Abstract
Erectile dysfunction (ED) is the most common sexual dysfunction disease in adult males. ED can be caused by many factors, such as vascular disease, neuropathy, metabolic disturbances, psychosocial causes, and side effects of medications. Although current oral phosphodiesterase type 5 inhibitors can achieve a certain effect, they cause temporary dilatation of blood vessels with no curative treatment effects. Emerging targeted technologies, such as stem cell therapy, protein therapy, and low-intensity extracorporeal shock wave therapy (Li-ESWT), are being used to achieve more natural and long-lasting effects in treating ED. However, the development and application of these therapeutic methods are still in their infancy, and their pharmacological pathways and specific mechanisms have not been fully discovered. This article reviews the preclinical basic research progress of stem cells, proteins, and Li-ESWT therapy, as well as the current status of clinical application of Li-ESWT therapy.
Keywords: Erectile dysfunction, Extracorporeal shockwave therapies, Proteins, Regenerative medicine, Stem cell
Graphical Abstract
INTRODUCTION
Erectile dysfunction (ED) refers to the continuous inability of the penis to achieve or maintain a sufficient erection to complete a satisfactory sexual life [1]. It is estimated to affect approximately 15% of males annually [2] and is expected to affect approximately 320 million ED patients by 2025 [3]. As first-line drugs for the treatment of ED, oral phosphodiesterase type 5 (PDE5) inhibitors promote penile erection by enhancing cavernosal smooth muscle relaxation and vasodilation. However, approximately 50% of patients with severe vascular or neurogenic ED, such as diabetes, cardiovascular disease, and metabolic syndrome, have little or no response to this treatment option [4,5]. Although certain treatment modalities for ED, such as low-intensity extracorporeal shock wave therapy (Li-ESWT), platelet-rich plasma, and stem cell therapy, exert some curative effects, but they are still in their infancy. Developing new targeted technologies based on a deeper understanding of the molecular mechanisms involved in the pathogenesis of ED is needed to cure ED patients who do not respond to current medical therapy and to restore natural erection and normal sex life. To do this, a systematic understanding of the current state of preclinical and clinical research on the regenerative technology to restore damaged erectile tissue is necessary. Of those regenerative therapies, only Li-ESWT is currently used for ED patients, whereas stem cell and protein therapy have not been launched as treatment methods. Therefore, this article only reviews the preclinical research status of stem cell and protein therapy in the field of ED for the past two decades, and addresses preclinical and clinical results of Li-ESWT.
PRECLINICAL RESEARCH OF ED BIOTHERAPY
1. Cell therapy
1) Overview of cell therapy
Cell therapy is the transfer of cellular material into a patient for medical purposes through injection, transplantation, or implantation methods [6]. Cell therapy has many advantages, such as the safety of autologous stem cells, and the diverse functions of stem cells, enabling them to provide faster treatment and recovery [7]. Cell therapy is broadly divided into stem cell and non-stem cell-based single-cell and multi-cell therapies, spanning multiple therapeutic areas, such as regenerative medicine, immune diseases, and cancer [6]. Currently, most cell-based therapies are still in clinical trial phases I and II [8]. Additionally, there is little research on non-stem cell-based cell therapy, such as peripheral blood mononuclear cells [9]. Therefore, in this review, we only describe the past 20 years of research related to preclinical stem cell-based therapies for ED and their associated strengths and limitations. We also put forward possible new target cells and development research opinions.
2) Stem cell-based therapy in ED
After a systematic literature review in PubMed, we found approximately 321 publications on stem cell therapy for ED in the past two decades (2002–2022). Among them, 13 were clinical trials, 205 were research articles, 1 was a meta-analysis, and 113 were review articles. After sorting, we developed five general study categories, such as adipose-derived stem cells (ADSCs), mesenchymal stem cells (MSCs), urine-derived stem cells (USCs), placental stem cells (PSCs), muscle-derived stem cells (MDSCs). Here, only some of the most representative papers are listed as shown in Table 1 [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27].
Table 1. Preclinical cell therapy on ED biotherapeutics.
| Cell type | Source of stem cell | Type of ED | Parameter of therapy | Reference |
|---|---|---|---|---|
| ADSCs | Bilateral groin, inguinal area, epididymal adipose tissue, human adipose tissues | Type 1 diabetic ED, CNI-ED, age-associated ED | ICP-MAP, smooth muscle, nNOS, cavernosum pyroptosis, VEGFA, eNOS, growth factor | [10,11,12,13,14] |
| MSCs | Human umbilical cord, human gingiva, bone marrow | Type 1 diabetic ED, type 2 diabetic ED, CNI-ED, age-associated ED | ICP-MAP, microRNA sequencing, growth factor, eNOS. nNOS, TGF-β, smooth muscle, collagen | [15,16,17,18,19,20,21] |
| USCs | Urine | Type 2 diabetic ED, CNI-ED | ICP-MAP, morphometric assessment, smooth muscle, nNOS, collagen | [22,23] |
| PSCs | Human placental | CNI-ED | ICP-MAP, nNOS, eNOS, MPG fluorescence. | [24,25] |
| MDSCs | Gastrocnemius, abdominal muscles | Type 2 diabetic ED | Tunel assay, smooth muscle, urethral pressure profile measurement, blood vessel formation, angiogenic capacity | [26,27] |
ED, erectile dysfunction; ADSC, adipose-derived stem cell; MSC, mesenchymal stem cell; USC, urine-derived stem cell; PSC, placental stem cell; MDSC, muscle-derived stem cell; CNI, cavernous nerve injury; ICP-MAP, intracavernosal pressure-mean arterial pressure; VEGFA, vascular endothelial growth factor A; eNOS, endothelial nitric oxide synthase; nNOS, neuronal nitric oxide synthase; TGF-β, transforming growth factor-beta; MPG, major pelvic ganglion.
For ADSCs-based ED treatment, He et al. [10] showed that injection of extracted ADSCs from the bilateral groin area can repair damaged corpus cavernosum nerves and restore ED to a certain extent, while injections of ADSCs modified by overexpression of VEGF and Smad7 can prevent penile fibrosis, reduce collagen deposition, and improve relaxation properties of smooth muscle cells, thereby restoring erectile function in bilateral cavernous nerve injury (BCNI)-induced ED rats. In addition, Zhang et al. [11] and Cheng et al. [12] also isolated ADSCs from epididymal adipose tissues and human adipose tissues [11,13,14], and these isolated ADSCs can also restore erectile function in different ED models, such as type 1 diabetic, neurotic, and age-associated ED.
For MSCs-based ED treatment, most MSCs were extracted from human umbilical cords, gingivae, and bone marrows [15,16,17,18,19,20,21]. Feng et al. [15], Kang et al. [16], Mukti et al. [17], Sun et al. [18], and Wu et al. [19,20] reported that transplanting human umbilical cord-derived MSCs can improve erectile function in type 1 diabetes patients through dysregulated miRNAs, increased production of growth factors, curative effects on inflammatory response and structural improvement, and attenuation of diabetes-induced ferroptosis in cavernous smooth muscle cells. Wu et al. [19] reported that human gingiva-derived MSC transplantation could significantly improve CNI-related ED by reducing fibrosis and increasing neuronal nitric oxide synthase (nNOS) expression. Liu et al. [21] also found that microRNA-145-engineered bone marrow-derived MSCs effectively attenuated age-related ED by increasing smooth muscle content in penile tissues.
For other stem cell-based therapies, Yang et al. [22] and Galhom et al. [23] showed that transplantation of human USCs prevented impairment of erectile function and cavernous structures in a CNI-related ED rat model through neuroprotection, increased smooth muscle content, reducing fibrosis and apoptosis. Gu et al. [24] and Dou et al. [25] found that human PSC therapy can effectively erectile function after pelvic neurovascular injury. Masouminia et al. [26] and Nakajima et al. [27] reported that injection of MDSCs from pre-diabetic rats into the corpus cavernosum improved erectile function, but autologous transplantation of MDSCs in chronic type 2 diabetic patients may not be effective and should be reprogrammed in vitro or replaced by allogeneic stem cells from non-diabetic sources.
Collectively, stem cell therapy in ED is mainly based on ADSCs and MSCs, while USCs-, PSCs- and MDSCs-based therapy only account for a small proportion. However, most stem-based treatments can only restore ED to a certain extent. Most stem cells require further modification to be more effective in restoring ED from various causes. Therefore, more types of effective stem cells or engineered stem cells are needed to treat ED. Recently, cavernous pericytes have been recognized for their central role in vascular maintenance and neurovascular regeneration in ED [28,29,30]. Pericyte also have stem cell-like properties and differentiate into adipocytes, chondrocytes, osteoblasts and granulocytes [31]. Therefore, we believe that using pericytes or pericyte differentiated cells may become a breakthrough in ED treatment.
2. Protein therapy
1) Overview of protein therapy
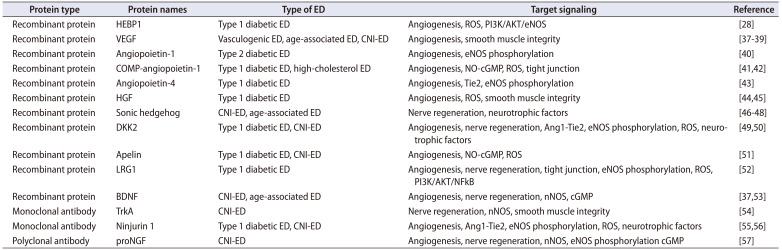
Since the first recombinant protein therapeutic drug--human insulin came out in 1978, about 239 protein therapeutic drugs have been approved by the United States Food and Drug Administration (FDA) and used clinically [32,33]. Many therapeutic proteins are in clinical trials. Initially, protein therapies were used rarely, but their use and frequency have recently dramatically increased [34]. Protein therapy has many advantages; depending on its high specificity, autologously produced proteins are well-tolerated and less likely to cause an immune response, making them an alternative to gene therapy for diseases with gene mutations or deletions [32]. Therefore, protein therapy is accepted and plays an important role in almost all areas of medicine, but this therapy is still in its infancy. Additionally, about 79 therapeutic monoclonal antibodies (mAbs), which is the main form of therapeutic protein, have been approved by the FDA [35,36]. This article summarizes some of the per-clinical research results of protein therapy for ED, summarizes currently prescribed 15 proteins or mAbs (Table 2) [28,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57], and categorizes them according to their roles in angiogenesis and nerve regeneration.
Table 2. Preclinical protein therapy on ED biotherapeutics.
| Protein type | Protein names | Type of ED | Target signaling | Reference |
|---|---|---|---|---|
| Recombinant protein | HEBP1 | Type 1 diabetic ED | Angiogenesis, ROS, PI3K/AKT/eNOS | [28] |
| Recombinant protein | VEGF | Vasculogenic ED, age-associated ED, CNI-ED | Angiogenesis, smooth muscle integrity | [37,38,39] |
| Recombinant protein | Angiopoietin-1 | Type 2 diabetic ED | Angiogenesis, eNOS phosphorylation | [40] |
| Recombinant protein | COMP-angiopoietin-1 | Type 1 diabetic ED, high-cholesterol ED | Angiogenesis, NO-cGMP, ROS, tight junction | [41,42] |
| Recombinant protein | Angiopoietin-4 | Type 1 diabetic ED | Angiogenesis, Tie2, eNOS phosphorylation | [43] |
| Recombinant protein | HGF | Type 1 diabetic ED | Angiogenesis, ROS, smooth muscle integrity | [44,45] |
| Recombinant protein | Sonic hedgehog | CNI-ED, age-associated ED | Nerve regeneration, neurotrophic factors | [46,47,48] |
| Recombinant protein | DKK2 | Type 1 diabetic ED, CNI-ED | Angiogenesis, nerve regeneration, Ang1-Tie2, eNOS phosphorylation, ROS, neurotrophic factors | [49,50] |
| Recombinant protein | Apelin | Type 1 diabetic ED, CNI-ED | Angiogenesis, NO-cGMP, ROS | [51] |
| Recombinant protein | LRG1 | Type 1 diabetic ED | Angiogenesis, nerve regeneration, tight junction, eNOS phosphorylation, ROS, PI3K/AKT/NFkB | [52] |
| Recombinant protein | BDNF | CNI-ED, age-associated ED | Angiogenesis, nerve regeneration, nNOS, cGMP | [37,53] |
| Monoclonal antibody | TrkA | CNI-ED | Nerve regeneration, nNOS, smooth muscle integrity | [54] |
| Monoclonal antibody | Ninjurin 1 | Type 1 diabetic ED, CNI-ED | Angiogenesis, Ang1-Tie2, eNOS phosphorylation, ROS, neurotrophic factors | [55,56] |
| Polyclonal antibody | proNGF | CNI-ED | Angiogenesis, nerve regeneration, nNOS, eNOS phosphorylation cGMP | [57] |
ED, erectile dysfunction; VEGF, vascular endothelial growth factor; COMP, cartilage oligomeric matrix protein; HGF, hepatocyte growth factor; BDNF, brain-derived neurotrophic factor; DKK2, dickkopf WNT signaling pathway inhibitor 2; HEBP1, heme-binding protein 1; LRG1, leucine-rich alpha-2-glycoprotein 1; TrkA, tyrosine receptor kinases; proNGF, precursor for nerve growth factor; CNI, cavernous nerve injury; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; ROS, reactive oxygen species; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; NFkB, Nuclear factor kappa B; nNOS, neuronal nitric oxide synthase.
2) Recombinant protein therapy in ED
Recombinant protein is a type of protein drug obtained through recombinant DNA or recombinant RNA technology, which has the advantages of more significant curative effect, lower toxicity, and fewer side effects [58,59]. After a systematic literature review of recombinant protein therapies in ED, we found that the use of recombinant proteins to treat ED is still in its infancy. Most protein therapies utilize angiogenic growth factors and neurotrophic factors. For example, Hsieh et al. [37] and Rogers et al. [38] initially showed that a recombinant VEGF protein or a combination of VEGF plus BDNF protein significantly restored erectile function in rat models of vasculogenic and neurotic ED. Subsequently, Park et al. [39] validated these effects of VEGF in an age-associated ED rat model. Jin et al. [40,41], Ryu et al. [42], Kwon et al. [43], Das et al. [44], and Liu et al. [45] also found that repeated intracavernous injections of angiopoietins, such as angiopoietin-1, COMP-angiopoietin-1, angiopoietin-4, and hepatocyte growth factor, can restore erectile function through inducing cavernous angiogenesis, NO-cGMP activity, and tight junction expression, as well as decreasing reactive oxygen species (ROS) production in several types of diabetic ED mice. In addition, sonic hedgehog (Shh) cascade is a critical regulator of erectile function, which is significantly reduced in diabetic and neurotic ED conditions. The Shh cascade functions to establish and maintain the sinusoidal morphology of the penis. There have been 23 related studies on ED treatment by regulating the Shh signaling pathway. The pathway has been studied in most detail by the Podlasek CA group [46,47,48]. More recently, Yin et al. [50,52,60] evaluated some pro-angiogenic proteins in various ED models by several in vivo and in vitro experiment approaches. The most representative of these is the injection of DKK2 and LRG1 recombinant proteins, which prove that these proangiogenic proteins can promote the regeneration of penile blood vessels and nerves, reduce the production of ROS, and finally restore erectile function in different ED models [49,50,51,52,60]. Throughout these studies, it is not difficult to find that most therapeutic candidate proteins are angiogenic and neurotrophic factors [53]. However, due to some limitations of protein therapeutics, such as high production costs, they could not be used as orally administered drugs, and large proteins could not effectively penetrate tissues to reach their targets. Therefore, most of them are currently concentrated in the preclinical stage. More candidate proteins need to be developed in the future, which will make important contributions to our understanding of ED mechanisms and the development of therapeutic drugs.
3) Antibody therapy in ED
Neutralizing mAbs are B cell-derived recombinant proteins. Due to their higher safety, lower toxicity, higher specificity, and single biological function, they have recently emerged as major contributors to the biopharmaceutical market [61]. However, most antibody therapeutic drugs are used for cancer, asthma, arthritis, transplant rejection, and infectious diseases. Only three studies on the use of neutralizing antibodies in treating ED have been published. Lin et al. [54] demonstrated that intracavernosal injections of tyrosine kinase receptor type 1 mAbs (TrkA-mAbs) significantly inhibits sympathetic nerve regeneration, stimulates parasympathetic nerve regeneration, ultimately promoting erectile function in CNI-induced ED rats. Yin et al. [55,56] found that intracavernosal blocking ninjurin 1 expression in diabetic and CNI-induced ED mice significantly rescued erectile function through inducing Ang1-Tie2 signaling, increasing neurotrophic factors expression, and reducing ROS production. Chung et al. [57] demonstrated that neutralizing antibodies to proNGF also can rescue erectile function by regulating the neurotrophic and angiogenic factors in CNI-induced ED mice. mAb preparation is time-consuming (6–9 months) and expensive, and the production system is applicable only to mice and rats. The current antibody-based drug therapy is still in its infancy, so finding more new target antigens is crucial for developing future antibody-based therapeutic drugs for ED.
3. Li-ESWT
1) Overview of Li-ESWT therapy
ESWT originated from high-intensity extracorporeal shock wave lithotripsy (ESWL), and recent studies have shown that Li-ESWT, can have more beneficial effects on human tissues [62]. First, Li-ESWT uses less energy than traditional ESWL [63,64], making it safer, noninvasive, and with fewer side effects. Second, Li-ESWT is simple to operate and relatively easy to operate. Third, the Li-ESWT treatment interval is long and the treatment time is short. Thus, patients can save a lot of time or money [65]. Because of these beneficial properties, Li-ESWT has become a treatment of choice for many conditions, including musculoskeletal disorders [66,67], wound healing [68], urological disorders [69], and restoration of erectile function [70,71,72]. This article summarizes some of the preclinical research results of Li-ESWT for ED, as shown in Table 3 [73,74,75,76,77,78,79,80,81].
Table 3. Preclinical shock wave therapy on ED biotherapeutics.
| Species | Type of ED | Parameter of therapy | Target signaling | Treatment protocol | Reference |
|---|---|---|---|---|---|
| Rat | Type 1 diabetic ED | ICP-MAP, nNOS, eNOS phosphorylation, endothelial and smooth muscle content | Nerve regeneration, recruitment of endogenous MSCs, TGF-β1/Smad/CTGF, SDF-1 signaling pathway | 300 shocks each time, three times a week for two weeks | [73,74,75] |
| Rat | Type 1 diabetic ED | ICP-MAP, eNOS phosphorylation, endothelial and smooth muscle content | nNOS, eNOS expression and VEGF signaling pathway | 300 shocks each time, three times a week for two weeks | [76] |
| Rat | Type 2 diabetic ED | ICP-MAP | NO/cGMP Pathway | 300 shocks at a frequency of 2 Hz, 2 sessions/week for three weeks, repeated after a three week no-treatment interval | [77] |
| Rat | CNI-ED | ICP-MAP, nNOS, schwann cell | nNOS, Erk1/2 signaling pathway | 300 shocks each time, two times a week for four weeks | [78,79,80] |
| Rat | Age-associated ED | Angiogenesis, progenitor cells, schwann cell | Erk1/2 signaling pathway | 300 or 500 shocks each time, two times a week for four weeks | [81] |
ED, erectile dysfunction; ICP-MAP, intracavernosal pressure-mean arterial pressure; nNOS, neuronal nitric oxide synthase; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; MSC, mesenchymal stem cell; TGF-β, transforming growth factor-beta; CTGF, connective tissue growth factor; SDF-1, stromal cell-derived factor-1; VEGF, vascular endothelial growth factor.
2) Li-ESWT in ED: preclinical studies
Lei et al. [73], Liu et al. [74], and Qiu et al. [75] demonstrated that Li-ESWT can partially ameliorate ED in type 1 diabetic rats by recruiting endogenous MSCs, possibly by inducing nNOS-positive nerve regeneration, endothelium, and smooth muscle content in the penis. Using the same rat model, Jeong et al. [76] found that Li-ESWT increased endothelial cell regeneration and reduced smooth muscle atrophy by increasing nNOS, eNOS expression, and VEGF signaling pathway. Furthermore, Assaly-Kaddoum et al. [77] showed that Li-ESWT not only significantly improved erectile function in a type 2 diabetes model, such as in Goto-Kakizaki rats, but also can be used together with PDE5i to treat ED. However, the mechanism by which Li-ESWT improves erectile function in Goto-Kakizaki rats is not mediated in a NO/cGMP-dependent manner. To date, most preclinical studies using Li-ESWT for ED have been performed in rats with diabetes-induced ED, and only a few studies have used aging and neurovascular ED models. In these studies [78,79,80,81], Behr-Roussel et al. [78], Peng et al. [79], Wang et al. [80], and Lin et al. [81] investigated the effect of Li-ESWT in two neurovascular ED models (BCNI and internal pudendal artery injury models) and an aging induced ED model. They found that Li-ESWT could induce angiogenesis and tissue regeneration in damaged penile areas by recruiting endogenous progenitor cells and proliferating Schwann cells, ultimately successfully improving erectile function. However, most studies on Li-ESWT for ED have focused on clinical trials, and further experiments are needed to refine factors, such as shock wave conditions, treatment duration, and treatment interval. Additionally, little is known on specific signaling pathways by which Li-ESWT restores erectile function, as all animal experiments were performed on rats. As mice studies have the advantages of low operating costs, relatively stable embryonic cells, and the flexibility of various genetic manipulations and gene editing, if relevant experiments can be performed on mice, it may be possible to better understand detailed signaling pathways of Li-ESWT in promoting erectile function.
3) Li-ESWT in ED: clinical studies
Clinical pilot data on Li-ESWT in ED patients were first reported by Vardi et al. [82] in 2010. It reported that 20 patients with vasculogenic ED treated twice a week for 3 weeks, which was repeated after a rest period of 3 weeks. In this study, the efficiency of Li-ESWT showed a significant increase in the International Index of Erectile Function (IIEF) after 1 month and maintained good results after 6 months. After this study was published, several clinical trial studies on Li-ESWT were published. However, Li-ESWT treatment protocols have not yet been established, leading to differences among studies. The most frequently used Li-ESWT setup method was an energy density of 0.09 mJ/mm2 with 1,500–2,000 pulses per section, which was used by Vardi et al. [82]. In addition, some conflicting results on Li-ESWT also have been reported [83,84,85]. Particularly, there are questions about long-term results. For example, Yee et al. [85] reported that there was no statistical differences in IIEF and Erectile Hardness Score (EHS) scores after 13 weeks of Li-ESWT in 29 each in the experimental group and the control group, with settings similar to those of Vardi et al. [82].
Therefore, to analyze these conflicting results, meta-analyses of RCTs on Li-ESWT for ED have been published (Table 4) [86,87,88,89,90,91]. First, a total of 833 patients from 14 studies were included in the meta-analysis published by Lu et al. [86], which showed that patients who underwent Li-ESWT had significantly improved IIEF (weighted mean difference [WMD]: 2.00; 95% confidence interval [CI], 0.99–3.00; p<0.0001) and EHS (risk difference: 0.16; 95% CI, 0.04–0.29; p=0.01) scores compared with the control. Since then, several meta-analyses have been published. We summarized the results of meta-analysis [86,87,88,89,90,91] in Table 4.
Table 4. Meta-analysis of Li-ESWT clinical data on ED.
| Included studies | Results | Summarized | Reference | |
|---|---|---|---|---|
| Total 14 studies including 833 patients (7 RCTs and 7 non-RCTs) | IIEF (total) | WMD: 2.00; 95% CI, 0.99–3.00; p<0.001 | 1. Most of these studies presented encouraging results, regardless of variation in Li-ESWT setup parameters or treatment protocols. 2. The patients with mild moderate ED had better therapeutic efficacy. 3. Therapeutic efficacy of Li-ESWTs could last at least 3 mo. |
[86] |
| IIEF after 1 mo | WMD: 0.37; 95% CI, -1.45–2.19; p=0.690 | |||
| IIEF after 3 mo | WMD: 2.71; 95% CI, 1.51–3.91; p<0.001 | |||
| EHS after 1 mo | RD: 0.47; 95% CI, 0.38–0.56; p<0.001 | |||
| EHS after 3 mo | RD: 0.16; 95% CI, 0.04–0.29; p=0.010 | |||
| Total 9 RCTs including 637 patients | IIEF (total) | WMD: 2.54; 95% CI, 0.83–4.25; p=0.040 | 1. Li-ESWT could significantly improve the IIEF and EHS of patients with ED. 2. Lower energy density (0.09 mJ/mm2), increased the number of pulses (3,000 pulses), and shorter total treatment courses (<6 wk) was showed better therapeutic efficacy. |
[87] |
| IIEF after 3 mo | WMD: 4.15; 95% CI, 1.40–6.90; p=0.003 | |||
| EHS after 3 mo | RD: 0.16; 95% CI, 0.03–0.28; p=0.010 | |||
| Total 5 studies including 460 patients (3 RCTs and 2 non-RCTs) (Only ED patients after radical prostatectomy) | IIEF (post op baseline) | WMD: 0.02; 95% CI, -0.28–0.32; p=0.900 | Li-ESWT showed a statistically significant effect on early recovery in penile rehabilitation of ED following RP. | [88] |
| IIEF after 3-4 mo | WMD: -3.14; 95% CI, -5.73–0.55; p=0.020 | |||
| IIEF after 9-12 mo | WMD: -5.37; 95% CI, -12.42–1.69; p=0.140 | |||
| Total 10 RCTs including 873 patients | IIEF | WMD: 3.97; 95% CI, 2.09–5.84; p<0.001 | This provided results showing that Li-ESWT significantly improves erectile function in patients with vasculogenic ED. | [89] |
| From EHS ≤2 to EHS ≥3 | OR: 0.16; 95% CI, 0.03–0.28; p=0.010 | |||
| Peak systolic velocity | WMD: 4.12; 95% CI, 2.30–5.94; p≤0.001 | |||
| Total 16 RCTs including 1,064 patients | IIEF after 1 mo | WMD: 3.18; 95% CI, 1.38–4.98; p=0.005 | 1. Li-ESWT could significantly increase IIEF and EHS in ED patients, especially in moderate ED group. 2. This suggest that treatment plans with an energy density of 0.09 mJ/mm2 and pulses number of 1,500 to 2,000 are more beneficial in ED patients. |
[90] |
| IIEF after 3 mo | WMD: 3.01; 95% CI, 2.04–3.98; p<0.001 | |||
| IIEF after 6 mo | WMD: 3.20; 95% CI, 2.49–3.92; p<0.001 | |||
| From EHS ≤2 to EHS ≥3 | OR: 5.07; 95% CI, 1.78–14.44; p=0.002 | |||
| Total 15 studies (4 RCTs and 11 non-RCTs) | IIEF (only RCTs) | RR: 2.50; 95% CI, 0.74–8.45; p=0.140 | 1. Li-ESWT, as a noninvasive treatment, has potential short-term therapeutic effect on patients with organic ED. 2. Nine-week protocol with energy density of 0.09 mJ/mm2 and 1,500 pluses seemed to have better therapeutic effect |
[91] |
| EHS (only RCTs) | RR: 8.31; 95% CI, 3.88–17.78; p<0.001 | |||
Li-ESWT, low-intensity extracorporeal shock wave therapy; ED, erectile dysfunction; RCT, randomized clinical trial; IIEF, International Index of Erectile Function; EHS, Erectile Hardness Score; WMD, weighted mean difference; CI, confidence interval; RD, risk differences; RR, risk ratio; OR, odds ratio; RP, radical prostatectomy.
As aforementioned, the treatment protocol for Li-ESWT is different for each study. In the published RCT studies, the energy density was used 0.09 mJ/mm2 or 0.1–0.2 mJ/mm2, and the pulses per section were 600, 1,500–2,000, or >3,000. Also, the duration of treatment is different. In this respect, several meta-analyses performed subgroup analyses. The most recently published meta-analysis by Yao et al. [90] performed subgroup analysis on energy density and pulses per section. They reported that a treatment setting plan with an energy density of 0.09 mJ/mm2 and a per-section pulse of 1,500–2,000 was more beneficial for IIEF recovery in ED patients. However, since these results are not a direct comparison between each group, care must be taken in interpreting it. Moreover, the results of different meta-analyses show different results [86,87]. Therefore, further research on the treatment protocol of Li-ESWT is needed to obtain higher evidence.
Most studies on Li-ESWT were studies on vasculogenic ED and Peyronie’s disease, but a study on Li-ESWT for ED after radical prostatectomy (RP) was conducted for the first time by Frey et al. [92] They reported that Li-ESWT was effective in ED recovery in 18 patients who underwent bilateral nerve-sparing. Since then, studies by ED after RP have been published, but there is no large-scale RCT study yet. Recently, a meta-analysis of ED after RP was performed by Rho et al. [88]. The results showed a statistically significant effect of Li-ESWT on early ED recovery at 3–4 months (WMD: -2.04; 95% CI, -3.72 to -0.35; p<0.020) from penile rehabilitation in ED after RP, although there was no statistical difference in the long-term results at 9–12 months (WMD: -5.37; 95% CI, -12.42 to 1.69; p=0.140).
The analysis of the results of Li-ESWT in ED treatment has limitations. In each RCT, there are differences in the patient population, such as treatment protocols that have not been established and whether recruited patients respond to PDE5i. Also, there is a limit to interpreting the duration of Li-ESWT effects. There is a lot of controversy about the effect, especially after 12 months. However, Li-ESWT has no major reported side effects, and although the long-term results are controversial, most meta-analyses show that Li-ESWT is effective in the short-term period. Therefore, it can be considered as an additional alternative treatment to ED treatment.
4. Combination regenerative therapy
Although there is currently no standardized treatment protocol for Li-ESWT for ED, such as energy settings, treatment intervals, duration, suitable disease types, etc [93]. However, the role of Li-ESWT in promoting stem/progenitor cell differentiation, proliferation and traction is unquestionable [94]. Many studies showed that human ADSCs, bone marrow-derived MSCs and human MSCs proliferation and differentiation were significantly activated by Li-ESWT treatment [95,96]. Furthermore, a study by Catalano et al. [97] demonstrated that combination of autologous human ADSCs and Li-ESWT therapy was shown to improve bone tissue repair in tissue engineering procedures. Furthermore, Schuh et al. [98] showed that Li-ESWT could be a promising tool to improve the quality of ADSCs for cell therapy in regenerative medicine applications. In fact, several studies have investigated the combination effect of Li-ESWT with stem cell therapy for ED treatment. For example, Jeon et al. [99] showed that combining Li-ESWT with ADSCs therapy was superior to treatment alone in restoring damaged nerves and improving corpus cavernosum angiogenesis. More recently, Shin et al. [100] showed that Li-ESWT combined with bone marrow-derived MSC therapy significantly improved erectile function and penile blood compared to monotherapy. However, research on the combined treatment of ED with Li-ESWT and stem cells is still in its infancy, and a large amount of research data is needed to formulate the best regimen to maximize the therapeutic effect. Larger clinical trials with longer follow-up are needed to assess efficacy, safety, and efficacy.
CONCLUSIONS
With the advancements regarding our understanding of the pathogenesis of ED at the molecular and cellular levels, there are many emerging targets for curing this condition. In terms of preclinical research, some progress has been made in stem cell therapy, protein therapy, and Li-ESWT, and Li-ESWT has also been applied in the clinical stage. Although the preclinical results by use of variety of stem cells, angiogenic and neurotrophic growth factor protein therapy, and mAbs are very promising, these candidate therapeutics should be proven with clinical trials. With collaborations with biopharmaceutical companies, we hope that regenerative therapy mainly targeting therapeutic angiogenesis and neural regeneration would be a future treatment modality to overcome the limitations of currently available ED therapeutics.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING: This research was supported by Inha University Research Grant (Ji-Kan Ryu).
- Research conception and design: Doo Yong Chung, Ji-Kan Ryu, and Guo Nan Yin.
- Drafting of the manuscript: Doo Yong Chung and Guo Nan Yin.
- Critical revision of the manuscript: Ji-Kan Ryu and Guo Nan Yin.
- Obtaining funding: Ji-Kan Ryu.
- Supervision: Ji-Kan Ryu and Guo Nan Yin.
- Approval of the final manuscript: all authors.
References
- 1.Muneer A, Kalsi J, Nazareth I, Arya M. Erectile dysfunction. BMJ. 2014;348:g129. doi: 10.1136/bmj.g129. [DOI] [PubMed] [Google Scholar]
- 2.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–463. [PubMed] [Google Scholar]
- 3.Jackson G. Erectile dysfunction and cardiovascular disease. Arab J Urol. 2013;11:212–216. doi: 10.1016/j.aju.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein I, Chambers R, Tang W, Stecher V, Hassan T. Real-world observational results from a database of 48 million men in the United States: relationship of cardiovascular disease, diabetes mellitus and depression with age and erectile dysfunction. Int J Clin Pract. 2018;72:e13078. doi: 10.1111/ijcp.13078. [DOI] [PubMed] [Google Scholar]
- 5.Schulster ML, Liang SE, Najari BB. Metabolic syndrome and sexual dysfunction. Curr Opin Urol. 2017;27:435–440. doi: 10.1097/MOU.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 6.El-Kadiry AE, Rafei M, Shammaa R. Cell therapy: types, regulation, and clinical benefits. Front Med (Lausanne) 2021;8:756029. doi: 10.3389/fmed.2021.756029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HJ, Park JS. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Dev Reprod. 2017;21:1–10. doi: 10.12717/DR.2017.21.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisson I, Green E, Sharpe M, Herbert C, Hyllner J, Mount N. Landscape of current and emerging cell therapy clinical trials in the UK: current status, comparison to global trends and future perspectives. Regen Med. 2015;10:169–179. doi: 10.2217/rme.14.71. [DOI] [PubMed] [Google Scholar]
- 9.Terlizzese G, Stubinski R, Casini A, Clerici G, Sangiorgi G. A case report of pudendal arteries angioplasty with sirolimus drug-coated balloon and drug-eluting stent associated with intracavernous autologous peripheral blood mononuclear cells injection for untreatable vasculogenic erectile dysfunction. Eur Heart J Case Rep. 2021;5:ytab244. doi: 10.1093/ehjcr/ytab244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He L, Yu T, Xiao Y, Huang Y, Guan Y, Zhao F, et al. Co-overexpression of VEGF and Smad7 improved the therapeutic effects of adipose-derived stem cells on neurogenic erectile dysfunction in the rat model. Andrologia. 2022;54:e14538. doi: 10.1111/and.14538. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Nie P, Yang W, Ma X, Chen Z, Wei H. Lipopolysaccharide-preconditioned allogeneic adipose-derived stem cells improve erectile function in a rat model of bilateral cavernous nerve injury. Basic Clin Androl. 2022;32:5. doi: 10.1186/s12610-022-00156-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J, Zheng Z, Tang W, Shao J, Jiang H, Lin H. A new strategy for stem cells therapy for erectile dysfunction: adipose-derived stem cells transfect Neuregulin-1 gene through superparamagnetic iron oxide nanoparticles. Investig Clin Urol. 2022;63:359–367. doi: 10.4111/icu.20220016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Yang Q, Xie Y, Deng C, Liu G, Zhang X. Comparative study of different transplantation methods of adipose tissue-derived stem cells in the treatment of erectile dysfunction caused by cavernous nerve injury. Andrologia. 2021;53:e13950. doi: 10.1111/and.13950. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Zhang Y, Zang G, Wang T, Yu Z, Wang S, et al. Adipose-derived stem cells improve erectile function partially through the secretion of IGF-1, bFGF, and VEGF in aged rats. Andrology. 2018;6:498–509. doi: 10.1111/andr.12483. [DOI] [PubMed] [Google Scholar]
- 15.Feng H, Liu Q, Deng Z, Li H, Zhang H, Song J, et al. Human umbilical cord mesenchymal stem cells ameliorate erectile dysfunction in rats with diabetes mellitus through the attenuation of ferroptosis. Stem Cell Res Ther. 2022;13:450. doi: 10.1186/s13287-022-03147-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang J, Song Y, Zhang Z, Wang S, Lu Y, Liu X. Identification of key microRNAs in diabetes mellitus erectile dysfunction rats with stem cell therapy by bioinformatic analysis of deep sequencing data. World J Mens Health. 2022;40:663–677. doi: 10.5534/wjmh.210147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukti AI, Ilyas S, Warli SM, Putra A, Rasyid N, Munir D, et al. Umbilical cord-derived mesenchymal stem cells improve TGF-β, α-SMA and collagen on erectile dysfunction in streptozotocin-induced diabetic rats. Med Arch. 2022;76:4–11. doi: 10.5455/medarh.2022.76.4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Luo L, Li J. LncRNA MALAT1 facilitates BM-MSCs differentiation into endothelial cells via targeting miR-206/VEGFA axis. Cell Cycle. 2020;19:3018–3028. doi: 10.1080/15384101.2020.1829799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Chen Z, Zhong F, Yang W, Ouyang X, Ma X, et al. Transplantation of human gingiva-derived mesenchymal stem cells ameliorates neurotic erectile dysfunction in a rat model. Front Bioeng Biotechnol. 2021;9:630076. doi: 10.3389/fbioe.2021.630076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu JH, Wang DY, Sheng L, Qian WQ, Xia SJ, Jiang Q. Human umbilical cord Wharton's jelly-derived mesenchymal stem cell transplantation could improve diabetic intracavernosal pressure. Asian J Androl. 2022;24:171–175. doi: 10.4103/aja.aja_33_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Cui Y, Lin H, Hu D, Qi T, Wang B, et al. MicroRNA-145 engineered bone marrow-derived mesenchymal stem cells alleviated erectile dysfunction in aged rats. Stem Cell Res Ther. 2019;10:398. doi: 10.1186/s13287-019-1509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Q, Chen X, Zheng T, Han D, Zhang H, Shi Y, et al. Transplantation of human urine-derived stem cells transfected with pigment epithelium-derived factor to protect erectile function in a rat model of cavernous nerve injury. Cell Transplant. 2016;25:1987–2001. doi: 10.3727/096368916X691448. [DOI] [PubMed] [Google Scholar]
- 23.Galhom RA, Korayem HE, Ibrahim MA, Abd-Eltawab Tammam A, Khalifa MM, Rashwan EK, et al. Urine-derived stem cells versus their lysate in ameliorating erectile dysfunction in a rat model of type 2 diabetes. Front Physiol. 2022;13:854949. doi: 10.3389/fphys.2022.854949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu X, Thakker PU, Matz EL, Terlecki RP, Marini FC, Allickson JG, et al. Dynamic changes in erectile function and histological architecture after intracorporal injection of human placental stem cells in a pelvic neurovascular injury rat model. J Sex Med. 2020;17:400–411. doi: 10.1016/j.jsxm.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Dou L, Matz EL, Gu X, Shu F, Paxton J, Song J, et al. Non-invasive cell tracking with brighter and red-transferred luciferase for potential application in stem cell therapy. Cell Transplant. 2019;28:1542–1551. doi: 10.1177/0963689719885078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masouminia M, Gelfand R, Kovanecz I, Vernet D, Tsao J, Salas R, et al. Dyslipidemia is a major factor in stem cell damage induced by uncontrolled long-term type 2 diabetes and obesity in the rat, as suggested by the effects on stem cell culture. J Sex Med. 2018;15:1678–1697. doi: 10.1016/j.jsxm.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima N, Tamaki T, Hirata M, Soeda S, Nitta M, Hoshi A, et al. Purified human skeletal muscle-derived stem cells enhance the repair and regeneration in the damaged urethra. Transplantation. 2017;101:2312–2320. doi: 10.1097/TP.0000000000001613. [DOI] [PubMed] [Google Scholar]
- 28.Yin GN. Pericyte-derived heme-binding protein 1 promotes angiogenesis and improves erectile function in diabetic mice. Investig Clin Urol. 2022;63:464–474. doi: 10.4111/icu.20220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin GN, Das ND, Choi MJ, Song KM, Kwon MH, Ock J, et al. The pericyte as a cellular regulator of penile erection and a novel therapeutic target for erectile dysfunction. Sci Rep. 2015;5:10891. doi: 10.1038/srep10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin GN, Wu J, Cui Y, Lin C, Shi L, Gao ZL, et al. Transcriptional profiling of mouse cavernous pericytes under high-glucose conditions: implications for diabetic angiopathy. Investig Clin Urol. 2021;62:100–110. doi: 10.4111/icu.20200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed TA, El-Badri N. Pericytes: the role of multipotent stem cells in vascular maintenance and regenerative medicine. Adv Exp Med Biol. 2018;1079:69–86. doi: 10.1007/5584_2017_138. [DOI] [PubMed] [Google Scholar]
- 32.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 33.Usmani SS, Bedi G, Samuel JS, Singh S, Kalra S, Kumar P, et al. THPdb: database of FDA-approved peptide and protein therapeutics. PLoS One. 2017;12:e0181748. doi: 10.1371/journal.pone.0181748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu H, Zhou Q, He J, Jiang Z, Peng C, Tong R, et al. Recent advances in the development of protein-protein interactions modulators: mechanisms and clinical trials. Signal Transduct Target Ther. 2020;5:213. doi: 10.1038/s41392-020-00315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM, et al. Phage display derived monoclonal antibodies: from bench to bedside. Front Immunol. 2020;11:1986. doi: 10.3389/fimmu.2020.01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27:1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh PS, Bochinski DJ, Lin GT, Nunes L, Lin CS, Lue TF. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int. 2003;92:470–475. doi: 10.1046/j.1464-410x.2003.04373.x. [DOI] [PubMed] [Google Scholar]
- 38.Rogers RS, Graziottin TM, Lin CS, Kan YW, Lue TF. Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int J Impot Res. 2003;15:26–37. doi: 10.1038/sj.ijir.3900943. [DOI] [PubMed] [Google Scholar]
- 39.Park K, Ahn KY, Kim MK, Lee SE, Kang TW, Ryu SB. Intracavernosal injection of vascular endothelial growth factor improves erectile function in aged rats. Eur Urol. 2004;46:403–407. doi: 10.1016/j.eururo.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 40.Jin HR, Kim WJ, Song JS, Piao S, Choi MJ, Tumurbaatar M, et al. Intracavernous delivery of a designed angiopoietin-1 variant rescues erectile function by enhancing endothelial regeneration in the streptozotocin-induced diabetic mouse. Diabetes. 2011;60:969–980. doi: 10.2337/db10-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin HR, Kim WJ, Song JS, Piao S, Tumurbaatar M, Shin SH, et al. Intracavernous delivery of synthetic angiopoietin-1 protein as a novel therapeutic strategy for erectile dysfunction in the type II diabetic db/db mouse. J Sex Med. 2010;7:3635–3646. doi: 10.1111/j.1743-6109.2010.01925.x. [DOI] [PubMed] [Google Scholar]
- 42.Ryu JK, Kim WJ, Koh YJ, Piao S, Jin HR, Lee SW, et al. Designed angiopoietin-1 variant, COMP-angiopoietin-1, rescues erectile function through healthy cavernous angiogenesis in a hypercholesterolemic mouse. Sci Rep. 2015;5:9222. doi: 10.1038/srep09222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon MH, Ryu JK, Kim WJ, Jin HR, Song KM, Kwon KD, et al. Effect of intracavernous administration of angiopoietin-4 on erectile function in the streptozotocin-induced diabetic mouse. J Sex Med. 2013;10:2912–2927. doi: 10.1111/jsm.12278. [DOI] [PubMed] [Google Scholar]
- 44.Das ND, Yin GN, Choi MJ, Song KM, Park JM, Limanjaya A, et al. Effectiveness of intracavernous delivery of recombinant human hepatocyte growth factor on erectile function in the streptozotocin-induced diabetic mouse. J Sex Med. 2016;13:1618–1628. doi: 10.1016/j.jsxm.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Liu T, Peng Y, Jia C, Fang X, Li J, Zhong W. Hepatocyte growth factor-modified adipose tissue-derived stem cells improve erectile function in streptozotocin-induced diabetic rats. Growth Factors. 2015;33:282–289. doi: 10.3109/08977194.2015.1077825. [DOI] [PubMed] [Google Scholar]
- 46.Choe S, Kalmanek E, Bond C, Harrington DA, Stupp SI, McVary KT, et al. Optimization of sonic hedgehog delivery to the penis from self-assembling nanofiber hydrogels to preserve penile morphology after cavernous nerve injury. Nanomedicine. 2019;20:102033. doi: 10.1016/j.nano.2019.102033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podlasek CA. Sonic hedgehog, apoptosis, and the penis. J Sex Med. 2009;6 Suppl 3(Suppl 3):334–339. doi: 10.1111/j.1743-6109.2008.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, et al. Sonic hedgehog cascade is required for penile postnatal morphogenesis, differentiation, and adult homeostasis. Biol Reprod. 2003;68:423–438. doi: 10.1095/biolreprod.102.006643. [DOI] [PubMed] [Google Scholar]
- 49.Ghatak K, Yin GN, Choi MJ, Limanjaya A, Minh NN, Ock J, et al. Dickkopf2 rescues erectile function by enhancing penile neurovascular regeneration in a mouse model of cavernous nerve injury. Sci Rep. 2017;7:17819. doi: 10.1038/s41598-017-17862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin GN, Jin HR, Choi MJ, Limanjaya A, Ghatak K, Minh NN, et al. Pericyte-derived Dickkopf2 regenerates damaged penile neurovasculature through an Angiopoietin-1-Tie2 Pathway. Diabetes. 2018;67:1149–1161. doi: 10.2337/db17-0833. [DOI] [PubMed] [Google Scholar]
- 51.Kwon MH, Tuvshintur B, Kim WJ, Jin HR, Yin GN, Song KM, et al. Expression of the apelin-APJ pathway and effects on erectile function in a mouse model of vasculogenic erectile dysfunction. J Sex Med. 2013;10:2928–2941. doi: 10.1111/jsm.12158. [DOI] [PubMed] [Google Scholar]
- 52.Yin GN, Kim DK, Kang JI, Im Y, Lee DS, Han AR, et al. Latrophilin-2 is a novel receptor of LRG1 that rescues vascular and neurological abnormalities and restores diabetic erectile function. Exp Mol Med. 2022;54:626–638. doi: 10.1038/s12276-022-00773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin G, Shindel AW, Fandel TM, Bella AJ, Lin CS, Lue TF. Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int. 2010;105:114–120. doi: 10.1111/j.1464-410X.2009.08647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin G, Li H, Zhang X, Wang J, Zaid U, Sanford MT, et al. Novel therapeutic approach for neurogenic erectile dysfunction: effect of neurotrophic tyrosine kinase receptor type 1 monoclonal antibody. Eur Urol. 2015;67:716–726. doi: 10.1016/j.eururo.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Yin GN, Choi MJ, Kim WJ, Kwon MH, Song KM, Park JM, et al. Inhibition of Ninjurin 1 restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. Proc Natl Acad Sci U S A. 2014;111:E2731–E2740. doi: 10.1073/pnas.1403471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin GN, Kim WJ, Jin HR, Kwon MH, Song KM, Choi MJ, et al. Nerve injury-induced protein 1 (Ninjurin-1) is a novel therapeutic target for cavernous nerve injury-induced erectile dysfunction in mice. J Sex Med. 2013;10:1488–1501. doi: 10.1111/jsm.12129. [DOI] [PubMed] [Google Scholar]
- 57.Chung DY, Song KM, Choi MJ, Limanjaya A, Ghatak K, Ock J, et al. Neutralizing antibody to proNGF rescues erectile function by regulating the expression of neurotrophic and angiogenic factors in a mouse model of cavernous nerve injury. Andrology. 2021;9:329–341. doi: 10.1111/andr.12873. [DOI] [PubMed] [Google Scholar]
- 58.Buckel P. Recombinant proteins for therapy. Trends Pharmacol Sci. 1996;17:450–456. doi: 10.1016/s0165-6147(96)01011-5. [DOI] [PubMed] [Google Scholar]
- 59.Lau JL, Dunn MK. Therapeutic peptides: historical perspectives, current development trends, and future directions. Bioorg Med Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 60.Yin GN, Ock J, Choi MJ, Limanjaya A, Ghatak K, Song KM, et al. Gene expression profiling of mouse cavernous endothelial cells for diagnostic targets in diabetes-induced erectile dysfunction. Investig Clin Urol. 2021;62:90–99. doi: 10.4111/icu.20200119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsumoto K, Isozaki Y, Yagami H, Tomita M. Future perspectives of therapeutic monoclonal antibodies. Immunotherapy. 2019;11:119–127. doi: 10.2217/imt-2018-0130. [DOI] [PubMed] [Google Scholar]
- 62.Guo J, Hai H, Ma Y. Application of extracorporeal shock wave therapy in nervous system diseases: a review. Front Neurol. 2022;13:963849. doi: 10.3389/fneur.2022.963849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crevenna R, Mickel M, Keilani M. Extracorporeal shock wave therapy in the supportive care and rehabilitation of cancer patients. Support Care Cancer. 2019;27:4039–4041. doi: 10.1007/s00520-019-05046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramon S, Español A, Yebra M, Morillas JM, Unzurrunzaga R, Freitag K, et al. [Current evidences in shockwave treatment. SETOC (Spanish Society of Shockwave Treatment) recommendations] Rehabilitacion (Madr) 2021;55:291–300. doi: 10.1016/j.rh.2021.02.002. Spanish. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg D, Andriessen A, Gold M. Radial shockwave therapy for male erectile rejuvenation in a dermatology and/or medical aesthetic practice. J Cosmet Dermatol. 2019;18:1596–1600. doi: 10.1111/jocd.13022. [DOI] [PubMed] [Google Scholar]
- 66.Moya D, Ramón S, Schaden W, Wang CJ, Guiloff L, Cheng JH. The role of extracorporeal shockwave treatment in musculoskeletal disorders. J Bone Joint Surg Am. 2018;100:251–263. doi: 10.2106/JBJS.17.00661. [DOI] [PubMed] [Google Scholar]
- 67.Simplicio CL, Purita J, Murrell W, Santos GS, Dos Santos RG, Lana JFSD. Extracorporeal shock wave therapy mechanisms in musculoskeletal regenerative medicine. J Clin Orthop Trauma. 2020;11(Suppl 3):S309–S318. doi: 10.1016/j.jcot.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang N, Yu X, Ma Y. Radial extracorporeal shock wave therapy in a patient with decubitus ulcer after spinal cord injury: a case report. Am J Transl Res. 2020;12:2093–2098. [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang D, Wang YL, Gong DX, Zhang ZX, Yu XT, Ma YW. Radial extracorporeal shock wave therapy as a novel agent for benign prostatic hyperplasia refractory to current medical therapy. Am J Mens Health. 2019;13:1557988319831899. doi: 10.1177/1557988319831899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mason MM, Pai RK, Masterson JM, Lokeshwar SD, Chu KY, Ramasamy R. Low-intensity extracorporeal shockwave therapy for diabetic men with erectile dysfunction: a systematic scoping review. Andrology. 2023;11:270–281. doi: 10.1111/andr.13197. [DOI] [PubMed] [Google Scholar]
- 71.Bocchino AC, Pezzoli M, Martínez-Salamanca JI, Russo GI, Lo Giudice A, Cocci A. Low-intensity extracorporeal shock wave therapy for erectile dysfunction: myths and realities. Investig Clin Urol. 2023;64:118–125. doi: 10.4111/icu.20220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeong HC, Bae WJ, Zhu GQ, Jeon SH, Choi SW, Kim SJ, et al. Synergistic effects of extracorporeal shockwave therapy and modified Ojayeonjonghwan on erectile dysfunction in an animal model of diabetes. Investig Clin Urol. 2019;60:285–294. doi: 10.4111/icu.2019.60.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei H, Xin H, Guan R, Xu Y, Li H, Tian W, et al. Low-intensity pulsed ultrasound improves erectile function in streptozotocin-induced type I diabetic rats. Urology. 2015;86:1241. e11–1241. e18. doi: 10.1016/j.urology.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Zhou F, Li GY, Wang L, Li HX, Bai GY, et al. Evaluation of the effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-induced diabetic rats. Int J Mol Sci. 2013;14:10661–10673. doi: 10.3390/ijms140510661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu X, Lin G, Xin Z, Ferretti L, Zhang H, Lue TF, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–746. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeong HC, Jeon SH, Qun ZG, Kim KS, Choi SW, Bashraheel F, et al. Effects of next-generation low-energy extracorporeal shockwave therapy on erectile dysfunction in an animal model of diabetes. World J Mens Health. 2017;35:186–195. doi: 10.5534/wjmh.17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Assaly-Kaddoum R, Giuliano F, Laurin M, Gorny D, Kergoat M, Bernabé J, et al. Low intensity extracorporeal shock wave therapy improves erectile function in a model of type II diabetes Independently of NO/cGMP pathway. J Urol. 2016;196:950–956. doi: 10.1016/j.juro.2016.03.147. [DOI] [PubMed] [Google Scholar]
- 78.Behr-Roussel D, Giuliano F. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. Transl Androl Urol. 2016;5:977–979. doi: 10.21037/tau.2016.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng D, Tan Y, Reed-Maldonado AB, Lin G, Lue TF. Molecular mechanism of action of low-intensity extracorporeal shockwave therapy for regenerating penile and peripheral nerves. Turk J Urol. 2020 doi: 10.5152/tud.2020.20419. [DOI] [PubMed] [Google Scholar]
- 80.Wang HS, Ruan Y, Banie L, Cui K, Kang N, Peng D, et al. Delayed low-intensity extracorporeal shock wave therapy ameliorates impaired penile hemodynamics in rats subjected to pelvic neurovascular injury. J Sex Med. 2019;16:17–26. doi: 10.1016/j.jsxm.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin G, Reed-Maldonado AB, Wang B, Lee YC, Zhou J, Lu Z, et al. In Situ activation of penile progenitor cells with low-intensity extracorporeal shockwave therapy. J Sex Med. 2017;14:493–501. doi: 10.1016/j.jsxm.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 82.Vardi Y, Appel B, Jacob G, Massarwi O, Gruenwald I. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58:243–248. doi: 10.1016/j.eururo.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 83.Kitrey ND, Gruenwald I, Appel B, Shechter A, Massarwa O, Vardi Y. Penile low intensity shock wave treatment is able to shift PDE5i nonresponders to responders: a double-blind, sham controlled study. J Urol. 2016;195:1550–1555. doi: 10.1016/j.juro.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 84.Poulakis V, Skriapas K, de Vries R, Dillenburg W, Ferakis N, Witzsch U, et al. Extracorporeal shockwave therapy for Peyronie’s disease: an alternative treatment? Asian J Androl. 2006;8:361–366. doi: 10.1111/j.1745-7262.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 85.Yee CH, Chan ES, Hou SS, Ng CF. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: a prospective, randomized, double-blinded, placebo controlled study. Int J Urol. 2014;21:1041–1045. doi: 10.1111/iju.12506. [DOI] [PubMed] [Google Scholar]
- 86.Lu Z, Lin G, Reed-Maldonado A, Wang C, Lee YC, Lue TF. Low-intensity extracorporeal shock wave treatment improves erectile function: a systematic review and meta-analysis. Eur Urol. 2017;71:223–233. doi: 10.1016/j.eururo.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 87.Man L, Li G. Low-intensity extracorporeal shock wave therapy for erectile dysfunction: a systematic review and Meta-analysis. Urology. 2018;119:97–103. doi: 10.1016/j.urology.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Rho BY, Kim SH, Ryu JK, Kang DH, Kim JW, Chung DY. Efficacy of low-intensity extracorporeal shock wave treatment in erectile dysfunction following radical prostatectomy: a systematic review and meta-analysis. J Clin Med. 2022;11:2775. doi: 10.3390/jcm11102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sokolakis I, Hatzichristodoulou G. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: a systematic review and meta-analysis of randomised controlled trials. Int J Impot Res. 2019;31:177–194. doi: 10.1038/s41443-019-0117-z. [DOI] [PubMed] [Google Scholar]
- 90.Yao H, Wang X, Liu H, Sun F, Tang G, Bao X, et al. Systematic review and meta-analysis of 16 randomized controlled trials of clinical outcomes of low-intensity extracorporeal shock wave therapy in treating erectile dysfunction. Am J Mens Health. 2022;16:15579883221087532. doi: 10.1177/15579883221087532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zou ZJ, Tang LY, Liu ZH, Liang JY, Zhang RC, Wang YJ, et al. Short-term efficacy and safety of low-intensity extracorporeal shock wave therapy in erectile dysfunction: a systematic review and meta-analysis. Int Braz J Urol. 2017;43:805–821. doi: 10.1590/S1677-5538.IBJU.2016.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frey A, Sønksen J, Fode M. Low-intensity extracorporeal shockwave therapy in the treatment of post-prostatectomy erectile dysfunction: a pilot study. Scand J Urol. 2016;50:123–127. doi: 10.3109/21681805.2015.1100675. [DOI] [PubMed] [Google Scholar]
- 93.Yuan F, Wang Y, Ma Z, Jing M, You Y, Yu X, et al. Low-intensity extracorporeal shockwave therapy for erectile dysfunction: an overview of systematic reviews. Transl Androl Urol. 2021;10:3684–3696. doi: 10.21037/tau-21-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leone L, Raffa S, Vetrano M, Ranieri D, Malisan F, Scrofani C, et al. Extracorporeal Shock Wave Treatment (ESWT) enhances the in vitro-induced differentiation of human tendon-derived stem/progenitor cells (hTSPCs) Oncotarget. 2016;7:6410–6423. doi: 10.18632/oncotarget.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raabe O, Shell K, Goessl A, Crispens C, Delhasse Y, Eva A, et al. Effect of extracorporeal shock wave on proliferation and differentiation of equine adipose tissue-derived mesenchymal stem cells in vitro. Am J Stem Cells. 2013;2:62–73. [PMC free article] [PubMed] [Google Scholar]
- 96.Suhr F, Delhasse Y, Bungartz G, Schmidt A, Pfannkuche K, Bloch W. Cell biological effects of mechanical stimulations generated by focused extracorporeal shock wave applications on cultured human bone marrow stromal cells. Stem Cell Res. 2013;11:951–964. doi: 10.1016/j.scr.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Catalano MG, Marano F, Rinella L, de Girolamo L, Bosco O, Fortunati N, et al. Extracorporeal shockwaves (ESWs) enhance the osteogenic medium-induced differentiation of adipose-derived stem cells into osteoblast-like cells. J Tissue Eng Regen Med. 2017;11:390–399. doi: 10.1002/term.1922. [DOI] [PubMed] [Google Scholar]
- 98.Schuh CM, Heher P, Weihs AM, Banerjee A, Fuchs C, Gabriel C, et al. In vitro extracorporeal shock wave treatment enhances stemness and preserves multipotency of rat and human adipose-derived stem cells. Cytotherapy. 2014;16:1666–1678. doi: 10.1016/j.jcyt.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Jeon SH, Shrestha KR, Kim RY, Jung AR, Park YH, Kwon O, et al. Combination therapy using human adipose-derived stem cells on the cavernous nerve and low-energy shockwaves on the corpus cavernosum in a rat model of postprostatectomy erectile dysfunction. Urology. 2016;88:226.e1–226.e9. doi: 10.1016/j.urology.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 100.Shin D, Jeon SH, Tian WJ, Kwon EB, Kim GE, Bae WJ, et al. Extracorporeal shock wave therapy combined with engineered mesenchymal stem cells expressing stromal cell-derived factor-1 can improve erectile dysfunction in streptozotocin-induced diabetic rats. Transl Androl Urol. 2021;10:2362–2372. doi: 10.21037/tau-21-79. [DOI] [PMC free article] [PubMed] [Google Scholar]