Abstract
People with unilateral transfemoral amputation using socket prostheses are at increased risk for developing osteoarthritis in both the residual hip and intact lower-limb joints. Osseointegrated prostheses are a surgical alternative to socket prostheses that directly attach to the residual femur via a bone-anchored implant, however their multi-joint loading effect is largely unknown. Our objective was to establish how osseointegrated prostheses influence joint loading during walking. Motion capture data (kinematics, ground reaction forces) were collected from 12 participants at baseline, with socket prostheses, and 12-months after prosthesis osseointegration during overground walking at self-selected speeds. Subject-specific musculoskeletal models were developed at each timepoint relative to osseointegration. Internal joint moments were calculated using inverse dynamics, muscle and joint reaction forces (JRFs) were estimated with static optimization. Changes in internal joint moments, JRFs, and joint loading-symmetry were compared using statistical parametric mapping before and after osseointegration. Amputated limb hip flexion moments and anterior JRFs decreased during terminal stance (; respectively), while amputated limb hip abduction moments increased during mid-stance , amputated hip rotation moment changed from internal to external throughout early stance . Intact limb hip extension and knee flexion moments (; respectively), superior and resultant knee JRFs (; respectively) decreased during the loading response following prosthesis osseointegration. These results may indicate that the direct loading transmission of these novel prostheses create a more typical mechanical environment in bilateral joints, which is comparable with loading observed in able-bodied individuals and could decrease the risk of development or progression of osteoarthritis.
Keywords: Osseointegrated prostheses, musculoskeletal modeling, above-knee amputation, socket prostheses, gait
1. Introduction
The number of individuals living with major limb amputation in the United States is expected to exceed 4 million adults by the year 2050 (Ziegler-Graham et al., 2008). Mobility is substantially compromised within this population which detrimentally impacts their overall quality of life, especially those with transfemoral amputation (TFA) (Gailey et al., 2008). The two most common causes of reduced mobility are chronic complications from ill-fitting socket-prostheses (Dillingham et al., 2001; Penn-Barwell, 2011; Reddy et al., 1981) and secondary comorbidities, such as low back pain or osteoarthritis (OA) (Highsmith et al., 2019; Morgenroth et al., 2012). Prosthesis osseointegration is a novel alternative that involves surgical fixation to the residual limb through a bone-anchored implant, designed to alleviate complications related to socket fit (Brånemark et al., 2014, 2001). However, there is still no consensus regarding the effect of these novel prostheses on secondary comorbidities for people with TFA.
Socket prosthesis users with unilateral TFA adopt biomechanical compensations due to the loss of the ankle and knee joints, and altered force transmission between the ground and residual limb. For instance, people with TFA commonly increase lateral trunk bending (i.e., compensated Trendelenberg), which positions their center of mass over the base of support to aid in stability due to weakened hip abductors (Carse et al., 2020; Goujon-Pillet et al., 2008; Jaegers et al., 1995). During ipsilateral push-off, patients with TFA commonly increase hip flexion demand to facilitate limb advancement without active ankle plantarflexion (Jaegers et al., 1995; Schaarschmidt et al., 2012; Seroussi et al., 1996). While necessary, habitual compensatory biomechanics increase the risk of secondary comorbidities in this population as chronically altered joint loading has been linked to articular cartilage damage (Block and Shakoor, 2009; Felson, 2013; Michaud et al., 2000; Nolan et al., 2003; Schaarschmidt et al., 2012). Individuals with unilateral TFA are 18 and 14 times more likely to develop knee and hip OA, respectively, compared to the general population (Struyf et al., 2009).
Although the musculoskeletal system is inherently asymmetrical after TFA, optimizing limb loading-symmetry remains a primary rehabilitation focus. However, there is conflicting evidence regarding which limb is over- or under-loaded (Castro et al., 2014; Nolan et al., 2003; Nolan and Lees, 2000; Schaarschmidt et al., 2012). Because socket prostheses alter the load transmission from the ground throughout the residual limb (Heitzmann et al., 2020; Mak et al., 2001), other regions of the body that are not directly affected by the amputation can be negatively influenced (e.g., low back); a concept known as regional interdependence (Sueki et al., 2013; Wainner et al., 2007). While improving loading-symmetry remains a clinical priority, achievement is challenging due to altered load transmission from socket prostheses.
Osseointegrated prostheses are an alternative to socket prostheses that directly attach to the residual limb via bone-anchored implant in the intramedullary canal of the residual limb (Brånemark et al., 2014, 2001). Early reports from transfemoral osseointegrated prosthesis users show high satisfaction levels and improved clinical outcomes (i.e., mobility, quality of life) compared to socket prosthesis users (Hagberg and Brånemark, 2009; Tranberg et al., 2011; Van De Meent et al., 2013). Notably, osseointegration improves force transmission between the ground and residual limb, resulting in more natural amputated limb loading compared to socket prostheses (Robinson et al., 2020; Thomson et al., 2019). We have recently demonstrated improved amputated limb hip muscle function during dynamic activities (Gaffney et al., 2022), however the effects of transfemoral osseointegrated prostheses on joint loading are not clearly defined.
Our objective was to determine the multi-joint loading changes in people with unilateral TFA before and 12-months after prosthesis osseointegration using musculoskeletal modeling. We hypothesized that osseointegration would normalize bilateral joint loading, resulting in improved hip joint loading-symmetry.
2. Methods
2.1. Participants
Twelve people scheduled to undergo unilateral, transfemoral prosthesis osseointegration were enrolled in this study (Table 1). Potential participants were excluded if their amputations had non-traumatic causes, there was history of substance abuse, acute systemic infections, unstable heart conditions, or candidates were undergoing cancer treatment. All study participants received press-fit implants in two surgical stages (OTN BV Implants, The Netherlands). During the first stage the fixture was implanted into the residual femur intramedullary canal, followed by 6 weeks of non-weight bearing allowing bony ingrowth between the implant and residual bone. The second stage created a stoma at the distal residual limb and secured a transcutaneous dual cone adaptor for prothesis connection. After the second stage, participants followed identical 3-week rehabilitation protocols, progressing from static prosthesis loading to dynamic load bearing exercises in preparation for community ambulation (Leijendekkers et al., 2017). Participants underwent a computed tomography (CT) scan prior to the first surgical stage to aid in surgical planning.
Table 1.
Demographic information of patient cohort including age (years), body-mass index before and after osseointegration (kg/m2), and time spent with a socket prosthesis before undergoing osseointegration (years).
| Patient ID | Sex | Age (yr) | BMI (kg/m2) Pre-OI |
BMI (kg/m2) Post-OI |
Time since Amputation (years) |
|---|---|---|---|---|---|
| Pt01 | F | 57 | 23.5 | 23.5 | 2 |
| Pt02 | F | 55 | 17.4 | 18.0 | 38 |
| Pt03 | M | 53 | 29.6 | 28.3 | 8 |
| Pt04 | F | 37 | 27.8 | 28.3 | 5 |
| Pt05 | F | 38 | 25.4 | 25.1 | 32 |
| Pt06 | M | 38 | 20.7 | 23.1 | 7 |
| Pt07 | M | 48 | 30.8 | 30.7 | 15 |
| Pt08 | F | 48 | 28.0 | 28.0 | 32 |
| Pt09 | F | 48 | 23.5 | 24.4 | 18 |
| Pt10 | F | 48 | 27.6 | 29.5 | 28 |
| Pt11 | M | 48 | 27.8 | 27.3 | 5 |
| Pt12 | M | 51 | 26.8 | 28.0 | 2 |
| Mean ± SD | - | 47.4 ± 6.6 | 25.7 ± 3.9 | 26.2 ± 3.5 | 16 ± 13.2 |
Each participant visited the laboratory for two motion capture data collections relative to the first surgical stage (~2 days before (baseline) and 12-months following). During baseline collection, participants wore their own socket-suspended protheses including an ischial containment socket, microprocessor knee, and dynamic carbon-fiber response foot. At the 12-month collection, participants wore their own prostheses with the same knee and foot componentry including a torque control adapter distally connected to the osseointegrated implant. Participants provided informed, written consent of an approved protocol by the Colorado Multiple Institutional Review Board prior to baseline collection.
2.2. Data Collection and Processing
Three-dimensional reconstructions of the residual femur were created using CT images via semi-automated thresholding for segmentation (Amira 2020.1, ThermoFisher, Waltham, MA) (Fig. 1a).
Figure 1.
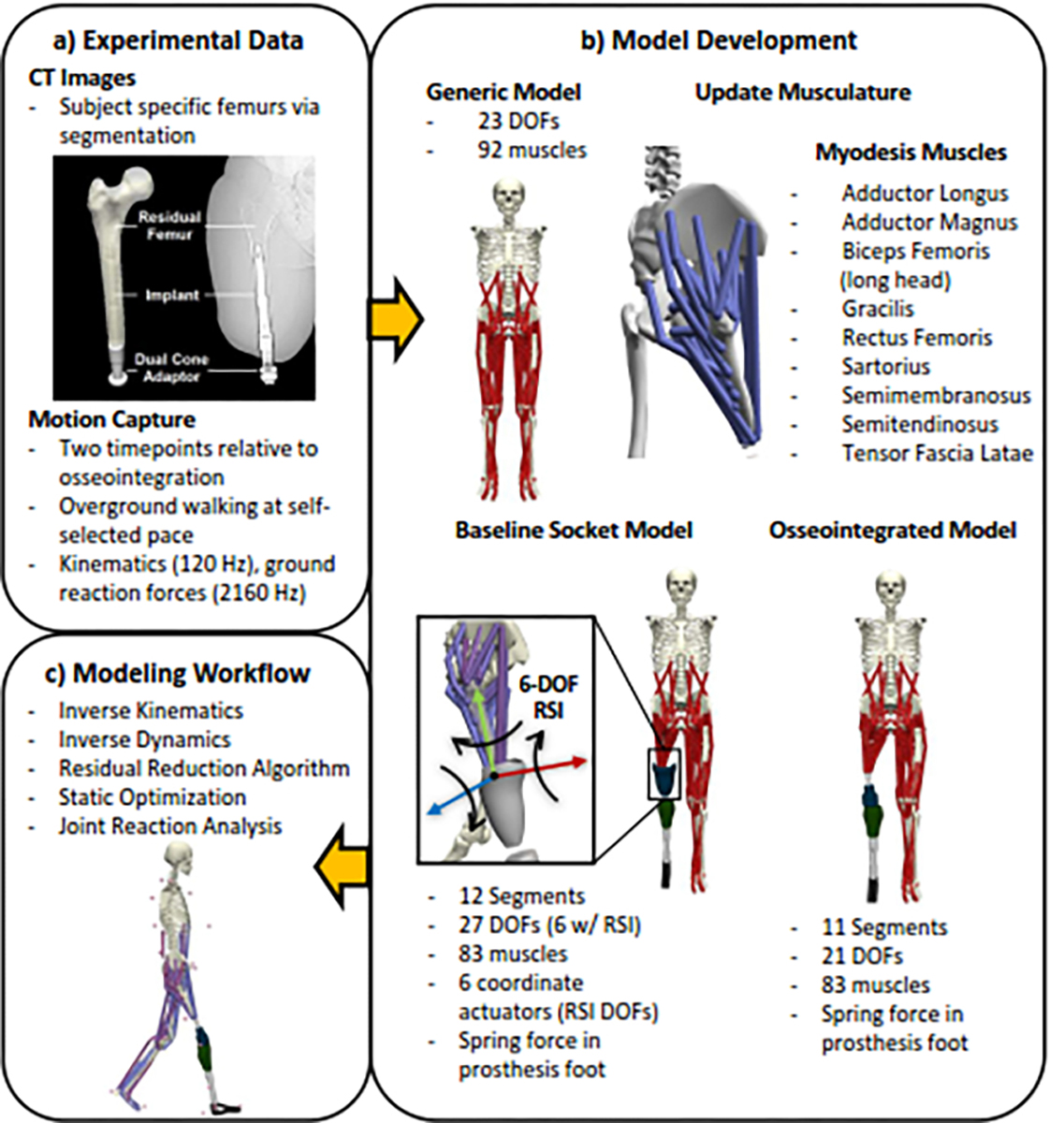
(a) CT images and experimental motion capture data were used to modify an existing generic model. (b) Subject-specific musculoskeletal models with a simulated myodesis were created for each patient before and after osseointegration. (c) Workflow in OpenSim to determine kinetics, and joint loading.
Motion capture data were recorded from participants during overground walking at self-selected speed (Fig. 1a). Kinematics were recorded from 38 reflective markers using eight infrared cameras (Vicon, Centennial, CO), ground reaction forces (GRF) were collected from six embedded force platforms (Bertec, Columbus, OH). Trajectory and analog data were filtered using a low-pass, fourth-order Butterworth filter ( and 20 Hz, respectively). Cutoff frequencies were determined via residual analysis (Winter, 2009).
2.3. Musculoskeletal Modeling
Separate subject-specific musculoskeletal models were created for each participant before and after prosthesis osseointegration by modifying an existing, generic model with 23 degrees of freedom (DOF) and 92 muscles within OpenSim (Lai et al., 2017; Rajagopal et al., 2016) (Fig. 1b). Each model was non-uniformly scaled using markers placed on anatomical landmarks. The generic pelvis and residual limb femur geometries were imported into Amira and scaled using corresponding scale factors. Reconstructed residual femurs were placed at the subject-specific hip joint centers, defined by the center of a sphere fit to the femoral head (Harris et al., 2017). Proximal hip musculature insertions were updated using a human anatomy atlas (Harris et al., 2017; Netter, 1951; Song et al., 2019). Ankle and uniarticular knee musculature were removed from the amputated limbs of all models. A myodesis procedure, which involves surgically suturing amputated limb musculature to the distal residual femur in a manner that attempts to preserve the muscle’s original line of action as closely as possible, was simulated by inserting biarticular hip/knee model musculature onto the distal reconstructed femurs using established methods (Harandi et al., 2020a, 2020b; Jones and Fey, 2021; Ranz et al., 2017) (Fig. 1b). Residual limb muscle properties (optimal fiber length, tendon slack length) were linearly scaled from generic model values to match updated insertion sites (Song et al., 2020). Residual limb proximal/distal diameters and length measurements (femoral head center to distal limb) from CT images were used to update the residual limb inertial properties, assuming uniform tissue density (Robinson et al., 2020). Generic tibial and foot geometries were replaced by generic prosthesis geometries with shanks modeled as cylinders and feet modeled as triangular prisms to define inertial properties, assuming carbon fiber material with constant density (Robinson et al., 2020). A spring force at the prosthesis ankle was added to the model’s force-set to simulate an energy storage and return foot (Silverman and Neptune, 2014).
Models for each timepoint followed this workflow, but differed in modeling the residual limb and prosthesis interface. Baseline socket models included a 6 DOF joint at the residual limb-socket interface (RSI) (Fig. 1b) located at the distal residual limb (LaPrè et al., 2018). Osseointegrated prosthesis models assumed rigid connections between the prosthesis and residual limb, as previously defined (Robinson et al., 2020) (Fig. 1b).
2.4. Modeling Workflow
Motion capture data were analyzed during three bilateral stance periods (heel strike to toe off) (Fig. 1c). Stance phases were defined using Visual3D’s automatic gait event labeling, which uses Event Target Pattern Recognition (TPR) signals to identify gait events based off kinematic and kinetic data (Hreljac and Marshall, 2000; Stanhope et al., 1990). Joint angles were calculated using inverse kinematics via weighted least squared minimization between experimental and virtual model markers. A residual reduction algorithm was performed to maintain dynamic consistency between the model and experimental data by minimizing non-physical residual forces and moments through small, controlled motion perturbations and slight adjustments to the model’s mass parameters (Delp et al., 2007). Static optimization was used to estimate muscle forces by solving for the sum of moments from individual muscle forces while minimizing the sum of squared muscle activations (Anderson and Pandy, 2001). GRFs and estimated muscle forces were used to analyze the anteroposterior, superior, mediolateral, and resultant joint reaction forces (JRFs) of bilateral hips and the intact limb knee (Steele et al., 2012). Hip JRFs were expressed in the pelvis frame of reference, representing the force interaction on the acetabulum (Harris et al., 2017; Song et al., 2020, 2019). Intact limb knee JRFs were expressed in the femoral frame of reference.
Model validation was performed according to previously established methods, including minimizing residual forces and moments (Hicks et al., 2015). Furthermore, the baseline socket model was validated through comparison to experimentally measured RSI forces (Schwarze et al., 2013) (Supplemental Figure 1).
2.5. Symmetry
Hip joint loading-symmetry was calculated using the Normalized Symmetry Index (NSI), which serves as a universal index for assessing symmetry in a clinical context by accounting for trial-to-trial magnitude variability (Queen et al., 2020). However, the standard NSI was adapted from calculating at a discrete timepoint to continuously calculate loading-symmetry at all timepoints across bilateral stance periods of gait:
| (1) |
where ‘’ is the total number of trials, ‘’ is the timepoint normalized to a percent of stance phase, ‘Int’ is intact limb loading, ‘Amp’ is amputated limb loading, ‘’and ‘’ are the absolute maximums and minimums across both limbs and all trials at . The NSI at each timepoint was determined using the difference between ‘Int’ and ‘Amp’ loading. Inter-trial variability was accounted for by dividing the bilateral loading difference by a “global range” at respective timepoints , determined from ‘Max’ and ‘Min’, then multiplied by 100 to normalize the index and averaged across trials (Eq. (1)). Symmetry index values ranged from −100 to +100 (representing full asymmetry governed by the amputated or intact limb, respectively) and zero indicated perfect symmetry.
2.6. Statistical Analysis
Dependent variables included trunk (two DOF), bilateral hip (three DOF) and intact knee (one DOF) internal joint moments, bilateral hip and intact knee JRFs, and hip joint loading-symmetry throughout stance period. All dependent variables were normalized to the stance period. Ensemble averages of each dependent variable were compared before (in socket) and after osseointegration using one-dimensional statistical parametric mapping (SPM) (Pataky et al., 2015, 2013). Gait speed at each timepoint relative to prosthesis osseointegration was included as a covariate in the SPM through a general linear model (GLM) (Savage et al., 2021), which is analogous to a one-way ANCOVA (Pataky, 2012). With gait speed included as a covariate, the GLM was ran as a paired t-test (two-tailed inference, ). All SPM analyses were implemented in Matlab (Matlab 2022a, Mathworks, Nattick, MA) using open-sourced spm1d code (www.spm1d.org).
3. Results
3.3. Walking Speed
Self-selected gait speed decreased by 8.6 ± 14.6%, from 1.07 ± 0.15 m/s at baseline with socket prostheses to 0.96 ± 0.14 m/s twelve-months after osseointegration .
3.4. Internal Joint Moments
Twelve-months following prosthesis osseointegration, intact limb hip extension and knee flexion moments during loading response decreased compared to baseline values within the socket (, respectively) (Fig. 2a). The amputated limb hip flexion moment decreased during pre-swing while the hip abduction moment increased during mid-stance (Fig. 2b). The hip rotation moment shifted from internal to external direction during loading response and through mid-stance (Fig. 2b). Finally, the sagittal lumbar bending moment shifted from flexion to extension 12-months after prosthesis osseointegration .
Figure 2.
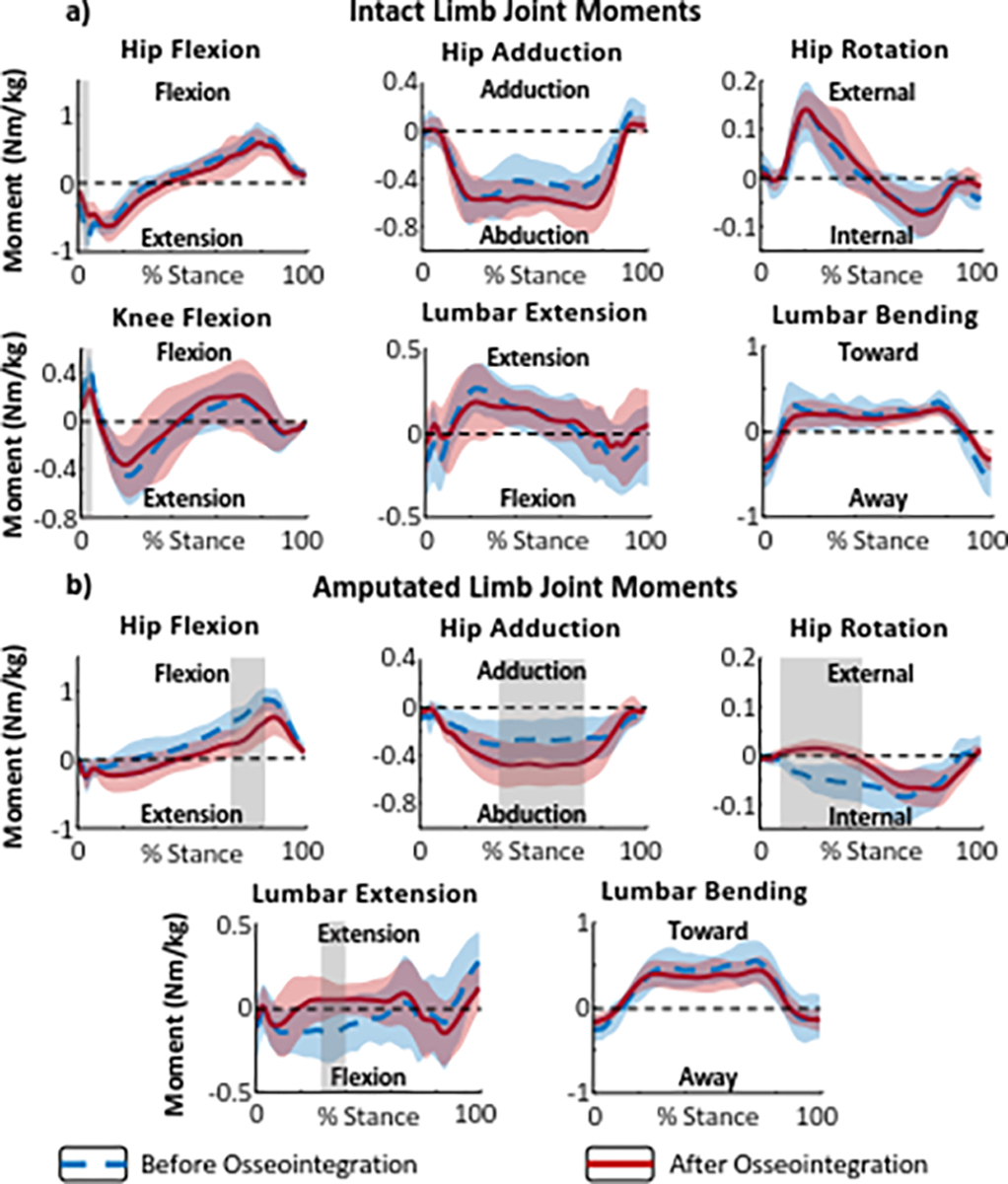
Internal joint moments during stance phases of level walking before and after osseointegration. (a) Intact limb hip, knee, and lumbar joint moments in stance phase. (b) Amputated limb hip and lumbar joint moments in stance phase. Dashed blue lines represent baseline (with socket) data and solid red lines represent data for 12 months post-osseointegration surgery. The red and blue shaded areas represent ± 1 standard deviation from the means. The gray shaded areas indicate a statistically significant difference (determined using SPM) between the mean moments at baseline and 12 months after osseointegration.
3.5. Joint Reaction Forces
Compared to baseline values with socket prostheses, the intact knee superior and resultant JRFs decreased during initial contact 12-months after prosthesis osseointegration (, respectively) (Fig. 3b). The amputated limb superior and resultant hip JRF increased during loading response (, respectively) (Fig. 3c). Finally, the amputated limb anterior hip JRF decreased during pre-swing 12-months after prosthesis osseointegration (Fig. 3c). Estimated muscle forces contributing to hip JRF can be found in Supplemental Figure 2.
Figure 3.
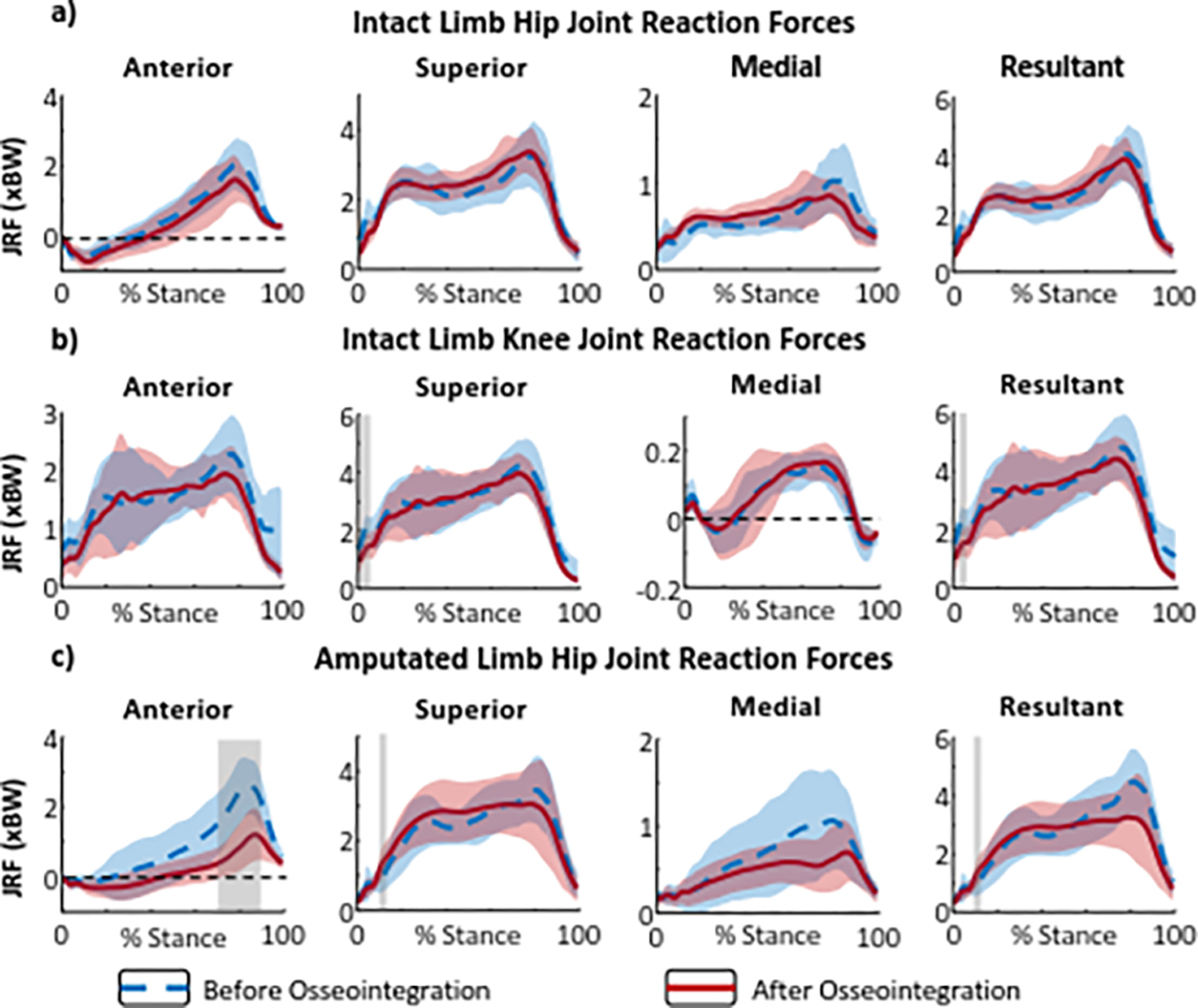
Bilateral joint reaction force (JRF) components and resultant during stance phases of level walking before and after osseointegration. (a) Intact limb hip, (b) intact limb knee, (c) and amputated limb hip JRF. Dashed blue lines represent baseline (with socket) data and solid red lines represent data for 12 months post-osseointegration surgery. The red and blue shaded areas represent ± 1 standard deviation from the means. The gray shaded areas indicate a statistically significant difference (determined using SPM) between the mean JRF at baseline and 12 months after osseointegration.
3.6. Joint Loading-Symmetry
Joint loading-symmetry changed following prosthesis osseointegration, and was dependent upon direction and timing within the stance period. The superior hip JRF symmetry increased (smaller NSI) during loading response and the anterior hip JRF decreased (larger NSI) during pre swing 12-months after prosthesis osseointegration (Fig. 4).
Figure 4.
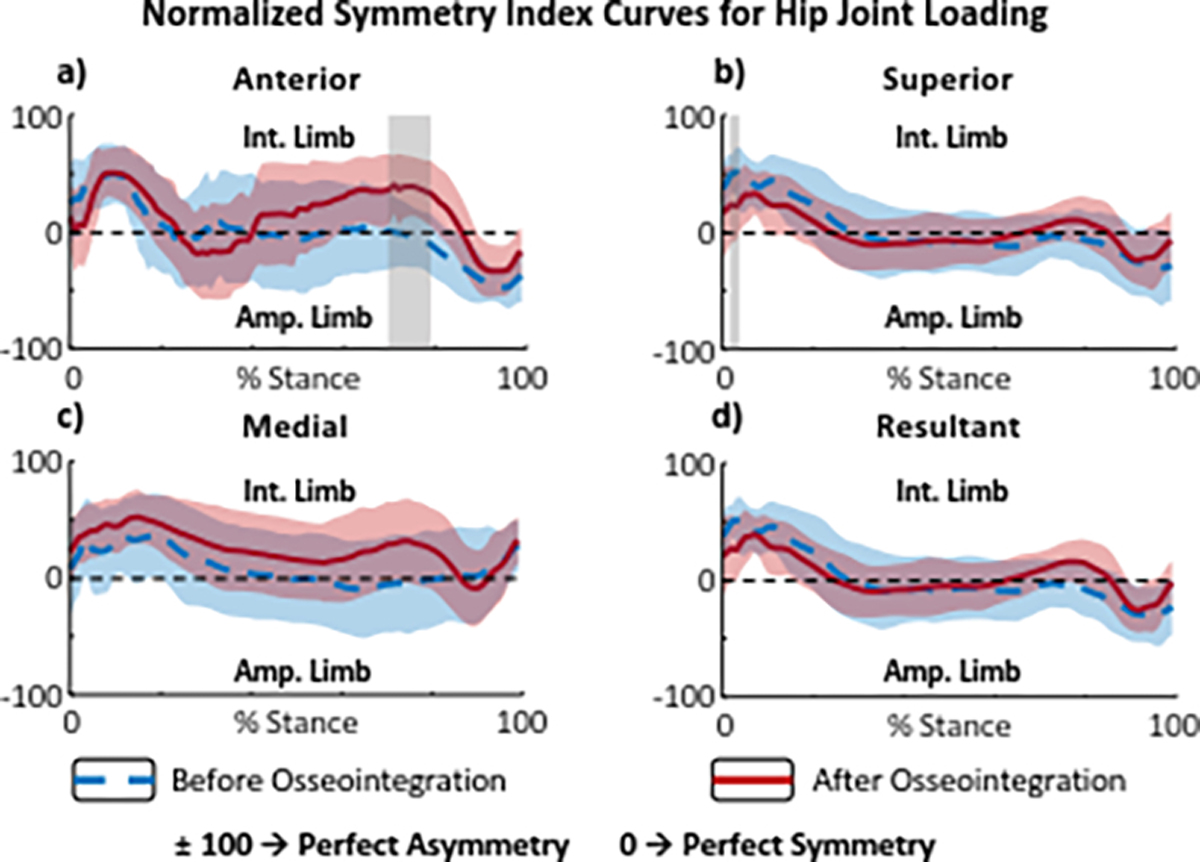
Waveform symmetry curves of bilateral hip joint loading during stance phases of level walking before and after prosthesis osseointegration. (a) Anterior, (b) superior, (c) medial, (d) and resultant JRF symmetry waveforms between the intact and amputated limbs. A value of zero indicates perfect symmetry while +100 and −100 indicate perfect asymmetry, overloading the intact or amputated limbs respectively. Dashed blue lines show the baseline loading symmetry trend with socket prostheses, solid red lines show the loading symmetry trend 12 months after prosthesis osseointegration. The red and blue shaded areas represent ± 1 standard deviation from the means. The gray shaded areas indicate a statistically significant difference (determined using SPM) between symmetry trends at baseline and 12 months after osseointegration.
4. Discussion
The purpose of this study was to quantify the effect of osseointegrated prostheses on lower-limb joint loading in individuals with unilateral TFA. When using osseointegrated prostheses, the amputated limb hip flexion moment and anterior hip JRF decreased during terminal stance, while the hip abduction moment increased during mid-stance compared to using socket prostheses. Furthermore, the intact limb hip and knee internal joint moments and knee joint loading decreased at initial contact when using an osseointegrated prosthesis but were otherwise unchanged and comparable with loading observed in able-bodied individuals (Harris et al., 2017). These joint loading changes may benefit the mechanical environment of the joints and, over long-term cyclic loading, may lower the risk of amputated limb OA in this population (Morgenroth et al., 2012; Struyf et al., 2009). Although our results demonstrated improvements in hip joint loading-symmetry in early stance, we also observed increased asymmetric hip joint loading in late stance after prosthesis osseointegration. These findings may help inform the timing of symmetry training within rehabilitation after unilateral prosthesis osseointegration. To our knowledge, this is the first study to longitudinally quantify the effects of osseointegrated prostheses on joint loading compared to socket prostheses.
Our results demonstrated that internal hip joint moments and hip JRF in the sagittal plane were reduced in the amputated limb 12-months after transfemoral prosthesis osseointegration compared to baseline values using socket prostheses. The internal hip flexion moment and anterior JRF in the amputated limb decreased during terminal stance and pre-swing of the amputated limb, which may indicate less demand being placed on the hip flexor muscles to advance the limb. People with TFA commonly compensate for the lack of amputated limb ankle propulsion by increasing hip flexor force to reposition the GRF vector posteriorly to the knee joint to generate forward propulsion (Jaegers et al., 1995; Koehler-McNicholas et al., 2016; Schaarschmidt et al., 2012; Seroussi et al., 1996). Increased hip flexor muscle force will directly increase anterior joint loading, as joint loading is predominantly muscle driven (Correa et al., 2010). Therefore, the significant decreases in hip anterior JRF after osseointegration, which are more comparable with magnitudes observed in able-bodied individuals (Harris et al., 2017; Layton et al., 2022), may demonstrate a more kinetically efficient forward propulsion strategy that is less reliant on hip flexors.
Muscle function in the amputated hip showed improvement after osseointegration. The amputated hip abduction moment significantly increased during mid-stance when using osseointegrated prostheses compared to baseline. The medial JRF decreased, but was not statistically significant due to high baseline variability which we attribute to varied participant mobility levels, evidenced by variable stabilizing movement patterns. People with unilateral TFA using socket prostheses improve stability by increasing step width (Heitzmann et al., 2020; Jaegers et al., 1995), thereby increasing the hip abduction angle and moment arm of the primary hip abductors. Because abductor muscle forces are the primary contributors to hip joint loading (Correa et al., 2010), any changes in force production will significantly influence hip joint loading. Previous work has demonstrated that simulating reduced hip abductor force resulted in increased hip joint loading (Valente et al., 2013). The authors attributed this to increased forces from the surrounding musculature (e.g., rectus femoris, iliopsoas) required to stabilize the hip joint in the simulated absence of hip abductor forces. Muscle weakness is commonly observed in people with TFA, which is a known etiological factor to OA as the mechanical environment of the joint changes (Amaro et al., 2007; Heitzmann et al., 2020; Hurley, 1999; Jaegers et al., 1995; Shakoor et al., 2008). We have recently shown increased amputated hip abductor muscle force during sitting (Gaffney et al., 2022) and overground walking after osseointegration (Supplemental Figure 2). Increased abductor muscle forces using osseointegrated prostheses may decrease the compensatory demand from surrounding hip musculature, resulting in the reduced medial hip JRF.
We also observed the amputated limb hip rotation moment switched from an internal to external rotational direction during early to mid-stance 12-months after prosthesis osseointegration. This directional change in hip rotation moment of the amputated limb after prosthesis osseointegration more closely resembles the intact limb hip rotation moment and previous results in a healthy population (Gronley and Perry, 1984; Perry, 1992; Ren et al., 2008; Schache and Baker, 2007). As we presented internal joint moments, a surrogate representation of muscle forces, this change is supported by the increase in amputated limb gluteal muscle forces observed after osseointegration (Supplemental Figure 2). At baseline, the internally directed hip rotation moment on the amputated limb throughout stance period may indicate inability to control the amputated limb loading within the socket. The gluteal muscles are eccentrically active with increased forces, that aid in power absorption during limb loading, contributing to the hip rotation moment. We interpret these changes to indicate greater stabilizing muscle control during amputated limb loading after prosthesis osseointegration. Thus, our results may show that osseointegrated prostheses restore the biomechanical environment of the hip joint through changes in muscle forces that could help mitigate the risk of secondary comorbidities, such as hip OA.
Osseointegrated prostheses also influenced intact limb joint loading. This can be observed in the decreased hip extension and knee flexion moments and decreased knee resultant and superior JRFs of the intact limb at heel strike. Schaarschmidt et al. (2012) found that people with unilateral TFA using socket prostheses increased braking impulses applied to the intact limb. The authors concluded that these impulses were used for deceleration without controlled weight transfer from the amputated limb (commonly referred to as ‘falling’ onto the intact limb). We observed a significant decrease in the anterior component of the braking impulse corresponding to shock absorption during heel strike that occurs during between-limb weight transfer (Supplemental Figure 3) (Marasovic et al., 2009). Repetitive loading at a high rate (i.e., high braking impulses) can cause micro-damage to sub-chondral bone, leading to degenerative changes in articular cartilage and increasing the risk of OA development (Morgenroth et al., 2012). Our results may demonstrate improved control during weight transfer from the amputated limb, where osseointegrated prosthesis users are less likely to ‘fall’ onto their intact limb. These decreased braking impulses may help normalize the mechanical environment of the intact limb joints and reduce the risk of OA development, especially the intact knee which shows 18-fold greater prevalence of OA development compared to able-bodied individuals (Struyf et al., 2009).
Our results showed that changes in joint loading-symmetry were dependent on gait cycle phase. Specifically, hip joint loading was more symmetrical during loading response of early stance, while more asymmetrical during terminal stance and pre-swing when using osseointegrated prostheses compared to socket prostheses. We attribute decreased between-limb asymmetry during loading response to a more direct load transmission from osseointegrated prostheses into the residual femur, potentially resulting in improvements in osseoperception and osseoproprioception (Hagberg et al., 2008; Örgel et al., 2021; Robinson et al., 2020). Osseointegration provides unique proprioceptive feedback to people with amputation, which improves sensation in the amputated limb, allowing greater control during weight transfer and leading to the increased symmetry observed during loading response. This may be indicated by the increased hip JRF on the amputated limb during loading response after osseointegration. Contrary to reduced asymmetry in early stance, hip joint loading asymmetry increased during pre-swing; primarily driven by lack of change in intact limb hip joint loading while amputated limb anterior hip JRF decreased significantly after prosthesis osseointegration. Considering the observed amputated limb hip muscle force normalization and associated persistence of between-limb hip loading asymmetry, our results indicate that loading-symmetry in pre-swing is not influenced by prosthesis osseointegration. However, future work is necessary to investigate the relative advantages and disadvantages of hip loading changes (or lack thereof) throughout stance. As rehabilitation standards after osseointegration continue to be developed, these findings may inform future work in targeted load-symmetry training across different phases of gait to account for inherent differences between limbs and optimize ambulation strategies.
This investigation had several limitations that should be considered. First, this study utilized a sample size of convenience, participants were varied in their baseline functional levels and reasons for undergoing prosthesis osseointegration. Variable physical function likely results in variability in compensatory movement patterns, which may have hindered our ability to detect statistical significance in joint loading with this sample size. Second, walking speed was not controlled for across timepoints or participants, which can influence joint loading (Lerner et al., 2014). However, while the observed decrease in walking speed after prosthesis osseointegration (mean decrease of 0.11 m/s) was slightly higher than the threshold of minimal clinically important difference in older adults (0.1 m/s), it was less than the minimal detectable change of older adults with mobility problems (0.13 m/s) (Bohannon et al., 2014; Chui et al., 2012; Perera et al., 2006). Thus, in conjunction with the lack of bilateral changes in joint loading after prosthesis osseointegration, we do not interpret the magnitude of this change in gait speed to be indicative of a reduction in physical function that would influence joint biomechanics. Third, patient-specific muscle strengths were not modeled as strength data for these participants were not available. Muscle weakness is well documented in people with TFA due to limb disuse within sockets (Henson et al., 2021; Jaegers et al., 1995). Prior work has demonstrated that musculoskeletal model outputs are highly sensitive to muscle parameter perturbations such as max isometric force, which represents an individual muscle’s strength (Scovil and Ronsky, 2006; Shepherd et al., 2022). We were also not able to measure prostheses inertial properties at respective timepoints relative to osseointegration, and therefore could not include this in our musculoskeletal models. While we focused on the stance phase in this study, the limb becomes more dynamic during terminal stance to prepare for forward propulsion of the limb. Future work will explore how strength changes affect joint loading after prosthesis osseointegration. Finally, muscle forces in this study were determined using OpenSim’s Static Optimization tool, which does not account for synergist muscle co-contraction. However, previous studies have demonstrated that this tool can produce hip muscle forces that are similar to normal gait as well as modeling muscle and joint forces for people with TFA (Anderson and Pandy, 2001; Correa et al., 2010; Harandi et al., 2020a, 2020b; Robinson et al., 2020). Therefore, we believe that utilizing a different muscle optimization tool would not affect the results produced in this study.
In conclusion, we found that people with unilateral TFA using osseointegrated prostheses adopted a more normative strategy for forward propulsion compared to socket prostheses. Despite the biomechanical improvements observed in this study, our results show that this population may still benefit from targeted rehabilitation strategies to further improve joint loading-symmetry. These preliminary results may indicate that transfemoral osseointegrated prostheses create a more normative mechanical environment in the amputated hip joint. Future work is needed to determine if this decreases the risk of OA development.
Acknowledgements
This project was supported by the University of Colorado Osseointegration Research Consortium, the National Institutes of Health (K01AR080776 and UL1TR002535), and the University of Colorado Denver Office of Research Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, or the United States Government. We thank Galen Roda for her assistance with volumetric image segmentation.
Footnotes
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaro A, Amado F, Duarte JA, Appell HJ, 2007. Gluteus medius muscle atrophy is related to contralateral and ipsilateral hip joint osteoarthritis. Int J Sports Med 28, 1035–1039. 10.1055/s-2007-965078 [DOI] [PubMed] [Google Scholar]
- Anderson FC, Pandy MG, 2001. Static and dynamic optimization solutions for gait are practically equivalent. J Biomech 34, 153–161. 10.1016/S0021-9290(00)00155-X [DOI] [PubMed] [Google Scholar]
- Block JA, Shakoor N, 2009. The Biomechanics of Osteoarthritis: Implications for Therapy. Curr Rheumatol Rep 11, 15–22. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Dpt PT, Ncs E, Glenney SS, Dpt Gcs PT, 2014. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract 20, 295–300. 10.1111/JEP.12158 [DOI] [PubMed] [Google Scholar]
- Brånemark R, Berlin Ö, Hagberg K, Bergh P, Gunterberg B, Rydevik B, 2014. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation A PROSPECTIVE STUDY OF 51 PATIENTS The implant (OPRA Implant System (Osseointegrated Prostheses. Bone Joint J 96, 106–113. 10.1302/0301-620X.96B1 [DOI] [PubMed] [Google Scholar]
- Brånemark R, Brånemark P-I, Rydevik B, Myers RR, 2001. Osseointegration in skeletal reconstruction and rehabilitation: A review and the VA San Diego Healthcare System. J Rehabil Res Dev 38, 175–181. [PubMed] [Google Scholar]
- Carse B, Scott H, Brady L, Colvin J, 2020. A characterisation of established unilateral transfemoral amputee gait using 3D kinematics, kinetics and oxygen consumption measures. Gait Posture 75, 98–104. 10.1016/J.GAITPOST.2019.09.029 [DOI] [PubMed] [Google Scholar]
- Castro M.P. de, Soares D, Mendes E, Machado L, 2014. Plantar pressures and ground reaction forces during walking of individuals with unilateral transfemoral amputation. PM and R 6, 698–707.e1. 10.1016/j.pmrj.2014.01.019 [DOI] [PubMed] [Google Scholar]
- Chui K, Hood E, Klima D, 2012. Meaningful change in walking speed. Top Geriatr Rehabil 28, 97–103. 10.1097/TGR.0B013E3182510195 [DOI] [Google Scholar]
- Correa TA, Crossley KM, Kim HJ, Pandy MG, 2010. Contributions of individual muscles to hip joint contact force in normal walking. J Biomech 43, 1618–1622. 10.1016/J.JBIOMECH.2010.02.008 [DOI] [PubMed] [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG, 2007. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng 54, 1940–1950. 10.1109/TBME.2007.901024 [DOI] [PubMed] [Google Scholar]
- Dillingham TR, Pezzin LE, Mackenzie EJ, Burgess AR, 2001. Use and Satisfaction with Prosthetic Devices Among Persons with Trauma-Related Amputations A Long-Term Outcome Study. Am J Phys Med Rehabil 80, 563–571. [DOI] [PubMed] [Google Scholar]
- Felson DT, 2013. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage 21, 10–15. 10.1016/J.JOCA.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney BMM, Vandenberg NW, Davis-Wilson HC, Christiansen CL, Roda GF, Schneider G, Johnson T, Stoneback JW, 2022. Biomechanical compensations during a stand-to-sit maneuver using transfemoral osseointegrated prostheses: A case series. Clinical Biomechanics 98, 105715. 10.1016/j.clinbiomech.2022.105715 [DOI] [PubMed] [Google Scholar]
- Gailey R, Allen K, Castles J, Kucharik J, Roeder M, 2008. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J Rehabil Res Dev 45, 15–30. 10.1682/JRRD.2006.11.0147 [DOI] [PubMed] [Google Scholar]
- Goujon-Pillet H, Sapin E, Fodé P, Lavaste F, 2008. Three-Dimensional Motions of Trunk and Pelvis During Transfemoral Amputee Gait. Arch Phys Med Rehabil 89, 87–94. 10.1016/J.APMR.2007.08.136 [DOI] [PubMed] [Google Scholar]
- Gronley JK, Perry J, 1984. Gait analysis techniques. Rancho Los Amigos Hospital gait laboratory. Phys Ther 64, 1831–1838. 10.1093/PTJ/64.12.1831 [DOI] [PubMed] [Google Scholar]
- Hagberg K, Brånemark R, 2009. One hundred patients treated with osseointegrated transfemoral amputation prostheses-Rehabilitation perspective. J Rehabil Res Dev 46, 331–344. 10.1682/JRRD.2008.06.0080 [DOI] [PubMed] [Google Scholar]
- Hagberg K, Häggström E, Jönsson S, Rydevik B, Brånemark R, 2008. Osseoperception and Osseointegrated Prosthetic Limbs, Psychpoprosthetics. Springer, London. [Google Scholar]
- Harandi VJ, Ackland DC, Haddara R, Cofré Lizama LE, Graf M, Galea MP, Lee PVS, 2020a. Individual muscle contributions to hip joint-contact forces during walking in unilateral transfemoral amputees with osseointegrated prostheses. Comput Methods Biomech Biomed Engin 23, 1071–1081. 10.1080/10255842.2020.1786686 [DOI] [PubMed] [Google Scholar]
- Harandi VJ, Ackland DC, Haddara R, Lizama LEC, Graf M, Galea MP, Lee PVS, 2020b. Gait compensatory mechanisms in unilateral transfemoral amputees. Med Eng Phys 77, 95–106. 10.1016/j.medengphy.2019.11.006 [DOI] [PubMed] [Google Scholar]
- Harris MD, MacWilliams BA, Bo Foreman K, Peters CL, Weiss JA, Anderson AE, 2017. Higher medially-directed joint reaction forces are a characteristic of dysplastic hips: A comparative study using subject-specific musculoskeletal models. J Biomech 54, 80–87. 10.1016/J.JBIOMECH.2017.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann DWW, Leboucher J, Block J, Günther M, Putz C, Götze M, Wolf SI, Alimusaj M, 2020. The influence of hip muscle strength on gait in individuals with a unilateral transfemoral amputation. PLoS One 15, 1–16. 10.1371/journal.pone.0238093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson DP, Edgar C, Ding Z, Sivapuratharasu B, Le Feuvre P, Finnegan ME, Quest R, McGregor AH, Bull AMJ, 2021. Understanding lower limb muscle volume adaptations to amputation. J Biomech 125. 10.1016/j.jbiomech.2021.110599 [DOI] [PubMed] [Google Scholar]
- Hicks JL, Uchida TK, Seth A, Rajagopal A, Delp SL, 2015. Is My Model Good Enough? Best Practices for Verification and Validation of Musculoskeletal Models and Simulations of Movement. J Biomech Eng 137, 1–24. 10.1115/1.4029304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith MJ, Goff LM, Lewandowski AL, Farrokhi S, Hendershot BD, Hill OT, Rábago CA, Russell-Esposito E, Orriola JJ, Mayer JM, 2019. Low back pain in persons with lower extremity amputation: a systematic review of the literature. Spine Journal. 10.1016/j.spinee.2018.08.011 [DOI] [PubMed] [Google Scholar]
- Hreljac A, Marshall RN, 2000. Algorithms to determine event timing during normal walking using kinematic data. J Biomech 33, 783–786. 10.1016/S0021-9290(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Hurley MV, 1999. THE ROLE OF MUSCLE WEAKNESS IN THE PATHOGENESIS OF OSTEOARTHRITIS. Rheumatic Disease Clinics of North America 25, 283–298. 10.1016/S0889-857X(05)70068-5 [DOI] [PubMed] [Google Scholar]
- Jaegers SMHJ, Arendzen JH, De Jongh HJ, 1995. Prosthetic Gait of Unilateral Transfemoral Amputees: A Kinematic Study. Arch Phys Med Rehabil 76, 736–743. [DOI] [PubMed] [Google Scholar]
- Jones RF, Fey NP, 2021. Femur Abduction Associated with Transfemoral Amputation Alters the Profile of Lumbopelvic Mechanical Loads During Generalized End-Limb Loading; Femur Abduction Associated with Transfemoral Amputation Alters the Profile of Lumbopelvic Mechanical Loads During Generalized End-Limb Loading. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). 10.1109/EMBC46164.2021.9630149 [DOI] [PubMed] [Google Scholar]
- Koehler-McNicholas SR, Lipschutz RD, Gard SA, 2016. The biomechanical response of persons with transfemoral amputation to variations in prosthetic knee alignment during level walking. J Rehabil Res Dev 53, 1089–1106. 10.1682/JRRD.2014.12.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AKM, Arnold AS, Wakeling JM, 2017. Why are Antagonist Muscles Co-activated in My Simulation? A Musculoskeletal Model for Analysing Human Locomotor Tasks. Ann Biomed Eng 45, 2762–2774. 10.1007/s10439-017-1920-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPrè AK, Price MA, Wedge RD, Umberger BR, Sup FC, 2018. Approach for gait analysis in persons with limb loss including residuum and prosthesis socket dynamics. Int J Numer Method Biomed Eng 34, 1–11. 10.1002/cnm.2936 [DOI] [PubMed] [Google Scholar]
- Layton R, Messenger N, Stewart T, 2022. Characteristics of hip joint reaction forces during a range of activities. Med Eng Phys 108. [DOI] [PubMed] [Google Scholar]
- Leijendekkers RA, van Hinte G, Nijhuis-van der Sanden MW, Staal JB, 2017. Gait rehabilitation for a patient with an osseointegrated prosthesis following transfemoral amputation. Physiother Theory Pract 33, 147–161. 10.1080/09593985.2016.1265620 [DOI] [PubMed] [Google Scholar]
- Lerner ZF, Haight DJ, Demers MS, Board WJ, Browning RC, 2014. The Effects of Walking Speed on Tibiofemoral Loading Estimated Via Musculoskeletal Modeling HHS Public Access. J Appl Biomech 30, 197–205. 10.1123/jab.2012-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak AF, Zhang M, Boone DA, 2001. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: A review. J Rehabil Res Dev 38, 161–174. [PubMed] [Google Scholar]
- Marasovic T, Cecic M, Zanchi V, 2009. Analysis and Interpretation of Ground Reaction Forces in Normal Gait. WSEAS Transactions on Systems 8, 1105–1114. [Google Scholar]
- Michaud SB, Gard SA, Childress DS, 2000. A preliminary investigation of pelvic obliquity patterns during gait in persons with transtibial and transfemoral amputation. J Rehabil Res Dev 37, 1–10. [PubMed] [Google Scholar]
- Morgenroth DC, Gellhorn AC, Suri P, 2012. Osteoarthritis in the Disabled Population: A Mechanical Perspective. PM&R 4, S20–S27. 10.1016/J.PMRJ.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Netter, 1951. Netter Atlas of Human Anatomy English, Gastroenterology.
- Nolan L, Lees A, 2000. The functional demands on the intact limb during walking for active trans-femoral and trans-tibial amputees. Prosthet Orthot Int 24, 117–125. 10.1080/03093640008726534 [DOI] [PubMed] [Google Scholar]
- Nolan L, Wit A, Dudziñski K, Lees A, Lake M, Wychowañski M, 2003. Adjustments in gait symmetry with walking speed in trans-femoral and trans-tibial amputees. Gait Posture 17, 142–151. 10.1016/S0966-6362(02)00066-8 [DOI] [PubMed] [Google Scholar]
- Örgel M, Elareibi M, Graulich T, Krettek Christian, Neunaber C, Aschoff H-H, Ranker Alexander, Winkelmann Marcel, 2021. Osseoperception in transcutaneous osseointegrated prosthetic systems (TOPS) after transfemoral amputation: a prospective study. Arch Orthop Trauma Surg. 10.1007/s00402-021-04099-1 [DOI] [PubMed] [Google Scholar]
- Pataky TC, 2012. One-dimensional statistical parametric mapping in Python. Comput Methods Biomech Biomed Engin 15, 295–301. 10.1080/10255842.2010.527837 [DOI] [PubMed] [Google Scholar]
- Pataky TC, Robinson MA, Vanrenterghem J, 2013. Vector field statistical analysis of kinematic and force trajectories. J Biomech 46, 2394–2401. 10.1016/J.JBIOMECH.2013.07.031 [DOI] [PubMed] [Google Scholar]
- Pataky TC, Vanrenterghem J, Robinson MA, 2015. Zero- vs. one-dimensional, parametric vs. non-parametric, and confidence interval vs. hypothesis testing procedures in one-dimensional biomechanical trajectory analysis. J Biomech 48, 1277–1285. 10.1016/J.JBIOMECH.2015.02.051 [DOI] [PubMed] [Google Scholar]
- Penn-Barwell JG, 2011. Outcomes in lower limb amputation following trauma: A systematic review and meta-analysis. Injury 42, 1474–1479. 10.1016/J.INJURY.2011.07.005 [DOI] [PubMed] [Google Scholar]
- Perera S, Mody SH, Woodman RC, Studenski SA, 2006. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 54, 743–749. 10.1111/J.1532-5415.2006.00701.X [DOI] [PubMed] [Google Scholar]
- Perry J, 1992. Gait Analysis: Normal and Pathological Function, Handbook of Clinical Neurology. 10.1016/B978-0-444-63916-5.00007-0 [DOI] [Google Scholar]
- Queen R, Dickerson L, Ranganathan S, Schmitt D, 2020. A novel method for measuring asymmetry in kinematic and kinetic variables: The normalized symmetry index. J Biomech 99, 109531. 10.1016/j.jbiomech.2019.109531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranz EC, Wilken JM, Gajewski DA, Neptune RR, 2017. The influence of limb alignment and transfemoral amputation technique on muscle capacity during gait. Comput Methods Biomech Biomed Engin 20, 1167–1174. 10.1080/10255842.2017.1340461 [DOI] [PubMed] [Google Scholar]
- Reddy NP, Cochran GVB, Krouskop TA, 1981. Interstitial fluid flow as a factor in decubitus ulcer formation. J Biomech 14, 879–881. 10.1016/0021-9290(81)90015-4 [DOI] [PubMed] [Google Scholar]
- Ren L, Jones RK, Howard D, 2008. Whole body inverse dynamics over a complete gait cycle based only on measured kinematics. J Biomech 41, 2750–2759. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Safai L, Harandi VJ, Graf M, Lizama LEC, Lee PVS, Galea MP, Khan F, Tse KM, Ackland DC, 2020. Load response of an osseointegrated implant used in the treatment of unilateral transfemoral amputation: An early implant loosening case study. Clinical Biomechanics 73, 201–212. 10.1016/j.clinbiomech.2020.01.017 [DOI] [PubMed] [Google Scholar]
- Savage TN, Saxby DJ, Pizzolato C, Diamond LE, Murphy NJ, Hall M, Spiers L, Eyles J, Killen BA, Suwarganda EK, Dickenson EJ, Griffin D, Fary C, O’Donnell J, Molnar R, Randhawa S, Reichenbach S, Tran P, Wrigley TV, Bennell KL, Hunter DJ, Lloyd DG, 2021. Trunk, pelvis and lower limb walking biomechanics are similarly altered in those with femoroacetabular impingement syndrome regardless of cam morphology size. Gait Posture 83, 26–34. 10.1016/J.GAITPOST.2020.10.002 [DOI] [PubMed] [Google Scholar]
- Schaarschmidt M, Lipfert SW, Meier-Gratz C, Scholle HC, Seyfarth A, 2012. Functional gait asymmetry of unilateral transfemoral amputees. Hum Mov Sci 31, 907–917. 10.1016/J.HUMOV.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Schache AG, Baker R, 2007. On the expression of joint moments during gait. Gait Posture 25, 440–452. 10.1016/J.GAITPOST.2006.05.018 [DOI] [PubMed] [Google Scholar]
- Schwarze M, Hurschler C, Seehaus F, Oehler S, Welke B, 2013. Loads on the prosthesis-socket interface of above-knee amputees during normal gait: Validation of a multi-body simulation. J Biomech 46, 1201–1206. 10.1016/j.jbiomech.2013.02.005 [DOI] [PubMed] [Google Scholar]
- Scovil CY, Ronsky JL, 2006. Sensitivity of a Hill-based muscle model to perturbations in model parameters. J Biomech 39, 2055–2063. 10.1016/J.JBIOMECH.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Seroussi RE, Gitter A, Czerniecki JM, Weaver K, 1996. Mechanical Work Adaptations of Above-Knee Amputee Ambulation. Arch Phys Med Rehabil 77, 1209–1214. [DOI] [PubMed] [Google Scholar]
- Shakoor N, Furmanov S, Nelson DE, Li Y, Block JA, 2008. Pain and its relationship with muscle strength and proprioception in knee OA: Results of an 8-week home exercise pilot study. Journal of Musculoskeletal Neuronal Interactions 8, 35–42. [PubMed] [Google Scholar]
- Shepherd MC, Gaffney BMM, Song K, Clohisy JC, Nepple JJ, Harris MD, 2022. Femoral version deformities alter joint reaction forces in dysplastic hips during gait. J Biomech 135, 111023. 10.1016/J.JBIOMECH.2022.111023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AK, Neptune RR, 2014. Three-dimensional knee joint contact forces during walking in unilateral transtibial amputees. J Biomech 47, 2556–2562. 10.1016/j.jbiomech.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Song K, Anderson AE, Weiss JA, Harris MD, 2019. Musculoskeletal models with generic and subject-specific geometry estimate different joint biomechanics in dysplastic hips. Comput Methods Biomech Biomed Engin 22, 259–270. 10.1080/10255842.2018.1550577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Gaffney BMM, Shelburne KB, Pascual-Garrido C, Clohisy JC, Harris MD, 2020. Dysplastic hip anatomy alters muscle moment arm lengths, lines of action, and contributions to joint reaction forces during gait. J Biomech 110. 10.1016/J.JBIOMECH.2020.109968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope SJ, Kepple TM, McGuire DA, Roman NL, 1990. Kinematic-based technique for event time determination during gait. Med Biol Eng Comput 355–360. [DOI] [PubMed] [Google Scholar]
- Steele KM, DeMers MS, Schwartz MH, Delp SL, 2012. Compressive tibiofemoral force during crouch gait. Gait Posture 35, 556–560. 10.1016/J.GAITPOST.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf PA, van Heugten CM, Hitters MW, Smeets RJ, 2009. The Prevalence of Osteoarthritis of the Intact Hip and Knee Among Traumatic Leg Amputees. Arch Phys Med Rehabil 90, 440–446. 10.1016/J.APMR.2008.08.220 [DOI] [PubMed] [Google Scholar]
- Sueki DG, Cleland JA, Wainner RS, 2013. A regional interdependence model of musculoskeletal dysfunction: research, mechanisms, and clinical implications. Journal of Manual & Manipulative Therapy 21, 90–102. 10.1179/2042618612Y.0000000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S, Lu W, Zreiqat H, Li JJ, Tetsworth K, al Muderis M, 2019. Proximal Bone Remodeling in Lower Limb Amputees Reconstructed With an Osseointegrated Prosthesis. J Orthop Res 37, 2524–2530. 10.1002/jor.24445 [DOI] [PubMed] [Google Scholar]
- Tranberg R, Zügner R, Kärrholm J, 2011. Improvements in hip- and pelvic motion for patients with osseointegrated trans-femoral prostheses. Gait Posture 33, 165–168. 10.1016/J.GAITPOST.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Valente G, Taddei F, Jonkers I, 2013. Influence of weak hip abductor muscles on joint contact forces during normal walking: probabilistic modeling analysis. J Biomech 46, 2186–2193. 10.1016/J.JBIOMECH.2013.06.030 [DOI] [PubMed] [Google Scholar]
- Van De Meent H, Hopman MT, Frölke JP, 2013. Walking ability and quality of life in subjects with transfemoral amputation: A comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil 94, 2174–2178. 10.1016/J.APMR.2013.05.020 [DOI] [PubMed] [Google Scholar]
- Wainner RS, Whitman JM, Cleland JA, Flynn TW, 2007. Regional interdependence: A musculoskeletal examination model whose time has come. Journal of Orthopaedic and Sports Physical Therapy 37, 658–660. 10.2519/JOSPT.2007.0110 [DOI] [PubMed] [Google Scholar]
- Winter DA, 2009. Biomechanics and Motor Control of Human Movement, Fourth Edition, John Wiley & Sons, Inc. 10.29057/xikua.v1i1.1175 [DOI] [Google Scholar]
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R, 2008. Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 89, 422–429. 10.1016/J.APMR.2007.11.005 [DOI] [PubMed] [Google Scholar]


