RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (no. 1U01 CA180964), NCATS (Center for Clinical and Translational Sciences) Grant (no. UL1 TR000371), and the MD Anderson Cancer Center Support Grant (no. P30 CA016672). We also acknowledge the team at DrawImpacts for help in creating Figure.
Footnotes
Conflict of Interest: AD reports advisory board/consultant position with Sanofi, Amgen and Foundation Medicine. VS reports grants from Eli Lilly/LOXO Oncology, Blueprint Medicines Corporation, Turning Point Therapeutics, Boston Pharmaceuticals; and grants from Helsinn Pharmaceuticals during the conduct of the study; in addition, V. Subbiah reports a grant and advisory board/consultant position with Eli Lilly/Loxo Oncology during the conduct of the study; research grants from Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP, University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, Pharmamar, Medimmune; an advisory board/consultant position with Helsinn, Incyte, QED Pharma, Daiichi-Sankyo, Signant Health, Novartis, Relay therapeutics, Roche, Medimmune; travel funds from Pharmamar, Incyte, ASCO, ESMO; other support from Medscape; all outside the submitted work.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Doe-Tetteh SA, Camp SY, Reales D, Crowdis J, Noronha AM, Wolff B, et al. Overcoming barriers to tumor genomic profiling through direct-to-patient outreach. Clin Cancer Res 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subbiah V, Kurzrock R. Challenging Standard-of-Care Paradigms in the Precision Oncology Era. Trends Cancer 2018;4:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbiah V, Wirth LJ, Kurzrock R, Pazdur R, Beaver JA, Singh H, et al. Accelerated approvals hit the target in precision oncology. Nature Medicine 2022;28:1976–9. [DOI] [PubMed] [Google Scholar]
- 4.Adashek JJ, Menta AK, Reddy NK, Desai AP, Roszik J, Subbiah V. Tissue-Agnostic Activity of BRAF plus MEK Inhibitor in BRAF V600–Mutant Tumors. Molecular cancer therapeutics 2022;21:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadik H, Pritchard D, Keeling D-M, Policht F, Riccelli P, Stone G, et al. Impact of clinical practice gaps on the implementation of personalized medicine in advanced non–small-cell lung cancer. JCO Precision Oncology 2022;6:e2200246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolgin E Drivers of change. Nature 2020;587:S16–S7. [DOI] [PubMed] [Google Scholar]
- 7.Sweeney NW, Ahlstrom JM, Fonseca R, Davies FE, Thompson MA. HealthTree Cure Hub: a patient-derived, patient-driven clinical cancer information platform used to overcome hurdles and accelerate research in multiple myeloma. JCO clinical cancer informatics 2022;6:e2100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai A, Warner J, Kuderer N, Thompson M, Painter C, Lyman G, et al. Crowdsourcing a crisis response for COVID-19 in oncology. Nature cancer 2020;1:473–6. [DOI] [PubMed] [Google Scholar]
- 9.Desai A, Sirohi B, Mathew A. Clinical trial access in low-and middle-income countries: a case study on India. Cancer Investigation 2021;39:685–9. [DOI] [PubMed] [Google Scholar]
- 10.Desai A, Kuderer NM, Lyman GH. Aligning cancer clinical trials with cancer burden: need for greater global leadership, resources, and vision. JAMA oncology 2021;7:357–8. [DOI] [PubMed] [Google Scholar]
- 11.Moyers JT, Subbiah V. Think Globally, Act Locally: Globalizing Precision Oncology. Cancer discovery 2022;12:886–8. [DOI] [PubMed] [Google Scholar]
- 12.Chiang RS, Desai A, Glover MJ, Hui G, Ramchandran KJ, Wakelee H, et al. Racial Diversity and Reporting in United States Food and Drug Administration Registration Trials for Thoracic Malignancies from 2006 to 2020. Cancer Investigation 2023;41:43–7. [DOI] [PubMed] [Google Scholar]
- 13.Subbiah V The next generation of evidence-based medicine. Nature Medicine 2023:1–10. [DOI] [PubMed] [Google Scholar]
- 14.Desai A, Subbiah V. COVID-19 pandemic and cancer clinical trial pandemonium: finding the silver lining. Journal of immunotherapy and precision oncology 2021;4:64–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martins I, Ribeiro IP, Jorge J, Gonçalves AC, Sarmento-Ribeiro AB, Melo JB, et al. Liquid biopsies: applications for cancer diagnosis and monitoring. Genes 2021;12:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai AP, Scheckel CJ, Soderberg LC, Jensen CJ, Orme JJ, Tella SH, et al. Economic Cost and Sustainability of Oral Therapies in Precision Oncology. JCO Oncology Practice 2022;18:e1247–e54. [DOI] [PubMed] [Google Scholar]


