Abstract
The prevalence of food allergy has been increasing globally, and comes with a heavy burden not just economically, but also on quality of life. Although oral immunotherapy (OIT) is effective at inducing desensitization to food allergens, it has several limitations that weaken its success. Limitations include long duration of build-up, especially if used for multiple allergens, and high rate of reported adverse events. Furthermore, OIT may not be effective in all patients. Efforts are underway to identify additional treatment options, either as monotherapy or in combination, to treat food allergy or enhance safety and efficacy of OIT. Biologics such as omalizumab and dupulimab, which already have FDA approval for other atopic conditions have been the most studied, but additional biologics and novel strategies are emerging.
In this review we discuss therapeutic strategies including IgE inhibitors, IgE disruptors, IL-4 and IL-13 inhibitors, anti-alarmins, JAK1 and BTK inhibitors, and nanoparticles and the data surrounding their application in food allergy and highlight their potential.
Keywords: Food allergy, oral immunotherapy, biologics, omalizumab, dupilumab, treatment, IgE antibodies, IgE disruptors, alarmin inhibitors, JAK inhibitors, BTK inhibitors, nanoparticles
Introduction
The increasing prevalence and substantial quality of life and economic burden of food allergy (FA)1–3 highlights the need for the development of more effective therapeutic strategies. While therapeutic strategies including sublingual and epicutaneous immunotherapy have been investigated, oral immunotherapy is the most widely used strategy due to its effectiveness and ease of use4. Many studies have demonstrated that OIT can safely and effectively desensitize patients to many allergens leading to the FDA approval of the oral immunotherapy (OIT) product, peanut (Arachis hypogaea) Allergen Powder-dnfp for the treatment of peanut allergies in 20205–10. However, OIT is a slow, allergen-specific process, and adverse events (AEs) to OIT are common, especially during the initial build up phase11, 12. Furthermore, 43%−86% of children with FA in the United States are allergic to more than one food, making traditional OIT in a sequential manner a slow and laborious process if treatment is sought for all the allergenic foods5, 13–15. Combined with the burden and stress of daily consumption of the allergic food during OIT can make long-term adherence difficult12. Therefore, increasing efforts are underway to identify therapeutic options which are focused on targeting the allergic pathways driving FA.
Novel therapeutic strategies are aiming to treat FA by targeting many different steps of the allergic cascade (Figure 1,2). The disruption of the epithelium, can lead to the exposure of epithelial immune cells to allergens which can trigger the release of alarmins such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP). These cytokines are the targets of anti-alarmin antibodies such as etokimab and tezepelumab. Alarmins drive the differentiation of naïve T cells into Th2 cells resulting in the release of a second wave of inflammatory cytokines including IL-4 and IL-13. IL-4 and IL-13 can bind to the IL-4 receptor alpha (IL-4Rα) on B cells (the therapeutic target for dupilumab), which together with the co-stimulatory interaction of the CD40 ligand on T cells, induces the production of immunoglobulin E antibodies (IgE), one of the primary drivers of allergic reactions (targeted by anti-IgE antibodies). Allergen-specific IgE crosslinks to FcεRI receptors on mast cells and basophils, sensitizing them to the allergen, which is the primary target for IgE disruptors. Subsequent allergen exposure to these sensitized mast cells and basophils triggers degranulation and the release of mediators of the allergic cascade such as histamine through pathways including the Bruton tyrosine kinase (BTK) and Janus kinase (JAK) pathways (targeted by abatacept and abrocitinib) to induce allergic reactions. Most of these therapeutic strategies have been studied as adjuvants to OIT, with a few of them being investigated as monotherapies. Furthermore, one trial is even investigating the combination of two biologics (omalizumab and dupilumab) with adjunct OIT. Here we summarize the potential of allergic pathway inhibitors and discuss promising combinations for FA treatment (Table 1).
Figure 1.
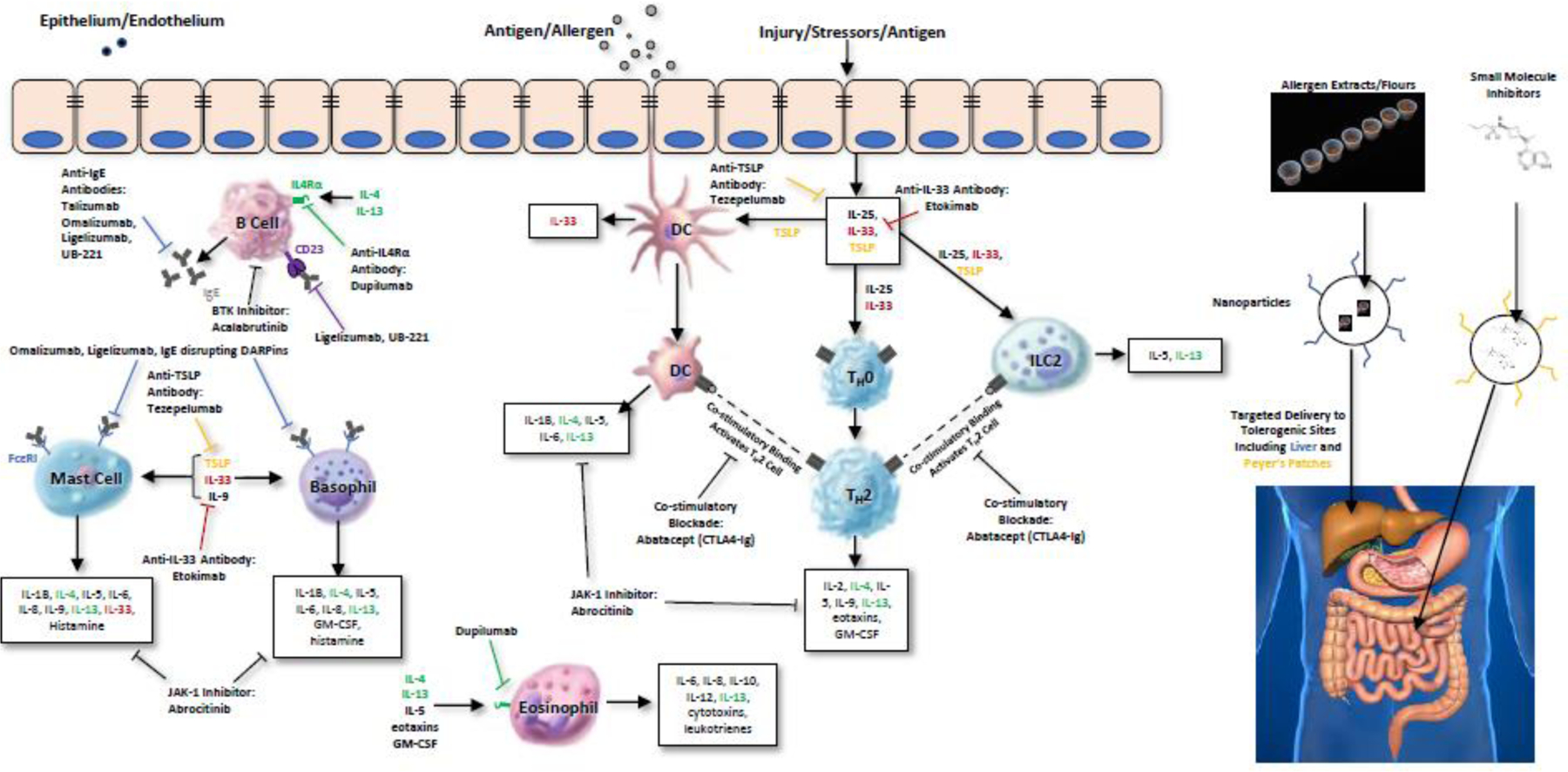
FA pathways targeted by therapeutics. Common pathways targeted by therapeutic strategies in FA.
Figure 2.
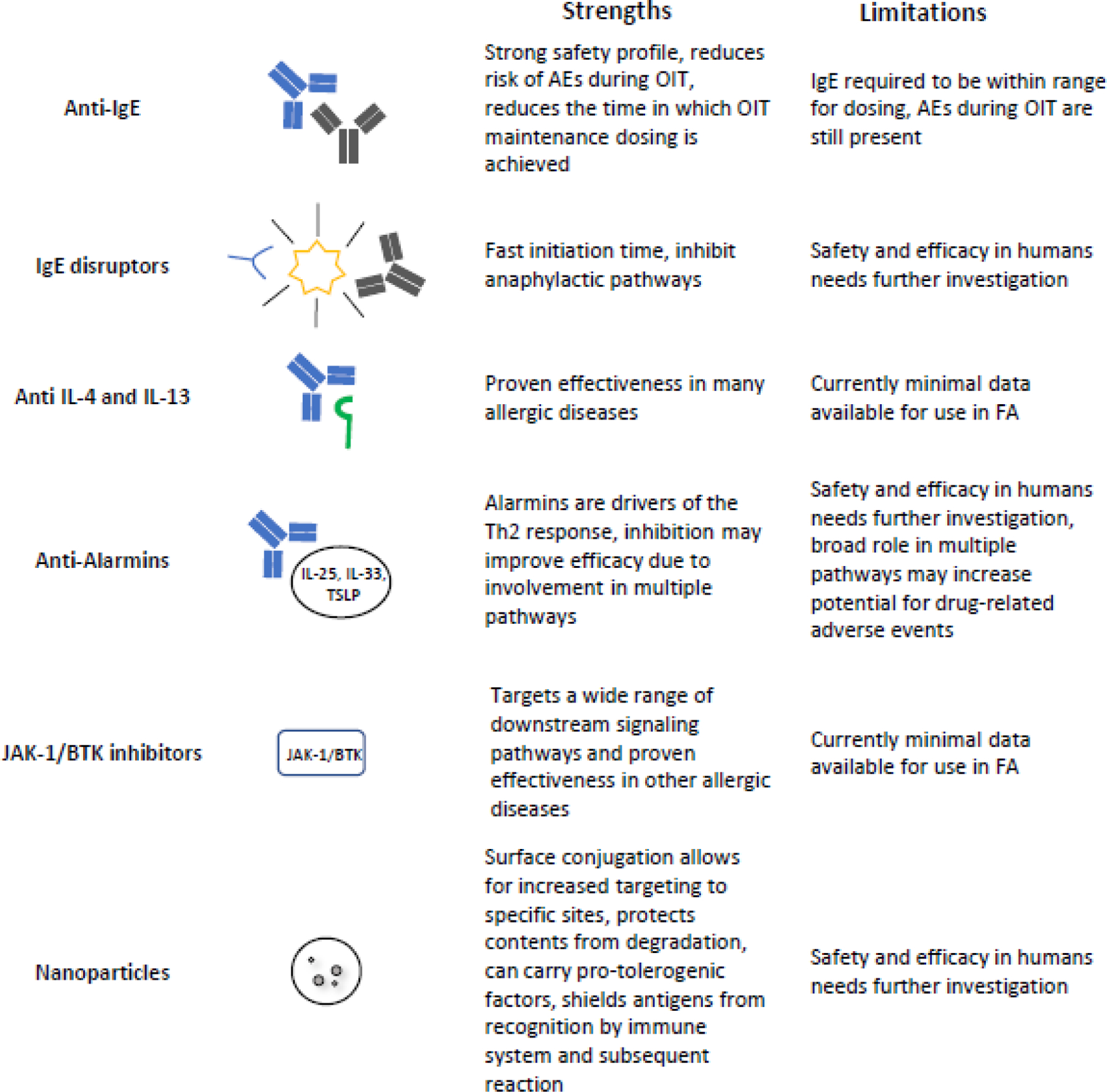
Strengths and limitations of therapeutic strategies in FA.
Table 1. Ongoing Trials of Novel Therapeutic Agents in FA.
| Intervention | Population | Dosage and Duration | Trial Number(s) | |
|---|---|---|---|---|
| Anti-IgE | a6–18 yo with allergy to 1 or more foods | aSubcutaneous injection of 75 or 150 mg omalizumab every 2 or 4 weeks depending on weight and IgE for 3–6 months | a NCT04037176 | |
| Omalizumab | b18–70 yo with food allergy | bSubcutaneous injections of 300 mg omalizumab every 2 week for 12 weeks | b NCT03964051 | |
| Omalizumab + peanut OIT | 7–25 yo with peanut allergy | Not specified. Likely Subcutaneous injection of 75 mg or 150 mg omalizumab either every 2 weeks or every 4 weeks depending on weight and IgE for 16 to 20 weeks | NCT01781637 | |
| Omalizumab +/− multi-allergen OIT | 1–56 yo with peanut allergy + 2 additional food allergies (milk, egg, wheat, cashew, hazelnut, walnut) | Subcutaneous injection of 75 mg or 150 mg omalizumab either every 2 weeks or every 4 weeks depending on weight and IgE for 16 to 20 weeks | NCT03881696 | |
| Omalizumab + multi-allergen OIT | 6–25 yo with multi-food allergy (≥3 foods) | Subcutaneous injection of 0.008 mg/kg every 2 weeks or 0.016 mg/kg omalizumab every 4 weeks for 16 weeks | NCT04045301 | |
| Ligelizumab | 6–57 yo with peanut allergy | Subcutaneous injection of 120 mg or 240 mg ligelizubmab every 4 weeks |
NCT04984876
NCT05678959 |
|
| Anti-IL4Rα | Dupilumab | 4–50 yo with cow’s milk allergy | Dupilumab injected every 2 weeks for 18 weeks | NCT04148352 |
| Anti-IgE + Anti-IL4Rα | Omalizumab + dupilumab +/− multi-allergen OIT | 5–55 yo with peanut allergy + 1–2 other food allergies | Subcutaneous injection of 0.008 mg/kg every 2 weeks or 0.016 mg/kg omalizumab every 4 weeks depending on weight and IgE for 8 weeks followed by subcutaneous injection of dupilumab every 2 weeks for 24 weeks following guidelines for treatment of AD | NCT03679676 |
| Anti-JAK | Abrocitinib | 18–50 yo with food allergy | Daily dosing of 100 mg or 200 mg abrocitinib for 4 months | NCT05069831 |
| Anti-BTK | Acalabrutinab | 18+ yo with peanut or tree nut allergy | Twice daily oral dosing of 100 mg acalabrutinib oral capsules for 2 days | NCT05038904 |
| Passive Blockade | Abatacept | 14–50 yo with peanut allergy | Intravenous abatacept following recommended dosages from the monograph for 24 weeks | NCT04872218 |
Anti-IgE Therapy
Talizumab (TNX-901)
Most allergic reactions are mediated by immunoglobulin E (IgE) antibodies, making them an attractive target in allergic diseases such as FA. Upon contact with an allergen, IgE antibodies specific to that allergen are produced that then bind to receptors such as FcεRI on mast cells and basophils. This binding sensitizes these cells to the allergen and causes the release of allergic mediators, such as histamine, upon subsequent exposures to the allergen. Anti-IgE antibodies are the most well studied class of FA therapeutics, the earliest of which was the humanized monoclonal IgE antibody talizumab (TNX-901). TNX-901 binds to the CHε3 region of IgE that normally binds to FcεRI receptors on mast cells, basophils, and dendritic cells to reduce symptoms of IgE-mediated allergic reactions by decreasing degranulation of mast cells and basophils16. Anti-IgE antibodies reduce IgE levels regardless of allergen specificity, and hence can be useful for treating patients with multiple food allergies. In 2003, Leung et al published the results of a trial evaluating the use of 12 weeks of TNX-901 therapy in eighty-four 12 to 60-year-old patients with peanut hypersensitivity. TNX-901 was well-tolerated and raised the reaction-eliciting dose from a baseline threshold of 178–436 mg to an exit threshold of 2627 mg17. The success of these early studies demonstrated the potential of anti-IgE antibodies and sparked the development of next generation anti-IgE antibodies.
Omalizumab
The monoclonal anti-IgE antibody omalizumab is the most well studied anti-IgE therapeutic in FA, and is FDA-approved to treat moderate-to-severe persistent asthma, chronic spontaneous urticaria, and nasal polyps. Omalizumab has demonstrated safety and efficacy in facilitating desensitization to allergenic volumes beyond accidental ingestion as a monotherapy18, 19 and in conjunction with multi-allergen OIT.
Similar to TNX-901, omalizumab binds preferentially to an epitope in the CHε3 portion of IgE that prevents binding with FcεRI receptors on effector cells such as mast cells, basophils, and dendritic cells, reducing circulating IgE20 and basophil and mast cell activation21. Lower concentrations of circulating IgE reduces the expression of FcεRI receptors on mast cells and basophils22. Additionally, omalizumab can remove IgE bound to FcεRI on plasmacytoid dendritic cells and reinstate their ability to produce regulatory T cells and IFN-α23.
In the setting of FA therapy, typically omalizumab is administered prior to OIT initiation to reduce AEs or symptoms related to IgE-mediated allergic reactions during buildup and maintenance. Clinical trials with omalizumab have shown promising results with decreased time needed to reach maintenance OIT dosing, higher cumulative tolerated thresholds to allergens, and increased safety of OIT for single food allergens, such as milk or peanut24–26. However, as 30–86% of children in the US have more than 1 FA5, 13–15, omalizumab is more often used in FA clinical trials to facilitate OIT to 2 or more foods (multi-OIT)27–29. In the MAP-X phase II trial of 4–15 year-old children allergic with 2–5 foods, omalizumab elevated the ability to pass an oral food challenge to at least 2 g food protein for 2 or more foods after 36 weeks (83% vs 33% without omalizumab, OR: 10, CI: 1.8–58.3, p= 0.004) and decreased time to maintenance (hazard ratio 5.36, 95% CI: 1.8, 15.99)27. Omalizumab also improved the safety of multi-OIT by reducing the number of doses associated with AEs from 68% without omalizumab to 27% with omalizumab (p=0.008)27. In a phase I trial by Bégin et. al in 2014 with 4–15 year old multi-allergenic children, pre-treatment with omalizumab reduced time to maintenance dose to 18 weeks28 as compared to 85 weeks without omalizumab30 In addition to improving multi-OIT safety and efficacy, several long-term follow-up studies show improvements in patient and parental quality of life when omalizumab is used alone (Pediatric Quality of Life Inventory increased from 65 ± 7.39 to 90 ± 4.54 for patients P < .001)19 or in conjunction with multi-OIT (parental QoL; MD: −0.76, P = 0.02, patients’ QoL; MD: 2.00, P < 0.001)29. The increasing prevalence of self-administration for omalizumab has made it more convenient, and cost studies have demonstrated its cost-effectiveness31. These traits combined with the improvements in safety, efficacy, and quality of life makes omalizumab an appealing treatment option for patients with FA.
In addition to the use of omalizumab as an adjunct to multi-OIT, preliminary studies have demonstrated that omalizumab also has potential as a monotherapy. In a study of patients with severe allergic asthma and food allergies treated with omalizumab alone there was an 8.6-fold increase in reaction threshold dose, as well as a decrease in reactions due to accidental ingestions from 47% to 2%19. In a phase II trial of omalizumab in peanut allergic patients by Sampson et. al in 2011, 44.4% of omalizumab-treated patients could tolerate more than 1000mg of peanut flour during an oral food challenge after 24 weeks of omalizumab treatment18. These findings demonstrate that omalizumab has potential for use outside the context of OIT. Ongoing clinical trials with omalizumab that are investigating its use as a monotherapy include the prospective OUtMATCH (NCT03881696), phase III study for patients with multi-FA which is comparing the safety and efficacy of omalizumab monotherapy in stage I and omalizumab in conjunction with multi-OIT in stage II, then assessing the ability to transition to real-life food equivalents in stage III. OUtMATCH also has the potential to address whether cessation of omalizumab while on OIT increases the risk of allergic AE’s in participants.
Ligelizumab
Ligelizumab is a next generation monoclonal anti-IgE antibody with ~88-fold higher binding affinities for free IgE, compared to omalizumab20. Unlike omalizumab, ligelizumab preferentially inhibits IgE binding to FcεRI on effector cells by recognizing an epitope that spans across the Fc domain Cε3 regions of human IgE and shows greater overlap with the binding site of FcεRI20. Ligelizumab has a high potency in blocking IgE/ FcεRI interactions, a major disease driver in food allergy, and neutralizes circulating IgE resulting in a strong reduction in inflammatory mediators released from mast cells32. In addition, ligelizumab has a greater potential to inhibit the binding of free IgE to plasmacytoid dendritic cells and restore their ability to generate FOXP3+ Tregs than omalizumab33. In chronic spontaneous urticaria, ligelizumab induced a complete response in 46.5–53.1% after 12–52 weeks of ligelizumab, which increased to 75.8% (95% confidence interval, 69.9–81.3) after an additional 52-week extension study34. Although AEs were common with 84.1% of patients having at least 1 AE, most were either mild (41.6%) or moderate (35.8%) and resolved by the following day, highlighting the tolerability of anti-IgE therapeutics. It is unclear at this time whether one is superior to the other; while ligelizumab is very effective at inhibiting the binding of IgE to FcεRI and basophil activation, it has a reduced ability to prevent IgE binding to CD2335–37. While omalizumab can remove IgE from the surface of plasmacytoid dendritic cells, ligelizumab is unable to remove IgE from the surface of pDCs from atopic donors33. Given these findings, there seems to be some context specificity as to whether omalizumab or ligelizumab is more effective and further research is needed to fully characterize what is the best anti-IgE for which patients. Given ligelizumab’s effects on reducing pro-inflammatory mediators and its potential to prevent food-induced allergic response and anaphylaxis, it is currently being studied for use in FA at 2 different doses 240 mg and 120 mg given subcutaneously every 4 weeks in a 52-week phase III study in 6–55 year-old peanut-allergic patients to determine if ligelizumab monotherapy is a safe and effective treatment for peanut allergy by increasing the threshold of tolerance to at least 600 mg of peanut protein (NCT04984876) after 52 weeks of use.
UB-221
UB-221 is another exciting upcoming anti-IgE antibody, which is 8 times more effective at inhibiting free IgE and 3 times stronger at inhibiting IgE binding to RBL SX-38 cells than omalizumab38. In addition, UB-221 can also inhibit IgE-CD23 complexes about 4–10 fold more effectively than ligelizumab, while omalizumab is not effective at dissociating already formed IgE-CD23 complexes and functions more by preventing binding of IgE to CD23 . Due to its inhibition of IgE-CD23 complexes, UB-221 reduces both the RNA and protein expression of IgE to a greater extent than either ligelizumab or omalizumab. UB-221 was found to be safe up to a maximum dose of 10 mg/kg in a phase I clinical trial (NCT03632291) of 15 patients with chronic spontaneous urticaria, with no serious AEs or dose-limiting toxicities38. In light of these promising findings, UB-221 has great potential in FA.
IgE disruptors
Prior to allergen binding, IgE and FcεRI form stable complexes on mast cells and basophils which primes them to initiate the allergic cascade upon allergen exposure. Anti-IgE therapies are generally better at inhibiting free-IgE as opposed to disrupting IgE-FcεRI complexes (which have a half-life of around 8 days), so they have a slow initiation time and are thus typically given for several months before the initiation of OIT28, 39. IgE disruptors such as those based on designed ankyrin repeat protein (DARPin) scaffolds are a newer therapeutic approach that is expected to act much quicker by disrupting pre-formed IgE:FcεRI complexes as well prevent binding of IgE to FcεRI, with kinetic studies demonstrating dissociation of IgE:FcεRI complexes within a few minutes40, 41. In a recent study, in mouse models DARPins reduced both free and mast and basophil bound IgE, while omalizumab only reduced free IgE, thus DARPins could reduce the risk of allergic reactions by inhibiting the sensitization of mast cells and basophils to allergens41. Furthermore, DARPins could also inhibit anaphylactic signaling pathways in mouse and human cells, suggesting that DARPins have potential as a rescue therapy as well40, 41. The potential for fast initiation and inhibition of anaphylactic pathways makes IgE disruption a promising strategy for targeting IgE, however further studies are needed to assess their potential in the clinic and compare their efficacy to anti-IgE antibodies.
Anti IL-4 and IL-13
Dupilumab
Allergic inflammation is largely driven by IL-4 and IL-13 signaling through their receptor, IL-4 receptor alpha (IL-4Rα), making IL-4Rα a promising therapeutic target for allergic diseases42. The human monoclonal antibody Dupilumab targeting the IL-4Rα receptor has shown great promise in many allergic diseases and has been FDA approved for several conditions, including moderate to severe AD, moderate to severe asthma, chronic rhinosinusistis with nasal polyposis, and eosinophilic esophagitis43. The success of dupilumab in these conditions has sparked interest in investigating the potential of dupilumab in FA. Additionally, a recent study has also shown large decreases in sIgE for all food allergens in patients being treated with dupilumab for AD, estimating decreases of at least 80% after 3 years of treatment44, highlighting the potential for dupilumab in FA.
Two clinical trials of dupilumab have been completed in children in 6–17 years-old with peanut allergy, however the results have not yet been announced. In the trial NCT03793608 dupilumab monotherapy was used in a single arm study with 25 participants. In the trial NCT03682770, at total of 149 participants received either placebo or dupilumab injections, in addition to adjunct peanut OIT with AR101. Other ongoing clinical trials include the MAGIC trial, a phase II trial of dupilumab as an adjunct to milk OIT in individuals aged 4–50 years who are allergic to cow’s milk (NCT04148352). In addition to adjunct studies, the COMBINE study utilizes a combination of omalizumab and dupilumab to target multiple allergenic pathways (NCT03679676). While the aim of this study is to evaluate the efficacy and safety of mOIT in patients receiving omalizumab in combination with dupilumab, this study also includes a cohort of placebo/dupilumab to compare results in this treatment arm to omalizumab/dupilumab as well as omalizumab/placebo in order to gain insight on the efficacy of dupilumab monotherapy. Considering the success of dupilumab in other allergic indications, there are high hopes for its application in FA.
Anti-alarmins
Defects in the epithelial barrier allow allergens to interact with immune cells in the epithelilium leading to the production of alarmins such as the epithelial cytokines thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 which initiate an allergic inflammatory response45. Therefore, therapeutics targeting alarmins are being investigated as potential treatment options for FA46.
Tezepelumab is an anti-TSLP antibody that lowers the rates of asthma exacerbations and improves lung function47. It has also been investigated in combination therapy with subcutaneous allergen immunotherapy in patients with cat allergy, and augments the efficacy of allergen immunotherapy, making anti-TSLP medications potential candidates for future FA trials48.
IL-33 is a pro-inflammatory cytokine that acts upstream of IgE and mediates B-class switching to IgE49. In a phase II clinical trial by Chinthrajah et. al in 2019 on the anti-IL-33 antibody etokimab, a single dose allowed 73% and 57% of patients to tolerate a cumulative dose of 275 mg peanut protein by days 15 and 45 respectively in comparison to 0% in the placebo control group46. Furthermore, there was a significant increase in cumulative tolerated dose from baseline to day 15 with etokimab (275 mg vs. 175 mg, P = 0.001), but not placebo (25 mg vs 75 mg, P = 0.63), suggesting that etokimab has potential for the treatment of FA.
Preliminary studies have also investigated the potential of targeting alarmins to prevent the development of FA. Interestingly, mouse studies indicate that inhibition of TSLP, IL-33, or IL-25 alone is insufficient to prevent egg allergy development, suggesting that these cytokines compensate for each other in FA50. However, a cocktail of monoclonal antibodies targeting all 3 cytokines could inhibit the development of egg allergy. This highlights the importance of combinatorial approaches in FA, demonstrating how blocking multiple targets can achieve much more effective results than targeting a single pathway. However more research is needed on the effectiveness of blocking alarmins in other allergic models.
JAK-1/BTK inhibitors
IgE and FcεRI form stable complexes on mast cells and basophils prior to antigen binding. Bruton tyrosine kinase (BTK) is essential for signaling through FcεRI, which is a key driver for allergic reactions, especially anaphylactic reactions. BTK inhibitors (BTKis) are currently approved for the treatment of B cell malignancies, however they have shown promise in rapidly and transiently preventing IgE-mediated reactions51. BTKi-mediated inhibition of the FcεRI signaling pathway and inflammatory cytokine production of mast cells and basophils, reduces the risk of AEs during OIT and can prevent IgE-mediated anaphylaxis51, 52. Furthermore, the BTKi, acalabrutinib, prevented moderate IgE-mediated anaphylaxis with two oral doses of pretreatment in humanized mice52, suggesting that BTKis could also be used as a prevention therapy. A phase II study is currently underway to examine the ability of 4 oral doses of 100 mg of acalabrutinib to prevent anaphylaxis in 10 adults during an oral food challenge to peanut or tree nut (NCT05038904).
Janus kinase (JAK) is a key enzyme class that is required for signaling through many pathogenic cytokines, making them attractive targets for many diseases. JAK inhibitors have been FDA approved in other allergic diseases and have potential for use in FA. In 2022, the US FDA approved the selective inhibitor of JAK-1 enzyme, abrocitinib (CIBINQQ®), for adults with refractory, moderate-to-severe AD, which has the potential to concurrently treat AD and FA without the need for injections 53. A phase I trial of abrocitinib in 40 adults is ongoing which will measure changes in basophil activation and skin prick test size after four months of 100 mg of daily abrocitinib to determine its potential for use in food allergy therapy (NCT05069831). In addition to abrocitinib, JAK inhibitors baricitinib and upadacitinib have shown in promise in AD and warrant study in FA as well54.
Nanoparticles
Nanoparticles are attractive vectors for therapeutics for many diseases and are being investigated for use in FA. While OIT continues to struggle with long-term adherence and safety concerns with the potential for systemic AEs, advancements with nanoparticle use in pre-clinical trials offer a potentially safer, more efficient alternative by targeted delivery of allergens to tolerogenic environments such as the liver or intestines55. Encapsulation of allergens in nanoparticles for oral administration also serves a protective function in shielding cargo from degradation via enzymatic or acidic processes, allowing for enhanced delivery to tolerogenic sites such as the Peyer’s Patches in the intestine56. This is particularly important for delivery of T cell epitopes, which are otherwise sensitive to degradation, that have the potential of modulating immune responses without the risk of IgE cross-linking and have shown promising capabilities to inhibit the allergic response through mechanisms including the induction of Foxp3+ regulatory T cells55, 57. In addition to allergens, nanoparticles may also be loaded with drugs to enhance the delivery of drugs such as curcumin, rapamycin, and other novel therapeutics such as Dnmt3aos smart silencer55, 58, 59.
The use of nanoparticles as a potentially safer and more effective long-term alternative to OITs has shown promising results in mainly pre-clinical studies and a few clinical trials. Murine model studies, immunotherapy using polyanhydride, peanut-encapsulating nanoparticles that could diffuse through the mucus layer of the intestines resulted in less severe anaphylaxis symptoms and higher survival rates compared to the free peanut, highlighting the increased safety of immunotherapy with nanoparticles compared to immunotherapy with non-encapsulated allergen extracts while also offering the benefit of lower dosing compared to OIT without nanoparticles60, 61. Peanut extract encapsulation resulted in safer delivery of allergen with only 2–3 doses, resulting in the suppression of Th2-mediated disease and decreased cytokines from mast cell degranulation62. These findings highlight the potential of nanoparticles for the treatment of FA. An ongoing phase II clinical trial (NCT05250856) is the first study of its kind to use an intravenously administered biodegradable nanoparticle encapsulating purified peanut extract, CNP-201, to reprogram the immune system of patients who have peanut allergies and reduce the potential risk of severe allergic reactions.
Passive blockade with monoclonal antibodies to allergen-specific epitopes
While IgE-inhibitors and disruptors target the entire IgE pathway, other strategies have investigated targeting specific allergens by creating a passive blockade with monoclonal antibodies to allergen-specific IgG. A recent phase I clinical trial of the REGN5713/14/15 antibody cocktail targeting the birch allergen Bet v 1 showed that a single dose could reduce the total nasal symptom score areas under the curve by −1.17 at day 8 with significant differences in SPT wheals in comparison to placebo that lasted for at least 2 months63. Although AEs were common (83.3–100% of patients), most were mild with only 0–16.7% of patients experiencing any severe AE during the study with no serious AEs. These findings suggest that this strategy holds potential for application in FA.
Conclusion
In summary there are many novel therapeutic strategies being investigated for the treatment of FA. Although omalizumab is the most well studied anti-IgE, it is not the only IgE inhibitor with promising potential. Both ligelizumab and UB-221 target IgE through unique moieties, which carry their own advantages and disadvantages. For instance, while ligelizumab has a strong binding affinity for free IgE, it is less effective at disrupting IgE-FcεRI complexes. Likewise, UB-221 is able to efficiently inhibit IgE-CD23 complexes. A cocktail of anti-IgE antibodies would thus be able to target the IgE signaling pathway from multiple angles and may prove to be more effective than using any anti-IgE antibody alone. All three of these antibodies are in ongoing clinical trials, and it would be of great interest to develop clinical trials assessing their combinatorial use alongside OIT with the hope of enhancing the safety of OIT.
Anti-IgE antibodies may also benefit from combination with IgE-disrupting DARPins alongside OIT. Current protocols for omalizumab-facilitated multi-OIT typically give omalizumab for about a month before OIT initiation. The ability of DARPins to dissociate IgE:FcεRI complexes within a few minutes40, 41, could vastly speed up the initiation of OIT. The combination of both DARPins and anti-IgE antibodies could take advantage of the quick initiation speed of DARPins with the long-lasting and well-studied safety of anti-IgE antibodies such as omalizumab to further enhance the speed of multi-OIT.
Nanoparticles offer an interesting alternative to OIT, with the potential for reduced risk of AEs which could be combined with other therapeutic strategies that are used in conjunction with OIT such as omalizumab or dupilumab to further reduce the risk of AEs. Encapsulation in nanoparticles would not only enhance the stability of the allergen to the target site but would also decrease the risk of reactions occurring in transit, while anti-IgE or dupilumab could reduce the risk of reactions at the target site. In addition, therapies such as DARPins, acalabrutinib, and abrocitinib could be loaded into nanoparticles to enhance their delivery to specific sites and to protect them from premature degradation.
Most biologics are being explored to be used either as monotherapy or as adjunct therapy with OIT. At this time there has been only 1 study (COMBINE, NCT03679676) investigating the potential of using these therapeutic strategies in combination with each other. Utilizing both omalizumab and dupilumab to treat multi-food allergic children. Targeting allergic pathways from multiple approaches has the potential for synergistic effects.
Many of the therapies described still need greater characterization for implementation in FA including, how safe and effective they are, what is the ideal dose and timing for usage in FA, and use what is the best patient population for their use. Furthermore, these therapeutics also hold great potential for use in combination with each other, with or without OIT. Future research into the potential synergy of these therapies with each other could greatly improve FA therapies.
Key Messages.
OIT is effective at inducing desensitization in FA, but it is slow, AEs are common but mostly mild, and is not effective for everyone.
IgE inhibitors are the most well studied biologic for FA which can reduce the risk of AEs and facilitate multi-OIT.
Several therapies such as dupilumab that are FDA approved for other allergic disease are currently under investigation for their application in FA.
Although each of these therapeutic strategies has shown promising potential individually as adjuvants for OIT, there is also great potential for synergy with their combinatorial use.
Acknowledgment
We would like to thank Andrew R. Chin for helping with the review and submission of this manuscript.
Funding:
This work was made possible by financial support from the Sean N. Parker Center for Allergy and Asthma Research at Stanford University, NIH grants 5UM1AI130839 and 5U19AI104209-09.
Abbreviations/Acronyms:
- AEs
Adverse events
- AD
Atopic dermatitis
- BTK
Bruton tyrosine kinase
- BTKis
Inhibitors of Bruton tyrosine kinase
- DARPin
Designed ankyrin repeat protein
- FA
Food allergy
- IgE
Immunoglobulin E
- JAK
Janus kinase
- IL-4Rα
IL-4 receptor alpha
- Multi-OIT
Oral immunotherapy to 2+ allergens
- OIT
Oral immunotherapy
- TNX-901
Talizumab
- TSLP
Thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Dr. Long reports consultant fees from COUR Pharmaceuticals. Dr. Chinthrajah reports grants from NIAID, CoFAR, Aimmune, DBV Technologies, Astellas, Regeneron, Stanford Maternal and Child Health Research Institute (MCHRI), and FARE. She is an Advisory Board Member at Alladapt Therapeutics, Novartis, Genentech, Sanofi, Allergenis, and Intrommune Therapeutics. Dr. Sindher reports grants from NIH, Regeneron, DBV Technologies, Aimmune, Novartis, CoFAR, and FARE. She is an Advisory member at Genentech and DBV Technologies. All other authors indicate no conflicts of interest.
References
- 1.Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw Open 2019;2:e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr 2013;167:1026–1031. [DOI] [PubMed] [Google Scholar]
- 3.Shaker MS, Schwartz J, Ferguson M. An update on the impact of food allergy on anxiety and quality of life. Curr Opin Pediatr 2017;29:497–502. [DOI] [PubMed] [Google Scholar]
- 4.Sindher SB, Long A, Chin AR, Hy A, Sampath V, Nadeau KC, et al. Food allergy, mechanisms, diagnosis and treatment: Innovation through a multi-targeted approach. Allergy 2022. [DOI] [PubMed] [Google Scholar]
- 5.Investigators PGoC Vickery BP, Vereda A Casale TB, Beyer K, du Toit G, et al. AR101 Oral Immunotherapy for Peanut Allergy. N Engl J Med 2018;379:1991–2001. [DOI] [PubMed] [Google Scholar]
- 6.Begin P, Winterroth LC, Dominguez T, Wilson SP, Bacal L, Mehrotra A, et al. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol 2014;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.J OBH, Beyer K, Abbas A, Fernandez-Rivas M, Turner PJ, Blumchen K, et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child Adolesc Health 2020;4:728–739. [DOI] [PubMed] [Google Scholar]
- 8.Bird JA, Spergel JM, Jones SM, Rachid R, Assa’ad AH, Wang J, et al. Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J Allergy Clin Immunol Pract 2018;6:476–485 e473. [DOI] [PubMed] [Google Scholar]
- 9.Jones SM, Kim EH, Nadeau KC, Nowak-Wegrzyn A, Wood RA, Sampson HA, et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet 2022;399:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol 2017;139:173–181 e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brozek JL, Firmino RT, Bognanni A, Arasi S, Ansotegui I, Assa’ad AH, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guideline update - XIV - Recommendations on CMA immunotherapy. World Allergy Organ J 2022;15:100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinthrajah RS, Purington N, Andorf S, Long A, O’Laughlin KL, Lyu SC, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2019;394:1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:e9–17. [DOI] [PubMed] [Google Scholar]
- 14.Chinthrajah RS, Purington N, Andorf S, Rosa JS, Mukai K, Hamilton R, et al. Development of a tool predicting severity of allergic reaction during peanut challenge. Ann Allergy Asthma Immunol 2018;121:69–76.e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischer DM, Greenhawt M, Sussman G, Bégin P, Nowak-Wegrzyn A, Petroni D, et al. Effect of Epicutaneous Immunotherapy vs Placebo on Reaction to Peanut Protein Ingestion Among Children With Peanut Allergy: The PEPITES Randomized Clinical Trial. JAMA 2019;321:946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadeau KC, Kohli A, Iyengar S, DeKruyff RH, Umetsu DT. Oral immunotherapy and anti-IgE antibody-adjunctive treatment for food allergy. Immunol Allergy Clin North Am 2012;32:111–133. [DOI] [PubMed] [Google Scholar]
- 17.Leung DY, Sampson HA, Yunginger JW, Burks AW Jr., Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003;348:986–993. [DOI] [PubMed] [Google Scholar]
- 18.Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, et al. A phase II, randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol 2011;127:1309–1310 e1301. [DOI] [PubMed] [Google Scholar]
- 19.Fiocchi A, Artesani MC, Riccardi C, Mennini M, Pecora V, Fierro V, et al. Impact of Omalizumab on Food Allergy in Patients Treated for Asthma: A Real-Life Study. J Allergy Clin Immunol Pract 2019;7:1901–1909 e1905. [DOI] [PubMed] [Google Scholar]
- 20.Gasser P, Tarchevskaya SS, Guntern P, Brigger D, Ruppli R, Zbaren N, et al. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun 2020;11:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manohar M, Dunham D, Gupta S, Yan Z, Zhang W, Minnicozzi S, et al. Immune changes beyond Th2 pathways during rapid multifood immunotherapy enabled with omalizumab. Allergy 2021;76:2809–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corren J, Shapiro G, Reimann J, Deniz Y, Wong D, Adelman D, et al. Allergen skin tests and free IgE levels during reduction and cessation of omalizumab therapy. J Allergy Clin Immunol 2008;121:506–511. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Abente J, Benito-Villalvilla C, Jaumont X, Pfister P, Tassinari P, Palomares O. Omalizumab restores the ability of human plasmacytoid dendritic cells to induce Foxp3(+)Tregs. Eur Respir J 2021;57. [DOI] [PubMed] [Google Scholar]
- 24.MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol 2017;139:873–881 e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandstrom J, Vetander M, Sundqvist AC, Lilja G, Johansson SGO, Melen E, et al. Individually dosed omalizumab facilitates peanut oral immunotherapy in peanut allergic adolescents. Clin Exp Allergy 2019;49:1328–1341. [DOI] [PubMed] [Google Scholar]
- 26.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 2016;137:1103–1110 e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol 2018;3:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begin P, Dominguez T, Wilson SP, Bacal L, Mehrotra A, Kausch B, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin Immunol 2014;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuberbier T, Wood RA, Bindslev-Jensen C, Fiocchi A, Chinthrajah RS, Worm M, et al. Omalizumab in IgE-mediated food allergy: A systematic review and meta-analysis. J Allergy Clin Immunol Pract 2022. [DOI] [PubMed] [Google Scholar]
- 30.Hill DA, Dudley JW, Spergel JM. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J Allergy Clin Immunol Pract 2017;5:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yee CSK, Albuhairi S, Noh E, El-Khoury K, Rezaei S, Abdel-Gadir A, et al. Long-Term Outcome of Peanut Oral Immunotherapy Facilitated Initially by Omalizumab. J Allergy Clin Immunol Pract 2019;7:451–461 e457. [DOI] [PubMed] [Google Scholar]
- 32.Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy 2014;44:1371–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benito-Villalvilla C, de la Rocha-Munoz A, Lopez-Abente J, Eggel A, Bottoli I, Severin T, et al. Ligelizumab impairs IgE-binding to plasmacytoid dendritic cells more potently than omalizumab and restores IFN-alpha production and FOXP3(+) Treg generation. Allergy 2022. [DOI] [PubMed] [Google Scholar]
- 34.Maurer M, Gimenez-Arnau A, Bernstein JA, Chu CY, Danilycheva I, Hide M, et al. Sustained safety and efficacy of ligelizumab in patients with chronic spontaneous urticaria: A one-year extension study. Allergy 2022;77:2175–2184. [DOI] [PubMed] [Google Scholar]
- 35.Maurer M, Gimenez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. Ligelizumab for Chronic Spontaneous Urticaria. N Engl J Med 2019;381:1321–1332. [DOI] [PubMed] [Google Scholar]
- 36.Gauvreau GM, Arm JP, Boulet LP, Leigh R, Cockcroft DW, Davis BE, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol 2016;138:1051–1059. [DOI] [PubMed] [Google Scholar]
- 37.Russo F, Cortonesi G, Lazzeri L, Taddeucci P, Rubegni P. A case of chronic spontaneous urticaria unresponsive to ligelizumab successfully treated with omalizumab. Dermatol Ther 2022;35:e15859. [DOI] [PubMed] [Google Scholar]
- 38.Kuo BS, Li CH, Chen JB, Shiung YY, Chu CY, Lee CH, et al. IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms. J Clin Invest 2022;132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sindher SB, Kumar D, Cao S, Purington N, Long A, Sampath V, et al. Phase 2, randomized multi oral immunotherapy with omalizumab ‘real life’ study. Allergy 2022. [DOI] [PubMed] [Google Scholar]
- 40.Eggel A, Baravalle G, Hobi G, Kim B, Buschor P, Forrer P, et al. Accelerated dissociation of IgE-FcepsilonRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J Allergy Clin Immunol 2014;133:1709–1719 e1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pennington LF, Gasser P, Brigger D, Guntern P, Eggel A, Jardetzky TS. Structure-guided design of ultrapotent disruptive IgE inhibitors to rapidly terminate acute allergic reactions. J Allergy Clin Immunol 2021;148:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sastre J, Davila I. Dupilumab: A New Paradigm for the Treatment of Allergic Diseases. J Investig Allergol Clin Immunol 2018;28:139–150. [DOI] [PubMed] [Google Scholar]
- 43.Administration UFaD. Dupixent Prescribing Information (revised June 2022): US Food and Drug Administration; 2022. [Google Scholar]
- 44.Spekhorst LS, van der Rijst LP, de Graaf M, van Megen M, Zuithoff NPA, Knulst AC, et al. Dupilumab has a profound effect on specific-IgE levels of several food allergens in atopic dermatitis patients. Allergy 2022. [DOI] [PubMed] [Google Scholar]
- 45.Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol 2016;137:984–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chinthrajah S, Cao S, Liu C, Lyu SC, Sindher SB, Long A, et al. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N Engl J Med 2021;384:1800–1809. [DOI] [PubMed] [Google Scholar]
- 48.Corren J, Larson D, Altman MC, Segnitz RM, Avila PC, Greenberger PA, et al. Effects of combination treatment with tezepelumab and allergen immunotherapy on nasal responses to allergen: A randomized controlled trial. J Allergy Clin Immunol 2023;151:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sampath V, Sindher SB, Zhang W, Nadeau KC. New treatment directions in food allergy. Ann Allergy Asthma Immunol 2018;120:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khodoun MV, Tomar S, Tocker JE, Wang YH, Finkelman FD. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J Allergy Clin Immunol 2018;141:171–179 e171. [DOI] [PubMed] [Google Scholar]
- 51.Dispenza MC. The Use of Bruton’s Tyrosine Kinase Inhibitors to Treat Allergic Disorders. Curr Treat Options Allergy 2021;8:261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dispenza MC, Krier-Burris RA, Chhiba KD, Undem BJ, Robida PA, Bochner BS. Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J Clin Invest 2020;130:4759–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fadlalmola HA, Albadrani MS, Elhusein AM, Mohamedsalih WE, Swamy VDS, Mamanao DM. Effectiveness and Safety of Abrocitinib in Patients with Moderate-to-Severe Atopic Dermatitis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Dermatol Res Pract 2021;2021:8382761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan H, Jia H, Xia T, Zhang D. Comparative efficacy and safety of abrocitinib, baricitinib, and upadacitinib for moderate-to-severe atopic dermatitis: A network meta-analysis. Dermatol Ther 2022;35:e15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Q, Wang X, Liu X, Liao YP, Chang CH, Mei KC, et al. Antigen- and Epitope-Delivering Nanoparticles Targeting Liver Induce Comparable Immunotolerance in Allergic Airway Disease and Anaphylaxis as Nanoparticle-Delivering Pharmaceuticals. ACS Nano 2021;15:1608–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gamazo C, Gastaminza G, Ferrer M, Sanz ML, Irache JM. Nanoparticle based-immunotherapy against allergy. Immunotherapy 2014;6:885–897. [DOI] [PubMed] [Google Scholar]
- 57.Hong J, Gao Q, Xiao X, Cao H, Yuan R, Liu Z, et al. T cell epitope of arginine kinase with CpG co-encapsulated nanoparticles attenuates a shrimp allergen-induced Th2-bias food allergy. Biosci Biotechnol Biochem 2020;84:804–814. [DOI] [PubMed] [Google Scholar]
- 58.Pei W, Li X, Bi R, Zhang X, Zhong M, Yang H, et al. Exosome membrane-modified M2 macrophages targeted nanomedicine: Treatment for allergic asthma. J Control Release 2021;338:253–267. [DOI] [PubMed] [Google Scholar]
- 59.Liang J, Dong X, Yang A, Zhu D, Kong D, Lv F. A dual fluorescent reverse targeting drug delivery system based on curcumin-loaded ovalbumin nanoparticles for allergy treatment. Nanomedicine 2019;16:56–68. [DOI] [PubMed] [Google Scholar]
- 60.Brotons-Canto A, Gamazo C, Martin-Arbella N, Abdulkarim M, Matias J, Gumbleton M, et al. Evaluation of nanoparticles as oral vehicles for immunotherapy against experimental peanut allergy. Int J Biol Macromol 2018;110:328–335. [DOI] [PubMed] [Google Scholar]
- 61.Brotons-Canto A, Gamazo C, Martin-Arbella N, Abdulkarim M, Gumbleton M, Quincoces G, et al. Mannosylated Nanoparticles for Oral Immunotherapy in a Murine Model of Peanut Allergy. J Pharm Sci 2019;108:2421–2429. [DOI] [PubMed] [Google Scholar]
- 62.Hughes KR, Saunders MN, Landers JJ, Janczak KW, Turkistani H, Rad LM, et al. Masked Delivery of Allergen in Nanoparticles Safely Attenuates Anaphylactic Response in Murine Models of Peanut Allergy. Front Allergy 2022;3:829605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gevaert P, De Craemer J, De Ruyck N, Rottey S, de Hoon J, Hellings PW, et al. Novel antibody cocktail targeting Bet v 1 rapidly and sustainably treats birch allergy symptoms in a phase 1 study. J Allergy Clin Immunol 2022;149:189–199. [DOI] [PubMed] [Google Scholar]


