Abstract
Background:
Radon may have a role in obstructive lung disease outside its known carcinogenicity. Little is known about radon’s effects on asthma morbidity.
Objective:
To determine the effect of radon on fractional exhaled nitric oxide (FENO), asthma symptom-days, and lung function in inner-city asthmatic school-children.
Methods:
Two hundred ninety-nine school-aged asthmatic children enrolled in the School Inner-City Asthma Study (SICAS-1) were followed. One and two-month averaged radon was assessed using a spatiotemporal model predicting zip code-specific monthly exposures. FENO and spirometry were measured twice during the academic year. Asthma symptoms were assessed four times during the academic year. The interaction between indoor radon exposure (Bq/m3) and seasonality predicting log-transformed FENO, FEV1% predicted, FVC% predicted, FEV1/FVC, and asthma symptom-days was evaluated.
Results:
Participants with high radon exposure had greater change in FENO from warm to cold periods compared to low radon exposure (interaction p=0.0013). Participants with >50th percentile radon exposure experience significant FENO increase from warm to cold weather (β=0.29 [95% CI: 0.04,0.54], p=0.0240). We report a positive association between radon 1-month moving average (IRR=1.01, p=0.0273) and 2-month moving average (IRR=1.01, p=0.0286) with maximum asthma symptom-days (n=299, obs=1,167).
Conclusions:
In asthmatic children, radon may be associated with increased asthma morbidity, suggesting radon may be a modifiable environmental risk factor for airway inflammation.
Keywords: Asthma, radon, environmental exposure, exhaled nitric oxide, obstructive lung disease
INTRODUCTION
Asthma is the most common non-communicable chronic disease in children worldwide,1 affecting approximately 14% of children globally2 and one of 11 (7.1 million) children in the United States.3 Environmental factors, such as allergen and air pollution exposure, are known to cause morbidity, disability, and healthcare utilization in children with asthma. Identifying potential environmental exposures and testing remediation strategies that improve asthma morbidity in children remains an unmet need.
Radon, an ubiquitous radioactive gas formed by the decay of uranium radionuclides naturally present in rocks in the earth’s crust,4 is well-known for its carcinogenic effects but more recent data suggests it is associated with chronic obstructive pulmonary disease (COPD) non-cancer morbidity and mortality, as well.5, 6 In buildings with inadequate ventilation, radon can accumulate to harmful concentrations indoors. Driven by pressure differences, radon gas traverses from the soil into homes through porous block walls, cracks, joints, or utility openings. The radioactive products of radon are known to bind fine particles, gain entry to the respiratory tract, and can deposit on the bronchial epithelium, exposing neighboring lung cells to radiation.7 Radon and radon progeny, the radioactive decay products of radon, yield oxygen free radicals and hydrogen peroxide in airway samples at doses within the range of acceptable home radon levels.
We recently found that residential radon concentrations were associated with asthma diagnosis and school absenteeism in an unselected cohort of urban school-aged children screened for enrollment in the School Inner City Asthma Study (SICAS).8 There are no studies to date examining the effects of radon exposure on children with asthma. Based on recent data demonstrating radon exposure is associated with non-cancer related COPD morbidity and mortality,5, 6 we hypothesized that residential radon exposure would be associated with asthma morbidity and increased airway inflammation. We tested this hypothesis in the well-characterized asthma population enrolled in the School Inner-City Asthma Study by determining the effect of environmental radon on airway inflammation, measured by fractional exhaled nitric oxide (FENO), asthma symptoms, and lung function.
METHODS
Study population and Design
SICAS was a prospective cohort study of the effect of school classroom environmental exposures on asthma morbidity conducted in children (ages 4–15 years) with persistent asthma attending inner-city schools in the northeast United States from 2008–2013. Full details of the study design and selection of the SICAS-1 study cohort have been described elsewhere.9 Briefly, children with asthma attending participating schools were included if they had children with physician diagnosis of asthma with either symptoms of exacerbation in the last year or controller medication (Figure 1). In the Spring prior to the study year, screening surveys were distributed to participating schools. Asthma symptoms were assessed by phone survey every 3 months through the following academic year, and spirometry and FENO measurements were obtained in the school twice during the academic year and linked to the Fall/Spring environmental sampling. Prior to enrollment, written informed consent was provided from the participant’s guardian, and assent was obtained from the participant. The SICAS study was approved by the Boston Children’s Hospital institutional review board.
Figure 1.
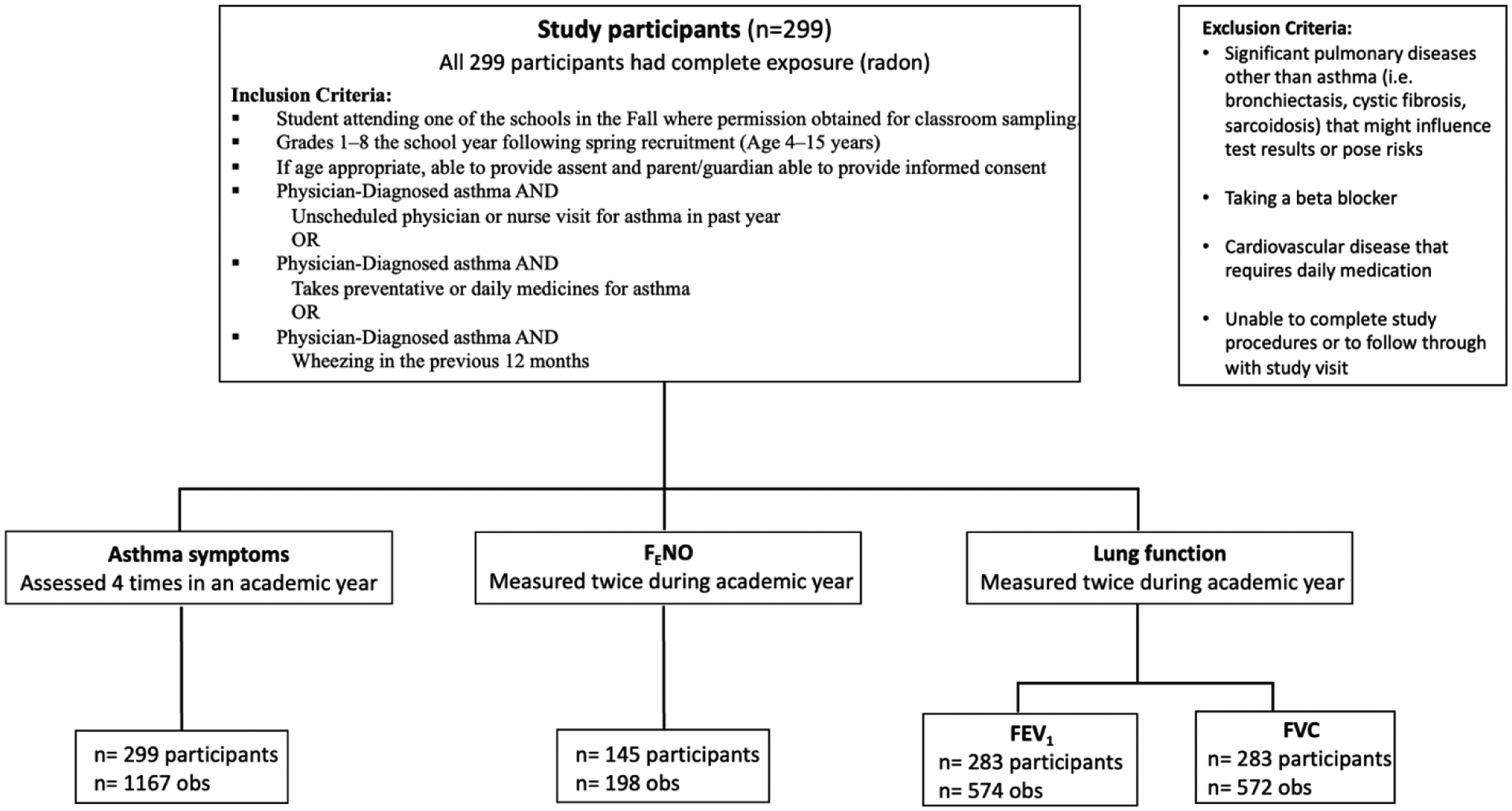
Inclusion and Exclusion Criteria with Number of participants and observations for variables (Exposure and Outcomes)
Flowchart shows the inclusion and exclusion criteria for the study and the number of study participants and observations, highlighting that all study participants (n=299) had complete exposure (radon). The outcomes with number of observations and study participants are also shown.
Study procedures
Baseline study participant characteristics, which assessed medical history, sociodemographic information, and baseline symptoms, were obtained at a research clinic visit during the summer prior to the academic year by questionnaire. Aeroallergen sensitization testing was assessed by serum specific IgE (ImmunoCAP, PhadiaAB, Uppsala, Sweden) or allergy skin testing (MultiTest device, Lincoln Diagnostics, Decatur, IL) to the following aeroallergens: cat, dog, mouse pelt, rat pelt, house dust mite (Dermatophagoides pteronyssinus and Dermatophagoides farinae), German cockroach (Blatella germanica), Alternaria tenius, Aspergillus, Penicillium, Cladosporium, ragweed mix, oak tree pollen, and timothy grass pollen (Greer, Lenoir, North Carolina). Sensitization was defined by specific-IgE level of ≥0.35 kU/L or a wheal size 3 mm or larger than the negative saline control on skin prick testing. “Any sensitization” was a term to specify participants with ≥1 sensitization to an allergen.
FENO assessment was not part of the initial parent study design and only collected in years 4 and 5 of the study. FENO was measured by the Niox Mino device (Aerocrine, Solna, Sweden). Spirometry (Koko spirometer, nSpire Health, Inc., Longmont, CO, USA) was performed according to ATS guidelines.10
Exposure assessment
Residential radon exposure was assessed using a spatiotemporal model predicting monthly radon levels (Bq/m3) for each ZIP Code Tabulation Area (ZCTA) based on home zip code where children in the SICAS study lived.7 Radon exposure data was contemporaneous with other study measurements. Full details of the model design have been described elsewhere.7 In brief, the prediction model was based on 363,783 field measurements in the Greater Boston area and used a two-stage ensemble-based machine learning method to estimate monthly ZIP Code-level average basement radon concentrations based on meteorological, geological, architectural, and socioeconomic factors. The temporal resolution of the model is monthly and the spatial resolution of the model was by zip code.7 Monthly PM2.5, NO2, and O3 measurements from contemporaneously collected samples from the local EPA central monitoring site were utilized in multipollutant models.
Outcome measures
FENO measurement (ppb) was performed per standardized methodology according to ATS guidelines.11 Asthma symptom outcomes were measured as maximum symptom-days, a validated asthma epidemiologic outcome12 which has been used in previous urban home- and school-based studies.13–16 It is defined by the greatest result of (1) number of days with wheezing, cough, or chest tightness, (2) number of days on which the child had to discontinue play activities or slow down due to wheezing, cough, or chest tightness, or (3) number of nights with wheezing, cough, or chest tightness leading to disturbed sleep in the 2 weeks prior to survey. The primary spirometry outcome was the ratio of forced expiratory volume in 1 second (FEV1) per forced vital capacity ratio (FEV1/FVC), which is the most sensitive marker of airflow obstruction in children with asthma.17, 18 Forced expiratory volume in one second (FEV1) percent predicted and forced vital capacity (FVC) percent predicted were calculated with NHANES III reference equations,19 the standard at the time of the SICAS study.
Statistical analysis
Subject characteristics at baseline were assessed with descriptive statistics. Moving averages of indoor radon exposure (Bq/m3) at the home zip code were calculated relative to the health outcome visit date as 1-month moving radon average and 2-month moving radon average. The 1-month moving average was defined as: (radon concentration during month of outcome visit + previous month)/2, and the radon 2-month moving average was defined as (radon concentration during month of outcome visit + previous 2 months)/3. For each moving average, we performed a repeated measures linear mixed effects model to evaluate the relationship between indoor radon exposure (Bq/m3) and log-transformed (to approximate normal distribution) FENO, FEV1/FVC, FEV1 percent predicted, and FVC percent predicted. For each model, we tested effect modification of seasonal period (defined as Fall/Winter for September-February and Spring/Summer for March-August) and “any sensitization,” and kept the interaction term in the model if it was significant (p<0.05). Evaluation of opposite seasons (fall/winter vs spring/summer), linked to the enrolled child, allowed assessment of seasonal epidemiology of asthma exacerbations which tend to follow the viral infection prevalence in the community. In all statistical models we adjusted for age, BMI, sex, ICS controller medication use, any sensitization, race, and household income <$25,000. A repeated measures generalized linear mixed model with a Quasi-Poisson distribution to account for overdispersion in count data was used to examine the association between residential radon exposure and asthma symptom-days. In our models, we specify a random effect for subject to account for repeated outcome measures. Statistical computations were performed using SAS software, version 9.4 (SAS Institutes). All tests were 2-tailed, and findings considered significant for p <0.05.
We conducted several sensitivity analyses to assess potential confounding of ambient PM2.5, NO2 and O3 on radon’s effect. We tested the association between each pollutant and health outcome separately, adjusting for all covariates included in the original models. Pollutants significantly associated with each outcome were included with radon in a final adjusted model. We tested the correlation between radon and pollutants to assess potential for multicollinearity in adjusted models.
RESULTS
The analysis included 299 elementary school-aged children with asthma enrolled in the School Inner-City Asthma Study (SICAS-1). Participants were largely Black or Hispanic and had fairly high poverty levels, with 43% reporting household income <$25,000 (Table 1). Of our cohort, 70% reported allergic sensitization, and 30% were on ICS controller medication. Mean FENO was 21 ppb, mean FEV1 % predicted 101%, FVC % predicted 100%, mean FEV1/FVC ratio 0.9, and mean max asthma symptom-days 2.9. One hundred forty-five participants had complete data for radon exposure and FENO measurements for analysis (198 observations) due to later addition of FENO to study assessments.
Table 1.
Study participant characteristics at baseline (n=299)
| Variable | Description | N=299 |
|---|---|---|
| Age | Mean (SD) | 8.5 (1.8) |
| Female, n (%) | 142 (47.5%) | |
| Race or ethnic group, n (%) | Black | 111 (37.1%) |
| Mixed | 52 (17.4%) | |
| Other | 124 (41.5%) | |
| White | 12 (4.0%) | |
| Hispanic, n (%) | 104 (34.8%) | |
| Household Income <$25,000, n (%) | No | 126 (42.1%) |
| Yes | 128 (42.8%) | |
| Unknown | 45 (15.1%) | |
| BMI Percentile | Mean (SD) | 74.6 (27.1) |
| BMI Category, n (%) | Underweight | 4 (1.3%) |
| Normal | 150 (50.2%) | |
| Overweight | 39 (13.0%) | |
| Obese | 106 (35.5%) | |
| Allergic Sensitization ≥ 1 allergen, n (%) | 214 (71.6%) | |
| ICS Controller Medication Use, n (%) | 91 (30.4%) | |
| F E NO (ppb) † | Mean (SD) | 21.0 (22.5) |
| FEV1 % predicted‡ | Mean (SD) | 101.1 (19.2) |
| FVC % predicted § | Mean (SD) | 100.0 (17.6) |
| FEV 1 /FVC ¶ | Mean (SD) | 0.9 (0.1) |
| Maximum asthma symptom-days # | Mean (SD) | 2.9 (4.1) |
FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; FENO: fractional exhaled Nitric Oxide.
Maximum asthma symptom-days = in the 2 weeks prior to each follow-up survey, the greatest result of the following three variables: 1) number of days with wheezing, cough, or chest tightness, 2) number of days on which child had to slow down or discontinue play activities due to wheezing, chest tightness, or cough, 3) number of nights with wheezing, cough, or chest tightness leading to disturbed sleep due to child’s asthma
total n included (%): 95 (31.8%),
total n included (%): 261 (87.6%),
total n included (%): 261 (87.6%),
total n included (%): 261 (87.6%),
total n included (%): 284 (99.3%)
Levels of indoor radon exposure (Bq/m3) are shown in Table 2 as a distribution of quantiles of radon percentile based on geospatial modeling at participants’ home zip code. Median radon level was 49.75 Bq/m3, with interquartile range of 33.67 to 83.50 Bq/m3.
Table 2.
Quantiles of indoor radon exposure (Bq/m3) for 1-month moving average
| Level | Quantile |
|---|---|
| 100% Max | 83.50 |
| 75% Q3 | 54.69 |
| 50% Median | 49.75 |
| 25% Q1 | 45.42 |
| 0% Min | 33.67 |
In adjusted analysis, we found a significant interaction of season in the relationship between radon and FENO (p-value for interaction <0.01) when radon was dichotomized at the 50th percentile of exposure. Higher radon was significantly associated with greater change in FENO from warm to cold seasonal periods compared to low radon exposure (n=145, obs=198, p=0.0099; Above 50th percentile radon exposure: β=0.29 [95% CI: 0.04,0.54], p=0.0240; Below 50th percentile radon exposure: β=−0.19 [95% CI: −0.43,0.06], p=0.1313) (Figure 2, Table S1 in Supplementary Material).
Figure 2. Radon is associated with seasonal change in FENO in school-age children with asthma.
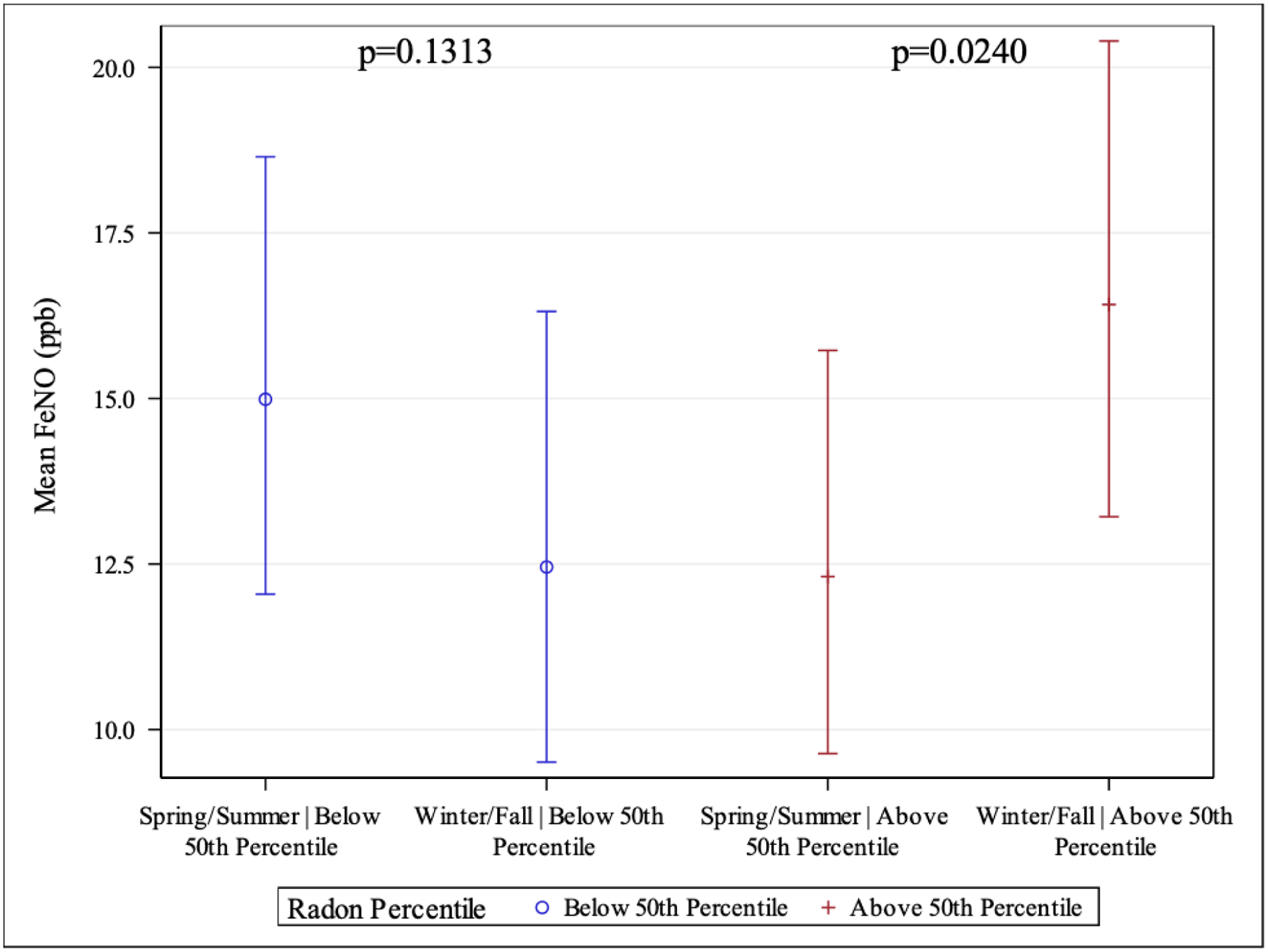
Interaction between indoor radon exposure and seasonal period predicting log-transformed FENO for 1-month moving average. Participants with high radon exposure (>50th percentile radon; red bars) had greater change in FENO from warm (Spring/Summer) to cold periods (Winter/Fall) compared to low radon exposure (<50th percentile radon; blue bars) (interaction p=0.0013). Interaction between >50th percentile radon exposure over 1-month moving average and seasonal period (n=198, p=0.0099). Above 50th percentile (β= 0.29 [95% CI: 0.04, 0.54], p=0.0240). Below 50th percentile (β= −0.19 [95% CI: −0.43, 0.06], p=0.1313).
All 299 participants had complete data for radon exposure and asthma symptom-days. In adjusted analysis, radon exposure was significantly associated with increase in maximum symptom-days, the main symptom-based outcome for both the 1-month moving average (IRR=1.014, 95% CI [1.002,1.027], p=0.0273) and 2-month moving average (IRR=1.015, 95% CI [1.002,1.028], p=0.0286) of radon exposure (n=299, obs=1,167) (Table 3).
Table 3.
Multivariate regression modeling predicting maximum asthma symptom-days by radon 1-month and 2-month moving average.
| Radon 1-month moving average |
Radon 2-month moving average |
|||
|---|---|---|---|---|
| IRR | p-value, 95% CI |
IRR | p-value, 95% CI |
|
| Maximum Asthma Symptom-Daysa | 1.014 | p=0.0273, 1.002 to 1.027 |
1.015 | p=0.0286, 1.002 to 1.028 |
Main effects of indoor radon exposure predicting maximum asthma symptom-days in school-age children with asthma show a positive association between radon 1-month moving average (IRR=1.014, 95% CI [1.002,1.027], p=0.0273) and radon 2-month moving average (IRR=1.015, 95% CI [1.002,1.028], p=0.0286) with maximum symptom-days (n=299, obs=1,167). Multivariate model: Maximum symptoms-days adjusted for Age, Race, Gender, BMI, ICS controller medication use, allergic sensitization, and household income <$25,000.
IRR = Incidence rate ratio, CI = Confidence interval
Maximum asthma symptom-days = in the 2 weeks prior to each follow-up survey, the greatest result of the following three variables: 1) number of days with wheezing, cough, or chest tightness, 2) number of days on which child had to slow down or discontinue play activities due to wheezing, chest tightness, or cough, 3) number of nights with wheezing, cough, or chest tightness leading to disturbed sleep due to child’s asthma
For maximum symptom-days and lung function outcomes, there was no effect modification by season or allergic sensitization. Radon exposure was not significantly associated with any spirometry outcomes.
We found a strong correlation between 1-month moving averages of radon, PM2.5, NO2, and O3, which was similar for 1- and 2-month moving averages (Tables S2 and S3 in Supplementary Material). In sensitivity analysis assessing confounding of the radon effect by PM2.5, NO2, and O3 on outcomes, we found that results were consistent for almost all models with the exception of O3 and maximum symptom-days in which O3 mitigated the effect of radon (Table S4 in Supplementary Material). However, the high collinearity of the exposures should be noted (Table S2 in Supplementary Material).
DISCUSSION
Radon is a modifiable environmental exposure with recent evidence suggesting a relationship to COPD and asthma. For the first time, we demonstrate a relationship between residential radon exposure and airway inflammation and asthma symptoms among school-aged children with asthma. We demonstrate a temporal association of indoor radon exposure with increase in asthma symptoms and a seasonal relationship with FENO. Participants with higher radon exposure had greater change in FENO from warm to cold periods compared to low radon exposure. These findings suggest radon exposure may be an important environmental risk factor for airway inflammation, identifying a novel, modifiable environmental risk factor for asthma morbidity in children.
This study has several strengths. Our sample size included 299 children in a well-characterized asthma population and represented a diverse group of subjects, particularly with regard to race and ethnicity. Several notable findings are highlighted by these analyses. First, levels of radon exposure in our study were all greater than the natural outdoor level of radon (14.8 Bq/m3), which is the level U.S. Congress has set as the target indoor radon level per the Indoor Radon Abatement Act of 1988,20 though lower compared to the EPA recommended action level of 148 Bq/m3 advised for indoor radon mitigation.21, 22 Despite this, we found a clear relationship between higher radon exposure with increased asthma symptom-days and higher FENO levels. Our findings suggest radon is an important contributor to asthma morbidity at levels below existing EPA guidelines, which were developed specifically based on lung cancer risk associated with radon.23 In addition, due to lower proximity to the ground, smaller lungs, and faster breathing rates in children, which may draw radon and other air pollutants deeper into children’s lungs, young children may experience even higher levels of radon exposure.23, 24
Second, we report elevated FENO with cold compared to warm seasons for participants with higher indoor radon exposure and found increased asthma symptoms associated with radon exposure. FENO measures airway inflammation, with higher levels associated with asthma exacerbations and lung function decline. In chronic inflammatory airway disease, including asthma, standard tests such as FEV1 reversibility or provocation tests are only indirectly associated with airway inflammation, with spirometry measuring airflow limitation, while FENO offers an advantage of directly reflecting the extent of airway inflammation.25 Therefore, the absence of finding an association with spirometry may be a reflection of the sensitivity of the measure rather than lack of airway disease.
It is known radon levels may fluctuate seasonally, with indoor levels typically higher during winter months,26 at a time when windows and doors are more often shut limiting natural ventilation. Additionally, use of heaters leads to warm air which can drive pressure differences in the home causing radon gas from soil in the ground to be entrained from beneath homes.27 At the same time, cold weather can cause the ground to become more compact and create pressure for radon to escape from the ground beneath homes through cracks, holes, or porous entry points in the foundation. With the combination of increased exposure levels and increased time spent indoors in cold weather, it is not surprising we found radon was associated with seasonal change in FENO, where participants with higher radon exposure (>50th percentile radon, Figure 2) had greater change in FENO from warm to cold periods compared to low radon exposure (<50th percentile radon). While the levels of FENO in our study were generally within what may be considered normal range [Mean ppb (SD) 21.0 (22.5), range 5.0–143 ppb], the highly significant difference in levels indicates a true biological and clinically relevant effect that is further supported by symptomatic relationships in asthma found in our study.
While we found a significant association of radon exposure with airway inflammation and asthma symptoms, there was lack of a significant association of radon with lung function via spirometry in our study, which may be due to several factors. Previous studies assessing spirometric measurements and bronchodilator response in children have described poor sensitivity in the pediatric population, thus highlighting challenges in pediatric asthma to measure clinically significant change in lung function.28–30 Additional studies have also found that in children with asthma, lung function tends to be normal even among those with severe asthma.31 Furthermore, asthma is assessed by multiple measures, with phenotypic research in asthma highlighting various domains of asthma presentation and treatment responses, with variable emphasis on lung function abnormalities, exacerbations, inflammatory biomarkers, and symptoms.32, 33
We did not find effect modification by allergic sensitization in the relationship between radon exposure and any of our asthma outcomes suggesting that the presence of atopy is not an important part of the mechanism by which radon exerts its health effects. This is supported by other data that has demonstrated radon to cause oxidative stress in cell culture34,35 and in vivo murine models.36 In this study, oxidative stress induction was evidenced by increased protein expression of Nrf-2 and its down-stream antioxidant proteins, which are consistent with changes in oxidative stress indices and likely to play a significant role.36 Additionally, human exposure studies examining the associations between particle radioactivity and biomarkers of oxidative stress and inflammation have demonstrated increase in biomarkers of oxidative stress and inflammation, such as increases in interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and P-selectin.37 These findings, together with our current study results, suggest radon’s respiratory effects are not mediated by conventional TH2 inflammatory mechanisms which are primarily implicated in pediatric asthma. This study utilized the clinically relevant measures of atopy. Measures of non-TH2 biomarkers was beyond the scope of this analysis but will be important to evaluate in future analyses.
There are a few limitations that should be noted. We were limited in our ability to assess the effect of co-pollutants as the parent study did not collect in-home dust or personal pollution samples. Our residential radon models are based on zipcode-based geospatial models. We performed sensitivity analyses to assess potential confounding by ambient PM2.5, NO2, and O3 and found largely consistent relationships between radon and our health outcomes, even despite a high degree of collinearity of the exposures. We recognize that direct measurement of radioactivity inside the classrooms using scintillation, ionization chamber or solid-state detection methods would offer more specific personalized measures of exposure. However, these measurements were not included in the SICAS protocol. Nevertheless, ZCTA offers validated spatiotemporal estimates for residential radon. Future studies should include directly measured radon, and co-pollutants to fully understand how these personal exposures affect respiratory health. As with any epidemiologic exposure study, the effect of unmeasured confounders is a risk.
We acknowledge we did not have complete data for the FENO outcome due to its late addition to the study protocol. However, these limited data do not suggest bias, as it would be considered missing completely at random due to study design rather than related to participant characteristics; the associations that we were able to find despite limited number of observations is indicative of the strength of the findings. Finally, we used modeled radon exposure in the absence of directly measured exposure; however, the model is derived from measured in-home values and has been validated.7 Future studies directly measuring radon may serve to complement our findings and further illuminate radon’s effects on asthma.
CONCLUSIONS
Residential radon has not previously been described as an environmental risk factor for asthma morbidity.5 Our work demonstrates radon is associated with increased asthma symptoms and elevated FENO in inner-city children with asthma, suggesting radon exposure may be an important novel and modifiable environmental risk factor for asthma morbidity in children. While the levels of FENO in our study were generally within what may be considered normal range [Mean ppb (SD) 21.0 (22.5), range 5.0–143 ppb], the highly significant difference in levels indicates a true biological and clinically relevant effect that is further supported by symptomatic relationships in asthma found in our study.
Supplementary Material
Acknowledgments
We thank the following companies for their generous donations. Lincoln Diagnostics, Inc., Decatur, IL, USA, Multi-Test II devices; Greer, Inc, Lenoir, NC allergenic extracts for skin testing. Thermo Fisher, Inc. ImmunoCAP® testing. Monaghan Medical, Inc aerochambers, and Aeorcrine, Inc., NiOx Machines.
We also thank the Community Schools, the participants, and their families for the support they provided for the study.
Funding:
NIH T32 AI 007512
NIH U01 AI 152033
NIH K24 AI 106822
NIH R01 ES030100
NIH K23 ES031663
NIH U01 AI 160087
Summary:
Dr. Banzon is supported by T32 AI 007512 from the National Institutes of Health.
Dr. Gaffin is supported by R01 ES030100 from the National Institutes of Health. Dr. Hauptman is supported by K23 ES031663 from the National Institutes of Health. Dr. Phipatanakul is supported by the grants U01 AI 152033, U01 AI 160087, and K24 AI 106822 from the National Institutes of Health and Allergy Asthma Awareness Initiative, Inc. Dr. Phipatanakul reports consultancy fees from Genentech, Novartis, Regeneron, Sanofi, GSK, and Astra Zeneca for therapeutics related to asthma, outside the submitted work.
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
This publication was made possible by U.S. EPA grant RD-835872. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. EPA. Further, U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Abbreviations:
- Bq/m3
Becquerels per cubic meter
- BMI
body mass index
- FENO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- FEV1/FVC
percent of forced expiratory volume in 1 second over forced vital capacity
- ICS
inhaled corticosteroid
- IRR
incidence rate ratio
- NO2
nitrogen dioxide
- O3
ozone
- PFT
pulmonary function test
- PM2.5
particulate matter ≤2.5 μm in diameter
- SICAS
School Inner-City Asthma Study
- ZCTA
ZIP Code Tabulation Area
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest:
The authors have no conflict of interest relevant to this article to disclose.
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18(11):1269–78. [DOI] [PubMed] [Google Scholar]
- 2.Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol. 2014;49(5):430–4. [DOI] [PubMed] [Google Scholar]
- 3.Asthma Facts—CDC’s National Asthma Control Program Grantees. Atlanta, GA: Centers for Disease Control and Prevention, Services USDoHaH; 2013. [Google Scholar]
- 4.Nyhan MM, Rice M, Blomberg A, Coull BA, Garshick E, Vokonas P, et al. Associations between ambient particle radioactivity and lung function. Environ Int. 2019;130:104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner MC, Krewski D, Chen Y, Pope CA 3rd, Gapstur SM, Thun MJ. Radon and nonrespiratory mortality in the American Cancer Society cohort. Am J Epidemiol. 2012;176(9):808–14. [DOI] [PubMed] [Google Scholar]
- 6.Vieira CLZ, Koutrakis P, Huang S, Grady S, Hart JE, Coull BA, et al. Short-term effects of particle gamma radiation activities on pulmonary function in COPD patients. Environ Res. 2019;175:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Blomberg AJ, Stern RA, Kang CM, Papatheodorou S, Wei Y, et al. Predicting Monthly Community-Level Domestic Radon Concentrations in the Greater Boston Area with an Ensemble Learning Model. Environ Sci Technol. 2021;55(10):7157–66. [DOI] [PubMed] [Google Scholar]
- 8.Mukharesh L, Greco KF, Banzon T, Koutrakis P, Li L, Hauptman M, et al. Environmental radon and childhood asthma. Pediatr Pulmonol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phipatanakul W, Bailey A, Hoffman EB, Sheehan WJ, Lane JP, Baxi S, et al. The school inner-city asthma study: design, methods, and lessons learned. J Asthma. 2011;48(10):1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 11.Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med1999. p. 2104–17. [DOI] [PubMed] [Google Scholar]
- 12.Wu TD, Perzanowski M, Peng RD, Wise RA, Balcer-Whaley S, Newman M, et al. Validation of the maximum symptom day among children with asthma. J Allergy Clin Immunol. 2019;143(2):803–5.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai PS, Sheehan WJ, Gaffin JM, Petty CR, Coull BA, Gold DR, et al. School Endotoxin Exposure and Asthma Morbidity in Inner-city Children. Chest. 2015;148(5):1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheehan WJ, Permaul P, Petty CR, Coull BA, Baxi SN, Gaffin JM, et al. Association Between Allergen Exposure in Inner-City Schools and Asthma Morbidity Among Students. JAMA Pediatr. 2017;171(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–80. [DOI] [PubMed] [Google Scholar]
- 17.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170(4):426–32. [DOI] [PubMed] [Google Scholar]
- 18.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol. 2006;118(5):1040–7. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. [DOI] [PubMed] [Google Scholar]
- 20.EPA. Radon: A Citizen’s Guide to Radon: US Environmental Protection Agency; 2016. [Available from: https://www.epa.gov/sites/default/files/2016-12/documents/2016_a_citizens_guide_to_radon.pdf.
- 21.George AC. The history, development and the present status of the radon measurement programme in the United States of America. Radiat Prot Dosimetry. 2015;167(1–3):8–14. [DOI] [PubMed] [Google Scholar]
- 22.EPA. U.S. Environmental Protection Agency What is EPA’s Action Level for Radon and What Does it Mean? 2022. [Available from: https://www.epa.gov/radon/what-epas-action-level-radon-and-what-does-it-mean.
- 23.Toxicological Profile for Radon Atlanta, GA, USA: Agency for Toxic Substances and Disease Registry; 2012. [Available from: https://www.atsdr.cdc.gov/toxprofiles/tp145.pdf. [PubMed] [Google Scholar]
- 24.Bateson TF, Schwartz J. Children’s response to air pollutants. J Toxicol Environ Health A. 2008;71(3):238–43. [DOI] [PubMed] [Google Scholar]
- 25.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellenbenz KR, Shakya KM. Spatial and temporal variations in indoor radon concentrations in Pennsylvania, USA from 1988 to 2018. J Environ Radioact. 2021;233:106594. [DOI] [PubMed] [Google Scholar]
- 27.(ATSDR) Agency for Toxic Substances and Disease Registry. Case Study in Environmental Medicine: Radon Toxicity 2010. [Available from: https://www.atsdr.cdc.gov/csem/radon/where_found.html. [Google Scholar]
- 28.Galant SP, Morphew T, Amaro S, Liao O. Value of the bronchodilator response in assessing controller naïve asthmatic children. J Pediatr. 2007;151(5):457–62, 62.e1. [DOI] [PubMed] [Google Scholar]
- 29.Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005;60(1):13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, et al. Diagnostic accuracy of the bronchodilator response in children. J Allergy Clin Immunol. 2013;132(3):554–9.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J Allergy Clin Immunol Pract. 2018;6(2):545–54.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bossley CJ, Fleming L, Ullmann N, Gupta A, Adams A, Nagakumar P, et al. Assessment of corticosteroid response in pediatric patients with severe asthma by using a multidomain approach. J Allergy Clin Immunol. 2016;138(2):413–20.e6. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick AM, Moore WC. Severe Asthma Phenotypes - How Should They Guide Evaluation and Treatment? J Allergy Clin Immunol Pract. 2017;5(4):901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1–2):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Q, Fang L, Chen B, Zhang H, Wu Q, Zhang H, et al. Radon induced mitochondrial dysfunction in human bronchial epithelial cells and epithelial-mesenchymal transition with long-term exposure. Toxicol Res (Camb). 2019;8(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xin L, Sun J, Zhai X, Chen X, Wan J, Tian H. Repeated radon exposure induced lung damage via oxidative stress-mediated mitophagy in human bronchial epithelial cells and mice. Environ Toxicol Pharmacol. 2022;90:103812. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Nyhan MM, Wilker EH, Vieira CLZ, Lin H, Schwartz JD, et al. Recent exposure to particle radioactivity and biomarkers of oxidative stress and inflammation: The Framingham Heart Study. Environ Int. 2018;121(Pt 2):1210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


