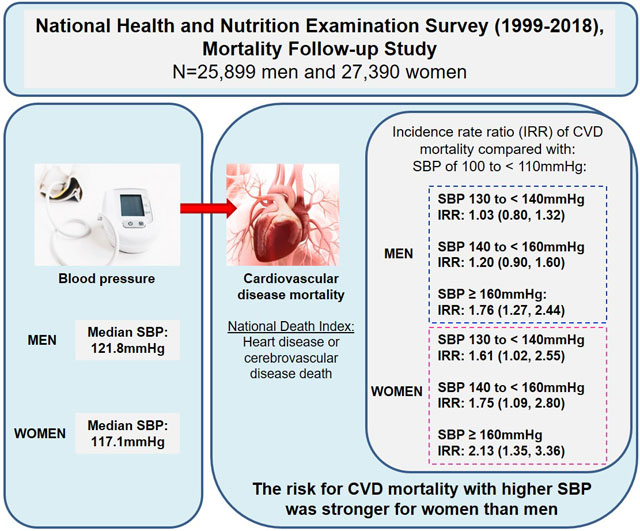
Abstract
Background:
Most research examining the association between blood pressure (BP) and cardiovascular disease (CVD) is sex-agnostic. Our goal was to assess sex-specific associations between BP and CVD mortality.
Methods:
We combined ten cycles of the National Health and Nutrition Examination Survey (1999–2018), N=53,289. Blood pressure was measured three times and averaged. Data were linked to National Death Index data and CVD mortality through December 31 2019 was defined from ICD-10 codes. We estimated sex-stratified, multivariable-adjusted incidence rate ratios (IRRs) for CVD mortality.
Results:
Over a median follow-up of 9.5 years, there were 2,405 CVD deaths. Associations between categories of SBP and DBP with CVD mortality differed by sex (p<0.01). Among men, compared with SBP of 100 to < 110 mmHg, CVD mortality was 76% higher with SBP ≥ 160 mmHg (IRR: 1.76, 95% CI: 1.27, 2.44). Among women, compared with SBP 100 to < 110 mmHg, CVD mortality was 61% higher with SBP 130–139 mmHg (IRR: 1.61, 95% CI: 1.02, 2.55), 75% higher with SBP 140–159 mmHg (IRR: 1.75, 95% CI: 1.09, 2.80), and 113% higher with SBP ≥ 160 mmHg (IRR: 2.13, 95% CI: 1.35, 3.36). Compared with DBP 70 to < 80 mmHg, CVD mortality was higher with DBP < 70 mmHg and DBP ≥ 80mmHg among men, and higher with DBP < 50 mmHg and DBP ≥ 80mmHg among women.
Conclusions:
The association between BP and CVD mortality differed by sex, with increased CVD mortality risk present at lower levels of systolic blood pressure among women compared with men.
Keywords: blood pressure, cardiovascular disease mortality, sex differences
Graphical Abstract
INTRODUCTION
High blood pressure is an important risk factor for the development of cardiovascular disease (CVD), the leading cause of mortality in the United States (US).1 Data from the National Health and Nutrition Examination Survey (NHANES) suggests that 45.6% of the US population has hypertension,2 affecting men more commonly than women (48.6% vs. 42.9%, respectively).2 However, differences in the prevalence of hypertension by sex are not uniform across the life course; among US adults at ≥65 years of age, women are more likely to have hypertension than men.3
Several studies4–8 have suggested that the association between BP and CVD outcomes differs by sex. Meta-analyses of observational studies show that the association between systolic BP (SBP) and diastolic BP (DBP) with CVD mortality is stronger in women compared to men.9, 10 Although hypertension guidelines do not endorse sex-based BP recommendations,11 prior findings4–8 suggest that further investigation of sex-specific BP targets may be warranted.
A 2022 multidisciplinary statement from the European Society of Cardiology12 and a recent review,13 highlighted that CVD complications start at different BP levels in women compared to men, questioning the current practice of using similar BP levels for the identification of elevated BP and hypertension in both sexes. Further elucidating these relationships may have implications for the management of hypertension and subsequent risk of cardiovascular outcomes. However, there is a paucity of sex-specific data on the relationship between BP and CVD,12 and even fewer studies conducted among US populations.4 To address this gap, we examined the association between BP levels and CVD mortality stratified by sex, using NHANES 1999–2000 through 2017–2018 cycles, linked to National Death Index (NDI) data.
METHODS
All data have been made publically available by the National Center for Health Statistics and can be accessed at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Study Population
NHANES is a cross-sectional survey designed to evaluate the health and nutritional status of non-institutionalized adults and children. Results can be weighted to provide nationally representative estimates for the non-institutionalized US population. NHANES was conducted continuously from 1999 to 2020 in two year cycles.14 Each cycle included different participants. Data collection included household interviews and physical examinations conducted in a mobile examination center. The current analysis included data from 25,899 adult male and 27,390 adult female NHANES participants from ten survey cycles (1999–2000 through 2017–2018) with BP measurements and mortality status assessed, see figure S1. The National Center for Health Statistics institutional review board approved all of the study procedures and written informed consent was obtained from all adult participants prior to commencement.
Blood Pressure
BP measurements were taken three times during the NHANES physical examination. Trained physicians obtained these measurements using a stethoscope and a mercury sphygmomanometer (1999–2016)15 or an Omron HEM-907XL automated oscillometric device (2017–2018)16 with appropriately-sized upper arm cuffs. Readings were obtained after a five-minute rest period with 30 second intervals in between readings. The three readings were averaged. We calibrated the BP measured in the 2017–2018 NHANES to account for differences between mercury sphygmomanometer and oscillometric devices.17 We calibrated the measurements by adding 1.1 mmHg for men or 2.1 mmHg for women to SBP values and 0.9 mmHg for men or 0.2 mmHg for women to DBP values obtained by the automated oscillometric device.17 SBP was categorized as follows: <100 mmHg, 100 to <110 mmHg, 110 to <120 mmHg, 120 to <130 mmHg, 130 to <140 mmHg, 140 to <160 mmHg, and ≥160 mmHg. DBP was categorized as follows: <50 mmHg, 50 to <60 mmHg, 60 to <70 mmHg, 70 to <80 mmHg, 80 to <90 mmHg, 90 to <100 mmHg, and ≥100 mmHg.
Mortality
The National Center for Health Statistics has made NDI data publicly available for linkage to US-based population surveys such as NHANES.18 NDI data are matched with survey participants through social security numbers, names, birthdates, and other personal identifiable information.19 NHANES data were merged with de-identified NDI data with death information ascertained through December 31, 2019.18 Cause-specific mortality was classified by primary cause of death according to recorded ICD-10 codes.20 CVD mortality reported in this analysis includes diseases of the heart and cerebrovascular diseases. Follow-up time from medical examination date until death or censor was recorded in years.
Other Variables
Demographic information was collected during the interview portion of the survey. Age, sex, race/ethnicity, education level (less than high school (HS) or at least HS or equivalent), and annual household income (<$55,000 per year, or ≥$55,000 per year) were self-reported. Individuals also self-reported health behaviors such as smoking status or use of antihypertensive and diabetes medications. Participants self-reported whether they participated in any vigorous or moderate physical activity in a typical week. Physical activity was defined as none (no vigorous or moderate physical activity), moderate (moderate physical activity only), or high (vigorous physical activity). Body mass index (BMI) was calculated as measured weight (kg)/measured height (m2). Diabetes was defined as: 1) self-reported diabetes or 2) self-reported diabetes medication use or 3) measured fasting glucose ≥ 126 mg/dL. Total cholesterol (mg/dL) was measured from a fasting blood draw. Using serum creatinine values (mg/dL), calibrated for applicable years,21 estimated glomerular filtration rate (eGFR) was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation22 and dichotomized as < or ≥ 60 ml/min per 1.73 m2. We defined prevalent CVD via self-reported history of congestive heart failure, coronary heart disease, angina, heart attack, or stroke.
Statistical Analysis
We described sex-specific population characteristics stratified by category of SBP. We used chi-square tests for proportions and ANOVAs for continuous variables to test whether characteristics differed by SBP category. We plotted kernel density histograms showing the population distribution of SBP and DBP by sex, also calculating the median values and interquartile range. Using Poisson regression models, we calculated sex-stratified, age-adjusted incidence rates (IRs) for CVD mortality per 1,000 person years (PY) at each category of SBP and DBP. We also compared each age-adjusted IR to the IR of a given reference category of SBP and DBP. For a SBP reference level, we selected the lowest BP range23 which included at least 10% of the sample of men and women (SBP 100 to <110mmHg). For DBP, we chose the highest DBP range considered normal BP (70 to < 80 mmHg) based on prior studies indicating that low DBP is associated with CVD.24 We used survey-weighted Cox Proportional regression models with spline terms and graphically depicted the age-adjusted association between continuous BP with CVD mortality. We preselected knots based on inflection points informed from IRs. Specifically, we placed knots at SBP of 110 and 120 mmHg for men or 100 and 110 mmHg for women. Knots for DBP were equivalent for men and women (at 70 and 80 mmHg). We selected SBP of 105 mmHg and DBP of 75 mmHg as the reference BP for our figures given that these values represented the midpoint of our reference BP categories (defined above). Finally, using Poisson models, we calculated multivariable-adjusted IR ratios (IRRs) for CVD mortality overall and by cause specific CVD mortality adjusting for age, sex, race/ethnicity, educational attainment, household income, smoking status, BMI, physical activity, diabetes, total cholesterol, eGFR < 60 ml/min per 1.73 m2, use of antihypertensive medications, and prior CVD. We also tested the multiplicative interaction between sex with both categories of SBP and DBP for CVD mortality using Poisson regression models.
We conducted four sensitivity analyses to determine whether our results were consistent among participants with a minimum three months25 of follow-up time prior to death (N=53,220), adults without prior CVD (n=47,809), adults not taking antihypertensive medication (n=40,424), and adults 50 years or older (n=24,202). We repeated the Poisson regression models to estimate IRs and IRRs in these sub-populations. All analyses accounted for the complex stratified survey design of NHANES including incorporation of mobile examination center survey weights. We used SUDAAN (v.11) and R software (3.6.0) for all analyses.
RESULTS
The median age of the population was 43.7 years (Quartile (Q) 1: 30.4, Q3:57.3) for men and 45.5 years (Q1: 31.5, Q3:59.5) for women, with 68% of both men and women being non-Hispanic White (Tables 1 and 2). Median SBP was 117.1 mmHg (Q1: 107.3, Q3:130.6) for women and 121.8 mmHg (Q1: 113.3, Q3: 131.8) for men (Figure 1). For both men and women, individuals with higher SBP were more likely to be older, a Black person, have less than a HS education, have lower income, non-smoker, be less physical active, and have CVD risk factors, including prior CVD (p values all < 0.01).
Table 1:
Characteristics of US adult men overall and stratified by systolic blood pressure, National Health and Nutrition Examination Survey, 1999–2018.
| Systolic Blood Pressure range (mmHg) | P-Value | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men N=25,899 (100%) |
<100 n=666 (2.6%) |
100-<110 n=3,084 (12.8%) |
110-<120 n=6,920 (29.0%) |
120-<130 n=6,467 (26.5%) |
130-<140 n=4,136 (15.1%) |
140-<160 n=3,432 (11.0%) |
≥160 n=1,194 (3.0%) |
||||||||||
| % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | ||
| Age, mean | 45.2 | 0.2 | 44.2 | 0.8 | 40.0 | 0.4 | 40.7 | 0.3 | 43.7 | 0.3 | 49.5 | 0.4 | 56.7 | 0.4 | 62.6 | 0.6 | <0.01 |
| Age group | |||||||||||||||||
| 18–34 | 31.7 | 0.5 | 33.5 | 2.2 | 42.6 | 1.2 | 40.6 | 0.9 | 33.9 | 0.8 | 22.5 | 0.9 | 10.1 | 0.8 | 3.8 | 1.0 | <0.01 |
| 35–49 | 28.5 | 0.4 | 28.3 | 2.3 | 31.1 | 1.1 | 30.7 | 0.8 | 31.0 | 0.8 | 27.1 | 0.9 | 19.8 | 0.9 | 15.0 | 1.5 | |
| 50–64 | 24.1 | 0.4 | 22.9 | 2.4 | 17.1 | 1.0 | 20.0 | 0.7 | 22.7 | 0.7 | 28.7 | 1.0 | 37.6 | 1.3 | 33.0 | 2.0 | |
| 65+ | 15.7 | 0.3 | 15.3 | 1.7 | 9.2 | 0.6 | 8.7 | 0.5 | 12.3 | 0.5 | 21.7 | 0.7 | 32.5 | 1.1 | 48.3 | 2.0 | |
| Race/Ethnicity | |||||||||||||||||
| Non Hispanic White | 68.4 | 1.0 | 73.3 | 2.0 | 67.9 | 1.4 | 67.6 | 1.1 | 69.1 | 1.2 | 68.6 | 1.4 | 68.9 | 1.4 | 63.9 | 2.3 | <0.01 |
| Non Hispanic Black | 10.4 | 0.5 | 7.4 | 1.0 | 8.5 | 0.5 | 8.9 | 0.5 | 10.0 | 0.6 | 11.9 | 0.8 | 13.9 | 0.9 | 18.4 | 1.6 | |
| Hispanic | 14.4 | 0.8 | 12.7 | 1.3 | 16.1 | 1.1 | 15.4 | 0.8 | 14.9 | 1.0 | 13.6 | 0.9 | 11.0 | 0.9 | 11.4 | 1.3 | |
| Other | 6.8 | 0.3 | 6.7 | 1.0 | 7.5 | 0.7 | 8.0 | 0.5 | 6.0 | 0.4 | 5.9 | 0.6 | 6.2 | 0.5 | 6.3 | 1.1 | |
| < HS Education | 18.5 | 0.5 | 20.5 | 1.8 | 18.0 | 1.0 | 16.9 | 0.7 | 17.6 | 0.6 | 19.6 | 0.9 | 20.9 | 0.9 | 26.0 | 1.6 | <0.01 |
| Income < $55,000 | 45.7 | 0.8 | 49.6 | 2.6 | 45.1 | 1.4 | 43.9 | 1.1 | 42.5 | 1.0 | 46.9 | 1.4 | 51.9 | 1.4 | 60.6 | 2.3 | <0.01 |
| Current smoker | 23.6 | 0.5 | 25.4 | 2.3 | 24.6 | 1.0 | 24.4 | 0.9 | 23.2 | 0.7 | 23.0 | 0.9 | 21.5 | 1.0 | 23.0 | 1.8 | <0.01 |
| No physical activity | 39.4 | 0.6 | 41.3 | 2.6 | 36.0 | 1.2 | 35.3 | 0.9 | 39.1 | 0.9 | 41.3 | 1.2 | 48.2 | 1.3 | 53.8 | 2.2 | <0.01 |
| BMI (kg/m 2 ), mean | 28.5 | 0.1 | 26.3 | 0.3 | 26.5 | 0.1 | 27.6 | 0.1 | 28.9 | 0.1 | 29.9 | 0.2 | 30.1 | 0.2 | 29.8 | 0.3 | <0.01 |
| Diabetes | 11.2 | 0.3 | 14.6 | 2.0 | 6.7 | 0.6 | 7.6 | 0.4 | 9.7 | 0.4 | 15.1 | 0.7 | 20.0 | 1.0 | 22.7 | 1.6 | <0.01 |
| Total cholesterol, mean | 192.7 | 0.5 | 179.9 | 2.3 | 185.2 | 0.9 | 189.7 | 0.6 | 194.4 | 0.8 | 197.5 | 1.0 | 199.5 | 0.9 | 199.2 | 2.3 | <0.01 |
| eGFR < 60 ml/min per 1.73 m 2 | 4.5 | 0.2 | 8.0 | 1.3 | 2.1 | 0.3 | 2.7 | 0.2 | 2.9 | 0.2 | 5.6 | 0.7 | 10.2 | 0.7 | 16.9 | 1.4 | <0.01 |
| BP med use | 19.5 | 0.4 | 21.8 | 2.3 | 9.2 | 0.7 | 11.6 | 0.5 | 17.6 | 0.7 | 26.7 | 1.0 | 38.7 | 1.1 | 48.0 | 1.9 | <0.01 |
| Prior CVD | 9.3 | 0.2 | 15.1 | 1.7 | 7.8 | 0.6 | 6.4 | 0.4 | 7.5 | 0.4 | 11.2 | 0.6 | 14.8 | 0.8 | 23.9 | 1.4 | <0.01 |
BMI: body mass index; BP: blood pressire; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; HS: High school; SE: standard error
P-Value < 0.05 indicates that the given characteristic differs across categories of systolic blood pressure using ANOVA for means or chi-square tests for proportions.
Table 2:
Characteristics of US adult women overall and stratified by systolic blood pressure, National Health and Nutrition Examination Survey, 1999–2018.
| Systolic Blood Pressure range (mmHg) | P-Value | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women N=27,390 (100%) |
<100 n=2,250 (8.5%) |
100-<110 n=5,926 (22.4%) |
110-<120 n=6,535 (25.0%) |
120-<130 n=4,605 (17.7%) |
130-<140 n=3,130 (11.2%) |
140-<160 n=3,208 (10.5%) |
≥160 n=1,736 (4.7%) |
||||||||||
| % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | ||
| Age, mean | 46.8 | 0.2 | 36.2 | 0.4 | 37.0 | 0.3 | 41.4 | 0.3 | 50.0 | 0.3 | 57.2 | 0.4 | 62.5 | 0.3 | 68.8 | 0.4 | <0.01 |
| Age group | |||||||||||||||||
| 18–24 | 29.2 | 0.5 | 51.8 | 1.4 | 49.1 | 1.0 | 38.4 | 0.9 | 18.1 | 0.7 | 7.2 | 0.6 | 2.3 | 0.3 | 0.6 | 0.2 | <0.01 |
| 25–44 | 27.8 | 0.4 | 30.4 | 1.3 | 32.8 | 1.0 | 32.4 | 0.8 | 30.5 | 1.0 | 21.3 | 1.0 | 15.9 | 0.9 | 7.4 | 1.0 | |
| 45–64 | 24.2 | 0.4 | 13.9 | 1.2 | 13.7 | 0.7 | 19.5 | 0.6 | 32.5 | 1.1 | 39.5 | 1.2 | 34.8 | 1.0 | 25.1 | 1.4 | |
| 65+ | 18.8 | 0.4 | 3.9 | 0.5 | 4.3 | 0.3 | 9.7 | 0.5 | 18.9 | 0.8 | 32.0 | 1.0 | 47.0 | 1.1 | 67.0 | 1.6 | |
| Race/Ethnicity | |||||||||||||||||
| Non Hispanic White | 67.8 | 1.1 | 67.5 | 1.2 | 65.3 | 1.2 | 67.2 | 1.2 | 69.7 | 1.3 | 70.1 | 1.4 | 69.5 | 1.5 | 66.2 | 1.8 | <0.01 |
| Non Hispanic Black | 11.9 | 0.6 | 7.7 | 0.6 | 9.9 | 0.6 | 11.8 | 0.7 | 12.1 | 0.8 | 14.2 | 0.9 | 14.9 | 1.0 | 16.6 | 1.4 | |
| Hispanic | 13.5 | 0.8 | 16.3 | 1.0 | 17.3 | 1.0 | 14.4 | 0.9 | 11.4 | 0.8 | 9.4 | 0.7 | 9.7 | 0.9 | 11.2 | 1.3 | |
| Other | 6.9 | 0.3 | 8.5 | 0.8 | 7.6 | 0.5 | 6.6 | 0.4 | 6.8 | 0.5 | 6.3 | 0.6 | 5.9 | 0.5 | 6.0 | 0.7 | |
| < HS Education | 17.3 | 0.4 | 14.0 | 1.0 | 15.4 | 0.6 | 15.2 | 0.6 | 16.4 | 0.7 | 19.2 | 0.9 | 22.6 | 1.0 | 30.7 | 1.4 | <0.01 |
| Inc < $55,000 | 50.6 | 0.8 | 46.5 | 1.6 | 46.4 | 1.1 | 48.9 | 1.0 | 49.8 | 1.1 | 53.5 | 1.3 | 58.1 | 1.4 | 67.3 | 1.8 | <0.01 |
| Smoker | 18.2 | 0.4 | 20.6 | 1.4 | 19.7 | 0.7 | 19.8 | 0.7 | 18.0 | 0.7 | 15.9 | 0.8 | 15.0 | 0.8 | 11.9 | 1.0 | <0.01 |
| Physical activity | 44.5 | 0.6 | 37.6 | 1.4 | 37.5 | 0.9 | 40.2 | 0.9 | 45.8 | 1.1 | 52.7 | 1.3 | 56.6 | 1.3 | 62.4 | 1.3 | <0.01 |
| BMI (kg/m 2 ), mean | 28.8 | 0.1 | 24.8 | 0.1 | 26.8 | 0.1 | 28.9 | 0.1 | 30.5 | 0.2 | 31.2 | 0.2 | 30.1 | 0.2 | 29.2 | 0.2 | <0.01 |
| Diabetes | 9.8 | 0.2 | 2.8 | 0.4 | 4.2 | 0.3 | 7.2 | 0.4 | 11.6 | 0.6 | 16.0 | 0.8 | 18.3 | 0.7 | 22.8 | 1.3 | <0.01 |
| Total cholesterol, mean | 198.1 | 0.4 | 186.8 | 1.2 | 189.1 | 0.7 | 194.9 | 0.8 | 202.4 | 0.7 | 207.0 | 1.1 | 211.3 | 1.0 | 212.5 | 1.5 | <0.01 |
| eGFR < 60 ml/min per 1.73 m 2 | 6.6 | 0.2 | 2.7 | 0.4 | 2.2 | 0.2 | 3.7 | 0.3 | 6.3 | 0.5 | 10.2 | 0.6 | 14.8 | 0.8 | 26.0 | 1.4 | <0.01 |
| BP med use | 22.5 | 0.4 | 6.1 | 0.6 | 7.1 | 0.5 | 13.6 | 0.6 | 25.5 | 0.9 | 40.6 | 1.2 | 50.1 | 1.2 | 57.4 | 1.7 | <0.01 |
| Prior CVD | 7.4 | 0.2 | 3.7 | 0.5 | 3.2 | 0.3 | 4.7 | 0.4 | 7.6 | 0.4 | 10.9 | 0.6 | 15.9 | 0.8 | 21.3 | 1.4 | <0.01 |
BMI: body mass index; BP: blood pressire; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; HS: High school; SE: standard error
P-Value < 0.05 indicates that the given characteristic differs across categories of systolic blood pressure using ANOVA for means or chi-square tests for proportions.
Figure 1:
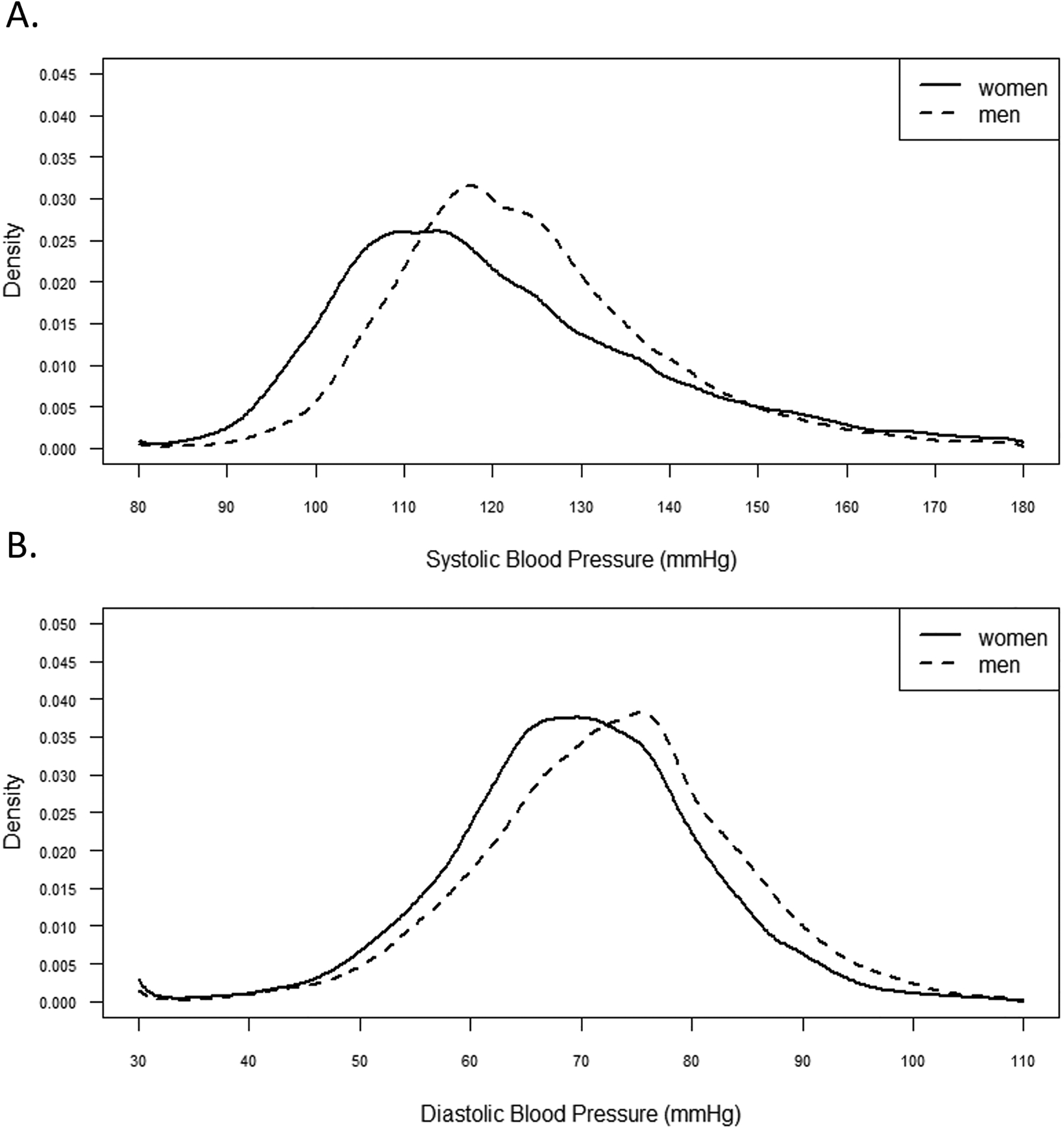
Kernal density distribution of population blood pressure by sex, National Health and Nutrition Examination Survey, 1999–2018.
Age adjusted associations between blood pressure and CVD
After a median follow-up of 9.5 years, 7,690 people (10.9%) died of any cause and 2,405 people (3.2%) died of CVD (1,981 heart disease deaths and 424 cerebrovascular disease deaths). The age-adjusted CVD mortality rate was lowest with SBP between 110 to <120mmHg for men and 100 to <110 mmHg for women (Figure 2). The age adjusted CVD mortality rate was lowest with DBP between 70 to <80mmHg for both men and women. Figure 3 (panel A) depicts the age-adjusted association between continuous SBP and CVD mortality for men and women. Compared with a SBP of 105 mmHg, a higher risk of CVD mortality was present at a SBP >122 mmHg for women or >134 mmHg for men (depicted by the second crossing of the x-axis for each curve). Compared with a DBP of 75mmHg, increased risk of CVD mortality was present at similar DBP values for women (>80mmHg) and men (>81mmHg) (Figure 3, panel B).
Figure 2:
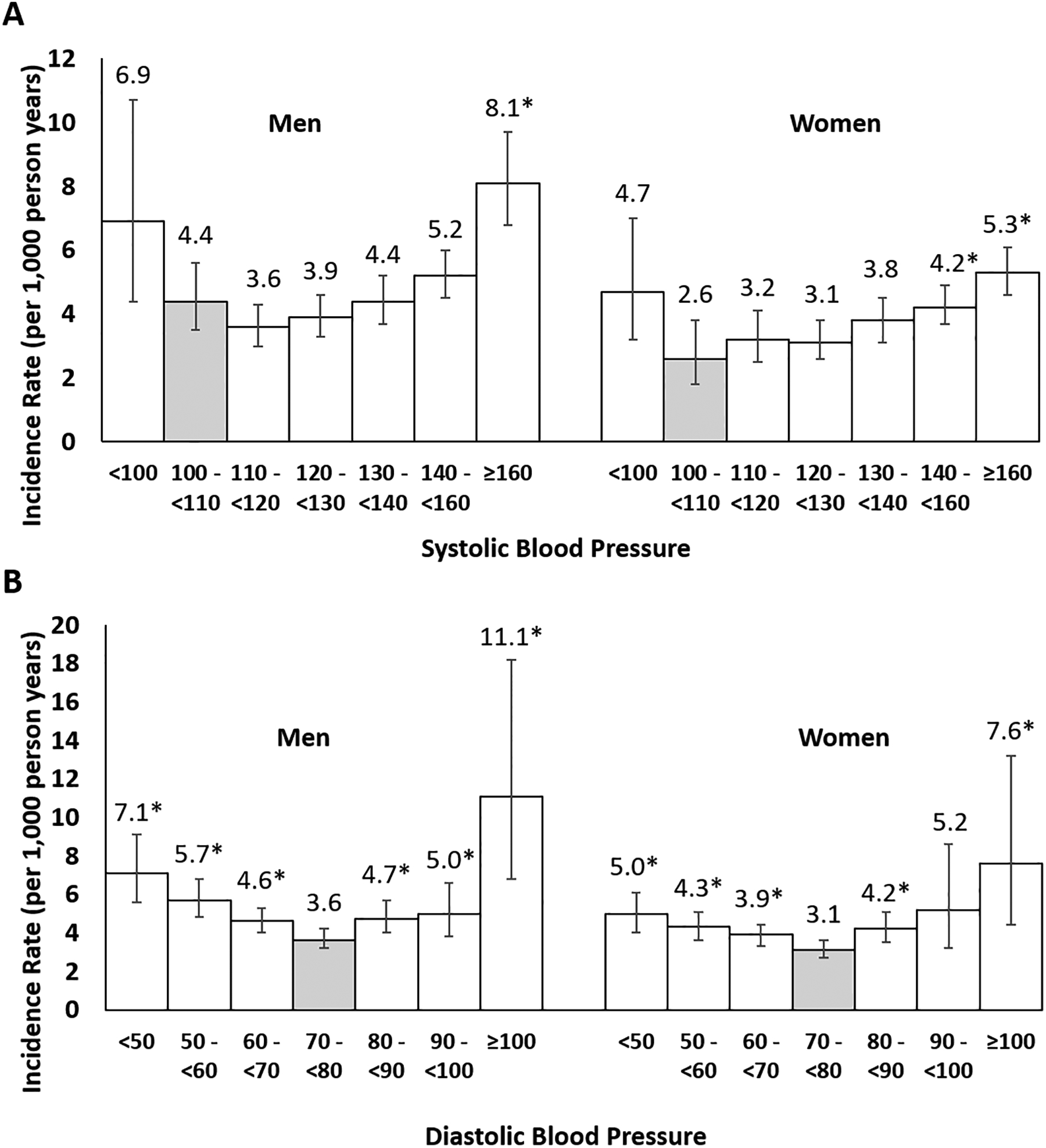
Age adjusted incidence rate of CVD mortality by blood pressure category among US adult men and women, National Health and Nutrition Examination Survey, 1999–2019.
*Indicates estimate differs significantly (p<0.05) from the reference category highlighted in grey.
For consistency, the same reference category was selected for men and women. Among men, the reference category for systolic blood pressure does not represent the lowest CVD mortality risk category. Compared with a reference category of systolic blood pressure 110 to < 120 mmHg, risk of CVD mortality is significantly greater among men with systolic blood pressure between 140 to <160 mmHg and ≥ 160 mmHg.
Figure 3:
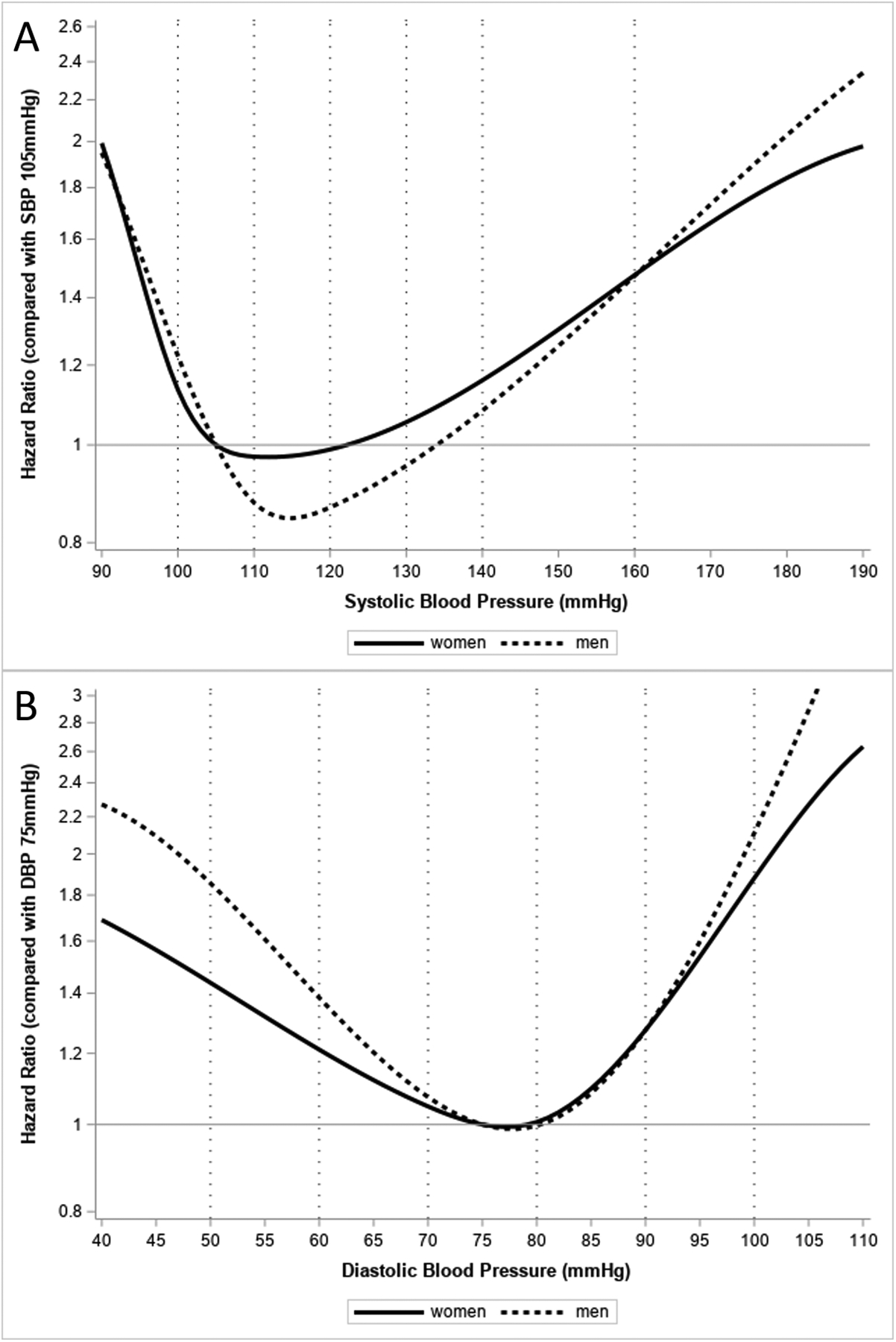
Age adjusted associations between blood pressure and CVD mortality among US adult men and women, National Health and Nutrition Examination Survey, 1999–2019.
Panel A depicts the age adjusted association between SBP with CVD mortality. SBP was modeled as a continuous variable using splines with knots placed at SBP 100 and 110mmHg for women or SBP 110 and 120mmHg for men. Each point along the curve represents the age adjusted hazards ratio for CVD mortality comparing the given SBP value to the reference SBP of 105mmHg.
Panel B depicts the age adjusted association between DBP with CVD mortality. DBP was modeled as a continuous variable using splines with knots placed at DBP 70 and 80mmHg for both men and women. Each point along the curve represents the age adjusted hazards ratio for CVD mortality comparing the given DBP value to the reference DBP of 75mmHg.
Multivariable adjusted associations between blood pressure and CVD
In multivariable adjusted Poisson models, the association between categories of SBP and DBP with CVD, both differed significantly by sex (p values < 0.05). In a multivariable-adjusted model (Table 3), compared to men with SBP between 100 to <110 mmHg, the risk of CVD mortality was greater for men with SBP ≥160 mmHg (IRR: 1.76, 95% CI: 1.27, 2.4). Compared to women with SBP between 100 to <110 mmHg, the risk of CVD mortality was greater for women with SBP 130 to < 140 mmHg (IRR: 1.61, 95% CI: 1.02, 2.55), SBP 140 to < 160 mmHg (IRR: 1.75, 95% CI: 1.09, 2.80), and SBP ≥160 mmHg (IRR: 2.13, 95% CI: 1.35, 3.36). Compared with men who had DBP between 70 to <80 mmHg, the risk of CVD mortality was greater among men with: DBP <50 mmHg (IRR: 1.54, 95% CI: 1.15, 2.05); DBP 50 to <60 mmHg (IRR: 1.35, 95% CI: 1.08, 1.70); DBP 60 to <70 mmHg (IRR: 1.22, 95% CI: 1.02, 1.46); DBP 80 to <90 mmHg (IRR: 1.31, 95% CI: 1.02, 1.67); and DBP ≥100 mmHg (IRR: 2.47, 95% CI: 1.41, 4.34). Compared with women who had DBP between 70 to <80 mmHg, the risk of CVD mortality was greater among women with: DBP <50 mmHg (IRR: 1.37, 95% CI: 1.06, 1.77); DBP 80 to <90 mmHg (IRR: 1.45, 95% CI: 1.14, 1.84); and DBP ≥100 mmHg (IRR: 2.45, 95% CI: 1.28, 4.70). Cause specific CVD mortality results showed increased risk of mortality beginning at a lower SBP among women compared with men for diseases of the heart (table S1), but not for cerebrovascular disease (table S2) mortality.
Table 3:
Multivariable adjusted associations between blood pressure and CVD Mortality among US adult men and women, National Health and Nutrition Examination Survey, 1999–2019.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Blood Pressure Category | Number of CVD deaths | IRR | 95% CI | Number of CVD deaths | IRR | 95% CI |
| SBP (mmHg) | ||||||
| <100 | 35 | 1.14 | 0.64, 2.04 | 33 | 1.79 | 0.96, 3.36 |
| 100 - <110 | 98 | 1.00 | 42 | 1.00 | ||
| 110 - <120 | 186 | 0.88 | 0.66, 1.19 | 119 | 1.33 | 0.81, 2.19 |
| 120 - <130 | 231 | 0.95 | 0.70, 1.29 | 134 | 1.36 | 0.85, 2.17 |
| 130 - <140 | 267 | 1.03 | 0.80, 1.32 | 171 | 1.61* | 1.02, 2.55 |
| 140 - <160 | 333 | 1.20 | 0.90, 1.60 | 270 | 1.75* | 1.09, 2.80 |
| ≥160 | 208 | 1.76* | 1.27, 2.44 | 278 | 2.13* | 1.35, 3.36 |
| DBP (mmHg) | ||||||
| <50 | 99 | 1.54* | 1.15, 2.05 | 119 | 1.37* | 1.06, 1.77 |
| 50 - <60 | 246 | 1.35* | 1.08, 1.70 | 197 | 1.12 | 0.89, 1.42 |
| 60 - <70 | 370 | 1.22* | 1.02, 1.46 | 307 | 1.19 | 0.98, 1.45 |
| 70 - <80 | 362 | 1.00 | 246 | 1.00 | ||
| 80 - <90 | 179 | 1.31* | 1.02, 1.67 | 124 | 1.45* | 1.14, 1.84 |
| 90 - <100 | 74 | 1.16 | 0.87, 1.55 | 37 | 1.53 | 0.83, 2.82 |
| ≥100 | 28 | 2.47* | 1.41, 4.34 | 17 | 2.45* | 1.28, 4.70 |
CI: Confidence interval; DBP: diastolic blood pressure; IR: Incidence rate (per 1,000 person years); IRR: Incidence rate ratio; SBP: systolic blood pressure
Incidence rates are age adjusted
Incidence rate ratios are adjusted for age, race/ethnicity, education, income, smoking status, physical activity, BMI, diabetes, total cholesterol, eGFR<60, use of hypertension medication, and prior CVD.
Indicates estimate differs from the reference category (p<0.05)
For consistency, the same reference category was selected for men and women. Among men, the reference category for systolic blood pressure does not represent the lowest CVD mortality risk category. Compared with a reference category of systolic blood pressure 110 to < 120 mmHg, risk of CVD mortality is significantly greater among men with systolic blood pressure between 140 to <160 mmHg and ≥ 160 mmHg in age adjusted and multivariable adjusted models.
Sensitivity analyses
Results from sensitivity analyses restricted to participants who did not die within three months of their study visit (table S3) or without prevalent CVD (table S4), showed increased risk of CVD mortality beginning at a lower SBP among women compared with men. Among participants not taking antihypertensive medication (table S5) and among adults age 50 years or older (table S6) the results were no longer statistically significant.
DISCUSSION
In a nationally representative study of over 50,000 adult men and women followed for 9.5 years, different associations were present between BP and CVD mortality for women and men. In multivariable-adjusted, sex-stratified models, increased risk of CVD mortality was present at a lower range of SBP for women than men. For DBP, we found a curvilinear association between DBP and CVD mortality for both men and women. However, at non-elevated DBP (DBP < 80 mmHg), increased risk of CVD mortality was present at a higher range of DBP for men than women.
The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) ACC/AHA BP guideline lowered BP thresholds for the diagnosis of hypertension by 10 mmHg such that elevated BP is SBP 120–129 mmHg and DBP <80 mmHg, and stage I hypertension is SBP 130–139 mmHg or DBP 80–89 mmHg.11 Although there is robust evidence to suggest that SBP ≥120 mmHg or DBP ≥80 mmHg is associated with an increased risk of CVD,26 27–29 there is less clarity regarding the optimal lower range of BP.30–32 While data from the current study depict higher risk of CVD mortality at SBP < 100mmHg compared with SBP between 100 to <110 mmHg, we recognize that low SBP may be a marker of overt CVD33 as 15% of men and 4% of women in this category reported prior CVD. Using data from healthy participants of the Multi-Ethnic Study of Atherosclerosis study, Whelton et. al found that, there was a stepwise increase in the presence of traditional CVD risk factors, coronary artery calcium, and overt atherosclerotic CVD starting with a SBP of 90 mmHg.34 Additionally, studies show that indigenous people with limited exposure to Western lifestyles are able to maintain SBP <100 mmHg with little to no increase with age,23, 35 suggesting that the optimal range of BP may be lower than what is defined as normal BP by the 2017 ACC/AHA BP guideline.11 However, whether there are sex-based differences in what is normal or optimal BP has garnered limited attention.36
The Framingham Heart Study found that compared with SBP < 120mmHg, SBP between 130 to < 140mmHg was associated with an increased risk of CVD by 60% among men or 150% among women, but these sex-stratified estimates were not significantly different.30 Other findings from observational data such as the Prospective Studies Collaboration, a meta-analysis of 61 observational studies,31 found sex differences in the association between SBP with stroke and ischemic heart disease mortality, although the authors concluded that these differences were not substantial. Consistent with these studies and a 2008 meta-analysis of randomized trials showing no sex differences in the relation between use of antihypertension medication and CVD,37 the 2017 ACC/AHA BP guideline states that there is no evidence to support sex-based recommendations for initiating BP lowering treatment.11 However, the European Society of Cardiology12 has attributed this lack of evidence to an absence of specifically designed clinical trials to evaluate sex differences in BP targets. While the lower on average BP for women versus men is well-described2, 38 at least prior to menopause, BP trajectories over time differ by sex, with women experiencing a higher pulsatile load over the life course compared with men.39 Additionally, women exhibit steeper increases in BP beginning as early as the third decade, which persist throughout the life span.40 Compared with pre-menopausal women, menopausal women exhibit an increase in plasma renin41 and sympathetic nerve activity,42 as well as a decrease in nitric oxide,43 which serves as a vasodilator. It has been posited that conditions common among women such as auto-immune diseases, obesity, and preeclampsia, may amplify cardiovascular complications if co-morbid with hypertension.44 Finally, women have smaller coronary artery size45 and aortic root dimensions compared with men, even after accounting for body size.46 These hormonal and anatomical differences may contribute to sex differences in the association between BP and CVD, and a number of observational studies4–8 show that risk of CVD increases at lower thresholds among women compared with men.
In a Norwegian cohort, compared with normal BP, stage 1 hypertension was associated with doubling of acute coronary syndrome risk for women in mid-life, but not for men.7 Likewise, using pooled data from over 27,000 CVD-free participants of four community based US cohorts, Ji et. al4 found that compared with SBP < 100mmHg, risk for CVD begins at lower SBP in women (100–109 mmHg) than in men (130–139 mmHg). Similar sex-specific results were also observed for myocardial infarction, heart failure, and stroke.4 Our study showed similar sex-specific patterns but with CVD mortality as an outcome. From our multivariable-adjusted models, compared with SBP between 100 to <110 mmHg, increased risk of CVD mortality was present with SBP ≥130 mmHg among women but only with SBP ≥160 mmHg among men. These findings should be interpreted cautiously as they only demonstrate differences in the association of SBP and CVD mortality between men and women. The lack of significant findings among men, particularly at SBP levels at 140 to < 160 mmHg, is attributed to the selected reference category (100 to < 110 mmHg) which does not reflect the lowest mortality rate among men (110 to < 120 mmHg), as demonstrated in Figure 1. While our findings for DBP show lowest CVD mortality rates with DBP 70 to <80 mmHg for both men and women, results from our multivariable models suggest subtle distinctions in the association between DBP with CVD mortality, particularly at non-elevated DBP. Compared with DBP between 70 to <80 mmHg, increased risk of CVD mortality was present with DBP ≥ 80 mmHg for both men and women or DBP < 70 mmHg for men. However, DBP in the range of 50 to < 60 mmHg or 60 to < 70 mmHg was not associated with CVD mortality among women, corroborating recent work from the Women’s Health Initiative showing increasing mortality risk around DBP < 50 mmHg.47 The results from our study and others,4–8 showing sex differences in the association between BP and CVD mortality, support the need for continued investigation to determine whether sex-based BP targets are indeed warranted, particularly for SBP.
This study has some notable strengths. The study population is large, and includes a multi-racial/ethnic sample that is representative of the non-institutionalized US population. Additionally, participants were followed for an average of ten years with mortality/primary cause of death ascertained through 2019, reflecting the contemporaneous nature of the data. This study has a few potential limitations. First, we were restricted to BP measurements from one visit. Interoffice BP variability is common,48, 49 however research-grade BP was measured three times using standardized protocols and averaged, consistent with recommendations for in-office BP measurement.50 Second, our data were cross-sectional and we could not account for BP burden across the life course,39 which may exert a different effect than BP measured at one point in time.51 Additionally, primary cause of death was based on ICD-10 codes and not adjudicated, therefore subject to potential misclassification. Furthermore, despite the large number of events, we were underpowered to detect sex-specific differences in cerebrovascular disease mortality, due to the relatively low number of events. Given the high prevalence of CVD in the sample, it is also likely that many adults had subclinical CVD that we were unable to assess or account for. To address this, we conducted sensitivity analyses among participants without prior CVD and participants with at least three months of follow-up prior to death, given that BP can drop immediately preceding death.52 Results from both these analyses were consistent with our main findings showing increased CVD mortality risk beginning at lower SBP ranges among women compared to men. Additionally, to address BP changes that occur during menopause,53 we also restricted our analyses to individuals age 50 or older. In this sub-population analysis, effect sizes among women were similar to our main results, though not significant. Finally, our observational study could not fully account for residual confounding.
Supplementary Material
PERSPECTIVES.
In a representative sample of US adult men and women, increased risk of CVD mortality was present starting at lower levels of SBP for women than men. At non-elevated DBP (<80 mmHg), CVD mortality risk was present starting at higher levels of DBP for men than women. These findings highlight different patterns in the association between BP and CVD mortality by sex. While these results are compelling, they are hypothesis generating- confirmatory studies will require other cohorts without CVD that include repeat BP measurements, non-fatal CVD event, and longer follow-up.
Novelty and Relevance.
What is new?
In a nationally representative cohort of 25,899 US men and 27,390 US women, from multivariable adjusted models, increase in CVD mortality risk at higher blood pressure began at lower levels of systolic blood pressure among women compared with men.
What is relevant?
Patterns in the relationship between blood pressure and CVD mortality differ by sex.
Clinical/pathophysiological implications:
Though guideline recommendations for the management of hypertension are sex-agnostic, the stronger association of SBP and CVD mortality in women versus men in the current study supports further investigation of sex-specific management of hypertension.
Sources of Funding:
Dr. Elfassy is currently supported by the National Institute on Minority Health and Health Disparities (K01MD014158) and a Katz Family Endowed Professorship. Dr. Yang is supported by the University of Washington Asian Health Initiative and Carl and Renee Behnke Endowed Professorship.
Abbreviations:
- ACC
American College of Cardiology
- AHA
American Heart Association
- BP
blood pressure
- BMI
body mass index
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- eGFR
estimated glomerular filtration rate
- NDI
National Death Index
- NHANES
National Health and Nutrition Examination Survey
- HS
high school
- IR
incidence rate
- IRR
incidence rate ratio
- PY
person-years
- Q
quartile
- SBP
systolic blood pressure
- US
United States
Footnotes
Disclosure:
The authors have no relevant disclosures to report.
REFERENCES
- 1.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics—2023 update: A report from the american heart association. Circulation. 2023;147:e93–e621 [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr., et al. Potential us population impact of the 2017 acc/aha high blood pressure guideline. Circulation. 2018;137:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald M, Hertz RP, Unger AN, Lustik MB. Prevalence, awareness, and management of hypertension, dyslipidemia, and diabetes among united states adults aged 65 and older. J Gerontol A Biol Sci Med Sci. 2009;64:256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, et al. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation. 2021;143:761–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boggia J, Thijs L, Hansen TW, Li Y, Kikuya M, Björklund-Bodegård K, et al. Ambulatory blood pressure monitoring in 9357 subjects from 11 populations highlights missed opportunities for cardiovascular prevention in women. Hypertension. 2011;57:397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermida RC, Ayala DE, Mojón A, Fontao MJ, Chayán L, Fernández JR. Differences between men and women in ambulatory blood pressure thresholds for diagnosis of hypertension based on cardiovascular outcomes. Chronobiol Int. 2013;30:221–232 [DOI] [PubMed] [Google Scholar]
- 7.Kringeland E, Tell GS, Midtbø H, Igland J, Haugsgjerd TR, Gerdts E. Stage 1 hypertension, sex, and acute coronary syndromes during midlife: The hordaland health study. Eur J Prev Cardiol. 2022;29:147–154 [DOI] [PubMed] [Google Scholar]
- 8.Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: Cohort study of uk biobank participants. BMJ. 2018;363:k4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. The Lancet. 2002;360:1903–1913 [DOI] [PubMed] [Google Scholar]
- 10.Han M, Chen Q, Liu L, Li Q, Ren Y, Zhao Y, et al. Stage 1 hypertension by the 2017 american college of cardiology/american heart association hypertension guidelines and risk of cardiovascular disease events: Systematic review, meta-analysis, and estimation of population etiologic fraction of prospective cohort studies. Journal of hypertension. 2020;38:573–578 [DOI] [PubMed] [Google Scholar]
- 11.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115 [DOI] [PubMed] [Google Scholar]
- 12.Gerdts E, Sudano I, Brouwers S, Borghi C, Bruno RM, Ceconi C, et al. Sex differences in arterial hypertension: A scientific statement from the esc council on hypertension, the european association of preventive cardiology, association of cardiovascular nursing and allied professions, the esc council for cardiology practice, and the esc working group on cardiovascular pharmacotherapy. European Heart Journal. 2022;43(46):4777–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman N, Ching SM, Konradi AO, Nuyt AM, Khan T, Twumasi-Ankrah B, et al. Arterial hypertension in women: State of the art and knowledge gaps. Hypertension. 2023; 10.1161/HYPERTENSIONAHA.122.20448 [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics.National Health and Nutrition Examination Survey Overview. https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/NHANES_Overview_Brochure.pdf. Published 2020. Accessed November 15th, 2022.
- 15.National Health and Nutrition Examination Survey(NHANES). Physician Examination Procedures Manual. 2015. Accessed November 15th 2022. https://wwwn.cdc.gov/Nchs/Data/Nhanes/2015-2016/Manuals/2015_Physician_Examination_Procedures_Manual.pdf
- 16.National Health and Nutrition Examination Survey(NHANES). Physician Examination Procedures Manual. 2018. Accessed November 15th 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2018-Physician-Examination-Manual-508.pdf
- 17.Ostchega Y, Zhang G, Sorlie P, Hughes JP, Reed-Gillette DS, Nwankwo T, et al. Blood pressure randomized methodology study comparing automatic oscillometric and mercury sphygmomanometer devices: National health and nutrition examination survey, 2009–2010. Natl Health Stat Report. 2012:1–15 [PubMed] [Google Scholar]
- 18.National Center for Health Statistics HCHS Data Linkage. Continuous NHANES Public-use Linked Mortality Files, 2019. https://www.cdc.gov/nchs/data-linkage/mortality-public.htm. Published 2022. Accessed November 15th 2022.
- 19.National Center for Health Statistics. Office of Analysis and Epidemiology. The Linkage of National Center for Health Statistics Survey Data to the National Death Index. Linked Mortality File (LMF): Methodology Overview and Analytic Considerations. 2015. [Google Scholar]
- 20.National Center for Health Statistics. National Death Index user’s guide. Hyattsville, MD: 2013 [Google Scholar]
- 21.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, et al. Calibration of serum creatinine in the national health and nutrition examination surveys (nhanes) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926 [DOI] [PubMed] [Google Scholar]
- 22.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin c-based equations to estimate gfr without race. N Engl J Med. 2021;385:1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller NT, Noya-Alarcon O, Contreras M, Appel LJ, Dominguez-Bello MG. Association of age with blood pressure across the lifespan in isolated yanomami and yekwana villages. JAMA Cardiology. 2018;3:1247–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franklin SS, Gokhale SS, Chow VH, Larson MG, Levy D, Vasan RS, et al. Does low diastolic blood pressure contribute to the risk of recurrent hypertensive cardiovascular disease events? Hypertension. 2015;65:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Supiano MA, Pajewski NM, Williamson JD. Systolic blood pressure and mortality: Role of reverse causation. J Am Geriatr Soc. 2018;66:205–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright JT Jr., Whelton PK, Reboussin DM. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2016;374:2294. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang H, et al. Prehypertension and incidence of cardiovascular disease: A meta-analysis. BMC Medicine. 2013;11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Su L, Cai X, Mai W, Wang S, Hu Y, et al. Association of all-cause and cardiovascular mortality with prehypertension: A meta-analysis. American Heart Journal. 2014;167:160–168.e161 [DOI] [PubMed] [Google Scholar]
- 29.Guo X, Zhang X, Guo L, Li Z, Zheng L, Yu S, et al. Association between pre-hypertension and cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Current Hypertension Reports. 2013;15:703–716 [DOI] [PubMed] [Google Scholar]
- 30.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297 [DOI] [PubMed] [Google Scholar]
- 31.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913 [DOI] [PubMed] [Google Scholar]
- 32.Kannel WB, Vasan RS, Levy D. Is the relation of systolic blood pressure to risk of cardiovascular disease continuous and graded, or are there critical values? Hypertension. 2003;42:453–456 [DOI] [PubMed] [Google Scholar]
- 33.Tsimploulis A, Lam PH, Arundel C, Singh SN, Morgan CJ, Faselis C, et al. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiology. 2018;3:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelton SP, McEvoy JW, Shaw L, Psaty BM, Lima JAC, Budoff M, et al. Association of normal systolic blood pressure level with cardiovascular disease in the absence of risk factors. JAMA Cardiology. 2020;5:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He J, Tell GS, Tang Y-C, Mo P-S, He G-Q. Effect of migration on blood pressure: The yi people study. Epidemiology. 1991;2 [DOI] [PubMed] [Google Scholar]
- 36.Gillis EE, Sullivan JC. Sex differences in hypertension: Recent advances. Hypertension. 2016;68:1322–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. European Heart Journal. 2008;29:2669–2680 [DOI] [PubMed] [Google Scholar]
- 38.Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: Data from NHANES III. J Gend Specif Med. 2001;4:10–13, 20 [PubMed] [Google Scholar]
- 39.Cheng S, Xanthakis V, Sullivan LM, Vasan RS. Blood pressure tracking over the adult life course. Hypertension. 2012;60:1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, et al. Sex differences in blood pressure trajectories over the life course. JAMA Cardiology. 2020;5:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. Postmenopausal hypertension: Role of the renin-angiotensin system. Hypertension. 2010;56:359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vongpatanasin W Autonomic regulation of blood pressure in menopause. Semin Reprod Med. 2009;27:338–345 [DOI] [PubMed] [Google Scholar]
- 43.Majmudar NG, Robson SC, Ford GA. Effects of the menopause, gender, and estrogen replacement therapy on vascular nitric oxide activity. J Clin Endocrinol Metab. 2000;85:1577–1583 [DOI] [PubMed] [Google Scholar]
- 44.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: Why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138:198–205 [DOI] [PubMed] [Google Scholar]
- 45.Dickerson JA, Nagaraja HN, Raman SV. Gender-related differences in coronary artery dimensions: A volumetric analysis. Clin Cardiol. 2010;33:E44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lønnebakken MT, Izzo R, Mancusi C, Losi MA, Stabile E, Rozza F, et al. Aortic root dimension and arterial stiffness in arterial hypertension: The campania salute network. J Hypertens. 2016;34:1109–1114 [DOI] [PubMed] [Google Scholar]
- 47.Haring B, McGinn AP, Kamensky V, Allison M, Stefanick ML, Schnatz PF, et al. Low diastolic blood pressure and mortality in older women. Results from the Women’s Health Initiative long life study. American Journal of Hypertension. 2022;35:795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickering TG, Gerin W, Schwartz JE, Spruill TM, Davidson KW. Franz volhard lecture: Should doctors still measure blood pressure? The missing patients with masked hypertension. J Hypertens. 2008;26:2259–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pickering TG, James GD, Boddie C, Harshfield GA, Blank S, Laragh JH. How common is white coat hypertension? JAMA. 1988;259:225–228 [PubMed] [Google Scholar]
- 50.Drawz PE, Ix JH. Bp measurement in clinical practice: Time to sprint to guideline-recommended protocols. J Am Soc Nephrol. 2018;29:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen NB, Khan SS. Blood pressure trajectories across the life course. Am J Hypertens. 2021;34:234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravindrarajah R, Hazra NC, Hamada S, Charlton J, Jackson SHD, Dregan A, et al. Systolic blood pressure trajectory, frailty, and all-cause mortality >80 years of age. Circulation. 2017;135:2357–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension. Hypertension. 2008;51:952–959 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


