Abstract
Objective
Few studies focus on upper limbs in bilateral cerebral palsy (CP) despite potential bimanual deficits. Electroencephalography (EEG) was utilized to investigate brain mechanisms underlying upper limb tasks in bilateral CP and typical development (TD) and relationships to function.
Methods:
26 (14 CP; 12 TD) completed the Box and Blocks Test and transport task with paper, sponge or mixed blocks, while recording EEG and motion data.
Results:
Group effects for path time, path length and Box and Blocks Test revealed bimanual deficits. Four sensorimotor-related EEG clusters were identified. Group effects were found in premotor and dominant motor clusters with greater beta event-related desynchronization (ERD) in CP. Hand and hand by group effects were found in the dominant motor cluster, showing greater ERD with the more affected hand in CP. Condition effects were prominent in the posterior parietal cluster with higher ERD reflecting greater difficulty in force modulation.
Conclusions:
Higher brain activation associated with greater bimanual deficits is similar to our lower limb findings but contrasts studies in TD or unilateral CP linking higher ERD to greater proficiency.
Significance:
Bilateral CP shows overreliance on the dominant hemisphere with the less functional hand and higher brain activity presumably related to excessive intracortical connectivity.
Keywords: brain injury, brain imaging, pediatric, upper limb, movement, event-related desynchronization
1.0. Introduction
Cerebral palsy (CP) is a group of movement disorders typically caused by brain injuries or malformations before birth or during the perinatal period (Krageloh-Mann and Horber, 2007; Robinson et al., 2009; Towsley et al., 2011). CP can affect one (unilateral) or both (bilateral) sides of the body as well as lower and upper limbs depending on the location and timing of the cortical insult (Graham et al., 2016) For children affected in their upper limbs, studies have found they tend to exhibit slower, less coordinated arm movements and difficulty modulating finger forces, all essential components for manual function(Gordon et al., 2000; Gordon and Duff, 1999). Causes for upper limb dysfunction in CP include muscle weakness, diminished sensation, increased muscle tone, and abnormal muscle synergies (Eliasson et al., 1991; Eliasson et al., 2006; Gordon and Duff, 1999; Koman et al., 2004). Far more studies on upper limb function have been focused on children with unilateral CP than those with bilateral CP, even though the latter may potentially experience significant functional limitations with both hands. The goal of this study was to examine performance during a complex upper limb task in children with bilateral CP compared to an age-matched neurotypical group (designated here as typically developing or TD) with simultaneous recording of electroencephalography (EEG) to evaluate task-related brain activation. To our knowledge, this is the first EEG study on upper limb function focused exclusively on those with bilateral CP.
Advancements in the understanding of the brain mechanisms underlying functional motor deficits in CP were made possible through the emergence of neuroimaging technologies. Brain activation during upper limb tasks in CP has been examined using different imaging modalities with the preponderance of studies utilizing functional magnetic resonance imaging (fMRI) and focusing on children with unilateral CP. Several studies have examined how activation patterns differ when using the paretic compared to the non-paretic hand in unilateral CP or to patterns in typically developing children (Inuggi et al., 2018; Kukke et al., 2015; Van de Winckel et al., 2013; Vandermeeren et al., 2003; Weinstein et al., 2018) Depending on the imaging modality as well as the cohort being studied, sensorimotor cortical representation for the paretic hand in unilateral CP may be most prominent in the ipsilateral (non-lesioned) hemisphere, retained to varying degrees in the contralesional hemisphere, and/or demonstrate a more bilateral activation pattern than seen with the non-paretic hand or either hand of those without brain injury. The functional relevance of these different patterns has also been explored, e.g. Vandermeeren et al. (2003) found that greater activation of the non-injured hemisphere when using the paretic hand in unilateral CP was associated with better hand function. While this may be an adaptive response to the loss of pathways in the injured hemisphere in order to enable the paretic hand to function, it is not necessarily optimal. Araneda et al. (2021) demonstrated that the best outcomes after intensive motor training in unilateral CP were correlated with a decrease in brain activity on the non-lesioned hemisphere and an increase on the lesioned hemisphere. In one of the few EEG studies in this cohort, Inuggi et al. (2018) examined 13 children with unilateral CP and 15 with TD during a reach to grasp task with each hand. Mu band suppression or decrease in alpha power in motor-related brain areas is a defining EEG characteristic during movement. A decrease in power in a certain frequency band is referred to as event-related desynchronization (ERD). Inuggi et al. (2018) found no significant group differences in ERD, but did find an interaction between group and level of function with post hoc results showing that mu ERD magnitude was lower in the central (sensorimotor) region during movement execution but greater in the frontocentral region during movement planning in CP compared to TD, but only when using the more affected arm. They further correlated activation in the lesioned hemisphere with hand function and found that lower ERD magnitude was related to poorer function.
Jongsma et al. (2020) evaluated mu power changes in each hemisphere when using each hand in 45 with TD and 15 with unilateral CP during motor execution, motor imagery and action observation, with the strongest ERD response seen in motor execution. In TD, over the central regions, mu ERD was observed in the contralateral hemisphere during movement with event-related synchronization (ERS) which is an increase in power relative to a resting baseline, in the ipsilateral one, interpreted as a, sign of inter-hemispheric inhibition, with the response similar across hands. In CP, only the uninjured hemisphere showed significant ERD with hand use that was lower in magnitude with the more, compared to the less, affected hand.
Others found no group differences in spectral power between unilateral CP and TD during a hand task in mu or beta frequencies but did find lower coherence between motor-related brain regions in CP (Kukke et al., 2015; Kulak and Sobaniec, 2005). Other functional imaging studies in unilateral CP have demonstrated that brain activation during motor tasks is more widespread, extending more anteriorly (premotor regions) and posteriorly (posterior parietal regions) compared to patterns from those with TD (Kurz et al., 2017; Lee et al., 2013).
Far less is known about cortical activation patterns in those with bilateral CP and there are few, if any, fMRI studies involving motor tasks in this cohort. A recent study by Himmelman (2020) on a large structural MRI dataset collected by the Surveillance of CP in Europe concluded that bilateral brain injuries are associated with greater motor severity than unilateral ones due to greater interference with early network building. Obtaining high quality MRI images in children with greater neurological injury is more challenging, especially when they are asked to perform a motor task in the scanner. Consequently, other modalities not as sensitive to motion artifact have been utilized, although also to a limited extent. In contrast to MRI, magnetoencephalography (MEG) does not elicit startle responses, which are particularly problematic in CP. A MEG study by Hoffman et al. (2019) during a button press task in 12 children with bilateral CP compared to an age-matched group with TD found no group difference in beta ERD magnitude (14–26 Hz) during movement in the sensorimotor hand areas across groups and no interaction, even though hand function was poorer in the group with CP. They did find a low gamma event -related synchronization (ERS) at the point of movement execution and post-movement beta rebound or ERS both of which were weaker in CP than in TD, and which were correlated with poorer motor outcomes.
A previous study from our laboratory (de Campos et al., 2020) utilized functional near-infrared spectroscopy (fNIRS) to evaluate brain activation in the sensorimotor cortical region during hand squeezing in both unilateral and bilateral CP compared to TD. The results in bilateral CP demonstrated that brain activation magnitude and extent increased dramatically with poorer hand function, specifically for those in Manual Ability Classification System [MACS] Level III compared to MACS I and II, the two highest functional levels, and was largely bilateral in this unilateral task. Both EEG and fNIRS are particularly well-suited to measure brain activity during functional movement because the electrodes or optodes are placed on and move with the head, greatly expanding the ability to study children with more significant motor impairments. No quantitative EEG studies have been conducted during upper limb motor tasks in a cohort restricted to only those with bilateral CP.
In this study, we combined EEG with quantitative measures of upper limb motion and motor performance to address the following aims: 1) evaluate and compare motor task performance and cortical activation during a complex upper limb task in children with bilateral CP and TD; and 2) relate task performance to brain activation outcomes in the group with CP. Participants performed a block transport task similar to the Box and Blocks Test, which is a clinically validated measurement tool to assess hand function (Zapata-Figueroa and Ortiz-Corredor, 2022), while EEG was recorded. We further challenged participants by including blocks with two different compositions, either a firm sponge or easily compressible origami paper, which were visually indistinguishable. Three trial conditions were conducted: two with each block type presented separately and one in which they were presented together in a randomly mixed order so that participants could not anticipate finger forces prior to the grasp. We hypothesized that the cohort with CP would have worse motor performance than their peers, especially on the easily compressible paper and mixed block trials based on previous reports on difficulty modulating finger forces. We further anticipated that any group differences in EEG would be more prominent in the paper and mixed block trials and finally that individual differences in motor performance in CP would be correlated with differences in ERD magnitude in mu and beta frequency bands and in ERS magnitude in the low gamma frequency band around movement onset in motor-related cortical regions. The ultimate goal of this study is to inform cortical mechanisms and management of upper limb functional deficits in bilateral CP.
2.0. Methods
2.1. Participants
This study was conducted based on the Code of Ethics of the Declaration of Helsinki, and approved by the Institutional Review Board of the National Institutes of Health (Protocol # 13-CC-0110). All participants and a parent provided informed assent and consent, respectively. Inclusion criteria were a diagnosis of bilateral CP, age 5–17 years, and the ability to reach and grasp objects with both hands (MACS level I-III). The MACS scale comprises five levels with higher levels indicating greater impairment. MACS Level I indicates that the child can handle objects easily and successfully, while a MACS Level V means the child is dependent on their caregiver for upper limb tasks (Eliasson, 2006). Children with uncontrolled seizures were excluded. Additionally, all were asked to refrain from caffeine for 24 hours prior to the study. While visual acuity and IQ were not measured, we confirmed with participants and their families prior to enrollment that they were able to see well enough (with correction as needed) to perform the task and were capable of complying with study instructions.
A total of 32 participants, 16 with bilateral CP and 16 age-matched peers with typical development (TD), were recruited. Two participants with CP and four with TD were excluded, due to an inability to perform the task as instructed (2 TD/1 CP) or poor EEG data quality (2 TD/1 CP). The final dataset included 14 participants with CP (6 males, 8 females; average age: 13.5 ± 2.7) and 12 with TD (7 males, 5 females; average age: 14.0 ± 2.5). Mean age was not significantly different between groups (p = 0.40). The etiology of bilateral CP was periventricular leukomalacia (PVL) secondary to preterm birth for the majority of participants. Demographic data for included participants are summarized in Table 1.
Table 1:
Participant demographics.
| Participant | Age (years) | Handedness | Sex | MACS Level | Gestational Age (weeks) | Etiology |
|---|---|---|---|---|---|---|
| CP01 | 14.4 | R | M | II | 38 | Dyskinetic CP of unknown etiology |
| CP02 | 16.0 | L | F | I | 31 (twin) | PVL |
| CP03 | 17.2 | R | F | I | 28 | PVL |
| CP04 | 14.3 | L | M | I | 29 (twin) | PVL suspected (no MRI) |
| CP06 | 6.5 | R | M | I | 37 | Spastic CP (abnormal MRI; unknown cause) |
| CP07 | 14.4 | L | F | I | 29 (twin) | IVH |
| CP08 | 14.4 | R | F | II | 39–40 | Dystonic CP w/ R hand congenital deformity |
| CP09 | 12.7 | R | F | II | 29 | PVL |
| CP10 | 9.8 | R | F | III | 28 | PVL |
| CP11 | 14.0 | L | M | I | 39 | Spastic CP (normal MRI) |
| CP13 | 14.6 | R | F | III | 25 (twin) | PVL |
| CP14 | 9.3 | R | M | I | 30 | PVL |
| CP15 | 13.3 | L | F | II | 34–35 | PVL |
| CP16 | 13.0 | R | M | II | 31 | PVL |
| CP (n=14) | 13.5±2.7 | 9R/5L | 6M/8F | 7I/5II/3III | ||
| TD01 | 15.5 | R | F | - | - | - |
| TD02 | 13.4 | R | F | - | - | - |
| TD03 | 16.0 | R | M | - | - | - |
| TD05 | 15.0 | R | M | - | - | - |
| TD06 | 18.2 | R | M | - | - | - |
| TD07 | 15.5 | L | M | - | - | - |
| TD08 | 10.5 | R | F | - | - | - |
| TD11 | 15.5 | R | F | - | - | - |
| TD12 | 12.4 | L | F | - | - | - |
| TD13 | 12.4 | R | M | - | - | - |
| TD14 | 14.1 | R | M | - | - | - |
| TD16 | 9.7 | R | M | - | - | - |
| TD (n=12) | 14.0±2.5 | 10R/2L | 7M/5F |
CP = cerebral palsy, TD = typically developing, R = right-hand dominant, L = left-hand dominant, M = male, F = female, MACS level = Manual Ability Classification System [Levels I – III]. PVL = periventricular leukomalacia, MRI = magnetic resonance imaging, IVH = Intraventricular hemorrhage.
2.2. Experimental Procedures
Prior to EEG and motion capture data collection, the Box and Blocks Test of upper limb coordination was administered for each hand, while the child sat comfortably at a table opposite the examiner. The number of blocks transported in one minute was recorded.
Reflective markers were then placed bilaterally on the dorsal midpoints of the wrists, over the second and fifth metacarpals, and on the fingernails of the thumb and index fingers. These markers were tracked by a 12-camera motion capture system (Vicon Motion Systems, Denver, CO). Digital videos were also captured to evaluate task performance.
We used a 64-channel, wireless, active EEG system (Brain Products, Morrisville, NC, United States) and positioned the cap according to the 10–20 international system. Electrode impedances were checked to ensure they were below 20 kΩ. EEG data were referenced to FCz and collected at 1000 Hz. First, two minutes of quiet, seated rest were collected.
During the experiment, participants were instructed to start and end each trial with the designated hand placed on the circle in the center of the table (the rest position). As soon as the start cue was given, they were instructed to transfer the 3 blocks placed in 3 squares on the side of the table corresponding to the designated hand, as quickly as possible one at a time to the corresponding square on the opposite side, starting with the closest block first. (See Figure 1) EEG and motion capture data were collected simultaneously using a trigger to ensure temporal alignment. Each participant performed 15 trials (45 total blocks) for each of three conditions with each hand in the same order across participants: (1) a mix of paper and sponge blocks, (2) paper blocks alone, and (3) sponge blocks alone. For the mixed condition, a random block order was predetermined to minimize anticipatory force adjustments. Children were given the opportunity to rest between conditions and were also told that they could ask to rest at any time between trials as needed.
Figure 1:

Experimental task overview. For each trial, children started with the designated hand in the center circle and once instructed to move, grasped a block starting with the one closest to them, transported the block across midline, and released it in the corresponding square on the opposite side of the table. This was repeated for the other hand.
All experiments were conducted in the middle of the day, either late morning or early afternoon depending on family preference and under controlled conditions (standardized instructions and identical set-up for each participant in a quiet room with minimal distractions) that were consistent throughout the study.
2.2.1. Behavioral Processing
2.2.1.1. Video Data
We videotaped all trials and visually identified errors in block handling, some of which led to a failed transport. Data from trials with failed transports were removed from the analysis.
2.2.1.2. Motion Capture Analysis
Motion capture data were processed using Visual 3D (C-Motion Inc, Germantown, MD). Grasp and release events were identified based on local minimum hand velocities. Additionally, data were averaged within individuals separately for the paper and sponge condition, as well as separately for the paper and sponge blocks from within the mixed trial.
2.2.1.3. EEG Processing
EEG data were processed in MATLAB with the EEGLAB toolbox (MathWorks, Natick, MA). Line noise (60 and 120 Hz) was removed using the Cleanline function. Channels were additionally removed if they exhibited a flatline for longer than 5 seconds, insufficient correlation with their neighboring channels (r < 0.7), and/or noise contamination exceeding a kurtosis of 4 standard deviations from the mean. If a channel was removed for a participant in one condition, it was removed for all other conditions. All datasets were then merged and down-sampled to 250 Hz before applying artifact subspace reconstruction (ASR), which removes non-stereotypical artifacts (Mullen et al., 2013).
Next, we removed any remaining noisy time periods via visual inspection before performing AMICA Independent Component Analysis (ICA). In order to match EEG data to corresponding brain regions, we aligned electrode positions to the standard MNI brain template (Montreal Neurological Institute, Quebec, Canada). The EEGLAB DIPFIT algorithm was subsequently used to generate best-fit equivalent-current dipoles for each independent component (IC) (Oostenveld and Oostendorp, 2002). We subsequently removed ICs with topographical sparseness greater than 5 or dipole residual variance greater than 20% (Bulea et al., 2015; Melnik et al., 2017). We applied the ICA sphering and weighting matrices from the merged file to the individual condition datasets, which did not have ASR applied, to minimize potential loss of cortical data. We rejected non-cortical components using the ICLabel plug-in and visual inspection of the topography, dipoles, and power spectra for each IC (Pion-Tonachini et al., 2019). Time series data from the remaining cortical components were epoched from 500 ms before to 1900 ms after grasp, which retained 96% of all successful transports. The 2-minute rest baseline trials were epoched every 2400 ms, to match the length of the experimental epochs (−500 ms to 1900 ms).
K-means clustering was performed to analyze group-level data. Before clustering, we reversed (left to right) the location of the dipoles of left-hand dominant participants, to align precomputed event-related potentials (ERPs), power spectra, inter-trial coherence (ITC), and scalp maps by dominant and non-dominant hemispheres. Data were then preclustered using Principal Component Analysis (PCA). Time-based information included power spectra (2 to 50 Hz), ERPs (low pass filtered at 20 Hz), and ITC values. Each of these measures was assigned 3 PCA dimensions and a weight of 1 for clustering. Dipole locations were assigned 3 PCA dimensions and a weight of 3.
Clusters were pruned by manually rejecting non-cortical components based on visual inspection of the power spectra and dipoles. For statistical analyses, ERD outcomes (in decibels [dB]) within clusters were split by group (TD and CP) and further subdivided by hand (dominant and non-dominant) and condition (paper, sponge, mixed paper, and mixed sponge). Grasp occurred at time 0 for all epochs. Epoch time-frequency data from the newtimef function were time-warped so that release time occurred at the median within each group. Participants with TD had a median release time of 530 ms, and those with CP had a median release time of 740 ms. For the rest and block transport trials, the power in each IC was calculated by multiplying the time frequency data by its complex conjugate and converting to decibels. We created event-related spectral perturbations (ERSPs) relative to rest by dividing the power in the experimental condition by the mean power per frequency for the rest condition. For each IC and condition, we calculated the mean ERSP across all epochs. For each cluster and participant, minimum event-related desynchronization (ERD) values, i.e. the largest magnitude ERD values. were extracted from the mu (6–12 Hz) and beta (15–30 Hz) frequency bands over the transport period. If a cluster had multiple ICs from one participant, an average ERD was computed across ICs. We initially extracted the largest magnitude event-related synchronization (ERS) from the entire transport period as we had done for the mu and beta bands in the low gamma frequency band (30–50 Hz). Based on Cheyne et al. (2008) who reported that gamma ERS has been shown to be temporal in nature, occurring very close to movement onset, and visual inspection of our ERSP results showing oscillating power including both ERD and ERS throughout the movement, we decided to limit our focus to gamma ERS during task onset. We extracted the maximum ERS values for each participant separated by condition, hand, and cluster surrounding the initial grasp movement at time 0.
2.2.2. Statistical Analysis
Motion capture outcomes (path time, path length), Box and Blocks scores and EEG outcomes (minimum mu and beta ERDs and maximum ERS in the low gamma band at the onset of the movement) were analyzed using the IBM Statistical Package for the Social Sciences (SPSS) V28. A repeated measures general linear mixed model was performed for each frequency band in each cluster, to quantify the effects of hand, condition, and group. If a main or interaction effect was significant (p<0.05), post hoc analyses were performed using independent samples t-tests for between group differences and paired samples t-tests for within-group differences. Pearson correlation analysis was performed to relate behavioral and EEG outcome measures for participants with CP (p<0.05).
3.0. Results
3.1. Motion capture, video and clinical results
There was a significant main effect for group in the Box and Blocks Test (p <0.001); children with TD transported a greater number of blocks with both hands compared to the corresponding hand in children with CP. The non-dominant hand in CP also transferred fewer blocks compared to their dominant hand.
There were no significant differences between or within groups for the number of successful transports across conditions and hands. The mean number of successful transports out of 45 total transports ranged from a low of 37.6 (84%) for the CP non-dominant paper condition to a high of 43.1 (96%) for the TD dominant and non-dominant sponge conditions, with a range across hands and conditions of 37.6 to 42.6 transports in CP and 42.5 to 43.1 transports in TD.
Significant main effects for group were found for both path time and distance (p <0.001 and p = 0.03, respectively), showing that children with CP had longer path times and path lengths when transporting blocks with each hand. Additionally, there was a significant hand by group interaction for path time (p =0.03) explained by the fact that children with CP took longer to transport blocks with their non-dominant versus dominant hand, whereas the TD group had similar timing across hands. Table 2 reports mean values for these outcomes by group and hand There were no condition by group interaction effects.
Table 2:
Mean ± standard deviation for transport oath time in seconds (s) and length in meters (m) by group, hand, and condition and the Box and Blocks Test by group and hand.
| Test | Hand | Condition | TD | CP | |
|---|---|---|---|---|---|
| Path Time (s) | Dominant | Paper | 0.54 ± 0.10 | 0.76 ± 0.21 | |
| Sponge | 0.53 ± 0.11 | 0.75 ± 0.20 | |||
| Mixed: Paper | 0.57 ± 0.09 | 0.81 ± 0.25 | |||
| Mixed: Sponge | 0.59 ± 0.09 | 0.80 ± 0.21 | |||
| Non-dominant | Paper | 0.57 ± 0.16 | 0.98 ± 0.40 | ||
| Sponge | 0.54 ± 0.12 | 0.98 ± 0.48 | |||
| Mixed: Paper | 0.57 ± 0.10 | 1.05 ± 0.45 | |||
| Mixed: Sponge | 0.57 ± 0.10 | 1.03 ± 0.43 | |||
| Path Length (m) | Dominant | Paper | 0.45 ± 0.03 | 0.46 ± 0.04 | |
| Sponge | 0.45 ± 0.04 | 0.47 ± 0.04 | |||
| Mixed: Paper | 0.46 ± 0.03 | 0.47 ± 0.04 | |||
| Mixed: Sponge | 0.46 ± 0.03 | 0.47 ± 0.04 | |||
| Non-dominant | Paper | 0.46 ± 0.04 | 0.48 ± 0.06 | ||
| Sponge | 0.46 ± 0.05 | 0.49 ± 0.09 | |||
| Mixed: Paper | 0.46 ± 0.03 | 0.49 ± 0.07 | |||
| Mixed: Sponge | 0.46 ± 0.04 | 0.49 ± 0.07 | |||
| Box and Blocks | Dominant | 57.9 ± 8.9 | 41.0 ± 13.0 | ||
| Non-dominant | 57.7 ± 7.5 | 34.6 ±14.8 |
TD = typically developing, CP = cerebral palsy.
3.2. EEG Results
Resting power in the dominant and non-dominant hemispheres was computed across groups with the means shown in Table 3. The group with CP had significantly lower resting power in the non-dominant hemisphere mu band compared to TD (p<0.001). There were no significant group differences in beta or low gamma resting power.
Table 3:
Resting power in the mu (6 – 12 Hz) and beta (15 – 30 Hz) frequency bands for (dominant and non-dominant sensorimotor clusters across groups with the only significant difference noted in bold. Resting power is in decibels (dB) and calculated as the mean power within each frequency band.
| Cluster | TD Mu Resting Power (dB) | CP Mu Resting Power (dB) | TD Beta Resting Power (dB) | CP Beta Resting Power (dB) | TD Gamma Resting Power (dB) | CP Gamma Resting Power (dB) |
|---|---|---|---|---|---|---|
| Dominant | 21.2 ± 0.8 | 21.2 ± 2.4 | 12.4 ± 2.6 | 12.3 ± 2.0 | 5.9 ± 2.8 | 7.1 ± 3.2 |
| Non-Dominant | 21.7 ± 1.0 | 19.4 ± 1.0 | 12.1 ± 2.2 | 12.3 ± 2.1 | 5.5 ± 3.3 | 5.9 ± 4.2 |
TD = typically developing, CP = cerebral palsy.
Four motor-related clusters were identified: dominant motor, non-dominant motor, premotor, and posterior parietal. Figure 2 shows the topographical map of these four clusters along with the number of TD and CP participants within each cluster.
Figure 2:
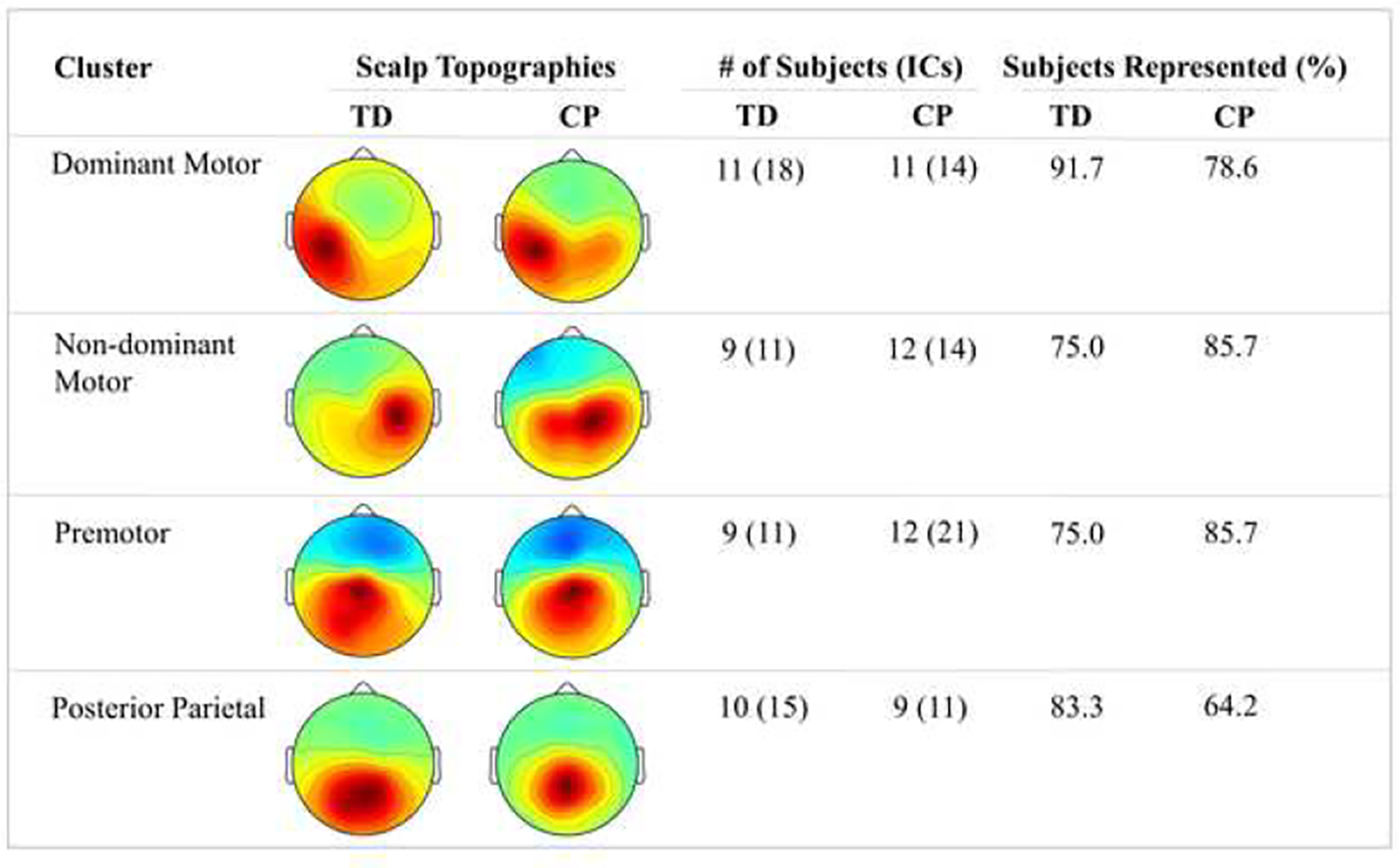
Overview of electroencephalography (EEG) sensorimotor clusters identified during block transport task with the number of independent components (ICs) and percent (%) of participants in each group who demonstrated significant activation in that cluster for the group with typical development (TD) and the group with cerebral palsy (CP).
A detailed summary of EEG findings across group, hand, and condition for motor-related clusters are presented in Figure 3 and Table 4. Only the non-dominant cluster showed no significant main or interaction effects in either frequency band. Supplemental Table 1 provides the mean EEG results by cluster, hand, condition, group, and frequency.
Figure 3:
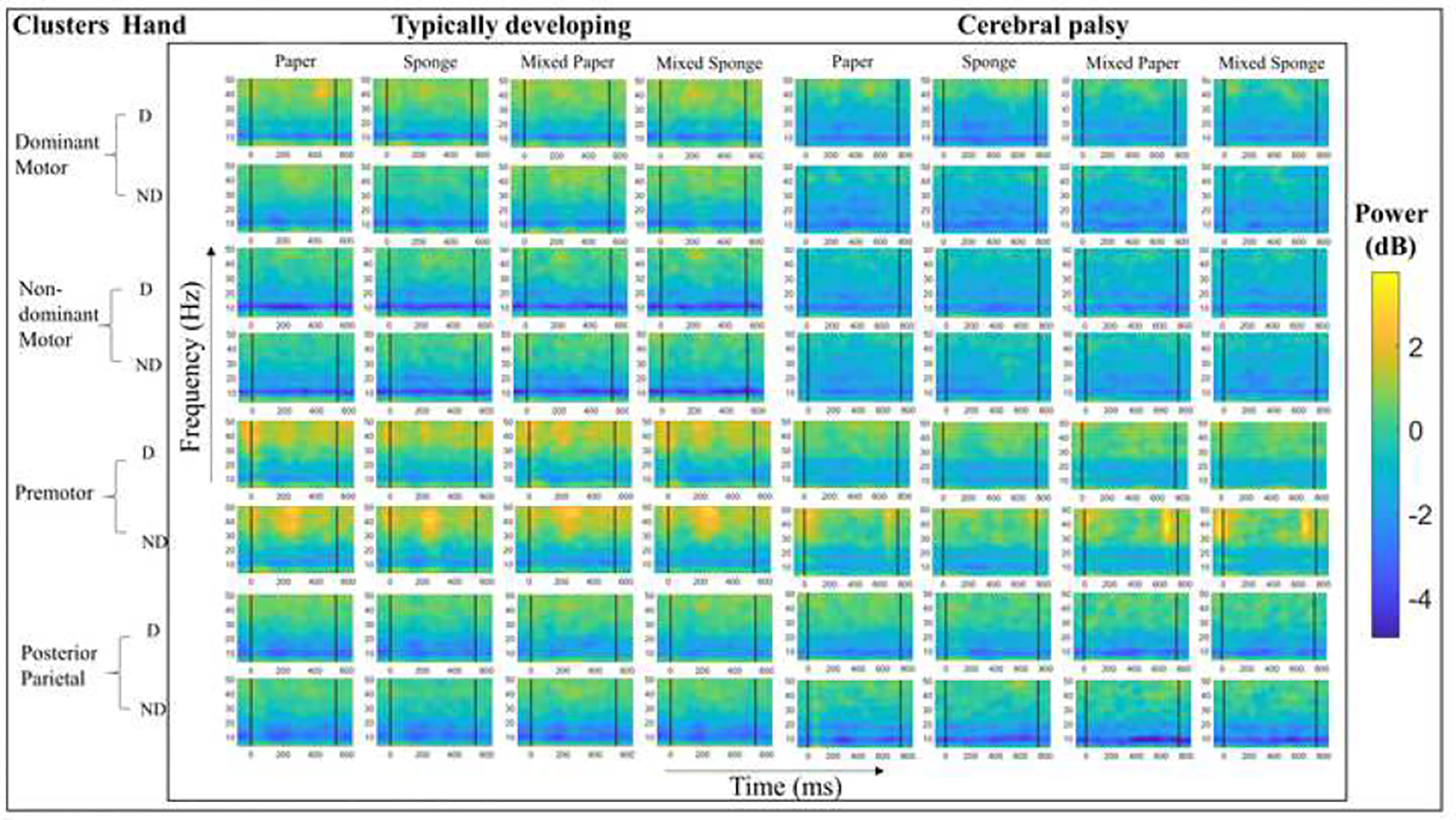
Event-related spectral perturbations (ERSP) within sensorimotor brain regions for each condition, separated by group. ERSP power bar limits (in decibels [dB]) were standardized across all clusters. Vertical bars on ERSPs indicate the grasp and release events, with all plots time-warped to the median transport time value in milliseconds (ms).
Table 4:
Summary of significant (p < 0.05) main and interaction effects for the minimum event-related desynchronization or ERD values in the mu and beta frequency bands and maximum event-related synchronization or ERS values in the low gamma frequency band for each cluster, hand, condition and group.
| Cluster | Frequency | Group | Hand | Hand * Group |
Condition | Condition * Group |
|---|---|---|---|---|---|---|
| Premotor |
Mu
Beta Gamma |
- 0.03 - |
- - |
- - - |
- - - |
- - - |
| Dominant Motor |
Mu
Beta Gamma |
- 0.02 - |
0.03 0.03 0.03 |
0.04 - - |
- <0.001 0.03 |
- - |
| Non – Dominant Motor |
Mu
Beta Gamma |
- - - |
- - - |
- - - |
- - 0.007 |
- - - |
| Posterior Parietal |
Mu
Beta Gamma |
- - - |
- - - |
- - - |
0.01 <0.001 0.01 |
0.01 0.01 - |
Groups: typically developing (TD), cerebral palsy (CP), hands: dominant, non-dominant, conditions: sponge, paper, mixed: sponge, mixed: paper.
3.2.1. Group Effects
No significant between group differences were found in the mu band ERD magnitude for any of the clusters. However, since the resting mu power was significantly lower in the non-dominant hemisphere in CP, we additionally performed the mixed model analysis for the non-dominant motor cluster mu band with baseline mu power as a covariate. A main effect for hand was found with post hoc analyses, revealing that the ERD magnitude was significantly less for the dominant hand in CP (6.08 ± 2.7 dB) compared to the non-dominant hand (7.2 ± 3.6 dB) and to the corresponding hand in TD (7.1 ± 3.0 dB).
Main effects for group were found in the beta band within the premotor and dominant motor clusters, with CP having overall greater magnitude ERD than TD. No significant between group differences were found in the low gamma band ERS magnitude around the time of grasp for any of the clusters.
3.2.2. Hand Effects
Main and interaction effects for hand were only found in the dominant motor cluster. In the mu and beta bands, there were significant main effects for hand (p = 0.03) in both and a significant hand by group interaction (p = 0.04) in the mu band and a trend in the beta band (p = 0.06). In the mu band, the mean ERD magnitudes for the non-dominant hands (TD: −5.8 +/− 3.0 dB; CP: −7.3 +/ 3.1 dB) were greater than those for the dominant hands (TD: −5.7 +/− 2.0 dB; CP: −5.8 +/− 2.0 dB), with the more pronounced magnitude in the CP group in the non-dominant hand leading to the group by hand interaction, In the beta band, a similar but non-significant pattern was present (non-dominant hand - TD: −4.1 +/− 1.3 dB; CP: −6.8 +/− 3.1 dB’ dominant hand - TD: −3.9 +/− 1,1 dB; CP: −4.9 +/− 1.7 dB).
In the low gamma band ERS, a main effect for hand was found in the dominant motor cluster. Post-hoc analysis revealed greater mean gamma ERS magnitude when using the non-dominant hand across conditions, but the mixed sponge condition was the only one that showed a significant difference (p = 0.01), although a similar non-significant trend was found for the paper and mixed paper conditions (p=0.08 for both).
3.2.3. Condition Effects
In the mu band, there was a main effect for condition (p = 0.01) and a condition by group interaction (p = 0.01) only in the posterior parietal region (see Figure 4A). Post hoc analyses to compare conditions within hands for both the TD and CP groups revealed that in their dominant hand, children with CP exhibited greater ERD magnitude for: mixed sponge (−6.8 +/− 3.2 dB) compared to sponge (−5.5 +/− 2.7dB; p = 0.003), mixed paper (−7.1 +/− 3.3 dB) compared to sponge (−5.5 +/− 2.7dB; p = 0.01), and mixed sponge (−6.8 +/−3.2 dB) compared to paper (−5.8 +/− 3.1 dB; p = 0.03). In their non-dominant hand, children with CP exhibited greater ERD magnitude during the mixed paper (−8.11 +/− 2.7 dB) condition compared to sponge (−6.2 +/− 2.9 dB; p = 0.02) and paper (−7.2 +/−2.9 dB) compared to sponge (−6.2 +/− 2.9 dB; p = 0.05). In contrast, children with TD only exhibited a greater ERD magnitude for mixed paper (−7.1 +/− 3.3 dB) compared to mixed sponge (−6.6 +/−3.4 dB) for their non-dominant hand (p = 0.02).
Figure 4:
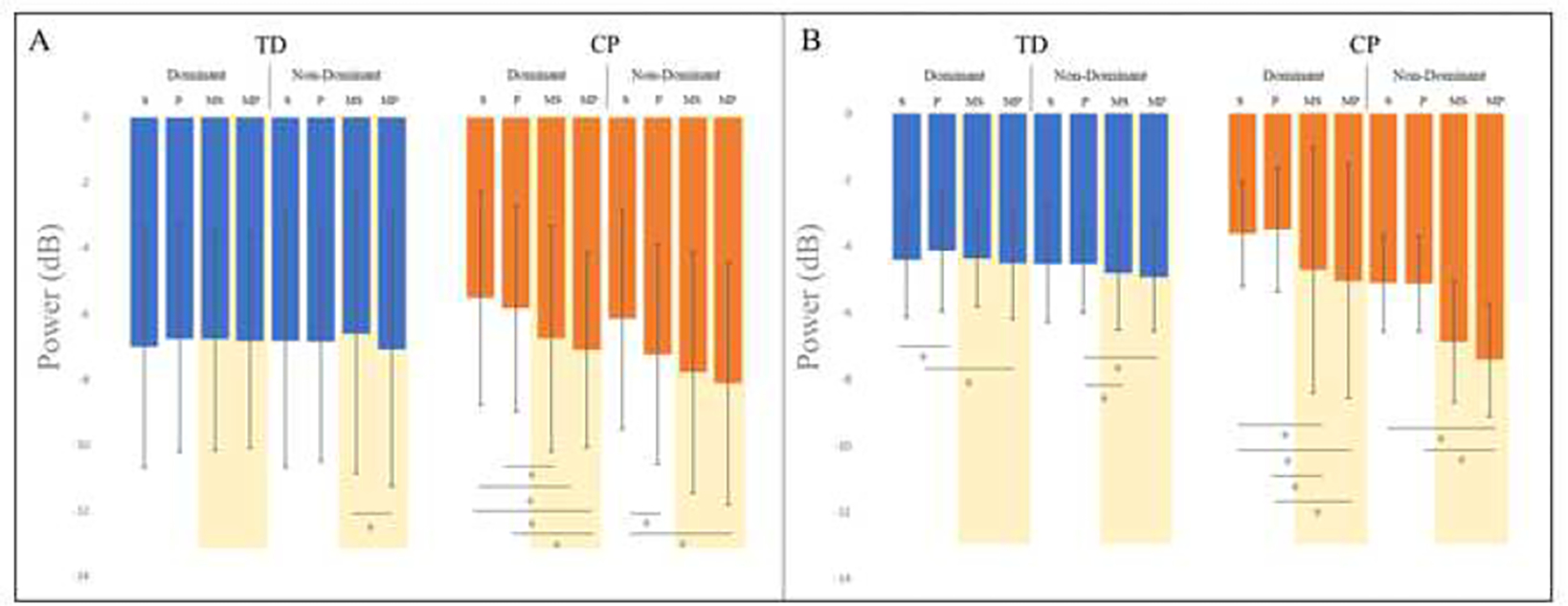
Minimum event-related desynchronization (ERD) magnitudes (in decibels [dB]) by group, hand and condition for the posterior parietal cluster for the mu (Figure 4A) and beta (Figure 4B) frequency bands, with significant post hoc differences for the within group comparisons of those with typical development (TD) and those with cerebral palsy (CP) indicated. Conditions are: S = sponge, P = paper, MS = mixed sponge, and MP = mixed paper with the two mixed conditions shaded.
In the beta band, there was a main effect for condition in the dominant motor and posterior parietal clusters (p < 0.001, p < 0.001) as well as a condition by group interaction in the posterior parietal cluster (p = 0.01). The condition by group effect is explained by the finding that children with CP exhibit notably greater ERD magnitude for mixed conditions (see Figure 4B). In the dominant hand, children with CP exhibited greater ERD magnitude in the mixed conditions compared to the separate conditions: mixed sponge (−4.7 +/− 2.0 dB) compared to sponge (−3.6 +/− 1.0 dB; p = 0.04), mixed sponge (−4.7 +/ 2.0 dB) compared to paper (−3.5 +/− 1.4 dB; p = 0.03), mixed paper (−5.0 +/−1.8 dB) compared to sponge (−3.6 +/1.0 dB)(p = 0.01), and mixed paper (−5.0 +/− 1.8 dB) compared to paper (−3.5 +/− 1.4 dB; p = 0.01). They also exhibited greater magnitude ERD in their non-dominant hand for the mixed paper (−7.4 +/− 4.6 dB) condition compared to paper (−5.1 +/− 2.0 dB; p = 0.02). Children with TD exhibited greater ERD magnitude in their dominant hand for mixed paper (−4.5 +/−1.6 dB) compared to paper (−4.1 +/− 1.5 dB; p = 0.01) and sponge (−4.4 +/− 1.8 dB) compared to paper (−4.1 +/− 1.5 dB; (p = 0.04). In their non-dominant hand, children with TD exhibited greater magnitude ERD for mixed paper (−4.9 +/− 0.5 dB) and mixed sponge (−4.8 +/− 1.5 dB) compared to paper (−4.5 +/− 1.8 dB; p = 0.03 and p = 0.01, respectively).
A main effect for condition was present in the non-dominant motor (p = 0.007), dominant motor (p = 0.03) and posterior parietal regions (p = 0.01). Post hoc analysis revealed that in the non-dominant motor cluster, ERS magnitude was greater when using the non-dominant hand for: mixed sponge [1.8 ± 1.9 dB] compared to paper [1.2 ± 1.5 dB] (p = < 0.001) and sponge [1.2 ± 1.9 dB] (p = 0.003). In the dominant motor region, ERS magnitude was greater when using the dominant hand for: mixed paper [2.2 ± 1.6 dB] compared to paper [1.7 ± 1.4 dB] (p = 0.05) and sponge [1.8 ± 1.4 dB] (p = 0.02). For the non-dominant hand, greater ERS magnitude was present during: mixed sponge [1.7 ± 1.8 dB] compared to sponge [1.3 ± 1.6 dB](p = 0.03), as well as mixed paper [1.8 ± 1.6 dB] compared to paper [1.4 ± 1.3 dB](p = 0.04) and sponge [1.3 ± 1.6 dB] (p = 0.002). Finally, in the posterior parietal region, ERS magnitude was greater when using the dominant hand for: mixed paper [2.0 ± 1.1 dB] compared to sponge [1.6 ± 1.1 dB] (p = 0.05), mixed sponge [2.2 ± 1.0 dB] compared to sponge [1.6 ± 1.1 dB] p = 0.005), and paper [2.0 ± 0.9 dB] compared to sponge [1.6 ± 1.1 dB] (p = 0.02). The ERS magnitude was greater with the non-dominant hand for: mixed paper [2.3 ± 1.4 dB] compared to sponge [1.7 ± 1.3 dB] (p = 0.002) and paper [1.6 ± 1.2 dB] (p = <0.001), as well as mixed sponge [2.3 ± 1.3 dB] compared to sponge[1.7 ± 1.3 dB] p = 0.01) and paper [1.6 ± 1.2 dB] (p = 0.004).
3.3. EEG and Behavioral Correlations in the Group with CP
A total of 48 EEG outcome measures ([4 clusters] X [3 frequency bands] X [4 conditions]) were correlated with the Box and Blocks, path time, and path length measures separately for the dominant and non-dominant hands. All four clusters exhibited significant correlations between EEG activity and behavioral measures. For the non-dominant hand, 20 wof 48 correlations were significant and only 5 of 48 correlations were significant for the dominant hand. Significant correlations were predominantly from the mixed conditions and in the beta band, and higher magnitude ERD values related to poorer motor performance in all cases with the exception of gamma ERS magnitude, which had an inverse correlation in the premotor cluster when using the dominant hand with paper blocks. Table 5 provides a summary of correlations.
Table 5:
Pearson correlation coefficients (r) for all significant results (p < 0.05) between electroencephalography (EEG) and behavioral outcomes (Box and Blocks Test (BBT), transport path length in meters [m] and time in milliseconds [ms]) for individuals with cerebral palsy (CP)EEG outcomes were the minimum event-related desynchronization (ERD) in decibels (dB) for each hand, condition, and mu and beta frequency bands, and the maximum event-related synchronization (ERS) in the low gamma frequency band (30–50 Hz) around the time of the grasp, within clusters. Correlations were only computed between behavioral and EEG outcome measures from the same hand and/or condition. Lower values on transport path length and time indicated better performance. Values (noted with *) between 0.50 and 0.70 are considered moderately correlated (lighter shading) and those above 0.70 are considered highly correlated (darker shading).
| Cluster | Frequency | Hand | Condition | Path Time | Path Length | Box and Blocks |
|---|---|---|---|---|---|---|
| Non-dominant | Mu | ND | Mixed: Paper | −0.48 | −0.39 | 0.61* |
| ND | Mixed: Sponge | −0.54 | −0.67* | 0.58 | ||
| Beta | ND | Paper | −0.46 | −0.31 | 0.71* | |
| ND | Mixed: Paper | −0.67* | −0.80** | 0.68* | ||
| ND | Mixed: Sponge | −0.72** | −0.86** | 0.68* | ||
| Gamma | ND | Mixed: Sponge | 0.48 | 0.63* | −0.22 | |
| Dominant | Mu | ND | Mixed: Paper | −0.37 | −0.60* | 0.36 |
| Beta | ND | Paper | −0.68* | −0.64* | 0.59 | |
| ND | Mixed: Paper | −0.82** | −0.88* | 0.74* | ||
| ND | Mixed: Sponge | −0.78** | −0.82** | 0.68* | ||
| Gamma | ND | Mixed: Sponge | 0.56 | 0.66* | −0.43 | |
| Posterior Parietal | Beta | D | Mixed: Sponge | −0.61 | −0.67* | −0.56 |
| ND | Paper | −0.65 | −0.76* | 0.59 | ||
| ND | Mixed: Paper | −0.75* | −0.91** | 0.69 | ||
| ND | Mixed: Sponge | −0.68* | −0.89** | 0.62 | ||
| Gamma | D | Sponge | 0.32 | 0.67 | −0.25 | |
| Premotor | Mu | ND | Paper | −0.71** | −0.51 | 0.58* |
| ND | Mixed: Paper | −0.80** | −0.82** | 0.57 | ||
| ND | Mixed: Sponge | −0.72** | −0.85** | 0.57 | ||
| Beta | D | Paper | −0.57* | −0.40 | 0.16 | |
| D | Sponge | −0.23 | −0.26 | 0.66* | ||
| ND | Paper | −0.81** | −0.66* | 0.60* | ||
| ND | Mixed: Paper | −0.86** | −0.87** | 0.68* | ||
| ND | Mixed: Sponge | −0.75** | −0.91** | 0.56 | ||
| Gamma | D | Paper | −0.58* | −0.53 | 0.34 |
D = Dominant hand, ND = Non-dominant hand.
4.0. Discussion
The goal of this study was to examine brain activity during a challenging upper limb task in children with bilateral CP as compared to controls without CP. Ideally, mobile brain imaging studies should utilize an integrative approach that simultaneously records brain and motor data, which is possible across a broad range of functional movements (Jungnickel and Gramann, 2016). Unlike the majority of previous studies using EEG in CP, here we utilized source localization techniques that grouped cortical sources into clusters based on anatomical location and timing of activation as opposed to using specific electrodes or sets of electrodes to represent brain regions. This may be especially important when evaluating those with brain injuries who may present with altered anatomy.
Children with unilateral CP typically have one highly functional upper limb with bimanual difficulties largely attributable to difficulties in the opposite limb, whereas children with bilateral CP may have significant deficits in both upper limbs, limiting both unimanual and bimanual function. This was demonstrated in our cohort with bilateral CP who were significantly slower and less directed in their movements as a group in both upper limbs during the task, as well as on the Blocks and Box test, compared to typically developing controls. As part of the first aim, to evaluate and compare motor task performance and cortical activation during a complex upper limb task in children with bilateral CP and TD, we also expected to detect significant behavioral differences between block compositions, which were not found. However, group differences with block compositions were found in EEG outcomes and were related to performance differences across participants with CP. This underscores the value of portable neuroimaging technologies to capture information otherwise not accessible from behavioral data alone. The condition in which the blocks were randomly mixed so that children could not anticipate how much force to exert required considerably more cortical resources in those with CP, especially during use of the more affected hand, which likely has poorer sensorimotor function as shown previously in CP.
Independent component analysis combined with source localization and clustering techniques now allow for the identification and analysis of cortical sources representing specific functional brain processes. Using data from both participant groups, we identified four motor-related clusters: premotor, dominant motor, non-dominant motor, and posterior parietal with similar proportional representation in each group. These four regions represent the frontoparietal network that has been previously identified to support the planning and execution of complex motor tasks (Thorpe et al., 2016) such as the one examined here. In our earlier EEG studies on treadmill walking in unilateral CP and typically developing children (George et al., 2021; Short et al., 2020) and adults (Bulea et al., 2015), we also identified activation in this same network. However, in our unilateral CP studies in contrast to this study in bilateral CP, the non-dominant motor cluster was less represented in CP given the asymmetrical nature of the brain injury (Short et al., 2020).
The concept of a frontoparietal network involved in goal-directed motor tasks is well-established, but it is still an active area of research to more fully understand the contribution of the included brain regions and how they interact. Corbetta and Shulman (2002) discussed “attentional sets” during goal-directed activities that involve the frontoparietal brain regions in both visual identification of a target (perceptual set) and formulation of a response to reach a target (motor set). They proposed that these are controlled mainly by top-down cognitive processes but can be modified by external stimuli (bottom-up). An MRI study by Gallivan et al. (2011) also identified what they referred to as a parieto-frontal network during a task that involved reaching for one of two different cubes or only touching the side of a cube. They described the integration of visuomotor and attention-related processes in specific parts of this network during motor planning that differentiated the three conditions (mainly frontal regions) and others (e.g. sensorimotor cortex) that were more active during movement execution. However, an EEG study by Naranjo et al. (2007) evaluating temporal dynamics instead found that activation in the frontal and parietal brain regions during a reaching task was more simultaneous than sequential supporting a parallel processing system or network where feedback circuits play an important role even before movement onset. Ogawa et al. (2022) presented a more recent and nuanced description of the contributions of different brain regions to what they termed the frontoparietal motor network, with the pre-motor regions more active during movement planning, primary motor and sensory areas during movement execution, and the parietal regions critical for formulating the spatial coordinates for limb movements required to reach the targets.
Interestingly in this study, we were able to identify group differences in the pre-motor and dominant motor regions, which were more prominent when using the more affected hand in CP. The greater amount of ERD in CP showed an overreliance on those regions for both motor planning and execution. A recent study in stroke by Hordacre et al. (2021) found that impairment of the dominant hand in stroke, which was found in our cohort with bilateral CP, is associated with greater activation in the frontoparietal network that was inversely related to dominant corticospinal tract integrity and was believed to be compensatory. This is consistent with the finding by Burton et al. (2009) of increased intracortical connectivity in bilateral CP due to fewer descending motor pathways.
EEG results revealed main effects or interactions across groups, hands, or conditions in three of the four clusters and in mu, beta, and gamma frequency bands, with specific results varying by cluster. The only cluster not showing group, hand, or condition differences in the mu and beta bands was the non-dominant motor cluster. Interestingly, there was no main effect of group for the mu band ERD or low gamma ERS magnitudes in any of the clusters, but there was a main effect for hand and a hand by group interaction in the dominant motor cluster. The non-dominant hand data from both groups in the dominant motor cluster had greater mean ERD magnitude compared to when the dominant hand was used, with this difference more pronounced in CP. This is in contrast to the non-dominant cluster where a difference in activation across hands in CP was not evident. These results suggest more reliance on the dominant motor cluster for sensorimotor tasks or alternately less inhibition to that hemisphere in CP during more affected hand movement as suggested in unilateral CP studies (Jongsma et al., 2020). The group with CP also had greater activation in the beta band in the premotor cluster compared to TD, illustrating the need for greater cortical resources in CP during movement.
The posterior parietal cluster in both the mu and beta bands had a significant main effect for condition and a condition by group interaction for ERD throughout the task. These effects were explained by the CP group notably showing higher magnitude ERD in the more challenging mixed condition. For the gamma band, differences across conditions were seen in all but the pre-motor cluster with an interaction of condition with group only found in the parietal cortex, which represented greater difficulty in CP during the mixed condition.
This finding of the prominent involvement of the posterior parietal region across block conditions is not surprising given its role in planning movements and integrating visual and sensory information with movement to facilitate actions like reaching and grasping, with greater activation magnitude associated with a higher degree of attention during a task (Tzagarakis et al., 2010; Wilhelm et al., 2021). This result reflects the need for those with CP to focus more attention on grasp planning when they were not certain whether objects were easily compressible (Gordon and Duff, 1999).
Increased beta band activation magnitude has been shown in previous studies of children with cerebral palsy (Kurz et al., 2020; Kurz et al., 2017; Short et al., 2020). In the 2017 Kurz study using MEG, 13 children with bilateral cerebral palsy and 15 children with TD performed a target-matching task with the knee, and the children with CP were found to have greater beta ERD magnitude in the primary motor and inferior parietal regions during movement planning and execution. Additionally, greater beta ERD magnitude was correlated to worse behavioral performance (Kurz et al., 2017). In their MEG study from 2020, the Kurz group again found an increase in beta ERD was associated with worse behavioral performance during walking for both the planning and execution stages in children with bilateral cerebral palsy. In the present study, poorer performance in CP was consistently associated with greater ERD magnitude in both the mu and beta bands almost exclusively when using the non-dominant hand (with the exception of one significant correlation found for the dominant hand) and during the mixed condition. Correlation magnitudes were notably quite high (ranged from 0.58 to 0.91), reflecting the functional relevance of the higher activation magnitudes in CP.
Our finding relating greater mu ERD magnitude to poorer function is in contrast to other studies such as Inuggi et al. (2018) which showed that in the non-dominant hemisphere in unilateral CP, less mu ERD magnitude correlated with poorer function. Their finding makes sense for those with loss of brain tissue in one hemisphere with the developmental potential to utilize and thereby retain ipsilateral pathways in the non-inured hemisphere that would otherwise diminish over time. It has also been demonstrated in healthy adults and infants that greater experience or ability in a specific skill tends to be associated with higher activation in the mu band (Thorpe et al., 2016). One potential explanation for the seemingly contradictory mu band correlations in bilateral CP may be that there needs to be a sufficient level of activation that supports skilled movement, beyond which increased activation may be more maladaptive. When there is a bilateral brain injury, the process of networking building is disrupted compared to the situation when one hemisphere is intact (Himmelmann et al., 2020). The brain injury in bilateral CP often involves diffuse white matter injury, with evidence of reduced pathways between the cortex and the lower brain centers such as the thalamus or with spinal motor neurons. The timing of the damage in individuals with bilateral CP, many of whom are born preterm and sustain brain damage in the third trimester, differentially affects the subplate neurons, which adversely reduces thalamocortical connectivity. This was hypothesized to lead to increased intracortical connectivity instead (Burton et al., 2009). Interestingly, in this cohort of children with bilateral CP, damage to the non-dominant motor cluster may not have been so extensive as to correlate with less functionality, as has been reported in unilateral CP (Inuggi et al., 2018). Instead less function was correlated with greater activation in that hemisphere, likely due to a maladaptive increase in connections within that hemisphere, as hypothesized. Also, of note here, although both hands in the bilateral CP cohort had functional limitations, correlations were largely with the non-dominant hand. While this might be a function of range truncation due to less impairment in the dominant hand, it may also be related to specific patterns of brain development or organization in the face of bilateral injury that are not yet well understood.
4.1. Limitations
Adequate sample sizes for these types of analyses have not been well-established, and given that EEG data are noisy, a large number of trials as well as subjects are needed to increase the ability to detect group differences as well as differences in other factors such as hand or condition. However, while our sample size here was fairly modest, a large number of significant EEG differences as well as correlations were uncovered. These were found even though we performed a general linear model including all data within each cluster, which by design increased the p-value to account for multiple comparisons.
Another possible limitation is the inclusion of data across the entire transport task, rather than evaluating separate events such as grasp and release, both of which have been shown to be difficult for children with CP who have upper limb impairments. Given that we were examining multiple factors already, we decided not to isolate these events here, although we plan to examine each of those components more closely in the future.
We were additionally limited by not applying individualized mu and beta frequency ranges, given that prior studies have shown interindividual variation of mu and beta frequency activity due to age and cognitive deficits (Cuevas et al., 2014; George et al., 2021; Klimesch, 1999). Although subject-specific frequency ranges were not applied to this dataset, we did include lower frequency values (6 and 7 Hz) not normally associated with traditional mu (8 – 12 Hz) to account for younger subjects and those with CP who may exhibit lower peak mu frequencies (Cuevas et al., 2014; George et al., 2021)
5.0. Conclusions
This is one of if not the first EEG study examining brain activation during an upper limb task exclusively in children with bilateral CP. It provided novel insights distinguishing the patterns and their functional correlates from those seen previously in unilateral CP, suggesting that brain development and organization may differ substantially when both hemispheres are injured. A dominant versus non-dominant hand and hemisphere still emerges, but the interplay between these warrants closer examination in this population.
The ultimate goal of these investigations is to inform existing and future therapeutic interventions in this population. One obvious conclusion is that upper limb deficits warrant far greater clinical attention in this population. It is also possible that EEG characteristics such as increased beta ERD magnitudes may ultimately be used as biomarkers to diagnose CP if these are similarly evident earlier in development. However, the confounding data across studies on the level of brain activation in specific brain regions and whether it relates to better or poorer skill also warrants rigorous longitudinal studies ideally during motor skill acquisition in those with and without brain injuries. As in unilateral CP, we anticipate that individualized patterns of brain activation may also be uncovered in bilateral CP. If this is the case, we recommend that baseline EEG evaluations be performed prior to prescribing motor training paradigms in this cohort as well. Finally, we anticipate that there will be greater use of EEG as an outcome measure of efficacy as a result of therapeutic intervention, with specific attention as to whether positive behavioral change is related to increased or reduced brain activation magnitude and extent, i.e., plasticity in specific regions post-training.
Supplementary Material
Highlights.
First EEG study evaluating brain mechanisms during upper limb tasks in bilateral CP
Children with bilateral CP demonstrate overreliance on their dominant motor region when using their more affected hand
Greater movement-related EEG power changes in CP are associated with greater task difficulty and poorer motor performance
Acknowledgments
This work was funded by the Intramural Research Program of the NIH Clinical Center (Protocol #: 13-CC-0110).
Footnotes
Disclosure
None of the authors has any disclosures or conflicts related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araneda R, Dricot L, Ebner-Karestinos D, Paradis J, Gordon AM, Friel KM, et al. (2021). Brain activation changes following motor training in children with unilateral cerebral palsy: An fMRI study. Ann Phys Rehabil Med, 64(3), 101502. 10.1016/j.rehab.2021.101502 [DOI] [PubMed] [Google Scholar]
- Bulea TC, Kim J, Damiano DL, Stanley CJ, and Park HS (2015). Prefrontal, posterior parietal and sensorimotor network activity underlying speed control during walking. Front Hum Neurosci, 9. 10.3389/fnhum.2015.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Dixit S, Litkowski P, and Wingert JR (2009). Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosens Mot Res, 26(4), 90–104. 10.3109/08990220903335742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, and Bostan AC (2008). Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. NeuroImage, 42(1), 332–342. 10.1016/j.neuroimage.2008.04.178 [DOI] [PubMed] [Google Scholar]
- Corbetta M, and Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Cuevas K, Cannon EN, Yoo K, and Fox NA (2014). The Infant EEG Mu Rhythm: Methodological Considerations and Best Practices. Dev Rev, 34(1), 26–43. 10.1016/j.dr.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos AC, Sukal-Moulton T, Huppert T, Alter K, and Damiano DL (2020). Brain activation patterns underlying upper limb bilateral motor coordination in unilateral cerebral palsy: an fNIRS study. Dev Med Child Neurol, 62(5), 625–632. 10.1111/dmcn.14458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson AC, Gordon AM, and Forssberg H (1991). Basic co-ordination of manipulative forces of children with cerebral palsy. Dev Med Child Neurol, 33(8), 661–670. 10.1111/j.1469-8749.1991.tb14943.x [DOI] [PubMed] [Google Scholar]
- Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, et al. (2006). The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol, 48(7), 549–554. 10.1017/S0012162206001162 [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Valyear KF, Pettypiece CE, and Culham JC (2011). Decoding action intentions from preparatory brain activity in human parieto-frontal networks. J Neurosci, 31(26), 9599–9610. 10.1523/JNEUROSCI.0080-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George KA, Damiano DL, Kim Y, and Bulea TC (2021). Mu Rhythm during Standing and Walking Is Altered in Children with Unilateral Cerebral Palsy Compared to Children with Typical Development. Dev Neurorehabil, 24(1), 8–17. 10.1080/17518423.2020.1756005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A, Charles J, and Duff S (2000). Fingertip Forces During Object Manipulation in Children with Hemiplegic Cerebral Palsy. II: Bilateral Coordination. Pediatr Phys Ther, 12(4), 195–196. https://www.ncbi.nlm.nih.gov/pubmed/17091032 [PubMed] [Google Scholar]
- Gordon AM, and Duff SV (1999). Fingertip forces during object manipulation in children with hemiplegic cerebral palsy. I: anticipatory scaling. Dev Med Child Neurol, 41(3), 166–175. 10.1017/s0012162299000353 [DOI] [PubMed] [Google Scholar]
- Graham HK, Rosenbaum P, Paneth N, Dan B, Lin JP, Damiano DL, et al. (2016). Cerebral palsy. Nat Rev Dis Primers, 2, 15082. 10.1038/nrdp.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelmann K, Horber V, Sellier E, De la Cruz J, Papavasiliou A, Krageloh-Mann I, et al. (2020). Neuroimaging Patterns and Function in Cerebral Palsy-Application of an MRI Classification. Front Neurol, 11, 617740. 10.3389/fneur.2020.617740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RM, Wilson TW, and Kurz MJ (2019). Hand Motor Actions of Children With Cerebral Palsy Are Associated With Abnormal Sensorimotor Cortical Oscillations. Neurorehabil Neural Repair, 33(12), 1018–1028. 10.1177/1545968319883880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordacre B, Lotze M, Jenkinson M, Lazari A, Barras CD, Boyd L, et al. (2021). Frontoparietal involvement in chronic stroke motor performance when corticospinal tract integrity is compromised. Neuroimage Clin, 29, 102558. 10.1016/j.nicl.2021.102558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuggi A, Bassolino M, Tacchino C, Pippo V, Bergamaschi V, Campus C, et al. (2018). Ipsilesional functional recruitment within lower mu band in children with unilateral cerebral palsy, an event-related desynchronization study. Exp Brain Res, 236(2), 517–527. 10.1007/s00221-017-5149-3 [DOI] [PubMed] [Google Scholar]
- Jongsma MLA, Steenbergen B, Baas CM, Aarts PB, and van Rijn CM (2020). Lateralized EEG mu power during action observation and motor imagery in typically developing children and children with unilateral Cerebral Palsy. Clin Neurophysiol, 131(12), 2829–2840. 10.1016/j.clinph.2020.08.022 [DOI] [PubMed] [Google Scholar]
- Jungnickel E, and Gramann K (2016). Mobile Brain/Body Imaging (MoBI) of Physical Interaction with Dynamically Moving Objects. Front Hum Neurosci, 10, 306. 10.3389/fnhum.2016.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev, 29(2–3), 169–195. 10.1016/s0165-0173(98)00056-3 [DOI] [PubMed] [Google Scholar]
- Koman LA, Smith BP, and Shilt JS (2004). Cerebral palsy. Lancet, 363(9421), 1619–1631. 10.1016/S0140-6736(04)16207-7 [DOI] [PubMed] [Google Scholar]
- Krageloh-Mann I, and Horber V (2007). The role of magnetic resonance imaging in furthering understanding of the pathogenesis of cerebral palsy. Dev Med Child Neurol, 49(12), 948. 10.1111/j.1469-8749.2007.00948.x [DOI] [PubMed] [Google Scholar]
- Kukke SN, de Campos AC, Damiano D, Alter KE, Patronas N, and Hallett M (2015). Cortical activation and inter-hemispheric sensorimotor coherence in individuals with arm dystonia due to childhood stroke. Clin Neurophysiol, 126(8), 1589–1598. 10.1016/j.clinph.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak W, and Sobaniec W (2005). Quantitative EEG analysis in children with hemiparetic cerebral palsy. NeuroRehabilitation, 20(2), 75–84. https://www.ncbi.nlm.nih.gov/pubmed/15920299 [PubMed] [Google Scholar]
- Kurz MJ, Bergwell H, Spooner R, Baker S, Heinrichs-Graham E, and Wilson TW (2020). Motor beta cortical oscillations are related with the gait kinematics of youth with cerebral palsy. Ann Clin Transl Neurol, 7(12), 2421–2432. 10.1002/acn3.51246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Proskovec AL, Gehringer JE, Heinrichs-Graham E, and Wilson TW (2017). Children with cerebral palsy have altered oscillatory activity in the motor and visual cortices during a knee motor task. Neuroimage Clin, 15, 298–305. 10.1016/j.nicl.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Lee DR, Shin YK, Lee NG, Han BS, and You SJ (2013). Comparative neuroimaging in children with cerebral palsy using fMRI and a novel EEG-based brain mapping during a motor task--a preliminary investigation. NeuroRehabilitation, 32(2), 279–285. 10.3233/NRE-130845 [DOI] [PubMed] [Google Scholar]
- Melnik A, Hairston WD, Ferris DP, and Konig P (2017). EEG correlates of sensorimotor processing: independent components involved in sensory and motor processing. Sci Rep, 7(1), 4461. 10.1038/s41598-017-04757-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen T, Kothe C, Chi YM, Ojeda A, Kerth T, Makeig S, et al. (2013). Real-time modeling and 3D visualization of source dynamics and connectivity using wearable EEG. Annu Int Conf IEEE Eng Med Biol Soc, 2013, 2184–2187. doi: 10.1109/EMBC.2013.6609968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo JR, Brovelli A, Longo R, Budai R, Kristeva R, and Battaglini PP (2007). EEG dynamics of the frontoparietal network during reaching preparation in humans. NeuroImage, 34(4), 1673–1682. 10.1016/j.neuroimage.2006.07.049 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Shimobayashi H, Hirayama JI, and Kawanabe M (2022). Asymmetric directed functional connectivity within the frontoparietal motor network during motor imagery and execution. NeuroImage, 247, 118794. 10.1016/j.neuroimage.2021.118794 [DOI] [PubMed] [Google Scholar]
- Oostenveld R, and Oostendorp TF (2002). Validating the boundary element method for forward and inverse EEG computations in the presence of a hole in the skull. Hum Brain Mapp, 17(3), 179–192. 10.1002/hbm.10061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion-Tonachini L, Kreutz-Delgado K, and Makeig S (2019). ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage, 198, 181–197. 10.1016/j.neuroimage.2019.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MN, Peake LJ, Ditchfield MR, Reid SM, Lanigan A, and Reddihough DS (2009). Magnetic resonance imaging findings in a population-based cohort of children with cerebral palsy. Dev Med Child Neurol, 51(1), 39–45. 10.1111/j.1469-8749.2008.03127.x [DOI] [PubMed] [Google Scholar]
- Short MR, Damiano D, Kim Y, and Bulea TC (2020). Children With Unilateral Cerebral Palsy Utilize More Cortical Resources for Similar Motor Output During Treadmill Gait. Front Hum Neurosci, 14, 36. 10.3389/fnhum.2020.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe SG, Cannon EN, and Fox NA (2016). Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Clin Neurophysiol, 127(1), 254–269. 10.1016/j.clinph.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towsley K, Shevell MI, Dagenais L, and Consortium R (2011). Population-based study of neuroimaging findings in children with cerebral palsy. Eur J Paediatr Neurol, 15(1), 29–35. 10.1016/j.ejpn.2010.07.005 [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, and Pellizzer G (2010). Beta-band activity during motor planning reflects response uncertainty. J Neurosci, 30(34), 11270–11277. 10.1523/JNEUROSCI.6026-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Winckel A, Verheyden G, Wenderoth N, Peeters R, Sunaert S, Van Hecke W, et al. (2013). Does somatosensory discrimination activate different brain areas in children with unilateral cerebral palsy compared to typically developing children? An fMRI study. Res Dev Disabil, 34(5), 1710–1720. 10.1016/j.ridd.2013.02.017 [DOI] [PubMed] [Google Scholar]
- Vandermeeren Y, Sebire G, Grandin CB, Thonnard JL, Schlogel X, and De Volder AG (2003). Functional reorganization of brain in children affected with congenital hemiplegia: fMRI study. NeuroImage, 20(1), 289–301. 10.1016/s1053-8119(03)00262-3 [DOI] [PubMed] [Google Scholar]
- Weinstein M, Green D, Rudisch J, Zielinski IM, Benthem-Muniz M, Jongsma MLA, et al. (2018). Understanding the relationship between brain and upper limb function in children with unilateral motor impairments: A multimodal approach. Eur J Paediatr Neurol, 22(1), 143–154. 10.1016/j.ejpn.2017.09.012 [DOI] [PubMed] [Google Scholar]
- Wilhelm RA, Threadgill AH, and Gable PA (2021). Motor Preparation and Execution for Performance Difficulty: Centroparietal Beta Activation during the Effort Expenditure for Rewards Task as a Function of Motivation. Brain Sci, 11(11), 1442. 10.3390/brainsci11111442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata-Figueroa V, and Ortiz-Corredor F (2022). Assessment of Manual Abilities Using the Box and Block Test in Children with Bilateral Cerebral Palsy. Occup Ther Int, 2022, 9980523. 10.1155/2022/9980523 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


