Abstract
Peptides have unique characteristics that make them highly desirable as therapeutic agents. The physicochemical and proteolytic stability profiles determine peptides’ therapeutic potential. Multiple strategies to enhance the therapeutic profile of peptides are emerging. They include chemical modifications, such as cyclization, substitution with D-amino acids, peptoids formation, N-methylation, side chain halogenation, and incorporation in delivery systems. There have been recent advances in approaches to discover peptides having these modifications to attain desirable therapeutic properties. We critically review these recent advancements in therapeutic peptide development.
Keywords: Peptides, Cyclization, D-amino acid, N-methylation, Peptoid, stability
Peptide therapeutics
In the last few years, significant progress has been made in the use of peptides for the treatment and diagnosis of various diseases. Specifically, the approval of oral semaglutide (Rybelsus®) in 2019 for treating type 2 diabetes marked a significant milestone in the advancement of peptide therapeutics.[1] Peptides can act as either agonists or antagonists by binding to receptors on the cell surface. They can also access the intracellular spaces through either clathrin-mediated endocytosis or clathrin-independent endocytosis (Text Box 1). Since 2019, at least 15 therapeutic peptides have been approved for clinical use (Table 1) [2-4], and several therapeutic peptide candidates are currently undergoing clinical trials to evaluate their safety and effectiveness in treating various diseases (Table 2).
Box 1. Amino acid scanning and truncation
Amino acid scanning is a technique used in the study of structure-activity relationship of bioactive peptides. This technique involves sequentially substituting individual amino acids in a peptide sequence with a scanning amino acid, one at a time. The commonly used amino acids for scanning include alanine, proline, glycine, leucine, and valine. However, alanine scanning is the most widely used. Alanine scanning is generally preferable because alanine has a small and chemically inert methyl side chain that does not alter the secondary structure of peptides. This technique has been successfully employed in identifying crucial amino acid residues in peptides. For instance, Zhang and colleagues utilized alanine scanning on a 10-mer wild type H3K4me3 peptide to identify crucial amino acid residues that are necessary for binding with the PHD3 protein (Figure IA and IC) [75]. The alanine scanning data revealed that amino acids at positions 2, 3, and 4 played important roles in maintaining activity. Specifically, substituting the amino acid at position 4 with alanine resulted in a significant increase in the Ki value of the peptide. Furthermore, using the alanine scanning data, the authors employed peptide truncation strategy in designing a series of truncated peptides. The objective of peptide truncation is not solely to create highly stable peptides. In general, shortening the length of a linear peptide can improve its stability by reducing the number of bonds that are susceptible to enzymatic degradation. Additionally, reducing the size of a peptide can increase its cell permeability and facilitate binding into smaller pockets. The combination of alanine scanning with peptide truncation resulted in the development of shorter peptides that maintained sufficient inhibitory activity.
Overall, alanine scanning is one of the most widely used strategies for identifying the particular amino acid residues that contribute to the structure, stability, and function of a peptide. One limitation of amino acid scanning is that it is only feasible for short peptides, and longer peptides can pose challenges for scanning. By contrast, site-specific mutagenesis, which uses recombinant DNA technology to alter specific amino acids in proteins, has been employed to map regions of interest in longer peptides and proteins [76].
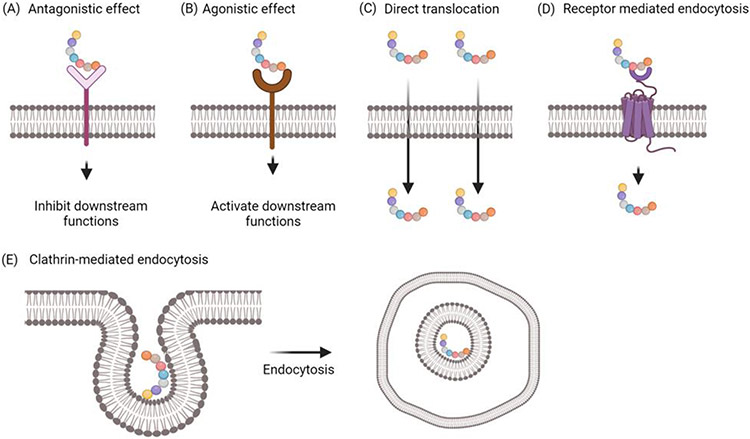
Box 2. Mechanisms of action of peptides
Therapeutic applications of peptides have been growing in recent years due to their advantages, such as high specificity, high affinity to targets, and minimal side effects [77]. Peptide drugs act on cells through various mechanisms (Figure II). Some peptides enter cells and exert their action in the cytoplasm, while others bind to cell-surface receptors. However, due to the poor membrane permeability of peptides, only a small proportion of peptides can enter the cells [78,79]. Cyclic peptides or cell-penetrating peptides are more efficient in crossing the membrane [80]. These peptides may traverse the membrane through passive diffusion, the formation of pores in the membrane, or endocytosis. Peptides that are hydrophobic in nature are more likely to directly cross the cell membrane [80]. In addition, positively charged peptides can gain entry to the intracellular region of cells through clathrin-mediated endocytosis or clathrin-independent endocytosis [81]. Peptides can also exert their action by binding to cell-surface receptors. When peptides bind to receptors, the ligand-receptor signaling is altered. Agonistic peptides can enhance downstream cellular functions, while antagonistic peptides can block or inhibit downstream cellular functions [82].
Table 1.
FDA-approved therapeutic peptides from 2019 to 2022
| Peptide (Brand, Approval Year) |
Mechanism of Action |
Indication | Administration Route |
Chemical Structure | Major Structural Features |
|---|---|---|---|---|---|
| Tirzepatide (Mounjaro, 2022) | Glucagon-like peptide-1 agonist | Type 2 diabetes | Subcutaneous injection |

|
Non-natural amino acid substitution, lipidation |
| Lutetium Lu-177 vipivotide tetraxetan (Pluvicto, 2022) | PSMA targeting | Prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer | Intravenous injection/infusion |

|
Modification with urea |
| Melphalan flufenamide (Pepaxto, 2021) | DNA Alkylating agent | Multiple myeloma and amyloid light-chain amyloidosis | Intravenous injection |

|
Amino peptidase liable peptide drug conjugate. Non-natural amin acids |
| Voclosporin (Lupkynis, 2021) | Calcineurin inhibitor | lupus nephritis | Oral |

|
D-amino acids N-alkylation Non-natural amino acid replacements |
| Vosoritide (Voxzogo, 2021) | C-type natriuretic peptide analog | Achondroplasia | Subcutaneous injection |

|
Cyclization |
| Pegcetacoplan (Empaveli, 2021) | Binds to complement protein C3 and its activation fragment C3b | Paroxysmal nocturnal hemoglobinuria | Subcutaneous injection |

|
Cyclization PEGylation Non-natural amino acids |
| Dasiglucagon (Zegalogue, 2021) | Glucagon analog | Hypoglycemia in diabetics | Subcutaneous injection | H-His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Aib-Ala-Arg-Ala-Glu-Glu-Phe-Val-Lys-Trp-Leu-Glu-Ser-Thr-OH Aib – Amino isobutyric acid | Non-natural amino acid |
| Piflufolastat-F18 (Pylarify, 2021) | PSMA targeting | Positron emission tomography (PET) of prostate-specific membrane antigen (PSMA)-positive lesions in men with prostate cancer | Intravenous injection |

|
Urea based modification |
| Difelikefalin (Korsuva, 2021) | Kappa opioid receptor agonist | Pruritus associated with chronic kidney disease | Intravenous injection |

|
D-amino acids Non-natural amino acid |
| 9 Odevixibat (Bylvay, 2021) | Inhibitor of ileal bile acid transporter | Pruritus in patients aged over 3 months with progressive familial intrahepatic cholestasis | Oral |

|
Non-natural amino acids |
| 10 Setmelanotide (Imcivree. 2020) | Melanocortin-4 receptor (MC4R) activator | Chronic weight management | Subcutaneous injection |

|
D-amino acid Cyclization |
| 11 64Cu-DOTATATE (Detectnet, 2020) | Somatostatin receptor agonist | Scintigraphic imaging | Intravenous injection |

|
D-amino acid substitution |
| 12 68Ga-PSMA-11 (Detectnet, 2020) | Prostate-specific membrane antigen (PSMA) | Diagnosis of recurrent prostate carcinoma | Intravenous injection |

|
Urea based modification |
| 13 Afamelanotide (Scenesse, 2019) | Melanocortin 1 receptor agonist | Erythropoietic protoporphyria | Subcutaneous implant | Ac-Ser-Tyr-Ser-Nle-Glu-His-D-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | D-amino acid N-alkylation |
| 14 Semaglutide (Rybelsus. 2019) | glucagon-like peptide-1 agonist | Type 2 diabetes | Oral |

|
Non-natural amino acid Lipidation |
| 15 Bremelanotide (Vyleesi, 2019) | Melanocortin receptor agonist | Hypoactive sexual desire disorder (HSDD) | Subcutaneous injection |

|
D-amino acid N-alkylation Cyclization |
Table 2.
Peptides drugs in Active Clinical trials
| Name of drug |
Chemical Structure | Major Structural Features |
Clinical trial Phase |
Disease |
|---|---|---|---|---|
| OPT101 | Structure undisclosed | 15-merpeptide | Phase 1 | Safety and tolerability in healthy individuals |
| BMN 111 (Vosoritide) |

|
cyclic analogue of C-type natriuretic peptide (CNP) | Phase 3 | Achondroplasia |
| Apraglutide | H-His-Gly-Asp-Gly-Ser-Phe-Ser-Asp-Glu-Ahx-D-Phe-Thr-Ile-Leu-Asp-Leu-Leu-Ala-Ala-Arg-Asp-Phe-Ile-Asn-Trp-Leu-Ile-Gln-Thr-Lys-Ile-Thr-Asp-NH2 Ahx: amino caproic acid |
33- mer peptide D-amino acid and N-alkylation | Phase 2 | Short Bowel Syndrome |
In contrast to most small molecule drugs, peptides exhibit greater efficacy with well-defined mechanisms of action, thus making them relatively easier to identify. Peptides also have unique characteristics that render them highly desirable as therapeutic agents. For example, compared to monoclonal antibodies, peptides have lower production costs, superior tumor penetration, lower immunogenicity, greater flexibility in structural modification and formulation. However, the therapeutic potential of peptides is limited by their susceptibility to proteolytic degradation by peptidases, [5,6] which leads to a short half-life and rapid elimination from the body. Peptides face challenges in achieving therapeutic concentrations in vivo due to their susceptibility to degradation. As a result, most therapeutic peptides are administered systemically, which results in poor patient compliance. To overcome the challenges of degradation, various strategies, such as chemical modification and incorporation in delivery systems, have been adopted.
The first step in discovering peptide therapeutics is to identify a potent peptide candidate, either from a library or from a natural source (Figure 1). Recent new approaches in peptide phage display, mRNA display, artificial intelligence, and sourcing from natural peptides have shown promises in expanding the repertoire of libraries for identifying peptides with desirable properties and potency. In this review, we present a critical discussion on the latest advancements in peptide drug discovery using phage and mRNA display technologies, as well as emerging technologies that aim to address the challenges associated with the further development of these peptide candidates.
Figure 1: Approaches for discovering and modifying therapeutic peptides.
Therapeutic peptides can be identified through screening phage display libraries, mRNA display libraries, and peptide libraries. Peptides can also be discovered from natural sources or through computer-aided design (utilizing artificial intelligence and machine learning). The bioactivity and stability of the lead peptides discovered can be further improved by implementing various chemical modifications, including cyclization, conversion to D-amino acids, peptoid formation, and N-alkyl peptide formation.
Discovery and optimization of functional peptides
Peptide phage and mRNA display technologies
The primary advantage of employing display technologies, such as phage display and mRNA display, in peptide drug discovery is the ability to generate large libraries that can be readily manipulated according to specific requirements. These libraries enable the screening of a large pool of natural peptides or modified peptides against a target of interest with a short time frame [7,8].
Peptide phage display has been widely applied in the discovery of various targeting and therapeutic peptides. It was first reported by George Smith in 1985, when he described the display of peptides on the surface of filamentous phages via fusion with the viral capsid protein [9]. In recognition of his work on phage display of peptides and antibodies, Dr. Smith was awarded the Nobel Prize in Chemistry in 2018. Biopanning is an affinity selection procedure to identify peptides from a phage display library (Figure 2). It allows the screening of natural peptides from large phage display libraries encoding approximately 1011 candidates and has been widely employed to identify therapeutic biomolecules [10]. While biopanning is efficient in identifying peptides that bind to a specific protein, it cannot select peptides that binds to a specific region of the target protein to block its interaction with its receptor protein. Recently, Liu et al. have developed a novel phage display biopanning to discover an anti-PD-L1 peptide that inhibits the PD-1/PD-L1 interaction. A negative selection approach was incorporated, where the phages were initially incubated with the PD-1/PD-L1 protein complex to remove phages that bind to off-target regions of the PD-L1. The unbound phages were then transferred to a separate well coated with PD-L1. This modification of the conventional phage biopanning helps to minimize the selection of non-specific phages that bind outside the relevant target interface [11]. The peptide underwent macrocyclization scanning to enhance its stability and blocking activity against the PD-1/PD-L1 interaction [12]. This new biopanning strategy can be used to identify peptides that modulate aberrant protein-protein interactions, which are associated with many disorders such as cancer, neurodegenerative disease, and infectious diseases [13].
Figure 2. Schematic diagram of the biopanning procedure using a peptide phage display library.
First, the target protein is immobilized and then incubated with a phage library. The unbound phages are removed by washing, leaving only the phages that have bound to the target. The bound phages are eluted and amplified using host bacteria. The amplified phages are then used for subsequent rounds of biopanning, which is repeated 3-4 times. Finally, DNA sequencing is performed to identify the sequences of the peptides that bind to the target.
Phage display can be modified through bicycle peptide phage display and mirror-image phage display to screen peptide candidates with higher specificity and higher affinity. For example, Zhou et al. used a mirror-image phage display biopanning to identify the D-peptide DTBP-3, which binds to T cell immunoreceptor with Ig and ITIM domains (TIGIT) and blocks its interaction with its ligand, poliovirus receptor (PVR) [14]. Phage biopanning can also be conducted in vivo to identify tissue-specific peptides. However, the two major challenges are off-target binding and the enrichment of parasitic peptide sequences, as the current in vivo biopanning procedures cannot determine the biodistribution of specific phages in multiple tissues. Keliko et al., employed bioinformatics tools to analyze the high-throughput sequencing (HTS) data to access the differential binding of peptide phages in different organs during biopanning. This led to the discovery of novel peptides that have lung and brain homing properties [15].
mRNA display is an in vitro selection technique used for discovering peptide or protein ligands that can bind to specific targets. It is a genotype-phenotype conjugate, where each molecule in an mRNA library is tagged with its encoding peptide via a puromycin linker. These large mRNA-peptide complexes can be screened against various targets to identify potential hits. These hits are subsequently isolated and amplified using reverse transcription and PCR. The process is repeated multiple times to enrich the mRNA sequences encoding peptides that selectively bind to the target of interest [16]. The advantages of mRNA display include a large library size (1012-1014), the ability to screen macrocyclic peptides, and the incorporation of non-natural amino acids in peptide variants [8,17]. In particular, mRNA display has been extensively used to identify macrocyclic peptides, which typically exhibit greater protease resistance and higher affinity than linear peptides.[16,17] For example, using mRNA display, a 9-mer macrocyclic peptide that binds to human STING, an important protein in innate immunity, was identified. This peptide contains both natural and non-natural amino acids and binds to STING with a high affinity (KD 3.4 nM) [18]. In another study, mRNA display was used to identify a highly selective cyclized peptide that binds to the MAGE-A4 protein with an IC50 of 21 nM and inhibit its interaction with RAD18, a DNA repair protein. This peptide can be potentially used to sensitize cancer cells to chemotherapy.[19] Furthermore, the Random Nonstandard Peptide Integrated Discovery (RaPID) mRNA display technology was employed to discover several potent cyclic peptide inhibitors that exhibit nanomolar IC50 values against human FXIIa. [20].
Chemical libraries of peptides
While phage display and mRNA display have been widely used to identify peptides, incorporating a wide variety of non-natural amino acids in these methods remains a challenge. In contrast, chemical libraries of peptides, such as one-bead-one-compound (OBOC) library and DNA-encoded library (DEL), can overcome this limitation.[21] However, the major drawback of these chemical libraries is their relative small diversity (~106) of peptide variants. As a result, recent efforts have focused on developing larger chemical libraries of peptides for screening. For example, Quartararo et al. utilized different peptide synthesis methods, including fast-flow and solid-phase peptide synthesis, to create an ultra-large library of ~108 random synthetic peptides. A magnetic bead capture-based affinity selection-mass spectrometry (AS-MS) was then used to identify high-affinity 14-3-3γ-binding peptides with KD values ranging from 3-19 nM. This library was also used to identify p53-like peptides that bind to the oncoprotein mouse double minute 2 (MDM2) [21]. In another study, Kalafatovic and colleagues employed an algorithm-supported methodology based on a multi-objective genetic algorithm to design a peptide library using an evolutionary advanced computing approach. This strategy resulted in a library with good diversity in terms of amino acid and molecular mass [22].
Peptide libraries are valuable tools for drug discovery scientists and can be either random or focused. A focused library typically comprises a systematic combination of a range of peptides with common designs, such as alanine scanning, overlapping peptides, truncation, and positional scanning. Focused libraries are advantageous because screening efforts are not wasted on non-functional space, thereby increasing the chances of discovering useful drug candidates. They can also assist researchers in avoiding peptide sequences that may be problematic and promote the advancement of lead candidates [23,24]. On the other hand, random libraries may allow for the accidental discovery of peptides with previously unpredicted pharmacokinetic and pharmacodynamic profiles [25]. For bioactivity screening, peptides in the libraries can be generated either genetically or through chemical synthesis. The development of automated solid phase peptide synthesis and the availability of more efficient purification systems have made synthetic peptide libraries more prevalent.
Application of artificial intelligence in peptide drug discovery
Various advanced approaches to designing therapeutic peptides through computational methods have been described previously [26,27]. A peptide scaffold with a desired bioactivity (a seed peptide) or a peptide sequence designed from scratch (de novo) is used as a starting point. In silico methods, such as machine learning, and deep learning approaches, are used to iterate and optimize the peptide sequences. These methods have been used to identify bioactive peptides. For example, Haney and coworkers designed a quantitative structure-activity relationship (QSAR) model to design antimicrobial peptides with an 80% prediction accuracy [28]. Recently, Capecchi et al. utilized machine learning models to identify membrane-disruptive antimicrobial peptides. In addition to discovering peptides with improved antimicrobial activity, the model was able to identify peptides with minimal toxicity to human erythrocytes, thereby overcoming a significant challenge faced by previous efforts in discovering antimicrobial peptides [29]
Isolation and optimization of peptides from natural sources
Nature has proven to be a reliable source of therapeutic peptides, as living organisms possess numerous peptides that are essential for biological functions. These peptides can be utilized in their natural form or modified for use as therapeutics [30,31]. Several blockbuster therapeutic peptides that functions as antimicrobials, immune modulators, antioxidants, and hormone regulators have been discovered from various natural sources [32,33]. For example, a series of GLP-1 analogs were developed by modifying them with different fatty acids to increase their plasma half-life via albumin binding. This led to the discovery of Ozempic (semaglutide), which can be administered once a week and was approved by the FDA for the treatment of type 2 diabetes [34]. Building on these concepts, scientists at Novo Nordisk developed the amylin analog, cagrilintide, which is currently in clinical trials for the management of obesity. To address the challenge of fibrillation that is associated with human amylin, the scientists designed new analogs based on the structure of rodent amylins, which do not form fibrils [35].
Rational design of peptide drugs
Rational design is a strategy that can be used at any stage of the peptide drug discovery process. When implemented in the early stages of lead identification, instead of relying on high-throughput assays, rational design allows researchers to utilize a targeted and smaller library of peptides that may be easier to manipulate. Through the application of relevant theories, extrapolation of structure-activity relationships, and utilization of computational models, researchers can create a reduced chemical space. This eliminates the uncertainties associated with random peptide libraries, resulting in a refined collection of peptides with more predictable bioactivity and pharmacokinetics [36]. For example, Zhou et at. used an in silico approach to develop bispecific fusion peptides that target domains II and IV of HER2, leading to a potent and selective binding to HER2-positive cells. These peptides were conjugated with camptothecin to form a peptide-drug conjugate for cancer therapy. The fusion peptides exhibited greater stability than single targeting peptides and enhanced antitumor activity compared to camptothecin alone. [37]. The increased availability of crystal structures of protein-protein interactions that mediate crucial biological processes has significantly improved the process of rational peptide drug design. For instance, Yin et al. discovered a PD-1 mimetic peptide MOPD-1, which binds to PD-L1 with nanomolar affinity and blocks the PD-1/PD-L1 interaction. Unlike most mimetic peptides, MOPD-1 was designed based on the mimicry of the interface of an affinity-optimized parent protein, rather than the native PD-1 protein [38].
Strategies to enhance the stability and bioactivity of peptides
While lead peptides may hold promise as therapeutic agents, they may not be sufficiently stable or potent to qualify as successful drug candidates. To enhance the bioactivity and stability of peptides, researchers have explored various chemical modification techniques, including cyclization, D-amino acid substitution, peptoids formation, N-methylation, halogenation of side chains, and terminus protection [39,40]. Although some of the modifications have been successful in developing peptide drugs, cyclization has emerged as the most successful and widely adopted strategy for optimizing the bioactivity of peptides. This is because cyclization has been shown to offer significant advantages to peptide drugs. Therefore, it is not surprising that cyclic peptides account for two-thirds of all approved therapeutic peptides [41].
Cyclization
Cyclization is a commonly used and relatively simple chemical strategy to impart several favorable characteristics to linear peptides and peptidomimetics, such as increased target specificity and selectivity, enhanced stability, and high cell permeability [42]. Because of the fixed geometry of cyclic peptides, the entropic cost of binding is reduced, which allows them to bind more efficiently and selectively to targets. In contrast, linear peptides have high conformational flexibility, which can result in promiscuous and off-target interactions and an increased likelihood of undesirable side effects [43]. There are several types of cyclization, such as end-to-end (head-to-tail) cyclization, side chain to side chain cyclization, and end-to-side chain cyclization (formed between either of the terminals and an amino acid side chain). Other variants of cyclization include bicyclic and stapled peptides. Figure 3 summarizes the different cyclization formats.
Figure 3: Major cyclization formats of therapeutic peptides.
When creating a cyclic peptide, several important parameters, such as length, stability, and linkage rigidity must be considered. There are various methods for cyclizing peptides. (A) end-to-end cyclic peptide. (B) end-to-side chain cyclic peptide. (C) stapled peptide. (D) bicyclic peptide. (E) side chain-to-side chain cyclic peptide. (F) Macrocyclization scanning. The two terminal amino acids in a linear peptide are replaced with cysteine. Then, the position of one or both cysteine residues is sequentially altered, followed by the formation of a disulfide bond between the two cysteine residues, leading to the formation of a macrocyclic peptide.
Cyclic peptides can be used as therapeutic agents in the modulation of protein-protein interactions, RNA binding, enzyme inhibition, imaging, and diagnostics. As a result, the use of macrocyclic peptides is expected to grow exponentially [44]. Various innovative approaches for the design, synthesis, and evaluation of cyclic peptide have been recently reported. For example, our group utilized a novel macrocyclization approach (Figure 3F) in the discovery of cyclic peptides that exert anti-tumor activity by blocking the PD-1/PD-L1 interaction. The cyclic derivatives exhibited about a 34-fold improvement in PD-L1 blocking activity and up to a 4-fold increase in serum stability compared to the parent linear peptide. Cyclization resulted in a peptide derivative with improved bioactivity, enhanced proteolytic stability and superior therapeutic efficacy even at a low dosage of 0.5 mg/kg [12]. This study demonstrated that macrocyclization scanning is a valuable tool in developing peptide drugs, especially when the structural characterization of the peptide-receptor complex is limited.
D-amino acids
Natural peptides and proteins in the body are composed of L-amino acids, which are vulnerable to enzyme degradation. In contrast, D-amino acids are more resistant to these enzymes, which typically recognize the structures of L-amino acids. Therefore, replacing L-amino acids with D-amino acids can be employed to improve the proteolytic stability of therapeutic peptides.[45] Moreover, D-amino acid substitution can reduce the generation of anti-PEG antibody and the toxicity of peptide-PEG conjugates in animals.[46] In general, peptides can be fully or partially substituted with D-amino acids. However, it is important to note that while substituting L-amine acids with D-amino acids increases the proteolytic stability of a peptide, it may also impact the bioactivity of the peptide. This is because introducing D-amino acids can alter the peptide’s conformation and affect its interaction with its target. Therefore, it is crucial to ensure that the incorporation of a D-amino acid does not significantly alter the peptide’s secondary structure. One approach is to limit the substitution of D-amino acids to the peptide termini.
The D-amino acid scan is a commonly used method for investigating how the stereochemistry of amino acids affects the function and structure of peptides. For example, a D-amino acid scan was employed to identify the critical stereocenters of α-Conotoxin RgIA, which is a nicotinic acetylcholine receptor antagonist. While most analogues showed a reduction in biological activity, analogue 13 retained full activity and exhibited increased stability against enzymatic degradation in human serum and simulated intestinal fluid (SIF) [47]. Similarly, a partially substituted analog, D-Arg-W3R6, was discovered from the antimicrobial peptide W3R6. It exhibited the same antimicrobial activity as the parent peptide, while the completely substituted D-enantiomer, D-W3R6, showed decreased activity. Both D-Arg-W3R6 and D-W3R6 showed improved proteolytic stability. [48].
Several strategies have been developed for generating fully substituted D-peptides. One approach is to first discover an L-peptide and then convert it to a retro-inverso-D-amino acid based peptide. Recently, there has been growing interest in using the mirror image phage display technology to directly discover D-peptides [14,49]. For instance, Adaligil et al. discovered D-peptides with high antibacterial activity and good safety.[49] The same technology has also led to the discovery of a D-peptide that blocks the immune checkpoint TIGIT, which could serve as a potential cancer immunotherapy agent.[14] The main limitation of this technology is the production of target proteins in D-form. Computational design is another new strategy for de novo designing D-form peptides. For example, Yang et al. de novo designed D-peptides that bind to tumor necrosis factor-α (TNFα) and inhibit its interaction with its receptor 1 (TNRFR1)[50].
Peptoids
Peptoids are peptidomimetics that contain N-substituted glycines, which provide improved proteolytic stability due to the absence of amide bonds in their backbone. Peptoids have a similar structure motif as peptides and can be readily synthesized using solid-phase synthesis techniques [51]. Peptoids can be discovered by substituting amino acids with peptoid residues. For example, Kessler et al. introduced N-substituted glycines to collagen-mimetic peptides (CMPs), resulting in a series of highly stabilized triple-helical collagen mimetic peptoids. These peptoids possess the potential to be used as collagen-mimetic therapeutics and materials [52]. Another approach to discovering peptoids involves screening a combinatorial library of peptoids against a specific target. Using the peptoid library agar diffusion (PLAD) assay, Spicer et al. discovered a tripeptoid called AEC5, which exhibited promising antifungal activity against C. neoformans and eliminated all viable fungal cells within a few hours [53]. In another study, a one-bead-one-compound (OBOC) library of cyclic peptoid was utilized to discover cyclic peptoid inhibitors of cyclophilin D as potential neuroprotective agents. The peptoid I11 was identified as the most effective anti-inflammatory peptoid with minimum cytotoxicity. It exhibited a great permeability across the blood-brain barrier (BBB), indicating its potential to modulate mitochondrial function in neuronal cells [54]. Due to their high proteolytic stability, peptoids are more promising than peptides in combinatorial library screening.
N-methylation
Alkylation of the nitrogen atom in peptide amide bonds is an important chemical modification for improving the drug-ability and pharmacokinetic properties (i.e., absorption, half-life, and bioavailability) of therapeutic peptides. Among the various N-alkylation methods, N-methylation is the most widely used because of its versatility and ease of formation. It increases steric hindrance and enables peptides to adopt a cis confirmation more readily [55]. For instance, McBrayer and colleagues demonstrated that N-methylation confers significant proteolytic stability to peptides. N-methylation increased the half-life of E. faecalis fsr quorum sensing modulating peptides by more than 6-fold [56].
The concept of sequential backbone N-methylation has been used to study the functional selectivity of human urotensin II and related peptides to their targets. The results revealed that the location of the N-C bond can affect the peptide’s biological activity, highlighting the crucial role of hydrogen-bond interactions in the bioactivity of these endogenous peptides [57]. Another study evaluated N-methylated peptide inhibitors of Angiotensin converting enzyme (ACE), neutral endopeptidase (NEP) and aminopeptidase N (APN) using their natural substrates. N-methylation resulted in peptide derivatives with enhanced activity against hypertensive, hypertrophic, and fibrosis [58]. The hurdles of the backbone N-methylation approach are the difficulty in synthesizing N-methyl building blocks chemically as well as the inefficiency in coupling during peptide synthesis. Recently, researchers have developed a chemoenzymatic strategy to overcome this limitation. They achieved N-methylation by conjugating peptides to the catalytic scaffold of a borosin-type methyltransferase. The peptides conjugated to the transferase showed efficient N-methylation, and the resulting N-methylated peptide can be cleaved from the scaffold [59].
Side chain halogenation
Halogenation is an emerging method for modulating the pharmacological activities of organic molecules including peptides. Among the halogens, iodine, bromine, chlorine, and fluorine have been incorporated into various drug molecules to improve their bioactivity. In particular, fluorine and chlorine have been extensively used in drug discovery efforts. Halogenation is generally associated with improved cell membrane permeability, enhanced target selectivity, and reduced side effects [60]. For example, the introduction of halotryptophans in RGD peptides increased their affinity and specificity toward integrin αvβ3 [61]. Similarly, the effects of substituting various halogens on the anti-microbial activity of peptoids were studied. It was revealed that substitution with chlorine or bromine resulted in enhanced antimicrobial activity against Gram-positive bacteria, whereas fluorination did not exert a significant effect. While halogenation can confer favorable properties to peptide drugs, substituting polar groups with halogens may increase the peptide’s hydrophobicity, which can causes aggregation and loss of activity [62].
Other chemical modifications of therapeutic peptides have also been explored but less frequently in the development for clinically useful peptides. Among these are reduced peptide bonds, the use of amide bond surrogates, termini protection, lipidation, PEGylation, and other polymer-peptide conjugations. These modifications have been elaborated in other references [63-65].
Delivery systems for peptides
Instead of chemical modifications, there is a growing interest in adopting delivery systems to protect peptides from the enzymes in the body. Some of these drug carries include nanoparticles, liposomes, dendrimers, micelles [66], polyelectrolyte complexes [67], hydrogels [68], biomimetic and bio-derived nanocomposites, which have been covered extensively in other references [69]. Nanomaterials have been utilized for targeted delivery of both therapeutic peptides and proteins. Physicochemical properties, such as particle size, shape, surface charge, and porosity, are crucial for the structure-function relationship of nanoparticles in an effective drug delivery system [70]. The concept of incorporating permeation enhancers in the formulation of peptide drugs has gained renewed attention following the FDA’s approval of oral semaglutide (Rybelsus®) in 2019. Co-formulating semaglutide, a GLP-1 analog, with salcaprozate sodium (SNAC) increases oral bioavailability and allows for oral administration. However, challenges such as poor stability in the gastrointestinal tract still remain, and more efforts are needed to further improve the bioavailability of oral peptides [34].
Peptides have also been utilized as delivery systems on their own. For example, self-assembled peptide drug delivery systems (PDDs) have been applied to transport therapeutic agents to various targets, including tumors. A comprehensive review of PDDs have been reported elsewhere [71]. Peptide-drug conjugates have also been explored as novel drug delivery systems in cancer treatment. These peptides may have several functions, such as exerting therapeutic effect, acting as a drug carrier, promoting targeted drug release, and enhancing in vivo drug distribution [72].
In addition to traditional delivery methods, several advanced delivery technologies like microneedle, inhalation, microcapsules, electroporation, sonophoresis, and iontophoresis are currently being investigated for their potential to revolutionize the delivery of bioactive peptides [73]. These advanced technologies aim to improve the stability, bioavailability, and targeted delivery of peptides, thus enhancing their therapeutic efficacy.
Concluding Remarks and Perspective
The interest in using peptides as pharmaceutical agents has grown tremendously over the years because peptides possess unique characteristics that make them highly desirable as therapeutics. Despite the considerable success of therapeutic peptides, their widespread use is still limited, in part, due to their susceptibility to enzymatic degradation. The stability and bioactivity of peptide drugs can be significantly improved by introducing conformational constraints, peptide bond mimics, unnatural amino acids, or non-peptidic scaffolds. Besides improving enzymatic stability, the incorporation of non-natural amino acids can also decrease immunogenicity. A recent study demonstrated that peptides containing non-natural amino acids exhibited reduced binding to HLA class II molecules, resulting in decreased T-cell stimulation [74].
However, it is essential to note that each strategy has its limitations, and no single approach can achieve all the desirable characteristics of an ideal therapeutic peptide (see Outstanding Questions). For example, while D-amino acid substitution can significantly improve the proteolytic stability of peptides, it may also compromise the biological activity of modified peptides. Similarly, replacing polar groups with halogens may increase the hydrophobicity of peptides, thus increasing the risk of aggregation in biological systems. Despite this, a combination of different chemical modifications, coupled with appropriate delivery technologies, may potentially generate significantly improved peptides. The development of peptide drugs is a long, expensive, and labor-intensive process. The use of in-silico methods, such as machine learning and deep learning, can aid in predicting and optimizing peptide bioactivity and stability. Artificial intelligence-powered strategies are expected to facilitate fast and cost-effective peptide drug development with high accuracy. In summary, the field of peptide therapeutics is rapidly expanding within the pharmaceutical industry, with many milestones yet to be achieved. This has generated a lot of enthusiasm among pharmaceutical companies and scientists.
Outstanding questions.
What are the commonly used optimization strategies to improve the stability and bioactivity of therapeutic peptides?
How will data-driven computational approaches, such as machine learning and deep learning, impact the development and advancement of peptide-based therapies?
Where will the field of peptide therapeutics be in the next five years?
Figure I. Alanine scanning and truncation to study the structure-activity relationship of the H3K4me3 peptide.
(A) Alanine scanning of the wild type H3K4me3 10-mer peptide. The amino acid residues within the peptide sequence are systematically replaced with alanine. It helps to identify which amino acids play a crucial role in the structure, interaction, stability, and function of the peptide. (B) The flanking amino acids of the peptide sequence are removed from the C-terminus. Truncation helps to identify the shortest sequence that maintains its functional activity. (C) The inhibition constant (Ki) of the peptides derived from alanine scanning or truncation. Reproduced from the reference [75] with permission from the Royal Society of Chemistry.
Figure II. Cellular uptake mechanisms of bioactive peptides.
(A) Peptides binds to the extracellular domain of receptors. After binding to the receptor, the downstream function is mediated depending on the nature of the peptide drug. Peptides with blocking effect mediate inhibitory signal to inhibit normal physiological functions. (B) Peptides with agonistic effect enhance downstream functions. (C) Direct translocation. Cell-penetrating peptides cross the cell barrier via direct translocation. (D) Receptor-mediated endocytosis. Binding of peptides to the surface receptor forms a receptor-peptide complex, which is then internalized into the cell. (E) Clathrin-mediated endocytosis. Peptides enter the cells through clathrin-mediated endocytosis.
Highlights.
Over the last half-decade, scientists and clinicians have witnessed remarkable success of therapeutic peptides both in the diagnosis and treatment various diseases.
Although peptide drugs have major advantages over other therapeutic modalities, poor physicochemical and proteolytic stability profiles are major setbacks that undercut their clinical application.
An assortment of different chemical modifications coupled with suitable delivery technologies could potentially generate greatly improved peptide drugs that can be translated to the clinic.
FDA approval of oral semaglutide (Rybelsus®) in 2019 for the management of type II diabetes marked a key milestone in the advancement peptide therapeutics.
Acknowledgements
This work is supported by the National Institutes of Health (R01CA23109, R01AA021510, R01GM121798, and R01CA271592).
Glossary
- End-to-end cyclization:
The formation of an amide bond between amino acid residues at the N- and C-termini. It is often referred to as backbone-to-backbone cyclization
- Side chain-to-side chain cyclization:
The formation of an amide, disulfide, or other bonds between amino acid side chains
- End-to-side chain cyclization:
At least one trifunctional amino acid is required, and a bond is formed between the trifunctional amino acid and one terminus of the peptide
- Stapled peptides:
Short α-helical peptides constrained by a synthetic brace, usually in the form of hydrocarbon linkers
- Bicyclic peptides:
Highly constrained peptides with two macrocyclic rings that are created by connecting the peptide to a small chemical connector at the center of the molecular scaffold
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer Statement
None.
References
- 1.Karasek D (2022) Oral semaglutide - Rybelsus(R), the first GLP-1 receptor agonist for oral use in clinical practice. Vnitr Lek 68, 89–95 [PubMed] [Google Scholar]
- 2.AI Shaer D et al. (2022) 2021 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals (Basel) 15. 10.3390/ph15020222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Musaimi O et al. (2021) 2020 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals (Basel) 14. 10.3390/ph14020145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullard A (2023) 2022 FDA approvals. Nature reviews. Drug Discovery, [DOI] [PubMed] [Google Scholar]
- 5.Thakur R et al. (2022) Peptides as Diagnostic, Therapeutic, and Theranostic Tools: Progress and Future Challenges. Crit Rev Ther Drug Carrier Syst 40, 49–100. 10.1615/CritRevTherDrugCarrierSyst.2022040322 [DOI] [PubMed] [Google Scholar]
- 6.Pechenov S et al. (2021) Development of an orally delivered GLP-1 receptor agonist through peptide engineering and drug delivery to treat chronic disease. Sci Rep 11, 22521. 10.1038/s41598-021-01750-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand T et al. (2021) Phage display technique as a tool for diagnosis and antibody selection for coronaviruses. Current microbiology 78, 1124–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton MS et al. (2019) In vitro selection of peptides and proteins—advantages of mRNA display. ACS synthetic biology 9, 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GP (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228, 1315–1317 [DOI] [PubMed] [Google Scholar]
- 10.Barderas R and Benito-Peña E (2019) The 2018 Nobel Prize in Chemistry: phage display of peptides and antibodies. Analytical and bioanalytical chemistry 411, 2475–2479 [DOI] [PubMed] [Google Scholar]
- 11.Liu H et al. (2019) Discovery of low-molecular weight anti-PD-L1 peptides for cancer immunotherapy. Journal for ImmunoTherapy of Cancer 7, 270. 10.1186/s40425-019-0705-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fetse J et al. (2022) Discovery of Cyclic Peptide Inhibitors Targeting PD-L1 for Cancer Immunotherapy. Journal of Medicinal Chemistry, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H et al. (2020) Recent advances in the development of protein-protein interactions modulators: mechanisms and clinical trials. Signal Transduct Target Ther 5, 213. 10.1038/s41392-020-00315-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X et al. (2020) A Novel D Peptide Identified by Mirror-image Phage Display Blocks TIGIT/PVR for Cancer Immunotherapy. Angewandte Chemie International Edition, [DOI] [PubMed] [Google Scholar]
- 15.Pleiko K et al. (2021) In vivo phage display: identification of organ-specific peptides using deep sequencing and differential profiling across tissues. Nucleic acids research 49, e38–e38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y et al. (2018) RNA display methods for the discovery of bioactive macrocycles. Chemical reviews 119, 10360–10391 [DOI] [PubMed] [Google Scholar]
- 17.Peacock H and Suga H (2021) Discovery of de novo macrocyclic peptides by messenger RNA display. Trends in Pharmacological Sciences 42, 385–397 [DOI] [PubMed] [Google Scholar]
- 18.Lin CW et al. (2021) A Selection of Macrocyclic Peptides That Bind STING From an mRNA-Display Library With Split Degenerate Codons. Angewandte Chemie 133, 22822–22827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming MC et al. (2022) Discovery and Structural Basis of the Selectivity of Potent Cyclic Peptide Inhibitors of MAGE-A4. J Med Chem 65, 7231–7245. 10.1021/acs.jmedchem.2c00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford DJ et al. (2021) Potent Cyclic Peptide Inhibitors of FXIIa Discovered by mRNA Display with Genetic Code Reprogramming. J Med Chem 64, 7853–7876. 10.1021/acs.jmedchem.1c00651 [DOI] [PubMed] [Google Scholar]
- 21.Quartararo AJ et al. (2020) Ultra-large chemical libraries for the discovery of high-affinity peptide binders. Nature communications 11, 3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalafatovic D et al. (2019) Algorithm-supported, mass and sequence diversity-oriented random peptide library design. Journal of cheminformatics 11, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponsel D et al. (2011) High affinity, developability and functional size: the holy grail of combinatorial antibody library generation. Molecules 16, 3675–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxter D et al. (2014) Library construction, selection and modification strategies to generate therapeutic peptide-based modulators of protein-protein interactions. Future Medicinal Chemistry 6, 2073–2092 [DOI] [PubMed] [Google Scholar]
- 25.Mahon CM et al. (2013) Comprehensive interrogation of a minimalist synthetic CDR-H3 library and its ability to generate antibodies with therapeutic potential. Journal of molecular biology 425, 1712–1730 [DOI] [PubMed] [Google Scholar]
- 26.Cardoso MH et al. (2020) Computer-Aided Design of Antimicrobial Peptides: Are We Generating Effective Drug Candidates? Frontiers in Microbiology 10. 10.3389/fmicb.2019.03097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basith S et al. (2020) Machine intelligence in peptide therapeutics: A next-generation tool for rapid disease screening. Medicinal research reviews 40, 1276–1314 [DOI] [PubMed] [Google Scholar]
- 28.Haney EF et al. (2018) Computer-aided Discovery of Peptides that Specifically Attack Bacterial Biofilms. Scientific Reports 8, 1871. 10.1038/s41598-018-19669-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capecchi A et al. (2021) Machine learning designs non-hemolytic antimicrobial peptides. Chemical science 12, 9221–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasasty V et al. (2018) Natural peptides in drug discovery targeting acetylcholinesterase. Molecules 23, 2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshiro KG et al. (2019) Bioactive peptides against fungal biofilms. Frontiers in microbiology 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sable R et al. (2017) Peptides, peptidomimetics, and polypeptides from marine sources: A wealth of natural sources for pharmaceutical applications. Marine drugs 15, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gewehr MC et al. (2020) Peptides from Natural or Rationally Designed Sources Can Be Used in Overweight, Obesity, and Type 2 Diabetes Therapies. Molecules 25, 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau J et al. (2015) Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. Journal of medicinal chemistry 58, 7370–7380 [DOI] [PubMed] [Google Scholar]
- 35.Kruse T et al. (2021) Development of cagrilintide, a long-acting amylin analogue. Journal of Medicinal Chemistry 64, 11183–11194 [DOI] [PubMed] [Google Scholar]
- 36.Manzanares P et al. (2019) Improving health-promoting effects of food-derived bioactive peptides through rational design and oral delivery strategies. Nutrients 11, 2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J et al. (2022) In Silico Exploration and Biological Evaluation of Bispecific Peptides Derived from Anti-HER2 Antibodies and Peptide–Camptothecin Conjugates for HER2-Positive Breast Cancer. Journal of Medicinal Chemistry 65, 15123–15139 [DOI] [PubMed] [Google Scholar]
- 38.Yin H et al. (2021) Rational Design of Potent Peptide Inhibitors of the PD-1: PD-L1 Interaction for Cancer Immunotherapy. Journal of the American Chemical Society 143, 18536–18547 [DOI] [PubMed] [Google Scholar]
- 39.Buckley ST et al. (2016) Chemically modified peptides and proteins - critical considerations for oral delivery. Tissue Barriers 4, e1156805. 10.1080/21688370.2016.1156805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakob AK et al. (2019) Backbone modifications in peptidic inhibitors of flaviviral proteases. Bioorganic & medicinal chemistry letters 29, 1913–1917 [DOI] [PubMed] [Google Scholar]
- 41.Zhang H and Chen S (2022) Cyclic peptide drugs approved in the last two decades (2001–2021). RSC Chemical Biology 3, 18–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zorzi A et al. (2017) Cyclic peptide therapeutics: past, present and future. Current opinion in chemical biology 38, 24–29 [DOI] [PubMed] [Google Scholar]
- 43.Jamieson AG et al. (2013) Peptide Scanning for Studying Structure-Activity Relationships in Drug Discovery. Chemical biology & drug design 81, 148–165 [DOI] [PubMed] [Google Scholar]
- 44.Choi J-S and Joo SH (2020) Recent Trends in Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol Ther (Seoul) 28, 18–24. 10.4062/biomolther.2019.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domhan C et al. (2019) Replacement of l-Amino Acids by d-Amino Acids in the Antimicrobial Peptide Ranalexin and Its Consequences for Antimicrobial Activity and Biodistribution. Molecules 24, 2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sylvestre M et al. (2021) Replacement of L-amino acid peptides with D-amino acid peptides mitigates anti-PEG antibody generation against polymer-peptide conjugates in mice. J Control Release 331, 142–153. 10.1016/j.jconrel.2021.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren J et al. (2019) D-amino acid substitution of α-conotoxin RGIA identifies its critical residues and improves the enzymatic stability. Marine drugs 17, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y et al. (2019) Antimicrobial activity, membrane interaction and stability of the D-amino acid substituted analogs of antimicrobial peptide W3R6. Journal of Photochemistry and Photobiology B: Biology 200, 111645. [DOI] [PubMed] [Google Scholar]
- 49.Adaligil E et al. (2019) Discovery of Peptide Antibiotics Composed of D-Amino Acids. ACS chemical biology 14, 1498–1506 [DOI] [PubMed] [Google Scholar]
- 50.Yang W et al. (2019) Computational design and optimization of novel d-peptide TNF α inhibitors. FEBS letters 593, 1292–1302 [DOI] [PubMed] [Google Scholar]
- 51.Eckhauser ML (1990) The neodymium-YAG laser and gastrointestinal malignancy. Arch Surg 125, 1152–1154. 10.1001/archsurg.1990.01410210078012 [DOI] [PubMed] [Google Scholar]
- 52.Kessler JL et al. (2021) Peptoid residues make diverse, hyperstable collagen triple-helices. Journal of the American Chemical Society 143, 10910–10919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spicer SK et al. (2019) Toward a clinical antifungal peptoid: Investigations into the therapeutic potential of AEC5. Biopolymers 110, e23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hyun S et al. (2021) One-bead-one-compound screening approach to the identification of cyclic peptoid inhibitors of cyclophilin D as neuroprotective agents from mitochondrial dysfunction. Chem Commun (Camb) 57, 2388–2391. 10.1039/d0cc08268f [DOI] [PubMed] [Google Scholar]
- 55.Li Y et al. (2021) Improvement on Permeability of Cyclic Peptide/Peptidomimetic: Backbone N-Methylation as A Useful Tool. Mar Drugs 19. 10.3390/md19060311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McBrayer DN et al. (2019) N-methylation of amino acids in gelatinase biosynthesis-activating pheromone identifies key site for stability enhancement with retention of the Enterococcus faecalis fsr quorum sensing circuit response. ACS infectious diseases 5, 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merlino F et al. (2019) Functional selectivity revealed by N-methylation scanning of human urotensin II and related peptides. Journal of medicinal chemistry 62, 1455–1467 [DOI] [PubMed] [Google Scholar]
- 58.Savitha MN et al. (2020) Combinatorial inhibition of Angiotensin converting enzyme, Neutral endopeptidase and Aminopeptidase N by N-methylated peptides alleviates blood pressure and fibrosis in rat model of dexamethasone-induced hypertension. Peptides 123, 170180. [DOI] [PubMed] [Google Scholar]
- 59.Zheng Y et al. (2022) Bioconjugate Platform for Iterative Backbone N-Methylation of Peptides. ACS Catal 12, 14006–14014. 10.1021/acscatal.2c04681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sana B et al. (2022) Halogenation of Peptides and Proteins Using Engineered Tryptophan Halogenase Enzymes. Biomolecules 12. 10.3390/biom12121841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kemker I et al. (2021) Tuning the Biological Activity of RGD Peptides with Halotryptophansdagger. J Med Chem 64, 586–601. 10.1021/acs.jmedchem.0c01536 [DOI] [PubMed] [Google Scholar]
- 62.Molchanova N et al. (2020) Halogenation as a tool to tune antimicrobial activity of peptoids. Scientific reports 10, 14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boutureira O and Bernardes G.a.J. (2015) Advances in chemical protein modification. Chemical reviews 115, 2174–2195 [DOI] [PubMed] [Google Scholar]
- 64.Jambunathan K and K Galande A (2014) Design of a serum stability tag for bioactive peptides. Protein and peptide letters 21, 32–38 [DOI] [PubMed] [Google Scholar]
- 65.Diao L and Meibohm B (2013) Pharmacokinetics and pharmacokinetic–pharmacodynamic correlations of therapeutic peptides. Clinical pharmacokinetics 52, 855–868 [DOI] [PubMed] [Google Scholar]
- 66.Acar H et al. (2017) Molecular engineering solutions for therapeutic peptide delivery. Chemical Society Reviews 46, 6553–6569 [DOI] [PubMed] [Google Scholar]
- 67.Borro BC and Malmsten M (2019) Complexation between antimicrobial peptides and polyelectrolytes. Advances in colloid and interface science 270, 251–260 [DOI] [PubMed] [Google Scholar]
- 68.Doostmohammadi M et al. (2019) Hydrogels For Peptide Hormones Delivery: Therapeutic And Tissue Engineering Applications. Drug Des Devel Ther 13, 3405–3418. 10.2147/DDDT.S217211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du AW and Stenzel MH (2014) Drug Carriers for the Delivery of Therapeutic Peptides. Biomacromolecules 15, 1097–1114. 10.1021/bm500169p [DOI] [PubMed] [Google Scholar]
- 70.Saleh T and Shojaosadati SA (2016) Multifunctional nanoparticles for cancer immunotherapy. Human vaccines & immunotherapeutics 12, 1863–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J et al. (2020) Self-assembled peptide drug delivery systems. ACS Applied Bio Materials 4, 24–46 [DOI] [PubMed] [Google Scholar]
- 72.Zhu Y-S et al. (2021) Peptide–drug conjugate-based novel molecular drug delivery system in cancer. Trends in pharmacological sciences 42, 857–869 [DOI] [PubMed] [Google Scholar]
- 73.Thotakura N et al. (2018) Advanced approaches of bioactive peptide molecules and protein drug delivery systems. Current pharmaceutical design 24, 5147–5163 [DOI] [PubMed] [Google Scholar]
- 74.Azam A et al. (2021) Introduction of Non-natural Amino Acids Into T-Cell Epitopes to Mitigate Peptide-Specific T-Cell Responses. Front Immunol 12, 637963. 10.3389/fimmu.2021.637963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang MY et al. (2022) Covalent labeling of a chromatin reader domain using proximity-reactive cyclic peptides. Chemical Science 13, 6599–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fosgerau K and Hoffmann T (2015) Peptide therapeutics: current status and future directions. Drug discovery today 20, 122–128 [DOI] [PubMed] [Google Scholar]
- 77.Apostolopoulos V et al. (2021) A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 26. 10.3390/molecules26020430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamers C (2022) Overcoming the shortcomings of peptide-based therapeutics. Future Drug Discovery 4, FDD75. 10.4155/fdd-2022-0005 [DOI] [Google Scholar]
- 79.Ruseska I and Zimmer A (2020) Internalization mechanisms of cell-penetrating peptides. Beilstein journal of nanotechnology 11, 101–123. 10.3762/bjnano.11.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madani F et al. (2011) Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys 2011, 414729. 10.1155/2011/414729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dougherty PG et al. (2019) Understanding Cell Penetration of Cyclic Peptides. Chem Rev 119, 10241–10287. 10.1021/acs.chemrev.9b00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li CM et al. (2021) Novel Peptide Therapeutic Approaches for Cancer Treatment. Cells 10. 10.3390/cells10112908 [DOI] [PMC free article] [PubMed] [Google Scholar]