Abstract
Background:
Respiratory dysfunction is a common complication of stroke, with an incidence of over 60%. Despite the high prevalence of stroke-induced respiratory dysfunction (SIRD) how disordered breathing influences recovery and cognitive outcomes after ischemic stroke is unknown. We hypothesized that stroke induces chronic respiratory dysfunction, breathing instability, and apnea in mice, which would contribute to higher mortality and greater post-stroke cognitive deficits.
Methods:
Mice were subjected to a 60-minute transient middle cerebral artery occlusion (MCAO) or permanent distal MCAO. Whole body plethysmography was performed on C57BL/6 young (2–3 months) and aged (20 months) male and female mice. Animals were exposed to a variety of gas conditions to assess the contribution of peripheral and central chemoreceptors. A battery of cognitive tests was performed to examine behavioral function.
Results:
MCAO led to disordered breathing characterized by hypoventilation and apneas. Cognitive decline correlated with the severity of disordered breathing. Distal permanent MCAO, which produces a smaller cortical infarct, also produced breathing disorders and cognitive impairment, but only in aged mice.
Conclusion:
Our data suggest that post-stroke apnea is associated with cognitive decline and highlights the influence of aging on breathing disorders after stroke. Therefore, treatment of respiratory instability may be a viable approach to improving cognitive outcomes after stroke.
Graphical Abstract
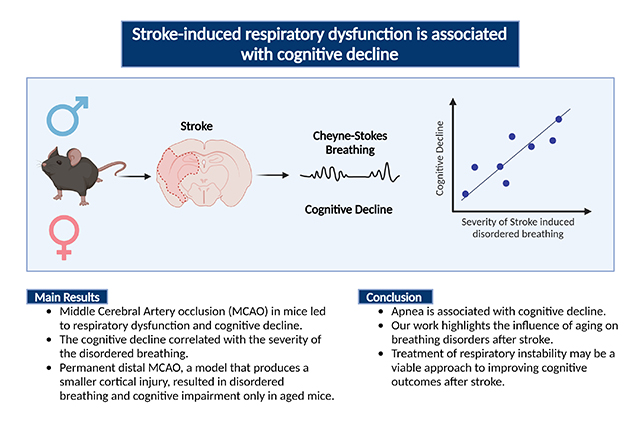
Introduction:
Stroke continues to be the leading cause of long-term disability in the United States.1 In 2012, stroke care cost an astounding 77.1 billion dollars, and due to the aging of the US population, this number is expected to triple by 2030 to 183 billion.2 Although acute mortality from stroke has declined due to improvements in medical care, this has led to a growing number of stroke survivors in our communities. Therefore, enhancing functional recovery and preventing further disability in stroke patients is critical.
It is well documented that respiratory dysfunction, particularly during sleep, is common following stroke and is associated with a worse prognosis.3–8 For example, more than 60% of individuals suffering a first stroke develop respiratory dysfunction characterized by apneas and hypoventilation3, and over 80% of stroke survivors initially diagnosed with stroke-disordered breathing had continued deficits even three years after their initial stroke.9 Despite the high prevalence of stroke-induced respiratory dysfunction (SIRD), little is known about the mechanism by which stroke affects breathing in part due to lack of animal models of this disorder. This is significant because SIRD is associated with higher one-year mortality and worse functional outcome at 3 months and 12 months following stroke.5,10 Currently the only treatments available for SIRD are physical interventions designed to maintain airway patency i.e. continuous positive airway pressure (CPAP). Unfortunately, this type of intervention is only marginally successful and not well-tolerated, and patient adherence is poor.5 Furthermore, decreased cognitive performance also correlates with respiratory dysfunction.11 Therefore, there is an urgent need to understand the cellular mechanisms contributing to SIRD.
Advanced chronological age is the most important non-modifiable risk factor for stroke.12 More than 80% of strokes occur in individuals over the age of 6513,14. Age is an independent predictor of poor outcome after stroke, and older patients have both higher in-hospital mortality and poorer functional outcomes after an ischemic event.13,15 Stroke is a sexually dimorphic disease, with women having a lower incidence of stroke in the age group of 55–75, until the age of 85 when women have a higher incidence.16,17 The majority of human studies report a blunting of age-dependent hypoxic and hypercapnic ventilatory responses.18,19 However, there is conflicting evidence regarding sex-associated change in the control of breathing.20,21 Sleep apnea has a higher prevalence among men of all ages, especially in elderly men who have a higher incidence of all forms of sleep apnea: central, mixed, and obstructive.22 Interestingly, elderly females with sleep apnea exhibit increased white matter loss and changes in structural integrity in several brain regions compared to age-matched males.23 The incidence of SIRD has never been evaluated in both sexes in pre-clinical models. The main goal of this study was to establish a mouse model of SIRD in both young and aged mice to investigate the link between SIRD and post-stroke cognitive decline.
Materials and Methods
The data supporting the findings of this study are available from the corresponding authors upon reasonable request. Methodological details beyond the descriptions below are provided in the online-only data supplement.
All experiments were performed according to NIH guidelines for the care and use of animals approved by the University of Texas Health Science Center Houston Institutional Animal Care and Use Committee. and reported in accordance with the ARRIVE guidelines. Mice were randomly assigned to either a 60-minute transient middle cerebral artery occlusion (MCAO), a permanent distal MCAO or corresponding sham surgery. Whole body plethysmography was performed on C57BL/6 young (2–3 months) and aged (20 months) male and female mice. All tests were conducted by investigators blinded to surgical condition. Statistics were performed using GraphPad Prism 9, and presented as means ± SEM for all experiments.
Results
MCAO produces disordered breathing characterized by hypoventilation and apneas
To determine if MCAO produces disordered breathing, 6–8-week-old male mice were subjected to 60 minutes of MCAO or sham surgery. To establish baseline respiratory activity, mice underwent plethysmography testing 1-day prior to surgery. On day 3-post surgery mice underwent plethysmography to identify the phenotype of SIRD. Interestingly, stroke mice exhibited a pattern of respiratory activity strikingly similar to the Cheyne-Stokes-like pattern of breathing observed in stroke patients (Fig. 1A). Compared to baseline, only stroke mice exhibited reduced respiratory activity after surgery compared to their baseline (Fig. 1B). This change was not seen in mice undergoing sham surgery (suture placed but not advanced in the MCA). The average respiratory frequency was suppressed. This was accompanied by an increase in the variability of respiratory frequency in stroke mice compared to sham, p<0.0001 (Fig. 1B) but with no change in tidal volume (TV) (ml/g) (p=0.1) (Fig. 1C), thus minute ventilation (MV) (ml/min/kg) was decreased compared to sham (p<0.01, Fig. 1D). Stroke mice displayed a noticeable increase in number of apneic events compared to sham animals under room air conditions, p<0.0001 (Fig. 1E). Coinciding with this respiratory phenotype, we also found 3 days after surgery that stroke mice breathing room air had reduced arterial PaO2 (p<0.05) (Table S1) and showed hemoglobin O2 saturation (SpO2) levels fluctuating between 94–96% but would frequently drop to between 80–90% during apneic events (Fig. 1F). Aged mice also showed saturation drops (Fig. S1) and the dropping appears more frequent and severe. Minute ventilatory response to CO2 is slightly blunted, evident by the right shift of the curve following 60-minute MCAO in young male mice. The slope of the minute ventilatory response to CO2 is unchanged following MCAO, (slope sham=0.38, stroke=0.34, p=0.77, Fig. S2-A). To understand if MCAO disrupts the hypoxic ventilatory response, mice underwent plethysmography recordings with exposure to 21% O2 (room air) followed by 10% O2. On day 3 post surgery, minute ventilatory response to hypoxia of stroke mice was blunted (p<0.05), yet chemosensitivity to hypoxia remained unchanged, (slope sham=0.03, stroke=0.04), and the difference between the slopes was not significant, (p=0.73, Fig. S2-B). PaCO2 measurements suggest that stroke mice retain CO2 as a result of disordered breathing (p=0.052), while arterial pH remained unchanged (p=0.63) (Table S1).
Figure 1. Phenotype of stroke-induced respiratory dysfunction.
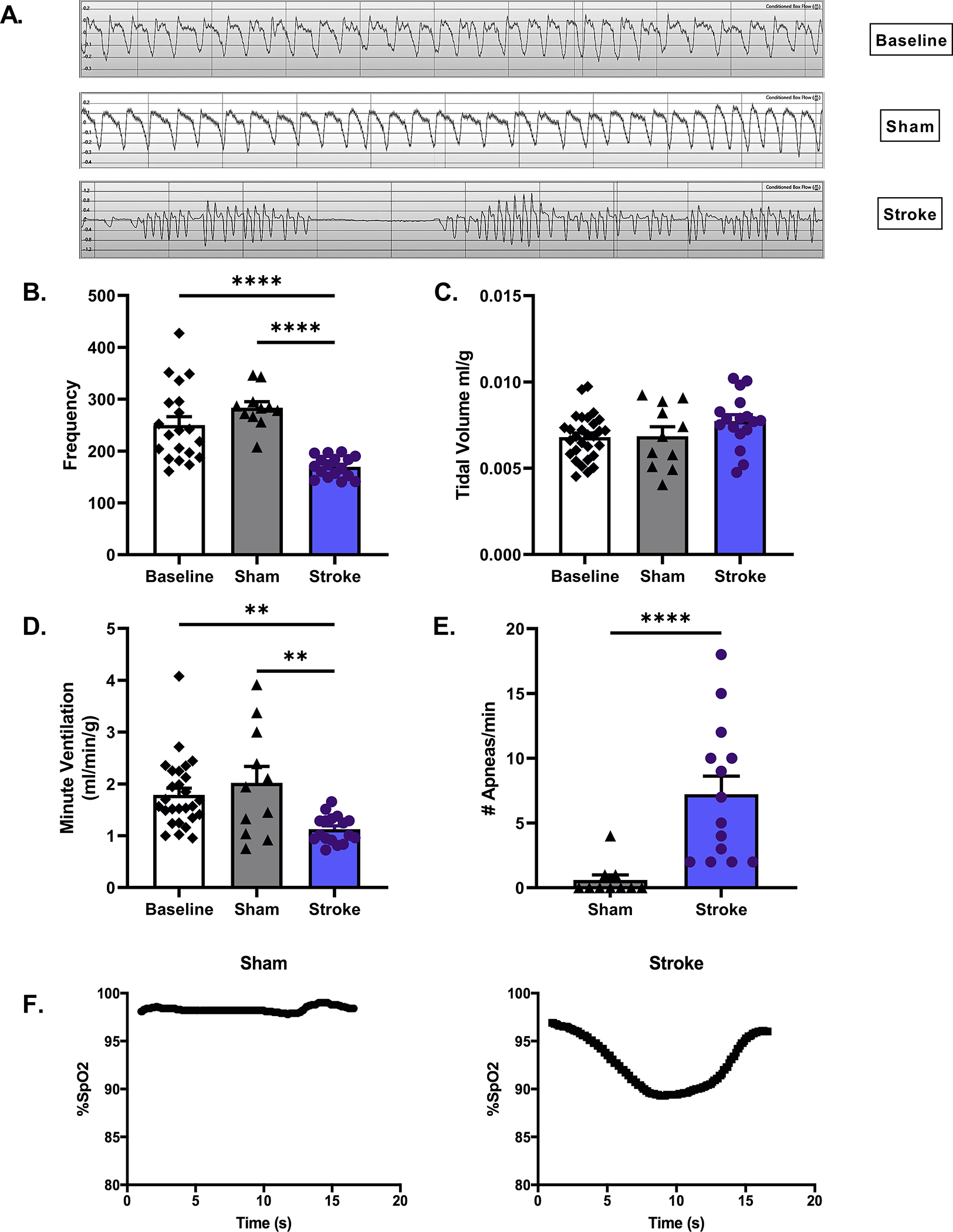
Whole body plethysmography was performed on stroke and sham young male mice day 3 post surgery. Representative respiratory waveform tracings of mice at baseline, sham and stroke are shown at 1 second intervals (A). Under room air conditions respiratory frequency, measured in breaths per minute, decreased in stroke compared to baseline & sham p<0.0001, (B), while TV (ml/g) increased although not significantly, p=0.28 (C). The product of frequency and TV, MV (ml/min/g), decreased, following MCAO, suggesting hypoventilation, p<0.01 (D). Number of apneas per minute markedly increased following stroke, Mann-Whitney test, p<0.0001 (E). B-E: stroke n=14–17, sham n=10–11 Stroke mice showed hemoglobin O2 saturation (SpO2) levels fluctuating between 94–96% but would frequently drop to between 80–90% during apneic events (F).
To rule out brainstem neuronal cell death as a causative factor of disordered breathing, tissue slices were assessed for histological damage at day 3 post-stroke. No cell death or neuronal degeneration was observed in the brainstem on either TTC staining or Fluorojade staining (Fig. S3). To determine if SIRD corresponds with changes in metabolic activity, we measured O2 consumption as a proxy of metabolic activity. We found no difference in O2 consumption between stroke and sham mice up to 6 weeks post-surgery (Fig. S4) (p = 0.24).
Age and sex are biological variables effecting post-stroke respiratory activity:
Age is an independent predictor of poor outcomes as older individuals have both higher in hospital mortality and worse functional and cognitive outcomes following an ischemic event.32 Age may also affect the SIRD phenotype we observed in young mice. Therefore, we performed a comprehensive assessment of the effects of age on respiratory physiology in stroke and sham mice. Consistent with the hypoventilatory phenotype observed in young mice, we found that aged male stroke mice in room air (day 3 post-surgery) display decreases in both respiratory frequency (p<0.01, Fig. 2A) and MV (p<0.001, Fig. 2B) compared to sham. These animals also showed a reduced respiratory output over CO2 values ranging from 0–7% CO2 but with no change in slope of the ventilatory response to CO2 (p=0.21, Fig. 2C), further suggesting stroke disrupts baseline breathing but not chemoreceptor gain. Consistent with the phenotype seen in young stroke mice, aged stroke animals also exhibited apneas (p<0.01) (Fig. 2D). In response to hypoxic conditions, there is an effect of stroke on MV (p<0.05) but the oxygen sensitivity is not perturbed (slope sham 0.04±0.06 vs. stroke 0.02±0.02, p=0.66, Fig. 2E).
Figure 2. Respiratory parameters of post-MCAO in aged males.
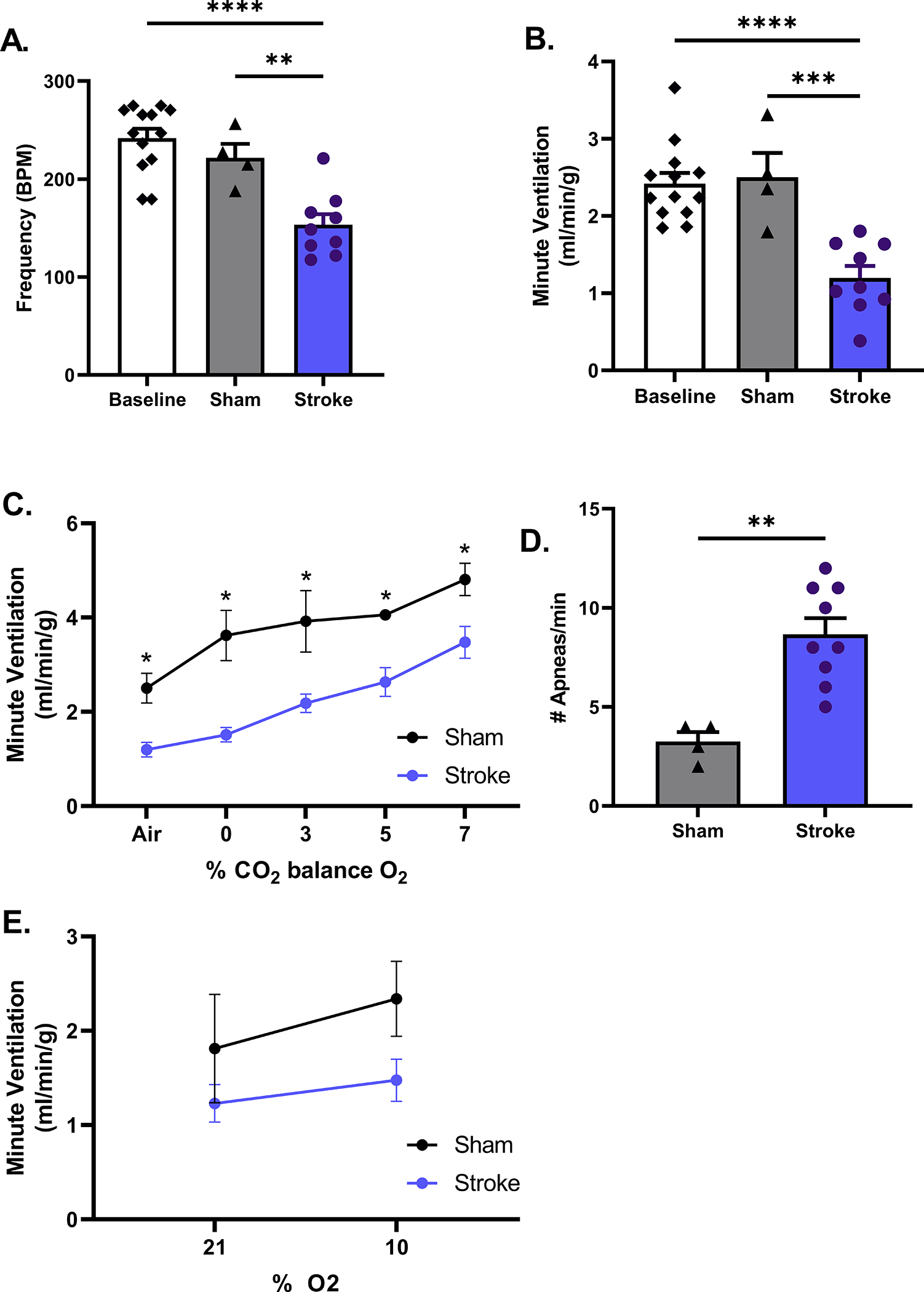
Respiratory phenotype of aged males (18–20 months) at day 3 that underwent MCAO is similar to young males. Respiratory frequency is decreased compared to baseline & sham, p<0.01 (A) resulting in diminished MV under room air conditions compared, p<0.001 (B). These animals also showed a reduced respiratory output over CO2 values ranging from 0–7% CO2 but with no change in slope of the ventilatory response to CO2, p=0.21 (C). Aged stroke animals also exhibited more apneas, p<0.01 (D). In response to hypoxic conditions, there is an effect of stroke on MV (p<0.05) but oxygen sensitivity is not perturbed (slope: p=0.68, E). A-E stroke n=9, sham n= 4.
To investigate respiratory dysfunction following stroke in females, we subjected 18–20-month-old female mice to MCAO and assessed respiratory parameters on day 3 post-stroke. Consistent with the results from aged male mice, aged female mice also showed a decrease in MV compared to sham (p<0.05, Fig. 3A) but in the absence of an apnea phenotype (p=0.07, Fig. 3B). We also found that aged female mice had higher baseline MV under room air conditions and in response to hypercapnia compared to aged males (p<0.05, Fig. 3C). Consistent with this, aged female stroke mice also showed a higher level of O2 consumption compared to aged-matched stroke male mice (p<0.05, Fig. 3E). Conversely, despite starting from a lower baseline level of activity, aged male mice showed a more severe decrease in MV following stroke compared to aged-matched female mice (p<0.001, Fig. 3D). These results show that aged male mice are more prone to baseline respiratory problems following stroke compared to age-matched female mice. Variations in infarct did not account for the discrepancies in MV observed between sexes, as seen by percent atrophy at day 7 (p=0.44, Fig. 3F).
Figure 3. Effect of sex on stroke-induced respiratory dysfunction.
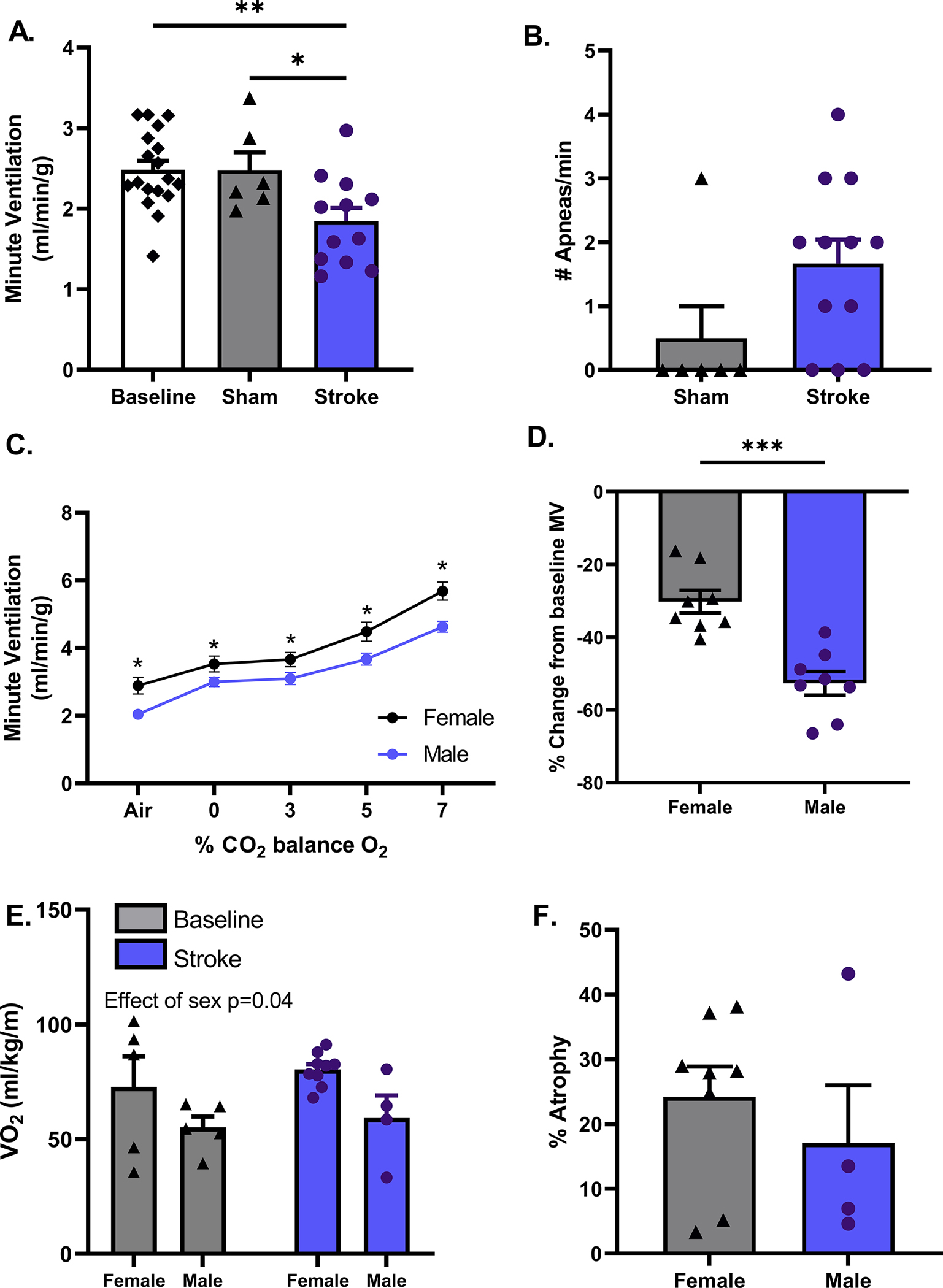
Aged female mice undergoing MCAO display a decrease in MV on day 3 following surgery, compared to baseline & sham p<0.05 (A). Despite having no increase in incidence of apnea, Mann-Whitney test, p=0.07 (B). A, B baseline n=18, stroke n=12, sham n=6. At baseline, aged females display a higher MV under room air conditions and in response to hypercapnia p<0.05, male n=9, female n=12 (C). Aged males displayed a greater percent change in MV from baseline following stroke, males p<0.001, male n=8, female n=8 (D). Metabolic activity was increased in females compared to males at baseline, effect of sex p=0.04, whereas stroke had no effect on metabolic activity in either sex (E). Variations in infarcts did not account for the discrepancies in MV observed between sexes, male atrophy, p=0.44 (F). E-F male n=4–5, female n=5–9
MCAO results in progressive cognitive decline:
Sham and stroke mice underwent cognitive assessment utilizing Barnes maze on days 21 and 42 post surgery. On day 21-post surgery stroke mice showed no difference in time to find the escape hole compared to sham mice (Fig. 4A). However, on day 42-post surgery, stroke mice performed significantly worse than shams in both escape time (p<0.01) and total errors made prior to finding escape hole (p<0.05, Fig. 4B). More importantly stroke mice performed progressively worse on day 42 compared to day 21, taking longer to escape and making more total errors (p<0.05), suggestive of progressive cognitive decline. Consistent with these findings, stroke mice also had poorer performance on Novel Object Recognition Test and in Contextual Fear Conditioning. Stroke mice spent reduced time with novel object compared to sham on day 28 (p<0.01, Fig. 4C). On day 42, stroke mice had less freezing behavior compared to sham in the fear conditioning arena, reflecting cognitive deficits (p<0.01, Fig. 4D).
Figure 4. MCAO results in cognitive decline.
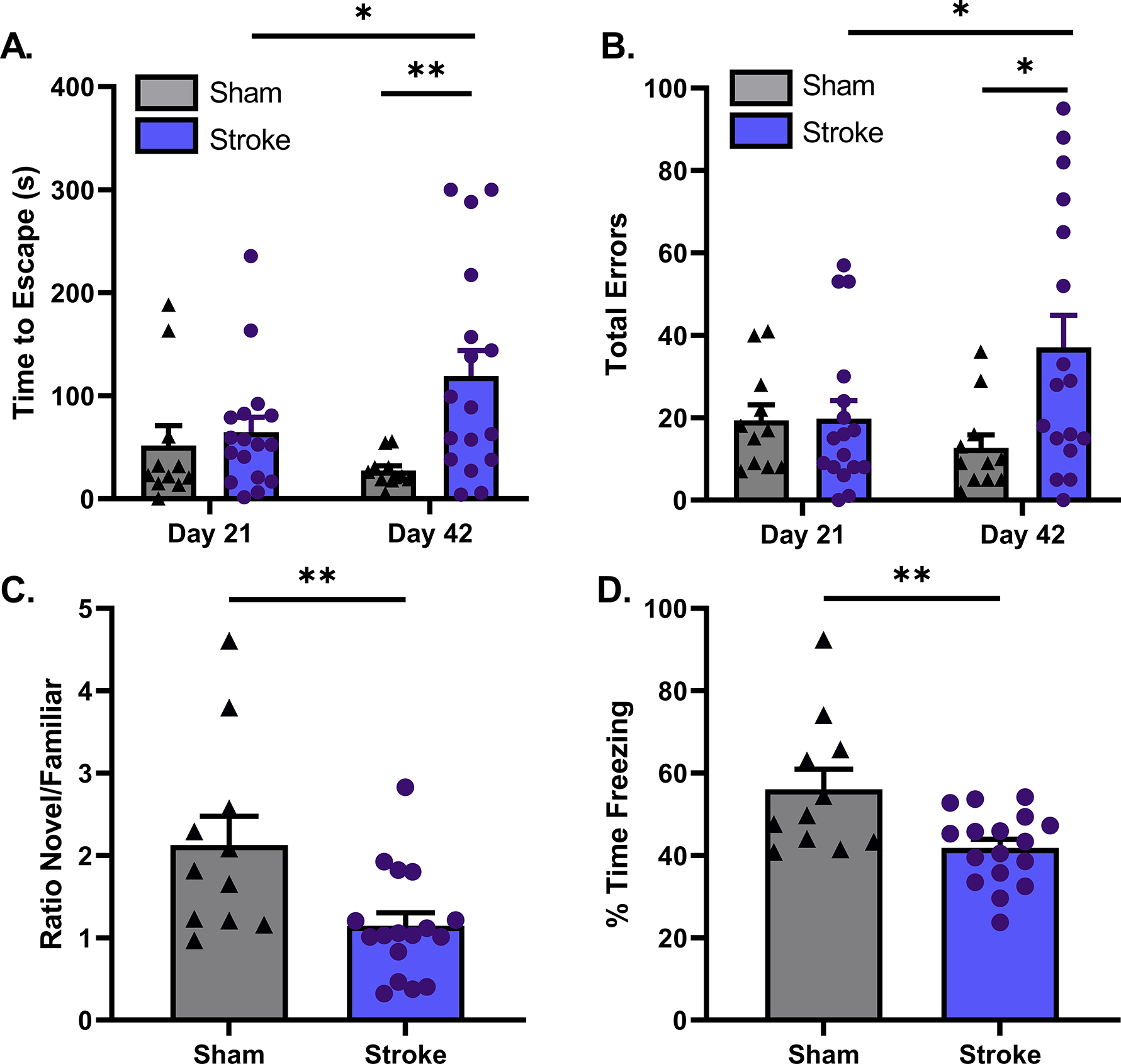
During Barnes maze, stroke mice showed no difference in time to find the escape hole compared to sham mice on day 21 post surgery. On day 42 post surgery, stroke mice perform worse than sham, and also had increasing time to find the escape hole compared to day 21 after stroke, p<0.01 (A). Interestingly, on day 21 stroke mice make approximately the same number of errors as sham do prior to finding the escape hole, yet on day 42, stroke mice not only make more errors than sham but more errors than they did on day 21, suggesting progressive cognitive decline (p=0.01) (B). Findings of day 28 NORT also are indicative of cognitive decline, stroke mice spend less time with novel object in relation to familiar object when compared to sham, ratio of time, p<0.01 (C). stroke mice spend less time displaying freezing behavior during contextual fear condition on day p<0.01 (D). Stroke n=17, sham n=11.
Progressive cognitive decline correlates with the severity of disordered breathing:
Following plethysmography assessment on day 42-post surgery in young male mice, we found that stroke mice could be stratified into two groups based on the number of apneas per minute. The minor group presented with 5 or less apneas a minute, while the severe group had 5 or more apneas a minute. The determination to separate groups based on the number of apneas a minute was adapted from American Academy of Sleep Medicine criteria used in humans.33 Consistent with day 3 findings, we found no indications of alteration in chemosensitivity as measured by ventilatory response to CO2 (p=0.51, Fig. 5B). After stratifying stroke mice based on severity of respiratory dysfunction, cognitive function was reevaluated based on these criteria. Surprisingly, we found that mice displaying severe form of disordered breathing exhibit signs of progressive cognitive decline while those with minor apneas did not. Performance on the Barnes maze was consistent with this progressive cognitive decline. Interestingly, statistical analysis revealed that animals with the most severe disordered breathing made more errors on day 42 (p<0.01, Fig 5C). Again, on Barnes maze there was an effect of time to escape in the stroke cohort as a whole (D21 vs. D42, p<0.05). Statistical analysis showed that the severe group had increasing escape time over the 6 weeks of testing (p<0.05, Fig. 5D).
Figure 5. Evolution of the SIRD phenotype and Progressive cognitive decline correlates with the severity of SIRD in young male mice.
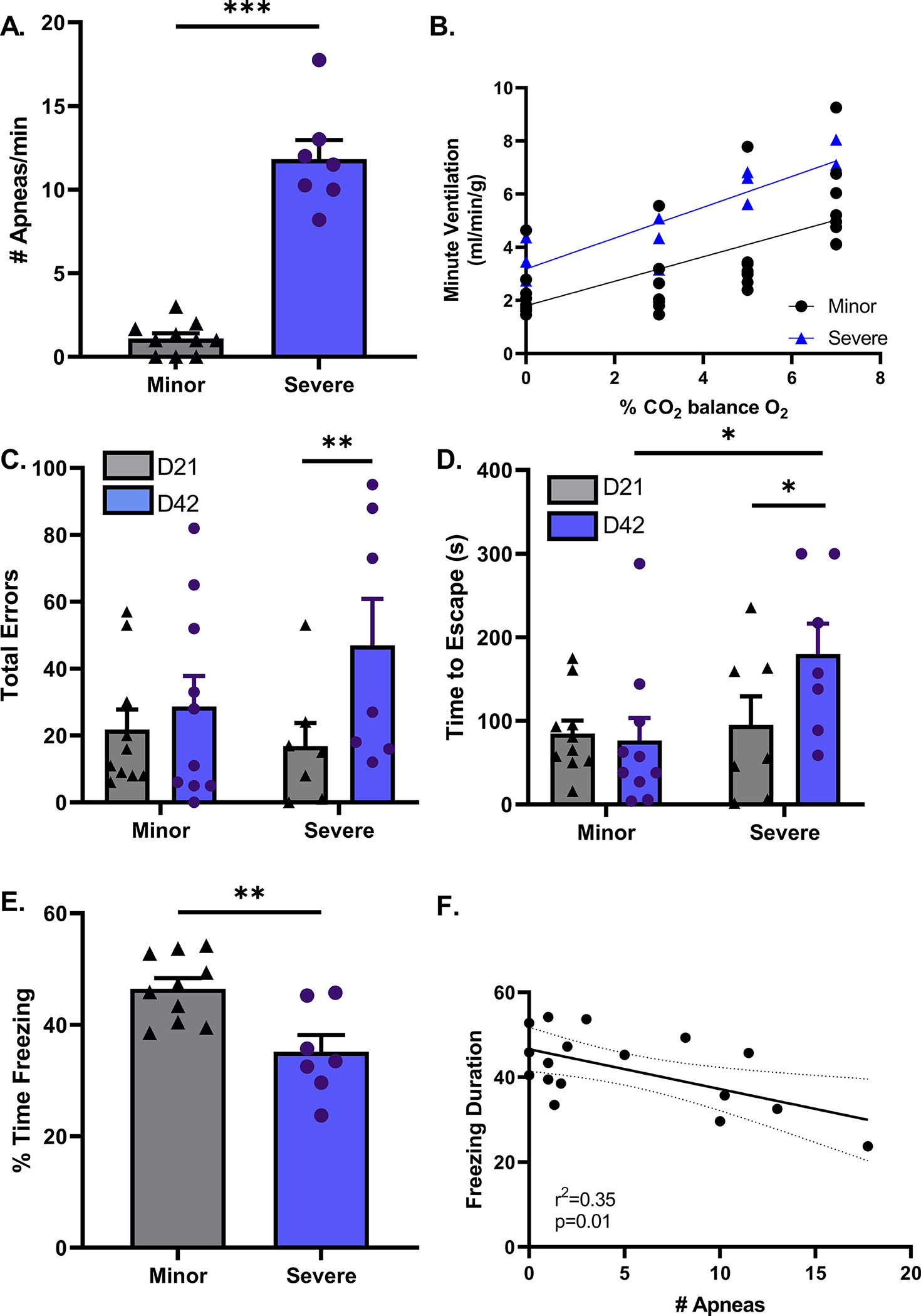
On day 42 post surgery, young stroke mice were stratified into 2 groups based on number of apneas per minute. The minor group experience 5 or less while the severe group had 5 or more apneas a minute (A). Central chemosensitivity did not differ between minor or severe groups, p=0.51 (B). Barnes maze performance was similar on day 21 between minor and severe groups in time to escape and number of errors made. On day 42-post stroke, the minor group is consistent in their performances on day 21. The severe group not only took longer to find the escape hole (p<0.05), but also made more errors than they did on day 21 (p<0.05) (C & D). During contextual fear condition testing, the severe group displays less freezing behavior than the minor group suggesting cognitive impairment, (P<0.01) (E). Linear regression analysis found a negative correlation between the number of apneas and cognitive performance measured as freezing time during contextual fear conditioning test, p=0.01 (F). minor n=10, severe n=7.
These findings were consistent with data from contextual fear conditioning. Mice with the most severe disordered breathing had lower freezing on day 42-post stroke (P<0.01, Fig. 5E). Linear regression analysis of percent time freezing compared to number of apneas per minute found a significant non-zero slope (p=0.01), indicating a negative correlation between apneas and freezing behavior (Fig. 5F).
To rule out infarct variability amongst the two groups of disordered breathing mice we measured infarct at day 42. No differences were observed in the quantification of cerebral atrophy between minor and severe groups after sacrifice on day 42 (p=0.66) (Fig. S5-A). Performance on corner test, a measurement of sensory motor function, was the same for both groups of mice when they were assessed at day 3 post-stroke (p=0.35). (Fig. S5-B)
Distal MCAO produced breathing disorder in aged but not in young:
Distal MCAO was then used to determine if a smaller cortical infarct also produced disordered breathing or cognitive decline. This model leads to an isolated cortical infarct, with minimal cerebral edema, remote from the brainstem respiratory nuclei. Plethysmography assessment in young males on days 3, 21 or 42-post surgery found no indication of disordered breathing, hypoventilation (p=0.97) or apneas (p=0.78), day 3 data shown in Fig. 6A and 6B. Cognitive performance, assessed by Barnes Maze (p=0.7) and NORT (p=0.57) showed no significant differences between stroke and sham groups (Fig. 6 C&D). However, when distal MCAO was induced in aged males, stroke led to apnea and cognitive impairment assessed 42 days after stroke (Fig. 6E, p<0.01 & Fig. 6F, p<0.05), demonstrating the critical role of aging in stroke disordered breathing phenotype. Interestingly, pdMCAO did not reduce breathing frequency and MV in aged (data not shown).
Figure 6. Distal MCAO produced apnea and cognitive impairment in aged but not in young mice.
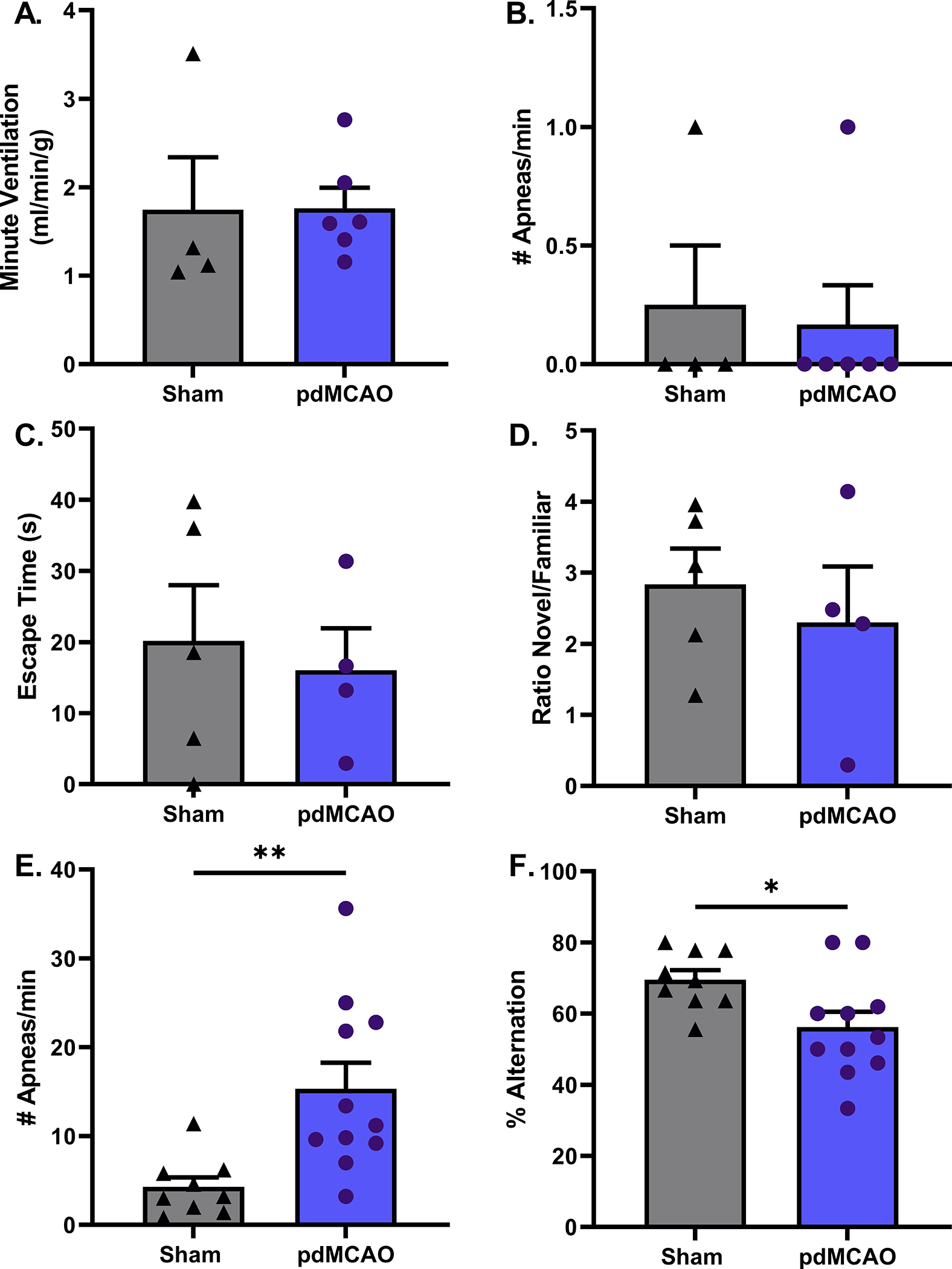
Young sham and stroke mice had similar MV and number of apneas per minute (A and B). Young sham and stroke mice had similar escape times on Barnes maze on day 42, p=0.7 (C). Both groups of young mice showed a significant preference for novel object, indicative of normal cognitive performance, p=0.57 (D). sham n=4–5, stroke n=4–6 for young mice. In aged mice, distal MCAO produced increased number of apneas, p<0.01 (E) and also showed a reduced percentage of alternations in y-maze test, p=0.02 (F). sham n=9, stroke n=11 for aged mice.
Discussion:
Breathing is maintained by a negative feedback regulator designed to preserve blood gas homeostasis; where central and peripheral chemoreceptors form the feedback portion of this control loop by adjusting the rate and depth of breathing in response to changes in tissue CO2/H+ and O2. A common type of disordered breathing, Cheyne-Stokes respiration, is thought to result from hyper-activation of the chemoreflex. Not surprisingly, direct lesions to brainstem respiratory centers can severely disrupt breathing.34,35 However, patients with hemispheric strokes (the most common type of stroke) also develop disordered breathing, and damage from these strokes do not directly affect the brainstem. Additionally, there is a lack of correlation between respiratory dysfunction and stroke location and/or severity.36 These results suggest that a non-specific mechanism underlies respiratory dysfunction in stroke. Furthermore, neural networks outside the lesion area are largely preserved, suggesting cellular elements of the respiratory network are particularly sensitive to stroke-induced insults.
Our review of the literature discovered only one experimental report of stroke altering ventilatory patterns. Koo et al. reported that following transient 60-minute left middle cerebral artery occlusion, mice displayed an increase in the coefficients of variation for respiratory frequency, TV and MV 24 hours after stroke. They concluded that ischemic stroke could develop a characteristic-breathing pattern similar to Cheyne-Stokes respiration.37 Albeit a significant finding, this work did not fully characterize the respiratory phenotype induced by MCAO and lacked any discussion of apnea, the fundamental feature of stroke-disordered breathing and a major contributor to hypoxia and cognitive decline. Additionally, advanced chronological age is the most important non-modifiable risk factor for stroke and is an independent predictor of poor outcome after stroke. To date no studies have been undertaken to investigate either age or sex as a biological variable in the study of stroke induced respiratory dysfunction.
We found that transient 60-minute MCAO produced respiratory dysfunction characterized by apneas and hypoventilation in both young and aged male mice, recapitulating the respiratory phenotype observed in human stroke patients. As direct brainstem lesions result in respiratory dysfunction, we examined the brains and found no evidence of neuronal brainstem cell death. Interestingly, the use of distal MCAO model only produced increased apnea and cognitive impairment in aged mice, suggesting the importance of aging in stroke-induced breathing disorder and cognitive impairment.
Respiratory constancy relies on a delicate balance of chemoreceptor gain, plant gain and temporal control of brain/heart blood flow. The Cheyne-Stokes respiratory pattern observed in humans is proposed to be a result of alterations in the sensitivity of the chemoreflex.38 In this model of SIRD the slope of the ventilatory response to CO2 and hypoxia remains unchanged, suggesting that the intrinsic ability of these neuronal chemoreceptor populations remains intact, yet the output of these groups is altered. Time delays in the blood circulating between lungs and chemoreceptor populations may contribute to this blunted response, potentially due to local or systemic alterations in vascular reactivity or cardiac variability. As seen in the representative O2 saturation curve shown in Figure 1F, the decline takes about 6–8 seconds until reaching a trough. We tried to perform concurrent plethysmography with SpO2 collar measurements to explore the relationship between apneas and hypoxic events. However, the DSI plethysmography system does not support this technology. Local variations in astrocyte reactivity, basement membrane restructuring and fibrosis are potential candidates for disrupted neural vascular communication and require further exploration.
Our 6-week studies reproduced findings of prior experiments reporting cognitive deficits following ischemic stroke.39,40 Cognitive deficits following MCAO may be detected as early as 3 days following ischemia and persist for months in rodents and for years in humans.41 Our current study found indications of progressive cognitive decline in spatial learning and memory, a hippocampal dependent task, over a six-week period following MCAO. The hippocampus receives blood supply via the anterior choroidal artery and the posterior communication artery42, both of which are unaffected by MCAO. This suggests that other factors are contributing to post stroke cognitive decline such as inflammation, disordered breathing and systemic hypoxia.43
Over six weeks, mice underwent weekly plethysmography studies to assess respiratory function after stroke. Based on the Apnea-Hypopnea Index (AHI) used in humans to report the number of apneas an hour during sleep. Mice were stratified as either minor (less than 5 apneas a minute) or severe (5 or more apneas a minute). Respiratory tracings from mice in the severe group represent a Cheyne-Stokes like form of periodic breathing, with a waxing and waning of TV. While the minor group displays a stable respiratory pattern, displaying improvements from day 3 tracings. Variations in TV in the severe group suggest it is the effect of ventilation on blood gases that contribute to development of apneas. Respiratory chemoreceptors contribute to both respiratory rate and TV based on stimulus of CO2/H+, the increases in TV on a breath-to-breath basis eliminate larger volumes of CO2, decreasing the CO2 reserve until CO2 levels drop below apneic threshold resulting in apnea. We can infer that CO2 levels begin to drop by the decreases in TV observed in the one or two breaths preceding an apnea. Increases in plant gain are predicted to destabilize breathing by decreasing the CO2 reserve. Breath-by-breath measurements of TV and exhaled CO2 will confirm our conclusions that MCAO increases plant gain therefore destabilizing breathing. Collectively, our data suggests that ischemic stroke decreases basal respiratory activity in response to both hypoxia and hypercapnia, increasing the incidence of apneas resulting in a feed forward loop of respiratory dysfunction.
Aged male mice display a higher level of respiratory activity, reflective of an increase in MV (normalized to body weight), which may be explained by changes in metabolic activity that accompany aging. The SIRD phenotype witnessed in aged males was similar to that of young. Slopes of ventilatory response to 10% inspired O2 were not significantly different, but it remains possible that aged mice are less responsive to changes in O2, leading to more apneas. At baseline, aged females display an increased MV under room air conditions compared to males, which is explained by increased metabolic activity. Interestingly, aged females have a less pronounced disordered breathing phenotype despite having similar sized infarcts as aged males. This may partly explain why aged male mice have worse functional outcomes and higher post stroke mortality than females. The hormonal and chromosomal contributions to sex differences in stroke are an area of ongoing investigation. For example, ischemia induced astrocyte reactivity is differentially regulated by age and sex. Increased levels of IL-6, IL-1b and TNF-α were observed in aged male astrocytes compared to astrocytes derived from females.44,45 There are likely many mechanisms contributing to the sex-based variations in respiratory dysfunction following ischemia. The long-term consequences of apnea may differ greatly based on the age and sex of the animal, and this may contribute to increased mortality and worse outcomes following ischemia.
Pain and inflammation may lead to tachypnea, facilitating the development of abnormal respiratory patterns. To ensure that these factors were not contributing to the breathing phenotypes seen after stroke, we quantified breathing frequency at baseline, before sham or stroke surgery. There was no difference between baseline and sham surgery in breathing frequency, across age and sex. We also performed both MCAO sham surgeries and pdMCAO (distal MCAO) on young male (2 months old) (pdMCAO + MCAO sham) concurrently. We found that there was no Cheyne-Stokes phenotype or difference in frequency or apnea between baseline and day 3 post-surgery (Fig. S6). Therefore, it is unlikely that inflammation or pain are responsible for the changes in breathing phenotypes seen after stroke.
In this work, we developed a model of SIRD. We found that abnormal respiratory patterns occur after stroke, and these are maladaptive and correlate with progressive cognitive decline. Although this work highlights the correlation between apnea-induced hypoxia and cognitive decline, further work determines the mechanisms involved. This work recapitulated the findings of numerous clinical studies reporting a negative correlation between the incidence of apnea and cognitive function in humans. Establishing a murine model to study stroke induced respiratory dysfunction lays the groundwork for further investigations into the mechanisms of disordered breathing and the development of therapies to prevent respiratory dysfunction and reduce cognitive decline. Use of this model could help identify novel treatments that could be used to stabilize breathing and improve overall patient health and quality of life.
Supplementary Material
Acknowledgement:
Our work was generously supported by NIH grants AG069466-01A1 (JL and LDM), NS094543 (LDM), TL1TR000369 (AP), 4TL1TR369-10 (MH) and 17PRE33410369 (MH).
Non-standard Abbreviations and Acronyms:
- SIRD
Stroke-induced Respiratory Dysfunction
- MCAO
Middle cerebral artery occlusion
- TV
Tidal Volume
- MV
Minute Ventilation
- NORT
Novel object recognition test
Footnotes
Disclosures:
None.
References
- 1.Centers for Disease Control and Prevention (CDC). Prevalence and most common causes of disability among adults–United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rochester CL, Mohsenin V. Respiratory complications of stroke. Semin Respir Crit Care Med. 2002;23:248–260. [DOI] [PubMed] [Google Scholar]
- 4.Bassetti C, Aldrich MS, Quint D. Sleep-disordered breathing in patients with acute supra- and infratentorial strokes. A prospective study of 39 patients. Stroke. 1997;28:1765–1772. [DOI] [PubMed] [Google Scholar]
- 5.Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke. 1996;27:252–259. [DOI] [PubMed] [Google Scholar]
- 6.Hudgel DW, Devadatta P, Quadri M, Sioson ER, Hamilton H. Mechanism of sleep-induced periodic breathing in convalescing stroke patients and healthy elderly subjects. Chest. 1993;104:1503–1510. [DOI] [PubMed] [Google Scholar]
- 7.Rowat AM, Dennis MS, Wardlaw JM. Central periodic breathing observed on hospital admission is associated with an adverse prognosis in conscious acute stroke patients. Cerebrovasc Dis. 2006;21:340–347. [DOI] [PubMed] [Google Scholar]
- 8.Patrizz A Modeling Post Stroke Respiratory Dysfunction, Apneas and Cognitive Decline [Internet]. 2017;Available from: https://digitalcommons.library.tmc.edu/utgsbs_dissertations/825
- 9.Cadilhac DA, Thorpe RD, Pearce DC, Barnes M, Rochford PD, Tarquinio N, Davis SM, Donnan GA, Pierce RJ, SCOPES II Study Group. Sleep disordered breathing in chronic stroke survivors. A study of the long term follow-up of the SCOPES cohort using home based polysomnography. J Clin Neurosci. 2005;12:632–637. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Suri JC, Manocha R. Study of association of severity of sleep disordered breathing and functional outcome in stroke patients. Sleep Med. 2017;34:50–56. [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc. 2001;49:1622–1627. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, Goldstein LB, Gorelick PB, Howard G, Kittner SJ, et al. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk factors. Stroke. 1997;28:1507–1517. [DOI] [PubMed] [Google Scholar]
- 13.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. [DOI] [PubMed] [Google Scholar]
- 14.Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. [DOI] [PubMed] [Google Scholar]
- 15.Forti P, Maioli F, Procaccianti G, Nativio V, Lega M-V, Coveri M, Zoli M, Sacquegna T. Independent predictors of ischemic stroke in the elderly: prospective data from a stroke unit. Neurology. 2013;80:29–38. [DOI] [PubMed] [Google Scholar]
- 16.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. [DOI] [PubMed] [Google Scholar]
- 18.Brischetto MJ, Millman RP, Peterson DD, Silage DA, Pack AI. Effect of aging on ventilatory response to exercise and CO2. J Appl Physiol. 1984;56:1143–1150. [DOI] [PubMed] [Google Scholar]
- 19.Kronenberg RS, Drage CW. Attenuation of the ventilatory and heart rate responses to hypoxia and hypercapnia with aging in normal men. J Clin Invest. 1973;52:1812–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holley HS, Behan M, Wenninger JM. Age and sex differences in the ventilatory response to hypoxia and hypercapnia in awake neonatal, pre-pubertal and young adult rats. Respir Physiol Neurobiol. 2012;180:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenninger JM, Olson EB Jr, Cotter CJ, Thomas CF, Behan M. Hypoxic and hypercapnic ventilatory responses in aging male vs. aging female rats. J Appl Physiol. 2009;106:1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hader C, Schroeder A, Hinz M, Micklefield GH, Rasche K. Sleep disordered breathing in the elderly: comparison of women and men. J Physiol Pharmacol. 2005;56 Suppl 4:85–91. [PubMed] [Google Scholar]
- 23.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Benashski SE, Venna VR, McCullough LD. Effects of metformin in experimental stroke. Stroke. 2010;41:2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129:239–257. [DOI] [PubMed] [Google Scholar]
- 27.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- 28.Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol. 1994;267:R1371–7. [DOI] [PubMed] [Google Scholar]
- 29.Patil SS, Sunyer B, Höger H, Lubec G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res. 2009;198:58–68. [DOI] [PubMed] [Google Scholar]
- 30.Uliana DL, Hott SC, Lisboa SF, Resstel LBM. Dorsolateral periaqueductal gray matter CB1 and TRPV1 receptors exert opposite modulation on expression of contextual fear conditioning. Neuropharmacology. 2016;103:257–269. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrell MJ, Heywood P, Moosavi SH, Guz A, Stevens J. Unilateral focal lesions in the rostrolateral medulla influence chemosensitivity and breathing measured during wakefulness, sleep, and exercise. J Neurol Neurosurg Psychiatry. 1999;67:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devereaux MW, Keane JR, Davis RL. Automatic respiratory failure associated with infarction of the medulla. Report of two cases with pathologic study of one. Arch Neurol. 1973;29:46–52. [DOI] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 37.Koo BB, Strohl KP, Gillombardo CB, Jacono FJ. Ventilatory patterning in a mouse model of stroke. Respir Physiol Neurobiol. 2010;172:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffin J The role of the central chemoreceptors: a modeling perspective. Respir Physiol Neurobiol. 2010;173:230–243. [DOI] [PubMed] [Google Scholar]
- 39.Chin Y, Kishi M, Sekino M, Nakajo F, Abe Y, Terazono Y, Hiroyuki O, Kato F, Koizumi S, Gachet C, et al. Involvement of glial P2Y1 receptors in cognitive deficit after focal cerebral stroke in a rodent model. J Neuroinflammation. 2013;10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Huang R, Shetty RA, Thangthaeng N, Liu R, Chen Z, Sumien N, Rutledge M, Dillon GH, Yuan F, et al. Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol Dis. 2013;59:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Wadley VG. Trajectory of Cognitive Decline After Incident Stroke. JAMA. 2015;314:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erdem A, Yaşargil G, Roth P. Microsurgical anatomy of the hippocampal arteries. J Neurosurg. 1993;79:256–265. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep. 2012;12:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HEW, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Differ. 2011;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


