Abstract
Background:
Glycated albumin (GA) is a short-term measure of glycemic control. Several studies have demonstrated an inverse association between body mass index (BMI) and GA, which may affect its performance as a biomarker of hyperglycemia. We investigated cross-sectional associations between GA and multiple measures of adiposity, and compared its performance as a glycemic biomarker by obesity status, in a nationally representative sample of U.S. adults.
Methods:
We measured GA in adults from the 1999–2004 National Health and Nutrition Examination Survey. Separately in adults with and without diabetes, we assessed associations of GA with adiposity measures (BMI, waist circumference, trunk fat, total body fat, and fat mass index) in sex-stratified multivariable regression models. We compared sensitivity and specificity of GA to identify elevated HbA1c, by obesity status.
Results:
In covariate-adjusted regression models, all adiposity measures were inversely associated with GA in adults without diabetes (β=−0.48 to −0.22 %-point GA per 1 SD adiposity measure; n=9,750) and with diabetes (β=−1.73 to −0.92 %-point GA per SD). Comparing adults with versus without obesity, GA exhibited lower sensitivity (43% vs 54%) with equivalent specificity (99%) to detect undiagnosed diabetes (HbA1c≥6.5%). Among adults with diagnosed diabetes (n=1,085), GA performed well to identify above target glycemia (HbA1c≥7.0%), with high specificity (>80%) overall but lower sensitivity in those with versus without obesity (81% vs 93%).
Conclusions:
Inverse associations between GA and adiposity were present in people with and without diabetes. GA is highly specific but may not be sufficiently sensitive for diabetes screening in adults with obesity.
Keywords: adiposity, diabetes, diagnosis, glycated albumin, glycated hemoglobin, obesity
Introduction
Glycated albumin (GA) is formed by nonenzymatic glycation of albumin and is a shorter-term marker of glycemic control as compared to hemoglobin A1c (HbA1c) (1). GA responds more rapidly to changes in glycemic control, reflecting average glucose concentrations over approximately the past 2–3 weeks, versus 2–3 months for HbA1c. Whereas HbA1c requires a whole blood sample and is affected by red blood cell turnover, GA can be measured in plasma or serum and is unaffected by conditions that affect hemoglobin or red blood cell turnover, such as anemias and some hemoglobin variants. The U.S. Food and Drug Administration approved GA for clinical use for the management of diabetes in 2017. However, there are no current guidelines for diagnosing prediabetes and diabetes with GA, and diagnostic cut-points have not been established.
Adiposity is associated with insulin resistance (2); therefore, people with greater adiposity are more likely to have hyperglycemia and high levels of glycemic biomarkers. Positive associations of adiposity with fasting glucose and HbA1c are well-established (3–7). Yet several studies have reported a perplexing inverse association between GA and body mass index (BMI) (3–10). Inverse associations between GA and visceral fat mass (11,12) and waist circumference (13) have also been demonstrated. These observations are primarily derived from small study samples, in mostly Asian populations. Whether these findings are generalizable to the U.S. population is not known. Furthermore, the impact of the inverse association on diabetes diagnosis and glycemic monitoring has not been evaluated. Given that obesity has a high prevalence in the U.S. and is a strong risk factor for diabetes, understanding the association between adiposity and GA is essential to guide its use in diabetes care.
In this study, we assessed the associations between GA and several measures of total and regional adiposity in a nationally representative sample of U.S. adults, stratified by sex and diabetes status. Adiposity measures included BMI and waist circumference, which are clinically accessible indirect measures of total and central adiposity; percent total body and trunk fat, which are direct measures of total and central adiposity; and fat mass index, a height-adjusted measure of total adiposity. We also compared the sensitivity and specificity of GA for detecting hyperglycemia (defined by elevated HbA1c) in adults with and without obesity.
Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional study conducted continuously to monitor the health of the noninstitutionalized U.S. civilian population. Participants are selected using a complex, stratified, multistage probability cluster sampling strategy to obtain a nationally representative sample. The National Center for Health Statistics ethics review board approved the stored serum study and participants provided informed consent.
We measured GA in stored serum samples from 1999–2004 NHANES participants who attended the in-person examination, provided blood samples, and consented to their use for future research. We restricted our study population to non-pregnant adults aged ≥20 years with stored samples that had not previously undergone a freeze-thaw cycle (Supplemental Figure 1) (14). We excluded participants with missing C-reactive protein (CRP), HbA1c, GA, or covariates and those with BMI<18.5 kg/m2 due to small numbers of participants. Adiposity measurements for some individuals were missing (15,16), resulting in a sample of 10,835 adults.
Anthropometric and Adiposity Measurements
Anthropometric and adiposity measurements were obtained by trained staff at the Mobile Examination Center. Weight was measured using a digital scale with minimal clothing and shoes removed. BMI was calculated as the ratio of weight (in kilograms) to height (in meters) squared. Waist circumference was measured along the horizontal plane level with the intersection of the right iliac crest and midaxillary line. Total body fat and trunk fat mass were measured using whole body dual-energy x-ray absorptiometry (DXA) scans taken with a Hologic QDR-4500A fan-beam densitometer (Hologic, Inc., Bedford, Massachusetts). Percent total body fat and percent trunk fat mass were calculated as fat mass (total body or trunk) divided by total mass (whole body or truncal region), multiplied by 100. Fat mass index was calculated as the ratio of fat mass (in kilograms) to height (in meters) squared. Multiply imputed DXA datasets were used, per National Center for Health Statistics recommendations, to obtain unbiased estimates with accurate standard errors (15).
Laboratory Measurements
We measured total and glycated albumin in serum specimens obtained through the NHANES Biospecimen Program (17). Assays were performed at the University of Maryland School of Medicine (Baltimore, MD) between 2018 and 2020 using the method of Asahi Kasei Pharma (Lucica-GA-L) (18) adapted to the Siemens Dimension Vista 1500 (Siemens Healthcare Diagnostics). GA was expressed as a percentage of total albumin [glycated albumin (g/dL)/serum albumin (g/dL)/1.14*100 + 2.9] (18).
Other biomarkers were measured according to the original 1999–2004 NHANES protocols. HbA1c was measured at the University of Missouri-Columbia by boronate affinity high performance liquid chromatography using on a Primus CLC 330 and Primus CLC 385 (Primus Corporation, Kansas City, MO) (19). CRP was measured to the nearest 0.01 mg by latex-enhanced nephelometry at the University of Washington Medical Center using a Dade Behring Nephelometer (Dade Behring Diagnostics Inc., Somerville, NJ) (20). Estimated glomerular filtration rate (eGFR) was calculated using the creatinine- and cystatin C-based 2021 Chronic Kidney Disease Epidemiology Collaboration formula without race (21).
Diabetes Definitions
We determined diabetes status based on self-reported diagnosis by a doctor or health professional. Among people without diagnosed diabetes, we defined undiagnosed diabetes as HbA1c ≥6.5% and prediabetes as HbA1c ≥5.7% (22). Among people with diagnosed diabetes, we defined glycemic control as HbA1c <7.0% (23).
Statistical Analyses
We stratified analyses by diagnosed diabetes status and by sex, as the statistical distributions of adiposity measures are shifted (mean percent total body and trunk fat are higher, mean waist circumference is lower) and wider (larger standard deviations) in women versus men, and the regional distribution of body fat differs by sex (24). We present participant characteristics by National Institute of Health BMI classifications of normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obesity (30–34.9, 35–39.9, and ≥40 kg/m2) (25). We calculated weighted Pearson’s correlations between GA, HbA1c, and adiposity measures, and visualized unadjusted associations using restricted cubic splines with four knots (at the 5th, 35th, 65th, and 95th percentiles).
We used multivariable linear regression to assess associations of GA and HbA1c with each measure of adiposity, modeled linearly per weighted sex-specific standard deviation. To quantify the average adiposity-associated separation between GA and HbA1c, we also assessed associations between adiposity measures and the ratio of GA to HbA1c (GA:HbA1c). A positive association indicates that the difference in GA is greater than the difference in HbA1c as adiposity increases (i.e. more GA is present relative to HbA1c), whereas a negative association indicates that the difference in GA is less than that of HbA1c as adiposity increases (less GA is present relative to HbA1c). Model 1 adjusted for covariates associated with adiposity and GA: age (continuous), self-identified race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other), eGFR (modeled as a linear spline with a knot at 60 mL/min/1.73 m2), and smoking status (current, former, never). Model 2 included model 1 covariates and additionally controlled for hypothesized mediators of the relationship between adiposity and GA: albumin (g/dL; model 2a) or natural log-transformed CRP (mg/dL; model 2b). We also calculated weighted Pearson’s correlations between GA and albumin, GA and log-transformed CRP, and absolute GA (in g/dL) and albumin.
Among people without diabetes, we evaluated the performance (sensitivity, specificity) of GA to identify people with prediabetes or undiagnosed diabetes at GA percentiles corresponding to the diagnostic HbA1c cut-points (5). Among people with diagnosed diabetes, we compared GA to “above-target” HbA1c (≥7.0%).
As a sensitivity analysis, we repeated analyses after additionally excluding participants with conditions that could affect serum albumin turnover (severe hypoalbuminemia [<3 g/L]; severe kidney disease [eGFR <30 mL/min/1.73 m2 or albumin-creatinine ratio >300 mg/g] or kidney failure; self-reported thyroid disorder; and self-reported liver disease or positive test for viral hepatitis) or red blood cell turnover (anemia, defined as hemoglobin <13.0 g/dL in men, <12.0 g/dL in women (26)).
Analyses were performed using Stata/SE 17.0. All statistical tests were two-sided and the significance level was set at alpha=0.05.
Data Availability
The datasets analyzed in the current study are publicly available online (27).
Results
Of the 10,835 sample participants included in our analyses, 1,085 self-reported a diabetes diagnosis. GA and HbA1c were highly correlated in the overall study population (r=0.77), with moderate correlation in people without diabetes (r=0.52) and a stronger correlation in people with diabetes (r=0.87) (Supplemental Figure 2).
Adults without diabetes
Among adults without diabetes, mean GA and GA:HbA1c were, respectively, 12.9% (SE: 0.1%) and 2.32 (SE: 0.01) in those with obesity and 13.4% (SE: 0.1%) and 2.51 (SE: 0.01) in adults without obesity. Mean GA was lower and HbA1c was higher at higher BMI categories in both men and women (Table 1). All adiposity measures were negatively correlated with GA (r=−0.06 to −0.19) and GA:HbA1c (r=−0.27 to −0.42) and positively correlated with HbA1c (r=0.22 to 0.30) (Supplemental Table 1). Inverse correlations between adiposity and GA were stronger in women versus men for all measures, whereas sex-specific correlations between adiposity and HbA1c were similar.
Table 1.
Characteristics of U.S. adults without diabetes (n=9,750) and with diabetes (n=1,085) by BMI category, in NHANES 1999–2004.a
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| 18.5–24.9 kg/m2 | 25–29.9 kg/m2 | 30–34.9 kg/m2 | ≥35 kg/m2 | 18.5–24.9 kg/m2 | 25–29.9 kg/m2 | 30–34.9 kg/m2 | ≥35 kg/m2 | |
| Without Diabetes | ||||||||
| Unweighted N | 1515 | 2100 | 930 | 395 | 1646 | 1504 | 946 | 714 |
| Age, y | 41.1 (0.5) | 45.2 (0.4) | 45.3 (0.7) | 43.0 (0.8) | 43.8 (0.4) | 47.9 (0.6) | 47.2 (0.5) | 45.1 (0.7) |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 70.1 (2.0) | 73.4 (1.8) | 75.4 (1.8) | 70.3 (2.9) | 78.0 (1.6) | 71.1 (2.6) | 68.0 (2.5) | 66.2 (2.6) |
| Non-Hispanic Black | 11.6 (1.1) | 8.1 (0.9) | 8.6 (1.0) | 14.2 (2.1) | 6.7 (0.9) | 10.3 (1.3) | 14.8 (1.7) | 20.0 (2.2) |
| Mexican American | 7.4 (0.9) | 8.2 (1.0) | 8.6 (1.4) | 6.8 (1.5) | 4.8 (0.7) | 7.1 (1.0) | 7.8 (1.2) | 5.8 (1.0) |
| Other | 11.0 (1.5) | 10.3 (1.5) | 7.3 (1.2) | 8.6 (2.0) | 10.4 (1.2) | 11.5 (2.0) | 9.4 (1.9) | 8.1 (2.1) |
| Glycated albumin, % | 13.3 (0.1) | 13.2 (0.1) | 12.9 (0.1) | 12.7 (0.1) | 13.7 (0.1) | 13.3 (0.1) | 13.0 (0.1) | 12.8 (0.1) |
| HbA1c, % | 5.3 (0.0) | 5.4 (0.0) | 5.6 (0.0) | 5.7 (0.0) | 5.2 (0.0) | 5.4 (0.0) | 5.5 (0.0) | 5.6 (0.0) |
| Categorized HbA1c | ||||||||
| ≤5.7% | 88.1(1.1) | 80.3 (0.9) | 70.4 (2.0) | 56.4 (2.7) | 90.6 (0.9) | 79.7 (1.3) | 68.5 (1.5) | 69.3 (2.3) |
| 5.7–6.4% | 11.6 (1.1) | 18.0 (0.8) | 25.4 (1.8) | 37.8 (3.0) | 9.1 (0.9) | 18.5 (1.3) | 29.4 (1.6) | 27.2 (2.3) |
| ≥6.5% | 0.4 (0.1) b | 1.7 (0.3) | 4.2 (0.7) | 5.9 (1.2) | 0.3 (0.1) b | 1.8 (0.4) | 2.1 (0.5) | 3.4 (0.7) |
| GA:HbA1c | 2.5 (0.0) | 2.4 (0.0) | 2.3 (0.0) | 2.2 (0.0) | 2.6 (0.0) | 2.5 (0.0) | 2.4 (0.0) | 2.3 (0.0) |
| C-reactive protein, mg/dL | 0.1 (0.0–0.2) | 0.1 (0.1–0.3) | 0.2 (0.1–0.4) | 0.4 (0.2–0.6) | 0.1 (0.1–0.3) | 0.2 (0.1–0.5) | 0.4 (0.2–0.8) | 0.6 (0.4–1.2) |
| Albumin, g/dL | 4.8 (0.0) | 4.8 (0.0) | 4.7 (0.0) | 4.5 (0.0) | 4.6 (0.0) | 4.5 (0.0) | 4.4 (0.0) | 4.3 (0.0) |
| Smoking status | ||||||||
| Never | 42.4 (1.8) | 43.1 (1.6) | 41.9 (2.0) | 50.8 (3.3) | 57.0 (1.6) | 56.7 (1.8) | 59.3 (2.4) | 55.5 (2.3) |
| Former | 20.9 (1.1) | 31.1 (1.3) | 34.4 (2.0) | 30.6 (3.0) | 19.5 (1.1) | 22.6 (1.5) | 18.5 (1.6) | 23.8 (2.2) |
| Current | 36.7 (1.6) | 25.8 (1.3) | 23.7 (2.1) | 18.5 (1.9) | 23.5 (1.5) | 20.7 (1.4) | 22.2 (1.7) | 20.7 (2.3) |
| eGFR, mL/min/1.73 m2 | 112 (101–122) | 109 (96–119) | 107 (96–116) | 108 (97–117) | 111 (99–121) | 107 (94–118) | 105 (91–118) | 104 (90–115) |
| With Diabetes | ||||||||
| Unweighted N | 107 | 227 | 133 | 97 | 72 | 157 | 135 | 157 |
| Age, y | 58.6 (1.9) | 58.9 (1.1) | 58.1 (1.4) | 54.4 (1.4) | 62.2 (1.9) | 62.8 (2.0) | 59.3 (1.5) | 54.5 (1.4) |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 61.3 (8.1) | 59.4 (5.2) | 70.0 (5.2) | 81.4 (3.8) | 65.6 (8.1) | 60.0 (6.1) | 65.9 (4.9) | 56.2 (4.5) |
| Non-Hispanic Black | 12.3 (3.0) | 12.9 (2.5) | 13.2 (2.8) | 7.1 (1.8) | 6.8 (2.7) b | 16.0 (3.8) | 18.2 (3.7) | 23.0 (3.5) |
| Mexican American | 8.5 (2.0) | 9.7 (2.2) | 5.1 (1.6) b | 3.0 (1.3) b | 4.6 (1.6) b | 8.5 (2.3) | 9.3 (1.9) | 8.2 (1.9) |
| Other | 17.8 (6.5) b | 18.1 (4.3) | 11.7 (4.5) b | 8.5 (3.7) b | 23.0 (7.3) b | 15.6 (5.1) b | 6.6 (3.0) b | 12.6 (3.4) |
| Glycated albumin, % | 22.6 (0.9) | 19.7 (0.7) | 19.0 (0.7) | 17.1 (0.5) | 20.6 (1.0) | 20.5 (1.0) | 18.7 (0.5) | 18.0 (0.5) |
| HbA1c, % | 7.9 (0.3) | 7.4 (0.2) | 7.6 (0.3) | 7.2 (0.2) | 7.5 (0.3) | 7.6 (0.3) | 7.4 (0.1) | 7.4 (0.1) |
| Categorized HbA1c | ||||||||
| HbA1c <7.0% | 37.5 (7.0) | 51.5 (5.0) | 49.2 (5.8) | 44.1 (6.4) | 49.4 (7.4) | 46.8 (6.1) | 49.6 (5.5) | 48.7 (4.5) |
| HbA1c ≥7.0% | 62.5 (7.0) | 48.5 (5.0) | 50.8 (5.8) | 55.9 (6.4) | 50.6 (7.4) | 53.2 (6.1) | 50.4 (5.5) | 51.3 (4.5) |
| GA:HbA1c | 2.8 (0.0) | 2.6 (0.1) | 2.5 (0.0) | 2.3 (0.0) | 2.7 (0.1) | 2.7 (0.0) | 2.5 (0.0) | 2.4 (0.0) |
| C-reactive protein, mg/dL | 0.1 (0.1–0.2) | 0.2 (0.1–0.4) | 0.3 (0.2–0.5) | 0.4 (0.2–0.6) | 0.1 (0.1–0.3) | 0.3 (0.2–0.6) | 0.4 (0.2–0.7) | 0.9 (0.5–1.5) |
| Albumin, g/dL | 4.6 (0.1) | 4.6 (0.0) | 4.5 (0.0) | 4.4 (0.0) | 4.6 (0.1) | 4.3 (0.0) | 4.3 (0.0) | 4.2 (0.0) |
| Smoking status | ||||||||
| Never | 29.5 (6.6) | 34.5 (4.9) | 33.8 (5.0) | 43.4 (5.5) | 57.9 (7.8) | 61.3 (4.6) | 65.7 (4.4) | 46.4 (4.6) |
| Former | 24.2 (5.3) | 47.2 (4.7) | 42.2 (4.7) | 38.6 (5.8) | 23.0 (7.0) b | 24.0 (3.7) | 17.2 (4.4) | 32.1 (4.9) |
| Current | 46.2 (7.3) | 18.4 (3.8) | 24.0 (5.4) | 18.0 (5.7) b | 19.0 (5.3) | 14.7 (4.1) | 17.1 (4.3) | 21.5 (4.6) |
| eGFR, mL/min/1.73 m2 | 94 (75–111) | 100 (74–113) | 100 (79–111) | 97 (87–108) | 95 (66–113) | 91 (68–110) | 92 (74–110) | 92 (73–111) |
Values are mean (SE) or median (IQR) for continuous variables, and % (SE) for categorical variables. BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; IQR, interquartile range; SE, standard error
Imprecise estimate; SE >30% of estimate
Albumin (measured as part of the Asahi Kasei Pharma GA method) was positively correlated with absolute GA in g/dL (men: r=0.26, women: r=0.36), but inversely correlated with percentage GA (men: r=−0.26, women: r=−0.16). GA was weakly correlated with CRP (men, r=0.03; women, r=−0.08).
When BMI was modeled continuously using splines, unadjusted associations between adiposity and GA, HbA1c, and GA:HbA1c appeared approximately linear (Supplemental Figure 3). Each adiposity measure was inversely associated with GA when modeled linearly (men: β=−0.12 to −0.18%-point GA per 1 SD adiposity; women: β=−0.29 to −0.34%-point GA per 1 SD adiposity; Supplemental Table 2). Inverse associations persisted after adjusting for age, race/ethnicity, smoking status, and eGFR (model 1; Figure 1). Adiposity measures were positively associated with HbA1c (men: β=0.12 to 0.14 %-point HbA1c per 1 SD adiposity; women: β=0.11 to 0.15 %-point per 1 SD adiposity), and these associations also persisted after controlling for model 1 covariates. The observed inverse association between adiposity and GA:HbA1c indicated greater discordance between GA and HbA1c with increasing adiposity, such that relatively less GA than HbA1c was present as adiposity increased. Further adjustment for CRP (model 2a) or albumin (model 2b) did not materially alter associations GA, HbA1c, or GA:HbA1c.
Figure 1.
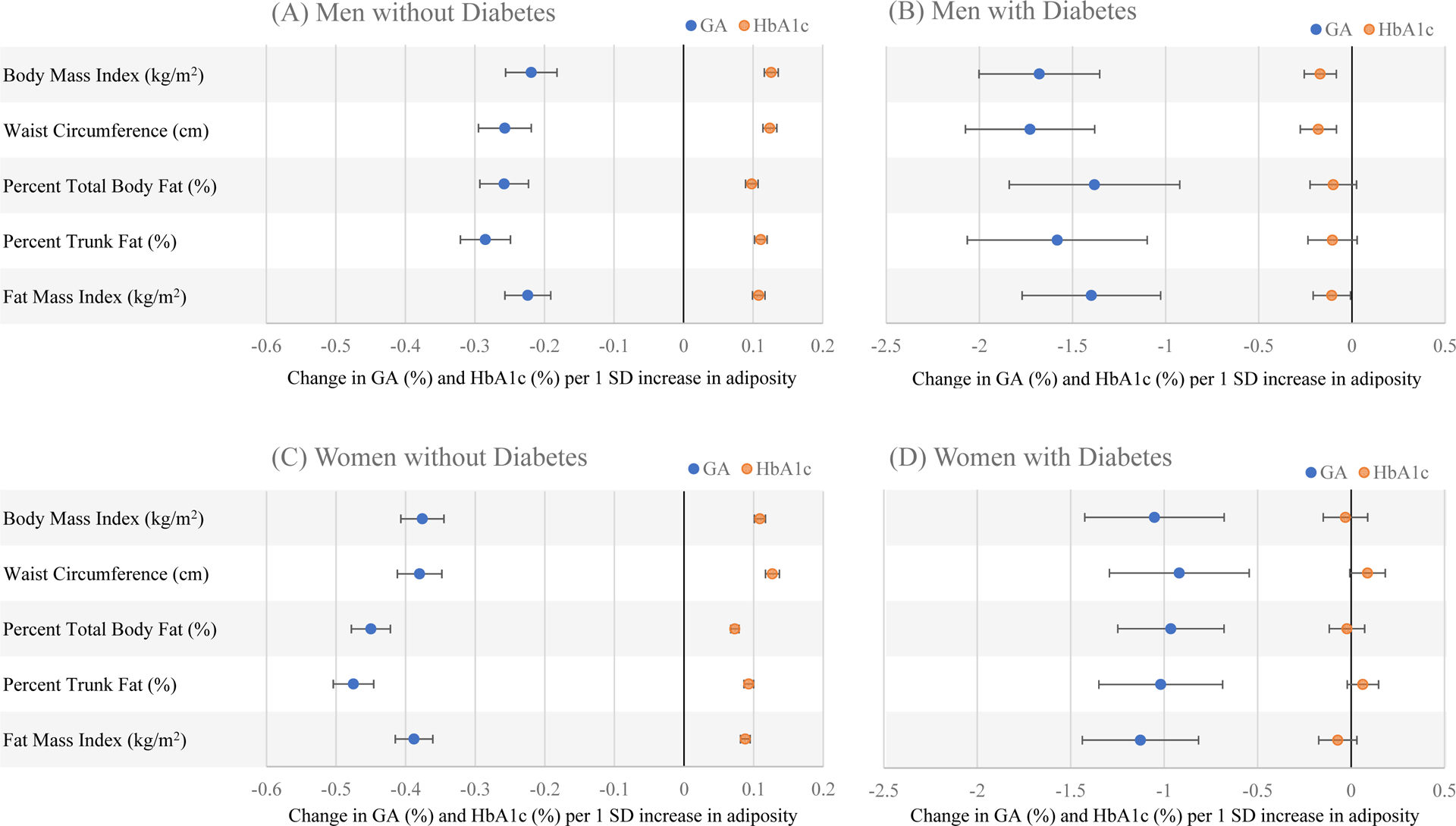
Adjusted beta coefficients (95% confidence intervals) from multivariable linear regression of GA and HbA1c on adiposity measures (body mass index, waist circumference, percent total body fat, percent trunk fat, and fat mass index), in (A) men without diabetes, (B) men with diabetes, (C) women without diabetes, and (D) women with diabetes, NHANES 1999–2004. Adjusted for age, race/ethnicity, eGFR, and smoking status. GA, glycated albumin; HbA1c, hemoglobin A1c; NHANES, National Health and Nutrition Examination Survey; SD, standard deviation
The GA value corresponding to the same percentile as HbA1c 5.7% (80th percentile) was 14.2%. GA performed worse for detecting prediabetes (HbA1c≥5.7%) among people with, versus without, obesity (Table 2). Only 30% of adults with obesity who had HbA1c ≥5.7% had comparably elevated GA (≥14.2%), versus 39% of adults without obesity. However, specificity was higher in adults with versus without obesity, such that 92% of adults with obesity who had HbA1c <5.7% had GA<14.2%, versus 81% of adults without obesity. GA also had poorer performance for identifying undiagnosed diabetes (HbA1c≥6.5%) in people with versus without obesity. The GA value corresponding to HbA1c 6.5% (98th percentile) was 17.6%. Among people with obesity, 43% of those with HbA1c ≥6.5% had elevated GA (≥17.6%) whereas 54% of people without obesity who had elevated HbA1c had elevated GA. This GA cut-point had comparably high specificity (99%) for identifying undiagnosed diabetes (HbA1c ≥6.5%) regardless of obesity status.
Table 2.
Sensitivity and specificity of glycated albumin to detect elevated HbA1c among adults without diagnosed diabetes (n=9,750) and with diagnosed diabetes (n=1,085), NHANES 1999–2004.
| Population | Outcome | GA cutpoint | Subgroup | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|
| Without Diabetes | Prediabetes (HbA1c≥5.7%) | 14.2% | BMI <30 kg/m2 | 0.39 (0.35–0.44) | 0.81 (0.77–0.84) |
| BMI ≥30 kg/m2 | 0.30 (0.25–0.35) | 0.92 (0.90–0.93) | |||
| Diabetes (HbA1c≥6.5%) | 17.6% | BMI <30 kg/m2 | 0.54 (0.42–0.66) | 0.99 (0.98–0.99) | |
| BMI ≥30 kg/m2 | 0.43 (0.32–0.55) | 0.99 (0.98–0.99) | |||
| With Diabetes | Glycemic control (HbA1c≥7.0%) | 17.4% | BMI <30 kg/m2 | 0.93 (0.87–0.96) | 0.81 (0.72–0.87) |
| BMI ≥30 kg/m2 | 0.81 (0.73–0.87) | 0.89 (0.83–0.92) |
BMI, body mass index; CI, confidence interval; GA, glycated albumin; HbA1c, hemoglobin A1c
Adults with diabetes
Among adults with diabetes, mean GA and GA:HbA1c were 18.2% (SE: 0.3%) and 2.44 (SE: 0.02), respectively, in those with obesity and 20.6% (SE: 0.6%) and 2.69 (SE: 0.04) in those without obesity. Mean GA was lower at higher BMI categories in men and women (Table 1). Mean HbA1c also tended to decrease with increasing BMI in men and was relatively constant across BMI in women. In men, adiposity measures were inversely correlated with GA (r=−0.26 to −0.30), HbA1c (r=−0.10 to −0.13), and GA:HbA1c (r=−0.34 to −0.39). In women, adiposity measures were inversely correlated with GA (r=−0.13 to −0.16) and GA:HbA1c (r=−0.28 to −0.39) but not HbA1c (r=−0.03 to 0.03).
Albumin correlated positively with absolute GA (in g/dL; men: r=0.12, women: r=0.16) and inversely with percentage GA (men: r=−0.12, women: r=−0.10). GA did not correlate with CRP (men: r=0.01, women: r<0.01).
When associations between BMI and GA, HbA1, and GA:HbA1c were modeled continuously using splines, severe deviations from linearity were not apparent (Supplemental Figure 4). In multivariable regression models, adiposity measures remained inversely associated with GA after adjusting for model 1 covariates (men: β=−1.38 to −1.73; women: β=−0.92 to −1.13; Figure 1; Supplemental Table 2). Adiposity measures were not significantly associated with HbA1c in men (β=−0.10 to −0.18; P>0.05) or women (β=−0.07 to 0.09; P>0.05). In both men and women, the inverse association between adiposity and the ratio GA:HbA1c indicated that GA underestimated glycemic exposure relative to HbA1c to a greater degree as adiposity increased. Observed associations between adiposity and GA and GA:HbA1c persisted after adjustment for CRP (model 2a) or albumin (model 2b).
The GA value corresponding to HbA1c ≥7.0% (48th percentile) was 17.4%. Sensitivity and specificity of GA to identify HbA1c ≥7.0% were high (>80%) regardless of obesity status though sensitivity was lower in people with, versus without, obesity (81% vs. 93%; Table 2).
Sensitivity Analysis
Inverse associations between all adiposity measures with GA and GA:HbA1c persisted after exclusion of people with severe hypoalbuminemia, severe kidney disease, thyroid disorders, liver disease, viral hepatitis, and anemia (Supplemental Table 3), and the performance of GA to detect hyperglycemia in people with obesity was not improved (Supplemental Table 4).
Discussion
In this nationally representative sample of U.S. adults, all measures of adiposity were inversely associated with GA, and GA exhibited lower sensitivity as a biomarker of hyperglycemia in people with, versus without, obesity. Relative to HbA1c, an established marker of long-term glycemic exposure, GA may underestimate average glucose concentrations in people who are overweight or obese.
Inverse associations between BMI and GA have previously been reported in adults (3–7,9–12,28–30) and children (8,31). While BMI is an easily and commonly used estimate of body composition, it does not precisely measure body fat or its distribution. Therefore, we also examined associations with regional anthropometric measurements (waist circumference) and direct measurements of body fat (DXA). Among adults without diabetes, all adiposity measures were inversely associated with GA and positively associated with HbA1c. The adiposity-associated discrepancy between GA versus HbA1c, represented by the ratio GA:HbA1c, was of similar magnitude for all adiposity measures in men but somewhat stronger for measures of central adiposity (waist circumference and percent trunk fat) in women. Similarly, among adults with diabetes, all adiposity measures were inversely associated with GA and GA:HbA1c, and the inverse associations were stronger for percent trunk fat in women. These findings suggest that greater central adiposity may be associated with the most profound discrepancy between GA versus HbA1c, at least in women, but the adiposity-associated divergence between GA and HbA1c occurs with all adiposity measures.
One of the prevailing hypotheses for the inverse association between adiposity and GA is that the pro-inflammatory activity of adipose tissue increases albumin turnover, resulting in a lower observed percentage of GA in circulation (4). Greater adiposity, particularly central adiposity, is associated with increased systemic inflammation, commonly measured by CRP (32,33). However, CRP only weakly correlated with GA in our population and controlling for CRP did not appreciably alter the associations between GA and adiposity, suggesting that inflammation does not explain the observed inverse association.
We also considered the impact of albumin concentration on GA. Albumin influences its own rate of turnover, such that catabolism slows when plasma concentrations are low (34). This longer persistence in circulation prolongs exposure to plasma glucose and results in greater glycation (34). We observed an inverse correlation between albumin concentration and percentage glycated albumin, consistent with previous studies (3,11). The lower albumin concentrations associated with increasing adiposity would plausibly predict greater glycation as adiposity increases, yet the opposite was observed in our study. Controlling for albumin concentration did not correct the inverse association between adiposity and GA, suggesting that the inverse GA-adiposity association is not likely explained by albumin concentration. However, we cannot exclude the possibility that albumin turnover increases with adiposity, independently of inflammation or albumin concentration, and thereby results in lower GA.
An alternative explanation for the inverse association between GA and adiposity is that glycation may be impaired by obesity-related changes in the local molecular environment surrounding albumin. In vitro glycation of proteins is slower in sera from adults with, versus without, obesity (35,36) and is enhanced by removal of fatty acids (37), which may alter amino group reactivity with glucose (38,39). Increased circulating free fatty acids released from lipolysis of insulin-resistant adipose tissue in obesity could explain adiposity-associated interference of albumin glycation (39). However, we were unable to explore this hypothesis in our study as circulating free fatty acid concentrations were not assessed.
In addition to confirming the inverse association between BMI and GA in U.S. adults, we extended this work to other measures of adiposity and compared the sensitivity and specificity of GA to detect hyperglycemia by obesity status. As diagnostic cut-points have not yet been established, we defined potential cut-points at percentiles corresponding to clinical HbA1c thresholds for glycemic monitoring and diagnosis. Our results suggest that the performance of GA as a measure of hyperglycemia is influenced by obesity status. Among adults without diabetes, sensitivity of GA to identify prediabetes or diabetes was lower in adults with obesity. Diagnosing prediabetes or diabetes based on GA will result in more missed cases among people with, versus without, obesity. Among adults with diabetes, GA had lower sensitivity and higher specificity for identifying poor glycemic control in adults with obesity, though sensitivity was high (>80%) regardless of obesity status. Thus, GA may be an acceptable alternative to HbA1c for glycemic monitoring in diabetes.
A strength of this study was, first, the use of a large diverse sample representing a wide range of adiposity, with application of appropriate statistical methods to obtain nationally representative estimates. Second, we utilized several measures of adiposity, spanning from commonly used but less precise anthropometric measurements to DXA measurements of whole body and regional fat. All measurements were obtained by trained staff using standardized procedures. Third, we confirmed our findings in a sensitivity analysis excluding persons with conditions known to affect red blood cell or albumin turnover to rule out metabolic aberrations that could explain the discordance between HbA1c and GA, and we adjusted for factors associated with adiposity and glycemic markers that could confound observed associations. Potential limitations include, first, assessment of GA in frozen serum samples stored for approximately 20 years. However, stability of GA in samples frozen for 19–23 years has previously been confirmed (40), and we restricted analyses to “pristine” samples that had not previously undergone a freeze-thaw cycle (14). Second, the observational nature of our study prohibited inferences regarding the causal effect of adiposity on GA and limited our ability to elucidate the physiology of this inverse association. Finally, we only examined diagnostic performance of GA relative to HbA1c, as this was the only diagnostic biomarker measured in the full (non-fasting) NHANES sample. However, HbA1c is perhaps the most appropriate biomarker for comparison with GA, as both result from the glycation of proteins and are indirect biomarkers of chronic hyperglycemia.
In conclusion, we found an inverse association between GA and adiposity that was independent of inflammation and albumin concentration. GA was highly specific but may not be sufficiently sensitive for screening purposes in adults with obesity, who represent the majority of Americans most likely to develop diabetes. Future studies investigating how albumin turnover, circulation, and glycation kinetics are altered in the context of obesity are needed to clarify the mechanism by which adiposity affects GA.
Supplementary Material
Impact Statement.
Glycated albumin (GA) is inversely associated with multiple measures of adiposity in a nationally representative sample of U.S. adults with and without diabetes and exhibits lower sensitivity to identify elevated HbA1c in people with obesity. GA is highly specific but may not be sufficiently sensitive for diabetes screening in adults with obesity, who represent the majority of Americans most likely to develop diabetes.
Funding:
This work was funded by a grant from the Foundation for the National Institutes of Health Biomarkers Consortium to the Johns Hopkins Bloomberg School of Public Health (PI: Elizabeth Selvin). The Foundation for the National Institutes of Health received support for this project from Abbott Laboratories, AstraZeneca, Johnson & Johnson, the National Dairy Council, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin was also supported by NIH/NHLBI grant K24 HL152440. Dr. Sullivan was supported by NIH/NHLBI grant T32 HL007024. Reagents for the glycated albumin assay were donated by the Asahi Kasei Pharma Corporation.
Abbreviations:
- GA
glycated albumin
- HbA1c
hemoglobin A1c
- BMI
body mass index
- NHANES
National Health and Nutrition Examination Survey
- CRP
C-reactive protein
- DXA
dual-energy x-ray absorptiometry
- eGFR
estimated glomerular filtration rate
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: R.H. Christenson, The Journal of Applied Laboratory Medicine, AACC. E. Selvin, Diabetes Care; Diabetologia; American Diabetes Association; American Heart Association.
Consultant or Advisory Role: R.H. Christenson, Quidel Medical, Roche Diagnostics, Siemens Healthineers, Beckman Coulter, Sphingotech, Pixcell Medical.
Honoraria: E. Selvin receives payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate on measurements of glycemic control and screening tests for type 2 diabetes. R.H. Christenson has received payments from Siemens Healthineers, Roche Diagnostics, Beckman Coulter, Sphingotech GHB, and Quidel Medical.
Patents: none declared
Stocks: none declared
References
- 1.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep 2014;14:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev 2013;34:463–500. [DOI] [PubMed] [Google Scholar]
- 3.Koga M, Matsumoto S, Saito H, Kasayama S. Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J 2006;53:387–91. [DOI] [PubMed] [Google Scholar]
- 4.Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clinica Chimica Acta 2007;378:48–52. [DOI] [PubMed] [Google Scholar]
- 5.Selvin E, Warren B, He X, Sacks DB, Saenger AK. Establishment of community-based reference intervals for fructosamine, glycated albumin, and 1,5-anhydroglucitol. Clin Chem 2018;64:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon AK, Juraschek SP, Ballantyne CM, Steffes MW, Selvin E. Comparative associations of diabetes risk factors with five measures of hyperglycemia. BMJ Open Diabetes Res Care 2014;2:e000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Mo Y, Ma X, Ying L, Zhu W, Wang Y, et al. Associations of body mass index with glycated albumin and glycated albumin/glycated hemoglobin A1c ratio in Chinese diabetic and non-diabetic populations. Clinica Chimica Acta 2018;484:117–21. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura R, Kanda A, Sano H, Matsudaira T, Miyashita Y, Morimoto A, et al. Glycated albumin is low in obese, non-diabetic children. Diabetes Res Clin Pract 2006;71:334–8. [DOI] [PubMed] [Google Scholar]
- 9.Miyashita Y, Nishimura R, Morimoto A, Matsudaira T, Sano H, Tajima N. Glycated albumin is low in obese, type 2 diabetic patients. Diabetes Res Clin Pract 2007;78:51–5. [DOI] [PubMed] [Google Scholar]
- 10.Koga M, Hirata T, Kasayama S, Ishizaka Y, Yamakado M. Body mass index negatively regulates glycated albumin through insulin secretion in patients with type 2 diabetes mellitus. Clinica Chimica Acta 2015;438:19–23. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Ma X, Hao Y, Yang R, Ni J, Xiao Y, et al. Serum glycated albumin is inversely influenced by fat mass and visceral adipose tissue in Chinese with normal glucose tolerance. PLoS One 2012;7:e51098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maruo S, Motoyama K, Hirota T, Kakutani Y, Yamazaki Y, Morioka T, et al. Visceral adiposity is associated with the discrepancy between glycated albumin and HbA1c in type 2 diabetes. Diabetol Int 2020;11:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Ma X, Shen Y, Wang Y, Zhou J, Bao Y. Increasing waist circumference is associated with decreased levels of glycated albumin. Clinica Chimica Acta 2019;495:118–22. [DOI] [PubMed] [Google Scholar]
- 14.Daya NR, Rooney MR, Tang O, Coresh J, Christenson RH, Selvin E. Glycated albumin in pristine and non-pristine stored samples in the National Health and Nutrition Examination Survey (NHANES) 1999–2004. J Appl Lab Med 2022;7:916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics, Centers for Disease Control and Prevention. NHANES 1999–2006 DXA multiple imputation data files. https://wwwn.cdc.gov/Nchs/Nhanes/Dxa/Dxa.aspx (Accessed August 2022)
- 16.National Center for Health Statistics, Centers for Disease Control and Prevention. NHANES 1999–2000: body measures data documentation, codebook, and frequencies. https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/BMX.htm (Accessed August 2022)
- 17.McQuillan GM, McLean JE, Chiappa M, Lukacs SL. National Health and Nutrition Examination Survey biospecimen program: NHANES III (1988–1994) and NHANES 1999–2014. Vital Health Stat 2 2015;170:1–14. [PubMed] [Google Scholar]
- 18.Kohzuma T, Yamamoto T, Uematsu Y, Shihabi ZK, Freedman BI. Basic performance of an enzymatic method for glycated albumin and reference range determination. J Diabetes Sci Technol 2011;5:1455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES 1999–2000: glycohemoglobin data documentation, codebook, and frequencies. https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/LAB10.htm (Accessed August 2022)
- 20.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES 1999–2000: C-Reactive Protein (CRP) data documentation, codebook, and frequencies. https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/LAB11.htm (Accessed August 2022)
- 21.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. , American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care 2023;46 Suppl:S19–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. , American Diabetes Association. 6. Glycemic targets: standards of care in diabetes—2023. Diabetes Care 2023;46 Suppl:S97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 25.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. Obes Res 1998;6:51S–209S. [PubMed] [Google Scholar]
- 26.Cappellini MD, Motta I. Anemia in clinical practice-definition and classification: does hemoglobin change with aging? Semin Hematol 2015;52:261–9. [DOI] [PubMed] [Google Scholar]
- 27.National Center for Health Statistics. Glycated albumin, beta-2 microglobulin, cystatin C (surplus) (SSCARD_A). https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/SSCARD_A.htm (Accessed September 2022)
- 28.Hsu P, Ai M, Kanda E, Yu NC, Chen HL, Chen HW, et al. A comparison of glycated albumin and glycosylated hemoglobin for the screening of diabetes mellitus in Taiwan. Atherosclerosis 2015;242:327–33. [DOI] [PubMed] [Google Scholar]
- 29.Ikezaki H, Furusyo N, Ihara T, Hayashi T, Ura K, Hiramine S, et al. Glycated albumin as a diagnostic tool for diabetes in a general Japanese population. Metabolism 2015;64:698–705. [DOI] [PubMed] [Google Scholar]
- 30.Freitas PAC, Hernandez MK, Camargo JL. Factors associated with glycated albumin in adults without diabetes. Med Pharm Rep 2021;94:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace AS, Rooney MR, Brady TM, Echouffo-Tcheugui J, Christenson R, Grams ME, et al. The performance of glycated albumin as a biomarker of hyperglycemia and cardiometabolic risk in children and adolescents in the United States. Pediatr Diabetes 2021;23:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-reactive protein to abdominal adiposity. Am J Cardiol 2010;106:56–61. [DOI] [PubMed] [Google Scholar]
- 33.Schlecht I, Fischer B, Behrens G, Leitzmann MF. Relations of visceral and abdominal subcutaneous adipose tissue, body mass index, and waist circumference to serum concentrations of parameters of chronic inflammation. Obes Facts 2016;9:144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schleicher ED, Olgemoller B, Wiedenmann E, Gerbitz KD. Specific glycation of albumin depends on its half-life. Clin Chem 1993;39:625–8. [PubMed] [Google Scholar]
- 35.Broussolle C, Tricot F, Garcia I, Orgiazzi J, Revol A. Evaluation of the fructosamine test in obesity: consequences for the assessment of past glycemic control in diabetes. Clin Biochem 1991;24:203–9. [DOI] [PubMed] [Google Scholar]
- 36.Ardawi MSM, Nasrat HAN, Bahnassy AA. Fructosamine in obese normal subjects and type 2 diabetes. Diabet Med 1994;11:50–6. [DOI] [PubMed] [Google Scholar]
- 37.Mereish KA, Rosenberg H, Cobby J. Glucosylated albumin and its influence on salicylate binding. J Pharm Sci 1982;71:235–8. [DOI] [PubMed] [Google Scholar]
- 38.Garlick R, Mazer J. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem 1983;258:6142–6. [PubMed] [Google Scholar]
- 39.Vergès B, Rouland A, Baillot-Rudoni S, Brindisi MC, Duvillard L, Simoneau I, et al. Increased body fat mass reduces the association between fructosamine and glycated hemoglobin in obese type 2 diabetes patients. J Diabetes Investig 2021;12:619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathan DM, Steffes MW, Sun W, Rynders GP, Lachin JM. Determining stability of stored samples retrospectively: the validation of glycated albumin. Clin Chem 2011;57:286–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in the current study are publicly available online (27).


