Abstract
Studies of cellular and cytokine profiles have contributed to the inflammation hypothesis of schizophrenia; however, precise markers of inflammatory dysfunction remain elusive. A number of proton magnetic resonance spectroscopy (1H-MRS) studies in patients with first-episode psychosis (FEP) have shown higher brain levels of metabolites such as glutamate, myo-inositol (mI) and choline-containing compounds (tCho), suggesting neuroinflammation. Here, we present peripheral inflammatory profiles in antipsychotic-naive FEP patients and age-and-sex matched healthy controls, as well as cortical glutamate, mI and tCho levels using 1H-MRS. Inflammatory profiles were analyzed using cytokine production by peripheral blood mononuclear cells, that were either spontaneous or stimulated, in 48 FEP patients and 23 controls. 1H-MRS of the medial prefrontal cortex was obtained in 29 FEP patients and 18 controls. Finally, 16 FEP patients were rescanned after 4 weeks of treatment (open-label) with Risperidone. FEP patients showed a higher proportion of proinflammatory Th1/Th17 subset, and an increased spontaneous production of Interleukin (IL)-6, IL-2 and IL-4 compared with the control group. Results obtained from 1H-MRS showed no significant difference in either glutamate, mI or tCho between FEP and control groups. At baseline, CD8% showed a negative correlation with glutamate in FEP patients; after 4 weeks of risperidone treatment, the FEP group exhibited a decrease in glutamate levels which positively correlated with CD4+ T cells. Nevertheless, these correlations did not survive correction for multiple comparisons. FEP patients show evidence of immune dysregulation, affecting both the innate and adaptive immune response, with a predominantly Th2 signature. These findings, along with the changes produced by antipsychotic treatment, could be associated with both systemic and central inflammatory processes in schizophrenia.
Keywords: Psychosis, peripheral blood mononuclear cells, cytokines, inflammation, magnetic resonance spectroscopy
1. Introduction
Psychosis is a complex phenomenon of multifactorial etiology that can be conceptualized as a neurodevelopmental disorder that manifests after multiple hits at critical stages, affecting a variety of systems, until the appearance of symptoms (Lieberman and First, 2018). One of these hits is neuroinflammation, which has become increasingly relevant, as recent research provides evidence for the association between abnormalities of the immune system and the pathophysiology of psychosis (Feigenson et al., 2014; Meyer, 2013; Miller et al., 2011; Muller et al., 2012). Specifically, subclinical inflammation of the central nervous system (CNS) and failures in immune regulation are present in patients with schizophrenia: neuroinflammation may lead to alterations in white matter, failures in neuronal connectivity and thus to the onset of psychosis (Najjar and Pearlman, 2015), even in individuals at clinical high-risk (Goldsmith et al., 2019; Mondelli et al., 2023). Emerging evidence suggests that changes in signaling protein expression of PBMCs, which might reflect cell signaling networks in the brain immune cells (i.e. microglia) (van Rees et al., 2018), can predict clinical outcomes in schizophrenia (Lago et al., 2022). Studies show that T lymphocytes transmigrate to the CNS and are responsible for the release of cytokines, neurogenesis modulation, cognitive deficits, as well as altered behavior (de Miranda et al., 2017; Kipnis et al., 2004). Additionally, another process in which cytokines are released in CNS is microglia activation. It could contribute to apoptosis, inhibition of neurogenesis, and white matter abnormalities in patients with recent onset and relapse stages of schizophrenia (de Witte et al., 2014; Garcia-Bueno et al., 2014). Other studies suggests that cytokines may also impact some neurotransmitter systems; inflammatory cytokines that activate the kynurenine pathway, both peripherally and centrally, pass the blood-brain barrier, activating immune responses in the CNS (Schwarcz and Stone, 2017). TNF-α and IFN-γ, for instance, act as inducers of indolamine 2,3-dioxygenase (IDO)-1 expression (Schwarcz and Stone, 2017). IDO-1 is involved in the conversion of tryptophan to kynurenine (KA). In brain, downstream metabolization of KA occurs through divergent routes, especially in microglia and astrocytes; in the latter, KA is metabolized by kynurenine aminotransferases to kynurenic acid, which is a well-established antagonist of all ionotropic glutamate receptor subtypes (Skorobogatov et al., 2021). It has been hypothesized that abnormally elevated kynurenic acid levels may result in glutamate hypofunction by sustained N-methyl-D-aspartate (NMDA) receptor antagonism (Skorobogatov et al., 2021), which has been associated with the onset of psychotic symptoms and cognitive and social impairments in schizophrenia (Javitt, 2007). This NMDA receptor alteration is also associated with dysfunction of parvalbumin containing γ-aminobutyric acid (GABA) interneurons, resulting in excessive glutamate release (Cohen et al., 2015). Proton Magnetic Resonance Spectroscopy (1H-MRS) allows the quantification of intracellular and extracellular glutamate, and other metabolites such as myo-inositol (mI) and total choline (phosphocholine + choline; tCho).
The medial prefrontal cortex (mPFC) has been a frequent target of 1H-MRS studies in schizophrenia since it has been implicated in processes that are altered in the disorder, such as reward, decision making, social processing, and episodic memory (de la Vega et al., 2016). Although some 1H-MRS studies have reported elevations of glutamatergic compounds in the mPFC of unmedicated schizophrenia (Kegeles et al., 2012) and antipsychotic-naive first-episode psychosis (FEP) patients (Cen et al., 2020; de la Fuente-Sandoval et al., 2018; Simmonite et al., 2023; Yang et al., 2015), these results were not supported by a recent mega-analysis (Merritt et al., 2023). Even though studies of the mPFC in patients with schizophrenia have found no differences in tCho or mI compared to controls (Birur et al., 2020; Bojesen et al., 2021; de la Fuente-Sandoval et al., 2018; Iwata et al., 2018; Kraguljac et al., 2012; Theberge et al., 2002), our group has previously reported neurometabolic differences in the striatum of antipsychotic-naive FEP patients (de la Fuente-Sandoval et al., 2013; Plitman et al., 2016). Increased levels of mI and tCho, which are present in higher concentrations within glial cells than in neurons, were found. These metabolites have been proposed to reflect glial activation (Chang et al., 2014; Inglese et al., 2003; Miller et al., 1993), which is commonly associated with a neuroinflammatory response (Chang et al., 2013). In the present study, we compared glutamate, mI and tCho (measured by 1H-MRS), in the mPFC of individuals with FEP and assessed immunophenotypic characterization of the peripheral blood mononuclear cells (PBMCs) and cytokines in serum and cell culture supernatant. This could be relevant since most of the available literature used immunoassay to profile static cytokine levels. Also, these studies consist of cross-sectional measurements mainly conducted in medicated patients.
These measures were repeated after 4 weeks of antipsychotic treatment, since these medications could normalize differences in inflammatory markers between FEP patients and controls (Kelsven et al., 2020). Finally, all results were compared with a group of age- and sex-matched healthy controls. We hypothesized to find increased glutamate levels, as well as pro-inflammatory cytokines in FEP individuals in comparison to healthy controls. After antipsychotic treatment in FEP patients, we expected a tendency towards normalization of glutamate and pro-inflammatory cytokines. Finally, we hypothesized positive correlations between glutamate, tCho and mI with pro-inflammatory cytokines before antipsychotic treatment.
2. Materials and Methods
2.1. Participants and clinical assessments
Forty-eight antipsychotic-naive FEP patients were recruited through the inpatient and outpatient services of the Instituto Nacional de Neurología y Neurocirugía in Mexico City (INNN). For the control group, 23 age-and-sex matched subjects were recruited. Inclusion and Exclusion criteria are described in the Supplementary Material.
A subsample of 29 FEP patients and 18 controls was scanned for 1H-MRS. At enrollment FEP patients were evaluated with the Positive and Negative Syndrome Scale (PANSS), and then, after the 1H-MRS session, treated with oral risperidone for 4 weeks, with an open flexible regimen based on clinical judgment, starting with 1mg and up to 5mg per day. Sixteen patients were rescanned after four weeks. The control group was only scanned at the initial evaluation.
The Ethics and Scientific Committees of the INNN approved this study. All participants provided written informed consent. For participants younger than 18 years of age, consent was obtained from both parents.
2.2. Immunophenotypic characterization of the peripheral blood mononuclear cells subsets
At the time of recruitment (baseline) and one month after the antipsychotic treatment, twenty milliliters of venous peripheral blood to each FEP patient were drew. Then, PBMCs were isolated by density gradients after centrifugation with Ficoll-Paque (GE Healthcare, Chicago, Illinois, USA). After two washes with phosphate buffered saline (PBS) (Fisher scientific, Waltham Massachusetts, USA), viability was assessed using the Zombie Aqua staining (Biolegend, San Diego, California, USA). Afterwards, the cells were washed twice with 5% fetal bovine serum (FBS) (Fisher scientific, Waltham Massachusetts, USA) in PBS. For the immunophenotypic characterization, PBMCs were stained with the following fluorochrome-coupled antibodies: CD3-APC/Fire 750, CD19-APC, CD4-Alexa Fluor 488, CD8-PE/Dazzle 594, CD25-PE, FoxP3-Brilliant Violet 421, CD56-PE, NKp46-Briliiant Violet 605, CD14-PerCP, CD16-APC (Biolegend, San Diego, California, USA). To evaluate the CD4+ T helper subsets, PBMCs were stimulated with 50 ng/mL phorbol myristate acetate (PMA), 1 μ/mL ionomycin (Sigma-Aldrich, Saint Louis, Missouri, USA) and monensin (BD Biosciences, Franklin Lakes, New Jersey, USA) for five hours. After two washes with 5% FBS in PBS, cells were fixed and permeabilized using the cytofix/cytoperm kit (BD Biosciences, Franklin Lakes, New Jersey, USA) according to the manufacturer’s instructions. The following intracytoplasmic cytokines with fluorochrome coupled antibodies were assessed: IFN-γ-APC, Interleukin (IL) −4-PE, and IL-17A-Brilliant Violet 421 (Biolegend, San Diego, California, USA). One million events from all the samples were acquired in a LSR Fortessa flow cytometer (BD Biosciences, Franklin Lakes, New Jersey, USA).
The proportions of the following cell subsets were quantified using the gating strategy described in Figure 1 of the Supplementary Material: B cells (CD3-, CD19+), CD8+ T cells (CD3+, CD8+), CD4+ T cells (CD3+, CD4+), regulatory T cells (Tregs) (CD3+, CD4+, CD25hi, FoxP3+), classical monocytes (CD14++, CD16-), intermediate monocytes (CD14+, CD16+), non-classical monocytes (CD14+, CD16++). NK cells were defined as CD3-, NKp46+ and further divided into CD56hi and CD56dim. The proportions of T helper subsets were characterized as follow: Th1 (CD4+, IFN-γ+), Th2 (CD4+, IL-4+), Th17 (CD4+, IFN-17A+), Th1/17 (CD4+, IFN-γ+, IL-17A+). For lymphoid subsets, the percentages refer to the proportion of live cells in the total lymphocyte gate; for the monocytic subsets, refers to the proportion of live cells in the monocyte gate according to the gating strategy. The analysis was performed using the FlowJo v10.8.1 software.
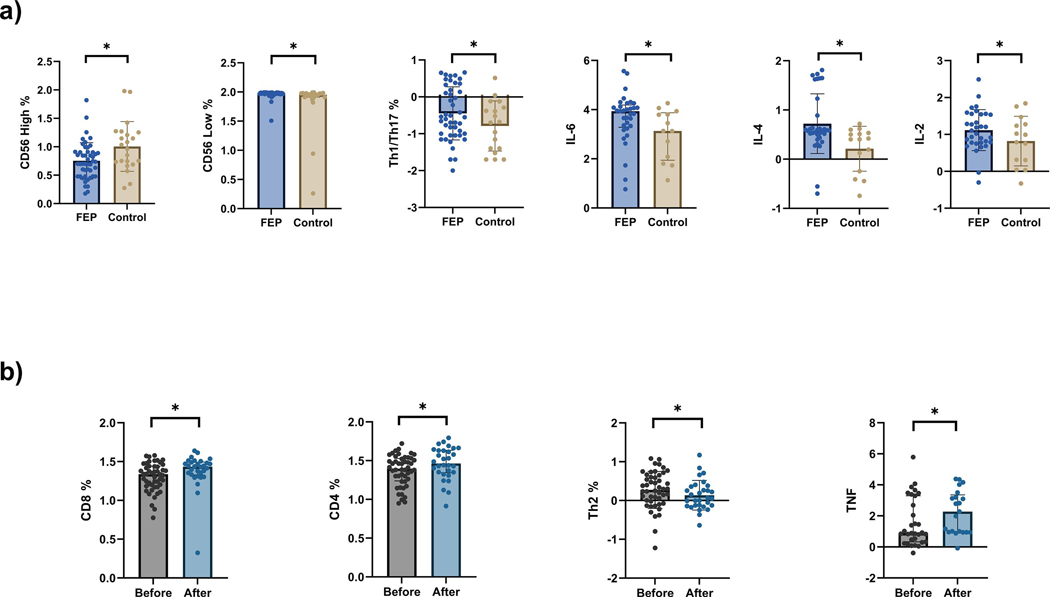
Figure 1.
Scatter plots of a) Inflammatory markers of never medicated first episode of psychosis subjects compared to healthy controls. b) Inflammatory markers of first episode of psychosis before and after 4 weeks of risperidone treatment. FEP, first-episode psychosis group; Th1, Type 1 helper T cells; Th17, T helper 17 cells; IL-6, interleukin 6; IL-4, interleukin 4; IL-2, interleukin 2; Th2, Type 2 helper T cells; TNF, Tumor necrosis factor. Data shown in the Figure has been log transformed for a better visualization. * p < 0.05.
2.3. Assessment of cytokines responses
Two million PBMCs were cultured during 72 hours with and without stimulation with 50 ng/mL PMA and 1 μ/mL ionomycin. Afterwards, samples were centrifugated for 10 minutes at 1600 rpm. The supernatant was recovered and stored at −80ºC until further analysis. The concentrations of IL17A, IFN-γ, TNF, IL-10, IL-6, IL-4 and IL-2 were evaluated in serum and in cell culture supernatants using the human Th1/Th2/Th17 cytometric bead array (CBA) kit (BD Biosciences, Franklin Lakes, New Jersey, USA) according to the manufacturer’s instructions. Samples were analyzed using the FCAP array v3.0 software.
2.4. Image acquisition
Magnetic Resonance studies were performed at the INNN on a 3T scanner (Magnetom Skyra, Siemens, Germany), using a 20-channel phased array transmit/receive-head coil. First, we acquired a high-resolution T1-weighted three-dimensional magnetization-prepared rapid acquisition with gradient echo (MPRAGE; TE=5ms, TR=12 ms, inversion time=450 ms, flip angle=20⁰, FOV=25.6 cm, 256×256 matrix, 186 slices, slice thickness=1 mm), oriented above and parallel to the anterior commissure-posterior commissure line. For the acquisition of 1H-MRS, the MPRAGE structural image was reformatted to sagittal and coronal views for voxel placement. 1H-MRS spectra was obtained using point-resolved spectroscopy (PRESS, TE=35 ms, TR=2000 ms; spectral width=5000 Hz; 4096 data points used; 128 water-suppressed and 16 water-unsuppressed averages) in a 2.5×2.5×2.5 cm voxel centered in the mPFC bilaterally (portions of Brodmann areas 10, 24, 32, and the pregenual anterior cingulate cortex).
2.5. 1H-MRS analysis
Water-suppressed spectra were analyzed using LCModel, version 6.3–1L (Provencher, 2001), detailed in the Supplementary Material. Metabolite levels were corrected for the cerebrospinal fluid (CFS) fraction of the voxel (Gasparovic et al., 2006). Metabolites fits with a %SD Cramér–Rao lower bound exceeding 20% spectra or with a full-width at half maximum exceeding 12 Hz as reported by LCModel were considered poor quality and were excluded from further analyses.
2.6. Data analysis
Parametric and non-parametric tests were used accordingly based on data distribution previously examined with normality tests (Shapiro-Wilk). Demographic and clinical characteristics between FEP and control groups were compared using χ2 test for nominal variables, and Student t test or Mann-Whitney U for numerical continuous variables. Data is shown using means with standard deviation, or medians with interquartile range, respectively. Systemic inflammatory profile and neurometabolites were also analyzed comparing means or medians, Student t test or Mann-Whitney U. Comparison of FEP patients before and after treatment was done with paired t test or Wilcoxon test, depending on data distribution. Statistical significance level for these variables was established at p<0.05. Finally, exploratory correlations between systemic inflammatory profile variables, 1H-MRS neurometabolites, and PANSS scores were examined using Pearson or Spearman linear correlation tests. The statistical threshold for these exploratory correlations was set p<0.016 (p<0.05/3) for the neurometabolites (glutamate, mI and tCho), and at p<0.012 (p<0.05/4) for the clinical scores (PANSS Total score and Positive, Negative, and General Psychopathology subscales). All statistical analyses were performed using GraphPad Prism, version 9.3.1.
3. Results
3.1. Sociodemographic and clinical characteristics
Demographic and clinical characteristics of study participants are shown in Table 1. No statistical differences were found between groups for age, gender, tobacco or cannabis use. The FEP patients had less education (t70=5.22, p<0.001), and parental education (U=326, p=0.01) in comparison to the control group. The mean (SD) PANSS total scores for the FEP group at baseline were 107.1 (20.2) and 64.8 (20.9) after 4 weeks of antipsychotic treatment (t31=8.71, p<0.01). The mean (SD) daily dose of risperidone after 4 weeks was 2.38 (0.72) mg.
Table 1.
Demographic and clinical characteristics of study participants.
| Characteristic | Mean (SD) | Statistic | p value | |
|---|---|---|---|---|
| FEP (n=48) | Controls (n=23) | |||
| Age, years | 30.7 (12.2) |
31.6 (12.4) |
U=521 | 0.71 |
| Female, No. (%) | 19 (0.40) | 13 (0.57) | χ2=1.80 | 0.18 |
| Education, years | 11.21 (3.48) |
15.55 (2.58) |
t=5.22 | <0.001 |
| Parental education, years | 9.23 (4.85) |
12.73 (4.98) |
U=326 | 0.01 |
| Tobacco (ever used) | 18 out of 48 |
5 out of 23 |
χ2=1.76 | 0.18 |
| Cannabis (ever used) | 20 out of 48 |
6 out of 23 |
χ2=1.62 | 0.20 |
| Duration of untreated psychosis, mean (SD) [range], weeks | 232 (415) [1–1729] |
NA | NA | NA |
| PANSS total score | 107.1 (20.2) |
NA | NA | NA |
| PANSS subscale score | ||||
| Positive symptoms | 28.98 (5.01) |
NA | NA | NA |
| Negative symptoms | 25.68 (8.03) |
NA | NA | NA |
| General psychopathology | 52.43 (10.75) |
NA | NA | NA |
FEP, first-episode psychosis group; SD, standard deviation; PANSS, Positive and Negative Syndrome Scale; NA, not applicable.
3.2. Altered immune function in first episode of psychosis
Differences on the systemic inflammatory profile were found between participants with FEP and controls at baseline (Figure 1a, Supplementary Table 1). FEP patients had a higher proportion of the proinflammatory Th1/Th17 subset compared to controls (U=325, p=0.03). Also, FEP patients had an increased production of IL-6 (U=193, p=0.02), IL-2 (U=196, p=0.03), and IL-4 (U=144, p<0.01) compared to controls. The percentage of NK CD56high cells was found lower in the FEP patients (U=334, p=0.01), while having an increased percentage of NK CD56low cells (U=328, p=0.01), when compared to controls (Figure 1a).
3.3. Inflammatory profile after antipsychotic treatment
Following 4 weeks of antipsychotic treatment, significant changes on several inflammatory markers were observed (Figure 1b, Supplementary Table 2): Increased percentage of CD8+ cells (Pre vs Post treatment, t29=2.77, p=0.01), CD4+ lymphocytes % (Pre vs Post treatment, t29=3.05, p<0.01) and TNF levels (Pre vs Post treatment, W= −102, p=0.04). On the other hand, Th2 levels decreased after treatment (Pre vs Post treatment, W=303, p<0.001). No differences were found in either Th1/Th17 subset, IL-6, IL-2, and IL-4 after treatment.
3.4. 1H-MRS results
1H-MRS data for 7 participants (data for 3 patients and 4 controls) was rejected from analyses due to poor fitting. Results showed a trend toward significance for higher glutamate levels in FEP compared to controls groups, (t38=1.95, p=0.06) (Supplementary Table 3). After 4 weeks of risperidone treatment (Supplementary Table 4), FEP patients were found to have decreased glutamate levels (t15=3.21, p<0.01). No differences in tCho or mI were observed between the FEP and control groups at baseline or in FEP before and after treatment.
3.5. Relationship between Inflammatory markers and clinical measures
In FEP patients at baseline, CD8% correlated negatively with glutamate (r24=−0.43, p=0.03). After 4 weeks of antipsychotic treatment, glutamate levels correlated positively with CD4% (r13=0.57, p=0.03), but not with CD8% (r13=−0.03, p=0.92) (Supplementary Figure 2). Regarding clinical measures, at 4 weeks total PANSS score negatively correlated with induced IL-17 production after 4 weeks of treatment (r18= −0.52, p=0.02). No other clinical correlations were observed. None of the reported correlations survived after correction for multiple comparisons.
4. Discussion
In the present study, we found that untreated FEP patients are characterized by an immune dysregulation that affects both the innate (IL-6, NK cells) and adaptive (Th1/Th17 subset) immune response, and that alterations of the innate (TNF) and adaptive (CD8+, CD4+, and Th2 cells) immune response are modified after antipsychotic treatment. Previous studies have demonstrated the presence of a proinflammatory response in schizophrenia, where a higher neutrophil to lymphocyte ratio (Hughes and Ashwood, 2020) and a differential pro-inflammatory gene expression were found (Sainz et al., 2013; van Kesteren et al., 2017). Indeed, intracellular signaling alterations in PBMCs have been associated with polygenic risk of schizophrenia and clinical outcomes (Lago et al., 2022). In the present study, we found a higher proportion of the proinflammatory Th1/Th17 subset, an increased production of IL-6 upon mitogenic stimulation, and an increased spontaneous release of IL-6 and IL-2 in PBMCs from FEP patients. Most human studies published to date in psychosis have measured plasma-circulating cytokine levels, instead of in vitro cell producing cytokines like in our study. While our results contribute to this literature niche, caution is warranted when discussing them in the context of current literature due to the previously reported difference between these two measurements. Moreover, the relation between plasma-circulating and cell produced cytokines might vary depending on several immune-altering factors (Jason et al., 2001). For instance, other studies have reported, using enzyme-linked immunosorbent assay and by cytometric analysis, increased IL-6 levels in treatment-naive FEP patients and recently diagnosed schizophrenia (Azizi et al., 2019; Noto et al., 2014). Aside from being elevated in serum and in CSF, IL-6 has been considered as a “state marker”, since its increase has been related to a longer duration of the disease and greater symptomatic severity in positive, negative, and cognitive symptoms (Reale et al., 2021). Moreover, increased IL-6 has also been associated with reduced activity of glutamate decarboxylase, involved in GABA production (Behrens et al., 2008), which has been hypothesized to lead to psychotic symptomatology (Guidotti et al., 2000). Although our results were obtained from cell production, it is tempting to consider that if serum IL-6 levels were also measured, they might have been also elevated as previously reported (Sahbaz et al., 2020). Ideally, future studies will continue to explore possible connections between different measurements of IL-6 in psychosis. At present, it seems that changes in the metabolic dynamics of glutamate in CNS are relevant since, in addition to neurotoxic effects (Plitman et al., 2014), it has been reported that changes in glutamatergic transmission may precede to alterations in downstream neurotransmitter systems such as dopamine (Stone et al., 2007). In turn, neurotransmitters such as dopamine are capable of modulating T cell functions. In support of this observation, genetic variants of some dopamine receptors have been shown to be associated with CD4+ T cell counts (Sahbaz et al., 2020).
On the other hand, other studies have found a Th2 predominance in psychotic disorders (Maino et al., 2007; Roomruangwong et al., 2020). In line with this finding, we found an increased spontaneous production of IL-4 in FEP PBMCs as previously described in male patients with FEP and subjects at clinical high-risk for psychosis (Karanikas et al., 2017). Although the role of IL-2 in psychotic disorders remains controversial, with studies showing both increased (Mahendran et al., 2004; Na and Kim, 2007) and decreased (Tan et al., 2015) levels (probably in relation to treatment status), our findings are in accordance with previous studies that shown a higher production of IL-2 in PBMCs of treatment-naive schizophrenia patients (Cazzullo et al., 2002; O’Donnell et al., 1996). Moreover, increased IL-2 is related to a better cognitive performance and milder symptomatology in schizophrenia (Hope et al., 2015; Kogan et al., 2018). Higher levels of the soluble IL-2 receptor (sIL-2R) have been observed in patients with FEP (Wang et al., 2020), and has been proposed as a potential marker of treatment refractoriness (Gilmore et al., 2004).
To the best of our knowledge, the increased proportion of CD56low NK cells and Th1/Th17 has not been previously reported in FEP patients. Natural killer cells acquire the CD56low phenotype after their exposure to IL-2 and promote a Th2 phenotype after they are exposed to IL-4 (Moretta et al., 2008). To the best of our knowledge, only one study has evaluated NK cells in FEP, finding increased markers of activation but deficient function (Tarantino et al., 2021). Furthermore, our study highlights the expansion of the Th1/Th17 in FEP as a proinflammatory marker, which has been associated with decreased Treg function in autoimmune diseases (Ulivieri and Baldari, 2013). One previous study with flow cytometric analyses also found higher percentages of Th17 cells in patients with schizophrenia under antipsychotic treatment (Drexhage et al., 2011). Interestingly, the same study found that higher Treg percentages correlated with a better clinical outcome, contributing further to the already proposed importance of Treg function (and its association with Th17 cells) in schizophrenia (Corsi-Zuelli et al., 2021). Such association, as well as the role of NK cells and Th1/Th17 subsets in the pathogenesis of FEP, require further investigation.
In accordance with our data, Steiner et al. found an increased number of total T cells and CD4+ T lymphocytes after 6 weeks of antipsychotic treatment (Steiner et al., 2010), suggesting a neuroprotective role (Byram et al., 2004). We found that after treatment, the Th2 subset decreased (Maino et al., 2007), as did glutamatergic compounds levels, as previously reported (de la Fuente-Sandoval et al., 2013; de la Fuente-Sandoval et al., 2018). Interestingly, the initial negative correlation between glutamate and CD8+ was not found after treatment, while the positive the correlation between glutamate levels and CD4+ was present after treatment, possibly highlights the immunoregulatory function of antipsychotics, and the possible glutamatergic mediation of neuroinflammation. However, these correlations did not survive correction for multiple comparisons, and it is important to clarify that such comparisons differ in sample size due to the reduced number of scans after treatment, which is a limitation on interpreting the significance of these correlations. Several study limitations need to be considered: First, clinical factors, such as smoking and cardiovascular disease, are known to modify inflammatory markers (Asthana et al., 2010; Danesh et al., 1998; El-Zayadi, 2006; Hirano, 2021). In our study we were not able to control these factors given the initial design of the study. Second, the groups could not be matched for education; this finding is common since patients become ill before reaching their full academic potential (Crossley et al., 2022). Third, we did not include cognitive evaluations; therefore, we could not address the possible effect of inflammatory markers on cognitive domains. Fourth, assessment of direct glutamate neurotransmission is not possible with 1H-MRS, since this technique is unable to differentiate neurotransmitter or vesicular and metabolic pools.
In conclusion, the studied cohort of treatment-naive FEP patients presented an enhanced Th2 signature and proinflammatory cytokine response in comparison to healthy controls. Additionally, the comparison of these parameters before and after treatment allowed us to detect the immunomodulatory effects of risperidone, which reduced this Th2 phenotype, along with glutamate levels, and enhanced the immune function measured as a higher capacity to produce cytokines in vitro.
Supplementary Material
Highlights.
First episode psychosis patients (FEP) and matched healthy controls were studied.
FEP patients showed higher T helper type (Th)1 / Th17 subset.
FEP patients showed increased pro-inflammatory interleukin (IL)-6, IL-2 and IL-4.
Acknowledgments
This project was supported by Consejo Nacional de Ciencia y Tecnología, Mexico, (CONACyT) Grant No. 261895 to Camilo de la Fuente-Sandoval, National Institutes of Health (NIH) Grant No. R21 MH102374 to Kristin S. Cadenhead and Camilo de la Fuente-Sandoval, and CONACyT’s Sistema Nacional de Investigadores (SNI) support to Pablo León-Ortiz, Francisco Reyes-Madrigal, Jiram Torres-Ruíz, Diana Gómez-Martín and Camilo de la Fuente-Sandoval.
Role of the Funding sources
CONACyT, NIH and SNI had no further role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online.
Declaration of Competing Interest
Pablo León-Ortiz and Francisco Reyes-Madrigal have received speaking fees from Janssen (Johnson & Johnson) outside the submitted work. The rest of the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH, 2010. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. Am Heart J 160, 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, Zavaran Hosseini A, Soudi S, Noorbala AA, 2019. Alteration of Serum Levels of Cytokines in Schizophrenic Patients before and after Treatment with Risperidone. Iran J Allergy Asthma Immunol 18, 262–268. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL, 2008. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28, 13957–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birur B, Kraguljac NV, VerHoef L, Morgan CJ, Jindal RD, Reid MA, Luker A, Lahti AC, 2020. Neurometabolic correlates of 6 and 16 weeks of treatment with risperidone in medication-naive first-episode psychosis patients. Transl Psychiatry 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen KB, Broberg BV, Fagerlund B, Jessen K, Thomas MB, Sigvard A, Tangmose K, Nielsen MO, Andersen GS, Larsson HBW, Edden RAE, Rostrup E, Glenthoj BY, 2021. Associations Between Cognitive Function and Levels of Glutamatergic Metabolites and Gamma-Aminobutyric Acid in Antipsychotic-Naive Patients With Schizophrenia or Psychosis. Biol Psychiatry 89, 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ, 2004. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci 24, 4333–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzullo CL, Sacchetti E, Galluzzo A, Panariello A, Adorni A, Pegoraro M, Bosis S, Colombo F, Trabattoni D, Zagliani A, Clerici M, 2002. Cytokine profiles in schizophrenic patients treated with risperidone: a 3-month follow-up study. Prog Neuropsychopharmacol Biol Psychiatry 26, 33–39. [DOI] [PubMed] [Google Scholar]
- Cen H, Xu J, Yang Z, Mei L, Chen T, Zhuo K, Xiang Q, Song Z, Wang Y, Guo X, Wang J, Jiang K, Xu Y, Li Y, Liu D, 2020. Neurochemical and brain functional changes in the ventromedial prefrontal cortex of first-episode psychosis patients: A combined functional magnetic resonance imaging-proton magnetic resonance spectroscopy study. Aust N Z J Psychiatry 54, 519–527. [DOI] [PubMed] [Google Scholar]
- Chang L, Jiang C, Cunningham E, Buchthal S, Douet V, Andres M, Ernst T, 2014. Effects of APOE epsilon4, age, and HIV on glial metabolites and cognitive deficits. Neurology 82, 2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Munsaka SM, Kraft-Terry S, Ernst T, 2013. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 8, 576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Tsien RW, Goff DC, Halassa MM, 2015. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res 167, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi-Zuelli F, Deakin B, de Lima MHF, Qureshi O, Barnes NM, Upthegrove R, Louzada-Junior P, Del-Ben CM, 2021. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health 17, 100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Alliende LM, Czepielewski LS, Aceituno D, Castaneda CP, Diaz C, Iruretagoyena B, Mena C, Mena C, Ramirez-Mahaluf JP, Tepper A, Vasquez J, Fonseca L, Machado V, Hernandez CE, Vargas-Upegui C, Gomez-Cruz G, Kobayashi-Romero LF, Moncada-Habib T, Arango C, Barch DM, Carter C, Correll CU, Freimer NB, McGuire P, Evans-Lacko S, Undurraga E, Bressan R, Gama CS, Lopez-Jaramillo C, de la Fuente-Sandoval C, Gonzalez-Valderrama A, Undurraga J, Gadelha A, 2022. The enduring gap in educational attainment in schizophrenia according to the past 50 years of published research: a systematic review and meta-analysis. Lancet Psychiatry 9, 565–573. [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Appleby P, Peto R, 1998. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279, 1477–1482. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, Alvarado-Alanis P, Ramirez-Bermudez J, Graff-Guerrero A, 2013. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 70, 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Jung-Cook H, Solis-Vivanco R, Graff-Guerrero A, Shungu DC, 2018. Prefrontal and Striatal Gamma-Aminobutyric Acid Levels and the Effect of Antipsychotic Treatment in First-Episode Psychosis Patients. Biol Psychiatry 83, 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T, 2016. Large-Scale Meta-Analysis of Human Medial Frontal Cortex Reveals Tripartite Functional Organization. J Neurosci 36, 6553–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miranda AS, Zhang CJ, Katsumoto A, Teixeira AL, 2017. Hippocampal adult neurogenesis: Does the immune system matter? J Neurol Sci 372, 482–495. [DOI] [PubMed] [Google Scholar]
- de Witte L, Tomasik J, Schwarz E, Guest PC, Rahmoune H, Kahn RS, Bahn S, 2014. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res 154, 23–29. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJ, Drexhage HA, 2011. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol 14, 746–755. [DOI] [PubMed] [Google Scholar]
- El-Zayadi AR, 2006. Heavy smoking and liver. World J Gastroenterol 12, 6098–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson KA, Kusnecov AW, Silverstein SM, 2014. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev 38, 72–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bueno B, Bioque M, Mac-Dowell KS, Barcones MF, Martinez-Cengotitabengoa M, Pina-Camacho L, Rodriguez-Jimenez R, Saiz PA, Castro C, Lafuente A, Santabarbara J, Gonzalez-Pinto A, Parellada M, Rubio G, Garcia-Portilla MP, Mico JA, Bernardo M, Leza JC, 2014. Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull 40, 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA, 2006. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55, 1219–1226. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM, 2004. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology 29, 1221–1229. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, Haroon E, Miller AH, Addington J, Bearden C, Cadenhead K, Cannon T, Cornblatt B, Mathalon D, McGlashan T, Seidman L, Tsuang M, Woods SW, Walker EF, Perkins DO, 2019. Association of baseline inflammatory markers and the development of negative symptoms in individuals at clinical high risk for psychosis. Brain Behav Immun 76, 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E, 2000. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 57, 1061–1069. [DOI] [PubMed] [Google Scholar]
- Hirano T, 2021. IL-6 in inflammation, autoimmunity and cancer. Int Immunol 33, 127–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope S, Hoseth E, Dieset I, Morch RH, Aas M, Aukrust P, Djurovic S, Melle I, Ueland T, Agartz I, Ueland T, Westlye LT, Andreassen OA, 2015. Inflammatory markers are associated with general cognitive abilities in schizophrenia and bipolar disorder patients and healthy controls. Schizophr Res 165, 188–194. [DOI] [PubMed] [Google Scholar]
- Hughes HK, Ashwood P, 2020. Overlapping evidence of innate immune dysfunction in psychotic and affective disorders. Brain Behav Immun Health 2, 100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M, Li BS, Rusinek H, Babb JS, Grossman RI, Gonen O, 2003. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med 50, 190–195. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Nakajima S, Plitman E, Mihashi Y, Caravaggio F, Chung JK, Kim J, Gerretsen P, Mimura M, Remington G, Graff-Guerrero A, 2018. Neurometabolite levels in antipsychotic-naive/free patients with schizophrenia: A systematic review and meta-analysis of (1)H-MRS studies. Prog Neuropsychopharmacol Biol Psychiatry 86, 340–352. [DOI] [PubMed] [Google Scholar]
- Jason J, Archibald LK, Nwanyanwu OC, Byrd MG, Kazembe PN, Dobbie H, Jarvis WR, 2001. Comparison of serum and cell-specific cytokines in humans. Clin Diagn Lab Immunol 8, 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, 2007. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol 78, 69–108. [DOI] [PubMed] [Google Scholar]
- Karanikas E, Manganaris S, Ntouros E, Floros G, Antoniadis D, Garyfallos G, 2017. Cytokines, cortisol and IGF-1 in first episode psychosis and ultra high risk males. Evidence for TNF-alpha, IFN-gamma, TauNF-beta, IL-4 deviation. Asian J Psychiatr 26, 99–103. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC, 2012. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69, 449–459. [DOI] [PubMed] [Google Scholar]
- Kelsven S, de la Fuente-Sandoval C, Achim CL, Reyes-Madrigal F, Mirzakhanian H, Domingues I, Cadenhead K, 2020. Immuno-inflammatory changes across phases of early psychosis: The impact of antipsychotic medication and stage of illness. Schizophr Res 226, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M, 2004. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A 101, 8180–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan S, Ospina LH, Kimhy D, 2018. Inflammation in individuals with schizophrenia - Implications for neurocognition and daily function. Brain Behav Immun 74, 296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti AC, 2012. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res 203, 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago SG, Tomasik J, van Rees GF, Rustogi N, Vazquez-Bourgon J, Papiol S, Suarez-Pinilla P, Crespo-Facorro B, Bahn S, 2022. Peripheral lymphocyte signaling pathway deficiencies predict treatment response in first-onset drug-naive schizophrenia. Brain Behav Immun 103, 37–49. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, First MB, 2018. Psychotic Disorders. N Engl J Med 379, 270–280. [DOI] [PubMed] [Google Scholar]
- Mahendran R, Mahendran R, Chan YH, 2004. Interleukin-2 levels in chronic schizophrenia patients. Ann Acad Med Singap 33, 320–323. [PubMed] [Google Scholar]
- Maino K, Gruber R, Riedel M, Seitz N, Schwarz M, Muller N, 2007. T- and B-lymphocytes in patients with schizophrenia in acute psychotic episode and the course of the treatment. Psychiatry Res 152, 173–180. [DOI] [PubMed] [Google Scholar]
- Merritt K, McCutcheon RA, Aleman A, Ashley S, Beck K, Block W, Bloemen OJN, Borgan F, Boules C, Bustillo JR, Capizzano AA, Coughlin JM, David A, de la Fuente-Sandoval C, Demjaha A, Dempster K, Do KQ, Du F, Falkai P, Galinska-Skok B, Gallinat J, Gasparovic C, Ginestet CE, Goto N, Graff-Guerrero A, Ho BC, Howes O, Jauhar S, Jeon P, Kato T, Kaufmann CA, Kegeles LS, Keshavan MS, Kim SY, King B, Kunugi H, Lauriello J, Leon-Ortiz P, Liemburg E, McIlwain ME, Modinos G, Mouchlianitis E, Nakamura J, Nenadic I, Ongur D, Ota M, Palaniyappan L, Pantelis C, Patel T, Plitman E, Posporelis S, Purdon SE, Reichenbach JR, Renshaw PF, Reyes-Madrigal F, Russell BR, Sawa A, Schaefer M, Shungu DC, Smesny S, Stanley JA, Stone J, Szulc A, Taylor R, Thakkar KN, Theberge J, Tibbo PG, van Amelsvoort T, Walecki J, Williamson PC, Wood SJ, Xin L, Yamasue H, McGuire P, Egerton A, Investigators H.M.i.S., 2023. Variability and magnitude of brain glutamate levels in schizophrenia: a meta and mega-analysis. Mol Psychiatry 10.1038/s41380-023-01991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, 2013. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 42, 20–34. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B, 2011. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD, 1993. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology 187, 433–437. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Blackman G, Kempton MJ, Pollak TA, Iyegbe C, Valmaggia LR, Amminger P, Barrantes-Vidal N, Bressan R, van der Gaag M, de Haan L, Krebs MO, Nordentoft M, Ruhrmann S, Riecher-Rossler A, Rutten BPF, Sachs G, Koutsouleris N, McGuire P, 2023. Serum Immune Markers and Transition to Psychosis in Individuals at Clinical High Risk. Brain Behav Immun 10.1016/j.bbi.2023.03.014. [DOI] [PubMed] [Google Scholar]
- Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L, 2008. NK cells at the interface between innate and adaptive immunity. Cell Death Differ 15, 226–233. [DOI] [PubMed] [Google Scholar]
- Muller N, Myint AM, Schwarz MJ, 2012. Inflammation in schizophrenia. Adv Protein Chem Struct Biol 88, 49–68. [DOI] [PubMed] [Google Scholar]
- Na KS, Kim YK, 2007. Monocytic, Th1 and th2 cytokine alterations in the pathophysiology of schizophrenia. Neuropsychobiology 56, 55–63. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, 2015. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 161, 102–112. [DOI] [PubMed] [Google Scholar]
- Noto C, Ota VK, Gouvea ES, Rizzo LB, Spindola LM, Honda PH, Cordeiro Q, Belangero SI, Bressan RA, Gadelha A, Maes M, Brietzke E, 2014. Effects of risperidone on cytokine profile in drug-naive first-episode psychosis. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MC, Catts SV, Ward PB, Liebert B, Lloyd A, Wakefield D, McConaghy N, 1996. Increased production of interleukin-2 (IL-2) but not soluble interleukin-2 receptors (sIL-2R) in unmedicated patients with schizophrenia and schizophreniform disorder. Psychiatry Res 65, 171–178. [DOI] [PubMed] [Google Scholar]
- Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, Chavez S, Gomez-Cruz G, Leon-Ortiz P, Graff-Guerrero A, 2016. Elevated Myo-Inositol, Choline, and Glutamate Levels in the Associative Striatum of Antipsychotic-Naive Patients With First-Episode Psychosis: A Proton Magnetic Resonance Spectroscopy Study With Implications for Glial Dysfunction. Schizophr Bull 42, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Nakajima S, de la Fuente-Sandoval C, Gerretsen P, Chakravarty MM, Kobylianskii J, Chung JK, Caravaggio F, Iwata Y, Remington G, Graff-Guerrero A, 2014. Glutamate-mediated excitotoxicity in schizophrenia: a review. Eur Neuropsychopharmacol 24, 1591–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW, 2001. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14, 260–264. [DOI] [PubMed] [Google Scholar]
- Reale M, Costantini E, Greig NH, 2021. Cytokine Imbalance in Schizophrenia. From Research to Clinic: Potential Implications for Treatment. Front Psychiatry 12, 536257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, Maes M, 2020. The Role of Aberrations in the Immune-Inflammatory Response System (IRS) and the Compensatory Immune-Regulatory Reflex System (CIRS) in Different Phenotypes of Schizophrenia: the IRS-CIRS Theory of Schizophrenia. Mol Neurobiol 57, 778–797. [DOI] [PubMed] [Google Scholar]
- Sahbaz C, Zibandey N, Kurtulmus A, Duran Y, Gokalp M, Kirpinar I, Sahin F, Guloksuz S, Akkoc T, 2020. Reduced regulatory T cells with increased proinflammatory response in patients with schizophrenia. Psychopharmacology (Berl) 237, 1861–1871. [DOI] [PubMed] [Google Scholar]
- Sainz J, Mata I, Barrera J, Perez-Iglesias R, Varela I, Arranz MJ, Rodriguez MC, Crespo-Facorro B, 2013. Inflammatory and immune response genes have significantly altered expression in schizophrenia. Mol Psychiatry 18, 1056–1057. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Stone TW, 2017. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 112, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonite M, Yao B, Welsh RC, Taylor SF, 2023. Increased rostral medial frontal GABA+ in early psychosis is obscured by levels of negative affect. Schizophr Res 252, 46–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorobogatov K, De Picker L, Verkerk R, Coppens V, Leboyer M, Muller N, Morrens M, 2021. Brain Versus Blood: A Systematic Review on the Concordance Between Peripheral and Central Kynurenine Pathway Measures in Psychiatric Disorders. Front Immunol 12, 716980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Jacobs R, Panteli B, Brauner M, Schiltz K, Bahn S, Herberth M, Westphal S, Gos T, Walter M, Bernstein HG, Myint AM, Bogerts B, 2010. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci 260, 509–518. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS, 2007. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. J Psychopharmacol 21, 440–452. [DOI] [PubMed] [Google Scholar]
- Tan Y, Li Y, Tan S, Wang Z, Yang FD, Cao B, Zunta-Soares GB, Soares JC, Zhang XY, 2015. Increased interleukin-2 serum levels were associated with psychopathological symptoms and cognitive deficits in treatment-resistant schizophrenia. Schizophr Res 169, 16–21. [DOI] [PubMed] [Google Scholar]
- Tarantino N, Leboyer M, Bouleau A, Hamdani N, Richard JR, Boukouaci W, Ching-Lien W, Godin O, Bengoufa D, Le Corvoisier P, Barau C, Ledudal K, Debre P, Tamouza R, Vieillard V, 2021. Natural killer cells in first-episode psychosis: an innate immune signature? Mol Psychiatry 26, 5297–5306. [DOI] [PubMed] [Google Scholar]
- Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, Neufeld RW, Rogers J, Pavlosky W, Schaefer B, Densmore M, Al-Semaan Y, Williamson PC, 2002. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry 159, 1944–1946. Ulivieri, C., Baldari, C.T., 2013. T-cell-based immunotherapy of autoimmune diseases. Expert Rev Vaccines 12, 297–310. [DOI] [PubMed] [Google Scholar]
- van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, Kahn RS, Sommer IE, 2017. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry 7, e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rees GF, Lago SG, Cox DA, Tomasik J, Rustogi N, Weigelt K, Ozcan S, Cooper J, Drexhage H, Leweke FM, Bahn S, 2018. Evidence of microglial activation following exposure to serum from first-onset drug-naive schizophrenia patients. Brain Behav Immun 67, 364–373. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wei Y, Edmiston EK, Womer FY, Zhang X, Duan J, Zhu Y, Zhang R, Yin Z, Zhang Y, Jiang X, Wei S, Liu Z, Zhang Y, Tang Y, Wang F, 2020. Altered structural connectivity and cytokine levels in Schizophrenia and Genetic high-risk individuals: Associations with disease states and vulnerability. Schizophr Res 223, 158–165. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhu Y, Song Z, Mei L, Zhang J, Chen T, Wang Y, Xu Y, Jiang K, Li Y, Liu D, 2015. Comparison of the density of gamma-aminobutyric acid in the ventromedial prefrontal cortex of patients with first-episode psychosis and healthy controls. Shanghai Arch Psychiatry 27, 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.