Abstract
Proximity to telomeres (i) and high adenine and thymine (A+T) content (ii) are two factors associated with high mutation rates in human chromosomes. We have previously shown that more than 100 human genes when mutated to cause congenital hydrocephalus (CH) meet either factor (i) or (ii) at 91% matching, while two factors are poorly satisfied in human genes associated with familial Parkinson’s disease (fPD) at 59%. Using the sets of mouse, rat, and human chromosomes, we found that 7 genes associated with CH were located on the X chromosome of mice, rats, and humans. However, genes associated with fPD were in different autosomes depending on species. While the contribution of proximity to telomeres in the autosome was comparable in CH and fPD, high A+T content played a pivotal contribution in X-linked CH (43% in all three species) than in fPD (6 % in rodents or 13 % in humans). Low A+T content found in fPD cases suggests that PARK family genes harbor roughly 3 times higher chances of methylations in CpG sites or epigenetic changes than X-linked genes.
Keywords: X-linked hydrocephalus, mutations, proximity to telomeres, A+T content, CpG sites, Parkinson’s disease
1. Introduction
Hydrocephalus is one of the most frequently encountered brain diseases, characterized by excessive accumulation of cerebrospinal fluid (CSF) in the intra- and extra-ventricular spaces (Rekate, 2009; Zeineddine et al., 2020). As published in 2018, the prevalence of hydrocephalus was approximately 85 of 100,000 individuals globally and varied by age (Isaacs et al., 2018). When birth defect such as spina bifida was included, the prevalence of hydrocephalus increased to 88 per 100,000 children, and the highest prevalence was found in the elderly at 175 out of 100,000 (Isaacs et al., 2018). The pooled incidence of congenital hydrocephalus (CH) was varied by geographic regions where it was highest in Latin America (316 per 100,000 births) and lowest in North America (68 per 100,000 births) (Dewan et al., 2018).
Depending on the timing of detection, hydrocephalus is classified into acquired, congenital (Dewan et al., 2018), and normal pressure hydrocephalus (NPH). Typical symptoms used as criteria in determining the diagnosis include ventricular volume and intracranial pressure (ICP) (Limbrick and Park, 2006). Acquired hydrocephalus, which develops at the time of birth or later, arises from meningitis, a tumor, injury, or disease that blocks the absorption of CSF in the brain. CH is diagnosed when it is present at birth due to genetic mutations associated with neural tube defects and mother’s infection during pregnancy (Gillman et al., 1948). Although NPH has been reported in children and adults (Williams et al., 2022), more cases of seniors at age >65 years are found as secondary and/or idiopathic NPH (Mechelli et al., 2022; Yang et al., 2021).
Most deleterious mutations (101 of 108) associated with CH (McKnight et al., 2021) are detected in the autosome (chromosome; chr 1 through 22) except those (7 of 108) on the X chromosome (McKnight et al., 2021). Neuronal cell adhesion molecule (CAM), L1CAM, on the X chromosome, is a well-known causative gene associated with CH or L1 syndrome (Fransen et al., 1995; Gao et al., 2022; Jouet et al., 1995; Jouet et al., 1994; Kanemura et al., 2006; Yamasaki et al., 2011; Zhao and Siu, 1996). The L1CAM gene mutations that cause CH leads to an L1 protein that cannot facilitate various neuronal functions. In fact, genes that affect brain function and genes that control fertility are preferentially located on the human X chromosome (Vicoso and Charlesworth, 2006). Consistent with X-linked human diseases, which usually affects only males (Christodoulou et al., 2019; Dennis et al., 2019; Franco and Ballabio, 2006; Fukae et al., 2017; Gill et al., 2013; Sabo et al., 2019), X linked hydrocephalus is also more likely to be found in males (Edwards, 1961; Edwards et al., 1961) because they are hemizygous for X chromosome alleles (Libert et al., 2010).
Along with L1CAM, we previously investigated other X-linked genes associated with CH on the human chromosome (McKnight et al., 2021). AP1S2 gene, for instance, which encodes AP-1 complex subunit sigma-2 protein, is highlighted with L1CAM as causative genes on the X chromosome (Shaheen et al., 2017), evoking CH (Ballarati et al., 2012; Borck et al., 2008; Cacciagli et al., 2014; Cappuccio et al., 2019; Huo et al., 2019; Luan et al., 2019; Saillour et al., 2007; Tarpey et al., 2006; Zhu et al., 2022; Zhu et al., 2021). ALG13, which heterodimerizes with asparagine-linked glycosylation 14 homolog, is associated with CH as well in addition to Lennox-Gastaut syndrome and epileptic encephalopathy (Epi et al., 2013). A syndromic gene similar to the one found in Wistar polycystic kidney rats (Shim et al., 2019) whose mutations cause CH, is orofaciodigital syndrome type I (OFD1) gene, which is also associated with Joubert syndrome and polycystic kidney phenotype (Field et al., 2012). Zic Family Member 3 (ZIC3), a transcription factor that regulates early stages of the left-right axis formation, is also on the X chromosome and, when mutated, causes Dandy-Walker malformation, neural tube defects, and CH (Grinberg and Millen, 2005). Filamin A, alpha (FLNA), if mutated, is also known to result in periventricular heterotopia and CH (Sheen et al., 2004). Coffin-Lowry syndrome gene, ribosomal protein S6 kinase alpha-3 (RPS6KA3), which is on the X chromosome, is included as one of those genes susceptible to CH as well (Kousi and Katsanis, 2016). Whether these X-linked genes continue to evoke CH consistent with the prior reports (Epi et al., 2013; Field et al., 2012; Gao et al., 2022; Grinberg and Millen, 2005; Kousi and Katsanis, 2016; Sheen et al., 2004; Zhu et al., 2022) in the future, however, is a different story. We conjectured that the significance of these genes as a causal factor of CH (Epi et al., 2013; Field et al., 2012; Gao et al., 2022; Grinberg and Millen, 2005; Kousi and Katsanis, 2016; Sheen et al., 2004; Zhu et al., 2022) might depend on where X-linked genes are located with respect to their telomeres or nucleotide compositions (Lucas et al., 2021; McKnight et al., 2021; Raines et al., 2022; White et al., 2022) in human chromosomes (Nusbaum et al., 2006).
While the main cue of inherited X-linked hydrocephalus is genetics (Guo et al., 2020; Hu et al., 2019; Izumi et al., 2022; Kong et al., 2020; Tripolszki et al., 2021), the significance of epigenetics has been recently demonstrated in mice where the control of accelerating aging and reversing these aging effects are achieved (Yang et al., 2023). The reversibility of aging in their mouse model supports the claim that epigenetic instruction drives the process of aging, not mutations in DNA sequences (Yang et al., 2023). Given all this reversibility of aging in mice shown in one generation, earlier research has highlighted three factors associated with high mutation rates (Nusbaum et al., 2006) over generations from parents (F1) to the next (F2 offspring) (Terekhanova et al., 2017). This at the same time opens new hypotheses stating whether the reversibility of aging can be comparably effective in humans as shown in the mouse study (Yang et al., 2023), and if the molecular techniques reversing the process of aging are therapeutically applicable to age-related conditions such as sporadic Parkinson’s disease (sPD) (Baertsch et al., 2022; Behbahanipour et al., 2019; Chen et al., 2020; Chen et al., 2021; Choi et al., 2020a; Choi et al., 2020b; Chowdhury et al., 2023; Derya et al., 2019; Drouin-Ouellet et al., 2022; Ekimova et al., 2020; Grzybowski and Kanclerz, 2020; Howard et al., 2022; Juarez-Flores et al., 2020; Kouli and Williams-Gray, 2022; Lason et al., 2023; Liu et al., 2023; Liu et al., 2021; Rani et al., 2022; Russo and Riessland, 2022; Shindo et al., 2021; Tamano et al., 2019; Wolfrum et al., 2022).
Proximity to telomeres (i) and high adenine and thymine (A+T) content (ii) are two factors associated with high mutation rate in human chromosomes (McKnight et al., 2021; Nusbaum et al., 2006; White et al., 2022). We have previously shown that 108 human genes when mutated to cause CH meet either factor (i) or (ii) at 91% match, while two factors show a lower matching rate at 59% in human genes associated with familial Parkinson’s disease (fPD) (McKnight et al., 2021). However, if genomic characteristics found in human chromosomes are consistent across species ensuring minimization of any discrepancy due to species differences often questioned surrounding the clinical trials (Hollenberg, 2000; Langston, 2017; Toutain et al., 2010), of which outcomes differed from a preclinical prediction (Yiannopoulou et al., 2019) and how many genes on the sex chromosome mediate the pathophysiology of hydrocephalus are not well-understood. Using 100 genes from the same list that we reported previously (McKnight et al., 2021), we identified the genetic loci associated with CH and fPD on the X chromosome and autosome, respectively, in three species of mice, rats, and humans. Then, we assessed whether any X chromosome genes associated with CH would show consistent chromosomal characteristics across three species, satisfying either factor (i) or (ii) in mice, rats, and humans. Given the prior reports (Chang et al., 1994; Miyata et al., 1987; Vicoso and Charlesworth, 2006) that if spermatogenesis is more mutagenic than oogenesis, the X chromosome is subjected to a lower mutation rate than the autosome, we investigated the genomic characteristics of X-linked CH genes focusing on their relative mutability by two factors. To this end, we examined whether multiple X chromosome genes associated with CH, if druggable as a pharmacological target, harbor a consistent mutability in mouse, rat, and/or human genome.
2. Methods
2.1. Quantifying the Proximity to a Telomere
To quantitively determine the approximate proximities to a telomere in million bases (Mb), we obtained human, mouse, and rat genomic information using the NCBI Genome Data Viewer (https://www.ncbi.nlm.nih.gov/genome/gdv/). In each sample obtained, we observed the following: coordinates (or locus) of each gene and its telomere, which chromosome number the genetic locus was found, the distance between each gene and its telomere, and the corresponding GenBank accession number that begins with “NM_ _ _ _ _” in a separate spreadsheet as an electronic file. This allowed us to view the full exon sequences of the chosen gene at the open nucleotide database (https://www.ncbi.nlm.nih.gov/nucleotide/).
2.2. Calculating A+T content
After obtaining the complete sequences from the nucleotide database, we used two independent GC content calculators as mutually complementary backup tools when one server is down. These are available online at https://www.sciencebuddies/org and https://www.biologicscorp.com/tools/GCContent/#.XvctCi-z2uV to derive the A+T content percentage of the nucleotides in each desired gene. From this data, the appropriate structures of adenine (A) and thymine (T) were determined along with the complete base-pair sizes of the nucleotide (Lucas et al., 2021; McKnight et al., 2021; Raines et al., 2022).
2.3. Theoretical Basis of Approximating the Proximity to a Telomere
The suggestions by Nusbaum and colleagues (Nusbaum et al., 2006) were used where high mutation rates were displayed in human chromosomes. In the study presented, the previous method was employed as the proximity to a telomere in a gene data was collected (Lucas et al., 2021; McKnight et al., 2021; Raines et al., 2022). The A+T content of all genes listed was also calculated (McKnight et al., 2021) by comparing each species’ (mouse, rat, and human) chromosome. The location of the gene and the locus of its telomere per each species was described by using the following conditions:
If the recombination frequency is less than or equal to 50 centimorgan (cM), then the genes are linked.
If the recombination frequency is greater than 50 cM, then the genes are not linked.
For the data stated above, 1 cM is equal to 1 Mb (Hastbacka et al., 1992).
2.4. Statistical analysis
Statistical methods and plots applicable from Prism (version 9.3.0, GraphPad Software Inc.) were used, which enabled us to organize heatmap plots and bar graphs of the quantitative data obtained with the genome data viewer and GC content calculator. The non-parametric test was applied with the understanding that this approach allowed a more conservative conclusion than the parametric tests in which random assignment of treatments to samples and Gaussian distribution were expected. As a result, a Mann-Whitney test and Kruskal-Wallis test were adopted for two group and three group comparisons, respectively. The difference between the data sets was considered significant at P<0.05; P values are noted in the figures and legends as *P<0.05, **P<0.01, and ***P<0.005.
3. Results
X-linked hydrocephalus genes vs. sixteen fPD genes in rodents and humans
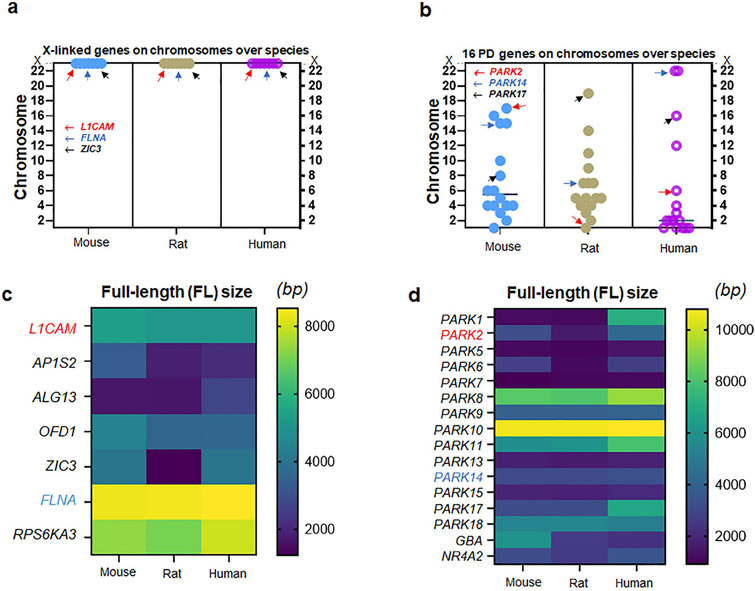
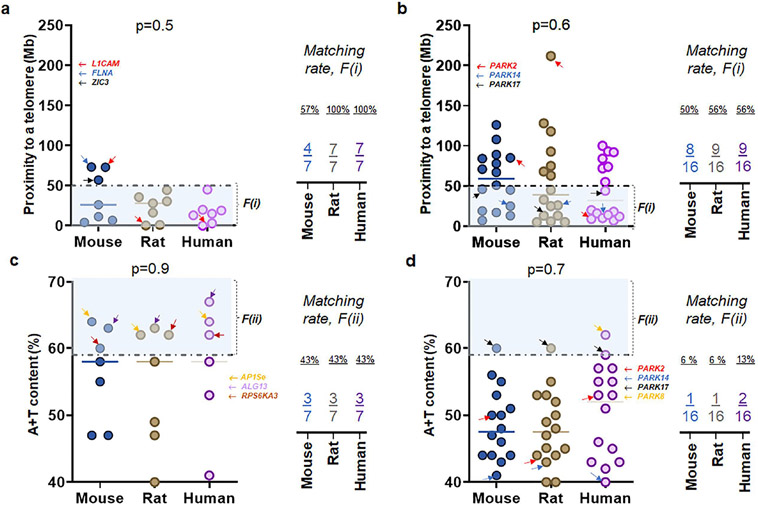
To compare X-linked hydrocephalus genes with genes associated with fPD, we investigated sex chromosome genes previously known to cause CH (McKnight et al., 2021) by two factors, F(i) and F(ii) (McKnight et al., 2021; Nusbaum et al., 2006). Of 108 genes associated with CH (McKnight et al., 2021), we found that seven genes on human sex (X) chromosome pertain to X-linked hydrocephalus (Emmert et al., 2019; Ferese et al., 2016; Guo et al., 2020; Kong et al., 2020; Marx et al., 2012; Ochando et al., 2016; Serikawa et al., 2014; Tuysuz et al., 2022; Zhou et al., 2022). When these seven X-linked genes were checked at mouse and rat chromosomes, there is no exception that X-linked genes remained in the same sex chromosomes of rodents (Fig. 1a). When sixteen human genes previously surveyed in fPD were identified in rodent chromosomes, however, their chromosomal locations were not necessarily identical in the autosomes of mouse and rat genome (Fig. 1b). The full-length (FL) sizes of nucleotide (gene transcripts) for X-linked genes (Fig. 1c) as well as genes associated with fPD (Fig. 1d) were not always identical over three species of mouse, rat, and human chromosomes (Fig. 1c-d). When the first factor, F(i), were taken into consideration, we found that seven X-linked genes associated with CH demonstrated a higher matching rate at 57 % (mouse), 100 % (rat), and 100 % (human) than sixteen fPD genes at 50 % (mouse), 56 % (rat), and 56 % (human) over three species (Fig. 2a-b). As the second factor, F(ii), were assessed, seven X-linked genes associated with CH displayed an even higher matching rate at 43 % (all three species) than sixteen fPD genes at 6 % (mouse and rat) and 13 % (human) over three species (Fig. 2c-d) (Fig. S1-S2; Table S1).
Figure 1. Genes associated with X-linked CH vs. those with fPD.
(a) Locations of seven genes associated with X-linked CH over three species of mice, rats, and humans. Note that all seven genes including L1CAM, FLNA, and ZIC3 are located on the X chromosomes of all three species (b) Locations of sixteen genes associated with familial PD (fPD) over three species of mice, rats, and humans. Note that all sixteen genes including PARK2, PARK14, and PARK17 are located somewhat differently on the autosomes over three species of mice, rats, and humans (c) The full-length (FL) size of seven genes (transcripts) associated with X-linked CH over three species. Note that L1CAM and FLNA show consistent molecular sizes in three species while other genes differ in humans as compared to rodents (d) The FL size of sixteen gene transcripts associated with fPD over three species. Note that PARK2 shows a different length depending on the species, while PARK14 consistent with PARK5, PARK7, PARK9, PARK10, PARK13, and PARK15 demonstrates a consistent FL size over three species.
Figure 2. Uniqueness of X-linked hydrocephalus genes by two factors of i) proximity to telomeres and ii) high A+T content.
(a) Proximity to telomeres of seven X-linked genes associated with CH over three species. Note that seven human genes have evolved in a way meeting proximity to telomeres or the first factor, F(i), associated with high mutation rate as 7 of 7 human genes have proximity to telomeres at less than 50 Mb as compared to those of mice. Interestingly, seven genes on rat X chromosomes show a similar feature to humans unlike mouse genes. (b) Proximity to telomeres of sixteen fPD genes over three species. (c) A + T content of seven X-linked genes associated with CH over three species show a consistent matching rate between each gene and the second factor, F(ii), associated with high mutation rates in human chromosome. Arrows in yellow, purple, and brown indicate APISe, ALG13, and RPS6KA3, respectively. (c) A + T content of sixteen genes associated with fPD over three species show a different matching rate between each gene and the second factor, F(ii) due primarily to PARK8 in humans. Arrows in red, blue, black, and yellow indicate PARK2, PARK14, PARK17, and PARK8, respectively.
X-linked hydrocephalus genes vs. genes associated with CH on the autosome
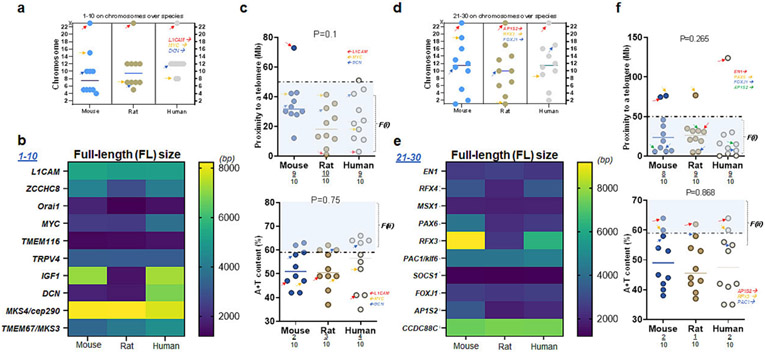
We next investigated seven X-linked hydrocephalus genes along with the remainder of >90 genes associated with CH located in the autosomes of three species. In the first set (#1-10), chromosomal locations of each gene were not always consistent across three species except those on the X chromosomes. For example, TBX1 gene was located on chr 16 in mice but it is no longer on chr 16 but elsewhere in the autosome of rats and humans. L1CAM in rodent and human genome was found on the X chromosome, while nine other genes were elsewhere in the autosome (Fig. 3a). The full-length (FL) size of transcript in this set (#1-10) was different except for three genes: L1CAM, TMEM116, and TRPV4 over mouse, rat, and human genome (Fig. 3b). The relative mutability of L1CAM was low as compared to 9 other genes in mice as it is >50 Mb distant from its telomere, while L1CAM satisfied F(i) in rats and humans (Fig. 3c top). On the other hand, less than a half of ten genes satisfied high A+T content or F(ii) in three species in which L1CAM failed to meet F(ii) in three species (Fig. 3c bottom). Overall, the relative mutability of L1CAM is consistent in rats, compared to humans, but not necessarily in mice (Fig. 3c).
Figure 3. L1CAM and AP1S2: their proximity to telomeres and A+T content.
(a) Locations of ten genes associated with CH over three species of mice, rats, and humans. Note that L1CAM is located on the X chromosomes of all three species (b) The full-length (FL) size of ten genes (transcripts) over three species. Note that L1CAM, TMEM116, and TRPV4 show consistent molecular sizes in three species while other genes differ in humans as compared to rodents (c) Proximity to telomeres of ten genes over three species. Note that ten human genes investigated in this list (1-10) have evolved in a way meeting proximity to telomeres or the first factor, F(i), associated with high mutation rate as 9 of 10 human genes have proximity to telomeres at less than 50 Mb as compared to those of mice and rats (upper plot). A + T content of ten genes over three species demonstrate 2-4 of 10 genes meeting the second factor, F(ii), associated with high mutation rate (lower plot). Arrows in red, orange, and blue indicate L1CAM, MYC, and DCN, respectively (scatter plots in c). (d) Locations of the next ten genes associated with CH over three species of mice, rats, and humans. Note also that AP1S2 is located on the X chromosome of all three species (e) The FL size of ten genes over three species. Note that EN1, MSX1, PAC1, SOCS1, FOXJ1, and CCDC88CL1CAM show consistent molecular sizes in three species while four other genes differ in humans as compared to rodents (f) Proximity to telomeres of another ten genes over three species. Note that the majority of ten human genes investigated in this list (21-30) have evolved in a way meeting proximity to telomeres or the first factor, F(i), associated with high mutation rate as 8-9 of 10 human genes have proximity to telomeres at <50 Mb as compared to those of mice and rats (upper plot). A + T content of ten genes over three species demonstrate 1-2 of 10 genes meeting F(ii) associated with high mutation rate (lower plot). Arrows in red, orange, blue, and green indicate EN1, PAX6, FOXJ1, and AP1S2, respectively (Upper plot in c); arrows in red, orange, and blue indicate AP1S2, RFX3, and PAC1, respectively (Lower plot in c).
In another set (#21-30) among the remainder of the list (Fig. S3-S8; Table S2-S4), chromosomal locations of each gene were somewhat similar across the species and that AP1S2 gene was on sex chromosome of all three species. Consistent with L1CAM, AP1S2 in rodent and human genome was found on the X chromosome, while nine other genes were distributed on the autosomes (Fig. 3d). By color-coding, it became evident that the nucleotide size of genes in this set (#21-30) differed except for four genes: EN1, MSX1, SOCS1, and CCDC88C over three species (Fig. 3e). The relative mutability of AP1S2 as measured by proximity to telomeres was consistent across mouse, rat, and human genome (Fig. 3f top). Further, AP1S2 met F(ii) in all three species as well (Fig. 3f bottom). By two factors, the relative mutability of AP1S2 on the X chromosome is consistent in mouse, rat, and human genome (Fig. 3f).
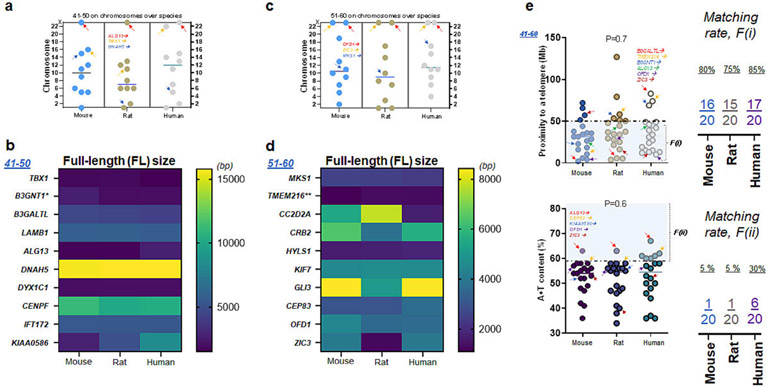
Next, chromosomal locations of the ten genes were comparable across the species (Table S5-S7). Here, ALG13 gene was detected on sex chromosome. Consistent with L1CAM and AP1S2, ALG13 in rodent and human genome was located on the X chromosome, while nine other genes were found on other chromosomes (Fig. 4a). The FL size of transcript in this set (#41-50) was not always consistent as shown in KIAA0586 over mouse, rat, and human genome (Fig. 3b). In the subsequent list of genes (#51-60), OFD1 and ZIC3 were located on the X chromosome of all three species, while nine other genes were on the autosomes (Fig. 4c). The nucleotide size of transcript in this set (#51-60) was different except for four genes: MKS1, TMEM216, HYLS1, and KIF7 over mouse, rat, and human genome (Fig. 4d). Combining these two sets (#41-60), the relative mutability of OFD1 was consistent across all three species satisfying F(i), while ZIC3 met F(i) in rats and humans (Fig. 4e top). On the other hand, OFD1 and ZIC3 on the X chromosome did not satisfy F(ii) in all three species (Fig. 4e bottom). By two factors, the relative mutability of OFD1 and ZIC3 is either moderately (ZIC3) or fully (OFD1) consistent in mouse, rat, and human genome (Fig. 3c).
Figure 4. ALG13, OFD1, and ZIC1: their proximity to telomeres and A+T content.
(a) Chromosome number of ten (#41-50) genes associated with CH over three species of mice, rats, and humans. Note that ALG13 is located on the X chromosome of all three species (b) The FL size of ten genes (#41-50) over three species. Note that TBX1, LAMB1, DNAH5, DYC1C1, and IFT172 show consistent molecular sizes in three species while other genes differ in humans as compared to rodents (c) Locations of the fourth tier (#51-60) genes associated with CH over three species of mice, rats, and humans. Note that OFD1 and ZIC1 are located on the X chromosome of all three species (d) The FL size of ten genes (transcripts) over three species. Note that MKS1, TMTM216, HYLS1, and KIF7 show consistent molecular sizes in three species while other genes differ in humans as compared to rodents (e) Proximity to telomeres of the next twenty genes over three species. Note that more than a half of twenty human genes investigated in this list (41-60) have evolved in a way meeting proximity to telomeres or F(i) associated with high mutation rate as 17 of 20 human genes have proximity to telomeres at <50 Mb as compared to those of mice and rats (upper plot). A + T content of twenty genes (#41-60) over three species demonstrate 1 (rodents) or 6 (human) of 10 genes meeting the second factor, F(ii), associated with high mutation rate (lower plot). Arrows in red, orange, and blue indicate L1CAM, MYC, and DCN, respectively (scatter plots in c).
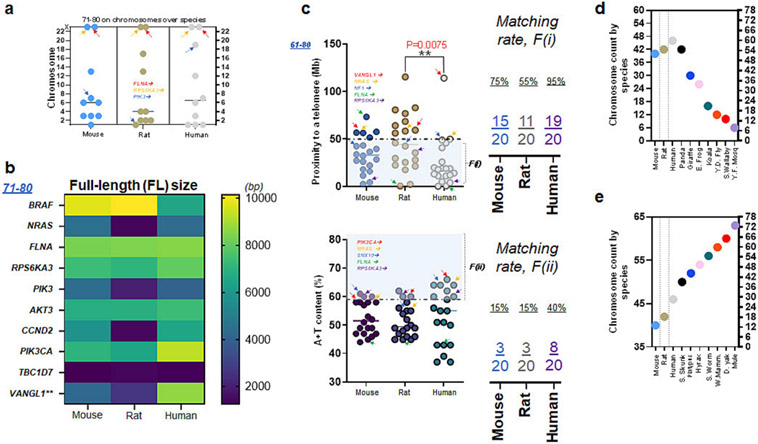
In the following set (#71-80) among the remainder of the list (Fig. S9-S10; Table S8-S11), chromosomal locations of ten genes were somewhat inconsistent across the species. We found that FLNA and RPS6KA3 are sex chromosome genes. Consistent with L1CAM, FLNA and RPS6KA3 were on the X chromosome, while eight other genes were on the autosome (Fig. 5a). The FL size of transcript in this set (#71-80) was inconsistent, while FLNA showed a consistent nucleotide size (Fig. 5b). Altogether (#61-80), the relative mutability of RPS6KA3 was consistent across all three species meeting F(i), while FLNA satisfied F(i) in rats and humans (Fig. 5c top). In contrast, FLNA failed to meet F(ii) but RPS6KA3 satisfied F(ii) in all three species (Fig. 5c bottom). Overall, the relative mutability of RPS6KA3 and FLNA is either thoroughly (RPS6KA3) or moderately (FLNA) consistent in all three species (Fig. 5c).
Figure 5. FLNA and RPS6KA3: their proximity to telomeres and A+T content.
(a) Chromosome number of ten (#71-80) genes associated with CH in mice, rats, and humans. Note that FLNA and RPS6KA3 are located on the X chromosome of all three species (b) The FL size of ten genes (#71-80) in mice, rats, and humans. Note that FLNA and TBC1D7 show consistent molecular sizes in three species while other genes differ in humans as compared to rodents (c) Proximity to telomeres in twenty genes (#61-80) including two X chromosome genes over three species. Note that more than a half of twenty human genes in this list ($61-80) have evolved in a way meeting proximity to telomeres or F(i) as 18 of 20 human genes have proximity to telomeres at <50 Mb as compared to those of mice and rats. Arrows in red, orange, blue, green, and purple indicate VANGL1, NRAS, NF1, FLNA, and RPS6KA3, respectively (upper plot). A + T content of twenty genes (#61-80) over three species demonstrate a limited number of 20 genes meeting the second factor, F(ii). Arrows in red, orange, blue, green, and purple indicate PIK3CA, NRAS, SNX10, FLNA, and RPS6KA3, respectively (lower plot). (d) Chromosome counts of frequently used laboratory animals (mice & rats) and humans as compared to other species such as Panda to yellow fever mosquito (Y.F. mosq). Including Panda (42), other species shown here have fewer number of chromosomes than humans at 46 chromosomes (23 pairs). (e) Chromosome counts of frequently used laboratory animals and humans as compared to other species such as Skunk to mule. Except mouse and rat, other species such as mule (72) shown here have more chromosomes than humans.
Discussion
Given the identities of the human genes associated with CH (McKnight et al., 2021), one would wonder if these X-linked genes are similarly located on the sex chromosomes of rodents such as mice and rats, which are preferred laboratory animals (White et al., 2022). If so, would they maintain the same genomic characteristics, which may be pivotal in developing phenotypes of CH without exceptions across species? Here, we have demonstrated that genes associated with CH on the X chromosome harbor a consistent mutability across three species.
The result shown in this study provides a novel way of delineating genes associated with CH per mouse, rat, and human genome. Recently, it has been reported that there is telomere transfer in T cells (Bird, 2022; Lanna et al., 2022) and that the intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory (Lanna et al., 2022). Thus, if genetic characteristics of 1) chromosomal location/number and the FL size, 2) proximity to telomeres, and 3) A+T content are consistent across 3 species as shown in seven X chromosome genes, consequences of telomere change or transfer (Bird, 2022; Lanna et al., 2022) over the lifespan of species are much more feasibly predicted than genes with different chromosomal characteristics such as those located on the autosomes.
The present study also echoes the idea that it is worth focusing on genes located on the X chromosome as a drug target (Raines et al., 2022). If not, would it be more helpful to target a downstream effector gene signaling associated with the causative genes for X-linked CH? CH in males vs. CH in females may have a different approach when it comes to X-linked CH. Our data further generate new hypothesis: If the seven genes on the X chromosome can be the biomarkers of CH or acquired hydrocephalus, would it be consistent across species as compared to 101 other causative genes associated with CH (McKnight et al. 2021)? If consistent, what additional studies can help better understand the pathogenic mechanisms of hydrocephalus (Shim and Madsen, 2018; Shim et al., 2019; White et al., 2022)?
One of the unique features observed in the sex chromosome is a different mutation rate. Most mutational changes in DNA are found to occur through replication errors during meiosis (Maekawa et al., 1998) and/or mitosis (Murata et al., 2000; Peng et al., 1996). As such, the mutation rate per generation is anticipated to increase as cells divide in egg and sperm cells, provided that only mutations in the sex cells are transmitted to the next generation (Arcila et al., 2020; Fu, 2001; Hanlon et al., 2019; Honma et al., 2014; Kang and Marjoram, 2011; Meyer et al., 2017; Zhang et al., 2022). In gonochoric species, males and females have different ways of making a mature haploid germ cell, which leads to a different cell division. For instance, in mammals, generation of sperms requires more cell divisions than that of oocytes, so that the mutation rate in the male gametes is likely to be higher than the female (Hurst and Ellegren, 1998). This effect is determined in part by the average ages of males and females at reproduction, as the mutation rate per sex is the total counts of mutations arising from individuals at all reproductively active ages (Segurel et al., 2014).
Hydrocephalus of primarily adults or NPH is a condition with close to normal intracranial pressure (ICP), which is rarely found in neonatal and pediatric patients (Barnett et al., 1987; Engel et al., 1979; Hill and Volpe, 1981; Stein et al., 1972; Torkelson et al., 1985). Most cases of NPH are found in the elderly patients (Ahn et al., 2020; Ates Bulut et al., 2019; Ates Bulut et al., 2021; Borzage et al., 2021; Buyukgok et al., 2021; Diniz et al., 2012; He et al., 2021; He et al., 2020; Ishikawa et al., 1994; Isik et al., 2019; Isik and Soysal, 2015; Kang et al., 2016; Kang et al., 2018; Kaya et al., 2022; Khan et al., 2017; Kita et al., 2019; Koo et al., 2021; Marumoto et al., 2012; Mechelli et al., 2022; Oike et al., 2021; Onder, 2020; Onder and Arslan, 2020), although not much is known about genetic contributions to the pathophysiology of NPH. A few previous reports have raised the possibility that genetic mutations alone on the autosome and/or interactions with an environmental risk contribute to the development of NPH (Cusimano et al., 2011; Morimoto et al., 2019; Sato et al., 2016). Furthermore, there are prior reports demonstrating the cases with X-linked NPH (Katsuragi et al., 2000; Koch et al., 2009). Hence, seven human genes on the X chromosome assessed in this study may contribute to the development of NPH in a multifactorial way (McAllister, 2012).
Unlike seven X-linked genes associated with CH (100%), sixteen fPD genes shown in this study demonstrated substantially lower matches by proximity to telomeres (56%) and/or high A+T content (13%) in humans. This poor match was attributable to the polygenic nature involving genetic and epigenetic contribution to the disease (McKnight et al., 2021). As high A+T content is associated with genetic contribution as in high mutation rates, the remainder nucleotide of cytosine and guanine (CG), may have a different role. Regions of DNA called ‘CG’ or CpG sites contain a cytosine nucleotide followed by a guanine nucleotide along the 5’ to 3’ direction (Jabbari and Bernardi, 2004). CpG sites occur often in genomic regions defined as CpG islands. In addition, cytosines in CpG dinucleotides can be methylated to form 5-methylcytosines. In mammalians, roughly 80% of CpG cytosines are methylated (Guo et al., 2014; Jabbari and Bernardi, 2004; Lee et al., 2020; Nishioka et al., 2016). DNA methylation GrimAge is shown to predict lifespan and healthspan (Lu et al., 2019). Taken together, the poor match of two factors with Parkinson’s disease might be better explained if the other side of a coin or ‘CpG’ is inspected. Recent studies addressing DNA methylation on the CpG sites suggest that epigenetic contribution including methylation of cytosines or CpG sites with increasing age should be taken into consideration.
Conclusion
We found that X-linked hydrocephalus genes screened by two factors, namely, i) proximity to telomeres and ii) high A+T content show the complete match at 100% in rat and human chromosome.
X-linked hydrocephalus genes are better matched by two factors than PARK family proteins and two other genes known to be associated with fPD. The data presented in this study suggest that at least all seven X chromosome genes associated with CH (Fig. 1-5; Table S1-S11), if druggable as a pharmacological target, harbor a consistent mutability in mouse, rat, and/or human chromosome.
Unlike mammalian genes located in the autosomes, genetic loci vulnerable to deleterious mutations leading to CH and/or other type(s) of pleiotropic conditions located in the X chromosomes are relatively more consistent in terms of their nucleotide size, proximity to telomeres, and A + T content as assessed in mouse, rat, and/or human chromosome.
The poor match of two factors with Parkinson’s disease suggests that high A+T content associated with high mutation rates is no longer a key determinant in this type of disease. Instead, low A+T or ‘CG’, particularly, methylations in this region (CG or CpG) might be critical, providing a clue to how genetic and epigenetic instruction might contribute to age-related diseases.
Supplementary Material
Highlight.
High mutation rate is associated with (i) proximity to telomeres and (ii) A+T content.
X-linked hydrocephalus genes met two factors with a relatively high A+T content.
Genes associated with Parkinson’s disease (PD) poorly met two factors with low A+T.
Low A+T content suggests that PD genes are vulnerable to CpG sites methylation.
Acknowledgements
We thank Raegan Halley, Tessa Gardner, Hannah Rollins, Nicholas Mamaligas, and Xavier Lewis for valuable comments on the manuscript. We wish to acknowledge the Marshall University Genomics Core for providing access to shared instrumentation. The Genomics Core is supported by funding from the WV-INBRE grant (NIH P20GM103434), the COBRE ACCORD grant (P20GM121299) and the West Virginia Clinical and Translational Science Institute (WV-CTSI) grant (2U54GM104942).
Funding:
This work was supported by the state of West Virginia startup fund to faculty members at Marshall University (to JWS). We thank for the Faculty Research and Travel award by the West Virginia Space Grant Consortium (WVSGC) sponsored by NASA. This research was also made possible by the NASA Established Program to Stimulate Competitive Research, Grant # 80NSSC22M0027 (to JWS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests.
Supplementary Material: The Supplementary Material for this article can be found online at:
References:
- Ahn BJ, Lee M, Ju H, Im K, Kwon KY, 2020. An elderly man with normal pressure hydrocephalus presenting with asymmetric parkinsonism in the upper and lower extremities. Geriatr Gerontol Int 20, 791–792. [DOI] [PubMed] [Google Scholar]
- Arcila ME, Yang SR, Momeni A, Mata DA, Salazar P, Chan R, Elezovic D, Benayed R, Zehir A, Buonocore DJ, Rekhtman N, Lin O, Ladanyi M, Nafa K, 2020. Ultrarapid EGFR Mutation Screening Followed by Comprehensive Next-Generation Sequencing: A Feasible, Informative Approach for Lung Carcinoma Cytology Specimens With a High Success Rate. JTO Clin Res Rep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates Bulut E, Karabay N, Soysal P, Isik AT, 2019. An elderly patient with Alzheimer's disease, normal pressure hydrocephalus and traumatic brain injury: presented with behavioral symptoms similar to behavioral variant frontotemporal dementia. Int J Neurosci 129, 623–626. [DOI] [PubMed] [Google Scholar]
- Ates Bulut E, Soysal P, Isik AT, 2021. Reply to comment on 'An elderly patient with Alzheimer's disease, normal pressure hydrocephalus and traumatic brain injury: presented with behavioral symptoms similar to behavioral variant frontotemporal dementia? Int J Neurosci 131, 317–318. [DOI] [PubMed] [Google Scholar]
- Baertsch HC, Bhatt NK, Giliberto JP, Dixon C, Merati AL, Sauder C, 2022. Quantification of Vocal Fold Atrophy in Age-Related and Parkinson's Disease-Related Vocal Atrophy. Laryngoscope. [DOI] [PubMed] [Google Scholar]
- Ballarati L, Cereda A, Caselli R, Maitz S, Russo S, Selicorni A, Larizza L, Giardino D, 2012. Deletion of the AP1S2 gene in a child with psychomotor delay and hypotonia. Eur J Med Genet 55, 124–127. [DOI] [PubMed] [Google Scholar]
- Barnett GH, Hahn JF, Palmer J, 1987. Normal pressure hydrocephalus in children and young adults. Neurosurgery 20, 904–907. [DOI] [PubMed] [Google Scholar]
- Behbahanipour M, Peymani M, Salari M, Hashemi MS, Nasr-Esfahani MH, Ghaedi K, 2019. Expression Profiling of Blood microRNAs 885, 361, and 17 in the Patients with the Parkinson's disease: Integrating Interaction Data to Uncover the Possible Triggering Age-Related Mechanisms. Sci Rep 9, 13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird L, 2022. Gift of life: APC to T cell telomere transfer. Nat Rev Immunol 22, 653. [DOI] [PubMed] [Google Scholar]
- Borck G, Molla-Herman A, Boddaert N, Encha-Razavi F, Philippe A, Robel L, Desguerre I, Brunelle F, Benmerah A, Munnich A, Colleaux L, 2008. Clinical, cellular, and neuropathological consequences of AP1S2 mutations: further delineation of a recognizable X-linked mental retardation syndrome. Hum Mutat 29, 966–974. [DOI] [PubMed] [Google Scholar]
- Borzage M, Saunders A, Hughes J, McComb JG, Bluml S, King KS, 2021. The First Examination of Diagnostic Performance of Automated Measurement of the Callosal Angle in 1856 Elderly Patients and Volunteers Indicates That 12.4% of Exams Met the Criteria for Possible Normal Pressure Hydrocephalus. AJNR Am J Neuroradiol 42, 1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukgok D, Ozdemir O, Unal TC, Barlas O, 2021. When to Assess: Cognitive Impact of Ventriculoperitoneal Shunt Operation in Elderly Adults with Normal Pressure Hydrocephalus. World Neurosurg 154, e302–e312. [DOI] [PubMed] [Google Scholar]
- Cacciagli P, Desvignes JP, Girard N, Delepine M, Zelenika D, Lathrop M, Levy N, Ledbetter DH, Dobyns WB, Villard L, 2014. AP1S2 is mutated in X-linked Dandy-Walker malformation with intellectual disability, basal ganglia disease and seizures (Pettigrew syndrome). Eur J Hum Genet 22, 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio G, Torella A, Mastrangelo M, Carducci C, Nigro V, Tudp, Brunetti-Pierri N, Leuzzi V,2019. AP1S2-truncating variant in a patient with severe neurodevelopmental disorder and cerebral folate deficiency. Acta Paediatr 108, 564–565. [DOI] [PubMed] [Google Scholar]
- Chang BH, Shimmin LC, Shyue SK, Hewett-Emmett D, Li WH, 1994. Weak male-driven molecular evolution in rodents. Proc Natl Acad Sci U S A 91, 827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HM, Zhang MM, Wang YL, 2020. [Association of age-related white matter hyperintensity with brain atrophy and cognitive impairment in patients with Parkinson's disease]. Zhonghua Yi Xue Za Zhi 100, 3397–3401. [DOI] [PubMed] [Google Scholar]
- Chen PJ, Wan L, Lai JN, Chen CS, Chen JJ, Yen WM, Chiu LT, Hu KC, Tien PT, Lin HJ, 2021. Increased risk of Parkinson's disease among patients with age-related macular degeneration. BMC Ophthalmol 21, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Jahng WJ, Park SM, Jee D, 2020a. Association of Age-Related Macular Degeneration on Alzheimer or Parkinson Disease: A Retrospective Cohort Study. Am J Ophthalmol 210, 41–47. [DOI] [PubMed] [Google Scholar]
- Choi S, Park SM, Jahng WJ, Jee D, 2020b. Reply to Comment on: Association of Age-Related Macular Degeneration on Alzheimer or Parkinson Disease: A Retrospective Cohort Study. Am J Ophthalmol 213, 320–321. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Wu G, Lu ZH, Kumar R, Ledeen R, 2023. Age-Related Decline in Gangliosides GM1 and GD1a in Non-CNS Tissues of Normal Mice: Implications for Peripheral Symptoms of Parkinson's Disease. Biomedicines 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou P, Tanteles G, Nikolaou N, Katsimbris I, Stefaniotou M, 2019. Intrafamilial Phenotype Variability in Two Male Siblings, With X-linked Juvenile Retinoschisis and Dorzolamide Treatment Effect in the Natural History of the Disease. Med Hypothesis Discov Innov Ophthalmol 8, 11–15. [PMC free article] [PubMed] [Google Scholar]
- Cusimano MD, Rewilak D, Stuss DT, Barrera-Martinez JC, Salehi F, Freedman M, 2011. Normal-pressure hydrocephalus: is there a genetic predisposition? Can J Neurol Sci 38, 274–281. [DOI] [PubMed] [Google Scholar]
- Dennis PM, Raghanti MA, Meindl RS, Less E, Henthorn E, Devlin W, Murray S, Meehan T, Kutinsky I, Murphy H, 2019. Cardiac disease is linked to adiposity in male gorillas (Gorilla gorilla gorilla). PLoS One 14, e0218763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derya D, Kang J, Kwon DY, Wallraven C, 2019. Facial Expression Processing Is Not Affected by Parkinson's Disease, but by Age-Related Factors. Front Psychol 10, 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan MC, Rattani A, Mekary R, Glancz LJ, Yunusa I, Baticulon RE, Fieggen G, Wellons JC, Park KB, Warf BC, 2018. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg, 1–15. [DOI] [PubMed] [Google Scholar]
- Diniz J, Diniz A, Ingham SJ, Carvalho AC, Frisoli A Jr., 2012. Normal pressure hydrocephalus associated with delirium in an elderly man who had undergone aortic valve replacement surgery. J Am Geriatr Soc 60, 2182–2183. [DOI] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Legault EM, Nilsson F, Pircs K, Bouquety J, Petit F, Shrigley S, Birtele M, Pereira M, Storm P, Sharma Y, Bruzelius A, Vuono R, Kele M, Stoker TB, Ottosson DR, Falk A, Jakobsson J, Barker RA, Parmar M, 2022. Age-related pathological impairments in directly reprogrammed dopaminergic neurons derived from patients with idiopathic Parkinson's disease. Stem Cell Reports 17, 2203–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JH, 1961. The syndrome of sex-linked hydrocephalus. Arch Dis Child 36, 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JH, Norman RM, Roberts JM, 1961. Sex-linked hydrocephalus. Report of a family with 15 affected members. Arch Dis Child 36, 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekimova IV, Guzeev MA, Simonova VV, Pastukhov YF, 2020. [Age-related differences in sleep disturbances in rat models of preclinical Parkinson's disease]. Zh Nevrol Psikhiatr Im S S Korsakova 120, 26–33. [DOI] [PubMed] [Google Scholar]
- Emmert AS, Vuong SM, Shula C, Lindquist D, Yuan W, Hu YC, Mangano FT, Goto J, 2019. Characterization of a novel rat model of X-linked hydrocephalus by CRISPR-mediated mutation in L1cam. J Neurosurg 132, 945–958. [DOI] [PubMed] [Google Scholar]
- Engel M, Carmel PW, Chutorian AM, 1979. Increased intraventricular pressure without ventriculomegaly in children with shunts: "normal volume" hydrocephalus. Neurosurgery 5, 549–552. [DOI] [PubMed] [Google Scholar]
- Epi KC, Epilepsy Phenome/Genome, P., Allen AS, Berkovic SF, Cossette P, Delanty N, Dlugos D, Eichler EE, Epstein MP, Glauser T, Goldstein DB, Han Y, Heinzen EL, Hitomi Y, Howell KB, Johnson MR, Kuzniecky R, Lowenstein DH, Lu YF, Madou MR, Marson AG, Mefford HC, Esmaeeli Nieh S, O'Brien TJ, Ottman R, Petrovski S, Poduri A, Ruzzo EK, Scheffer IE, Sherr EH, Yuskaitis CJ, Abou-Khalil B, Alldredge BK, Bautista JF, Berkovic SF, Boro A, Cascino GD, Consalvo D, Crumrine P, Devinsky O, Dlugos D, Epstein MP, Fiol M, Fountain NB, French J, Friedman D, Geller EB, Glauser T, Glynn S, Haut SR, Hayward J, Helmers SL, Joshi S, Kanner A, Kirsch HE, Knowlton RC, Kossoff EH, Kuperman R, Kuzniecky R, Lowenstein DH, McGuire SM, Motika PV, Novotny EJ, Ottman R, Paolicchi JM, Parent JM, Park K, Poduri A, Scheffer IE, Shellhaas RA, Sherr EH, Shih JJ, Singh R, Sirven J, Smith MC, Sullivan J, Lin Thio L, Venkat A, Vining EP, Von Allmen GK, Weisenberg JL, Widdess-Walsh P, Winawer MR, 2013. De novo mutations in epileptic encephalopathies. Nature 501, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferese R, Zampatti S, Griguoli AM, Fornai F, Giardina E, Barrano G, Albano V, Campopiano R, Scala S, Novelli G, Gambardella S, 2016. A New Splicing Mutation in the L1CAM Gene Responsible for X-Linked Hydrocephalus (HSAS). J Mol Neurosci 59, 376–381. [DOI] [PubMed] [Google Scholar]
- Field M, Scheffer IE, Gill D, Wilson M, Christie L, Shaw M, Gardner A, Glubb G, Hobson L, Corbett M, Friend K, Willis-Owen S, Gecz J, 2012. Expanding the molecular basis and phenotypic spectrum of X-linked Joubert syndrome associated with OFD1 mutations. Eur J Hum Genet 20, 806–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco B, Ballabio A, 2006. X-inactivation and human disease: X-linked dominant male-lethal disorders. Curr Opin Genet Dev 16, 254–259. [DOI] [PubMed] [Google Scholar]
- Fransen E, Lemmon V, Van Camp G, Vits L, Coucke P, Willems PJ, 1995. CRASH syndrome: clinical spectrum of corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraparesis and hydrocephalus due to mutations in one single gene, L1. Eur J Hum Genet 3, 273–284. [DOI] [PubMed] [Google Scholar]
- Fu YX, 2001. Estimating mutation rate and generation time from longitudinal samples of DNA sequences. Mol Biol Evol 18, 620–626. [DOI] [PubMed] [Google Scholar]
- Fukae J, Fujioka S, Yanamoto S, Mori A, Nomi T, Hatano T, Fukuhara K, Ouma S, Hattori N, Tsuboi Y, 2017. Serum uric acid level is linked to the disease progression rate in male patients with multiple system atrophy. Clin Neurol Neurosurg 158, 15–19. [DOI] [PubMed] [Google Scholar]
- Gao S, Zhao X, Zhao G, Dai P, Kong X, 2022. Analysis of L1CAM gene mutation and imaging appearance in three Chinese families with L1 syndrome: Three case reports. Mol Genet Genomic Med 10, e2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HK, Kumar HC, Cheng CK, Ming CC, Nallusamy R, Yusoff NM, Mohamad SB, Ripen AM, Dhaliwal JS, Murad S, 2013. X-linked chronic granulomatous disease in a male child with an X-CGD carrier, Klinefelter brother. Asian Pac J Allergy Immunol 31, 167–172. [DOI] [PubMed] [Google Scholar]
- Gillman J, Gilbert C, Gillman T, 1948. A preliminary report on hydrocephalus, spina bifida and other congenital anomalies in the rat produced by trypan blue; the significance of these results in the interpretation of congenital malformations following maternal rubella. S Afr J Med Sci 13, 47–90. [PubMed] [Google Scholar]
- Grinberg I, Millen KJ, 2005. The ZIC gene family in development and disease. Clin Genet 67, 290–296. [DOI] [PubMed] [Google Scholar]
- Grzybowski A, Kanclerz P, 2020. Comment on: Association of Age-Related Macular Degeneration on Alzheimer or Parkinson Disease: A Retrospective Cohort Study. Am J Ophthalmol 213, 320. [DOI] [PubMed] [Google Scholar]
- Guo D, Shi Y, Jian W, Fu Y, Yang H, Guo M, Yong W, Chen G, Deng H, Qin Y, Liao W, Yao R,2020. A novel nonsense mutation in the L1CAM gene responsible for X-linked congenital hydrocephalus. J Gene Med 22, e3180. [DOI] [PubMed] [Google Scholar]
- Guo JU, Su Y, Shin JH, Shin J, Li H, Xie B, Zhong C, Hu S, Le T, Fan G, Zhu H, Chang Q, Gao Y, Ming GL, Song H, 2014. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat Neurosci 17, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon VCT, Otto SP, Aitken SN, 2019. Somatic mutations substantially increase the per-generation mutation rate in the conifer Picea sitchensis. Evol Lett 3, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastbacka J, de la Chapelle A, Kaitila I, Sistonen P, Weaver A, Lander E, 1992. Linkage disequilibrium mapping in isolated founder populations: diastrophic dysplasia in Finland. Nat Genet 2, 204–211. [DOI] [PubMed] [Google Scholar]
- He D, Tan SQ, Lan LF, Fan YH, 2021. [Cognitive and gait dysfunction in the elderly caused by age-related cerebral small vessel disease and idiopathic normal pressure hydrocephalus: a case series of three patients]. Zhonghua Yi Xue Za Zhi 101, 1093–1096. [DOI] [PubMed] [Google Scholar]
- He WJ, Zhou X, Long J, Xu QZ, Huang XJ, Jiang J, Xia J, Yang G, 2020. Idiopathic Normal Pressure Hydrocephalus and Elderly Acquired Hydrocephalus: Evaluation With Cerebrospinal Fluid Flow and Ventricular Volume Parameters. Front Aging Neurosci 12, 584842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Volpe JJ, 1981. Normal pressure hydrocephalus in the newborn. Pediatrics 68, 623–629. [PubMed] [Google Scholar]
- Hollenberg NK, 2000. Implications of species difference for clinical investigation: studies on the renin-angiotensin system. Hypertension 35, 150–154. [DOI] [PubMed] [Google Scholar]
- Honma H, Hirai M, Nakamura S, Hakimi H, Kawazu S, Palacpac NM, Hisaeda H, Matsuoka H, Kawai S, Endo H, Yasunaga T, Ohashi J, Mita T, Horii T, Furusawa M, Tanabe K, 2014. Generation of rodent malaria parasites with a high mutation rate by destructing proofreading activity of DNA polymerase delta. DNA Res 21, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard SL, Grenet D, Bellumori M, Knight CA, 2022. Measures of motor segmentation from rapid isometric force pulses are reliable and differentiate Parkinson's disease from age-related slowing. Exp Brain Res 240, 2205–2217. [DOI] [PubMed] [Google Scholar]
- Hu S, Wang L, Liu N, Kong X, 2019. [Analysis of L1CAM gene mutation in pedigrees with X-linked genetic hydrocephalus]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 36, 465–467. [DOI] [PubMed] [Google Scholar]
- Huo L, Teng Z, Wang H, Liu X, 2019. A novel splice site mutation in AP1S2 gene for X-linked mental retardation in a Chinese pedigree and literature review. Brain Behav 9, e01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst LD, Ellegren H, 1998. Sex biases in the mutation rate. Trends Genet 14, 446–452. [DOI] [PubMed] [Google Scholar]
- Isaacs AM, Riva-Cambrin J, Yavin D, Hockley A, Pringsheim TM, Jette N, Lethebe BC, Lowerison M, Dronyk J, Hamilton MG, 2018. Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS One 13, e0204926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Kikuchi H, Hirai O, 1994. [Idiopathic normal pressure hydrocephalus in the aged]. No Shinkei Geka 22, 309–315. [PubMed] [Google Scholar]
- Isik AT, Kaya D, Ates Bulut E, Dokuzlar O, Soysal P, 2019. The Outcomes Of Serial Cerebrospinal Fluid Removal In Elderly Patients With Idiopathic Normal Pressure Hydrocephalus. Clin Interv Aging 14, 2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik AT, Soysal P, 2015. Neuroleptic malignant syndrome in an elderly patient with normal pressure hydrocephalus overlapping corticobasal degeneration. Am J Alzheimers Dis Other Demen 30, 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi R, Takahashi H, Kanemura Y, Shofuda T, Yoshioka E, Narumi R, Matsubara S, 2022. Adducted thumb may not be mandatory for prenatal diagnosis of X-linked hydrocephalus in early second trimester. Taiwan J Obstet Gynecol 61, 353–355. [DOI] [PubMed] [Google Scholar]
- Jabbari K, Bernardi G, 2004. Cytosine methylation and CpG, TpG (CpA) and TpA frequencies. Gene 333, 143–149. [DOI] [PubMed] [Google Scholar]
- Jouet M, Moncla A, Paterson J, McKeown C, Fryer A, Carpenter N, Holmberg E, Wadelius C, Kenwrick S, 1995. New domains of neural cell-adhesion molecule L1 implicated in X-linked hydrocephalus and MASA syndrome. Am J Hum Genet 56, 1304–1314. [PMC free article] [PubMed] [Google Scholar]
- Jouet M, Rosenthal A, Armstrong G, MacFarlane J, Stevenson R, Paterson J, Metzenberg A, Ionasescu V, Temple K, Kenwrick S, 1994. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat Genet 7, 402–407. [DOI] [PubMed] [Google Scholar]
- Juarez-Flores DL, Ezquerra M, Gonzalez-Casacuberta I, Ormazabal A, Moren C, Tolosa E, Fucho R, Guitart-Mampel M, Casado M, Valldeoriola F, de la Torre-Lara J, Munoz E, Tobias E, Compta Y, Garcia-Garcia FJ, Garcia-Ruiz C, Fernandez-Checa JC, Marti MJ, Grau JM, Cardellach F, Artuch R, Fernandez-Santiago R, Garrabou G, 2020. Disrupted Mitochondrial and Metabolic Plasticity Underlie Comorbidity between Age-Related and Degenerative Disorders as Parkinson Disease and Type 2 Diabetes Mellitus. Antioxidants (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemura Y, Okamoto N, Sakamoto H, Shofuda T, Kamiguchi H, Yamasaki M, 2006. Molecular mechanisms and neuroimaging criteria for severe L1 syndrome with X-linked hydrocephalus. J Neurosurg 105, 403–412. [DOI] [PubMed] [Google Scholar]
- Kang CJ, Marjoram P, 2011. Inference of population mutation rate and detection of segregating sites from next-generation sequence data. Genetics 189, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Choi W, Yoon U, Lee JM, Lee HW, 2016. Abnormal White Matter Integrity in Elderly Patients with Idiopathic Normal-Pressure Hydrocephalus: A Tract-Based Spatial Statistics Study. Eur Neurol 75, 96–103. [DOI] [PubMed] [Google Scholar]
- Kang YS, Park EK, Kim JS, Kim DS, Thomale UW, Shim KW, 2018. Efficacy of endoscopic third ventriculostomy in old aged patients with normal pressure hydrocephalus. Neurol Neurochir Pol 52, 29–34. [DOI] [PubMed] [Google Scholar]
- Katsuragi S, Teraoka K, Ikegami K, Amano K, Yamashita K, Ishizuka K, Miyakawa T, 2000. Late onset X-linked hydrocephalus with normal cerebrospinal fluid pressure. Psychiatry Clin Neurosci 54, 487–492. [DOI] [PubMed] [Google Scholar]
- Kaya D, Erken N, Ontan MS, Altun ZS, Isik AT, 2022. The applause sign in elderly patients with idiopathic normal pressure hydrocephalus. Appl Neuropsychol Adult 29, 893–898. [DOI] [PubMed] [Google Scholar]
- Khan SH, Rather TA, Sinha S, 2017. Cerebral Spinal Fluid Cisternography in Normal Pressure Hydrocephalus of the Elderly. Indian J Nucl Med 32, 250–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita D, Park C, Hayashi Y, 2019. Aqueductal Developmental Venous Anomaly Presenting with Mimic Symptoms of Idiopathic Normal Pressure Hydrocephalus in an Elderly Patient: A Case Report. NMC Case Rep J 6, 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HJ, Nanev D, Becker K, 2009. [Acute behaviour disorder in a patient with X-linked hydrocephalus with normal pressure]. Wien Med Wochenschr 159, 62–64. [DOI] [PubMed] [Google Scholar]
- Kong W, Wang X, Zhao J, Kang M, Xi N, Li S, 2020. A new frameshift mutation in L1CAM producing X-linked hydrocephalus. Mol Genet Genomic Med 8, e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo AB, Elsamadicy AA, Lin IH, David WB, Reeves BC, Santarosa C, Cord B, Malhotra A, Kahle KT, Matouk CC, 2021. Patient Risk Factors Associated With 30- and 90-Day Readmission After Ventriculoperitoneal Shunt Placement for Idiopathic Normal Pressure Hydrocephalus in Elderly Patients: A Nationwide Readmission Study. World Neurosurg 152, e23–e31. [DOI] [PubMed] [Google Scholar]
- Kouli A, Williams-Gray CH, 2022. Age-Related Adaptive Immune Changes in Parkinson's Disease. J Parkinsons Dis 12, S93–S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousi M, Katsanis N, 2016. The Genetic Basis of Hydrocephalus. Annu Rev Neurosci 39, 409–435. [DOI] [PubMed] [Google Scholar]
- Langston JW, 2017. The MPTP Story. J Parkinsons Dis 7, S11–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanna A, Vaz B, D'Ambra C, Valvo S, Vuotto C, Chiurchiu V, Devine O, Sanchez M, Borsellino G, Akbar AN, De Bardi M, Gilroy DW, Dustin ML, Blumer B, Karin M, 2022. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat Cell Biol 24, 1461–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lason W, Jantas D, Leskiewicz M, Regulska M, Basta-Kaim A, 2023. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer's and Parkinson's Diseases: A Narrative Review. Cells 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Saito Y, Park SJ, Nakai K, 2020. Existence and possible roles of independent non-CpG methylation in the mammalian brain. DNA Res 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C, Dejager L, Pinheiro I, 2010. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 10, 594–604. [DOI] [PubMed] [Google Scholar]
- Limbrick DD Jr., Park TS, 2006. Occult hydrocephalus in children with cerebral palsy. Neurosurgery 58, E590; author reply E590. [DOI] [PubMed] [Google Scholar]
- Liu JY, Ma LZ, Wang J, Cui XJ, Sheng ZH, Fu Y, Li M, Ou YN, Yu JT, Tan L, Lian Y, 2023. Age-Related Association Between APOE varepsilon4 and Cognitive Progression in de novo Parkinson's Disease. J Alzheimers Dis 91, 1121–1132. [DOI] [PubMed] [Google Scholar]
- Liu M, Luo YJ, Gu HY, Wang YM, Liu MH, Li K, Li J, Zhuang S, Shen Y, Jin H, Chen J, Mao CJ, Liu CF, 2021. Sex and onset-age-related features of excessive daytime sleepiness and night-time sleep in patients with Parkinson's disease. BMC Neurol 21, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, Assimes TL, Ferrucci L, Horvath S, 2019. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 11, 303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan W, Ding Y, Ma S, Ruan H, Wang J, Lu F, 2019. Long noncoding RNA LINC00518 acts as a competing endogenous RNA to promote the metastasis of malignant melanoma via miR-204-5p/AP1S2 axis. Cell Death Dis 10, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas HB, McKnight I, Raines R, Hijazi A, Hart C, Lee C, Kim DG, Li W, Lee PHU, Shim JW,2021. Factors Associated with Mutations: Their Matching Rates to Cardiovascular and Neurological Diseases. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Sugano K, Kashiwabara H, Ushiama M, Fujita S, Ohkura H, Kakizoe T, 1998. Point mutations of ornithine decarboxylase gene are an infrequent event in colorectal cancer but a missense mutation was found in a replication error positive patient with hMSH2 germline mutation. Jpn J Clin Oncol 28, 383–387. [DOI] [PubMed] [Google Scholar]
- Marumoto K, Koyama T, Hosomi M, Kodama N, Miyake H, Domen K, 2012. Diffusion tensor imaging in elderly patients with idiopathic normal pressure hydrocephalus or Parkinson's disease: diagnosis of gait abnormalities. Fluids Barriers CNS 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx M, Diestel S, Bozon M, Keglowich L, Drouot N, Bouche E, Frebourg T, Minz M, Saugier-Veber P, Castellani V, Schafer MK, 2012. Pathomechanistic characterization of two exonic L1CAM variants located in trans in an obligate carrier of X-linked hydrocephalus. Neurogenetics 13, 49–59. [DOI] [PubMed] [Google Scholar]
- McAllister JP 2nd, 2012. Pathophysiology of congenital and neonatal hydrocephalus. Semin Fetal Neonatal Med 17, 285–294. [DOI] [PubMed] [Google Scholar]
- McKnight I, Hart C, Park IH, Shim JW, 2021. Genes causing congenital hydrocephalus: Their chromosomal characteristics of telomere proximity and DNA compositions. Exp Neurol 335, 113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Quattrone A, Nistico R, Crasa M, La Torre D, Vescio B, Quattrone A, 2022. Blink reflex recovery cycle distinguishes patients with idiopathic normal pressure hydrocephalus from elderly subjects. J Neurol 269, 1007–1012. [DOI] [PubMed] [Google Scholar]
- Meyer R, Soellner L, Begemann M, Dicks S, Fekete G, Rahner N, Zerres K, Elbracht M, Eggermann T, 2017. Targeted Next Generation Sequencing Approach in Patients Referred for Silver-Russell Syndrome Testing Increases the Mutation Detection Rate and Provides Decisive Information for Clinical Management. J Pediatr 187, 206–212 e201. [DOI] [PubMed] [Google Scholar]
- Miyata T, Hayashida H, Kuma K, Mitsuyasu K, Yasunaga T, 1987. Male-driven molecular evolution: a model and nucleotide sequence analysis. Cold Spring Harb Symp Quant Biol 52, 863–867. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Yoshida S, Kinoshita A, Satoh C, Mishima H, Yamaguchi N, Matsuda K, Sakaguchi M, Tanaka T, Komohara Y, Imamura A, Ozawa H, Nakashima M, Kurotaki N, Kishino T, Yoshiura KI, Ono S, 2019. Nonsense mutation in CFAP43 causes normal-pressure hydrocephalus with ciliary abnormalities. Neurology 92, e2364–e2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Iwao K, Miyoshi Y, Nagasawa Y, Ohta T, Shibata K, Oda K, Wada H, Tominaga S, Matsuda Y, Ohsawa M, Nakamura Y, Shimano T, 2000. Molecular and biological analysis of carcinoma of the small intestine: beta-catenin gene mutation by interstitial deletion involving exon 3 and replication error phenotype. Am J Gastroenterol 95, 1576–1580. [DOI] [PubMed] [Google Scholar]
- Nishioka K, Kishida T, Masui S, Mazda O, 2016. De novo CpG methylation on an artificial chromosome-like vector maintained for a long-term in mammalian cells. Biotechnol Lett 38, 731–740. [DOI] [PubMed] [Google Scholar]
- Nusbaum C, Mikkelsen TS, Zody MC, Asakawa S, Taudien S, Garber M, Kodira CD, Schueler MG, Shimizu A, Whittaker CA, Chang JL, Cuomo CA, Dewar K, FitzGerald MG, Yang X, Allen NR, Anderson S, Asakawa T, Blechschmidt K, Bloom T, Borowsky ML, Butler J, Cook A, Corum B, DeArellano K, DeCaprio D, Dooley KT, Dorris L 3rd, Engels R, Glockner G, Hafez N, Hagopian DS, Hall JL, Ishikawa SK, Jaffe DB, Kamat A, Kudoh J, Lehmann R, Lokitsang T, Macdonald P, Major JE, Matthews CD, Mauceli E, Menzel U, Mihalev AH, Minoshima S, Murayama Y, Naylor JW, Nicol R, Nguyen C, O'Leary SB, O'Neill K, Parker SC, Polley A, Raymond CK, Reichwald K, Rodriguez J, Sasaki T, Schilhabel M, Siddiqui R, Smith CL, Sneddon TP, Talamas JA, Tenzin P, Topham K, Venkataraman V, Wen G, Yamazaki S, Young SK, Zeng Q, Zimmer AR, Rosenthal A, Birren BW, Platzer M, Shimizu N, Lander ES, 2006. DNA sequence and analysis of human chromosome 8. Nature 439, 331–335. [DOI] [PubMed] [Google Scholar]
- Ochando I, Vidal V, Gascon J, Acien M, Urbano A, Rueda J, 2016. Prenatal diagnosis of X-linked hydrocephalus in a family with a novel mutation in L1CAM gene. J Obstet Gynaecol 36, 403–405. [DOI] [PubMed] [Google Scholar]
- Oike R, Inoue Y, Matsuzawa K, Sorimachi T, 2021. Screening for idiopathic normal pressure hydrocephalus in the elderly after falls. Clin Neurol Neurosurg 205, 106635. [DOI] [PubMed] [Google Scholar]
- Onder H, 2020. An elderly patient with Alzheimer's disease, normal pressure hydrocephalus and traumatic brain injury: presented with behavioural symptoms similar to behavioural variant frontotemporal dementia? Int J Neurosci 130, 562–563. [DOI] [PubMed] [Google Scholar]
- Onder H, Arslan G, 2020. The Utility of Serial Cerebrospinal Fluid Removal in Elderly Patients with Idiopathic Normal-Pressure Hydrocephalus? J Mov Disord 13, 166–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Chen G, Du M, Singh N, Isaacson PG, Pan L, 1996. Replication error phenotype and p53 gene mutation in lymphomas of mucosa-associated lymphoid tissue. Am J Pathol 148, 643–648. [PMC free article] [PubMed] [Google Scholar]
- Raines R, McKnight I, White H, Legg K, Lee C, Li W, Lee PHU, Shim JW, 2022. Drug-Targeted Genomes: Mutability of Ion Channels and GPCRs. Biomedicines 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani L, Sahu MR, Mondal AC, 2022. Age-related Mitochondrial Dysfunction in Parkinson's Disease: New Insights Into the Disease Pathology. Neuroscience 499, 152–169. [DOI] [PubMed] [Google Scholar]
- Rekate HL, 2009. A contemporary definition and classification of hydrocephalus. Semin Pediatr Neurol 16, 9–15. [DOI] [PubMed] [Google Scholar]
- Russo T, Riessland M, 2022. Age-Related Midbrain Inflammation and Senescence in Parkinson's Disease. Front Aging Neurosci 14, 917797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo MC, Blain M, McCulloch D, Glasgow HL, Sengupta DJ, Le T, Cookson BT, Pottinger PS, Liles WC, Graham SM, 2019. Back Pain in a 23-Year-Old Male With X-Linked Chronic Granulomatous Disease. Open Forum Infect Dis 6, ofz449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillour Y, Zanni G, Des Portes V, Heron D, Guibaud L, Iba-Zizen MT, Pedespan JL, Poirier K, Castelnau L, Julien C, Franconnet C, Bonthron D, Porteous ME, Chelly J, Bienvenu T, 2007. Mutations in the AP1S2 gene encoding the sigma 2 subunit of the adaptor protein 1 complex are associated with syndromic X-linked mental retardation with hydrocephalus and calcifications in basal ganglia. J Med Genet 44, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takahashi Y, Kimihira L, Iseki C, Kato H, Suzuki Y, Igari R, Sato H, Koyama S, Arawaka S, Kawanami T, Miyajima M, Samejima N, Sato S, Kameda M, Yamada S, Kita D, Kaijima M, Date I, Sonoda Y, Kayama T, Kuwana N, Arai H, Kato T, 2016. A Segmental Copy Number Loss of the SFMBT1 Gene Is a Genetic Risk for Shunt-Responsive, Idiopathic Normal Pressure Hydrocephalus (iNPH): A Case-Control Study. PLoS One 11, e0166615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segurel L, Wyman MJ, Przeworski M, 2014. Determinants of mutation rate variation in the human germline. Annu Rev Genomics Hum Genet 15, 47–70. [DOI] [PubMed] [Google Scholar]
- Serikawa T, Nishiyama K, Tohyama J, Tazawa R, Goto K, Kuriyama Y, Haino K, Kanemura Y, Yamasaki M, Nakata K, Takakuwa K, Enomoto T, 2014. Prenatal molecular diagnosis of X-linked hydrocephalus via a silent C924T mutation in the L1CAM gene. Congenit Anom (Kyoto) 54, 243–245. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Sebai MA, Patel N, Ewida N, Kurdi W, Altweijri I, Sogaty S, Almardawi E, Seidahmed MZ, Alnemri A, Madirevula S, Ibrahim N, Abdulwahab F, Hashem M, Al-Sheddi T, Alomar R, Alobeid E, Sallout B, AlBaqawi B, AlAali W, Ajaji N, Lesmana H, Hopkin RJ, Dupuis L, Mendoza-Londono R, Al Rukban H, Yoon G, Faqeih E, Alkuraya FS, 2017. The genetic landscape of familial congenital hydrocephalus. Ann Neurol 81, 890–897. [DOI] [PubMed] [Google Scholar]
- Sheen VL, Basel-Vanagaite L, Goodman JR, Scheffer IE, Bodell A, Ganesh VS, Ravenscroft R, Hill RS, Cherry TJ, Shugart YY, Barkovich J, Straussberg R, Walsh CA, 2004. Etiological heterogeneity of familial periventricular heterotopia and hydrocephalus. Brain Dev 26, 326–334. [DOI] [PubMed] [Google Scholar]
- Shim JW, Madsen JR, 2018. VEGF Signaling in Neurological Disorders. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JW, Territo PR, Simpson S, Watson JC, Jiang L, Riley AA, McCarthy B, Persohn S, Fulkerson D, Blazer-Yost BL, 2019. Hydrocephalus in a rat model of Meckel Gruber syndrome with a TMEM67 mutation. Sci Rep 9, 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo K, Morishima Y, Suwa Y, Fukao T, Kurita T, Satake A, Tsuchiya M, Ichinose Y, Hata T, Koh K, Nagasaka T, Takiyama Y, 2021. Age-related changes in blood pressure and heart rates of patients with Parkinson's disease. J Clin Hypertens (Greenwich) 23, 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BM, Fraser RA, Tenner MS, 1972. Normal pressure hydrocephalus: complication of posterior fossa surgery in children. Pediatrics 49, 50–58. [PubMed] [Google Scholar]
- Tamano H, Nishio R, Morioka H, Furuhata R, Komata Y, Takeda A, 2019. Paraquat as an Environmental Risk Factor in Parkinson's Disease Accelerates Age-Related Degeneration Via Rapid Influx of Extracellular Zn(2+) into Nigral Dopaminergic Neurons. Mol Neurobiol 56, 7789–7799. [DOI] [PubMed] [Google Scholar]
- Tarpey PS, Stevens C, Teague J, Edkins S, O'Meara S, Avis T, Barthorpe S, Buck G, Butler A, Cole J, Dicks E, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jones D, Menzies A, Mironenko T, Perry J, Raine K, Richardson D, Shepherd R, Small A, Tofts C, Varian J, West S, Widaa S, Yates A, Catford R, Butler J, Mallya U, Moon J, Luo Y, Dorkins H, Thompson D, Easton DF, Wooster R, Bobrow M, Carpenter N, Simensen RJ, Schwartz CE, Stevenson RE, Turner G, Partington M, Gecz J, Stratton MR, Futreal PA, Raymond FL, 2006. Mutations in the gene encoding the Sigma 2 subunit of the adaptor protein 1 complex, AP1S2, cause X-linked mental retardation. Am J Hum Genet 79, 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terekhanova NV, Seplyarskiy VB, Soldatov RA, Bazykin GA, 2017. Evolution of Local Mutation Rate and Its Determinants. Mol Biol Evol 34, 1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkelson RD, Leibrock LG, Gustavson JL, Sundell RR, 1985. Neurological and neuropsychological effects of cerebral spinal fluid shunting in children with assumed arrested ("normal pressure") hydrocephalus. J Neurol Neurosurg Psychiatry 48, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutain PL, Ferran A, Bousquet-Melou A, 2010. Species differences in pharmacokinetics and pharmacodynamics. Handb Exp Pharmacol, 19–48. [DOI] [PubMed] [Google Scholar]
- Tripolszki K, Sasaki E, Hotakainen R, Kassim AH, Pereira C, Rolfs A, Bauer P, Reardon W, Bertoli-Avella AM, 2021. An X-linked syndrome with severe neurodevelopmental delay, hydrocephalus, and early lethality caused by a missense variation in the OTUD5 gene. Clin Genet 99, 303–308. [DOI] [PubMed] [Google Scholar]
- Tuysuz B, Ercan-Sencicek AG, Ozer E, Goc N, Yalcinkaya C, Bilguvar K, 2022. Severe Phenotype in Patients with X-linked Hydrocephalus Caused by a Missense Mutation in L1CAM. Turk Arch Pediatr 57, 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicoso B, Charlesworth B, 2006. Evolution on the X chromosome: unusual patterns and processes. Nat Rev Genet 7, 645–653. [DOI] [PubMed] [Google Scholar]
- White H, Webb R, McKnight I, Legg K, Lee C, Lee PHU, Spicer OS, Shim JW, 2022. TRPV4 mRNA is elevated in the caudate nucleus with NPH but not in Alzheimer's disease. Front Genet 13, 936151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Nagel SJ, Golomb J, Jensen H, Dasher NA, Holubkov R, Edwards RJ, Luciano MG, Zwimpfer TJ, Katzen H, Moghekar A, Wisoff JH, McKhann GM, Hamilton MG, 2022. Safety and effectiveness of the assessment and treatment of idiopathic normal pressure hydrocephalus in the Adult Hydrocephalus Clinical Research Network. J Neurosurg, 1–13. [DOI] [PubMed] [Google Scholar]
- Wolfrum P, Fietz A, Schnichels S, Hurst J, 2022. The function of p53 and its role in Alzheimer's and Parkinson's disease compared to age-related macular degeneration. Front Neurosci 16, 1029473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Nonaka M, Suzumori N, Nakamura H, Fujita H, Namba A, Kamei Y, Yamada T, Pooh RK, Tanemura M, Sudo N, Nagasaka M, Yoshioka E, Shofuda T, Kanemura Y, 2011. Prenatal molecular diagnosis of a severe type of L1 syndrome (X-linked hydrocephalus). J Neurosurg Pediatr 8, 411–416. [DOI] [PubMed] [Google Scholar]
- Yang HW, Lee S, Yang D, Dai H, Zhang Y, Han L, Zhao S, Zhang S, Ma Y, Johnson MF, Rattray AK, Johnson TA, Wang G, Zheng S, Carroll RS, Park PJ, Johnson MD, 2021. Deletions in CWH43 cause idiopathic normal pressure hydrocephalus. EMBO Mol Med 13, e13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Hayano M, Griffin PT, Amorim JA, Bonkowski MS, Apostolides JK, Salfati EL, Blanchette M, Munding EM, Bhakta M, Chew YC, Guo W, Yang X, Maybury-Lewis S, Tian X, Ross JM, Coppotelli G, Meer MV, Rogers-Hammond R, Vera DL, Lu YR, Pippin JW, Creswell ML, Dou Z, Xu C, Mitchell SJ, Das A, O'Connell BL, Thakur S, Kane AE, Su Q, Mohri Y, Nishimura EK, Schaevitz L, Garg N, Balta AM, Rego MA, Gregory-Ksander M, Jakobs TC, Zhong L, Wakimoto H, El Andari J, Grimm D, Mostoslavsky R, Wagers AJ, Tsubota K, Bonasera SJ, Palmeira CM, Seidman JG, Seidman CE, Wolf NS, Kreiling JA, Sedivy JM, Murphy GF, Green RE, Garcia BA, Berger SL, Oberdoerffer P, Shankland SJ, Gladyshev VN, Ksander BR, Pfenning AR, Rajman LA, Sinclair DA, 2023. Loss of epigenetic information as a cause of mammalian aging. Cell 186, 305–326 e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH, 2019. Reasons for Failed Trials of Disease-Modifying Treatments for Alzheimer Disease and Their Contribution in Recent Research. Biomedicines 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineddine HA, Dono A, Kitagawa R, Savitz SI, Choi HA, Chang TR, Ballester LY, Esquenazi Y, 2020. Endoscopic Third Ventriculostomy for Hydrocephalus Secondary to Extraventricular Obstruction in Thalamic Hemorrhage: A Case Series. Oper Neurosurg (Hagerstown) 19, 384–392. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yang Q, Ai H, Huang L, 2022. Revisiting the evolutionary history of pigs via De Novo mutation rate estimation in a three-generation pedigree. Genomics Proteomics Bioinformatics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Siu CH, 1996. Differential effects of two hydrocephalus/MASA syndrome-related mutations on the homophilic binding and neuritogenic activities of the cell adhesion molecule L1. J Biol Chem 271, 6563–6566. [DOI] [PubMed] [Google Scholar]
- Zhou H, Yu Q, Li Y, Fu F, Li R, Chen G, Wang D, Lu Y, Yang X, Li D, Liao C, 2022. Case Report: Two Novel L1CAM Mutations in Two Unrelated Chinese Families With X-Linked Hydrocephalus. Front Genet 13, 810853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Wang M, Xu Y, Zhang J, Yang F, Yang Z, 2022. Identification of a 5 bp duplicate in the AP1S2 gene of an individual with X-linked intellectual disability. Neurogenetics 23, 179–185. [DOI] [PubMed] [Google Scholar]
- Zhu L, Chen Y, Chen M, Wang W, 2021. Mechanism of miR-204-5p in exosomes derived from bronchoalveolar lavage fluid on the progression of pulmonary fibrosis via AP1S2. Ann Transl Med 9, 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.