1. Introduction
Uveitis is an ocular inflammatory disease that can lead to visual complications and permanent vision loss, if not promptly detected and treated [1,2]. The etiology of uveitis includes infections, autoimmune conditions, and autoinflammatory disorders. Juvenile idiopathic arthritis (JIA) is the most common systemic disease associated with uveitis in childhood [3]. However, in many cases, investigations for a systemic association are unrevealing, rending a diagnosis of “idiopathic uveitis”.
JIA is the most common pediatric rheumatic disease, and up to 20% of patients develop associated uveitis depending on the population studied [1]. Further, approximately 50% of patients still experience ocular complications despite the availability of effective medications [3]. Expert consensus guidelines generally recommend a step-ladder approach to treat JIA-associated uveitis (JIA-U) with corticosteroids as first-line agent followed by steroid-sparing systemic treatment with methotrexate. Methotrexate is combined with tumor necrosis factor alpha inhibitors (TNFi) (adalimumab or infliximab) if vision-threatening disease is present at initial presentation or sequentially if the patient has an inadequate response to methotrexate. [1,2] TNFi have dramatically changed the uveitis prognosis in children[4,5]. However, 15–30% of patients still do not achieve inactive uveitis on anti-TNFi [4,5]. No consensus exists regarding the subsequent treatment of those who fail methotrexate and TNFi at above standard doses or increased frequency[1–3]. Based on limited evidence, biologics that target specific molecules of inflammation such as anti-interleukin (IL)-6 (tocilizumab), CTLA4 inhibitors (abatacept), and antiCD20 (rituximab) have been recommended, but there is still no preferred agent [1,2]. However, some cases of uveitis are recalcitrant to all of these biologics, thus there is a need for alternative therapy. As the etiology and pathogenesis of JIA-U remain poorly understood, new targeted therapy using Janus Kinase Inhibitors (JAK-I) has been trialed. JAK-I are small molecules administered orally, include baricitinib, tofacitinib or upadacitinib, and are potential alternatives for children with autoimmune disorders such as JIA-U[6–8].
2. Role of JAK Pathway or Rationale for Janus Kinase inhibition
In uveitis, dysregulated activation of the immune system leads to hyper-activation of T Cells (Th1 and Th17) and B cell subsets[7]. Studies in models of experimental autoimmune uveitis and in biospecimens such as aqueous humor and tears highlight the crucial role of Th1 and Th17 as in the development of uveitis, triggering the inflammatory cascade[7,9]. The secretion of inflammatory mediators such as cytokines (IL-6, TNF-α, interferon-gamma [IFN-γ], IL-2, and IL-17), and chemokines amplify the inflammatory cascade. Recruitment of additional inflammatory cells and increased cytokines and chemokines locally leads to a consequent breakdown of the blood retina barrier[7,8]. Cytokines and chemokines interact with their receptors resulting in receptor oligomerization with subsequent activation and phosphorylation of JAK. Subsequently, JAK phosphorylates the signal transducer and activator of transcription (STATs), a superfamily of DNA binding proteins (STAT1, 2, 3, 4, 5A, 5B, and 6)[7,8]. After phosphorylation, STAT will then dimerize and translocate to the nucleus where it regulates the expression of specific genes critical for enhancing inflammation [7]. Different ligand and receptors activate different subtypes of JAK, which then influence inflammatory cell proliferation, development, differentiation, migration and apoptosis [7]. In this context, Janus Kinase acts as a signaling pathway mediator for cytokines and chemokines by transducing the signals affecting and regulating the immune response6.
Humans have multiple JAK enzymes, JAK1, JAK2, JAK3, and tyrosine kinase (TYK2); three of the four JAK enzymes are ubiquitously expressed, while JAK3 is restricted to immune cells [7,8]. Thus, inhibition of the JAK pathway may play a key role in regulating the inflammatory and autoimmune response pathway in uveitis.
3. Current evidence for the treatment of JIA associated uveitis with JAKi
Current evidence on the use of JAK-I in the treatment JIA-U remain scarce, with most focused on adult patients with a history of JIA-U (Table 1)[10–12]. As shown in Table 1, in two case reports and one case series of four patients, all six patients treated with JAK-I had uveitis that was recalcitrant to several non-biologic and biologic disease modifying anti-rheumatic drugs (DMARDs) including anti-TNF, anti-IL6 or antiCD20, with many developing ocular complications[10–12]. After JAK-I (3 baricitinib, 2 tofacitinib, and 1 upadacitinib) was initiated, all patients achieved ocular disease control. Moreover, a systematic review published in 2021 reported the effectiveness of JAK-I not only in adult patients with JIA-U, but also in other types of uveitis and ocular inflammatory conditions including autoimmune scleritis.[6,13]
Table 1:
Current evidence about the efficacy of Jak-inhibitors in uveitis.
| Miserocchi et al., Clinical Rheumatology, 2020 (12) | 4 adults with JIA-U | 3 baricitinib (1 pt: 5mg/daily 2 pts: 4 mg/daily) 1 pt: tofacitinib 5mg × 2 / daily |
INF, ADA, Leflu, MTX, TCZ, AZA, ABA, RTX | Inactive JIA-U | 3 pts: arthritis controlled (1 tofacitinib, 2 baricitinib) 1 pt arthritis not controlled (baricitinib) |
| Bauermann et al., Ocul Immunol Inflamm 2019 (11) | 1 adult JIA-U | 1 tofacitinib (5 mg × 2/daily | MTX, ADA, RTX, GOL, INF, cyclosporine, TCZ, MMF | Control of JIA-U | Not reported |
| Baquet-Walscheid et al., Ocul immunol Inflamm 2022 (10) | 1 JIA-U | 1 upadacitinib (15 mg/daily) | MTX, cyclosporine, ADA, IFN, TCZ, tofacitinib | Inactive JIA-U | Arthritis inactive |
JIA-U Juvenile idiopathic arthritis-associated uveitis, Leflu leflunomide, MTX methotrexate, TCZ tocilizumab, AZA Azathioprine, ABA abatacept, RTX rituximab, MMF mycophenolate mofetil, GOL golimumab, INF infliximab, ADA adalimumab
Recently, the results of a randomized clinical trial (RCT) that evaluated the effectiveness of tofacitinib in the treatment of polyarticular JIA led to FDA approval for the treatment of JIA[14]. Although this RCT did not evaluate the effectiveness on uveitis, notably no patients experienced flares of or new onset uveitis during the observation period[14].
To date, there is an ongoing multicenter, phase 3 clinical trial evaluating the efficacy of Baricitinib for pediatric chronic anterior uveitis associated with JIA or ANA (+) idiopathic disease. This trial will include up to 40 patients ages 2 to < 18 years with an inadequate response or intolerance to methotrexate (NCT04088409)[15]. In this trial conducted by open-label Bayesian design, children will be randomly assigned (1:1) to either baricitinib or adalimumab, which is currently considered the reference drug for the treatment of pediatric uveitis. Moreover, 20 additional patients who are methotrexate or biologic resistant will be assigned to baricitinib. However, the results are still pending as of this publication.
4. Conclusion & Expert Opinion
Because of the sight-threatening nature of uveitis in children, specifically in JIA-U, the availability of effective therapeutic agents is critical to prevent permanent vision loss. As highlighted, targeting the JAK pathway, a key kinase in the transduction of signal of inflammatory cascade, may potentially lead to the control of inflammation in several inflammatory diseases, including JIA-U. JAK-I has already shown to be effective treatment for JIA, but data on JIA-U are lacking.
Studies in adult patients demonstrate that JAK-I is effective in patients with inflammatory ocular disease recalcitrant to standard treatment, specifically in JIA-U[6,10–12]. However, where JAK-I fits in pediatric uveitis treatment algorithms is unclear. Clarification is needed regarding the optimal time to start JAK-I, dosing strategies, and indications. Subsequent therapy in children with JIA-U who have an inadequate response to first-line treatment remains unclear. Although abatacept, tocilizumab, golimumab, and rituximab are recommended as next options, there is no preference for which agent to start. Further, there are no recommendations for when and whether to initiate JAKi. The multicenter phase 3 trial Juve-Bright (NCT04088409) comparing baricitinib to adalimumab may better elucidate the role of JAKi for JIA-associated chronic anterior uveitis (CAU) and ANA+, idiopathic CAU phenotypes.[15] If more children on baricitinib meet the primary and secondary endpoints compared to adalimumab, baricitinib may be indicated earlier, after methotrexate failure/intolerance or failure of TNFi agents. (Figure 1). Further studies are needed to determine if there are specific populations that better respond to JAKi such as those with certain subtypes of uveitis based on biologic pathways or who have distinct complications such as cystoid macular edema, and whether combination therapy is more effective.
Figure 1:
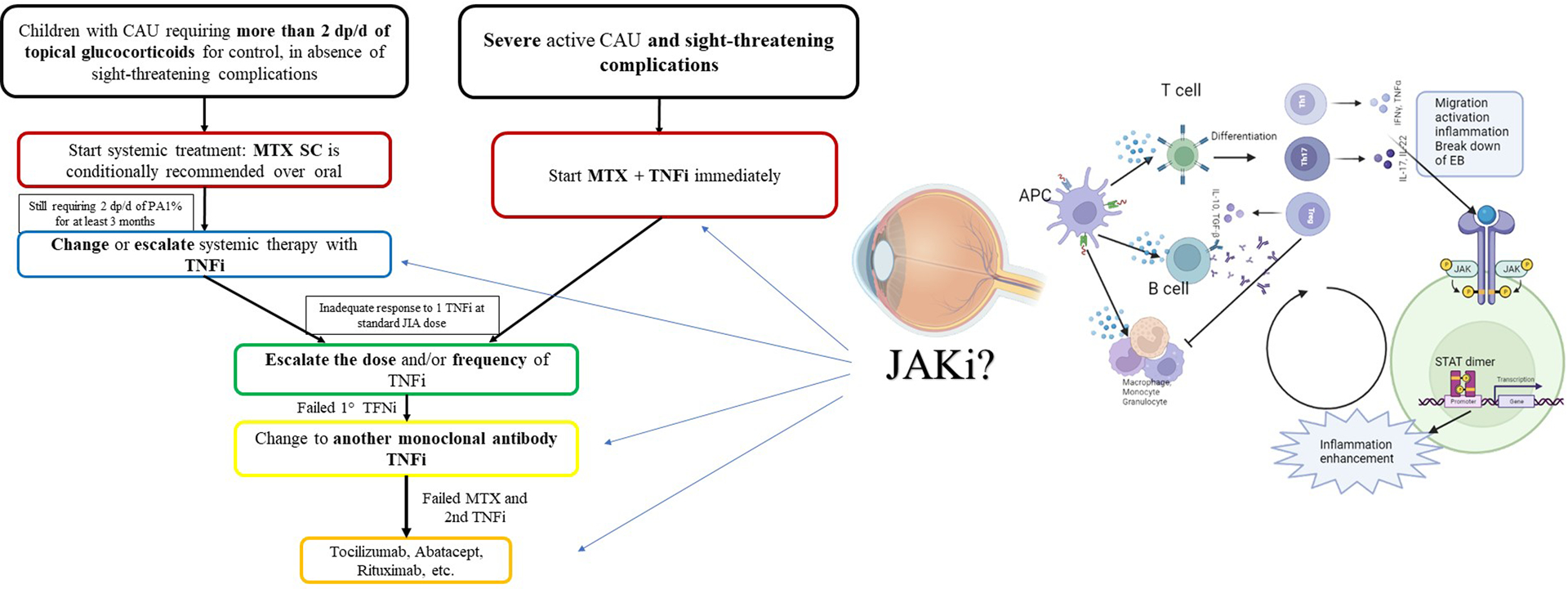
Proposed therapeutic approach based on the 2019 American College of Rheumatology recommendations for the treatment of JIA associated uveitis.
List of abbreviations: APC antigen presenting cell, CAU chronic anterior uveitis, d daily, dp drops, EB endothelial barrier, os oral, JAKi Janus Kinase inhibitor, JIA Juvenile Idiopathic Arthritis, MTX Methotrexate, PA prednisone acetate, sc subcutaneous, TNFi Tumor Necrosis factor alpha inhibitor.
Results have been encouraging for adult patients with JIA-U[6,10–12]. When considering dosage of JAKi, we must be cognizant of the fact that many cases of uveitis require higher than standard JIA treatment doses. ACR and CARRA expert consensus groups recommend escalating infliximab and adalimumab to doses as high as 20 mg/kg/dose q4 weeks or weekly respectively[1]. The optimal dose of JAKi for pediatric uveitis is unknown. In our practice, we have successfully used tofacitinib at standard JIA dosing to treat uveitis (unpublished). However, the uveitis phenotype, presence of active ocular complications, systemic disease activity, and resistance to standard dosing will likely dictate and personalize future treatment strategies. The side effect profile of higher dosing, and long-term systemic effects of JAKi on children is also relatively unknown. Thus, the potential benefits must be weighed against systemic risks of infection, cardiovascular disease, and a patient’s individual risk factors. A recent RCT by Ruperto et al. reported a favorable safety profile of tofacitinib in children with JIA wherein there were only a few cases of increased levels of liver enzymes, upper respiratory tract infections and reactivation of herpes zoster [14]. Moreover, targeting a more restricted pattern of JAK might be beneficial in order to prevent side effects as hepatotoxicity as recently demonstrated in children [16,17].
Timely vaccination for varicella zoster in patients without previous immunization or who are not up to date with their vaccination might be considered prior to JAK-I initiation as recommended in adulthood [18].Vaccine recommendations have been published by the ACR for JIA which may be applicable to children with uveitis on similar treatment [19].
In conclusion, JAK-I has shown to be effective for children with JIA and as potentially promising treatment of ocular inflammatory disease. The oral route of administration may also be of benefit in the pediatric population, as likely to be better tolerated than injectable routes of administration. In addition, it is less burdensome to families than a hospital infusion. However, this must be weighed against adherence to daily administration as lapses in treatment in combination with short half-life may pose challenges to long-term disease control. Overall, in the absence of long-term efficacy and safety data, we remain cautious, but optimistic. We will engage in shared decision making with patient and families, sharing the available evidence for or against JAKi, taking into account individual patient factors when selecting JAK-I treatment..
Funding
S Angeles-Han is supported by the NIH National Eye Institute under award numbers R01EY030521 and R01EY034565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1**.Angeles-Han ST, Ringold S, Beukelman T et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis care & research. 71(6), 703–716 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]; Updated recommendations for the treatment of JIA-associated uveitis by the American College of Rheumatology for North American
- 2**.Foeldvari I, Maccora I, Petrushkin H et al. New and updated recommendations for the treatment of juvenile idiopathic arthritis associated uveitis and idiopathic chronic anterior uveitis. Arthritis care & research (2022) [DOI] [PubMed] [Google Scholar]; Updated recommendations for the treatment of JIA-associated uveitis by the American College of Rheumatology by the Multinational Interdisciplinary Working Group for Uveitis in Childhood (MIWGUC) for Europe
- 3.Angeles-Han ST, Srivastava SK. Screening, Monitoring, and Treating Children With Juvenile Idiopathic Arthritis-Associated Uveitis: Visualizing Better Outcomes. The Journal of rheumatology (2022) [DOI] [PubMed] [Google Scholar]
- 4.Maccora I, Fusco E, Marrani E,et al. Changing evidence over time: updated meta-analysis regarding anti-TNF efficacy in childhood chronic uveitis. Rheumatology (Oxford, England). 60(2), 568–587 (2021) [DOI] [PubMed] [Google Scholar]
- 5.Ramanan AV, Dick AD, Jones AP et al. Adalimumab plus Methotrexate for Uveitis in Juvenile Idiopathic Arthritis. The New England journal of medicine. 376(17), 1637–1646 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Wen J, Hu H, Chen M, et al. Role of Janus Kinase (JAK) Inhibitor in Autoimmune Ocular Inflammation: A Systematic Review. Journal of immunology research. 2021, 2324400 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su Y, Tao T, Liu X, et al. JAK-STAT signaling pathway in non-infectious uveitis. Biochemical pharmacology. 204, 115236 (2022) [DOI] [PubMed] [Google Scholar]
- 8*.Damsky W, Peterson D, Ramseier J et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. The Journal of allergy and clinical immunology. 147(3), 814–826 (2021) [DOI] [PubMed] [Google Scholar]; Important review about the emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases
- 9.Amadi-Obi A, Yu C-R, Liu X et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 13(6), 711–718 (2007) [DOI] [PubMed] [Google Scholar]
- 10.Baquet-Walscheid K, Heinz C, Rath T, et al. Beneficial Effect of Upadacitinib in an Adult Patient with Juvenile Idiopathic Arthritis-associated Uveitis after Unsatisfactory Response to Tofacitinib: A Case Report. Ocular immunology and inflammation, 1–2 (2022) [DOI] [PubMed] [Google Scholar]
- 11.Bauermann P, Heiligenhaus A, Heinz C. Effect of Janus Kinase Inhibitor Treatment on Anterior Uveitis and Associated Macular Edema in an Adult Patient with Juvenile Idiopathic Arthritis. Ocular immunology and inflammation. 27(8), 1232–1234 (2019) [DOI] [PubMed] [Google Scholar]
- 12.Miserocchi E, Giuffrè C, Cornalba M,et al. JAK inhibitors in refractory juvenile idiopathic arthritis-associated uveitis. Clinical rheumatology. 39(3), 847–851 (2020) [DOI] [PubMed] [Google Scholar]
- 13.Paley MA, Karacal H, Rao PK, et al. Tofacitinib for refractory uveitis and scleritis. American journal of ophthalmology case reports. 13, 53–55 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Ruperto N, Brunner HI, Synoverska O et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet (London, England). 398(10315), 1984–1996 (2021) [DOI] [PubMed] [Google Scholar]; Pivotal trial about the effectiveness of tofacitinib in polyarticular JIA.
- 15**.Ramanan AV, Guly CM, Keller SY et al. Clinical effectiveness and safety of baricitinib for the treatment of juvenile idiopathic arthritis-associated uveitis or chronic anterior antinuclear antibody-positive uveitis: study protocol for an open-label, adalimumab active-controlled phase 3 clinical trial (JUVE-BRIGHT). Trials. 22(1), 689 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]; Discontinued head to head clinical trial of baricitinib versus adalimumab in JIA associated uveitis and anterior chronic uveitis
- 16.Marcuzzi A, Rimondi E, Melloni E et al. New Applications of JAK/STAT Inhibitors in Pediatrics: Current Use of Ruxolitinib. Pharmaceuticals (Basel, Switzerland). 15(3) (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerrigan SA, McInnes IB. JAK Inhibitors in Rheumatology: Implications for Paediatric Syndromes? Current rheumatology reports. 20(12), 83 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke B, Yates M, Adas M et al. The safety of JAK-1 inhibitors. Rheumatology (Oxford, England). 60(Suppl 2), ii24–ii30 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onel KB, Horton DB, Lovell DJ et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: Recommendations for Nonpharmacologic Therapies, Medication Monitoring, Immunizations, and Imaging. Arthritis & rheumatology (Hoboken, N.J.). 74(4), 570–585 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]


