Abstract
Adolescent substance use is a significant public health problem and there is a need for effective substance use preventions. To develop effective preventions, it is important to identify neurobiological risk factors that predict increases in substance use in adolescence and to understand potential sex differences in risk mechanisms. The present study used functional magnetic resonance imaging and hierarchical linear modeling to examine negative emotion- and reward-related neural responses in early adolescence predicting growth in substance use to middle adolescence in 81 youth, by sex. Adolescent neural responses to negative emotional stimuli and monetary reward receipt were assessed at age 12–14. Adolescents reported on substance use at age 12–14 and at 6 month, and 1, 2, and 3 year follow-ups. Adolescent neural responses did not predict initiation of substance use (yes/no), but, among users, neural responses predicted growth in substance use frequency. For girls, heightened right amygdala responses to negative emotional stimuli in early adolescence predicted growth in substance use frequency through middle adolescence. For boys, blunted left nucleus accumbens and bilateral ventromedial prefrontal cortex responses to monetary reward predicted growth in substance use frequency. Findings suggest different emotion and reward-related predictors of the development of substance use for adolescent girls versus boys.
Keywords: emotion, reward, adolescence, fMRI, substance use, sex differences
1. Introduction
Substance use increases dramatically during adolescence [1] and adolescent substance use (SU) can have significant consequences. Adolescent SU is associated with greater rates of impaired driving, violent behaviors, risky sex, and suicide during adolescence [2,3]. Furthermore, adolescent SU, particularly early and frequent/heavy SU, predicts risk for future substance use disorders (SUDs) and psychological problems into adulthood [4,5]. Notably, adolescent girls show a faster progression to SUDs and greater acute consequences of SU than boys [6]. Given the consequences of adolescent SU, it is important to understand risk factors for SU and to examine sex differences in risk factors to inform SU prevention programs. Two potential SU risk factors that are rapidly developing during adolescence are negative emotion- and reward-related brain function. The present study examined adolescents’ negative emotion- and reward-related neural responses predicting the longitudinal development of SU from early to middle adolescence, by adolescent sex.
1.1. Negative Emotion
Several theories propose that negative emotional reactivity plays a role in the development of SU [7,8]. These propose that individuals who experience high negative emotional arousal may seek out substances to down-regulate arousal. This may occur particularly in adolescence. During this developmental period, emotion-related neurobiological systems are rapidly changing, with heightened sensitivity of neural systems supporting emotional arousal (e.g., limbic regions) and protracted development of prefrontal systems that support regulation of emotional arousal [9]. This leads to a developmental period of heightened emotional arousal and reactivity and greater emotional intensity. Also during adolescence, youth initiate and escalate in substance use (and other risk behaviors). Thus, theorists have proposed that links between neural emotional reactivity and SU may be particularly salient in adolescence [9–12]. Several behavioral studies have examined associations between emotional arousal and SU in adolescence and support that they are connected, with studies finding that higher reported negative emotional arousal correlates with greater adolescent SU [e.g., 13].
Initial studies have also examined negative emotion-related neural function and adolescent SU. Several brain regions are involved in processing negative emotional stimuli. The present study focused on two commonly found regions that are specifically involved in supporting emotional reactivity: the amygdala (involved in rapid detection and reactivity) and anterior insula [AI] (involved in interoceptive awareness and reactivity). Two initial studies with adolescents found that higher amygdala reactivity to negative emotional stimuli longitudinally predicted earlier alcohol initiation [14] and cross-sectionally predicted alcohol and cannabis use disorder symptoms [15] in 12–18 year olds and one study found higher AI reactivity predicted current SU in 12–14 year old girls [16]. This supports that heightened neural reactivity to negative emotional stimuli may be associated with adolescent SU, though more research is needed.
1.2. Reward
Theorists have also proposed that altered reward sensitivity may lead to risk for SU [17]. In adolescence, reward systems in the brain are rapidly developing, with heightened sensitivity and reactivity of fronto-striatal circuits that support reactivity to reward [10,11]. At the same time, behaviorally, adolescence is a time of seeking out rewards and new sensations, and increasing SU and other risk behaviors [11]. Thus, theorists have proposed that changing and heightened neural reward system activation during adolescence may lead youth to seek out new sensations, including positive risk-taking and also including SU and negative risk behaviors [10,17–19]. Consistent with this, behavioral studies have found that higher self-reported reward seeking is correlated with greater adolescent SU [e.g., 20]. Alternatively, some youth may develop chronically blunted reward system activation. This pattern of blunted reward arousal could lead to SU in adolescence through youth seeking out substances to up-regulate low reward activation, consistent with reward deficiency theories of SU [21].
Several studies have examined neural responses to monetary reward and adolescent SU. Several brain regions are involved in reward processing. The present study focused on two commonly found regions that are specifically involved in reactivity to reward – the nucleus accumbens [NAc] (a ventral striatal region involved in motivation/salience reactivity) and ventromedial prefrontal cortex [vmPFC] (involved in salience reactivity with connections to NAc). Three studies with adolescents found that heightened NAc and/or vmPFC reactivity to monetary reward anticipation and outcome predicted current and future SU in adolescents aged 9–24 [22–24]. In contrast, four studies with adolescents found that blunted NAc reactivity (and, for two studies, also putamen reactivity) to monetary reward anticipation and outcome predicted current and future SU in adolescents aged 14–18 [15,25–27]. In sum, existing studies suggest that NAc and vmPFC reward responses are related to adolescent SU, with some finding heightened responses to reward and some finding blunted responses to reward predicting adolescent SU behavior.
1.3. Sex Differences
Sex differences in pathways to SU are important to consider for several reasons. First, girls and boys show different courses and consequences of SU [6,28]. For example, girls/women show greater intoxication and functional impairment consequences from SU and show a faster progression from initial use to SU problems than boys/men [6,28]. Second, theorists have proposed that there may be sex differences in risk factors for SU, with negative emotion and stress-related risk factors more commonly leading to SU for girls and with reward and sensation seeking risk factors more common for boys [12,29]. These sex differences in risk factors could be due to biological differences (e.g., hormone effects) and/or gender differences, with girls often socialized to more freely express negative emotions and boys to seek out new sensations [12]. Despite these differences, sex differences in the development of SU are understudied [28,29] and so more work is needed in this area.
As noted above, it has been theorized that the relationship between heightened neural response to negative emotion and SU may be stronger for girls than boys. Girls show higher stress reactivity, depressive symptoms, and negative emotional reactivity than boys [30,31]. Thus, girls may be more likely to take a pathway to SU through negative emotional reactivity [12,29]. Consistent with this, behavioral studies have found that negative emotional arousal predicts SU more strongly for adolescent girls than boys [32]. Few studies of neural negative emotion processing and SU consider sex differences, however a few support this pathway. Heightened neural reactivity to negative emotional imagery in adults has been associated with SUDs in women but not men [33]. And, one study with adolescents that examined sex differences found that higher anterior insula reactivity to negative emotional stimuli correlated with greater SU in 12–14 year old girls but not boys [16]
Theorists have also proposed that the association between altered reward system responses and SU may be stronger for boys than girls [12,29]. Boys report higher sensation seeking [34] and higher externalizing symptoms than girls [35], behaviors which are associated with neural reward responses [e.g., 36] and boys may show more sensitive reward system reactivity than girls [29]. Thus, boys may have a pattern of being more responsive to and seeking out rewards and sensations than girls, leading boys to be more likely to take a pathway to SU through seeking out substances to enhance their reward arousal [29,37]. Consistent with a reward pathway to SU for boys, studies have found that externalizing symptoms, which involve heightened sensation-seeking and reward sensitivity, predict SU more strongly for boys than girls [5]. Few studies have examined sex differences in neural reward reactivity predicting adolescent SU. In the only study with adolescents to our knowledge that examined sex differences, heightened NAc/ventral striatal reactivity to reward anticipation at age 16 predicted alcohol use two years later for adolescent boys [37]. This finding may suggest that neural reactivity to anticipating a reward may lead to SU, particularly for boys. The present study will contribute to this literature by examining sex differences in emotion and reward related risk factors for SU in adolescence.
1.4. Present Study
In sum, emerging work suggests that emotion- and reward-related neural function are important for adolescent SU. However, this work typically is cross-sectional and does not consider sex differences. The present study used fMRI and longitudinal methodology to examine neural reactivity to negative emotional and reward stimuli at age 12–14 (when youth have been minimally exposed to substances) predicting growth in SU through age 15–17 (when youth escalate SU), by sex. First, we hypothesized that higher neural reactivity (in amygdala and AI) to negative emotional stimuli would predict increases in SU, particularly for girls. Second, we hypothesized that altered (heightened or blunted) neural reactivity to monetary reward (in NAc and vmPFC) would predict increases in SU, particularly for boys.
2. Method
2.1. Participants
Participants were eighty one 12–14 year olds. Participants were recruited from a larger behavioral study of sex differences, emotion, and the development of SU to participate in an additional MRI session. Race/ethnicity was representative of the local community (for demographic information, see Table 1). Families were recruited for the larger study through flyers and mailings in a suburban area in the mid-Atlantic United States. Inclusion criteria for the larger study were age 12–14, IQ >= 80 for adolescent, and adequate English proficiency to complete questionnaires. Exclusion criteria were history of psychotic disorder for adolescent. The first 81 youth in the larger study who were eligible and interested were recruited for the MRI session. Inclusion criteria for the MRI session were that the adolescent was MRI safe and had no history of congenital brain defect or significant traumatic brain injury. We included youth with psychiatric medication use (n = 9, taking ADHD or anti-depressant medications), to include youth with a range of symptoms. Youth taking medication were asked to maintain their usual schedule of use.
Table 1.
Demographic and Substance Use (SU) Information
| Baseline | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 81 | ||||||||||||||||||||
|
| ||||||||||||||||||||
| Demogra phics | M | SD | ||||||||||||||||||
|
|
||||||||||||||||||||
| Age | 12.62 | 0.72 | ||||||||||||||||||
| N | % | |||||||||||||||||||
| Male | 42 | 51.9% | ||||||||||||||||||
| White | 59 | 72.8% | ||||||||||||||||||
|
| ||||||||||||||||||||
| Baseline | 6-Month Follow-up | 1-Year Follow-up | 2-Year Follow-up | 3-Year Follow-up | ||||||||||||||||
| n = 81 | n = 80 | n = 79 | n = 79 | n = 78 | ||||||||||||||||
|
| ||||||||||||||||||||
| Any SU | n | % | n | % | n | % | n | % | n | % | ||||||||||
| 14 | 17.3 | 13 | 16.3 | 14 | 17.3 | 15 | 18.5 | 32 | 39.59 | |||||||||||
| SU Freq* (for users) | M | SD | M | SD | M | SD | M | SD | M | SD | ||||||||||
| 0.54 | 0.87 | 0.49 | 0.88 | 0.48 | 0.78 | 0.98 | 1.80 | 2.10 | 2.17 | |||||||||||
|
| ||||||||||||||||||||
| Baseline | 6-Month Follow-up | 1-Year Follow-up | 2-Year Follow-up | 3-Year Follow-up | ||||||||||||||||
| girls | boys | girls | boys | girls | boys | girls | boys | girls | boys | |||||||||||
|
| ||||||||||||||||||||
| Any SU | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| 9 | 23.1 | 5 | 11.9 | 8 | 20.5 | 5 | 11.9 | 11 | 28.2 | 3 | 7.1 | 7 | 17.9 | 8 | 19.0 | 19 | 48.7 | 13 | 31.0 | |
|
| ||||||||||||||||||||
| SU Freq* (for users) | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| 0.55 | 0.85 | 0.53 | 0.93 | 0.51 | 0.91 | 0.47 | 0.87 | 0.71 | 0.92 | 0.18 | 0.39 | 1.02 | 1.95 | 0.91 | 1.61 | 2.22 | 2.10 | 1.92 | 2.32 | |
Note. White = Non-Hispanic White, SU= Substance Use, Freq = frequency
SU frequency is based on YRBS scoring bands (0 = 0 days, 1 = 1–2 days, 3 = 3–9 days, 4 = 20–39 days, 5 = 40 or more days), summed across substances.
2.2. Procedure
Families attended three sessions at baseline (from 2013–2015). In the first two, adolescents and parents completed questionnaires, interviews, tasks, and urine screens assessing SU and other constructs. In the third, adolescents completed the MRI scan. The baseline sessions were about 1–2 weeks apart (for 4 adolescents, MRI sessions were delayed 4–6 months due to orthodontic braces). Following baseline, adolescents returned for 6 month and 1, 2, and 3 year follow-ups, during which they completed questionnaires, interviews, and urine screens. Retention to follow-ups was high, with 99% attending 6-month, 98% attending 1 and 2 year, and 96% attending 3 years. Procedures were approved by the University Institutional Review Board and informed consent and assent were obtained.
2.3. MRI Measures
At the MRI session, adolescents completed practice items for the fMRI tasks on a computer outside of the scanner. They then completed the MRI scan, which included the emotion task, reward task, and a T1-weighted structural scan, on a Siemens 3T Allegra MR scanner with a standard single-channel birdcage head coil.
2.3.1. Emotion task.
Adolescents viewed negative emotional, neutral, and positive emotional images from the International Affective Picture System (IAPS) [38]. We selected negative images from a prior study of adolescents, which were developmentally appropriate for and elicited amygdala and AI activation in adolescents [39]. We selected developmentally appropriate neutral and positive IAPS pictures matched to the negative pictures on subject type and color. IAPS pictures were presented in an event-related design in a randomized order, with trial order and timing determined with Optseq2. Eighty-one trials (27 negative, 27 neutral, 27 positive) were presented across 3 runs. Each trial consisted of viewing a picture (4s), youth rating emotions with a button box (4s), and an inter-trial interval jittered between 2s and 12s. Analyses focused on negative trials minus neutral trials to focus on neural responses to negative emotional stimuli (versus to viewing and rating stimuli generally).
2.3.2. Reward task.
The reward task was an event-related card-guessing task developed for adolescents [40]. This task has been shown to elicit NAc and medial PFC responses in adolescents [40]. Twenty-four trials were presented in one run in pseudo-random order, with 12 possible win trials (6 actual wins, 6 neutral) and 12 possible loss trials (6 losses, 6 neutral). During each trial, the participant: 1) sees a question mark and guesses via button press whether the value of a card will be higher or lower than 5 (4s), 2) is told the trial type (possible win or possible loss, by viewing shuffling hands with an up or down arrow) and anticipates feedback (6s), and 3) sees the card (500ms) and receives outcome feedback (win, loss, or neutral) (500ms). After each of these events, the participant views a fixation cross for 9s. Participants are told before the task that they will receive $1 for each win and lose 50 cents from possible earnings for each loss. Trials actually have predetermined outcomes and each participant was given a “win” of $6 at the end of the study session. Analyses focused on possible win-win outcome trials minus possible win-neutral outcome trials. Because both had the same anticipation period, the contrast focuses on the difference between win versus neutral outcome.
2.3.3. MRI analyses.
The hemodynamic response during the tasks was collected using gradient-echo echoplanar images (GE-EPI) (TR/TE: 2250/30ms; flip = 70o; FOV: 192mm; matrix size: 64 × 64; 40 axial 3mm thick/1mm gap slices). For structural imaging, we acquired a whole-head anatomical scan using a T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) pulse sequence (TR/TE = 2300/3ms; FOV = 260mm; matrix size = 256 × 256; 160 1mm thick slices).
MRI data were analyzed with FSL 5.0 and MATLAB, using a standard preprocessing and analysis pipeline [see also 16]. Data were motion corrected, slice time corrected, and B0 unwarped. Data were smoothed with a 6mm full width half maximum (FWHM) kernel and temporally filtered. Data were coregistered to each participant’s mean MPRAGE and then normalized to the Montreal Neurological Institute (MNI) template. Runs with motion > 3mm at any TR in any direction were excluded (6 adolescents in the emotion task were excluded-thus the N for emotion task analyses is 75). Two adolescents had only 1–2 TR spikes of between 3 and 6mm in the emotion task and we kept those participants in the data after scrubbing the spikes with FSL motion outlier function. One adolescent did not complete the reward task due to a computer malfunction, thus the N for reward task analyses is 80.
Next, first-level General Linear Models (GLMs) were run with FSL’s fMRI Expert Analysis Tool (FEAT). Blood-oxygen-level-dependent (BOLD) signal at each voxel was modeled using generalized least squares with a voxel-wise, temporally and spatially regularized autocorrelation model, drift fit with Gaussian-weighted running line smoother (96 s FWHM). These models included timing of events of interest (for each condition) and motion correction parameters as regressors. These regressors were convolved with double gamma functions to create explanatory variables.
2.4. Questionnaire Measures
In a private room in the lab, adolescents reported on their SU in lifetime at each time-point (baseline, 6-month, and 1, 2, and 3 year follow-up) on a questionnaire (the Youth Risk Behavior Survey 2011 National Version (YRBS) [41] and on a structured interview (the Teen Addiction Severity Index (T-ASI) [42]. The YRBS asks about number of days in lifetime using substances for 11 substances (e.g., alcohol, marijuana, inhalants) and number of days in past 30 using nicotine. For each substance, the YRBS is scored as 0 (never used), 1 (used 1–2 days), 2 (3–9 days), 4 (20–39 days), or 5 (40 or more days). We summed these scores across all substances to create an overall SU frequency score. The T-ASI asks about past 30 day SU. The T-ASI identified 3 additional youth who denied use on YRBS. For these youth, we coded their T-ASI data using the YRBS scoring and used this data in analyses. We also collected urine drug screens, but those did not identify additional users who did not report use on YRBS or T-ASI.
2.5. Analysis Plan
2.5.1. ROIs.
Analyses focused on responses in a-priori ROIs (amygdala and AI for emotion task and NAc and vmPFC for reward task). ROIs were created from FSL’s Harvard-Oxford atlas for amygdala and NAc and Automated Anatomical Labels for AI and vmPFC, in MNI standard space. Coefficient of parameter estimate (COPE) values for negative and neutral trials (in the emotion task) and for reward outcome and neutral outcome trials (in the reward task) were extracted and averaged across these subject-specific ROIs. We calculated the difference between negative-neutral trials (for the emotion task) and the difference between reward outcome-neutral outcome trials (for reward task).
2.5.2. Covariates.
We considered the following as covariates if they predicted growth in SU in HLMs: age at baseline, race, psychiatric medication use, and pubertal stage. None of these predicted SU growth, so no covariates were included in analyses.
2.5.3. Main analyses.
We created growth models in HLM 8.0 software, with extracted ROI responses to negative emotion (-neutral) and to reward outcome (-neutral) predicting growth in SU over time. As is common in studies of adolescent SU, the SU frequency variable was zero-inflated. Thus, we conducted HLMs using a zero-inflated modeling approach. We first examined growth in dichotomous use/no use. Then, we restricted analysis to users (youth who used at one or more time-point- n = 42) and examined growth in the continuous SU frequency variable.
In the growth models we created, on Level 1, the HLMs predicted change in the SU outcome variable (yes/no SU or SU frequency) over time. Time was modeled as years since baseline (0, 0.5, 1, 2, and 3 years). For analyses predicting the yes/no SU categorical outcome, the HLM used Bernouli (0/1) modeling. On Level 2, person-attributes including the ROI response score (amygdala/AI response to negative – neutral images, NAc/vmPFC response to reward – neutral outcome), sex (girl = 0, boy = 1), and the ROI response score X sex interaction were entered as having effects on both the intercept and slope. Four models were run for the emotion task (R amygdala, L amygdala, R AI, L AI responses) and four models were run for the reward task (R NAc, L NAc, R vmPFC, L vmPFC responses) and we used FDR correction for multiple ROIs within each task. In each HLM, random effects were estimated to allow variability across individuals in both their intercepts and growth rates. For significant ROI activation X sex interactions, we plotted growth in SU separately for boys and girls with high/low (±1SD) ROI response and conducted separate HLMs for boys and girls.
3. Results
3.1. Preliminary Analyses
Continuous variables were examined for outliers (values greater than or less than 3 SDs from the mean). Amygdala, AI, and vmPFC responses and SU frequency each had 1–4 outlier cases, all of which were above the mean. To reduce the impact of extreme outliers, these cases were winsorized (set to equal 3 SDs above the mean), consistent with other studies of neural activation measures [e.g., 43]. After winsorization, skewedness was < 2.0. We tested sex differences in ROI responses and these were not significant.
Behavioral ratings.
For the emotion task, the mean negative rating for negative images was 3.36 on a 1–4 scale (SD = .46) and for neutral pictures was 1.48 (SD = .44) (difference: t = 28.73, p < .001). Two adolescents were missing some behavioral button presses for the emotion task (50%- 75% of button presses were missing) and two adolescents were missing some of the behavioral button presses for the reward task. This could reflect lack of attention to the task. Given this, we conducted a sensitivity analysis excluding these youth (see below).
3.2. Substance Use
As shown in Table 1, 14 (17.3%) of youth reported SU at baseline, consistent with rates in community studies [e.g., 44]. 97.6% of the users used alcohol, 35.7% marijuana, 19.0% nicotine, 7.1% inhalants, 4.8% benzodiazepines, and 2.4% used LSD, methamphetamine, or sedatives (we excluded SU due to medications). SU rates increased from 17.3% at baseline to 40.0% at 3 year follow-up. Of the users, mean SU frequency scores increased from 0.54 (SD = 0.87) at baseline, corresponding to less than 1 day of use, to 2.17 (SD = 2.17) at 3 year follow-up, corresponding to about 3–9 days of use. There were not significant sex differences in SU at baseline, 6 month, 2 year or 3 year follow-ups. There was a significant sex difference in SU 1 year follow-up, with girls showing higher SU rates (Chi2 = 3.91, p = .05) and frequency (t = 2.50, p = .02) than boys.
SU rates and frequency both increased slowly at first from baseline to 1 year follow-up, then more quickly from 1 and 2 year to 3 year follow-up. We tested a linear growth model (years), but, given this pattern, we also tested a model that included a quadratic term (years2). Both the linear and quadratic terms had significant fixed effects on SU (both yes/no SU and SU frequency). Both the linear and quadratic terms also had significant random effects for growth in SU, however the linear time variable had a random effects variance component that was 10 times greater than the quadratic time variable, suggesting that most of the between person differences in growth were well accounted for by the linear component. Consistent with Raudenbush and Bryk’s [45] recommendation to be parsimonious with random effects, we added years2 to the HLM models described above as a fixed effect, but not as a random effect.
Nine youth in the study were inconsistent reporters (reported lifetime SU at one of the time-points, but then denied at the next follow-up[s]). Inconsistent reporting is common in studies of adolescent SU [46]. To address this, we conducted secondary sensitivity analyses. First, we tested the HLMs excluding these 9 youth.
Second, we tested HLMs with a “carry forward” variable that used the last reported SU value carried forward to future time points.
3.3. Negative Emotion to Yes/No SU
There were no significant amygdala or AI response to emotion X sex interactions or main effects on the intercept or on growth in the yes/no SU variable.
3.4. Negative Emotion to SU Frequency
For youth who used substances, there was a significant R amygdala response X sex interaction effect on growth in SU frequency (b = −0.06, SE = 0.02, FDR-corrected p = .02) (see Table 2). In addition, there was a significant main effect of higher R amygdala response predicting greater growth in SU frequency over time (b = 0.05, SE = 0.01, FDR-corrected p = .004). There were no significant R amygdala response X sex interactions or main effects on the SU frequency intercept.
Table 2.
Results of Hierarchical Linear Modeling Analyses with ROI Response to Negative Emotional Stimuli Predicting Growth in Substance Use (SU) Frequency, for Users
| R Amygdala to SU Frequency | L Amygdala to SU Frequency | R AI to SU Frequency | L AI to SU Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Fixed Effects | B | SE | p | B | SE | p | B | SE | P | B | SE | P |
|
| ||||||||||||
| For Intercept: | ||||||||||||
| Intercept | 0.59 | 0.17 | .001 | 0.57 | 0.17 | .002 | 0.60 | 0.17 | .001** | 0.60 | 0.17 | <001*** |
| Sex | 0.003 | 0.28 | .99 | 0.06 | 0.29 | .83 | 0.03 | 0.27 | .92 | −0.02 | 0.27 | .95 |
| ROI Resp | −0.01 | 0.02 | .64 | −0.01 | 0.02 | .52 | −0.004 | 0.01 | .74 | 0.01 | .01 | .17 |
| Sex X ROI Resp |
−0.003 | 0.03 | .92 | −0.01 | 0.03 | .68 | −0.003 | 0.01 | .81 | −0.01 | 0.01 | .31 |
| For Slope: Intercept | −0.40 | 0.2 | .16 | −0.38 | 0.27 | .16 | −0.37 | 0.29 | .21 | −0.37 | 0.29 | .21 |
| Sex | 0.06 | 0.26 | .82 | 0.01 | 0.22 | .98 | 0.03 | 0.25 | .91 | −0.32 | 0.26 | .90 |
| ROI Resp | 0.05 | 0.01 | .002** | 0.05 | 0.01 | <001*** | 0.01 | 0.02 | .61 | −0.001 | 0.02 | .94 |
| Sex X ROI Resp |
−0.06 | 0.02 | .004** | −0.03 | 0.02 | .11 | −.003 | .02 | .89 | 0.002 | 0.02 | .92 |
| For Slope2: | ||||||||||||
| 0.29 | 0.0 | <001*** | 0.29 | 0.0 | <001*** | 0.29 | 0.0 | <001*** | 0.29 | 0.7 | <001*** | |
| Intercept | 8 | 8 | 8 | 9 | ||||||||
|
| ||||||||||||
| Random Effects | Var | SD | P | Var | SD | P | Var | SD | P | Var | SD | P |
|
|
||||||||||||
| Intercept | 0.35 | 0.59 | .001** | 0.35 | 0.59 | .001** | 0.37 | 0.61 | <001*** | 0.36 | 0.60 | <001*** |
| Slope | 0.44 | 0.66 | <001*** | 0.43 | 0.66 | <001*** | 0.37 | 0.60 | <001*** | 0.55 | 0.74 | <001*** |
| Level-1 Error | 0.74 | 0.86 | 0.74 | 0.86 | 0.74 | 0.86 | 0.74 | 0.86 | ||||
Note. AI = anterior insula; SU = substance use; ROI = region of interest; Resp = response; B = unstandardized coefficient; Var = Variance component. This analysis uses youth with emotion task data who used substances (n = 38). Uncorrected p values are presented here. FDR-corrected p values for significant main effects or interactions are presented in the text.
p < .05
p < .01
p < .001
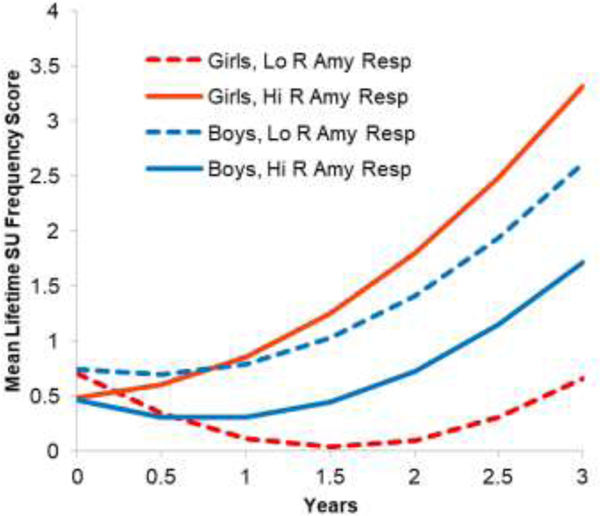
To follow up the R amygdala response to negative emotion X sex interaction, we plotted the interaction and conducted HLMs for girls and boys. As shown in Figure 1, for girls, higher R amygdala responses predicted greater increases in SU frequency. Girls at one SD above the mean in R amygdala responses increased in SU frequency scores from 0.5 (about 1 day in lifetime) to 3.3 (about 25 days in lifetime) from baseline to 3 year follow-up, whereas the girls at 1 SD below the mean stayed at 0.7 in SU frequency scores from baseline to 3 year. For boys, high and low R amygdala responses predicted similar growth in SU frequency. Boys at 1 SD above the mean in response increased 1.3 points and boys at 1 SD below the mean increased 1.9 points from baseline to 3 year. In the separate follow-up HLMs, for girls, higher R amygdala response to negative emotional stimuli significantly predicted greater increases in SU frequency over time (b = 0.05, SE = 0.01, p = .003). For boys, R amygdala response did not predict growth in SU frequency (b = −0.01, SE = 0.01, p = .39).
Figure 1.
Interaction Growth Curve Plots for Right Amygdala Response to Negative Emotional Stimuli by Sex Predicting Increases in Substance Use Frequency from Baseline through 3 Year Follow-up, for Users
Note. Lo = low; Hi = high; R = right; Amy= Amygdala; Resp= response; SU = substance use. SU frequency is based on YRBS scoring bands (0 = 0 days, 1 = 1–2 days, 3 = 3–9 days, 4 = 20–39 days, 5 = 40 or more days), summed across substances. This analysis is with youth with emotion task data who used substances (n = 38). The lines indicate mean growth for boys and girls with high (1 SD above the mean) or low (1 SD below the mean) in amygdala responses.
For youth who used substances, there was not a significant L amygdala response X sex interaction effect on growth in SU frequency. However, there was a significant main effect of higher L amygdala response predicting greater growth in SU frequency over time (b = 0.05, SE = 0.01, FDR-corrected p = .004) (see Table 2). There were no significant L amygdala response X sex interactions or main effects on the SU frequency intercept.
AI response X sex interactions and AI main effects on intercept or growth in SU frequency were not significant.
3.5. Reward to Yes/No SU
There were no significant NAc or vmPFC response to reward X sex interactions or main effects on the intercept or growth in the yes/no SU variable.
3.6. Reward to SU Frequency
For youth who used substances, there was a significant L NAc X sex interaction effect on growth in SU frequency (b = −0.03, SE = 0.01, FDR-corrected p = .02) (see Table 3).
Table 3.
Results of Hierarchical Linear Modeling Analyses with ROI Response to Reward Predicting Growth in Substance Use (SU) Frequency, for Users
| R NAc to SU Frequency | L NAc to SU Frequency | R vmPFC to SU Frequency | L vmPFC to SU Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Fixed | B | SE | P | B | SE | P | B | SE | p | B | SE | P |
|
|
||||||||||||
| Effects | ||||||||||||
|
| ||||||||||||
| For Intercep: | ||||||||||||
| Intercept | 0.64 | 0.16 | <001*** | 0.66 | 0.20 | .002** | 0.67 | 0.18 | <001*** | 0.69 | 0.20 | .002** |
| Sex | −0.25 | 0.24 | .32 | −0.30 | 0.27 | .27 | −0.31 | 0.25 | .22 | −0.36 | 0.31 | .25 |
| ROI Resp | 0.004 | 0.01 | .72 | 0.001 | 0.01 | .88 | −0.005 | 0.01 | .66 | −0.01 | .01 | .40 |
| Sex X ROI Resp |
0.01 | 0.01 | .55 | 0.01 | 0.01 | .37 | 0.03 | 0.01 | .02* | 0.02 | 0.01 | .11 |
| For Slope: Intercept | −.40 | 0.29 | .18 | −.48 | 0.28 | .10 | −0.38 | 0.28 | .18 | 0.41 | 0.29 | .17 |
| Sex | 0.18 | 0.31 | .57 | 0.38 | 0.25 | .15 | 0.14 | 0.24 | .57 | 0.22 | 0.26 | .42 |
| ROI Resp |
−0.003 | 0.01 | .79 | 0.005 | 0.01 | .57 | −0.001 | 0.01 | .95 | 0.002 | 0.01 | .82 |
| Sex X | −0.02 | .01 | .27 | −.03 | .01 | .01* | −.04 | .02 | .03* | −.03 | .01 | .01* |
| ROI Resp For Slope2: Intercept | 0.29 | 0.07 | <001*** | .28 | .07 | <001*** | 0.29 | 0.07 | <001*** | |||
|
| ||||||||||||
| Random Effects | Var | SD | P | Var | SD | P | Var | SD | P | Var | SD | P |
|
|
||||||||||||
| Intercept | 0.32 | 0.56 | <001*** | 0.32 | 0.56 | .001** | 0.26 | 0.51 | .004 | 0.29 | 0.54 | .002** |
| Slope | 0.44 | 0.67 | <001*** | 0.39 | 0.62 | <001*** | 0.37 | 0.60 | <001*** | 0.39 | 0.63 | <001*** |
| Level-1 Error |
0.70 | 0.83 | 0.69 | 0.83 | 0.70 | 0.83 | 0.69 | 0.83 | ||||
Note. R = right; L = left; SU = substance use; ROI = region of interest; Resp = response; B = unstandardized regression coefficient; Var = Variance component. This analysis uses n = 41- youth with reward task data who used substances. Uncorrected p values are presented here. FDR-corrected p values for significant main effects or interactions are presented in the text.
p < .05
p < .01
p < .001
There were no significant L NAc response X sex interactions or main effects on the SU frequency intercept.
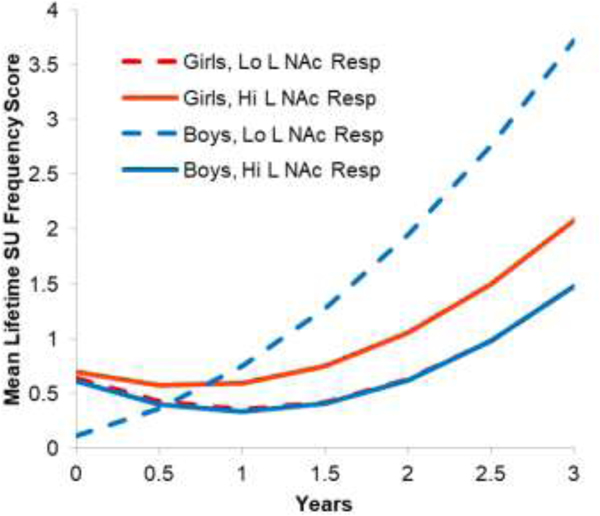
To follow up the L NAc response to reward X sex interaction, we plotted the interaction and conducted HLMs for girls and boys. As shown in Figure 2, for boys, lower L NAc activation predicted greater increases in SU frequency. Boys at one SD below the mean in L NAc responses increased in SU frequency scores from about 0.1 (about zero days) to 3.7 (about 31 days in lifetime) from baseline to 3 year, whereas the boys at 1 SD above the mean only increased from 0.6 (about 1 day in lifetime) to 1.5 (about 5 days in lifetime). For girls, high and low L NAc response predicted similar growth in SU. Girls at 1 SD above the mean in L NAc response increased 1.4 points and girls at 1 SD below the mean in L NAc response increased 0.9 points in lifetime SU frequency scores from baseline to 3 year. In the separate HLMs, for boys, lower L NAc response to reward predicted greater increases in SU frequency (b= −0.02, SE = 0.01, p = .002). For girls, L NAc response did not predict growth in SU (b= −0.01, SE = 0.01, p = .48).
Figure 2.
Interaction Growth Curve Plots for Left NAc Response to Reward by Sex Predicting Increases in Substance Use Frequency from Baseline through 3 Year Follow-up, for Users
Note. Lo = low; Hi = high;L = left; NAc = Nucleus Accumbens; Resp= response; SU = substance use. SU frequency is based on YRBS scoring bands (0 = 0 days, 1 = 1–2 days, 3 = 3–9 days, 4 = 20–39 days, 5 = 40 or more days), summed across substances. This analysis is with youth with reward task data who used substances (n = 41). The lines indicate mean growth for boys and girls with high (1 SD above the mean) or low (1 SD below the mean) in NAc responses.
There were no significant R NAc response X sex interactions or main effects on the intercept or on growth in SU frequency.
For youth who used substances, there was a significant L vmPFC X sex interaction effect on growth in SU frequency (b= −0.03, SE = 0.01, FDR-corrected p = .02) (see Table 3). There were no significant L vmPFC X sex interaction or main effects on the SU frequency intercept.
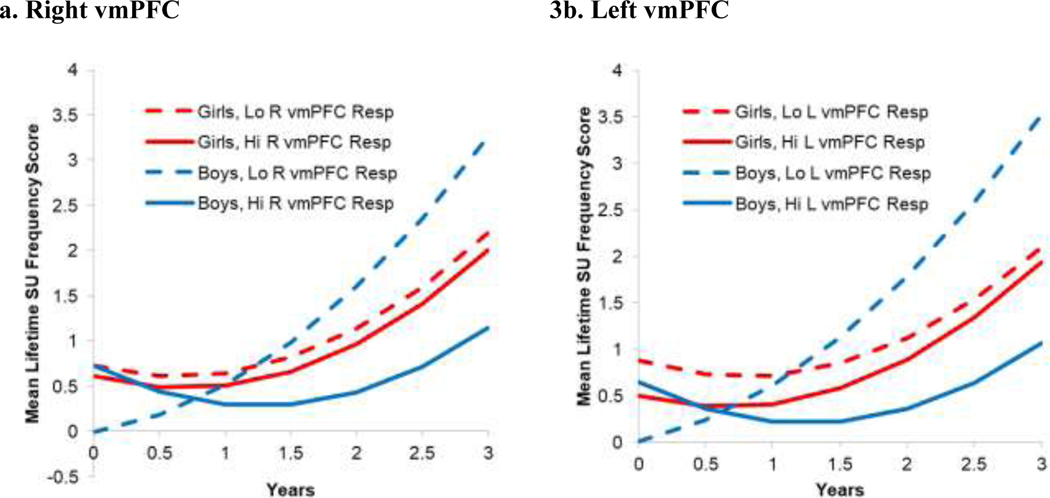
To follow up the L vmPFC response X sex interaction, we plotted the interaction and conducted HLMs for girls and boys. As shown in Figure 3, for boys, lower L vmPFC response to reward predicted greater increases in SU frequency. Boys at one SD below the mean in L vmPFC responses increased in SU frequency scores from 0.0 (zero days) to 3.5 (about 25 days in lifetime) from baseline to 3 year, whereas the boys at 1 SD above the mean only increased from 0.6 (about 1 day in lifetime) to 1.1 (1–2 days in lifetime). For girls, high and low L vmPFC response predicted similar growth in SU. Girls at 1 SD above the mean in response increased 1.4 points and girls at 1 SD below the mean in response increased 1.2 points from baseline to 3 year. In the separate HLMs, for boys, lower L vmPFC response to reward predicted greater increases in SU frequency (b= 0.02, SE = 0.01, p = .001). For girls, L vmPFC response did not predict growth in SU (b= −0.01, SE = 0.01, p =.09).
Figure 3.
Interaction Growth Curve Plots for Right and Left vmPFC Responses to Reward by Sex Predicting Increases in Substance Use Frequency from Baseline through 3 Year Follow-up, for Users
Note. Lo = low; Hi = high; R = right; L = left; vmPFC = ventromedial prefrontal cortex; Resp=response; SU = substance use. SU frequency is based on YRBS scoring bands (0 = 0 days, 1 = 1–2 days, 3 = 3–9 days, 4 = 20–39 days, 5 = 40 or more days), summed across substances. This analysis is with youth with reward task data who used substances (n = 41). The lines indicate mean growth for boys and girls with high (1 SD above the mean) or low (1 SD below the mean) in vmPFC responses.
For R vmPFC responses, for youth who used substances, there was a significant R vmPFC X sex interaction effect on growth in SU frequency (b= −0.04, SE = 0.01, FDR-corrected p = .045), as shown in Table 3. There was also a significant R vmPFC response X sex interaction effect on the intercept, but this fell out of significance with FDR correction (b= 0.03, SE = 0.01, FDR-corrected p = .09).
To follow up the R vmPFC response X sex interaction, we plotted the interaction and conducted HLMs for girls and boys. As shown in Figure 3, for boys, lower R vmPFC response to reward predicted greater increases in SU frequency over time. Boys at one SD below the mean in R vmPFC responses increased in SU frequency scores from 0.0 (zero days in lifetime) to 3.3 (about 19 days in lifetime) from baseline to 3 year, whereas boys at 1 SD above the mean only increased from 0.7 (about 1 day in lifetime) to 1.1 (1–2 days in lifetime). For girls, high and low R vmPFC response predicted similar growth in SU. Girls at 1 SD above the mean in response increased 1.4 points and girls at 1 SD below the mean in response increased 1.5 points from baseline to 3 year. In the separate HLMs, for boys, lower R vmPFC response to reward predicted greater increases in SU frequency (b= −0.04, SE = 0.01, p < .001). For girls, R vmPFC response did not predict growth in SU frequency (b= −0.02, SE = 0.01, p = .11).
3.7. Sensitivity Analyses
We re-ran the HLMs excluding the 9 youth who were inconsistent SU reporters and with SU carried forward and excluding the 2 youth with excessive missing behavioral ratings. Findings did not change. ROI response main effects and response X sex interactions that were significant remained significant.
4. Discussion
This was the first study to examine sex differences in emotion- and reward-related neural responses predicting growth in SU from early to middle adolescence in a longitudinal study. This contributes to the literature by allowing understanding of early neural risk factors for SU before youth have had significant substance use effects on brain function. The study also examined sex differences, which is important given that girls may take different pathways to SU than boys [6]. This study found that neural responses did not predict growth in SU initiation (yes/no) from early to middle adolescence, but did predict growth in the frequency of SU (among users) from early to middle adolescence. Initiation of SU by middle adolescence may be a more normative process, whereas escalating frequency of SU may reflect risk for SU problems. If this is the case, altered neural responses may be more predictive of problematic than normative SU.
The present study found a right amygdala response to negative emotional stimuli by sex interaction predicting growth in SU frequency, with heightened right amygdala responses in early adolescence predicting increases in SU frequency to middle adolescence for girls but not boys. The study also found left NAc and bilateral vmPFC response to reward by sex interactions predicting growth in SU frequency, with blunted NAc and vmPFC responses in early adolescence predicting increases in SU frequency through middle adolescence for boys but not girls. Overall, our findings suggest different pathways to increases in SU frequency from early to middle adolescence for girls versus boys.
4.1. Negative Emotion
As noted above, in analyses of youth that used substances, heightened right amygdala responses to negative emotional stimuli predicted growth in SU frequency for girls, but not boys. For left amygdala, there was a main effect for heightened left amygdala response to negative emotional stimuli predicting growth in SU frequency. The left amygdala by sex interaction was not significant (p = .11), but there may have been a similar sex-differentiated pattern for left amygdala that was just out of significance due to insufficient power (which could potentially be due to signal-to-noise-ratio inconsistencies between the ROIs).
Our findings of heightened amygdala response to negative emotional stimuli predicting increases in SU is consistent with initial prior research with adolescents [14,15]. The finding that this was stronger for girls (for right amygdala at least) adds to the literature [along with one other study - 16] by suggesting that this neural risk factor may be stronger for girls. Interestingly, our findings were significant for amygdala but not AI. One interpretation of this is that this processing is operating particularly for initial automatic reactivity to negative emotion rather than subjective interpretation of emotion.
Thus, our findings suggest that adolescent girls with higher reactivity to negative emotional stimuli or situations may be at risk for increasing in frequency of SU in adolescence. This may suggest that girls are more likely to take a self-medication or internalizing pathway to SU. In response to negative emotional events, girls may be more likely to experience high negative emotional reactivity and arousal. In adolescence, as youth (and particularly girls) are faced with mounting negative stressors, girls may feel heightened negative emotional arousal and may use substances to down-regulate this arousal. Higher amygdala reactivity to negative emotional stimuli (and high negative emotions and rumination on negative emotions) is also associated with depressive symptoms [e.g., 47] and depressive symptoms are more common for adolescent girls [31] and are a predictor of SU [7]. Future research can examine internalizing symptoms as a mediator between heightened amygdala reactivity and SU in girls. The present findings should be interpreted with caution, however, given that we only found results in the part of the zero inflated model that restricted analyses to users and examined SU frequency.
Thus, more research is needed in samples with a greater number of substance users.
4.2. Reward
For analyses of youth that used substances, blunted left NAc and bilateral vmPFC responses to reward outcome predicted growth in SU frequency for boys, but not for girls. . This may indicate that reward responses are not important risk factors for SU for girls (as noted above, negative emotionality may be a more a stronger pathway for girls). However, it may be that, whereas girls’ responses to reward outcome may not be important, girls’ responses to reward anticipation might be important for SU. This was observed in one study [37] that found that blunted neural response to reward anticipation in middle adolescence predicted alcohol use in adolescent girls. Our task did not allow us to examine reward anticipation separately from outcome, so we were unable to test the effects of neural response to reward anticipation on development of SU.
Nonetheless, our finding that blunted responses to reward outcome predicted SU for boys but not girls is an important addition to the literature and may suggest that boys take a pathway to SU characterized by blunted reactivity to reward. Our finding adds to one other study of sex differences [37] which found that heightened ventral striatal response to reward anticipation and average vmPFC responses to reward outcome predicted alcohol use for boys. Our study was only able to examine reward outcome and found that blunted NAc and vmPFC responses to reward outcome predicted SU. Taken together, for boys, higher ventral striatal reactivity to anticipating reward but lower ventral striatal and average or lower vmPFC reactivity to reward outcome may be a combination that leads to motivation to seek rewards and SU. In everyday life, this may present as boys showing a pattern of excitement when anticipating a reward, but being more easily let down when receiving the reward. It could be that boys use substances at higher frequency in an effort to fulfill their need for reward.
Boys may show a pattern of lower responses to receiving rewards in childhood and then, in adolescence, may use substances (as they become available to them) in order to up-regulate feelings of reward or sensation. This suggests that boys may take a sensation-seeking or reward-seeking pathway to escalation of SU. This may also reflect that boys are more likely to take an externalizing pathway to SU. Consistent with this, boys show higher externalizing symptoms than girls in childhood and externalizing symptoms have been linked to blunted neural responses to reward [36] and have been shown to predict SU [7]. Thus, boys’ blunted reward reactivity may lead to externalizing symptoms in childhood (to increase feelings of reward), which then may lead to risk for increasing SU in adolescence as part of a pattern of acting out behaviors. Future research may consider externalizing symptoms as a mediator between blunted reward reactivity and SU for boys. Notably, the present study findings of sex differences in reward predictors of SU frequency should be viewed with caution given that the sample for the analyses of SU frequency was small. Future studies with larger samples of users, particularly studies that can separate reward anticipation from reward outcome, are needed to further test sex differences in reward-related pathways to SU.
4.3. Limitations and Future Directions
The present study’s strength is that it is the first to examine sex differences in neural responses in well-established negative emotion and reward processing tasks and in theoretically-grounded ROIs predicting escalation of SU longitudinally over three years during a critical developmental period from early to middle adolescence. Neural activation being measured when SU is low (or non-existent) is a strength because we can more strongly conclude that neural patterns are an initial vulnerability factor for SU rather than a possible consequence of SU. A significant limit is that our sample size was small, and even smaller for analyses that were limited to the users. Finding samples of substance using (or at least, substance use reporting) youth is a common challenge in community-based SU studies [48]. The present study findings, however, are consistent with theory and an emerging body of behavioral and neuroimaging findings suggesting different emotion- and reward-related pathways to SU in girls and boys [e.g., 29]. The present study findings provide intriguing targets/patterns that can be tested in larger samples. For example, future research can test our model of sex differences in early adolescent neural risk factors with the Adolescent Brain Cognitive Development (ABCD) dataset once the SU data for middle adolescence is released. Another limit is that our sample was mostly White, which may limit generalizability to other groups, groups which may have differing sex roles and expectations or different patterns of life experiences [49]. In addition, our measure of sex was obtained from a dichotomous boy/girl question and thus does not necessarily reflect gender identity, which is also important to consider in the development of SU.
5. Conclusions
In sum, we found sex differences in negative emotion- and reward-related neural responses predicting longitudinal growth in SU frequency from early to middle adolescence. For girls, higher right amygdala responses to negative emotional stimuli in early adolescence predicted increases in SU frequency to middle adolescence. In contrast, for boys, blunted NAc and vmPFC responses to receiving rewards in early adolescence predicted increases in SU frequency to middle adolescence. If these findings are replicated in larger samples, this would have significant implications. It would suggest a need to consider sex differences in developing SU prevention programs. For example, SU prevention programs may attend to reducing heightened negative emotional arousal particularly for girls and to increasing reward responses particularly for boys.
Highlights.
Adolescents’ neural responses during negative emotion and reward processing predicted longitudinal growth in substance use frequency over three years in adolescence.
Heightened amygdala responses to negative emotional stimuli predicted increases in substance use from early to middle adolescence for girls, but not boys.
Blunted striatal and ventromedial prefrontal cortex responses to reward predicted increases in substance use from early to middle adolescence for boys, but not girls.
Findings suggest sex differences in neural predictors of substance use risk during adolescence.
Acknowledgments:
The authors gratefully acknowledge the study sponsor, the participating families, and the study research assistants, particularly Fran Faundez, Alexandra Martelli, Juliana Jacangelo, and Corynne Ross.
Funding:
Support for this project was provided by the National Institute on Drug Abuse (NIDA) through grants R01-DA-033431 and R01-DA-033431-S1 (PI: Chaplin) and grant F31- DA051154 (PI: Gonçalves).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declarations of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME, Monitoring the Future national survey results on drug use, 1975–2021: Overview, key findings on adolescent drug use, Institute for Social Research, University of Michigan, Ann Arbor. (2022). 10.7302/4142. [DOI] [Google Scholar]
- [2].National Institute on Alcohol Abuse and Alcoholism (NIAAA), Underage Drinking, Alcohol’s Effects on Health. (2023). [Google Scholar]
- [3].Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Smith GT, Giedd J, Dahl RE, Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age, Pediatrics. 121 Suppl 4 (2008) S273–289. 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brook DW, Brook JS, Zhang C, Cohen P, Whiteman M, Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders, Arch Gen Psychiatry. 59 (2002) 1039–1044. 10.1001/archpsyc.59.11.1039. [DOI] [PubMed] [Google Scholar]
- [5].Chassin L, Pitts SC, Prost J, Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes, Journal of Consulting and Clinical Psychology. 70 (2002) 67–78. 10.1037/0022-006X.70.1.67. [DOI] [PubMed] [Google Scholar]
- [6].Amaro H, Blake SM, Schwartz PM, Flinchbaugh LJ, Developing theory-based substance abuse prevention programs for young adolescent girls, The Journal of Early Adolescence. 21 (2001) 256–293. 10.1177/0272431601021003002. [DOI] [Google Scholar]
- [7].Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S, An internalizing pathway to alcohol use and disorder, Psychol Addict Behav. 25 (2011) 390–404. 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khantzian EJ, The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence, Am J Psychiatry. 142 (1985) 1259–1264. 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- [9].Powers A, Casey BJ, The Adolescent Brain and the Emergence and Peak of Psychopathology, Journal of Infant, Child, and Adolescent Psychotherapy. 14 (2015) 3–15. 10.1080/15289168.2015.1004889. [DOI] [Google Scholar]
- [10].Chambers RA, Taylor JR, Potenza MN, Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability, Am J Psychiatry. 160 (2003) 1041–1052. 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dahl RE, Adolescent Brain Development: A Period of Vulnerabilities and Opportunities. Keynote Address, Annals of the New York Academy of Sciences. 1021 (2004) 1–22. 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- [12].Chaplin TM, Niehaus C, Gonçalves SF, Stress reactivity and the developmental psychopathology of adolescent substance use, Neurobiol Stress. 9 (2018) 133–139. 10.1016/j.ynstr.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Measelle J, Stice E, Springer D, A Prospective Test of the Negative Affect Model of Substance Use and Abuse: Moderating Effects of Social Support, Psychol Addict Behav. 20 (2006) 225–233. 10.1037/0893-164X.20.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elsayed NM, Kim MJ, Fields KM, Olvera RL, Hariri AR, Williamson DE, Trajectories of Alcohol Initiation and Use During Adolescence: The Role of Stress and Amygdala Reactivity, J Am Acad Child Adolesc Psychiatry. 57 (2018) 550–560. 10.1016/j.jaac.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aloi J, Blair KS, Crum KI, Meffert H, White SF, Tyler PM, Thornton LC, Mobley AM, Killanin AD, Adams KO, Filbey F, Pope K, Blair RJR, Adolescents show differential dysfunctions related to Alcohol and Cannabis Use Disorder severity in emotion and executive attention neuro-circuitries, NeuroImage: Clinical. 19 (2018) 782–792. 10.1016/j.nicl.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chaplin TM, Poon JA, Thompson JC, Hansen A, Dziura SL, Turpyn CC, Niehaus CE, Sinha R, Chassin L, Ansell EB, Sex-Differentiated Associations among Negative Parenting, Emotion-Related Brain Function, and Adolescent Substance Use and Psychopathology Symptoms, Soc Dev. 28 (2019) 637–656. 10.1111/sode.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Geier CF, Adolescent cognitive control and reward processing: implications for risk taking and substance use, Horm Behav. 64 (2013) 333–342. 10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- [18].Telzer EH, Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation, Dev Cogn Neurosci. 17 (2016) 57–67. 10.1016/j.dcn.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ, Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents, J Neurosci. 26 (2006) 6885–6892. 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Knyazev GG, Behavioural activation as predictor of substance use: mediating and moderating role of attitudes and social relationships, Drug Alcohol Depend. 75 (2004) 309–321. 10.1016/j.drugalcdep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- [21].Blum K, Cull JG, Braverman ER, Comings DE, Reward deficiency syndrome, American Scientist. 84 (1996) 132–146. [Google Scholar]
- [22].Braams BR, Peper JS, van der Heide D, Peters S, Crone EA, Nucleus accumbens response to rewards and testosterone levels are related to alcohol use in adolescents and young adults, Dev Cogn Neurosci. 17 (2016) 83–93. 10.1016/j.dcn.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cope LM, Martz ME, Hardee JE, Zucker RA, Heitzeg MM, Reward activation in childhood predicts adolescent substance use initiation in a high-risk sample, Drug Alcohol Depend. 194 (2019) 318–325. 10.1016/j.drugalcdep.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bertocci MA, Bebko G, Versace A, Iyengar S, Bonar L, Forbes EE, Almeida JRC, Perlman SB, Schirda C, Travis MJ, Gill MK, Diwadkar VA, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson DA, Frazier TW, Arnold LE, Fristad MA, Youngstrom EA, Horwitz SM, Findling RL, Phillips ML, Reward-related neural activity and structure predict future substance use in dysregulated youth, Psychol Med. 47 (2017) 1357–1369. 10.1017/S0033291716003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nees F, Witt SH, Dinu-Biringer R, Lourdusamy A, Tzschoppe J, Vollstädt-Klein S, Millenet S, Bach C, Poustka L, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, Büchel C, Conrod PJ, Frank J, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Mann K, Martinot J-L, Paus T, Pausova Z, Robbins TW, Smolka MN, Rietschel M, Schumann G, Flor H, IMAGEN consortium BDNF Val66Met and reward-related brain function in adolescents: role for early alcohol consumption, Alcohol. 49 (2015) 103–110. 10.1016/j.alcohol.2014.12.004. [DOI] [PubMed] [Google Scholar]
- [26].Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Feldstein Ewing SW, Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents, Dev Cogn Neurosci. 16 (2015) 5–15. 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot J-L, Paus T, Poline J-B, Robbins TW, Rietschel M, Smolka M, Ströhle A, Struve M, Loth E, Schumann G, Büchel C, IMAGEN Consortium, Lower ventral striatal activation during reward anticipation in adolescent smokers, Am J Psychiatry. 168 (2011) 540–549. 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- [28].Fox HC, Sinha R, Sex Differences in Drug-Related Stress-System Changes: Implications for Treatment in Substance-Abusing Women, Harv Rev Psychiatry. 17 (2009) 103–119. 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hammerslag LR, Gulley JM, Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use, Behavioural Brain Research. 298 (2016) 15–26. 10.1016/j.bbr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chaplin TM, Aldao A, Gender differences in emotion expression in children: a meta-analytic review, Psychol Bull. 139 (2013) 735–765. 10.1037/a0030737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hankin BL, Mermelstein R, Roesch L, Sex differences in adolescent depression: stress exposure and reactivity models, Child Dev. 78 (2007) 279–295. 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- [32].Chaplin TM, Visconti KJ, Molfese PJ, Susman EJ, Klein LC, Sinha R, Mayes LC, Prenatal cocaine exposure differentially affects stress responses in girls and boys: Associations with future substance use, Development and Psychopathology. 27 (2015) 163. 10.1017/S0954579414000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Potenza MN, Hong KA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R, Neural correlates of stressinduced and cue-induced drug craving: influences of sex and cocaine dependence, Am J Psychiatry. 169 (2012) 406–414. 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shulman EP, Harden KP, Chein JM, Steinberg L, Sex differences in the developmental trajectories of impulse control and sensation-seeking from early adolescence to early adulthood, J Youth Adolesc. 44 (2015) 1–17. 10.1007/s10964-014-0116-9. [DOI] [PubMed] [Google Scholar]
- [35].Kimonis ER, Frick PJ, McMahon RJ, Conduct and oppositional defiant disorders, in: Mash EJ, Barkely RA (Eds.), Child Psychopathology, Guilford Press, New York, NY, 2014: pp. 145–179. [Google Scholar]
- [36].Gatzke-Kopp LM, Beauchaine TP, Shannon KE, Chipman J, Fleming AP, Crowell SE, Liang O, Johnson LC, Aylward E, Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders, Journal of Abnormal Psychology. 118 (2009) 203–213. 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Swartz JR, Weissman DG, Ferrer E, Beard SJ, Fassbender C, Robins RW, Hastings PD, Guyer AE, Reward-Related Brain Activity Prospectively Predicts Increases in Alcohol Use in Adolescents, J Am Acad Child Adolesc Psychiatry. 59 (2020) 391–400. 10.1016/j.jaac.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lang PJ, Bradley MM, Cuthbert BN, International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual, University of Florida, Gainesville, FL, 2008. [Google Scholar]
- [39].McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JDE, Ochsner KN, The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults, Soc Cogn Affect Neurosci. 7 (2012) 11–22. 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE, Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder, Am J Psychiatry. 166 (2009) 64–73. 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Center for Disease Control (CDC), National Youth Risk Behavior Survey, 2011 National Version [Instrument], (2011). https://www.cdc.gov/healthyyouth/data/yrbs/files/2011/pdf/2011_xxh_questionnaire.pdf.
- [42].Kaminer Y, Bukstein O, Tarter RE, The Teen-Addiction Severity Index: rationale and reliability, Int J Addict. 26 (1991) 219–226. 10.3109/10826089109053184. [DOI] [PubMed] [Google Scholar]
- [43].Baranger DAA, Lindenmuth M, Nance M, Guyer AE, Keenan K, Hipwell AE, Shaw DS, Forbes EE, The longitudinal stability of fMRI activation during reward processing in adolescents and young adults, Neuroimage. 232 (2021) 117872. 10.1016/j.neuroimage.2021.117872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kristjansson AL, Kogan SM, Mann MJ, Smith ML, Juliano LM, Lilly CL, James JE, Does early exposure to caffeine promote smoking and alcohol use behavior? A prospective analysis of middle school students, Addiction. 113 (2018) 1706–1713. 10.1111/add.14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Raudenbush SW, Bryk AS, Hierarchical Linear Models: Applications and Data Analysis Methods, 2nd edition, SAGE Publications, Inc, Thousand Oaks, 2002. [Google Scholar]
- [46].Broman MJ, Bista S, Broman CL, Inconsistency in Self-Reporting the Use of Substances over Time, Subst Use Misuse. 57 (2022) 1356–1364. 10.1080/10826084.2022.2083168. [DOI] [PubMed] [Google Scholar]
- [47].Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, Bischoff-Grethe A, Lansing AE, Brown G, Strigo IA, Wu J, Paulus MP, Adolescents with major depression demonstrate increased amygdala activation, J Am Acad Child Adolesc Psychiatry. 49 (2010) 42–51. 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sullivan RM, Wade NE, Wallace AL, Tapert SF, Pelham WE, Brown SA, Cloak CC, Ewing SWF, Madden PAF, Martz ME, Ross JM, Kaiver CM, Wirtz HG, Heitzeg MM, Lisdahl KM, Substance use patterns in 9 to 13-year-olds: Longitudinal findings from the Adolescent Brain Cognitive Development (ABCD) study, Drug and Alcohol Dependence Reports. 5 (2022) 100120. 10.1016/j.dadr.2022.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Durik AM, Hyde JS, Marks AC, Roy AL, Anaya D, Schultz G, Ethnicity and gender stereotypes of emotion, Sex Roles: A Journal of Research. 54 (2006) 429–445. 10.1007/s11199-006-9020-4. [DOI] [Google Scholar]