Abstract
Since its discovery more than 85 years ago, ferritin has principally been known as an iron storage protein. However, new roles, beyond iron storage, are being uncovered. Novel processes involving ferritin such as ferritinophagy and ferroptosis and as a cellular iron delivery protein not only expand our thinking on the range of contributions of this protein but present an opportunity to target these pathways in cancers. The key question we focus on within this review is whether ferritin modulation represents a useful approach for treating cancers. We discussed novel functions and processes of this protein in cancers. We are not limiting this review to cell intrinsic modulation of ferritin in cancers, but also focus on its utility in the trojan horse approach in cancer therapeutics. The novel functions of ferritin as discussed herein realize the multiple roles of ferritin in cell biology that can be probed for therapeutic opportunities and further research.
Keywords: Ferritin, cancer, iron, nanocarrier
1. Introduction
Ferritin has long been established as an iron storage protein with a central role in iron regulation. In mammals ferritin is composed of 24-subunits and consists of two distinct subunits, H (Heavy, 21 kDa) and L (Light, 19 kDa) chains1,2. The H subunit (FTH1) has ferroxidase activity, which converts Fe2+ to Fe3+ for storage inside the shell, and L subunit (FTL) has a nucleation site for iron. Ferritin H and L subunits are encoded by two different genes, and the proteins co-assemble in various ratios (isoferritins) with a tissue-specific distribution. For example, H chain is expressed highly in the heart, whereas L-chain expressed predominantly in the liver3. In the brain, the oligodendrocytes, microglia, and neurons express ferritin4. Oligodendrocytes express equal amounts of both H and L subunits, whereas microglia are enriched in L-ferritin, and neurons predominantly have the H-subunit of ferritin. In physiological states, ferritin can store nearly 2000 Fe3+. When the cavity is completely saturated, ferritin can store up to 4500 Fe3+ This form of iron storage prevents peroxide and reactive oxygen species production which occurs typically via a Fenton reaction between Fe2+ and H2O2, making ferritin not only a crucial part of the cellular iron trafficking machinery but also an important cellular defense against stress and inflammation5–9. Sequestering iron and converting it to a non-toxic Fe3+ thereby protecting nucleic acids, proteins, and lipids from ROS, is crucial for cell survival as increased ROS could lead to cell death. This is further highlighted by the observation that homozygous murine knock outs of H-ferritin are embryonically lethal10.
Our laboratory has been focusing on ferritin, especially H-ferritin as a potential irondelivery protein11. Furthermore, our and other studies also demonstrated that ferritin is a multi-functional protein with possible roles in proliferation, angiogenesis, antioxidation, and immunosuppression12. In this review, we discuss structure, ferritin uptake and release followed by regulation of ferritin synthesis and an overview of types of ferritins. Additionally, we will be focusing H-ferritin’s role in cancers and its potential as a therapeutic target and as a targeting molecule in cancer therapeutics.
1.1. Ferritin Structure
The H and L subunits are essentially made up of 4 α- helices, A, B, C and D, which form the main bundle and a 5th C-terminal small α-helix, E (Fig. 1). The helices are assembled in a 4-3-2 symmetry to form a hollow spherical cage with an inner and outer diameter of 7~8 nm and 12~14 nm respectively and a thickness of 2~2.5 nm13,14. The symmetrical assembly of helices leaves openings aka channels at 3- and 4-fold subunit junctions which are lined with hydrophilic and hydrophobic residues allowing iron entry-exit and exchange of electron transport respectively15,16. Fe2+ ions enter the protein via the hydrophilic channels and are guided towards the Glu and His rich ferroxidase centers of the H-subunit which oxidize 2 Fe2+ to 2 Fe3+ at a time. Fe3+ ions being unstable at the ferroxidase center migrate to a, Glu rich, nucleation center of the L-subunit6,17–19.
Fig. 1. Structure of Ferritin.
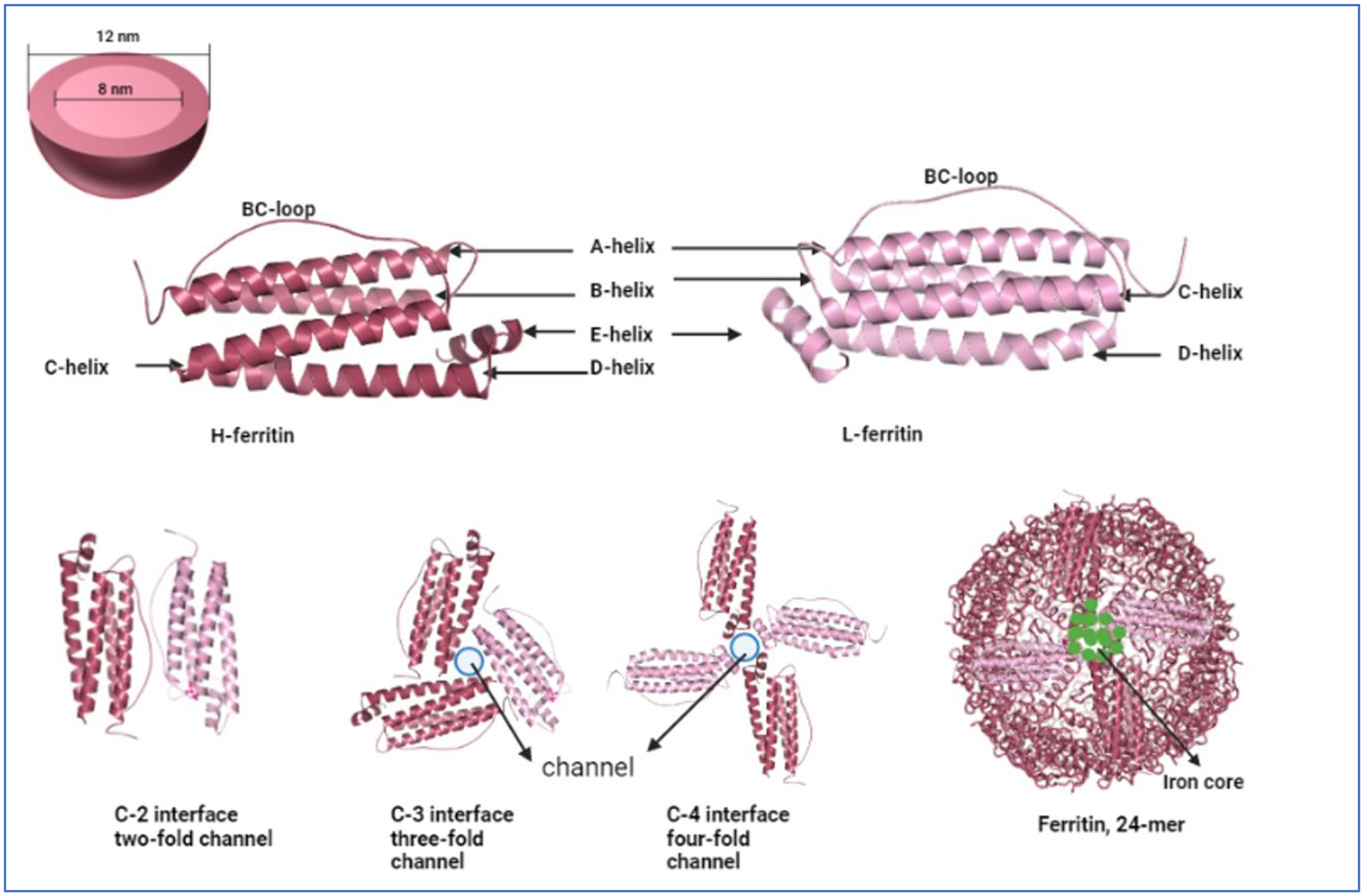
(A) A representation of ferritin as a sphere showing the inner core and outer core diameter. (B) Graphical representation of the identical ferritin H- and L- peptides. Images were built in PyMOL 2.5.4 using ferritin heavy chain PDB ID 1FHA and ferritin light chain PDB ID 2FFX. (C) The various interfaces of the heavy and light chain ferritin. The three- and four-fold channels represent the open channels through which iron atoms can flow in and out of the structure. Finally, a 24-mer representation of the ferritin structure containing iron atoms is also presented. The image is inspired from the review by Chenxi Zhang et al13.
Iron mineralization grows at the nucleation centers inside the shell of the protein accommodating up to 4500 oxygen- and hydroxyl-bridged iron atoms6,16. Mineralized iron in ferritin is in the form of a crystalline ferric oxyhydroxide, (Fe3+)2O3•0.5H2O, typically accompanied by phosphate in ratio of ≥10:1. Iron mineralization grows at the nucleation centers inside the shell of the protein accommodating up to 4500 oxygen- and hydroxyl-bridged iron atoms6,16.
1.2. H-Ferritin Uptake and release
Several studies have indicated H-ferritin is taken up into the brain parenchyma11 and its trafficking across the blood brain barrier (BBB) via endothelial cells20–23. One of the important functions of ferritin being taken from extracellular environment is to deliver iron. Our laboratory has previously established that H-ferritin can replace transferrin as an iron source for oligodendrocytes24. In addition to oligodendrocytes, H-ferritin uptake has been observed in hepatocytes, reticulocytes, lymphoid cells, and erythroid precursor cells25,26. The mechanism of H-ferritin uptake is reported via a receptor mediated binding and endocytic uptake of this protein (Fig. 2)27. Some of the receptors implicated in its uptake, by cells, include TfR128, Tim120,29, Tim2 (rodent models)30,31, CXCR432, and Scara 5 (for FTL specifically)33–35. Some studies focusing on the downstream fate of ferritin, post uptake, have shown ferritin entering the endosomal and lysosomal compartments in MOLT4 T-lymphoblast cell line28.
Fig. 2. Ferritin uptake and release.

Schematic of ferritin uptake and its downstream journey. Left: Ferritin H-chain binding and uptake has been shown to be mediated by receptors TfR1, Tim-1 (human), and Tim-2 (mice). Ferritin L-chain has been shown to bind Scara5 and CXCR4 receptors. Post binding at the cell surface, ferritin is endocytosed into the endosomes. While not all receptors are required simultaneously for endocytosis of ferritin, the schematic represents information from the various reports that have identified the presence of the receptors in question in the endosomes. The hypothesized fate of ferritin post endocytosis is shown. The dotted lines from the endosomal compartments to the endolysosome compartment represent one of the hypothesis for ferritin fate: which states that ferritin enters the lysosome and is degraded due to the acidic milieu of the compartment. Degradation of ferritin causes the release of the stored iron atoms which subsequently leave the compartment and become part of the labile iron pool. Another hypothesis is that ferritin leaves the endosomal compartment as is and becomes part of the intracellular ferritin levels. Intracellular ferritin can be shuttled to the lysosome, via NCOA4, during iron deplete conditions or transported to the nucleus or ubiquitinated and degraded in proteasome. Mitochondrial ferritin (MtFt) is shown in this figure for completeness. See text for discussion. Right: Ferritin has been shown to be secreted via two regulated pathways: (1) secretory autophagy/lysosomal secretory pathway or (2) encapsulation in exosomes, released extracellularly via multivesicular bodies. This image was created by using BioRender.
However, there is a gap in our current understanding of the uptake pathway and the downstream fate of ferritin in cancer cells36. For example, does ferritin enter the endo-lysosomal compartment and release iron in the acidic milieu of that compartment? How do the cells handle the surge in iron (as exogenous ferritin carries anywhere from 100 to 1000 times as much iron as transferrin)? If ferritin is broken down in the lysosomes, do the individual polypeptides of ferritin assemble back into the nanocage and are recycled? All in all, it seems clear that endocytosis of ferritin results in increased intracellular iron suggesting a more efficient iron delivery system at play in times of high iron demand, such as during neural development, rapid growth, and cancers11. Riding on this potential of efficient delivery (of iron) to cells ferritin has been extensively studied for its use as a nanoparticle that can deliver chemotherapeutics to various cancer cells in vivo. It is even more attractive as a nanoparticle delivery agent especially in brain tumors because it can easily cross the BBB. We will discuss this delivery potential of ferritin detail later in this article.
While uptake of ferritin is believed to deliver iron to the cells it is also released from cells potentially as a signal regarding local cellular iron status that can be communicated to other cells or as a mechanism to decrease the cellular iron content. As stated later in this article, newer research has suggested that some of the extracellular ferritin is part of extracellular vesicles which are now beginning to emerge as signaling vesicles between the cells. Because cancer cell signaling in crucial for cell survival, proliferation, drug-and radio resistance ferritin release might emerge as a critical signaling mechanism.
1.3. Regulation
Cellular iron status is the primary regulator of ferritin synthesis inside the cells37. Regulation by iron mainly occurs at the post-transcriptional level when mRNA binding proteins, iron regulatory protein (IRP) 1 and 2, bind to the 5’ stem loop structure on ferritin mRNA to inhibit its translation. The 5’ stem loop structure aka iron responsive element (IRE) is highly conservative in nature and is found on other proteins that are regulated by iron levels (for example, transferrin receptor) as well. During iron scarcity cells rely on iron content stored in their ferritin and allow ferritin degradation by proteasomal or lysosomal machinery to balance the intracellular iron levels. In such iron scarce situations additional ferritin translation is unnecessary and cells accomplish this translation blocking using IRP1/IRP2 binding to 5’ IREs. IRP1 usually exists as a cytosolic aconitase in presence of abundant iron but assumes an open conformation that allows it to bind to IRE. Both FTH1 and FTL, encoded by different highly conserved genes having 3 introns and 4 exons, encode the highly conserved 5’ IRE38,39. Both IRP1 and IRP2 have tissue specific expressions and regulate FTH and FTL in a highly context dependent manner in cancers.
For example, FTH1 but not FTL is specifically downregulated by IRP2 in breast and prostate cancer cell lines40,41. Post transcriptional regulation by miRNAs, binding to mRNA 3’ untranslated regions (UTRs) to inhibit translation or induce degradation of the transcript, is also known. miR335 for example, has been shown to directly bind to FTH1 transcript and direct it for degradation42. Unlike post transcriptional regulation, transcriptional regulation of the ferritin genes particularly FTH1 are less well elucidated. Tissue specific transcription activation of FTH1 gene was shown to be mediated by cyclic AMP and hemin via a 0.1kb B- site located upstream of transcription initiation sequence. However, much work needs to be done to understand the transcription factors binding to the B-site and the various regulators regulating FTH1 transcription43. Apart from iron, pro-inflammatory cytokines (TNFα and IL-1α), growth factors, hormones, oxidative stress, hypoxia, non-coding RNAs (ncRNAs), and some proteins such as p53 have been shown to play a role in regulating ferritin levels in an iron-independent manner44,45. Regulation by these factors is briefly discussed below.
1.3.1. Regulation by oxidative stress
Ferritin is an antioxidant and has a cytoprotective roles against iron catalyzed ROS and oxidative stress. Hence, its regulation by oxidative stress is predictable. Oxidative stress proteins such as Nrf2 activate ferritin gene transcription via the conserved antioxidant/electrophile response element (ARE). FTH1 ARE consists of two activator protein 1 (AP1) binding sites, upstream of the transcription start site. While FTL ARE consists of an additional Maf recognition element (MARE) which makes it highly responsive to oxidative stress; even more than by iron levels46.
Nrf2 directly binds to the AP1 binding sites to activate FTH1 and FTL transcription. Binding of Nrf2 to FTH1 ARE however is highly dependent on epigenetic methylation. In human HaCaT keratinocytes for example methylation of histone H4R3 and H3R17 in the FTH1 ARE region and subsequent binding of protein arginine (R) methyltransferases 1 and 4 (PRMT1 and PRMT4) to the methylated histones drives the activation of transcription47,48. Like Nrf2, other b-zip transcription factors, JunD and JunB, are also involved in binding FTH1 ARE and activating its transcription in HepG2 hepatocarcinoma cells43,49. In erythroleukemia, transcription factor regulatory protein PIAS3 (protein inhibitor of activated STAT3) indirectly activates FTH1 transcription by inhibiting the DNA binding capacity of a repressor, ATF1 (Activating Transcription Factor 1), on ARE sequence49. These studies demonstrate that various oxidative stress responsive proteins potently activate FTH1 in various cancer cells.
1.3.2. Regulation by hypoxia
Hypoxia is a common pathological feature of cancers. Hypoxia promotes tumor malignancy, EMT, angiogenesis and drug resistance. In U87 and U251 glioma cells, FTL was shown to be upregulated under hypoxia and hypoxia inducible FTL was a positive regulator of epithelial mesenchymal transition (EMT). In these cells, Hypoxia-inducible factor 1-alpha (HIF 1α) directly bound to the HRE-3 region on the FTL promoter to activate its transcription50. HIF1α is upregulated in hypoxia and is implicated in promoting tumor growth and metastasis in multiple tumors including glioma, breast, hepatocellular, and hypernephroma51. FTH1 regulation by HIF1A is complex. FTH1 is known to stabilize HIF1A by reducing PHD (HIF prolyl hydroxylase that targets HIF1A for proteasomal degradation) activity through deprivation of Fe2+. Additionally, FTH1 interacts with FIH (factor inhibiting HIF inhibits the transcriptional activity of HIF-1α) and activates its hydroxylase activity that inhibits HIF1A. Thus, FTH1 has opposing effect on two negative regulators of HIF1A. In hypoxic human primary macrophages FTH1 and mitochondrial ferritin expression was elevated and protected these cells from iron induced cell death52. In cancer cells, K562, however, hypoxia only marginally activated FTH1 translation by reducing the IRE-IRP interactions. In the same study, cobalt chloride, a hypoxia mimetic, was shown to reduce FTH1 expression by increasing the IRE-IRP interactions, resulting in translational block of the FTH1 mRNA53. These studies depict a reciprocal regulation between hypoxia/HIF1A and ferritin and further highlight the differences in use of hypoxia mimetics such as cobalt chloride versus hypoxic conditions developed via maintaining 1% O2 gas conditions. A point to remember here is that hypoxia in the tumor microenvironment is a complex state that employs distinct mechanisms for expression of various genes including ferritin genes by targeting both DNA and RNA regulatory elements. While FTH1 might play a role in protecting normal cells from hypoxic damage, cancer cells on the other hand might utilize this same machinery to their advantage in sustaining and promoting tumorigenesis processes.
1.3.3. Regulation by oncogenes and tumor suppressors
One of the first reports of an oncogene regulating FTH1 came in 1993, from a report by Y Tsuji et al., describing preferential repression of FTH1 but not FTL by E1A oncogene in fibroblast cells54. Consistent with this report, proto-oncogene c-myc was also shown to repress FTH1 and stimulate IRP2 expression in highly tumorigenic, c-myc–transformed, Epstein-Barr virus–immortalized B cells55. Another report in U937 cells, a pro-monocytic, human myeloid leukemia cell line, showed c-myc downregulation corresponding with upregulation of FTH1 levels38. C-myc, a proto-oncogene and a transcription factor, is often hyper-activated in cancers. One way to explain reduction in ferritin in presence of c-myc (and other oncogenes described above) is to look at ferritin’s classical role in the cells which is to sequester iron. Repressing ferritin causes an increase in the labile iron pool (LIP) which is considered actively available iron for cellular processes56. In cancer cells, which are often known to be ‘iron addicted’, intracellular iron funds multiple different processes such as cellular proliferation, invasion, migration, and drug metabolism57. Increase in LIP might thus ultimately feed into cancer cell progression mechanisms. This idea has support in a related study showing c-myc expression increasing TfR1 expression suggesting an increase in iron uptake, and thereby LIP, in cancer cells58.
Tumor suppressor p53 regulates cell cycle and apoptosis. Cellular stress, including DNA damage, hypoxia, ribosomal stress, and loss of adhesion activates p53. Once activated p53 can lead to activation of genes that promote growth arrest, cell death, or senescence and repress certain genes that mediate cell proliferation, growth and metastasis59,60. The relationship between p53 and FTH1 is anything but simple. On one hand excess p53 downregulates FTH1 gene via a trimeric transcription factor, NF-Y, in HeLa cells61. On the other, in lung cancer cells, H1299, excess p53 upregulates FTH1 via reducing its binding to IRP159. FTH1 in return also regulates p53. In HEK293T, H1299, and MCF7 cell lines, FTH1 was shown to bind directly with p53 and upregulate its levels during hydrogen peroxide mediated oxidative stress62. In a more recent report, FTH1 upregulated p53 levels, in non-small cell lung cancer (NSCLC) cells, via miR-125b63. These studies suggest that FTH1 perhaps has a tumor suppressive role in conjunction with p53 and can be targeted for specific cancer therapeutics to enhance this activity.
1.3.4. Regulation by non-coding (ncRNA) and pseudogenes
The role of ncRNAs, especially miRNAs, in regulating gene expression, has been extensively studied and continues to attract attention of researchers in cancer and other diseases. miRNAs are 19–25 nucleotides long and can modulate gene expression by inhibiting translation or inducing degradation of mRNA transcripts. A single miRNA could regulate multiple genes and it has been suggested that around 60% of the protein coding genome is regulated by miRNAs. Specific patterns of miRNA expression (miRNome) during different stages, subtypes, tissue- cell type specific, have been explored for cancer diagnosis, prognosis, and therapeutics64,65. Reciprocal regulation exists between miRNAs and FTH1. FTH1 regulates let-7g, let-7f, let-7i and miR-125b expression, and their down-stream genes, in K562 erythroleukemia cell line. While oncogenic miRNA (oncomiRs) miR-200b targets FTH1 expression in highly aggressive breast cell line MDA-MB-231. Moreover, downregulation of FTH1 by miR200b increased the sensitivity of the breast cancer cells to doxorubicin chemotherapy66. miR-335 has been shown to target FTH1 and induce ferroptotic cell death in Parkinson’s disease42. A recent study in prostate cancer identified FTH1 and its pseudogenes to be top targets of oncogenic miRNA, miR638, hinting at a larger miRNA:gene:pseudogene network. Pseudogenes are ncRNA transcripts that act as a sponge for the miRNAs that are targeted to their ancestral genes and thus possess competing endogenous RNAs (ceRNA) activity. Apart from miRNAs, long non-coding RNAs (lncRNA), circular RNAs (circ RNA), pseudogenes, and some mRNAs could compete for and sequester shared miRNAs thus displaying ceRNA activity. Some other oncogenic miRNAs targeting the FTH1 gene:pseudgene network in prostate cancer are miR-19b-3p, 19b-1–5p, 181a-5p, 210–3p, 362–5p and 616–3p67. Interestingly, this network of miRNAs that target FTH1 and its pseudogenes is targetable. In prostate cancer cell lines, FTH1 and pseudogenes have a tumor suppressive role and hence targeting miRNAs that degrade FTH1 gene and pseudogenes could prove to be a beneficial therapeutic strategy67.
Apart from the above discussed modes of regulation, growth hormones, cytokines, inflammation also regulate ferritin expression. The readers are guided to the excellent review on these additional regulatory mechanisms by F. M. Torti et al38. These different regulatory pathways of ferritin highlight the diversity and complexity of its post-transcriptional regulation. Considering the intra and inter- tumoral heterogeneity and specialized roles of ferritin in the different cells in the tumor microenvironment (TME) these different modes of regulation seem very plausible.
1.4. Types of Ferritin
Ferritin mainly exists in the cytosolic compartment of the cells. However, it has also been observed in the nuclear and mitochondrial compartments68–71. Extracellularly, ferritin has been reported in the serum, urine, synovial and cerebrospinal fluids10,72,73. The presence of ferritin in different sub-cellular and extracellular regions provides evidence of its multi-functional capacity which is discussed in this review further below.
1.4.1. Cytosolic ferritin
Cytosolic ferritin is the most abundant form of ferritin. The structure and function of ferritin discussed above are of a typical cytosolic ferritin.
1.4.2. Mitochondrial ferritin
Mitochondrial ferritin, as the name suggests, is located, specifically in the mitochondrion. Mitochondrial ferritin (MtFt) resembles H-ferritin subunit in structure and function but lacks the IRE sequence and is synthesized as a precursor that is targeted to mitochondria with the help of a 60 amino acid leader sequence74,75. The leader sequence is processed inside the mitochondria yielding a 22 kDa H-ferritin like protein which assembles into a 24 subunit homopolymer shell possessing ferroxidase activity76. Since, MtFt lacks IRE sequence, it is found to be regulated by epigenetic mechanisms activated in response to oxidative stress77. MtFt functions to regulate mitochondrial iron levels thereby reducing the reactive oxygen species (ROS) formation and instigating a cytoprotective effect on the cells. MtFt is maintained at low levels in normal cells as higher levels siphon off iron from cytosol to mitochondria, leading to a reduced availability of iron in cytosol, causing an iron deficient phenotype. However, higher expression of MtFt is observed in tissues that have high oxygen consumption and increased metabolic activity such as testes, brain, and erythrocytes74,78. Higher expressions of MtFt have also been observed in disease states such as sideroblastic anemia, refractory anemia with ringed sideroblasts, and in in vivo cancer cells79. In normal cells, MtFt functions to regulate mitochondrial iron levels thereby reducing the reactive oxygen species (ROS) formation and instigating a cytoprotective effect on the cells. For example, cytoprotective effects of MtFt were observed when its overexpression inhibited erastin induced ferroptosis (iron induced cell death) in SH-SY5Y, neuroblastoma, cells. Overexpression of MtFt even rescued transgenic drosophila, fed an erastincontaining diet, from dying as compared to the drosophila with normal MtFt levels80.
In cancer cells, however, MtFt plays an important role in being a tumor suppressor. For example, MtFt was found to be overexpressed in the HeLa and leukemic (K562) cells causing apoptosis of cells via cytosolic iron deprivation induced activation of Janus kinase 2 (JAK2) - signal transducer and activator of transcription 5 (STAT5) pathway71,79,81. Tumor inhibitory effects of high MtFt levels, likely due to cytosolic iron deprivation, were also observed in non-small cell lung carcinoma (H1299) cells82. High expressions of MtFt in neuroblastoma cell lines (SH-SY5Y) were shown to have an inhibitory effect on cellular proliferation. In SH-SY5Y, MtFt caused upregulation of tumor suppressors p53 and N-myc downstream-regulated gene-1 (NDRG1) and downregulation tumor promotors such as C-myc, N-myc and p-Rb75. Another example of tumor suppressor function of MtFt was observed when ovarian cancer cells overcame cisplatin resistance in presence roflumilast induced up-regulation of MtFt via the activation of cAMP/PKA/CREB signals83,84. Thus, MtFt plays key roles in iron metabolism impacting important cellular functions such as proliferation, apoptosis, ferroptosis, iron metabolism and oxidative stress management.
1.4.3. Nuclear ferritin
Nuclear ferritin has been observed in some cell types such as macrophages, hepatocytes, reticular, muscle, and nerve cells. It has also been observed in some brain tumors and glial cell lines in vitro. Unlike mitochondrial ferritin, nuclear ferritin is translated from the same mRNA that generates cytosolic ferritin. However, O-linked glycosylation of the six putative glycosylation sites on the nuclear ferritin sets it apart from the cytosolic ferritin and at the same time it enables it to translocate into the nucleus via an ATP dependent mechanism57,69. Since nuclear ferritin is produced from the same mRNA as FTH1, its expression is also primarily regulated by the IRE-IRP system. Functionally, nuclear ferritin protects cells from the UV and iron-induced oxidative stresses. In 2002, our group showed for the first time, that nuclear ferritin binds to DNA and protects it from iron-induced oxidative damage by sequestering excess iron70,85,86. Structurally, nuclear ferritin, like mitochondrial ferritin, is mainly composed of the H-subunits. However, unlike mitochondrial ferritin which has a leader sequence, nuclear ferritin lacks a nuclear localization signal (NLS)87. Even though no specific NLS has been found on the nuclear ferritin, a separate transporter called ferritoid which contains an NLS has been shown transporting nuclear ferritin in the corneal epithelium (CE) cells. Ferritoid mediated transport of nuclear ferritin is believed to be specific to CE cells. CE cells are in fact believed to be highly tolerant to UV mediated DNA damage due to the protective effect of nuclear ferritin88. Protective role of nuclear ferritin led our group to perform studies on the role of nuclear ferritin in cancer cells where we have shown that reducing ferritin levels in the cancer cells increases their sensitivity to radiation and chemotherapy89,90. Thus, nuclear ferritin seems to be facilitating tumorigenesis.
1.4.4. Serum and other extracellular ferritins
Extracellular ferritin is the secreted form of ferritin present in various body fluids such as serum, urine, synovial, and cerebrospinal fluid. Serum ferritin (SF) is an extracellular ferritin that is the most clinically assessed biomarker as its concentrations may reflect systemic iron levels with < 100 ng/ml corelating with iron deficiency and > 800 ng/ml corelating with iron overload. SF, a 24-mer protein that resembles L-ferritin immunologically and structurally, is thus, a marker of choice when iron deficiency or anemia is suspected91–94. SF levels are also upregulated in many other diseases with inflammatory biology including COVID-19 infections95, autoimmune diseases96, and cancers97. High SF levels are in fact reported in numerous cancers including glioblastoma, neuroblastoma, breast, renal, and cervical cancers and is often associated with poor survival in these patients57. Nevertheless, because increased levels of SF are reported during inflammatory conditions, which often confounds several diseases, much caution must be administered in using SF as a disease detection marker95. Iron saturation levels of SF are a matter of controversy in the existing literature as differing reports have been published. One group demonstrated that hemochromatosis patients have SF which is iron poor while another demonstrated that hemochromatosis patients have iron rich SF98,99. In general, however, it can be summarized that SF is found to be saturated with iron from anywhere between 5% to almost 50% depending on certain factors such as inflammation or developmental stage. Inflammation in general causes SF to have low iron saturation due to an obstruction in the mobilization of iron from the reticuloendothelial system. In terms of relative iron content, SF is known to carry less iron atoms when compared to liver ferritin. Liver ferritin can carry 4500 iron atoms100 while SF can carry ~ 700 iron atoms. Although SF carries less iron atoms relative to other cytoplasmic ferritins it still carries higher iron atoms than Transferrin (TF) protein which can carry only two iron atoms26.
Many cell types including hepatocytes and macrophages have been shown to secrete ferritin in normal and disease states. Bone marrow derived macrophages secrete extracellular ferritin that is composed of 50% H- and 50% L- subunits. This composition is slightly different from the typical SF, which is primarily of the L subunit type, suggesting that macrophage secreted ferritin could be carrying a larger iron payload than the typical SF101. Tumor associated macrophages (TAMs) in certain cancers such as melanoma and breast cancer have been shown to secrete ferritin into the tumor microenvironment102,103. In breast cancer, secreted ferritin from TAMs, induced by tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL-1β), was found to stimulate proliferation of the cancer cells102–104. Taken together, this raises the possibility that released/secreted ferritin, with higher H- subunit composition, could be functioning as an iron delivery protein during times when cells need considerably more iron, such as during development, growth, and in iron addicted tumors (tumor cells), than what can be delivered by just TF.
Ferritin in cerebrospinal fluid (CSF) is a secreted, extracellular, form of ferritin that has been shown to be elevated in certain inflammatory neurologic disease and in patients with malignant involvement of the CNS. In 2008, a group from Brazil showed that CSF ferritin levels were highest in group of patients with malignant infiltration as compared to 4 other groups; (1) that had no malignancy, (2) with inflammatory neurological diseases, (3) with neurocysticercosis, (4) and with acute bacterial meningitis. This data led to the conclusion that CNS ferritin could be used as an adjuvant biomarker for diagnosing CNS malignant infiltrations105–107.
A portion of the SF has been shown to be glycosylated and binds to concanavalin A91,108. Because of glycosylation of SF, classical endoplasmic reticulum (ER) - Golgi secretory pathway is believed to be involved in secretion of some portion of SF. However, some other reports have shown a lack of secretion signal on SF ruling out the classical endoplasmic reticulum - Golgi secretory pathway101. In such cases, two non-classical secretory pathways that have been identified, so far, for ferritin are: a lysosomal secretory pathway demonstrated by Cohen et al. in 2010 and a multivesicular body-exosome pathway described by Truman-Rosentsvit et al. in 2018102,109–111. Ferritin is found in exosomes of neuroblastoma cells112,113 and more recently in exosomes released from brain endothelial cells114. Thus, secretion/release via lysosomal secretory, exosomal, or ER-Golgi pathway, all organelle-based, regulated pathways, suggests that ferritin’s presence in the extracellular milieu is not a product of cell damage or death but a regulated signaling mechanism. All in all, iron content of the ferritin, its mode of release, cell type from which it is released, and the target cell type where it is taken up might drive the function of the released ferritin. For example, ferritin released in extracellular vesicles might play a significant role in cell-cell signaling or might be ousted from the cancer cells as a way to export iron out of the cells to avoid iron mediated cell death (ferroptosis)111. Whereas ferritin released from macrophages, containing high iron content, might play a role in iron delivery to the neighboring tumor cells in the tumor microenvironment. Thus, there are multiple functions to ferritin that are released via multiple different pathways showing the ability of cells including cancer cells to fine tune mechanisms that help drive their survival and growth.
2. Ferritin in cancer
Dysregulation of iron metabolism has been associated with multiple cancers. Frequently termed as “iron addicted”, cancer cells have evolved to regulate the expression of multiple iron metabolism proteins to not only suffice their abnormal iron demands but to utilize them in various capacities exceeding their specified roles115. Ferritin protein and mRNA levels are reportedly dysregulated in many cancers including breast103, glioblastoma116, and prostate117. The significance of altered ferritin levels in cancer is highlighted in the following section and a schematic overview of the cancer processes affected by FTH1 is also presented (Fig. 3).
Fig. 3. Ferritin in cancer.
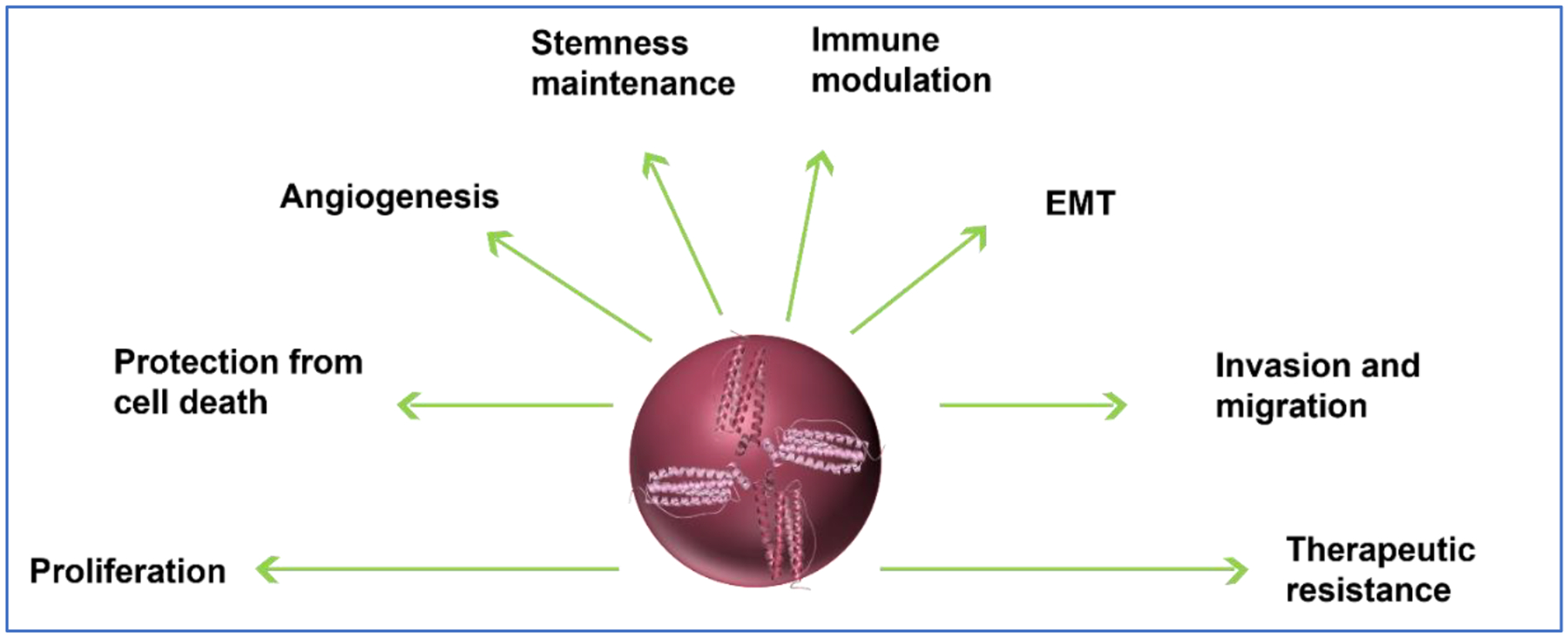
Schematic of a broad overview of ferritin’s impact on multiple processes in cancer.
2.1. Functional role of ferritin in cancer
Functionally ferritin impacts various cellular processes in cancer cells ranging from proliferation, apoptosis, and EMT. Its individual role in cellular processes is summarized below.
2.1.1. Proliferation
Uncontrolled proliferation is a hallmark of cancer cells. Ferritin has been shown to support proliferation of cells irrespective of its iron content. Breast cancer cell lines such as MCF7 and T47D, for example, were shown to proliferate in presence of ferritin in an iron independent manner103. Upregulated FTH1 expression is associated with poor survival in multiple cancers, such as, Brain lower grade glioma (LGG), Acute myeloid leukemia (LAML), Head and neck squamous cell carcinoma (HNSC), and uveal melanoma (UVM). A recent study in hepatocellular carcinoma (HCC) cells, HCCLM3 and MHCC97H, showed that upregulated FTH1 expression enhanced cell proliferation by reducing peroxide and ROS levels118. FTL levels are also associated with cancer proliferation. For example, in Glioblastoma (GBM) FTL is upregulated, and it promotes cell growth via deactivation of pro-apoptotic GADD45A/JNK pathway. FTL was shown to bind GADD45A further suggesting that it could be inhibiting dimerization, essential for activation of GADD45A. In the same study, FTL was shown to be associated with upregulation of c-myc and cyclin D1 which further contributed to promoting proliferation of the cells119. In HeLa cells, upregulation of FTL levels was shown to increase proliferation in an iron - independent manner120. FTL was also shown to promote proliferation of osteosarcoma, MG-63, cells via GADD45/JNK pathway regulation121. It is not just the intracellular expression of ferritin that affects proliferation. As described in the previous section, extracellular ferritin secreted from TAMs also stimulates cancer cell proliferation via tumor microenvironment reprogramming. All in all, ferritin behaves as a positive regulator of proliferation in many different cancers.
2.1.2. Apoptosis
While many cancer cells have increased proliferation in presence of increased FTH1, association of FTH1 with tumor suppressor p53 has been shown to decrease the proliferation in H460 and A549 non-small-cell lung cancer (NSCLC) cells. In the H460 and A549 cell lines, FTH1 was shown to upregulate the expression of p53 by downregulating a p53 inhibitor miR125b via an indirect epigenetic mechanism. Increased p53 in presence of increased FTH1 led to induction of apoptotic pathway in these cells63,122. In human erythroleukemic K562 cell line high MtFt led to increased apoptosis in cells through cytosolic iron sequestration and JAK2/STAT5 pathway activation79. Contrasting the apoptotic activity, downregulation of the FTH1 at transcriptional and translational levels, by using antisense oligonucleotides, led to specific increase in apoptosis in MCF7 breast cancer cell line, suggesting FTH1’s role in protecting the cancer cells from apoptotic cell death123. In another report, increased expression of a ferroxidase mutant and wild type FTH1 in HeLa cells was shown to rescue from TNFα induced apoptosis in these cells124. Further suggesting that FTH1 participates in anti-apoptotic activity in some cancer cells irrespective of its iron content potentially by physically binding and inhibiting apoptotic activators. Indeed, in a separate report, in 293T cells, FTH1 was shown to bind and inhibit key apoptotic activating protein Bax125. In melanoma cells derived from a primary cutaneous melanoma, FTL downregulation was shown to inhibit cell proliferation in vitro and cell growth in vivo and increase sensitivity to apoptosis126. All this evidence suggests that although ferritin has a context dependent role in different cancers it primarily behaves as an oncogenic protein in protecting cancer cells from apoptotic cell death and supporting cell growth and proliferation.
2.1.3. Epithelial-mesenchymal transition (EMT)
EMT is a process whereby epithelial cells take on mesenchymal features i.e., they acquire fibroblast-like phenotype with reduced adhesive and augmented motility. Regulated EMT is mainly seen during development and adult tissue regeneration stages. Whereas in cancers, EMT is highly deregulated allowing cancer metastasis and invasion127. In lung cancer cell line A549, transforming growth factor beta 1 (TGFβ1) triggered EMT was enhanced in presence of autophagic degradation of FTH1. It was shown that autophagic degradation of FTH1 induced iron-driven oxidant injury that caused a feed forward loop which further increased autophagy during EMT128,129. FTH1 downregulation was shown to induce EMT in breast cancer cell lines MCF7 and H460 as well. In MCF7 and H460 cells, FTH1 silencing was associated with activation of two crucial EMT regulating pathways; c-x-c motif chemokine 4 (CXC4)/c-x-c motif chemokine ligand 12 (CXCL12) CXCR4/CXCL12 and iron/ROS. In contrast to FTH1, FTL levels were observed to either increase or decrease the metastatic, proliferative, and invasive capacities of the cancers in a context specific manner. For example, in osteosarcoma cancer cell line, MG-63, high FTL levels decreased the metastatic potential via a reduction in metastatic protein players such as CDH2 and Vimentin121. Whereas, in GBM, FTL downregulation repressed EMT via AKT/GSK3β/β-catenin signaling both in vitro and in vivo130. A process closely dependent on acquisition of EMT features in the cancer cells is invasion capacity131. Ferritin affects EMT in cancer cells suggesting that it might directly or indirectly (through iron delivery) affect the invasion capacity of the cells as well.
2.1.4. Angiogenesis
New growth in vascular network is required for cancer cell progression, dissemination, attainment of nutrients and oxygen, and removal of waste products. New growth of blood vessels from the existing ones, aka angiogenesis, depends on several activating and inhibiting factors. FTH1 has been shown to have either inhibitory or activating roles in angiogenesis depending on its interactions with these factors. For example, in human prostate cancer xenograft model, FTH1 was shown to support vessel growth by binding to and subsequently inhibiting the 22 amino acid antiangiogenic subdomain of HKa132,133. On the other hand, in colon cancer cell lines, HCT116 and SW480, FTH1 was shown to repress the HIF1α transcriptional activity in normoxic and hypoxic conditions134. HIF1α is an oxygen sensitive transcriptional mediator of hypoxia which regulates angiogenesis via activating factors such vascular endothelial growth factor (VEGF), placental growth factor (PIGF), and angiopoietins135. The current literature on the relationship of FTH1 is contradictory and the impact may relate to its iron status. For example, FTH1 activity on blood vessels was shown to be independent of iron.132
2.1.5. Therapeutic resistance
Normal cells activate various endogenous stress mitigation pathways (SMPs) to survive when they are exposed to stressors such as DNA damage, starvation, toxins, infections, etc. Cancer cells utilize these endogenous SMPs to survive when they are exposed to stressors such as those coming from a variety of cancer therapeutics. These SMPs are not limited to individual cells but also encompass heterotypic cell-cell signaling which is required for maintaining the right tumor microenvironment that can support in mitigating the stresses. Genetic modulations such as upregulation of multi drug resistant (MDR) genes, reinforcement of DNA repair systems, and rewiring of the cell signaling pathways such as incapacitation of cell death pathways, and fortification of antioxidant defenses are some of the SMPs evolved by cancer cells136. Intrinsic drug resistance mechanisms include pre-existing clones and some genetic mutations while acquired drug resistance mechanisms include non-genetic modifications such as cancer cell plasticity, and microenvironment modulations. Drug efflux, inactivation and/or alterations in the drug targets are also commonly acquired drug resistance mechanisms136,137. Since ferritin is an important player in antioxidant defense, many cancer cells utilize altered ferritin expression to survive the stresses from various cancer therapeutics such as chemo-, radio-, and immunotherapies. One of the first reports of FTH1 involvement in cancer resistance came from a study in leukemia cells that had enhanced resistance to oxidants in presence of elevated FTH1 levels138. FTL is also implicated in conferring resistance against oxidants in melanoma cells126. In erythroleukemia cells, increased FTH1 levels increased resistance to oxidative stress via increasing expression of multidrug resistance mutation 1 a (MDR1a) protein139. Nuclear ferritin’s ability to protect the DNA is involved in resistance against DNA alkylating agents such as doxorubicin, BCNU, and cisplatin in glioma, breast, and leukemia cells66,89,140,141. Given nuclear ferritin’s role in protecting the DNA from damage, our laboratory has established its role in conferring radioresistance in U251 glioma cells in vitro and in vivo142. Another report of FTH1’s involvement in radioresistance comes from a recent study of lipid droplets (LDs) in breast, bladder, lung, neuroglioma, and prostate cancer cells. In this study, it was shown that increased number of LDs, carrying elevated FTH1, was associated with radioresistance in these cells143. While FTH1 seems to be conferring radio resistance in some cancers, its absence in certain others such as ovarian cancer cells, SKOV3, activates NF-κB induced chemoresistance144. Based on these reports modulating FTH1 levels in cancer cells might be a mechanism to alleviate therapeutic resistance in cancers.
2.1.6. Cancer initiating cells
Cancer initiating cells aka cancer stem cells (CSCs) have been shown to be iron addicted. CSCs demonstrate increased iron uptake, and storage along with a reduced export. There is considerable evidence that suggests that iron is essential for maintaining stem- cell phenotype. High FTH1 expression was recently associated with CSC features in breast145, prostate145, and cholangiocarcinomas146. FTH1 downregulation in GBM CSCs has been shown to inhibit it’s in vivo tumorigenic potential. In these cells, FTH1-STAT3-FOXM1 axis was uncovered to be maintaining stemness and survival116. Reports from our laboratory have also confirmed FTH1’s role in survival and stemness of GBM CSCs90.
3. Ferritin in cancer therapy
Ferritin is an attractive target for cancer therapy. It has been shown that siRNA mediated downregulation of FTH1 in HeLa cells147, and human glioma cells89 increases chemosensitivity. FTH1 downregulation by siRNA also showed selective radiation sensitivity of GBM CSCs90. Thus, cellular processes that influence ferritin levels such as ferritinophagy and ferroptosis may be targetable for therapeutic purposes. Below, we discuss the potential of targeting these pathways for cancer therapy and further discuss the potential of ferritin itself aiding in transporting therapeutics to tumors.
3.1. Ferritinophagy
Ferritinophagy is the selective autophagy process whereby nuclear receptor coactivator 4 (NCOA4) binds to ferritin to traffic it to the lysosomes where it is degraded, and iron stored within it released148. NCOA4 is crucial for iron homeostasis at cellular and systemic levels as dysregulation of NCOA4 renders cells susceptible to ferroptosis. During selective autophagy, degradation cues guide the selective receptors, such as NCOA4, to recognize, bind and link their bound cargo to the autophagosomal membrane by interacting with lipidated ATG8 proteins (LC3/GABARAP). NCOA4 binds directly with a conserved c-terminal domain on FTH1 subunit. NCOA4 is regulated by the iron levels in the cells. During iron replete conditions, NCOA4 is recognized by a multifunctional ubiquitin E3 ligase, HERC2, and directed for degradation via the ubiquitin–proteasome system149. NCOA4 mRNA and protein levels have been shown to be relevant to carcinogenesis in many different cancers. In ovarian cancer for example, NCOA4 mRNA and protein levels were upregulated in multiple malignant subtypes; serous, serous papillary, and mucinous. A knockdown of NCOA4 in the malignant ovarian cancer cells increased FTH1 levels which was accompanied by reduced cell survival150. Recently, upregulation of NCOA4 has also been reported in patient derived and murine PDAC models of pancreatic ductal adenocarcinoma (PDAC). Ablation of NCOA4 in these PDAC models, improved survival and delayed tumor progression emphasizing the critical role of NCOA4 mediated ferritinophagy in survival and tumor progression151. Low NCOA4 levels, on the other hand, were recently discovered to be associated with high grade malignancy, poor survival, and defective immune cell infiltration in clear cell renal carcinoma (ccRCC). NCOA4 depletion in ccRcc increased FTH1 levels and lowered the occurrence of ferroptosis (iron mediated cell death)152. Thus, an imbalance in FTH1 levels due to dysregulated NCOA4 plays a crucial role in carcinogenesis and ferroptosis. NCOA4 from the ferritinophagy pathway can thus be targeted depending on the cancer type, to initiate mechanisms that trigger cancer cell death. Further evidence and importance of NCOA4/FTH1 mediated ferritinophagy in ferroptotic cell death is provided below.
3.2. Ferroptosis
Ferroptosis is a recently discovered form of regulated, iron-dependent, cell death pathway. Membrane damage due to oxidation of polyunsaturated fatty acid (PUFA)-containing phospholipids because of a reduction in glutathione peroxidase (GPX4) activity and accumulation of redox active iron are the trademarks of ferroptotic cell death153. Multiple different cellular pathways such as KEAP1/NRF2, iron metabolism, EMT, cell adhesion, ferritinophagy, and RAS/MAPK, and cell intrinsic molecules such as p53, GPX4, and system xc− regulate ferroptotic cell death153. Small molecules that can inhibit GPX4 or system xc− (an antiporter which imports cystine into the cells) such as (1S, 3R)-RSL3 and erastin, respectively, have been shown to induce ferroptosis154. One of the first reports of ferroptotic cell death induction by RSL3 and erastin came from a screen to identify small molecules that can selectively be lethal to RAS-mutant tumor cells (hence the name RAS-selective lethal (RSL) compounds)155,156. There are various other small molecules that can induce ferroptosis via multiple different routes that have been covered in excellent reviews by Dixon and Stockwell153, Mou et al.157, and Lu et al.154. Clinically, immunotherapy and a combination of immuno- and radiotherapy have been shown to synergistically induce cancer cell death via ferroptosis158,159. Administering drugs that might trigger ferroptosis in drug resistant cancer cells is an area that is actively under research. There are three main pathways that can be targeted to induce ferroptosis to reverse drug resistance cancers: (1) canonical GPX4-regulated pathway, (2) iron metabolism pathway, (3) and lipid metabolism pathway160. One of the ways iron metabolism pathway can be targeted is via modulating labile iron pool of the cells. For example, dihydroartemisinin, an antimalarial drug, was found to increase labile iron pool in cisplatin resistant pancreatic ductal adenocarcinoma consequently triggering ferroptototic cell death161. Similarly, inhibitors of the divalent metal transporter 1 (DMT1), a membrane protein which allows translocation of ferrous iron (Fe2+) to the cytosol following iron endocytosis, were shown to increase labile iron pool and subsequently trigger ferroptosis in drug resistant breast cancer stem cells162. Another way to target iron metabolism pathway to induce ferroptosis is via modulating FTH1 levels. One of the first reports of involvement of FTH1 in ferroptosis comes from a study by N. D. Yang et al. where an anti-malarial repurposed for cancer therapy, artesunate, was reported to induce ferroptosis in HeLa and hepatocellular carcinoma, HepG2, cells. In that study, overexpression of FTH1 directly and indirectly, via knockdown of NCOA4, was able to rescue artesunate mediated ferroptosis163,164. Artesunate was found to induce ferroptosis, in a similar manner, in the highly apoptotic resistant pancreatic cancer cells as well165. A role for FTH1 in ferroptosis was further confirmed in a study by Y. Q. Wang et al. that showed that overexpressing FTH1 in neuronal cells inhibited erastin induced ferroptosis80,166. In another report degradation of NCOA4 in pancreatic and fibrosarcoma cancer cells was shown to suppress ferroptosis167. In GBM mice models, downregulation of COPZ1 (Coatomer protein complex subunit zeta 1) was shown to induce ferroptosis by increasing NCOA4 mediated ferritinophagy downregulation of FTH1. Thus, COPZ1/NCOA4/FTH1 axis could be another target for destroying GBM cells168. Taken together, high FTH1 levels protect cancer cells from ferroptotic cell death and therapeutic strategies that can downregulate FTH1 levels, thereby triggering ferroptosis, might prove to be a promising target for cancer therapeutics.
3.3. Ferritin Nanoparticles
Ferritin’s use as nanoparticles is an area that has expanded in the past few decades. Detailed descriptions on ways to engineer ferritin for its use as a therapeutic carrier can be found in literature169–174. Here we provide an overview on ferritin and its various uses in cancers. As described previously, 24-mer ferritin has a hollow spherical core where iron atoms are stored in a mineralized form. The self- assembling property of the ferritin 24-mers, into a spherical shape, is highly pH dependent. Where extreme pH of 2–3 or 10–12 causes the spherical shell to decompose into subunits, physiological pH causes the re-assembly of the protein into a spherical shell. This renders ferritin a highly useful drug carrier as it is easy to package the drug in it with mere pH manipulations. Apart from easy packaging some of the other properties that make ferritin an ideal nanocarrier are: (1) robust tumor targeting ability: ferritin is highly permeable through the vasculature and is often enriched at the tumor site. Speculated reasoning behind this enrichment is the uptake of ferritin by tumors for their superior iron needs. Indeed, we have recently demonstrated that FTH1 binds to GBMs, and the amount of binding is sexdependent (Revised Manuscript under review) providing novel clinical considerations for using ferritin as a therapeutic target, (2) excellent biocompatibility and non-immunogenicity: ferritin is highly conserved among various organisms38. Moreover, recombinant human ferritin can also be easily expressed in E.coli and scaled up as required, (3) good stability: ferritin can withstand high temperatures up to 75 °C for 10 min. It is also highly stable in presence of various denaturants175,176, (4) easily modifiable: lysine and cysteine residues on the outer surface of ferritin allow easy modifications to obtain better targeting capabilities. For example, EGF ligand has been conjugated on the surface of recombinant human ferritin to target it to the EGFR receptors in breast cancers, (5) hydrophobic drug packaging: the inner surface of the protein allows packaging of hydrophobic drugs as well and since most of the cancer therapeutics are hydrophobic in nature it can carry a vast variety of hydrophobic molecules177–180, (6) versatility: ferritin is straightforwardly genetically and chemically modifiable increasing its versatile use in medical therapeutics, imaging, diagnostics, bioelectronics, and non-medical applications such as water purification, and as a bioactuator for potential development as artificial muscles181,182. This can open doors to combined photo- and chemotherapy treatments. All in all, ferritin has the potential to become an ideal drug carrier for various chemotherapeutics (Fig. 4)183–185.
Fig. 4. Ferritin in nanotherapeutics.
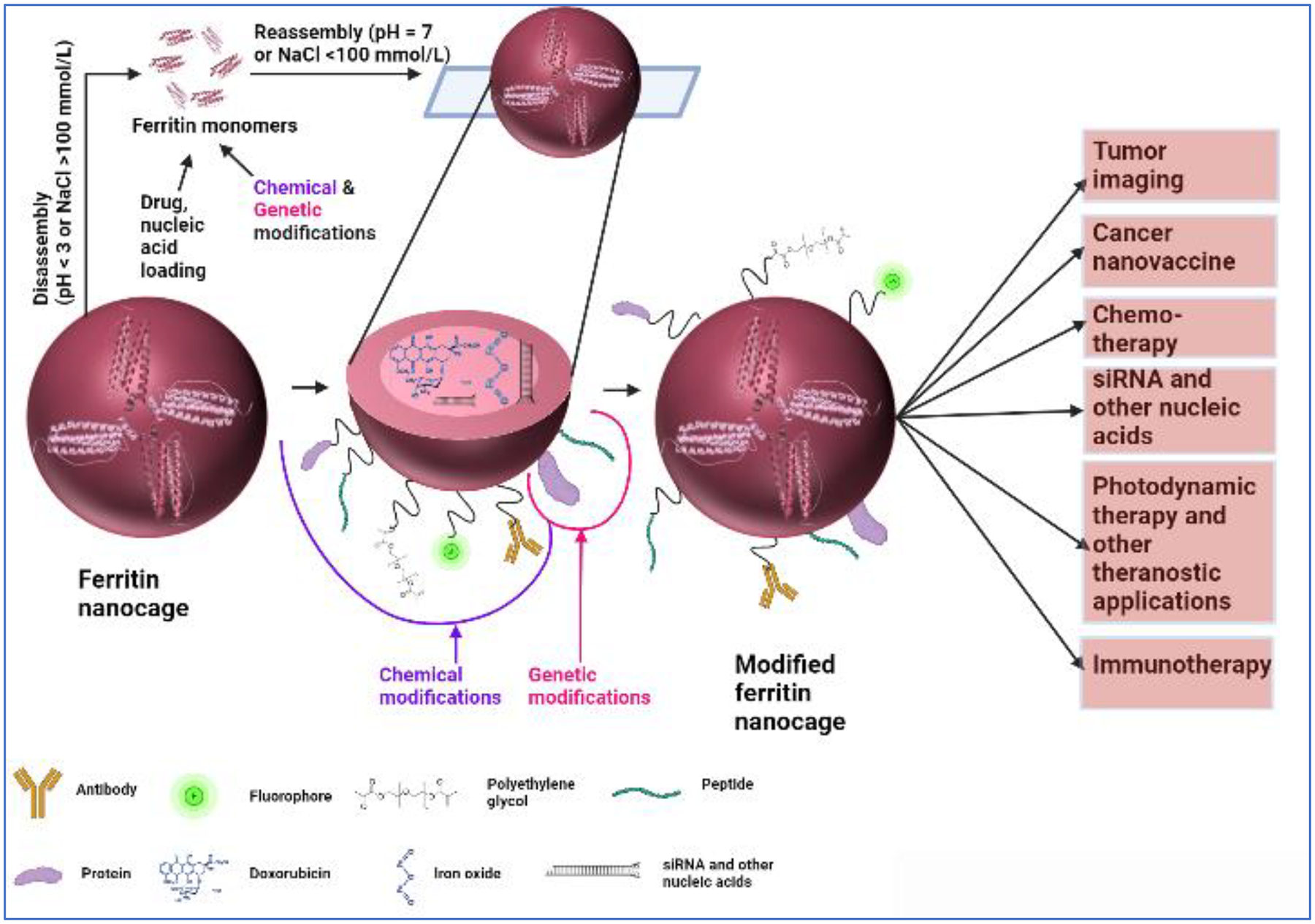
Ferritin 24-mer hollow sphere can be utilized in a trojan horse approach to deliver chemotherapeutics to tumors. Specific targeting of the ferritin protein can be achieved by genetically or chemically attaching peptide or peptide ligand molecules that bind to tumor specific receptors. Apart from peptides a variety of other molecules such as polyethylene glycol, fluorophores, and antibodies can be attached to ferritin surface. Furthermore, ferritin nanocage can also be loaded with imaging agents, iron oxide, and nucleic acids such as siRNA. Loading and modifying ferritin with these different molecules makes it useful in a wide variety of applications such as tumor imaging, photodynamic and immunotherapy, and tumor nano-vaccines. This presentation was created with the help of BioRender.
There are numerous reports of ferritin being used as a nanocage drug carrier for a variety of chemotherapeutics in different cancers186. Typically, metal containing drugs such as cisplatin, Gd-DO3A, and deferoxamine B are encapsulated in the ferritin nanocage via passive loading i.e., allowing the molecules to enter ferritin via its six hydrophobic channels and eight hydrophilic channels at room temperature. In a study in U87 GBM cells, doxorubicin conjugated with Cu2+ is packaged, via hydrophilic channels, in surface engineered human ferritin molecules. It was shown that doxorubicin-Cu2+ complex packaged this way in ferritin had significantly enhanced pharmacokinetics such as prolonged circulation and half-life, reduced non-specific cardiotoxicity, and much higher uptake in the tumor cells187. Similarly, Gefitinib, a tyrosine kinase inhibitor was passively encapsulated, via hydrophobic channels, within human heavy chain apoferritin. Gefitinib packaged in H-ferritin nanocage demonstrated increased cellular uptake and potency, against HER2 overexpressing SKBR3 cell line, as compared to Gefitinib alone188. Release of the chemotherapeutic drugs from ferritin is also mediated by these hydrophobic and hydrophilic channels/pores. In a study by Luo et al., daunomycin was packaged with poly-L-aspartic acid (PLAA) to enhance its sustained release from the hyaluronic acid (HA) surface modified ferritin. Daunomycin packaged HA-ferritin allowed specific binding of ferritin to CD44 markers on A549 lung cancer cells and improved sustained release of the drug from the nanocarrier189. Apart from passive loading, disassembly (and reassembly) of ferritin, to load drugs, in presence of 8M urea and extreme pH conditions has also been reported. Fat soluble drugs such as curcumin, paclitaxel, etc. have been packaged using such dissociation methods180.
Because of its ability to traffic across the blood brain barrier (BBB), via receptors such as Tim1 and TfR, ferritin could prove effective in carrying drugs across the BBB to treat various neurological diseases. Treatment of many of the diseases of the brain is challenged by permeability of drugs across the BBB. In fact, it is well known that only 2% of the pharmaceutical compounds can cross the BBB. In highly aggressive brain tumors such as neuroblastoma or glioblastoma, ferritin thus, represents newer opportunities to explore. As an example, Huang et al. had recently shown a 2- fold increase in mice glioma tumor targeting capability, after crossing BBB, of H ferritin nanocarriers packed with doxorubicin. The H-ferritin carriers in the study were genetically modified to carry a surface peptide that targets α2β1 integrins190. In another study, by Chen et al., ferritin packed with doxorubicin was shown to reliably cross the BBB and increase survival period of mice treated with the doxorubicin in H-ferritin nanocarrier (30 days) as compared to doxorubicin alone (19 days)191. These examples highlight the potential of using ferritin to package drugs for brain related ailments including lethal glioblastomas. As mentioned, our studies have demonstrated FTH11 binding to GBMs (Revised Manuscript under review) and uptake into orthotopic animal models192. In further support of the potential to use FTH1 delivery to the brain in non-tumor diseases, we have demonstrated that FTH1 is taken up into the brain in normal mice where the uptake patterns are influenced by both age and sex193. Thus, the data strongly support the exploration of ferritin as a mechanism for therapeutic interventions strategies for the brain.
Apart from chemotherapeutics ferritin is being studied for carrying nucleic acids as well. Because of the labile nature of siRNA its delivery in the in vivo systems has been hampered. Ferritin encapsulated delivery of siRNA not only protects it from extracellular nuclease degradation but also from scavenging activity of immune cells. Ongoing efforts for using naïve and modified ferritins for siRNA delivery are proving to be successful in many different cancers. For example, a study by Li et al., has shown that siRNA against insulin receptor (anti-InsR) can be easily packaged in ferritin via a pH modulated disassembly-reassembly method. Anti-InsRferritin complexes were then shown to transfect human colon adenocarcinoma cell line, Caco-2, with high efficiency194. Recently, Yuan et al. have developed genetically modified H-ferritin carriers containing cationic inner surface. Arginine mutations in the inner surface of the protein presented robust electrostatic forces that enhanced the siRNA encapsulation efficacy of the nanoparticle195. Chemical modifications of the inner surface by chemoselective conjugation of cationic piperazine-based compounds have also been proven to be highly efficient in encapsulation and delivery of siRNA to cancer cells196. Ferritin is thus emerging as a nanocarrier of choice for nucleic acids.
Use of ferritin nanocarriers is not limited to carrying chemotherapeutics, and anti-tumor siRNAs. Ferritin nanocarriers have been developed for various imaging methods such as computer tomography (CT), magnetic resonance imaging MRI, and near-infrared fluorescence (NIRF) imaging. For example, Zhao et al., developed magnetoferritin probes loaded with iron oxide in 125I radionuclide- surface modified ferritin for multimodal imaging in tumors of living mice197. In another example, gadolinium- loaded ferritin was developed to image endothelial cells in tumors by contrast MRI198. Ferritins loaded and/or conjugated with fluorophores are not only being studied for advanced diagnostic imaging but for image-guided surgery of cancers as well. For example, indocyanine loaded (ICG) loaded ferritin was shown to trace cancer cells in a preclinical model of breast cancer. Since ICG is already used in the clinics for lymph node mapping and NIR fluorescence image-guided surgery (FGS), its encapsulation in the ferritin increases its potential to be used in FGS in cancers199. As mentioned previously, ferritin is highly versatile, and that can be seen in the study by Lin et al. in which they developed a hybrid ferritin nanoparticle that is activated in presence of tumor matrix metalloproteinases (MMPs). These hybrid nanoparticles are developed by generating two different ferritin peptides, one that is conjugated with infrared activated Cy5.5 fluorophore and a second type that has a black hole quencher (BHQ-3) conjugation. Upon encountering the tumor MMPs the quencher molecule is released, and the fluorophore fluoresces in presence of NIRF200.
Ferritin carrying ICG has been explored in cancer photothermal therapy (PTT) as well. ICG is a, FDA approved photothermal agent that can absorb near-infrared light and convert it into heat. This high then induces apoptosis a characteristic that can prove crucial in cancer therapy201. ICG derivative, IR820, packaged in ferritin was shown to achieve 100% tumor elimination, without any evident non-specific toxicity, in subcutaneous mice model of breast cancer202. Combination of PTT with chemotherapy is another tool that has drawn interest in cancer therapy. A very interesting example of this comes from a recent study by Lin et al. They developed grenade-like nanoparticles, covered in NIR dye, that upon laser irradiation burst open into smaller cluster warheads which are basically ferritin nanocages carrying doxorubicin. Release of doxorubicin from these nanoparticles is further controlled by low pH found in tumors and within the acidic compartments of tumor cells203. Use of ferritin as a carrier for hydrophobic photosensitizers is also gaining traction for photodynamic cancer therapy (PDT). Photosensitizers generate ROS, in the presence of oxygen, when activated by specific light. High amounts of ROS can kill tumor cells, destroy tumor vasculature and/or activate the immune system to kill tumor cells180.
A surprising use of ferritin nanocarrier came from a study, in 2006, when Li et al. first reported HIV1 Tat antigen functionalization on the outer surface of ferritin. Since then, several studies have shown ferritin nanoparticle use in antigen/vaccine delivery. A more comprehensive review on the vaccine development using ferritin nanoparticle can be found in the review by Rodrigues et al204. More recently, there have been numerous studies that have shown ferritin nanoparticles carrying SARS-CoV-2/COVID-19 vaccine to induce robust innate immune activity in hosts205–209. One of these SARS-CoV-2- vaccines, by Joyce et al., is active in phase I clinical trial (ClinicalTrials.gov ID NCT04784767). Besides SARS-CoV-2, ferritin nanoparticle based vaccines against Influenza and Epstein Barr Virus have also moved to phase I clinical trials (ClinicalTrials.gov ID NCT04579250 and NCT04645147). Combining drug resistance and ferroptosis in ferritin delivery, iron saturated ferritin nanoparticles carrying doxorubicin were recently shown to activate ferroptotic cell death in leukemia, CRC, breast, liver, cervical, and lung cancer cells210.
4. Conclusions
Ferritin is a protein that wears multiple hats when it comes to cancers and other potential medical uses. While it performs its classical role of storing iron and protecting DNA inside the nucleus quite efficiently, ferritin’s role as a tumor suppressor or an oncogene, depending on the type of cancer, presents it as a potential target for cancer therapy. Cell-cell communication via exosomes in the tumor microenvironment is also a recent novel concept for ferritin. One of the most beneficial roles of ferritin is emerging in its use as a biological nanocarrier for delivering chemotherapeutics. The current state of the art of the scientific interrogation into the versatility of ferritin is likely just the tip of the iceberg when it comes to understanding the role of this unique protein in cancer and its dynamic roles in both a target and trojan horse for treating cancers.
Acknowledgements
James Connor and Bhavyata Shesh are supported by P01CA245705 by National Institutes of Health. We would like to thank Dr. Kondiah Palsa for providing his insights into this research article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kim M, Rho Y, Jin KS, Ahn B, Jung S, Kim H, et al. PH-dependent structures of ferritin and apoferritin in solution: Disassembly and reassembly. Biomacromolecules. 2011;12(5):1629–1640. doi: 10.1021/BM200026V/ASSET/IMAGES/LARGE/BM-2011-00026V_0009.JPEG [DOI] [PubMed] [Google Scholar]
- 2.Lawson DM, Artymiuk PJ, Yewdall SJ, Smith JMA, Livingstone JC, Treffry A, et al. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nat 1991 3496309. 1991;349(6309):541–544. doi: 10.1038/349541A0 [DOI] [PubMed] [Google Scholar]
- 3.Cairo G, Rappocciolo E, Tacchini L, Schiaffonati L. Expression of the genes for the ferritin H and L subunits in rat liver and heart. Evidence for tissue-specific regulations at pre- and posttranslational levels. Biochem J. 1991;275(Pt 3):813. doi: 10.1042/BJ2750813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pino JMV, da Luz MHM, Antunes HKM, Giampá SQ d. C, Martins VR, Lee KS. Iron-restricted diet affects brain ferritin levels, dopamine metabolism and cellular prion protein in a regionspecific manner. Front Mol Neurosci. 2017;10:145. doi: 10.3389/FNMOL.2017.00145/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koorts AM, Viljoen M. Ferritin and ferritin isoforms I: Structure-function relationships, synthesis, degradation and secretion. Arch Physiol Biochem. 2007;113(1):30–54. doi: 10.1080/13813450701318583 [DOI] [PubMed] [Google Scholar]
- 6.Bradley JM, Moore GR, Le Brun NE. Mechanisms of iron mineralization in ferritins: one size does not fit all. J Biol Inorg Chem. 2014;19(6):775–785. doi: 10.1007/S00775-014-1136-3 [DOI] [PubMed] [Google Scholar]
- 7.Everett J, Brooks J, Lermyte F, O’Connor PB, Sadler PJ, Dobson J, et al. Iron stored in ferritin is chemically reduced in the presence of aggregating Aβ(1–42). Sci Rep. 2020;10(1). doi: 10.1038/S41598-020-67117-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, et al. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011;18(5):829–840. doi: 10.1038/cdd.2010.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torti SV, Kwak EL, Miller SC, Miller LL, Ringold GM, Myambo KB, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263(25):12638–12644. doi: 10.1016/S0021-9258(18)37801-3 [DOI] [PubMed] [Google Scholar]
- 10.Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23(3):95–104. doi: 10.1016/J.BLRE.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher J, Devraj K, Ingram J, Slagle-Webb B, Madhankumar AB, Liu X, et al. Ferritin: A novel mechanism for delivery of iron to the brain and other organs. Am J Physiol - Cell Physiol. 2007;293(2):641–649. doi: 10.1152/AJPCELL.00599.2006/ASSET/IMAGES/LARGE/ZH00080753030006.JPEG [DOI] [PubMed] [Google Scholar]
- 12.Alkhateeb AA, Connor JR. The significance of ferritin in cancer: Anti-oxidation, inflammation and tumorigenesis. Biochim Biophys Acta - Rev Cancer. 2013;1836(2):245–254. doi: 10.1016/J.BBCAN.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Zhang X, Zhao G. Ferritin Nanocage: A Versatile Nanocarrier Utilized in the Field of Food, Nutrition, and Medicine. Nanomaterials. 2020;10(9):1–25. doi: 10.3390/NANO10091894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honarmand Ebrahimi K, Hagedoorn PL, Hagen WR. Unity in the biochemistry of the iron-storage proteins ferritin and bacterioferritin. Chem Rev. 2015;115(1):295–326. doi: 10.1021/CR5004908/ASSET/IMAGES/LARGE/CR-2014-004908_0023.JPEG [DOI] [PubMed] [Google Scholar]
- 15.Gorobets O, Gorobets S, Koralewski M. Physiological origin of biogenic magnetic nanoparticles in health and disease: From bacteria to humans. Int J Nanomedicine. 2017;12:4371–4395. doi: 10.2147/IJN.S130565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levisq S, Luzzagoll A, Cesarenill G, Cozzis A, Franceschinellis F, Albertiniss A, et al. Mechanism of Ferritin Iron Uptake: Activity of the H-chain and Deletion Mapping of the Ferrooxidase Site. J Biol Chem. 1988;263(34):180–18092. doi: 10.1016/S0021-9258(19)81326-1 [DOI] [PubMed] [Google Scholar]
- 17.Tosha T, Behera RK, Ng HL, Bhattasali O, Alber T, Theil EC. Ferritin protein nanocage ion channels: Gating by N-terminal extensions. J Biol Chem. 2012;287(16):13016–13025. doi: 10.1074/JBC.M111.332734/ATTACHMENT/F06702AB-C159-4039-B2C2FDCC830E0812/MMC1.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33(10):940–959. doi: 10.1016/S1357-2725(01)00063-2 [DOI] [PubMed] [Google Scholar]
- 19.Theil EC. Ferritin protein nanocages use ion channels, catalytic sites, and nucleation channels to manage iron/oxygen chemistry. Curr Opin Chem Biol. 2011;15:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiou B, Neal EH, Bowman AB, Lippmann ES, Simpson IA, Connor JR. Endothelial cells are critical regulators of iron transport in a model of the human blood–brain barrier. J Cereb Blood Flow Metab. 2019;39(11):2117. doi: 10.1177/0271678X18783372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiou B, Neal EH, Bowman AB, Lippmann ES, Simpson IA, Connor JR. Pharmaceutical iron formulations do not cross a model of the human blood-brain barrier. PLoS One. 2018;13(6). doi: 10.1371/JOURNAL.PONE.0198775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson IA, Ponnuru P, Klinger ME, Myers RL, Devraj K, Coe CL, et al. A novel model for brain iron uptake: introducing the concept of regulation. J Cereb Blood Flow Metab. 2015;35(1):48. doi: 10.1038/JCBFM.2014.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duck KA, Connor JR. Iron uptake and transport across physiological barriers. Biometals. 2016;29(4):573. doi: 10.1007/S10534-016-9952-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todorich B, Zhang X, Connor JR. H-ferritin is the major source of iron for oligodendrocytes. Glia. 2011;59(6):927–935. doi: 10.1002/GLIA.21164 [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto S, Kawabata H, Masuda T, Uchiyama T, Mizumoto C, Ohmori K, et al. H-Ferritin Is Preferentially Incorporated by Human Erythroid Cells through Transferrin Receptor 1 in a Threshold-Dependent Manner. PLoS One. 2015;10(10):e0139915. doi: 10.1371/JOURNAL.PONE.0139915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiou B, Connor JR. Emerging and Dynamic Biomedical Uses of Ferritin. Pharmaceuticals. 2018;11(4). doi: 10.3390/PH11040124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalgaonkar S, Lönnerdal B. Receptor-mediated uptake of ferritin-bound iron by human intestinal Caco-2 cells. J Nutr Biochem. 2009;20(4):304. doi: 10.1016/J.JNUTBIO.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Fang CJ, Ryan JC, Niemi EC, Lebrón JA, Björkman PJ, et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci U S A. 2010;107(8):3505–3510. doi: 10.1073/PNAS.0913192107/SUPPL_FILE/PNAS.200913192SI.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiou B, Lucassen E, Sather M, Kallianpur A, Connor J. Semaphorin4A and H-ferritin utilize Tim-1 on human oligodendrocytes: A novel neuro-immune axis. Glia. 2018;66(7):1317–1330. doi: 10.1002/GLIA.23313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J, Seaman WE, Di X, Wang W, Willingham M, Torti FM, et al. Iron Uptake Mediated by Binding of H-Ferritin to the TIM-2 Receptor in Mouse Cells. PLoS One. 2011;6(8):e23800. doi: 10.1371/JOURNAL.PONE.0023800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todorich B, Zhang X, Slagle-Webb B, Seaman WE, Connor JR. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem. 2008;107(6):1495–1505. doi: 10.1111/J.14714159.2008.05678.X [DOI] [PubMed] [Google Scholar]
- 32.Li R, Luo C, Mines M, Zhang J, Fan GH. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem. 2006;281(49):37616–37627. doi: 10.1074/JBC.M607266200 [DOI] [PubMed] [Google Scholar]
- 33.Yu B, Cheng C, Wu Y, Guo L, Kong D, Zhang Z, et al. Interactions of ferritin with scavenger receptor class A members. J Biol Chem. 2020;295(46):15727–15741. doi: 10.1074/JBC.RA120.014690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendes-Jorge L, Ramos D, Valenҫa A, López-Luppo M, Pires VMR, Catita J, et al. L-Ferritin Binding to Scara5: A New Iron Traffic Pathway Potentially Implicated in Retinopathy. PLoS One. 2014;9(9):e106974. doi: 10.1371/JOURNAL.PONE.0106974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, et al. Scara5 Is a Ferritin Receptor Mediating Non-Transferrin Iron Delivery. Dev Cell. 2009;16(1):35–46. doi: 10.1016/J.DEVCEL.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akinc A, Battaglia G. Exploiting Endocytosis for Nanomedicines. Cold Spring Harb Perspect Biol. 2013;5(11). doi: 10.1101/CSHPERSPECT.A016980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zahringer J, Baliga BS, Munro HN. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976;73(3):857. doi: 10.1073/PNAS.73.3.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99(10):3505–3516. doi: 10.1182/BLOOD.V99.10.3505 [DOI] [PubMed] [Google Scholar]
- 39.Sammarco MC, Ditch S, Banerjee A, Grabczyk E. Ferritin L and H Subunits Are Differentially Regulated on a Post-transcriptional Level. J Biol Chem. 2008;283(8):4578–4587. doi: 10.1074/JBC.M703456200 [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Deng Z, Hatcher H, Miller LD, Di X, Tesfay L, et al. IRP2 regulates breast tumor growth. Cancer Res. 2014;74(2):497–507. doi: 10.1158/0008-5472.CAN-13-1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng Z, Manz DH, Torti SV, Torti FM. Iron-responsive element-binding protein 2 plays an essential role in regulating prostate cancer cell growth. Oncotarget. 2017;8(47):82231–82243. doi: 10.18632/ONCOTARGET.19288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Si W, Li Z, Tian Y, Liu X, Ye S, et al. miR-335 promotes ferroptosis by targeting ferritin heavy chain 1 in in vivo and in vitro models of Parkinson’s disease. Int J Mol Med. 2021;47(4):112. doi: 10.3892/IJMM.2021.4894/HTML [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuji Y. JunD activates transcription of the human ferritin H gene through an antioxidant response element during oxidative stress. Oncogene. 2005;24(51):7567. doi: 10.1038/SJ.ONC.1208901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ringoldj GM, Myamboll KB, Young11 AP, Torti FM. The Molecular Cloning and Characterization of Murine Ferritin Heavy Chain, a Tumor Necrosis Factor-inducible Gene. J Biol Chem. 1988;263(25):12638–12614. doi: 10.1016/S0021-9258(18)37801-3 [DOI] [PubMed] [Google Scholar]
- 45.Wei Y, Miller SC, Tsuji Y, Torti SV., Torti FM. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169(1):289–296. doi: 10.1016/0006291X(90)91466-6 [DOI] [PubMed] [Google Scholar]
- 46.MacKenzie EL, Tsuji Y. Elevated Intracellular Calcium Increases Ferritin H Expression Through an NFAT-Independent Posttranscriptional Mechanism Involving mRNA Stabilization. Biochem J. 2008;411(1):107. doi: 10.1042/BJ20071544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pietsch EC, Chan JY, Torti FM, Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J Biol Chem. 2003;278(4):2361–2369. doi: 10.1074/JBC.M210664200 [DOI] [PubMed] [Google Scholar]
- 48.Huang BW, Ray PD, Iwasaki K, Tsuji Y. Transcriptional regulation of the human ferritin gene by coordinated regulation of Nrf2 and protein arginine methyltransferases PRMT1 and PRMT4. FASEB J. 2013;27(9):3763. doi: 10.1096/FJ.12-226043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwasaki K, Hailemariam K, Tsuji Y. PIAS3 interacts with ATF1 and regulates the human ferritin H gene through an antioxidant-responsive element. J Biol Chem. 2007;282(31):22335–22343. doi: 10.1074/JBC.M701477200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Gao L, Zhan N, Xu P, Yang J, Yuan F, et al. Hypoxia induced ferritin light chain (FTL) promoted epithelia mesenchymal transition and chemoresistance of glioma. J Exp Clin Cancer Res. 2020;39(1):1–17. doi: 10.1186/S13046-020-01641-8/FIGURES/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, et al. The Expression and Distribution of the Hypoxia-Inducible Factors HIF-1α and HIF-2α in Normal Human Tissues, Cancers, and Tumor-Associated Macrophages. Am J Pathol. 2000;157(2):411. doi: 10.1016/S00029440(10)64554-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36. doi: 10.1016/J.REDOX.2020.101670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang BW, Miyazawa M, Tsuji Y. Distinct Regulatory Mechanisms of the Human Ferritin Gene by Hypoxia andHypoxia Mimetic Cobalt Chloride at the Transcriptional and Post-transcriptionalLevels. Cell Signal. 2014;26(12):2702. doi: 10.1016/J.CELLSIG.2014.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsuji Y, Kwak E, Saika T, Torti SV, Torti$ FM. Preferential repression of the H subunit of ferritin by adenovirus E1A in NIH-3T3 mouse fibroblasts. J Biol Chem. 1993;268(10):7270–7275. doi: 10.1016/S0021-9258(18)53172-0 [DOI] [PubMed] [Google Scholar]
- 55.Wu KJ, Polack A, Dalla-Favera R. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science (80-). 1999;283(5402):676–679. doi: 10.1126/SCIENCE.283.5402.676/SUPPL_FILE/983675S3_THUMB.GIF [DOI] [PubMed] [Google Scholar]
- 56.Lv H, Shang P. The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Metallomics. 2018;10(7):899–916. doi: 10.1039/C8MT00048D [DOI] [PubMed] [Google Scholar]
- 57.Brown RAM, Richardson KL, Kabir TD, Trinder D, Ganss R, Leedman PJ. Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front Oncol. 2020;10:476. doi: 10.3389/FONC.2020.00476/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Donnell KA, Yu D, Zeller KI, whan Kim J, Racke F, Thomas-Tikhonenko A, et al. Activation of Transferrin Receptor 1 by c-Myc Enhances Cellular Proliferation and Tumorigenesis. Mol Cell Biol. 2006;26(6):2373. doi: 10.1128/MCB.26.6.2373-2386.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang F, Wang W, Tsuji Y, Torti SV, Torti FM. Post-transcriptional Modulation of Iron Homeostasis during p53-dependent Growth Arrest. J Biol Chem. 2008;283(49):33911. doi: 10.1074/JBC.M806432200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389(6648):300–305. doi: 10.1038/38525 [DOI] [PubMed] [Google Scholar]
- 61.Faniello MC, Di Sanzo M, Quaresima B, Baudi F, Di Caro V, Cuda G, et al. p53-mediated downregulation of H ferritin promoter transcriptional efficiency via NF-Y. Int J Biochem Cell Biol. 2008;40(10):2110–2119. doi: 10.1016/J.BIOCEL.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 62.Lee JH, Jang H, Cho EJ, Youn HD. Ferritin binds and activates p53 under oxidative stress. Biochem Biophys Res Commun. 2009;389(3):399–404. doi: 10.1016/J.BBRC.2009.08.125 [DOI] [PubMed] [Google Scholar]
- 63.Biamonte F, Battaglia AM, Zolea F, Oliveira DM, Aversa I, Santamaria G, et al. Ferritin heavy subunit enhances apoptosis of non-small cell lung cancer cells through modulation of miR-125b/p53 axis. Cell Death Dis 2018 912. 2018;9(12):1–10. doi: 10.1038/s41419-018-1216-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pontrelli P, Accetturo M, Gesualdo L. miRNome analysis using real-time PCR. Methods Mol Biol. 2014;1186:201–232. doi: 10.1007/978-1-4939-1158-5_11 [DOI] [PubMed] [Google Scholar]
- 65.Biamonte F, Zolea F, Bisognin A, Di Sanzo M, Saccoman C, Scumaci D, et al. H-FerritinRegulated MicroRNAs Modulate Gene Expression in K562 Cells. PLoS One. 2015;10(3). doi: 10.1371/JOURNAL.PONE.0122105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shpyleva SI, Tryndyak VP, Kovalchuk O, Starlard-Davenport A, Chekhun VF, Beland FA, et al. Role of ferritin alterations in human breast cancer cells. Breast Cancer Res Treat. 2011;126(1):63–71. doi: 10.1007/S10549-010-0849-4 [DOI] [PubMed] [Google Scholar]
- 67.Chan JJ, Kwok ZH, Chew XH, Zhang B, Liu C, Soong TW, et al. A FTH1 gene:pseudogene:microRNA network regulates tumorigenesis in prostate cancer. Nucleic Acids Res. 2018;46(4):1998. doi: 10.1093/NAR/GKX1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alkhateeb AA, Connor JR. Nuclear ferritin: A new role for ferritin in cell biology. Biochim Biophys Acta. 2010;1800(8):793–797. doi: 10.1016/J.BBAGEN.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 69.Surguladze N, Patton S, Cozzi A, Fried MG, Connor JR. Characterization of nuclear ferritin and mechanism of translocation. Biochem J. 2005;388(Pt 3):731–740. doi: 10.1042/BJ20041853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Surguladze N, Thompson KM, Beard JL, Connor JR, Fried MG. Interactions and Reactions of Ferritin with DNA. J Biol Chem. 2004;279(15):14694–14702. doi: 10.1074/JBC.M313348200 [DOI] [PubMed] [Google Scholar]
- 71.Corsi B, Cozzi A, Arosio P, Drysdale J, Santambrogio P, Campanella A, et al. Human mitochondrial ferritin expressed in HeLa cells incorporates iron and affects cellular iron metabolism. J Biol Chem. 2002;277(25):22430–22437. doi: 10.1074/JBC.M105372200 [DOI] [PubMed] [Google Scholar]
- 72.Bahr TM, Christensen RD, Ward DM, Meng F, Jackson LK, Doyle K, et al. Ferritin in serum and urine: A pilot study. Blood Cells Mol Dis. 2019;76:59–62. doi: 10.1016/J.BCMD.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petzold A, Worthington V, Appleby I, Kerr ME, Kitchen N, Smith M. Cerebrospinal fluid ferritin level, a sensitive diagnostic test in late-presenting subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2011;20(6):489–493. doi: 10.1016/J.JSTROKECEREBROVASDIS.2010.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drysdale J, Arosio P, Invernizzi R, Cazzola M, Volz A, Corsi B, et al. Mitochondrial ferritin: A new player in iron metabolism. Blood Cells Mol Dis. 2002;29(3):376–383. doi: 10.1006/bcmd.2002.0577 [DOI] [PubMed] [Google Scholar]
- 75.Shi ZH, Shi FF, Wang YQ, Sheftel AD, Nie G, Zhao YS, et al. Mitochondrial ferritin, a new target for inhibiting neuronal tumor cell proliferation. Cell Mol Life Sci. 2015;72(5):983–997. doi: 10.1007/S00018-014-1730-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levi S, Corsi B, Bosisio M, Invernizzi R, Volz A, Sanford D, et al. A human mitochondrial ferritin encoded by an intronless gene. J Biol Chem. 2001;276(27):24437–24440. doi: 10.1074/JBC.C100141200 [DOI] [PubMed] [Google Scholar]
- 77.Guaraldo M, Santambrogio P, Rovelli E, Di Savino A, Saglio G, Cittaro D, et al. Characterization of human mitochondrial ferritin promoter: identification of transcription factors and evidences of epigenetic control. Sci Reports 2016 61. 2016;6(1):1–11. doi: 10.1038/srep33432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santambrogio P, Biasiotto G, Sanvito F, Olivieri S, Arosio P, Levi S. Mitochondrial Ferritin Expression in Adult Mouse Tissues. J Histochem Cytochem. 2007;55(11):1129. doi: 10.1369/JHC.7A7273.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santambrogio P, Erba BG, Campanella A, Cozzi A, Causarano V, Cremonesi L, et al. Overexpression of mitochondrial ferritin affects the JAK2/STAT5 pathway in K562 cells and causes mitochondrial iron accumulation. Haematologica. 2011;96(10):1424–1432. doi: 10.3324/HAEMATOL.2011.042952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang YQ, Chang SY, Wu Q, Gou YJ, Jia L, Cui YM, et al. The Protective Role of Mitochondrial Ferritin on Erastin-Induced Ferroptosis. Front Aging Neurosci. 2016;8(DEC). doi: 10.3389/FNAGI.2016.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nie G, Sheftel AD, Kim SF, Ponka P. Overexpression of mitochondrial ferritin causes cytosolic iron depletion and changes cellular iron homeostasis. Blood. 2005;105(5):2161–2167. doi: 10.1182/BLOOD-2004-07-2722 [DOI] [PubMed] [Google Scholar]
- 82.Nie G, Chen G, Sheftel AD, Pantopoulos K, Ponka P. In vivo tumor growth is inhibited by cytosolic iron deprivation caused by the expression of mitochondrial ferritin. Blood. 2006;108(7):2428–2434. doi: 10.1182/BLOOD-2006-04-018341 [DOI] [PubMed] [Google Scholar]
- 83.Gong S, Chen Y, Meng F, Zhang Y, Wu H, Wu F. Roflumilast restores cAMP/PKA/CREB signaling axis for FtMt-mediated tumor inhibition of ovarian cancer. Oncotarget. 2017;8(68):112341. doi: 10.18632/ONCOTARGET.22866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gong S, Chen Y, Meng F, Zhang Y, Li C, Zhang G, et al. Roflumilast enhances cisplatin-sensitivity and reverses cisplatin‐resistance of ovarian cancer cells via cAMP/PKA/CREB‐FtMt signalling axis. Cell Prolif. 2018;51(5). doi: 10.1111/CPR.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cai C, Ching A, Lagace C, Linsenmayer T. Nuclear ferritin-mediated protection of corneal epithelial cells from oxidative damage to DNA. Dev Dyn. 2008;237(10):2676–2683. doi: 10.1002/DVDY.21494 [DOI] [PubMed] [Google Scholar]
- 86.Cai CX, Birk DE, Linsenmayer TF. Nuclear ferritin protects DNA from UV damage in corneal epithelial cells. Mol Biol Cell. 1998;9(5):1037–1051. doi: 10.1091/MBC.9.5.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thompson KJ, Fried MG, Ye Z, Boyer P, Connor JR. Regulation, mechanisms and proposed function of ferritin translocation to cell nuclei. J Cell Sci. 2002;115(10):2165–2177. doi: 10.1242/JCS.115.10.2165 [DOI] [PubMed] [Google Scholar]
- 88.Linsenmayer TF, Beazley KE, Cai CX, Canner JP, Fitch JM, Kubilus JK, et al. Corneal Epithelial Nuclear Ferritin and Its Transporter Ferritoid Afford Unique Protection to DNA from UV Light and Reactive Oxygen Species. Oxidative Stress Appl Basic Res Clin Pract. Published online 2015:39–66. doi: 10.1007/978-1-4939-1935-2_3 [DOI] [Google Scholar]
- 89.Liu X, Madhankumar AB, Slagle-Webb B, Sheehan JM, Surguladze N, Connor JR. Heavy chain ferritin siRNA delivered by cationic liposomes increases sensitivity of cancer cells to chemotherapeutic agents. Cancer Res. 2011;71(6):2240–2249. doi: 10.1158/0008-5472.CAN-10-1375/656456/P/HEAVY-CHAIN-FERRITIN-SIRNA-DELIVERED-BY-CATIONIC [DOI] [PubMed] [Google Scholar]
- 90.Ravi V, Madhankumar AB, Abraham T, Slagle-Webb B, Connor JR. Liposomal delivery of ferritin heavy chain 1 (FTH1) siRNA in patient xenograft derived glioblastoma initiating cells suggests different sensitivities to radiation and distinct survival mechanisms. PLoS One. 2019;14(9):e0221952. doi: 10.1371/JOURNAL.PONE.0221952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum Ferritin: Past, Present and Future. Biochim Biophys Acta. 2010;1800(8):760. doi: 10.1016/J.BBAGEN.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Daru J, Colman K, Stanworth SJ, De La Salle B, Wood EM, Pasricha SR. Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr. 2017;106(Suppl 6):1634S. doi: 10.3945/AJCN.117.155960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shalitin S, Carmi D, Weintrob N, Phillip M, Miskin H, Kornreich L, et al. Serum ferritin level as a predictor of impaired growth and puberty in thalassemia major patients. Eur J Haematol. 2005;74(2):93–100. doi: 10.1111/J.1600-0609.2004.00371.X [DOI] [PubMed] [Google Scholar]
- 94.Harrison-Findik DD. Gender-related variations in iron metabolism and liver diseases. World J Hepatol. 2010;2(8):302–310. doi: 10.4254/wjh.v2.i8.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kappert K, Jahić A, Tauber R. Assessment of serum ferritin as a biomarker in COVID-19: bystander or participant? Insights by comparison with other infectious and non-infectious diseases. 10.1080/1354750X20201797880. 2020;25(8):616–625. doi: [DOI] [PubMed] [Google Scholar]
- 96.Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D’Cruz DP, Shoenfeld Y. The Hyperferritinemic Syndrome: Macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11(1):1–11. doi: 10.1186/1741-7015-11185/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramírez-Carmona W, Díaz-Fabregat B, Yuri Yoshigae A, Musa de Aquino A, Scarano WR, de Souza Castilho AC, et al. Are Serum Ferritin Levels a Reliable Cancer Biomarker? A Systematic Review and Meta-Analysis. 10.1080/0163558120211982996. 2021;74(6):19171926. doi: [DOI] [PubMed] [Google Scholar]
- 98.Arosio P, Yokota M, Drysdale JW. Characterization of serum ferritin in iron overload: possible identity to natural apoferritin. Br J Haematol. 1977;36(2):199–207. doi: 10.1111/J.13652141.1977.TB00640.X [DOI] [PubMed] [Google Scholar]
- 99.Ten Kate J, Wolthuis A, Westerhuis B, van Deursen C. The iron content of serum ferritin: physiological importance and diagnostic value. Eur J Clin Chem Clin Biochem. 1997;35(1):53–56. doi: 10.1515/CCLM.1997.35.1.53 [DOI] [PubMed] [Google Scholar]
- 100.Anderson ER, Shah YM. Iron homeostasis in the liver. Compr Physiol. 2013;3(1):315. doi: 10.1002/CPHY.C120016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meyron-Holtz EG, Moshe-Belizowski S, Cohen LA. A possible role for secreted ferritin in tissue iron distribution. J Neural Transm. 2011;118(3):337–347. doi: 10.1007/s00702-011-0582-0 [DOI] [PubMed] [Google Scholar]
- 102.Gray CP, Franco AV, Arosio P, Hersey P. Immunosuppressive effects of melanoma-derived heavy-chain ferritin are dependent on stimulation of IL-10 production. Int J cancer. 2001;92(6):843–850. doi: 10.1002/IJC.1269 [DOI] [PubMed] [Google Scholar]
- 103.Alkhateeb AA, Han B, Connor JR. Ferritin stimulates breast cancer cells through an iron-independent mechanism and is localized within tumor-associated macrophages. Breast Cancer Res Treat. 2013;137(3):733–744. doi: 10.1007/S10549-012-2405-X [DOI] [PubMed] [Google Scholar]
- 104.Gray CP, Arosio PHP. Association of Increased Levels of Heavy-Chain Ferritin with Increased CD4+ CD25+ Regulatory T-Cell Levels in Patients with Melanoma1 | Citedby Results | Clinical Cancer Research | American Association for Cancer Research. Clinical Cancer Research. Published 2003. Accessed November 25, 2022. https://aacrjournals.org/clincancerres/crossrefcitedby/203600 [PubMed] [Google Scholar]
- 105.De Almeida SM, Da Cunha DS, Yamada E, Doi EM, Ono M. Quantification of cerebrospinal fluid ferritin as a biomarker for CNS malignant infiltration. Arq Neuropsiquiatr. 2008;66(3 B):720–724. doi: 10.1590/S0004-282X2008000500022 [DOI] [PubMed] [Google Scholar]
- 106.Koskiniemi M Malignancy markers in the cerebrospinal fluid. Eur J Pediatr. 1988;148(1):3–8. doi: 10.1007/BF00441801 [DOI] [PubMed] [Google Scholar]
- 107.Sato Y, Honda Y, Asoh T, Oizumi K, Ohshima Y, Honda E. Cerebrospinal fluid ferritin in glioblastoma: Evidence for tumor synthesis. J Neurooncol. 1998;40(1):47–50. doi: 10.1023/A:1006078521790 [DOI] [PubMed] [Google Scholar]
- 108.Ghosh S, Hevi S, Chuck SL. Regulated secretion of glycosylated human ferritin from hepatocytes. Blood. 2004;103(6):2369–2376. doi: 10.1182/BLOOD-2003-09-3050 [DOI] [PubMed] [Google Scholar]
- 109.Truman-Rosentsvit M, Berenbaum D, Spektor L, Cohen LA, Belizowsky-Moshe S, Lifshitz L, et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood. 2018;131(3):342–352. doi: 10.1182/BLOOD-2017-02-768580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116(9):1574–1584. doi: 10.1182/BLOOD-2009-11-253815 [DOI] [PubMed] [Google Scholar]
- 111.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, et al. Prominin2 Drives Ferroptosis Resistance by Stimulating Multivesicular Body/Exosome-Mediated Iron Export. Dev Cell. 2019;51(5):575. doi: 10.1016/J.DEVCEL.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mrowczynski OD, Madhankumar AB, Slagle-Webb B, Lee SY, Zacharia BE, Connor JR. HFE genotype affects exosome phenotype in cancer. Biochim Biophys acta Gen Subj. 2017;1861(8):1921–1928. doi: 10.1016/J.BBAGEN.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 113.Mrowczynski OD, Zacharia BE, Connor JR. Exosomes and their implications in central nervous system tumor biology. Prog Neurobiol. 2019;172:71–83. doi: 10.1016/J.PNEUROBIO.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 114.Palsa K, Baringer SL, Shenoy G, Simpson IA, Connor JR. Exosomes are involved in iron transport from human blood-brain barrier endothelial cells and are modified by endothelial cell iron status. J Biol Chem. 2023;299(2):102868. doi: 10.1016/j.jbc.2022.102868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, Ning G, et al. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene 2017 3629. 2017;36(29):4089–4099. doi: 10.1038/onc.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schonberg DL, Miller TE, Wu Q, Flavahan WA, Das NK, Hale JS, et al. Preferential Iron Trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell. 2015;28(4):441–455. doi: 10.1016/j.ccell.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu C, Zhao H, Luo C, Lei T, Zhang M. Knockdown of ferritin heavy chain (FTH) inhibits the migration of prostate cancer through reducing S100A4, S100A2, and S100P expression. Transl Cancer Res. 2020;9(9):5418–5429. doi: 10.21037/TCR-19-2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu W, Zhou C, Jing Q, Li Y, Yang J, Yang C, et al. FTH promotes the proliferation and renders the HCC cells specifically resist to ferroptosis by maintaining iron homeostasis. Cancer Cell Int. 2021;21(1):1–18. doi: 10.1186/S12935-021-02420-X/FIGURES/7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu T, Li Y, Liu B, Zhang S, Wu L, Zhu X, et al. Expression of Ferritin Light Chain (FTL) Is Elevated in Glioblastoma, and FTL Silencing Inhibits Glioblastoma Cell Proliferation via the GADD45/JNK Pathway. PLoS One. 2016;11(2):e0149361. doi: 10.1371/JOURNAL.PONE.0149361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cozzi A, Corsi B, Levi S, Santambrogio P, Biasiotto G, Arosio P. Analysis of the biologic functions of H- and L-ferritins in HeLa cells by transfection with siRNAs and cDNAs: evidence for a proliferative role of L-ferritin. Blood. 2004;103(6):2377–2383. doi: 10.1182/BLOOD-200306-1842 [DOI] [PubMed] [Google Scholar]
- 121.Yu GH, Fu L, Chen J, Wei F, Shi WX. Decreased expression of ferritin light chain in osteosarcoma and its correlation with epithelial-mesenchymal transition. Eur Rev Med Pharmacol Sci. 2018;22(9):2580–2587. doi: 10.26355/EURREV_201805_14951 [DOI] [PubMed] [Google Scholar]
- 122.Zhang J, Chen X. p53 Tumor Suppressor and Iron Homeostasis. FEBS J. 2019;286(4):620. doi: 10.1111/FEBS.14638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang DC, Jiang X, Elliott RL HJ. Antisense ferritin oligonucleotides inhibit growth and induce apoptosis in human breast carcinoma cells - PubMed. Anticancer Res. Published online 2002:22(3):1513–24. Accessed November 27, 2022. https://pubmed.ncbi.nlm.nih.gov/12168831/ [PubMed] [Google Scholar]
- 124.Cozzi A, Levi S, Corsi B, Santambrogio P, Campanella A, Gerardi G, et al. Role of iron and ferritin in TNFα-induced apoptosis in HeLa cells. FEBS Lett. 2003;537(1–3):187–192. doi: 10.1016/S0014-5793(03)00114-5 [DOI] [PubMed] [Google Scholar]
- 125.Chang CM, Fitch ME, Yuan M, Parslow TG, Shu HG. Bax can associate with ferritin heavy chain (FHC) resulting in inhibition of bax-mediated apoptosis. Int J Radiat Oncol. 2001;51(3):189. doi: 10.1016/s0360-3016(01)02166-6 [DOI] [Google Scholar]
- 126.Baldi A, Lombardi D, Russo P, Palescandolo E, De Luca A, Santini D, et al. Ferritin Contributes to Melanoma Progression by Modulating Cell Growth and Sensitivity to Oxidative Stress. Clin Cancer Res. 2005;11(9):3175–3183. doi: 10.1158/1078-0432.CCR-04-0631 [DOI] [PubMed] [Google Scholar]
- 127.Ribatti D, Tamma R, Annese T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl Oncol. 2020;13(6):100773. doi: 10.1016/J.TRANON.2020.100773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sioutas A, Vainikka LK, Kentson M, Dam-Larsen S, Wennerström U, Jacobson P, et al. Oxidantinduced autophagy and ferritin degradation contribute to epithelial–mesenchymal transition through lysosomal iron. J Inflamm Res. 2017;10:29. doi: 10.2147/JIR.S128292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang KH, Tian HY, Gao X, Lei WW, Hu Y, Wang DM, et al. Ferritin heavy chain-mediated iron homeostasis and subsequent increased reactive oxygen species production are essential for epithelial-mesenchymal transition. Cancer Res. 2009;69(13):5340–5348. doi: 10.1158/00085472.CAN-09-0112 [DOI] [PubMed] [Google Scholar]
- 130.Liu J, Gao L, Zhan N, Xu P, Yang J, Yuan F, et al. Hypoxia induced ferritin light chain (FTL) promoted epithelia mesenchymal transition and chemoresistance of glioma. J Exp Clin Cancer Res. 2020;39(1):1–17. doi: 10.1186/S13046-020-01641-8/FIGURES/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Son H, Moon A. Epithelial-mesenchymal Transition and Cell Invasion. Toxicol Res. 2010;26(4):245. doi: 10.5487/TR.2010.26.4.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coffman LG, Parsonage D, D’Agostino R, Torti FM, Torti SV. Regulatory effects of ferritin on angiogenesis. Proc Natl Acad Sci U S A. 2009;106(2):570–575. doi: 10.1073/PNAS.0812010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tesfay L, Huhn AJ, Hatcher H, Torti FM, Torti SV. Ferritin Blocks Inhibitory Effects of TwoChain High Molecular Weight Kininogen (HKa) on Adhesion and Survival Signaling in Endothelial Cells. PLoS One. 2012;7(7):e40030. doi: 10.1371/JOURNAL.PONE.0040030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jin P, Kang J, Lee MK, Park JW. Ferritin heavy chain controls the HIF-driven hypoxic response by activating the asparaginyl hydroxylase FIH. Biochem Biophys Res Commun. 2018;499(3):475481. doi: 10.1016/J.BBRC.2018.03.173 [DOI] [PubMed] [Google Scholar]
- 135.Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res Int. 2015;2015. doi: 10.1155/2015/549412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Labrie M, Brugge JS, Mills GB, Zervantonakis IK. Therapy resistance: opportunities created by adaptive responses to targeted therapies in cancer. Nat Rev Cancer 2022 226. 2022;22(6):323–339. doi: 10.1038/s41568-022-00454-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nie Z, Chen M, Gao Y, Huang D, Cao H, Peng Y, et al. Ferroptosis and Tumor Drug Resistance: Current Status and Major Challenges. Front Pharmacol. 2022;13:1647. doi: 10.3389/FPHAR.2022.879317/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin F, Girotti AW. Elevated ferritin production, iron containment, and oxidant resistance in hemin-treated leukemia cells. Arch Biochem Biophys. 1997;346(1):131–141. doi: 10.1006/ABBI.1997.0300 [DOI] [PubMed] [Google Scholar]
- 139.Epsztejn S, Glickstein H, Picard V, Slotki IN, Breuer W, Beaumont ZIC C. H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties - PubMed. Blood. Accessed December 10, 2022. https://pubmed.ncbi.nlm.nih.gov/10552971/ [PubMed] [Google Scholar]
- 140.Chekhun VF, Lukyanova NY, Burlaka AP, Bezdenezhnykh NA, Shpyleva SI, Tryndyak VP, et al. Iron metabolism disturbances in the MCF-7 human breast cancer cells with acquired resistance to doxorubicin and cisplatin. Int J Oncol. 2013;43(5):1481–1486. doi: 10.3892/IJO.2013.2063 [DOI] [PubMed] [Google Scholar]
- 141.Wu J, Liu H, Zhang G, Gu L, Zhang Y, Gao J, et al. Antileukemia Effect of Ciclopirox Olamine Is Mediated by Downregulation of Intracellular Ferritin and Inhibition β-Catenin-c-Myc Signaling Pathway in Glucocorticoid Resistant T-ALL Cell Lines. PLoS One. 2016;11(8). doi: 10.1371/JOURNAL.PONE.0161509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Connor JR. Role of H-Ferritin in Radiosensitivity of Human Glioma Cells. Cancer Biol Treat. 2016;3(1):1–10. doi: 10.24966/CBT-7546/100006 [DOI] [Google Scholar]
- 143.Tirinato L, Marafioti MG, Pagliari F, Jansen J, Aversa I, Hanley R, et al. Lipid droplets and ferritin heavy chain: A devilish liaison in human cancer cell radioresistance. Elife. 2021;10. doi: 10.7554/ELIFE.72943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aversa I, Chirillo R, Chiarella E, Zolea F, Di Sanzo M, Biamonte F, et al. Chemoresistance in H-Ferritin Silenced Cells: The Role of NF-κB. Int J Mol Sci. 2018;19(10). doi: 10.3390/IJMS19102969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rychtarcikova Z, Lettlova S, Tomkova V, Korenkova V, Langerova L, Simonova E, et al. Tumorinitiating cells of breast and prostate origin show alterations in the expression of genes related to iron metabolism. Oncotarget. 2017;8(4):6376. doi: 10.18632/ONCOTARGET.14093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Raggi C, Gammella E, Correnti M, Buratti P, Forti E, Andersen JB, et al. Dysregulation of Iron Metabolism in Cholangiocarcinoma Stem-like Cells. Sci Reports 2017 71. 2017;7(1):1–12. doi: 10.1038/s41598-017-17804-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cozzi A, Corsi B, Levi S, Santambrogio P, Biasiotto G, Arosio P. Analysis of the biologic functions of H- and L-ferritins in HeLa cells by transfection with siRNAs and cDNAs: evidence for a proliferative role of L-ferritin. Blood. 2004;103(6):2377–2383. doi: 10.1182/BLOOD-2003-06-1842 [DOI] [PubMed] [Google Scholar]
- 148.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nat 2014 5097498. 2014;509(7498):105–109. doi: 10.1038/nature13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mancias JD, Kimmelman AC. Mechanisms of Selective Autophagy in Normal Physiology and Cancer. J Mol Biol. 2016;428(9):1659–1680. doi: 10.1016/J.JMB.2016.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rockfield S, Flores I, Nanjundan M. Expression and function of nuclear receptor coactivator 4 isoforms in transformed endometriotic and malignant ovarian cells. Oncotarget. 2018;9(4):5344. doi: 10.18632/ONCOTARGET.23747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Santana-Codina N, Del Rey MQ, Kapner KS, Zhang H, Gikandi A, Malcolm C, et al. NCOA4-mediated ferritinophagy is a pancreatic cancer dependency via maintenance of iron bioavailability for iron-sulfur cluster proteins. Cancer Discov. 2022;12(9):2180. doi: 10.1158/2159-8290.CD-220043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mou Y, Wu J, Zhang Y, Abdihamid O, Duan C, Li B. Low expression of ferritinophagy-related NCOA4 gene in relation to unfavorable outcome and defective immune cells infiltration in clear cell renal carcinoma. BMC Cancer. 2021;21(1):1–12. doi: 10.1186/S12885-020-07726Z/FIGURES/7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dixon SJ, Stockwell BR. The Hallmarks of Ferroptosis. 10.1146/annurevcancerbio-030518-055844. 2019;3(1):35–54. doi: [DOI] [Google Scholar]
- 154.Lu B, Chen XB, Ying MD, He QJ, Cao J, Yang B. The Role of Ferroptosis in Cancer Development and Treatment Response. Front Pharmacol. 2017;8(JAN). doi: 10.3389/FPHAR.2017.00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245. doi: 10.1016/J.CHEMBIOL.2008.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. Published online 2012. doi: 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, et al. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1). doi: 10.1186/S13045-019-0720-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.CD-19-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8+ T cells regulate tumor ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270. doi: 10.1038/S41586-019-1170-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21(1):1–12. doi: 10.1186/S12943-022-01530-Y/FIGURES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Du J, Wang X, Li Y, Ren X, Zhou Y, Hu W, et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 2021;12(7). doi: 10.1038/S41419-021-03996-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Turcu AL, Versini A, Khene N, Gaillet C, Cañeque T, Müller S, et al. DMT1 Inhibitors Kill Cancer Stem Cells by Blocking Lysosomal Iron Translocation. Chem – A Eur J. 2020;26(33):7369–7373. doi: 10.1002/CHEM.202000159 [DOI] [PubMed] [Google Scholar]
- 163.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: Process and function. Cell Death Differ. Published online 2016. doi: 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Di Yang N, Tan SH, Ng S, Shi Y, Zhou J, Tan KSW, et al. Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J Biol Chem. 2014;289(48):33425–33441. doi: 10.1074/JBC.M114.564567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2(5):517–532. doi: 10.18632/ONCOSCIENCE.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen X, Yu C, Kang R, Tang D. Iron Metabolism in Ferroptosis. Front Cell Dev Biol. 2020;8:1089. doi: 10.3389/FCELL.2020.590226/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Y, Kong Y, Ma Y, Ni S, Wikerholmen T, Xi K, et al. Loss of COPZ1 induces NCOA4 mediated autophagy and ferroptosis in glioblastoma cell lines. Oncogene. 2021;40(8):1425. doi: 10.1038/S41388-020-01622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fan K, Jia X, Zhou M, Wang K, Conde J, He J, et al. Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma. ACS Nano. 2018;12(5):4105–4115. doi: 10.1021/acsnano.7b06969 [DOI] [PubMed] [Google Scholar]
- 170.Palombarini F, Di Fabio E, Boffi A, Macone A, Bonamore A. Ferritin Nanocages for Protein Delivery to Tumor Cells. Molecules. 2020;25(4). doi: 10.3390/MOLECULES25040825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reutovich AA, Srivastava AK, Arosio P, Bou-Abdallah F. Ferritin nanocages as efficient nanocarriers and promising platforms for COVID-19 and other vaccines development. Biochim Biophys acta Gen Subj. 2023;1867(3). doi: 10.1016/J.BBAGEN.2022.130288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Palombarini F, Masciarelli S, Incocciati A, Liccardo F, Di Fabio E, Iazzetti A, et al. Self-assembling ferritin-dendrimer nanoparticles for targeted delivery of nucleic acids to myeloid leukemia cells. J Nanobiotechnology. 2021;19(1). doi: 10.1186/S12951-021-00921-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Theil EC. Ferritin protein nanocages—the story. Nanotechnol perceptions. 2012;8(1):7. doi: 10.4024/N03TH12A.NTP.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jiang B, Jia X, Ji T, Zhou M, He J, Wang K, et al. Ferritin nanocages for early theranostics of tumors via inflammation-enhanced active targeting. Sci China Life Sci. 2022;65(2):328–340. doi: 10.1007/S11427-021-1976-0/METRICS [DOI] [PubMed] [Google Scholar]
- 175.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275(3):161–203. doi: 10.1016/0005-2728(96)00022-9 [DOI] [PubMed] [Google Scholar]
- 176.Santambrogios P, Levis S, Arosiosq P, Palagis L, Vecchioq G, Lawsonll DM, et al. Evidence that a salt bridge in the light chain contributes to the physical stability difference between heavy and light human ferritins. 1992;287:14077–14083. doi: 10.1016/S0021-9258(19)49681-6 [DOI] [PubMed] [Google Scholar]
- 177.Bellini M, Mazzucchelli S, Galbiati E, Sommaruga S, Fiandra L, Truffi M, et al. Protein nanocages for self-triggered nuclear delivery of DNA-targeted chemotherapeutics in Cancer Cells. J Control Release. 2014;196:184–196. doi: 10.1016/J.JCONREL.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 178.Kang S, Oltrogge LM, Broomell CC, Liepold LO, Prevelige PE, Young M, et al. Controlled assembly of bifunctional chimeric protein cages and composition analysis using noncovalent mass spectrometry. J Am Chem Soc. 2008;130(49):16527–16529. doi: 10.1021/JA807655T [DOI] [PubMed] [Google Scholar]
- 179.Belletti D, Pederzoli F, Forni F, Vandelli MA, Tosi G, Ruozi B. Protein cage nanostructure as drug delivery system: magnifying glass on apoferritin. 10.1080/1742524720171243528. 2016;14(7):825–840. doi: [DOI] [PubMed] [Google Scholar]
- 180.Sun X, Hong Y, Gong Y, Zheng S, Xie D. Bioengineered Ferritin Nanocarriers for Cancer Therapy. Int J Mol Sci. 2021;22(13). doi: 10.3390/IJMS22137023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Khoshnejad M, Parhiz H, Shuvaev VV., Dmochowski IJ, Muzykantov VR. Ferritin-based drug delivery systems: Hybrid nanocarriers for vascular immunotargeting. J Control Release. 2018;282:13–24. doi: 10.1016/j.jconrel.2018.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bellini M, Riva B, Tinelli V, Rizzuto MA, Salvioni L, Colombo M, et al. Engineered Ferritin Nanoparticles for the Bioluminescence Tracking of Nanodrug Delivery in Cancer. Small. 2020;16(39):2001450. doi: 10.1002/SMLL.202001450 [DOI] [PubMed] [Google Scholar]
- 183.Zhang B, Tang G, He J, Yan X, Fan K. Ferritin nanocage: A promising and designable multimodule platform for constructing dynamic nanoassembly-based drug nanocarrier. Adv Drug Deliv Rev. 2021;176. doi: 10.1016/J.ADDR.2021.113892 [DOI] [PubMed] [Google Scholar]
- 184.Mohanty A, Parida A, Raut RK, Behera RK. Ferritin: A Promising Nanoreactor and Nanocarrier for Bionanotechnology. ACS Bio Med Chem Au. 2022;2(3):258–281. doi: 10.1021/ACSBIOMEDCHEMAU.2C00003/ASSET/IMAGES/LARGE/BG2C00003_0010.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yin S, Davey K, Dai S, Liu Y, Bi J. A critical review of ferritin as a drug nanocarrier: Structure, properties, comparative advantages and challenges. Particuology. 2022;64:65–84. doi: 10.1016/J.PARTIC.2021.04.020 [DOI] [Google Scholar]
- 186.Truffi M, Fiandra L, Sorrentino L, Monieri M, Corsi F, Mazzucchelli S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol Res. 2016;107:57–65. doi: 10.1016/J.PHRS.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 187.Zhen Z, Tang W, Chen H, Lin X, Todd T, Wang G, et al. RGD Modified Apoferritin Nanoparticles for Efficient Drug Delivery to Tumors. ACS Nano. 2013;7(6):4830. doi: 10.1021/NN305791Q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kuruppu AI, Zhang L, Collins H, Turyanska L, Thomas NR, Bradshaw TD. An Apoferritin-based Drug Delivery System for the Tyrosine Kinase Inhibitor Gefitinib. Adv Healthc Mater. 2015;4(18):2816–2821. doi: 10.1002/ADHM.201500389 [DOI] [PubMed] [Google Scholar]
- 189.Luo Y, Wang X, Du D, Lin Y. Hyaluronic acid-conjugated apoferritin nanocages for lung cancer targeted drug delivery. Biomater Sci. 2015;3(10):1386–1394. doi: 10.1039/C5BM00067J [DOI] [PubMed] [Google Scholar]
- 190.Huang CW, Chuang CP, Chen YJ, Wang HY, Lin JJ, Huang CY, et al. Integrin α2β1-targeting ferritin nanocarrier traverses the blood–brain barrier for effective glioma chemotherapy. J Nanobiotechnology. 2021;19(1):1–17. doi: 10.1186/S12951-021-00925-1/FIGURES/10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen Z, Zhai M, Xie X, Zhang Y, Ma S, Li Z, et al. Apoferritin Nanocage for Brain Targeted Doxorubicin Delivery. Mol Pharm. 2017;14(9):3087–3097. doi: 10.1021/acs.molpharmaceut.7b00341 [DOI] [PubMed] [Google Scholar]
- 192.Shesh Bhavyata (Pandya). Iron Profiling in GBMs. Penn State University; 2023. Pending [Google Scholar]
- 193.Chiou B, Neely EB, Mcdevitt DS, Simpson IA, Connor JR. Transferrin and H-ferritin involvement in brain iron acquisition during postnatal development: impact of sex and genotype. J Neurochem. 2020;152(3):381. doi: 10.1111/JNC.14834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li L, Muñoz-Culla M, Carmona U, Lopez MP, Yang F, Trigueros C, et al. Ferritin-mediated siRNA delivery and gene silencing in human tumor and primary cells. Biomaterials. 2016;98:143–151. doi: 10.1016/J.BIOMATERIALS.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 195.Yuan Z, Wang B, Teng Y, Ho W, Hu B, Boakye-Yiadom KO, et al. Rational design of engineered H-ferritin nanoparticles with improved siRNA delivery efficacy across an in vitro model of the mouse BBB. Nanoscale. 2022;14(17):6449–6464. doi: 10.1039/D1NR07880A [DOI] [PubMed] [Google Scholar]
- 196.Pediconi N, Ghirga F, Del Plato C, Peruzzi G, Athanassopoulos CM, Mori M, et al. Design and Synthesis of Piperazine-Based Compounds Conjugated to Humanized Ferritin as Delivery System of siRNA in Cancer Cells. Bioconjug Chem. 2021;32(6):1105–1116. doi: 10.1021/ACS.BIOCONJCHEM.1C00137/ASSET/IMAGES/LARGE/BC1C00137_0004.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhao Y, Liang M, Li X, Fan K, Xiao J, Li Y, et al. Bioengineered Magnetoferritin Nanoprobes for Single-Dose Nuclear-Magnetic Resonance Tumor Imaging. ACS Nano. 2016;10(4):4184–4191. doi: 10.1021/ACSNANO.5B07408/SUPPL_FILE/NN5B07408_SI_001.PDF [DOI] [PubMed] [Google Scholar]
- 198.Crich SG, Bussolati B, Tei L, Grange C, Esposito G, Lanzardo S, et al. Magnetic resonance visualization of tumor angiogenesis by targeting neural cell adhesion molecules with the highly sensitive gadolinium-loaded apoferritin probe. Cancer Res. 2006;66(18):9196–9201. doi: 10.1158/0008-5472.CAN-06-1728 [DOI] [PubMed] [Google Scholar]
- 199.Sitia L, Sevieri M, Bonizzi A, Allevi R, Morasso C, Foschi D, et al. Development of TumorTargeted Indocyanine Green-Loaded Ferritin Nanoparticles for Intraoperative Detection of Cancers. ACS Omega. 2020;5(21):12035–12045. doi: 10.1021/ACSOMEGA.0C00244/ASSET/IMAGES/LARGE/AO0C00244_0007.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lin X, Xie J, Zhu L, Lee S, Niu G, Ma Y, et al. Hybrid Ferritin Nanoparticles as Activatable Probes for Tumor Imaging. Angew Chem Int Ed Engl. 2011;50(7):1569. doi: 10.1002/ANIE.201006757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.The unique physiology of solid tumors: opportunities (and problems) for cancer therapy - PubMed. Accessed December 4, 2022. https://pubmed.ncbi.nlm.nih.gov/9537241/ [PubMed]
- 202.Huang P, Rong P, Jin A, Yan X, Zhang MG, Lin J, et al. Dye Loaded Ferritin Nanocages for Multimodal Imaging and Photothermal Therapy. Adv Mater. 2014;26(37):6401. doi: 10.1002/ADMA.201400914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lin CY, Shieh MJ. Near-Infrared Fluorescent Dye-Decorated Nanocages to Form Grenade-like Nanoparticles with Dual Control Release for Photothermal Theranostics and Chemotherapy. Bioconjug Chem. 2018;29(4):1384–1398. doi: 10.1021/ACS.BIOCONJCHEM.8B00088/SUPPL_FILE/BC8B00088_SI_001.PDF [DOI] [PubMed] [Google Scholar]
- 204.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. Published online 2008. doi: 10.1016/j.tcb.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Joyce MG, King HAD, Elakhal-Naouar I, Ahmed A, Peachman KK, Cincotta CM, et al. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates. Sci Transl Med. 2022;14(632):5735. doi: 10.1126/SCITRANSLMED.ABI5735/SUPPL_FILE/SCITRANSLMED.ABI5735_DATA_FILE_S1.ZIP [DOI] [PubMed] [Google Scholar]
- 206.Carmen JM, Shrivastava S, Lu Z, Anderson A, Morrison EB, Sankhala RS, et al. SARS-CoV-2 ferritin nanoparticle vaccine induces robust innate immune activity driving polyfunctional spikespecific T cell responses. npj Vaccines 2021 61. 2021;6(1):1–18. doi: 10.1038/s41541-021-00414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Powell AE, Zhang K, Sanyal M, Tang S, Weidenbacher PA, Li S, et al. A Single Immunization with Spike-Functionalized Ferritin Vaccines Elicits Neutralizing Antibody Responses against SARS-CoV-2 in Mice. ACS Cent Sci. 2021;7(1):183–199. doi: 10.1021/ACSCENTSCI.0C01405/ASSET/IMAGES/LARGE/OC0C01405_0004.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Masoomi Nomandan SZ, Azimzadeh Irani M, Hosseini SM. In silico design of refined ferritinSARS-CoV-2 glyco-RBD nanoparticle vaccine. Front Mol Biosci. 2022;9:972. doi: 10.3389/FMOLB.2022.976490/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Johnston SC, Ricks KM, Lakhal-Naouar I, Jay A, Subra C, Raymond JL, et al. A SARS-CoV-2 Spike Ferritin Nanoparticle Vaccine Is Protective and Promotes a Strong Immunological Response in the Cynomolgus Macaque Coronavirus Disease 2019 (COVID-19) Model. Vaccines. 2022;10(5):717. doi: 10.3390/VACCINES10050717/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yang R, Li Y, Wang X, Yan J, Pan D, Xu Y, et al. Doxorubicin loaded ferritin nanoparticles for ferroptosis enhanced targeted killing of cancer cells. RSC Adv. 2019;9(49):28548–28553. doi: 10.1039/C9RA04478G [DOI] [PMC free article] [PubMed] [Google Scholar]


