Abstract
Purpose:
The goal of the current study was to identify longitudinal changes in urinary metabolites following IR exposure and to determine potential alleviation of radiation toxicities by administration of recombinant APC formulations.
Materials and Methods:
Female adult WAG/RijCmcr rats were irradiated with 13.0 Gy leg-out partial body X-rays; longitudinally collected urine samples were subject to LC-MS based metabolomic profiling. Sub-cohorts of rats were treated with three variants of recombinant APC namely, rat wildtype (WT) APC, rat 3K3A mutant form of APC, and human WT APC as two bolus injections at 24 and 48 hours post IR.
Results:
Radiation induced robust changes in the urinary profiles leading to oxidative stress, severe dyslipidemia, and biosynthesis of PUFAs, glycerophospholipids, sphingolipids, and steroids. Alterations were observed in multiple metabolic pathways related to energy metabolism, nucleotide biosynthesis and metabolism that were indicative of disrupted mitochondrial function. On the other hand, sub-cohorts of rats that were treated with rat wildtype-APC showed alleviation of radiation toxicities, in part, at the 90-day time point, while rat 3K3A-APC showed partial alleviation of radiation induced metabolic alterations 14 days after irradiation.
Conclusions:
Taken together, these results show that augmenting the Protein C pathway and activity via administration of recombinant APC may be an effective approach for mitigation of radiation induced normal tissue toxicity.
Keywords: Radiation injury, activated protein C (APC), WAG/RijCmcr rat, metabolomics, lipidomics, biomarkers
Introduction
Management of large populations at risk of exposure to ionizing radiation (IR) in the event of a radiological scenario requires emergency preparedness to identify, treat and manage exposed individuals. Exposure to IR triggers a cascade of altered physiological events affecting a network of cellular responses at the metabolic, proteomic as well as at the genomic level. Mapping the physiological response to IR by characterizing metabolic perturbations will augment the development of biomarkers panels that would help assess absorbed dose and predict the development of radiation injuries to help strategize the administration of radiation countermeasures. The National Institute of Allergy and Infectious Diseases (NIAID) radiation and nuclear countermeasures program promotes discoveries to study the mechanisms of radiation-induced injuries and to develop medical countermeasures (MCM) to treat the acute (gastrointestinal, hematopoietic injuries) and delayed effects of radiation (lungs, heart, kidneys, brain injuries) that may occur from a nuclear attack or terrorist event (Fish et al. 2016). Hence a high throughput biomonitoring and diagnostic platform that is easily deployable, rapid, reproducible, reliable, and appropriate to triage and permit rapid assessment of many individuals in a mass casualty setting would be of utmost importance. Through the last couple of decades, several groups have reported on the identification and subsequent development of biomarkers panels for acute radiation syndrome (ARS) (Singh et al. 2016).
Despite continual efforts to develop radiation countermeasures, the only USFDA approved safe and effective medical countermeasures against unwanted radiation exposures are drugs largely repurposed from their original oncology indications (Singh, Garcia, et al. 2017; Singh, Hanlon, et al. 2017; Singh and Seed 2017). Radio injury protectors or mitigators such as Activated protein C (APC) are administered a priori to protect soldiers, first responders, or civilians in anticipation of radiation exposure or cancer patients undergoing radiotherapy, or immediately after exposure to prevent worsening of late-stage symptoms (Stone et al. 2004). However, currently there is no US FDA approved radioprotector available for either hematopoietic ARS (H-ARS) or gastrointestinal ARS (GI-ARS)(Singh and Seed 2017). APC is a vitamin-K dependent natural protein that has anti-coagulant and anti-inflammatory properties and is known to improve endothelial function through the activation of several endothelial cell surface receptors (Griffin et al. 2018). APC has previously been shown to have potent radiation mitigative effects in a mouse model of the acute radiation syndrome (Geiger et al. 2012). The APC mutant 3K3A-APC was created with reduced anti-coagulant properties (<10% of wild-type (WT) APC) but full cytoprotective properties (Williams et al. 2012). However, it is now believed that the beneficial characters of APC are partially independent from its anticoagulant activity (Wang and Li 2009). APCs are known to exert potent radiation-mitigative effects by improving endothelial cell function (Griffin et al. 2007).
The goal of the current study was to identify longitudinal changes in urinary metabolites following IR exposure and to determine potential alleviation of radiation toxicities by administration of recombinant APC formulations. We used the well characterized WAG/RijCmcr female rat model of partial body irradiation (leg-out PBI) to prolong survival. This model is accurate for the radiation induced occurrence of dose-, time- and sex-dependent sequelae representing GI, hematopoietic, lung and kidney toxicities after leg-out PBI (Fish et al. 2016). This model optimally mimics injuries from a nuclear accident or radiological attack, which are rising threats to public health (D. J. Barnett 2006). The prolonged survival of animals makes it feasible to study the effects of radioprotective agents such as thrombomodulin activated protein kinase C (APC).
Female adult WAG/RijCmcr rats were irradiated with 13.0 Gy leg-out partial body X-rays; longitudinally collected urine samples were subject to LC-MS based metabolomic profiling. Sub-cohorts of rats were treated with three variants of recombinant APC namely, rat wildtype (WT) APC, rat 3K3A mutant form of APC, and human WT APC as two bolus injections at 24 and 48 hours post IR. The APC variant 3K3A APC used in this study was designed to retain normal cell signaling activities and cytoprotective properties, with reduced anti-coagulant properties (Williams et al. 2012). We found that exposure to radiation induced perturbations in rat urinary profiles that was indicative of dyslipidemia and disruption of the metabolic pathways related to energy production and oxidative stress. The treatment with APC post IR exposure was observed to offer significant protection from radiation injury especially with the rat 3k3a version of recombinant APC.
1. Materials and methods:
2a. Animal care and irradiation protocols:
(i). Animals and groups:
All animal protocols were approved by the Institutional Animal Care and Use Committees (IACUC) at the Medical College of Wisconsin, Milwaukee. WAG/RijCmcr female rats were irradiated at about 11-12 week of age (~155 grams). To test the effect of radiation on urinary composition and the radioprotection exhibited by APC variants, a cohort of rats was randomized into 1) No irradiation, vehicle (n=8), 2) 13.0 Gy leg–out PBI (n=10), 3) 13.0 Gy leg–out PBI (n=10) with human WT APC, 4) 13.0 Gy leg–out PBI (n=10) with rat WT APC, 5) 13.0 Gy leg–out PBI (n=10) with variant rat 3k3a APC. The protocols used for leg-out PBI were similar as reported previously (Fish et al. 2020). Briefly, rats were restrained and irradiated without the use of anesthetics. One of the hind limbs of each rat was externalized carefully and shielded with a 0.25-inch lead block. An X-RAD 320kV orthovoltage X-ray system (Precision X-Ray, North Branford, Connecticut) was operated at 320 kVp and 13 mA with a half value layer of 1.4 mm Cu with a dose-rate of 1.69 Gy/min for a total dose of 13.0 Gy. Radiation was delivered posterior-to-anterior to the rat. Dosimetry was performed as described by Medhora et al (Medhora et al. 2014). All rats received supportive care including hydration by daily subcutaneous injection of saline 40 mL/kg/day from days 2-10, and antibiotics (enrofloxacin) at 10 mg/kg/day from days 2-14 given in the drinking water. Powdered food was added to the cages from days 35 to 70 after irradiation due to tooth loss that resolved by day 70.
(ii). APC treatment:
Rat WT APC and the variant 3k3a-APC, and the human WT APC were synthesized by Dr. Griffin’s laboratory and made available to us for this study by Dr. Fernandez (Scripps research institute, La Jolla, CA). All the 3 APC stock solutions were supplied as aliquots in a stabilization buffer (10 mM sodium citrate, 300 mM sodium chloride solution at a pH of 6.0). The drugs were stored at −80 °C until use. The APCs were administered to rats by intravenous (IV) injection at 24 and 48 hours after irradiation. On the day of administration, the drugs were diluted to the appropriate concentration with Dulbecco’s Phosphate-Buffered Saline as follows: rat WT APC (0.2 mgkg−1), rat variant 3K3A APC (0.2 mgkg−1) and human WT APC (0.6 mgkg−1). The vehicle group received equivalent dilutions of stabilization buffer made in DPBS.
(iii). Urine collection:
At 24 hours, 14-, 30- and 90-days post irradiation, rats were placed individually in metabolic cages for 24 hours and urine samples were collected. The urine samples were centrifuged at 13,000 rpm at 4 °C for 10 minutes and the supernatant was stored frozen at −80 °C until analyses.
2b. Mass spectrometry solvents and reagents:
All LC-MS grade solvents including acetonitrile and water were purchased from Fisher Optima grade, Fisher Scientific. High purity formic acid (99%) was purchased from Thermo-Scientific. Debrisoquine and 4-nitrobenzoic acid were purchased from Sigma-Aldrich. EquiSPLASH® LIPIDOMIX® quantitative mass spec internal standard and 15:0-18:1-d7-PA, C15 Ceramide-d7 (d18:1-d7/15:0) and 18:1 Chol (D7) ester were purchased from Avanti polar lipids. Internal standard for free fatty acid (FFA), dihydroceramides (DCER), hexosylceramides (HCER), lactosylceramides (LCER) were purchased from Sciex as Lipidyzer platform kit. The LC-MS data acquisition details are provided in Supplementary data.
2. Results:
3a. IR exposure induced distinct metabolic profiles:
In this study, adult female Wag/RijCmcr rats were irradiated (leg-out PBI) with sham or 13.0 Gy of X-rays to test the effect of radiation on urinary composition and further a sub-cohort was administered with recombinant APC at 24 and 48 hours post IR to determine potential alleviation of radiation injury. For doing this, a cohort of rats was randomized into five groups as detailed: 1) no irradiation, vehicle (n=8), 2) 13.0 Gy leg–out PBI (n=10), 3) 13.0 Gy leg–out PBI (n=10) with human WT APC, 4) 13.0 Gy leg–out PBI (n=10) with rat WT APC, 5) 13.0 Gy leg–out PBI (n=10) with rat 3k3a APC. Urine samples were collected longitudinally at 1, 14, 30- and 90-days following irradiation and APC treatment and subjected to LC-MS based metabolomic profiling. (Figure 1A). LC-MS data deconvolution was performed using XCMS software that yielded approximately 2316 and 1432 features in electrospray positive and negative modes, respectively (Gowda et al. 2014). Data normalization and log transformation was performed prior to multivariate analysis. Binary group comparisons between sham and irradiated urine samples at days 1 and 90 showed a clear distinction between the two groups (Figure 1B). Urinary profiles that showed robust metabolic dysregulation at day 1 post IR, stabilized for most part by 14 and 30 days, possibly reflecting homeostasis (Dimova et al. 2008; Desouky et al. 2019). However, we observed apparent metabolic shifts at 90 days, possibly indicating the development of late effects of acute radiation exposure (DEARE) (Supplementary Table 1). Figure 1C depicts the longitudinal trend (from day 1 to day 90) of significantly dysregulated metabolites. Metabolic dysregulations at day 1 post-IR, were visualized as volcano plots (Figure 2A) with a fold-change (FC) criterion of ≥2.0 and p-value < 0.05 (FDR adjusted), most of these alterations returned to near sham levels on days 14 and 30 (Figure 2B & C). Most of metabolites and lipids which were downregulated at day 1 showed upregulations at day 90 (Figure 2D). Accurate mass based putative identification of the 3931 significantly dysregulated features was performed by tandem mass spectrometry (MS/MS) validation. Putative annotation was done using the CEU Mass Mediator RESTful API service. Thereafter, 306 dysregulated features were putatively identified using fragmentation pattern matching by performing searches in NIST 2017 MS/MS database with a ppm error of less than 10 (Lin et al. 2014; Cooper et al. 2019). The putatively identified metabolome included broad classes of small polar molecules and lipids as detailed in Supplementary Table 2. We also used in-house developed targeted multiple reaction monitoring (MRM) based quantitative metabolomics and lipidomic analytical methodologies for the identification and quantitation of metabolites that exhibited radiation induced alteration in their abundance levels. Data processing followed by quality control measures provided us with 250 metabolites and 490 lipids in metabolomics and lipidomic mode, respectively. With the criteria of FC ⩾2.0, p-value < 0.05, (FDR adjusted), most of the dysregulations were observed at day 90 indicating delayed effects of IR (Supplementary Table 3).
Figure 1.
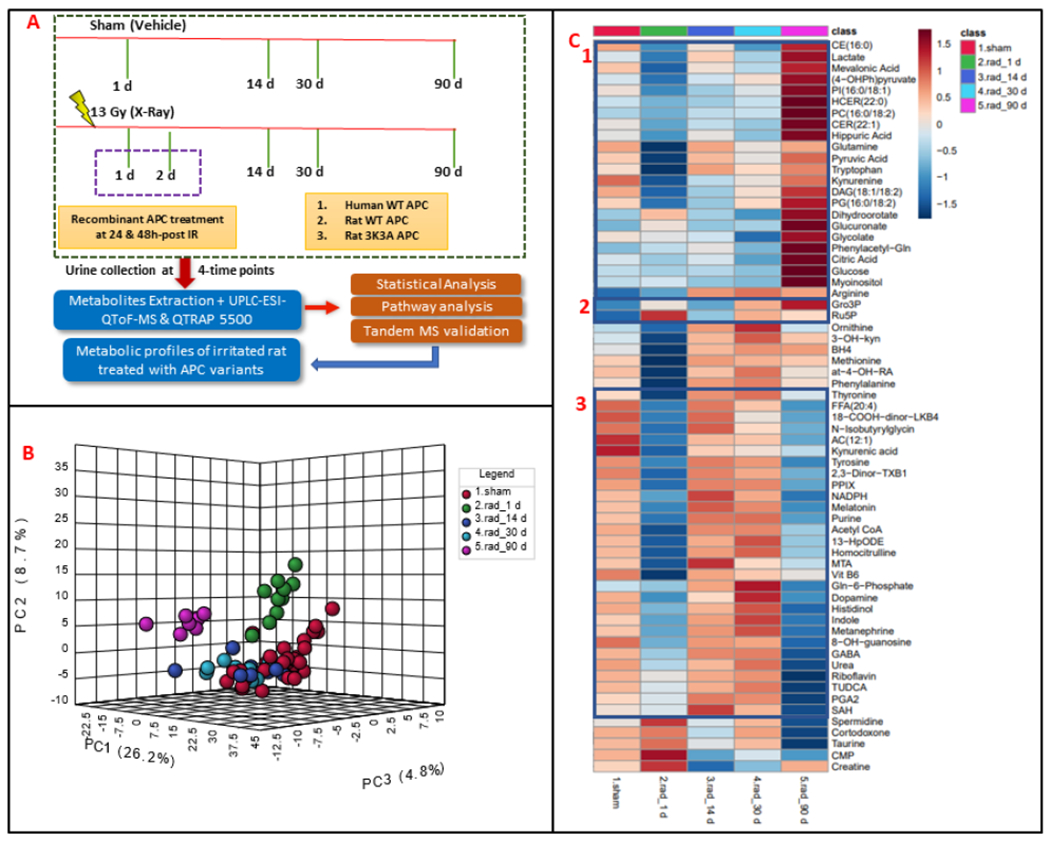
Panel A. Experimental and analytical design of the study. Urine samples collected from Wag/RijCmcr female rats that received either sham or 13 Gy of X-Ray radiation and processed for LC-MS based metabolomic and lipidomic analyses. Panel B. Three-dimensional Principal Component Analysis (PCA) plot demonstrating group separation between sham and irradiated animals as a function of time, post-irradiation. Panel C. Heatmap showing alterations in abundance of urinary metabolites at days 1, 14, 30 and 90 post-irradiation.
Figure 2. Volcano plots showing dysregulated urinary metabolites at day 1 (Panel A), day 14 (Panel B), 1 month (Panel C) and 3-months (Panel D) post-irradiation.
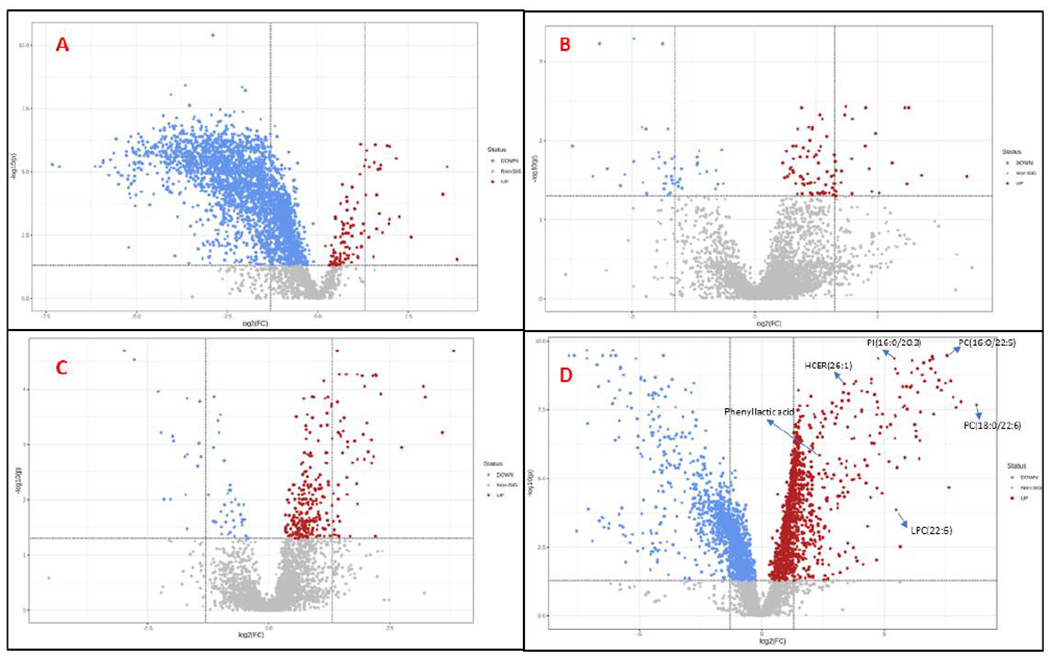
Each dot represents a putative metabolite; statistically significant changes comparing sham vs irradiated groups include adjusted p-value (<0.05) on the X-axis and fold change of ≤ 2 on the Y-axis. Gray: indicates no significant change in metabolite abundance, Red: Statistically significant upregulation, Blue: Red: Statistically significant downregulation
IR appeared to induce dyslipidemia, as observed by alterations in the abundance of several lipid classes, the major ones being fatty acyls, phospholipids, glycerolipids, bile-acids, steroids, and sphingolipids among others. Most of the lipid classes exhibited oscillatory abundance post-IR (downregulation at day 1, which mostly normalized at 14 and 30 days and showed significant upregulation again at day 90 and (Figure 1C). However, the downregulations of lipids such as steroids and fatty acyls at day 1 were maintained at 90 days. The longitudinal trends for lipidomic dysregulations are shown in Figure 3. Another important parameter of IR-induced oxidative stress is the relative composition of phospholipids such as PC/LPC (Angelini et al. 2014). The PC/LPC ratio was observed to fall significantly, indicating radiation-induced oxidative stress mediated conversion of PCs into LPCs (Figure S1).
Figure 3. Radiation causes dyslipidemia in rats.
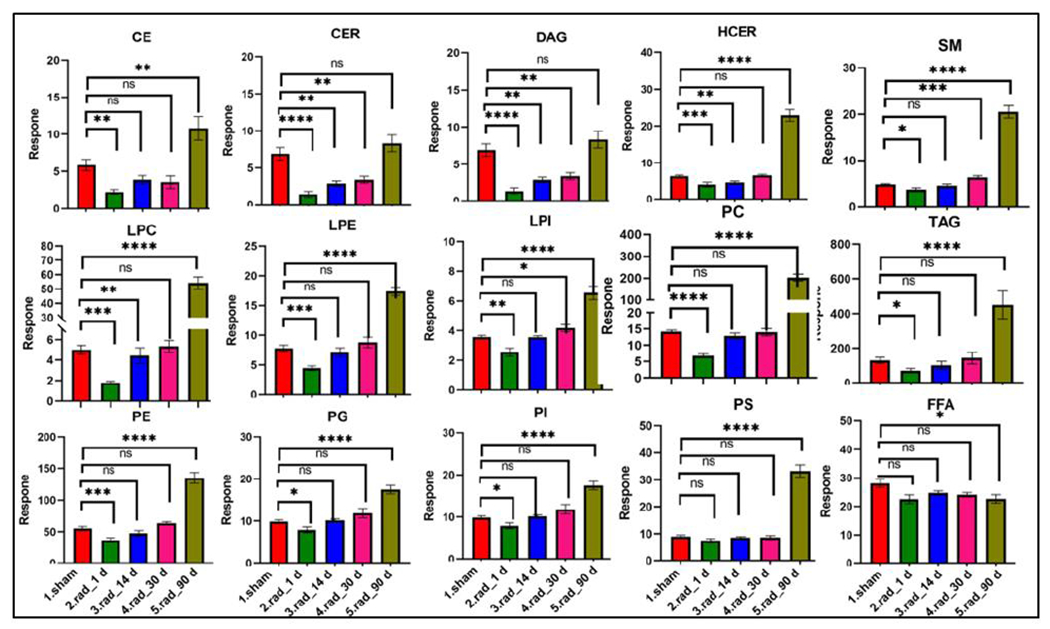
Box-plot showing normalized abundance of broad classes of lipids as a function of time at d1, d14, d30 and d90 post irradiated with 13.0 Gy of X-ray. ns = p > 0.05, * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001 and **** = p ≤ 0.0001.
Subsequently, metabolomics analysis indicated differential abundance of a broad variety of small polar molecules upon IR exposure. The longitudinal trends of metabolic dysregulations are classified in three main segments of heatmap (Figure 1C). Segment 1 details the downregulations at day 1 that mostly dissipated at 14 and 30 days however, showed significant upregulation at day 90. The above-mentioned trends for glycolysis and TCA cycle were evident from alterations in urinary levels of pyruvate and citrate, respectively. Amino acids like tryptophan and kynurenine showed significant upregulations at day 90. Segment 2 of the heatmap shows metabolites having stable response to radiation and were observed upregulated at day 1 and day 90 includes metabolites of pentose phosphate pathway like ribulose-5-phosphate. Segment 3 of heatmap highlights the metabolites which showed stable response to radiation impact and were observed as downregulated at day 1 and day 90 includes metabolites of hexosamine biosynthesis pathway (HBP) like glucosamine-6-phosphate. HBP is one of the key pathways that link the metabolic sensing and cellular signaling. The WAG/RijCmcr rat model is well known to exhibit radiation induced intestinal injuries and exhibit the altered gut microbiome (Li et al. 2020; Fernandes et al. 2021; Liu et al. 2021). Remarkably gut injury was evident with the significantly downregulated levels of shikimate pathway metabolites such as 4-aminobenzoic acid which is an intermediate in the bacterial biosynthesis of folic acid by the gut microbiota.
Significant downregulations were observed for the biomolecules modified by radiation exposure including 8-hydroxyguanosine, and nitro-tyrosine (NT). Upon irradiation, the modified biomolecules could be generated by hydroxyl radicals that are produced via radiolysis of water molecules in vascular endothelium while generation of methionine sulfoxide indicates elevated oxidative stress burden post irradiation. Similarly, NT could be a result of increased nitrative/nitrosative stress. Further, we performed Mummichog pathway analysis of dysregulated metabolites in urine samples of irradiated rats. Multiple pathways showed alterations at days 1 through 90 including purine and pyrimidine metabolism, biosynthesis and metabolism of amino acids, sphingolipids and glycerophospholipids metabolism. The alterations in bile acid biosynthesis, glycerolipid metabolism and carnitine shuttle were specific to delayed radiation injury and showed up at 90 days (Figure 4A, Supplementary Table 4). Taken together, LC-MS based global and targeted metabolomics and targeted lipidomics approach, used herein, showed dysregulations in metabolic profiles that may be indicative of radiation damage. We found acute and long-term sequelae of radiation damage that has been reported in human radiation exposure case studies (Fish et al. 2016).
Figure 4.
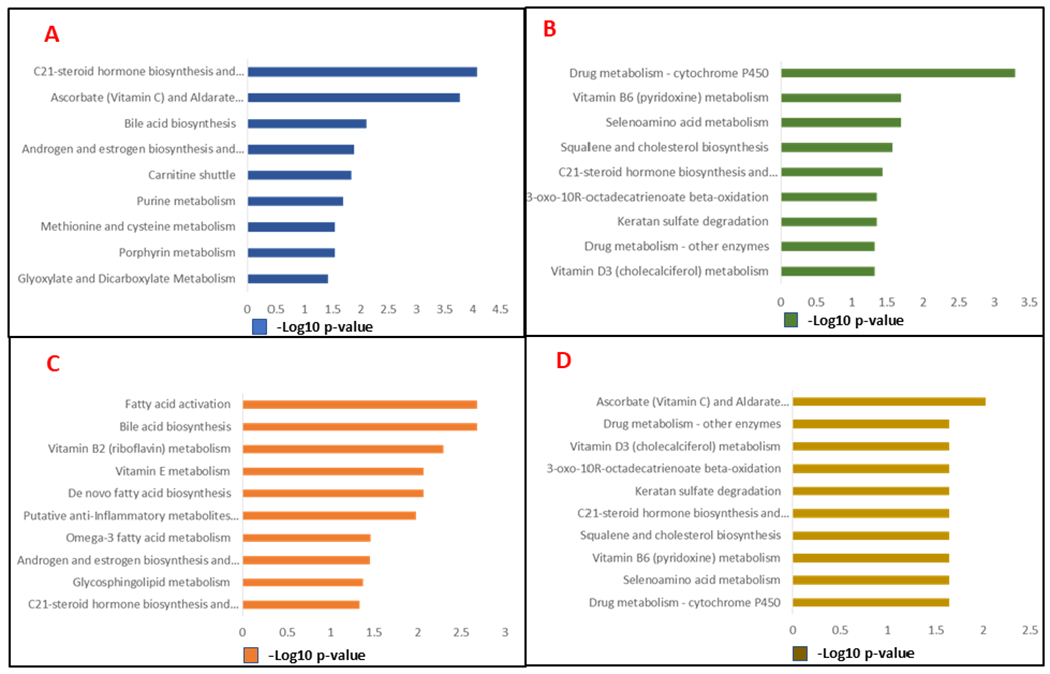
Mummichog (v2.06) based pathway enrichment analysis results for changes at 90-days post irradiation (panel A); 90-days after treatment with human WT APC (panel B); 90-days after treatment with rat WT APC (panel C); 90-days after treatment with rat 3K3A APC (panel D).
3b. Alleviation of radiation toxicity by APC administration
APCs along with causing the alteration of gene expression profiles also possess anti-inflammatory and antiapoptotic activities. Further effects include antithrombotic actions and cytoprotective activities with the net effect of maintaining the health and integrity of the vasculature via endothelial barrier stabilization. The direct cytoprotective role requires APC binding to the endothelial protein C receptor (EPCR) and activating protease activated receptor-1 (PAR-1) (Wang and Li 2009). We asked whether IR-induced changes in metabolic profiles were mitigated by treatment with APC. We observed significant restoration of lipid metabolism. A variety of lipid classes and small metabolites were observed to be corrected by APC treatments, a few of them are shown in Figure 5.
Figure 5.
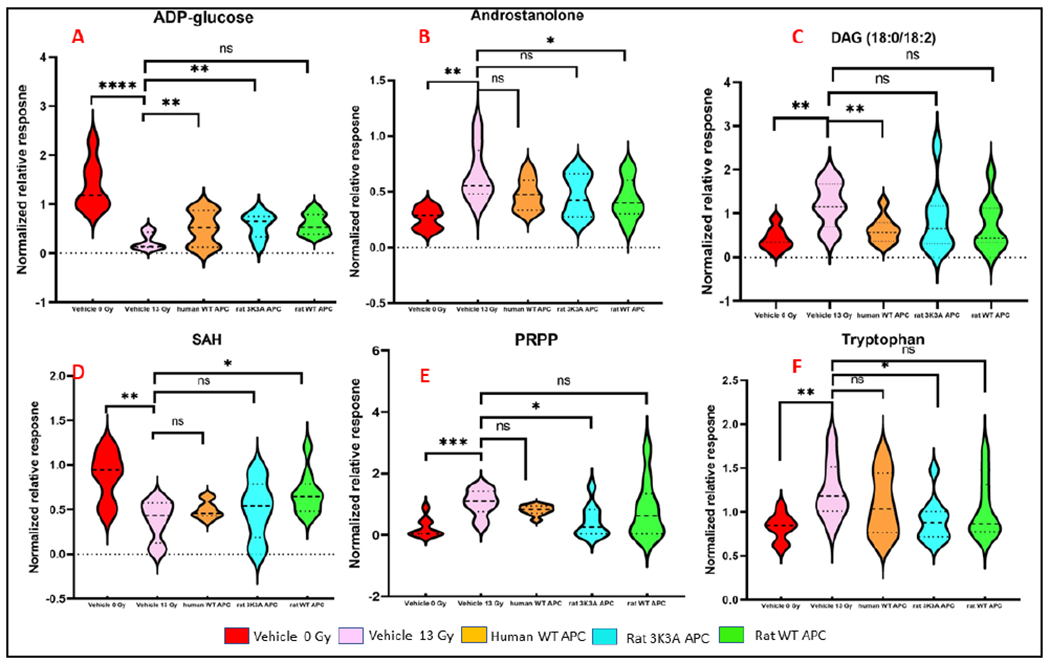
APC treatment results in partial alleviation of metabolic dysregulations at 90 days post-irradiation (Panels A-D); 14 days (Panels E-F). ns = p > 0.05, * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001 and **** = p ≤ 0.0001.
(i). Early alleviation of radiation effects by rat 3K3A APC:
At day 14, rat 3K3A variant of APC was most effective in partially restoring urinary metabolic levels to near-normal abundance for the metabolites including tryptophan, PRPP, nicotinuric acid, 15S-HpEDE, 9-OH-PGF2α, while rat WT mitigated the effects of IR on PRPP, Trp, 15S-HpEDE. Human WT APCs treatment managed to recover the levels of 11-DHC, PKC-substrate to near normal at day 14 post IR (Supplementary Table 5). Rat 3K3A also induced a total recovery for androstanolone at 30 d. Pathway analysis revealed that rat 3K3A APC impacted the conversion of valine, leucine, and isoleucine degradation, C21-steroid hormone biosynthesis and metabolism post 14-days treatment with rat 3K3A APC.
(ii). Rat WT APC mitigates delayed effects of IR:
Of the three recombinant versions of APCs studied herein, rat WT APC was observed as most effective at 90 d, where 4 metabolites (S-adenosylhomocysteine (SAH), androstanolone, ADP-glucose, Di-HETE) exhibited near sham levels while the dysregulations were corrected significantly for other 8 metabolites as detailed in Supplementary Table 3. Human WT APC restored the level of DAG (18:0/18:2), while rat 3K3A restored the levels of 5 metabolites (ADP-glucose, LCER 18:1, N-acetyl alanine, leucine, isoleucine) to near sham levels at 90-days post-IR (Supplementary Table 5, Figure 5). Mummichog pathway analyses showed protection in bile acid biosynthesis, C21-steroid hormone biosynthesis and metabolism, de novo fatty acid biosynthesis, glycosphingolipid metabolism post 90-days treatment with the mentioned three APC recombinants.
Discussion:
Accidental or intentional exposure to ionizing radiation is a major, worldwide public health concern that requires substantial efforts to manage and treat exposed individuals. In the present study, we report on metabolomics and lipidomic profiling on urine samples from female WAG/RijCmcr rats exposed to 13.0 Gy of leg-out X-ray PBI. The metabolic profiling study had two objectives: (i) studying urinary profiles post-IR, and (ii) assessing the potential radio injury mitigation activity of APC treatment.
The observed inflammatory phenotype in the urinary profiles of irradiated rats is suggestive of severe dyslipidemia. Lipids could be appropriate biomarkers to study long term effects of IR that are proportional to lipoxidative stress intensity (Pannkuk et al. 2017). Glycerophospholipid metabolism is considered as the most significant pathway among the IR-responsive lipids (Maceyka and Spiegel 2014). SMs, as important components of lipid raft domain within the plasma membrane are essential for cell signaling and the endothelial cell stress response (Mathias et al. 1998; Hammad 2011; Peter Slotte 2013). However, IR-induced oxidative stress could result into enzymatic hydrolysis of sphingomyelins by activation of acid sphingomyelinase and generate ceramides (Kolesnick 1991). Elevated levels of ceramides could disrupt lipid rafts and impair cellular signaling. Ceramides are significant radiation injury biomarkers and IR-induced elevation in ceramide levels have been detected in many pathological conditions (Yun et al. 2020; Choi et al. 2021).
Free fatty acids are vital endogenous molecules for cellular energy metabolism, and the decrease in their levels following IR is most likely a protective mechanism of the cells to meet their energy demands (Munir et al. 2019). Downregulated levels of fatty acyls and oxylipins as observed in this study could be related to either downregulated PUFA levels or high cellular antioxidant content (Morton et al. 1979; Cheeseman et al. 1988; Das 2006a, 2006b). Among bile-constituents, taurine is major, and a well-known marker of IR induced oxidative stress (Tyburski et al. 2008). Bile salts facilitate the absorption of fatty acids and cholesterol from dietary sources in the GI tract, however, disrupted due to alterations in gut microbiota following radiation exposure.
Upregulation in TCA cycle play a pivotal role in oncogenesis and inflammation. TCA cycle appeared as an actionable pathway in immunopathology and, the dysfunctions of TCA cycle in cancer are known to cause metabolic vulnerabilities (Scagliola et al. 2020). Citrate is reported to reduce hyperglycaemia-induced endothelial inflammation and abolishing endothelial dysfunction (Bryland et al. 2012). The dysregulations in HBP observed in this study could affect nucleotide biosynthesis and metabolism to impede DNA biosynthesis and repair. N-acetylation of glucosamine is reported as impaired in patients with inflammatory bowel disease, indicating response of gut tissue to inflammation under oxidative stress conditions (Burton and Anderson 1983). The perturbations in fatty acid biosynthetic and metabolic pathways could limit the supply for mitochondrial fatty-acid beta-oxidation that could subsequently result in dysregulated acetyl CoA levels, as observed. Acetyl CoA is the main feed to the TCA cycle, followed by ETS, and physiological dysfunction of mitochondria could disrupt the energy metabolism (Boroughs and DeBerardinis 2015; Cucchi et al. 2021). Pyruvic acid is reported to positively affect angiogenic cascade, DNA synthesis, migration, and tube formation in bovine aortic endothelial cells. Furthermore, mRNA expression of fibroblast growth factor receptor-2 and vascular endothelial growth factor was enhanced by pyruvic acid (Lee et al. 2001).
The prominent upregulation of shikimic acid pathway indicate IR-induced intestinal injury resulting in disturbances across related physiological events (Sun et al. 2020). Following metabolism by the disrupted gut microbiota, higher urinary levels of phenylacetylglutamine are suggestive of kidney dysfunction (Barrios et al. 2015). Perturbations in the oxalate and glyoxylate metabolism could also cause hyperoxaluria, a condition with elevated urinary levels of oxalate causing the buildup of calcium oxalate in the urine, and thus the eventual formation of kidney stones, a key cause of nephrolithiasis (Holmgren et al. 1978; Bhasin et al. 2015). Kidney malfunction due to late radiation effects is known characteristic of WAG/RijCmcr rat model post 90 days of IR exposure (Moulder John E. and Fish 1997; Moulder J. E. 2014; Fish et al. 2016).
IR-induced perturbations in amino acids can have a potential impact on both, protein levels and DNA damage repair (Cucchi et al. 2021). Amino acids are essential for maintaining intact endothelial functions, which include cell proliferation, regulation of blood flow and vascular tone, coagulation and fibrinolysis, and metabolism of a variety of macromolecules. Low tyrosine levels in the study may indicate kidney damage which is a well-known symptom of radiation exposure (Schrier 2007). Additionally, dysregulated abundance of S-Nitroso-L-glutathione, arginine and homoarginine that are known to improve endothelial functions by enhancing the production of NO, could further worsen the impacts of oxidative stress (Huynh and Chin-Dusting 2006; de Oliveira et al. 2008; Grosse et al. 2020).
APC is hypothesized to mitigate IR-induced damage (Geiger et al. 2012). The vascular endothelium is one of the most sensitive tissues to radiation damage, contributing to injury in organ systems that show loss of structure and function as early as a few days (gut and bone marrow) or months to years (brain, heart, lung and kidneys) after radiation (Hopewell et al. 1993; Wang et al. 2007; Baselet et al. 2019). APC has been shown to mitigate radiation injury by improving endothelial cell function. Wild-type APC has both anti-coagulant properties and binds to several receptors on the endothelial cell surface to alter endothelial signaling and function (Griffin et al. 2007; Pendurthi and Rao 2018). The treatment with recombinant APC was found to partially alleviate metabolic dysregulation caused by IR exposure. While rat WT APC appeared as the best radiation injury mitigative variant at day 90, rat 3K3A showed maximal radio mitigative effect at 14 days especially via significant restoration of lipid profiles. Corrections were also observed for nicotinuric acid, carnitines and regulated glycolysis suggest normalized energy metabolism and possibly repaired tissue injury. APC was found to restrict abnormal degradation of branched-chain amino acids including isoleucine and leucine. Isoleucine has been reported to down-regulate angiogenesis via inhibition of vascular endothelial growth factor while regular leucine metabolism could help prevent endothelial dysfunction in hyperglycemic conditions (Murata and Moriyama 2007). Since vascular impairments and in turn cognitive impairments are observed due to perturbed tryptophan metabolism, bringing Trp and SAH to near sham levels could assist in maintaining the endothelial function intact (Kwiatkowska et al. 2020; Mahalakshmi et al. 2022). Near sham Trp levels also indicates alleviation of GI injury since Trp is a key marker of gut homeostasis and its metabolism is closely related to intestinal microbiome. Taken together, these findings suggest that the APC treatments alleviated vascular endothelial dysfunction. Further studies are ongoing to better understand radiation mitigation by APC.
3. Conclusions:
Urinary metabolic profiles were used to characterize metabolic perturbations up to 90 days following IR, in a partial body irradiation model of adult female WAG/RijCmcr rats. Our data show that radiation causes metabolic perturbations some of which are restored by systemic repair processes to help achieve homeostasis. However, over time, new metabolic changes become apparent that may be suggestive of tissue or organ damage manifesting as late toxicities. We further show that administration of recombinant APC may be used effectively for alleviation of late tissue toxicities by optimizing the dose and treatment regimens.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the Metabolomics Shared Resource at Georgetown University (Washington, DC, USA) partially supported by NIH/NCI/CCSG grant P30-CA051008.
Funding
The study was supported by grants 1U01AI148308-01 and 5U01AI133561-04 from NIH/NIAID to MB and AKC and P20 GM109005 from NIH/NIGMS to MB.
Footnotes
Supplementary material
Supplementary Figures, Tables and LC-MS methods are provided in the Supplementary Information File.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Angelini R, Vortmeier G, Corcelli A, Fuchs B. 2014. A fast method for the determination of the PC/LPC ratio in intact serum by MALDI-TOF MS: an easy-to-follow lipid biomarker of inflammation. Chem Phys Lipids. 183:169–175. [DOI] [PubMed] [Google Scholar]
- Barrios C, Beaumont M, Pallister T, Villar J, Goodrich JK, Clark A, Pascual J, Ley RE, Spector TD, Bell JT et al. 2015. Gut-Microbiota-Metabolite Axis in Early Renal Function Decline. PLoS One. 10(8):e0134311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselet B, Sonveaux P, Baatout S, Aerts A. 2019. Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell Mol Life Sci. 76(4):699–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin B, Urekli HM, Atta MG. 2015. Primary and secondary hyperoxaluria: Understanding the enigma. World J Nephrol. 4(2):235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroughs LK, DeBerardinis RJ. 2015. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 17(4):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryland A, Wieslander A, Carlsson O, Hellmark T, Godaly G. 2012. Citrate treatment reduces endothelial death and inflammation under hyperglycaemic conditions. Diab Vasc Dis Res. 9(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AF, Anderson FH. 1983. Decreased incorporation of 14C-glucosamine relative to 3H-N-acetyl glucosamine in the intestinal mucosa of patients with inflammatory bowel disease. Am J Gastroenterol. 78(1):19–22. [PubMed] [Google Scholar]
- Cheeseman KH, Emery S, Maddix SP, Slater TF, Burton GW, Ingold KU. 1988. Studies on lipid peroxidation in normal and tumour tissues. The Yoshida rat liver tumour. Biochem J. 250(1):247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. 2021. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol. 18(10):701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BT, Yan X, Simon-Manso Y, Tchekhovskoi DV, Mirokhin YA, Stein SE. 2019. Hybrid Search: A Method for Identifying Metabolites Absent from Tandem Mass Spectrometry Libraries. Anal Chem. 91(21):13924–13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchi D, Gibson A, Martin SA. 2021. The emerging relationship between metabolism and DNA repair. Cell Cycle. 20(10):943–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett CLP DJ, Blodgett DW, Wierzba RK, and Links JM. 2006. Understanding radiologic and nuclear terrorism as public health threats: preparedness and response perspectives. J Nucl Med. 47(10):9. [PubMed] [Google Scholar]
- Das UN. 2006a. Essential Fatty acids - a review. Curr Pharm Biotechnol. 7(6):467–482. [DOI] [PubMed] [Google Scholar]
- Das UN. 2006b. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 1(4):420–439. [DOI] [PubMed] [Google Scholar]
- de Oliveira CP, de Lima VM, Simplicio FI, Soriano FG, de Mello ES, de Souza HP, Alves VA, Laurindo FR, Carrilho FJ, de Oliveira MG. 2008. Prevention and reversion of nonalcoholic steatohepatitis in OB/OB mice by S-nitroso-N-acetylcysteine treatment. J Am Coll Nutr. 27(2):299–305. [DOI] [PubMed] [Google Scholar]
- Desouky O, Ding N, Zhou G. 2019. Targeted and non-targeted effects of ionizing radiation. Journal of Radiation Research and Applied Sciences. 8(2):247–254. [Google Scholar]
- Dimova EG, Bryant PE, Chankova SG. 2008. Adaptive response: some underlying mechanisms and open questions. Genetics and Molecular Biology. 31(2):396–408. [Google Scholar]
- Fernandes A, Oliveira A, Soares R, Barata P. 2021. The Effects of Ionizing Radiation on Gut Microbiota, a Systematic Review. Nutrients. 13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish BL, Gao F, Narayanan J, Bergom C, Jacobs ER, Cohen EP, Moulder JE, Orschell CM, Medhora M. 2016. Combined Hydration and Antibiotics with Lisinopril to Mitigate Acute and Delayed High-dose Radiation Injuries to Multiple Organs. Health Phys. 111(5):410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish BL, MacVittie TJ, Szabo A, Moulder JE, Medhora M. 2020. WAG/RijCmcr rat models for injuries to multiple organs by single high dose ionizing radiation: similarities to nonhuman primates (NHP). Int J Radiat Biol. 96(1):81–92. [DOI] [PubMed] [Google Scholar]
- Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, Fernandez JA, Cancelas JA, Ryan MA, Kustikova O et al. 2012. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 18(7):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda H, Ivanisevic J, Johnson CH, Kurczy ME, Benton HP, Rinehart D, Nguyen T, Ray J, Kuehl J, Arevalo B et al. 2014. Interactive XCMS Online: simplifying advanced metabolomic data processing and subsequent statistical analyses. Anal Chem. 86(14):6931–6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JH, Fernandez JA, Gale AJ, Mosnier LO. 2007. Activated protein C. J Thromb Haemost. 5 Suppl 1:73–80. [DOI] [PubMed] [Google Scholar]
- Griffin JH, Zlokovic BV, Mosnier LO. 2018. Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 132(2):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse GM, Schwedhelm E, Worthmann H, Choe CU. 2020. Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications. Int J Mol Sci. 21(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad SM. 2011. Blood sphingolipids in homeostasis and pathobiology. Adv Exp Med Biol. 721:57–66. [DOI] [PubMed] [Google Scholar]
- Holmgren G, Hornstrom T, Johansson S, Samuelson G. 1978. Primary hyperoxaluria (glycolic acid variant): a clinical and genetical investigation of eight cases. Ups J Med Sci. 83(1):65–70. [DOI] [PubMed] [Google Scholar]
- Hopewell JW, Calvo W, Jaenke R, Reinhold HS, Robbins ME, Whitehouse EM. 1993. Microvasculature and radiation damage. Recent Results Cancer Res. 130:1–16. [DOI] [PubMed] [Google Scholar]
- Huynh NN, Chin-Dusting J. 2006. Amino acids, arginase and nitric oxide in vascular health. Clin Exp Pharmacol Physiol. 33(1-2):1–8. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN. 1991. Sphingomyelin and derivatives as cellular signals. Progress in Lipid Research. 30(1):1–38. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska I, Hermanowicz JM, Mysliwiec M, Pawlak D. 2020. Oxidative Storm Induced by Tryptophan Metabolites: Missing Link between Atherosclerosis and Chronic Kidney Disease. Oxid Med Cell Longev. 2020:6656033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Moon EJ, Lee SW, Kim MS, Kim KW, Kim YJ. 2001. Angiogenic activity of pyruvic acid in in vivo and in vitro angiogenesis models. Cancer Res. 61(8):3290–3293. [PubMed] [Google Scholar]
- Li Y, Yan H, Zhang Y, Li Q, Yu L, Li Q, Liu C, Xie Y, Chen K, Ye F et al. 2020. Alterations of the Gut Microbiome Composition and Lipid Metabolic Profile in Radiation Enteritis. Front Cell Infect Microbiol. 10:541178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HR, Liao CC, Lin TC. 2014. Improved identification of multiple drugs of abuse and relative metabolites in urine samples using liquid chromatography/triple quadrupole mass spectrometry coupled with a library search. Rapid Commun Mass Spectrom. 28(19):2043–2053. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu C, Yue J. 2021. Radiotherapy and the gut microbiome: facts and fiction. Radiat Oncol. 16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Spiegel S. 2014. Sphingolipid metabolites in inflammatory disease. Nature. 510(7503):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalakshmi AM, Paneyala S, Ray B, Essa MM, Dehhaghi M, Heng B, Guillemin GJ, Babu Chidambaram S. 2022. Alterations in Tryptophan Metabolism Affect Vascular Functions: Connected to Ageing Population Vulnerability to COVID-19 Infection? Int J Tryptophan Res. 15:11786469221083946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S, Pena LA, Kolesnick RN. 1998. Signal transduction of stress via ceramide. Biochem J. 335 (Pt 3):465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Wu Q, Molthen RC, Jacobs ER, Moulder JE, Fish BL. 2014. Model development and use of ACE inhibitors for preclinical mitigation of radiation-induced injury to multiple organs. Radiat Res. 182(5):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RE, Hartz JW, Reitz RC, Waite BM, Morris HP. 1979. The acyl-CoA desaturases of microsomes from rat liver and the Morris 7777 hepatoma. Biochim Biophys Acta. 573(2):321–331. [DOI] [PubMed] [Google Scholar]
- Moulder JE. 2014. 2013 Dade W. Moeller lecture: medical countermeasures against radiological terrorism. Health Phys. 107(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE, Fish BL. 1997. Age Dependence of Radiation Nephropathy in the Rat. Radiation Research. 147(3). [PubMed] [Google Scholar]
- Munir R, Lisec J, Swinnen JV, Zaidi N. 2019. Lipid metabolism in cancer cells under metabolic stress. Br J Cancer. 120(12):1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Moriyama M. 2007. Isoleucine, an essential amino acid, prevents liver metastases of colon cancer by antiangiogenesis. Cancer Res. 67(7):3263–3268. [DOI] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Singh VK, Fornace AJ. 2017. Lipidomic Signatures of Nonhuman Primates with Radiation-Induced Hematopoietic Syndrome. Sci Rep. 7(1):9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendurthi UR, Rao LVM. 2018. Endothelial cell protein C receptor-dependent signaling. Curr Opin Hematol. 25(3):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter Slotte J. 2013. Molecular properties of various structurally defined sphingomyelins -- correlation of structure with function. Prog Lipid Res. 52(2):206–219. [DOI] [PubMed] [Google Scholar]
- Scagliola A, Mainini F, Cardaci S. 2020. The Tricarboxylic Acid Cycle at the Crossroad Between Cancer and Immunity. Antioxid Redox Signal. 32(12):834–852. [DOI] [PubMed] [Google Scholar]
- Schrier RW 2007. Diseases of the Kidney & Urinary Tract. 8th Edition, Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- Singh VK, Garcia M, Seed TM. 2017. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part II. Countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status. Int J Radiat Biol. 93(9):870–884. [DOI] [PubMed] [Google Scholar]
- Singh VK, Hanlon BK, Santiago PT, Seed TM. 2017. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part III. Countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int J Radiat Biol. 93(9):885–906. [DOI] [PubMed] [Google Scholar]
- Singh VK, Newman VL, Romaine PL, Hauer-Jensen M, Pollard HB. 2016. Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Rev Mol Diagn. 16(1):65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Seed TM. 2017. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int J Radiat Biol. 93(9):851–869. [DOI] [PubMed] [Google Scholar]
- Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS et al. 2004. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Radiat Res. 162(6):711–728. [DOI] [PubMed] [Google Scholar]
- Sun B, Wang X, Liu X, Wang L, Ren F, Wang X, Leng X. 2020. Hippuric Acid Promotes Renal Fibrosis by Disrupting Redox Homeostasis via Facilitation of NRF2-KEAP1-CUL3 Interactions in Chronic Kidney Disease. Antioxidants (Basel). 9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburski JB, Patterson AD, Krausz KW, Slavik J, Fornace AJ Jr., Gonzalez FJ, Idle JR. 2008. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiat Res. 170(1):1–14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Boerma M, Fu Q, Hauer-Jensen M. 2007. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 13(22):3047–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li J. 2009. Activated protein C: a potential cardioprotective factor against ischemic injury during ischemia/reperfusion. Am J Transl Res. 1(4):381–392. [PMC free article] [PubMed] [Google Scholar]
- Williams PD, Zlokovic BV, Griffin JH, Pryor KE, Davis TP. 2012. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr Pharm Des. 18(27):4215–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H, Sun L, Wu Q, Zong G, Qi Q, Li H, Zheng H, Zeng R, Liang L, Lin X. 2020. Associations among circulating sphingolipids, beta-cell function, and risk of developing type 2 diabetes: A population-based cohort study in China. PLoS Med. 17(12):e1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


