Abstract
Background:
Cirrhosis, the end-result of liver injury, has a high global mortality. Access to diagnosis and treatment varies worldwide, but these variations’ impact on mortality is unclear. Therefore, we aimed to determine predictors of mortality in inpatients with cirrhosis using a global consortium focusing on cirrhosis-related and access-related variables.
Methods:
In this prospective observational study, the CLEARED Consortium followed cirrhosis inpatients at 90 tertiary-care hospitals in 25 countries across 6 continents. Consecutive patients >18 years of age admitted non-electively without COVID-19, or advanced malignancy were enrolled. We ensured equitable participation by limiting enrollment to a maximum of 50 patients per site. Presentation, disease severity, hospital management, and 30-day post-discharge mortality data were collected from patients and their medical records. Primary outcomes were inpatient and 30-day post-discharge mortality and liver transplant (LT). Sites were surveyed regarding availability and access to diagnostic and treatment services, and World bank income classifications [high (HIC), upper-middle (UMIC), low/low-middle income (LMIC)] of participating countries were used. Multi-variable models controlling for demographics, disease etiology, and severity were created.
Findings:
Patients were recruited between 5th November 2021 through 31st August 2022. Complete inpatient data were obtained for 3884 patients [mean age 55.9 years, 2486 (64%) men, 1413 (36.4%) HIC, 1757(45.2%) UMIC, 714 (18.4%) LMIC], with 410 lost to follow-up after hospital discharge. Inpatient and 30-day post-discharge mortality was lowest in HICs [110 (8%); and 179 (14%), p<0.001] compared to UMIC [182 (10%); and 267 (17%)] and LMICs [158 (22%); and 204 (30%), p<0.001]. Being from a non-HIC center was also associated with lower LT rates during hospitalization [HIC 59 (4%), vs. UMIC 28 (2%) and LMICs 14 (2%), p<0.001] and at 30-days post-discharge [HIC 110 (10%), vs. UMIC 57 (4%) and LMICs 16 (2%), p<0.001]. Higher mortality amongst non-HIC patients was also observed on multi-variable analysis for inpatient [adjusted OR (AOR) 2.54 LMIC, 2.14 for UMIC versus HIC] and 30-day mortality [AOR 1.84 LMIC, 1.95 UMIC versus HIC] (p<0.0001). Similarly, not being in a HIC was associated with lower odds of inpatient LT [AOR and 95% CI 0.21 (0.10, 0.41) LMIC, 0.41 (0.24, 0.69) UMIC vs. HIC)], and for 30-day post-discharge LT [AOR and 95% CI 0.21 (0.11, 0.40) LMIC, 0.58 (0.39, 0.85) UMIC vs. HIC], Site survey results showed that non-HIC centers had lower access to important medications (rifaximin, albumin, terlipressin) and interventions (emergency endoscopy, LT, intensive care, and palliative care).
Interpretation:
Inpatients with cirrhosis treated in lower-income countries have significantly higher mortality independent of usual medical risk factors, likely due to disparities in access to essential diagnostic and treatment services. These results should encourage researchers and policymakers to consider access to services and medications when evaluating cirrhosis-related outcomes.
Funding:
NIH and VA
BACKGROUND:
Chronic liver disease and cirrhosis are significant causes of morbidity and mortality worldwide,1 and this burden is projected to grow over time2. Chronic liver disease accounts for 2 million or 4% of deaths annually and is also the 11th leading cause of mortality worldwide1. Cirrhosis progresses from a compensated to a decompensated stage with complications such as ascites, variceal bleeding, hepatic encephalopathy (HE), acute kidney injury (AKI), and increased infection risk; the proximate cause of mortality in most patients is organ failure.3 These inpatients require resource-intensive management strategies that could be variable worldwide in affordability and access. In the US in 2016, the cost involved in treating liver disease was $32.5 billion, with almost 70% used for inpatient or emergency department care, but it is unclear whether the significant expenditure in the global North is associated with better outcomes than in patients admitted in resource-poor countries4. Most cirrhosis studies are from the global North or are regional5–7 and have not considered the availability and affordability of diagnostic and treatment modalities or cultural or social factors. Disparities in liver disease diagnosis, management, and outcomes among underserved populations have been identified in the United States,8. Still, global prospectively collected data are sparse and needed to inform approaches to improving patient outcomes. We initiated the Chronic Liver disease Evolution And Registry for Events and Decompensation (CLEARED) Consortium with the aim to determine predictors of mortality in hospitalized patients with cirrhosis across all populated continents using prospectively collected data.
METHODS:
Study design and participants:
The CLEARED Consortium has two principal investigators (PIs) from USA and India, steering committee members from Australia, Brazil, Canada, Ethiopia, Mexico, China, Turkey, the UK, and the USA, and 90 participating clinical sites located in all 6 populated continents. This is a prospective observational cohort study across 90 centers and 25 countries of consecutive hospitalized patients with cirrhosis who provided written informed consent. To ensure equity and adequate representation, we only allowed maximum of 50 patients per site (Tables S1/2). We included subjects who were >18 years with confirmed cirrhosis (liver biopsy, decompensation, or other locally validated methods) admitted non-electively and who were either able to consent or had an authorized representative available. We excluded those with an unclear cirrhosis diagnosis, prisoners, hepatocellular cancer (HCC) without loco-regional control, and COVID-19 at any time.
Procedures:
Informed consent and protocol documents were translated into local languages and approved by all local ethics committees. Consecutive potential subjects were evaluated through chart review and approached by study coordinators. We collected data from admission through 30-day post-discharge after informed consent (appendix). Data were collected by the study coordinators and site investigators at enrollment, discharge, and 30-day follow-up using patient reports and chart reviews. Data were then uploaded in a de-identified manner to the data coordinating center at Virginia Commonwealth University.
Admission data collected included demographics, including self-reported gender with options that included male or female, country of origin, liver disease severity (MELD-Na score) and etiology (as determined by local investigators, which did not include performing viral serology specifically for this study) of cirrhosis, medications used, co-morbid conditions, reason(s) for admission, transplant listing, prior cirrhosis-related history over the last six months and prior complications, infections and hospitalizations. These were gathered using the patient report and chart reviews. During hospitalization, we collected data regarding cirrhosis severity. organ dysfunction including ventilation, vasopressor use, intensive care unit (ICU) transfer, infections, other decompensating events, death, and liver transplant (LT). After discharge, we collected data regarding readmission, death, and LT.
We divided countries of origin into high-income (HIC), upper-middle income (UMIC), and low/lower-middle income (LIC/LMIC) using World Bank definitions9. Infections were diagnosed using published definitions10 (appendix page 3). The primary outcomes of interest were mortality and LT while admitted and 30-day post-discharge. Other outcomes evaluated were organ dysfunction, defined as stage ≥2 AKI, grade ≥3 HE as per West Haven criteria, and need for ventilation or vasopressor support11 (appendix page 3); and length of stay (LOS), nosocomial infections, hospice referral and ICU transfer as well as discharge MELD-Na and, 30-day re-admissions.
Follow-up data collection was performed using a combination of chart review, patient calls, and evaluation of medical records. Transfer to hospice was equated to death since patient calls after hospice transfer were discouraged. Finally, a standardized survey (appendix page 16) was sent to all PIs, inquiring about patients managed at their centers regarding insurance coverage, resource, and medication availability such as rifaximin, terlipressin, intravenous albumin, as well as emergency endoscopy, LT, and hospice/palliative care services. The PIs were also asked to assess the ability of their average patient to afford investigations, ICU admission, medications, LT, and palliative care. The data monitoring center sent this survey via REDCAP to the PIs of all sites that had enrolled subjects on 6th August 2022 with a month for completion, and a reminder was sent a month later.
Statistical analysis:
Continuous variables were summarized using means and standard deviations or, where appropriate, medians and interquartile ranges, while all categorical data were summarized using percentages and frequencies. Group comparisons were made using two-sample t-tests/one-way ANOVA or Mann-Whitney U-tests/Kruskal Wallis tests where appropriate for continuous variables, and chi-square tests/Fisher’s Exact test for categorical variables. For analysis of two groups and normally distributed data, the two-sample t-test, and for three or more groups and normally distributed data, the one-way ANOVA was utilized for comparisons. If the data were not normally distributed, the Mann-Whitney U-test was used for group comparisons involving two groups while the Mann-Whitney U-test extension, the Kruskal-Wallis test, was utilized when three or more groups were compared. With regards to categorical data group comparisons, whenever 25% or more of the cells in the corresponding contingency tables had an expected value less than 5, the Fisher’s exact test was utilized; otherwise, the chi-Square test was utilized. The statistical methods above were used to compare those who survived or received LT versus those who died or were referred to hospice by patient and disease-related factors, to compare outcomes between HIC, UMIC and LMIC patients, and to analyze the PI survey. Multivariable logistic regression models were developed using a modified purposeful selection of covariate procedure since it was a fixed time-period where the outcomes were studied12. Variables that would be available at admission and had a significance level of α = 0.25 were considered for inclusion as well as considered clinically relevant by the steering committee (demographics, cirrhosis severity on admission, events related to cirrhosis and infections over the last 6 months, medications and co-morbid conditions, reasons for admission, alcohol and viral-related etiology and country income status). A backward elimination procedure was then utilized, with an α = 0.25 level to stay in the model. Variables were removed manually with the goal of parsimony by keeping only variables that were significant at the α = 0.05 significance level. Once this parsimonious model was arrived at, all previously removed variables were added back one at a time and retained only if they achieved the α = 0.05 significance level (appendix page 1) in the final model. The multi-variable approach above was used to predict inpatient and 30-day post-discharge mortality and LT. Linearity assumptions were also tested (appendix page 2).
All analyses were performed using SAS 9.4, and unless otherwise specified, an α = 0.05 significance level was used for all tests. Due to the need to balance equitable participation and workload across sites, the limited funding for this study, and the lack of pilot data, the steering committee decided on a maximum of 50 patients per site. There is no statistical justification of the sample size per se.
Role of the funding source:
The funding source had no role in data collection, data analysis, data interpretation, or writing of the report.
RESULTS:
We approached 4395 patients; 511 were excluded for various reasons (Figure 1), leaving 3884 patients who fulfilled the eligibility criteria and had complete inpatient data. Subjects were recruited between 5th November 2021 through 31st August 2022 from 90 centers with a median of 49 patients per center (IQR 43–50 patients/center). The highest number of patients were from China, followed by North America, India, and Turkey (Figure S1/Table S2). 24 sites were from China, 23 from North America, 11 India, 9 Middle East, 7 each from Australia, and South America, 6 from Africa and 3 sites from the rest of Asia.
Figure 1:
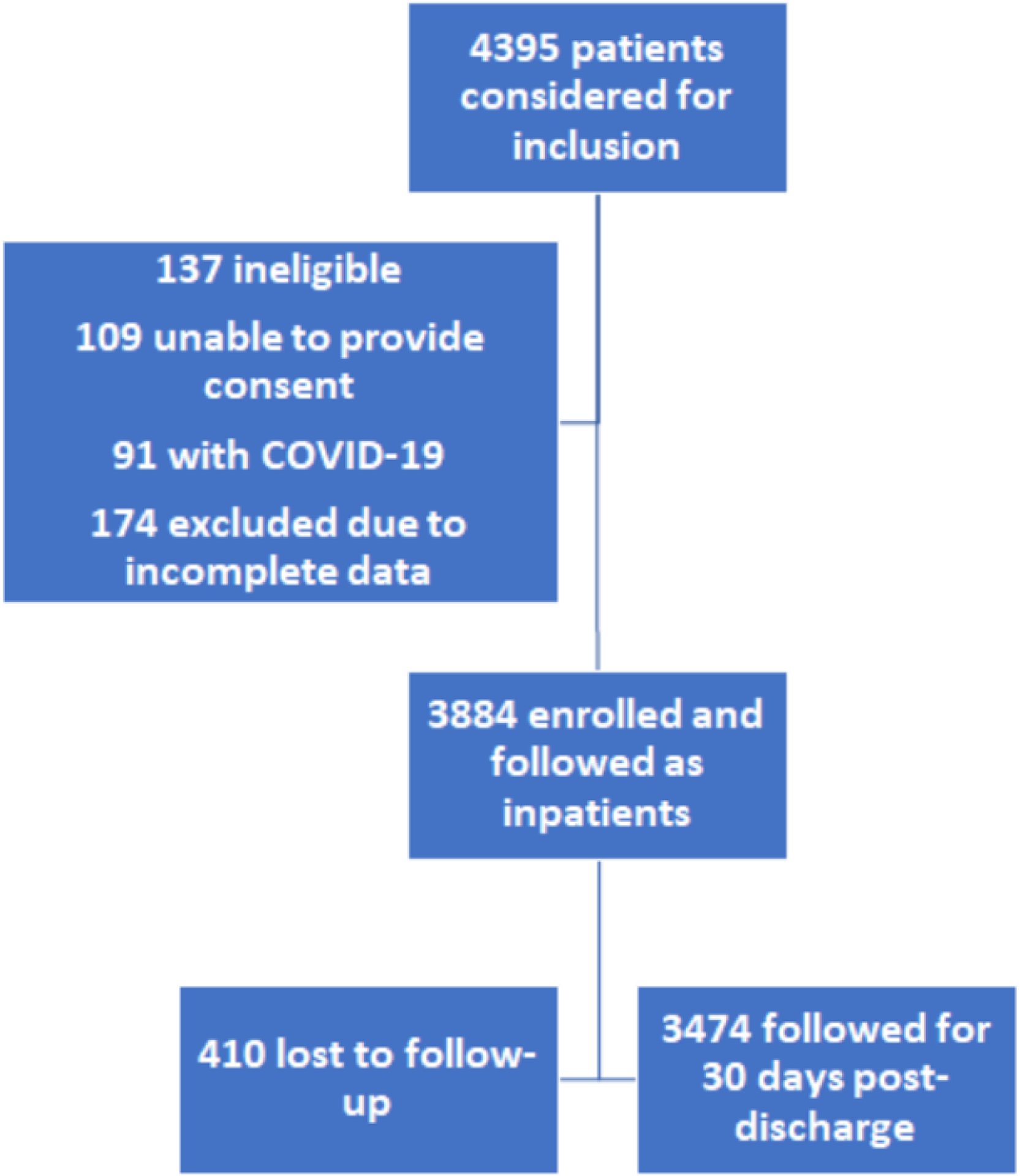
Flow of subjects through the study
The mean age was 55.9±13.3 years, and 64% were men. Within the previous 6 months, 1942 (50%) had experienced hospitalizations,777 (20%) had infections, 1049 (27%) HE, 621 (16%) AKI, 427 (11%) needed paracentesis, 311(8%) had hydrothorax, and 194 (5%) had HCC. 1413 (36.4%) patients were from HIC, 1,757 (45.2%) from UMIC, 629 were from LMIC, and 85 were from LIC (18.4% combined) countries. Since the LIC numbers were relatively low, we combined LMIC and LICs for all comparisons.
During the index admission, the main reasons for admission were liver-related, primarily anasarca (1437, 37%), followed by HE (1126, 29%), GI bleeding (971, 25%), and AKI (932, 24%). 817 (21%) had infection-related admissions [ SBP (350, 43% of infections), then respiratory (233, 28% of infections), and urinary tract infections (194, 24% of infections)]. Common liver-unrelated admissions were respiratory (466, 12%), cardiac (389, 10%), and orthopedic (311, 8%) admissions unrelated to infection. The median admission MELD-Na score was 21 (IQR 15–27). The most common etiology was alcohol (1589, 41%) followed by hepatitis B (805, 21%), and NAFLD (686, 18%). 687 (18%) were on hepatitis B antiviral therapy on admission; no other antiviral use was noted.
The median length of stay was 9 (IQR 5–16) days, during which 738 (19%) needed ICU transfer, 1359 (35%) developed AKI or worsening of AKI, 505 (13%) developed grade 3–4 HE, 427 (11%) required vasopressors, 389 (10%) needed mechanical ventilation and 505 (13%) developed nosocomial infections. The inpatient mortality was 466 (12%), 39 (1%) were transferred to hospice, and 117 (3%) received an in-hospital LT. At day 30 post-discharge, 410 patients were lost to follow-up, a total of 625 (18%) of the remaining 3474 patients died, 973 (28%) were readmitted, and 208 (6%) received LT. The lost to follow-up patients were not responsive to phone calls, and did not have hospice transfer, or follow-up on chart review.
When we comparedthose who survived or received LT versus died or went to hospice, we found that those who had died or needed hospice had worse liver disease severity and were taking medications associated with advanced cirrhosis (rifaximin, lactulose, SBP prophylaxis, Table 1); however, variceal bleeding, prior hospitalizations, HCC, use of beta-blockers, diuretics, proton pump inhibitors (PPI), and statin use were not different, and use of HBV anti-viral drugs was protective (Table 2). Infections and liver-related admissions, in-hospital outcomes of AKI, ICU transfer, nosocomial infections, vasopressor use, and length of stay (LOS) were higher in those who had died/went to hospice versus not. There was a lower death rate in HICs compared to LMICs or UMICs during hospitalization and at 30-day follow-up. When we compared HIC, UMIC and LMIC patients, we found that 14ore patients in the HIC group had prior hospitalizations and infections, HE, hyponatremia, and ascites within 6 months, while prior variceal bleeding was higher in LMIC (Table 2, Figure 2A). Prior HCC and transplant listings were similar in UMIC and HIC patients but lower in LMIC. Alcohol and NAFLD etiologies were highest in HIC, while viral and cryptogenic etiologies were highest in UMIC. HIC patients were older and had lower MELD-Na on admission versus LMICs. Although most admissions across countries were liver-related, the relative proportion of liver-unrelated reasons for admission was higher in HICs (Table S3). When reasons for admission were evaluated, specific liver-related causes (GI bleeding, HE, hepatitis B flare, and drug-induced liver injury) and SBP were highest, while other infections (C.difficile, intra-abdominal) were lowest in LMICs. Respiratory infections were highest in UMICs. All other infections and unrelated liver causes were similar across countries.
Table 1:
Differences in Patients who survived/transplanted versus those who died or were sent to hospice**
| In-hospital outcomes (n=3884) | 30-day post-discharge outcomes (n=3474)* | |||||||
|---|---|---|---|---|---|---|---|---|
| Survived/LT (n = 3,377) | Died/hospice (n = 507) | P value | Survived/LT (n = 2824) | Died (n = 650) | P value | |||
| Age (years) (Mean (Std)) | 55.91 (13.27) | 55.67 (13.22) | 0.71 | Age (years) (Mean (Std)) | 55.41 (13.18) | 56.30 (13.71) | 0.12 | |
| Sex | 0.97 | Sex | 0.56 | |||||
| Female | 1209 (35.8%) | 182 (36%) | Female | 994 (35%) | 221 (34%) | |||
| World Bank Classification | <0.0001 | World Bank Classification | <0.0001 | |||||
| High Income | 1277 (37.8%) | 136 (27%) | High Income | 1065 (37.7%) | 179 (28%) | |||
| Cirrhosis etiology | Cirrhosis etiology | |||||||
| Hepatitis C | 336 (10%) | 42 (8%) | 0.24 | Hepatitis C | 271 (10%) | 53 (8%) | 0.25 | |
| Alcohol | 1359 (40.2%) | 230 (45%) | 0.03 | Alcohol | 1158 (41.0%) | 286 (44%) | 0.16 | |
| Non-alcoholic fatty liver disease | 592 (18%) | 94 (19%) | 0.58 | Non-alcoholic fatty liver disease | 483 (17%) | 112 (17%) | 0.94 | |
| Hepatitis B | 745 (22%) | 60 (12%) | <0.0001 | Hepatitis B | 628 (22%) | 104 (16%) | 0.0004 | |
| Cryptogenic | 254 (8%) | 44 (9%) | 0.36 | Cryptogenic | 214 (8%) | 56 (9%) | 0.37 | |
| Others | 551 (16%) | 101 (20%) | 0.04 | Others | 462 (16%) | 127 (20%) | 0.051 | |
| Prior AKI | 507 (15%) | 135 (27%) | <0.0001 | Prior AKI | 403 (14%) | 170 (26%) | <0.0001 | |
| Prior Hydrothorax | 282 (8%) | 37 (7%) | 0.43 | Prior Hydrothorax | 249 (9%) | 54 (8%) | 0.69 | |
| Cirrhosis history | Cirrhosis history | |||||||
| Prior LVP in 6M | 352 (10%) | 74 (15%) | 0.005 | Prior LVP in 6M | 286 (10%) | 92 (14%) | 0.003 | |
| Hospitalized in 6M | 1692 (50.1%) | 253 (50%) | 0.96 | Hospitalized in 6M | 1430 (50.6%) | 339 (52%) | 0.47 | |
| Prior HE in 6M | 865 (25%) | 191 (38%) | <0.0001 | Prior HE in 6M | 722 (26%) | 231 (36%) | <0.0001 | |
| Variceal bleed in 6M | 966 (29%) | 143 (28%) | 0.86 | Variceal bleed in 6M | 797 (28%) | 182 (28%) | 0.92 | |
| Transplant listed? | 330 (10%) | 62 (12%) | 0.08 | Transplant listed? | 285 (10%) | 59 (9%) | 0.44 | |
| Infected in the Past 6M | 624 (18%) | 144 (28%) | <0.0001 | Infected in the Past 6M | 525 (19%) | 181 (28%) | <0.0001 | |
| Prior HCC in 6M | 166 (5%) | 31 (6%) | 0.25 | Prior HCC in 6M | 132 (5%) | 46 (7%) | 0.01 | |
| Admission details | Index Admission details | |||||||
| Medications on admission | Medications on admission | |||||||
| Betablockers | 1056 (31%) | 146 (29%) | 0.26 | Betablockers | 839 (30%) | 196 (30%) | 0.82 | |
| Lactulose | 1325 (39%) | 302 (60%) | <0.0001 | Lactulose | 1083 (38.3%) | 365 (56%) | <0.0001 | |
| Rifaximin | 784 (23%) | 175 (35%) | <0.0001 | Rifaximin | 654 (23%) | 215 (33%) | <0.0001 | |
| Diuretics | 1778 (52.7%) | 254 (50%) | 0.2805 | Diuretics | 1447 (51.2%) | 341 (52%) | 0.5740 | |
| PPI | 1440 (42.6%) | 222 (44%) | 0.5800 | PPI | 1186 (41.9%) | 284 (44%) | 0.3990 | |
| Statins | 337 (10%) | 44 (9%) | 0.3633 | Statins | 268 (10%) | 61 (9%) | 0.9389 | |
| SBP Prophylaxis | 427 (13%) | 82 (16%) | 0.0270 | SBP Prophylaxis | 345 (12%) | 107 (16%) | 0.0036 | |
| HBV Antivirals | 638 (19%) | 49 (10%) | <0.0001 | Antivirals | 537 (19%) | 84 (13%) | 0.0003 | |
| Infection Admission | 624 (18%) | 201 (40%) | <0.0001 | Infection Admission | 513 (18%) | 242 (37%) | <0.0001 | |
| Liver Related Admission | 3050 (90%) | 477 (94%) | 0.006 | Liver Related Admission | 2546 (90.3%) | 610 (94%) | 0.003 | |
| MELD-Na (Median, IQR) | 20 (14, 26) | 29 (24, 33) | <0.0001 | MELD-Na (Median, IQR) | 20 (14, 25) | 28 (23, 33) | <0.0001 | |
| Outcomes | Outcomes on index admission | |||||||
| In-hospital AKI | 947 (29%) | 394 (79%) | <0.0001 | Hospital AKI | 762 (28%) | 467 (73%) | <0.0001 | |
| Nosocomial infection | 293 (10%) | 161 (34%) | <0.0001 | Nosocomial infection | 243 (9%) | 174 (29%) | <0.0001 | |
| ICU Transfer | 444 (13%) | 278 (55%) | <0.0001 | ICU Transfer | 359 (13%) | 293 (45%) | <0.0001 | |
| Grade 3–4 HE | 252 (7%) | 232 (46%) | <0.0001 | Grade 3–4 HE | 201 (7%) | 252 (39%) | <0.0001 | |
| Ventilation | 162 (5%) | 217 (43%) | <0.0001 | Ventilation | 131 (5%) | 227 (35%) | <0.0001 | |
| Vasopressor use | 177 (5%) | 268 (53%) | <0.0001 | Vasopressor use | 143 (5%) | 281 (43%) | <0.0001 | |
| LOS (Median, IQR) | 9 (5, 15) | 11 (6, 21) | <0.0001 | LOS (Median, IQR) | 9 (5, 15) | 11 (6, 20) | <0.0001 | |
| Discharge MELD-Na (Median, IQR) | 17 (13, 23) | 32 (26, 39) | <0.0001 | Discharge MELD-Na (Median, IQR) | 17 (13, 22) | 30 (25, 38) | <0.0001 | |
| Inpatient LT | 116 (3%) | 0 (0%) | <0.0001 | LT within 30 days | 208 (6%) | 0 (0%) | <0.0001 | |
HCV: hepatitis C virus, NAFLD: non-alcoholic fatty liver disease, HBV: hepatitis B virus, HE: hepatic encephalopathy, SBP: spontaneous bacterial peritonitis, ICU: intensive care unit, PPI: proton pump inhibitors, LOS: length of stay, LVP: large volume paracentesis, MELD-Na: model for end-stage liver disease sodium [presented as median (range)], HCC: hepatocellular cancer.
Includes everyone who was not lost to follow-up.
Unless otherwise specified, all data is reported as percentage (frequency)
Table 2:
Comparison between patients in low/low middle, upper middle and high-income countries
| % (Freq) (Unless specified) | L/LMIC (n=714) | UMIC (n = 1757) | HIC (n = 1413) | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) (Mean (Std)) | 48.51 (13.43) | 56.69 (12.80) | 58.58 (12.38) | <0.0001 | |||||
| Sex | <0.0001 | ||||||||
| Female | 171 (24%) | 638 (36%) | 582 (41%) | ||||||
| Diabetes | 178 (25%) | 472 (27%) | 498 (35%) | <0.0001 | |||||
| Cirrhosis etiology | |||||||||
| Hepatitis C | 39 (5%) | 156 (9%) | 186 (13%) | <0.0001 | |||||
| Alcohol | 315 (44%) | 463 (26%) | 811 (53%) | <0.0001 | |||||
| Non-alcoholic fatty liver disease | 126 (18%) | 169 (10%) | 391 (24%) | <0.0001 | |||||
| Hepatitis B | 129 (18%) | 608 (35%) | 68 (5%) | <0.0001 | |||||
| Cryptogenic | 65 (9%) | 178 (10%) | 55 (4%) | <0.0001 | |||||
| Others | 115 (16%) | 331 (19%) | 206 (15%) | 0.01 | |||||
| Cirrhosis history | |||||||||
| Prior LVP in 6M | 104 (15%) | 131 (7%) | 191 (14%) | <0.0001 | |||||
| Hospitalized in 6M | 283 (40%) | 845 (48%) | 817 (58%) | <0.0001 | |||||
| Prior HE in 6M | 187 (26%) | 377 (21%) | 492 (35%) | <0.0001 | |||||
| Variceal bleed in 6M | 249 (35%) | 523 (30%) | 337 (24%) | <0.0001 | |||||
| Transplant listed? | 52 (7%) | 178 (10%) | 162 (11%) | 0.01 | |||||
| Infected in the Past 6M | 122 (17%) | 298 (17%) | 348 (25%) | <0.0001 | |||||
| Prior HCC in 6M | 24 (3%) | 101 (6%) | 72 (5%) | 0.049 | |||||
| Prior hyponatremia | 118 (17%) | 219 (12%) | 331 (24%) | <0.0001 | |||||
| Prior ascites | 470 (66%) | 1038 (59.1%) | 980 (69%) | <0.0001 | |||||
| Admission Medications | |||||||||
| Betablockers | 269 (38%) | 466 (27%) | 467 (33%) | <0.0001 | |||||
| Lactulose | 448 (63%) | 517 (29%) | 662 (47%) | <0.0001 | |||||
| Rifaximin | 291 (41%) | 267 (15%) | 401 (28%) | <0.0001 | |||||
| Diuretics | 320 (45%) | 894 (51%) | 818 (58%) | <0.0001 | |||||
| Proton Pump Inhibitors | 367 (51%) | 513 (29%) | 782 (55%) | <0.0001 | |||||
| Statins | 26 (4%) | 82 (5%) | 273 (19%) | <0.0001 | |||||
| SBP Prophylaxis | 209 (29%) | 128 (7%) | 172 (12%) | <0.0001 | |||||
| HBV antivirals | 127 (18%) | 497 (28%) | 63 (4%) | <0.0001 | |||||
| Admission details | |||||||||
| Infection Admission | 188 (26%) | 362 (21%) | 275 (19%) | <0.0001 | |||||
| Liver Related Admission | 672 (94%) | 1653 (94.1%) | 1202 (85.1%) | <0.0001 | |||||
| MELD-Na (Median, IQR)) | 27 (20, 32) | 19 (13, 25) | 21 (16, 27) | <0.0001 | |||||
| Inpatient Outcomes | |||||||||
| Acute kidney injury development | 275 (42%) | 489 (28%) | 577 (42%) | <0.0001 | |||||
| Intensive care unit transfer | 252 (36%) | 180 (10%) | 290 (21%) | <0.0001 | |||||
| Grade 3–4 Hepatic encephalopathy | 130 (18%) | 183 (10%) | 171 (12%) | <0.0001 | |||||
| Ventilation | 109 (15%) | 120 (7%) | 150 (11%) | <0.0001 | |||||
| Vasopressor use | 129 (18%) | 183 (10%) | 133 (9%) | <0.0001 | |||||
| Nosocomial Infection | 108 (16%) | 181 (11%) | 165 (13%) | 0.007 | |||||
| LOS (Median, IQR)) | 8 (5, 12) | 10 (6, 17) | 8 (5, 16) | <0.0001 | |||||
| Inpatient mortality | 158 (22%) | 182 (10%) | 110 (8%) | <0.0001 | |||||
| Hospice transfer | 3 (0%) | 28 (2%) | 26 (2%) | <0.0001 | |||||
| Inpatient LT | 14 (2%) | 28 (2%) | 59 (4%) | <0.0001 | |||||
| Discharge MELD-Na (Median, IQR)) | 24 (13) | 17 (11) | 19 (11) | <0.0001 | |||||
| 30-day outcomes | |||||||||
| Lost to Follow-Up at 30 Days | 40 (6%) | 201 (11%) | 169 (12%) | <0.0001 | |||||
| Readmissions | 94 (18%) | 337 (25%) | 409 (36%) | <0.0001 | |||||
| Liver transplant | 16 (3%) | 57 (4%) | 110 (10%) | <0.0001 | |||||
| Mortality | 204 (30%) | 267 (17%) | 179 (14%) | <0.0001 |
P-values indicate differences between the three groups, HCV: hepatitis C virus, NAFLD: non-alcoholic fatty liver disease, HBV: hepatitis B virus, SBP: spontaneous bacterial peritonitis, LOS: length of stay, Std: standard deviation, MELD-Na: model for end-stage liver disease sodium, L/LMIC: low and lower middle income countries, UMIC: upper middle income countries, HIC: high income countries according to the World Bank classification.
Figure 2:
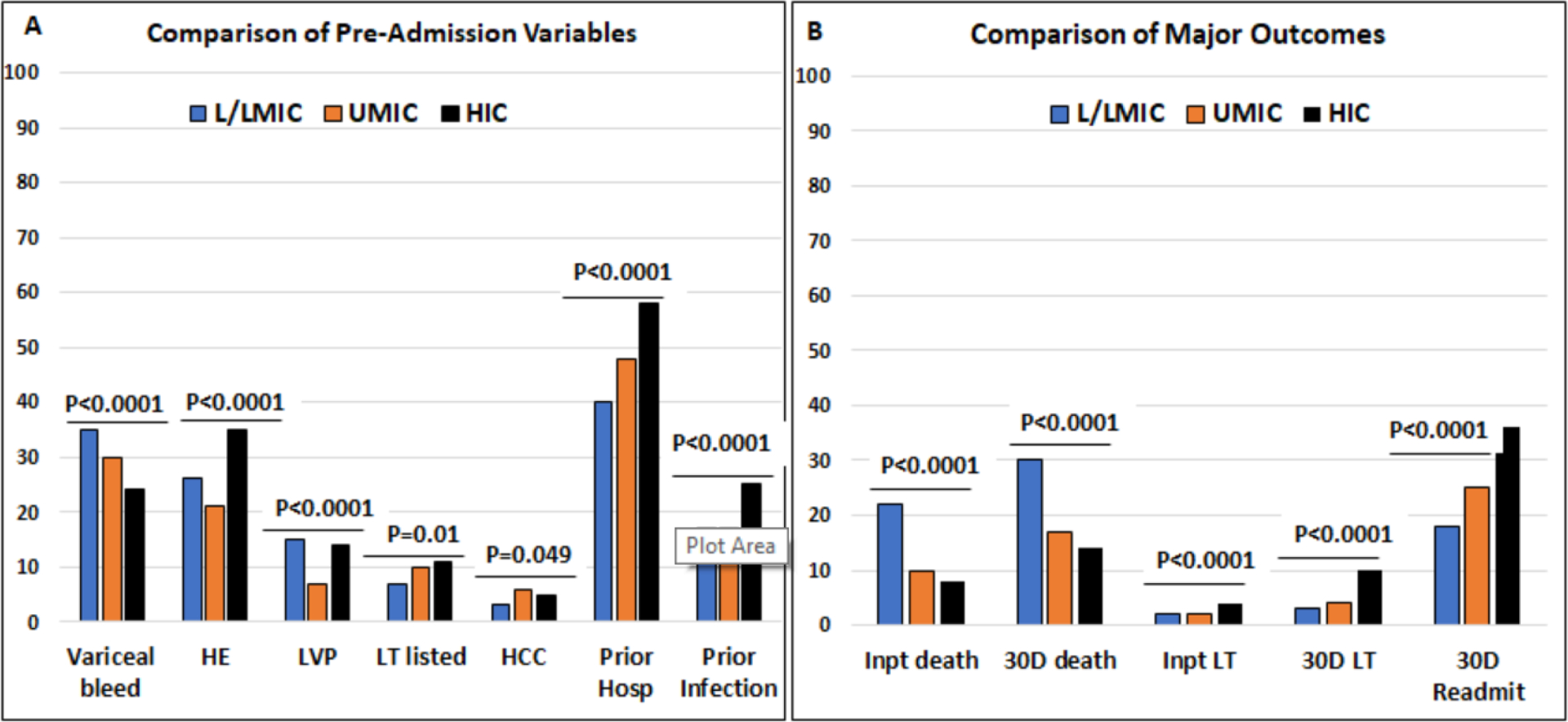
Comparison of proportion of subjects in each country type according to the World Bank Classification. L/LMIC: low and low-middle income countries, UMIC: Upper-middle income countries, HIC: high-income countries Y axis is percentage of patients in each country grouping. Comparisons performed using Chi-square tests. ***=p<0.001, **=p<0.01, *=p<0.05.
Figure 2A: important pre-admission variables within 6 months of this admission compared between groups. VB: variceal bleeding, HRS: hepatorenal syndrome, Hyponat: hyponatremia, HE: hepatic encephalopathy, HCC: hepatocellular cancer, LT listed? listed for liver transplant, LVP: large volume paracentesis, Hosp: hospitalization.
Figure 2B: Comparison of major outcomes within the hospitalization and 30-days post-discharge. Inpt: inpatient, 30D: 30-day post-discharge, LT: liver transplant, Readmit: readmission
Proportion discharged to hospice, and in-hospital mortality were similar between UMIC and HIC, but LT was highest in HICs (Figure 2B). Hospice transfer was lowest in LMIC, while nosocomial infection and mortality were highest in those patients. Similar patterns were seen at 30-days, where LT was highest, and mortality was lowest in HIC. However, as shown in figure 2B, the 30-day readmission rate was the highest in HICs despite a lower lost-to-follow-up rate in LMICs.
Site investigator survey analysis showed that most insurances were national except for centers in India, the United States, and Mexico, where private insurance rates were relatively higher (Table 3). LT was available or easily accessible in most centers except for some African sites; Australian sites had access to LT in other hospitals in their system covered by public insurance. However, some African (n=6), Chinese (n=8), Middle Eastern (n=4), and South American (n=4) sites did not have complete insurance coverage for LT and could not offer it to all patients. Similarly, apart from North America, Australia, sites in Asia other than China/India, and European sites, most patients could not afford ICU. Most sites, apart from those in Africa and one site in Mexico and China respectively, had 24-hour endoscopy services. Indian, Chinese, and African sites had the lowest palliative care and hospice facilities availability. In most sites in India, China, and Africa, only a minority of patients could afford palliative care .
Table 3:
Survey of principal investigators across 95 sites regarding availability and affordability of cirrhosis-related interventions and management strategies
| Site location | China | India | North America* | Europe | Australia | Africa | South America | Middle East | Rest of Asia |
|---|---|---|---|---|---|---|---|---|---|
| No. of sites (n=95) | 24 | 11 | 23 | 6 | 7 | 5 | 7 | 9 | 3 |
| National Insurance | 24 | 2 | 12 | 6 | 7 | 4 | 5 | 9 | 2 |
| Private Insurance | 0 | 9 | 11 | 0 | 0 | 1 | 2 | 0 | 1 |
| LT available in center? | 16 | 10 | 19 | 3 | 1 | 1 | 7 | 6 | 3 |
| >50% of pts can afford LT | 2 | 0 | 15 | 3 | 6 | 0 | 3 | 4 | 2 |
| Number of ICU beds (Median, IQR) ** | 45 (18, 92) | 60 (40, 80) | 30 (22, 54) | 35 (19, 80) | 40 (24, 58) | 8 (5, 10) | 32 (28, 106) | 50.0 (14, 66) | 20 (15, 40) |
| >50% pts can afford ICU | 11 | 2 | 16 | 3 | 6 | 1 | 3 | 4 | 3 |
| Endoscopy after hours available? | 23 | 11 | 22 | 6 | 7 | 1 | 7 | 9 | 3 |
| >50% forgo tests | 1 | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 |
| Rifaximin available? | 24 | 11 | 23 | 6 | 7 | 3 | 7 | 9 | 2 |
| >50% can afford rifaximin | 13 | 6 | 13 | 3 | 6 | 1 | 2 | 6 | 1 |
| Terlipressin available? | 23 | 11 | 8 | 6 | 7 | 2 | 7 | 9 | 3 |
| >50% can afford terlipressin | 22 | 7 | 4 | 3 | 6 | 0 | 3 | 4 | 2 |
| >50% can afford albumin | 21 | 5 | 16 | 3 | 5 | 1 | 3 | 4 | 3 |
| Somatostatin or Octreotide available? | 23 | 11 | 23 | 6 | 7 | 2 | 7 | 9 | 3 |
| >50% can afford somatostatin or octreotide | 22 | 6 | 18 | 3 | 6 | 1 | 3 | 4 | 3 |
| Palliative care available | 12 | 4 | 22 | 6 | 7 | 2 | 7 | 6 | 2 |
| >50% can afford palliative care | 7 | 1 | 17 | 3 | 6 | 0 | 3 | 1 | 2 |
includes USA (n=12 sites), Canada (n=3 sites), and Mexico (n=8 sites), LT: liver transplantation, ICU: intensive care unit, Somatostatin and octreotide are medications needed for treating variceal hemorrhage,
p=0.01 for ICU beds. All Canadian sites (n=3) and all but one Mexican site (n=7) had national insurance coverage while only one US-based site of 12 had national insurance.
Regarding medications, most countries, apart from a few in rest of Asia and Africa, had rifaximin available. However, rifaximin was considered unaffordable for more patients in Africa, South America, and rest of Asia compared with other centers. Somatostatin and octreotide were available at all sites except in Africa and one Chinese site. According to site investigators, these agents were largely affordable for most patients in China, Australia, North America, and sites in Asia other than China and India, but not elsewhere. A minority of patients in Indian, African, South American, and Middle Eastern sites could afford IV albumin, in the site investigators’ opinion. Terlipressin was not available in USA and Canada. In the remaining sites, patients from Australian, Chinese and rest of Asian (apart from India) sites were judged more likely to be able to afford terlipressin.
Multi-variable analyses:
Inpatient mortality was associated with higher age and admission MELD-Na, prior HCC or infections, and lactulose use, as well as not being from a HIC, were associated with higher odds of inpatient mortality [adjusted OR (AOR) and 95% CI 2.54 (1.82, 3.54) LMIC, 2.14 (1.61, 2.84) for UMIC versus HIC, Figure 2A, table S4]. Patients who were already on diuretics, SBP prophylaxis, and HBV antivirals on admission, together with transplant listing and prior hospitalizations, were associated with lower odds of mortality (Figure 3A, table S4).
Figure 3:
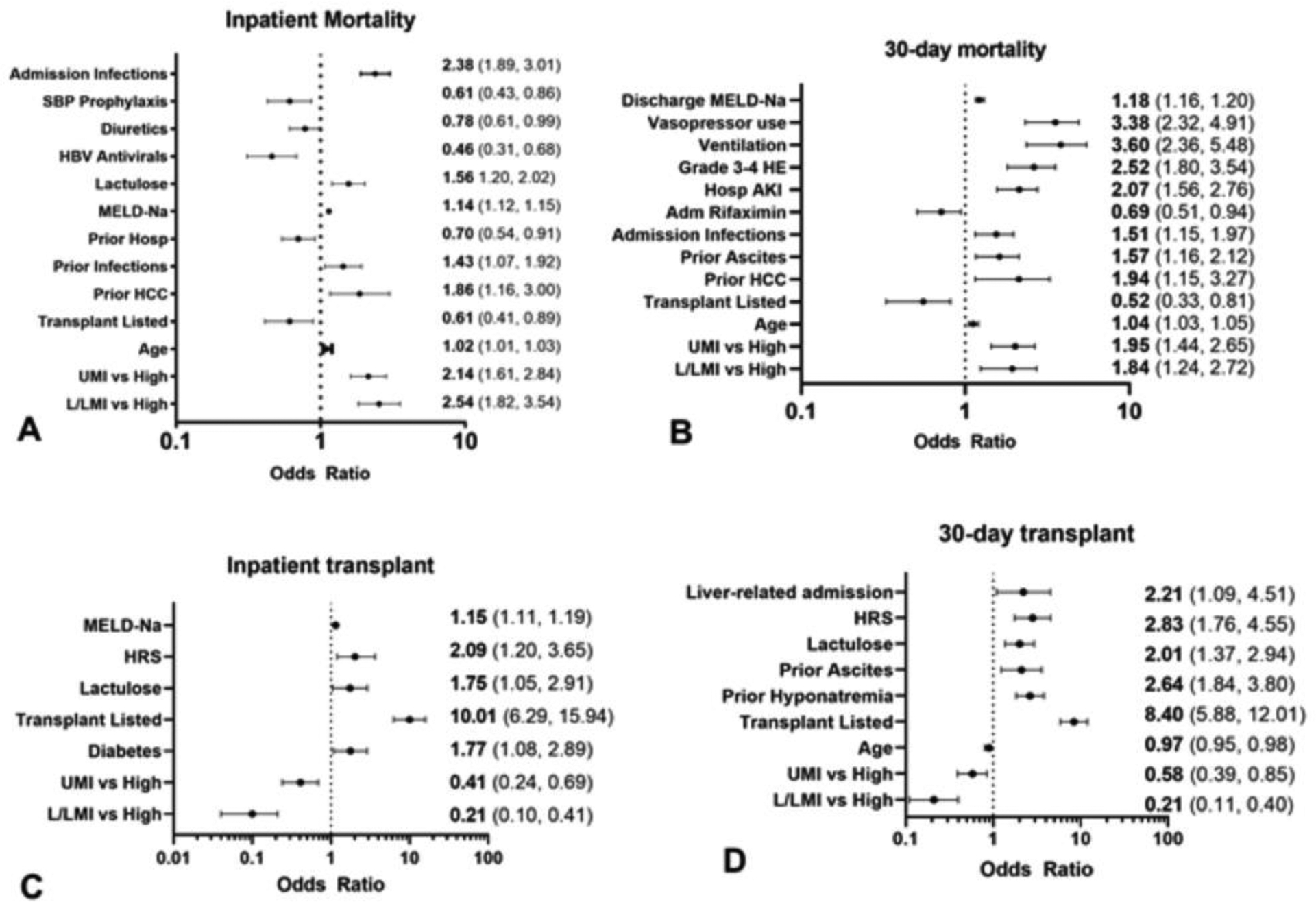
Forest plots for logistic regression for inpatient and 30-day post-discharge mortality and liver transplant L/LMIC: low and low-middle income countries, UMIC: Upper-middle income countries, HIC: high-income countries, HCC: hepatocellular cancer, AKI: acute kidney injury, Hosp: during hospitalization, HE: hepatic encephalopathy, HRS: hepatorenal syndrome, MELD-Na: model for end-stage liver disease sodium
Figure 3A: Inpatient mortality odds ratios and 95% CI
Figure 3B: 30-day mortality odds ratios and 95% CI
Figure 3C: Inpatient liver transplant odds ratios and 95% CI
Figure 3D: 30-day liver transplant odds ratios and 95% CI
30-day post-discharge mortality was associated with higher age, prior ascites, prior HCC, admission for infection and negative outcomes during hospitalization (ventilation, HE 3–4, vasopressor use), MELD-Na at discharge and not being in a HIC (AOR and 95%CI 1.84 (1.24, 2.72) LMIC, 1.95 (1.44, 2.65) UMIC versus HIC) were associated with higher odds of 30-day post-discharge mortality. In contrast, transplant listing and being on rifaximin were associated with lower odds of this outcome (Figure 3B, Table S4).
Inpatient LT was affected by not being in a HIC was associated with lower odds of inpatient LT(AOR and 95% CI 0.21 (0.10, 0.41) LMIC, 0.41 (0.24, 0.69) UMIC vs. HIC), while being listed for LT, admission MELD-Na, hepatorenal syndrome, lactulose use, and diabetes were associated with higher odds of inpatient LT (Figure 3C, Table S5).
LT within 30-days post-discharge was associated with lower age and not being in a HIC reduced the odds for receiving LT (AOR and 95% CI 0.21 (0.11, 0.40) LMIC, 0.58 (0.39, 0.85) UMIC vs. HIC), while the opposite was observed for those with hepatorenal syndrome, ascites, lactulose use, hyponatremia, and liver-related index admission (Figure 3D, Table S5).
DISCUSSION:
Cirrhosis represents an important intersection between medical factors and social determinants of health that culminate in liver injury and organ dysfunction. Major causes of liver disease such as obesity, excess alcohol, and viral hepatitis have an increasingly important global footprint2,8. Management of patients with cirrhosis includes optimal outpatient care to reduce preventable admissions and meticulous inpatient care that spans several specialties. However, variations in the etiology of cirrhosis, patient ability to access or afford important diagnostic and treatment modalities, and healthcare infrastructure may influence cirrhosis outcomes. Thus, the need for a global study to address these determinants. While variations in mortality globally may be intuitive, data quantifying these variations to understand the relative importance of contributing factors is key to making progress toward better patient outcomes globally.
In this worldwide consortium of patients hospitalized with complications of cirrhosis, there were significant differences in outcomes based on location. Not being in a high-income country significantly increased the risk of inpatient and 30-day mortality independent of demographics, in-hospital course, and cirrhosis severity, likely due to disparities in access to ICU care, diagnostics, medical therapies, and liver transplant. This is important because there is a high burden of cirrhosis in Africa and Asia, where patients and centers have varying capabilities and access to interventions and medications required for optimizing outcomes13,14.
Previous studies of inpatients with cirrhosis focused on specific regions and used pre-specified definitions of covariates5,6,15, or were based on global databases rather than prospective data collection. A global perspective using prospectively collected data, which accounts for the availability and affordability of resources required to prevent mortality is lacking. Our data demonstrate that where the patient is located, a broad corollary of the availability of outpatient and inpatient resources, is one of the critical determinants of mortality regardless of other, established cirrhosis outcome predictors16. In our analysis, access to LT is a significant factor associated with 30-day mortality, and globally, many patients do not have access to or cannot afford this lifesaving procedure17. In addition to liver transplant, which requires major governmental commitment, societal acceptance, and medical infrastructure, there were significant disparities in access to diagnostic methods, ICU care, endoscopy, and medications18. The variation in ICU bed availability and affordability is an essential but unrecognized factor in cirrhosis outcomes19. This is compounded by variations in access to services such as 24-hour endoscopy, the availability of medications such as rifaximin, terlipressin, albumin, octreotide, and somatostatin20–22. Even within HICs, there are access issues related to availability, affordability, and/or insurance coverage for medications such as rifaximin and terlipressin,22 as well as barriers to transplant and other lifesaving procedures17. These are novel aspects with respect to cirrhosis, which has continued to be a major burden in all parts of the world.
Several aspects of these data show the importance of robust outpatient healthcare, which could influence the need for hospitalizations across regions. HBV antiviral therapies, diuretics, SBP prophylaxis, and rifaximin are all components of good outpatient care, which were associated with a lower inpatient or 30-day mortality on multi-variable analysis23. We found that patients in LMICs were more likely to be admitted with GI bleeding, hepatitis B flare, and SBP, all of which may have been mitigated by outpatient prophylactic strategies24. Reflecting this potential lack of outpatient care, compounded by a lack of personal financial resources in LMICs and therefore seeking of treatment at later stages of the disease, the MELD score on admission was also higher in LMICs. In contrast, we observed higher 30-day readmissions in HICs. This could be due to several factors, including better linkage to post-hospitalization outpatient care25 and closer outpatient monitoring, and different criteria for admission.25,26 Lastly, we found a disparity in the use of hospice and palliative care services,27 which were more often used in HI countries and lowest in African, Chinese, and Indian sites. Cultural practices towards sick and elderly family members could affect hospice referral in these countries28,29 The availability of hospice infrastructure could also be a negative factor in hospice transfer. Ultimately, our data highlight the disparities across almost all aspects of cirrhosis care, including inpatient and pre- and post-hospitalization outpatient services and access. These results should inform the need to develop appropriate models that predict chronic liver disease outcomes, including access to services, medications, and coverage and a more granular approach to social determinants of health. Our findings are novel because they span large parts of the globe that have been largely under-represented in previous research using prospective data collection. We are arguably the first to determine the impact of center location on the prediction of outcomes in cirrhosis2,13. The study is limited since we only included inpatients and used institution-level rather than individual data regarding access to diagnostic and treatment modalities, precluding incorporation of this data in multivariable models. We relied mainly on academic medical centers, introducing a source of bias. Many countries in this work do not have population-level hospitalization databases available, and we recruited study sites with specialists interested in caring for patients with cirrhosis. We also did not elicit patient-level financial resource information, urban/rural residence, or substance abuse details which could have enhanced our interpretation further. Thus, our findings likely underestimate the actual disparities in global cirrhosis outcomes, particularly regarding regions where specialist care is often unavailable. However, in future research we do intend to assess patient-level resources and access to important elements of care. While access to inpatient and outpatient treatment resources, including LT, could explain most of the disparities between HIC versus LMIC and UMIC countries, other relevant factors could be related to socio-cultural practices, patient-level variability in income, social support and social support and insight into a disease that were not explicitly assessed. The study design and analysis could have also allowed unmeasured confounders. We enrolled subjects during the pandemic but excluded those with COVID-19 and ensured contemporaneous data collection.
The current global experience focuses on a neglected aspect of outcomes of inpatients with cirrhosis, namely the location of care and the ability of the patient to access or afford modalities needed for optimum management. Not being in a high-income country significantly increased the risk of inpatient and 30-day mortality independent of demographics, medications, in-hospital course, and cirrhosis severity. This is most likely due to disparities in access to ICU care, diagnostics, medical therapies, and liver transplant, which should be accounted for in global models. Changes in public policies to allow improved access to even a few of these important resources may improve outcomes for patients with cirrhosis. As non-communicable diseases increasingly account for morbidity and mortality worldwide, our finding of income-related disparities at a country level in short-term cirrhosis mortality highlights the need to improve access to diagnostic, preventative, and treatment resources for liver disease in resource-limited contexts.
Supplementary Material
Research in context.
Evidence before this study
Mortality is high in hospitalized patients with cirrhosis and is associated with the severity of liver disease, infections, and organ dysfunction. However, based on PubMed literature review in the English language from 2010 through 2022 focused on terms “cirrhosis”, “disparities”, “global” “outcomes”, “mortality”, “prospective”, “transplant”, we found that studies of hospitalized patients with cirrhosis have not evaluated the impact of resource limitations on cirrhosis management and mortality on a global scale using prospectively collected data.
Added value of this study
The CLEARED consortium prospectively enrolled and followed 3884 inpatients with cirrhosis from 90 centers across six continents. We found that receiving care in a lower-income country doubled inpatient and 30-day mortality rates independent of known medical risk factors. Access to and affordability of services such as intensive care, liver transplant, and important medications showed a wide variation across centers and were associated with mortality.
Implications of all the available evidence
Global cirrhosis mortality is high and due in large part to limited access to diagnostic and therapeutic modalities. Therefore, researchers and policymakers should consider access to services and medications when evaluating cirrhosis-related outcomes.
Acknowledgement:
partly supported by Veterans Affairs Merit Review 2I0CX001076 and McGuire Research Institute to JSB; Dr. Thacker’s efforts were supported in part by award No. UL1TR002649 from the National Institutes of Health’s National Center for Advancing Translational Science. Dr. Alvares-da-Silva’s work was supported in part by the National Council for Scientific and Technological Development (CNPq), Brazil.
Footnotes
Declaration of interests: none for any author
Data sharing:
The individual data collected will not be made available due to restrictions from ethics boards.
References:
- 1.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol 2019; 70(1): 151–71. [DOI] [PubMed] [Google Scholar]
- 2.Vento S, Cainelli F. Chronic liver diseases must be reduced worldwide: it is time to act. Lancet Glob Health 2022; 10(4): e471–e2. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj JS, Kamath PS, Reddy KR. The Evolving Challenge of Infections in Cirrhosis. N Engl J Med 2021; 384(24): 2317–30. [DOI] [PubMed] [Google Scholar]
- 4.Ma C, Qian AS, Nguyen NH, et al. Trends in the Economic Burden of Chronic Liver Diseases and Cirrhosis in the United States: 1996–2016. Am J Gastroenterol 2021; 116(10): 2060–7. [DOI] [PubMed] [Google Scholar]
- 5.O’Leary JG, Reddy KR, Garcia-Tsao G, et al. NACSELD Acute-on-Chronic Liver Failure (NACSELD-ACLF) Score Predicts 30-Day Survival in Hospitalized Patients with Cirrhosis. Hepatology 2018. [DOI] [PubMed] [Google Scholar]
- 6.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013; 144(7): 1426–37, 37 e1–9. [DOI] [PubMed] [Google Scholar]
- 7.Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int 2019; 13(4): 353–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kardashian A, Serper M, Terrault N, Nephew LD. Health disparities in chronic liver disease. Hepatology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html.
- 10.Bajaj JS, O’Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology 2012; 56(6): 2328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jalan R, Gines P, Olson JC, et al. Acute on Chronic Liver Failure. J Hepatol 2012. [DOI] [PubMed] [Google Scholar]
- 12.Hosmer DW Jr., Lemeshow S, Sturdivant RX. Applied Logistic Regression. Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 13.Vento S, Dzudzor B, Cainelli F, Tachi K. Liver cirrhosis in sub-Saharan Africa: neglected, yet important. Lancet Glob Health 2018; 6(10): e1060–e1. [DOI] [PubMed] [Google Scholar]
- 14.Golabi P, Paik JM, AlQahtani S, Younossi Y, Tuncer G, Younossi ZM. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: Data from Global Burden of Disease 2009–2019. J Hepatol 2021; 75(4): 795–809. [DOI] [PubMed] [Google Scholar]
- 15.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009; 3(1): 269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44(1): 217–31. [DOI] [PubMed] [Google Scholar]
- 17.Nephew LD, Serper M. Racial, Gender, and Socioeconomic Disparities in Liver Transplantation. Liver Transpl 2021; 27(6): 900–12. [DOI] [PubMed] [Google Scholar]
- 18. https://data.worldbank.org/indicator/SH.MED.BEDS.ZS.
- 19.Sen-Crowe B, Sutherland M, McKenney M, Elkbuli A. A Closer Look Into Global Hospital Beds Capacity and Resource Shortages During the COVID-19 Pandemic. J Surg Res 2021; 260: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362(12): 1071–81. [DOI] [PubMed] [Google Scholar]
- 21.Wong F, Pappas SC, Curry MP, et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med 2021; 384(9): 818–28. [DOI] [PubMed] [Google Scholar]
- 22.Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018; 391(10138): 2417–29. [DOI] [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018; 69(2): 406–60. [DOI] [PubMed] [Google Scholar]
- 24.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VIIF. Baveno VII - Renewing consensus in portal hypertension. J Hepatol 2022; 76(4): 959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanwal F, Asch SM, Kramer JR, Cao Y, Asrani S, El-Serag HB. Early Outpatient Follow-up and 30-day Outcomes in Patients Hospitalized with Cirrhosis. Hepatology 2016. [DOI] [PubMed] [Google Scholar]
- 26.Tapper EB. Challenge accepted: Confronting readmissions for our patients with cirrhosis. Hepatology 2016; 64(1): 26–8. [DOI] [PubMed] [Google Scholar]
- 27.Rogal SS, Hansen L, Patel A, et al. AASLD Practice Guidance: Palliative care and symptom-based management in decompensated cirrhosis. Hepatology 2022; 76(3): 819–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen JJ, Dingley C, Yoo JW, et al. Sociocultural Factors Associated with Awareness of Palliative Care and Advanced Care Planning among Asian Populations. Ethn Dis 2020; 30(3): 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson KS. Racial and ethnic disparities in palliative care. J Palliat Med 2013; 16(11): 1329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual data collected will not be made available due to restrictions from ethics boards.


