Abstract
Background:
Chimeric antigen receptor-engineered (CAR) T-cell therapy remains limited by significant toxicities such as cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS). The optimal management of severe and/or refractory CRS/ICANS remains ill-defined. Anakinra has emerged as a promising agent based on preclinical data, but its safety and efficacy in CAR T-cell patients remains unknown.
Objectives:
Our primary objective was to evaluate the safety of anakinra to treat refractory CRS and ICANS after CAR T-cell therapy. Our secondary objective was to evaluate the impact of key treatment, patient, and disease-related variables on time to CRS/ICANS resolution and treatment-related mortality (TRM).
Study design:
We retrospectively analyzed the outcomes of 43 patients with B-cell or plasma cell malignancies treated with anakinra for refractory CRS or ICANS at 9 institutions in the United States and Spain between 2019 and 2022. Cause-specific Cox regression was used to account for competing risks. Multivariable cause-specific Cox regression was used to estimate the effect of the anakinra dose on outcomes while minimizing treatment allocation bias by including age, CAR-T product, prelymphodepletion (pre-LD) ferritin and performance status.
Results:
Indications for anakinra treatment were as follows: grade ≥2 ICANS with worsening or lack of symptom improvement despite treatment with high-dose corticosteroids (n=40), grade ≥2 CRS with worsening symptoms despite treatment with tocilizumab (n=3).
Anakinra treatment was feasible and was safe; anakinra discontinuation due to anakinra-related side effects was only reported in 3 patients (7%). The overall response rate (ORR) to CAR T-cell therapy was 77%. The cumulative incidence of TRM in the whole cohort at day-28 and day-60 after CAR T-cell infusion was 7% (95%CI, 2-17) and 23% (95%CI, 11-38), respectively. The cumulative incidence of TRM at day-28 after anakinra initiation was 0% and 47% (95%CI, 20-70) in the high-dose (>200mg/day administered intravenously [IV]) and low-dose (100-200mg/day administered subcutaneously or IV) anakinra patients, respectively. The median cumulative incidence of CRS/ICANS resolution from the time of anakinra initiation was 7 days in patients who received high-dose anakinra and was not reached in patients who received low-dose anakinra due to the high TRM in this group. Univariate Cox modeling suggested shorter time to CRS/ICANS resolution in high-dose anakinra patients (HR, 2.19; 95%CI, 0.94-5.12; p=0.069).
In a multivariable Cox model for TRM including age, CAR-T product, pre-LD ferritin and pre-LD KPS, higher anakinra dose remained associated with lower TRM (HR = 0.41 per 1mg/kg/day increase; 95% CI, 0.17-0.96; p=0.039. The only factor independently associated with time to CRS/ICANS resolution in a multivariable Cox model including age, CAR-T product, pre-LD ferritin, and anakinra dose, was higher pre-LD KPS HR = 1.05 per 10% increase; 95%CI, 1.01-1.09; p=0.02).
Conclusion:
Anakinra treatment for refractory CRS or ICANS was safe at doses up to 12mg/kg/day IV. We observed an ORR of 77% after CAR T-cell therapy despite anakinra treatment, suggesting limited impact of anakinra on CAR T-cell efficacy. Higher anakinra dose may be associated with faster CRS/ICANS resolution and was independently associated with lower TRM. Prospective comparative studies are needed to confirm our findings.
Keywords: CAR T-cell, anakinra, toxicity, ICANS, CRS
Introduction:
Chimeric antigen receptor-engineered (CAR) T-cells are now established immunotherapy for refractory B-cell and plasma-cell malignancies. Several CAR T-cell products are now FDA-approved for relapsed or refractory (R/R) acute B-cell acute lymphoblastic leukemia (B-ALL)1,2, large B cell lymphoma (LBCL)3,4, follicular lymphoma (FL)5, mantle cell lymphoma (MCL)6 and multiple myeloma (MM)7,8 While CAR T-cell therapy is revolutionizing the management of these patients, cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS), limiting its use to large academic centers and leading to high resource utilization. There is a critical need to identify safe and effective second-line therapies when tocilizumab and corticosteroids fail to control CRS and ICANS after CAR T-cell therapy9-11. Recently, the recombinant interleukin-1 receptor antagonist (IL-1RA) anakinra has emerged as a promising approach after failure of tocilizumab and/or corticosteroids to prevent or treat severe or refractory CRS, ICANS and hemophagocytic lymphohistiocytosis (HLH)12,13. In two preclinical murine models, anakinra could prevent CRS in immunodeficient Beige mice14, and both CRS and ICANS in humanized SGM3 mice15.
Given the limited data to date regarding the safety, efficacy, and optimal dosing regimen of anakinra to treat CAR T-cell-related toxicities, we conducted a multicenter retrospective analysis including 43 patients with B-cell or plasma-cell malignancies treated with anakinra for refractory CRS and/or ICANS after CAR T-cell therapy.
Methods:
Study design and patient selection
We retrospectively analyzed data from 43 patients with B-cell or plasma cell malignancies treated at 9 institutions in the United States (n=2) and Spain (n=7) between 2019 and 2022. This study was conducted with approval of the Fred Hutchinson Cancer Research Center Institutional Review Board and in accordance with the Declaration of Helsinki.
Anakinra treatment
All patients received anakinra for refractory CRS and/or ICANS after CAR T-cell therapy. The anakinra dose, administration route, and treatment duration were at the discretion of the treating physician. Anakinra was administered subcutaneously (SC; n=21) or intravenously (IV; n=20) or both (n=2). Dose groups were defined based on the bimodal shape of the distribution of the anakinra dose (Figure S1): high-dose anakinra, >200mg/day IV (n=28); low-dose anakinra, 100-200mg/day SC or IV (n=15)
Toxicity assessment and management
Peak CRS and ICANS severity after CAR T-cell therapy were graded retrospectively by chart review according to the ASTCT consensus grading system16. CRS and ICANS management were at the discretion of the treating physicians, in accordance with the product-specific risk evaluation and mitigation strategies (REMS) guidelines for patients treated per standard of care using commercial CAR-T cell products (n=37) and using protocol-specific recommendations for patients treated on a clinical trial (n=6).
Definitions:
A pre-lymphodepletion (pre-LD) bulky disease was defined as having at minimum a mass larger than 5cm.17-19
Disease response assessments
We applied the Lugano 2014 classification and the International Myeloma Working Group criteria for patients with non-Hodgkin lymphoma and multiple myeloma, respectively.
Statistical analyses
Descriptive statistics were provided to summarize the patient and disease characteristics, including median, interquartile range (Q1, Q3) for continuous variables, and counts and percentages for categorical variables (Table 1).
Table 1.
Patient and disease characteristics
| Low Dose (N=15) |
High Dose (N=28) |
Total (N=43) |
|
|---|---|---|---|
| Age | |||
| Median [Q1,Q3] | 51.0 [32.0,63.0] | 63.5 [50.0,71.0] | 58.0 [44.5,67.5] |
| Disease type | |||
| DLBCL | 10 (66.7%) | 15 (53.6%) | 25 (58.1%) |
| MCL | 0 (0%) | 7 (25.0%) | 7 (16.3%) |
| B-ALL | 2 (13,4%) | 3 (10.7%) | 5 (11,6%) |
| PMBCL | 3 (20.0%) | 0 (0%) | 3 (7.0%) |
| FL | 0 (0%) | 2 (7.1%) | 2 (4.7%) |
| MM | 0 (0%) | 1 (3.6%) | 1 (2.3%) |
| CAR T-cell product | |||
| Axi-cel | 12 (80.0%) | 8 (28.6%) | 20 (46.5%) |
| Tisa-cel | 2 (13.3%) | 6 (21.4%) | 8 (18.6%) |
| Brexu-cel | 0 (0%) | 6 (21.4%) | 6 (14.0%) |
| Liso-cel | 0 (0%) | 3 (10.7%) | 3 (7.0%) |
| Investigational JCAR021 | 0 (0%) | 3 (10.7%) | 3 (7.0%) |
| Investigational JCAR14 | 1 (6.7%) | 0 (0%) | 1 (2.3%) |
| Investigational JCAR017 | 0 (0%) | 1 (3.6%) | 1 (2.3%) |
| Investigational BCMA-targeted | 0 (0%) | 1 (3.6%) | 1 (2.3%) |
| Weight (kg) | |||
| Median [Q1,Q3] | 67.6 [60.0,75.0] | 73.5 [64.8,82.3] | 73.0 [64.5,80.2] |
| Pre-LD KPS (%) | |||
| Median [Q1,Q3] | 80.0 [65.0,90.0] | 80.0 [80.0,90.0] | 80.0 [77.5,90.0] |
| MD | 0 (0%) | 3 (10.7%) | 3 (7.0%) |
| Pre-LD bulky disease | |||
| No | 8 (53.3%) | 16 (57.1%) | 24 (55.8%) |
| Yes | 7 (46.7%) | 12 (42.9%) | 19 (44.2%) |
| Pre-LD bone marrow involvement | |||
| No | 12 (86%) | 15 (60%) | 27 (62%) |
| Yes | 2 (14%) | 8 (32%) | 10 (23%) |
| MD | 0 (0%) | 2 (8%) | 2 (5%) |
| Pre-LD ferritin μg/L) | |||
| Median [Q1,Q3] | 1720 [705,3400] | 534 [236,1370] | 809 [310,2270] |
| MD | 1 (6.7%) | 6 (21.4%) | 7 (16.3%) |
| Pre-LD platelet (G/L) | |||
| Median [Q1,Q3] | 93.0 [43.5,244] | 106 [58.0,158] | 103 [47.0,163] |
| Pre-LD LDH (UI/L) | |||
| Median [Q1,Q3] | 609 [288,1300] | 216 [168,392] | 295 [192,579] |
| MD | 0 (0%) | 1 (3.6%) | 1 (2.3%) |
| Pre-LD Albumin (g/L) | |||
| Median [Q1,Q3] | 39.0 [29.8,40.8] | 34.0 [27.8,40.0] | 35.5 [28.0,40.0] |
| MD | 1 (6.7%) | 0 (0%) | 1 (2.3%) |
| Pre-LD CRP (mg/mL) | |||
| Median [Q1,Q3] | 8.75 [2.50,14.4] | 8.54 [3.68,26.9] | 8.54 [3.22,16.7] |
| MD | 1 (6.7%) | 10 (35.7%) | 11 (25.6%) |
Abbreviations: KPS, Karnofsky Pergormance Status ; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MM, multiple myeloma; MCL, mantle cell lymphoma, PMBCL, primary mediastinal B-cell lymphoma; axi-cel, axicabtagene ciloleucel; tisa-cel, tisagenlecleucel; brexu-cel, brexucabtagene autoleucel; liso-cel, lisocabtagene maraleucel; LD, lymphodepletion; MD, missing data.
Cumulative incidence estimates of time to CRS/ICANS resolution and treatment-related mortality (TRM) from the time of anakinra initiation were computed using the Kalbfleisch and Prentice method. For time to CRS/ICANS resolution, death of any cause was modeled as the competing risk. For TRM, disease relapse or progression was modeled as the competing risk. Univariate and multivariable analyses of both time to CRS/ICANS resolution and TRM were modeled using cause-specific Cox regression. Time to CRS/ICANS resolution was defined as the time from CAR T-cell infusion left-truncated at the time of anakinra initiation until CRS/ICANS symptom resolution (first day of grade 0 stable for at least three consecutive days), whichever happened last, censoring for death from any cause. TRM, was defined as the time from CAR T-cell infusion left-truncated at the time of anakinra initiation until death, censoring for disease progression or relapse. TRM models included the anakinra dose as a continuous variable (daily dose of anakinra in mg/kg/day) since no event occurred in the high-dose anakinra group, precluding the models from converging. To minimize confounding, we built a directed diacyclic graph (DAG; Figure S2) including hypothesized confounders (i.e., patient and disease-related variables that might have impacted both the choice of the anakinra dose and outcomes). DAGs are an established framework to address confounding based on observational non-randomized data 20-23. Using the R package Dagitty 24, the following variables were selected as the minimal adjustment set of covariates: age, pre-LD Karnosfky performance status (KPS), pre-LD ferritin, CAR T-cell product type. Generalized estimating equations (GEE) with unstructured correlation matrix were computed to evaluate the longitudinal relationship between the anakinra dose and ICANS grade. All analyses were performed using RStudio (R version 3.6.2).
Results
Patient, disease, and CAR T-cell treatment characteristics (Table 1)
Median age at the time of CAR T-cell infusion was 58 (range, 10-77), 3 pediatric patients were included aged of 10, 12 and 15 years old. Disease type was diffuse large B cell lymphoma (DLBCL) (n=25, 58%), MCL (n=7, 16%), B-ALL (n=5, 12%), primary mediastinal large B-cell lymphoma (PBMCL; n=3, 7%), FL (n=2, 5%), or MM (n=1, 2%). Prior central nervous system (CNS) history was reported in 6 patients (14%; CNS involvement by lymphoma, n=3; stroke, n=2; frontal meningioma, n=1). The median pre-LD LDH serum concentration was 295 UI (range, 102-2074) and pre-LD bulky disease (largest lesion diameter ≥ 5cm) was noted in 19 patients (44%). Twenty patients received axicabtagene ciloleucel (axi-cel) (47%) per the FDA label, eight patients received tisagenlecleucel (19%), six patients received brexucabtagene autoleucel (14%), three patients received lisocabtagene maraleucel (7%) while 6 patients received an investigational CD19 (n=5) or BCMA-targeted (n=1) CAR T-cell product. The median follow-up after CAR T-cell infusion was 62 days (range, 6-474).
CRS and ICANS characteristics, severity, and treatment course (Table 2)
Table 2.
CRS, ICANS, treatment characteristics
| Characteristic | Anakinra dosing | p-value2 | ||
|---|---|---|---|---|
| Low, N = 151 | High, N = 281 | Total, N=43 | ||
| Peak CRS grade (ASTCT 2019) | 2 (1, 2) 1-3 | 2 (1, 2) 0-4 | 2 (1, 2) 0-4 | 0.4 |
| Peak ICANS grade (ASTCT 2019) | 4 (4, 4) 0-5 | 4 (3, 4) 0-4 | 4 (3, 4) 0-5 | 0.069 |
| Time to first anakinra administration (days) | 9 (8, 14) 6-41 | 8 (6, 11) 4-22 | 8 (7, 12) 4-41 | 0.13 |
| Corticosteroid treatment duration (days) | 13 (4, 24) 1-51 | 12 (8, 20) 3-43 | 12 (8, 21,5) 1-51 | >0.9 |
| Cumulative steroid dose (mg, dexamethasone equivalence) | 610 (395, 2,175) 25-8,000 | 562 (233, 945) 120-2,700 | 598 (260,1040) 25-8,000 | 0.3 |
| Cumulative dose of anakinra (mg) | 700 (450-1600) 100-12600 | 4200 (2600-5700) 400-18000 | 2800 (1125-4950) 100-18000 | 0.0001 |
| Duration of anakinra treatment (days) | 6 (4.5-10) 1-44 | 7.5 (4.75-12.2) 1-29 | 7 (4.5-11.5) 1-44 | 0.41 |
| Hospital location | ||||
| Spain | 11 (73%) | 7 (25%) | 18 (42%) | 0.004 |
| US | 4 (27%) | 21 (75%) | 25 (58%) | |
Median (IQR) Minimum-Maximum; n (%)
Wilcoxon rank sum test used for continuous variables; Fisher's exact test used for categorical variables;
Abbreviations: CRS, cytokine release syndrome; ICANS, Immune effector cell-associated neurotoxicity syndrome, LDH : lactate dehydrogenase, MD : missing data
Median peak CRS grade was 2 (range, 0-4) and median CRS duration was 4 days (range, 3-18). Vasopressor use was reported in 6 patients. Median peak ICANS grade was 4 (range, 0-5), and median ICANS duration was 6 days (range, 1-38), respectively. Encephalopathy with depressed level of consciousness was reported in 31 patients (76%), while seizures were reported in 8 (19%). Seven patients (16%) developed cerebral edema.
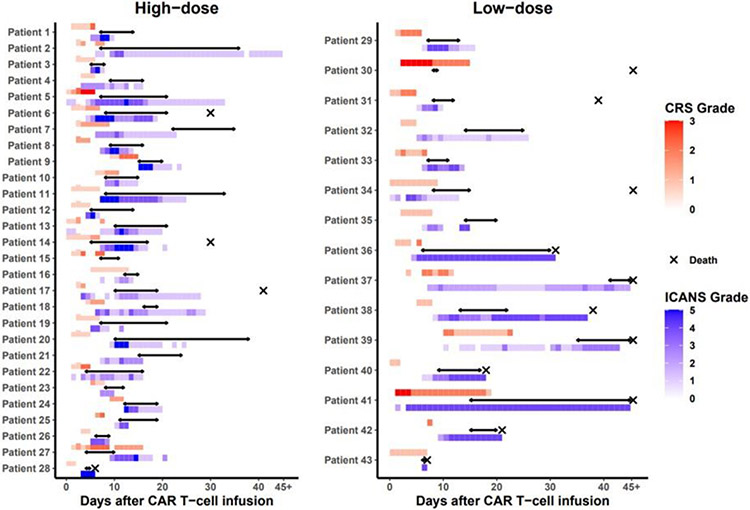
Indications for anakinra treatment were as follows: grade ≥2 ICANS with worsening or lack of symptom improvement despite treatment with high-dose corticosteroids (n=40), grade ≥2 CRS with worsening symptoms despite treatment with tocilizumab (n=3). All patients received anakinra concurrently with corticosteroids. Anakinra was initiated at the peak of ICANS severity in 30 patients (70%) and in 7 patients (16%) after peak of severity. Peak of toxicity was observed after anakinra for patients (14%) of patients. Individual patient clinical courses are shown in Figure 1.
Figure 1. Individual treatment courses categorized by anakinra dose.
Red bar, CRS grade; blue bar, ICANS grade; black arrow, anakinra treatment; cross, death. Abbreviations: CAR, chimeric antigen receptor; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome
The median time from CAR T-cell infusion to anakinra initiation was 8 days (range, 4-41), and from CRS or ICANS onset, whichever occurred last prior to anakinra initiation, was 3 (range, 0-32). Anakinra was initiated within 24 hours of an increase in the corticosteroids dose in 22 patients (51%). Anakinra and corticosteroids were simultaneously initiated in 8 patients (9%). Twelve patients (27%) received additional therapies for CRS or ICANS after anakinra initiation including siltuximab (n=11), intrathecal chemotherapy (n=2), etoposide (n=1) or ruxolitinib (n=1).
Safety
Treatment modifications due to presumed anakinra-related adverse events were reported in three patients: administration route changed from SC to IV due to a subcutaneous hematoma (n=1), and anakinra discontinuation due to elevated liver enzymes (n=2). In the 15 deceased patients, causes of death were as follows: disease progression (n=6), infection (n=5), ICANS (n=4).
Anti-tumor efficacy
We observed anti-tumor responses (partial or complete) to CAR T-cell therapy in 31 of 40 patients evaluable for response (77%; B-ALL, n=5/5 [100%]; DLBCL, n=17/25 [68%]; MCL, n=5/7 [71%]; MM, n=1/1 [100%]; PMBCL, n=1/3[33%]). Three patients (7%) died prior to disease restaging.
Comparisons of patient characteristics and outcomes between subjects treated with high-dose versus low-dose anakinra (Table 1 and 2)
Patients receiving high-dose anakinra were older compared to those who received low-dose anakinra (64 versus 51; p=0.018). We measured higher pre-LD ferritin and LDH in patients treated with low-dose anakinra compared to patients treated with high-dose (ferritin 534μg/L versus 1,724μg/L; p= 0,049 and LDH 216U/L versus 609U/L; p=0.002). The median time to anakinra initiation from CRS or ICANS onset was comparable in patients receiving high-dose compared to low-dose anakinra (8 versus 9 days, respectively; p=0.13). The median peak CRS grade was 2 in both high and low-dose groups (interquartile range [IQR], 1-2; p=0.4). The median peak ICANS was 4 in both high and low-dose groups (IQR, 4-4 versus 3-4, respectively; p=0.07). Duration of corticosteroid treatment was comparable in high and low-dose anakinra patients (median, 12 days versus 13 days, respectively; p>0.9). The median duration of anakinra treatment in high-dose was versus low-dose patients was also comparable (7.5 versus 6 days; p=0.41). American patients were more likely to receive high-dose anakinra compared to Spanish patients (75% versus 21%, respectively; p=0.004).
Univariate analyses of factors associated with TRM (Table 3) and time to CRS/ICANS resolution (Table 4)
Table 3.
Univariate cause-specific Cox model for TRM
| Characteristic | HR1 | 95% CI1 | p-value |
|---|---|---|---|
| CAR T cell Product (axi-cel/brexu-cel vs other) | 6.06 | 0.76, 48.6 | 0.090 |
| Age | 1.00 | 0.97, 1.04 | 0.954 |
| Pre-LD KPS (%) | 0.94 | 0.90, 0.98 | 0.004 |
| Pre-LD Ferritin (log10) | 2.25 | 0.88, 5.79 | 0.092 |
| Pre-LD LDH (log10) | 4.23 | 1.26, 14.2 | 0.020 |
| Pre-LD Albumin (log10) | 19.9 | 0.02, 15,974 | 0.381 |
| Pre-LD Bulky Disease | 3.31 | 0.82, 13.4 | 0.093 |
| CRS or ICANS grade at anakinra initiation | 1.20 | 0.63, 2.31 | 0.577 |
| Time to anakinra initiation from CAR T-cell infusion | 1.14 | 1.05, 1.24 | 0.002 |
| Time to anakinra initiation from CRS/ICANS onset | 1.15 | 1.06, 1.26 | 0.001 |
| Anakinra daily dose (mg/kg/day) | 0.41 | 0.22, 0.78 | 0.007 |
HR = Hazard Ratio, CI = Confidence Interval
Abbreviations: axi-cel, axicabtagene ciloleucel; brexu-cel, brexucabtagene autoleucel; LDH, lactate dehydrogenase; LD, lymphodepletion; CAR, chimeric antigen receptor; CRS, cytokine release syndrome, ICANS, Immune effector cell-associated neurotoxicity syndrome; TRM, treatment-related mortality
Table 4.
Univariate cause-specific Cox model for time to CRS/ICANS resolution
| Characteristic | HR1 | 95% CI1 | p-value |
|---|---|---|---|
| CAR T cell Product (axi-cel/brexu-cel vs other) | 0.70 | 0.35, 1.39 | 0.304 |
| Age | 1.00 | 0.98, 1.01 | 0.616 |
| Pre-LD KPS (%) | 1.04 | 1.01, 1.08 | 0.007 |
| Pre-LD Ferritin (log10) | 0.61 | 0.33, 1.12 | 0.112 |
| Pre-LD LDH (log10) | 0.36 | 0.10, 1.27 | 0.111 |
| Pre-LD Albumin (log10) | 0.76 | 0.29, 2.00 | 0.577 |
| Pre-LD Bulky Disease | 0.52 | 0.25, 1.07 | 0.074 |
| CRS or ICANS grade at anakinra initiation | 0.89 | 0.64, 1.23 | 0.468 |
| Time to anakinra initiation from CAR T-cell infusion | 0.98 | 0.90, 1.07 | 0.667 |
| Time to anakinra initiation from CRS/ICANS onset | 0.94 | 0.84, 1.05 | 0.260 |
| Anakinra dose (High vs Low) | 2.19 | 0.94, 5.12 | 0.069 |
| Anakinra daily dose (mg/kg/day) | 1.10 | 0.97, 1.24 | 0.136 |
HR = Hazard Ratio, CI = Confidence Interval
Abbreviations: axi-cel, axicabtagene ciloleucel; brexu-cel, brexucabtagene autoleucel ; LDH, lactate dehydrogenase; LD, lymphodepletion; CAR, chimeric antigen receptor ; CRS, cytokine release syndrome, ICANS, Immune effector cell-associated neurotoxicity syndrome; TRM, treatment-related mortality
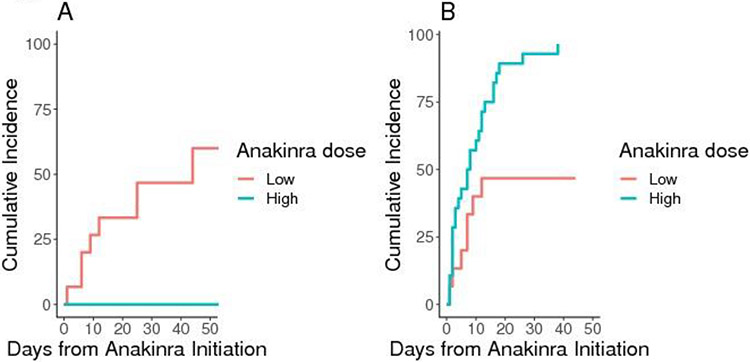
The cumulative incidence of TRM in the whole cohort at day-28 and day-60 after CAR T-cell infusion was 7% (95%CI, 2-17) and 23% (95%CI, 11-38), respectively. The cumulative incidence of TRM at day-28 after anakinra initiation was 0% (95%CI, not evaluable) and 47% (95%CI, 20-70) in the high-dose and low-dose anakinra patients, respectively (Figure 2A). Higher anakinra dose was associated with lower TRM (HR per 1mg/kg/day increase, 0.41; 95%CI, 0.22-0.78; p=0.007). The association between the log-hazard of TRM as a function of the anakinra dose – modeled as a continuous variable – is shown in Figure S3. Lower pre-LD KPS (HR, 0.94; 95%CI, 0.90-0.98; p=0.004), higher pre-LD LDH (HR, 4.23; 95%CI, 1.26-14.18; p=0.020), longer time from CAR-T infusion to anakinra initiation (HR, 1.14; 95%CI, 1.05-1.24, p=0.002), and longer time from CRS/ICANS onset to anakinra initiation (HR, 1.15; 95%CI, 1.06-1.26; p=0.001) were associated with higher TRM.
Figure 2. TRM (A) and time to CRS/ICANS resolution (A) after anakinra initiation.
A, cumulative incidence of TRM, defined as the time from anakinra initiation to death with disease progression or relapse as the competing risk.; B, cumulative incidence of CRS/ICANS symptom resolution, whichever happened last, from the time of anakinra initiation with death from any cause as the competing risk Abbreviations: CRS, cytokine release syndrome; ICANS, Immune Effector Cell-Associated Neurotoxicity Syndrome; TRM, treatment-related mortality.
The median time to CRS/ICANS resolution from anakinra initiation was 7 days in patients who received high-dose anakinra and was not reached in patients who received low-dose anakinra (Figure 2B). In a univariate cause-specific Cox model, high-dose anakinra was associated with faster CRS/ICANS resolution, although we could not exclude a null effect at the 0.05 level (HR, 2.19; 95%CI, 0.94-5.12; p=0.069). Higher pre-LD KPS was also associated with faster time to CRS/ICANS resolution (HR, 1.04; 95%CI, 1.01-1.08; p=0.007).
Multivariable modeling to estimate the effect of the anakinra dose on TRM and time to CRS/ICANS resolution after adjustment for potential confounders.
We hypothesized the effect of the anakinra dose on outcomes could be confounded by patient, disease, and treatment-related variables (Figure S2).
In a multivariable Cox model for TRM including age, CAR-T product, pre-LD ferritin and pre-LD KPS, higher anakinra dose remained associated with lower TRM (HR = 0.41 per 1mg/kg/day increase; 95% CI, 0.17-0.96; p=0.039; Table S1).
In a multivariable Cox model including age, CAR-T product, pre-LD ferritin, and anakinra dose, a higher pre-LD KPS remained associated with faster CRS/ICANS resolution (HR = 1.05 per 10% increase; 95%CI, 1.01-1.09; p=0.02; Table S2).
Longitudinal modeling of the effect of anakinra dose on ICANS grade
Since time to CRS/ICANS resolution may not capture clinically meaningful improvements, e.g., in case of prolonged grade 1 toxicities, we fitted a GEE model to estimate the longitudinal impact of the anakinra dose on ICANS severity. We observed that high-dose anakinra was associated with lower ICANS grade over time (beta = − 0.89, 95% CI −1.5 to −0.3; p=0.002). Locally weighted scatter plot smoothing (LOESS) also suggested lower ICANS grade over time and faster resolution in patients receiving high-dose anakinra (Figure S4).
Discussion
CAR T-cell therapy achieves high efficacy in patients with lymphoid or plasma-cell malignancies but is limited by significant toxicities such as CRS and ICANS. In a subset of patients, standard treatment with tocilizumab and corticosteroids fails to reverse CRS or ICANS symptoms. As such, there is an urgent need to better characterize the second-line management of CRS and ICANS. IL-1 is known as having an important role in severe systemic inflammation and its association with CAR T-cell toxicities has been established in murine models 14,15, non-human primates 25 and in humans 26. Despite limited clinical data, a growing number of practitioners have started using the IL-1RA anakinra to treat CRS or ICANS after failure of tocilizumab or corticosteroids. Yet the safety, efficacy, and optimal dosing of anakinra in this setting remains ill-defined. Hence, we performed the first and largest multicenter retrospective analysis including 43 patients treated anakinra for refractory CRS and/or ICANS after CAR T-cell therapy.
First, treatment with anakinra was safe and feasible, without dose or administration route modification in 40 patients (93%). In univariate analyses, higher anakinra dose and shorter time to anakinra initiation – from both CAR T-cell infusion or CRS/ICANS onset – were associated with lower TRM. A multivariable Cox model including potential confounders suggested a higher anakinra dose was independently associated with lower TRM. Superior CNS penetration of high-dose IV anakinra may have contributed to this finding. Anakinra is a relatively large molecule (17kDa) with low CNS penetration, and high serum concentrations of anakinra are needed to achieve neuroprotective concentrations in the cerebrospinal fluid.27. Taken together, this suggests early initiation of high-dose IV anakinra may be beneficial to improves the outcomes of patients developing refractory CRS/ICANS. While our univariate analyses suggested an association between high-dose anakinra and faster time to CRS/ICANS resolution (HR, 2.19; 95%CI, 0.94-5.12; p=0.069), it became undetermined in multivariable analysis (HR, 1.89; %CI, 0.65-5.46; p=0.24). This could reflect confounding of the effect of anakinra dose from covariates, or low statistical power.
Although a theoretical concern is that anakinra treatment may impair CAR T-cell-mediated anti-tumor efficacy, we observed high response rates to CAR T-cell therapy [overall response rate; 77% [high-dose anakinra, 85%] CR rate; 49% [high-dose anakinra, 63%]). While preclinical studies did not show a detrimental impact of anakinra on anti-tumor effects and in vivo CAR T-cell persistence 15, further research is needed to evaluate the impact of anakinra on duration of response and in vivo CAR T-cell kinetics.
Our univariate and multivariable analyses also suggested lower pre-LD KPS, impacted by disease control, patient comorbidities and frailty, was strongly associated with more prolonged toxicities and higher TRM, which could have contributed to the significantly higher TRM (47% at day-28 after anakinra initiation) in patients treated with low-dose anakinra. The expected TRM in this patient population is unknown. A limited number of case series including patients treated with anakinra have been reported to date, with low sample size precluding robust TRM estimation. A study conducted at the Massachusetts General Hospital Cancer Center reported on 14 patients treated with anakinra at the dose of 100-200mg/day for steroid-refractory ICANS with or without CRS28. One patient died of neurotoxicity on day 29 and three patients died of infection. Another case series from the MD Anderson Cancer Center reported on the use of low-dose subcutaneous anakinra in eight LBCL patients treated with axi-cel13. Non-disease-related deaths were reported in 5 of 8 patients (63%). Investigators at the National Cancer Institute reported encouraging results using subcutaneous high-dose anakinra (5-8mg/kg/day) in 8 patients presenting with delayed HLH manifestations outside of CRS in pediatric B-ALL treated with CD22 CAR T cells on a clinical trial29. All patients had resolution of HLH-like toxicities (short-term outcomes for anakinra-treated patients were not reported specifically). Last, Kennedy et al. reported on macrophage activation syndrome (MAS)-like manifestations following BCMA-directed CAR T-cell therapy, for which 10 of 55 patients received anakinra. Although early mortality was not reported, the 1-year overall survival was 65.2% vs 90.6% and relapse-free survival was 35.4% vs 54.7% for patients with MAS-like compared to those without MAS-like features, respectively.30 We speculate our higher TRM may reflect the inclusion of a more frail population with more aggressive disease. More work is needed to better define CAR-T-related toxicity refractoriness and the expected TRM in this specific patient population.
To our knowledge, our work is the first evaluating specifically the impact of the anakinra dose on TRM and time to CRS/ICANS resolution after CAR T-cell therapy.
We acknowledge the limitations related to the retrospective nature of our work, susceptible to selection and treatment allocation bias due to unmeasured confounders. The higher TRM observed in the low-dose anakinra group may have reflected higher patient frailty not adequately captured by the KPS. In addition, anakinra treatment was associated in most patients with concurrent corticosteroids (new initiation or dose increase), which could have confounded our assessments of the efficacy of anakinra on CRS and ICANS symptoms.
In conclusion, anakinra treatment was safe, at doses up to 12mg/kg/day. Early treatment with high-dose anakinra may be associated with lower TRM compared to low-dose anakinra in patients with refractory CRS/ICANS. While this is encouraging, high-dose IV anakinra remained associated with high cumulative corticosteroid usage and prolonged hospitalizations, suggesting prophylactic treatment may be a more promising approach. Our group (NCT04359784) and others (NCT04148430, NCT04150913, NCT04432506) are currently evaluating prophylactic low-dose anakinra to prevent or treat CRS and ICANS after CAR T-therapy.
Supplementary Material
Key points:
High-dose anakinra (up to 12mg/kg/day) for refractory CRS/ICANS was safe
Higher anakinra dose may be associated with faster CRS/ICANS resolution and was independently associated with lower TRM
Acknowledgments
The authors thank the Fred Hutchinson Cancer Center Cell Processing Facility and Cell Therapy Laboratory, the staff of the Bezos Family Immunotherapy Clinic, the Oregon Health and Science University Cell Therapy Clinical, Administrative and Laboratory Programs, and the Grupo Español de Trasplante Hematopoyético y Terapia Celular.
J.G. acknowledges the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA015704-45).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Results presented in part at the 2021 ASH, 2022 ASTCT and 2022 EBMT Annual Meetings
Conflicts of interest
NG, ECL, QVW, JMV, RH, NMC, APM, SW, EK: nothing to disclose ; PB: Consultancy and Honoraria: Allogene, Amgen, BMS, Kite/Gilead, Miltenyi biomedicine, Incyte, Novartis, Pfizer ; GI : Consultancy and Honoraria from Novartis, Roche, Kite/Gilead, Bristol-Myers Squibb, Abbvie, Janssen, Sandoz, AstraZeneca, Miltenyi ; MK : Consultancy and Honoraria from Novartis, Kite/Gilead, AstraZeneca, Pfizer ; JLRO : Honoraria from Kite/Gilead, Novartis, Janssen, Sanofi, Bristol-Myers-Squibb ; LLC : Honoraria : Gilead, consulting fees : Gilead, Novartis ; VOM : declares receiving travel grants and/or advisory fees from Kite, Janssen, BMS and Novartis ; RTM : advisor or consultant for Artiva, CRISPR Therapeutics, Incyte, and Novartis; reports honoraria from Bristol-Myers Squibb/Celgene, Incyte, Intellia, and Kite; research support from BMS and Novartis ; ERN : Consultant : Novartis ; AJC: Research funding: Harpoon, Sanofi, BMS, Janssen, Nektar, Abbvie Consultancy: Abbvie, BMS, Allogene, EUSA, GSK, Janssen, Secura Bio Steering committee membership: Adaptive ; DJG : Bristol Myers Squibb: Research Funding, Membership on a Board or Advisory Committee, Patents & Royalties ; Cellectar Biosciences: Research Funding ; GSK Membership on a Board or Advisory Committee ;Janssen Biotech: Research Funding, Membership on a Board or Advisory Committee ; Juno Therapeutics: Research Funding, Patents & Royalties ; Legend Biotech: Consultancy ; Neoleukin Therapeutics: Membership on a Board or Advisory Committee ; Seattle Genetics: Research Funding, Membership on a Board or Advisory Committee ; SpringWorks Therapeutics: Research Funding ; AVH ; honoraria from Bristol Myers Squibb and Novartis; receives research funding from Juno Therapeutics, a Bristol Myers Squibb Company, and Nektar Therapeutics ; DGM : has received research funding paid to his institution and honoraria from Juno Therapeutics, a BMS Company, Celgene, a BMS Company and Kite Pharma. He has participated in ad hoc advisory board meetings and received honoraria from Amgen, BMS, Genentech, Gilead Sciences, Incyte, Janssen, Legend Biotech, Mustang Bio, MorphoSys, Novartis, Pharmacyclics and Umoja. He has rights to royalties from Fred Hutch for patents licensed to Juno/BMS, is a member of the A2 Biotherapeutics Scientific Advisory Board with stock options and compensation and is a member of the Navan Technologies Scientific Advisory Board with stock options and compensation ; CJT : Research funding: Juno Therapeutics/BMS, Nektar Therapeutics. Scientific and Clinical Advisory Boards: Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, T-CURX, Myeloid Therapeutics, ArsenalBio, Century Therapeutics, Kyverna. Ad hoc advisory boards/consulting (last 12 months): GlaxoSmithKline, Decheng Capital, Nektar Therapeutics, Allogene, Sobi, Legend Bio, Syncopation Life Sciences. Stock-options: Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, ArsenalBio. Patents: CJT has the right to receive payment from Fred Hutch as an inventor on patents related to CAR T-cell therapy ; JG : Ad hoc consultant, having received honoraria : Sobi, Legend Biotech, Janssen, Kite Pharma, MorphoSys, Research Funding: Sobi, Juno Therapeutics (a BMS company), Celgene (a BMS company), Angiocrine Bioscience
Data Sharing Statement
Data available upon request to the corresponding author.
References
- 1.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Research C for DE and. FDA approves brexucabtagene autoleucel for relapsed or refractory B-cell precursor acute lymphoblastic leukemia. FDA. Published online January 31, 2022. Accessed May 31, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-brexucabtagene-autoleucel-relapsed-or-refractory-b-cell-precursor-acute-lymphoblastic [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91–103. doi: 10.1016/S1470-2045(21)00591-X [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. The Lancet. 2021;398(10297):314–324. doi: 10.1016/S0140-6736(21)00933-8 [DOI] [PubMed] [Google Scholar]
- 8.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019;380(18):1726–1737. doi: 10.1056/NEJMoa1817226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021;56(3):552–566. doi: 10.1038/s41409-020-01134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J. A Phase II Study of Prophylactic Anakinra to Prevent CRS and Neurotoxicity in Patients Receiving CD19 CAR T Cell Therapy for Relapsed or Refractory Lymphoma. In: ASH; 2021. Accessed December 14, 2021. https://ash.confex.com/ash/2021/webprogram/Paper150431.html [Google Scholar]
- 13.Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy–associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123–3127. doi: 10.1182/bloodadvances.2020002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giavridis T, van der Stegen SJ, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. doi: 10.1038/s41591-018-0036-4 [DOI] [PubMed] [Google Scholar]
- 16.Pennisi M, Jain T, Santomasso BD, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4(4):676–686. doi: 10.1182/bloodadvances.2019000952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607–5615. doi: 10.1182/bloodadvances.2020003001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ababneh H, Frigault M, Ng AK, Patel CG. 18FDG PET/CT Parameters for the Prediction of CAR T-Cell Therapy Response among Patients with Large B-Cell Lymphoma. Int J Radiat Oncol Biol Phys. 2022; 114(3):S84. doi: 10.1016/j.ijrobp.2022.07.489 [DOI] [Google Scholar]
- 19.Qi S, Milgrom S, Dabaja B, et al. Two distinct prognostic groups in advanced-stage Hodgkin lymphoma revealed by the presence and site of bulky disease. Blood Adv. 2020;4(9):2064–2072. doi: 10.1182/bloodadvances.2019001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–298. doi: 10.1093/aje/kws412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro DD, Msaouel P. Causal Diagram Techniques for Urologic Oncology Research. Clin Genitourin Cancer. 2021;19(3):271.e1–271.e7. doi: 10.1016/j.clgc.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 22.VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34(3):211–219. doi: 10.1007/s10654-019-00494-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauthier J, Gazeau N, Hirayama AV, et al. Impact of CD19 CAR T-cell product type on outcomes in relapsed or refractory aggressive B-NHL. Blood. Published online April 19, 2022:blood.2021014497. doi: 10.1182/blood.2021014497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package “dagitty.” Int J Epidemiol. 2016;45(6):1887–1894. doi: 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 25.Taraseviciute A, Tkachev V, Ponce R, et al. Chimeric Antigen Receptor T Cell–Mediated Neurotoxicity in Nonhuman Primates. Cancer Discov. 2018;8(6):750–763. doi: 10.1158/2159-8290.CD-17-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santomasso BD, Park JH, Salloum D, et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018;8(8):958–971. doi: 10.1158/2159-8290.CD-17-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gueorguieva I, Clark SR, McMahon CJ, et al. Pharmacokinetic modelling of interleukin-1 receptor antagonist in plasma and cerebrospinal fluid of patients following subarachnoid haemorrhage. Br J Clin Pharmacol. 2008;65(3):317–325. doi: 10.1111/j.1365-2125.2007.03026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wehrli M, Gallagher K, Chen YB, et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J Immunother Cancer. 2022;10(1):e003847. doi: 10.1136/jitc-2021-003847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(17):1938–1950. doi: 10.1200/JCO.19.03279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy VE, Wong C, Huang CY, et al. Macrophage activation syndrome-like (MAS-L) manifestations following BCMA-directed CAR T cells in multiple myeloma. Blood Adv. 2021;5(23):5344–5348. doi: 10.1182/bloodadvances.2021005020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon request to the corresponding author.