Abstract
Purpose
To define transcutaneous medial plantar nerve stimulation (T-MPNS) as a new neuromodulation method and assess the efficacy of T-MPNS on quality of life (QoL) and clinical parameters associated with incontinence in women with idiopathic overactive bladder (OAB).
Materials and Methods
Twenty-one women were included in this study. All women received T-MPNS. Two self-adhesive surface electrodes were positioned with the negative electrode near the metatarsal-phalangeal joint of the great toe on the medial aspect of the foot and the positive electrode 2 cm inferior-posterior of the medial malleolus (in front of the medio-malleolar-calcaneal axis). T-MPNS was performed 2 days a week, 30 minutes a day, for a total of 12 sessions for 6 weeks. Women were evaluated for incontinence severity (24-h pad test), 3-day voiding diary, symptom severity (Overactive Bladder Questionnaire [OAB-V8]), QoL (Quality of Life-Incontinence Impact Questionnaire [IIQ-7]), positive response and cure-improvement rates, and treatment satisfaction at baseline and at the 6th week.
Results
Statistically significant improvement was found in the severity of incontinence, frequency of voiding, incontinence episodes, nocturia, number of pads, symptom severity, and QoL parameters at the 6th week compared with baseline. Treatment satisfaction, treatment success, and cure or improvement rates were found to be high at the 6th week.
Conclusions
T-MPNS was first described in the literature as a new neuromodulation method. We conclude that T-MPNS is effective on both clinical parameters and QoL associated with incontinence in women with idiopathic OAB. Randomized controlled multicenter studies are needed to validate the effectiveness of T-MPNS.
Keywords: Medial plantar nerve, Neuromodulator receptor, Overactive bladder, Transcutaneous nerve stimulation, Urinary incontinence
Graphical Abstract
INTRODUCTION
Stimulation of an area of the innervation system with electrical currents may alter the nervous behavior of other systems and cause changes in bladder function by stimulating peripheral nerves [1,2]. The pudendal nerve, dorsal genital nerve, and tibial nerve (TN) are examples of peripheral nerves that can affect bladder behavior.
Percutaneous and transcutaneous TN stimulation (PTNS and TTNS) have been reported to be effective in patients with idiopathic overactive bladder (OAB) in different studies [3,4,5,6,7,8,9,10,11,12,13]. The European Association of Urology (EAU) Guidelines on the management of non-neurogenic female lower urinary tract symptoms recommend considering PTNS as an option to improve idiopathic OAB in women who have not benefited from anticholinergic drugs (strong recommendation) [4]. In some cases, however, it may be difficult to perform the TTNS and PTNS techniques. Some conditions, such as congenital or acquired impaired integrity of the skin or soft tissue loss or deformity of the inner part of the ankle where the transcutaneous or percutaneous application will be performed, can make these applications impossible. In our clinical practice, when we could not stimulate the TN, we identified the medial plantar nerve (MPN) as a new region for stimulation. Stimulation of the TN may also be difficult in patients with advanced peripheral tibial edema, pretibial myxedema, lymphedema, or lipoedema of the lower extremities or in those with morbid obesity.
The lower urinary tract is innervated by the lumbar, sacral, and coccygeal segmental nerves originating from L2-S4. After exiting the spinal cord, afferent and efferent fibers form an extensive network that innervates all the pelvic organs. The MPN (L4-5) is one of the terminal branches of the TN (L4-S3) [1]. Transcutaneous MPN stimulation (T-MPNS) is the electrostimulation of the lumbosacral roots that activate the lumbosacral plexus, which controls the visceral organs and the pelvic floor muscles, thereby improving bladder control. However, the exact working mechanism of T-MPNS is unknown.
Our study is the first in which T-MPNS was defined as a new peripheral neuromodulation method and its efficacy investigated in women with idiopathic OAB. In this study, we aimed to assess the efficacy of T-MPNS on quality of life (QoL) and clinical parameters associated with incontinence in women with idiopathic OAB.
MATERIALS AND METHODS
This study was a prospective, single-arm (before and after) trial. The trial was conducted at the Urogynecological Rehabilitation Unit of the Pamukkale University Faculty of Medicine between June 2020 and June 2022. This trial was approved by the Institutional Review Board of Pamukkale University (60116787-020/29686). Women gave written consent to participate.
As previously published, PTNS succeeds in reducing incontinence episodes in 71% of cases compared with 0% after sham PTNS [11]. A level of significance of 95% (α=5%), a power of 80% (ß=0.20), and an expected improvement of incontinence episodes were expressed as a decrease of 50% or greater in incontinence episodes in 24 hours in the previous study [11]. We determined the optimum sample size in our study to be 19 cases. To account for possible withdrawals (10%), we enrolled 21 women.
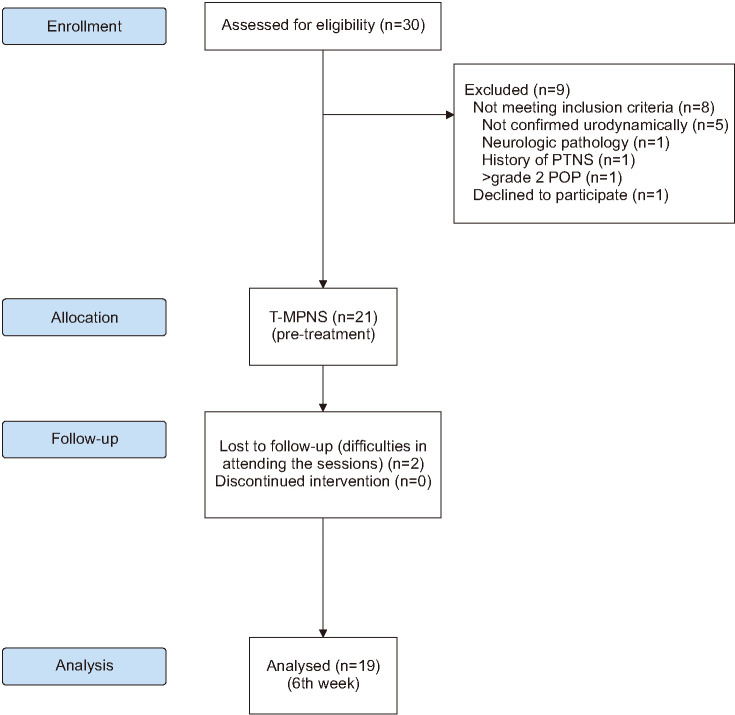
We recruited 30 women with a complaint of OAB who were referred to the Urogynecological Rehabilitation Unit. Women who experienced urgency and urge incontinence with leakage on the way to the toilet were considered to have wet-OAB. The inclusion and exclusion criteria are shown in Table 1. Twenty-one women were included in this study; the planned treatment duration was 6 weeks (Fig. 1). All women received T-MPNS.
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Women over the age of 18 years with clinical diagnosis of idiopathic wet-OAB | History of neuromodulation therapy such as PTNS, TTNS |
| Intolerant or unresponsive to antimuscarinics or oral ß3 adrenoreceptor agonist (mirabegron)and discontinued after at least 4 weeks | Pregnancy or intention to become pregnant during the study |
| Able to give written, informed consent | Current vulvovaginitis or urinary tract infections or malignancy |
| Able to understand the procedures, advantages, and possible adverse effects | Previous urogynecological surgery within 3 months |
| Anatomic or posttraumatic malformations or skin disorders of plantar medial nerve region on inner foot that do not permit application of the electrodes | |
| More than stage 2 according to the POP-Q | |
| Cardiac pacemaker or implanted defibrillator | |
| Neurogenic bladder, signs of neurologic abnormalities at objective examination; history of the peripheral or central neurologic pathology | |
| Ultrasonographic evidence of PVR volume more than 100 mL |
OAB, overactive bladder; PTNS, percutaneous tibial nerve stimulation; TTNS, transcutaneous tibial nerve stimulation; POP-Q, pelvic organ prolapse quantification; PVR, post-void residual urine.
Fig. 1. Flow diagram of the study. PTNS, percutaneous tibial nerve stimulation; POP, pelvic organ prolapse; T-MPNS, transcutaneous medial plantar nerve stimulation.
1. Transcutaneous medial plantar nerve stimulation
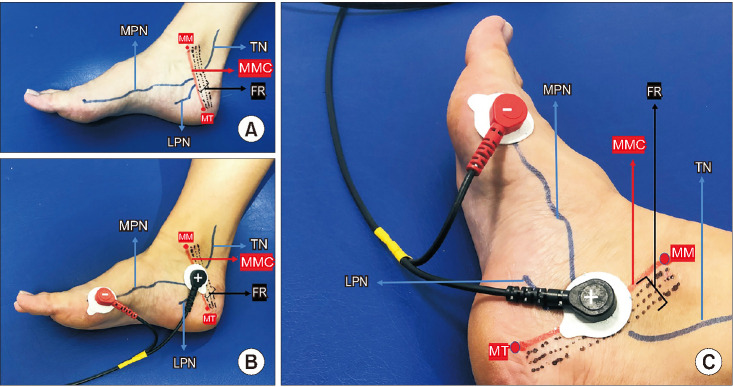
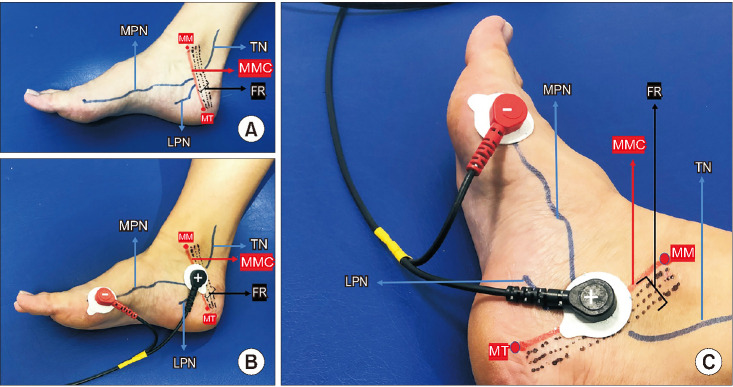
Two self-adhesive surface electrodes were positioned with the negative electrode near the metatarsal-phalangeal joint of the great toe on the medial aspect of the foot and the positive electrode about 2 cm inferior-posterior of the medial malleolus (in front of the medio-malleolar-calcaneal [MMC] axis) according to the topographic anatomy and the electrophysiological nerve conduction of the MPN (Fig. 2). Correct positioning was determined by noting a hallux reaction (plantar flexion of the big toe or, rarely, fanning of the fingers) [14,15,16,17,18]. The stimulation protocol was applied at a continuous mode of 20 Hz and a pulse width of 200 µs [7,9,13]. The intensity level of the stimulation current (range, 0-50 mA) was determined after correct positioning was achieved according to the comfort level of the participant. These electrodes did not need to be adjusted during treatment sessions. T-MPNS sessions were performed twice a week for 6 weeks. Each session lasted 30 minutes. The intervention included a 12-session T-MPNS treatment program.
Fig. 2. (A) Medial aspect of the foot showing TN, MPN, and MMC axis. (B) Electrode positioning for T-PMNS (medial aspect). (C) Electrode positioning for T-PMNS (medial-posterior aspect). MPN, medial plantar nerve; TN, tibial nerve; MMC, medio-malleolar-calcaneal axis; FR, flexor retinaculum; LPN, lateral plantar nerve; MM, medial malleolus; MT, medial tubercle of calcaneum; T-MPNS, transcutaneous medial plantar nerve stimulation.
T-MPNS sessions were performed by a physician. Participants who did not attend more than 20% of the sessions were excluded from the study. During the treatment, all women were advised to continue medical treatment not related to incontinence, and no women were recommended lifestyle modifications for incontinence.
2. Evaluation parameters
The frequency of voiding, incontinence episodes, nocturia, and the number of pads used were collected from the 3-day bladder diary. The primary outcome measure was improvement in incontinence episodes (positive response rate) [11]. Women with a 50% or greater reduction in incontinence episodes were considered positive responders [11,13]. The 24-hour pad test was performed to assess the severity of incontinence [19]. The Overactive Bladder Questionnaire (OAB-V8) was used to evaluate symptom severity [20,21]. The Quality of Life-Incontinence Impact Questionnaire (IIQ-7) was used to evaluate QoL related to incontinence [22].
No incontinence in the 24-hour pad test was considered a cure. Improvement was assessed as a 50% or greater reduction in wet weight compared with baseline measurements in the 24-hour pad test [19]. Women rated their satisfaction with the change in urinary incontinence on a 5-point Likert scale (5, very satisfied; 1, very unsatisfied) [23].
All the evaluation parameters were performed by the physician during the initial visit and were repeated at the 6th week (end of the treatment). All women were questioned about adverse effects such as pain and numbness in the feet and toes.
The TREND Statement checklist recommended for nonrandomized controlled studies was used in this study (https://www.cdc.gov/trendstatement/pdf/trendstatement_trend_checklist.pdf).
3. Statistics
Because the distributions were not normal and the variances of the quantitative variables were nonhomogeneous, nonparametric tests were used in the statistical evaluation. Wilcoxon signed-rank tests were used for intragroup comparison of parameters at different times. The accepted statistical significance level was 5%.
RESULTS
Two women withdrew because of difficulties attending the sessions. The flow chart and the demographic data at baseline are shown in Fig. 1 and Table 2, respectively.
Table 2. Demographic data of patients (n=19).
| Variable | T-MPNS | |
|---|---|---|
| Age (y) | 61.52±8.70 | |
| Height (cm) | 158.10±4.29 | |
| Weight (kg) | 75.05±9.53 | |
| Duration of incontinence (mo) | 66.94±37.64 | |
| Education | ||
| Primary | 10 (52.6) | |
| High school | 6 (31.6) | |
| >High school | 3 (15.8) | |
| Smoking | ||
| No | 16 (84.2) | |
| Yes | 3 (15.8) | |
| Cup of tea/daily (cup) | ||
| 1–2 | 15 (78.9) | |
| ≥3 | 4 (21.1) | |
| Cup of coffee/daily (cup) | ||
| No | 7 (36.8) | |
| 1–2 | 6 (31.6) | |
| ≥3 | 6 (31.6) | |
| Alcohol intake | ||
| No | 18 (94.7) | |
| Yes | 1 (5.3) | |
| Delivery | ||
| No | - | |
| 1–3 | 11 (57.9) | |
| ≥4 | 8 (42.1) | |
| Delivery type | ||
| NSVD | 18 (94.7) | |
| Cesarean delivery | 1 (5.3) | |
| Episiotomy | ||
| No | 15 (78.9) | |
| Yes | 4 (21.1) | |
| Menopausal status | ||
| Premenopausal | - | |
| Postmenopausal | 19 (100.0) | |
| HRT use | ||
| No | 12 (63.2) | |
| Yes | 7 (36.8) | |
Values are presented as mean±standard deviation or number (%).
T-MPNS, transcutaneous medial plantar nerve stimulation; NSVD, normal spontaneous vaginal delivery; HRT, hormone replacement therapy.
Statistically significant improvement was found in the severity of incontinence, incontinence episodes, frequency of voiding, nocturia, number of pads, symptom severity, and QoL parameters at the end of the treatment compared with baseline (p<0.001) (Table 3).
Table 3. Comparison of assessment variables of the patients.
| Variable | Pre-treatment | 6th week | p-value |
|---|---|---|---|
| Severity of incontinence (Pad test) | 55 (115–25) | 7 (14–1) | <0.001 |
| Frequency | 12 (15–10) | 7 (8–6) | <0.001 |
| Nocturia | 3 (4–2) | 1 (2–1) | <0.001 |
| Incontinence episodes | 4 (7–3) | 1 (2–1) | <0.001 |
| Number of pads | 3 (4–2) | 1 (1–0) | <0.001 |
| Symptom severity (OAB-V8) | 28 (33–22) | 6 (8–4) | <0.001 |
| Quality of life (IIQ7) | 11 (16–5) | 2 (3–1) | <0.001 |
Values are presented as median (ınterquartile range); IQR=3rd quartile–1st quartile.
OAB-V8, Overactive Bladder Questionnaire; IIQ-7, Incontinence Impact Questionnaire.
p-value by Wilcoxon signed-rank test.
Cure and improvement, positive response, and treatment satisfaction (very satisfied and satisfied) rates were high (respectively, 68.4%, 68.4%, and 73.7%) at the end of treatment (Table 4).
Table 4. Treatment satisfaction, positive response, and cure-improvement rates at the end of treatment.
| Variable | T-MPNS (n=19) | |
|---|---|---|
| Treatment satisfaction | ||
| Very satisfied | 9 (47.4) | |
| Satisfied | 5 (26.3) | |
| Equally satisfied and unsatisfied | 1 (5.3) | |
| Unsatisfied | 4 (21.1) | |
| Very unsatisfied | - | |
| Positive response rate | ||
| Yes | 13 (68.4) | |
| No | 6 (31.6) | |
| Cure-improvement rate | ||
| Cure | 3 (15.8) | |
| Improvement | 10 (52.6) | |
| No change | 6 (31.6) | |
Values are presented as number (%).
T-MPNS, transcutaneous medial plantar nerve stimulation.
No serious adverse events were reported in any participants.
DISCUSSION
In this prospective, single-arm (before and after) trial, we investigated the effectiveness of T-MPNS on QoL and clinical parameters associated with incontinence in women with idiopathic OAB. We observed significant improvement in all parameters (incontinence severity, frequency of voiding, incontinence episodes, nocturia, number of pads, symptom severity, and QoL). Moreover, treatment satisfaction, treatment success, and cure/improvement rates were high.
The present study is important because it is the first study to define T-MPNS as a peripheral neuromodulation method and to evaluate its effectiveness in women with idiopathic OAB. Our findings show that T-MPNS was effective on both clinical parameters and QoL in women with idiopathic OAB. In prospective randomized controlled trials evaluating the effectiveness of PTNS and TTNS in patients with idiopathic OAB, a wide variety of assessment parameters have been used to evaluate treatment success [7,9,11,12,13]. In these studies, it was stated that treatment success rates ranged from 54.5% to 89% for PTNS and from 68% to 95% for TTNS. In our study, the positive response rate (68.4%), which was evaluated as a 50% reduction in incontinence episodes, was compatible with other studies. However, interpretation of the results of our study should take into consideration that the study was not controlled.
In our study, we used previous PTNS and TTNS studies of OAB to select the stimulation parameters and the number and frequency of sessions [7,8,9,10,11,12,13]. Previous studies reported no local or systemic adverse effects related to the transcutaneous technique [4,6,7,8,9,10]. In our study, transcutaneous electrodes were well tolerated, and there were no serious complications in women treated with T-MPNS, which is compatible with other transcutaneous stimulation studies [4,6,7,8,9,10]. The adherence to treatment in our study (90.4%) was compatible with reports in other studies (Peters et al. [12], for PTNS, 87.0%; Manríquez et al. [8], for TTNS, 91.4%; Ramírez-García et al. [10], overall, 89.7%).
The full mechanism of action of TN stimulation is still not fully understood, and the therapy was initially viewed with skepticism by physicians. This was until Finazzi-Agrò et al. [11] and Peters et al. [12] demonstrated true efficacy in two randomized placebo-controlled trials. This therapy is currently included in the guidelines for OAB treatment [4,24]. In our study, T-MPNS has been defined for the first time, and the results obtained can be an exciting start for T-MPNS. Stimulating the MPN affects lower urinary tract function. The exact working mechanism of T-MPNS is unknown. The mechanism of action of bladder neuromodulation is unclear, but the effect is possibly mediated through a combination of increasing cerebral endorphins, stimulation of somatic sacral and lumbar afferent fibers, and activation of efferent fibers to the striated urethral sphincter, all of which inhibit detrusor activity [25]. For now, it is clear that physicians will also be skeptical of T-MPNS. Therefore, randomized controlled (placebo/sham) studies are needed to prove the effectiveness of T-MPNS.
Knowledge of the topographic anatomy of the TN and the electrophysiological nerve conduction technique of the MPN may aid in correct use of T-MPNS (Fig. 2). The MPN and lateral plantar nerves are terminal branches of the TN in the tarsal tunnel. The topographic anatomy of the TN in the tarsal tunnel has been discussed by other authors [14,15,16,17]. Using a reference line called the MMC axis, which extends from the tip of the medial malleolus to the medial tubercle of the calcaneum, Bilge et al. [14] divided the location of the division of the TN into three types (Fig. 2). Type 1, 2, and 3 represent bifurcations proximal to the reference line but in the tarsal tunnel (within the 2-cm range, at the line, and distal to the posterior tarsal tunnel, respectively). Awari and Vatsalaswamy [17] also have added a type IV in the classification for the nerve dividing above the flexor retinaculum. i.e., 2 cm or more proximal to the MMC axis (Fig. 2). Concerning the percentage of cadaveric feet, which shows the various types of division of the TN regarding the MMC axis, 84% were type 1 and 16% were type 4. Their study also showed the most common division level of the TN in the tarsal tunnel to be level 4, which means the division lies in the range of 6 mm to 10 mm above the distal border of the flexor retinaculum [17]. In light of the results, the TN is widely predicted to branch before the reference line (MMC axis). In other words, the MMC axis already states that the MPN is divided from the TN. This is the main reason the proximal (positive) electrode is placed on the MMC axis in this method.
The scientific and clinical importance of our study results are as follows: (i) Our study describes for the first time T-MPNS as a new peripheral neuromodulation method in women with idiopathic OAB; (ii) T-MPNS was shown to be clinically effective in the treatment of women with idiopathic OAB; (iii) Randomized sham-controlled studies are needed to validate the effectiveness of T-MPNS; (iv) When such evidence is obtained, T-MPNS may be a candidate for use as an alternative treatment option in patients with OAB.
There are some limitations in our study. Unfortunately, the study was not controlled and the results could be influenced by a placebo effect. Other limitations of this study are the lack of data on urodynamics and long-term follow-up of women. Another limitation is that it was a single-center study.
CONCLUSIONS
T-MPNS was defined for the first time as a new neuromodulation method. We conclude that T-MPNS was effective on both clinical parameters and QoL associated with incontinence in women with idiopathic OAB in the short term. Randomized placebo/sham-controlled multicenter studies are needed to validate the effectiveness of T-MPNS.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING: None.
- Research conception and design: Necmettin Yildiz.
- Data acquisition: Rafet Sonmez.
- Statistical analysis: Necmettin Yildiz.
- Data analysis and interpretation: all authors.
- Drafting of the manuscript: Necmettin Yildiz.
- Revision of the manuscript: Necmettin Yildiz.
- Approval of the final manuscript: all authors.
References
- 1.de Groat WC, Yoshimura N. Anatomy and physiology of the lower urinary tract. Handb Clin Neurol. 2015;130:61–108. doi: 10.1016/B978-0-444-63247-0.00005-5. [DOI] [PubMed] [Google Scholar]
- 2.de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol. 2015;5:327–396. doi: 10.1002/cphy.c130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moossdorff-Steinhauser HF, Berghmans B. Effects of percutaneous tibial nerve stimulation on adult patients with overactive bladder syndrome: a systematic review. Neurourol Urodyn. 2013;32:206–214. doi: 10.1002/nau.22296. [DOI] [PubMed] [Google Scholar]
- 4.Harding CK, Lapitan MC, Arlandis S, Bø K, Cobussen-Boekhorst H, Costantini E, et al. The European Association of Urology (EAU) Guidelines. EAU Guidelines on management of non-neurogenic female lower urinary tract symptoms [Internet] EAU; 2022. Available from: https://uroweb.org/guidelines/non-neurogenic-female-luts. [Google Scholar]
- 5.Burton C, Sajja A, Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn. 2012;31:1206–1216. doi: 10.1002/nau.22251. [DOI] [PubMed] [Google Scholar]
- 6.Ammi M, Chautard D, Brassart E, Culty T, Azzouzi AR, Bigot P. Transcutaneous posterior tibial nerve stimulation: evaluation of a therapeutic option in the management of anticholinergic refractory overactive bladder. Int Urogynecol J. 2014;25:1065–1069. doi: 10.1007/s00192-014-2359-0. [DOI] [PubMed] [Google Scholar]
- 7.Booth J, Hagen S, McClurg D, Norton C, MacInnes C, Collins B, et al. A feasibility study of transcutaneous posterior tibial nerve stimulation for bladder and bowel dysfunction in elderly adults in residential care. J Am Med Dir Assoc. 2013;14:270–274. doi: 10.1016/j.jamda.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Manríquez V, Guzmán R, Naser M, Aguilera A, Narvaez S, Castro A, et al. Transcutaneous posterior tibial nerve stimulation versus extended release oxybutynin in overactive bladder patients. A prospective randomized trial. Eur J Obstet Gynecol Reprod Biol. 2016;196:6–10. doi: 10.1016/j.ejogrb.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Schreiner L, dos Santos TG, Knorst MR, da Silva Filho IG. Randomized trial of transcutaneous tibial nerve stimulation to treat urge urinary incontinence in older women. Int Urogynecol J. 2010;21:1065–1070. doi: 10.1007/s00192-010-1165-6. [DOI] [PubMed] [Google Scholar]
- 10.Ramírez-García I, Blanco-Ratto L, Kauffmann S, Carralero-Martínez A, Sánchez E. Efficacy of transcutaneous stimulation of the posterior tibial nerve compared to percutaneous stimulation in idiopathic overactive bladder syndrome: randomized control trial. Neurourol Urodyn. 2019;38:261–268. doi: 10.1002/nau.23843. [DOI] [PubMed] [Google Scholar]
- 11.Finazzi-Agrò E, Petta F, Sciobica F, Pasqualetti P, Musco S, Bove P. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol. 2010;184:2001–2006. doi: 10.1016/j.juro.2010.06.113. [DOI] [PubMed] [Google Scholar]
- 12.Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol. 2010;183:1438–1443. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Sonmez R, Yildiz N, Alkan H. Efficacy of percutaneous and transcutaneous tibial nerve stimulation in women with idiopathic overactive bladder: a prospective randomised controlled trial. Ann Phys Rehabil Med. 2022;65:101486. doi: 10.1016/j.rehab.2021.101486. [DOI] [PubMed] [Google Scholar]
- 14.Bilge O, Ozer MA, Govsa F. Neurovascular branching in the tarsal tunnel. Neuroanatomy. 2003;2:39–41. [Google Scholar]
- 15.Joshi SS, Joshi SD, Athavale SA. Anatomy of tarsal tunnel and its applied significance. J Anat Soc India. 2006;55:52–56. [Google Scholar]
- 16.Torres AL, Ferreira MC. Study of the anatomy of the tibial nerve and its branches in the distal medial leg. Acta Ortop Bras. 2012;20:157–164. doi: 10.1590/S1413-78522012000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awari P, Vatsalaswamy P. Topographic anatomy of tibial nerve and its terminal branches in relation with the posterior tarsal tunnel with clinical correlations. Int J Anat Res. 2020;8:7371–7377. [Google Scholar]
- 18.Preston DC, Shapiro BE. Electromyography and neuromuscular disorders: clinical-electrophysiologic correlations. 3rd ed. Elsevier Saunders; 2013. Section IV, routine lower extremity nerve conduction techniques; pp. 115–124. [Google Scholar]
- 19.O’Sullivan R, Karantanis E, Stevermuer TL, Allen W, Moore KH. Definition of mild, moderate and severe incontinence on the 24-hour pad test. BJOG. 2004;111:859–862. doi: 10.1111/j.1471-0528.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 20.Tarcan T, Mangır N, Özgür MÖ, Akbal C. OAB-V8 overactive bladder questionnaire validation study. Urology Bull. 2012;21:113–116. [Google Scholar]
- 21.Acquadro C, Kopp Z, Coyne KS, Corcos J, Tubaro A, Choo MS, et al. Translating overactive bladder questionnaires in 14 languages. Urology. 2006;67:536–540. doi: 10.1016/j.urology.2005.09.035. Erratum in: Urology 2007;69:202. [DOI] [PubMed] [Google Scholar]
- 22.Cam C, Sakalli M, Ay P, Cam M, Karateke A. Validation of the short forms of the incontinence impact questionnaire (IIQ-7) and the urogenital distress inventory (UDI-6) in a Turkish population. Neurourol Urodyn. 2007;26:129–133. doi: 10.1002/nau.20292. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Choi HW, Cho HJ, Hwang TK, Kim JC. The influence of preoperative bladder outlet obstruction on continence and satisfaction in patients with stress urinary incontinence after midurethral sling. Int Neurourol J. 2010;14:267–271. doi: 10.5213/inj.2010.14.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bo K, Frawley HC, Haylen BT, Abramov Y, Almeida FG, Berghmans B, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36:221–244. doi: 10.1002/nau.23107. [DOI] [PubMed] [Google Scholar]
- 25.Groen J, Bosch JL. Neuromodulation techniques in the treatment of the overactive bladder. BJU Int. 2001;87:723–731. doi: 10.1046/j.1464-410x.2001.02219.x. [DOI] [PubMed] [Google Scholar]