Abstract
Background:
The American Heart Association recently released a new cardiovascular health (CVH) metric, Life’s Essential 8 (LE8) for health promotion. However, the association between levels of LE8 and the risk of CVD outcomes is not known from a large prospective cohort. We aim to analyze the relationship between CVH, indicated by LE8, and risks of coronary heart disease (CHD), stroke, and cardiovascular disease (CVD). Moreover, we sought to test whether the genetic susceptibility to CHD or stroke could be modified by LE8.
Methods:
A total of 137,794 participants free of CVD from the UK Biobank were included. CVH was scored using LE8 and categorized as low, moderate, and high.
Results:
During a median of 10 years, 8,595 CVD cases (6,968 CHD and 1,948 strokes) were documented. A higher LE8 score was associated with remarkably lower risks of CHD, stroke, and CVD (p<0.001 for all). Comparing the high CVH to the low CVH, the HRs (95% CI) were 0.34 (0.30, 0.38) for CHD, 0.45 (0.37, 0.54) for stroke, and 0.36 (0.33, 0.40) for CVD. Moreover, the model with LE8 achieved higher accuracy and outperformed the model with Life’s Simple 7 for CHD, stroke, and CVD (p<0.001 for all). The protective associations of the LE8 score with CVD outcomes were more pronounced among women (p-interaction <0.001 for CHD and 0.0013 for CVD, respectively), and among younger adults (p-interaction <0.001, 0.007, and <0.001 for CHD, stroke, and CVD, respectively). In addition, a significant interaction was found between the genetic risk of CHD and the LE8 score (p-interaction <0.001). The inverse association was stronger among those with a lower genetic risk of CHD.
Conclusions:
High level of CVH, defined by LE8, was associated with significantly lower risks of CHD, stroke, and CVD.
Keywords: Cardiovascular health, Gene-Lifestyle interaction, Cohort study
Graphical Abstract:
Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality and morbidity in the U.S. and worldwide.1,2 Reducing the burden of CVD continues to be a pivotal public health focus. Of note, the risk of CVD is largely driven by unhealthy lifestyles, such as an unhealthy diet, physical inactivity,3,4 and unhealthy sleep habits.5,6 To improve cardiovascular health (CVH), the American Health Association (AHA) formulated a novel construct of CVH.7 Recently, the AHA released an enhanced approach to assess CVH: Life’s essential 8 (LE8).8 Sleep health, indicated by optimal habitual sleep duration, is incorporated as a new metrics component, on basis of the accumulating studies that consistently link sleep duration with cardiometabolic health and health outcomes.5,6 Moreover, sleep duration is closely related to each of the original 7 components of CVH and adds independent value to the overall CVH.9–11 Therefore, with sleep health being adopted, the LE8 metrics may hold great potential for better improving CVH among the general population. However, supportive data is still lacking.
In addition, it is well-recognized that both genetic and lifestyle factors contribute to the risk of CVD.12 Genome-wide association studies have successfully identified genetic variants that are associated with CVD outcomes.13,14 Previous studies have shown that genetic susceptibility to CVD could be attenuated by adhering to a healthy lifestyle, though the results were not entirely consistent.12,15–17 We hypothesized that CVH defined by LE8 might interact with genetic susceptibility in relation to CVD risk.
In the current study, we sought to evaluate the association between the level of CVH, as defined using the LE8 score, and the incident CVD outcomes among adult participants from the UK Biobank. We also tested the interaction between genetic predisposition to CHD or stroke and LE8, as well as the joint association of LE8 and genetic factors on the risk of CVD.
Materials and Methods
All data and materials that support the findings of this study are available at the UK biobank. Qualified researchers who have been trained in human subject confidentiality protocols may request the access to the UK Biobank (https://www.ukbiobank.ac.uk/researchers/).
Study design and population
The UK biobank is a large, prospective cohort study in the United Kingdom, which provides in-depth genetic and health information.18,19 The details of the study design have been reported previously.19 Briefly, over 500,000 middle-aged (age range: 37–73) participants were recruited between 2006 and 2010 from 22 assessment centers across England, Scotland, and Wales. Participants provided a wide range of health-related information through touchscreen questionnaires, physical examinations, and biological samples. The 24-hour dietary recall questionnaire was distributed to participants who provided email addresses at the initial visit. A total of 210,962 participants completed at least one 24-hour dietary recall of the previous day between 2009 and 2012. The study was approved by both the National Health Service (NHS) National Research Service (ref: 11/NW/0382) and the Institutional Review Board of Tulane University (study number: 2018–1872). All participants provided written informed consent.
In the current study, we defined the time when the first 24-hour diet recall was completed as the baseline. Among participants with at least one typical 24-hour diet recall, we excluded participants with CHD or stroke at baseline, and those with missing information on one or more components of LE8, leaving a total of 137,794 participants for the analyses. Compared to the entire UK biobank participants, those who provided 24-hour dietary recall data were with high socioeconomic status and less likely to have concurrent diseases (Table S1). When analyzing genetic data, we only included 136,659 participants with complete genetic data for the analyses.
Cardiovascular health metrics (CVH) assessment with Life’s Essential 8 (LE8)
LE8 is a mix of behavioral (healthy diet, participation in physical activity, avoidance of nicotine, healthy sleep, and healthy weight) and biological (healthy levels of blood lipids, blood glucose, and blood pressure) targets.8 Healthy diet was evaluated by the Dietary Approaches to Stop Hypertension (DASH) diet score using the information collected from the 24-hour dietary recall.20 Frequency and duration of moderate and vigorous physical activity, nicotine exposure, sleep duration, and medication use were assessed by touchscreen questionnaires at baseline. Height and body weight were measured during the initial assessment center visit; body mass index (BMI) was calculated as weight in kilograms divided by squared height in meters (kg/m2). Total cholesterol was measured by CHO-POD analysis and high-density cholesterol was measured by enzyme immunoinhibition on a Beckman Coulter AU5800. Glycated hemoglobin (HbA1c) was measured by HPLC analysis on a Bio-Rad VARIANT II Turbo. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice by an Omron device, and averaged values were used for analyses. The details of the scoring algorithm according to AHA are shown in Table S2. Each component ranges from 0 to 100 points; the aggregate score is also scaled from 0 to 100 points. We categorized the CVH scores according to the AHA advisory as follows: high CVH (80–100); moderate CVH (50–79); and low CVH (0–49).8 We also calculated a behavior subscale based on the behavioral metrics (diet, physical activity, nicotine exposure, sleep health, and BMI) and a biological subscale based on the biological metrics (blood lipids, blood glucose, and blood pressure).
Assessment of the outcomes
The diagnosis of CVD, including CHD and stroke, was defined by information from the International Statistical Classification of Diseases and Related Health Problems 9th (ICD-9) and 10th (ICD-10) revisions (through hospital admissions), self-report, and death registries. Incident CHD was defined by ICD-10 code I20-I25; incident stroke was defined by ICD-10 code I60–64. Incident CVD was defined as the composite outcomes of CHD and stroke.
Covariates assessment
Age, sex, and ethnicity were obtained from baseline. Townsend deprivation index (based on participant’s postcode, a higher score indicates higher degree of deprivation) was obtained from local NHS Primary Care Trust registries and the name of the recruitment center. Alcohol intake was self-reported as never, special occasions only, 1–3 times/month, once or twice/week, 3–4 times/week, and daily or almost daily. Education, average household income, and use of medications were assessed by the touchscreen questionnaire during the initial assessment center visit. Sedentary behavior time was calculated as the sum of time spent on television watching, computer use (not at work), and driving.21
Calculation of genetic risk score
Genotyping was performed by the UK biobank team by two similar arrays: UK BiLEVE Axiom array and UK Biobank Axiom array. Detailed information is available elsewhere (http://www.ukbiobank.ac.uk/scientists-3/genetic-data/).22 We created a weighted genetic risk score (GRS) for CHD using 64 single-nucleotide polymorphisms (SNPs) identified to be associated with CHD from genome-wide association study (GWAS).13 Each SNP is coded as 0, 1, or 2 according to the number of risk alleles. The weight GRS was calculated by the widely used equation: GRS = (β1×SNP1 + β2×SNP2 + … + β64×SNP64) × (total number of SNPs/sum of the β coefficient). Similarly, a GRS for stroke was created using 30 SNPs identified from stoke GRS.14 A higher GRS indicates a higher genetic predisposition to CHD or stroke. We also categorized the GRS into low (Q1), medium (Q2-Q4), and high (Q5) based on quintile distributions of the GRS, according to previous studies.12
Statistical analysis
Baseline characteristics were presented as mean (SD) or median [interquartile range (IQR)] for continuous variables and N (%) for categorical variables according to the categories of CVH. Follow-up time was calculated from the baseline to the date of first diagnosis of CHD or stroke, or end of the follow-up (May 2021), whichever came first. Cox proportional hazard models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between LE8 and risk of CHD, stroke, and CVD. Proportional hazard assumption was tested by adding an interaction term of LE8 and log of the time variable in the model; we did not find violation of the assumption. Model 1 was adjusted for age, ethnicity, and sex. Model 2 was further controlled for assessment center, Townsend deprivation index, alcohol intake, sedentary behavior, average household income, and education levels. We repeated the analyses for behavior and biological subscale scores, with mutual adjustment for each other in the model. We also analyzed the association between each individual component of the LE8 and incidence of CVD outcomes, with all the 8 metrics simultaneously included in the model. Moreover, we compared the performance of LE8 with the previous assessment tool for CVH, Life’s simple 7 (LS7). The different survival model was assessed by the Harrell’s C-statistics.23 The methods proposed by Uno et al was further applied to examine the c-index difference between model with LE8 and model with LS7.24 We also conducted a sensitivity analysis by excluding sleep health from the LE8 score. To investigate whether the genetic predisposition could be offset by CVH, an interaction between LE8 and genetic factor was tested by adding a cross-product of LE8 and GRS into the model. Sex difference and age modification were also tested by adding an interaction term between LE8 and age or sex in the model. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc). All the statistical tests were 2-sided and p<0.05 was considered as statistically significant.
Results
Among the 137,794 participants with complete information on LE8 and no previous CVD, 52% were female, and the mean age was 56 years. Of all the study participants, 18.9% (N=26,071) met the high CVH criteria, and 74.3% (N=102,364) of the study participants were in the moderate CVH category, 6.8% (N=9,359) were in the low CVH category. Baseline characteristics of the participants according to low, moderate, and high cardiovascular health metrics (CVH) are shown in Table 1. Participants with a high CVH were younger, more likely to be women, had lower levels of deprivation, higher education, and higher average household income. Distribution of the overall LE8 score and each component of LE8 among the study participants are shown in Figure S1. The distribution of ideal status varied widely across LE8 metrics, ranging from 16% of participants having ideal status of blood pressure, to 85% of participants having ideal status of blood glucose. Fifty percent of participants had a poor status of healthy diet and more than 50% had a poor status of blood lipids.
Table 1.
Baseline characteristics according to the categories of LE8
| Life’s Essential 8 |
|||
|---|---|---|---|
| Low | Moderate | High | |
|
| |||
| Age, years | 56.2 (7.4) | 56.6 (7.8) | 53.7 (8.1) |
| Female, Y | 3,924 (41.9) | 52,758 (51.5) | 19,418 (74.5) |
| White, Y | 8,499 (91.3) | 93,428 (91.6) | 23,623 (90.8) |
| BMI, kg/m2 | 32.5 (5.6) | 27.1 (4.2) | 23.3 (2.5) |
| Townsend deprivation index | −1.6 [4.6] | −2.4 [3.6] | −2.4 [3.6] |
| College/University degree, Y | 2,665 (28.5) | 42,040 (41.1) | 14,293 (54.8) |
| Non-HDL, mg/dL | 183.6 (44.7) | 168.7 (39.6) | 140.5 (30.9) |
| Glucose, mg/dL | 99.5 (33.3) | 91.4 (17.0) | 87.7 (11.9) |
| HbA1c, % | 5.8 (3.0) | 5.4 (2.6) | 5.2 (2.5) |
| Dash diet score | 20.8 (4.0) | 24.7 (4.5) | 28.7 (3.9) |
| Sleep duration, h/day | 6.8 (1.4) | 7.2 (1.0) | 7.3 (0.8) |
| Moderate physical activity, min/week | 0.0 [30.0] | 90.0 [220.0] | 175.0 [280.0] |
| Vigorous physical activity, min/week | 0.0 [0.0] | 20.0 [100.0] | 60.0 [130.0] |
| Sedentary behavior time, h/day | 5.6 (2.6) | 4.4 (2.3) | 3.5 (2.1) |
| SBP, mmHg | 145.9 (16.9) | 138.9 (17.7) | 124.7 (15.6) |
| DBP, mmHg | 88.3 (10.0) | 82.9 (9.6) | 75.2 (8.3) |
| Alcohol intake | |||
| Daily or almost daily | 2,2255 (24.1) | 24,713 (24.2) | 4,972 (19.1) |
| Three or four times a week | 1,794 (19.2) | 25,637 (25.1) | 7,258 (27.8) |
| Once or twice a week | 2,077 (22.2) | 25,164 (24.6) | 6,980 (26.8) |
| One to three times a month | 1,179 (12.6) | 11,041 (10.8) | 2,928 (11.2) |
| Special occasions only | 1,304 (13.9) | 9,710 (9.5) | 2,362 (9.1) |
| Never | 744 (8.0) | 6,065 (5.9) | 1,566 (6.0) |
| Household income | |||
| Less than £18,000 | 1,893 (22.8) | 14,181 (15.4) | 2,599 (11.0) |
| £18,000 to £30,999 | 2,071 (24.9) | 22,913 (24.9) | 5,000 (21.1) |
| £31,000 to £51,999 | 2,307 (27.7) | 26,539 (28.9) | 6,879 (29.0) |
| £52,000 to £100,000 | 1,646 (19.8) | 22,104 (24.0) | 6,783 (28.6) |
| Greater than £100,000 | 401 (4.8) | 6,220 (6.8) | 2,472 (10.4) |
| Medication use | |||
| Cholesterol-lowering | 2,504 (26.9) | 14,266 (14.0) | 1,312 (5.1) |
| Antihypertensive | 3,452 (37.0) | 18,199 (17.9) | 1,023 (3.9) |
| Insulin treatment | 247 (2.7) | 728 (0.7) | 36 (0.1) |
Data are mean (SD), median [IQR], or N (%).
IQR: interquartile range; BMI: body mass index; Non-HDL: non-high-density-lipoprotein cholesterol; SBP, sysolic blood pressure; DBP, diastolic blood pressure.
During a median follow-up of 10 years, a total of 8,595 CVD cases (6,968 CHD and 1,948 stroke) were documented. The cumulative incidence showed graded relationships according to the levels of CVH categories during follow-up, particularly for CHD and CVD (Figure 1, Log-rank tests<0.001 for all). After adjustment for age, race, sex, a better CVH, indicated by a higher LE8 score, was associated with significantly lower risks of CHD, stroke, and CVD (Table 2, Model 1). The HR (95% CI) for each SD increment in LE8 score was 0.69 (0.67, 0.70) for CHD, 0.77 (0.73, 0.80) for stroke, and 0.71 (0.69, 0.72) for CVD. Further adjustment for assessment center, Townsend deprivation index, alcohol intake, sedentary behavior, average household income, and education did not appreciably attenuate the relationship (Table 2, Model 2). Compared to those with a low CVH category, participants with a moderate CVH had a 42% lower risk of developing CHD (HR: 0.58, 95% CI: 0.54, 0.62), a 35% lower risk of developing stroke (HR: 0.65, 95% CI: 0.57, 0.74), and a 40% lower risk of developing CVD (HR: 0.60, 95% CI: 0.56, 0.64). More strikingly, high CVH was associated with a 66% lower risk of CHD (HR: 0.34, 95% CI: 0.30, 0.38), a 55% lower risk of stroke (HR: 0.45, 95% CI: 0.37, 0.54), a 64% lower risk of CVD (HR: 0.36, 95% CI: 0.33, 0.40). In addition, both behavior and biological subscale scores were associated with lower risks of CHD, stroke, and CVD, similar to the overall LE8 score, but with attenuated magnitudes (Table 2). Models with each individual component of LE8 showed higher C-indexes compared to the base model (Table S5). Furthermore, the model with LE8 achieved higher accuracy and outperformed the model with LS7 (C-index: 0.7417 vs. 0.7345, p<0.001 for CHD, 0.7208 vs. 0.7165, p<0.001 for stroke, and 0.7339 vs. 0.7276, p<0.001 for CVD).
Figure 1.
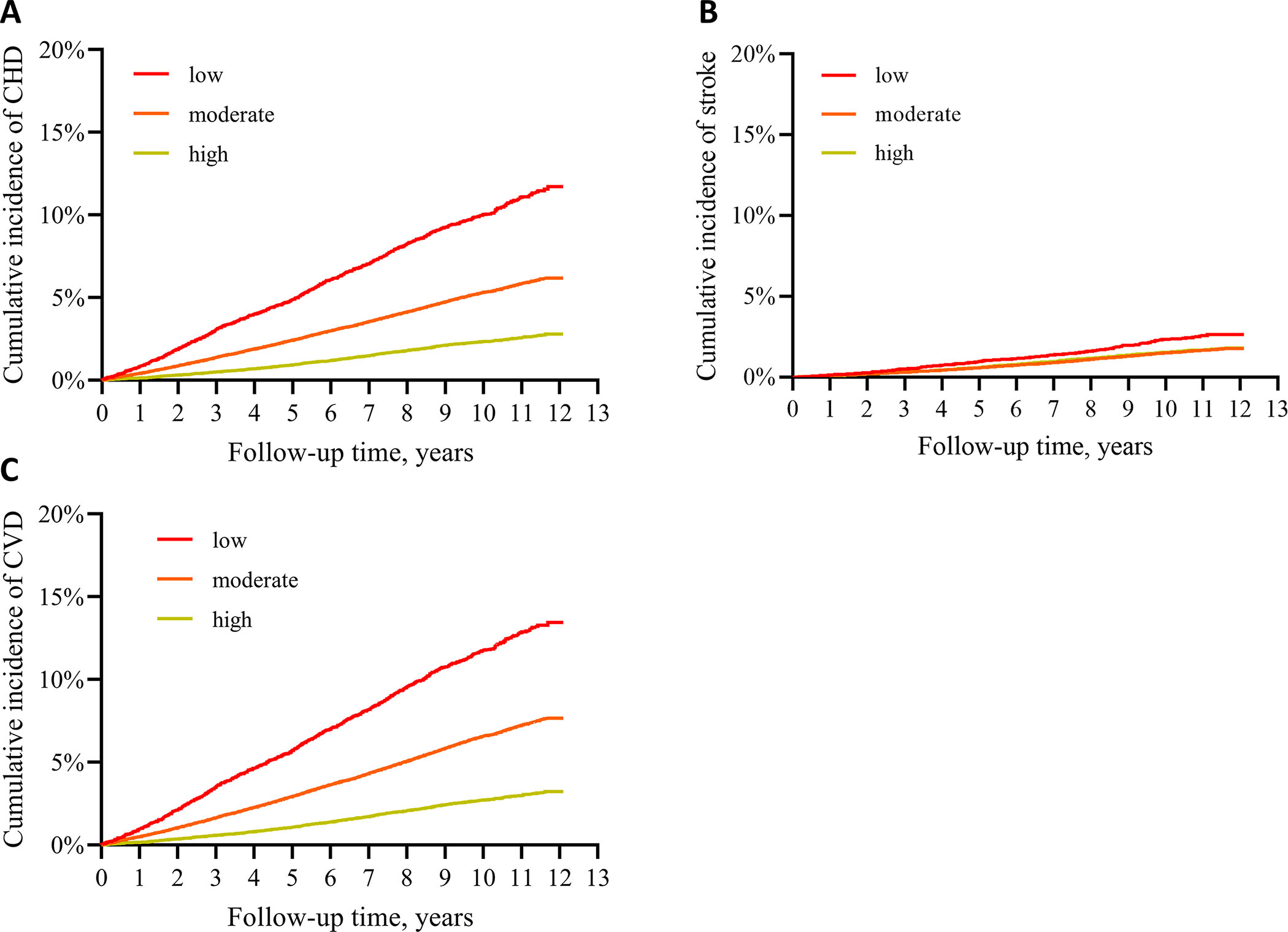
Cumulative incidence of CHD, stroke, and CVD according to CVH categories defined by LE8.
CHD: coronary heart disease; CVD: cardiovascular disease. Panel A: CHD; B: stroke, C: CVD. Log-rank tests p<0.001 for all.
Table 2.
Life’s essential 8 and incidence of CVD outcomes
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
|
||||
| HR (95%) | p | HR (95%) | p | |
|
| ||||
| CHD | ||||
|
| ||||
| Cardiovascular health metrics | ||||
| Low | ref | ref | ||
| Moderate | 0.52 (0.49, 0.56) | <0.001 | 0.58 (0.54, 0.62) | <0.001 |
| High | 0.29 (0.26, 0.32) | <0.001 | 0.34 (0.30, 0.38) | <0.001 |
| Per SD increment in LE8 | 0.69 (0.67, 0.70) | <0.001 | 0.71 (0.69, 0.73) | <0.001 |
| Subscale | ||||
| Per SD increment in behavior scale | 0.80 (0.78, 0.82) | <0.001 | 0.83 (0.81, 0.85) | <0.001 |
| Per SD increment in biological scale | 0.75 (0.73, 0.77) | <0.001 | 0.75 (0.73, 0.77) | <0.001 |
|
| ||||
| Stroke | ||||
|
| ||||
| Cardiovascular health metrics | ||||
| Low | ref | ref | ||
| Moderate | 0.61 (0.53, 0.69) | <0.001 | 0.65 (0.57, 0.75) | <0.001 |
| High | 0.40 (0.33, 0.48) | <0.001 | 0.45 (0.37, 0.54) | <0.001 |
| Per SD increment in LE8 | 0.77 (0.73, 0.80) | <0.001 | 0.79 (0.75, 0.82) | <0.001 |
| Subscale | ||||
| Per SD increment in behavior scale | 0.85 (0.81, 0.89) | <0.001 | 0.87 (0.83, 0.91) | <0.001 |
| Per SD increment in biological scale | 0.82 (0.78, 0.86) | <0.001 | 0.82 (0.78, 0.86) | <0.001 |
|
| ||||
| CVD | ||||
|
| ||||
| Cardiovascular health metrics | ||||
| Low | ref | ref | ||
| Moderate | 0.55 (0.51, 0.58) | <0.001 | 0.60 (0.56, 0.64) | <0.001 |
| High | 0.31 (0.29, 0.35) | <0.001 | 0.36 (0.33, 0.40) | <0.001 |
| Per SD increment in LE8 | 0.71 (0.69, 0.72) | <0.001 | 0.73 (0.72, 0.75) | <0.001 |
| Subscale | ||||
| Per SD increment in behavior scale | 0.82 (0.80, 0.83) | <0.001 | 0.85 (0.83, 0.87) | <0.001 |
| Per SD increment in biological scale | 0.76 (0.74, 0.78) | <0.001 | 0.76 (0.74, 0.78) | <0.001 |
CHD: coronary heart disease; CVD: cardiovascular heart disease.
Model 1 was adjusted for age, race, and sex.
Model 2: Model 1 + assessment center, Townsend deprivation index, alcohol intake, sedentary behavior, average household income, and education.
Behavior scale and biological scale were mutually adjusted for subscale analyses.
When we analyzed the individual component of LE8 and simultaneously included all the metrics in the model, high sleep health was associated with significant lower risks of CHD (HR: 0.73, 95% CI: 0.67, 0.80), stroke (HR: 0.81, 95% CI: 0.68, 0.96), and CVD (HR: 0.74, 95% CI: 0.69, 0.81), independent of the other 7 components (Table S3). Moreover, excluding sleep health in the LE8 showed an attenuated association with the risks of CHD, stroke, and CVD, compared with the full LE8 score. The differences in Uno’s concordance statistics compared to the full LE8 score indicated that excluding sleep from the LE8 had a lower model fit, particularly for CHD and CVD (Table S4).
We observed significant sex difference in the association of LE8 with risks of CHD and CVD (Table 3, p-interaction <0.001 for CHD, and p=0.0013 for CVD). Compared with men, women had greater benefit by having a high CVH defined by LE8. Among women, each SD increment in LE8 score was associated with an HR of 0.68 (95% CI: 0.65, 0.71) for CHD and 0.70 (95% CI: 0.68, 0.73) for CVD after adjustment for covariates, while among men, the HR was 0.73 (0.71, 0.76) for CHD and 0.75 (0.73, 0.77) for CVD. The associations between LE8 score and risk of stroke were similar among men and women. In addition, significant effect modification with age was detected such that the beneficial association between LE8 and the CVD outcomes were stronger when LE8 was measured at age ≤55 years versus age > 55 years (Figure S2, p-interaction <0.001, 0.007, and <0.001 for CHD, stroke, and CVD, respectively). The protective association appeared to be stronger among participants aged 55 and younger. The adjusted HR was 0.62 (0.59, 0.65) for CHD, 0.76 (0.69, 0.83) for stroke, and 0.64 (0.61, 0.67) for CVD. Among participants >55 years old, the adjusted HR was 0.75 (0.73, 0.77) for CHD, 0.80 (0.76, 0.84) for stroke, and 0.76 (0.74, 0.78) for CVD.
Table 3.
Hazard ratios of CVD outcomes according to LE8 in women and men
| Women | Men | p-int | |||
|---|---|---|---|---|---|
|
|
|||||
| HR (95%) | p | HR (95%) | p | ||
|
| |||||
| CHD | <0.001 | ||||
|
| |||||
| Cardiovascular health metrics | |||||
| Low | ref | ref | |||
| Moderate | 0.53 (0.46, 0.60) | <0.001 | 0.60 (0.55, 0.65) | <0.001 | |
| High | 0.29 (0.24, 0.34) | <0.001 | 0.39 (0.34, 0.45) | <0.001 | |
| Per SD increment in LE8 | 0.68 (0.65, 0.71) | <0.001 | 0.73 (0.71, 0.76) | <0.001 | |
| Subscale | |||||
| Per SD increment in behavior scale | 0.78 (0.75, 0.82) | <0.001 | 0.85 (0.83, 0.88) | <0.001 | |
| Per SD increment in biological scale | 0.76 (0.73, 0.80) | <0.001 | 0.75 (0.73, 0.78) | <0.001 | |
|
| |||||
| Stroke | 0.44 | ||||
|
| |||||
| Cardiovascular health metrics | |||||
| Low | ref | ref | |||
| Moderate | 0.69 (0.54, 0.89) | <0.001 | 0.63 (0.54, 0.75) | <0.001 | |
| High | 0.43 (0.32, 0.58) | <0.001 | 0.50 (0.39, 0.65) | <0.001 | |
| Per SD increment in LE8 | 0.77 (0.72, 0.83) | <0.001 | 0.80 (0.75, 0.85) | <0.001 | |
| Subscale | |||||
| Per SD increment in behavior scale | 0.86 (0.80, 0.92) | <0.001 | 0.88 (0.83, 0.93) | <0.001 | |
| Per SD increment in biological scale | 0.82 (0.76, 0.88) | <0.001 | 0.83 (0.78, 0.89) | <0.001 | |
|
| |||||
| CVD | 0.0013 | ||||
|
| |||||
| Cardiovascular health metrics | |||||
| Low | ref | ref | |||
| Moderate | 0.56 (0.49, 0.63) | <0.001 | 0.61 (0.57, 0.66) | <0.001 | |
| High | 0.32 (0.27, 0.37) | <0.001 | 0.41 (0.36, 0.47) | <0.001 | |
| Per SD increment in LE8 | 0.70 (0.68, 0.73) | <0.001 | 0.75 (0.73, 0.77) | <0.001 | |
| Subscale | |||||
| Per SD increment in behavior scale | 0.80 (0.77, 0.83) | <0.001 | 0.87 (0.85, 0.90) | <0.001 | |
| Per SD increment in biological scale | 0.77 (0.74, 0.81) | <0.001 | 0.76 (0.74, 0.79) | <0.001 | |
CHD: coronary heart disease; CVD: cardiovascular heart disease.
Model was adjusted for age, race, assessment center, Townsend deprivation index, alcohol intake, sedentary behavior, average household income, and education.
Behavior scale and biological scale were mutually adjusted for subscale analyses.
Intriguingly, we found that the genetic predisposition to CHD significantly modified the association between the LE8 score and the risk of CHD (Table 4, p-interaction <0.001). The inverse association between LE8 and CHD risk appeared to be stronger among those with a lower genetic risk of CHD (HRs in ascending GRS stratum: 0.68 (0.64, 0.72), 0.70 (0.67, 0.72), and 0.77 (0.73, 0.81), respectively). We did not find significant interaction between GRS for stroke and LE8 (p-interaction=0.43). The joint association of the LE8 score and CHD-GRS is shown in Figure S3. Compared with participants with a high genetic risk of CHD and low CVH profile, those with a low genetic risk of CHD and a high CVH had a 78% lower risk of developing CHD (HR: 0.22, 95% CI: 0.17, 0.28). A similar pattern was also observed for the joint categories of stroke GRS and categories of CVH for stroke incidence (Figure S4). Compared with participants with a high genetic risk of stroke and low CVH, those with a low genetic risk of stroke and a high CVH had a 64% lower risk of developing stroke (HR: 0.36, 95% CI: 0.24, 0.53).
Table 4.
Association between LE8 and incident CHD and stroke by stratum of the genetic risk scores
| CHD | Stroke | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| GRS | HR (95% CI) | P | p-interaction | HR (95% CI) | P | p-interaction |
| Q1 | 0.68 (0.64, 0.72) | <0.001 | <0.001 | 0.84 (0.75, 0.93) | <0.001 | 0.43 |
| Q2-Q4 | 0.70 (0.67, 0.72) | <0.001 | 0.79 (0.74, 0.84) | <0.001 | ||
| Q5 | 0.77 (0.73, 0.81) | <0.001 | 0.73 (0.66, 0.80) | <0.001 | ||
HR: hazard ratio; GRS: genetic risk score; Q1 is the lowest quintile.
HR were estimated per SD increment of the LE8.
Models were adjusted for age, race, sex, assessment center, Townsend deprivation index, alcohol intake, sedentary behavior, average household income, and education.
Discussion
In this large prospective cohort, we found that better CVH, defined by higher LE8 score, was associated with significantly lower risks of CHD, stroke, and CVD. Compared with participants with a low CVH, those with a high CVH had a 66%, 55%, and 64% lower risk of developing CHD, stroke, and CVD, respectively. The protective association was more evident among women and younger participants. In addition, the genetic predisposition to CHD significantly modified the association between LE8 score and risk of CHD. Participants with a lower genetic risk of CHD appeared to benefit more from a high LE8 score.
To our knowledge, this is the first study to examine the association of the new CVH metrics, defined by LE8, and the risk of CVD outcomes. Our results are consistent with previous reports using LS7 as the assessment for CVH.25–28 For example, in the Atherosclerosis Risk in Communities Study (ARIC) cohort (aged 45 to 64 years), there was a strong gradient of cumulative CVD incidence according to the number of ideal health metrics met.25 Such associations were also observed across ethnic background. In the Northern Manhattan Study, participants with ≥5 ideal CVH metrics had significantly lower risks of myocardial infarction, stroke, and CVD, compared with those with 0 or 1 ideal CVH metrics, across whites, blacks, and Caribbean Hispanics.26 Of note, the previous algorithm defined by LS7 was less sensitive to interindividual difference, due to its very simplified categories of ideal, intermediate, and ideal classification for each component. For example, individuals with 1 minute/week moderate to vigorous activity and individuals with 149 minutes/week vigorous activity would be categorized into the same intermediate group.8 The LE8 score, a more continuous scale (each component varies from 0–100), is designed to be more sensitive to interindividual differences.8 The improved performance of LE8 in predicting CVD risks could be explained by the more sophisticated scoring algorithm, as well as the inclusion of more relative factor that contribute the diseases risk, i.e., sleep health. Moreover, our sensitivity analysis showed that excluding sleep health from the CVH score, the association with CVD outcomes were all attenuated, indicating the newly included component, sleep health, indeed added independent values to the overall CVH.
Our stratified analyses showed that the protective associations were more pronounced among women. Such observation is consistent with previous studies, where women appeared to have a healthier lifestyle compared to men.29,30 The sex difference in CVD has long been noted.31,32 In line with our findings, previous study found that women presented with a more favorable CVH profile than do man, particularly at a younger age.33 In the Jackson Heart Study, it was also reported that the men had a higher CVD incidence rate across the number of ideal CVH metrics evaluated by LS7, compared to women.29 Similarly, in Kailuan community cohort (>100,000 participants, mean age 51 years old), the cumulative incidence rates of CVD were higher among men than women, regardless of the number of the ideal LS7 met.30 The reasons underpinning the sex differences in CVD outcomes are not fully understood. Factors, such as stress,34 hormones,35,36 and psychosocial factors,37–39 may contribute additionally to higher CVD incidence among men. This implies that women could benefit to a greater extent in having a higher level of CVH defined by AHA LE8 than men; more efforts are needed to improve the CVH profile among men.
Several prior studies have reported significant age effects whereby the CVD protections associated with attaining more healthy CVD metrics were more pronounced among younger compared with older middle age adults.40,41 In accordance with our results, Ramírez-Vélez et al. conducted a meta-regression of 12 prospective studies with 210,443 adults and showed a significant age effect on the association between CVH defined by LS7 and overall CVD incidence.40 The association was significantly stronger among younger participants.40 Another study conducted among 4,638 young adults (mean age 25) from Coronary Artery Risk Development in Young Adults Study (CARDIA) also reported a strong inverse association between CVH assessed by LS7 at late adolescent/young adult with CVD outcomes.42 Our results showed that a high level of healthy CVH, defined by LE8, may have greater impact particularly among young adults. This finding is of special importance because data suggest that young adults have experienced frustrating small decreases in CVD incidence.4,43
Intriguingly, for the first time, we found a significant interaction between the LE8 and genetic susceptibility to CHD. The observed protective association was more evident among participants with a lower genetic risk of CHD. The result is in line with previous studies on gene-environment interactions,16 suggesting that genetic factors and lifestyle factors jointly influence the risk of disease.44 Even though the magnitude of risk reduction is relatively low in high genetic risk group compared to low genetic risk group, our results demonstrated that the risk of developing CHD conferred by high genetic predisposition could still be largely offset by healthy lifestyle.
Our study also has limitations. First, the assessments of behavioral factors are mainly obtained by self-report. Information bias and misclassification are inevitable. Second, same as any population-based cohort study, the disease prevalence and incidence rates are generally lower due to the healthy volunteer bias.45,46 However, this would not affect the internal validity of our analyses. Third, most of the study participants were whites. Further investigations in other racial/ethnic groups are warranted. Fourth, LE8 was only calculated at one-time point. We could not account for potential lifestyle changes during the follow-up to the current analysis. The strengths of this study include the application of the new LE8 score to assess CVH, the large study sample, and a prospective ascertainment of CVD outcomes.
In conclusion, our findings in a large prospective cohort study indicate a strong graded inverse association between the LE8 defined CVH and risks of CHD, stroke, and CVD. Such associations are particularly pronounced among women, younger participants, and those with lower genetic risk. These findings highlight the importance of improving cardiovascular health by adopting the AHA LE8.
Supplementary Material
Highlights.
The American Heart Association recently released a new cardiovascular health (CVH) metric, Life’s Essential 8 (LE8) for health promotion and preservation. However, supportive data is lacking on the association between CVH defined by LE8 and cardiovascular disease (CVD) risk.
We found that higher cardiovascular health, indicated by a higher LE8 score, was associated with significantly lower risks of coronary heart disease (CHD), stroke, and CVD.
Moreover, the new LE8 score outperformed the previous cardiovascular health metrics, Life’s Simple 7.
In addition, we found genetic predisposition to CHD significantly modified the association between LE8 and the risk of CHD.
Acknowledgments:
The authors appreciate the participants in UK Biobank for their participation and contribution to the research. The study has been conducted using the UK Biobank Resource under Application 29256.
Funding:
The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383), the Fogarty International Center (TW010790), and Tulane Research Centers of Excellence Awards. Xiang Li was the recipient of the American Heart Association Predoctoral Fellowship Award (19PRE34380036).
Non-standard Abbreviations and Acronyms (alphabetic order)
- AHA
American Heart Association
- CHD
coronary heart disease
- CVD
cardiovascular disease
- CVH
cardiovascular health
- DBP
diastolic blood pressure
- GRS
genetic risk score
- LE8
Life’s Essential 8
- SBP
systolic blood pressure
- SNP
single nucleotide polymorphism
Footnotes
Disclosure: All the authors declare that there is no conflict of interest.
References:
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Ward JL, Azzopardi PS, Francis KL, et al. Global, regional, and national mortality among young people aged 10–24 years, 1950–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;398:1593–1618. doi: 10.1016/S0140-6736(21)01546-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet (London, England). 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 4.Gooding HC, Gidding SS, Moran AE, et al. Challenges and Opportunities for the Prevention and Treatment of Cardiovascular Disease Among Young Adults: Report From a National Heart, Lung, and Blood Institute Working Group. J Am Heart Assoc. 2020;9:e016115. doi: 10.1161/JAHA.120.016115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan M, Sun D, Zhou T, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. 2020;41:1182–1189. doi: 10.1093/eurheartj/ehz849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Xue Q, Wang M, et al. Adherence to a Healthy Sleep Pattern and Incident Heart Failure: A Prospective Study of 408 802 UK Biobank Participants. Circulation. 2021;143:97–99. doi: 10.1161/CIRCULATIONAHA.120.050792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makarem N, Castro-Diehl C, St-Onge MP, et al. Redefining Cardiovascular Health to Include Sleep: Prospective Associations With Cardiovascular Disease in the MESA Sleep Study. J Am Heart Assoc. 2022;11:e025252. doi: 10.1161/JAHA.122.025252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Onge M-P, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Q, Wang M, Zhou T, et al. The Lifestyle-Related Cardiovascular Risk Is Modified by Sleep Patterns. Mayo Clin Proc. 2022;97:519–530. doi: 10.1016/j.mayocp.2021.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khera A V, Emdin CA, Drake I, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med. 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikpay M, Goel A, Won H-H, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik R, Chauhan G, Traylor M, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdullah Said M, Verweij N, Van Der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK biobank study. JAMA Cardiol. 2018;3:693–702. doi: 10.1001/jamacardio.2018.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikkanen E, Gustafsson S, Ingelsson E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: Longitudinal analyses in the UK biobank study. Circulation. 2018;137:2583–2591. doi: 10.1161/CIRCULATIONAHA.117.032432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Y, Chen X, Han J, Jiang W, Natarajan P, Zhao H. Interactions Between Enhanced Polygenic Risk Scores and Lifestyle for Cardiovascular Disease, Diabetes, and Lipid Levels. Circ Genomic Precis Med. 2021;14:e003128. doi: 10.1161/CIRCGEN.120.003128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins R What makes UK Biobank special? Lancet (London, England). 2012;379:1173–1174. doi: 10.1016/S0140-6736(12)60404-8 [DOI] [PubMed] [Google Scholar]
- 19.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779–e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-Style Diet and Risk of Coronary Heart Disease and Stroke in Women. Arch Intern Med. 2008;168:713–720. doi: 10.1001/archinte.168.7.713 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Zhou T, Ma H, Liang Z, Fonseca VA, Qi L. Replacement of Sedentary Behavior by Various Daily-Life Physical Activities and Structured Exercises: Genetic Risk and Incident Type 2 Diabetes. Diabetes Care. 2021; 44:2403–10. doi: 10.2337/dc21-0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018; 562:203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell FEJ, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: [DOI] [PubMed] [Google Scholar]
- 24.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folsom AR, Yatsuya H, Nettleton JA, et al. Community Prevalence of Ideal Cardiovascular Health, by the American Heart Association Definition, and Relationship With Cardiovascular Disease Incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS V, Sacco RL. Ideal Cardiovascular Health Predicts Lower Risks of Myocardial Infarction, Stroke, and Vascular Death Across Whites, Blacks, and Hispanics. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low Prevalence of “Ideal Cardiovascular Health” in a Community-Based Population. Circulation. 2011;123:850–857. doi: 10.1161/CIRCULATIONAHA.110.980151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachman S, Peters RJG, Lentjes MAH, et al. Ideal cardiovascular health and risk of cardiovascular events in the EPIC-Norfolk prospective population study. Eur J Prev Cardiol. 2016;23:986–994. doi: 10.1177/2047487315602015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ommerborn MJ, Blackshear CT, Hickson DA, et al. Ideal Cardiovascular Health and Incident Cardiovascular Events: The Jackson Heart Study. Am J Prev Med. 2016;51:502–506. doi: 10.1016/j.amepre.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, Huang Z, Yang X, et al. Prevalence of Ideal Cardiovascular Health and Its Relationship With the 4-Year Cardiovascular Events in a Northern Chinese Industrial City. Circ Cardiovasc Qual Outcomes. 2012;5:487–493. doi: 10.1161/CIRCOUTCOMES.111.963694 [DOI] [PubMed] [Google Scholar]
- 31.Zucker DR, Griffith JL, Beshansky JR, Selker HP. Presentations of Acute Myocardial Infarction in Men and Women. J Gen Intern Med. 1997;12:79–87. doi: 10.1007/s11606-006-5001-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maas AHEM Appelman YEA. Gender differences in coronary heart disease. Netherlands Hear J Mon J Netherlands Soc Cardiol Netherlands Hear Found. 2010;18:598–602. doi: 10.1007/s12471-010-0841-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walli-Attaei M, Rosengren A, Rangarajan S, et al. Metabolic, behavioural, and psychosocial risk factors and cardiovascular disease in women compared with men in 21 high-income, middle-income, and low-income countries: an analysis of the PURE study. Lancet. 2022;400:811–821. doi: 10.1016/S0140-6736(22)01441-6 [DOI] [PubMed] [Google Scholar]
- 34.Murphy MO, Loria AS. Sex-specific effects of stress on metabolic and cardiovascular disease: are women at higher risk? Am J Physiol Regul Integr Comp Physiol. 2017;313:R1–R9. doi: 10.1152/ajpregu.00185.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007;297:1465–1477. [DOI] [PubMed] [Google Scholar]
- 36.Tunstall-Pedoe H Myth and paradox of coronary risk and the menopause. Lancet. 1998;351:1425–1427. [DOI] [PubMed] [Google Scholar]
- 37.Leimkühler AMM, Heller J, Paulus N-C. Subjective well-being and ‘male depression’in male adolescents. J Affect Disord. 2007;98:65–72. [DOI] [PubMed] [Google Scholar]
- 38.Rahman O, Strauss J, Gertler P, Ashley D, Fox K. Gender differences in adult health: an international comparison. Gerontologist. 1994;34:463–469. [DOI] [PubMed] [Google Scholar]
- 39.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–1246. doi: 10.1016/j.jacc.2007.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramírez-Vélez R, Saavedra JM, Lobelo F, Celis-Morales CA, Pozo-Cruz B del, García-Hermoso A. Ideal Cardiovascular Health and Incident Cardiovascular Disease Among Adults: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2018;93:1589–1599. doi: 10.1016/j.mayocp.2018.05.035 [DOI] [PubMed] [Google Scholar]
- 41.Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: A meta-analysis of prospective studies. Clin Cardiol. 2017;40:1339–1346. doi: 10.1002/clc.22836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perak AM, Ning H, Khan SS, et al. Associations of Late Adolescent or Young Adult Cardiovascular Health With Premature Cardiovascular Disease and Mortality. J Am Coll Cardiol. 2020;76:2695–2707. doi: 10.1016/j.jacc.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. Cardiovascular diseases (CVDs). Accessed October 30, 2021. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 44.Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet. 2005;6:287–298. doi: 10.1038/nrg1578 [DOI] [PubMed] [Google Scholar]
- 45.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enzenbach C, Wicklein B, Wirkner K, Loeffler M. Evaluating selection bias in a population-based cohort study with low baseline participation: the LIFE-Adult-Study. BMC Med Res Methodol. 2019;19:135. doi: 10.1186/s12874-019-0779-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


