Abstract
Objective
Diagnostic errors, termed “missed opportunities for improving diagnosis” (MOIDs), are known sources of harm in children but have not been well characterized in pediatric hospital medicine. Our objectives were to systematically identify and describe MOIDs among general pediatric patients who experienced hospital readmission, outline improvement opportunities, and explore factors associated with increased risk of MOID.
Patients and Methods
Our retrospective cohort study included unplanned readmissions within 15 days of discharge from a freestanding children’s hospital (October 2018-September 2020). Health records from index admissions and readmissions were independently reviewed and discussed by practicing inpatient physicians to identify MOIDs using an established instrument, SaferDx. MOIDs were evaluated using a diagnostic-specific tool to identify improvement opportunities within the diagnostic process.
Results
MOIDs were identified in 22 (6.3%) of 348 readmissions. Opportunities for improvement included: delay in considering the correct diagnosis (n=11, 50%) and failure to order needed test(s) (n=10, 45%). Patients with MOIDs were older (median age: 3.8 (IQR 1.5, 11.2) versus 1.0 (0.3, 4.9) years) than patients without MOIDs but similar in sex, primary language, race, ethnicity, and insurance type. We did not identify conditions associated with higher risk of MOID. Lower respiratory tract infections accounted for 26% of admission diagnoses but only 1 (4.5%) case of MOID.
Conclusions
Standardized review of pediatric readmissions identified MOIDs and opportunities for improvement within the diagnostic process, particularly in clinician decision-making. We identified conditions with low incidence of MOID. Further work is needed to better understand pediatric populations at highest risk for MOID.
INTRODUCTION
Diagnostic safety is an important domain of healthcare quality. Diagnostic error (DE) has been defined as “the failure to establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient,”1 regardless of patient harm.2 DEs have been associated with escalation of care and death in adult acute healthcare settings;3–8 however, DEs are often underreported and difficult to recognize.3 To promote a focus on safety, DEs have also been termed “missed opportunities for improving diagnosis” (MOIDs) in the quality improvement (QI) literature.3,9 The extent to which we can extrapolate adult diagnostic safety research to pediatrics is limited given different pathophysiology and disease burden among children.10 Thus, systematic approaches for identifying and evaluating pediatric MOIDs are needed to inform diagnostic quality and safety improvement interventions.10,11
A critical initial step in driving improvement efforts is identification of populations at increased risk for MOIDs.11 The incidence and patterns among adult patients were recently characterized; between 6–11% of adult patients with early (7-day) hospital readmissions were found to experience DEs.4,12 Though prior studies have not identified modifiable factors related to pediatric readmissions,13–15 readmissions represent a burden to patients, families, and the healthcare system; the role of MOID in pediatric readmissions has not been well characterized.16 Evaluating hospital readmissions may elucidate the incidence and characteristics of MOIDs within pediatric acute care and drive systems improvement. In this study, we sought to identify and describe MOIDs among pediatric patients who experienced hospital readmission and illustrate the associated diagnostic process improvement opportunities. We also sought to foster dialogue among pediatric physicians around diagnosis as part of our institution’s quality and safety improvement work and identify factors associated with MOIDs to inform future systematic screening approaches.
METHODS
Study setting and participants
This study was conducted at a freestanding academic children’s hospital with approximately 29,000 annual discharges, including 8,000 annual discharges from the general pediatrics service. Patient charts were reviewed if a patient was discharged from the general pediatrics service between October 1, 2018, and September 30, 2020, and had an unplanned readmission to the hospital (to any service) within 15 days of discharge. Planned readmissions (e.g., scheduled surgery) were excluded. We evaluated 15-day readmissions to align with the pediatric readmission literature, which has found readmissions within this timeframe to be more frequently modifiable than readmissions after 15 days.8,15,17–19 This study was reviewed by the institutional review board (IRB) at our institution and met criteria for exemption.
Review process
To leverage the expertise of practicing inpatient pediatric physicians, the study team was comprised of 13 hospital-based pediatricians, including one general pediatric resident, one pediatric infectious disease fellow (with board certification in general pediatrics), and 11 pediatric hospital medicine attending physicians (board certified in general pediatrics). Reviewers were offered maintenance of certification (MOC) credit for participation. To foster a common approach to case review, all reviewers participated in a one-hour training in identification of MOIDs led by a faculty member with expertise in QI, specifically diagnostic quality and safety, prior to commencing chart reviews. In keeping with our goals of fostering dialogue about diagnosis and building capacity in utilizing novel improvement tools, reviewers were encouraged to discuss cases as a group during weekly sessions which served to further develop reviewer skills and comfort in identifying MOIDs.
Each case was independently reviewed by two study team members. Case reviews occurred asynchronously, and responses were recorded in REDCap, a secure, web-based data collection platform.20 Reviewers were asked to consider all documentation in the electronic health record (EHR) pertaining to the episodes of care under review, including inpatient note documentation for the index admission and readmission, documentation from interval or preceding primary care and outpatient specialty visits, emergency department (ED) documentation, laboratory or radiology results, telephone encounters, vital sign data, medication administration, and prescription records. Reviewers documented the International Classification of Disease-Clinical Modification (ICD-CM) codes for the index admission and readmission final diagnoses. Reviewers did not evaluate cases in which they had provided direct clinical care. To assess feasibility of the review process, reviewers were also asked to estimate time spent on each case review.
Our use of standardized tools and language to identify cases with potential diagnostic delays and error were for the purposes of learning and continuous improvement, both locally and to contribute to the diagnostic medicine literature. These cases were reviewed by clinicians trained in analytic tools for possible opportunities in the diagnostic process, without consideration for any determination of a deviation in the standard of care.
Identifying MOIDs
For each case, reviewers completed SaferDx, a widely adopted instrument designed to guide and operationalize evaluation for presence or absence of MOIDs.9,21,22 SaferDx contains 13 statements relevant to the patient encounter. Reviewers indicate agreement with each statement on a scale from 1 (strongly disagree) to 7 (strongly agree). The final (13th) item states that “the episode of care under review has a missed opportunity to make a correct and timely diagnosis”8 based on all the preceding 12 items. Similarly, reviewers indicate agreement with this concluding statement on a 1 (strongly disagree) to 7 (strongly agree) scale. For our study, cases in which reviewers assigned a score of >4 to the concluding SaferDx statement were classified as containing a MOID.9 A neutral score of 4 was presented to reviewers as an option only if the reviewer could not decide about the presence of a MOID. Discordant reviews (in which one reviewer assigned a score <4 and the other reviewer assigned a score ≥4) and non-definitive reviews (in which both reviewers assigned a score of 4) were referred to a third reviewer within the study team for final determination. The case assignment process was performed at random with an equal number of cases assigned to each study review team member. Open forum discussion by the study team was employed to achieve consensus in cases where the third reviewer assigned a neutral score of 4.
Central to operationalizing the identification of MOID is the explicit cognizance (and avoidance of) hindsight bias. In both reviews and case discussions, there was deliberate attention paid to: (1) whether there was opportunity to improve diagnosis based on the information available to clinicians at the time, and (2) whether delays in diagnosis were present rather than progression or evolution of disease.
Characterizing diagnostic process improvement opportunities
All cases with a MOID were discussed by the study team to identify potential diagnostic process improvement opportunities, mapped to domains in the Modified Diagnostic Error and Evaluation Research (DEER) Taxonomy which classifies “failure points”23 (or opportunities for improvement) according to the phase in the diagnostic process in which they occur. The DEER taxonomy instrument illustrates the following phases of the diagnostic process: Access to Care, History, Physical Exam, Testing, Hypothesis Generation, Consultation, and Follow-up and delineates potential opportunities for improvement within each phase. The tool allows for designation of multiple opportunities for each case. Case discussions occurred during weekly virtual conferences in which reviewers presented a brief case synopsis and their corresponding SaferDx scores, followed by identification of pertinent Modified DEER Taxonomy diagnostic process domains. A minimum of one third of the study team was present for each discussion.
Data analysis
We calculated the incidence of MOID among unplanned general pediatric readmissions. Modified DEER Taxonomy diagnostic process opportunities were tabulated, and the median number of opportunities per instance of MOID was calculated.
Descriptive statistics were used to compare demographic and clinical characteristics of the readmission study population with and without MOIDs. We looked at demographic characteristics that have been previously studied in the adult readmission context4 including patient age, sex, preferred language, insurance, and a proxy equity measure using Social Vulnerability Index (SVI) category.24 Diagnostic disparities have been identified across conditions ranging from acute appendicitis25 to depression,26 and therefore we included race and ethnicity in our evaluation. To identify populations at increased risk for MOIDs and potentially refine future review criteria, we evaluated clinical characteristics including medical complexity defined in this study by presence of a complex chronic condition (CCC),27 admission and discharge ICD-CM codes, admission source (ED or hospital-to-hospital transfer), intensive care unit (ICU) admission during either index or readmission, time to readmission (0–7 vs. 8–15 days), median time to readmission, and hospital length of stay (LOS). ICD-CM codes were grouped using ICD-10 categories and description keywords. Because the study period spanned the beginning of the COVID-19 pandemic, with resultant changes to patient volumes and staffing which could have impacted diagnosis, we categorized episodes of care with an index admission date on or after March 1, 2020, as “during the pandemic” and evaluated this period separately. Differences were evaluated using Pearson’s chi-squared test. For continuous variables we computed the median and interquartile range. Analyses were completed at both the patient and the readmission level but are presented at the readmission level, in keeping with our objective of identifying clinical systems’ opportunities for improvement. Finally, reviewer estimates of review time per case were averaged. Data analysis was performed using R version 4.0.3 (Foundation for Statistical Computing, Vienna, Austria) and Stata 17 (StataCorp LP, College Station, TX).
RESULTS
Diagnostic process improvement opportunities
During the study period, there were 353 readmissions by 321 unique patients, including 5 planned readmissions excluded from review (Figure 1). Of the 348 cases reviewed with SaferDx, 22 (6.3%) MOIDs were identified (Table 1). Of the cases with MOIDs, potential diagnostic process improvement opportunities were identified in every Modified DEER Taxonomy domain except Access to Care (Figure 2). Cases with MOIDs had a median of 5 areas for potential diagnostic improvement. The most frequently identified diagnostic process improvement opportunities included: clinician assessment (weighing of a piece of history (n=10, 45%)), decision-making (ordering needed test(s) (n=10, 45%)), and hypothesis generation or diagnostic reasoning (considering the correct diagnosis (n=11, 50%), weighing of the correct diagnosis (n=10, 45%)). Example cases of each DEER taxonomy domain are illustrated in Table 2.
FIGURE 1.
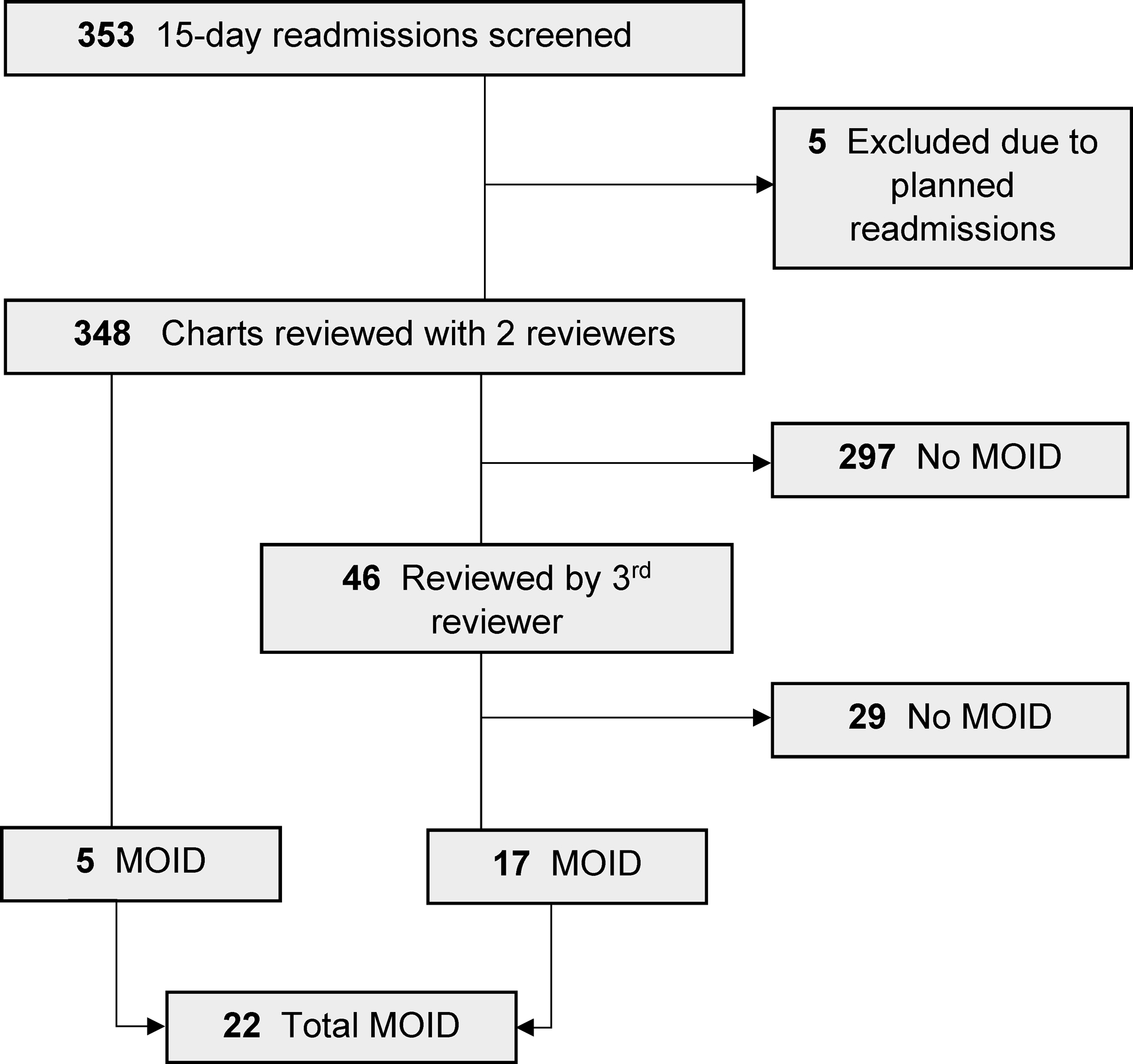
Overview of 15-day readmission chart reviews. MOID, missed opportunity for improving diagnosis
TABLE 1.
Demographic and Clinical Characteristics of General Pediatric Readmissions Within 15 Days of Discharge.
| Readmission characteristics | Total readmissions, | Readmissions without MOIDs, | Readmissions with MOIDs, | p-value * |
|---|---|---|---|---|
| N = 348 | N=326 | N=22 | ||
| Age, n (%) | 0.013 | |||
| <1 years | 167 (48.0) | 163 (50.0) | 4 (18) | |
| 2–5 years | 89 (25.6) | 81 (24.8) | 8 (36) | |
| >5 years | 92 (26.4) | 82 (25.2) | 10 (46) | |
| Sex, n (%) | 0.87 | |||
| Female | 164 (47.1) | 154 (47.2) | 10 (46) | |
| Male | 184 (52.9) | 172 (52.8) | 12 (55) | |
| Race, n (%) | 0.31 | |||
| Asian | 15 (4.3) | 13 (4.0) | 2 (9) | |
| Black | 133 (38.2) | 128 (39.3) | 5 (23) | |
| Multiracial or Other† | 54 (15.5) | 49 (15.0) | 5 (23) | |
| White | 146 (42.0) | 136 (41.7) | 10 (46) | |
| Ethnicity, n (%) | 0.97 | |||
| Hispanic or Latino | 46 (13.2) | 43 (13.2) | 3 (14) | |
| Not Hispanic or Latino | 301 (86.5) | 282 (86.5) | 19 (86) | |
| Refused | 1 (0.3) | 1 (0.3) | 0 (0.0) | |
| Preferred Language, n (%) | 0.084 | |||
| English | 319 (91.7) | 301 (92.3) | 18 (82) | |
| Non-English | 29 (8.3) | 25 (7.7) | 4 (18) | |
| Insurance, n (%) | 0.29 | |||
| Commercial/Other | 137 (39.4) | 126 (38.7) | 11 (50) | |
| Public | 211 (60.6) | 200 (61.3) | 11 (50) | |
| Complex chronic condition‡, n (%) | 101 (29.0) | 94 (28.8) | 7 (32) | 0.46 |
| Social Vulnerability Index (SVI)§, n (%) | 0.17 | |||
| Highest | 119 (34.2) | 109 (33.4) | 10 (46) | |
| Lowest | 75 (21.6) | 72 (22.1) | 3 (14) | |
| Medium high | 63 (18.1) | 62 (19.0) | 1 (5) | |
| Medium low | 88 (25.3) | 80 (24.5) | 8 (36) | |
| Missing | 3 (0.9) | 3 (0.9) | 0 (0) | |
| Index admission length of stay in days, median (IQR) | 2.1 (1.4–4.0) | 2.1 (1.4–4.2) | 1.6 (1.2–3.0) | 0.069 |
| Readmission length of stay in days, median (IQR) | 2.4 (1.5–5.1) | 2.3 (1.5–4.8) | 4.3 (2.0–11.9) | 0.023 |
| Time between admissions, n (%) | 0.13 | |||
| 0 to 7 days | 233 (67.0) | 215 (66.0) | 18 (82) | |
| 8 to 15 days | 115 (33.0) | 111 (34.0) | 4 (18) | |
| Admission source, n (%) | 0.039 | |||
| Direct admission¶ | 23 (6.6) | 23 (7.1) | 0 (0) | |
| Emergency department | 290 (83.3) | 271 (83.1) | 19 (86) | |
| Transfer from another hospital or facility | 14 (4.0) | 11 (3.4) | 3 (14) | |
| Missing | 21 (6.0) | 21 (6.4) | 0 (0) | |
| ICU stay during index admission, n (%) | 65 (19.3) | 63 (20.0) | 2 (9) | 0.27 |
| ICU stay during readmission, n (%) | 59 (18.0) | 55 (18.0) | 4 (18) | 1.00 |
| COVID-19 pandemic, n (%)** | 0.18 | |||
| Index admission date prior to 3/1/2020 | 235 (67.5) | 223 (68.4) | 12 (54.5) | |
| Index admission date on or after 3/1/2020 | 113 (32.5) | 103 (31.6) | 10 (45.5) |
MOID, missed opportunity for improving diagnosis; SVI, Social Vulnerability Index; IQR, interquartile range; ICU, intensive care unit.
This is an analysis of 348 readmissions by 321 patients.
Differences were evaluated using Pearson’s chi-squared test.
“Other” includes patients who did not disclose or who reported American Indian or Alaska Native, Asian, Indian, Multi-racial, Native Hawaiian or Other Pacific Islander or Other.
Based on Feudtner et al. 2014.26
Social Vulnerability Index (SVI) is a composite index created by the CDC based on the patient’s primary home address to quantify “social vulnerability” which is defined by the CDC as “potential negative effects on communities caused by external stresses on human health.”23
inbound transport type (that is not interfacility)
Readmissions where index admission occurred on or after March 1, 2020 were classified as “during the pandemic”
FIGURE 2. Potential diagnostic process improvement opportunities, by Diagnostic Error and Evaluation Research (DEER) taxonomy23 category and subcategory.
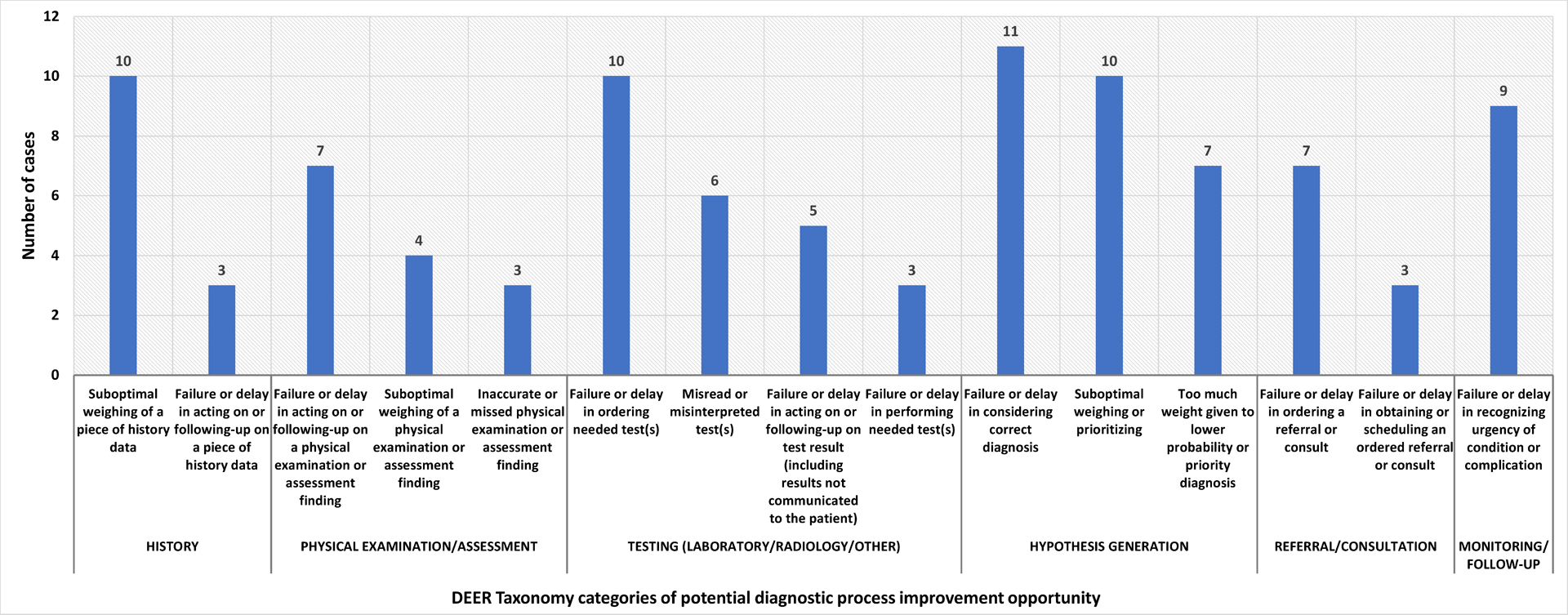
Excludes category and subcategories with <3 opportunities identified.
Cases could have multiple failure points within each category.
Each case had multiple potential diagnostic process opportunities, so these numbers do not sum to 22.
Table 2.
Representative case descriptions of potential opportunities for improvement for each Diagnostic Error and Evaluation Research (DEER) taxonomy* domain of the diagnostic process
| Diagnostic process phase where opportunities were identified | Case description | Initial diagnosis | Final diagnosis |
|---|---|---|---|
| History | Toddler with multiple hospitalizations for croup and remote history of intubation was admitted with stridor. Diagnosed with croup and required intensive care. Readmitted with recurrent symptoms. On otolaryngology evaluation found to have tracheal stenosis requiring balloon dilation. | Croup | Tracheal stenosis |
| Exam | Teenager with frequent emergency department visits for moderate persistent asthma presented with cough, tachypnea, and dyspnea. Initial ED exam noted decreased breath sounds but no wheeze. Diagnosed with acute asthma exacerbation but demonstrated limited improvement with bronchodilators. Readmitted with recurrent symptoms. Found to have symptomatic anemia requiring transfusion. Diagnosed with hyperventilation syndrome. | Mild intermittent asthma with (acute) exacerbation | Anemia and Hyperventilation syndrome |
| Testing | Small for gestational age neonate admitted for hyperbilirubinemia requiring phototherapy. Admission labs demonstrated marked metabolic acidosis. Readmitted soon after discharge with lethargy. Found to be hypoglycemic, hypothermic, with worsening acidosis. Newborn screen was positive for inborn error of metabolism. | Neonatal Physiologic Jaundice | Methylmalonic acidemia |
| Hypothesis | School aged child admitted with several weeks of headaches, emesis, and weight loss with remote history of minor head injury. Diagnosed with post-concussive symptoms and viral infection. Readmitted with worsening symptoms and new back pain. Imaging and lab testing demonstrated demyelinating disease. | Viral intestinal infection, unspecified | Anti-myelin oligodendrocyte glycoprotein (MOG) associated inflammatory encephalitis |
| Consults | Adolescent admitted with fever, periorbital edema, headache, back and abdominal pain. Found to have acute kidney injury and hypertension. Diagnosed with viral syndrome. Readmitted with worsening symptoms. Diagnosed with lymphoproliferative disorder. | Viral infection | Multi-centric Castleman Disease |
| Monitoring | Infant with multiple admissions for insufficient weight gain admitted with vomiting, inconsolability, and dehydration. Found to have hematochezia and hypocalcemia. Diagnosed with viral gastroenteritis. Readmitted soon after discharge with lethargy and required intensive care due to electrolyte derangements, found to have inflammatory bowel disease (IBD). | Infant malnutrition and Gastroenteritis | Very early onset inflammatory bowel disease (VEO-IBD) |
No cases were found to have opportunities for improvement in the Access to Care Phase
Schiff GD, Kim S, Abrams R, et al. Diagnosing Diagnosis Errors: Lessons from a Multi-institutional Collaborative Project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Advances in Patient Safety. Agency for Healthcare Research and Quality (US); 2005. Accessed October 23, 2019. http://www.ncbi.nlm.nih.gov/books/NBK20492
Patient characteristics
Readmitted patients who experienced MOIDs were older (median age 3.8 years, IQR: 1.5 to 11.2) than those without MOIDs (median age 1.0 year, IQR: 0.3 to 4.9), but otherwise demographically similar (Table 1). Patients with MOIDs experienced longer readmission LOS (median 4.3 days, IQR 2.0–11.9) than cases without (median 2.3 days, IQR 1.5–4.8) MOIDs. Although most (82%) MOIDs were associated with readmissions occurring within 0–7 days from discharge, the probability of having a MOID did not vary significantly among those readmitted within 0–7 days compared to those readmitted from 8–15 days (p=0.13). We evaluated demographic characteristics at both the encounter and the patient level; no notable differences in significance or magnitude existed.
MOIDs occurred across a range of conditions. Final readmission diagnoses included infectious processes such as septic arthritis, lymphadenitis with abscess, and cellulitis (n = 6, 22.7%), upper airway anomalies such as tracheal stenosis and laryngeal cleft (n=3, 13.6%), and cardiac etiologies (n= 2, 9.1%). No index admission diagnoses or diagnostic groups were significantly associated with MOIDs. However, lower respiratory tract conditions (including bronchiolitis, asthma exacerbation, and pneumonia) accounted for 91 (26%) of index admission diagnoses but only 1 (4.5%) case of MOID (p=0.02). Among those admitted for non-lower respiratory tract conditions (n=257), the incidence of MOIDs did not differ by age group (p=0.3).
Review process
Reviewers reported that using the SaferDx tool to reach a conclusion about the presence or absence of MOIDs took an average of 30 minutes per case. As each case was reviewed by two reviewers and 46 cases (13%) required a third review, this represented approximately 371 review hours (or 29 hours per reviewer over the study period). Limiting review to readmissions within 7 days of discharge and to non-lower respiratory tract conditions would have reduced the review burden by nearly half (from 348 charts to 179 charts) and identified 17 (78%) of 22 MOIDs, increasing the rate of MOID to 9.5%.
DISCUSSION
Using a systematic review process, we identified MOIDs in 6.3% of children who were discharged from the general pediatrics service and required readmission within 15 days of discharge. Potential diagnostic process improvement opportunities within our cohort were those related to clinician decision-making and consideration or prioritization of the correct diagnosis. We did not identify conditions at increased risk for MOIDs, however readmissions for lower respiratory tract infections were less likely to experience a MOID.
The identified rate of MOID among general pediatric readmissions in our study is similar to the DE rate identified in adult internal medicine readmissions (5.6%).4 It also aligns with rates identified among pediatric inpatients in the United Kingdom (5.0%)28 and among pediatric patients discharged from the ED who were subsequently hospitalized (4.8%).29 Given that nearly 3 million children experience hospitalization in the United States every year, these rates indicate an urgent need to develop diagnostic improvement strategies for acute care pediatrics. Our approach identified novel opportunities to improve diagnosis and potentially avert the harms associated with readmission in a subset of readmitted patients.
We used DEER taxonomy to classify potential diagnostic process improvement domains. Echoing the adult readmissions literature,4 the most frequently identified opportunities for improvement pertained to clinician assessment (ordering tests and hypothesis generation/diagnostic reasoning). This aligns with findings from a 2014 study of a British pediatric community hospital cohort, which identified cognitive factors (data gathering and formulation of a different diagnosis) as primary drivers of DE.28 While crafting specific interventions was not the focus of this study, our findings suggest that QI efforts targeting diagnostic reasoning (e.g., adoption of standardized processes to label diagnostic uncertainty30 or development of note templates that support recognition of alternative diagnoses) may be helpful in reducing MOID. Identification of these potential improvement domains provides focus for the systems-change efforts needed to promote and support diagnostic quality and safety at an individual, team, and systems level.10
We sought to identify demographic and clinical characteristics of readmitted children associated with increased risk of MOID. We observed overall age differences between readmitted patients who experienced MOIDs and those who did not, though the frequency of admissions due to respiratory conditions among infants (especially for admissions related to lower respiratory tract conditions) appears to drive these differences and is consistent with prior literature among unplanned pediatric admissions identified by an e-trigger.29 While we did not identify sociodemographic factors significantly associated with MOIDs, it is notable that 18% of MOIDs occurred in the 29 (8%) encounters in which patients or families identified as using a primary language other than English (LOE).31 Prior studies have noted that patients with language barriers are at particular risk for medical error (including DE),32–36 and we suggest the importance of further surveillance of MOIDs among this population.
One of our secondary objectives was to support dialogue about diagnostic improvement within a diverse group of practicing pediatricians. Mindful of the psychological safety of clinicians and to promote a focus on systems improvement, we shifted from “error” nomenclature to “potential missed opportunities.” We observed that although we used a standardized review strategy, 13% of cases required a third reviewer because of discordant or non-definitive scoring. Even among cases that were ultimately identified as having a MOID, many (17/22) required a third reviewer. This likely represents a combination of complexity or nuance in the particular cases in addition to the relative newness of this work. It also reflects the reality that even with a standardized tool like SaferDx, it can be difficult for individual reviewers to classify cases as having a MOID. In response to reviewer uncertainty, we established open discussion forums to come to group consensus about cases. We anticipate that as concepts of MOID become more familiar within pediatrics and at our institution, reviewers will be more definitive in their scoring.
Finally, we explored readmission factors that might improve the yield of our case review process. Pediatric autopsy data from studies in pediatric and neonatal critical care units have found high rates of missed sepsis and vascular events;37,38 however, these conditions occur less frequently in general pediatrics and did not occur in our sample. Electronic health record or administrative diagnostic code-based triggers for diagnostic review have been proposed as a strategy for identifying opportunities to improve diagnosis.[SPADE, Lam, Michaelson] In the absence of clear diagnostic groups associated with pediatric inpatient MOIDs, there may be a subset of pediatric hospital readmissions that are high yield in identifying opportunities to improve diagnosis (and could serve as an e-trigger for review). Limiting future reviews to 7-day readmissions and excluding readmissions for lower respiratory tract infections would have reduced the case review burden by nearly half, while identifying 78% of MOIDs. Narrowing the cohort of reviewed pediatric hospital readmission cases may be a pragmatic strategy for ongoing MOID surveillance, acknowledging the tradeoff of cases missed using narrower review criteria.
One strength of our study is the application of a standardized, 2-reviewer process which used an established instrument (SaferDx) to identify MOIDs coupled with case review via DEER taxonomy. Since Warwick et al25 published initial exploration of DE among pediatric inpatients in 2014, tools such as SaferDx have been developed to operationalize identification of MOID. Others have utilized SaferDx to evaluate pediatric diagnosis in critical care settings37–39 and emergency department settings;29,40 we utilized SaferDx and DEER taxonomy to identify process improvement opportunities among general pediatric inpatients. Our standardized review approach is readily replicable in other settings. Our 13-member review panel provided varied perspectives and robust clinical experiences; this review model allowed for the identification of a range of potential improvement opportunities and promoted dialogue within the practice community. Reflecting on two years of general pediatric readmissions allowed for evaluation of MOIDs across time, hospital care delivery model, and clinician.
Our findings should be contextualized within the limitations of our approach. First, we detected relatively few MOIDs and thus were limited in our ability to assess differences between groups. Identifying populations at low risk for MOID should allow for future review efforts to be more efficient, perhaps allowing for detection of a cohort large enough for us to evaluate drivers of MOID. Similarly, future work exploring approaches to pediatric hospital MOID identification in community hospital settings or across institutions will be important in terms of generalizability. We sought to mitigate the risk of hindsight bias from retrospective case review by engaging in multi-clinician, discussions in which this potential hazard was explicitly named and considered. Our reviews were limited by the information available in the EHR; important factors such as clinician cognitive load, family perspectives, and verbal communication were outside our scope. While we had access to records from our tertiary healthcare system, we were unable to detect MOIDs in which patients did not return to care, did not require readmission, or were readmitted at a different institution.
CONCLUSION
Using a standardized approach with SaferDx and DEER taxonomy, we identified MOIDs among general pediatric readmissions. We found common areas for diagnostic process improvement opportunities, namely clinician decision-making and diagnostic reasoning that confirm data trends in adult populations. We identified conditions at lower risk for MOID that will inform ongoing improvement work. Future studies across institutions and care settings can further describe populations of children at risk for MOID.
Supplementary Material
Acknowledgement:
The authors thank Kristi McNaughton, MHS for editing assistance.
Abbreviations:
- CCC
complex chronic condition
- DE
diagnostic error
- DEER
Diagnostic Error and Evaluation Research
- ED
emergency department
- EHR
electronic health record
- ICD-CM
International Classification of Disease-Clinical Modification
- ICU
intensive care unit
- IQR
interquartile range
- IRB
institutional review board
- QI
quality improvement
- LOE
language other than English
- LOS
length of stay
- MOID
missed opportunity for improving diagnosis
- SVI
Social Vulnerability Index
Footnotes
Conflict of Interest Disclosures (includes financial disclosures): The authors have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Balogh E, Miller BT, Ball J, Institute of Medicine (U.S.), eds. Improving Diagnosis in Health Care. The National Academies Press; 2015. [PubMed] [Google Scholar]
- 2.Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Amp Saf. 2015;24(2):103. doi: 10.1136/bmjqs-2014-003675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagn Berl Ger. 2015;2(2):97–103. doi: 10.1515/dx-2014-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. Published online August 4, 2020:bmjqs-2020-010896. doi: 10.1136/bmjqs-2020-010896 [DOI] [PubMed] [Google Scholar]
- 5.Newman-Toker DE. Diagnostic errors—the next frontier for patient safety. JAMA. 2009;301(10):1060. doi: 10.1001/jama.2009.249 [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. Published online August 9, 2017. doi: 10.1136/bmjqs-2017-006774 [DOI] [PubMed] [Google Scholar]
- 7.Khoo EM, Lee WK, Sararaks S, et al. Medical errors in primary care clinics--a cross sectional study. BMC Fam Pract. 2012;13:127. doi: 10.1186/1471-2296-13-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham KL, Auerbach AD, Schnipper JL, et al. Preventability of early versus late hospital readmissions in a national cohort of general medicine patients. Ann Intern Med. 2018;168(11):766–774. doi: 10.7326/M17-1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis. 2019;6(4):315–323. doi: 10.1515/dx-2019-0012 [DOI] [PubMed] [Google Scholar]
- 10.Marshall TL, Rinke ML, Olson APJ, Brady PW. Diagnostic error in pediatrics: a narrative review. Pediatrics. 2022;149(Suppl 3):e2020045948D. doi: 10.1542/peds.2020-045948D [DOI] [PubMed] [Google Scholar]
- 11.Perry MF, Melvin JE, Kasick RT, et al. The diagnostic error index: A quality improvement initiative to identify and measure diagnostic errors. J Pediatr. 2021;232:257–263. doi: 10.1016/j.jpeds.2020.11.065 [DOI] [PubMed] [Google Scholar]
- 12.Graham KL, Wilker EH, Howell MD, Davis RB, Marcantonio ER. Differences between early and late readmissions among patients: a cohort study. Ann Intern Med. 2015;162(11):741–749. doi: 10.7326/M14-2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auger KA. Progress(?) towards reducing pediatric readmission. J Hosp Med. Published online 2019. doi: 10.12788/jhm.3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest CB, Silber JH. Concept and measurement of pediatric value. Acad Pediatr. 2014;14(5):S33–S38. doi: 10.1016/j.acap.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 15.Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis: readmission analysis using fault tree. J Hosp Med. 2016;11(5):329–335. doi: 10.1002/jhm.2555 [DOI] [PubMed] [Google Scholar]
- 16.Sawicki JG, Nystrom D, Purtell R, Good B, Chaulk D. Diagnostic error in the pediatric hospital: a narrative review. Hosp Pract. Published online November 25, 2021:1–8. doi: 10.1080/21548331.2021.2004040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace SS, Keller SL, Falco CN, et al. An examination of physician-, caregiver-, and disease-related factors associated with readmission from a pediatric hospital medicine service. Hosp Pediatr. 2015;5(11):566–573. doi: 10.1542/hpeds.2015-0015 [DOI] [PubMed] [Google Scholar]
- 18.Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171–181. doi: 10.1542/peds.2012-0820 [DOI] [PubMed] [Google Scholar]
- 19.Berry JG, Gay JC. Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5(11):602–604. doi: 10.1542/hpeds.2015-0189 [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Mutairi A, Meyer AND, Thomas EJ, et al. Accuracy of the Safer Dx instrument to identify diagnostic errors in primary care. J Gen Intern Med. 2016;31(6):602–608. doi: 10.1007/s11606-016-3601-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agency for Healthcare Research and Quality. Measure Dx: A Resource To Identify, Analyze, and Learn From Diagnostic Safety Events.; 2022. Accessed September 30, 2022. https://www.ahrq.gov/patient-safety/settings/multiple/measure-dx.html
- 23.Schiff GD, Kim S, Abrams R, et al. Diagnosing Diagnosis Errors: Lessons from a Multi-institutional Collaborative Project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Advances in Patient Safety. Agency for Healthcare Research and Quality (US); 2005. Accessed October 23, 2019. http://www.ncbi.nlm.nih.gov/books/NBK20492/ [Google Scholar]
- 24.CDC/ATSDR Social Vulnerability Index 2018 Database US. Accessed May 12, 2022. https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html
- 25.Goyal MK, Chamberlain JM, Webb M, et al. Racial and ethnic disparities in the delayed diagnosis of appendicitis among children. Acad Emerg Med. Published online September 29, 2020:acem.14142. doi: 10.1111/acem.14142 [DOI] [PubMed] [Google Scholar]
- 26.Lukachko A, Olfson M. Race and the clinical diagnosis of depression in new primary care patients. Gen Hosp Psychiatry. 2012;34(1):98–100. doi: 10.1016/j.genhosppsych.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 27.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warrick C, Patel P, Hyer W, Neale G, Sevdalis N, Inwald D. Diagnostic error in children presenting with acute medical illness to a community hospital. Int J Qual Health Care. 2014;26(5):538–546. doi: 10.1093/intqhc/mzu066 [DOI] [PubMed] [Google Scholar]
- 29.Lam D, Dominguez F, Leonard J, Wiersma A, Grubenhoff JA. Use of e-triggers to identify diagnostic errors in the paediatric ED. BMJ Qual Saf. Published online March 22, 2022:bmjqs-2021-013683. doi: 10.1136/bmjqs-2021-013683 [DOI] [PubMed] [Google Scholar]
- 30.Marshall TL, Ipsaro AJ, Le M, et al. Increasing physician reporting of diagnostic learning opportunities. Pediatrics. 2021;147(1):e20192400. doi: 10.1542/peds.2019-2400 [DOI] [PubMed] [Google Scholar]
- 31.Yeboah D, McDaniel C, Lion KC. Language Matters: Why We Should Reconsider the Term “Limited English Proficiency.” Hosp Pediatr. 2023;13(1):e11–e13. doi: 10.1542/hpeds.2022-007014 [DOI] [PubMed] [Google Scholar]
- 32.Bell SK, Dong J, Ngo L, McGaffigan P, Thomas EJ, Bourgeois F. Diagnostic error experiences of patients and families with limited English-language health literacy or disadvantaged socioeconomic position in a cross-sectional US population-based survey. BMJ Qual Saf. Published online February 4, 2022:bmjqs-2021–013937. doi: 10.1136/bmjqs-2021-013937 [DOI] [PubMed] [Google Scholar]
- 33.Cohen AL, Rivara F, Marcuse EK, McPhillips H, Davis R. Are language barriers associated with serious medical events in hospitalized pediatric patients? Pediatrics. 2005;116(3):575–579. doi: 10.1542/peds.2005-0521 [DOI] [PubMed] [Google Scholar]
- 34.Flores G, Laws MB, Mayo SJ, et al. Errors in medical interpretation and their potential clinical consequences in pediatric encounters. Pediatrics. 2003;111(1):6–14. doi: 10.1542/peds.111.1.6 [DOI] [PubMed] [Google Scholar]
- 35.Flores G Language barriers and hospitalized children: are we overlooking the most important risk factor for adverse events? JAMA Pediatr. 2020;174(12):e203238. doi: 10.1001/jamapediatrics.2020.3238 [DOI] [PubMed] [Google Scholar]
- 36.Khan A, Yin HS, Brach C, et al. Association between parent comfort with English and adverse events among hospitalized children. JAMA Pediatr. 2020;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Custer JW, Winters BD, Goode V, et al. Diagnostic errors in the pediatric and neonatal ICU: a systematic review. Pediatr Crit Care Med. 2015;16(1):29–36. doi: 10.1097/PCC.0000000000000274 [DOI] [PubMed] [Google Scholar]
- 38.Cifra CL, Jones KL, Ascenzi JA, et al. Diagnostic errors in a PICU: insights from the morbidity and mortality conference. Pediatr Crit Care Med. 2015;16(5):468–476. doi: 10.1097/PCC.0000000000000398 [DOI] [PubMed] [Google Scholar]
- 39.Davalos MC, Samuels K, Meyer AND, et al. Finding diagnostic errors in children admitted to the PICU. Pediatr Crit Care Med. 2017;18(3):265–271. doi: 10.1097/PCC.0000000000001059 [DOI] [PubMed] [Google Scholar]
- 40.Mahajan P, Mollen C, Alpern ER, et al. An operational framework to study diagnostic drrors in emergency departments: findings from a consensus panel. J Patient Saf. 2021;17(8):570–575. doi: 10.1097/PTS.0000000000000624 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


