Abstract
Purpose:
Cisplatin-induced hearing loss (CIHL) is common and permanent. As compared to earlier otoprotectants, we hypothesized N-acetylcysteine (NAC) offers potential for stronger otoprotection through stimulation of glutathione production. This study tested the optimal dose, safety, and efficacy of NAC to prevent CIHL.
Patients and Methods:
In this nonrandomized, controlled Phase 1a/1b trial, children and adolescents newly diagnosed with non-metastatic, cisplatin-treated tumors received NAC intravenously four hours post-cisplatin. The trial performed dose-escalation across three dose levels to establish a safe dose that exceeded the targeted peak serum NAC concentration of 1.5 mM (as identified from preclinical models). Patients with metastatic disease or otherwise ineligible were enrolled in an observation-only/control arm. To evaluate efficacy, serial age-appropriate audiology assessments were performed. Integrated biology examined genes involved in glutathione metabolism and post-NAC glutathione concentrations.
Results:
Of 52 patients enrolled, 24 received NAC and 28 were in the control arm. The maximum tolerated dose was not reached; analysis of peak [NAC] identified 450 mg/kg as the recommended Phase 2 dose (RP2D). Infusion-related reactions were common. No severe adverse events occurred. Compared to the control arm, NAC decreased likelihood of CIHL at end of cisplatin therapy (odds ratio [OR] 0.13, 95% confidence interval [CI] 0.021-0.847, p=0.033) and recommendations for hearing intervention at end of study (OR 0.082, 95%CI 0.011-0.60, p=0.014). NAC increased glutathione; GSTP1 influenced risk for CIHL and NAC otoprotection.
Conclusion:
NAC was safe at the RP2D with strong evidence for efficacy to prevent CIHL, warranting further development as a next-generation otoprotectant.
Trial number:
Keywords: Cisplatin, Ototoxicity, Hearing Loss, Otoprotection, N-acetylcysteine, Neoplasm
Statement of Translational Relevance:
Cisplatin-induced hearing loss (CIHL) results from oxidant-mediated damage to the cochlea. Sodium thiosulfate (STS) successfully reduced CIHL in recently concluded randomized trials, the first thiol-class agent to do so. N-acetylcysteine (NAC) is a strong thiol antioxidant, but also acts as a glutathione prodrug, stimulating glutathione production to restore innate resistance to oxidant damage. In this non-randomized, controlled Phase 1a/1b trial, NAC demonstrated clear efficacy and safety signals as an otoprotectant, warranting further development in randomized trials. Although multiple otoprotective mechanisms have been proposed, NAC’s recognized role in glutathione metabolism, reinforced by the serum and pharmacogenomics findings, support targeting the antioxidant pathway for otoprotection, including further investigation into glutathione’s central role in CIHL and otoprotection. Moreover, with marked heterogeneity in tumor types treated and cisplatin-based chemotherapy regimens, this successful Phase 1 trial highlights the critical importance of selecting clinically relevant tumors and infusion methods in developing novel otoprotectants.
Introduction
Cisplatin is a platinum chemotherapy used to treat pediatric and adult solid tumors. While integral for cure, cisplatin has multiple off-target effects, commonly resulting in ototoxicity as well as myelosuppression, nephropathy, and peripheral neuropathy. In children, moderate to severe cisplatin-induced hearing loss (CIHL) impacting communication affects ~40% of exposed patients, with incidence exceeding 60% in certain patient subsets.(1) CIHL is permanent and progressive with continued cisplatin administration.(2) Children are particularly sensitive to the adverse impact of hearing loss, developing lifelong deficits in neurocognition, communication, social adaptation, and job and academic performance.(3–5) To mitigate the severity of CIHL, providers typically reduce or omit cisplatin dosing once ototoxicity becomes evident. This therapy-limiting approach potentially compromises disease cure and does not reverse existing cochlear damage. New otoprotective strategies have therefore pivoted to preventing cochlear damage through identifying mechanisms of ototoxicity and integrating targeted otoprotectant compounds into regimens.(6) Sodium thiosulfate (STS), a “thiol-class” otoprotectant, demonstrated efficacy in two international Phase 3 trials and is the only currently approved drug to prevent CIHL.(7,8) As with any first-in-class agent, STS offers benefit along with several opportunities for improvement. Nearly a third of patients receiving STS in the two trials still developed CIHL. STS also did not offer protection from cisplatin-induced nephrotoxicity or myelosuppression in either trial; indeed, STS may even have exacerbated cisplatin nephropathy.(8) Finally, STS infusions are complicated by infusion-related reactions, manifesting as vomiting, hypotension, and/or severe rigors.(8) Nonetheless, STS provided proof-of-principle for thiol-based otoprotection. A second-generation agent would offer the possibility of greater efficacy to prevent CIHL, reduced risk for multiple cisplatin toxicities, and improved tolerability of infusions.
To address these needs, we developed N-acetylcysteine (NAC) for a new role as a next-generation thiol-class otoprotectant. NAC was selected as a targeted agent to address a key mechanism of cisplatin cochlear damage.(6) The cochlea is a fixed system with little ability to eliminate cisplatin;(9) cisplatin depletes cochlear glutathione (GSH), causing free-radicals and oxidant damage, resulting in CIHL.(6,10–12) STS addresses this mechanism by forming STS-platinum adducts to reduce cisplatin exposure and by binding free radicals in the cochlea.(13,14) NAC similarly binds cisplatin (15) and free radicals,(16) but in response to oxidant stress and GSH depletion also serves as a GSH prodrug.(16) Thus, NAC has the additional capacity to directly respond to cisplatin-induced effects in the cochlea by restoring GSH synthesis and intrinsic antioxidant capacity. Both in vitro and in vivo testing of NAC supported its potential for cisplatin otoprotection,(17–21) and potentially marrow- and nephroprotection too.(22,23) NAC has the additional benefit of a long history of clinical usage, well-characterized pharmacokinetics, and patient data for efficacy to prevent nephrotoxicity from non-neoplastic nephrotoxins,(24) many with shared physiology with cisplatin kidney injury. (25,26) Of critical importance for its planned inclusion into frontline chemotherapy, in vitro and in vivo animal models of two pediatric and one adult tumor demonstrated no interaction between post-cisplatin NAC rescue and cisplatin efficacy.(19,27,28) From those preliminary data, we hypothesized that NAC is safe to incorporate into frontline chemotherapy and is a more effective otoprotectant, benefitting from its additional promotion of the glutathione pathway. We present here the results of a Phase I trial of the first use of NAC for otoprotection from cisplatin-based chemotherapy. The trial utilized a controlled design enabling concurrent evaluation of its biologically active dose, maximum tolerated dose, and preliminary evidence for efficacy.
Patients and Methods
Patient eligibility.
Eligible patients were 1-21 years old and newly diagnosed with a solid tumor requiring cisplatin chemotherapy. To receive NAC, treatment regimens included ≥2 cycles of cisplatin, a planned cumulative cisplatin dose ≥200 mg/m2, a cisplatin infusion time of ≤6 hours, and ≤2 consecutive days of cisplatin. Due to concerns for potential treatment interactions among those with metastatic disease who received STS on the COG ACCL0431 trial,(8,29) only patients with localized tumors were eligible to receive NAC rescue. However, for the included pediatric tumors, patients received identical cisplatin dosing irrespective of metastatic disease at presentation; patients with metastatic hepatic tumors may have received additional cycles of chemotherapy. Patients were required to have adequate renal function (GFR≥ 60 ml/min/1.73m2 or a serum creatinine <1.5x upper limit of normal for age), an electrocardiogram with a normal sinus rhythm and QTc <500, and adequate organ function as per the primary chemotherapy regimen. Patients were not eligible for NAC if they had metastatic tumors at diagnosis, known allergy to NAC, moderate or severe persistent asthma,(30) congenital arrythmia, performance score <50%, and/or were pregnant or had a breastfeeding infant. Use of amifostine was not allowed. Patients declining the treatment arm or not eligible to receive NAC, including those with metastatic disease, were eligible for enrollment into an observation-only control arm. Patients with any level of baseline hearing were eligible for trial.
Trial Design.
The trial utilized a non-randomized controlled Phase 1a/1b design with a concurrently enrolled prospective observation/control group. The primary endpoint for the trial was the dose of NAC necessary to exceed peak otoprotective serum concentrations observed in our preclinical animal model (1.5mM).(17,18) The second primary endpoint was to describe the acute toxicity of IV NAC infusions integrated into intensive multi-agent chemotherapy regimens. To determine the maximum-tolerated dose (MTD), the trial used a classic 3+3 dose-escalation scheme with three planned dose levels (DL): DL1 (225 mg/kg), DL2 (300 mg/kg), and DL3 (450 mg/kg) (Table 1). An intermediate dose level 2.5 (375 mg/kg) was prespecified if DL3 exceeded the MTD. The MTD was determined as the highest DL where ≤1/6 patients developed a dose-limiting toxicity (DLT). Following dose-escalation, an interim analysis utilized a dose-response non-linear ordinary least squares method with logarithmic transformation of the peak NAC and dose level to select the dose most likely to achieve target blood levels (≥1.5nM) as the recommended phase 2 dose (RP2D). An expansion cohort then collected additional toxicity and outcome data at the RP2D. The clinical trial was conducted according to the principles of the U.S. Common Rule. All human investigation was performed after approval by the Institutional Review Board (IRB). Written informed consent was obtained from all patients and/or their guardians prior to enrollment. The trial was monitored by the IRB, a Data and Safety Monitoring Committee, and the Food and Drug Administration (IND122400). The clinical trial was registered on ClinicalTrials.gov prior to first enrollment (NCT02094625).
Table 1:
Dose escalation schema and dose limiting toxicity
| Dose Level | Dose Limiting Toxicity | |
|---|---|---|
| n DLT/N DL | CTCAE v4.03 Term (Grade) | |
| Dose Level 1 = 225 mg/kg | 0/3 | No DLT |
| Dose Level 2 = 300 mg/kg | 1/6 | Allergy (3) |
| Interim Dose level 2.5 = 375 mg/kg | Not required | |
| Dose Level 3 = 450 mg/kg | 1/6 | Infusion Related Reaction (2) (precluding completion of NAC) |
DLT = dose limiting toxicity; DL = dose level. CTCAE = Common Terminology Criteria for Adverse Events (CTCAE) v4.03; NAC = N-acetylcysteine
Drug dosing and administration.
Due to its extensive first-pass metabolism, NAC was administered intravenously (IV) for this study. IV NAC was supplied as a sterile solution in single-dose glass vials containing 200mg/ml NAC in 30 ml (Cumberland Pharmaceuticals, Perrigo Pharmaceuticals). NAC was reconstituted using standard dilution per package insert. IV NAC rescue followed each dose of cisplatin. Based on preclinical data for efficacy and to avoid interactions with cisplatin cytotoxicity,(18,19,27) NAC was infused beginning four hours after completion of each cisplatin dose (sFig1). In the dose-escalation cohort, NAC was infused over ~30 minutes. Premedication consisted of methylprednisolone [2mg/kg, max dose 60 mg], diphenhydramine [1mg/kg, max dose 50 mg], and ranitidine [2mg/kg, max dose 50mg]. As described below, in the expansion cohort, infusion duration was extended from 30 to 60 minutes and a leukotriene receptor antagonist (monteleukast, 4-10 mg by age) was added to the premedication regimen. All patients received routine antiemetics for underlying highly emetogenic chemotherapy. For moderate or severe IRR, the NAC infusion was paused, additional antihistamine provided, and the infusion restarted at 50% the prior rate once symptoms subsided.
Ototoxicity Monitoring.
All patients underwent assessment for hearing loss at baseline, prior to every cisplatin-containing cycle, at end of chemotherapy (or prior to autologous stem cell transplant [ASCT], if applicable), and 1-year post-treatment (from end of therapy or day +365 of final ASCT). Following testing for middle-ear pathology, patients were assessed with distortion-product otoacoustic emissions (DPOAE) and conventional sound-field audiometry (utilizing visual reinforcement or conditioned play techniques for younger children). Hearing thresholds were measured at frequencies between 0.5 – 8 kHz and up to 12.5 kHz when available and per patient compliance. Patients unable to comply with audiometry were tested using tone-burst evoked auditory brainstem potentials (ABR) at frequencies between 0.5-6 kHz. Individualized recommendations for hearing interventions (FM/hearing assistive device, hearing aid, or cochlear implant) were provided by audiologists in accordance with American Academy of Audiology guidelines.(31) All audiology assessments underwent central review independently by two investigators (K.R.K., E.O.); ear-specific ototoxicity grades were assigned using the SIOP scale. The ototoxicity endpoint for the trial was specified as communicatively significant CIHL. This was defined using the consensus International Society of Paediatric Oncology (SIOP) Ototoxicity Scale (32) as Grade ≥2 (moderate or severe CIHL) in the better ear.
Assessment of dose-limiting toxicity & clinical response.
Toxicities in the trial were graded using the Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Dose-limiting toxicities (DLT) were defined as an adverse event probably or definitely attributed to NAC and not otherwise attributed to the underlying chemotherapy regimen. Specific DLT included any grade infusion-related reaction (IRR), nausea, emesis, and/or abdominal pain that prevented completion of the NAC infusion. Additional DLT criteria were seizures (any grade) and other Grade ≥3 non-hematological toxicity. IRR were considered a single toxicity for DLT assessment; individual components of the IRR were then described separately. In addition to the ototoxicity endpoint, clinical response to NAC was also evaluated for reduced nephrotoxicity and myelosuppression. Nephrotoxicity was assessed by incidence of Grade ≥3 acute kidney injury (AKI), comparison of maximum serum creatinine, and by requirement for electrolyte supplementation (i.e., from renal tubular injury). Myelosuppression was evaluated using a surrogate measure of cycle duration (days).
NAC and GSH concentrations.
Blood samples were collected from all patients for serial measurement of NAC and GSH levels at pre-cisplatin, pre-NAC, post-NAC (peak), and delayed (+2 hours from completion of NAC infusion) (sFig. 1). Samples from patients in the observation arm followed the corresponding timing (pre-cisplatin, +4 hours and +6 hours from end cisplatin). Blood from each patient was immediately placed in an ice bath, centrifuged, and serum frozen at −80C until time of assay. GSH and NAC were measured by spectrophotometric assay using the Calbiochem Glutathione Assay Kit (Millipore Sigma, Inc).
Candidate gene analyses.
Following enrollment, DNA was purified from patient saliva samples that were collected from patients in both the treatment and observation arms (Oragene®, DNA Genotek, Ottawa, Canada), and extracted using an automated DNA purification system (QiaSymphony). Candidate gene variants involved in glutathione metabolism that have been previously associated with CIHL (GSTP1, GSTT1, GSTM1, GSTM3, GSTA1, GPX5) were genotyped on an Illumina Infinium Global Screening Array (v2.0) with standard sample and variant quality control (QC), phasing, and imputation. Copy number variants for GSTM1 and GSTT1 were analyzed using a TaqMan CNV Assay (Thermo-Fisher).
Statistical Considerations.
The planned sample size for the intervention arm was 24 patients, with patients not required to complete the dose-escalation (maximum of 4 dose levels, 6 patients per dose-level) enrolled in the dose-expansion cohort. Patient demographics and treatment data were compared by the Wilcoxon Rank Sum test or Fisher’s Exact test. Primary analysis of efficacy from NAC for otoprotection utilized a logistic regression model to analyze risk of CIHL at end of chemotherapy (EOT), defined as post-cisplatin and prior to ASCT (as applicable), in patients receiving all planned doses of NAC and in the control group. Additional analysis examined the endpoint of an audiologist-recommended hearing intervention and used Cox proportional hazards models to assess time to CIHL. Patients who did not experience CIHL were censored at the time of the latest follow-up. Secondary analyses for otoprotection analyzed CIHL with an intent-to-treat approach (i.e., any NAC exposure). Endpoints for additional cisplatin toxicities were analyzed using linear (serum creatinine, cycle duration) and logistic regression (electrolyte supplementation). All multivariable models included age and either tumor type or starting cisplatin dose. Starting cisplatin dose was selected for the dose measure as it is a significant key predictor of CIHL (1), is independent of subsequent cisplatin dose modifications, and exhibits a direct 1:1 relationship with the ensuing NAC rescue dose. Survival from start of cisplatin to first event (disease progression or death) between treatment arms was summarized using Kaplan-Meier methods and compared with the Cox regression model. Candidate genes were analyzed for the endpoints of SIOP ≥2 hearing loss and for GSH levels in multivariable logistic and linear regression additive risk models, respectively, inclusive of age, cisplatin dose, and NAC. All analyses were two-sided and significance set at the 0.05 level. Statistical software STATA v17 and SAS v9.4 were used for the analyses.
Data availability
Data are available upon reasonable request to the corresponding author.
Results
Patient Characteristics and Treatment Data.
Fifty-two patients were enrolled into the study (2016-2020), 24 in the NAC arm and 28 in the observation/control arm (Table 2). The cohort included patients and tumors representative of the pediatric cisplatin-treated population, including hepatic tumors, brain tumors (medulloblastoma, atypical teratoid rhabdoid tumor), osteosarcoma, neuroblastoma, and germ cell tumors (sTable 1). Notably, nearly half of patients were <5 years old at the time of the first cisplatin dose, and the majority self-identified as Hispanic/Latinx. No significant differences were present in demographic or treatment variables between trial arms. At enrollment, 51/52 patients had SIOP grade <2 hearing in the better ear; the remaining patient had normal hearing as assessed by DPOAE. As per eligibility, the observation arm included additional tumor types with metastatic disease at diagnosis. There were no significant differences between patients enrolled in the dose escalation and dose expansion cohorts (sTable 2). In the NAC-treated arm, 23/24 patients completed their planned primary chemotherapy regimen (treatment death from surgical complication unrelated to NAC, n=1). In the observation arm, 24/28 completed their primary chemotherapy regimen (disease progression, n=4).
Table 2:
Description of study cohort
| Variable | NAC treated Cohort N (%) |
Observation arm n (%) |
p-value |
|---|---|---|---|
| Total | 24 (100) | 28 (100) | |
| Age at first Cisplatin dose, years | |||
| 0-5 | 9 (38) | 12 (43) | 0.485 |
| 6-10 | 7 (29) | 4 (14) | |
| ≥11 | 8 (33) | 12 (43) | |
| Sex | |||
| Male | 14 (58) | 19 (68) | 0.568 |
| Female | 10 (42) | 9 (32) | |
| Race | |||
| White | 20 (83) | 16 (57) | 0.087 |
| Black or African American | 1 (4) | 2 (7) | |
| Asian & Pacific Islander | 2 (8) | 2 (7) | |
| Other/Not Reported | 1 (4) | 8 (29) | |
| Ethnicity | |||
| Hispanic/Latinx | 13 (54) | 18 (64) | 0.573 |
| Not Hispanic/Latinx | 11 (46) | 10 (36) | |
| Tumor type | |||
| Hepatic tumor | 5 (21) | 4 (14) | 0.008 |
| CNS tumor | 9 (38) | 10 (36) | |
| Osteosarcoma | 10 (42) | 5 (18) | |
| Other | 0 (0) | 9* (32) | |
| Disseminated disease at diagnosis | |||
| No | 24 (100) | 6 (21) | < 0.001 |
| Yes | 0** (0) | 22 (79) | |
| Starting cisplatin dose/day, mg/m2 | |||
| Median (range) | 104.35 (75.2, 142.0) | 104.00 (68.5, 254.4) | 0.920 |
| Cumulative Cisplatin dose, mg/m2 | |||
| Median (range) | 460.85 (202.2, 952.8) | 399.25 (74.1, 603.2) | 0.069 |
| Reduction in cisplatin dose | |||
| No | 19 (79) | 22 (79) | 1.000 |
| Yes | 5 (21) | 6 (21) | |
| Pretreatment Cranial radiation | |||
| No | 19 (79) | 24 (86) | 0.716 |
| Yes | 5 (21) | 4 (14) | |
| Autologous stem cell transplant | |||
| No | 22 | 24 | 0.674 |
| Yes | 2 | 4† | |
| VPS prior to cisplatin | |||
| No | 18 (75) | 22 (79) | 1.000 |
| Yes | 6 (25) | 6 (21) |
Other diagnosis (n = 9): Germ cell tumor (5), Neuroblastoma (3), and NUT midline carcinoma (1); VPS = ventriculoperitoneal shunt;
Disseminated tumors were ineligible for NAC treatment.
2 did not receive hearing evaluations post-transplant.
Dose-escalation and toxicity.
The NAC dose was escalated as scheduled to DL3 (450 mg/kg), requiring 15 patients, and without reaching the MTD (Table 1). There was one DLT each at DL2 (Allergic reaction, Grade 3) and DL3 (IRR preventing NAC completion, Grade 2), both definitely attributed to NAC. The determination of an allergic reaction and not IRR in the patient at DL2 was established due to its prolonged nature and recurrence of isolated urticaria after the expected half-life of NAC. No serious adverse events (SAEs) were attributed to NAC. Protocol-defined targeted toxicities from NAC infusions are described in Table 3. IRR (any grade) were present in 64% (75/117) of infusions. In the dose expansion cohort (n=9), the addition of a leukotriene receptor antagonist reduced the incidence and severity of the bronchospasm component of the IRR. Overall, 19/24 (79%) NAC treated patients completed all planned NAC infusions, 12/15 (80%) completed all infusions at the RP2D, and 8/9 (89%) did so at the RP2D with the final recommended premedication regimen.
Table 3:
Targeted toxicities associated with NAC infusions (no grade 4-5 toxicities were observed)
| CTCAE Term (Reportable Grade) |
Dose-Escalation (69 infusions) |
Dose-Expansion (48 infusions)* |
||||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| n (% of infusions) | n (% of infusions) | n (% of infusions) | n (% of infusions) | n (% of infusions) | n (% of infusions) | |
| Infusion-related reaction (Any) | 20 (29) | 18 (26) | 0 (0) | 18 (38) | 19 (40) | 0 (0) |
| Flushing | 9 (13) | 0 (0) | 0 (0) | 13 (27) | 0 (0) | 0 (0) |
| Urticaria | 7 (10) | 0 (0) | 0 (0) | 9 (19) | 1 (2) | 0 (0) |
| Cough | 16 (23) | 7 (10) | 0 (0) | 4 (8) | 7 (15) | 0 (0) |
| Dyspnea | 0 (0) | 5 (7) | 0 (0) | 2 (4) | 0 (0) | 0 (0) |
| Facial Edema | 1 (1) | 0 (0) | 0 (0) | 2 (4) | 0 (0) | 0 (0) |
| Bronchospasm | 6 (9) | 2 (3) | 3 (4) | 1** (2) | 2** (4) | 0 (0) |
| Hypotension | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Allergic reaction (3-5) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Nausea (Any) | 9 (13) | 2 (3) | 0 () | 11 (23) | 3 (6) | 3 (6) |
| Vomiting (Any) | 13 (19) | 2 (3) | 0 (0) | 22 (46) | 7 (15) | 1 (2) |
| Abdominal Pain (Any) | 7 (10) | 1 (1) | 0 (0) | 3 (6) | 0 (0) | 0 (0) |
| Diarrhea (Any) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Prolonged INR (3-5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypofibrinogenemia (3-5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
45/48 infusions included final premedication regimen;
Subjective throat tightness, no other symptoms.
Efficacy for hearing protection.
Hearing was evaluable in 90% (47/52) of patients at EOT and 85% (44/52) at the post-therapy 12 month timepoint. At EOT, the proportion of patients who completed all NAC doses with SIOP ≥2 CIHL versus the observation group was 29% (5/17) versus 40% (10/25) (p=0.531). At the 12 month follow-up, the proportion of patients with SIOP ≥2 CHIL was 44% (8/18) vs 57% (12/21), respectively (p=0.527). In those with any NAC exposure, SIOP ≥2 CIHL was present in 32% (7/22) at EOT and 44% (10/23) at 12 months. In multivariable analysis adjusting for age and starting cisplatin dose, receiving NAC rescue following each dose of cisplatin significantly protected hearing at EOT (odds ratio [OR] 0.13, 95% confidence interval [CI] 0.021-0.847, adjusted p=0.033). (Table 4, Fig. 1). NAC remained significantly associated with otoprotection in a model substituting diagnosis for starting cisplatin dose (OR 0.15, 95%CI 0.022-0.961, p=0.045) (Table 4) and in the time-to-event analysis (sTable 3, sTable 4, sFig 2). At the 12 month timepoint, hearing interventions were recommended in 28% (5/18) versus 44% (12/27) of patients completing all doses of NAC versus those in the control arm (p=0.351). After adjusting for age and diagnosis, children who completed all NAC doses were less likely to be recommended a hearing intervention by this final time point (adjusted OR = 0.082, 95%CI 0.011-0.60, p=0.014). In the secondary intent-to-treat analysis including patients with any NAC exposure, a trend for otoprotection remained present in analyses of time to CIHL (adjusted for age/disease group, HR 0.438, 95%CI 0.174-1.085, p=0.073 and adjusted for age/cisplatin dose, HR 0.426, 95%CI 0.169-1.045, p=0.063).
Table 4:
Multivariable logistic regression models of SIOP ≥2 hearing loss at end of therapy
| Model #1: Cisplatin daily dose | Model #2: Disease group | ||||||
|---|---|---|---|---|---|---|---|
| Covariable | event/ total (n/n) |
Odds Ratio (95% CI) |
p-value | Covariable | event/ total (n/n) |
Odds Ratio (95% CI) |
p-value |
| Age, years | Age, years | ||||||
| 0-5 | 7/17 | 1.03 (0.09, 12.36) | 0.055 | 0-5 | 7/17 | 5.02 (0.52, 48.67) | 0.106 |
| 6-10 | 6/11 | 12.52 (1.16, 134.89) | 6-10 | 6/11 | 14.25 (1.21, 167.92) | ||
| ≥11 | 2/14 | Reference group | ≥11 | 2/14 | Reference group | ||
| Cisplatin Daily Dose, mg/m2 | n/a | 1.05 (1.00, 1.10) | 0.033 | Tumor type | |||
| CNS | 8/15 | 0.89 (0.10, 7.70) | 0.309 | ||||
| OST | 3/10 | 0.74 (0.05, 11.27) | |||||
| Other | 1/9 | 0.08 (0.004, 1.70) | |||||
| Hepatic | 3/8 | Reference group | |||||
| NAC | NAC | ||||||
| Yes | 5/17 | 0.13 (0.02, 0.85) | 0.033 | Yes | 5/17 | 0.15 (0.02, 0.96) | 0.045 |
| No | 10/25 | Reference group | No | 10/25 | Reference group | ||
NAC = N-acetylcysteine; CNS = central nervous system tumor, OST = osteosarcoma.
Figure 1. Predicted probability of SIOP Grade≥2 hearing loss with and without NAC.
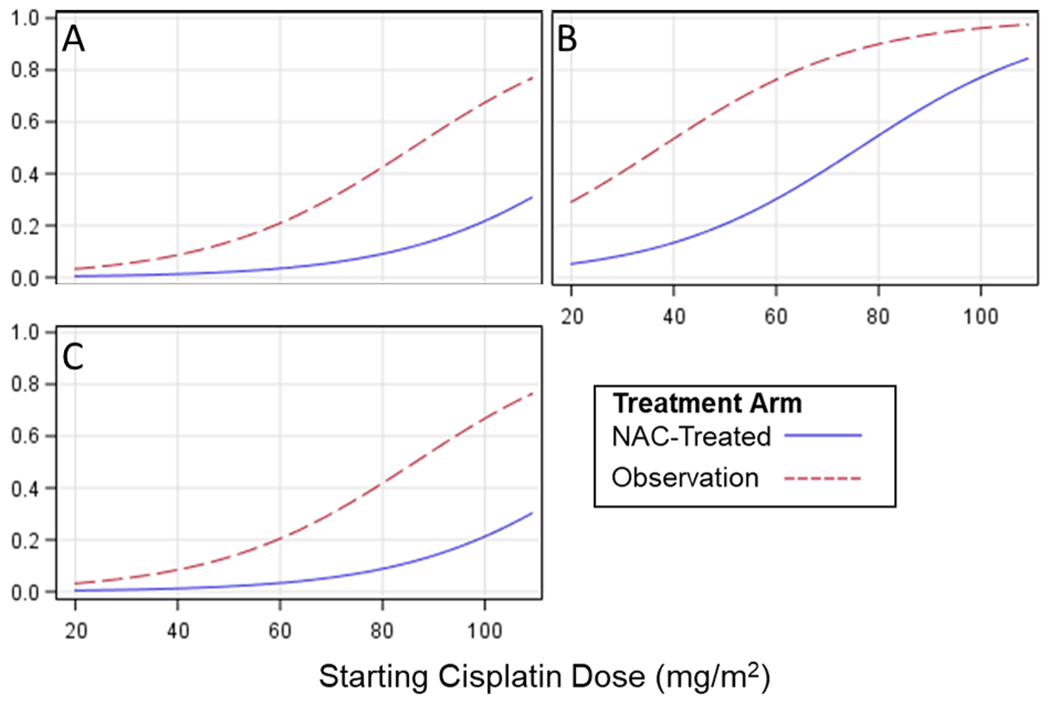
From multivariable logistic regression model for risk of developing SIOP Grade ≥2 hearing loss at the end of chemotherapy timepoint (i.e., after all cisplatin and prior to autologous stem cell transplant [as applicable]), the probability of developing hearing loss was reduced in patients receiving N-acetylcysteine (NAC) versus those with observation-only in the control arm. This difference was present within all three age groups and across the range of cisplatin dosing (mg/m2/day): (A) less than 5 years old, (B) 6-10 years old, (C) 11 years and older.
Serum NAC and GSH concentration.
Following NAC infusion, peak NAC concentrations exhibited a dose-response with increasing DL (Table 5). Planned interim analysis following dose-escalation determined a dose of 450 mg/kg (DL3) had the highest probability (P) of exceeding the target level of 1.5 mM as compared to 300 mg/kg (P=0.88 vs P=0.52). DL3 was therefore selected as the RP2D for further testing in the expansion cohort. Median peak GSH concentrations following NAC infusions were significantly higher for all three dose levels as compared to the observation group (DL1 p=0.026, p<0.0001 for DL2 and DL3). Peak GSH concentrations were significantly higher than pre-NAC trough within DL2 and DL3 (sFig. 3). At the delayed +2 hour timepoint, only GSH in DL3 remained borderline elevated compared to the observation group (p=0.073) and to the pre-NAC trough (p=0.010). Planned testing of NAC and GSH concentrations in the expansion cohort was precluded due to specimen degradation during the COVID-19 pandemic (sFig 4A, 4B).
Table 5:
NAC concentrations in dose escalation cohort
| Cohort | Trough [NAC], mM | Peak [NAC], mM | Achieved Target Concentration* | Delayed [NAC], mM | |||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Frequency | Median | Range | |
| Observation | NA | NA | 0 | 0 - 0 | 0/16 | 0 | 0 - 0 |
| DL1 (225mg/kg) | 0 | 0 - 0 | 0.67 | 0.24 – 0.70 | 0/3 | 0 | 0 - 0 |
| DL2 (300mg/kg) | 0 | 0 - 0 | 1.95 | 0.36 – 3.67 | 4/6 | 0 | 0 – 15 |
| DL3 (450mg/kg) | 0 | 0 - 0 | 3.71 | 1.47 – 8.91 | 5/6 | 0.25 | 0 – 0.68 |
Peak serum [NAC] >1.5mM within 15 minutes following infusion; see Methods.
Influence of glutathione gene variants.
In logistic analysis inclusive of age, cisplatin dose, and NAC treatment, genotyping for candidate genes demonstrated a significant association with GSTP1 105 A > G (rs1695, G effect allele) with risk for SIOP ≥2 CIHL at latest follow-up (OR = 17.62, 95%CI 1.53-203.6, p=0.022). In this model, receiving NAC rescue remained significantly associated with reduced CIHL (OR 0.01, 95%CI 0.0002-0.381, p=0.015). The effect allele was not significantly associated with increased GSH concentration immediately following NAC infusion (regression slope 0.022, 95%CI [−0.012]-0.056, p=0.217) nor at the delayed +2 hour time point (slope 0.006, 95%CI [−0.048]-0.063, p=0.817). The frequency of AA, AG, and GG were 0.30, 0.56, and 0.14 respectively. The remaining candidate glutathione pathway genes GSTM3, GSTA1, GSTT1, GPX5, and GSTM1 were not associated with CIHL nor GSH level (sTable 5).
Impact of NAC on kidneys, cycle duration, and tumor.
Three patients developed Grade ≥3 AKI (one in the intervention arm, two in the observation arm), all of which were transient and self-limited. In multivariable regression models, NAC was not significantly associated with protection from cisplatin-induced nephrotoxicity as assessed by elevations in maximum serum creatinine or need for electrolyte supplementation (sTable 6). After controlling for age and disease type, median duration of treatment cycle in NAC-treated patients was significantly shorter (+NAC 0.88 fewer days, 95%CI 0.777-0.992, adjusted p=0.038) (sTable 6). The identical model substituting cisplatin dose for disease type yielded consistent findings (+NAC 0.84 fewer days, 95%CI 0.732-0.968, adjusted p=0.016). Evaluation of progression-free survival (PFS) demonstrated no evidence of interference with cisplatin cytotoxicity in patients receiving NAC versus observation (1-year PFS 95.7%, 95%CI 72.9-99.4 versus 75.0%, 95%CI 54.6-87.2, log-rank test p=0.159 (sFig 5A, 5B).
Discussion
In this early-phase trial, we determined IV NAC to be safe to integrate into intensive multiagent chemotherapy at a biologically active otoprotective dose. The MTD was not reached at the highest dose-level tested (DL3, 450 mg/kg) and most patients achieved adequate peak NAC serum concentrations at this dose. Moreover, a clear efficacy signal for otoprotection from NAC was present in patients receiving cisplatin with NAC rescue versus those on the control arm receiving cisplatin alone. In multivariable analyses accounting for treatment and patient risk factors, NAC rescue following each dose of cisplatin resulted in an approximately 87% reduction in the likelihood of developing communicatively significant hearing loss following cisplatin-based chemotherapy, and an approximate 90% reduction in the likelihood of being recommended a hearing intervention following all therapy (i.e., hearing assistive device, hearing aid, cochlear implant). This strong protective effect from NAC was significant across all analyses, including whether the model controlled for diagnosis (as a surrogate of treatment factors) or the specific cisplatin dose, and whether it was compared at the end of chemotherapy or over the duration of the study period. NAC was also predicted to be similarly effective across all included ages in the trial, and with preserved otoprotection over a range of cisplatin dosing. Using a controlled Phase 1 trial design, we demonstrated NAC was safe, exceeded the biologic target established by preclinical studies, and possessed significant potential efficacy to prevent CIHL.
Currently, only intravenous STS is approved for cisplatin otoprotection.(33) We selected NAC for testing as a next generation otoprotectant due to several potential advantages, including (1) possibly increased efficacy compared with STS, (2) reduction in cisplatin non-cochlear toxicities, and (3) less infusion toxicity than STS. Direct comparisons with STS are limited as the agents were evaluated in separate trials, with different hearing endpoints. Nonetheless, within the context of a small controlled trial, NAC demonstrated a robust efficacy signal to protect hearing. Audiologist recommendations for a hearing intervention represent a real-world endpoint that captures not only hearing sensitivity, but also includes family perspectives and other factors (e.g., younger age, co-existing morbidities, developmental delays). NAC demonstrated strong efficacy for this pragmatic endpoint too. Although direct comparisons for efficacy are not possible, it is noteworthy that NAC-treated patients were treated with greater cumulative doses of cisplatin than patients in the ACCL0431 trial (median cumulative dose cisplatin <400 mg/m2) and at least equivalent to the SIOPEL6 trial (unreported, maximum dose by regimen 480 mg/m2). Rates of pre-cisplatin cranial irradiation were also higher in NAC-treated patients (20%) as compared to the ACCL0431 (6%) and SIOPEL-6 (0%) trials of STS. Higher cisplatin dose and prior irradiation would be expected to bias the NAC cohort toward greater risk for CIHL. Treatment risk factors in our cohort were comparable to patient enrolled into the two STS trials, and potentially predisposed our population to even greater risk for CIHL. In the context of an early phase trial, the significant decrease in the proportion of patients with communicatively significant CIHL is therefore notable, and the reduction in recommendations for hearing interventions even more striking.
NAC is poorly bioavailable with minimal tissue delivery, precluding use of an oral formulation to achieve the hypothesized otoprotective circulating concentrations.(17,34) Thus, a key finding from the trial was that infusion toxicity from IV NAC following chemotherapy was tolerable, and most patients completed planned infusions at the RP2D (~90%). IRR were common and consisted of urticaria, flushing, and nausea/vomiting. Nausea/vomiting were temporally related to the NAC infusions, though must be interpreted in the context of preceding highly emetogenic chemotherapy. Episodes of bronchospasm as an IRR component were rare, and following incorporation of the final premedication regimen, were only mild and subjective. All IRR symptoms resolved promptly with pausing the infusion and none resulted in serious toxicity. In the two randomized trials of STS in children,(7,8) nausea and vomiting were also common, and severe Grade ≥3 nausea/vomiting developed in fewer than 10% of patients in each trial. In both STS trials, incidence of nephropathy was increased by ~10% in the STS arm versus those in the control arm, as evidenced by electrolyte wasting (hypophosphatemia, hypokalemia). Thus, while incidence of nausea/vomiting from NAC was similar to that reported from STS, in contrast to published results from STS, no concern for renal injury was identified in patients receiving NAC. Interestingly, though the hypothesized nephroprotection from NAC (22) was also not observed, patients receiving NAC rescue experienced shorter duration of treatment cycles on average, a surrogate marker for reduced marrow toxicity. Interpretation of this finding for NAC is complicated by the proportion of patients with metastatic disease in the control arm, many of whom may have received higher intensity chemotherapy influencing marrow recovery. As STS did not demonstrate marrow protection from cisplatin, if this finding from NAC is validated in future studies, NAC may offer additional benefit for chemotherapy delivery, particularly in tumors where dose-compression is vital and in young adults where myelosuppression-induced delays complicate therapy delivery. Although this study was limited to a pediatric population, a similar toxicity profile from IV NAC was reported for this dose-range in a dose-escalation trial in adults without cancer.(35) Additional study is required to determine the toxicity profile for NAC rescue in adults receiving cisplatin chemotherapy. If comparably well-tolerated, NAC may offer benefit for young adults with cisplatin-treated tumors, such as those treated for testicular cancer where more than half of survivors report suffering from cisplatin-induced hearing loss. (36)
We had hypothesized that observed benefits from NAC would result from the added capacity to stimulate GSH production. However, it was unclear if NAC would be able to accomplish this in the context of intensive, GSH-depleting chemotherapy. Cisplatin itself has been demonstrated to profoundly deplete GSH in cochlear tissues.(10–12) Moreover, inherited variation in GSH metabolism profoundly impacts ability to respond to oxidant damage. Integrated biology in the trial therefore explored the influence of NAC on GSH production during chemotherapy as well as the influence of candidate genes related to GSH synthesis and activity. Despite being administered after intensive chemotherapy, NAC stimulated production of excess GSH at all dose-levels, with evidence for sustained GSH above baseline for two hours after NAC at the RP2D of 450 mg/kg. Exploratory analysis of candidate genes involved in GSH metabolism demonstrated a significant, additive effect for the GSTP1 rs1695 G allele (Ile105Val) in risk for developing CIHL. Polymorphisms in the GSTP1 gene are associated with reduced or absent catalytic enzyme activity.(37) Prior cohorts have reported polymorphisms in GSTP1 influence the risk for developing CIHL, supporting a role for GSH metabolism in the mechanism underlying CIHL (38,39). In multivariable analyses with and without adjustment for the GSTP1 rs1695 G allele, NAC demonstrated even stronger otoprotection after adjustment for its impact. Together, these findings support the role of GSH metabolism in CIHL and in NAC rescue. Interestingly, GSTP1 produces the glutathione-S-transferase Pi subtype, located only in Deiters’ cells in the cochlea.(40) Dieters’ cells support the sensory hair cells, are implicated in hair cell viability and death, and are the first cells to be affected by cisplatin.(41–43) While GSH is produced in multiple cochlear cells, GSTP1 was the only gene significant in a model containing NAC exposure. If validated in larger cisplatin+NAC treated cohorts, one might hypothesize that NAC may preferentially rescue the cochlea through GSH production within the hair cell support infrastructure affected earliest by cisplatin. Future studies exploring differences in GSH production by cochlear cell type in response to cisplatin and NAC may offer new insight into the pathophysiology of ototoxicity and targets for otoprotection.
There are several potential limitations of this study. First, though a strong efficacy signal for NAC otoprotection was detected, no direct comparisons with STS are possible, and observed otoprotection must be interpreted within the inherent limitations from the small sample size of a Phase 1 trial. However, the relatively small sample size of the Phase 1 setting was balanced through use of a prospective controlled design to provide stronger evidence of efficacy than typical single-arm early-phase trials. Analysis of the efficacy endpoint was additionally supported through robust audiology evaluations, including prospective, serial assessments strengthened via a rigorous central review process by two independent investigators. Nonetheless, treatment intensity from non-cisplatin chemotherapy may differ for those with metastatic disease in the control arm and innate differences between arms impacting trial endpoints cannot be entirely excluded. Second, due to inaccessibility of the cochlea for in vivo sampling in humans, only surrogate measures of the glutathione pathway were available to measure drug effect. Nonetheless, the combination of serum and pharmacogenomics implicating the GSH pathway, with past in vitro and explant data, all support further exploration of this mechanism as a key otoprotective pathway. Finally, though definitive demonstration of the absence of an interaction with chemotherapy efficacy is precluded by the small sample, there was no hint of compromised survival with NAC exposure. In combination with the preclinical animal models, these data support the safety of subsequent testing of NAC in future trials. A randomized trial to formally test efficacy from NAC should include all these considerations. Such a future trial would include direct comparisons with STS in the control arm, evaluate disease response and survival for theoretical chemotherapy interactions, incorporate annual long-term follow-up to assess differences in late-onset hearing loss, and integrate genomics and mechanistic aims to further advance our biologic understanding of cisplatin ototoxicity. Post-treatment tinnitus from cisplatin-induced cochlear injury is common, impacts patient-reported quality of life, (44) and also should be included as a key endpoint in such a trial. Lastly, as young adult patients are commonly treated with cisplatin, future trials of NAC and other otoprotectants should expand eligibility into this older age range to address this key toxicity in a broader population.
In the context of this Phase 1a/1b trial, NAC was safe to integrate into multi-agent chemotherapy regimens and possessed a tolerable infusion profile. Moreover, it demonstrated a robust efficacy signal to prevent CIHL in children with cisplatin-treated tumors. As NAC is a well-recognized GSH prodrug, and as further supported by the pharmacogenomics and integrated biology, glutathione metabolism is identified for future investigation as a potential central mechanism for ototoxicity and otoprotection. NAC has therefore met the key thresholds for further development and warrants ongoing testing in randomized trials as a second-generation systemic otoprotectant.
Supplementary Material
Acknowledgements:
This study was supported by the National Institutes of Health (NIH), National Institute on Deafness and Other Communication Disorders (NIH/NIDCD) under K23DC014291 (E. Orgel), and by the American Cancer Society under MRSG-15-194-01-TBG (E. Orgel). Database support for the trial was provided from the NIH National Center for Advancing Translational Science (NCATS) under UL1TR001855 and UL1TR000130. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The team would also like to thank the patients and families who participated in the trial, Dr. Richard Sposto for biostatistical insights into the trial design, and Mary Morrison-Barrios, the trial research coordinator for her dedication to the patients and to the trial that was instrumental to its success.
Footnotes
Conflicts of Interest: Dr. Rassekh: Legal consulting, patent case involving Sodium Thiosulfate and otoprotection. OHSU and Dr. Neuwelt, financial interest in technology licensed to Fennec Pharmaceuticals, a company that may have a commercial interest in the results of this research and technology. Dr. Orgel, consulting outside scope of this study: Jazz Pharmaceuticals, Servier Pharmaceuticals, Seagen Inc. The remaining authors declare no potential conflicts of interest.
References
- 1.Moke DJ, Luo C, Millstein J, Knight KR, Rassekh SR, Brooks B, et al. Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: a multi-institutional North American cohort study. Lancet Child Adolesc Health 2021;5(4):274–83 doi 10.1016/S2352-4642(21)00020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg 2007;15(5):364–9. [DOI] [PubMed] [Google Scholar]
- 3.Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children’s Oncology Group. Pediatrics 2007;120(5):e1229–36. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber JE, Gurney JG, Palmer SL, Bass JK, Wang M, Chen S, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol 2014;16(8):1129–36 doi 10.1093/neuonc/nou006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orgel E, O’Neil SH, Kayser K, Smith B, Softley TL, Sherman-Bien S, et al. Effect of Sensorineural Hearing Loss on Neurocognitive Functioning in Pediatric Brain Tumor Survivors. Pediatr Blood Cancer 2016;63(3):527–34 doi 10.1002/pbc.25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheth S, Mukherjea D, Rybak LP, Ramkumar V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Frontiers in Cellular Neuroscience 2017;11(338) doi 10.3389/fncel.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brock PR, Maibach R, Childs M, Rajput K, Roebuck D, Sullivan MJ, et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N Engl J Med 2018;378(25):2376–85 doi 10.1056/NEJMoa1801109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freyer DR, Chen L, Krailo MD, Knight K, Villaluna D, Bliss B, et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18(1):63–74 doi 10.1016/S1470-2045(16)30625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nature Communications 2017;8(1):1654 doi 10.1038/s41467-017-01837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lautermann J, Crann SA, McLaren J, Schacht J. Glutathione-dependent antioxidant systems in the mammalian inner ear: effects of aging, ototoxic drugs and noise. Hear Res 1997;114(1–2):75–82 doi 10.1016/s0378-5955(97)00154-8. [DOI] [PubMed] [Google Scholar]
- 11.Rybak LP, Whitworth C, Somani S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope 1999;109(11):1740–4 doi 10.1097/00005537-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Touliatos JS, Neitzel L, Whitworth C, Rybak LP, Malafa M. Effect of cisplatin on the expression of glutathione-S-transferase in the cochlea of the rat. Eur Arch Otorhinolaryngol 2000;257(1):6–9 doi 10.1007/pl00007509. [DOI] [PubMed] [Google Scholar]
- 13.Bijarnia RK, Bachtler M, Chandak PG, van Goor H, Pasch A. Sodium Thiosulfate Ameliorates Oxidative Stress and Preserves Renal Function in Hyperoxaluric Rats. PLOS ONE 2015;10(4):e0124881 doi 10.1371/journal.pone.0124881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sooriyaarachchi M, Narendran A, Gailer J. The effect of sodium thiosulfate on the metabolism of cis-platin in human plasma in vitro. Metallomics 2012;4(9):960–7 doi 10.1039/c2mt20076g. [DOI] [PubMed] [Google Scholar]
- 15.Sooriyaarachchi M, Narendran A, Gailer J. N-acetyl-L-cysteine modulates the metabolism of cis-platin in human plasma in vitro. Metallomics 2013;5(3):197–207 doi 10.1039/c3mt00012e. [DOI] [PubMed] [Google Scholar]
- 16.Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, et al. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free radical research 2018;52(7):751–62 doi 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 17.Dickey D, Muldoon L, Doolittle N, Peterson D, Kraemer D, Neuwelt E. Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin-induced toxicity in rat models. Cancer Chemother Pharmacol 2008;62(2):235–41 doi 10.1007/s00280-007-0597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickey D, Muldoon L, Kraemer D, Neuwelt E. Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Hear Res 2004;193(1-2):25–30 doi 10.1016/j.heares.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Muldoon LL, Wu YJ, Pagel MA, Neuwelt EA. N-acetylcysteine chemoprotection without decreased cisplatin antitumor efficacy in pediatric tumor models. J Neurooncol 2015;121(3):433–40 doi 10.1007/s11060-014-1657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feghali JG, Liu W, Van De Water TR. L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope 2001;111(7):1147–55 doi 10.1097/00005537-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, et al. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol 1997;18(5):559–71. [PubMed] [Google Scholar]
- 22.Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther 2005;314(3):1052–8 doi 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 23.Neuwelt EA, Pagel MA, Hasler BP, Deloughery TG, Muldoon LL. Therapeutic efficacy of aortic administration of N-acetylcysteine as a chemoprotectant against bone marrow toxicity after intracarotid administration of alkylators, with or without glutathione depletion in a rat model. Cancer Res 2001;61(21):7868–74. [PubMed] [Google Scholar]
- 24.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 2006;354(26):2773–82 doi 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 25.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel) 2010;2(11):2490–518 doi 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant Mechanisms in Renal Injury and Disease. Antioxidants & Redox Signaling 2016;25(3):119–46 doi 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu YJ, Muldoon LL, Neuwelt EA. The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J Pharmacol Exp Ther 2005;312(2):424–31 doi 10.1124/jpet.104.075119. [DOI] [PubMed] [Google Scholar]
- 28.Neuwelt EA, Pagel MA, Kraemer DF, Peterson DR, Muldoon LL. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther 2004;309(2):594–9 doi 10.1124/jpet.103.063347. [DOI] [PubMed] [Google Scholar]
- 29.Minasian LM, Frazier AL, Sung L, O’Mara A, Kelaghan J, Chang KW, et al. Prevention of cisplatin-induced hearing loss in children: Informing the design of future clinical trials. Cancer Medicine 2018;7(7):2951–9 doi 10.1002/cam4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007;120(5 Suppl):S94–138 doi 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 31.Ching T, Galster J, Grimes AM, Johnson C, Lewis DE, McCreery R, et al. American Academy of Audiology Clinical Practice Guidelines: Pediatric Amplification American Academy of Audiology. Volume 5-60. Reston, VA: American Academy of Audiology; 2013. [Google Scholar]
- 32.Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol 2012;30(19):2408–17 doi 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhillon S. Sodium Thiosulfate: Pediatric First Approval. Pediatric Drugs 2023;25(2):239–44 doi 10.1007/s40272-022-00550-x. [DOI] [PubMed] [Google Scholar]
- 34.Tenório M, Graciliano NG, Moura FA, Oliveira ACM, Goulart MOF. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants (Basel) 2021;10(6) doi 10.3390/antiox10060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dosa E, Heltai K, Radovits T, Molnar G, Kapocsi J, Merkely B, et al. Dose escalation study of intravenous and intra-arterial N-acetylcysteine for the prevention of oto- and nephrotoxicity of cisplatin with a contrast-induced nephropathy model in patients with renal insufficiency. Fluids Barriers CNS 2017;14(1):26 doi 10.1186/s12987-017-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez VA, Shuey MM, Jr PCD, Monahan PO, Fosså SD, Sesso HD, et al. Patient-Reported Functional Impairment Due to Hearing Loss and Tinnitus After Cisplatin-Based Chemotherapy. Journal of Clinical Oncology;0(0):JCO.22.01456 doi 10.1200/jco.22.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2001;482(1):21–6. [DOI] [PubMed] [Google Scholar]
- 38.Rednam S, Scheurer ME, Adesina A, Lau CC, Okcu MF. Glutathione S-transferase P1 single nucleotide polymorphism predicts permanent ototoxicity in children with medulloblastoma. Pediatr Blood Cancer 2013;60(4):593–8 doi 10.1002/pbc.24366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol 2007;25(6):708–14. [DOI] [PubMed] [Google Scholar]
- 40.el Barbary A, Altschuler RA, Schacht J. Glutathione S-transferases in the organ of Corti of the rat: enzymatic activity, subunit composition and immunohistochemical localization. Hear Res 1993;71(1-2):80–90 doi 10.1016/0378-5955(93)90023-t. [DOI] [PubMed] [Google Scholar]
- 41.Waissbluth S, Maass JC, Sanchez HA, Martínez AD. Supporting Cells and Their Potential Roles in Cisplatin-Induced Ototoxicity. Front Neurosci 2022;16:867034 doi 10.3389/fnins.2022.867034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramírez-Camacho R, García-Berrocal JR, Buján J, Martín-Marero A, Trinidad A. Supporting cells as a target of cisplatin-induced inner ear damage: therapeutic implications. Laryngoscope 2004;114(3):533–7 doi 10.1097/00005537-200403000-00027. [DOI] [PubMed] [Google Scholar]
- 43.Laurell G, Bagger-sjöbäck D. Degeneration of the Organ of Corti Following Intravenous Administration of Cisplatin. Acta Oto-Laryngologica 1991;111(5):891–8 doi 10.3109/00016489109138427. [DOI] [PubMed] [Google Scholar]
- 44.Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, Sesso HD, et al. Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. Journal of Clinical Oncology 2016;34(23):2712–20 doi 10.1200/JCO.2016.66.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request to the corresponding author.


