Abstract
The rapid and coordinated propagation of neural activity across the brain provides the foundation for complex behavior and cognition. Technical advances across neuroscience subfields have advanced understanding of these dynamics, but points of convergence are often obscured by semantic differences, creating silos of subfield-specific findings. In this Review, we describe how a parsimonious conceptualization of brain state as the fundamental building block of whole-brain activity offers a common framework to relate findings across scales and species. We present examples of the diverse techniques commonly used to study brain states associated with physiology and higher-order cognitive processes and discuss how integration across them will enable a more comprehensive, mechanistic characterization of the neural dynamics that are crucial to survival and disrupted in disease.
Keywords: neural dynamics, integrative neuroscience, multiscale analysis, spontaneous activity, arousal
Brain state as a framework to advance understanding of neural dynamics
The brain is a dynamic organ, continuously adapting in response to a changing world. Recent technical advances in recording methods and computational analysis have created an opportunity to better describe brain dynamics and relate them to brain function, but these approaches differ widely in temporal and spatial resolution, as well as in the nature of the measured signal (Fig. 1). These technical differences have driven subfield-specific definitions of “brain state,” yielding siloed, parallel lines of inquiry into how brain-wide activity changes to subserve behavior. Individually, these definitions and related constructs have enabled researchers to describe phenomena ranging from membrane potential fluctuations of single neurons[1] to the evolution—over seconds to minutes—of behaviorally relevant, whole-brain functional connectivity patterns in humans (e.g. [2]). But the picture offered by such work is incomplete. To further current understanding of the dynamic brain, we must integrate across these levels of analysis, capitalizing on the complementary insights they offer.
Figure 1. Systems and cognitive neuroscience techniques each offer partial, but complementary, insight into brain states.
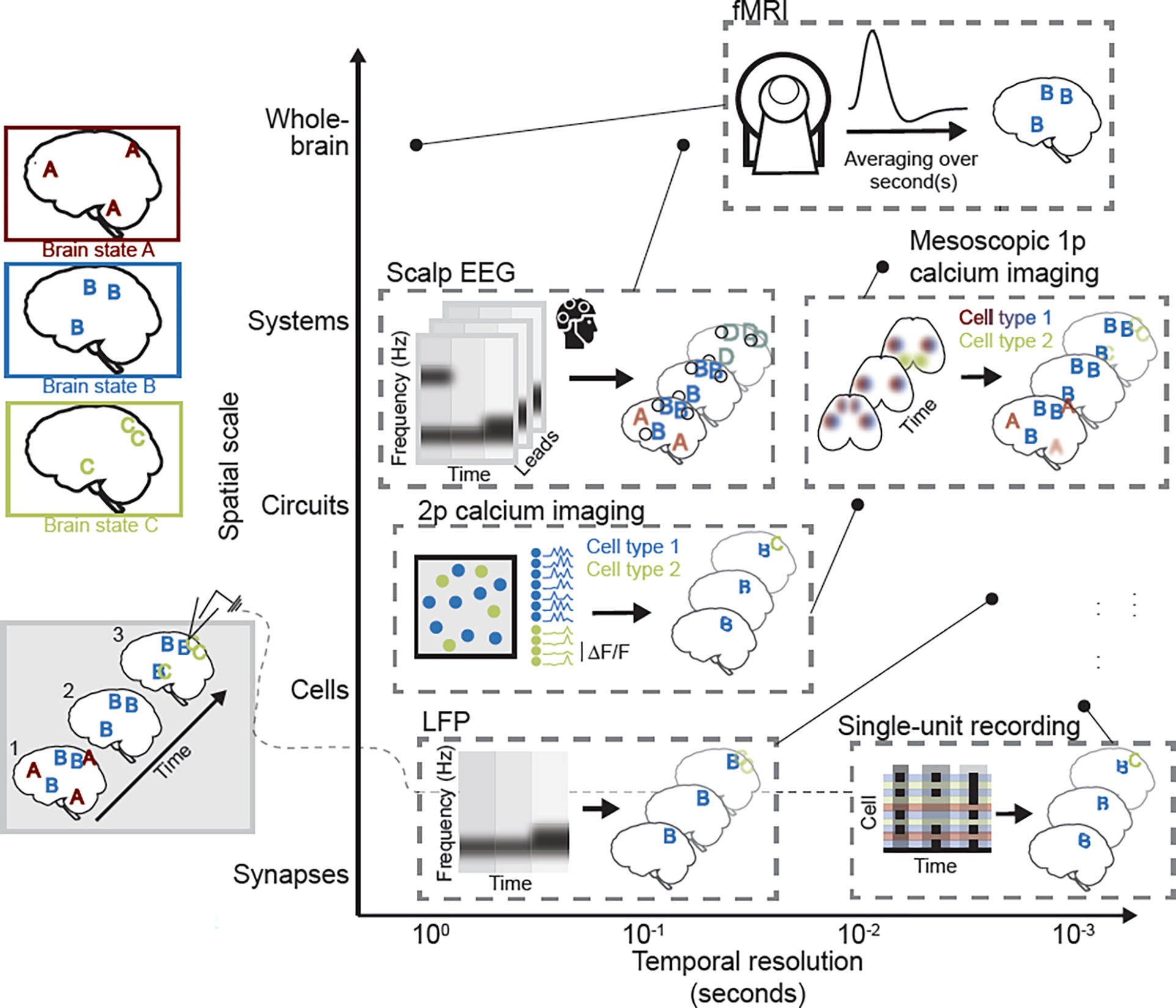
In this example, three schematic brain states, A, B, and C, are depicted (upper left). Each brain state is defined by a stereotyped pattern of whole-brain activity, and brain states can occur in isolation (timepoint 2; bottom left) or in combination (timepoints 1 and 3). Commonly used techniques are schematized and placed (see corresponding point) according to their approximate, relative temporal resolution and spatial scale (i.e., field of view size), as typically implemented. Single-unit recording: Schematized raster plot represents cell spiking at three time points. Limited field of view is represented by focal patterns, and cell response heterogeneity that may obscure patterns is represented by incomplete “B” and “C”. The identity of the cells is typically unknown. Two-photon (2p) calcium imaging suffers from many of the same limitations (represented by focal, partial brain state patterns and heterogeneous calcium signal at the individual cell level), but can offer insight into cell-type specificity of brain state expression (represented by colored cells, each of which preferentially responds during the brain state of that color). Local field potentials (LFPs) capture regional fluctuations in activity with high temporal resolution, and can be used, for instance, to assess changes in frequency of extracellular oscillations across brain states. Mesoscopic single-photon (1p) calcium imaging has slightly lower temporal resolution, but captures cell type-specific patterns of brain activity across the cortical mantle, yielding more complete representations of brain states (though with limited resolution of activity in deep structures, represented by blurred pattern elements). Scalp electroencephalography (EEG), like LFPs, captures brain state-associated differences in power across frequency bands, but suffers from imprecise source localization (represented by enlarged, blurred, and conflated pattern elements – i.e., pattern D that merges brain states B and C). Functional magnetic resonance imaging (fMRI), like EEG, offers whole-brain coverage in humans, but has low temporal resolution, such that brain state patterns will be effectively averaged over seconds. In this example, the only resolved brain state will thus be brain state B, which persists across all time points.
We argue not for a single spatiotemporal definition of a brain state (see Glossary), but rather for a shared, conceptual framework of a brain state as a pattern of brain activity or functional coupling that emerges from and has consequences for physiology and/or behavior. This definition transcends spatiotemporal scales and can be used to organize this vast and expanding literature, revealing points of convergence often obscured by semantics. It yields three criteria that must be met by all brain state research: 1. A brain state is a product of a specified physiological or cognitive state, 2. A brain state is characterized by a widely distributed pattern of activity or coupling, and 3. A brain state affects the organism’s future physiology and/or behavior. Implicit in this framework is the possibility that at any point in time, multiple brain states may be expressed, such that each snapshot of brain activity can be decomposed into these constituent parts. Further, these brain states are a neurobiological phenomenon, likely resulting from neuronal activity propagating across the structural scaffold of the brain and modified by neuromodulatory tone. This definition is consistent with, but not limited to, varied conceptualizations of brain state that have been recently reviewed: brain state as “a background of spontaneous, ongoing activity”[3] (see also [4]), as internal behavioral states (e.g., arousal) with neuronal correlates[5], and as “the amount of common fluctuation in population spiking activity”[6]. Finally, every technique for recording neural activity offers different—and, we argue, complementary—insight into these whole-brain patterns (Box 1). In fact, we suggest that a comprehensive characterization of a given brain state, as well as the disentangling of multiplexed brain states, can only be achieved by integrating across these levels of analysis.
Box 1: Data acquisition techniques.
Acquisition techniques at all levels of analysis offer insight into brain states, but when used in isolation, each offers only a partial view of brain dynamics (Fig. 1). In parallel, a range of analytical approaches exists to make sense of these data, and in many cases offers a means to bridge these levels of analysis (e.g., [125]).
Single-unit electrophysiological recordings offer high temporal and spatial resolution, permitting high-fidelity observation of a given cell’s movement through brain states. Insights gained from these recordings are limited, however, by cell response heterogeneity: a state-selective cell likely does not respond with perfect consistency—in firing rate or inter-spike interval—when that state is visited[131,132], and neighboring cells may be recruited by different brain states[28,30,31], an issue compounded by the technique’s lack of cell-type specificity. Further, measured signals lack regional, inter-regional, and whole-brain context. The technique thus offers a focal, partial view of relevant activity.
In-vivo two-photon calcium imaging, while an abstraction from spiking activity, offers the crucial benefit of cell-type specificity that can aid exploration of cell response heterogeneity. Like single-unit recordings, two-photon imaging offers a highly focal window into neural activity, devoid of broader context, although recent work has demonstrated the feasibility of significantly expanded spatial scale[133,134].
Local field potentials (LFPs) offer some context, capturing regional and local, interregional motifs with moderately high temporal resolution[135,136]. Single-photon mesoscopic calcium imaging, often likened to whole-brain LFPs, offers more complete insight into interregional interactions as well as cell-type specificity. It is, however, a further abstraction from spiking activity, and is largely limited to the cortical mantle (but see [137]).
Two commonly used human neuroscience techniques are scalp electroencephalography (EEG) and functional magnetic resonance imaging (fMRI). Their non-invasive nature makes them crucial for the study of brain state dynamics in humans, but they offer limited insight into mechanism. EEG has moderate temporal resolution and whole-cortex coverage, but poor spatial resolution that may obscure or conflate brain state patterns. FMRI has better spatial resolution than EEG but poor temporal resolution, likely “blurring” fast neural dynamics and resolving only pattern combinations with long dwell times and/or high recurrence (though “fast fMRI” can be used to resolve distinct arousal states[138]). Further, the blood-oxygen-level-dependent (BOLD) signal on which it is based is an indirect measure of neural activity that is confounded by many variables (e.g., heart and respiratory rate, neurovascular coupling, and neuroenergetics[139–142]), which may interact with behavioral states—and thus distort resolved brain states. Nevertheless, the ease of fMRI data acquisition during a variety of behavioral states makes it a critical tool to acquire large quantities of data suitable for studying dynamics at both short (e.g., [143]) and long (minutes to years) timescales in healthy and clinical populations.
The promise of multiscale, multimodal neuroscience has been recognized before—this philosophy has, for example, motivated the NIH BRAIN Initiative—but divergent conceptualization and operationalization of brain state has often proved limiting. In what follows, we propose that a shared concept of brain state can facilitate this work. Specifically, we describe, in general terms, the principles of organization and biological underpinnings of brain states across levels of analysis. We then apply the three proposed criteria to past studies, grouped based on commonly employed measures of physiological state (see section ‘Physiological changes are reflected in reproducible brain states’) or cognitive paradigms (see ‘The brain on task’ and Fig. 2). We show how these studies collectively contribute to the characterization of a common brain state across spatial and temporal scales (criterion 2; Fig. 3). Finally, when possible, we demonstrate how each of these brain states influences subsequent behavior and physiology (criterion 3). We seek not to exhaustively review a specific set of brain states (as has been done previously, e.g., [6]), but rather to develop a conceptual framework, supported by these examples, to reveal opportunities for an interdisciplinary study of brain dynamics. While the application of this framework to previous work demonstrates its validity and generalizability, its greatest utility lies in its guidance for future studies of brain state, as exemplified by several recent papers (see section ‘Using brain state to unify the study of neural dynamics’). We provide an example case to illustrate how, in the absence of multimodal data from a single study, focusing on brain state may still facilitate integration of results from independent studies using distinct modalities to characterize brain dynamics (Fig. 4). This approach is, crucially, prospective rather than retrospective: we provide recommendations to first identify a brain state of interest and consider its manifestation across scales and modalities, and then, within a single project, to pursue these predictions, directly mapping results across levels of analysis. Such work promises to yield a more complete description of functionally relevant, stereotyped brain states, with the ultimate goal of revealing the biology underlying brain function in health and disease (Box 2).
Figure 2. Using physiological states and cognitive paradigms to characterize corresponding brain states.
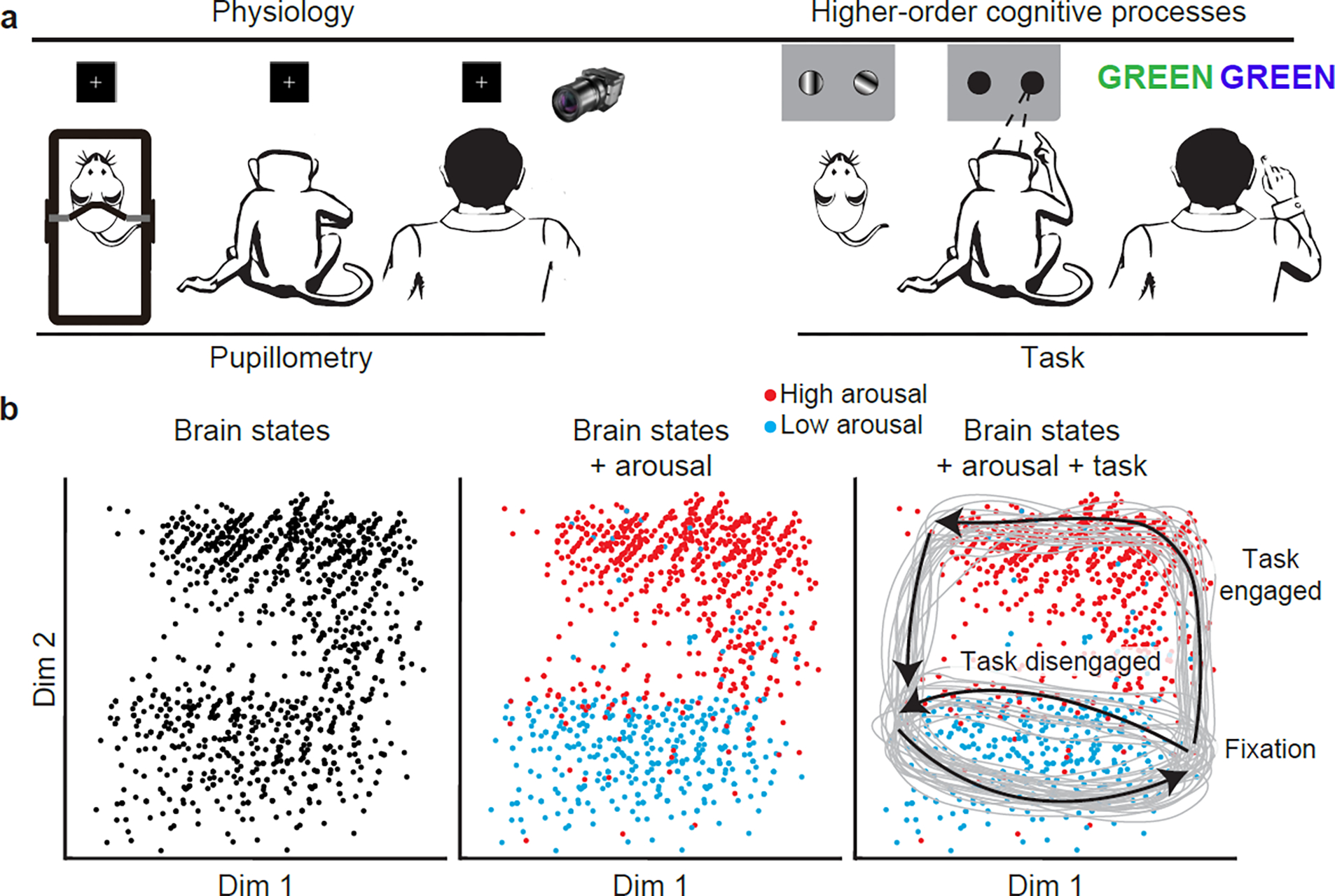
a, In this article, we discuss brain states that correspond to physiological states and cognitive paradigms. The former offers an opportunity to study spontaneous fluctuations in brain activity, but the lack of clearly defined behavioral state boundaries complicates interpretation of these changes (though studies have demonstrated the potential to segment behavioral sequences from spontaneous activity, e.g. [156]). Cognitive paradigms can offer clearer behavioral state boundaries. b, As represented in simulated neural data embedded via dimensionality reduction in a two-dimensional state space, clusters of similar time points are clearly visible, but cannot be labeled (left panel). With a distinct and supporting modality, such as pupillometry to index arousal (middle panel), one can observe the influence of physiology on whole-brain activity, identifying time points at which patterns correspond to high and low arousal. Further interpretation of these brain states is made possible by knowledge about the structure of the cognitive paradigm during which data were collected (right panel), revealing stereotyped trajectories through state space on high- and low-engagement trials, with low-arousal activity patterns more common on low-engagement trials, perhaps tracking decreased task performance. These boundaries likely do not fully explain the brain’s traversal through state space (task-defined states can also be affected by other factors), underscoring the need to study brain states at multiple temporal scales (Fig. 3). Nevertheless, each approach offers distinct and complementary insight into the nature and functional implications of neural dynamics. Dim, dimension.
Figure 3. Behavioral states evolve at varied temporal scales and interact at each time point.
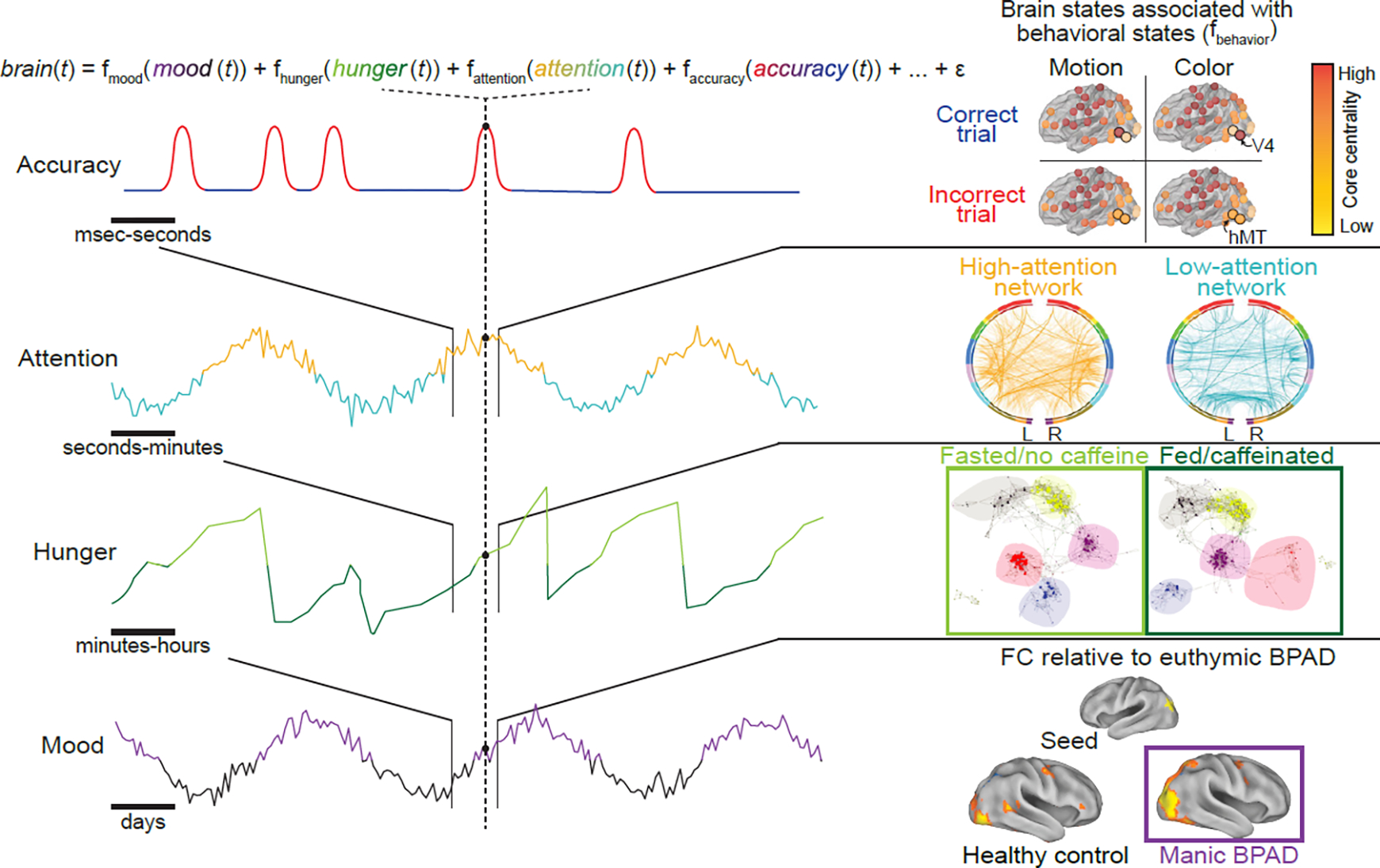
Left: four examples of behavioral (i.e., physiological and cognitive) state domains—mood, hunger, attention, and task performance—are depicted by simulated time courses. Each behavioral state unfolds on a different temporal scale, and brain activity at each moment in time reflects the combination of the behavioral states occurring and interacting at that time and the brain states underlying them. Right: examples of previous work studying patterns of brain activity (brain states) that correspond to each of these physiological and cognitive states in isolation. Top: Network reconfiguration tracks task performance, such that core centrality of task-relevant regions (area V4 on color discrimination trials and area hMT on motion discrimination trials) increases during preparation for trials on which participants respond correctly. Schematized depiction of results from[85]. Second from top: Functional connectivity patterns that predict low and high attention across two, independent datasets. Colored bars represent “lobes” (e.g., blue, temporal). Adapted from[86]. Second from bottom: Changes in whole-brain network organization, as measured using fMRI-based functional connectivity, in fed and fasted states. Each point represents a brain region, colored by network assignment (e.g., blue, visual network), with hub regions enlarged. Adapted from[157]. Bottom: differences in seed-based functional connectivity (FC) between bipolar mania and bipolar euthymia, and between healthy control and bipolar euthymia. Adapted from[158]. BPAD, bipolar affective disorder.
Figure 4. Using brain state to unify the study of neural dynamics.
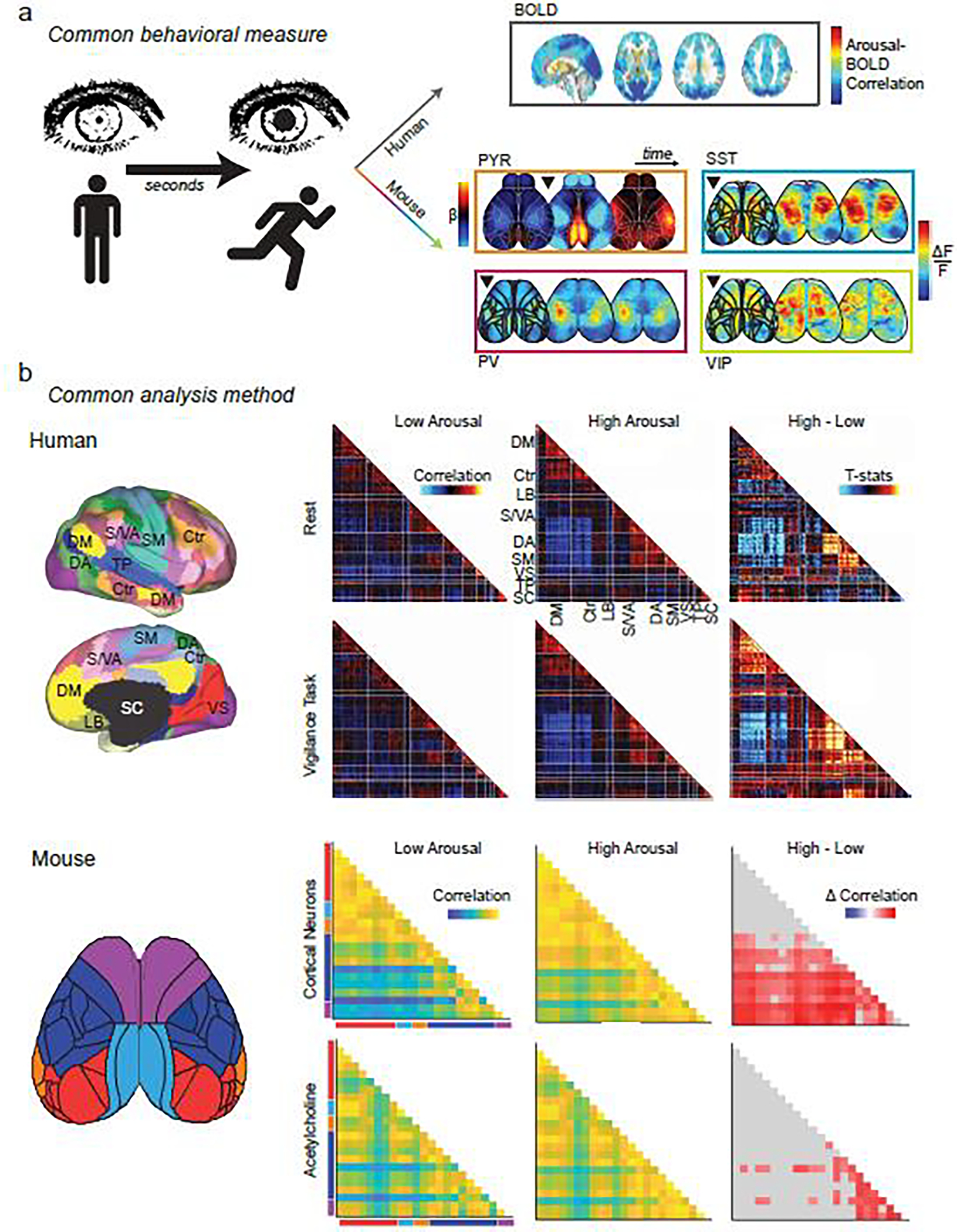
Common behavioral measures link the study of brain states across species: a, Changes in arousal measures (i.e. pupil size, movement) occur on the order of seconds. Top right: the slow dynamics of BOLD fluctuations on this time scale yield persistent (but whole-brain) patterns related to changes in arousal. Adapted from [159]. Bottom right: faster dynamics (each image represents changes over ~300 msec) can be captured with cell-type specificity using mesoscopic calcium imaging in mice. Adapted from [37] (PYR) and [66] (PV, SST, VIP). Black triangle represents onset of increased arousal. By matching behavioral states across species, more invasive, higher resolution techniques in animal models can be leveraged to reveal mechanistic insights into the complex behavioral and cognitive processes that can uniquely be studied in humans. Shared analytic methods also provide a tool to bridge disciplines: b, Whole-brain activity patterns can be divided into parcels within functionally defined regions (left) and pairwise correlations of parcels’ time courses yield functional connectivity (FC) matrices, whether from fMRI data in humans (left top) or from mesoscopic functional imaging data in mice (left bottom). FC can be calculated across physiological states (low versus high arousal) from data acquired during a given cognitive state (e.g. rest versus task) or from measures of neuronal activity or acetylcholine release. These FC matrices can be used to reveal common features of low- and high-arousal brain state patterns and yield mechanistic insight into how these states may be generated. For example, tasks amplify arousal-related FC increases in somato-motor networks in humans (right top and upper middle matrices). Adapted from [160]. Increases in arousal also cause correlated acetylcholine release and synchronize neuronal activity in analogous networks in mice (right lower middle and bottom matrices). Adapted from [27]. Human network labels: DM, default mode; Ctr: control; LB: limbic; S/VA: salience/ventral attention; DA: dorsal attention; SM: somato-motor; VS: visual; TP: temporo-parietal; SC: subcortical. Mouse network labels: red: visual; light blue: retrosplenial; orange: auditory; dark blue: somatosensory; purple: motor.
Box 2: Clinical applications.
A growing body of evidence suggests that brain state patterns are altered in disease (e.g., [144]); a more complete understanding of brain state dynamics thus holds the promise of shedding light on the neural bases and consequences of various neurological and neurodevelopmental conditions. Brain state dynamics in autism spectrum disorder (ASD) have been particularly well studied.
For example, there is growing evidence that spontaneous brain activity patterns differ in autistic people relative to typically developing (TD) individuals. In ASD-related mouse models, gamma band oscillatory EEG activity is increased[145] and decreased excitatory drive onto mPFC pyramidal cells results in reduced coordination of activity in delta and theta frequency bands[146]. Similar disruptions of oscillatory activity have been found in autistic people [147,148], though results are not always convergent (e.g., [149]). Recent work suggests a link between such disrupted oscillatory activity and connectivity alterations in ASD[150].
While not always feasible in populations with neurological and neurodevelopmental conditions, the use of cognitive paradigms to manipulate brain state can also reveal clinically relevant alterations in brain states. Consistent with findings at rest, altered oscillatory and functional connectivity patterns have also been demonstrated in ASD during various tasks, and found to track task performance[151]. Further, consistency of task-induced activity patterns may differ in ASD; less consistency of task-induced brain states in ASD than TD participants tracks severity of restrictive behaviors[152].
More generally, cross-species approaches provide a means to recapitulate, mechanistically characterize, and test interventions for brain state alterations observed in human disease. For instance, a study in Fmr1 knock-out mice, which recapitulate aspects of Fragile X syndrome (FXS), used a common visual discrimination paradigm to identify similar behavioral impairments in FXS individuals and the model mice, and then used the latter to show that these deficits are mediated by parvalbumin interneuron dysfunction[153]. Recent work using 15q dup mice, a model of autism, has revealed widespread hypoconnectivity compared to wild type mice during wakeful rest. D-cycloserine rescued social behaviors and partially restored connectivity patterns in affected mice[154]. Though it is unlikely that D-cycloserine will be efficacious for all autistic individuals[155], integration of ongoing brain state research across species may help define subgroups likely to benefit from it.
Such work demonstrates the promise of using interdisciplinary methods to characterize brain states and their causal mechanisms. Further, differences in the brain state repertoire across cognitive and disease processes may themselves be targets for intervention. Recent studies in mice[129] and humans[128] have used real-time feedback to train subjects to express large-scale patterns of brain activity, resulting in sustained changes in functional connectivity. Direct brain state manipulation has also been achieved (see ‘Establishment of causal links’). These technological innovations offer the exciting possibility of better understanding and potentially intervening in a range of clinically relevant processes.
The organization, biology, and measurement of brain states
There is growing evidence for stereotyped, transient, and meaningful whole-brain activity patterns at all levels of analysis. In humans, combined neuroimaging and electrophysiological methods have shown converging patterns of activity and dynamic functional connectivity, regardless of signal source (i.e., blood flow or electrical currents) or precision of spatial localization[7,8]. Paired measurements in animal models have further validated the robust relationship between neural activity, local field potential, and hemodynamics[9–11] (but see[12]) and the consistency of inter-region functional connectivity across modalities[13–15]. Work in rodents links these patterns to variation in fast and transient neuronal coactivation patterns[16,17]. Thus, brain states can be resolved using many measurement and analytical techniques, each offering limited, but complementary, insights into their cause, representation, and consequences (Box 1 and Fig. 1).
While largely inaccessible in humans, the neuronal underpinnings of brain states can be readily studied in animal models, reflecting the importance of merging findings across species and techniques. In rodents, for example, the biological bases of these spontaneously evolving whole-cortex brain states are increasingly well described. First, studies relating spontaneous activity patterns to detailed anatomical atlases[18] have demonstrated that recurring meso-scale activity motifs[19,20] are well, though not entirely, predicted by patterns of axonal projections. These results are consistent with the finding in humans that functional connectivity is constrained by structural connectivity[21,22]. Second, transitions between whole-brain activity patterns can be triggered by activation of neuromodulatory centers, such as the ventral tegmental area[23] and locus coeruleus[24], and the expression of specific patterns of activity may be a function of both the heterogeneous innervation of brain regions by neuromodulatory axons[25,26] and the divergent responses of those regions to neuromodulator release[24,27]. Third, while entire brain regions are recruited by these activity patterns, within local cortical (and subcortical[28,29]) circuits, neighboring neurons are often recruited by different patterns of cortical activity[30,31], suggesting that the distinct computations performed by individual neurons may be, at least in part, a function of the brain-wide functional networks to which they belong. Together, this work suggests a model of brain states as transient, anatomically constrained patterns of activity that are influenced by neuromodulatory tone, and that differentially recruit individual neurons while overall synchronizing distributed brain regions. This model helps rectify seemingly discrepant findings across modalities and species (Box 1). We turn next to recent and ongoing work to characterize the brain states that correspond to physiological and cognitive processes.
Physiological changes are reflected in reproducible brain states
We first discuss brain dynamics associated with fluctuating physiology, the bottom-up manifestation of homeostatic processes with effects throughout the body. Consistent with our proposed criteria, a growing literature (in humans[32] and animals[33]) suggests that there are recurring patterns of brain activity that emerge from physiological state, have a stereotyped representation in the brain, and have consequences for future behavior.
Physiological changes induce distinct brain states
While numerous physiological changes have been related to brain dynamics[5], the physiological state measure most commonly related to large-scale patterns of brain activity is arousal, typically quantified using markers such as heart and respiratory rates, skin conductance, and pupil diameter. Changes in pupil diameter occur on the order of seconds and are associated with alterations in brain activity measured at levels ranging from cellular membrane potential[1,34], to circuit-level synaptic and firing rate synchronization[35], to brain-wide network coupling[36,37], all of which comprise or reflect brain state dynamics. Pupillometry has similarly been used to track arousal in human fMRI studies[38,39]; recurring patterns of brain-wide activity have been associated with epochs of changing pupil diameter, but not the absolute size of the pupil[38], suggesting that, as in the mouse[35], spontaneous, whole-brain activity is highly sensitive to abrupt transitions in physiological state. Further, direct manipulation of pupil-indexed arousal through stimulation of neuromodulatory nuclei[40,41] or the vagal nerve[42–44] is sufficient to induce brain-wide changes in neural activity.
Neural representation of physiology-related brain states
Such physiology-induced brain state changes have a stereotyped representation in the brain at multiple levels of analysis. Using human neuroscience techniques, such as fMRI and EEG, arousal-related changes in distributed activity patterns can be resolved[45–47] and demonstrate convergent patterns across studies. Key emergent trends include a negative coupling between pupil diameter and brain activity in sensorimotor regions[48], and a positive coupling between pupil diameter and brain activity in higher-order areas, such as the frontoparietal network[38,48]. Further, increased network integration is a hallmark of increased arousal, as indexed by pupil size[39], consistent with the idea that increased arousal and related brain activity track increased attention and task performance[48], though likely only to a point[4] (see ‘Behavioral consequences’ below).
The animal literature offers complementary insights into arousal-related brain state expression, although integration across studies is limited by the variable use of movement and pupil diameter as state measures. Large-scale recordings of activity using high-density extracellular electrophysiology or cortex-wide optical imaging of fluorescent reporters have shown that patterns of spontaneous activity are high-dimensional (with dimensionality scaling with the resolution of the recording method; see[49]). While these patterns of activity are not specific to arousal states, they are preferentially expressed, such that arousal measures can be predicted from moment-to-moment fluctuations in activity[50,51] (and vice versa[36,37]) as well as from rapid changes in functional coupling between areas at the transitions between quiet wakefulness and elevated arousal[51,52]. Further, studies have demonstrated a postero-medial to antero-lateral gradient of arousal modulation in mouse cortex, with sensory and association areas in the posterior and medial cortex exhibiting greater modulation by arousal[30,31,37,50,51,53], consistent with previous studies in rodents performed within single brain regions, but in opposition to what has in many cases been described in humans. Recent theory-driven work has begun to address this apparent contradiction, demonstrating arousal-locked traveling waves in humans (using fMRI) and non-human primates (using electrocorticography) similar to those observed in rodents[13,17,54–56], and suggests a model for future multimodal investigation.
Behavioral consequences of physiologically-induced brain states
Physiology-related brain states profoundly affect the organism’s subsequent behavior. For example, optimal task performance occurs at intermediate levels of arousal in both animals and humans[4]. This is likely attributable to the permissive effect of arousal on task engagement and other ongoing cognitive processes, which elicit stereotyped brain states[57,58] that predict task performance and may be abolished at high arousal[1]. In humans, moderate arousal increases brain integration, facilitating task performance, an effect apparently driven by the catecholaminergic system[59]. Interestingly, in rodents, increasing arousal coincides with global cortical desynchronization (as in humans[60]), a population-level suppression in spontaneous activity[61] (although notably some cell-types exhibit higher levels of activity[62]), and increased task engagement[57,58]; an inverse relationship between arousal and BOLD and global signal amplitude has similarly been noted in humans[63,64]. Further, these physiologically-induced brain states can be directly modulated or reproduced – titrated vagal nerve stimulation improves motor learning through activation of the ascending cholinergic system[65], the influence of which is likely mediated by cortex-wide and subtype-specific effects of acetylcholine on different interneuron populations[66]. Arousal-related behavior changes have also been shown with activation of noradrenergic and other neuromodulatory deep brain nuclei[41]. Similar changes in brain-wide spontaneous neural activity and behavior have been shown for satiety signals across species[67–70], demonstrating how other homeostatic processes can drive behavior. Lastly, work probing individual differences[71] and circadian patterns[72] reinforces that spatially distributed, complex activity patterns reflect physiology-related brain states and hold potential for clinical translation (Box 2).
Limitations and outstanding questions
Without experimental manipulation, it is difficult to demonstrate that brain states both facilitate behavior and reflect changes in physiological states. For example, physiological recordings rely on measures that may index numerous processes with distinct relations to brain activity[73]. Other means to manipulate arousal, through pharmacology[74] and sleep deprivation (recently reviewed in [75]), can be used to study physiological brain states, but have limitations, including pharmacologic agents’ imprecise mechanisms of action and the non-physiologic nature of sleep deprivation. Similarly, observed dynamics in resting-state data, comprising much of the human work cited above, may be confounded by uncontrolled and unmeasured processes[37,76,77]. While direct manipulation to address related questions is increasingly feasible in model organisms, this is not often the case for human neuroscience (but see ‘Establishment of causal links’ below), limiting cross-species translation. These limitations highlight the opportunity that external manipulations present: inducing distinct patterns of brain activity using constrained tasks can offer additional insights into brain state across species.
The brain on task: brain states induced by cognitive processes
Given the challenges of studying physiology-associated brain states, externally modulated, controlled changes to an individual’s environment present an appealing framework for the study of brain states (Fig. 2). Such manipulations come in many forms; we focus on use of task paradigms to study cognitive processes of interest in both the human and animal literatures. Carefully designed tasks—traditional controlled experiments and, increasingly, naturalistic paradigms[78,79]—provide a more specific means to study brain state because they can be used both as brain state manipulations and as environmental context to interpret the behavioral consequences of brain state changes[2]. Tasks as manipulations provide a window into the neural representation of brain states, while tasks as environmental context provide a means to validate and interpret them.
Task-induced brain state changes are substantial and reliable
While the overall functional architecture of the brain has been found to be relatively stable across various task and resting states[80], task-induced changes in functional brain organization—at the whole task, block, or even trial levels—are nevertheless substantial[2,81,82], consistent[83], and relevant to the task at hand[84–88]. For example, long-range functional connectivity strength (“integration,” in graph theoretic terms) increases during cognitively demanding tasks[39,89–91] (but see[88]), tracking increases in arousal[92], particularly within and between cognitive control and task-relevant macroscale networks[93,94]. A growing body of interdisciplinary work suggests this load-dependent change in integration may result from bottom-up catecholaminergic activity[39,40,59,95,96] as well as top-down cortico-cortical feedback[85,97–99].
Neural representation of task-induced brain states
More generally, cognitive tasks limit the number of accessible brain states[2,100] (Fig. 2), as demonstrated, for example, by decreased functional connectivity variability during tasks relative to rest[94,101]. This constrained repertoire may explain the recent finding that, across a wide range of tasks, task-based functional connectivity better reveals brain-phenotype relationships than resting-state functional connectivity[86,102,103]. Recent work in rodents has shed further light on this. While trial-to-trial and inter-individual variability in cortical activity is best explained by putatively task-irrelevant behaviors, tasks both reveal and constrain such variability[37,97], and demonstrate how behavior may interact with cognitive demands to limit accessible brain states[37,104,105].
Whereas tasks have been shown to elicit (and require) specific whole-brain activity patterns, they also reliably induce cell- and circuit-level changes that have been extensively characterized in animal models. We propose that the concept of brain state links these fields, suggesting ways local changes scale to yield whole-brain patterns. For example, task-induced brain state changes manifest on the circuit level through oscillatory synchronization, as has been shown for high-level cognitive processes, such as working memory and attention (in both humans[106] and animal models[107,108]). Neurons representing an attended stimulus are transiently and locally synchronized[109–111], and in turn entrain downstream neuronal populations[112,113], amplifying representation of task-relevant information. Further, consistent with the observation that tasks constrain accessible brain states in humans, tasks induce patterns of brain activity that are important for sensory gating by spontaneous activity[114,115]. This suggests a potential mechanism by which tasks constrain brain states in a highly specific manner that modifies inter-areal effective connectivity[107,116].
Behavioral consequences of task-induced brain states
In addition to affecting brain states, tasks provide a key, of sorts, to decode them. That is, relating recurrent brain states to task events offers a means to interpret those activity patterns, tracking their relevance to ongoing cognitive processes and subsequent behaviors. This approach has been leveraged by human neuroscience, given its access to complex cognitive processes, with the consistent finding that distributed brain activity patterns, as captured by functional connectivity, stably and distinctly represent different cognitive tasks[83,117]. The cognitive processes invoked by each task can in turn be used to interpret such patterns; for example, changes in functional connectivity patterns reflect real-time task demands, with task-relevant brain areas transiently drawn into brain-wide networks related to task performance[84,85]. That task performance improves with greater expression of such task-relevant activity patterns validates their functional relevance, a finding that has been replicated in various domains and species[87,89,91,99,104,118].
These examples underscore the cognitive specificity of brain state changes, and together, this work illustrates both the utility of brain states to characterize task-related brain activity, and the utility of task paradigms to validate the behavioral consequences of resolved brain states.
Limitations and outstanding questions
While tasks can help resolve the nuanced interactions among physiological and cognitive states (hereafter, “behavioral states”), the beginning and end of a task manipulation are unlikely to clearly demarcate the beginning and end of discrete behavioral states, which are difficult to disentangle not least because they evolve on varying timescales (e.g., task demands superimposed on background physiological and cognitive states; Fig. 3). To date, the neural influence of each such behavioral state has largely been studied in isolation, but this approach fails to acknowledge the simultaneous expression of many behavioral states and corresponding brain states (Fig. 3).
We propose that this presents an opportunity for interdisciplinary approaches to integrate across the spatial scales and modalities from which we drew examples above to provide a more complete picture of these complex dynamics and to disentangle multiplexed brain states. By linking seemingly disparate approaches, a shared concept of a brain state offers an organizing framework for this work, to which we turn next.
Using brain state to unify the study of neural dynamics
In the preceding sections, we have highlighted the diverse ways in which physiological and cognitive processes can be studied across species using brain states. The field-specific techniques we have highlighted differ in temporal and spatial scale, in capacity to offer mechanistic or whole-brain insights, and in practical application (Box 1). And yet, a shared concept of brain state can relate seemingly disparate findings across these scales and disciplines. That is, brain states, as the fundamental unit of whole-brain neural dynamics, evolve and combine to yield observations at every temporal and spatial scale. Combining these methods of observation will thus permit a more comprehensive understanding of how and why the brain moves through stereotyped states. This will, in turn, shed light on the neurobiological underpinnings of the dynamic brain, of the behavioral states that these brain states subserve, and of disease-specific alterations in these dynamics (Box 2). In what follows, we highlight how such interdisciplinary work can use the concept of brain state to map and integrate results across levels of analysis, with a look to the future (see Outstanding Questions).
Outstanding questions.
Is there a finite “dictionary” of brain states that recur over time, and how should we interpret them in the context of continuously evolving, simultaneously expressed behavioral states?
Necessity: What features of a brain state are necessary to induce changes in behavior?
Sufficiency: Can inducing a brain state associated with a given physiological state or cognitive paradigm—in the absence of that physiological process or paradigm—cause comparable changes in behavior?
How often and completely must a pattern of activity recur to be considered a brain state, both within and across individuals, and does this criterion depend on data modality?
How do global brain states emerge from local phenomena?
To what extent do other species experience “human-specific” brain states, and which cognitive and physiological states can be equated for the study of corresponding brain states across species?
How can we best disentangle co-occurring brain states, leveraging complementary hypothesis- and data-driven techniques?
How can we use direct manipulation of brain activity—in animal models and human patients—to study brain states and develop novel clinical interventions?
Theoretical considerations
Each neuroscience technique, when used in isolation, offers a partial view of brain dynamics, from high-fidelity observation of a single neuron’s cycling through brain states to characterization of brain-wide activity patterns related to high-level cognitive processes over long timescales (Box 1). In combination, however, they hold the promise of more completely characterizing and interpreting brain states.
Such interdisciplinary work will require a willingness to think outside of the box about how brain states manifest across modalities[119] and species[120]. One could ask, for example, how local changes in single-unit activity and population synchrony scale up to yield corresponding whole-brain activity patterns for a given behavioral state. The experimental steps may also be reversed, using the advantages of human neuroscience techniques to identify regions or patterns of interest for more focal study of mechanism in animal models. Finally, one may explore analogous properties of the dynamic brain across modalities, such as network small-worldness[121].
Two crucial challenges in these lines of work are identifying recurring brain states over time, during apparently distinct behavioral states, and disentangling jointly expressed brain states. To date, the greatest progress has been made on the former, treating any given moment as representative of a single behavioral state, identifying corresponding brain states, and searching for the expression of those brain states in distinct behavioral states. In actuality, however, many behavioral—and corresponding brain—states are entangled at any given moment, and their separation is necessary for a complete, precise characterization of brain dynamics. This will require bridging levels of analysis, either at the data acquisition or analysis stage. We turn next to selected works that exemplify this, using them to clarify key, practical recommendations for the approach we propose.
Identification of recurrent brain states: Applied examples
Several recent studies, leveraging techniques at varying levels of spatial and temporal analysis within a single project, have demonstrated that brain states recur over time during distinct behavioral states. Using human fMRI-based functional connectivity, it was recently demonstrated that a brain state that predicts attention across individuals also predicts attention fluctuations within individuals across time and physiological states (e.g., light anesthesia and sedation)[74]. Others similarly demonstrated the recurrence of two distinct brain states in a highly sampled individual across time and physiological states (e.g., hunger and satiety)[122]. A strength of these human studies is the complexity of behavior that can be related to brain states, but insights into mechanism are limited. Conversely, a recent study used two model organisms—larval zebrafish and mice—to mechanistically characterize conserved neuromodulatory cell types that shape brain states corresponding to arousal, a relatively low-level physiological state[41].
Leveraging the strengths of both of these approaches by studying humans and model organisms together, a recent study first used human fMRI to identify a brain state induced by reward anticipation in an inferential reasoning task, and then invasive methods to both record and manipulate its expression in mice[123]. By using a common task and analysis method to link data across modalities and species, the authors were thus able to comprehensively characterize a conserved brain state underlying complex behavior.
We propose that a unifying concept of a brain state has the potential to make such interdisciplinary work widely accessible. No single study or research group must perform every stage of this process, but individual studies should be designed to identify common brain states. Examples include the collection of physiological data (e.g., pupillometry, facial movement) and implementation of tasks that are readily adaptable across species, and the development of analysis frameworks applicable across modalities. Such work will permit inter-species comparison of brain states to identify useful points of convergence. We leverage example data from unrelated papers to illustrate this point (Fig. 4). The use of a common physiological construct—arousal—permits changes in whole-brain activity in humans to be directly linked to fast, cell type-specific activity in comparable physiological states in the mouse (Fig. 4a). Alternatively, a common analytic approach, such as whole-brain functional connectivity calculation, similarly offers a bridge across levels of analysis. For example, functional connectivity patterns can be compared across species during matched arousal states; human study offers the opportunity to use complex arousal manipulations (e.g., vigilance task in sleep-deprived participants) with implications for cognition, while animal models again offer a route to mechanistic explanation (Fig. 4b). The examples illustrated in Figure 4 are intended not to make specific scientific claims, but to illustrate the notion of synthesizing data across modalities and species, even in unrelated experiments, using common measures and analysis tools.
Separation of jointly expressed brain states: Applied examples
All of these examples treat each moment in time as a single behavioral state, with a single corresponding brain state. As described above, however, a second critical challenge for the study of brain dynamics is disentangling the many brain—and behavioral—states that may be jointly expressed at any given time, but that have traditionally been studied in isolation (e.g., Fig. 3), particularly at rest[124]. Temporal decomposition techniques (e.g., [13,17,36]) are widely used to explore recurring, overlapping spatiotemporal activity patterns; blind source separation frees such analyses from theory-driven assumptions about the temporal evolution of brain states[125], but correspondingly poses challenges for neurobiological interpretation linking resolved patterns to physiological and cognitive processes. Efforts to decompose each timepoint into such interpretable brain states are crucial to accurately attribute brain activity patterns to a given behavioral state. This was recently achieved by performing near-whole-brain, chronic extracellular electrophysiological recordings in rodents, with application of machine learning methods to separate neurobiologically distinct, but jointly expressed, activity patterns (“electomes”) underlying stress vulnerability and stress-related dysfunction[126]. This study disentangles fast time-varying signals that contain information about slowly unfolding mood phenomena, and raises the question of what other unmodeled but co-occurring behavioral states could be identified using similar techniques.
Such work separates multiplexed brain states, which can then be identified and studied in other modalities. This need not be accomplished in a single experiment; our proposed, brain state-based approach provides a roadmap to combine observations across modalities and levels of analysis. This yields a common space to isolate distinct brain states by identifying and removing the neural manifestations of other co-occurring behavioral states (Fig. 3).
Establishment of causal links between brain and behavioral states
Finally, establishing a causal relationship between brain and behavioral states requires direct manipulation of brain states. Such manipulation may be invasive (e.g., via optogenetics or direct electrical stimulation in neurosurgical patients[127]) or noninvasive (e.g., via real-time, imaging-based neurofeedback[128,129], temporal interference[130], pharmacologic agents[74] or transcutaneous vagus nerve stimulation[44]). By manipulating circuits to achieve a given brain—and consequent behavioral—state, such work can both illuminate the neural dynamics underlying complex physiological and cognitive states and promote the use of inducible brain states for clinical intervention.
Concluding remarks
Each research community, from cellular, to systems, to cognitive, to clinical neuroscientists, motivated by the benefits and limitations of its available techniques, offers a different window into brain state. These perspectives are necessarily different—not because they are based on inherently different definitions of brain state, but rather because they often employ different techniques to study the same construct. Here, we aimed to reframe these efforts as complementary work toward a common goal: a more comprehensive understanding of brain states, the structured, reproducible, and behaviorally relevant patterns of brain activity that allow an individual to successfully move through a changing world.
Highlights:
Recent advances in neuroscientific data acquisition and analysis have permitted novel insight into neural dynamics.
These advances have motivated substantial interest in the concept of “brain state,” but approaches differ in spatial and temporal scale, yielding siloed lines of inquiry and subfield-specific definitions of a brain state.
We describe how a unified concept of brain state as a whole-brain activity pattern that emerges from and has consequences for physiology and/or behavior holds the promise of integrating across these levels of analysis, organizing fast-growing literatures and permitting a more comprehensive characterization of neural dynamics.
We explore how this conceptualization of brain state can guide future integrative work to reveal the biology underlying brain dynamics in health and disease.
Acknowledgements
This work was supported by funding from the NIH (GM007205 to ASG, CH, and DB, TR001864 to ASG, NS007224 to DB, EY029581 to DB, MH121095 to RTC and DS). The authors thank the members of the Constable, Higley, Crair, and Cardin labs for helpful discussions and Michael Higley for comments on the manuscript.
Glossary
- Behavioral state
Any state involving a discrete physiological (bottom-up and low-level) or cognitive (higher-order, e.g., as induced by a cognitive paradigm) process.
- Brain state
Recurring activity patterns distributed across the brain that emerge from physiological or cognitive processes. These patterns are neurobiological phenomena with functional (e.g., behavioral) relevance.
- Cognitive paradigm
Any experimental manipulation, ranging from traditional controlled to naturalistic experiments, providing a more specific means to study brain states associated with higher-order processes. Cognitive paradigms can be used as brain state manipulations and also offer environmental context that can be used to interpret the behavioral consequences of brain state changes.
- Physiological state
The bottom-up manifestation of homeostatic processes with effects throughout the body of the organism (e.g., metabolic [hunger, satiety], arousal-related, and immunological states).
Footnotes
Declaration of interests: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGinley MJ et al. (2015) Cortical Membrane Potential Signature of Optimal States for Sensory Signal Detection. Neuron 87, 179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Castillo J and Bandettini PA (2018) Task-based dynamic functional connectivity: Recent findings and open questions. NeuroImage 180, 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulet JFA and Crochet S (2018) The Cortical States of Wakefulness. Front. Syst. Neurosci. 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick DA et al. (2020) Neuromodulation of Brain State and Behavior. Annual Review of Neuroscience 43, 391–415 [DOI] [PubMed] [Google Scholar]
- 5.Flavell SW et al. (2022) The emergence and influence of internal states. Neuron 110, 2545–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris KD and Thiele A (2011) Cortical state and attention. Nature Reviews Neuroscience 12, 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C et al. (2013) EEG correlates of time-varying BOLD functional connectivity. Neuroimage 72, 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He BJ et al. (2008) Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc. Natl. Acad. Sci. U. S. A. 105, 16039–16044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz K et al. (2012) Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat. Methods 9, 597–602 [DOI] [PubMed] [Google Scholar]
- 10.Ma Y et al. (2016) Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons. Proc. Natl. Acad. Sci. U. S. A. 113, E8463–E8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logothetis NK et al. (2001) Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 [DOI] [PubMed] [Google Scholar]
- 12.Winder AT et al. (2017) Weak correlations between hemodynamic signals and ongoing neural activity during the resting state. Nat. Neurosci. 20, 1761–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui T et al. (2016) Transient neuronal coactivations embedded in globally propagating waves underlie resting-state functional connectivity. Proc. Natl. Acad. Sci. U. S. A. 113, 6556–6561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mateo C et al. (2017) Entrainment of Arteriole Vasomotor Fluctuations by Neural Activity Is a Basis of Blood-Oxygenation-Level-Dependent “Resting-State” Connectivity. Neuron 96, 936–948.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lake EMR et al. (2020) Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI. Nat. Methods 17, 1262–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsui T et al. (2019) Neuronal Origin of the Temporal Dynamics of Spontaneous BOLD Activity Correlation. Cereb. Cortex 29, 1496–1508 [DOI] [PubMed] [Google Scholar]
- 17.Aedo-Jury F et al. (2020) Brain states govern the spatio-temporal dynamics of resting-state functional connectivity. Elife 9, 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh SW et al. (2014) A mesoscale connectome of the mouse brain. Nature 508, 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohajerani MH et al. (2013) Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat. Neurosci. 16, 1426–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L et al. (2020) BRICseq Bridges Brain-wide Interregional Connectivity to Neural Activity and Gene Expression in Single Animals. Cell 182, 177–188.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorrentino P et al. (2021) The structural connectome constrains fast brain dynamics. Elife 10, e67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honey CJ et al. (2009) Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U. S. A. 106, 2035–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohani S et al. (2017) Unexpected global impact of VTA dopamine neuron activation as measured by opto-fMRI. Mol. Psychiatry 22, 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zerbi V et al. (2019) Rapid Reconfiguration of the Functional Connectome after Chemogenetic Locus Coeruleus Activation. Neuron 103, 702–718.e5 [DOI] [PubMed] [Google Scholar]
- 25.Kebschull JM et al. (2016) High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RNA. Neuron 91, 975–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X et al. (2017) Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. U. S. A. 115, 415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohani S et al. (2022) Spatiotemporally heterogeneous coordination of cholinergic and neocortical activity. Nat. Neurosci. 25, 1706–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao D et al. (2017) Mapping cortical mesoscopic networks of single spiking cortical or sub-cortical neurons. Elife 6, e19976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters AJ et al. (2021) Striatal activity topographically reflects cortical activity. Nature 591, 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clancy KB et al. (2019) Locomotion-dependent remapping of distributed cortical networks. Nat. Neurosci. 22, 778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barson D et al. (2020) Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits. Nat. Methods 17, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lurie DJ et al. (2020) Questions and controversies in the study of time-varying functional connectivity in resting fMRI. Netw. Neurosci. 4, 30–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engel TA and Steinmetz NA (2019) New perspectives on dimensionality and variability from large-scale cortical dynamics. Current Opinion in Neurobiology 58, 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reimer J et al. (2014) Pupil Fluctuations Track Fast Switching of Cortical States during Quiet Wakefulness. Neuron 84, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinck M et al. (2015) Arousal and Locomotion Make Distinct Contributions to Cortical Activity Patterns and Visual Encoding. Neuron 86, 740–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stringer C et al. (2019) Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musall S et al. (2019) Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci. 22, 1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider M et al. (2016) Spontaneous pupil dilations during the resting state are associated with activation of the salience network. Neuroimage 139, 189–201 [DOI] [PubMed] [Google Scholar]
- 39.Shine JM et al. (2016) The Dynamics of Functional Brain Networks: Integrated Network States during Cognitive Task Performance. Neuron 92, 544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi S et al. (2016) Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovett-Barron M et al. (2017) Ancestral Circuits for the Coordinated Modulation of Brain State. Cell 171, 1411–1423.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins L et al. (2021) Vagus nerve stimulation induces widespread cortical and behavioral activation. Curr. Biol. 31, 2088–2098 [DOI] [PubMed] [Google Scholar]
- 43.Mridha Z et al. (2021) Graded recruitment of pupil-linked neuromodulation by parametric stimulation of the vagus nerve. Nat. Commun. 12, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharon O et al. (2021) Transcutaneous Vagus Nerve Stimulation in Humans Induces Pupil Dilation and Attenuates Alpha Oscillations. J. Neurosci. 41, 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy PR et al. (2014) Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 35, 4140–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang C et al. (2016) Tracking brain arousal fluctuations with fMRI. Proc. Natl. Acad. Sci. U. S. A. 113, 4518–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kucyi A and Parvizi J (2020) Pupillary Dynamics Link Spontaneous and Task-Evoked Activations Recorded Directly from Human Insula. J. Neurosci. 40, 6207–6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breeden AL et al. (2017) Coupling between spontaneous pupillary fluctuations and brain activity relates to inattentiveness. Eur. J. Neurosci. 45, 260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avitan L and Stringer C (2022) Not so spontaneous: Multi-dimensional representations of behaviors and context in sensory areas. Neuron 110, 3064–3075 [DOI] [PubMed] [Google Scholar]
- 50.MacDowell CJ and Buschman TJ (2020) Low-dimensional spatiotemporal dynamics underlie cortex-wide neural activity. Curr. Biol. 30, 2665–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benisty H et al. (2022) Rapid fluctuations in functional connectivity of cortical networks encode spontaneous behavior. bioRxiv DOI: 10.1101/2021.08.15.456390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West SL et al. (2022) Wide-Field Calcium Imaging of Dynamic Cortical Networks during Locomotion. Cereb. Cortex 32, 2668–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimaoka D et al. (2018) Effects of Arousal on Mouse Sensory Cortex Depend on Modality. Cell Rep. 22, 3160–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raut RV et al. (2021) Global waves synchronize the brain’s functional systems with fluctuating arousal. Sci. Adv. 7, eabf2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis ZW et al. (2020) Spontaneous travelling cortical waves gate perception in behaving primates. Nat. 587, 432–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitra A et al. (2018) Spontaneous Infra-slow Brain Activity Has Unique Spatiotemporal Dynamics and Laminar Structure. Neuron 98, 297–305.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinmetz NA et al. (2019) Distributed coding of choice, action and engagement across the mouse brain. Nature 576, 266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobs EAK et al. (2020) Cortical state fluctuations during sensory decision making. Curr. Biol. 30, 4944–4955.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shine JM et al. (2017) Catecholaminergic manipulation alters dynamic network topology across cognitive states. Netw. Neurosci. 2, 381–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tononi G (2005) Consciousness, information integration, and the brain. Prog. Brain Res. 150, 109–126 [DOI] [PubMed] [Google Scholar]
- 61.McGinley MJ et al. (2015) Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron 87, 1143–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dipoppa M et al. (2018) Vision and Locomotion Shape the Interactions between Neuron Types in Mouse Visual Cortex. Neuron 98, 602–615.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olbrich S et al. (2009) EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage 45, 319–332 [DOI] [PubMed] [Google Scholar]
- 64.Wong CW et al. (2013) The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage 83, 983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bowles S et al. (2022) Vagus nerve stimulation drives selective circuit modulation through cholinergic reinforcement. Neuron 110, 2867–2885.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ren C et al. (2022) Global and subtype-specific modulation of cortical inhibitory neurons regulated by acetylcholine during motor learning. Neuron 110, 2334–2350.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y et al. (2000) The temporal response of the brain after eating revealed by functional MRI. Nature 405, 1058–1062 [DOI] [PubMed] [Google Scholar]
- 68.de Araujo IE et al. (2006) Neural Ensemble Coding of Satiety States. Neuron 51, 483–494 [DOI] [PubMed] [Google Scholar]
- 69.Livneh Y et al. (2017) Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546, 611–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allen WE et al. (2019) Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee K et al. (2022) Arousal impacts distributed hubs modulating the integration of brain functional connectivity. Neuroimage 258, 119364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orban C et al. (2020) Time of day is associated with paradoxical reductions in global signal fluctuation and functional connectivity. PLOS Biol. 18, e3000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joshi S and Gold JI (2020) Pupil Size as a Window on Neural Substrates of Cognition. Trends in Cognitive Sciences 24, 466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenberg MD et al. (2020) Functional connectivity predicts changes in attention observed across minutes, days, and months. Proc. Natl. Acad. Sci. U. S. A. 117, 3797–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tagliazucchi E and van Someren EJW (2017) The large-scale functional connectivity correlates of consciousness and arousal during the healthy and pathological human sleep cycle. NeuroImage 160, 55–72 [DOI] [PubMed] [Google Scholar]
- 76.Laumann TO et al. (2017) On the stability of BOLD fMRI correlations. Cereb. Cortex 27, 4719–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mortaheb S et al. (2022) Mind blanking is a distinct mental state linked to a recurrent brain profile of globally positive connectivity during ongoing mentation. Proc. Natl. Acad. Sci. 119, e2200511119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meer JNv.d. et al. (2020) Movie viewing elicits rich and reliable brain state dynamics. Nat. Commun. 11, 5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antony JW et al. (2021) Behavioral, Physiological, and Neural Signatures of Surprise during Naturalistic Sports Viewing. Neuron 109, 377–390.e7 [DOI] [PubMed] [Google Scholar]
- 80.Cole MW et al. (2014) Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohen JR (2018) The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. Neuroimage 180, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Willumsen A et al. (2022) Local networks from different parts of the human cerebral cortex generate and share the same population dynamic. Cereb. Cortex Commun. 3, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirer WR et al. (2012) Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 22, 158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chadick JZ and Gazzaley A (2011) Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat. Neurosci. 14, 830–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ekman M et al. (2012) Predicting errors from reconfiguration patterns in human brain networks. Proc. Natl. Acad. Sci. U. S. A. 109, 16714–16719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenberg MD et al. (2015) A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 19, 165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fornito A et al. (2012) Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc. Natl. Acad. Sci. U. S. A. 109, 12788–12793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pinto L et al. (2019) Task-Dependent Changes in the Large-Scale Dynamics and Necessity of Cortical Regions. Neuron 104, 810–824.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spadone S et al. (2015) Dynamic reorganization of human resting-state networks during visuospatial attention. Proc. Natl. Acad. Sci. U. S. A. 112, 8112–8117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shine JM and Poldrack RA (2018) Principles of dynamic network reconfiguration across diverse brain states. NeuroImage 180, 396–405 [DOI] [PubMed] [Google Scholar]
- 91.Kitzbichler MG et al. (2011) Cognitive Effort Drives Workspace Configuration of Human Brain Functional Networks. J. Neurosci. 31, 8259–8270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mäki-Marttunen V (2021) Pupil-based states of brain integration across cognitive states. Neuroscience 471, 61–71 [DOI] [PubMed] [Google Scholar]
- 93.Cocchi L et al. (2014) Complexity in Relational Processing Predicts Changes in Functional Brain Network Dynamics. Cereb. Cortex 24, 2283–2296 [DOI] [PubMed] [Google Scholar]
- 94.Hutchison RM and Morton JB (2015) Tracking the brain’s functional coupling dynamics over development. J. Neurosci. 35, 6849–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eldar E et al. (2013) The effects of neural gain on attention and learning. Nat. Neurosci. 16, 1146–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aston-Jones G and Cohen JD (2005) An Integrative Theory of Locus Coeruleus-Norepinephrine Function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 28, 403–450 [DOI] [PubMed] [Google Scholar]
- 97.Makino H et al. (2017) Transformation of Cortex-wide Emergent Properties during Motor Learning. Neuron 94, 880–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Kempen J et al. (2020) Top-down coordination of local cortical state during selective attention. Neuron 109, 894–904.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allen WE et al. (2017) Global Representations of Goal-Directed Behavior in Distinct Cell Types of Mouse Neocortex. Neuron 94, 891–907.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Churchland MM et al. (2010) Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat. Neurosci. 13, 369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elton A and Gao W (2015) Task-related modulation of functional connectivity variability and its behavioral correlations. Hum. Brain Mapp. 36, 3260–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Finn ES et al. (2017) Can brain state be manipulated to emphasize individual differences in functional connectivity? Neuroimage 160, 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Greene AS et al. (2018) Task-induced brain state manipulation improves prediction of individual traits. Nat. Commun. 9, 2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gilad A et al. (2018) Behavioral strategy determines frontal or posterior location of short-term memory in neocortex. Neuron 99, 814–828.e7 [DOI] [PubMed] [Google Scholar]
- 105.Gallero-Salas Y et al. (2021) Sensory and Behavioral Components of Neocortical Signal Flow in Discrimination Tasks with Short-Term Memory. Neuron 109, 135–148.e6 [DOI] [PubMed] [Google Scholar]
- 106.Hipp JF et al. (2011) Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron 69, 387–396 [DOI] [PubMed] [Google Scholar]
- 107.Buschman TJ and Miller EK (2007) Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315, 1860–1864 [DOI] [PubMed] [Google Scholar]
- 108.Gregoriou GG et al. (2009) High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324, 1207–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fries P et al. (2001) Modulation of Oscillatory Neuronal Synchronization by Selective Visual Attention. Science 291, 1560–1564 [DOI] [PubMed] [Google Scholar]
- 110.Womelsdorf T et al. (2006) Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 439, 733–736 [DOI] [PubMed] [Google Scholar]
- 111.Herman WX et al. (2019) A Switch and Wave of Neuronal Activity in the Cerebral Cortex During the First Second of Conscious Perception. Cereb. Cortex 29, 461–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buschman TJ and Kastner S (2015) Perspective From Behavior to Neural Dynamics: An Integrated Theory of Attention. Neuron 88, 127–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Desimone R and Duncan J (1995) Neural Mechanisms of Selective Visual Attention. Annu. Rev. Neurosci. 18, 193–222 [DOI] [PubMed] [Google Scholar]
- 114.Shimaoka D et al. (2019) The impact of bilateral ongoing activity on evoked responses in mouse cortex. Elife 8, e43533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lewis CM et al. (2016) Stimulus-induced visual cortical networks are recapitulated by spontaneous local and interareal synchronization. Proc. Natl. Acad. Sci. U. S. A. 113, E606–E615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bosman CA et al. (2012) Attentional Stimulus Selection through Selective Synchronization between Monkey Visual Areas. Neuron 75, 875–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gonzalez-Castillo J et al. (2015) Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns. Proc. Natl. Acad. Sci. U. S. A. 112, 8762–8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Braun U et al. (2015) Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc. Natl. Acad. Sci. U. S. A. 112, 11678–11683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johnston R et al. (2022) EEG signals index a global signature of arousal embedded in neuronal population recordings. eNeuro 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barron HC et al. (2021) Cross-species neuroscience: closing the explanatory gap. Philos. Trans. R. Soc. B Biol. Sci. 376, 20190633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bullmore E and Sporns O (2009) Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience 10, 186–198 [DOI] [PubMed] [Google Scholar]
- 122.Shine JM et al. (2016) Temporal metastates are associated with differential patterns of time-resolved connectivity, network topology, and attention. Proc. Natl. Acad. Sci. U. S. A. 113, 9888–9891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barron HC et al. (2020) Neuronal Computation Underlying Inferential Reasoning in Humans and Mice. Cell 183, 228–243.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leonardi N et al. (2014) Disentangling dynamic networks: Separated and joint expressions of functional connectivity patterns in time. Hum. Brain Mapp. 35, 5984–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Calhoun VD and de Lacy N (2017) Ten Key Observations on the Analysis of Resting-state Functional MR Imaging Data Using Independent Component Analysis. Neuroimaging Clin. N. Am. 27, 561–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hultman R et al. (2018) Brain-wide Electrical Spatiotemporal Dynamics Encode Depression Vulnerability. Cell 173, 166–180.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Inman CS et al. (2018) Direct electrical stimulation of the amygdala enhances declarative memory in humans. Proc. Natl. Acad. Sci. U. S. A. 115, 98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DeBettencourt MT et al. (2015) Closed-loop training of attention with real-time brain imaging. Nat. Neurosci. 18, 470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clancy KB and Mrsic-Flogel TD (2021) The sensory representation of causally controlled objects. Neuron 109, 677–689.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grossman N et al. (2017) Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 169, 1029–1041.E16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maimon G and Assad JA (2009) Beyond Poisson: Increased Spike-Time Regularity across Primate Parietal Cortex. Neuron 62, 426–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shadlen MN and Newsome WT (1998) The variable discharge of cortical neurons: Implications for connectivity, computation, and information coding. J. Neurosci. 18, 3870–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sofroniew NJ et al. (2016) A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. Elife 5, e14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stirman JN et al. (2016) Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain. Nat. Biotechnol. 34, 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Logothetis NK (2008) What we can do and what we cannot do with fMRI. Nature 453, 869–878 [DOI] [PubMed] [Google Scholar]
- 136.Murray JD et al. (2014) A hierarchy of intrinsic timescales across primate cortex. Nat. Neurosci. 17, 1661–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ackman JB et al. (2012) Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Setzer B et al. (2022) A temporal sequence of thalamic activity unfolds at transitions in behavioral arousal state. Nat. Commun. 13, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang C et al. (2008) Mapping and correction of vascular hemodynamic latency in the BOLD signal. Neuroimage 43, 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Birn RM et al. (2006) Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 31, 1536–1548 [DOI] [PubMed] [Google Scholar]
- 141.Shmueli K et al. (2007) Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage 38, 306–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Biswal B et al. (1997) Hypercapnia Reversibly Suppresses Low-Frequency Fluctuations in the Human Motor Cortex During Rest Using Echo-Planar MRI. J Cereb Blood Flow Metab 17, 301–308 [DOI] [PubMed] [Google Scholar]
- 143.Ogawa S et al. (2000) An approach to probe some neural systems interaction by functional MRI at neural time scale down to milliseconds. Proc. Natl. Acad. Sci. U. S. A. 97, 11026–11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bolton TAW et al. (2020) Tapping into Multi-Faceted Human Behavior and Psychopathology Using fMRI Brain Dynamics. Trends in Neurosciences 43, 667–680 [DOI] [PubMed] [Google Scholar]
- 145.Dhamne SC et al. (2017) Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol. Autism 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lazaro MT et al. (2019) Reduced Prefrontal Synaptic Connectivity and Disturbed Oscillatory Population Dynamics in the CNTNAP2 Model of Autism. Cell Rep. 27, 2567–2578.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Machado C et al. (2013) QEEG Spectral and Coherence Assessment of Autistic Children in Three Different Experimental Conditions. J. Autism Dev. Disord. 45, 406–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cornew L et al. (2012) Resting-state oscillatory activity in autism spectrum disorders. J. Autism Dev. Disord. 42, 1884–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rojas DC and Wilson LB (2014) γ-band abnormalities as markers of autism spectrum disorders. Biomarkers in Medicine 8, 353–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Buckley AW et al. (2015) State-Dependent Differences in Functional Connectivity in Young Children With Autism Spectrum Disorder. EBioMedicine 2, 1905–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.You X et al. (2013) Atypical modulation of distant functional connectivity by cognitive state in children with Autism Spectrum Disorders. Front. Hum. Neurosci. 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bolton TAW et al. (2020) Neural responses in autism during movie watching: Interindividual response variability co-varies with symptomatology. Neuroimage 216, 116571. [DOI] [PubMed] [Google Scholar]
- 153.Goel A et al. (2018) Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat. Neurosci. 21, 1404–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsurugizawa T et al. (2020) Awake functional MRI detects neural circuit dysfunction in a mouse model of autism. Sci. Adv. 6, eaav4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Minshawi NF et al. (2016) A randomized, placebo-controlled trial of d-cycloserine for the enhancement of social skills training in autism spectrum disorders. Mol. Autism 7, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Markowitz JE et al. (2018) The Striatum Organizes 3D Behavior via Moment-to-Moment Action Selection. Cell 174, 44–58.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Poldrack RA et al. (2015) Long-term neural and physiological phenotyping of a single human. Nat. Commun. 6, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brady RO et al. (2017) Differential brain network activity across mood states in bipolar disorder. J. Affect. Disord. 207, 367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goodale SE et al. (2021) fMRI-based detection of alertness predicts behavioral response variability. Elife 10, e62376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang C et al. (2016) Spontaneous eyelid closures link vigilance fluctuation with fMRI dynamic connectivity states. Proc. Natl. Acad. Sci. U. S. A. 113, 9653–9658 [DOI] [PMC free article] [PubMed] [Google Scholar]


