Abstract
Sex hormone concentrations, particularly testosterone, are primary determinants of sex-based differences in athletic and sports performance, and this relationship may inform fair competition and participation for athletes. This article describes the sex-based dichotomy in testosterone and the implications for sex-based differences in individual sports performance, including factors that relate to athletic performance for transgender individuals, and areas of future investigation.
Keywords: transgender, athletes, athletic performance, sex differences, testosterone
Summary for Table of contents:
Testosterone represents a key determinant of sex-based differences in athletic performance and must be considered for fair elite athletic competition.
INTRODUCTION
Throughout much of history, “sport meant men’s sport” (1). Women were first allowed to compete in Olympic games in 1900 (only in two sports), in track and field events in the 1928 Olympic games, but not until more recently for longer distance events such as the marathon (1984 Olympic games) and the 1500m freestyle swim (2020 Olympic games) (1, 2). Recognition of sex-based differences in athletic performance and promotion of fair competition for athletes in the female category are the primary basis for dichotomous sport categories. Although many sports are mixed sex during childhood, the onset of male puberty is associated with a sex-related divergence in athletic performance (3).
Sex verification testing in elite sports began in the 1940s, and due to the multidimensional nature of sex, identified individuals with intersex traits or differences of sex development (Table 1) (4). Both sex and gender are multi-dimensional (definitions in Table 1) (5), and throughout history, policies that determine who can compete in the female competition category have shifted focus among various sex traits, from physical exams (e.g. appearance of external genitalia), to karyotype analysis (chromosomal complement) or polymerase chain reaction for SRY testing, to hormone testing (testosterone concentrations in blood). While all of these are aspects of one’s biologic sex, testosterone concentrations, and the impact of testosterone on physical changes during masculinizing puberty, remain the single most significant contributor to sex differences in athletic performance (6). Therefore, policies that affect the participation of transgender athletes (individuals whose gender identity differs from birth sex) in elite sports have focused on who is eligible to complete in the female competition category (4, 7). This primarily impacts transgender women, those individuals who were born with a male sex and have a female gender identity (Table 1). Over time, there have been significant shifts in the International Olympic Committee’s (IOC) policies on participation of transgender women in elite female competition, from recommending gonadectomy/orchiectomy, legal recognition of sex plus hormone therapy in 2003 (7), to a gender identity aligned as a woman and suppressed serum total testosterone to <10 nmol/L in 2015 (note that adult female concentrations are, on average, <2.5 nmol/L) (8) to a new framework in 2021. The 2021 framework includes 10 principles: inclusion, prevention of harm, non-discrimination, and fairness, no presumption of advantage, evidence-based approach, primacy of health and bodily autonomy, stakeholder-centered approach, periodic reviews (9).
Table 1.
Definitions
| Terminology | |
|---|---|
| Sex | Biologic sex is multidimensional; the dimensions include: sex chromosomes, gonads, sex steroids, internal reproductive structures, external genitalia, secondary sex characteristics (5). While often binary (male or female sex), there are individuals with differences of sex development or intersex traits (see definition below). |
| Gender | Multidimensional psychosocial construct that encompasses gender identity, expression, social and cultural expectations about social status, characteristics, and behavior that are associated with sex traits and may change/evolve over time (5) |
| Cisgender | Gender identity aligns with birth sex |
| Transgender | Gender identity differs from birth sex |
| Difference/disorder of sex development (DSD)/intersex | Congenital conditions in which the development of the chromosomal, gonadal, or anatomic sex is atypical (outside the binary of male/female) |
| Gender expression | External manifestations of gender, expressed through name, pronouns, clothing, haircut, behavior, voice or other characteristics |
| Gender identity/experienced gender | One’s internal, deeply held sense of gender; not visible to others |
| Gender role | Behaviors, attitudes and personality traits that a society (in a given culture and historical period) designates as masculine or feminine and/or that society associates with the typical social role of men or women |
| Transgender man/male | Individuals designated female at birth who identify and live as men |
| Transgender woman/female | Individuals designated male at birth who identify and live as women |
| Transition for Transgender individuals | |
| Transition | Process during which persons change their physical, social and/or legal characteristics consistent with their affirmed gender identity. |
| Social transition | Process of "coming out” or disclosing one’s gender identity to friends, family and loved ones; may involve use of name/pronouns different from those at birth and/or a change in gender expression (e.g. clothing, hairstyle, etc.). |
| Medical transition | Initiation of medications to block endogenous secondary sex characteristics and/or induce development of secondary sex characteristics that algin with gender identity. May include surgeries to further align body with gender identity. Details outlined in guidelines (75, 126) and below. |
| Legal transition | Legally changing one’s name and/or gender on various identification documents; the ability to do so varies by state in the U.S. and country worldwide (127). |
| Gonadotropin releasing hormone (GnRH) agonist (puberty blockade) | Inhibition of the hypothalamic-pituitary-gonadal access, suppression of gonadotropins and sex steroids (testosterone/estradiol); pause endogenous puberty changes |
| Gender affirming hormone therapy (GAHT) | Hormones, including testosterone or estradiol, that are prescribed to eligible individuals to induce development of secondary sex characteristics that align with gender identity. Estradiol therapy results in feminizing secondary sex characteristics (breast development, body fat redistribution). Testosterone therapy results in masculinizing secondary sex characteristics (voice depending, facial/body hair growth, scalp hair loss, increased muscle mass/strength, body fat redistribution, cessation of menses). |
| Medications to block/suppress testosterone | Estradiol is typically used concurrently with a medication to block or suppress testosterone. Medications include: spironolactone (androgen receptor blockade), cyproterone acetate (inhibits of testosterone synthesis and action, not available in the U.S.), progestins (inhibits of pituitary-gonadal axis, suppresses testosterone), bicalutamide (androgen receptor antagonist), finasteride (blocks conversion of testosterone to dihydrotestosterone), and others. GnRH agonists may also be used to suppress testosterone (reviewed in (97)). |
| Gender affirming surgery | Surgery or surgeries to align one’s body with one’s gender identity. Feminizing surgeries may include: vaginoplasty, orchiectomy, breast augmentation, facial feminization surgery, tracheal shave. Masculinizing surgeries may include: chest masculinizing surgery (removal of breast tissue), hysterectomy, oophorectomy, metoidioplasty, phalloplasty. |
Data from Table 1 in the 2017 Endocrine Society Guidelines (75), Table 1 in (126), NIH Terms & Definitions (128) and National Academies (5). For a more comprehensive review of medications see (126). Note that this review does not cover DSD/intersex athletes, for additional history please refer to the following references (2, 16) and others.
The recommendations from the IOC are broad and devolve regulatory power to governing bodies associated with individual sports. In this context, individual sports are now tasked with creating polices specific for their own sports that align with the principles outlined by the IOC, and may vary widely (10). The impact of testosterone on athletic performance is known (6), and testosterone and other “anabolic androgenic steroids” are banned substances by the World Anti-Doping Agency (WADA) (without a Therapeutic Use Exemption) (11, 12). Therapeutic Use Exemptions enable athletes to use substances from the list of prohibited drugs (the doping list) to treat medical conditions. However, the boundaries between the use of prohibited pharmacological substances under the guise of a Therapeutic Use Exemption and “doping” may be blurred, and there is concern that Therapeutic Use Exemption represents a problem in anti-doping policy (13-15). Additionally, due to the performance enhancing effects of testosterone, inclusion of transgender athletes is balanced with maintaining fairness in elite athletic competition in the female category.
The purpose of this perspectives article is to review key physiological and medical insights related to sex-based physiology associated with exercise and athletic performance. We will review the aspects of medical transition some transgender individuals will undergo, and the available data on the impact of gender affirming medical therapy on athletic performance. We will divide major areas by key questions that informed our conceptual framework. Finally, we will chart future directions and testable hypotheses for the field. A review of individuals with differences of sex development, systematic reviews of the literature, and specific recommendations for sports policy are beyond the scope of this perspectives article (and have been reviewed elsewhere (2, 16)).
What are the key factors that contribute to sex differences in athletic performance?
We focus on sports determined by physiologically-based physical performance that require muscular power and/or endurance of individuals, and these sports are typically segregated on the basis of sex. In this section and the one following, “males” and “females” generally refers to one’s birth sex and the research reflects what is known in cisgender individuals (gender identify aligns with sex).
Before puberty, athletic performance is relatively similar between boys and girls (3, 17-19). Among elite youth swimmers under 10 years of age, girls are ~3% faster than boys (50m to 400m freestyle events) (17). In other sports such as youth elite track running (100m to 800 m) boys are ~3-6 % faster than girls prior to puberty (20). Several studies, however, demonstrate that, overall, girls are less active and have lower sports participation than boys throughout childhood (21), likely due to social factors (22).
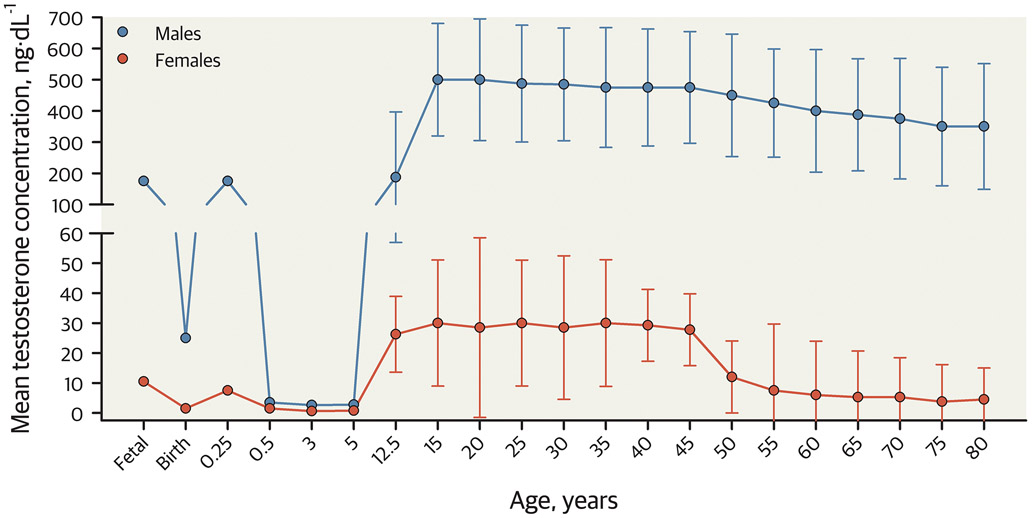
Sex divergence in athletic performance emerges near the onset of puberty among boys (3). Sex differences in strength and athletic performance (running, jumping, swimming, and more) emerge around 12 to 13 years of age, and these differences are associated with the increase in testosterone during puberty in males (3, 17). High concentrations of endogenous testosterone among males are the primary determinant of the large sex difference in athletic performance (17, 23, 24). Circulating testosterone concentrations increase in those with a male sex during puberty because the testes produce significantly more testosterone than prior to puberty, resulting in about 15-fold more testosterone among adolescent males than females of any age (3, 6, 23). There are clear and distinct non-overlapping distributions of testosterone by sex beginning at the onset of puberty, as shown in Figure 1 (23). The typical adult (among those ages 20-30 years) testosterone ranges are 13-71 ng/dL (0.59-2.46 nmol/L) among females and 264-916 ng/dL (9.16-31.79 nmol/L) among males (25), when measured using the gold standard for steroid hormone determination, liquid chromatography-tandem mass spectrometry (LC/MS-MS) (26). Measured testosterone can vary substantially based on the assay used. Testosterone can be measured in its free or bound form (total). Under physiologic conditions, the majority of testosterone is strongly bound to sex hormone binding globulin (SHBG), some is weakly bound to albumin and a small amount circulates as free testosterone (1-3%) (26, 27). Radioimmunoassays and chemiluminescence immunoassays are the most widely used assays for measuring total testosterone, but have limited accuracy with testosterone concentrations below 300 ng/dL (<10.4 nmol/L, the range in women and children), have interference from other androgens, and testosterone concentration is often overestimated (26, 28). Measurement of total testosterone by LC/MS-MS is more accurate, particularly in those with total testosterone concentrations below the adult male range (26). Note, as shown in Figure 1 that male infants have a transient surge of testosterone in early life, known as the minipuberty of infancy (female infants also have a surge of estradiol) (29), although the impact of the minipuberty on body composition, growth velocity and muscle mass remains an understudied area.
Figure 1. Approximate mean total testosterone concentrations of males and females across the lifespan.
Line and scatter plot displaying total testosterone concentrations of individuals with a male sex (blue lines and symbols) and female sex (red lines and symbols) across the lifespan. The estimate of error represents a 95% confidence interval around the mean. This figure shows the non-overlapping and significant sex-related dimorphism in endogenous testosterone concentrations that emerge during adolescence and persist throughout adulthood. Note the drop in testosterone concentrations at birth and the transient “minipuberty” of infancy in early post-natal life. This figure is drawn using data from (60, 129).
After puberty, males on average, are taller than females and have longer limbs; broader shoulders; narrower hips; larger, faster, and more powerful muscles; and less body fat than females (30-33). The muscles of males are faster contracting than females during maximal efforts (34) because the muscle fibers are larger, especially the fast myosin heavy chain fibers (Type II), so that collectively, the whole muscle is more powerful than that of females (32, 35-38). In addition, males have larger hearts and lungs, greater blood volume and more hemoglobin (oxygen carrying capacity in the blood) than females who are similarly active and trained (6, 30, 31, 39-41). These fundamental differences between biologic males and females that emerge at the onset of puberty confer sex-based differences in anatomy and physiology which enable elite males to outperform elite females in sports relying on physical capacity, as subsequently discussed.
What are the known sex differences in athletic performance in adults?
The primary justification to categorically dichotomize elite sport competitions based on sex is because males, on average, have several physiological advantages as delineated below—more muscular power, greater endurance, and anthropometric advantages. Males are faster, jump and throw/pitch further, jump higher, and are stronger and more powerful than females (6, 17, 42-48) (Figures 2 and 3). World records in the male and female categories provide insight into the profound sex-based differences in sports and athletic performance, largely independent of motivation and training. While there is considerable overlap between males and females in performance among non-elite participants, it is well established that the best males always outperform the best females when the sport relies on muscle power, muscle endurance, or aerobic power. Many sports rely on a combination of these attributes and skills. The differences between male and females during and after puberty are based on physiological and anatomical differences between the sexes (3, 6). Hence, males typically outperform females by 10-20% (Figure 3). Several performance characteristics that differ by sex are described below.
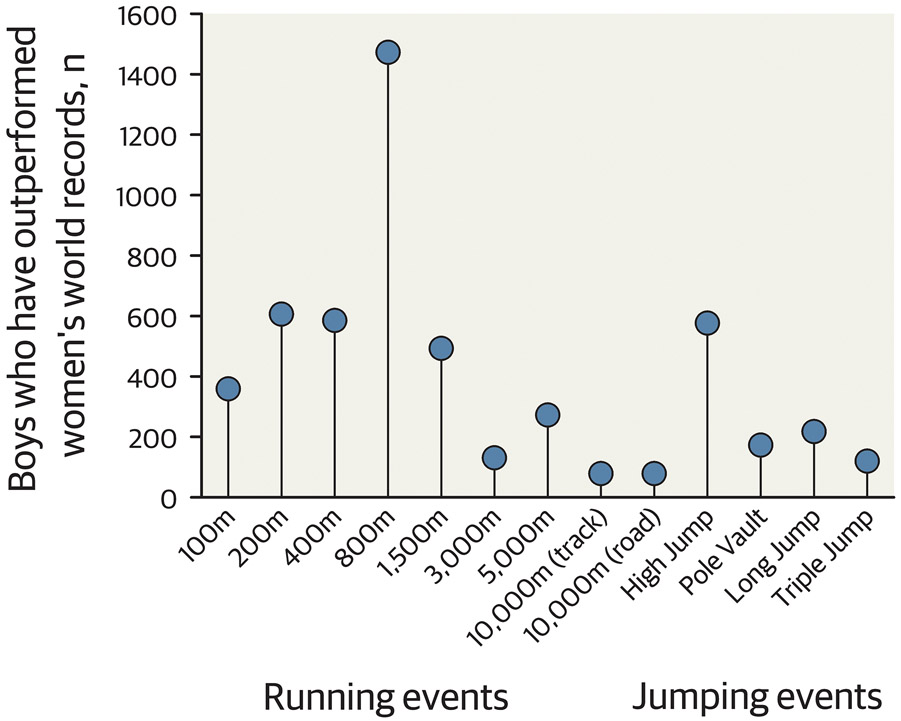
Figure 2. Number of adolescent boys <18 years who would beat the women’s world record time/distance.
This figure depicts the number of males under the age of 18 with a faster time or longer/higher distance than the female’s world record holder for each track and field event. Data retrieved from worldathletics.org.
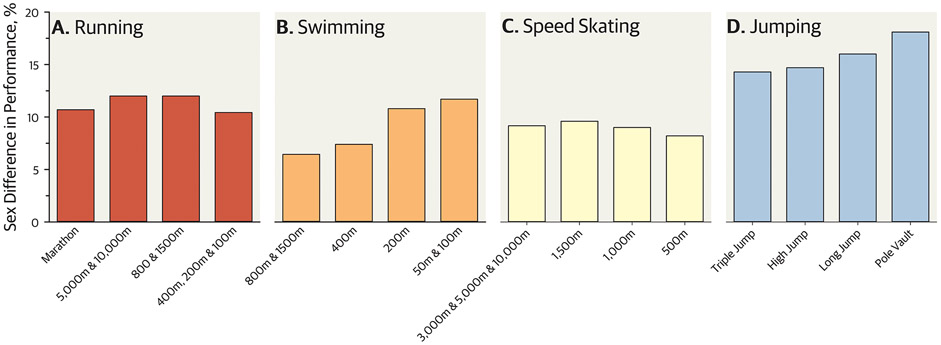
Figure 3. Sex differences in world record times/distances by sport.
This figure shows the percent difference between males and females (positive difference indicates males have better performance than females) in outdoor running (a), freestyle swimming (b), speed skating (c), and outdoor jumping (d) with males being faster or jumping longer/higher in all events. Records are as of November 1, 2022; data extracted from online publicly available databases.
Muscular power.
Muscular power is the product of muscle strength and speed. The muscle mass and power of males can be twice that of females across many age groups (35, 36, 49, 50); because the muscles of males are both larger and contract faster than females, which allows males to produce greater muscle force more quickly than females. There are larger sex-related advantages for males in upper body muscle mass, strength, and power than in the lower body (30, 36, 51, 52). The strength and power of limb muscles in females range from 50-60% that of males in the arm muscles such as the elbow flexor muscles, and ~60-80% in the lower limb muscles (36, 51-53). More powerful muscles for example, allows males to be faster over short distances, throw/pitch further, and jump higher and further than females.
Endurance.
Endurance and longer distance performance are directly related to the ability of the body to consume and utilize oxygen (39, 42). Because males have larger hearts (41), lungs and airways (54, 55), greater blood volume with more hemoglobin and oxygen carrying capacity than females across all abilities and training, the peak or maximal oxygen uptake (aerobic capacity or max) of males is typically ~10%-30% larger than that of females during swimming and running (39, 40) The larger max of males allows them to perform sports at higher velocities for longer periods of time compared with females (39, 56).
Anthropometrics and Body Composition.
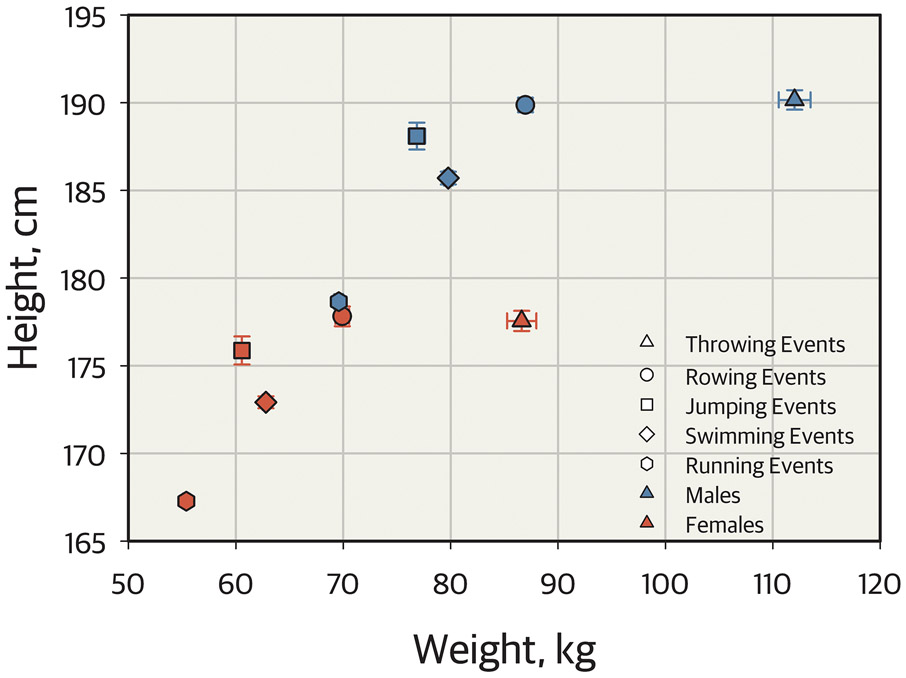
On average, males are taller with greater lean body mass and less fat than females (57, 58). The timing and magnitude of the pubertal growth spurt results in height differences between sexes. Peak height velocity occurs about two years earlier among girls compared to boys, and boys have a higher peak growth velocity and later growth plate closure (a process mediated by estradiol in both sexes) (56). Body composition also changes during puberty, with increased muscle mass in both sexes, but more so in boys, increased fat mass in girls and decreased fat mass in boys (59). Whether taller height of males is advantageous or disadvantageous varies by sports. These sex-based differences in anatomy and body composition underpin sex-based differences in many sports such as rowing, jumping, swimming, running as shown in Figure 4.
Figure 4. Key metrics of body size among male and female athletes competing in the 2012 London Olympic Games.
Bi-directional error scatter plots displaying height and weight of male (blue symbols) and female (red symbols) athletes competing in the 2012 London Olympic Games. This figure represents 272 athletes competing in Athletics throwing events (148 males, 124 females, triangles); 512 athletes competing in rowing events (330 males, 182 females, circles); 163 athletes competing in Athletics jumping events (81 males, 82 females, squares); 860 athletes competing in swimming events (445 males, 415 females, diamonds); and 1,344 athletes competing in Athletics running events (700 males, 644 females, hexagons). As determined using univariate analysis of variance, both height and weight are different between each sporting event (P < 0.001) and larger among males compared to females (P < 0.001). However, there are no interactions of sporting event and sex. This figure represents data from a public repository (130).
Testosterone concentrations.
In males, there is an association between endogenous testosterone concentrations and physical performance, among athletes and non-athletes (3, 6, 17, 60). In elite youth swimmers for example, testosterone increase that occurs in puberty explains 98% of the increase in performance of males (17). Females typically have considerably lower testosterone concentrations; testosterone concentrations in males are at least 15x higher by 18 years. Female track and field athletes with the highest tertile of free testosterone perform significantly better than those in the lowest tertile (61, 62), and serum testosterone concentrations in female athletes is correlated with explosive performance (63). In a randomized, double-blind, placebo-controlled trial, administration of 10 mg testosterone cream daily for 10 weeks in healthy young adult women resulted in an increase in aerobic and anaerobic performance (running time to exhaustion and the Wingate test, respectively), but no changes in squat jump, counter movement jump or knee extension (64). Studies in females that administered even small doses of testosterone for example, show significant increases in fiber cross sectional area for example after 10 weeks of administration (65, 66). Furthermore, males who undergo androgen-deprivation therapy (e.g. for prostate cancer) have modest losses in muscle mass (~2%) over 12 months (67), however, muscular strength and fitness can be mitigated by resistance exercise training (68, 69).
Androgens have been banned from most competitive sports since the 1970s (70, 71) unless there is a documented medical exemption. However, the administration of performance enhancing drugs to athletes is likely one of the largest pharmacological experiments in history. A number of ‘secret’ files which contain detailed documentation of systematic drug abuse in the German Democratic Republic provide key insights into the performance-enhancing effects of testosterone (and related agents) for elite athletes including both males and females (72). Thus, there is decisive evidence that exogenous testosterone can enhance sport performance in both sexes.
What are the medical and surgical interventions that transgender individuals may undergo, and what is the impact of these interventions on athletic performance?
Guidelines for medical and surgical interventions, including eligibility, requirements and expected effects are summarized in The World Professional Association for Transgender Health (WPATH) Standards of Care (73, 74) and the Endocrine Society Guidelines (75). In the following section and in Table 1, we will briefly review these interventions, their expected effects on physiology and known or putative effect on athletic performance. Transition for transgender individuals may involve one or many processes, both social and medical, that aim to align the outward self with one’s internal gender identity. A review of types of transition are outlined in Table 1. Overall, transition is an individualized process based on the individual’s gender goals. Medical transition, including medications used, may vary widely by the individual (including the country or state they live and which medications are available and covered by health insurance).
Puberty delaying medications.
Tanner or Sexual Maturity Rating Scale, is an objective, 5-point classification system (1 to 5) to describe the development and sequence of secondary sex characteristics during puberty (1=prepubertal, 5=puberty is complete) (76). The average age of pubertal onset is age 10-11 years among people with a female sex (range, 8 to 13 years), and 11-12 years among people with a male sex (range, 9 to 14 years) (56). For individuals with a male sex, outward virilization occurs towards the middle-to-latter half of puberty. Gonadotropin releasing hormone (GnRH) agonists are recommended both by WPATH and the Endocrine Society beginning at Tanner stage 2 for eligible transgender individuals to block puberty changes that do not align with the individual’s gender identity (73, 75, 77). Treatment with a GnRH agonist suppresses gonadotropins and sex steroids (testosterone or estradiol) after initial stimulation that can last for 1 to 2 weeks (78). GnRH agonists pause or halt development of secondary sex characteristics concordant with biologic sex and are not used indefinitely as monotherapy (75). Some individuals will continue GnRH agonist therapy and later start exogenous testosterone or estradiol to align with their gender identity and will never develop secondary sex characteristics based on their endogenous gonads.
GnRH agonists are on WADA’s prohibited list for their initial stimulation of gonadotropins (which is followed by suppression 1 to 2 weeks later, unlike GnRH antagonists) (11). There are limited studies available describing the longer-term impact of GnRH agonist therapy on athletic performance, but it is unlikely to be associated with enhanced sport performance. GnRH agonist use in transgender youth is associated with increased body fat and decrease in lean mass after initiation (79, 80), and compared to age- and BMI-matched control youth (81), may also result in worse insulin sensitivity (81). If started before skeletal maturity (aka growth plate closure), GnRH agonists will decrease linear growth and slow or prevent growth plate closure due to suppression of sex steroids, which are necessary for these aspects of puberty (82-84). GnRH agonist monotherapy results in decreased bone turnover and bone mineral apparent density Z-scores of the lumbar spine (85).
Gender affirming hormone therapy
Gender affirming hormone therapy (GAHT) refers to hormones that induce secondary sex characteristics to align the body with one’s gender identity. The Endocrine Society and WPATH guidelines recommend treatment with sex steroids (testosterone or estradiol) for eligible adolescents or adults (73, 75).
Masculinizing transition.
For eligible adolescents and adults, the Endocrine Society recommends a gradually increasing dose schedule of testosterone (75), which causes masculinization of the body with maximum effects after 2 to 5 years of treatment (73, 75). The most common adverse effect of testosterone is erythrocytosis/polycythemia (hematocrit >50%) (75). Some transgender men may have masculinizing surgeries. The most commonly performed surgery is masculinizing chest surgery to remove breast tissue, followed by hysterectomy (86).
Testosterone treatment after puberty among transgender men is associated with increased hemoglobin concentrations, lean body mass and muscle cross sectional area; reduced body fat; and no change in height (Figure 5) (87-90). These physical adaptations result in increased performance of upper and lower body strength and power by ~20% within one year of transition including handgrip strength, (88) the number of pushups able to be performed in 60 seconds, (91) and leg strength (89). The increases in strength are associated with the increase in muscle mass/lean mass (88, 89). These findings of enhanced physical performance, particularly in strength, due to increased muscle mass are consistent with the insight gained over the last 50 years on the effects androgens in athletes (92).
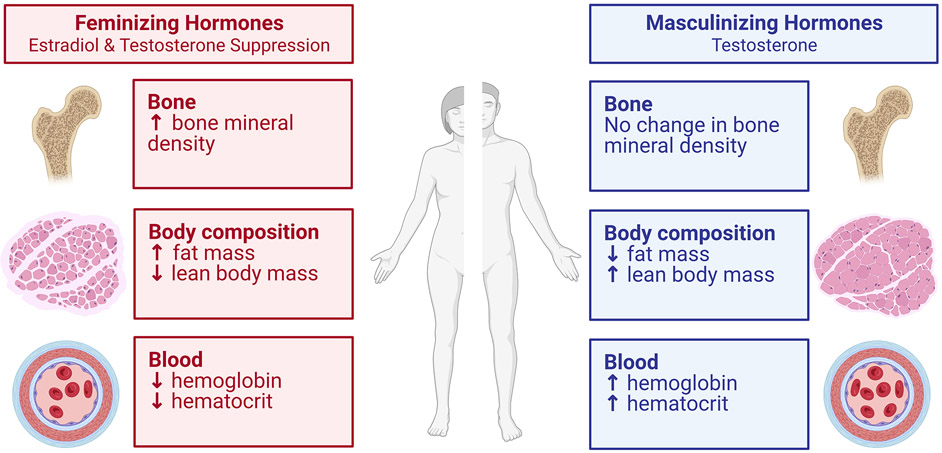
Figure 5. Impact of exogenous hormone therapy on factors relevant to athletic performance.
This figure was created from the known information to date extracted from several articles, systematic reviews and meta-analyses showing the impact of gender affirming hormone therapy (testosterone or estradiol) among transgender individuals on various factors relevant to athletic performance (90, 101, 131, 132). Note that changes may vary by individual, and type and duration of gender affirming hormone therapy. Created with BioRender.com.
Endurance performance also increases in transgender men after masculinizing GAHT, but the timeline does not appear to be as rapid in the change in strength and power. For example, running speed over 1.5 mile distance did not change after one year of masculinizing GAHT with testosterone, but running speed was improved by 16.4% after 2.5 years of masculinizing GAHT (91). This is despite the typically rapid increases (within 4 months) in hemoglobin with testosterone administration (87, 93). A recent study of transgender men in the U.S. Air Force showed that prior to testosterone therapy, transgender men had worse performance than cisgender men in the number of sit-ups and push-ups in one minute and their 1.5 mile run time, until 3 years of testosterone therapy, when performance was similar (94). Thus, comparison of the variations of adaptations in strength, and aerobic performance over time need to be clarified with further studies and for longer periods. Transgender men, on average, will not be as tall as cisgender men. Overall, transgender men can achieve performance levels within the range of cisgender males (91, 94), however, there is no evidence of transgender men outperforming cisgender males among elite athletes, although data on elite athletes are limited. There are no data on performance of those who had endogenous female puberty blocked with a GnRH agonist and subsequently started testosterone.
Feminizing transition.
For eligible adolescents and adults, the Endocrine Society recommends a gradually increasing dose schedule of oral or transdermal 17β-estradiol (75) which causes feminization of the body several months after treatment initiation with maximum effects after 2 to 3 years of treatment (73, 75).
Estradiol alone suppresses testosterone, although doses higher than what would be used as hormone replacement therapy in cisgender women may be used to achieve suppression (95). Estradiol therapy is typically paired with a medication to block or suppress testosterone to <50 ng/dL or 1.7 nmol/L, or within the female range, such as GnRH agonists (96), spironolactone, cyproterone acetate and others, as previously reviewed (97). Notably, the effectiveness of these agents in suppressing endogenous testosterone to typical female ranges varies, with only about 20% of transgender women on spironolactone achieving testosterone concentrations in the range recommended by the endocrine society of <50 ng/dL (1.74 nmol/L), compared to 90% of transgender women on cyproterone acetate (75, 98). GnRH agonists are very effective in testosterone suppression, so much so, that lower doses of estradiol may be used, but GnRH agonists are also the costliest way to suppress testosterone, with variable insurance coverage and availability (99). Individuals who undergo orchiectomy (surgical removal of testicles) no longer require testosterone suppression (as there is no longer any gonadal source of testosterone) and have testosterone concentrations within the cisgender female range (median 0.48 nmol/L) (100). Despite prior regulations that elite transgender athletes undergo orchiectomy to compete in the female competition category (7), there are limited studies evaluating orchiectomy vs. medical testosterone suppression or blockade on athletic performance. The details of medical regimens are included here, to be considered when interpreting the results of the studies in the following paragraphs.
GAHT (including estradiol with testosterone suppression) among transgender women (Figure 5) results in reductions in hemoglobin within 4 months to levels similar to cisgender females and modest reductions in muscle mass and increases in body fat (87, 93, 101). Longitudinal studies demonstrate reductions of lean body mass and muscle cross-sectional area in the first 12 to 36 months of GAHT (33, 101). These modest physiological changes are associated with small reductions or no change in limb strength assessed by hand grip or knee flexion/extension (101-103).
In contrast to the rather short time needed for the increase in muscle mass and strength in the transgender men in response to testosterone, suppression of testosterone among transgender women (who already completed masculinizing puberty) results in small or minimal reductions in strength after one year of GAHT (88, 89, 101). There are more data on handgrip strength than other muscles (101). Handgrip strength adaptations over the first 12 months of estradiol plus testosterone suppression range no change to a slight decline (of note, hormone therapy regimens varied) (101). One study on leg strength reported no significant changes in the leg muscles (knee extensor and knee flexors) over the first 12 months of estradiol therapy plus testosterone suppression with a GnRH agonist (89). The alterations in lean body mass, and strength are summarized in the recent reviews (33, 101). A review of changes seen with GAHT is illustrated in Figure 5.
There are minimal longitudinal studies on endurance performance and strength in transgender women on GAHT. Data is exceedingly sparse on transgender women athletes, but swimming performance may still surpass that of cisgender women after 2 years of GAHT (104). There have been two studies in the U.S. Airforce evaluating changes in physical performance (1.5 mile run, push-ups, sit-ups) following GAHT (91, 94). Prior to initiation of GAHT, transgender women outperformed than cisgender women (91). After one year of hormone therapy (various types of estradiol + spironolactone in 80%, spironolactone + finasteride in 14%, and a GnRH agonist in a minority), transgender women had reductions in physical performance, but retained advantages in sit-ups, push-ups and 1.5-mile run compared to cisgender women (91). These reductions in running performance are likely associated with lower hemoglobin that declines to levels similar to cisgender levels in the first 4-6 months of therapy (33, 101). A recent follow-up study of this cohort showed that transgender women had 1.5-mile run times within the cisgender female range after 2 years of GAHT. After 4 years of GAHT, the number of sit-ups in 1 minute among transgender women was within the range for cisgender females but retained better performance in the number of push-ups in 1 minute (although there were only 5 individuals assessed at the 4-year timepoint) (94). Estradiol and anti-androgen therapy results in relatively rapid declines in hemoglobin, and slower declines in strength and endurance. Limited data, primarily in non-athletes, suggests that after one year of GAHT, adult transgender women (who previously completed masculinizing puberty), may retain advantages in athletic performance compared to cisgender women. A longer duration of GAHT may result in further declines in strength and endurance, although studies and the number of participants followed for 2-4 years or more are limited.
In a recent cross-sectional study comparing non-athlete transgender women (on average of 14.4 ± 3.5 years), absolute peak was higher than cisgender women, but there were no differences in relative peak (average among transgender women was 33.5 mL/kg/min) (105). Handgrip strength between transgender women and cisgender women was not statistically significantly different; the mean value for transgender women was between that of the cisgender men and women (105). Thus, in transgender women who are not athletes (~14 years post transition), their absolute aerobic capacity is greater than cisgender women but not when normalized to body size or lean mass. Studies to determine if these findings are similar among athletes are needed.
Further studies should explore if there are differences by type of testosterone blockade/suppression used concurrently with estradiol, as spironolactone is not very effective at testosterone blockade/suppression, but is the most widely used medication in the U.S., compared to cyproterone acetate (more effective, not available in the U.S., used in Europe) or GnRH agonists (most effective, not widely accessible) (98, 99). Finally, additional information is also needed on the impact of orchiectomy on measures of athletic performance.
Possible legacy effects.
For athletes who start GAHT after puberty, there are some physical characteristics that do not change for both transgender men and transgender women. Most notably is height and limb length (33, 87), and these maybe accompanied by anatomy/physiology that scale with body size, although there have not been studies on factors such as heart, lung or airway size. Thus, transgender women will typically be taller with longer limbs than cisgender female competitors. Likewise, transgender men will likely remain smaller, on average, compared with cisgender men. Recent studies demonstrate that after 4-14 years of GAHT, run time and relative max may be within similar ranges among non-athlete transgender women and cisgender women (94, 105). Most studies are performed in non-athletes, and the impact of training on strength, endurance and other performance measures as individuals start GAHT is needed, as training may mitigate losses in muscle strength (69). Larger studies with more performance variables and among athletes across various sports requiring endurance and muscular power will provide more definitive answers on the duration and magnitude of possible legacy effects among transgender women who completed a masculinizing puberty prior to transition.
What are the socio-political issues facing transgender individuals in sports participation?
Despite the research and political focus on transgender individuals in elite sports, participation in elite sports by transgender athletes to date is a relatively rare occurrence. Some of the increased focus among sporting organizations is likely a result of the IOC’s 2021 framework (9) and the responsibilities of governing bodies for individual sports to create their own policies. In this section, we will highlight the barriers that limit many of the 1.6 million transgender people in the U.S. (106) from participation in recreational sport. Overall, transgender individuals are less physically active compared to their cisgender counterparts (107, 108). A systematic review found that the primary barrier to sports participation was “the lack of inclusive and comfortable environments” and prior negative experiences in sports (109). There are several internal barriers to physical activity and sports participation (gender incongruence, body dissatisfaction/dysphoria, anxiety about others’ reactions) and external barriers (changing and showering facilities, gender-specific sports related clothing and trouble concealing breasts or genitals, and team sports) (110). Factors that facilitate participation include: body satisfaction and changes, gender affirming surgery, safe spaces or “trans only environments” (110). Generally, transgender people feel more comfortable engaging in physical activity after than before transition (111).
There are also several other factors that may limit transgender individuals from participating in sports, which may impact physical and/or mental health (112). At baseline before any hormonal interventions, transgender individuals are more likely to be overweight or obese (113, 114) and have lower bone mineral density than their cisgender counterparts (85, 115-117). Forty percent of transgender adults and 35% of transgender youth have attempted suicide in their lifetime (86, 118), and have a high prevalence of depression, anxiety and eating disorders (119). In cisgender populations, more frequent physical activity and sports participation contribute to well-being and lower levels of anxiety and depression (120), although more research is needed on benefits for transgender individuals. Transgender people who are on GAHT, which is related to higher body satisfaction, are more engaged in physical activity than transgender people not on GAHT (108). Although, for transgender men, many avoid or limit physical activity and sports participation before chest masculinizing surgery (121). Finally, legislation that restricts participation based on birth sex (122) will further decrease health and fitness opportunities and stigmatize transgender individuals in youth in recreational sports.
FUTURE PERSPECTIVES
What are the implications for youth, recreational and elite sporting organizations?
Youth and recreational sport.
There are low participation rates of transgender youth in sports and many possible benefits, in an inclusive space, for mental and physical well-being. Given the small or minimal sex differences in performance prior to puberty (3, 17-19), there is strong evidence supporting that recreation youth sports can be inclusive of those who want to participate. After pubertal changes begin, sex segregation for sports involving endurance, power, and strength (factors associated with higher endogenous testosterone) allows adolescent girls and women to excel.
Elite sport.
Sport-specific safeguards must promote fair competition and participation for all athletes. Given the substantial evidence supporting that testosterone is strongly associated with sex-based differences in sports performance, testosterone may be an ideal biological marker to fairly distinguish competitive cohorts in most sports, but especially those athletic events that rely on strength, speed, power, or endurance. Thus, while striving for fair and inclusive policies for all athletes, the profound influence of testosterone on sports performance should be recognized with appropriate consideration. Prolonged testosterone suppression is associated with decreased hemoglobin, endurance and strength, although additional research, particularly in athletes, is needed. It is unknown how early pubertal blockade followed by estradiol therapy will impact athletic performance for future transgender athletes. Finally, it is not known whether gonadectomy or varying GAHT regimens impact performance. Suggestions for future areas of investigation are in Table 2.
Table 2.
Gaps in the literature and recommendations for future research
| Topic | Possible research questions |
|---|---|
| GnRH agonists |
|
| Gender affirming hormone therapy (GAHT, testosterone or estradiol) |
|
| Testosterone blockade/suppression |
|
| Gonadectomy |
|
| Policy and participation |
|
Heterogeneity of the current literature.
It is important to note that most studies evaluating body composition, strength, endurance, and other factors that may impact physical performance are convenience samples following non-athlete transgender individuals longitudinally before and after GAHT. GAHT treatments regimens vary substantially, even within certain studies (33, 91, 101), based on the country where the study was performed, medications available and covered by insurance, method of androgen blockade or suppression, patient preference, and gender goals. Many studies and meta-analyses pool data from individuals on hormone regimens that vary widely (101) or may not achieve testosterone concentrations in the female range (101, 123). Large multi-center studies are underway which may provide additional insights (124, 125). Although, studies in transgender athletes and the impact of training on measures related to exercise capacity are needed to further understand performance changes after transitioning and GAHT.
In conclusion, testosterone is the primary determinant of sex differences in athletic performance, and transgender women who completed masculinizing puberty prior to starting GAHT show some legacy effects of testosterone in strength and muscle mass, however, more studies beyond 2-3 years of treatment are warranted in athletes and non-athletes. Additional studies are needed to understand the impact of pubertal blockade (GnRH agonist therapy with future GAHT), hormone regimens (specifically which medications are used to block/suppress testosterone), and the impact of gonadectomy in adulthood on athletic performance. Finally, the impact of minority stress and early inclusion or exclusion in recreational sports on future physical and mental well-being and athletic performance are needed to inform on the health of transgender recreational and elite athletes.
Key Points.
Sex-based differences in athletic performance emerge at the onset of puberty and are the basis of binary sport categories and for fairness in the female competition category.
Testosterone is a key determinant of sex-based differences in athletic performance. In sports that rely on strength, speed, power, or endurance, elite males outperform elite females after the onset of puberty.
We describe the hormonal basis of sex differences in athletic performance, and highlight key perspectives related to transgender athletes, including aspects of medical transition. Feminizing and masculinizing hormone therapy alter hemoglobin concentrations, body composition, muscle size and strength, with limited studies on long-term athletic performance.
There are several barriers to recreational sports participation for non-athlete transgender individuals.
Research is needed to inform the long-term implications of endogenous or exogenous testosterone on sports performance among cisgender and transgender athletes to contribute to evidence-based guidelines for sport-specific safeguards that balance inclusion and fair competition.
Disclosure of funding:
NJN: NIH/NHLBI K23 HL151868; NIH/NIA U54 AG062319
Footnotes
Conflicts of Interest: NJN is a consultant for Neurocrine Biosciences, Inc. and Ionis Pharmaceuticals.
References
- 1.Harper J. Athletic gender. Law & Contemp Probs. 2017;80:139. [Google Scholar]
- 2.Harper J. Sporting gender: The history, science, and stories of transgender and intersex athletes: Rowman & Littlefield Publishers; 2019. [Google Scholar]
- 3.Handelsman DJ. Sex differences in athletic performance emerge coinciding with the onset of male puberty. Clin Endocrinol (Oxf). 2017;87(1):68–72. Epub 20170508. doi: 10.1111/cen.13350. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan CF. Gender verification and gender policies in elite sport: Eligibility and “fair play”. Journal of sport and social issues. 2011;35(4):400–19. [Google Scholar]
- 5.National Academies of Sciences E, Medicine. Measuring sex, gender identity, and sexual orientation. 2022. [PubMed] [Google Scholar]
- 6.Handelsman DJ, Hirschberg AL, Bermon S. Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance. Endocr Rev. 2018;39(5):803–29. doi: 10.1210/er.2018-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Committee IO. Statement of the Stockholm consensus on sex reassignment in sports 2003. Available from: https://olympics.com/ioc/news/ioc-approves-consensus-with-regard-to-athletes-who-have-changed-sex-1.
- 8.Committee IO. IOC Consensus Meeting on Sex Reassignment and Hyperandrogenism November 2015 2015. Available from: https://stillmed.olympic.org/Documents/Commissions_PDFfiles/Medical_commission/2015-11_ioc_consensus_meeting_on_sex_reassignment_and_hyperandrogenism-en.pdf.
- 9.Committee IO. IOC Framework on Fairness, Inclusion and Non-Discrimination on the Basis of Gender Identity and Sex Variations 2021. Available from: https://stillmed.olympics.com/media/Documents/News/2021/11/IOC-Framework-Fairness-Inclusion-Non-discrimination-2021.pdf?_ga=2.201127979.429328561.1651852798-298301940.1651852798. [DOI] [PubMed]
- 10.Mosier C transathlete.com [May/6/2022]. Available from: https://www.transathlete.com/.
- 11.Agency WA-D. World Anti-Doping Code International Standard Prohibited List 2022 2022. Available from: https://www.wada-ama.org/sites/default/files/resources/files/2022list_final_en.pdf.
- 12.Gerrard D, Pipe A. Therapeutic Use Exemptions. Med Sport Sci. 2017;62:55–67. Epub 20170601. doi: 10.1159/000460700. [DOI] [PubMed] [Google Scholar]
- 13.Fitch KD. Therapeutic use exemptions (TUEs) at the Olympic Games 1992-2012. Br J Sports Med. 2013;47(13):815–8. Epub 20130416. doi: 10.1136/bjsports-2013-092460. [DOI] [PubMed] [Google Scholar]
- 14.Overbye M, Wagner U. Between medical treatment and performance enhancement: an investigation of how elite athletes experience Therapeutic Use Exemptions. Int J Drug Policy. 2013;24(6):579–88. Epub 20130409. doi: 10.1016/j.drugpo.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Vernec A, Healy D. Prevalence of therapeutic use exemptions at the Olympic Games and association with medals: an analysis of data from 2010 to 2018. Br J Sports Med. 2020;54(15):920–4. Epub 20200506. doi: 10.1136/bjsports-2020-102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper J, Lima G, Kolliari-Turner A, Malinsky FR, Wang G, Martinez-Patino MJ, et al. The fluidity of gender and implications for the biology of inclusion for transgender and intersex athletes. Current sports medicine reports. 2018;17(12):467–72. [DOI] [PubMed] [Google Scholar]
- 17.Senefeld JW, Clayburn AJ, Baker SE, Carter RE, Johnson PW, Joyner MJ. Sex differences in youth elite swimming. PLoS One. 2019;14(11):e0225724. Epub 20191122. doi: 10.1371/journal.pone.0225724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuguchi S, Cunanan AJ, Suarez DG, Cedar WE, South MA, Gahreman D, et al. Performance Comparisons of Youth Weightlifters as a Function of Age Group and Sex. Journal of Functional Morphology and Kinesiology. 2021;6(3):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ervin RB, Fryar CD, Wang C-Y, Miller IM, Ogden CL. Strength and body weight in US children and adolescents. Pediatrics. 2014;134(3):e782–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkinson M, Linde J, Hunter S. Sex Differences in Performance of Elite Youth Track and Field Athletes. American College of Sports Medicine Annual Meeting; June 2023; Denver, CO.2023. [Google Scholar]
- 21.Hyde ET, Omura JD, Fulton JE, Lee SM, Piercy KL, Carlson SA. Disparities in Youth Sports Participation in the U.S., 2017-2018. Am J Prev Med. 2020;59(5):e207–e10. Epub 20200730. doi: 10.1016/j.amepre.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Boiché J, Plaza M, Chalabaev A, Guillet-Descas E, Sarrazin P. Social antecedents and consequences of gender-sport stereotypes during adolescence. Psychology of Women Quarterly. 2014;38(2):259–74. [Google Scholar]
- 23.Senefeld JW, Lambelet Coleman D, Johnson PW, Carter RE, Clayburn AJ, Joyner MJ. Divergence in Timing and Magnitude of Testosterone Levels Between Male and Female Youths. JAMA. 2020;324(1):99–101. Epub 2020/07/08. doi: 10.1001/jama.2020.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark RV, Wald JA, Swerdloff RS, Wang C, Wu FCW, Bowers LD, et al. Large divergence in testosterone concentrations between men and women: Frame of reference for elite athletes in sex-specific competition in sports, a narrative review. Clinical endocrinology. 2019;90(1):15–22. Epub 20180927. doi: 10.1111/cen.13840. [DOI] [PubMed] [Google Scholar]
- 25.Labcorps. Testosterone, Total, Serum, Mass Spectrometry [March/26/2022]. Available from: https://specialtytesting.labcorp.com/tests/500159/testosterone-total-serum-mass-spectrometry.
- 26.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. The Journal of Clinical Endocrinology & Metabolism. 2007;92(2):405–13. [DOI] [PubMed] [Google Scholar]
- 27.Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A Reappraisal of Testosterone's Binding in Circulation: Physiological and Clinical Implications. Endocr Rev. 2017;38(4):302–24. doi: 10.1210/er.2017-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghazal K, Brabant S, Prie D, Piketty M-L. Hormone immunoassay interference: a 2021 update. Annals of laboratory medicine. 2022;42(1):3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanciotti L, Cofini M, Leonardi A, Penta L, Esposito S. Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Frontiers in endocrinology. 2018;9:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. Journal of applied physiology. 2000;89(1):81–8. [DOI] [PubMed] [Google Scholar]
- 31.Bredella MA. Sex Differences in Body Composition. Adv Exp Med Biol. 2017;1043:9–27. Epub 2017/12/11. doi: 10.1007/978-3-319-70178-3_2. [DOI] [PubMed] [Google Scholar]
- 32.Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology. 2015;30(1):30–9. doi: 10.1152/physiol.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilton EN, Lundberg TR. Transgender Women in the Female Category of Sport: Perspectives on Testosterone Suppression and Performance Advantage. Sports medicine. 2021;51(2):199–214. Epub 2020/12/09. doi: 10.1007/s40279-020-01389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delgadillo JD, Sundberg CW, Kwon M, Hunter SK. Fatigability of the knee extensor muscles during high-load fast and low-load slow resistance exercise in young and older adults. Experimental gerontology. 2021;154:111546. Epub 2021/09/08. doi: 10.1016/j.exger.2021.111546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. The Journal of physiology. 2003;552(Pt 1):47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. European journal of applied physiology and occupational physiology. 1993;66(3):254–62. [DOI] [PubMed] [Google Scholar]
- 37.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, et al. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48(5):623–9. [DOI] [PubMed] [Google Scholar]
- 38.Porter MM, Stuart S, Boij M, Lexell J. Capillary supply of the tibialis anterior muscle in young, healthy, and moderately active men and women. J Appl Physiol. 2002;92(4):1451–7. [DOI] [PubMed] [Google Scholar]
- 39.Joyner MJ. Physiological limits to endurance exercise performance: influence of sex. The Journal of physiology. 2017;595(9):2949–54. doi: 10.1113/JP272268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen K, Johansen L, Secher NH. Influence of body mass on maximal oxygen uptake: effect of sample size. European journal of applied physiology. 2001;84(3):201–5. Epub 2001/04/26. doi: 10.1007/s004210170005. [DOI] [PubMed] [Google Scholar]
- 41.Riley-Hagan M, Peshock RM, Stray-Gundersen J, Katz J, Ryschon TW, Mitchell JH. Left ventricular dimensions and mass using magnetic resonance imaging in female endurance athletes. Am J Cardiol. 1992;69(12):1067–74. Epub 1992/04/15. doi: 10.1016/0002-9149(92)90865-v. [DOI] [PubMed] [Google Scholar]
- 42.Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol. 2008;586(1):35–44. Epub 20070927. doi: 10.1113/jphysiol.2007.143834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senefeld J, Smith C, Hunter SK. Sex Differences in Participation, Performance, and Age of Ultramarathon Runners. Int J Sports Physiol Perform. 2016;11(7):635–42. Epub 20151109. doi: 10.1123/ijspp.2015-0418. [DOI] [PubMed] [Google Scholar]
- 44.Hunter SK, Stevens AA. Sex differences in marathon running with advanced age: physiology or participation? Med Sci Sports Exerc. 2013;45(1):148–56. doi: 10.1249/MSS.0b013e31826900f6. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka H, Seals DR. Age and gender interactions in physiological functional capacity: insight from swimming performance. J Appl Physiol (1985). 1997;82(3):846–51. doi: 10.1152/jappl.1997.82.3.846. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka H, Seals DR. Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol. 2008;586(1):55–63. Epub 20070823. doi: 10.1113/jphysiol.2007.141879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keenan KG, Senefeld JW, Hunter SK. Girls in the boat: Sex differences in rowing performance and participation. PLoS One. 2018;13(1):e0191504. Epub 20180119. doi: 10.1371/journal.pone.0191504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan JJ, Knowlton RG, Hetzler RK, Woelke PL. Anthropometric characteristics and performance related predictors of success in adolescent pole vaulters. J Sports Med Phys Fitness. 1994;34(2):179–84. [PubMed] [Google Scholar]
- 49.Sundberg CW, Kuplic A, Hassanlouei H, Hunter SK. Mechanisms for the age-related increase in fatigability of the knee extensors in old and very old adults. Journal of applied physiology. 2018;125(1):146–58. doi: 10.1152/japplphysiol.01141.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alway SE, Grumbt WH, Gonyea WJ, Stray-Gundersen J. Contrasts in muscle and myofibers of elite male and female bodybuilders. J Appl Physiol. 1989;67(1):24–31. [DOI] [PubMed] [Google Scholar]
- 51.Senefeld J, Yoon T, Bement MH, Hunter SK. Fatigue and recovery from dynamic contractions in men and women differ for arm and leg muscles. Muscle & nerve. 2013;48(3):436–9. doi: 10.1002/mus.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senefeld J, Yoon T, Hunter SK. Age differences in dynamic fatigability and variability of arm and leg muscles: Associations with physical function. Experimental gerontology. 2017;87(Pt A):74–83. Epub 2016/12/19. doi: 10.1016/j.exger.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russ DW, Towse TF, Wigmore DM, Lanza IR, Kent-Braun JA. Contrasting influences of age and sex on muscle fatigue. Medicine and science in sports and exercise. 2008;40(2):234–41. doi: 10.1249/mss.0b013e31815bbb93. [DOI] [PubMed] [Google Scholar]
- 54.Ripoll JG, Guo W, Andersen KJ, Baker SE, Wiggins CC, Shepherd JRA, et al. Sex differences in paediatric airway anatomy. Experimental physiology. 2020;105(4):721–31. Epub 2020/02/01. doi: 10.1113/ep088370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dominelli PB, Molgat-Seon Y, Sheel AW. Sex Differences in the Pulmonary System Influence the Integrative Response to Exercise. Exercise and sport sciences reviews. 2019;47(3):142–50. Epub 2019/03/01. doi: 10.1249/JES.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 56.Biro FM, Chan Y-M. Normal puberty. UpToDate Duryea TK, Snyder PJ, Geffner MEMA: Upto Date Waltham. 2017.
- 57.Cavelaars A, Kunst AE, Geurts J, Crialesi R, Grötvedt L, Helmert U, et al. Persistent variations in average height between countries and between socio-economic groups: an overview of 10 European countries. Annals of human biology. 2000;27(4):407–21. [DOI] [PubMed] [Google Scholar]
- 58.Bredella MA. Sex differences in body composition. Sex and gender factors affecting metabolic homeostasis, diabetes and obesity. 2017:9–27. [Google Scholar]
- 59.Travers SH, Jeffers BW, Bloch CA, Hill J, Eckel R. Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. The Journal of Clinical Endocrinology & Metabolism. 1995;80(1):172–8. [DOI] [PubMed] [Google Scholar]
- 60.Senefeld JW, Coleman DL, Johnson PW, Carter RE, Clayburn AJ, Joyner MJ. Divergence in timing and magnitude of testosterone levels between male and female youths. JAMA. 2020;324(1):99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bermon S, Garnier P-Y. Serum androgen levels and their relation to performance in track and field: mass spectrometry results from 2127 observations in male and female elite athletes. British journal of sports medicine. 2017;51(17):1309–14. [DOI] [PubMed] [Google Scholar]
- 62.Bermon S, Hirschberg AL, Kowalski J, Eklund E. Serum androgen levels are positively correlated with athletic performance and competition results in elite female athletes. British journal of sports medicine. 2018;52(23):1531–2. [DOI] [PubMed] [Google Scholar]
- 63.Cardinale M, Stone MH. Is testosterone influencing explosive performance? The Journal of Strength & Conditioning Research. 2006;20(1):103–7. [DOI] [PubMed] [Google Scholar]
- 64.Hirschberg AL, Elings Knutsson J, Helge T, Godhe M, Ekblom M, Bermon S, et al. Effects of moderately increased testosterone concentration on physical performance in young women: a double blind, randomised, placebo controlled study. Br J Sports Med. 2020;54(10):599–604. Epub 20191015. doi: 10.1136/bjsports-2018-100525. [DOI] [PubMed] [Google Scholar]
- 65.Horwath O, Apró W, Moberg M, Godhe M, Helge T, Ekblom M, et al. Fiber type-specific hypertrophy and increased capillarization in skeletal muscle following testosterone administration in young women. Journal of applied physiology. 2020;128(5):1240–50. [DOI] [PubMed] [Google Scholar]
- 66.Bermon S. Androgens and athletic performance of elite female athletes. Current Opinion in Endocrinology, Diabetes and Obesity. 2017;24(3):246–51. [DOI] [PubMed] [Google Scholar]
- 67.Lee H, McGovern K, Finkelstein JS, Smith MR. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer. 2005;104(8):1633–7. [DOI] [PubMed] [Google Scholar]
- 68.Galvao DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Medicine & Science in Sports & Exercise. 2006;38(12):2045–52. [DOI] [PubMed] [Google Scholar]
- 69.Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. Journal of clinical oncology. 2003;21(9):1653–9. [DOI] [PubMed] [Google Scholar]
- 70.Wood RI, Stanton SJ. Testosterone and sport: current perspectives. Horm Behav. 2012;61(1):147–55. Epub 20111001. doi: 10.1016/j.yhbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saudan C, Baume N, Robinson N, Avois L, Mangin P, Saugy M. Testosterone and doping control. Br J Sports Med. 2006;40 Suppl 1(Suppl 1):i21–4. doi: 10.1136/bjsm.2006.027482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franke WW, Berendonk B. Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clin Chem. 1997;43(7):1262–79. [PubMed] [Google Scholar]
- 73.Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, et al. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People: World Professional Association for Transgender Health (WPATH); 2012 2012. [Google Scholar]
- 74.Coleman E, Radix A, Bouman W, Brown G, De Vries A, Deutsch M, et al. Standards of care for the health of transgender and gender diverse people, version 8. International Journal of Transgender Health. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102(11):3869–903. Epub 2017/09/26. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 76.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. Epub 1970/02/01. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ III, Spack NP, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94:3132–54. [DOI] [PubMed] [Google Scholar]
- 78.Roth CL, Brendel L, Rückert C, Hartmann K. Antagonistic and agonistic GnRH analogue treatment of precocious puberty: tracking gonadotropin concentrations in urine. Horm Res. 2005;63(5):257–62. Epub 20050701. doi: 10.1159/000086685. [DOI] [PubMed] [Google Scholar]
- 79.Schagen SE, Cohen-Kettenis PT, Delemarre-van de Waal HA, Hannema SE. Efficacy and Safety of Gonadotropin-Releasing Hormone Agonist Treatment to Suppress Puberty in Gender Dysphoric Adolescents. J Sex Med. 2016;13(7):1125–32. Epub 2016/06/19. doi: 10.1016/j.jsxm.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Klaver M, de Mutsert R, Wiepjes CM, Twisk JWR, den Heijer M, Rotteveel J, et al. Early Hormonal Treatment Affects Body Composition and Body Shape in Young Transgender Adolescents. J Sex Med. 2018;15(2):251–60. Epub 2018/02/10. doi: 10.1016/j.jsxm.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 81.Nokoff NJ, Scarbro SL, Moreau KL, Zeitler P, Nadeau KJ, Reirden D, et al. Body Composition and Markers of Cardiometabolic Health in Transgender Youth on Gonadotropin-Releasing Hormone Agonists. Transgend Health. 2021;6(2):111–9. Epub 20210416. doi: 10.1089/trgh.2020.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cutler GB Jr. The role of estrogen in bone growth and maturation during childhood and adolescence. J Steroid Biochem Mol Biol. 1997;61(3-6):141–4. [PubMed] [Google Scholar]
- 83.Sasano H, Uzuki M, Sawai T, Nagura H, Matsunaga G, Kashimoto O, et al. Aromatase in human bone tissue. Journal of Bone and Mineral Research. 1997;12(9):1416–23. [DOI] [PubMed] [Google Scholar]
- 84.Schulmeister C, Millington K, Kaufman M, Finlayson C, Olson-Kennedy J, Garofalo R, et al. Growth in Transgender/Gender-Diverse Youth in the First Year of Treatment With Gonadotropin-Releasing Hormone Agonists. Journal of Adolescent Health. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vlot MC, Klink DT, den Heijer M, Blankenstein MA, Rotteveel J, Heijboer AC. Effect of pubertal suppression and cross-sex hormone therapy on bone turnover markers and bone mineral apparent density (BMAD) in transgender adolescents. Bone. 2017;95:11–9. [DOI] [PubMed] [Google Scholar]
- 86.James SE, Herman JL, Rankin S, Keisling M, Mottet L, & Anafi M The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality. 2016. [Google Scholar]
- 87.Gooren LJ, Bunck MC. Transsexuals and competitive sports. Eur J Endocrinol. 2004;151(4):425–9. Epub 2004/10/13. doi: 10.1530/eje.0.1510425. [DOI] [PubMed] [Google Scholar]
- 88.Scharff M, Wiepjes CM, Klaver M, Schreiner T, T'Sjoen G, den Heijer M. Change in grip strength in trans people and its association with lean body mass and bone density. Endocr Connect. 2019;8(7):1020–8. Epub 2019/06/28. doi: 10.1530/EC-19-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wiik A, Lundberg TR, Rullman E, Andersson DP, Holmberg M, Mandić M, et al. Muscle Strength, Size, and Composition Following 12 Months of Gender-affirming Treatment in Transgender Individuals. J Clin Endocrinol Metab. 2020;105(3). Epub 2019/12/04. doi: 10.1210/clinem/dgz247. [DOI] [PubMed] [Google Scholar]
- 90.Klaver M, Dekker M, de Mutsert R, Twisk JWR, den Heijer M. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: a meta-analysis. Andrologia. 2017;49(5). Epub 2016/08/31. doi: 10.1111/and.12660. [DOI] [PubMed] [Google Scholar]
- 91.Roberts TA, Smalley J, Ahrendt D. Effect of gender affirming hormones on athletic performance in transwomen and transmen: implications for sporting organisations and legislators. British journal of sports medicine. 2020. Epub 2020/12/09. doi: 10.1136/bjsports-2020-102329. [DOI] [PubMed] [Google Scholar]
- 92.Bhasin S, Hatfield DL, Hoffman JR, Kraemer WJ, Labotz M, Phillips SM, et al. Anabolic-Androgenic Steroid Use in Sports, Health, and Society. Medicine and science in sports and exercise. 2021;53(8):1778–94. Epub 2021/07/16. doi: 10.1249/MSS.0000000000002670. [DOI] [PubMed] [Google Scholar]
- 93.Defreyne J, Vantomme B, Van Caenegem E, Wierckx K, De Blok CJM, Klaver M, et al. Prospective evaluation of hematocrit in gender-affirming hormone treatment: results from European Network for the Investigation of Gender Incongruence. Andrology. 2018;6(3):446–54. Epub 2018/03/31. doi: 10.1111/andr.12485. [DOI] [PubMed] [Google Scholar]
- 94.Chiccarelli E, Aden J, Ahrendt D, Smalley J. Fit Transitioning: When Can Transgender Airmen Fitness Test in Their Affirmed Gender? Military Medicine. 2022. [DOI] [PubMed] [Google Scholar]
- 95.Leinung MC, Feustel PJ, Joseph J. Hormonal treatment of transgender women with oral estradiol. Transgender health. 2018;3(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mamoojee Y, Seal LJ, Quinton R. Transgender hormone therapy: understanding international variation in practice. The Lancet Diabetes & Endocrinology. 2017;5(4):243–6. [DOI] [PubMed] [Google Scholar]
- 97.Angus LM, Nolan BJ, Zajac JD, Cheung AS. A systematic review of antiandrogens and feminization in transgender women. Clinical endocrinology. 2021;94(5):743–52. [DOI] [PubMed] [Google Scholar]
- 98.Burinkul S, Panyakhamlerd K, Suwan A, Tuntiviriyapun P, Wainipitapong S. Anti-androgenic effects comparison between cyproterone acetate and spironolactone in transgender women: a randomized controlled trial. The journal of sexual medicine. 2021;18(7):1299–307. [DOI] [PubMed] [Google Scholar]
- 99.Jensen RK, Jensen JK, Simons LK, Chen D, Rosoklija I, Finlayson CA. Effect of concurrent gonadotropin-releasing hormone agonist treatment on dose and side effects of gender-affirming hormone therapy in adolescent transgender patients. Transgender health. 2019;4(1):300–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Collet S, Gieles N, Wiepjes CM, Heijboer AC, Reyns T, Fiers T, et al. Changes in serum testosterone and adrenal androgen levels in transgender women with and without gonadectomy. J Clin Endocrinol Metab. 2022. Epub 20221006. doi: 10.1210/clinem/dgac576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harper J, O'Donnell E, Sorouri Khorashad B, McDermott H, Witcomb GL. How does hormone transition in transgender women change body composition, muscle strength and haemoglobin? Systematic review with a focus on the implications for sport participation. Br J Sports Med. 2021;55(15):865–72. Epub 20210301. doi: 10.1136/bjsports-2020-103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiik A, Lundberg TR, Rullman E, Andersson DP, Holmberg M, Mandic M, et al. Muscle strength, size, and composition following 12 months of gender-affirming treatment in transgender individuals. The Journal of Clinical Endocrinology & Metabolism. 2020;105(3):e805–e13. [DOI] [PubMed] [Google Scholar]
- 103.Heather AK. Transwoman Elite Athletes: Their Extra Percentage Relative to Female Physiology. International Journal of Environmental Research and Public Health. 2022;19(15):9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Senefeld JW, Hunter SK, Coleman D, Joyner MJ. Case Studies in Physiology: Male to Female Transgender Swimmer in College Athletics. J Appl Physiol (1985). 2023. Epub 20230317. doi: 10.1152/japplphysiol.00751.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alvares LAM, Santos MR, Souza FR, Santos LM, de Mendonça BB, Costa EMF, et al. Cardiopulmonary capacity and muscle strength in transgender women on long-term gender-affirming hormone therapy: a cross-sectional study. British Journal of Sports Medicine. 2022. [DOI] [PubMed] [Google Scholar]
- 106.Herman JL, Flores AR, O'Neill KK. How many adults and youth identify as transgender in the United States? 2022. Available from: https://williamsinstitute.law.ucla.edu/wp-content/uploads/Trans-Pop-Update-Jun-2022.pdf. [Google Scholar]
- 107.Muchicko MM, Lepp A, Barkley JE. Peer victimization, social support and leisure-time physical activity in transgender and cisgender individuals. Leisure/Loisir. 2014;38(3-4):295–308. doi: 10.1080/14927713.2015.1048088. [DOI] [Google Scholar]
- 108.Jones BA, Haycraft E, Bouman WP, Arcelus J. The Levels and Predictors of Physical Activity Engagement Within the Treatment-Seeking Transgender Population: A Matched Control Study. J Phys Act Health. 2018;15(2):99–107. Epub 20171125. doi: 10.1123/jpah.2017-0298. [DOI] [PubMed] [Google Scholar]
- 109.Jones BA, Arcelus J, Bouman WP, Haycraft E. Sport and transgender people: a systematic review of the literature relating to sport participation and competitive sport policies. Sports Medicine. 2017;47(4):701–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jones BA, Arcelus J, Bouman WP, Haycraft E. Barriers and facilitators of physical activity and sport participation among young transgender adults who are medically transitioning. International Journal of Transgenderism. 2017;18(2):227–38. [Google Scholar]
- 111.Elling-Machartzki A. Extraordinary body-self narratives: Sport and physical activity in the lives of transgender people. Leisure Studies. 2017;36(2):256–68. [Google Scholar]
- 112.Barrera E, Millington K, Kremen J. The Medical Implications of Banning Transgender Youth From Sport Participation. JAMA pediatrics. 2022;176(3):223–4. [DOI] [PubMed] [Google Scholar]
- 113.Fornander MJ, Roberts T, Egan AM, Moser CN. Weight Status, Medication Use, and Recreational Activities of Treatment-Naïve Transgender Youth. Childhood Obesity. 2021. [DOI] [PubMed] [Google Scholar]
- 114.Valentine A, Davis S, Furniss A, Dowshen N, Kazak AE, Lewis C, et al. Multicenter Analysis of Cardiometabolic-Related Diagnoses in Transgender and Gender Diverse Youth: a PEDSnet study. J Clin Endocrinol Metab. 2022. Epub 20220810. doi: 10.1210/clinem/dgac469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee JY, Finlayson C, Olson-Kennedy J, Garofalo R, Chan Y-M, Glidden DV, et al. Low bone mineral density in early pubertal transgender/gender diverse youth: Findings from the Trans Youth Care Study. Journal of the Endocrine Society. 2020;4(9):bvaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Joseph T, Ting J, Butler G. The effect of GnRH analogue treatment on bone mineral density in young adolescents with gender dysphoria: findings from a large national cohort. J Pediatr Endocrinol Metab. 2019;32(10):1077–81. doi: 10.1515/jpem-2019-0046. [DOI] [PubMed] [Google Scholar]
- 117.Klink D, Caris M, Heijboer A, van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin-releasing hormone analog treatment and cross-sex hormone treatment in adolescents with gender dysphoria. J Clin Endocrinol Metab. 2015;100(2):E270–5. Epub 20141126. doi: 10.1210/jc.2014-2439. [DOI] [PubMed] [Google Scholar]
- 118.Johns MM, Lowry R, Andrzejewski J, Barrios LC, Demissie Z, McManus T, et al. Transgender Identity and Experiences of Violence Victimization, Substance Use, Suicide Risk, and Sexual Risk Behaviors Among High School Students - 19 States and Large Urban School Districts, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(3):67–71. Epub 2019/01/25. doi: 10.15585/mmwr.mm6803a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nunes-Moreno M, Buchanan C, Cole FS, Davis S, Dempsey A, Dowshen N, et al. Behavioral Health Diagnoses in Youth with Gender Dysphoria Compared with Controls: A PEDSnet Study. J Pediatr. 2022;241:147–53.e1. Epub 20210924. doi: 10.1016/j.jpeds.2021.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McMahon EM, Corcoran P, O’Regan G, Keeley H, Cannon M, Carli V, et al. Physical activity in European adolescents and associations with anxiety, depression and well-being. European Child & Adolescent Psychiatry. 2017;26(1):111–22. doi: 10.1007/s00787-016-0875-9. [DOI] [PubMed] [Google Scholar]
- 121.Mehringer JE, Harrison JB, Quain KM, Shea JA, Hawkins LA, Dowshen NL. Experience of chest dysphoria and masculinizing chest surgery in transmasculine youth. Pediatrics. 2021;147(3). [DOI] [PubMed] [Google Scholar]
- 122.Equality Federation [January/14/22]. Available from: https://www.equalityfederation.org/state-legislation.
- 123.Jarin J, Pine-Twaddell E, Trotman G, Stevens J, Conard LA, Tefera E, et al. Cross-sex hormones and metabolic parameters in adolescents with gender dysphoria. Pediatrics. 2017;139(5). [DOI] [PubMed] [Google Scholar]
- 124.Kreukels BP, Haraldsen IR, De Cuypere G, Richter-Appelt H, Gijs L, Cohen-Kettenis PT. A European network for the investigation of gender incongruence: the ENIGI initiative. Eur Psychiatry. 2012;27(6):445–50. Epub 2010/07/14. doi: 10.1016/j.eurpsy.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 125.Quinn VP, Nash R, Hunkeler E, Contreras R, Cromwell L, Becerra-Culqui TA, et al. Cohort profile: Study of Transition, Outcomes and Gender (STRONG) to assess health status of transgender people. BMJ open. 2017;7(12):e018121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nokoff NJ. Medical Interventions for Transgender Youth. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al. , editors. Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000-2022, MDText.com, Inc.; 2000. [Google Scholar]
- 127.Center TL. [October/27/2022]. Available from: https://transgenderlawcenter.org/resources/id/state-by-state-overview-changing-gender-markers-on-birth-certificates.
- 128.Health NIo. Sexual & Gender Minority Terms and Definitions [cited 2022 September/27/2022]. Available from: https://www.edi.nih.gov/people/sep/lgbti/safezone/terminology.
- 129.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9(12):911–22. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Could you be an athlete? Olympics 2012 by age, weight and height [Internet]. Available from: https://www.theguardian.com/sport/datablog/2012/aug/07/olympics-2012-athletes-age-weight-height.
- 131.Singh-Ospina N, Maraka S, Rodriguez-Gutierrez R, Davidge-Pitts C, Nippoldt TB, Prokop LJ, et al. Effect of sex steroids on the bone health of transgender individuals: a systematic review and meta-analysis. The Journal of Clinical Endocrinology & Metabolism. 2017;102(11):3904–13. [DOI] [PubMed] [Google Scholar]
- 132.Rothman MS, Iwamoto SJ. Bone health in the transgender population. Clinical reviews in bone and mineral metabolism. 2019;17(2):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]