Abstract
Apolipoprotein A1 (APOA1) and APOB are the structural proteins of high-density lipoprotein and APOB-containing lipoproteins, such as low-density lipoprotein and very low-density lipoprotein, respectively. The four smaller APOCs (APOC1, APOC2, APOC3 and APOC4) are exchangeable apolipoproteins; they are readily transferred among high-density lipoproteins and APOB-containing lipoproteins. The APOCs regulate plasma triglyceride and cholesterol levels by modulating substrate availability and activities of enzymes interacting with lipoproteins and by interfering with APOB-containing lipoprotein uptake through hepatic receptors. Of the four APOCs, APOC3 has been best studied in relation to diabetes. Elevated serum APOC3 levels predict incident cardiovascular disease and progression of kidney disease in people with type 1 diabetes. Insulin suppresses APOC3 levels, and accordingly, elevated APOC3 levels associate with insulin deficiency and insulin resistance. Mechanistic studies in a mouse model of type 1 diabetes have demonstrated that APOC3 acts in the causal pathway of diabetes-accelerated atherosclerosis. The mechanism is likely due to the ability of APOC3 to slow the clearance of triglyceride-rich lipoproteins and their remnants, thereby causing an increased accumulation of atherogenic lipoprotein remnants in lesions of atherosclerosis. Less is known about the roles of APOC1, APOC2 and APOC4 in diabetes.
Graphical Abstrct
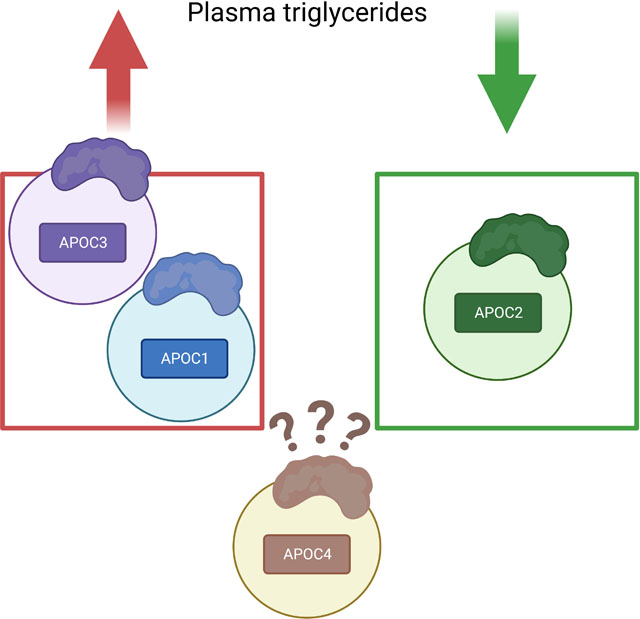
Introduction to the APOCs and diabetes
Diabetes has been known to be associated with dyslipidemia since ancient times when bloodletting was used as one of the treatment practices for the disease. A milky appearance of the blood was so common in patients with severe diabetes that it was used diagnostically.1 Much later, in 1935, Mann and Peters2 showed a lack of correlation between plasma cholesterol levels and levels of fatty acids and phospholipids in subjects with diabetes; differences we now know are due to elevated levels of triglyceride-rich lipoproteins (TRLs) in poorly controlled diabetes. With the knowledge that diabetes is associated with dyslipidemia and the realization that diabetic dyslipidemia might contribute to the increased risk of cardiovascular disease (CVD) that became apparent with the discovery and rapid initiation of insulin therapy, scientists started to investigate changes in specific proteins that bind to lipid to form lipoproteins – the apolipoproteins.
The history of apolipoprotein research is described in an excellent review article by Siri-Tarino and Krauss.3 A and B apolipoproteins were first identified, but other apolipoproteins were soon discovered in chylomicron preparations (chylomicrons are large TRLs secreted from the intestine during and immediately following a meal). These proteins were termed C apolipoproteins.4 It is now known that there are four apolipoprotein Cs, encoded by separate genes – APOC1, APOC2, APOC3 and APOC4 (Figure 1). The APOC1, APOC2 and APOC4 genes are part of the APOE/APOC1/APOC4/APOC2 gene cluster located on chromosome 19 in humans and on chromosome 7 in mice, whereas the APOC3 gene is part of the APOA1/APOC3/APOA4/APOA5 gene cluster on chromosome 11 in humans and chromosome 9 in mice.
Figure 1. The human APOC genes and proteins.
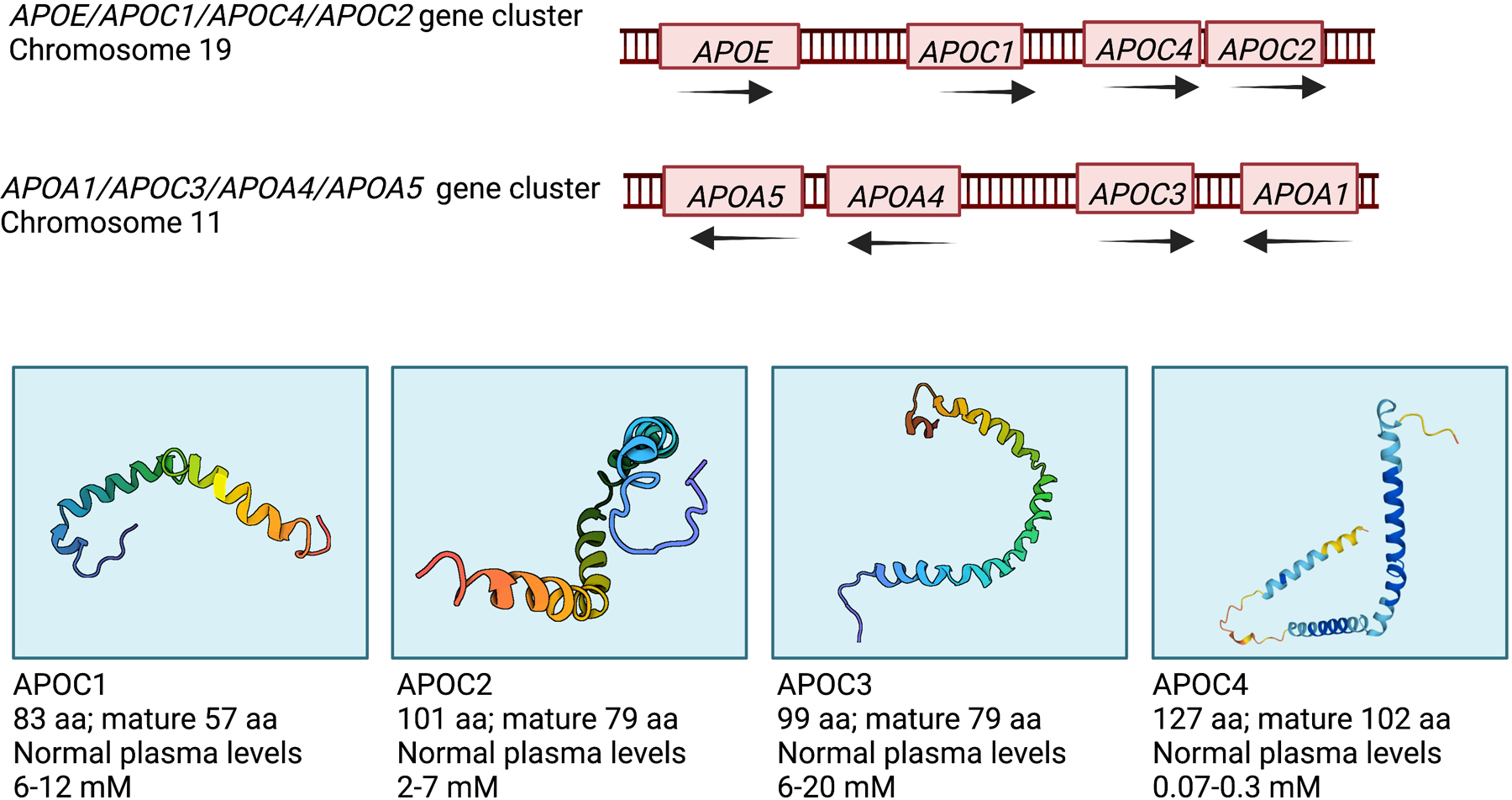
Two human gene clusters contain the four APOC genes (top). The APOC protein 3D structures (bottom) were retrieved from the US data center for the global Protein Data Bank (PDB) archive. The NMR-derived APOC1 (1IOJ), APOC2 (1O8T) and APOC3 (2JQ3) structures are based on the conformation of human APOCs in complexes with sodium dodecyl sulfate. The human APOC4 structure is predicted by the AlphaFold Protein Structure Database developed by DeepMind and EMBL-EBI (https://alphafold.ebi.ac.uk/). Plasma levels are based on quantification by Hortin et al.84 Created with BioRender.com
The four APOCs are small proteins secreted primarily by hepatocytes, which also secrete very low-density lipoprotein (VLDL). VLDLs are TRL particles secreted from the liver between meals. APOCs are added to the maturing VLDL particle in hepatocytes prior to secretion. The APOCs are also produced at lower levels by the intestine, which secretes chylomicrons into the lymph. Lymph chylomicrons are believed to acquire a significant amount of their APOC1, APOC2 and APOC3 cargo by transfer from lipoproteins filtered from plasma into lymph.5, 6 APOCs are also expressed by enterocytes in the intestine. Upregulation of Apoc2 gene expression in the jejunum following oil gavage7 as well as the localization of APOC3 and APOB in the villus tip regions8 suggest that APOC2 and APOC3 are involved in chylomicron generation.
Conversely, the APOCs can be transferred from TRLs (chylomicrons and VLDL) to HDL in circulation. As the TRL particle becomes smaller when its triglyceride cargo is lipolyzed by lipoprotein lipase (LPL), the “excess” phospholipid surface material is believed to be transferred to HDL, likely carrying APOCs and other exchangeable apolipoproteins with it.6 This mechanism is consistent with the very low levels of HDL in people with LPL deficiency. Richard Havel proposed that small amounts of APOCs are secreted from the liver in VLDL and then recycled repeatedly between VLDL and HDL before they are catabolized.6 Thus, the APOCs are exchangeable apolipoproteins, as opposed to APOA1 (the structural apolipoprotein of HDL) and APOB (the structural apolipoprotein of TRLs, TRL remnants, and LDL).
TRL levels are often increased in individuals with sub-optimally controlled diabetes and/or insulin resistance.9 Because of the increased APOC levels associated with elevated TRLs, and because insulin acutely suppresses large VLDL secretion,10 many studies have investigated the association between diabetes and the APOCs. For example, people with diabetes who also had increased levels of VLDL were found to exhibit an increase in the relative amount of APOC3 in plasma.11 While all four APOCs appear to play important roles in triglyceride metabolism, the role and regulation of APOC3 in the setting of diabetes is most well studied. Contrarily, almost nothing is known about APOC4.
Little is also known about the potential role of APOCs secreted by the intestine in the setting of diabetes. Early studies in rats suggested that the main APOCs produced by the intestine are APOC2 and APOC3.12 Studies on mice transgenic for APOC3 have suggested that APOC3 blunts basolateral uptake of lipids into enterocytes, perhaps by interfering with lipoprotein uptake through LDLR, thereby reducing chylomicron secretion into the lymph.13 However, the human relevance of these findings is uncertain because humans with APOC3 loss-of-function mutations show no differences in production rates of APOB48-containing chylomicrons.14
This review focuses on the roles and regulation of the APOCs in diabetes, and the emerging evidence that APOCs, in particular APOC3 and perhaps APOC2, might serve as drug targets for prevention of some of the vascular complications associated with type 1 diabetes (T1D) and type 2 diabetes (T2D).
Current methodological issues hamper studies of the role of APOCs and the lipoprotein particle populations they modulate
Reviewing the literature on APOCs and the lipoprotein particles they modulate, it is important to consider several methodological issues. First, because the APOCs (and many other apolipoproteins) are exchangeable, mouse overexpression models can “overload” the lipoprotein particle, thereby causing unintended biological consequences by the overexpressed APOC, displacing endogenous apolipoproteins15 or perhaps, blocking the access of enzymes to substrates or ligands to receptors,16 thus altering the composition and biological function of the particle. Issues of non-physiological alterations in composition and function of lipoproteins might also confound data interpretation on knockout models. Second, the method used for isolation of lipoprotein particles can alter the apolipoprotein composition ex vivo. For example, apolipoproteins can “fall off” the particle during ultracentrifugation. Alternatively, affinity chromatography e.g., using anti-APOC3 antibodies has been used to isolate APOC3-containing lipoproteins from human plasma.17 Such methods are informative in that different lipoprotein populations carrying different sets of apolipoproteins can be quantified, but this approach requires the availability of suitable and specific antibodies. Third, APOC1, APOC3 and APOC4 are believed to slow the clearance of TRLs and their remnants in part by interfering with APOE binding to the hepatic receptors, LDLR (low-density lipoprotein [LDL] receptor) and LRP1 (LDLR related receptor 1).18–20 The most widely used mouse models of atherosclerosis, Ldlr−/− and Apoe−/− mice, therefore have deficiencies in this mechanism. Moreover, mice differ from humans in several critical aspects related to lipoprotein metabolism. For example, mice are naturally deficient in CETP (cholesteryl ester transfer protein), which transfers triglycerides from TRLs and exchanges them for cholesteryl esters from HDL and vice versa in humans. These issues must be considered when interpreting apolipoprotein and lipoprotein studies based on mouse models.
Moreover, there are not yet standardized methods available to accurately quantify concentrations and sizes of TRLs and their remnants in large clinical trials.21, 22 Despite these methodological issues, many informative studies have revealed new information on APOCs and their relationships to diabetes.
APOC1
APOC1 is the smallest of the APOCs (Figure 1). In normolipidemic individuals the majority of APOC1 is present in HDL. However, with increased triglyceride levels, such as those often observed in people with T2D and people with suboptimally controlled T1D, the total plasma concentration and the proportion of APOC1 in TRLs increase.23 The altered partitioning of APOC1 is thought to be important as HDL-associated APOC1 and TRL-associated APOC1 appear to have different functions. For example, HDL-associated APOC1 serves to inhibit CETP activity24 (Figure 2) through a mechanism that may be due to APOC1’s ability to change the electrostatic charge of the HDL particle, resulting in a weaker HDL-CETP interaction.25 Thus, APOC1-mediated inhibition of CETP would result in triglyceride enrichment of TRLs and cholesterol-enriched HDL.
Figure 2. Functions of the four APOCs and their roles in diabetes.
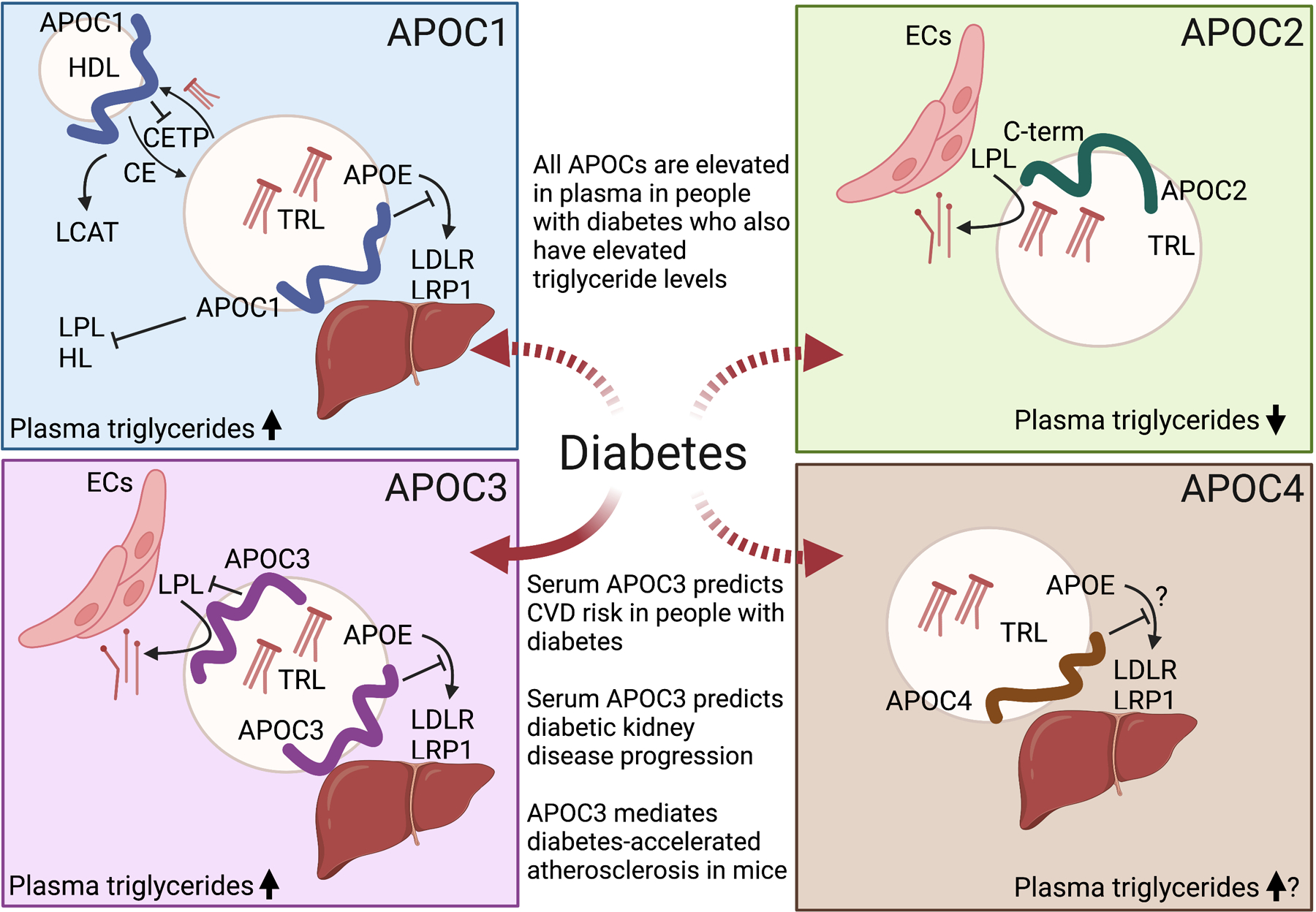
Plasma levels of all APOCs are higher in people and animals with elevated plasma triglycerides, such as often is the case in T2D and poorly controlled T1D. APOC1 (top, left) appears to have a number of functions. APOC1 associated with HDL inhibits the action of CETP. APOC1 also has been shown to inhibit a number of enzymatic activities, including LPL and hepatic lipase (HL) in vitro and when overexpressed, to activate LCAT, and to inhibit hepatic uptake of TRLs by preventing APOE binding to LDLR and LRP1. APOC2 (top, right) promotes LPL activity and therefore is an important mediator of triglyceride lowering. APOC3 (bottom, left) is the most studied APOC with the clearest evidence of an important role in diabetes. Elevated serum levels of APOC3 predict incident CVD and kidney disease progression in people with T1D, and APOC3 is a causal mediator of diabetes-accelerated atherosclerosis in a mouse model of T1D. The likely mechanism of action of APOC3 is through reduced clearance of TRLs and their remnants, leading to increased accumulation of atherogenic remnants in the artery wall. There is little information on the mechanism of action of APOC4 (bottom, right) and its potential role in diabetes. APOC4 overexpression in a mouse model leads to increased plasma triglycerides, likely by interfering with the hepatic uptake of TRLs. The dashed arrows indicate that the link to diabetes is largely unknown. ECs, endothelial cells. Created with BioRender.com
Transgenic mice overexpressing APOC1 and in vitro studies have revealed that APOC1 also appears to have other functions, including inhibition of the activity of several lipases26, 27 and at least in vitro, activation of lecithin-cholesterol acyl transferase (LCAT),28 an enzyme involved in converting small HDL particles into larger particles. In vitro, APOC1 bound to TRLs prevents LPL from hydrolyzing triglycerides due to the displacement of LPL from the particles.29 The inhibition of lipolysis is likely partly an in vitro phenomenon because mice overexpressing APOC1 do not exhibit impaired VLDL lipolysis; rather, the increase in plasma triglyceride levels is due to impaired hepatic uptake of VLDL and TRL remnants, likely by interfering with APOE-binding to LRP1, leading to increased levels of cholesterol-enriched VLDL, TRL remnants and LDL.19, 30 Mouse studies suggest that in situations where APOC1 increases levels of APOB lipoproteins, APOC1 plays a pro-atherogenic role,31, 32 but its overexpression has also been shown to protect against obesity and insulin resistance,30, 33 perhaps because of impaired fatty acid uptake in adipose tissue and skeletal muscle.33 Whether APOC1 plays a role in obesity or insulin resistance in humans is unclear.34
Studies in mice deficient in endogenous APOC1 have confirmed that APOC1 increases VLDL, TRL remnants and LDL triglyceride and cholesterol levels, without altering intestinal triglyceride absorption.35 Because these studies were performed in Apoe−/− mice, which have a defect in hepatic uptake of TRLs, endogenous APOC1 in this model was shown to act by increasing hepatic VLDL production and by reducing lipolysis via LPL inhibition. Thus, the triglyceride-elevating mechanism of APOC1 is multifactorial (Figure 2).
Plasma levels of APOC1 are elevated in people with T1D and T2D who also have elevated plasma triglycerides.34, 36 Similarly, an Ldlr−/− mouse model of T1D exhibits increased plasma levels of APOC1, concomitant with increased plasma triglyceride levels.37 However, T2D appears to increase APOC1 plasma levels in part independently of plasma triglyceride levels through an unknown mechanism.34 Moreover, APOC1 levels are negatively correlated with CETP activity in controls but not in people with hypertriglyceridemia, hypercholesterolemia or T1D or T2D.36, 38 It is possible that the loss of APOC1 inhibition associated with dyslipidemia is due to a relative redistribution of APOC1 to VLDL.38 In the case of diabetes-associated loss of CETP inhibition by APOC1, it has been hypothesized to be due to increased glycation of APOC1.36
Overall, there is still much we do not know about APOC1’s physiological function in different conditions, including in diabetes. Because of its pleiotropic effects, APOC1 may be unlikely to be a promising drug target for combating complications of diabetes.
APOC2
The principal function of APOC2 is much clearer than that of APOC1; APOC2 promotes the hydrolysis of circulating TRLs through the activation of LPL (Figure 2). The exact mechanism for how APOC2 enhances LPL activity is unclear. Most data support a model in which the C-terminus of APOC2 interacts with LPL to promote triglyceride hydrolysis.39, 40 Consistently, individuals with APOC2 deficiency or APOC2 loss-of-function mutations have severe hypertriglyceridemia, which can lead to pancreatitis,41 and APOC2-deficient mice also present with hypertriglyceridemia.42
People who have elevated plasma triglycerides also have increased levels of APOC2. Thus, individuals with T2D, who often have hypertriglyceridemia, exhibit elevated plasma APOC2 levels.43 Similarly, plasma APOC2 levels are elevated in an Ldlr−/− mouse model of T1D.37 The mechanism behind hypertriglyceridemia in the presence of elevated APOC2 levels could be due, in part, to parallel increases in APOC343–an LPL inhibitor–or to excess APOC2 interfering with the access of LPL to its substrates.16 Accordingly, individuals with diabetes and increased VLDL have been shown to exhibit an increase in the relative amount of APOC3, and a consequent decrease in the ratio of APOC2/APOC3 in VLDL.11
The most promising development in the area of APOC2 in relation to diabetes is the generation of APOC2 mimetic peptides that reduce plasma triglycerides. Consistent with the concept that the ratio of APOC2 to APOC3 is important in controlling plasma triglycerides, the APOC2 mimetic peptide, D6PV, has the ability to reduce plasma triglycerides in transgenic mouse models both via LPL activation and through APOC3 displacement.42 The displacement of APOC3 is believed to promote TRL metabolism by relieving the LPL-dependent and LPL-independent inhibitory effects of APOC3 on TRL clearance. The mimetic peptide could therefore provide an alternative strategy for reducing diabetes-associated complications by lowering TRLs and their remnants in individuals with diabetes.
APOC3
APOC3 is the most well-studied apolipoprotein in the APOC family (Figures 1–2). Like the other APOCs, the lipoprotein distribution of APOC3 is dependent on the metabolic state of an individual, with elevated levels in individuals with diabetes and elevated levels of TRLs. Moreover, APOC3 can be glycosylated and sialylated in humans, modulating its hepatic clearance,44 adding another layer of complexity. As with all apolipoproteins, plasma levels are a reflection of both production and clearance rates. Lipoprotein-bound APOC3 is cleared from circulation via receptor-mediated hepatic uptake while non-lipidated APOC3 is cleared via renal filtration.20, 45 Kinetic studies have shown that individuals with T2D have an increased APOC3 secretion rate that is associated with increased TRL levels.46 Diabetes also likely reduces the clearance rate of APOC3 by dampening LPL activity and, presumably, hepatic clearance of TRLs.37, 47
Early studies in isolated hepatocytes, mice deficient in hepatic insulin receptors, and diabetic mice treated with a bolus dose of insulin suggested that hyperglycemia and lack of insulin contribute to the increased hepatic Apoc3 mRNA and plasma APOC3 levels associated with diabetes.48–50 Subsequent studies showed that diabetes-associated hyperglycemia is not the main driver of the elevated plasma levels of APOC3 in a mouse model since normalizing blood glucose with a sodium-glucose co-transporter 2 inhibitor did not reduce plasma APOC3 levels, while intense insulin therapy normalized diabetes-associated increases in APOC3.51 The reduction in plasma APOC3 in response to insulin treatment of diabetic mice did not appear to occur via a direct decrease in hepatic Apoc3 transcription. This finding was unexpected because prior studies on mice overexpressing the transcription factor FOXO1 showed increased hepatic Apoc3 expression consistent with the ability of insulin to induce FOXO1 phosphorylation and nuclear exclusion.49 To further investigate the role of FOXO transcription factors, we subsequently demonstrated that deletion of all three of the hepatic FOXOs (FOXO1, 3 and 4) failed to suppress hepatic Apoc3 levels.51 Thus, the increased hepatic levels of Apoc3 mRNA in diabetic mice are likely to be explained by increased mRNA stability or other post-transcriptional mechanisms. The conclusion that increased transcription of the APOC3 gene is not the mechanism whereby T1D can cause to elevated plasma APOC3 levels is also consistent with findings that mutations in an insulin response element (T-455 -> C) in the human APOC3 promoter in individuals with T1D do not confer a reduction in either plasma APOC3 or triglycerides.52 The most consistent observation in humans with either T1D or T2D is that plasma APOC3 concentrations increase in tandem with plasma TRL levels. The mechanism might be related to lack of sufficient insulin signaling in the liver, either because portal vein insulin delivery is not mimicked by exogenous insulin treatment or because insulin resistance exists both in T1D and T2D. In fact, serum APOC3 levels in subjects with T1D associate negatively with insulin sensitivity.53 Further studies are needed to determine how APOC3 is regulated in diabetes, including transcriptional and post-transcriptional regulation and fractional production and clearance rates.
The seminal study by Pollin and colleagues first demonstrated that individuals with APOC3 loss-of-function mutations have reduced levels of plasma triglycerides and an apparent protection against CVD.54 Three separate studies on distinct cohorts of people with T1D in the US and Europe have now shown that serum levels of APOC3 predict cardiovascular events independent of traditional CVD risk factors.51, 55, 56 Using a mouse model of T1D, we further demonstrated that silencing APOC3 protects against diabetes-accelerated atherosclerosis, both measured as early lesion initiation as well as the expansion of the necrotic cores associated with more advanced atherosclerosis.51 Importantly, the beneficial effect of APOC3 lowering was observed despite the lack of effects of APOC3 silencing on hyperglycemia. The lesion stabilizing effect of APOC3 reduction was also reported in mice without diabetes, but only in mice in which APOC3 also regulated TRLs,57 suggesting that the pro-atherogenic effects of APOC3 are mediated by its effects on TRLs and their remnants.
APOC3 causes an elevation of plasma triglyceride levels by reducing the lipolysis and the hepatic clearance of TRLs and their remnants (Figure 2). This interpretation is consistent with the finding that mice expressing a human APOC3 transgene are hypertriglyceridemic and have reduced VLDL clearance.58 The mechanism behind APOC3-mediated inhibition of triglyceride lipolysis has been proposed to involve inhibition of LPL activity when LPL is bound to glycosylphosphatidylinositol-anchored HDL-binding protein 1 on endothelial cells,59 perhaps by blocking the access of LPL to TRL triglycerides. When APOC3 levels are very high, it might also work by displacing APOC2 from the surface of the TRLs.60 In addition, APOC3 inhibits the clearance of TRLs and their remnants through LDLR and LRP1,20 by a mechanism believed to be due to interference with APOE binding to those receptors. The relative contribution of these mechanisms differ in different conditions. In humans with a loss-of-function mutation in APOC3, a 50% reduction in plasma APOC3 levels results in increased fractional clearance rates of VLDL triglycerides and APOB and a higher conversion rate of VLDL remnants to LDL, suggesting increased LPL activity.61 The fractional clearance rates of both APOC3 and APOC2 were also higher in these subjects. On the other hand, suppression of plasma APOC3 leads to reduced plasma triglycerides in individuals with familial chylomicronemia syndrome and genetic deficiency of LPL, demonstrating that APOC3 also acts through an LPL-independent pathway.62 This finding, together with the finding that APOC3 can displace APOE from small VLDL15 supports the conclusion that APOC3 can increase plasma triglycerides and cholesterol independently of LPL, by inhibiting hepatic clearance of TRLs and their remnants.
The mechanism whereby suppression of APOC3 prevents atherosclerosis in the setting of diabetes is most likely due to its inhibition of clearance of TRLs and their remnants. Immunoreactive APOC3 accumulates at a greater extent in lesions in diabetic mice and co-localizes with APOB and APOE, suggesting accumulation of TRLs and/or TRL remnants, and this accumulation is prevented by silencing of APOC3 or by increasing hepatic clearance of TRLs.37, 51, 63 However, some studies have suggested a role for APOC3 beyond that of TRL lipolysis and clearance. For example, non-lipidated APOC3 can induce alternative NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome activation in isolated human and mouse monocytes.64, 65 Although it is tempting to speculate that elevated levels of inflammatory markers in humans or mice with diabetes may be due to elevated plasma APOC3, we recently demonstrated that the inflammasome-activating effect of non-lipidated APOC3 is lost when APOC3 is bound to lipid particles, as it is in circulation in people with and without diabetes.65 Furthermore, diabetic mice in which APOC3 was silenced did not have reduced plasma levels of IL-18 or IL-1β, pro-inflammatory cytokines released through inflammasome pathways, and APOC3 transgenic mice did not exhibit increased plasma levels of IL-1β or IL-18 despite the high levels of APOC3. Although it is possible that some APOC3 exists free of lipoproteins in tissues, these studies are consistent with the proposal that endogenous APOC3 does not act upstream of NLRP3 activation in diabetes. However, human studies show an intriguing interaction between an NLRP3 intron variant associated with increased NLRP3 activation and APOC3 plasma concentrations.66 Such a connection might be explained by a mechanism in which plasma APOC3 levels are regulated downstream of NLRP3 because recent mouse studies demonstrated reduced serum APOC3 levels in diabetic mice with hematopoietic NLRP3 deficiency.67 These results highlight the importance of considering the function of APOC3 in its physiological lipidated state, and support the notion that APOC3 acts to promote atherosclerosis primarily by its effects on TRLs and TRL remnants.
Reduced renal function is associated with increased plasma APOC3 levels,45, 68 and plasma levels of APOC3 in people with T1D are positively associated with nephropathy and retinopathy.52, 69 Interestingly, in a study of APOC3 as a predictor of incident CVD in participants with T1D in the FinnDiane cohort, APOC3 only predicted CVD in those who also had markers of diabetic kidney disease,56 suggesting that the close relationship between diabetic kidney disease and CVD includes APOC3. Plasma levels of APOC3 also predicted renal disease progression in people with T1D. Mechanistically, it is unclear how APOC3, or APOC3-mediated dyslipidemia might accelerate diabetic kidney disease, but APOC3 overexpression appears to worsen mild diabetic kidney disease in part by augmenting glomerular inflammation.70 It is currently difficult to disentangle the effects of APOC3 from those of elevated triglycerides and insulin resistance, both on CVD and diabetic kidney disease.
Some studies have suggested that APOC3 could play a role in development or worsening of insulin resistance because of the links among plasma APOC3, dyslipidemia, insulin resistance and diabetes.71, 72 Indeed, a small clinical trial investigating the triglyceride-lowering effects of volanesorsen (an APOC3 antisense oligonucleotide therapeutic) in subjects with T2D indicated that suppression of APOC3 resulted in improved insulin sensitivity.73 However, a larger randomized clinical trial on volanesorsen failed to detect improvements in insulin resistance (HOMA-IR) or HbA1c in subjects with T2D.74 On the other hand, studies in mice and isolated cells suggested that APOC3 is produced by pancreatic islet cells75 and that reducing APOC3 levels either systemically76 or locally in the islet increased the survival of beta cells.75 However, additional studies in which APOC3 was overexpressed demonstrated that APOC3 is insufficient in causing beta cell dysfunction.77 Although the possible direct or indirect effects of APOC3 in beta cell physiology need further study, current data do not unequivocally support a causative role for APOC3 in insulin resistance or incidence of T2D.
Overall, there is strong evidence that APOC3 is a biomarker and mediator of CVD and atherosclerosis, and likely of kidney disease, in the setting of T1D. Further studies are needed to address whether similar relationships are present in T2D.
APOC4
APOC4 is a relatively understudied member of the APOC family. The APOC4 gene was discovered and characterized nearly 25 years ago,78 but so far has not attracted much attention, perhaps because plasma levels of APOC4 are at least an order of magnitude lower than those of APOC1, APOC2 and APOC3 (Figure 1). APOC4 is found on circulating TRLs and HDL, like the other APOCs. Rabbit APOC4 exists in several sialoforms,79 but the physiological and pathological significance of these isoforms remain unknown.
Endogenous APOC4 is thought to increase plasma triglyceride levels because overexpression of human APOC4 at supraphysiological levels in mice causes elevated levels of VLDL.18 The mechanism of the elevated VLDL levels in APOC4 transgenic mice was suggested to be due to displacement of APOE, resulting in reduced hepatic clearance of VLDL, rather than to a reduction in lipase activity (Figure 2). Moreover, previous small studies have revealed that polymorphisms in the human APOC4 gene are associated with plasma triglyceride levels in women, although the contribution of APOC4 genetic variation to plasma triglycerides was only 2%.80 APOC4 polymorphisms have also been associated with mildly elevated triglycerides and an increased coronary artery disease risk in a Chinese Han population.81 The biological functions of endogenous APOC4 as well as its potential role in diabetes and diabetes complications need further study.
Clinical implications
If APOC3-mediated arterial retention of TRLs and their remnants indeed contributes causally to incident CVD in people with diabetes, APOC3 inhibition (or potentially the APOC2 mimetic peptide described above) might be a promising novel approach to CVD prevention in people with suboptimally controlled T1D, T2D, or insulin resistance. It is important to consider that APOC3 is not universally elevated in these conditions but rather is elevated in those with increased risk for incident CVD.
Several approaches to target APOC3 have been established, including siRNA, liver-targeted antisense oligonucleotides, blocking antibodies and peptides, all with great success in reducing plasma APOC3 levels and triglycerides. Importantly, blocking APOC3’s action would be predicted to increase both TRL lipolysis and hepatic removal of TRLs and TRL remnants, but not to reduce total levels of APOB100-containing lipoproteins.82
The necessity of lowering total levels of APOB-containing lipoproteins to achieve cardioprotection was recently highlighted by the PROMINENT trial (NCT03071692).83 This double-blind, randomized, controlled trial investigated the effect of pemafibrate, a highly selective PPARα modulator, on lipids and cardiovascular outcomes in participants with T2D who had mild-to-moderate hypertriglyceridemia. The results demonstrated that the incidence of CVD events was not lower among those who received pemafibrate than those who received placebo, although pemafibrate lowered triglycerides, VLDL cholesterol, and APOC3 levels by 26–28%.83 One likely explanation of the study’s futility is that the pemafibrate-mediated reductions in TRLs were accompanied by increases in plasma LDL-cholesterol and APOB levels, so that there was no overall benefit in non-HDL cholesterol and total cholesterol levels. The authors suggested that pemafibrate results in an increased conversion of TRLs to LDL, rather than an increased removal of these particles by the liver, which might have negated any benefit of reduction in TRL or remnant levels. This trial suggests that increased lipolysis of TRLs without also increasing hepatic removal of APOB lipoproteins, including TRL remnants and LDL, will not be a successful strategy for CVD prevention in people with diabetes. Therefore, when designing cardiovascular outcome trials on the effect of APOC3 inhibition, including in participants with diabetes and elevated APOC3 levels, strategies to ensure effective hepatic clearance of TRLs, TRL remnants and LDL should be carefully considered.
Highlights:
Apolipoprotein Cs (APOCs) are involved in the regulation of plasma triglycerides and cholesterol, and are increased in individuals who have diabetes and elevated triglyceride levels.
APOC1 and APOC3 increase plasma triglyceride levels, APOC2 decreases plasma triglycerides, but the role of endogenous APOC4 is largely unknown.
Elevated levels of serum APOC3 are associated with increased cardiovascular disease risk and kidney disease progression in individuals with type 1 diabetes.
Targeting the clearance of triglyceride-rich lipoproteins and their remnants via APOC3 inhibition or APOC2 mimetic peptides might be a promising approach for preventing cardiovascular diseases in individuals with diabetes, but such an approach will need to simultaneously increase the hepatic removal of APOB lipoproteins.
Sources of funding
Research in the authors’ laboratories is supported in part by grants from the National Institutes of Health P01HL151328, R35HL150754 (K.E.B.), R01DK121756 and American Diabetes Association grant 11–22-IBSPM-09 (J.E.K.), a postdoctoral fellowship award from the American Diabetes Association 9–18-CVD1–002 (V.K.) and a Predoctoral Fellowship Award from the American Heart Association 828090 (C.C.H.).
Abbreviations
- APO
apolipoprotein
- CETP
cholesteryl ester transfer protein
- CVD
cardiovascular disease
- LDLR
LDL receptor
- LRP1
LDL receptor related receptor 1
- LPL
lipoprotein lipase
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TRL
triglyceride-rich lipoprotein
- VLDL
very low-density lipoprotein
Footnotes
Disclosures
Dr. Bornfeldt serves on the scientific advisory board of Esperion Therapeutics.
References
- 1.Bloor WR. The lipoids (“fat”) of the blood in diabetes. J Biol Chem. 1916;26:417–430 [Google Scholar]
- 2.Mann EB, Peters JP. Serum lipoids in diabetes. J Clin Invest. 1935;14:579–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siri-Tarino PW, Krauss RM. The early years of lipoprotein research: From discovery to clinical application. J Lipid Res. 2016;57:1771–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodbell M, Frederickson DS. The nature of the proteins associated with dog ano human chylomicrons. J Biol Chem. 1959;234:562–566 [PubMed] [Google Scholar]
- 5.Schaefer EJ, Wetzel MG, Bengtsson G, Scow RO, Brewer HB Jr., Olivecrona T Transfer of human lymph chylomicron constituents to other lipoprotein density fractions during in vitro lipolysis. J Lipid Res. 1982;23:1259–1273 [PubMed] [Google Scholar]
- 6.Havel RJ, Kane JP, Kashyap ML. Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J Clin Invest. 1973;52:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buttet M, Poirier H, Traynard V, Gaire K, Tran TT, Sundaresan S, Besnard P, Abumrad NA, Niot I. Deregulated lipid sensing by intestinal cd36 in diet-induced hyperinsulinemic obese mouse model. PLoS One. 2016;11:e0145626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moor AE, Harnik Y, Ben-Moshe S, Massasa EE, Rozenberg M, Eilam R, Bahar Halpern K, Itzkovitz S. Spatial reconstruction of single enterocytes uncovers broad zonation along the intestinal villus axis. Cell. 2018;175:1156–1167 e1115 [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg HN. Lipoprotein physiology in nondiabetic and diabetic states. Relationship to atherogenesis. Diabetes Care. 1991;14:839–855 [DOI] [PubMed] [Google Scholar]
- 10.Adiels M, Westerbacka J, Soro-Paavonen A, Hakkinen AM, Vehkavaara S, Caslake MJ, Packard C, Olofsson SO, Yki-Jarvinen H, Taskinen MR, et al. Acute suppression of vldl1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia. 2007;50:2356–2365 [DOI] [PubMed] [Google Scholar]
- 11.Gabor J, Spain M, Kalant N. Composition of serum very-low-density and high-density lipoproteins in diabetes. Clin Chem. 1980;26:1261–1265 [PubMed] [Google Scholar]
- 12.Wu AL, Windmueller HG. Identification of circulating apolipoproteins synthesized by rat small intestine in vivo. J Biol Chem. 1978;253:2525–2528 [PubMed] [Google Scholar]
- 13.Li D, Rodia CN, Johnson ZK, Bae M, Muter A, Heussinger AE, Tambini N, Longo AM, Dong H, Lee JY, et al. Intestinal basolateral lipid substrate transport is linked to chylomicron secretion and is regulated by apoc-iii. J Lipid Res. 2019;60:1503–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taskinen MR, Bjornson E, Matikainen N, Soderlund S, Ramo J, Ainola MM, Hakkarainen A, Sihlbom C, Thorsell A, Andersson L, et al. Postprandial metabolism of apolipoproteins b48, b100, c-iii, and e in humans with apoc3 loss-of-function mutations. JCI Insight. 2022;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breyer ED, Le NA, Li X, Martinson D, Brown WV. Apolipoprotein c-iii displacement of apolipoprotein e from vldl: Effect of particle size. J Lipid Res. 1999;40:1875–1882 [PubMed] [Google Scholar]
- 16.Shachter NS, Hayek T, Leff T, Smith JD, Rosenberg DW, Walsh A, Ramakrishnan R, Goldberg IJ, Ginsberg HN, Breslow JL. Overexpression of apolipoprotein cii causes hypertriglyceridemia in transgenic mice. J Clin Invest. 1994;93:1683–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos H, Perlov D, Khoo C, Sacks FM. Distinct patterns of lipoproteins with apob defined by presence of apoe or apoc-iii in hypercholesterolemia and hypertriglyceridemia. J Lipid Res. 2001;42:1239–1249 [PubMed] [Google Scholar]
- 18.Allan CM, Taylor JM. Expression of a novel human apolipoprotein (apoc-iv) causes hypertriglyceridemia in transgenic mice. J Lipid Res. 1996;37:1510–1518 [PubMed] [Google Scholar]
- 19.Jong MC, Hofker MH, Havekes LM. Role of apocs in lipoprotein metabolism: Functional differences between apoc1, apoc2, and apoc3. Arterioscler Thromb Vasc Biol. 1999;19:472–484 [DOI] [PubMed] [Google Scholar]
- 20.Gordts PL, Nock R, Son NH, Ramms B, Lew I, Gonzales JC, Thacker BE, Basu D, Lee RG, Mullick AE, et al. Apoc-iii inhibits clearance of triglyceride-rich lipoproteins through ldl family receptors. J Clin Invest. 2016;126:2855–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chait A, Ginsberg HN, Vaisar T, Heinecke JW, Goldberg IJ, Bornfeldt KE. Remnants of the triglyceride-rich lipoproteins, diabetes, and cardiovascular disease. Diabetes. 2020;69:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bornfeldt KE. The remnant lipoprotein hypothesis of diabetes-associated cardiovascular disease. Arterioscler Thromb Vasc Biol. 2022;42:819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohn JS, Tremblay M, Batal R, Jacques H, Veilleux L, Rodriguez C, Bernier L, Mamer O, Davignon J. Plasma kinetics of vldl and hdl apoc-i in normolipidemic and hypertriglyceridemic subjects. J Lipid Res. 2002;43:1680–1687 [DOI] [PubMed] [Google Scholar]
- 24.Gautier T, Masson D, Jong MC, Duverneuil L, Le Guern N, Deckert V, Pais de Barros JP, Dumont L, Bataille A, Zak Z, et al. Apolipoprotein ci deficiency markedly augments plasma lipoprotein changes mediated by human cholesteryl ester transfer protein (cetp) in cetp transgenic/apoci-knocked out mice. J Biol Chem. 2002;277:31354–31363 [DOI] [PubMed] [Google Scholar]
- 25.Dumont L, Gautier T, de Barros JP, Laplanche H, Blache D, Ducoroy P, Fruchart J, Fruchart JC, Gambert P, Masson D, et al. Molecular mechanism of the blockade of plasma cholesteryl ester transfer protein by its physiological inhibitor apolipoprotein ci. J Biol Chem. 2005;280:38108–38116 [DOI] [PubMed] [Google Scholar]
- 26.Conde-Knape K, Bensadoun A, Sobel JH, Cohn JS, Shachter NS. Overexpression of apoc-i in apoe-null mice: Severe hypertriglyceridemia due to inhibition of hepatic lipase. J Lipid Res. 2002;43:2136–2145 [DOI] [PubMed] [Google Scholar]
- 27.Rouland A, Masson D, Lagrost L, Verges B, Gautier T, Bouillet B. Role of apolipoprotein c1 in lipoprotein metabolism, atherosclerosis and diabetes: A systematic review. Cardiovasc Diabetol. 2022;21:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soutar AK, Garner CW, Baker HN, Sparrow JT, Jackson RL, Gotto AM, Smith LC. Effect of the human plasma apolipoproteins and phosphatidylcholine acyl donor on the activity of lecithin: Cholesterol acyltransferase. Biochemistry. 1975;14:3057–3064 [DOI] [PubMed] [Google Scholar]
- 29.Larsson M, Vorrsjo E, Talmud P, Lookene A, Olivecrona G. Apolipoproteins c-i and c-iii inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J Biol Chem. 2013;288:33997–34008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shachter NS, Ebara T, Ramakrishnan R, Steiner G, Breslow JL, Ginsberg HN, Smith JD. Combined hyperlipidemia in transgenic mice overexpressing human apolipoprotein cl. J Clin Invest. 1996;98:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westerterp M, Van Eck M, de Haan W, Offerman EH, Van Berkel TJ, Havekes LM, Rensen PC. Apolipoprotein ci aggravates atherosclerosis development in apoe-knockout mice despite mediating cholesterol efflux from macrophages. Atherosclerosis. 2007;195:e9–16 [DOI] [PubMed] [Google Scholar]
- 32.Gautier T, Deckert V, Aires V, Le Guern N, Proukhnitzky L, Patoli D, Lemaire S, Maquart G, Bataille A, Xolin M, et al. Human apolipoprotein c1 transgenesis reduces atherogenesis in hypercholesterolemic rabbits. Atherosclerosis. 2021;320:10–18 [DOI] [PubMed] [Google Scholar]
- 33.Jong MC, Voshol PJ, Muurling M, Dahlmans VE, Romijn JA, Pijl H, Havekes LM. Protection from obesity and insulin resistance in mice overexpressing human apolipoprotein c1. Diabetes. 2001;50:2779–2785 [DOI] [PubMed] [Google Scholar]
- 34.Bouillet B, Gautier T, Aho LS, Duvillard L, Petit JM, Lagrost L, Verges B. Plasma apolipoprotein c1 concentration is associated with plasma triglyceride concentration, but not visceral fat, in patients with type 2 diabetes. Diabetes Metab. 2016;42:263–266 [DOI] [PubMed] [Google Scholar]
- 35.Westerterp M, de Haan W, Berbee JF, Havekes LM, Rensen PC. Endogenous apoc-i increases hyperlipidemia in apoe-knockout mice by stimulating vldl production and inhibiting lpl. J Lipid Res. 2006;47:1203–1211 [DOI] [PubMed] [Google Scholar]
- 36.Bouillet B, Gautier T, Blache D, Pais de Barros JP, Duvillard L, Petit JM, Lagrost L, Verges B. Glycation of apolipoprotein c1 impairs its cetp inhibitory property: Pathophysiological relevance in patients with type 1 and type 2 diabetes. Diabetes Care. 2014;37:1148–1156 [DOI] [PubMed] [Google Scholar]
- 37.Shimizu-Albergine M, Basu D, Kanter JE, Kramer F, Kothari V, Barnhart S, Thornock C, Mullick AE, Clouet-Foraison N, Vaisar T, et al. Crebh normalizes dyslipidemia and halts atherosclerosis in diabetes by decreasing circulating remnant lipoproteins. J Clin Invest. 2021;131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillois X, Gautier T, Bouillet B, Pais de Barros JP, Jeannin A, Verges B, Bonnet J, Lagrost L. Constitutive inhibition of plasma cetp by apolipoprotein c1 is blunted in dyslipidemic patients with coronary artery disease. J Lipid Res. 2012;53:1200–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacRaild CA, Howlett GJ, Gooley PR. The structure and interactions of human apolipoprotein c-ii in dodecyl phosphocholine. Biochemistry. 2004;43:8084–8093 [DOI] [PubMed] [Google Scholar]
- 40.Meyers NL, Larsson M, Olivecrona G, Small DM. A pressure-dependent model for the regulation of lipoprotein lipase by apolipoprotein c-ii. J Biol Chem. 2015;290:18029–18044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolska A, Dunbar RL, Freeman LA, Ueda M, Amar MJ, Sviridov DO, Remaley AT. Apolipoprotein c-ii: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis. 2017;267:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolska A, Lo L, Sviridov DO, Pourmousa M, Pryor M, Ghosh SS, Kakkar R, Davidson M, Wilson S, Pastor RW, et al. A dual apolipoprotein c-ii mimetic-apolipoprotein c-iii antagonist peptide lowers plasma triglycerides. Sci Transl Med. 2020;12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beliard S, Nogueira JP, Maraninchi M, Lairon D, Nicolay A, Giral P, Portugal H, Vialettes B, Valero R. Parallel increase of plasma apoproteins c-ii and c-iii in type 2 diabetic patients. Diabet Med. 2009;26:736–739 [DOI] [PubMed] [Google Scholar]
- 44.Kegulian NC, Ramms B, Horton S, Trenchevska O, Nedelkov D, Graham MJ, Lee RG, Esko JD, Yassine HN, Gordts P. Apoc-iii glycoforms are differentially cleared by hepatic trl (triglyceride-rich lipoprotein) receptors. Arterioscler Thromb Vasc Biol. 2019;39:2145–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ooi EM, Chan DT, Watts GF, Chan DC, Ng TW, Dogra GK, Irish AB, Barrett PH. Plasma apolipoprotein c-iii metabolism in patients with chronic kidney disease. J Lipid Res. 2011;52:794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adiels M, Taskinen MR, Bjornson E, Andersson L, Matikainen N, Soderlund S, Kahri J, Hakkarainen A, Lundbom N, Sihlbom C, et al. Role of apolipoprotein c-iii overproduction in diabetic dyslipidaemia. Diabetes Obes Metab. 2019;21:1861–1870 [DOI] [PubMed] [Google Scholar]
- 47.Willecke F, Scerbo D, Nagareddy P, Obunike JC, Barrett TJ, Abdillahi ML, Trent CM, Huggins LA, Fisher EA, Drosatos K, et al. Lipolysis, and not hepatic lipogenesis, is the primary modulator of triglyceride levels in streptozotocin-induced diabetic mice. Arterioscler Thromb Vasc Biol. 2015;35:102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caron S, Verrijken A, Mertens I, Samanez CH, Mautino G, Haas JT, Duran-Sandoval D, Prawitt J, Francque S, Vallez E, et al. Transcriptional activation of apolipoprotein ciii expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol. 2011;31:513–519 [DOI] [PubMed] [Google Scholar]
- 49.Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. Foxo1 mediates insulin action on apoc-iii and triglyceride metabolism. J Clin Invest. 2004;114:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen M, Breslow JL, Li W, Leff T. Transcriptional regulation of the apoc-iii gene by insulin in diabetic mice: Correlation with changes in plasma triglyceride levels. J Lipid Res. 1994;35:1918–1924 [PubMed] [Google Scholar]
- 51.Kanter JE, Shao B, Kramer F, Barnhart S, Shimizu-Albergine M, Vaisar T, Graham MJ, Crooke RM, Manuel CR, Haeusler RA, et al. Increased apolipoprotein c3 drives cardiovascular risk in type 1 diabetes. J Clin Invest. 2019;129:4165–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein RL, McHenry MB, Lok KH, Hunter SJ, Le NA, Jenkins AJ, Zheng D, Semler AJ, Brown WV, Lyons TJ, et al. Apolipoprotein c-iii protein concentrations and gene polymorphisms in type 1 diabetes: Associations with lipoprotein subclasses. Metabolism. 2004;53:1296–1304 [DOI] [PubMed] [Google Scholar]
- 53.Buckner T, Shao B, Eckel RH, Heinecke JW, Bornfeldt KE, Snell-Bergeon J. Association of apolipoprotein c3 with insulin resistance and coronary artery calcium in patients with type 1 diabetes. J Clin Lipidol. 2021;15:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, et al. A null mutation in human apoc3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basu A, Bebu I, Jenkins AJ, Stoner JA, Zhang Y, Klein RL, Lopes-Virella MF, Garvey WT, Budoff MJ, Alaupovic P, et al. Serum apolipoproteins and apolipoprotein-defined lipoprotein subclasses: A hypothesis-generating prospective study of cardiovascular events in t1d. J Lipid Res. 2019;60:1432–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jansson Sigfrids F, Stechemesser L, Dahlstrom EH, Forsblom CM, Harjutsalo V, Weitgasser R, Taskinen MR, Groop PH, FinnDiane Study G. Apolipoprotein c-iii predicts cardiovascular events and mortality in individuals with type 1 diabetes and albuminuria. J Intern Med. 2022;291:338–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramms B, Patel S, Sun X, Pessentheiner AR, Ducasa GM, Mullick AE, Lee RG, Crooke RM, Tsimikas S, Witztum JL, et al. Interventional hepatic apoc-iii knockdown improves atherosclerotic plaque stability and remodeling by triglyceride lowering. JCI Insight. 2022;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aalto-Setala K, Fisher EA, Chen X, Chajek-Shaul T, Hayek T, Zechner R, Walsh A, Ramakrishnan R, Ginsberg HN, Breslow JL. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) ciii transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo ciii and reduced apo e on the particles. J Clin Invest. 1992;90:1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsson M, Allan CM, Jung RS, Heizer PJ, Beigneux AP, Young SG, Fong LG. Apolipoprotein c-iii inhibits triglyceride hydrolysis by gpihbp1-bound lpl. J Lipid Res. 2017;58:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boren J, Packard CJ, Taskinen MR. The roles of apoc-iii on the metabolism of triglyceride-rich lipoproteins in humans. Front Endocrinol (Lausanne). 2020;11:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reyes-Soffer G, Sztalryd C, Horenstein RB, Holleran S, Matveyenko A, Thomas T, Nandakumar R, Ngai C, Karmally W, Ginsberg HN, et al. Effects of apoc3 heterozygous deficiency on plasma lipid and lipoprotein metabolism. Arterioscler Thromb Vasc Biol. 2019;39:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, et al. Targeting apoc3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371:2200–2206 [DOI] [PubMed] [Google Scholar]
- 63.Kanter JE, Bornfeldt KE. Apolipoprotein c3 and apolipoprotein b colocalize in proximity to macrophages in atherosclerotic lesions in diabetes. J Lipid Res. 2021;62:100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zewinger S, Reiser J, Jankowski V, Alansary D, Hahm E, Triem S, Klug M, Schunk SJ, Schmit D, Kramann R, et al. Apolipoprotein c3 induces inflammation and organ damage by alternative inflammasome activation. Nat Immunol. 2020;21:30–41 [DOI] [PubMed] [Google Scholar]
- 65.Hsu CC, Shao B, Kanter JE, He Y, Vaisar T, Witztum JL, Snell-Bergeon J, McInnes G, Bruse S, Gottesman O, et al. Apolipoprotein c3 induces inflammasome activation only in its delipidated form. Nat Immunol. 2023;24:408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zewinger S, Reiser J, Jankowski V, Alansary D, Hahm E, Triem S, Klug M, Schunk SJ, Schmit D, Kramann R, et al. Reply to: Apolipoprotein c3 induces inflammasome activation only in its delipidated form. Nat Immunol. 2023;24:412–413 [DOI] [PubMed] [Google Scholar]
- 67.Hsu CC, Fidler TP, Kanter JE, Kothari V, Kramer F, Tang J, Tall AR, Bornfeldt KE. Hematopoietic nlrp3 and aim2 inflammasomes promote diabetes-accelerated atherosclerosis, but increased necrosis is independent of pyroptosis. Diabetes. 2023;In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attman PO, Samuelsson O, Alaupovic P. The effect of decreasing renal function on lipoprotein profiles. Nephrol Dial Transplant. 2011;26:2572–2575 [DOI] [PubMed] [Google Scholar]
- 69.Klein RL, McHenry MB, Lok KH, Hunter SJ, Le NA, Jenkins AJ, Zheng D, Semler A, Page G, Brown WV, et al. Apolipoprotein c-iii protein concentrations and gene polymorphisms in type 1 diabetes: Associations with microvascular disease complications in the dcct/edic cohort. J Diabetes Complications. 2005;19:18–25 [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Huang X, Xu P, Liu X, Zhou Z, Wang F, Li J, Wang Y, Xian X, Liu G, et al. Apolipoprotein c3 aggravates diabetic nephropathy in type 1 diabetes by activating the renal tlr2/nf-kappab pathway. Metabolism. 2021;119:154740. [DOI] [PubMed] [Google Scholar]
- 71.Sokooti S, Flores-Guerrero JL, Heerspink HJL, Connelly MA, Bakker SJL, Dullaart RPF. Triglyceride-rich lipoprotein and ldl particle subfractions and their association with incident type 2 diabetes: The prevend study. Cardiovasc Diabetol. 2021;20:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Croyal M, Wargny M, Chemello K, Chevalier C, Blanchard V, Bigot-Corbel E, Lambert G, Le May C, Hadjadj S, Cariou B. Plasma apolipoprotein concentrations and incident diabetes in subjects with prediabetes. Cardiovasc Diabetol. 2022;21:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Digenio A, Dunbar RL, Alexander VJ, Hompesch M, Morrow L, Lee RG, Graham MJ, Hughes SG, Yu R, Singleton W, et al. Antisense-mediated lowering of plasma apolipoprotein c-iii by volanesorsen improves dyslipidemia and insulin sensitivity in type 2 diabetes. Diabetes Care. 2016;39:1408–1415 [DOI] [PubMed] [Google Scholar]
- 74.Gouni-Berthold I, Alexander VJ, Yang Q, Hurh E, Steinhagen-Thiessen E, Moriarty PM, Hughes SG, Gaudet D, Hegele RA, O’Dea LSL, et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (compass): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9:264–275 [DOI] [PubMed] [Google Scholar]
- 75.Avall K, Ali Y, Leibiger IB, Leibiger B, Moede T, Paschen M, Dicker A, Dare E, Kohler M, Ilegems E, et al. Apolipoprotein ciii links islet insulin resistance to beta-cell failure in diabetes. Proc Natl Acad Sci U S A. 2015;112:E2611–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmberg R, Refai E, Hoog A, Crooke RM, Graham M, Olivecrona G, Berggren PO, Juntti-Berggren L. Lowering apolipoprotein ciii delays onset of type 1 diabetes. Proc Natl Acad Sci U S A. 2011;108:10685–10689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu YZ, Cheng X, Zhang T, Lee S, Yamauchi J, Xiao X, Gittes G, Qu S, Jiang CL, Dong HH. Effect of hypertriglyceridemia on beta cell mass and function in apoc3 transgenic mice. J Biol Chem. 2016;291:14695–14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allan CM, Walker D, Segrest JP, Taylor JM. Identification and characterization of a new human gene (apoc4) in the apolipoprotein e, c-i, and c-ii gene locus. Genomics. 1995;28:291–300 [DOI] [PubMed] [Google Scholar]
- 79.Zhang LH, Kotite L, Havel RJ. Identification, characterization, cloning, and expression of apolipoprotein c-iv, a novel sialoglycoprotein of rabbit plasma lipoproteins. J Biol Chem. 1996;271:1776–1783 [DOI] [PubMed] [Google Scholar]
- 80.Kamboh MI, Aston CE, Hamman RF. DNA sequence variation in human apolipoprotein c4 gene and its effect on plasma lipid profile. Atherosclerosis. 2000;152:193–201 [DOI] [PubMed] [Google Scholar]
- 81.Xu S, Cheng J, Li NH, Chen YN, Cai MY, Tang SS, Huang H, Zhang B, Cen JM, Yang XL, et al. The association of apoc4 polymorphisms with premature coronary artery disease in a chinese han population. Lipids Health Dis. 2015;14:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, et al. Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of apoc-iii. J Am Coll Cardiol. 2017;69:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Das Pradhan A, Glynn RJ, Fruchart JC, MacFadyen JG, Zaharris ES, Everett BM, Campbell SE, Oshima R, Amarenco P, Blom DJ, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med. 2022 [DOI] [PubMed] [Google Scholar]
- 84.Hortin GL, Sviridov D, Anderson NL. High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin Chem. 2008;54:1608–1616 [DOI] [PubMed] [Google Scholar]


