Abstract
Background:
The objective of this study was to evaluate the performance of the Healthy Aging Brain Care Monitor (HABC-M) as a patient-reported outcome tool to measure cognitive, functional, and psychological symptoms among older adults who sustained non-neurologic injuries requiring hospital admission.
Materials and Methods:
We used data from a multicenter randomized controlled trial to evaluate the utility of the HABC-M Self-Report version in older patients recovering from traumatic injuries. A total of 143 patients without cognitive impairment were included in the analysis. Cronbach’s alpha was used to measure the internal consistency, and Spearman’s rank correlation test was used to evaluate the relationship of the HABC-M with standard measures of cognitive, functional, and psychological outcomes.
Results:
The HABC-M subscales and the total scale showed satisfactory internal consistency (Cronbach’s alpha = 0.64 to 0.77). The HABC-M cognitive subscale did not correlate with the Mini-Mental State Examination. The HABC-M functional and psychological subscales correlated with corresponding standard reference measures (∣rs∣ = 0.24-0.59).
Conclusions:
The HABC-M Self-Report version is a practical alternative to administering multiple surveys to monitor functional and psychological sequelae in older patients recovering from recent non-neurologic injuries. Its clinical application may facilitate personalized, multidisciplinary care coordination among older trauma survivors without cognitive impairment.
Keywords: health-related quality of life, long-term care, multidisciplinary care team, patient-reported outcome measure, traumatic injury
INTRODUCTION
For many survivors of traumatic injuries, hospital discharge marks the beginning, not the end, of the arduous road to recovery. Injury survivors suffer from various cognitive, functional, behavioral, and psychological symptoms.1-9 At the end of one year, up to 50% of injury survivors do not return to work,1 and up to 40% report behavioral and psychological symptoms due to anxiety, depression, or post-traumatic stress disorder2-6 regardless of the injury mechanism7 or severity.8 Further, recovery after injury is not static, and patients experience different psychological and functional recovery trajectories in the year after injury.10
Over the past decade, collaborative care models have emerged as a promising approach to managing traumatic injury survivors with complex recoveries.11,12 One key component of effective collaborative care models is the ability to monitor cognitive, functional, behavioral, and psychological symptoms. The SF-36 is the most commonly used patient-reported outcome tool in the trauma literature.13,14 However, this was originally developed for community-dwelling younger adults aged less than 6215 and may not be useful among individuals with physical or cognitive impairment.16 Meanwhile, trauma-specific tools such as the Trauma Outcome Profile17 and the trauma-specific quality-of-life questionnaire18 lack cognitive domain and are often lengthy, limiting their widespread adoption. The brief version of the trauma-specific quality-of-life questionnaire19 is more practical although its clinical utility has not been described yet.
The Healthy Aging Brain Care Monitor (HABC-M) is a 27-item survey that evaluates cognitive, functional, behavioral, and psychological symptoms in 5 minutes.20 Both the Caregiver and Self-Report Versions of the HABC-M have been validated in older adults with cognitive or mood disorders21,22 and survivors of critical illness.20,23 They also have been used longitudinally in real-world clinical environments to care for these patients.24-26 These real-world implementations of the HABC-M demonstrate the potential adaptability of the HABC-M in different contexts including the longitudinal follow-up of trauma survivors. The objective of this study was to evaluate the performance of the HABC-M Self-Report version in patients who sustained traumatic injuries. We hypothesized that the HABC-M would demonstrate internal consistency and correlate well with widely used measures of cognitive, functional, behavioral, and psychological outcomes in older adults who sustained non-neurologic injuries requiring hospital admission.
MATERIALS AND METHODS
Study Design
This was a cross-sectional study of data from a multicenter randomized controlled trial that is evaluating the effectiveness of Trauma Medical Home, a collaborative care model to improve the recovery of trauma survivors. The study protocol of the Trauma Medical Home trial has been published elsewhere.26 In this trial, patients in the intervention arm receive longitudinal follow-up with multidisciplinary, collaborative care interventions after hospital discharge. The HABC-M Self-Report Version is administered at multiple time points to monitor multidomain symptoms, their severity, and response to treatment, serving as the guiding principle to personalize care until symptom resolution.
We used the HABC-M scores obtained during the first outpatient visits from patients randomized to the Trauma Medical Home intervention arm to examine the internal consistency and relationships with seven widely used measurement tools for cognitive, functional, behavioral, and psychological outcomes. The reporting of this study followed the Standards for Reporting of Diagnostic Accuracy Studies (STARD) statement.27 The institutional review board of Indiana University granted study approval (IRB number 1612690852).
Participants
Four level-one trauma centers in the United States participated in the trial. The participating sites (Indiana University Health Methodist Hospital, Sidney & Lois Eskenazi Hospital, St. Vincent Hospital in Indianapolis, IN, and University of Wisconsin Health University Hospital in Madison, WI) treat over 10,000 injured patients combined annually. A total of 448 English-speaking adults aged 50 years or older were recruited from October 2017 to September 2021. Patients were eligible if they were admitted to the hospital for traumatic injury, had an injury severity score28 of 9 or greater, and had access to a telephone after discharge. Patients were excluded if they had a significant head injury (defined as any intracranial blood on imaging studies or Glasgow Coma Scale score of less than 13), spinal cord injury with a persistent neurologic deficit upon hospital discharge, a stroke upon admission or while hospitalized, baseline cognitive impairment (defined as any mention of dementia or Alzheimer disease in the medical record), sensory impairment that would preclude active participation with study assessments or communications, a burn with total body surface area >10%, recent alcohol or drug use disorder (determined from the medical record and/or the Drug Abuse Screening Test or the Alcohol Use Disorders Screening Test C within the past 6 months), were incarcerated, had malignancy with less than 1 year of life expectancy, or resided greater than 50 miles away from the admitting trauma center. Research staff identified eligible patients on a consecutive basis and approached them for enrollment before discharge. All patients or their proxies who agreed to participate in the trial provided written consent. Participants randomized to the Trauma Medical Home arm who completed a baseline HABC-M assessment were included in this analysis.
HABC-M (Self-Report Version)
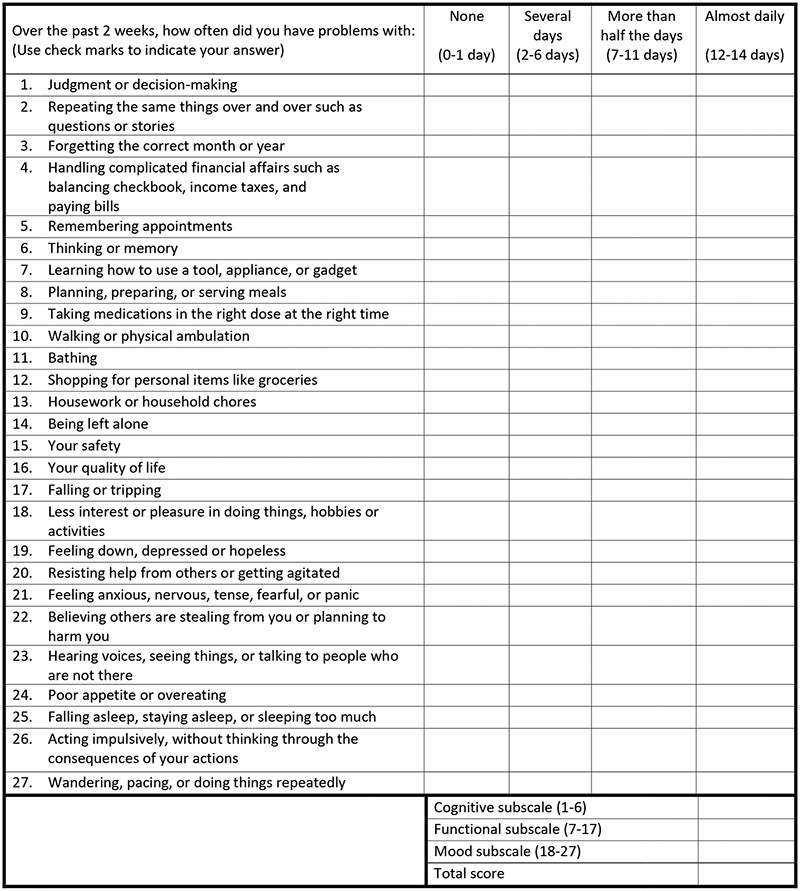
The HABC-M is a 27-item patient-reported outcome tool that evaluates cognitive, functional, behavioral, and psychological symptoms, originally developed by an interdisciplinary panel of dementia experts to measure and monitor dementia symptoms.21 It has good sensitivity to change over time among older adults with cognitive or mood disorders,21 and its use has expanded recently to survivors of critical care survivors to monitor multidomain symptoms after hospital discharge.20 Each item asks patients the frequency at which they experienced a symptom in the preceding two weeks rated on a 4-point ordinal scale: 0 = None (0-1 day), 1 = Several Days (2-6 days), 2 = More than half the days (7-11 days), 3 = Almost daily (12-14 days). There are six cognitive items, 11 functional items, and 10 behavioral and psychological items. Scores from each item are summed to calculate total scores for individual subscales and the total scale, with a higher score indicating a higher severity of symptoms (Figure 1). The Self-Report Version was chosen (as opposed to the Caregiver version) in the trial because enrolled patients were cognitively intact and did not require caregiver input.
Figure 1. Healthy Aging Brain care Monitor self-Report version.
None (0-1 day) = 0 point, Several Days (2-6 days) = 1 point, More than half the days (7-11 days) = 2 points, Almost daily (12-14 days) = 3 points
Cut scores of the HABC-M developed for older adults with cognitive or mood disorders are shown in Table 1 for reference. In the Trauma Medical Home trial, a reduction of at least 2 points in the subscale or at least 5 points in the total scale were considered a worsening of symptoms. Likewise, an increase of at least 2 points in the subscale or at least 5 points in the total scale were considered an improvement of symptoms.
Table 1.
HABC-M cut scores*
| Cognitive | Functional | Behavioral and Psychological |
Total | |
|---|---|---|---|---|
| Possible Range | 0-18 | 0-33 | 0-30 | 0-81 |
| Normal | ≤ 4 | ≤ 3 | ≤ 5 | ≤ 14 |
| Mild Symptoms | 5-8 | 4-6 | 6-7 | 15-23 |
| Moderate Symptoms | 9-11 | 7-11 | 8-11 | 24-35 |
| Severe Symptoms | ≥ 12 | ≥ 12 | ≥ 12 | ≥ 36 |
HABC-M, Healthy Aging Brain Care Monitor
These cut scores are based on data from older adults with mood disorders or cognitive impairment during their primary care visits.22 The application of these cut scores among injury survivors has not been tested.
Reference Standard Measures
We used the following seven outcome measures as the reference standards, each corresponding to either single or multiple subdomains of the HABC-M. These reference measures were chosen based on their common use and validation in the literature to capture the multifaceted effects of injuries on health-related quality of life.13,14
Mini-Mental State Examination (MMSE) is an 11-question instrument that measures patients’ cognitive function.29 A score of 24-30 indicates normal cognition. Short Form-36 Health Survey (SF-36) is the most used patient-reported outcome measure in the trauma literature13,14 that contains 36 questions. It calculates scores ranging from 0 to 100 in eight domains of health with a higher score indicating a better health status.30 Katz Index of Independence in Activities of Daily Living (Katz ADL) is a 6-item questionnaire that evaluates independence in the basic activities of daily living,31 and Lawton Instrumental Activities of Daily Living Scale (IADL) assesses independent living skills in 8 functional domains.32 Pain, Enjoyment of Life, and General Activity Scale (PEG) is a 3-item scale that asks about the pain severity and how much pain interfered with enjoyment of life and general activity on a scale of 0-10, with a higher score indicating a higher burden of pain.33 Hospital Anxiety and Depression Scale (HADS) is a tool developed to identify anxiety disorders and depression in nonpsychiatric clinical settings.34,35 It is divided into an anxiety subscale and a depression subscale, each containing seven items with a score range of 0-3 (total score range 0-21). Posttraumatic Stress Disorder (PTSD) Checklist-Civilian Version (PCL-C) is the most frequently used self-report measure of PTSD that contains a 17-item checklist of 1-5 Likert scale responses.36 The total score ranges from 17 to 85, with a higher score indicating an increased likelihood of PTSD.
The following reference measures were obtained at enrollment immediately prior to hospital discharge: the SF-36, Katz ADL, and Lawton IADL. The research assistants who collected these measures had access to electronic medical records and were not blinded to clinical information. A different research team member, a nurse care coordinator, administered the MMSE, PEG, HADS, PCL-C, and the HABC-M Self-Report Version to participants assigned to the intervention arm during the first home visit, which occurred within one month after hospital discharge (median, 16 [interquartile range, 28] days). The nurse care coordinator was blinded to the results of the three reference standards obtained at enrollment. No adverse events were reported from performing the tests.
Patient demographics, injury severity score, and Charlson Comorbidity Index were collected by a research assistant through a review of electronic medical records. Self-identified race was included in the data collection to better characterize the patient population included in this study. We categorized race into White, African-American, and Other, which included Asian, South Asian, Pacific Islander, and Native American.
Analysis
We calculated the following descriptive statistics for each HABC-M subscale and the total scale: range, median, mean, standard deviation, percentages of participants who scored at the floor (minimum score), and at the ceiling (maximum score). Cronbach’s alpha was used to estimate the internal consistency.37 We used Spearman’s rank correlation to evaluate the relationship of the HABC-M scores with the seven reference standards. Patients with missing data on HABC-M were excluded from this analysis. In addition, for three reference standards obtained prior to discharge (the SF-36, Katz ADL, and Lawton IADL), we only included data that were obtained within 14 days of the HABC-M administration. This decision was made to prioritize the comparison of assessments at closer time points because symptoms are known to change over time.10 The sample size calculation was only performed for the parent trial. All analyses were performed with SAS 9.4 (Cary, NC).
RESULTS
Participants
Two hundred sixteen patients were randomized to the Trauma Medical Home. Data from 143 patients were included in the analyses after excluding 73 who had incomplete measurements of the HABC-M due to loss to follow-up. The cohort had a mean age of 70 ± 10 years, and the majority identified themselves as non-Hispanic (99%) and White (89%). The median hospital length of stay was 7 [4-10] days. Fall was the most common mechanism of injury (53%), followed by motor vehicle crashes (29%), with a median injury severity score of 10 (interquartile range [IQR], 9-14). The patient characteristics of the 73 patients who were lost to follow-up were similar to those with a baseline HABC-M score except for the SF-36 role physical (Appendix). The results of the seven reference standard measures and the HABC-M are detailed in Table 2.
Table 2.
Distributions of Overall Patient Characteristics (n=143)
| % (n) or Median (IQR) | |
|---|---|
| Age | |
| 50-64 | 35% (50) |
| 65-79 | 44% (63) |
| 80+ | 21% (30) |
| Female | 52% (75) |
| Hispanic | 0.7% (1) |
| Race | |
| White | 89% (127) |
| African-American | 9% (13) |
| Other | 1% (2) |
| Hospital Length of Stay (days) | 7 (4-10) |
| Mechanism of Injury | |
| Fall | 53% (76) |
| Motor Vehicle Accident | 29% (42) |
| Other | 17% (25) |
| Injury Severity Score | 10 (9-14) |
| Charlson Comorbidity Index | 0 (0-2) |
| HABC-M | |
| Cognition | 0 (0-0) |
| Functional | 4 (1-9) |
| Behavioral and Psychological | 2 (0-5) |
| Total | 8 (3-13) |
| MMSEa | 27 (27-28) |
| SF-36b | |
| Physical Functioning | 20 (0-60) |
| Role Physical | 0 (0-75) |
| Role Emotional | 100 (33-100) |
| Pain | 35 (10-77.5) |
| Vitality | 50 (35-70) |
| Emotional Well Being | 76 (60-92) |
| General Health | 70 (45-85) |
| Social Functioning | 75 (37.5 – 100) |
| Katz ADLc | 6 (6-6) |
| Lawton IADLd | 8 (8-8) |
| PEGe | 4.2 (2-6.7) |
| HADSf | |
| Depression | 3 (1-4) |
| Anxiety | 2 (0-4) |
| PCL-Cg | 21 (18-24) |
IQR, interquartile range; HABC-M, Healthy Aging Brain Care Monitor; MMSE, Mini-Mental State Examination; SF-36, Short Form-36 Health Survey; Katz ADL, Katz Index of Independence in Activities of Daily Living; Lawton IADL, The Lawton Instrumental Activities of Daily Living Scale; PEG, Pain, Enjoyment of Life, and General Activity Scale; HADS, Hospital Anxiety and Depression Scale; PCL-C, Posttraumatic Stress Disorder Checklist-Civilian Version.
For MMSE, a score of 24-30 indicates normal cognition.29
For SF-36, each domain receives a score ranging from 0 to 100 with a higher score indicating a better health status.30
For Katz ADL, the score ranges from 0 to 6 with a higher score indicating a higher level of independence in the basic activities of daily living.31
For Lawton IADL, the score ranges from 0 to 8 with a higher score indicating a higher level of independence in daily living.32
For PEG, each item of the 3-item scale has a score range of 0 to 10. The average of the three items are used as the final score with a higher score indicating a higher burden of pain.33
For HADS, both the depression and anxiety subscales have a score range of 0 to 21, with a higher score indicating greater severity of symptoms.34,35
For PCL-C, the score ranges from 17 to 85, with a higher score indicating an increased likelihood of PTSD.36
Internal Consistency and Score Distributions
Cronbach’s alpha ranged from 0.64 to 0.75 for the HABC-M subscales, with the lowest value observed in the behavioral and psychological subscale (alpha = 0.64). Cronbach’s alpha for the total scale (27 items) was 0.77. The distributions of scores were positively skewed across all scales of the HABC-M. This pattern was most prominent in the cognitive subscale, where 78% of participants reported no cognitive symptoms. There were no subjects reaching the ceiling in any of the categories. (Table 3)
Table 3.
HABC-M score features: internal consistency reliability and score distributions (n=143)
| Cognitive | Functional | Behavioral and Psychological |
Total | |
|---|---|---|---|---|
| Number of Items | 6 | 11 | 10 | 27 |
| Cronbach’s Alpha | 0.75 | 0.72 | 0.64 | 0.77 |
| Possible Range | 0-18 | 0-33 | 0-30 | 0-81 |
| Observed Range | 0-11 | 0-26 | 0-15 | 0-35 |
| Median | 0 | 4 | 2 | 8 |
| Mean | 0.6 | 5.2 | 3.2 | 8.9 |
| Standard Deviation | 1.6 | 5.0 | 3.3 | 7.4 |
| % Floor | 78.3 | 20.3 | 27.3 | 12.6 |
| % Ceiling | 0 | 0 | 0 | 0 |
HABC-M, Healthy Aging Brain Care Monitor
Comparisons with the Reference Standards
Of the 143 patients with a HABC-M assessment, two did not complete MMSE, and one did not complete PEG and HADS due to refusal. Seventy-one and 69 patients completed the SF-36 and the two measures of ADL (Katz and Lawton) within 14 days of HABC-M assessment, respectively. Table 4 shows Spearman’s correlation coefficients of the HABC-M subscales and the total scale described with the seven reference standards. The HABC-M cognitive subscale and MMSE did not correlate (rs = 0.10), but the observed ranges of MMSE scores were small. The HABC-M functional subscale correlated with the SF-36 physical functioning domain and Lawton IADL (∣rs∣ = 0.24-0.25). The HABC-M behavioral and psychological subscale correlated with all corresponding reference standards (SF-36 emotional domain, PEG, HADS, and PCL-C) (∣rs∣ = 0.29-0.59). The HABC-M total scale correlated with SF-36 physical functioning, role physical, emotional well being, general health, social functioning domains, PEG, HADS, and PCL-C (∣rs∣ = 0.25-0.61).
Table 4.
Spearman’s Correlation of HABC-M and Reference Standards
| Cognitive | Functional | Behavioral and Psychological |
Total | |
|---|---|---|---|---|
| Administered at the same time as HABC-M | ||||
| MMSE (n=141) | 0.10 | −0.15 | −0.01 | −0.08 |
| PEG (n=142) | 0.19A | 0.29C | 0.34C | 0.38C |
| HADS (n=142) | ||||
| Anxiety | 0.36C | 0.18A | 0.59C | 0.43C |
| Depression | 0.23B | 0.46C | 0.57C | 0.61C |
| PCL-C (n=143) | 0.25B | 0.33C | 0.59C | 0.49C |
| Administered within 14 days of HABC-M | ||||
| SF-36 (n=71) | ||||
| Physical Functioning | −0.16 | −0.24A | −0.23 | −0.25A |
| Role Physical | −0.15 | −0.15 | −0.35B | −0.25A |
| Role Emotional | −0.14 | −0.11 | −0.25A | −0.22 |
| Pain | −0.12 | −0.05 | −0.19 | −0.10 |
| Vitality | −0.21 | −0.14 | −0.23 | −0.22 |
| Emotional Well Being | −0.21 | −0.26A | −0.29A | −0.29A |
| General Health | −0.26A | −0.32B | −0.37B | −0.42C |
| Social Functioning | −0.24A | −0.26A | −0.36B | −0.34B |
| Katz ADL (n=69) | −0.08 | −0.16 | −0.17 | −0.18 |
| Lawton IADL (n=69) | −0.08 | −0.25A | −0.08 | −0.15 |
p<0.05
p<0.01
p<0.001
HABC-M, Healthy Aging Brain Care Monitor; MMSE, Mini-Mental State Examination; SF-36, Short Form-36 Health Survey; Katz ADL, Katz Index of Independence in Activities of Daily Living; Lawton IADL, The Lawton Instrumental Activities of Daily Living Scale; PEG, Pain, Enjoyment of Life, and General Activity Scale; HADS, Hospital Anxiety and Depression Scale; PCL-C, Posttraumatic Stress Disorder Checklist-Civilian Version
DISCUSSION
This is the first study to evaluate the HABC-M Self-Report Version as a practical clinical tool to measure and monitor multidomain recovery among injury survivors. Our results show that the HABC-M had a satisfactory internal consistency. Among the HABC-M subscales, the behavioral and psychological subscale correlated best with the corresponding reference standards (HADS, PCL-C, and PEG) followed by a weaker correlation in the functional subscale with the SF-36 and Lawton IADL. We observed no correlation between the HABC-M cognitive subscale and MMSE.
To estimate the internal consistency reliability of the HABC-M, we used Cronbach’s alpha, which calculates the interrelatedness of items on a scale. We found alpha values ranging from 0.64 to 0.75 for the HABC-M subscales. These were considerably lower than values > 0.8 previously reported in other patient populations.20-23 The differences may represent the heterogeneous effects of traumatic injuries—a similar injury mechanism or injury severity score could affect patients’ anatomical regions and functions differently.8 While a much lower alpha value (for example, < 0.50) raises concerns that the scale does not reliably measure the same construct, an extremely high value (for example, > 0.95) is also undesirable because that suggests unnecessary repetition or overlap of items.38 Therefore, we deemed the above alpha values sufficient evidence of internal consistency reliability. Further investigation into the responses may identify symptom clusters by different injury patterns.
Correlation coefficients are affected by the observed ranges of values: a narrow range will result in a small correlation coefficient.39 This likely explains, at least in part, the lack of correlation we observed between MMSE and the HABC-M cognitive subscale. Given that the items on the cognitive subscale were developed to measure and monitor dementia symptoms over time, it is not surprising that most patients included in this study (those without cognitive impairment) scored 0 in the cognitive subscale (78%). Future studies of the HABC-M should include patients with varying cognitive functions to fully evaluate the utility of the cognitive subscale in the trauma population.
The HABC-M behavioral and psychological subscale correlated well with HADS and PCL-C (rs = 0.57-0.59, p < 0.001) and weakly with PEG (rs = 0.34, p < 0.001) and the corresponding SF-36 subdomains (role emotional, rs = −0.25, p < 0.05; emotional well being, rs = −0.29, p < 0.05). For the HABC-M functional subscale, the correlation with SF-36 physical functioning was weak (rs = −0.24, p < 0.05). This may be because the functional subscale included questions related to daily activities rather than strictly physical functioning, therefore measuring slightly different constructs (Figure 1). Interestingly, the HABC-M functional subscale had higher correlation coefficients with HADS depression (rs = 0.46, p < 0.001), PCL-C (rs = 0.33, p < 0.001), and SF-36 General Health (rs = −0.32, p < 0.01). This is consistent with the previous literature that higher levels of anxiety and depression were associated with lower cognitive and functional status.40-42 Overall, these findings support the use of the HABC-M in injury survivors with a caveat on the cognitive subscale as described above.
Monahan et al. and Wang et al. tested the HABC-M Self-Report Version in two different patient populations: primary care clinic patients with cognitive impairment or depression22 and intensive care unit survivors with cognitive, functional, behavioral, and psychological symptoms.20 As shown in Table 5, patients in the current study scored similarly to the primary care patients with slightly higher scores in the functional subscale (indicating greater physical impairments) but had an overall lower symptom burden compared with the intensive care unit survivors. Such comparisons, although not yet validated, may be an advantage of a multidomain patient-reported outcome instrument like the HABC-M, allowing quantitative assessment of the health-related quality of life of trauma survivors within a larger context.
Table 5.
Comparison of HABC-M Scores by Patient Populations
| HABC-M Self-Report |
Trauma patients (n = 143)A |
Primary care patients22 (n = 291)B |
Critical Care Recovery Center patients20 (n = 142)C |
|||
|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Mean | Median | |
| Cognition | 0.6 (1.6) | 0 [0-0] | 1.9 (2.9) | 0 [0-3] | 3.7 (4.1) | 2 [0-6] |
| Functional | 5.2 (5.0) | 4 [1-9] | 3.2 (4.5) | 2 [0-5] | 6.3 (6.8) | 3.3 [0.6-11] |
| Behavioral and Psychological | 3.1 (3.3) | 2 [0-5] | 3.2 (4.2) | 2 [0-5] | 6.4 (6.0) | 5 [1-11] |
| Total | 8.9 (7.4) | 8 [3-13] | 8.3 (10.3) | 4 [1-12] | 16.3 (14.5) | 12.5 [3.2-27.6] |
Values are expressed as mean (standard deviation) and median [interquartile range].
HABC-M, Healthy Aging Brain Care Monitor
Mean age 70 (standard deviation, 10) years.
Mean age 73 (standard deviation, 6) years. All patients in this study were age ≥65 years and had a diagnosis of cognitive impairment or depression.
Mean age 52 (standard deviation, 13) years. All patients were adult (age ≥18 years) survivors of intensive care unit with Mini-Mental State Examination scores > 17.
Since its increased recognition in the early 1990s, the literature has continually described the unmet needs of patients recovering from traumatic injuries.2,9,13,43 The recovery process is complex and unique to each patient; it involves social, physical, psychological, economic, and environmental factors and follows various recovery trajectories.10 This calls for a longitudinal, multidisciplinary patient support structure that can promptly address individual needs. Published consensus statements recommend measuring patient-reported outcomes at multiple time points after injuries.44,45 Yet, a recent systematic review revealed that most studies on health-related quality of life reported only a single measurement.13
The potential advantage of the HABC-M over the existing tools is its demonstrated feasibility in guiding patient management longitudinally.24-26 While the HABC-M may be inferior to trauma-specific tools in measuring symptoms specific to traumatic injuries, the overarching goal of injury follow-up is to enhance the overall health of the survivors. Trauma affects every aspect of life. And given that psychological disorders and dementia are known to be underdiagnosed among older adults,46,47 a practical tool like the HABC-M may uncover conditions prompting appropriate medical attention.
Our findings suggest that the HABC-M adequately captures functional, behavioral, and psychological symptoms of older injury survivors, all in one instrument. As piloted in the Trauma Medical Home trial, a simple multidomain clinical tool like the HABC-M could facilitate care coordination for trauma survivors.26 In the future, we plan to evaluate sensitivity to change and the potential use of the HABC-M as an actionable monitoring tool—just like measuring blood pressures—for the recovery of trauma patients.
Our study has several limitations. First, the SF-36, Katz ADL, and Lawton IADL were not collected at the same time as the HABC-M. Although we limited the data to those obtained within 14 days of the HABC-M in the analysis, this could have affected the correlation coefficients between these measures and the HABC-M. Second, our study did not evaluate the sensitivity of the HABC-M scores to change because follow-up data were unavailable at the time of this writing. Sensitivity to change is a key quality for a clinical tool intended to monitor patient symptoms over time. This aspect of HABC-M was previously validated in another patient population21 and we plan to evaluate this among injury survivors in the future.
Another limitation is that the current study excluded patients with cognitive impairment or significant head injury, which could explain the lack of correlation between the MMSE and the HABC-M cognitive subscale. A follow-up study should include trauma patients across various degrees of cognitive functioning and head injury, and use the Caregiver Version for those with moderate to severe impairments. Further, most of our cohort had moderate injury according to the injury severity score, so the applicability of the HABC-M among severely injured patients remains unknown. Our sample was not ethnically or racially diverse. Although this raised some caution for its application in underrepresented minorities, previous validation studies of the HABC-M demonstrated feasibility across diverse racial groups.20-23 Lastly, the HABC-M does not include measures of social support since the items are focused on patients’ symptoms. Because social support is essential in recovery from injury,48,49 healthcare providers using this tool should actively seek out information about patients’ social circumstances, especially when they score high on any of the HABC-M subscales.
CONCLUSIONS
In summary, the HABC-M Self-Report version may be a practical alternative to administering multiple surveys to monitor functional and psychological sequelae in older patients recovering from recent non-neurologic injuries. Its clinical application may facilitate personalized, multidisciplinary care coordination among trauma survivors without cognitive impairment.
Supplementary Material
HIGHLIGHTS.
The HABC-M was used in a randomized controlled trial to guide post-injury care.
The HABC-M may be a useful tool to monitor recovery after traumatic injuries.
The use of the HABC-M cognitive subscale requires validation in future studies.
DISCLOSURE
The study was supported by a grant from the National Institute on Aging [grant number: R01AG052493]. The authors report no propriety or commercial interest related to this work.
Appendix. Comparison of Samples with and without a Baseline HABC Monitor
| Baseline HABC Monitor (n=143) |
No Baseline HABC Monitor (n=73) |
P-value | |
|---|---|---|---|
| Age | 0.995 | ||
| 50-64 | 35.0% (50) | 35.6% (26) | |
| 65-79 | 44.1% (63) | 43.8% (32) | |
| 80+ | 21.0% (30) | 20.6% (15) | |
| Female | 52.4% (75) | 53.4% (39) | 0.892 |
| Hispanic | 0.7% (1) | 1.4% (1) | 0.638 |
| Race | 0.575 | ||
| White | 89.4% (127) | 91.8% (67) | |
| African-American | 9.2% (13) | 8.2% (6) | |
| Other | 1.4% (2) | 0.0% (0) | |
| Hospital Length of Stay | 7 (4-10) | 7 (4-10) | 0.856 |
| Mechanism of Injury | 0.452 | ||
| Fall | 53.2% (76) | 57.5% (42) | |
| Motor Vehicle Accident | 29.4% (42) | 31.5% (23) | |
| Other | 17.5% (25) | 11.0% (8) | |
| Injury Severity Score | 10 (9-14) | 10 (9-14) | 0.416 |
| Charlson Comorbidity Index | 0 (0-2) | 0 (0-1) | 0.973 |
| SF-36 | |||
| Physical Functioning | 20 (0-60) | 10 (5-55) | 0.963 |
| Role Physical | 0 (0-75) | 50 (0-100) | 0.029 |
| Role Emotional | 100 (33-100) | 100 (33-100) | 0.664 |
| Pain | 35 (10-77.5) | 45 (12.5-80) | 0.309 |
| Vitality | 50 (35-70) | 50 (30-65) | 0.515 |
| Emotional Well Being | 76 (60-92) | 80 (56-88) | 0.949 |
| General Health | 70 (45-85) | 60 (45-75) | 0.215 |
| Social Functioning | 75 (37.5 – 100) | 75 (37.5-100) | 0.613 |
| Katz ADL | 6 (6-6) | 6 (6-6) | 0.888 |
| Lawton IADL | 8 (8-8) | 8 (8-8) | 0.674 |
Values are expressed as % (n) or median (interquartile range).
SF-36, Short Form-36 Health Survey; Katz ADL, Katz Index of Independence in Activities of Daily Living; Lawton IADL, The Lawton Instrumental Activities of Daily Living Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was submitted for the 81st Annual Meeting of the American Association for the Surgery of Trauma and Clinical Congress for Acute Care Surgery which will be held in September 2022.
REFERENCES
- 1.Zatzick D, Jurkovich GJ, Rivara FP, et al. A national US study of posttraumatic stress disorder, depression, and work and functional outcomes after hospitalization for traumatic injury. Ann Surg. 2008;248(3):429–437. [DOI] [PubMed] [Google Scholar]
- 2.Michaels AJ, Michaels CE, Smith JS, Moon CH, Peterson C, Long WB. Outcome from injury: general health, work status, and satisfaction 12 months after trauma. J Trauma. 2000;48(5):841–848; discussion 848-850. [DOI] [PubMed] [Google Scholar]
- 3.Soberg HL, Bautz-Holter E, Roise O, Finset A. Mental health and posttraumatic stress symptoms 2 years after severe multiple trauma: self-reported disability and psychosocial functioning. Arch Phys Med Rehabil. 2010;91(3):481–488. [DOI] [PubMed] [Google Scholar]
- 4.Shih RA, Schell TL, Hambarsoomian K, Belzberg H, Marshall GN. Prevalence of posttraumatic stress disorder and major depression after trauma center hospitalization. J Trauma. 2010;69(6):1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziobrowski HN, Kennedy CJ, Ustun B, et al. Development and Validation of a Model to Predict Posttraumatic Stress Disorder and Major Depression After a Motor Vehicle Collision. JAMA Psychiatry. 2021;78(11):1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrera-Escobar JP, Seshadri AJ, Stanek E, et al. Mental Health Burden After Injury: It's About More than Just Posttraumatic Stress Disorder. Ann Surg. 2021;274(6):e1162–e1169. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman T, Foster K, Curtis K. Mental health following traumatic physical injury: an integrative literature review. Injury. 2013;44(11):1383–1390. [DOI] [PubMed] [Google Scholar]
- 8.Halcomb E, Daly J, Davidson P, Elliott D, Griffiths R. Life beyond severe traumatic injury: an integrative review of the literature. Aust Crit Care. 2005;18(1):17-18, 20-14. [DOI] [PubMed] [Google Scholar]
- 9.Schemitsch C, Nauth A. Psychological factors and recovery from trauma. Injury. 2020;51 Suppl 2:S64–s66. [DOI] [PubMed] [Google Scholar]
- 10.Zarzaur BL, Bell T. Trajectory subtypes after injury and patient-centered outcomes. J Surg Res. 2016;202(1):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zatzick D, Jurkovich G, Rivara FP, et al. A randomized stepped care intervention trial targeting posttraumatic stress disorder for surgically hospitalized injury survivors. Ann Surg. 2013;257(3):390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wade DT, King NS, Wenden FJ, Crawford S, Caldwell FE. Routine follow up after head injury: a second randomised controlled trial. J Neurol Neurosurg Psychiatry. 1998;65(2):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritschel M, Kuske S, Gnass I, et al. Assessment of patient-reported outcomes after polytrauma - instruments and methods: a systematic review. BMJ Open. 2021;11(12):e050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrzejowski P, Holch P, Giannoudis PV. Measuring functional outcomes in major trauma: can we do better? Eur J Trauma Emerg Surg. 2021. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Brook RH, Williams KN, Stewart AI, Davies-Avery A. Conceptualization and measurement of health for adults in the health insurance study. Located at: Vol 1. Model of health and methodology. Santa Monica, California: Rand Corporation, 1980. (Publication No R-1987/1-HEW.). [Google Scholar]
- 16.Andresen EM, Gravitt GW, Aydelotte ME, Podgorski CA. Limitations of the SF-36 in a sample of nursing home residents. Age Ageing. 1999;28(6):562–566. [DOI] [PubMed] [Google Scholar]
- 17.Attenberger C, Amsler F, Gross T. Clinical evaluation of the Trauma Outcome Profile (TOP) in the longer-term follow-up of polytrauma patients. Injury. 2012;43(9):1566–1574. [DOI] [PubMed] [Google Scholar]
- 18.Wanner JP, deRoon-Cassini T, Kodadek L, Brasel K. Development of a trauma-specific quality-of-life measurement. J Trauma Acute Care Surg. 2015;79(2):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera-Escobar JP, deRoon-Cassini T, Brasel K, et al. Development and validation of a revised trauma-specific quality of life instrument. J Trauma Acute Care Surg. 2020;88(4):501–507. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Allen D, Perkins A, et al. Validation of a New Clinical Tool for Post-Intensive Care Syndrome. Am J Crit Care. 2019;28(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monahan PO, Boustani MA, Alder C, et al. Practical clinical tool to monitor dementia symptoms: the HABC-Monitor. Clin Interv Aging. 2012;7:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monahan PO, Alder CA, Khan BA, Stump T, Boustani MA. The Healthy Aging Brain Care (HABC) Monitor: validation of the Patient Self-Report Version of the clinical tool designed to measure and monitor cognitive, functional, and psychological health. Clin Interv Aging. 2014;9:2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Jawed Y, Perkins A, et al. Healthy Aging Brain Care Monitor, Caregiver Version: Screening for Post-Intensive Care Syndrome. Am J Crit Care. 2022;31(2):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaMantia MA, Alder CA, Callahan CM, et al. The Aging Brain Care Medical Home: Preliminary Data. J Am Geriatr Soc. 2015;63(6):1209–1213. [DOI] [PubMed] [Google Scholar]
- 25.Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115(3):24–31; quiz 34, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz D, Meagher AD, Lindroth H, et al. A trauma medical home, evaluating collaborative care for the older injured patient: study protocol for a randomized controlled trial. Trials. 2020;21(1):655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker SP, O'Neill B, Haddon W Jr., Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 30.Kopjar B. The SF-36 health survey: a valid measure of changes in health status after injury. Inj Prev. 1996;2(2):135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. [DOI] [PubMed] [Google Scholar]
- 32.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 33.Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 35.Hung M, Bounsanga J, Tang P, Chen W, Cheng C. The factor structure of the hospital anxiety and depression scale in orthopedic trauma patients. J Clin Med Res. 2015;7(6):453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald SD, Calhoun PS. The diagnostic accuracy of the PTSD checklist: a critical review. Clin Psychol Rev. 2010;30(8):976–987. [DOI] [PubMed] [Google Scholar]
- 37.LJ C. Coefficient alpha and the internal structure of tests. Psychometrika.1951;16(3):297–334. [Google Scholar]
- 38.Cho E K S. Cronbach’s coefficient alpha: well known but poorly understood. Organizational Research Methods. 2015;18(2):207–230. [Google Scholar]
- 39.Schober P, Boer C, Schwarte LA. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg. 2018;126(5):1763–1768. [DOI] [PubMed] [Google Scholar]
- 40.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006; 166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 42.Oyesanya M, Harmer CJ, Young AH. Editorial: Cognition in Mood Disorders. Front Psychiatry. 2019;10:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sluys K, Häggmark T, Iselius L. Outcome and quality of life 5 years after major trauma. J Trauma. 2005;59(1):223–232. [DOI] [PubMed] [Google Scholar]
- 44.Ardolino A, Sleat G, Willett K. Outcome measurements in major trauma--results of a consensus meeting. Injury. 2012;43(10):1662–1666. [DOI] [PubMed] [Google Scholar]
- 45.Van Beeck EF, Larsen CF, Lyons RA, Meerding WJ, Mulder S, Essink-Bot ML. Guidelines for the conduction of follow-up studies measuring injury-related disability. J Trauma. 2007;62(2):534–550. [DOI] [PubMed] [Google Scholar]
- 46.Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17(9):769–781. [DOI] [PubMed] [Google Scholar]
- 47.Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of Dementia: an Observational Study of Patterns in Diagnosis and Awareness in US Older Adults. J Gen Intern Med. 2018;33(7):1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carr BW, Severance SE, Bell TM, Zarzaur BL. Perceived loss of social support after non-neurologic injury negatively impacts recovery. J Trauma Acute Care Surg. 2020;88(1):113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orlas CP, Herrera-Escobar JP, Hau KM, et al. Perceived social support is strongly associated with recovery after injury. J Trauma Acute Care Surg. 2021;91(3):552–558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.