Abstract
Objective:
The impact of involving peers on research engagement is largely unknown. The purpose of this pilot study, a part of a larger research, was to evaluate the impact of recovery peer involvement as a study team member on recruitment/retention of persons with lived experience of SUD during pregnancy and to assess participant perceptions about factors impacting engagement of this population and their children in research, especially brain magnetic resonance imaging (MRI).
Methods:
This study randomly assigned participants (1:1) to either Peer or Research Coordinator (RC) arms. Eligible participants were English-speaking adult, non-pregnant females with lived experience of substance use during pregnancy. Certified Peers were recruited word of mouth and completed study-specific training. The impact of trained, certified Peer versus RC on research engagement was assessed by between-arm comparison of retention rates. Quantitative and qualitative survey data on participant perceptions were summarized.
Results:
Thirty-eight individuals enrolled into the study (19 Peer, 19 RC). Peer versus RC had 7.2 times greater odds of completing Visit 2 (Fisher’s exact test; 95%CI: 1.2, 81.8; p = 0.03). The majority (70.4%) of respondents identified being accompanied by a peer and getting a tour of the MRI facility/procedures as ‘extremely’ helpful for improving participant comfort and engagement in future studies. Motivators of future research engagement also included creating a trusting, supportive, non-judgmental research environment, and linkages to treatment and other services.
Conclusion:
Findings support the notion that peers involved as research team members could boost research engagement among persons with substance use during pregnancy.
Keywords: Peer support, Addiction, Recruitment, Retention, Substance use disorder, Research engagement
1. Introduction
Addiction-related stigma and fear of legal consequences are barriers to care, especially during pregnancy [1,2]. Punitive policies and laws that threaten to separate parents and their children further deter pregnant persons and parents from seeking help for substance use disorders (SUDs). The practice of including trained peer support specialists to improve treatment engagement and outcomes in SUD is supported by a theoretical framework rooted in mutual self-help concepts and early research [3], and has been steadily increasing throughout the US, leading to more robust workforces in healthcare and recovery settings [4,5].
Trained peer support is considered an evidence-based practice [4] and is Medicaid-reimbursable in most US states [5,6]. Most states offer a SUD peer support credentialing, which requires specialized training preparing certified peer support specialists (“Peers”) to provide peer-to-peer coaching, linkages to treatment, recovery, and social welfare services, and assistance with SUD remission and recovery [4,5,7,8], all of which contribute to improved outcomes in SUD. Peers are uniquely positioned to create personable, trusting relationships as a result of their training and “lived experience”, which give them a deep understanding of individuals affected by SUD and the community they serve [4,5]. Peers can help address emotional and social barriers, which can deter individuals with SUD from engaging in treatment [9–13].
These barriers can present major obstacles to research participation in this population, particularly when research focuses on substance use and SUD during pregnancy [2,12,13]. Substance-using pregnant persons can face harsh social and legal implications, including criminal charges, arrest, and child custody loss, which reinforce the challenges of engaging them in research. Human subjects research regulations designed to protect vulnerable populations add complexity with unintended consequences of further limiting research on child development and well-being in higher-risk populations of pregnant persons or parents with addiction. Yet, research with vulnerable populations is imperative for identifying effective interventions and informing clinical care and public health policies designed to promote well-being and resilience in children and families affected by SUD. Overcoming ethical, legal and stigma-related barriers to research with substance-using populations requires rigorous methodology and additional safeguards to protect the anonymity and confidentiality of participants as well as offer protection from legal hazards [14].
While peer support offers promise for improving clinical and research outcomes [15–18], the impact of involving peers on research engagement among persons with lived experience of SUD during pregnancy or early parenting is largely unknown. The goal of this pilot randomized study, a part of the larger research, was 1) to evaluate the impact of peers involved as a study team member on research recruitment/retention of persons with lived experience of SUD during pregnancy, and 2) to assess their perceptions about factors impacting engagement of this population and their children in research, especially brain magnetic resonance imaging (MRI).
2. Material and methods
2.1. Design
This study was a self-contained pilot-study, nested within a larger project, funded by the National Institutes of Health Healthy Brain and Child Development (HBCD) Initiative, and focused on the development of research methods to optimally support a long-term birth cohort study involving peripartum persons with substance use, and their children and families. This next step, the large national HBCD Study, is now ongoing, with the goal to discern the impact of substance use and other factors on child neurocognitive development, health, and resilience [19].
This two parallel-arm randomized pilot assessed 1) impact of a recovery peer support specialist (Peer) on research engagement among the target population, and 2) perceptions of women with lived experience of drug use during pregnancy (“target population”) about barriers versus motivators to long-term birth cohort participation. Prior to their study engagement, potential participants were randomly assigned (1:1) to either the Peer or Research Coordinator (RC) arms, interacting only with either Peer or RC during the study. The study activities were completed remotely and included a brief introduction/enrollment contact, followed by Visit 1, with survey completion, and Visit 2, with survey completion and a “tour” of the MRI facility and its procedures pertaining to brain imaging in children. This pilot study used a convenience sample enrolled during the recruitment timeframe, from December 1, 2020 through March 31, 2021; the study visits were completed on April 7, 2021.
The pilot study design and procedures were reviewed by the Institutional Review Board (IRB) and deemed as posing no more than minimal risk, meeting criteria for an expedited review. All study materials were informed by the Stakeholder Advisory Committee, which met regularly as a part of the parent study. The Committee comprised 19 people representing diverse groups interacting with the target population, including clinicians, health systems, public health, child welfare, law enforcement and women with lived experience, as detailed elsewhere [12].
2.2. Settings
Recruitment was statewide. Direct methods involved contacting directors of addiction treatment and recovery programs requesting to pass the electronic study flyer to their staff and patients. Indirect methods involved posting the study flyer on two FacebookⓇ sites: Wisconsin Voices For Recovery (a statewide recovery community organization) and Madison Mom (group devoted to providing early motherhood-related resources). The flyer stated the study was “[…] recruiting women with previous lived experience to give their thoughts and opinions on possible barriers and facilitators to join and complete future studies,” and included contact information to leave a voicemail or send an email to the study team.
2.3. Participants
Eligibility was determined based on self-report. Eligible participants were English-speaking females 18 years old or older who were not pregnant, and had lived experience of drug use during pregnancy, defined as direct personal experience or experience as an ally (e.g., as a family member or close friend) for a woman who used drugs while pregnant. In addition, those with personal lived experience needed to self-report being stable in recovery or currently engaged in SUD treatment.
2.4. Randomization
Randomization (1:1) into either the Peer or RC groups was completed in blocks of 10, prepared by the RC as sealed assignment cards (five per arm) that were ‘mixed’ in an opaque jar and randomly drawn by the RC to determine and write down the randomization order, which was then consecutively assigned to potential participants based on their study contact date/time. Due to the nature of the study, the study personnel who interacted with participants were not blinded to the group status. Although participants were not blinded to the study personnel’s job titles and experiences, as the personnel introduced themselves as a peer versus a research coordinator, the purpose and random up-front assignment to the Peer versus RC arms were not explicitly discussed with (prospective) participants. Outcome data collection was completed by participants online, and supported by the study personnel, however, the research team did not directly collect the data. The investigators were blinded to the group status until the study was completed.
2.5. Study arms
2.5.1. RC arm
The two female RCs were members of the university-based research team, experienced in the conduct of clinical trials, with excellent track-record of interactions with study participants, including those with substance use. They did not have a formal educational background or lived experience with SUDs, and completed a two-hour online training developed by the study team on SUD recovery and effects on health and behavior, especially among peripartum women with SUD.
2.5.2. Peer arm
Three potential Peers were recruited through “word of mouth” through the study manager (FH) and Principal Investigator’s (AEZ) professional contacts in local SUD recovery organizations.
Peer eligibility criteria included female gender, lived experience of substance use while pregnant, being in recovery, and being state-certified as a Certified Peer Support Specialist [7]. The state certification included close to 50 h of training across four domains of core competencies (values; in-depth knowledge of recovery; roles and responsibilities; and skills), followed by a written exam [20,21].
The Peers completed a study-specific, three-part training, developed and conducted by the research team and led by the project manager. Part 1 included a two-hour interactive educational session consisting of: (1) information and discussion about research (what is research and its types? what is it for?), research terminology, and ethics/regulations guiding human subjects research; (2) three video-based mock scenarios of participant-research personnel interactions to illustrate high (attentive, active listening, non-judgmental, welcoming attitude) versus poor quality interactions; and (3) IRB training requirements for personnel involved in human subjects research. Part 2 involved Peer observation of the RC-conducted screening call and Visit 1. Part 3 included supervision by the study manager of at least two sessions led by the Peer. After successful completion of this training, the Peer was approved to independently conduct the study activities, with the study manager and RCs readily available by phone in case of questions or problems.
Of the three potential Peers, one dropped out after Part 2 of the training due to illness, and another after the training due to feeling overwhelmed by the technology skills necessary for virtual interaction with participants; one Peer became a research team member. She completed all IRB-required training prior to engaging in research.
2.6. Study procedures
Potential participants who contacted the study team and provided their contact information were randomly assigned to either Peer or RC groups, and had contact with either the Peer or RC during the study’s three virtual contacts/visits: (1) Phone call with potential participants (pilot study description; verbal consent; screening; enrollment; Visit 1 scheduling); (2) Visit 1 completed by Webex (description of the future birth cohort study, Qualtrics-based online survey); and (3) Visit 2 completed by Webex (virtual tour of the neuroimaging facility, neuroimaging-related education, Qualtrics-based online survey). Participants were reimbursed $50 per completed survey (totaling $100 for two surveys) in Walmart gift cards.
2.6.1. Initial contact, enrollment
Scripts for the study description, and participant screening and enrollment were identical across the study arms, with the exception of the RC introducing herself as a RC, and the Peer introducing herself as a person with lived experience. The study description included explanation of the pilot study and its activities, and emphasized that no identifying or protected health information would be collected. Those interested and agreeing to participate completed the eligibility screening. Eligible persons were enrolled after a verbal consent and scheduled for Visit 1.
2.6.2. Visit 1 (baseline)
During Visit 1, lasting approximately 45 min, the Peer or RC described, following the scripted protocol, a future potential long-term birth cohort study recruiting pregnant/postpartum persons with SUD and, thereafter, answered participant questions and made clarifications. Participants were then provided a link to an online survey in the chat box and requested to complete it, and Visit 2 was scheduled. If Visit 2 was scheduled more than three days out, participants were contacted to “check in” and remind about the visit.
2.6.3. Visit 2 (follow-up)
Visit 2 was completed on average within 2 weeks after Visit 1 and involved a live virtual tour of the research magnetic resonance imaging (MRI) facility using laptop-based video technology. The Peer virtually joined alongside the Peer group participants. The RC reminded participants about Visit 2, but did not join. The tour averaged 30 min and was led by the neuroimaging facility researcher for participants to learn about the safety and approach, ask questions about neuroimaging of infants and young children, and witness a mock scanner. Last, a link to an online survey was provided in the chat box to each tour participant.
2.7. Outcome measures
2.7.1. Research engagement
The impact of Peer versus RC on research engagement was assessed by rates of completion of each study Visit per arm, with attendance of Visit 2 serving as the main measure of engagement and retention.
2.7.2. Research barriers and facilitators
Visit 1 and Visit 2 surveys inquired about participant perspectives on the facilitators and barriers to research engagement, and ways to overcome the barriers, especially in long-term birth cohort involving peripartum persons with SUD.
The Visit 1 survey consisted of 23 questions, including five open-ended ones, on demographics (age, income range, race, ethnicity, marital status, education level), views on barriers and facilitators for joining future research, potential impact on research engagement of peer involvement, and quality of their interaction with the research team (1–5 Likert scale, 1 = least favorable, 5 = most favorable).
The Visit 2 survey consisted of 10 questions, including 3 open-ended ones, on participant views on acceptability of MRI of a child’s brain among the target population and suggestions for increasing its acceptability, and importance of having peers as study team members.
2.8. Analytical methods
The Fisher’s Exact Test (R software program [22]) compared the completion rates of the follow-up session between the Peer and the RC groups, with two-tailed p < 0.05 signifying the statistical significance level. Descriptive statistics (mean score ± standard deviation [SD], frequencies) described the quantitative survey data. Qualitative survey data from the open-ended questions were summarized using qualitative thematic analysis methods [23]. The responses were parsed into meaning units to describe the content; emergent themes and subsequent patterns of meaning were identified across participant responses for each of the main categories, and representative quotes were selected to illustrate each theme.
3. Results
3.1. Participant flow (Fig. 1)
Fig. 1.
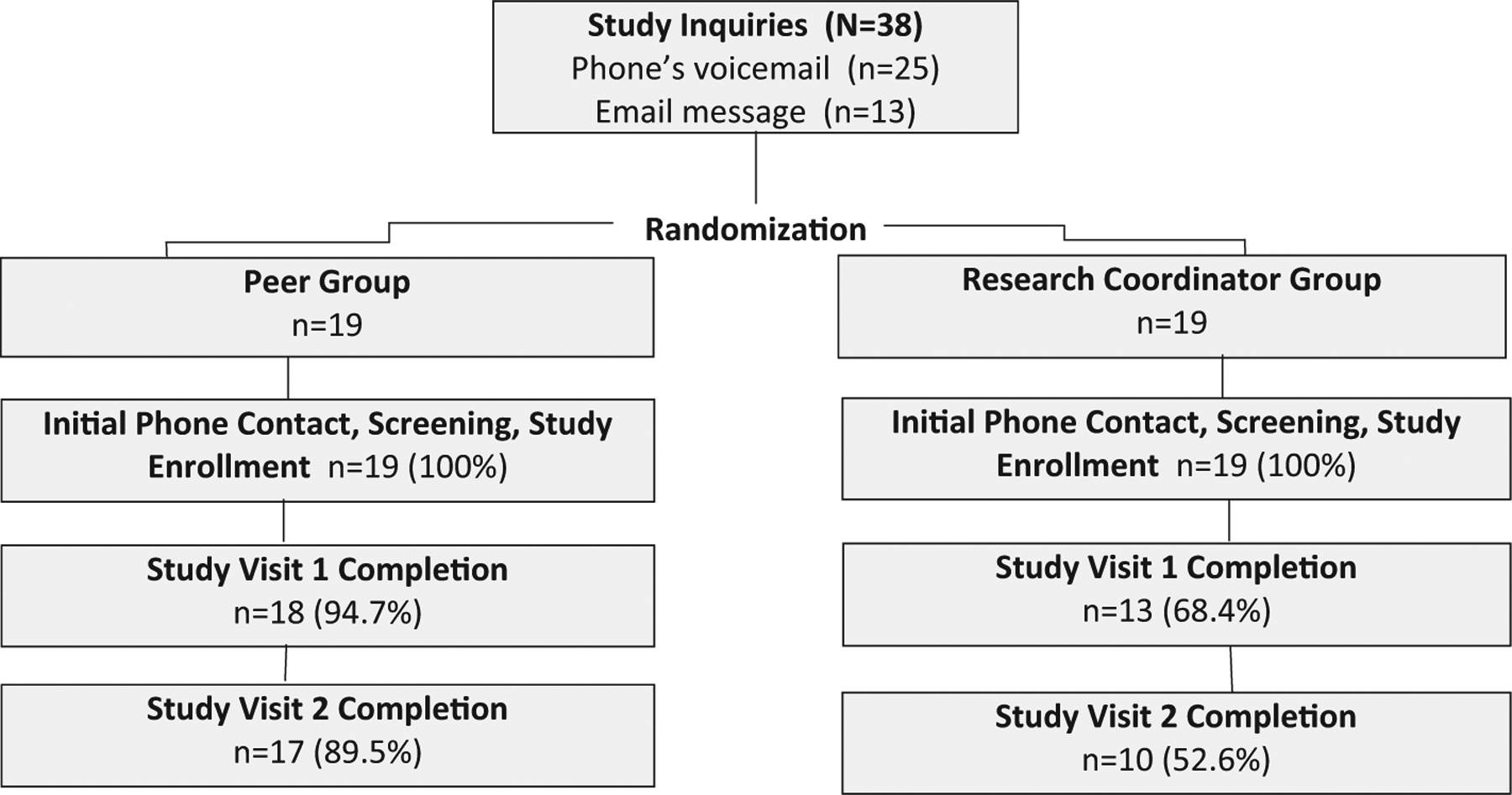
Study flow.
Thirty-eight individuals inquired about the study and provided their contact information (25 by phone, 13 by email) and were randomly assigned to the Peer (n = 19) or the RC (n = 19) groups. They were all reached by phone, found eligible, and, after verbal consent, enrolled into the study. Study Visit 1 was completed by 31 participants (18 Peer, 13 RC groups), and Visit 2 by 27 participants (17 Peer, 10 RC groups). Participants who did not complete Visit 1 or 2 activities were unreachable (i.e., lost to follow-up).
3.2. Participant characteristics (Table 1)
Table 1.
Demographic characteristics of participants completing Study Visit 1 surveys.
| Variable | Study sample (N = 31) |
|---|---|
| Age, years, mean (SD), range | 41.2 (13.7), 19–70 |
| Non-Hispanic ethnicity, # (%) | 31 (100) |
| Race, # (%) | |
| Black/African American | 18 (58.1) |
| White | 12 (38.7) |
| American Indian/Alaskan | 1 (3.2) |
| Marital status, # (%) | |
| Single | 22 (71.0) |
| Married / In a domestic partnership | 3 (9.7) |
| Widowed / Divorced / Separated | 6 (19.4) |
| Highest degree/level of school completed, # (%) | |
| Less than high school | 3 (9.7) |
| High school | 9 (23.0) |
| Some college, no degree | 11 (35.5) |
| Associate degree | 4 (12.9) |
| Bachelor’s degree | 2 (6.5) |
| Graduate degree | 2 (6.5) |
| Annual individual income, # (%) | |
| < $20,000 | 21 (67.7) |
| $20,000 – $34,999 | 7 (22.6) |
| $35,000 – $49,000 | 2 (6.5) |
| $50,000 – $74,999 | 1 (3.2) |
| $75,000 or more | 0 (0) |
Thirty-one participants completed the Visit 1 survey. They were, on average, middle-aged, and identified as non-Hispanic (100%), with majority reporting Black/African American (58.1%), followed by White (38.7%), and American Indian/Alaskan (3.2%) race, and were single (71.0%). Approximately one-third completed up to high school education (32.7%) versus ‘some college, no degree’ (35.5%). The majority (90.3%) reported an annual individual income <$35,000, with 67.7% reporting <$20,000.
3.3. Research engagement by group
In the Peer (n = 19) versus RC (n = 19) groups, 95% versus 68% completed Visit 1, and 90% versus 53% completed Visit 2 (Fig. 1). In the RC group, most dropouts (n = 6) occurred between the initial phone contact and Visit 1. Between-group comparison of Visit 2 completion rates indicated the Peer group had 7.2 times greater odds of completing Visit 2 compared to the RC group (Fisher’s exact test, p = 0.029, 95% Confidence Interval: 1.2, 81.8).
3.4. Factors promoting recruitment and retention: survey’s quantitative findings (Table 2)
Table 2.
Importance of specific incentives for recruiting and retaining the target population (n = 31).
| Mean (SD) | Not at all (1) n (%) | A little (2) n (%) | Somewhat (3) n (%) | Very (4) n (%) | Extremely (5) n (%) | |
|---|---|---|---|---|---|---|
| Importance of the following incentives for women to enroll in the future long-term study | ||||||
| Supplies for your baby (e.g. formula or diapers) | 4.7 (0.8) | 1 (3.3) | 0 (0) | 0 (0) | 4 (12.9) | 25 (80.6) |
| Knowing that your participation will help other children in the future | 4.8 (0.5) | 0 (0) | 0 (0) | 1 (3.2) | 5 (16.1) | 24 (77.4) |
| Healthcare services (e.g. addiction treatment, counseling, etc.) | 4.7 (0.5) | 0 (0) | 0 (0) | 0 (0) | 10 (32.3) | 20 (64.5) |
| Compensation (cash or check) | 4.3 (0.8) | 1 (3.2) | 0 (0) | 2 (6.5) | 15 (48.4) | 13 (41.9) |
| Gift cards (e.g., Walmart or Target) | 4.2 (0.8) | 0 (0) | 2 (6.5) | 1 (3.2) | 16 (51.6) | 11 (35.5) |
| Importance of the following incentives for women to stay engaged long-term in a future study | ||||||
| Having support from and access to a woman with lived experience who is a certified peer support specialist | 4.7 (0.4) | 0 (0) | 0 (0) | 0 (0) | 8 (25.8) | 23 (74.2) |
| Childcare services for when you have a study visit | 4.6 (0.7) | 0 (0) | 0 (0) | 3 (9.7) | 7 (22.6) | 21 (67.7) |
| Help with transportation (e.g., gas money) | 4.6 (0.7) | 0 (0) | 1 (3.2) | 1 (3.2) | 7 (22.6) | 21 (67.7) |
| Flexible scheduling | 4.6 (0.6) | 0 (0) | 0 (0) | 2 (6.5) | 7 (22.6) | 21 (67.7) |
| Being provided a list of affordable healthcare resources for you and your baby | 4.6 (0.6) | 0 (0) | 0 (0) | 2 (6.5) | 10 (32.3) | 19 (61.3) |
| Check-ins to see how you are doing throughout the study | 4.5 (0.8) | 0 (0) | 1 (3.2) | 2 (6.5) | 10 (32.3) | 18 (58.1) |
| Reminder texts or e-mails for when you have study visits | 4.5 (0.6) | 0 (0) | 0 (0) | 1 (3.2) | 14 (45.2) | 16 (51.6) |
SD: standard deviation.
The number of respondents (percentage) may not add to the total sample size (100%) due to missing data (e.g., declined or not provided).
Thirty-one participants completed the Visit 1 survey about factors that could impact research engagement. Participants reported receiving supplies for the baby, such as formula or diapers (80.6%), knowledge that their study participation could help others in the future (77.4%), and getting help with accessing healthcare services (64.5%) as their top three ‘extremely important’ recruitment incentives; reimbursement was viewed as “extremely important” for recruitment by approximately 40% of respondents.
Participants indicated support and access to a woman with lived experience who is a certified peer support specialist (74.2%), flexible scheduling of the study visits (67.7%), and assistance with transportation (67.7%) and childcare (67.7%) when attending the study visits as ‘extremely important’ for retention in a long-term study. A list of affordable healthcare resources for women and children (61.3%), regular check-ins with the research team (58.1%) and reminders about the upcoming study visits (51.6%) were viewed as ‘extremely important’ by over half of the respondents.
3.5. Comfort level with different study assessments: survey’s quantitative findings (Table 3)
Table 3.
Comfort level with baby’s brain imaging and other assessments during the study, and ways to increase comfort level with the imaging-based assessments.
| Mean (SD) | Not at all (1) n (%) | A little (2) n (%) | Somewhat (3) n (%) | Very (4) n (%) | Extremely (5) n (%) | |
|---|---|---|---|---|---|---|
| Visit 1. Comfort level with your baby’s brain imaging using MRI (n = 31) | ||||||
| 3.2 (1.2) | 4 (12.9) | 4 (12.9) | 10 (32.3) | 9 (29.0) | 4 (12.9) | |
| Visit 2. Comfort level among women with the following activities in the future study (n = 27) | ||||||
| Completing brain scans on the child from birth - 10 years old | 3.5 (0.9) | 0 (0) | 3 (11.1) | 11 (40.7) | 7 (25.9) | 5 (18.5) |
| Obtaining a blood sample from the child for testing | 3.4 (1.0) | 1 (3.7) | 3 (11.1) | 13 (48.1) | 4 (14.8) | 5 (18.5) |
| Obtaining a sample of meconium (your baby’s first bowel movement) for testing | 3.6 (1.0) | 1 (3.7) | 1 (3.7) | 12 (44.4) | 8 (29.6) | 5 (18.5) |
| Obtaining urine samples from yourself while pregnant for urine drug testing | 3.5 (1.3) | 2 (7.4) | 4 (14.8) | 8 (29.6) | 5 (18.5) | 8 (29.6) |
| Survey questions about your and your baby’s health history | 3.9 (0.8) | 0 (0) | 1 (3.7) | 8 (29.6) | 11 (40.7) | 7 (25.9) |
| Visit 2. Helpfulness of the following to make the MRI process better for future women (n = 27) | ||||||
| Having a tour of the scanning facility before signing up for the study | 4.3 (0.8) | 0 (0) | 1 (3.7) | 2 (7.4) | 11 (40.7) | 13 (48.1) |
| Knowing how the scan of my child’s brain may help others in the future | 4.4 (0.7) | 0 (0) | 0 (0) | 3 (11.1) | 9 (33.3) | 14 (51.9) |
| Added compensation (money/gift cards) for completing the brain scan | 4.2 (0.8) | 0 (0) | 1 (3.7) | 4 (14.8) | 11 (40.7) | 10 (37.0) |
| Having information about the safety of the brain scan | 4.5 (0.6) | 0 (0) | 0 (0) | 2 (7.4) | 9 (33.3) | 16 (59.3) |
| Having a peer support person with me at the scanning facility | 4.6 (0.7) | 0 (0) | 1 (3.7) | 1 (3.7) | 6 (22.2) | 19 (70.4) |
| Talking with other people whose babies have already done the scan | 4.4 (0.6) | 0 (0) | 0 (0) | 2 (7.4) | 12 (44.4) | 12 (44.4) |
SD: standard deviation.
The number of respondents (percentage) may not add to the total sample size (100%) due to missing data (e.g., declined or not provided).
Twenty-seven participants completed the Visit 2 surveys. The vast majority felt at least ‘somewhat’ comfortable with survey-based assessments (96.3%) and testing of the newborn’s meconium (92.6%). The respondents were slightly less comfortable with obtaining pregnant person’s urine sample for toxicology or child’s blood sample, with 77.8% and 81.5% of the respondents, respectively, reporting feeling at least ‘somewhat’ comfortable.
Following the MRI facility tour during Visit 2, 85.2% of the respondents felt at least ‘somewhat’ comfortable, and none felt uncomfortable with the child’s brain MRI-based assessment. Prior to the MRI facility tour, the Visit 1 surveys (n = 31) revealed a lower comfort level, with 74.2% of the respondents feeling at least ‘somewhat’ comfortable, and 12.9% feeling uncomfortable with their child’s brain MRI.
The majority (70.4%) of Visit 2 survey respondents identified the presence of a peer who accompanied study participants at the imaging facility as ‘extremely’ helpful for improving the MRI process and participant comfort level in future studies. Close to one-half also viewed the availability of information about safety of the brain scan (59.3%) and how the child’s brain scan could help others in the future (51.9%) and completing a tour of the scanning facility (48.1%) as ‘extremely’ helpful when deciding about enrolling in a study involving the MRI or agreeing to the MRI assessment. Talking with other people whose children had undergone the scan (44.4%) and receiving compensation for the completed scan (37.0%) were endorsed as ‘extremely’ helpful by a smaller proportion of participants.
3.6. Quality of interactions with research staff: survey’s quantitative findings
Participants provided high ratings across both groups for how clearly the study was explained (4.7 ± 0.4), how welcome they felt during the session (5.0 ± 0.2), and the extent to which the research staff displayed the qualities of trustworthiness (4.8 ± 0.5), understanding (4.8 ± 0.4) and accessibility (4.8 ± 0.4), without differences (independent samples t-test, p > 0.05) in these ratings between the RC and Peer groups.
3.7. Ways participants learned about the study: survey’s qualitative findings
Participant responses to the Visit 1 survey’s open-ended question indicated the vast majority heard about the study through “word of mouth” from their friends, family members, or current treatment or other service providers or agencies; only one person reported learning about the study through a flyer.
3.8. Potential additional ways to promote research engagement: survey’s qualitative findings
Participant comments in response to open-ended questions in Visit 1 (n = 31) and Visit 2 (n = 27) surveys fell into three overarching categories. These categories and their main themes are summarized below, with representative participant quotes presented in Table 4.
Table 4.
Additional ways to promote research engagement: qualitative comments provided in Visit 1 (n = 31) and Visit 2 (n = 27) surveys:
| Category 1: Other incentives for facilitating general research engagement (Visit 1) |
| Theme 1. Facilitate participant access to existing healthcare and community resources |
|
|
|
|
|
|
|
|
|
Theme 2. Enable learning about the child’s health and development |
|
|
|
|
|
Theme 3. Foster trusting, supportive research environment for the participants |
|
|
|
|
|
|
|
|
|
|
Theme 4. Other |
|
|
|
|
|
|
Category 2: Other ways for increasing participant comfort with brain MRI of their children for research purposes (Visit 2) |
|
Theme 1. Having the child accompanied by a parent or another trusted person |
|
|
|
Theme 2. Becoming familiar with the MRI safety and procedural details |
|
|
|
|
|
|
| Category 3: Other feedback (Visits 1 and 2) |
| Theme 1. Study assessing child’s health and development is very needed |
|
|
|
|
|
|
|
Theme 2. Importance of peer involvement |
|
|
|
|
|
|
Theme 3. Creating a safe, non-judgmental research environment |
|
|
|
3.8.1. Category 1: other incentives for facilitating general research engagement
When asked during Visit 1 about “other incentives” for promoting recruitment and retention, participants offered suggestions, which formed four main themes. Theme 1 described the need of facilitating connections for participants by the study team to healthcare and community resources. Theme 2 emphasized the importance of noting potential benefits of study participation by learning more about their child’s health and development and being promptly linked to existing services should any problems be detected. Theme 3 noted the value of creating a supportive, non-judgmental environment to foster trust and rapport between the study team and participants. Theme 4 included a variety of other suggestions for improving study engagement, such as regular check-ins to support the family and its emerging needs, staying in touch, and considering study reimbursement options. While some participants favored cash, others voiced concerns about receiving cash remuneration and, alternatively, suggested gift cards for groceries or child supplies.
3.8.2. Category 2: other ways for increasing comfort with child’s brain MRI
Comments on ways for improving participant comfort level with their child’s brain MRI for research purposes formed two broad themes. Theme 1 emphasized that parents are likely to feel uncomfortable leaving their child during the imaging with the unfamiliar research personnel; whereas allowing the parents or other trusted person (e.g., recovery peer specialist) to accompany the child would help increase parental comfort level with the MRI. Theme 2 noted that learning more about the MRI’s details and safety, completing the facility tour, and having questions answered by a neuroimaging researcher are critical for increasing MRI’s acceptability.
3.8.3. Category 3: other feedback
When requested to provide additional feedback, participants’ comments created three distinct themes. Theme 1 endorsed the sentiment that a study evaluating factors of children’s health and neuro-development is critically needed and important to the target population. Theme 2 identified the involvement of peers as important for initial contact, and for both signing up and staying engaged in a long-term study. Theme 3 emphasized the importance of creating a research environment for participants that feels safe and non-judgmental.
4. Discussion
Findings from this pilot study support the notion that peers with lived experience of substance use during pregnancy could boost research engagement among persons who use substances or have SUD during the perinatal period. Both the retention rates at Visits 1 and 2 were higher in the Peer compared to the RC group, with Peer group having 7.2 times greater odds of completing Visit 2 compared to the RC group, and participant survey responses identified the presence of peers as ‘extremely important’ for recruitment and retention.
To our knowledge, the impact of peer-conducted research recruitment and retention efforts has not been rigorously evaluated. Despite growing implementation in clinical settings [4], evidence on the effectiveness of peer services in improving health outcomes remains limited. Some systematic reviews noted potential salutary effects of peer involvement in clinical and research settings, including with hard-to-reach populations [18] and those with SUD [15,16], while others did not find sufficient evidence for the effectiveness of these services, including in SUD [24]. Unlike prior studies, our project did not assess the effects of a peer-delivered or peer-augmented intervention; rather, we included a peer, after additional training in the key components of human subjects research, as a research team member. “Replacing” a highly-trained research coordinator with a peer may not be appropriate for all tasks (e.g., consenting for higher-risk studies, more complex assessments, data/database management). However, if participant engagement in research could be boosted, peer involvement would represent an invaluable contribution since recruitment and retention pose challenges in clinical trials. Peers could be particularly helpful with research involving vulnerable, underserved populations, including substance-using pregnant persons or those with SUD [13,15].
Creating a trusting, supportive, non-judgmental research environment and linkages to treatment and other services were noted by our study participants as top incentives for research engagement among peripartum persons with substance use. Recovery peers could help facilitate both the positive research team-participant relationships and resource navigation [4,5,13]. Educating research staff about the science of addiction and related stigma, legal consequences, and sociocultural ramifications of substance use while pregnant, and modeling compassionate, non-stigmatizing interactions with participants are essential. Interestingly, both the Peer and the RCs in our study received stellar ratings from the Visit 1 participants on their interaction quality, suggesting that interaction characteristics are not the only mechanism underlying research engagement. It is possible that mistrust in research and researchers in general [25] could have contributed to the early attrition noted in the RC group, while the peer’s lived experience enhanced rapport and trust with participants, promoting retention.
Understanding that research participation might help their children and families (e.g., by confirming a child’s appropriate development or by offering linkages to existing services should any problems be found through research assessments) as well as future generations was identified as an extremely important motivator to research engagement. A recent qualitative study of women who used substances during pregnancy noted that shame and deep worries about potential harms of substance exposure made research that evaluates the impact of perinatal substance use on fetal/child development and early identification of potential problems (with referral to services as needed) of high importance to this population [13].
Our pilot study indicated that more in-depth knowledge about the study details, including “touring” of the more complex assessments, e.g., child’s brain MRI, and presence of trusted team members (e.g., peers), could help reduce parental discomfort while making the study more acceptable and increase participant willingness to engage in research involving such activities. Implementing robust research engagement efforts (e.g., availability of peers or staff for neuroimaging facility touring during recruitment) can be costly and less feasible. Yet, these services were viewed as more important research incentives than those routinely offered in clinical trials (e.g., remuneration, ‘check ins’). Research funds designated for engagement efforts will be critical for implementing participant-recommended incentives and overcoming the current recruitment and retention challenges [26]. Additionally, participant qualitative comments highlighted the dilemma often faced in SUD-focused research regarding the approach to remuneration for research participation; while cash-based reimbursements can be useful, they might also increase the risk of substance use [13].
The study’s main limitations included a small, convenience-based sample comprising persons with lived experience of SUD during pregnancy, and lack of blinding to the study “intervention” (i.e., assignment to Peer vs RC groups). The lack of requirement of being currently pregnant (while using substances) was driven by the need to reduce any harmful legal consequences, which could have impacted substance-using pregnant participants. Engagement and survey responses could have been different should substance-using pregnant participants had been a part of this study. In addition, our pilot study posed low-risk and overall low demands on participants; it featured a short follow-up duration, remote conduct (due to the COVID-19 pandemic restrictions), no treatment-oriented intervention or complex or burdensome procedures. It has also heavily focused on the MRI-related experiences and considerations during Visit 2. As such, although promising, our pilot results may not be generalizable to substance-using pregnant persons considering participation in clinical trials with more demanding, higher risk protocols. Our findings will need further corroboration from future carefully designed, blinded, randomized controlled trials to determine if the involvement of peers as research team members increases recruitment, retention, and/or study protocol adherence.
5. Conclusions
Involvement of recovery peer specialists as research team members has the potential to increase research recruitment and retention among persons who use substances perinatally. Increasing research engagement in this population can be achieved by building trusting research team-participant relationships, designing research to maximize benefits for participants and their children, producing knowledge that serves the well-being of future generations, and providing detailed explanations of the study procedures, especially for “riskier” activities.
Acknowledgements
We would like to thank Drs. Beth Planalp and Doug Dean III from the University of Wisconsin Waisman Center, and DaVita Walker from Safe Comminutes Madison-Dane County for making this study possible.
Funding
Research reported in this publication was supported by the National Institutes of Health (NIH) Helping to End Addiction Long-term (HEAL) HEALthy Brain and Child Development Initiative under award no. R34DA050270. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- IRB
Institutional Review Board
- RC
research coordinator
- SD
standard deviation
- SUD
substance use disorder
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- [1].Room R, Stigma, social inequality and alcohol and drug use, Drug Alcohol Rev. 24 (2) (2005) 143–155, 10.1080/09595230500102434 [DOI] [PubMed] [Google Scholar]
- [2].Stone R, Pregnant women and substance use: fear, stigma, and barriers to care, Health Justice 3 (2015) 2. [Google Scholar]
- [3].Best DW, Lubman DI, The recovery paradigm - a model of hope and change for alcohol and drug addiction, Aust. Fam. Physician 41 (8) (2012) 593–597. [PubMed] [Google Scholar]
- [4].Substance Abuse and Mental Health Services Administration (SAMHSA), Peer Support Workers for those in Recovery, SAMHSA, Sept 27, 2022. Accessed on Dec 31, 2022 at, https://www.samhsa.gov/brss-tacs/recovery-support-tools/peers. [Google Scholar]
- [5].Mental Health America (MHA), MHA Peer Support, MHA, 2022. accessed on Dec 31, 2022 at: https://www.mhanational.org/center-peer-support.
- [6].University of Michigan Behavioral Health Workforce Research Center, National Analysis of Peer Support Providers: Practice Settings, Requirements, Roles, and Reimbursement, UMSPH, Ann Arbor, MI, 2019. accessed on Dec 31, 2022 at: https://www.behavioralhealthworkforce.org/wp-content/uploads/2019/10/BHWRC-Peer-Workforce-Full-Report.pdf. [Google Scholar]
- [7].Peer Recovery Center of Excellence (PRCoE), Comparative Analysis of State Requirements for Peer Support Specialist Training and Certification in the United States, PRCoE, 2021. accessed on Dec 31, 2022 at: https://www.peerrecoverynow.org/focus-area/workforce.aspx. [Google Scholar]
- [8].Substance Abuse and Mental Health Services Administration (SAMHSA), Peers Supporting Recovery from Substance Use Disorders, SAMHSA, 09/27/2022. Accessed on May 10, 2023 at: https://www.samhsa.gov/sites/default/files/programs_campaigns/brss_tacs/peers-supporting-recovery-substance-use-disorders−2017.pdf. [Google Scholar]
- [9].Scott CK, et al. , Opioid recovery initiation: pilot test of a peer outreach and modified recovery management checkup intervention for out-of-treatment opioid users, J. Subst. Abus. Treat 86 (2018) 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Powell KG, et al. , Promoting opioid overdose prevention and recovery: an exploratory study of an innovative intervention model to address opioid abuse, Int. J. Drug Policy 64 (2019) 21–29. [DOI] [PubMed] [Google Scholar]
- [11].Waye KM, Goyer J, Dettor D, Mahoney L, Samuels EA, Yedinak JL, Marshall BDL, Implementing peer recovery services for overdose prevention in Rhode Island: an examination of two outreach-based approaches, Addict. Behav 89 (2019) 85–91, 10.1016/j.addbeh.2018.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goldstein E, Nervik K, Hagen S, Hilliard F, Turnquist A, Bakhireva LN, McDonald R, Ossorio PN, Lo J, Zgierska AE, A socioecological framework for engaging substance-using pregnant persons in longitudinal research: multi-stakeholder perspectives, Neurotoxicol. Teratol 87 (2021), 106997, 10.1016/j.ntt.2021.106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hilliard F, Goldstein E, Nervik K, Croes K, Ossorio PN, Zgierska AE, Voices of women with lived experience of substance use during pregnancy: a qualitative study of motivators and barriers to recruitment and retention in research, Fam. Commun. Health 46 (1) (2023) 1–12, 10.1097/fch.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ryan JE, Smeltzer SC, Sharts-Hopko NC, Challenges to studying illicit drug users, J. Nurs. Scholarsh 51 (4) (2019) 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bassuk EL, Hanson J, Greene RN, Richard M, Laudet A, Peer-delivered recovery support services for addictions in the United States: a systematic review, J. Subst. Abus. Treat 63 (2016) 1–9, 10.1016/j.jsat.2016.01.003. [DOI] [PubMed] [Google Scholar]
- [16].Georgie JM, Sean H, Deborah MC, Matthew H, Rona C, Peer-led interventions to prevent tobacco, alcohol and/or drug use among young people aged 11–21 years: a systematic review and meta-analysis, Addiction 111 (3) (2016) 391–407, 10.1111/add.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tracy K, Wallace SP, Benefits of peer support groups in the treatment of addiction, Subst. Abus. Rehabil 7 (2016) 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].National Institutes of Health Helping to End Addiction Long-termSM Initiative (NIH HEAL). HEALthy Brain and Child Development Study (HBCD), NIH HEAL Initiative, Accessed on May 10, 2023 at: https://heal.nih.gov/research/infants-and-children/healthy-brain, February 21, 2023. [Google Scholar]
- [19].Sokol R, Fisher E, Peer support for the hardly reached: a systematic review, Am. J. Public Health 106 (7) (2016), 10.2105/AJPH.2016.303180 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wisconsin Department of Health Services (WIDHS), Wisconsin Certified Peer Specialist Core Competencies, WIDHS, Sept 2019. accessed on Jan 23, 2023 at: https://www.dhs.wisconsin.gov/publications/p00972b.pdf. [Google Scholar]
- [21].Wisconsin Peer Specialists (WPS), Certified Peer Specialist, WPS, Madison, WI, 2023. accessed on Jan 23, 2023 at: https://www.wicps.org/certified-peer-specialist. [Google Scholar]
- [22].R Core Team R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2021. accessed on Jan 23, 2023 at: https://www.R-project.org. accessed on Jan 23, 2023 at: [Google Scholar]
- [23].Saldaña J, Coding Manual for Qualitative Researchers, Sage Publications Ltd, 2009. [Google Scholar]
- [24].Gormley MA, Pericot-Valverde I, Diaz L, Coleman A, Lancaster J, Ortiz E, Moschella P, Heo M, Litwin AH, Effectiveness of peer recovery support services on stages of the opioid use disorder treatment cascade: a systematic review, Drug Alcohol Depend. 229 (Pt B) (2021), 109123, 10.1016/j.drugalcdep.2021.109123 [DOI] [PubMed] [Google Scholar]
- [25].Corbie-Smith G, Thomas SB, St George DM, Distrust, race, and research, Arch. Intern. Med 162 (21) (2002) 2458–2463, 10.1001/archinte.162.21.2458 [DOI] [PubMed] [Google Scholar]
- [26].Fleury MJ, Djouini A, Huỳnh C, Tremblay J, Ferland F, Ménard JM, Belleville G, Remission from substance use disorders: a systematic review and meta-analysis, Drug Alcohol Depend. 168 (2016) 293–306, 10.1016/j.drugalcdep.2016.08.625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


