Abstract
Background:
HPV-related oropharyngeal cancer (HPV-OPC) screening is being explored in research studies, but strategies to identify an appropriate population are not established. We evaluated whether a screening population could be enriched for participants with oncogenic HPV biomarkers using risk factors for oral HPV.
Methods:
Participants were enrolled at Johns Hopkins Hospitals and Mount Sinai Icahn School of Medicine. Eligible participants were men aged ≥30 with ≥2 lifetime oral sex partners, with a personal history of anogenital dysplasia/cancer, or partners of HPV-related cancer patients. Oral rinse and serum samples were tested for oncogenic HPV DNA,RNA and E6,E7 antibodies respectively. Participants with any biomarker were considered “at-risk”.
Results:
Of 1108 individuals, 7.3% had any oncogenic oral HPV DNA and 22.9% had serum antibodies for oncogenic HPV E6 or E7. 17 participants (1.5%) had both oral and blood biomarkers. HPV16 biomarkers were rarer, detected in 3.7% of participants, including 20 with oral HPV16 DNA, and 22 with HPV16 E6 serum antibodies (n=1 with both). In adjusted analysis, living with HIV (aOR=2.65, 95%CI=1.60–4.40) and older age (66–86 vs 24–45 years; aOR=1.70, 95%CI=1.07–2.70) were significant predictors of being at-risk. Compared to the general population, prevalence of oral HPV16 (1.8% vs 0.9%), any oncogenic oral HPV DNA (7.3% vs. 3.5%) and HPV16 E6 antibodies (2.2% vs 0.3%) were significantly elevated.
Conclusion:
Enrichment by the eligibility criteria successfully identified a population with higher biomarker prevalence, including HPV16 biomarkers, which may be considered for screening trials. Most in this group are still expected to have low risk of OPC.
Keywords: HPV, screening, OPC, biomarker, oral sex, antibody
Introduction
Oropharyngeal cancer (OPC) is a relatively rare cancer with nearly 20,000 incident cases in the United States (US) in 20211,2, most (>80%) of those in men3,4. About 90% of all OPCs in the US are now caused by human papillomavirus (HPV).5 OPC incidence has increased among men consistently 2.7% per year in the US over 20 years.1 However, screening is currently not recommended for OPC. The majority of HPV-related OPC (HPV-OPC) cases are detected at the time of nodal involvement, not early when isolated to a small primary tumor in the tonsil or base of the tongue.6,7
Typical cancer screening strategies employ risk-stratification methods to enrich for people most likely to have a malignancy, and who could most benefit from screening.8,9 Therefore, critical to evaluating any future OPC screening scenario is determining whether risk-stratification methods effectively identify people at risk, especially in the context of a rarer cancer like HPV-OPC. However, the optimal risk-stratification approach for HPV-OPC screening is unclear.
Risk factors for oral HPV DNA, the precursor for HPV-OPC, include male sex, current smoking, and number of lifetime oral sex partners.10 Individuals living with HIV11,12, with a personal history of anogenital cancer or partner with HPV-related cancer13 also have an increased prevalence of oncogenic oral HPV DNA. These risk factors overlap with those for E6 antibodies and HPV-OPC.14–16 Therefore, we hypothesized we could use these risk factors to identify an enriched population with HPV biomarkers associated with HPV-OPC, which may have relevance for future screening populations.
Methods
Study Design and Population
This analysis uses baseline data from a multi-center prospective cohort study. Healthy participants considered at increased risk for HPV-OPC were eligible for enrollment in “Men and women Offering Understanding of Throat HPV” (MOUTH17) study based upon meeting ≥1 of the following eligibility criteria: men aged ≥30 years with ≥2 or more self-reported lifetime oral sex partners10, history of anogenital dysplasia or cancer, or having a partner/spouse with HPV-OPC or anogenital cancer. Enrollment occurred April 2017-January 2022, at Johns Hopkins Medicine (JHM; Baltimore and other MD sites) and Icahn School of Medicine at Mount Sinai (New York, New York). Participants recruited at other locations were excluded from analysis due to low enrollment (OHSU, n=12), or different eligibility criteria (MWCCS cohort)12.
The study was approved by the institutional IRBs of both sites and informed consent was obtained from all participants.
Eligibility for At-Risk Cohort
Oral rinse and serum samples were collected and tested for oncogenic HPV biomarkers. Participants positive for any of these markers were considered eligible for the follow-up cohort (called the “at-risk” cohort), with planned annual study visits for four more years. Participants were considered “at-risk” if they had: 1) any oncogenic HPV DNA or RNA (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 66, or 68) detected in oral rinse; and/or 2) serum E6 or E7 antibodies to any oncogenic HPV type.
Baseline Data Collection
Participants provided demographic, medical history and behavioral data via computer-assisted self-interview (CASI) survey in Redcap, completed at study visit or with an emailed personalized link. Oral rinse specimens were collected either in person or remotely (Supplemental Table). This study was conducted in part during the COVID-19 pandemic, which impacted data collection.
Sample Collection
Oral rinse collection, processing and testing are described elsewhere18. Briefly, oral and oropharyngeal exfoliated cells were collected using 10 mL saline by 30-second oral rinse and gargle and stored at 4°C until processed. Once processed, oral rinse samples were aliquoted (750μl) and stored at −80° until shipped to DDL Diagnostic Laboratory for testing.
Blood was collected in SST tubes and stored at 4°C until processed. Blood samples were centrifuged at 1,942×g for 15 minutes at 37°C to separate serum layer, which was stored at −80°C until tested. All samples were processed and stored at Johns Hopkins Biospecimen Repository.
Oral rinse testing
Samples were diluted by a factor of 2.5x (750 μl sample + 1125 μl dilution) and DNA was extracted using the NucliSENS easyMAG. HPV DNA testing was done by PCR amplification using SPF10 primer system (version 1). Samples positive by SPF10 were genotyped using the DNA Enzyme Immuno-assay (DEIA) detection system which evaluates the presence of oncogenic HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 66, as well as 12 non-oncogenic HPV types. HPV16 qPCR testing was also performed on samples with HPV16 detected by DEIA.
Besides DEIA HPV DNA testing, two additional genomic tests currently approved for cervical HPV testing19 were also performed. This included HPV DNA testing performed using Cobas (Roche) on all oral rinse samples and HPV E6/E7 mRNA detection using Aptima (Hologic) on all oral rinse samples positive by SPF10. Oral rinse samples positive by DEIA, Cobas or Aptima testing were considered eligible for the at-risk cohort.
Serum testing
Antibody testing was performed centrally by the German Cancer Research Center (DKFZ) using the multiplex serology method that uses glutathione S-transferase (GST) capture ELISA with fluorescent bead-based technology20. Serum was tested for E1, E2 antibodies of HPV types 16 and 18, and E6 and E7 oncogene antibodies for oncogenic HPV types 16,18,31,33,35,45,52,58. Standardized positivity thresholds based on HPV-negative controls were applied.21,22 One batch of results (with 269 samples) had statistically significantly higher seroprevalence of non-16 oncogenic HPV E7 results. Since batch effects of testing could not be excluded, these results were not used to determine eligibility.
Comparison of HPV biomarkers to general population
Oral oncogenic HPV DNA prevalence was compared in our study population to similar data collected in subjects aged 24–69 in NHANES, collected 2015–16 in NHANES, analyzed using NHANES weighted prevalence to represent the prevalence in the general U.S. population.23 Prevalence of E6 serum antibodies to HPV16 and to any oncogenic HPV strain were compared in our study population and controls in the HPV Cancer Cohort Consortium (HPVC3). HPVC3 is a collaboration of nine cohort studies with blood collected during 1972–2009, and antibody testing using the same methods;24 overall control prevalence in HPVC3 was previously published24 and sex-specific prevalence were provided by authors for use in this report.
Statistical analysis
Inclusion in the analytic population required at least partial completion of the baseline survey and completion of HPV biomarker testing for ≥1 specimens at the time of analysis; 65 individuals who were enrolled but did not yet have data collection and/or testing were excluded. The primary outcome for this analysis was eligibility for at-risk cohort. A secondary outcome was having HPV16-specific biomarkers.
To explore further enrichment by demographic or behavioral characteristics, we compared oral health, medical history, sexual history, and substance use behaviors between participants at-risk and not-at-risk. Chi squared test was used for categorical variables and K-sample equality-of-medians test for continuous variables.
Cumulative behavioral measures (amount used per day * years used) calculated included tobacco packyears, alcohol drinkyears, marijuana jointyears and sexual partners/10 years (sex-years)14. Biomarker prevalence estimates were reported overall and compared across enrollment criteria categories, using chi squared and Fisher’s exact tests. Univariate and multivariable logistic regression was used to explore characteristics associated with at-risk cohort eligibility. We compared biomarker prevalence in our study to available data in the general population using an equality of proportions test. Statistical analyses were conducted with Stata version 16.1.
Results
Among 1108 individuals in the MOUTH study, most (88.9%) participants were eligible based upon self-identification as a man 30 years or older, with a lifetime history of performing oral sex on at least two people (Table 1), and the remainder were eligible based on personal history of anogenital dysplasia/cancer (6.5%, n=72) or having a partner/spouse with an HPV-related cancer (9.5%, n=105). Participants were enrolled primarily in outpatient clinics of Otolaryngology-Head and Neck Surgery (n=680) and internet-based outreach (n=208) with some also enrolled in outpatient Urology (n=94), HIV care (n=48) and Executive Health (n=16) clinics, as well as referrals and community events (n=62).
Table 1.
Description of baseline study population*
| Study Population Overall | N (biomarker prevalence) | ||
|---|---|---|---|
|
| |||
| N (%) | At-Risk | Select HPV16 Biomarkers detected~ | |
|
| |||
| N=1108 | N=248 | N=41 | |
|
| |||
| Eligibility groups (some participants were eligible for multiple groups) | |||
| Men with 2+ lifetime oral sex partners | 985 (88.9) | 229 (23.3) | 37 (3.8) |
| Hx of genital or anal dysplasia/cancer | 72 (6.5) | 18 (25.0) | 3 (4.2) |
| Partner/spouse of person with: | 105 (9.5) | 17 (16.2) | 5 (4.8) |
| ICC or cervical dysplasia | 15 (1.4) | 3 (20.0) | 0 (0) |
| HPV-OPC | 72 (6.5) | 9 (12.5) | 4 (5.6) |
| Genital or anal cancer | 18 (1.6) | 5 (27.8) | 1 (5.6) |
| Biologic Sex | |||
| Men | 993 (89.6) | 231 (23.3) | 38 (3.8) |
| Women | 115 (10.4) | 17 (14.8) | 3 (2.6) |
| Race and Ethnicity | |||
| White, non-Hispanic | 791 (71.4) | 159 (20.1) | 30 (3.8) |
| Black, non- Hispanic | 179 (16.2) | 58 (32.4) | 7 (3.9) |
| Hispanic any race | 85 (7.7) | 16 (18.8) | 0 (0) |
| Other | 53 (4.8) | 15 (28.3) | 4 (7.6) |
| Study Site | |||
| Johns Hopkins Hospital | 1026 (92.6) | 228 (22.2) | 37 (3.6) |
| Mount Sinai Hospital | 82 (7.4) | 20 (24.4) | 4 (4.9) |
| Age in years | |||
| 24–45 | 255 (23.0) | 48 (18.8) | 8 (3.1) |
| 46–55 | 279 (25.2) | 55 (19.7) | 12 (4.3) |
| 56–65 | 358 (32.3) | 90 (25.1) | 16 (4.5) |
| 66–86 | 216 (19.5) | 55 (25.5) | 5 (2.3) |
| Education Level ^ | |||
| < High-school | 41 (3.7) | 5 (12.2) | 1 (2.4) |
| High school/GED | 129 (11.7) | 36 (27.9) | 1 (0.8) |
| Any college | 469 (42.5) | 116 (24.7) | 23 (4.9) |
| Advanced/professional degree | 465 (42.1) | 90 (19.4) | 16 (3.4) |
| Annual Family Income (in thousands USD) ^ | |||
| 0 – <15 | 83 (8.1) | 18 (21.7) | 2 (2.4) |
| 15–<50 | 110 (10.7) | 30 (27.3) | 4 (3.6) |
| 50–<100 | 210 (20.5) | 49 (23.3) | 9 (4.3) |
| 100–<200 | 355 (34.6) | 71 (20.0) | 12 (3.4) |
| ≥200 | 268 (26.1) | 55 (20.5) | 11 (4.1) |
| Ever received HPV vaccine (≥1 dose) | 40 (3.6) | 7 (17.5) | 1 (2.5) |
| Living with HIV | 97 (8.8) | 44 (45.4) | 8 (8.3) |
This population includes 99 subjects classified as Not at-risk based on oral rinse data only, since blood was not collected (N=82) or was collected but test results not yet available (N=17) at the time of this analysis. Population also includes 3 subjects were determined to be Not at-risk based on blood data only, since rinse was not collected (N=1) or was collected but not tested (N=2).
To be classified as positive in this group, needed to have either HPV16 oral DNA and/or HPV16 E6 seropositivity. 114 people who only had oral rinse (n=111) or only had blood (n=3) results for HPV16 were classified for this group by that single test.
Excludes people who reported “prefer not to answer” for this variable
Participants were predominantly male (89.6%), non-Hispanic White (71.4%), with college education (42.5%) or advanced degree (42.1%), and annual family income of ≥100,000 (60.7%). Women enrolled included 65 with history of genital or anal dysplasia/cancer and 59 spouses/long-term sexual partners of patients with an HPV-related cancer. Only 40 (3.6%) of these adult participants reported ever receiving an HPV vaccine; this included 24 men and 16 women, age range 24–66 years. Most participants were not in ages targeted for HPV vaccination, but there were 83 participants who were within catch-up HPV vaccine targeted ages when approved, of whom 16 (19.3%) reported history of HPV vaccination.
At least one oncogenic HPV biomarker was found in 248 (22.4%) participants; this was in oral (n=86), blood (n=179), and both oral and blood (n=17) samples. Less than half of these were HPV16-specific (95 of 248; Figure 1), the oncogenic type known to cause the majority of HPV-OPCs.5 Excluding HPV16 E7 seropositivity, which is thought to have a lower specificity and sensitivity than E6 seropositivity18,25,26, only 41 participants (3.7% of those screened) had HPV16-specific biomarkers, including 20 with oral HPV16 DNA and 22 with HPV16 E6 antibodies(Table 2). Only one participant had both HPV16 biomarkers(Figure 1). Pairwise comparison of concordance between rinse and serum biomarkers for HPV16 was poor for all comparisons (all kappa<0.14). Further, concordance between HPV16 E6 and E7 seropositivity was low, with only 2 of 77 participants with HPV16 antibodies being seropositive for both E6 and E7 (Figure 1).
Figure One.
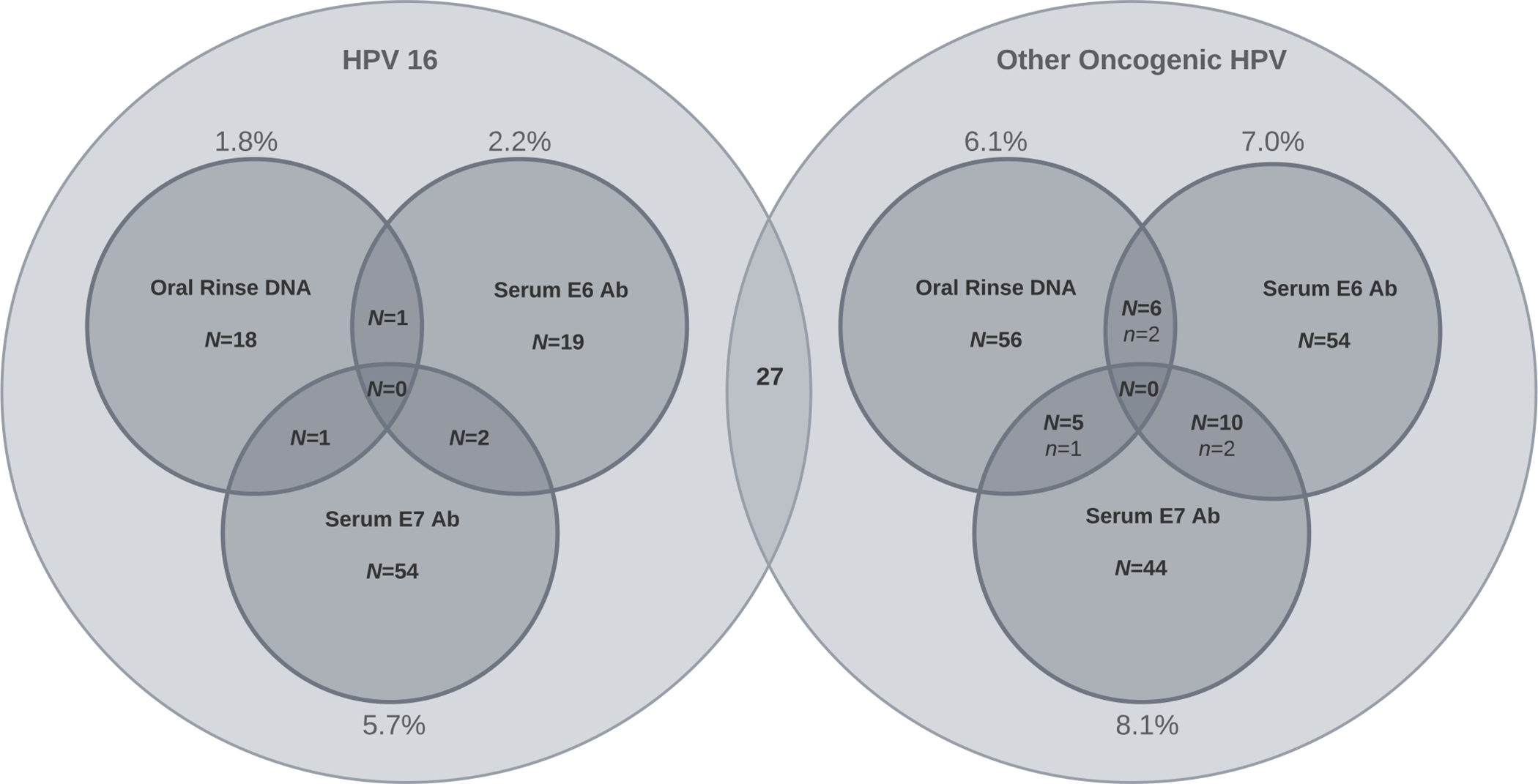
Depiction of number of people with oral and serum HPV biomarker positivity, by HPV type. This includes 95 people with HPV16 biomarkers on the left, and 175 people with ≥1 non-16 other oncogenic HPV biomarker on the right. This includes 27 people with both HPV16 and ≥1 non-16 oncogenic HPV markers (these 27 people are shown in both circles, ie circles are not mutually exclusive). Number of people who had positivity for multiple biomarkers is shown in intersection of the smaller circles with a large N. Number of people with multiple markers of the same HPV type are shown in the intersections with a small n).
Table 2.
| Biomarker | N (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| All participants N=1108 | History of anogenital dysplasia/ cancer N=72 | Partner of HPV related cancer case N=94 | Men 30 years and older with 2 or more lifetime oral sex partners N=942 | P-value~ | |
|
| |||||
| ORAL RINSE : Number tested | N=1105 | N=72 | N=94 | N=939 | |
| Any oncogenic HPV DNA | 81* (7.3) | 6 (8.3) | 6 (6.4) | 69 (7.4) | 0.89 |
| HPV16 DNA (by DEIA/LiPA or Cobas) | 20 (1.8) | 1 (1.4) | 1 (1.1) | 18 (1.9) | 0.81 |
| Any other oncogenic HPV DNA (by DEIA/LiPA or Cobas) | 67 (6.1) | 5 (6.9) | 5 (5.3) | 57 (6.1) | 0.91 |
| HPV 18 DNA (by DEIA/LiPA) | 7 (0.6) | 2 (2.8) | 0 | 5 (0.5) | 0.11 |
| HPV 31 DNA (by DEIA/LiPA) | 7 (0.6) | 1 (1.4) | 1 (1.1) | 5 (0.5) | 0.28 |
| HPV 33 DNA (by DEIA/LiPA) | 6 (0.5) | 2 (2.8) | 0 | 4 (0.4) | 0.08 |
| HPV 51 DNA (by DEIA/LiPA) | 15 (1.4) | 1 (1.4) | 1 (1.1) | 13 (1.4) | 0.97 |
| Any oncogenic HPV RNA (by Aptima)o | 20 (1.8) | 3 (4.2) | 0 | 17 (1.8) | 0.13 |
| Any oncogenic HPV DNA or RNA | 86 (7.8) | 7 (9.7) | 6 (6.4) | 73 (7.7) | 0.73 |
|
| |||||
| SERUM ANTIBODIES: Number tested | N=997 | N=63 | N=83 | N=851 | |
| Any oncogenic HPV E6 or E7# antibodies | 179* (22.9) | 12 (25.5) | 11 (18.3) | 156 (23.2) | 0.63 |
| HPV16 E6 or E7 | 77 (7.7) | 7 (11.1) | 6 (7.2) | 64 (7.5) | 0.58 |
| Other oncogenic HPV E6 or E7# | 119 (15.7) | 7 (15.6) | 7 (12.1) | 105 (16.0) | 0.73 |
|
| |||||
| HPV16 | |||||
| HPV16 E6 | 22 (2.2) | 2 (3.2) | 2 (2.4) | 18 (2.1) | 0.85 |
| HPV16 E7 | 57 (5.7) | 6 (9.5) | 4 (4.8) | 47 (5.5) | 0.39 |
| HPV16: ≥3 of: E1, E2, E6, E7 | 2 (0.2) | 0 | 0 | 2 (0.24) | 0.99 |
|
| |||||
| Other oncogenic HPV types | |||||
| Other oncogenic HPV E6 | 70 (7.0) | 2 (3.2) | 6 (7.2) | 62 (7.3) | 0.47 |
| Other oncogenic HPV E7# | 59 (8.1) | 5 (11.4) | 1 (1.8) | 53 (8.5) | 0.15 |
|
| |||||
| ELIGIBLE FOR AT-RISK COHORT | |||||
| Any oncogenic HPV: oral HPV DNA/RNA or serum E6 or E7 antibodies | 248 (22.4) | 18 (25.0) | 15 (16.0) | 215 (22.8) | 0.27 |
Oncogenic HPV strains were defined to include types 16,18,31,33,35,45,52, 58 (both serum and oral rinse), and types 39,51,56,59,66, 68 (tested in oral rinse only)
Eligibility group categories were made mutually exclusive for this table, using the following hierarchy for participants reporting multiple categories: History of anogenital dysplasia/cancer, partner of HPV related cancer case, men ≥30 years with ≥ 2 lifetime oral sex partners
This row excludes 3 samples with invalid results, thereby bringing total rinse samples with Aptima testing to N=1102
Excludes a subset of non-16 oncogenic HPV E7 results from one batch of test results, which were set to missing due to higher-than-expected prevalence.
P-value tested with chi square test for categorical variables. When any biomarker categories had <10 participants positive, Fishers exact tests used.
These include people that can be positive for either or both of the two rows listed below; as they are not counted twice here the total does not equal the sum of rows below
The prevalence of each HPV biomarker of interest is shown in Table 2. Biomarker prevalence did not vary by enrollment groups (P=0.27). While any oncogenic oral HPV DNA was detected among 7.3% of participants (n=81), oral HPV16 DNA was only found among 1.8% of participants (n=20). Oncogenic oral HPV RNA was detected among 20 participants, of which five had undetectable oncogenic HPV DNA.
Serum antibodies for oncogenic HPV E6 or E7 were detected in 22.9% (N=179) of participants (Table 2). The most common antibodies detected were to oncogenic HPV types other than HPV16, in 15.7% (N=119) of participants. HPV16 E6 and E7 antibodies were detected among 2.2% (N=22) and 5.7% (N=57) of participants, respectively. Antibodies to three or more HPV16 early proteins (E1, E2, E6, E7) were detected in only 0.2% of participants (N=2).
Next, we explored characteristics of at-risk participants compared to those not at-risk, to see whether they could be used to further enrich those at risk (Supplemental Table 2). Oral and physical health were similar in participants at-risk and not at-risk. This included similar low prevalence of infrequent dental visits (7.3% vs 5.9%) and less than daily tooth brushing (4.9% vs 5.0%), and similar prevalence of symptoms associated with pharyngeal inflammation or malignancy. Both groups also had similar medical histories, with no increase in history of genital warts, asthma, cardiovascular disease, or tonsillectomy in those at-risk. People living with HIV (PLWH) and those with a history of sexually transmitted infections (STI) were more likely to be at-risk (Supplemental Table 2). However, self-reported history of other autoimmune disorder was not associated with being at-risk.
Similarly, at-risk and not at-risk participants had comparable sexual histories and substance use (Table 3). Most at-risk participants (61.4%) had ≤10 lifetime oral sexual partners (vs 66.7% of those not at-risk). Further, 39.4% of at-risk participants had ≤5 lifetime oral sex partners (vs 43.3% of not at-risk). However, the higher range of oral sexual partners was more common among at-risk participants, with 12.6% reporting more than 50 lifetime oral sex partners (vs 7% of not at risk). The distribution of median number of lifetime oral sexual partners (P=0.14), sex-years (P=0.45) and first age of oral sex (P=0.48) were similar between the two groups (Table 3). However, higher number of oral sexual partners (≥20 vs 2–5 partners) was associated with increased oral oncogenic HPV prevalence (11.3% vs 5.8%, P=0.01) and HPV16 E6 seropositivity (4.9% vs 1.5%,P=0.02), Table 5). Median number of lifetime sexual partners (any act) was slightly higher in at-risk participants (15 vs 12, P=0.051). Tobacco, alcohol, coffee, and opioid use were not associated with being at-risk. Neither was ever or current marijuana use, although median joint-years was higher among at-risk than not at-risk marijuana users (3.7 vs 0.4, P=0.01).
Table 3.
Sexual history and substance use in study population, by at-risk determination based on HPV biomarker detection.
| N (%) | ||||
|---|---|---|---|---|
|
| ||||
| Characteristics | At-riska | Not at-riskb | P-valuec | Subset with HPV16 Biomarkers detectedd |
|
| ||||
| N=248 | N=860 | N=41 | ||
|
| ||||
| Lifetime number of people performed oral sex on | ||||
| ≤5 | 97 (39.4) | 371 (43.3) | 0.04 | 13 (32.5) |
| 6–10 | 54 (22.0) | 201 (23.4) | 9 (22.5) | |
| 11–50 | 64 (26.0) | 225 (26.3) | 13 (32.5) | |
| ≥51 | 31 (12.6) | 60 (7.0) | 5 (12.5) | |
| Marijuana use | ||||
| Never | 139 (56.0) | 459 (53.4) | 0.73 | 20 (48.8) |
| Former | 76 (30.7) | 285 (33.2) | 16 (39.0) | |
| Current | 33 (13.3) | 115 (13.4) | 5 (12.2) | |
| Cigarette use | ||||
| Never | 149 (60.1) | 512 (59.6) | 0.64 | 22 (53.6) |
| Former | 68 (27.4) | 255 (29.7) | 15 (36.6) | |
| Current | 31 (12.5) | 92 (10.7) | 4 (9.8) | |
| Ever e-cigarette use | 16 (6.5) | 76 (8.8) | 0.23 | 3 (7.3) |
| Current alcohol use | 145 (58.7) | 547 (63.7) | 0.15 | 28 (70.0) |
| Ever used an opioid pain reliever | 156 (72.2) | 516 (68.3) | 0.27 | 27 (84.4) |
|
| ||||
| Median (IQR) | Median (IQR) | |||
|
| ||||
| Lifetime number of people performed oral sex on | 8 (4,25) | 6 (4,15) | 0.14 | 9.0 (4.5,41.5) |
| Lifetime number of any sex partners | 15 (7,50) | 12 (7,30) | 0.051 | 24.5 (7.5,55) |
| Age first performed oral sex | 18 (16,21) | 18 (16,21) | 0.48 | 18.0 (16,21.5) |
| Sex-years | 4.5 (2.1,12.5) | 4.2 (2.0,8.8) | 0.45 | 7.2 (2.2,20.9) |
| Among ever marijuana users: Joint years | 3.7 (0.1, 23) | 0.4 (0, 4.3) | 0.01 | 3.2 (0.4, 25.3) |
| Among ever smokers: packyears | 15.8 (6.5, 32) | 13.4 (4.8, 25.2) | 0.37 | 15.6 (8.5, 26.6) |
| Among ever drinkers: Drink years | 10.3 (5.6, 44.4) | 14.7 (5.1, 38.4) | 0.25 | 7.5 (5.9,18.8) |
Among At-risk, N=17 had both positive rinse and blood, while 69 were categorized as positive based only on rinse, and 162 categorized as positive based only on blood.
Among those Not at-risk, N=758 were categorized based on both rinse and blood results being negative, 99 based on rinse only being negative (since blood not collected or results not yet available) and 3 based on blood only being negative (since rinse not collected or results not yet available).
P-value was tested for differences using chi-squared for categorical variables and K-sample equality-of-median tests for continuous variables
To be classified as positive in this group, needed to have either HPV16 oral DNA and/or HPV16 E6 seropositivity. 114 people who only had oral rinse (n=111) or only had blood (n=3) results for HPV16 were classified for this group by that single test.
Table 5.
Comparison of oncogenic HPV biomarkers in study population (MOUTH) to the general population (NHANES and HPV Cancer Cohort Consortium [HPVC3])
| Prevalence* | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Any oncogenic oral HPV DNA | Oral HPV16 DNA | Any serum oncogenic HPV E6 antibodies a | HPV16 E6 serum antibodies | |||||
|
| ||||||||
| MOUTH N=1105 |
NHANES b N=4,546 |
MOUTH N=1105 |
NHANES b N=4,546 |
MOUTH N=997 |
HPVC3 e N=5794 |
MOUTH N=997 |
HPVC3 e N=5794 |
|
|
| ||||||||
| Overall | 7.3%* | 3.5%* | 1.8%* | 0.9%* | 8.8% | 7.4% | 2.2%* | 0.3%* |
|
| ||||||||
| By Sex | ||||||||
| Men | 8.0%* | 5.8%* | 2.0% | 1.6% | 8.9% | 7.6% | 2.1%* | 0.3%* |
| Women | 1.7% | 1.2% | 0.0% | 0.25% | 7.9% | 6.9% | 3.0%* | 0.4%* |
|
| ||||||||
| By HIV Status | ||||||||
| Living with HIV c | 22.7% | -- | 5.2% | -- | 15.6% | 4.4% | -- | |
| Living without HIV | 5.9%* | 3.4%* | 1.5%* | 0.69%* | 8.2% | 2.0% | -- | |
|
| ||||||||
| By Number of People Performed Oral Sex on in Lifetime | ||||||||
| 0–1 d | 0% | 0.54% | 0% | 0.18% | 5.0% | 5.0% | -- | |
| 2–5 | 5.8%* | 3.9%* | 1.3% | 0.54% | 7.1% | 1.5% | -- | |
| 6–19 | 6.9%* | 3.6%* | 1.6% | 1.1% | 7.7% | 0.9% | -- | |
| ≥20 | 11.3%* | 9.2%* | 3.1% | 3.2% | 13.5% | 4.9% | -- | |
These prevalence estimates were significantly different (P<0.05)
Prevalence more than doubled when antibodies to any oncogenic E6 or E7 were included and was high among both MOUTH (22.9%) and HPVC3 (21.5%) suggesting low specificity when including E7 seropositivity.
weighted NHANES prevalence using 2015–16 NHANES data among 24 to 69 year olds
NHANES had <20 people living with HIV (PLWH) with oral HPV data available, so estimates are not presented in this group since they are underpowered
There were only 20 people in mouth with 0–1 lifetime oral sex partners (1 man and 19 women), as one of the largest eligibility groups was men with at least 2 lifetime oral sexual partners, but men and women with fewer partners could meet other eligibility group criteria to enter. Therefore estimates in this MOUTH category have high uncertainty rates; for example, seroprevalence of HPV16 E6 antibodies in this category is based upon 1 seropositive among 20.
Non-cancer controls in nine cohort studies in the United States, Europe, and Australia with blood samples collected between 1972 and 2009, matched by age, sex, study, and date of blood collection to cases who developed HPV-related cancer in that cohort (see Robbins et al. JCO. 2022). Median age of 57 years (range 18–86), 66% ever smokers.
In univariate analysis, sex, race, education, number of lifetime oral sexual partners, joint-years, and living with HIV were associated with increased odds of oncogenic HPV biomarker positivity (Table 4). Although the women included in the study had either a history of anogenital disease or a long-term partner with an HPV-related cancer, men were still more likely than women to have oncogenic HPV biomarkers detected (OR=1.75, P=0.04). In adjusted analysis (Table 4), only living with HIV (aOR=2.65, P<0.0001) and older age (66–86 years vs 24–45; aOR=1.70, P=0.02) were independent significant predictors of being considered at-risk. Sex, race, education, sexual behavior, and joint-years were no longer significant predictors after adjusting for other factors. Risk factors were similar in men and women (results not shown).
Table 4:
Predictors of positivity for any of the eligibility markers at baseline among 1108 participants in the MOUTH study
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
|
| |||||
| Variable | N | OR (95%CI) | P-value | aOR (95%CI) | P-value |
|
| |||||
| DEMOGRAPHIC | |||||
| Sex | |||||
| Women | 115 | Ref | |||
| Men | 993 | 1.75 (1.02, 2.98) | 0.04 | 1.61 (0.93, 2.81) | 0.09 |
| Race and Ethnicity | |||||
| White | 791 | Ref | Ref | ||
| Black | 179 | 1.9 (1.33, 2.72) | <0.001 | 1.41 (0.92, 2.15) | 0.11 |
| Hispanic | 85 | 0.92 (0.52,1.63) | 0.78 | 0.95 (0.53, 1.73) | 0.88 |
| Other | 53 | 1.56 (0.84, 2.92) | 0.16 | 1.79 (0.94, 3.44) | 0.08 |
| Age (in years) | |||||
| 24–45 | 255 | Ref | Ref | ||
| 46–55 | 279 | 1.06 (0.68, 1.63) | 0.79 | 1.00 (0.64, 1.57) | 0.99 |
| 56–65 | 358 | 1.44 (0.98, 2.14) | 0.07 | 1.34 (0.88, 2.03) | 0.17 |
| 66–86 | 216 | 1.47 (0.95, 2.28) | 0.08 | 1.70 (1.07, 2.70) | 0.02 |
| Education | |||||
| Advanced/Professional Degree | 465 | Ref | |||
| Any College | 469 | 1.37 (1.00, 1.86) | 0.05 | ||
| ≤High school/GED | 170 | 1.32 (0.87, 2.01) | 0.19 | ||
| SELF REPORTED MEDICAL HISTORY | |||||
| Living with HIV | 1100 | 3.27 (2.13, 5.02) | <0.001 | 2.65 (1.60, 4.40) | <0.0001 |
| Ever received HPV vaccine | 1108 | 0.73 (0.31, 1.66) | 0.45 | ||
| SEXUAL HISTORY | |||||
| Lifetime oral sex partners | |||||
| ≤5 | 468 | Ref | Ref | ||
| 6–10 | 255 | 1.03 (0.71, 1.49) | 0.88 | 0.99 (0.68, 1.46) | 0.98 |
| 11–50 | 289 | 1.09 (0.76, 1.55) | 0.64 | 1.02 (0.71, 1.47) | 0.91 |
| ≥51 | 91 | 1.97 (1.21, 3.21) | 0.006 | 1.49 (0.88, 2.50) | 0.14 |
|
| |||||
| SUBSTANCE USE | |||||
| Cigarette use | |||||
| Never | 661 | Ref | |||
| Former | 323 | 0.92 (0.66, 1.26) | 0.59 | ||
| Current | 123 | 1.16 (0.74, 1.80) | 0.52 | ||
| Pack years (per pack-year) | 1046 | 1.002 (0.99, 1.01) | 0.67 | ||
| Current alcohol use | 1106 | 0.81 (0.61, 1.08) | 0.15 | ||
| Ever Opioid Use | 972 | 1.20 (0.86 1.69) | 0.27 | ||
| Joint years | |||||
| 0 | 688 | Ref | |||
| >0–5 | 90 | 0.95 (0.55, 1.63) | 0.86 | ||
| >5–20 | 28 | 1.42 (0.61, 3.30) | 0.41 | ||
| >20 | 41 | 2.27 (1.18, 4.37) | 0.01 | ||
Lastly, we compared the prevalence of oncogenic HPV biomarkers in our study population to that in the general population to evaluate how well eligibility criteria for the study enriched for those at-risk. Prevalence of oncogenic oral HPV infection among study participants was twice that of the general U.S. population, as measured in NHANES (Table 5; 7.3% vs. 3.5%, P<0.001). Despite a greater proportion of men in the study population, oral HPV prevalence remained higher than the general population when stratified by sex (Table 5). The prevalence of HPV16 E6 antibodies (2.2% vs 0.3%, P<0.001, Table 5) was also elevated in the study population compared to control participants in HPVC3. Although prevalence was not significantly different for any oncogenic HPV E6 (8.8% vs 7.4%, P=0.12) or any oncogenic HPV E6 or E7 (22.9% vs 21.5%, P=0.32). Comparing men and women within our study population, HPV16 E6 prevalence was similar (2.1% vs 3.0%, P=0.20; similarly, in HPVC3 prevalence was no different by sex (0.3% vs 0.4%, P=0.36).
Discussion
This analysis was designed to evaluate how well we identified a subset of the population enriched with oncogenic HPV biomarkers, that is at-risk for development of OPC. We successfully identified many individuals with oncogenic oral HPV DNA or serum antibodies to oncogenic HPV, but prevalence of HPV16-specific biomarkers (which are thought to be most associated with HPV-OPC), was low. Prevalence of oral27,28 and serum29 oncogenic HPV biomarkers was elevated among all enrollment groups, compared to the general population (Table 5). Of note, the differences in prevalence between the MOUTH and NHANES study populations indicate successful enrichment for individuals at risk which reflects the eligibility criteria of the study. However, among those screened, there were no clear predictors of biomarker positivity, suggesting that stratification beyond the eligibility criteria of the study, by sexual behaviors or demographics, would not further enrich for a population at higher risk for HPV-OPC. Therefore, eligibility factors in this study may be used for future screening trials.
Among those screened, at-risk and not at-risk participants were similar in demographic and behavioral profiles. Indeed, some participants with low number of oral sex partners had the HPV biomarkers of interest, and 39.4% of at-risk participants reported 5 or fewer lifetime oral sexual partners. This underscores that while prevalence of oral HPV DNA, and thus HPV-OPC risk, increases with increasing number of lifetime oral sex partners, the majority (~80%) of HPV-OPC cases are people who have <10 lifetime oral sexual partners14. Thus, while higher range of sexual partners as a criteria may enrich for HPV-OPC biomarkers, it would likely exclude the majority of people who eventually develop HPV-OPC.
Additionally, oral health, medical histories, and symptoms of pharyngeal inflammation or malignancy were similar between those at-risk and those not at-risk. This is expected as symptoms of pharyngeal inflammation typically indicate the presence of lesions, and neither prevalent oncogenic HPV DNA nor HPV-related cancers at presentation are usually accompanied by symptoms. The diagnosis of HIV was the only medical factor associated with increased biomarker positivity. This is consistent with previous research suggesting living with HIV, and specifically HIV-related immunosuppression, is associated with increased oral HPV incidence and persistence12,30, as well as increased risk for anogenital HPV persistence and cancer31–33.
While oral HPV DNA is a recognized precursor to HPV-OPC,34 screening for it alone would have a poor balance of benefits and harms. The majority of oral HPV DNA clears, which would result in a high false positive rate and a low positive predictive value (PPV).18 Positivity of multiple markers could suggest increased risk, but concordance of biomarkers was low, consistent with prior studies.18 Only 17 people had both oral and serum HPV markers, and only one was positive for oral HPV16 DNA and HPV16 E6 antibodies. HPV16 E6 may exceed oral HPV in performance characteristics, due to its stability over the years between seroconversion and clinical presentation of disease35. The Hamburg study, the largest published screening study to date (n=4424), used seropositivity against HPV16 E6 and at least one other early protein to identify an at-risk population. Of 35 E6 seropositive subjects (median follow-up of 4.7 years), two advanced stage and one early stage case were identified, highlighting the limited PPV of serology.36
The number needed to screen (NNTS) to prevent one death is a metric used to evaluate screening implementation feasibility. For common cancers such as colon, prostate and breast cancer the NNTS is 1300–2500.37–39 However, If HPV16 E6 seroprevalence were used for screening, its low prevalence would imply a high NNTS. This may reduce feasibility, despite easier administration of a HPV16 E6 blood-based test compared to more invasive screening practices, as one would have to screen a large population to identify those at risk. A higher NNTS might be appropriate with assays having high specificity and predictive value. Multiple assays in concert or in sequence may identify a more enriched population at high-risk of malignancy. For example, methylation markers of oncogenesis and detection of ctDNA with E6 seropositivity may afford improved performance characteristics.40,41 Nevertheless, this study shows that behavioral characteristics used as entry criteria can be applied to screening studies to enrich for a population with biomarkers associated with increased risk of HPV-OPC. Defining risk eligibility informs who might benefit from screening or alternatively who is at increased risk for the malignancy of interest. Future studies are needed to explore whether alternate or additional biomarkers can be incorporated to improve enrichment of an at-risk population.
In conclusion, the entry criteria for this study (gender, sexual behavior, and personal history and exposure to HPV-related dysplasia/cancer) may be used to enrich for a population with higher HPV biomarker prevalence. Whether or not this level of enrichment is sufficient for screening in the future remains to be understood.
Supplementary Material
Acknowledgements:
Authors thank the HPV Cancer Cohort Consortium for sharing data on sex-specific HPV seroprevalence, and Maura Gillison and Anil Chaturvedi for oral HPV testing performed in NHANES and used as publicly available data for this analysis.
Funding:
Supported by R35DE026631 (NIDCR, NIH), and 5U01CA195603.
Footnotes
COI: These authors have no disclosures: AD, SRT, TT, HAR, MJ, CF, LS BAM. TW serves on advisory boards for MSD (Merck) Sharp & Dohme.
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
References
- 1.Damgacioglu H, Sonawane K, Zhu Y, et al. Oropharyngeal Cancer Incidence and Mortality Trends in All 50 States in the US, 2001–2017. JAMA Otolaryngol-- Head Neck Surg. 2022;148(2):155–165. doi: 10.1001/jamaoto.2021.3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2022. Published 2022. Accessed October 24, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf
- 3.Yom SS, Torres-Saavedra P, Caudell JJ, et al. Reduced-Dose Radiation Therapy for HPV-Associated Oropharyngeal Carcinoma (NRG Oncology HN002). J Clin Oncol Off J Am Soc Clin Oncol. 2021;39(9):956–965. doi: 10.1200/JCO.20.03128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris RL, Flamand Y, Weinstein GS, et al. Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311). J Clin Oncol Off J Am Soc Clin Oncol. 2022;40(2):138–149. doi: 10.1200/JCO.21.01752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott-Wittenborn N, D’Souza G, Tewari S, et al. Prevalence of human papillomavirus in head and neck cancers at tertiary care centers in the United States over time. Cancer. 2022;128(9):1767–1774. doi: 10.1002/cncr.34124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011 [DOI] [PubMed] [Google Scholar]
- 7.Malm IJ, Fan CJ, Yin LX, et al. Evaluation of proposed staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. Cancer. 2017;123(10):1768–1777. doi: 10.1002/cncr.30512 [DOI] [PubMed] [Google Scholar]
- 8.Marcus PM, Freedman AN, Khoury MJ. Targeted Cancer Screening in Average-Risk Individuals. Am J Prev Med. 2015;49(5):765–771. doi: 10.1016/j.amepre.2015.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomans-Kropp HA, Umar A. Cancer prevention and screening: the next step in the era of precision medicine. Npj Precis Oncol. 2019;3(1):1–8. doi: 10.1038/s41698-018-0075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Souza G, McNeel TS, Fakhry C. Understanding personal risk of oropharyngeal cancer: risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol. 2017;28(12):3065–3069. doi: 10.1093/annonc/mdx535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beachler DC, Weber KM, Margolick JB, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2012;21(1):122–133. doi: 10.1158/1055-9965.EPI-11-0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Souza G, Clemens G, Strickler HD, et al. Long-term Persistence of Oral HPV Over 7 Years of Follow-up. JNCI Cancer Spectr. 2020;4(5):pkaa047. doi: 10.1093/jncics/pkaa047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirghani H, Sturgis EM, Aupérin A, Monsonego J, Blanchard P. Is there an increased risk of cancer among spouses of patients with an HPV-related cancer: A systematic review. Oral Oncol. 2017;67:138–145. doi: 10.1016/j.oraloncology.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 14.Drake VE, Fakhry C, Windon MJ, et al. Timing, number and type of sexual partners associated with risk of oropharyngeal cancer. Cancer. 2021;127(7):1029–1038. doi: 10.1002/cncr.33346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner N, Mentzer AJ, Hill M, et al. Characterization of human papillomavirus (HPV) 16 E6 seropositive individuals without HPV-associated malignancies after 10 years of follow-up in the UK Biobank. EBioMedicine. 2020;62:103123. doi: 10.1016/j.ebiom.2020.103123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Waterboer T, Pawlita M, et al. Human Papillomavirus (HPV) 16 E6 seropositivity is elevated in subjects with oral HPV16 infection. Cancer Epidemiol. 2016;43:30–34. doi: 10.1016/j.canep.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Study MOUTH - Featured Clinical Trial: Johns Hopkins Kimmel Cancer Center. Men and women Offering Understanding of Throat HPV (MOUTH) Study. Supported by NIDCR R35 DE026631. Accessed August 31, 2022. http://bit.ly/MOUTHStudy [Google Scholar]
- 18.D’Souza G, Clemens G, Troy T, et al. Evaluating the Utility and Prevalence of HPV Biomarkers in Oral Rinses and Serology for HPV-related Oropharyngeal Cancer. Cancer Prev Res Phila Pa. 2019;12(10):689–700. doi: 10.1158/1940-6207.CAPR-19-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The American Cancer Society Guidelines for the Prevention and Early Detection of Cervical Cancer. Accessed August 31, 2022. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/cervical-cancer-screening-guidelines.html
- 20.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51(10):1845–1853. doi: 10.1373/clinchem.2005.052381 [DOI] [PubMed] [Google Scholar]
- 21.Clifford GM, Shin HR, Oh JK, et al. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2007;16(9):1874–1879. doi: 10.1158/1055-9965.EPI-07-0349 [DOI] [PubMed] [Google Scholar]
- 22.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708–2715. doi: 10.1200/JCO.2012.47.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NHANES 2015–2016 Data Documentation, Codebook, and Frequencies. Human Papillomavirus (HPV) - Oral Rinse (ORHPV_I). Accessed July 7, 2022. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/ORHPV_I.htm
- 24.Robbins HA, Ferreiro-Iglesias A, Waterboer T, et al. Absolute Risk of Oropharyngeal Cancer After an HPV16-E6 Serology Test and Potential Implications for Screening: Results From the Human Papillomavirus Cancer Cohort Consortium. J Clin Oncol. Published online June 14, 2022:JCO2101785. doi: 10.1200/JCO.21.01785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anantharaman D, Gheit T, Waterboer T, et al. Human papillomavirus infections and upper aero-digestive tract cancers: the ARCAGE study. J Natl Cancer Inst. 2013;105(8):536–545. doi: 10.1093/jnci/djt053 [DOI] [PubMed] [Google Scholar]
- 26.Hibbert J, Halec G, Baaken D, Waterboer T, Brenner N. Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis. Cancers. 2021;13(12):3010. doi: 10.3390/cancers13123010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaturvedi AK, Graubard BI, Broutian T, et al. NHANES 2009–2012 Findings: Association of Sexual Behaviors with Higher Prevalence of Oral Oncogenic Human Papillomavirus Infections in U.S. Men. Cancer Res. 2015;75(12):2468–2477. doi: 10.1158/0008-5472.CAN-14-2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PloS One. 2014;9(1):e86023. doi: 10.1371/journal.pone.0086023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang Kuhs KA, Anantharaman D, Waterboer T, et al. Human Papillomavirus 16 E6 Antibodies in Individuals without Diagnosed Cancer: A Pooled Analysis. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2015;24(4):683–689. doi: 10.1158/1055-9965.EPI-14-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Aar F, Mooij SH, van der Sande MAB, et al. Twelve-month incidence and clearance of oral HPV infection in HIV-negative and HIV-infected men who have sex with men: the H2M cohort study. BMC Infect Dis. 2014;14:668. doi: 10.1186/s12879-014-0668-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS Lond Engl. 2018;32(6):795–808. doi: 10.1097/QAD.0000000000001765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donà MG, Giuliani M, Rollo F, et al. Incidence and clearance of anal high-risk Human Papillomavirus infection and their risk factors in men who have sex with men living with HIV. Sci Rep. 2022;12(1):184. doi: 10.1038/s41598-021-03913-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly H, Chikandiwa A, Alemany Vilches L, Palefsky JM, de Sanjose S, Mayaud P. Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta-analysis. Lancet HIV. 2020;7(4):e262–e278. doi: 10.1016/S2352-3018(19)30434-5 [DOI] [PubMed] [Google Scholar]
- 34.Agalliu I, Gapstur S, Chen Z, et al. Associations of Oral α-, β-, and γ-Human Papillomavirus Types With Risk of Incident Head and Neck Cancer. JAMA Oncol. Published online January 21, 2016. doi: 10.1001/jamaoncol.2015.5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreimer AR, Ferreiro-Iglesias A, Nygard M, et al. Timing of HPV16-E6 antibody seroconversion before OPSCC: findings from the HPVC3 consortium. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(8):1335–1343. doi: 10.1093/annonc/mdz138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busch CJ, Hoffmann AS, Viarisio D, et al. Detection of stage I HPV-driven oropharyngeal cancer in asymptomatic individuals in the Hamburg City Health Study using HPV16 E6 serology - A proof-of-concept study. EClinicalMedicine. 2022;53:101659. doi: 10.1016/j.eclinm.2022.101659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317(7154):307–312. doi: 10.1136/bmj.317.7154.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabar L, Vitak B, Yen MFA, Chen HHT, Smith RA, Duffy SW. Number needed to screen: lives saved over 20 years of follow-up in mammographic screening. J Med Screen. 2004;11(3):126–129. doi: 10.1258/0969141041732175 [DOI] [PubMed] [Google Scholar]
- 39.Loeb S, Vonesh EF, Metter EJ, Carter HB, Gann PH, Catalona WJ. What is the true number needed to screen and treat to save a life with prostate-specific antigen testing? J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(4):464–467. doi: 10.1200/JCO.2010.30.6373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliano AR, Nedjai B, Lorincz AT, et al. Methylation of HPV 16 and EPB41L3 in oral gargles: Associations with oropharyngeal cancer detection and tumor characteristics. Int J Cancer. 2020;146(4):1018–1030. doi: 10.1002/ijc.32570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostareli E, Holzinger D, Bogatyrova O, et al. HPV-related methylation signature predicts survival in oropharyngeal squamous cell carcinomas. J Clin Invest. 2013;123(6):2488–2501. doi: 10.1172/JCI67010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


