Abstract
Trait anxiety diminishes with age, which may result from age-related decline in registering salient emotional stimuli and/or enhancement in emotion regulation. We tested the hypotheses in 88 adults 21 to 85 years of age and studied with fMRI of the Hariri task. Age-related decline in stimulus registration would manifest in delayed reaction time (RT) and diminished saliency circuit activity in response to emotional vs. neutral stimuli. Enhanced control of negative emotions would manifest in diminished limbic/emotional circuit and higher prefrontal cortical (PFC) responses to negative emotion. The results showed that anxiety was negatively correlated with age. Age was associated with faster RT and diminished activation of the medial PFC, in the area of the dorsal and rostral anterior cingulate cortex (dACC/rACC) – a hub of the saliency circuit – during matching of negative but not positive vs. neutral emotional faces. A slope test confirmed the differences in the regressions. Further, age was not associated with activation of the PFC in whole-brain regression or in region-of-interest analysis of the dorsolateral PFC, an area identified from meta-analyses of the emotion regulation literature. Together, the findings fail to support either hypothesis; rather, the findings suggest age-related automaticity in processing negative emotions as a potential mechanism of diminished anxiety. Automaticity results in faster RT and diminished anterior cingulate activity in response to negative but not positive emotional stimuli. In support, analyses of psychophysiological interaction demonstrated higher dACC/rACC connectivity with the default mode network, which has been implicated in automaticity in information processing. As age increased, individuals demonstrated faster RT with higher connectivity during matching of negative vs. neutral images. Automaticity in negative emotion processing needs to be investigated as a mechanism of age-related reduction in anxiety.
Keywords: Aging, Emotion, Face, Medial prefrontal cortex, fMRI, Automaticity
1. Introduction
Aging is associated with changes in cognitive and affective functions. Trait anxiety represents a tendency to experience negative emotions and a risk factor of mood disorders (Weger and Sandi, 2018). Older as compared to young and middle-aged adults showed lower susceptibility to anxiety disorders (Jorm, 2000). Of the anxiety symptoms, older adults reported comparable somatic symptoms but less worry (Brenes, 2006). Previous studies have reported age-related reduction in trait anxiety (Machado et al., 2019), consistent with the positivity effect – the observation that older vs. younger adults experience negative emotions to a lesser extent and “see” things more in positive lights (Carstensen et al., 2011; Charles et al., 2003; Mather, 2003; Reed et al., 2014).
The two mostly discussed theories of the positivity effect are the cognitive control hypothesis (CCH) and dynamic integration theory (DIT). CCH posits top-down regulation of negative emotion as the mechanism of positivity under Carstensen’s socioemotional selectivity theory, which allows the elderly to have more emotionally gratifying experiences. DIT explains the positivity effect from the perspective of age-related cognitive decline and neural degradation such that relative difficulty in processing negative emotion forces the elderly to focus on positive information (Carstensen and DeLiema, 2018; Reed and Carstensen, 2012). The evidence supporting either hypothesis is somewhat mixed (Barber and Kim, 2021). Further, how these processes may be associated with age-related changes in anxiety has not been thoroughly studied.
A widely used paradigm to query brain activation to negative emotional stimuli, the Hariri task requires participants to match one of two faces with the same emotional expression as the target face (Hariri et al., 2002) and reliably engages corticolimbic structures, including the amygdala and those of the saliency circuit (Foland-Ross et al., 2010; Preckel et al., 2019). Aging is associated with brain network reorganization (Sala-Llonch et al., 2015; Tomasi and Volkow, 2012) that may affect emotion processing. For instance, older relative to young adults (age>60 vs. <30 years) demonstrated higher prefrontal cortical and lower amygdala and posterior fusiform activity during negative emotion processing in the Hariri task (Tessitore et al., 2005). A study noted greater insula and reduced amygdala/hippocampus activations in older as compared to younger adults during angry versus neutral face processing (Fischer et al., 2005). Another study did not report higher cortical but noted reduced amygdala and hippocampus activity each during negative and positive emotion perception in older vs. young adults (Iidaka et al., 2002). Other studies did not observe age differences in amygdala activity during exposure to fearful vs. familiar neutral faces (Wright et al., 2007, 2006). A meta-analysis of studies pooled across affect experiences highlighted greater frontal, anterior insula, and anterior cingulate activations in older and greater amygdala, hippocampus, posterior insula and middle cingulate activations in younger adults, especially during negative emotion processing (MacCormack et al., 2020). Collectively, with some discrepancy the literature appears to support age-related, diminished and enhanced activity in the amygdala and frontal cortex, respectively, in accord with the CCH that the positivity effect arises from down-regulation of amygdala activity via frontal cortical signals (Hariri et al., 2003).
The DIT accounts for the positivity effect in terms of age-related reduction in cognitive capacity to react to and process negative emotions (Ruffman et al., 2008; Woods et al., 2015). Thus, with age, attention is biased towards positive and away from negative stimuli early in emotion perception (Gronchi et al., 2018; Hilimire et al., 2014; Houston et al., 2018). According to the DIT, one would hypothesize age-related delay in reaction time (RT) and reduction in neural responses to matching negative vs. neutral but relative sparing of positive vs. neutral emotional faces – an interactive effect of age and valence – in the Hariri task. Ex-tant studies have not systematically investigated age-related changes in RT in the Hariri task. Studies that employed other paradigms mostly reported faster RT in young compared to older adults (Birmingham et al., 2018; Fernandes et al., 2019; Izumika et al., 2022; Liao et al., 2017; Loi et al., 2021; Yankouskaya et al., 2014; Ziaei et al., 2021), but none examined the age effects on RT to emotional vs. neutral stimuli.
Here, we tested the two hypotheses by examining behavioral and neural responses to emotional faces in the Hariri task. Age-related cognitive decline as posited by the DIT would manifest in delayed RT along with reduced saliency/emotional circuit activity in processing negative but not positive vs. neutral emotions. Age-related enhancement in emotion regulation as posited by the CCH would manifest as higher prefrontal cortical activities during negative vs. neutral emotion processing. This effect may be less pronounced during positive vs. neutral emotion processing. We used the Hariri emotional face processing task, which to our knowledge is a well-validated and effective paradigm to probe facial affect processing. Although the task is not designed specifically to examine active emotion regulation, studies have reported and attributed frontal cortical activation (Kim et al., 2022; Sladky et al., 2022) to top-down regulation of emotion during individuals’ natural responses to emotional human faces (Sladky et al., 2022). A review recognizes and discusses implicit emotion regulations during emotional face processing (Braunstein et al., 2017). A broad literature has employed the Hariri task and noted altered emotion regulation activities in individuals with affective, including anxiety, disorders (Binelli et al., 2016; Li et al., 2020; Sokołowski et al., 2020; Townsend et al., 2010). Moreover, emotion regulation in older vs. young adults demonstrated higher frontal regulatory activity over saliency responses during exposure to negative emotions (Urry and Gross, 2010), even during simple emotion processing, including the Hariri, tasks, where participants were not explicitly instructed to regulate their emotions (Braunstein et al., 2017; Lindquist et al., 2012; MacCormack et al., 2020).
Individual trait anxiety may affect processing of negative emotions. Chronic exposure to inescapable stress leads to defective PFC control and aberrant amygdala activation along with the development of anxiety and depressive disorders (Quirk and Gehlert, 2003). Amygdala response was elevated to matching fearful relative to happy facial expressions, in correlation with trait anxiety and among people with anxiety disorders, as compared to controls (Fonzo et al., 2015). Another study likewise associated trait anxiety with higher amygdala and insula activation during exposure to emotional facial vs. shape stimuli (Stein et al., 2007). A meta-analysis found greater activity in the amygdala and insula in response to negative emotional stimuli in patients with anxiety disorders vs. comparison subjects (Etkin and Wager, 2007). During fear conditioning, trait anxiety was associated with higher amygdala activation to fear-eliciting cues and less recruitment of the ventrolat-eral PFC (Indovina et al., 2011) in young, healthy adults. In a meta-analysis, patients with post-traumatic stress disorder relative to controls showed hypoactivation of the dorsal anterior cingulate cortex (ACC), ventromedial PFC/rostral ACC and dorsomedial PFC, and hyperactivetion of the amygdala and insula during negative emotion processing (Etkin and Wager, 2007). A critical question is whether and how age-related changes in negative/positive emotion processing is associated with anxiety. One previous study observed age by stress interaction in the fusiform gyrus with enhanced responses to stress only in older adults (Everaerd et al., 2017); however, the interaction effect was not distin-guished or contrasted for negative vs. positive emotions. Here, we identified age-related regional responses to negative and positive emotions and associated these responses to anxiety.
To summarize, in the current study, we 1) confirmed age-related reduction in trait anxiety; 2) investigated how age influences the RT and regional responses during matching of negative and happy relative to neutral emotional faces in the Hariri task; and 3) in the case where we failed to identify age-related changes in PFC activity during exposure to emotional vs. neutral stimuli, conducted a meta-analysis of emotion regulation in tasks that employed facial stimuli and characterized how age influenced the regulatory activities. To anticipate, we observed that age was associated with faster RT and diminished activation of the medial PFC during matching of negative but not positive vs. neutral emotional faces. Further, activity of the dorsolateral PFC – the only regulatory region identified from meta-analyses – did not show significant age differences in identification of emotional vs. neutral faces. Not consistent with the CCH nor with DIT, these findings are discussed in terms of spontaneity or automaticity in negative emotion processing as a mechanism of age-related reduction in anxiety (Dolcos et al., 2014).
2. Materials and methods
2.1. Subjects and assessments
Eighty-eight healthy adults (43 women) 21 to 85 years of age volun-teered to participate in the study. Candidates were recruited from the greater New Haven, Connecticut, area. All participants were physically healthy with no major medical conditions. Those with current use of prescription medications or with a history of head injury or neurological illness were excluded. Other exclusion criteria included current or history of Axis I disorders according to the Structured Clinical Interview for DSM-IV (First et al., 1996). Candidates who reported current use of illicit substances or tested positive for cocaine, methamphetamine, opioids, marijuana, barbiturates, or benzodiazepines were not invited to participate. All participants were assessed with the State-Trait Anxiety Inventory (STAI). The trait anxiety scale (O scale or Form 2-M) contains 20 statements that measure how the subject feels in general (Spielberger, 1989). The STAI trait score ranged from 20 to 60 with a mean ± SD of 34.7 ± 10.3 in the current sample. The Human Investigation Committee at Yale School of Medicine approved the study procedures. All participants signed an informed consent prior to the study.
2.2. MRI protocol and behavioral task
Brain images were collected using multiband imaging with a 3-Tesla MR scanner (Siemens Trio, Erlangen, Germany). Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization. Anatomical 3D magnetization prepaired-rapid gradient echo (MPRAGE) image were next obtained with spin echo imaging in the axial plane parallel to the AC–PC line with TR = 1900 ms, TE = 2.52 ms, bandwidth = 170 Hz/pixel, field of view = 250 × 250 mm, matrix = 256 × 256, 176 slices with slice thickness = 1 mm and no gap. Functional, blood oxygen level-dependent (BOLD) signals were acquired with a single-shot gradient echoplanar imaging (EPI) sequence. Fifty-one axial slices parallel to the AC–PC line covering the whole brain were acquired with TR = 1000 ms, TE = 30 ms, bandwidth = 2290 Hz/pixel, flip angle = 62°, field of view = 210 × 210 mm, matrix = 84 × 84, 51 slices with slice thickness = 2.5 mm and no gap, 392 vol, and multiband acceleration factor = 3. Images from the first ten TRs at the beginning of each scan were discarded to ensure that only BOLD signals in steady-state equilibrium between RF pulsing and relaxation were included in data analyses.
In the Hariri task, 48 different images were used, with 12 (6 male and 6 female) each of happy, angry, fearful, and neutral emotional faces (Ekman and Freisen, 1976) in a block design. The target face was shown on the top and two faces either matching or not matching the target were shown at the bottom. Participants were asked to match one of two simultaneously presented faces with the target face by pressing a left or right buttons on their right or dominant hand (Fig. 1A). A session com-prised 10 s of dummy scans, followed by the instruction “choose one to match the picture at the top” for 2 s and 10 blocks. The 10 blocks included two each to match happy, angry, and fearful facial expressions interleaved with four to match neutral faces, in the sequence: one neutral block → two happy blocks → one neutral block → two angry blocks → one neutral block → two fearful blocks → one neutral block (Supplementary Figure S1). Each block started with a fixation period of 2 s, followed by 6 stimuli (3 images of each sex with the target affect) each lasting 6 s. The 6 stimuli were presented consecutively without inter-stimuli gap. The scan session last approximately 392 s (7 m). During imaging, subjects responded by pressing one of two buttons, allowing for the determination of accuracy and reaction time (RT). Subjects were told that the stimuli would be presented long enough for them to make an accurate match but were not explicitly instructed to respond as fast as possible. This allowed us to assess the natural preferences in emotion processing across subjects (Fakra et al., 2008). Previous studies have noted the positivity effect to be more evident when the experimental goals (e.g., fast RT), which may alter preferences and bias attention (Carstensen and DeLiema, 2018; Reed et al., 2014), were not revealed.
Fig. 1.
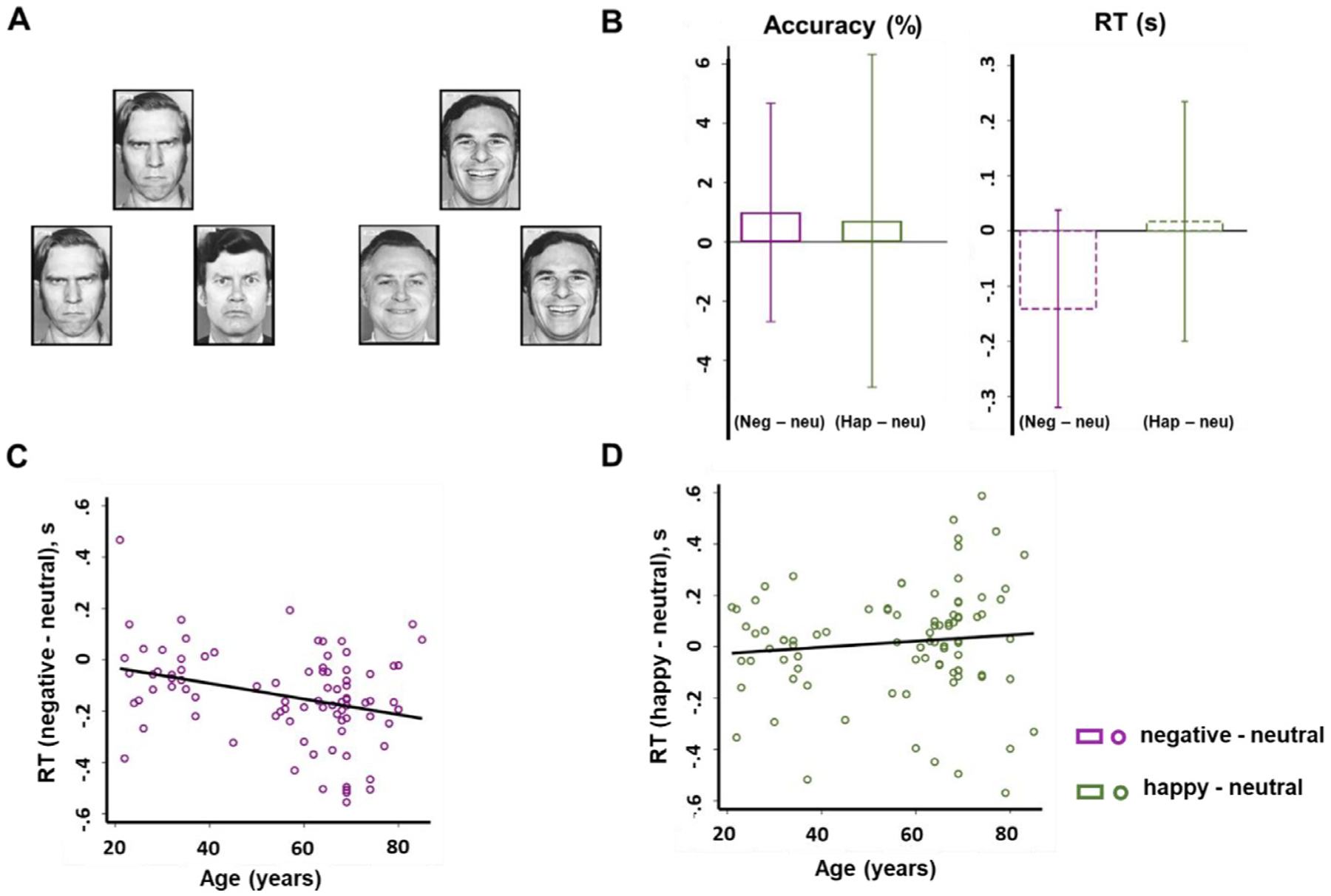
(A) Example negative and positive emotional pictures employed in the Hariri task; (B) the differences in accuracy (%) and reaction time (RT, s) between negative and neutral blocks (negative – neutral) and between happy and neutral blocks (happy – neutral) in bar plots of mean ± SD; (C) The differences in RT between negative and neutral blocks or RT (negative – neutral) were significantly and negatively correlated with age across participants; (D) The differences in RT between happy and neutral blocks or RT (happy – neutral) were not significantly correlated with age. Neg: negative, Hap: happy, Neu: neutral.
2.3. MRI data preprocessing and modeling
Data were analyzed with Statistical Parametric Mapping (SPM12, Wellcome Department of Imaging Neuroscience, University College London, U.K.), following our published routines (Li et al., 2021, 2020). Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were co-registered with the high-resolution structural image and segmented for normalization with affine registration followed by nonlinear transformation. The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. The resampled voxel size is 2.5 × 2.5 × 2.5 mm3. Finally, the images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum.
A statistical analytical block design was constructed for each individual subject using a general linear model (GLM) by convolving the canon-ical hemodynamic response function (HRF) with the boxcar function in SPM, separately for angry, fearful, happy, and neutral faces. Realignment parameters in all six dimensions were also entered in the model. The GLM estimated the component of variance that could be explained by each of the regressors.
In the first-level analysis, we combined blocks of angry and fearful face targets and constructed for each individual subject contrasts of “negative ˗ neutral face”, and “happy ˗ neutral face”, blocks to evaluate brain regions that responded differently to matching these images. The contrast images of the first-level analysis were used for group statistics. In random effects analyses, we performed a linear regression of the contrast images against age across all subjects, and one-sample t tests of the contrasts for the whole brain. Following current reporting standards, all results were evaluated at voxel p<0.001, uncorrected, in combination with cluster p<0.05, FWE corrected, on the basis of Gaussian random field theory (RFT) as implemented in SPM.
On the basis of the literature, we would specifically examine for amygdala and prefrontal cortical (PFC) activities that may be implicated in emotion processing and regulation. If these regional activities were not observed in whole-brain analyses, we would use the bilateral amygdala mask from the Automated anatomical labeling (AAL) atlas and conduct a meta-analysis (as described in Section 2.5) to identify the PFC area for region of interest (ROI) analyses.
In ROI analysis, we used MarsBaR (http://marsbar.sourceforge.net/) to derive for each individual subject the β contrasts of the functional ROIs identified from whole brain regression, the amygdala mask, and the PFC mask identified of the meta-analysis. In post-hoc analyses, we used slope test (Zar, 1999) to assess differences in the correlation of β and age for those clusters identified in “negative ˗ neutral face” and “happy ˗ neutral face” alone. Note that these analyses did not represent double-dipping as we identified the clusters in whole-brain analysis with a threshold, and the clusters showing a significant correlation, say in the regression of “negative ˗ neutral” vs. age may have just missed the threshold in “happy ˗ neutral” vs. age, and vice versa. Thus, we needed to perform slope tests to confirm the differences. We also assessed the correlations between the β’s and clinical/behavioral data, and, where appropriate, evaluated the interactive effects of variables.
To assess the sample size with a sufficient power (set at 80%), we performed power analysis using a web-based toolbox (www.neuropowertools.org; (Durnez et al., 2016)). We performed the analysis in a pilot sample of 15 subjects who were not included in the study sample (Supplement). We showed that a sample size of 40 would have 80% power for us to observe regional responses to both negative and positive vs. neutral face matching at p<0.05 FWE-corrected (Supplementary Figure S2, Table S1). Hence, with a sample size of 88 in the current study, we expected sufficient power for these effects.
2.4. Psychophysiological interaction (PPI)
We conducted a generalized gPPI analysis with the mPFC as a seed (identified from whole-brain age regressions; see Results) to explore age-related changes in functional connectivity during emotion processing. We followed the method as in our previous studies (Le et al., 2021; Wang et al., 2021). A PPI model was created for each subject with three regressors: the physiological variable which represents temporally filtered, mean-corrected and deconvolved time series of the seed region mPFC, the psychological variable which represents the task contrasts (e.g., negative vs. neutral), and a PPI variable that was computed as element-by-element product of deconvolved time series of the seed and contrast, followed by re-convolution with the HRF. The PPI images of each subject were used in random effect analyses, including a one-sample t-test and whole-brain age regression. We extracted the average functional connectivity (FC β) between the mPFC seed and clusters (if any) identified from one-sample t-test and assessed the correlations between the FC β’s and behavioral data.
2.5. Meta-analysis of neural correlates of emotion regulation
With some discrepancy, the literature suggested PFC activities during matching of negative vs. neutral faces in the Hariri task. In the scenario where we were not able to identify PFC activities, we would perform meta-analyses to identify the PFC region(s) for analyses to test the CCH. The meta-analysis would include variants of the Hariri task where participants are asked to label and match emotions and the contrast label > match was considered to involve down-regulating activities (Hariri et al., 2000). The meta-analysis also included studies with facial stimuli and with participants instructed specifically to down-regulate their emotions. Of note, previous meta-analyses included emotion-regulation studies that used mixed stimuli (e.g., emotional faces, complex scenes, emotional words, stressful contexts, etc.) that may vary in how they invoke the regulatory processes (Hung et al., 2018; Morawetz et al., 2020). Here, we focused only on studies with facial stimuli in the hope that the findings would be most relevant to the current study. A positive association between age and regulatory activity during ‘negative vs. neutral’ would support the CCH.
We followed the PRISMA guidelines and identified seven studies from earlier meta-analyses on emotion regulation (Morawetz et al., 2020), and emotion inhibition (Hung et al., 2018), and six more from PubMed search on 09/02/2022 (restricting search from 2017 to 2022) using the keywords (“face” or “emotion regulation” or “affective regulation” or “implicit emotion regulation” or “explicit emotion regulation” or “extrinsic emotion regulation” or “intrinsic emotion regulation” or “reappraisal” or “suppression” or “distraction” or “detachment”) AND (“fMRI” or “neuroimaging” or “functional magnetic resonance imaging”, or “functional MRI”) (Supplementary Figure S3). Study quality was assessed using a 10-point checklist (Supplementary Table S2) via an objective description of individual studies on demographic and clinical characteristics (Supplementary Table S3) and imaging methods (Supplementary Table S4), as with earlier meta-analyses. The selected studies were all of sufficient quality with a score of 10.
We performed activation likelihood estimation (ALE) meta-analysis (Eickhoff et al., 2012, 2009; Turkeltaub et al., 2012, 2002) with Gin-gerALE 3.0.2 (http://www.brainmap.org/ale/) on the 13 studies (611 participants; 68 activation foci, 69 deactivation foci) of emotion down-regulation (Supplementary Table S3). The methodological details were described in our recent work (Chaudhary et al., 2022) and in the Supplement. Meta-analytic convergence of activations and deactivations were analyzed separately. To achieve a balance between sensitivity and specificity, cluster-level inference was made using cluster-forming threshold of voxel-level p<0.001, uncorrected and the resulting supra-threshold clusters were compared to a null distribution of cluster sizes established by 1000 permutations of the data, at an FWE-corrected threshold of p<0.05.
We assessed publication bias using the “Fail-safe N” approach rec-ommended for ALE meta-analysis (Acar et al., 2018; Chaudhary et al., 2022; Degasperi et al., 2021). We identified mixed nature of emotion regulation tasks as potential source of heterogeneity but could not assess its effects on the current findings because the small number of homogeneous studies in each category did not allow the ALE analysis in homogenous sub-samples (Chaudhary et al., 2022; Degasperi et al., 2021). We also extracted the significant ALE clusters and assessed publication bias of the cluster peak with seed-based d mapping (SDM; https://www.sdmproject.com/; Albajes-Eizagirre et al., 2019a, 2019b), as described in the Supplement.
3. Results
3.1. Clinical and behavioral findings
Across emotions, the mean RTs of our participants ranged from 1.68 to 1.85 s, comparable to 1.08 to 2.37 s (Binelli et al., 2016; Kleinhans et al., 2010), and the mean accuracies ranged from 97.3% to 98.3%, also comparable to 93.6% to 100% (Cardoner et al., 2011; Contreras-Rodríguez et al., 2014), as reported in studies with similar experimental designs. We derived the differences in behavioral data of negative and happy vs. neutral trials – i.e., negative minus neutral or “Neg – Neu” and happy minus neutral or “Hap – Neu” – for analyses. Paired t-test showed no difference in accuracy rate between “Neg – Neu” and “Hap – Neu” (t = 0.49, p= 0.625). However, RT was significantly faster during matching of negative vs. neutral faces as compared to happy vs. neutral faces; i.e., “Neg – Neu” < “Hap – Neu” (t = −5.48, p < 0.001; Fig. 1B). The accuracy rate and RT of negative, happy, and neutral trials as well as the statistics of repeated measures ANOVA are shown in Supplementary Figure S4 and Table S5.
Across all subjects, age was significantly and negatively correlated with STAI trait score (r = −0.44, p < 0.001). RT (Neg – Neu) was significantly correlated with age across all subjects (r = −0.32, p < 0.003, Fig. 1C) whereas RT (Hap – Neu) did not correlate significantly with age (r = −0.10, p = −0.337, Fig. 1D). The difference in slope of the two regressions was significant (t = 2.66, p = 0.009), suggesting that 1-unit change in RT (Neg – Neu) with 1-unit change in age differed significantly from 1-unit change in RT (Hap – Neu) with the same. Neither accuracy (Neg – Neu) nor accuracy (Hap – Neu) was significantly correlated with age (r = 0.07, p = 0.491; r = −0.07, p = 0.509, respectively). None of the behavioral measures correlated significantly with STAI score (0.07<r’s<0.17, p’s>0.117).
3.2. Imaging findings
In voxelwise regression, age showed negative correlation with activation of a cluster in medial prefrontal cortex (mPFC; x = −10, y = −36, z = −16, voxel Z = −4.32, 483 voxels), in the area of dorsal and rostral anterior cingulate cortex (dACC/rACC), during identification of negative vs. neutral faces (Fig. 2A). No clusters showed positive correlation for the same contrast. No clusters showed responses to other contrasts in significant correlation with age.
Fig. 2.
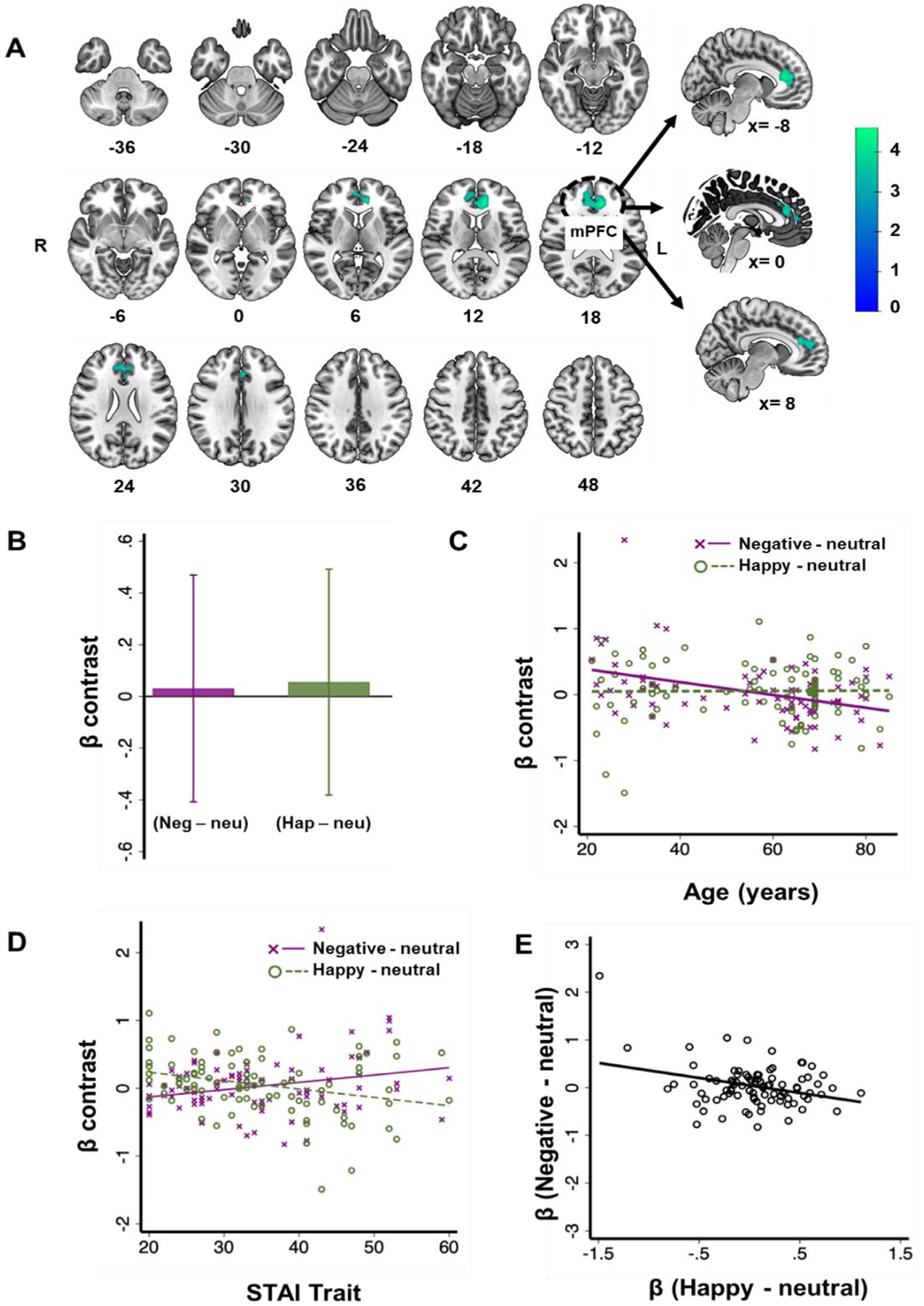
(A) The medial prefrontal cortex mPFC showed activity in negative correlation with age during identification of negative vs. neutral faces, at voxel p<0.001, uncorrected and cluster p<0.05, FWE-corrected. (B) Mean ± SD of the β contrast of mPFC for “negative – neutral” and “happy – neutral”. (C) The β contrast of mPFC for “negative – neutral” but not “happy – neutral” was significantly correlated with age. (D) The β contrast of mPFC for “negative – neutral” and “happy – neutral” correlated positively and negatively, respectively with STAI trait. (E) The β contrasts of mPFC for “negative – neutral” and “happy – neutral” were negatively correlated. R: Right, L: Left, Neg: negative, Neu: neutral, Hap: happy.
We extracted the β estimate of “Neg – Neu” and “Hap – Neu” of the mPFC cluster for all subjects. The two β estimates did not differ significantly (t = 0.32, p = 0.748, paired-sample t-test, Fig. 2B). As expected, the β estimate of “Neg – Neu” was significantly correlated with age (r = −0.44, p < 0.001); in contrast, the β contrast of “Hap – Neu” was not significantly correlated with age (r = 0.01, p = 0.933). Slope tests confirmed the difference in the slopes of the age regressions (t = 2.66,p = 0.009, Fig. 2C), suggesting that 1-unit (year) change in age was associated with 1-unit change in β (Neg – Neu) differently from the same with 1-unit change in β (Hap – Neu).
We also assessed the correlation between RT/accuracy (Neg/Hap – Neu) and β (Neg/Hap – Neu) of mPFC, and none of the correlations were significant (−0.11<r’s<0.08, 0.324<p’s<0.735).
Next, we assessed the correlation between anxiety scores and β (Neg/Hap – Neu) of the mPFC. The correlation of β (Neg – Neu) and STAI score was significant and positive (r = 0.25, p = 0.018). The correlation of β (Hap – Neu) and STAI score was also significant but negative (r = −0.29, p = 0.006). The difference in slope of the two regressions was significant (t = 3.70, p < 0.001, Fig. 2D).
Further, across participants, β (Neg – Neu) and β (Hap – Neu) were negatively correlated (r = 0.31, p = 0.003, Fig. 2E).
Notably, we did not observe amygdala activations in whole-brain regression against age. In an ROI analysis, neither β (Neg – Neu) nor β (Hap – Neu) of bilateral amygdala (AAL mask) was correlated significantly with age (r = −0.13, p = 0.213; r = −0.06, p = 0.552, respectively).
We showed the regional activations to negative vs. neutral, happy vs. neutral, and happy vs. negative faces in one-sample t tests of all subjects at voxel p<0.001, uncorrected in Supplementary Figure S5, with Table S6 summarizing the clusters that met cluster p<0.05, FWE-corrected.
3.3. MPFC functional connectivity
The mPFC cluster showed age-related reduction in responses to identification of negative vs. neutral emotional faces. With the mPFC cluster as the seed (physiological variable) and “negative – neutral” as the psychological variable, the gPPI analysis showed clusters in the default mode network (DMN), including bilateral angular gyrus, superior frontal gyrus, and precuneus (Fig. 3, Supplementary Table S7).
Fig. 3.
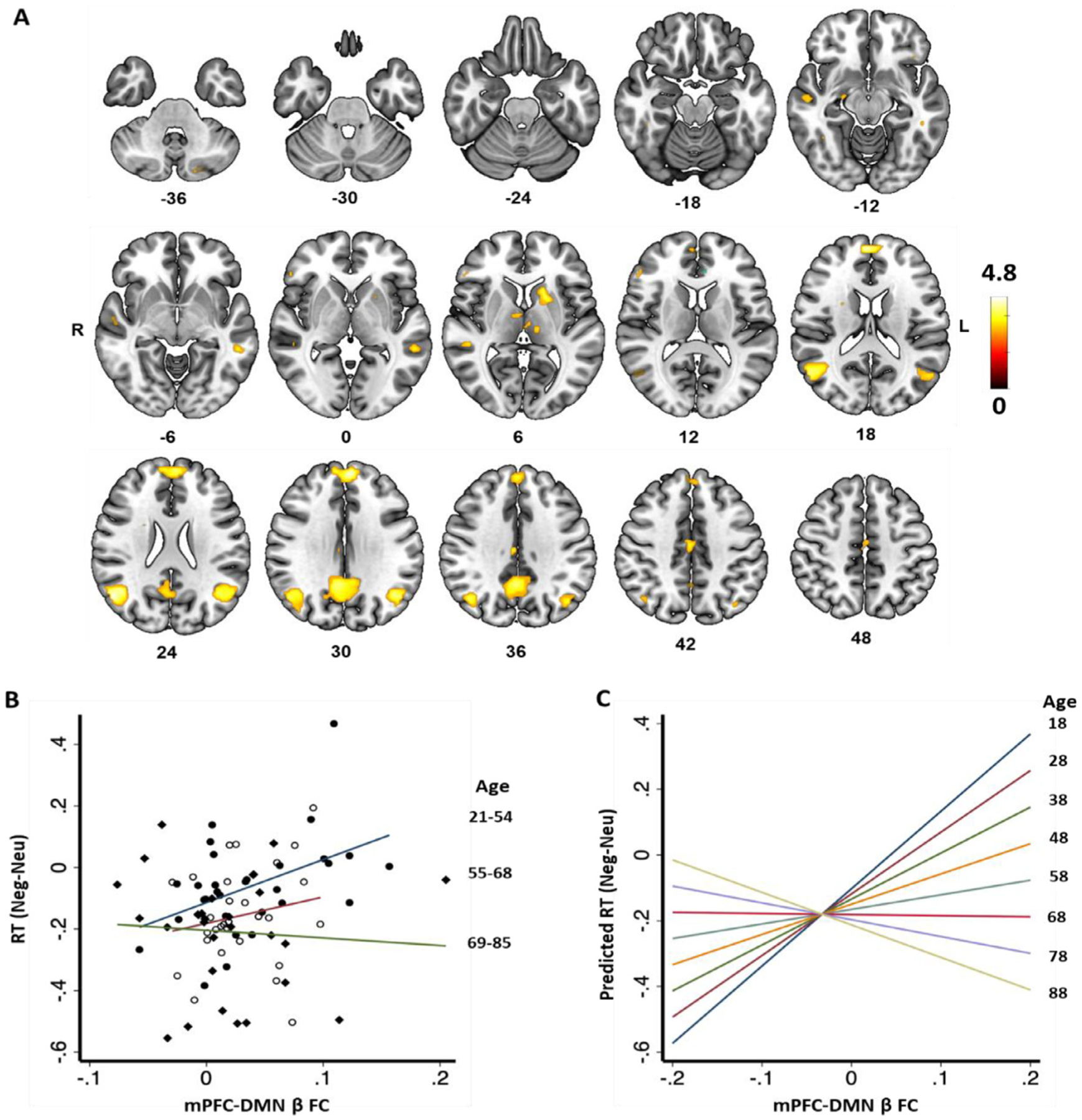
(A) GPPI showed functional connectivity (FC) between mPFC (seed) and regions in the default model network (DMN) during identification of negative vs. neutral faces (Neg-Neu), at voxel p<0.001, uncorrected; Color bars show voxel T values,. (B) Scatterplot of RT(Neg-Neu) against mPFC-DMN FC β for age groups 21–54 (n = 30), 55–68 (n = 29) and 69–85 years (n = 29) and (C) Regression of predicted RT(Neg-Neu) against mPFC-DMN FC β each with 10 years increases in age both to show significant interactive effects of age and FC β on RT.
We extracted the average functional connectivity (FC β) between mPFC and DMN clusters of negative vs neutral face and assessed its association with RT/accuracy (Neg – Neu), age, and STAI score. The FC β showed a significant correlation with RT (Neg – Neu) (r = 0.21, p = 0.049) but not with accuracy (Neg – Neu), age, or STAI score ( −0.10 <r’s < 0.15, 0.151 <p’s <0.337). As RT was significantly correlated both with FC β and age, we assessed the interaction of FC β and age on RT in the model: RT (Neg – Neu) = b1 × age + b2 × FC + β × b3 × age × FC β. The results showed significant interaction of age and FC β on RT (Neg – Neu) (t = −2.52, p = 0.014); as age increased, individuals demonstrated faster RT with higher FC β (Fig 3B, C).
We also conducted the gPPI analyses with the same seed region and “happy – neutral” as the psychological variable. The results showed significant clusters in bilateral postcentral gyrus (PoCG) at the same threshold (Supplementary Figure S6 and Table S7). Likewise, we computed the FC β of mPFC - PoCG in response to happy vs. neutral face and assessed its association with RT/accuracy (Hap – Neu), age, and STAI score. None of the correlations was significant (− 0.07 <r’s < 0.05, 0.708 <p’s <0.519).
Further, we did conduct whole-brain gPPI correlation with age with the mPFC seed and “negative-neutral” as the psychological variable. However, at the same threshold, the analysis did not yield any significant clusters. The same analysis for “happy-neutral” showed age in negative correlation with clusters in the right MTG/STG (x = 60, y = −36, z = 1, voxel Z = 5.39; x = 65, y = −26, z = 7, voxel Z = 4.95; x = 52, y = −21, z = 10, voxel Z = 4.59; 471 voxels) and SMG (x = 57, y = −53, z = 20, voxel Z = 4.45; x = 45, y = −51, z = 30, voxel Z = 4.20; x = 57, y = −41, z = 25, voxel Z = 3.88; 306 voxels) but the gPPI β did not show a significant correlation with RT/accuracy (Hap – Neu) or STAI score (0.10 <r’s < 0.15, 0.151 <p’s <0.337).
3.4. Meta-analysis of emotion regulation
In meta-analysis, the activation foci from studies of emotion regulation converged to a single cluster in the left dorsolateral prefrontal cortex (DLPFC; x = −46, y = 1, z = 51; 632 mm3; Supplementary Figure S7). The deactivation foci did not show significant convergence.
The Fail-safe N analysis showed the DLPFC cluster to be sufficiently robust and supported by desired minimum number of contributing studies. In accord, the funnel plot did not show asymmetry, and the bias-test was not significant (p = 0.344; Supplement and Figure S8). For com-pleteness, we have also provided a PRISMA checklist of meta-analysis as Supplementary Table S8.
We employed the DLPFC cluster as a mask and extracted the β estimates of “negative – neutral face” for a linear regression against age and anxiety score across the 88 subjects. We observed no significant correlation of the β with age (r = −0.01, p = 0.899) or with trait anxiety (r = 0.15, p = 0.169).
4. Discussion
Age was associated with lower anxiety and faster RT in matching negative vs. neutral emotional faces and as confirmed by slope test, this relationship is specific to negative emotions. Further, older adults achieved faster RT without sacrificing accuracy; the finding thus cannot be accounted for by age-related deficits in cognitive function or facial recognition. These findings alone are inconsistent with age-related changes in attentional engagement as a mechanism of the positivity effect, as posited by the DIT. Whole-brain regression did not reveal any clusters in positive correlation with age, even when evaluated with a more liberal threshold. ROI analyses of a mask of the DLPFC identified from meta-analyses did not show a significant correlation with age, either. Thus, age-related diminution in anxiety did not appear to be supported by enhanced PFC regulation of emotional reactivity as assessed by the Hariri task.
In whole-brain regression, a cluster in the dACC/rACC showed age-related reduction in activation during identification of negative but not positive vs. neutral emotional faces. The dACC/rACC represents a hub of the saliency circuit (Manza et al., 2016), suggesting that negative emotional faces are less salient for older relative to younger adults. Further, the dACC/rACC showed a positive psychophysiological interaction with the DMN, such that with increasing age, individuals demonstrated faster RT in identifying negative vs. neutral emotional faces in association with elevated dACC/rACC-DMN functional connectivity. The latter finding can be discussed in conjunction with previous reports of less deactivation and higher DMN connectivities with the development of automaticity during rule-based categorization (Shamloo and Helie, 2016), decision-making under predictable demands (Vatansever et al., 2017), or diminishing cognitive load during behavioral challenges (Jenkins, 2019). Further, anxiety correlated positively with dACC/rACC activity during negative emotion processing. Together, the behavioral and neural evidence suggests automaticity in negative emotion processing as a potential mechanism of age-associated reduction in anxiety. As people age, they process negative emotional faces more spontaneously and with less effort (Dolcos et al., 2014), a process that appears distinct from the declining trajectory of other cognitive abilities (Mather, 2012).
Although negative emotions are evolutionally readied for automatic processing (Okon-Singer et al., 2007; Rellecke et al., 2011), the age-related changes of automaticity in processing negative emotions may be considered more broadly with other experiments. For instance, despite overall cognitive decline, older and younger adults did equally well in learning to automate target identification in a feature search task (Anandam and Scialfa, 1999). When target identification requires memory retrieval, older adults showed longer processing time but achieved automatic processing, over practice, at equivalent rates (Madden and Nebes, 1980). Other studies similarly demonstrated age-invariant automaticity in processing learned events, including those that transpired in virtual reality (Voinescu and David, 2020). At the automatic stage of visual search of emotional stimuli, older but not younger adults were faster to detect emotionally negative, high-arousal targets (Leclerc and Kensinger, 2008). Another study demonstrated that older adults’ performance in recognizing facially expressed emotions were not affected by stereotype (ageism) threat, likely because emotion recognition is automatic and less susceptible to the cognitive load posed by the threats (Atkinson et al., 2020). Notably, a recent study reported positive versus negative categorization of faces of ambiguous valence to be faster in older and slower in young adults, and discussed role of information processing automaticity in manifesting the positivity effect (Petro et al., 2021). Adding to this literature, the current findings of age-related reduction in RT but not accuracy in identifying negative emotional faces may suggest that older adults are not only equally able to correctly register negative emotional faces but do so with relatively little effort, as compared to their younger counterparts. Collectively, these findings suggest greater automaticity in negative information processing with aging. Studies in combination with recording of event-related potentials (Holmes et al., 2009; Rellecke et al., 2011) and skin-conductance responses (Esteves et al., 1994) and potentially eye tracking (Berggren et al., 2012) may provide additional physiological markers of automaticity processing.
Activity within the dACC/rACC diminished with age during negative but not positive emotion processing. Many human and animal studies have implicated the ACC, a critical hub of the saliency circuit, in negative emotion processing (Etkin et al., 2011). Age-related diminution of ACC activity may suggest reduced saliency of negative information, in accord with previous findings of lower event-related potentials evoked to negative but not positive images (Kisley et al., 2007) and to loss but not gain during incentive processing (Samanez-Larkin et al., 2007) in older vs. young adults. The findings may also be considered along with earlier reports of age-related decreases in brain responses to negative stimuli (Wood and Kisley, 2006) and absence of negativity bias (Fairfield et al., 2022; Wood and Kisley, 2006). Other studies showed that older individuals are less likely to remember negative than positive emotional materials (Charles et al., 2003; Mather, 2003) and may pay less attention to negative images (Carstensen et al., 2000; Charles et al., 2001; Mather and Carstensen, 2003). As we argued earlier, higher ACC connectivity with the DMN suggests automaticity in negative emotion processing, as shown in faster RT in older adults. Thus, it remains to be clarified whether, despite more automatic registering of negative emotional stimuli (and hence faster processing), older adults may not neces-sarily take advantage of the registered information in memory encoding or other processes that require sustained attention. Notably, the current findings of dampened dACC/rACC response without concurrent elevation of control circuit activities do not refute the presence of emotion regulatory processes, which may accrue from life experiences in older individuals (John and Gross, 2004). It is possible that the regulatory processes would be elicited under behavioral conditions that are more challenging than face identification.
Unlike the great majority of studies discussed earlier, here, we did not observe significant correlation of age with amygdala response to negative vs. neutral emotional faces in whole-brain or ROI analysis. This finding is consistent with an earlier study of participants evaluating the perceptual features or meanings of emotional pictures (Ritchey et al., 2011), another of adults exposed to emotional video stimuli (Schweizer et al., 2019), and a more recent work of emotional Stroop task (Almdahl et al., 2021). Notably, among the studies of the Hariri (Tessitore et al., 2005) and other tasks that reported age-related reduction in amygdala response to negative emotional faces (Fischer et al., 2010, 2005; Gunning-Dixon et al., 2003; Iidaka et al., 2002) and images (Roalf et al., 2011), all except Gunning-Dixon et al. employed ROI analyses to evaluate age-related changes in amygdala activity. In con-sideration too is that many of the studies contrasted amygdala responses to negative emotional faces with those to neutral pictures rather than faces (Drabant et al., 2009; Fisher et al., 2009; Tessitore et al., 2005). Thus, it remains unclear whether the observed age effects on amygdala reactivity pertained more to facial rather than negative emotional processing. Finally, amygdala activation may habituate to repeated exposure to emotional stimuli (Geissberger et al., 2020). An earlier study noted amygdala habituation with faster RT in older adults such that the positivity bias hinders learning of detailed emotional information in the amygdala, whereas younger adults demonstrate the opposite (Petro et al., 2021). Thus, with reduced saliency of negative facial emotions we would expect habituated and diminished amygdala activity with age, an effect that may elude analyses that estimate mean fMRI response over a task block (Plichta et al., 2014). More studies are certainly warranted to investigate the roles of the amygdala in emotional aging.
The finding that age was negatively correlated with both trait anxiety and diminished dACC/rACC responses to negative emotions is consistent with a broad literature. For instance, neuroticism, a disposition to experience negative affect, including anxiety, was associated with higher dACC activity in response to “oddballs” in an oddball (Eisenberger et al., 2005) and emotional speech melody (Brück et al., 2011) task. Relative to controls, individuals with social anxiety disorder demonstrated higher ACC activity during processing of negative versus neutral faces (Amir et al., 2005). In children, higher ACC response to anticipation of fearful faces was associated with the severity of self-reported anxiety (Clauss et al., 2017). It should be noted that the coordinates reported in these and many other studies varied, likely due to differences in the behavioral paradigms to elicit negative emotions and regulation of negative emotions. As the ventral ACC may have more of a regulatory role, whereas dorsal-caudal ACC and mid-cingulate may be more in-volved in the appraisal and expression of negative emotions (Etkin et al., 2011), these areal responses would likely manifest differently in anxiety (Kim et al., 2011). Studies are needed to investigate the functional map of the mPFC in processing negative emotions and whether distinct regional activities may be implicated across varying behavioral challenges and in different clinical populations.
We considered a few limitations in the current study. First, participants were engaged in a small number of blocks, as with some of the previous studies of the Hariri task. Nonetheless, the current findings should be considered preliminary. Second, in meta-analysis, we could not evaluate heterogeneity due to the small number of studies employing similar fMRI tasks. Third, face processing is of evolutionary importance to emotional processing. However, we are exposed to negative emotional scenarios that do not involve facial stimuli, and studies are needed to evaluate the effects of age on how we process non-facial emotional stimuli. Fourth, the STAI trait score ranged from 20 to 60 in the current sample. Participants with higher STAI score are needed to better understand the effects of anxiety as well as the impact of age on anxiety, with the caveat that most people with the highest STAI scores have a clinical diagnosis of anxiety and depressive disorders and typically receive mediations for their conditions. Finally, behavioral contingen-cies that distinguish passive emotional exposure and active regulation of emotions within subjects would help in identifying regulatory activities and investigating the effects of age on the circuit activity.
In conclusion, age is associated with lower anxiety as well as faster RT and diminished dACC/rACC responses to identification of negative emotional faces. The findings are specific to identification of negative facial emotions and cannot be explained by age-related cognitive or motor decline. No brain regions, including the DLPFC identified of meta-analyses, showed higher activities in response to negative emotions. These findings thus dot not appear consistent with the DIT that older adults are less engaged with negative emotions (Gurera and Isaacowitz, 2019) because of cognitive decline or with the CCH that older adults better regulate negative emotions. Rather, the findings appear in accord with age-related development of automaticity in processing negative emotions.
Supplementary Material
Acknowledgments
The current study was supported by NIH grants R21AG067024 (Li), R01AG072893 (Li), R01CA218501 (Chao), as well as a VA Merit Award CX001301 (Chao). The NIH and VA are otherwise not responsible for the design of the study or data analyses and interpretation or in the decision to publish these findings.
Abbreviations:
- ALE
activation likelihood estimation
- CCH
cognitive control hypothesis
- DIT
dynamic integration theory
- dACC/rACC
dorsal and rostral anterior cingulate cortex
- fMRI
functional magnetic resonance imaging
- mPFC
medial prefrontal cortex
- PPI
psychophysiological interaction
Footnotes
Declaration of Competing Interest
Authors declare no conflicts of interest in the current work.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2023.120207.
Data availability
Data will be made available on request.
References
- Acar F, Seurinck R, Eickhoff SB, Moerkerke B, 2018. Assessing robustness against potential publication bias in Activation Likelihood Estimation (ALE) meta-analyses for fMRI. PLoS One 13, 1–23. doi: 10.1371/journal.pone.0208177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albajes-Eizagirre A, Solanes A, Fullana MA, Ioannidis JPA, Fusar-Poli P, Tor-rent C, Solé B, Bonnín CM, Vieta E, Mataix-Cols D, Radua J, 2019a. Meta-analysis of Voxel-Based Neuroimaging Studies using Seed-based d Mapping with Permutation of Subject Images (SDM-PSI). JoVE e59841. doi: 10.3791/59841. [DOI] [PubMed] [Google Scholar]
- Albajes-Eizagirre A, Solanes A, Vieta E, Radua J, 2019b. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage 186, 174–184. doi: 10.1016/j.neuroimage.2018.10.077. [DOI] [PubMed] [Google Scholar]
- Almdahl IS, Martinussen LJ, Agartz I, Hugdahl K, Korsnes MS, 2021. Inhibition of emotions in healthy aging: age-related differences in brain network connectivity. Brain Behav. 11, e02052. doi: 10.1002/brb3.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS, 2005. Increased activation of the anterior cingulate cortex during processing of dis-gust faces in individuals with social phobia. Biol. Psychiatry 57, 975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Anandam BT, Scialfa CT, 1999. Aging and the Development of Automaticity in Feature Search. Aging, Neuropsychol. Cogn 6, 117–140. doi: 10.1076/anec.6.2.117.787. [DOI] [Google Scholar]
- Atkinson L, Murray JE, Halberstadt J, 2020. Older Adults’ Emotion Recognition Ability Is Unaffected by Stereotype Threat. Front. Psychol 11, 605724. doi: 10.3389/fpsyg.2020.605724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SJ, Kim H, 2021. The Positivity Effect: A Review of Theories and Recent Findings. Multiple Pathways of Cognitive Aging: Motivational and Contextual Influences. Oxford University Press; doi: 10.1093/oso/9780197528976.003.0005. [DOI] [Google Scholar]
- Berggren N, Koster EHW, Derakshan N, 2012. The effect of cognitive load in emotional attention and trait anxiety: An eye movement study. J. Cogn. Psychol 24, 79–91. doi: 10.1080/20445911.2011.618450. [DOI] [Google Scholar]
- Binelli C, Muñiz A, Subira S, Navines R, Blanco-Hinojo L, Perez-Garcia D, Crippa J, Farré M, Pérez-Jurado L, Pujol J, Martin-Santos R, 2016. Facial emotion processing in patients with social anxiety disorder and Williams-Beuren syndrome: an fMRI study. J. Psychiatry Neurosci 41, 182–191. doi: 10.1503/jpn.140384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham E, Svärd J, Kanan C, Fischer H, 2018. Correction: Exploring emotional expression recognition in aging adults using the Moving Window Technique. PLoS One 13, e0208767. doi: 10.1371/journal.pone.0208767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein LM, Gross JJ, Ochsner KN, 2017. Explicit and implicit emotion regulation: a multi-level framework. Soc. Cogn. Affect. Neurosci 12, 1545–1557. doi: 10.1093/scan/nsx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes GA, 2006. Age differences in the presentation of anxiety. Aging Ment. Health 10, 298–302. doi: 10.1080/13607860500409898. [DOI] [PubMed] [Google Scholar]
- Brück C, Kreifelts B, Wildgruber D, 2011. Emotional voices in context: a neurobiological model of multimodal affective information processing. Phys. Life Rev 8, 383–403. doi: 10.1016/j.plrev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Cardoner N, Harrison BJ, Pujol J, Soriano-Mas C, Hernández-Ribas R, López-Solá M, Real E, Deus J, Ortiz H, Alonso P, Menchón JM, 2011. Enhanced brain responsiveness during active emotional face processing in obsessive compul-sive disorder. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 12, 349–363. doi: 10.3109/15622975.2011.559268. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, DeLiema M, 2018. The positivity effect: a negativity bias in youth fades with age. Curr. Opin. Behav. Sci 19, 7–12. doi: 10.1016/j.cobeha.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR, 2000. Emotional experience in everyday life across the adult life span. J. Pers. Soc. Psychol 79, 644–655. [PubMed] [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, Brooks KP, Nesselroade JR, 2011. Emotional experience improves with age: Evidence based on over 10 years of experience sampling. Psychol. Aging doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL, 2003. Aging and emotional memory: the forget-table nature of negative images for older adults. J. Exp. Psychol. Gen 132, 310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Charles ST, Reynolds CA, Gatz M, 2001. Age-related differences and change in positive and negative affect over 23 years. J. Pers. Soc. Psychol 80, 136–151. [PubMed] [Google Scholar]
- Chaudhary S, Zhornitsky S, Chao HH, van Dyck CH, Li C-SR, 2022. Cerebral Volumetric Correlates of Apathy in Alzheimer’s Disease and Cognitively Normal Older Adults: Meta-Analysis, Label-Based Review, and Study of an Independent Cohort. J. Alzheimers. Dis 85, 1251–1265. doi: 10.3233/JAD-215316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J, Rao U, Blackford J, 2017. 13. Anticipatory Anterior Cingulate Cortex Activity Predicts Development of Anxiety Symptoms in Inhibited Children. Biol. Psychiatry 81, S6. doi: 10.1016/j.biopsych.2017.02.024. [DOI] [Google Scholar]
- Contreras-Rodríguez O, Pujol J, Batalla I, Harrison BJ, Bosque J, Ibern-Regàs I, Hernández-Ribas R, Soriano-Mas C, Deus J, López-Solà M, Pifarré J, Menchón JM, Cardoner N, 2014. Disrupted neural processing of emotional faces in psychopathy. Soc. Cogn. Affect. Neurosci 9, 505–512. doi: 10.1093/scan/nst014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degasperi G, Cristea IA, Di Rosa E, Costa C, Gentili C, 2021. Parsing variability in borderline personality disorder: a meta-analysis of neuroimaging studies. Transl. Psychiatry 11, 314. doi: 10.1038/s41398-021-01446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos S, Katsumi Y, Dixon RA, 2014. The role of arousal in the spontaneous regulation of emotions in healthy aging: a fMRI investigation. Front. Psychol 5, 681. doi: 10.3389/fpsyg.2014.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ, 2009. Individual Differences in Typical Reappraisal Use Predict Amygdala and Prefrontal Responses. Biol. Psychiatry 65, 367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnez J, Degryse J, Moerkerke B, Seurinck R, Sochat V, Poldrack RA, Nichols TE, 2016. Power and sample size calculations for fMRI studies based on the prevalence of active peaks. bioRxiv 49429. doi: 10.1101/049429. [DOI] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT, 2012. Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT, 2009. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a randomeffects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp 30, 2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB, 2005. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cogn. Affect. Behav. Neurosci 5, 169–181. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Ekman P, Freisen WV, 1976. Pictures of facial affect. Consult. Psychol. Palo Alto. [Google Scholar]
- Esteves F, Dimberg U, öhman A, 1994. Automatically elicited fear: Conditioned skin conductance responses to masked facial expressions. Cogn. Emot 8, 393–413. doi: 10.1080/02699939408408949. [DOI] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci 15, 85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd D, Klumpers F, Oude Voshaar R, Fernández G, Tendolkar I, 2017. Acute Stress Enhances Emotional Face Processing in the Aging Brain. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging 2, 591–598. doi: 10.1016/j.bpsc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Fairfield B, Padulo C, Bortolotti A, Perfetti B, Mammarella N, Balsamo M, 2022. Do Older and Younger Adults Prefer the Positive or Avoid the Negative? Brain Sci. 12. doi: 10.3390/brainsci12030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O, 2008. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr. Res 100, 191–205. doi: 10.1016/j.schres.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Gonçalves AR, Pasion R, Ferreira-Santos F, Barbosa F, Martins IP, Marques-Teixeira J, 2019. Age-related decline in emotional perspective-taking: Its effect on the late positive potential. Cogn. Affect. Behav. Neurosci 19, 109–122. doi: 10.3758/s13415-018-00648-1. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer R, Gibbon M, Williams J, 1996. Structured Clinical Interview For DSM-IV Axis I disorders. American Psychiatric Press., Washington, DC. [Google Scholar]
- Fischer H, Nyberg L, Bäckman L, 2010. Age-related differences in brain regions supporting successful encoding of emotional faces. Cortex 46, 490–497. doi: 10.1016/j.cortex.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Bäckman L, 2005. Age-differential patterns of brain activation during perception of angry faces. Neurosci. Lett 386, 99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, Moses-Kolko EL, Berga SL, Hariri AR, 2009. Medial prefrontal cortex 5-HT(2A) density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb. Cortex 19, 2499–2507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Altshuler LL, Bookheimer SY, Lieberman MD, Townsend J, Pen-fold C, Moody T, Ahlf K, Shen JK, Madsen SK, Rasser PE, Toga AW, Thompson PM, 2010. Amygdala Reactivity in Healthy Adults Is Correlated with Prefrontal Cortical Thickness. J. Neurosci 30, 16673. doi: 10.1523/JNEUROSCI.4578-09.2010, LP –16678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Ramsawh HJ, Flagan TM, Sullivan SG, Letamendi A, Simmons AN, Paulus MP, Stein MB, 2015. Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. Br. J. Psychiatry 206, 206–215. doi: 10.1192/bjp.bp.114.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissberger N, Tik M, Sladky R, Woletz M, Schuler A−L, Willinger D, Windischberger C, 2020. Reproducibility of amygdala activation in facial emotion processing at 7T. Neuroimage 211, 116585. doi: 10.1016/j.neuroimage.2020.116585. [DOI] [PubMed] [Google Scholar]
- Gronchi G, Righi S, Pierguidi L, Giovannelli F, Murasecco I, Viggiano MP, 2018. Automatic and controlled attentional orienting in the elderly: A dual-process view of the positivity effect. Acta Psychol. (Amst) 185, 229–234. doi: 10.1016/j.actpsy.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE, 2003. Age-related differences in brain activation during emotional face processing. Neurobiol. Aging 24, 285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Gurera JW, Isaacowitz DM, 2019. Chapter 14 - Emotion regulation and emotion perception in aging: A perspective on age-related differences and similarities. In: Srinivasan NBT (Ed.), Emotion and Cognition. Elsevier, pp. 329–351. doi: 10.1016/bs.pbr.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC, 2000. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 11. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR, 2003. Neocortical mod-ulation of the amygdala response to fearful stimuli. Biol. Psychiatry 53, 494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR, 2002. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 17, 317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hilimire MR, Mienaltowski A, Blanchard-Fields F, Corballis PM, 2014. Age-related differences in event-related potentials for early visual processing of emotional faces. Soc. Cogn. Affect. Neurosci 9, 969–976. doi: 10.1093/scan/nst071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Nielsen MK, Tipper S, Green S, 2009. An electrophysiological investigation into the automaticity of emotional face processing in high versus low trait anxious individuals. Cogn. Affect. Behav. Neurosci 9, 323–334. doi: 10.3758/CABN.9.3.323. [DOI] [PubMed] [Google Scholar]
- Houston JR, Pollock JW, Lien M–C, Allen PA, 2018. Emotional arousal deficit or emotional regulation bias? An electrophysiological study of age-related differences in emotion perception. Exp. Aging Res 44, 187–205. doi: 10.1080/0361073X.2018.1449585. [DOI] [PubMed] [Google Scholar]
- Hung Y, Gaillard SL, Yarmak P, Arsalidou M, 2018. Dissociations of cognitive inhibition, response inhibition, and emotional interference: Voxelwise ALE meta-analyses of fMRI studies. Hum. Brain Mapp 39, 4065–4082. doi: 10.1002/hbm.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, Yonekura Y, 2002. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus 12, 352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Núñez-Elizalde AO, Dunn BD, Bishop SJ, 2011. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron 69, 563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumika R, Cabeza R, Tsukiura T, 2022. Neural Mechanisms of Perceiving and Sub-sequently Recollecting Emotional Facial Expressions in Young and Older Adults. J. Cogn. Neurosci 34, 1183–1204. doi: 10.1162/jocn_a_01851. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, 2019. Rethinking Cognitive Load: A Default-Mode Network Perspective. Trends Cogn. Sci 23, 531–533. doi: 10.1016/j.tics.2019.04.008. [DOI] [PubMed] [Google Scholar]
- John OP, Gross JJ, 2004. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. J. Pers 72, 1301–1333. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Jorm AF, 2000. Does old age reduce the risk of anxiety and depression? A review of epidemiological studies across the adult life span. Psychol. Med 30, 11–22. doi: 10.1017/s0033291799001452. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Knodt AR, Hariri AR, 2022. Meta-analytic activation maps can help identify affective processes captured by contrast-based task fMRI: the case of threat-related facial expressions. Soc. Cogn. Affect. Neurosci 17, 777–787. doi: 10.1093/scan/nsac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ, 2011. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res 223, 403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisley MA, Wood S, Burrows CL, 2007. Looking at the sunny side of life: age-related change in an event-related potential measure of the negativity bias. Psychol. Sci 18, 838–843. doi: 10.1111/j.1467-9280.2007.01988.x. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson J, Dawson G, Aylward E, 2010. Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia 48, 3665–3670. doi: 10.1016/j.neuropsychologia.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TM, Zhornitsky S, Wang W, Zhang S, Li C-SR, 2021. Problem drinking alters gray matter volume and food cue responses of the lateral orbitofrontal cortex. Addict. Biol 26, e12857. doi: 10.1111/adb.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA, 2008. Effects of age on detection of emotional information. Psychol. Aging doi: 10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- Li G, Chen Y, Le TM, Wang W, Tang X, Li C-SR, 2021. Neural correlates of individual variation in two-back working memory and the relationship with fluid intelli-gence. Sci. Rep 11, 9980. doi: 10.1038/s41598-021-89433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang S, Le TM, Tang X, Li C-SR, 2020. Neural responses to negative facial emotions: Sex differences in the correlates of individual anger and fear traits. Neuroimage 221, 117171. doi: 10.1016/j.neuroimage.2020.117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Wang K, Lin K, Chan RCK, Zhang X, 2017. Neural Temporal Dynamics of Facial Emotion Processing: Age Effects and Relationship to Cognitive Function. Front. Psychol 8, 1110. doi: 10.3389/fpsyg.2017.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF, 2012. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci 35, 121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi N, Ginatempo F, Manca A, Melis F, Deriu F, 2021. Faces emotional expressions: from perceptive to motor areas in aged and young subjects. J. Neurophysiol 126, 1642–1652. doi: 10.1152/jn.00328.2021. [DOI] [PubMed] [Google Scholar]
- MacCormack JK, Stein AG, Kang J, Giovanello KS, Satpute AB, Lindquist KA, 2020. Affect in the Aging Brain: A Neuroimaging Meta-Analysis of Older Vs. Younger Adult Affective Experience and Perception. Affect. Sci 1, 128–154. doi: 10.1007/s42761-020-00016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado L, Thompson LM, Brett CHR, 2019. Visual analogue mood scale scores in healthy young versus older adults. Int. Psychogeriatr 31, 417–424. doi: 10.1017/S1041610218000996. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Nebes RD, 1980. Aging and the development of automaticity in visual search. Dev. Psychol 16, 377–384. doi: 10.1037/0012-1649.16.5.377. [DOI] [Google Scholar]
- Manza P, Hu S, Chao HH, Zhang S, Leung H−C, Li C-SR, 2016. A dual but asymmetric role of the dorsal anterior cingulate cortex in response inhibition and switching from a non-salient to salient action. Neuroimage 134, 466–474. doi: 10.1016/j.neuroimage.2016.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, 2012. The emotion paradox in the aging brain. Ann. N. Y. Acad. Sci 1251, 33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, 2003. Aging and Emothinal Memory, Memory and. Oxford University Press, London. [Google Scholar]
- Mather M, Carstensen LL, 2003. Aging and attentional biases for emotional faces. Psychol. Sci 14, 409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Morawetz C, Riedel MC, Salo T, Berboth S, Eickhoff SB, Laird AR, Kohn N, 2020. Multiple large-scale neural networks underlying emotion regulation. Neurosci. Biobehav. Rev 116, 382–395. doi: 10.1016/j.neubiorev.2020.07.001. [DOI] [PubMed] [Google Scholar]
- Okon-Singer H, Tzelgov J, Henik A, 2007. Distinguishing between automaticity and attention in the processing of emotionally significant stimuli. Emotion 7, 147–157. doi: 10.1037/1528-3542.7.1.147. [DOI] [PubMed] [Google Scholar]
- Petro NM, Basyouni R, Neta M, 2021. Positivity effect in aging: evidence for the primacy of positive responses to emotional ambiguity. Neurobiol. Aging 106, 232–240. doi: 10.1016/j.neurobiolaging.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Grimm O, Morgen K, Mier D, Sauer C, Haddad L, Tost H, Esslinger C, Kirsch P, Schwarz AJ, Meyer-Lindenberg A, 2014. Amygdala habituation: a reliable fMRI phenotype. Neuroimage 103, 383–390. doi: 10.1016/j.neuroimage.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Preckel K, Trautwein F−M, Paulus FM, Kirsch P, Krach S, Singer T, Kanske P, 2019. Neural mechanisms of affective matching across faces and scenes. Sci. Rep 9, 1492. doi: 10.1038/s41598-018-37163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR, 2003. Inhibition of the amygdala: key to pathological states? Ann. N. Y. Acad. Sci 985, 263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Reed AE, Carstensen LL, 2012. The theory behind the age-related positivity effect. Front. Psychol 3, 339. doi: 10.3389/fpsyg.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AE, Chan L, Mikels JA, 2014. Meta-analysis of the age-related positivity effect: Age differences in preferences for positive over negative information. Psychol. Aging doi: 10.1037/a0035194. [DOI] [PubMed] [Google Scholar]
- Rellecke J, Palazova M, Sommer W, Schacht A, 2011. On the automaticity of emotion processing in words and faces: event-related brain potentials evidence from a superficial task. Brain Cogn 77, 23–32. doi: 10.1016/j.bandc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Bessette-Symons B, Hayes SM, Cabeza R, 2011. Emotion processing in the aging brain is modulated by semantic elaboration. Neuropsychologia 49, 640–650. doi: 10.1016/j.neuropsychologia.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Pruis TA, Stevens AA, Janowsky JS, 2011. More is less: emotion induced prefrontal cortex activity habituates in aging. Neurobiol. Aging 32, 1634–1650. doi: 10.1016/j.neurobiolaging.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffman T, Henry JD, Livingstone V, Phillips LH, 2008. A meta-analytic review of emotion recognition and aging: implications for neuropsychological models of aging. Neurosci. Biobehav. Rev 32, 863–881. doi: 10.1016/j.neubiorev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, Junqué C, 2015. Reorganization of brain networks in aging: a review of functional connectivity studies. Front. Psychol 6, 663. doi: 10.3389/fpsyg.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, Knutson B, 2007. Anticipation of monetary gain but not loss in healthy older adults. Nat. Neurosci 10, 787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Stretton J, Van Belle J, Price D, Calder AJ, Dalgleish T, 2019. Age-related decline in positive emotional reactivity and emotion regulation in a population-derived cohort. Soc. Cogn. Affect. Neurosci 14, 623–631. doi: 10.1093/scan/nsz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamloo F, Helie S, 2016. Changes in default mode network as automaticity develops in a categorization task. Behav. Brain Res 313, 324–333. doi: 10.1016/j.bbr.2016.07.029. [DOI] [PubMed] [Google Scholar]
- Sladky R, Hahn A, Karl I−L, Geissberger N, Kranz GS, Tik M, Kraus C, Pfabigan DM, Gartus A, Lanzenberger R, Lamm C, Windischberger C, 2022. Dynamic Causal Modeling of the Prefrontal/Amygdala Network During Processing of Emotional Faces. Brain Connect. 12, 670–682. doi: 10.1089/brain.2021.0073. [DOI] [PubMed] [Google Scholar]
- Sokołowski A, Folkierska-Żukowska M, Jednoróg K, Moodie CA, Dragan WŁ, 2020. The relationship between early and recent life stress and emotional expression processing: A functional connectivity study. Cogn. Affect. Behav. Neurosci 20, 588–603. doi: 10.3758/s13415-020-00789-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, 1989. State–Trait Anxiety Inventory: A comprehensive Bibliography. Consulting Psychologists Press, Palo Alto, CA. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP, 2007. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am. J. Psychiatry 164, 318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS, 2005. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 139, 9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, 2012. Aging and functional brain networks. Mol. Psychiatry 17, 549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JD, Eberhart NK, Bookheimer SY, Eisenberger NI, Foland-Ross LC, Cook IA, Sugar CA, Altshuler LL, 2010. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Res. 183, 209–217. doi: 10.1016/j.pscychresns.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA, 2002. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16, 765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P, 2012. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum. Brain Mapp 33, 1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Gross JJ, 2010. Emotion Regulation in Older Age. Curr. Dir. Psychol. Sci 19, 352–357. doi: 10.1177/0963721410388395. [DOI] [Google Scholar]
- Vatansever D, Menon DK, Stamatakis EA, 2017. Default mode contributions to automated information processing. Proc. Natl. Acad. Sci 114, 12821–12826. doi: 10.1073/pnas.1710521114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinescu A, David D, 2020. Age effects on recollection and automaticity in a fully im-mersive virtual reality environment. Annu. Rev. CyberTherapy Telemed 18, 111–115. [Google Scholar]
- Wang W, Zhornitsky S, Zhang S, Li C-SR, 2021. Noradrenergic correlates of chronic cocaine craving: neuromelanin and functional brain imaging. Neuropsychopharmacology 46, 851–859. doi: 10.1038/s41386-020-00937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger M, Sandi C, 2018. High anxiety trait: A vulnerable phenotype for stress-induced depression. Neurosci. Biobehav. Rev 87, 27–37. doi: 10.1016/j.neubiorev.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Wood S, Kisley MA, 2006. The negativity bias is eliminated in older adults: age-related reduction in event-related brain potentials associated with evaluative categorization. Psychol. Aging 21, 815–820. doi: 10.1037/0882-7974.21.4.815. [DOI] [PubMed] [Google Scholar]
- Woods DL, Wyma JM, Yund EW, Herron TJ, Reed B, 2015. Corrigendum: Age-related slowing of response selection and production in a visual choice reaction time task. Front. Hum. Neurosci. doi: 10.3389/fnhum.2015.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D, 2007. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer’s disease. Biol. Psychiatry 62, 1388–1395. doi: 10.1016/j.biopsych.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS, 2006. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiol. Aging 27, 361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Yankouskaya A, Rotshtein P, Humphreys GW, 2014. Interactions between Identity and Emotional Expression in Face Processing across the Lifespan: Evidence from Re-dundancy Gains. J. Aging Res 2014, 136073. doi: 10.1155/2014/136073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH, 1999. Biostatistical Analysis, 4th ed Prentice Hall, Upper Saddle River. [Google Scholar]
- Ziaei M, Arnold C, Ebner NC, 2021. Age-related Differences in Expression Recognition of Faces with Direct and Averted Gaze Using Dynamic Stimuli. Exp. Aging Res 47, 451–463. doi: 10.1080/0361073X.2021.1902459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


