Abstract
Background and Aims:
Tumor Necrosis Factor inhibitors (TNFi), including infliximab and adalimumab, are a mainstay of pediatric Crohn’s disease (PCD) therapy; however, non-response and loss of response is common. As combination therapy with methotrexate may improve response, we performed a multi-center, randomized, double-blind, placebo-controlled pragmatic trial to compare TNFi with oral methotrexate to TNFi monotherapy.
Methods:
PCD patients initiating infliximab or adalimumab were randomized in 1:1 allocation to methotrexate or placebo and followed for 12–36 months. The primary outcome was a composite indicator of treatment failure. Secondary outcomes included anti-drug antibodies (ADA) and patient reported outcomes (PROs) of pain interference and fatigue. Adverse events (AEs) and Serious AEs (SAEs) were collected.
Results:
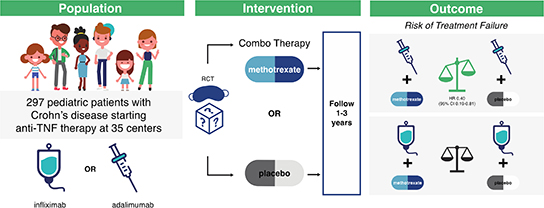
Of 297 participants (mean age 13.9 years, 35% female), 156 were assigned to methotrexate (110 infliximab initiators and 46 adalimumab initiators) and 141 to placebo (102 infliximab initiators and 39 adalimumab initiators). In the overall population, time to treatment failure did not differ by study arm (HR 0.69, 95% CI 0.45–1.05). Among infliximab initiators, there were no differences between combination and monotherapy (HR 0.93, 95% CI 0.55–1.56). Among adalimumab initiators, combination therapy was associated with longer time to treatment failure (HR 0.40, 95% CI 0.19–0.81). A trend towards lower ADA development in the combination therapy arm was not significant. [(infliximab OR 0.72 (0.49–1.07); adalimumab OR 0.71 (0.24–2.07)]. No differences in PROs were observed. Combination therapy resulted in more AEs but fewer SAEs.
Conclusions:
Among adalimumab but not infliximab initiators, PCD patients treated with methotrexate combination therapy experienced a 2-fold reduction in treatment failure with a tolerable safety profile.
Keywords: Crohn’s disease, children, anti-tumor necrosis factor alpha, infliximab, adalimumab
Graphical Abstract
Lay Summary
Tumor Necrosis Factor (TNF) inhibitors, including infliximab and adalimumab, are a mainstay of pediatric Crohn’s disease (PCD) therapy; however, non-response and loss of response is common. Combination therapy with methotrexate may improve response. We conducted a randomized clinical trial to compare TNFI combination therapy with methotrexate to TNFI alone. We found that combination therapy outperformed monotherapy for patients starting adalimumab but not infliximab.
Background
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) that affects approximately 600,000 Americans1 and 1.1 million Europeans2, costs $3.6 billion annually3 and results in substantial morbidity,4 absenteeism5 and diminished quality of life.6 Pediatric CD (PCD) is often more severe,7substantially impacting psychosocial and physical development.8
Anti-Tumor Necrosis Factor (TNF) biologics (infliximab and adalimumab) have revolutionized the treatment of PCD. However, despite robust efficacy, not all patients achieve remission, and many lose response over time.9 Combination therapy with a second immunosuppressive agent can improve response and prevent anti-drug antibodies (ADA) development10 which may contribute to loss of response.11 The risks of combination therapy include further immune suppression and a low, but well-described, risk of malignancy12.
In a landmark trial of adult CD, patients receiving combination therapy with infliximab and azathioprine had higher rates of remission and less frequent ADA than those treated with infliximab monotherapy.13 In PCD, methotrexate, is generally used in combination therapy, due to malignancy concerns with azathioprine. However, evidence to support oral methotrexate is lacking. A randomized trial of subcutaneous methotrexate with infliximab in adult CD14 found no differences in clinical outcomes. However, patients receiving combination therapy were less likely to develop ADA, raising the possibility that the trial was too short to observe differences resulting from ADA development.
Maximizing anti-TNF response is particularly important in PCD, as second-line treatments for adults are not FDA approved in children. Yet, the benefits and risks of anti-TNF combination therapy have not been well-established. We conducted a randomized, double-blind, multicenter, pragmatic clinical trial to compare the effectiveness and safety of anti-TNF in combination with low-dose, oral methotrexate versus monotherapy. We hypothesized that combination therapy would be more effective with tolerable safety.
Methods
Study Setting
We recruited participants at 35 U.S. centers participating in the ImproveCareNow Network15 between October 2018 and December 2021. The Institutional Review Board at Cincinnati Children’s Medical Center approved the study protocol.
Participants
Participants were < 21 years of age, ≥ 20kg , diagnosed with PCD by standard criteria,16 and initiating infliximab or adalimumab (or biosimilars). Exclusion criteria were: 1) prior anti-TNF treatment for PCD, 2) anti-TNF use for post-operative prophylaxis without active disease, 3) abdominal/pelvic abscess, 4) other methotrexate contraindications, 5) lack of stable address, 6) anticipated short follow-up, and 7) inability to provide assent and/or consent.
Intervention and Comparator
Our primary intervention was oral methotrexate or an identically matched placebo manufactured and tested by Temple CGMP Services (Philadelphia, PA) in addition to the anti-TNF agent. Selection and dosing of the anti-TNF agent was at the discretion of the treating physician in accordance with pragmatic trial design17. Therapeutic drug monitoring (TDM) and dose/interval adjustment were allowed.
For those in the active arm, oral methotrexate was administered with a weekly dose of 15 mg for children ≥ 40kg, 12·5 mg for children 30 to <40 kg, and 10 mg for children 20 to <30 kg. All participants received pre-treatment with ondansetron 4mg (or placebo) to prevent nausea and folic acid (1mg per day).
Study medications were dispensed by mail and refilled quarterly by a central investigational pharmacy (McKesson, Irving, TX).
Randomization and Masking
Randomization occurred within 42 days of anti-TNF initiation. We randomized participants with a computer-generated 1:1 allocation ratio, stratified by site and anti-TNF agent using constrained block sequences with a maximum imbalance of 3.18 Upon randomization, the treatment assignment was electronically sent to the study pharmacy directly. By necessity, the study central study pharmacy was unblinded. Participants, caregivers, study teams, the overall study principle investigator, and the lead statistician were blinded until completion of analysis.
Prior and Concomitant Medications
Immunomodulators were discontinued prior to randomization, if applicable. Patients treated with corticosteroids were initiated on a taper at the discretion of the treating physician. Other immunosuppressants or biologics were not permitted.
Study Outcomes
Primary Outcome
The primary outcome, an indicator of failure to achieve or maintain steroid-free remission, was defined by occurrence of any of the following: 1) Failure to achieve remission [Short Pediatric Crohn’s Disease Activity Index (SPCDAI) < 15] by week 26; 2) Failure to complete a steroid taper by week 16; 3) SPCDAI ≥ 15, attributed to active Crohn’s disease, at two or more consecutive visits beyond week 26; 4) Hospitalization or surgery for CD beyond week 26; 5) Use of corticosteroids for CD for ≥ 10 weeks cumulatively, beyond week 16; and 6) Discontinuation of anti-TNF and/or study drug for lack of effectiveness or toxicity.
Treatment de-escalation or discontinuation of anti-TNF or study medication for non-medical reasons was not considered a treatment failure.
Secondary Outcomes
We conducted a multi-stakeholder process to identify and prioritize a set of previously validated PROs from the NIH Patient Reported Outcome Measurement and Information System (PROMIS) that were most relevant to patients with PCD. In an initial phase, 42 children with CD, 70 parents, and 26 expert clinicians rated the importance of available PROMIS item banks. The domains of Pain Interference and Fatigue emerged as the highest priority. We next conducted semi-structured interviews with 37 patients and cognitive interviews with 14 patients to further explore their experiences with fatigue and pain. Based on concepts that participants identified as important, item understandability, psychometric evaluation of precision and coverage, and balance across different facets of each domain, we constructed 8-item short forms comprised of items selected from the PROMIS Fatigue and Pain Interference item banks.19 Prior data demonstrate these PROs are reliable, valid and responsive.20, 21Pre-specified measurement timepoints were approximately 1- and 2- years following randomization.
Serum was collected at approximately 26- and 91–104-weeks following randomization for measurement of ADAs. Samples were analyzed at two reference laboratories using both drug-sensitive [Progenika Biopharma (Derio, Spain)] and drug-tolerant [LabCorp (Calabasas, CA, USA)] assays.22 (Supplemental Methods)
Adverse Events (AE) and Serious Adverse Events (SAE), as described in Supplemental Methods, were reported by site investigators. Exacerbations of PCD were captured as treatment failures and were not required to be submitted as separate AEs.
Covariates
We recorded the following covariates, as assessed at baseline: participant age, gender, race, ethnicity, the anti-TNF agent used, SPCDAI score, physician global assessment of disease activity, disease location, current and prior perianal disease, current or prior use of prednisone and other steroid medications, prior use of MTX, prior use of 6 mercaptopurine or azathioprine, time from diagnosis (< 2 or ≥ 2 years), height, weight, BMI, albumin, hemoglobin, CRP, and ESR.
Participant Follow-up and Data Collection
Consistent with pragmatic trial design, follow-up occurred in the context of routine clinical care. Guidance for suggested follow-up intervals and assessments was included in the study protocol. Participants were followed for 104 weeks or until study termination (April 2021), after the last enrolled participant completed 52 weeks of follow-up. Participants were given the option to participate for an additional third year.
Study data was collected through the ImproveCareNow registry15, 23, described further in the Supplemental Methods. In addition, electronic Case Report Forms (CRF) were used to capture trial-specific data not already included in the registry. Site investigators provided oversight to ensure the accuracy, completeness, and timeliness of the data collection. In the event of incomplete or inconsistent data, correction and/or clarification was requested from the site. Sites ascertained individual components of the composite endpoint during routine office visits, at the time of hospitalization or surgery, or between encounters. When sites identified that a participant met one or more components of the primary endpoint, they indicated the outcome(s) met and date on a separate CRF that was reviewed and signed by the Site PI. Additionally, the study monitor and research project manager queried the ImproveCareNow Registry data and COMBINE CRFs regularly to identify any possible outcomes that were not yet identified by sites and asked the sites to confirm (or not) whether an endpoint had been met. Additionally, at each visit, site PIs were asked to confirm that the participant had not yet met a component of the primary endpoint and would continue on study treatment. Finally at the end of each participant’s follow-up or at the time or at the time of loss to follow-up or disenrollment, site PI’s also confirmed participants who had not met a study endpoint.
Statistical Analysis
All analyses were based on a modified intent to treat population, including participants who received at least one shipment of medication from the study pharmacy. We first described and compared the distributions of patient characteristics within treatment arms overall, and stratified by anti-TNF agent, using standard bivariate statistics.
To compare the distribution of time to treatment failure in the two arms, we computed log-rank tests stratified by anti-TNF agent prescribed (infliximab and adalimumab). Additionally, we developed a Cox model adjusting for anti-TNF (infliximab and adalimumab), site census region, and covariates that differed between treatment groups using a threshold of p<0.2.
We compared the average of PROMIS Pain Interference and Fatigue scores between treatment groups at week 52 and 104. We estimated the difference in mean PROMIS scores at 52 and 104 weeks by fitting mixed model for repeated measures (MMRM) to PROMIS scores at all available time points, adjusted for covariates used in our primary outcome analyses.
We next compared the proportion of positive ADA between treatment groups overall, and stratified by anti-TNF, using the chi square test. We considered patients with ADA detected at either/both timepoints on either/both assays as positive.
For all three secondary endpoints, we prespecified a threshold of p < 0.05/3 for determining statistical significance based on Bonferroni correction.
Finally, we summarized investigator-reported AEs and SAEs using standard descriptive statistics.
Pre-specified subgroup analyses
We explored Heterogeneity of Treatment Effects (HTE) by conducting a number of pre-specified subgroup analyses of our primary study endpoint. Subgroups considered included 1) time from diagnosis (< 2 or ≥ 2 years), 2) elevation of baseline CRP > 2X normal (include only if non-missing), 3) elevation of baseline sed rate or ESR using a cutoff of > 18, nonwhite versus white race, Hispanic versus non-Hispanic, Disease location (3 levels ileum only, colon only, and ileocolonic), and whether dose adjustment was performed over the course of follow-up (a surrogate for proactive therapeutic drug monitoring).
Missing Data
There was no missing data on the primary study endpoint, as we confirmed whether and when participants met (or not) one or more components of the primary composite endpoint as described above. Regarding the secondary endpoints of PROMIS measures, missing data were handled by fitting an MMRM model. To analyze the average of the PRO reported at week 52 and week 104, if the week 52 PRO was missing, we analyzed only the week 104 value and vice versa. For analyses of ADA, not all participants were able to provide a sample at both timepoints. Analyses were limited to provided samples. For all adjusted analyses, missing covariates were imputed using multiple imputation. We used SAS software (Cary NC) for all analyses.
Sample Size
We estimated a necessary sample size of 353 participants (Supplemental Methods) and set a recruitment target of 425 participants to explore heterogeneity of treatment effects. Due to slow recruitment, exacerbated by the COVID-19 pandemic, the study was discontinued prior to full enrollment with a final sample size of 297 participants. Based on the actual sample size our statistical power was 73% to detect a 15% difference in the primary outcome.
Patient and Stakeholder Engagement
Two parents (DW and LP) served as co-investigators from the time of proposal development through all phases of project implementation and were provided financial support for their time and effort. Additionally, the larger ICN Parent Working Group served as a study advisory board, affirming the importance and patient/family-centeredness of the overall study question, and providing input into the overall study design. All key design decisions were informed by stakeholder input, including the preference for individual-level versus cluster randomization, and the incorporation of a placebo-controlled design. After funding, the final study protocol was developed using a similar process of co-production.
As described above, we utilized a multi-stakeholder process to identify PROs most relevant to patients with PCD to serve as secondary outcomes for the trial.
We also incorporated meaningful patient and parent engagement in the development of recruitment materials. Parent co-investigators led the design of paper and web-based recruitment materials, including an animated video. Recruitment materials were also reviewed by parents and patients not associated with the research to assure balance and appeal. To further support recruitment, we developed shared decision making (SDM) tools to improve knowledge about the study, lower decisional conflict, and increase decisions that are congruent with patients’ values.24 Importantly, we elicited and incorporated the perspectives of patients, parents, and clinicians to iteratively refine components of the SDM process and related training materials as previously reported.25
Role of the Funding Sources
The study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Results
Study Population
Across 35 centers, we pre-screened 1905 patients, enrolled 321, and randomized 306, with 297 included in our modified ITT analysis. In total, 156 patients were assigned methotrexate (110 infliximab initiators and 46 adalimumab initiators) and 141 were assigned placebo (102 infliximab initiators and 39 adalimumab initiators). Median follow up in the methotrexate and placebo arms was 751 and 737 days, respectively (Figure 1).
Figure 1.
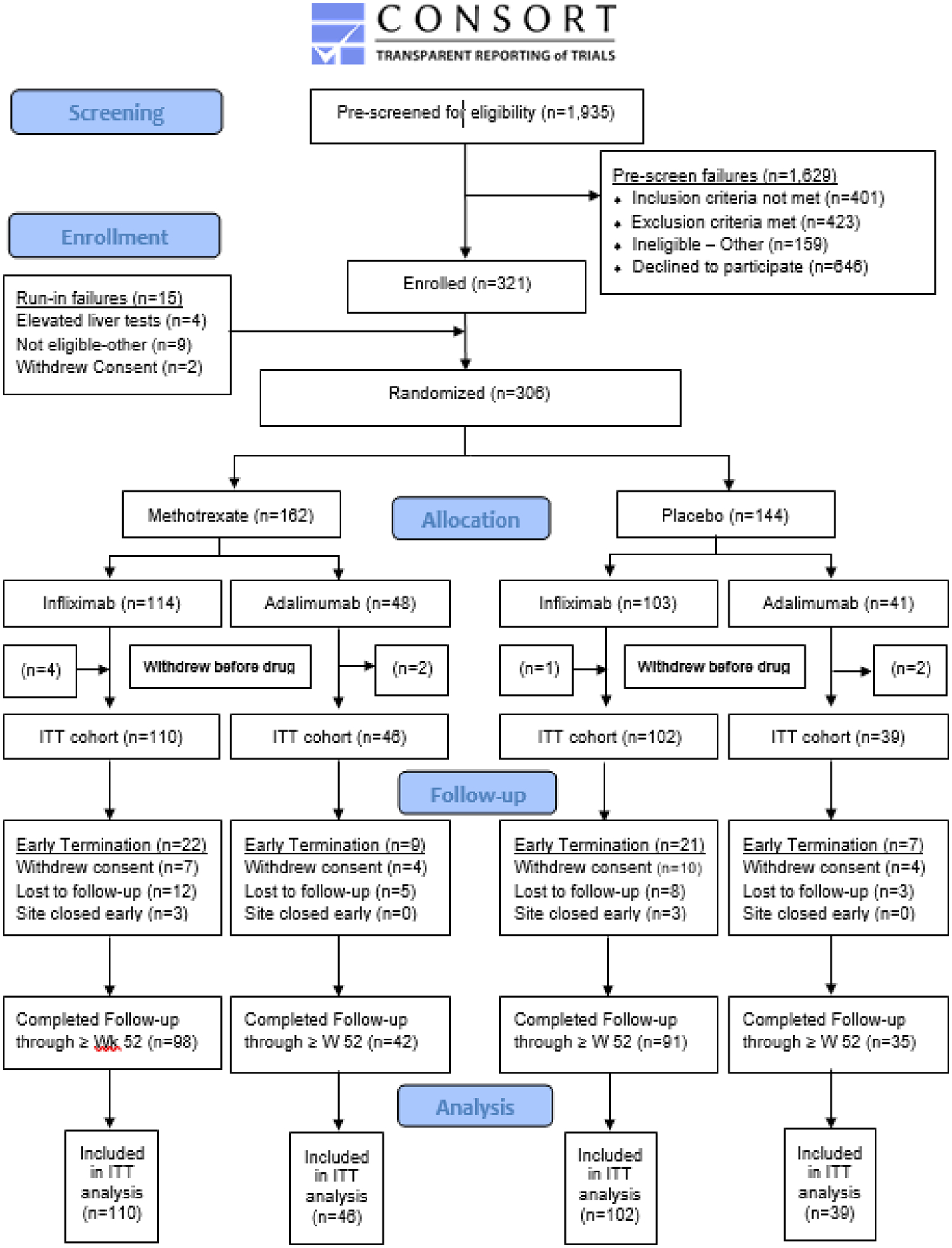
Participant Flow Diagram
Demographic and clinical characteristics of the study population overall and stratified by anti-TNF are provided in Tables 1 and Supplemental Tables 1a and 1b. The mean age was 13.9 years, 35% were female, and 82% white. Median time from diagnosis was 2 months. Median SPCDAI at enrollment was 15 (mild disease activity); 41% were on steroids at randomization. Participant characteristics were generally well-balanced between study arms.
Table 1.
Demographic and Clinical Characteristics of the Overall Study Population
| Demographics | All Patients | Combination Therapy (Active) | Monotherapy (Placebo) | P value | |||
|---|---|---|---|---|---|---|---|
| n | % or SD | n | % or SD | n | % or SD | ||
| Total Number of patients | 297 | 100% | 156 | 53% | 141 | 47% | |
| Female (N, %) | 104 | 35% | 53 | 33% | 51 | 36% | 0.72 |
| Mean age (SD) | 13.9 | 2.6 | 13.8 | 2.5 | 14.0 | 2.8 | 0.49 |
| Race (n, %) | |||||||
| Asian | 4 | 1% | 2 | 1% | 2 | 1% | 1.0 |
| Black / African American | 32 | 11% | 13 | 8% | 19 | 13% | 0.15 |
| White | 244 | 82% | 131 | 84% | 113 | 80% | 0.45 |
| Multi-race or Other | 13 | 4% | 8 | 5% | 5 | 4% | 0.51 |
| Ethnicity (n, %) | |||||||
| Hispanic or Latino | 8 | 3% | 5 | 3% | 3 | 2% | 0.73 |
| Not Hispanic or Latino | 285 | 97% | 150 | 97% | 138 | 98% | |
| Clinical Characteristics | |||||||
| Mean Height, z-score (SD) | −0.24 | 1.07 | −0.21 | 1.08 | −0.28 | 1.07 | 0.61 |
| Mean Weight, z-score (SD) | −0.25 | 1.12 | −0.27 | 1.14 | −0.23 | 1.10 | 0.74 |
| Mean BMI, z-score (SD) | −0.20 | 1.17 | −0.25 | 1.21 | −0.14 | 1.14 | 0.44 |
| Mean time from diagnosis in months (SD) | 8.9 | 15.6 | 8.1 | 16.0 | 9.7 | 19.2 | 0.46 |
| Disease Location – Lower GI (n, %) | |||||||
| None | 5 | 2% | 5 | 3% | 0 | 0% | |
| Ileum Only | 67 | 24% | 32 | 22% | 35 | 26% | 0.03 |
| Colon Only | 48 | 17% | 19 | 13% | 29 | 21% | |
| Ileocolonic | 161 | 57% | 88 | 61% | 73 | 53% | |
| Upper GI – Proximal (n, %) | 140 | 52% | 74 | 53% | 66 | 51% | 0.72 |
| Upper GI – Distal (n, %) | 70 | 28% | 36 | 28% | 34 | 28% | 0.99 |
| Perianal disease at enrollment (n, %) | 31 | 21% | 17 | 22% | 14 | 21% | 0.86 |
| History of perianal disease (n, %) | 85 | 29% | 43 | 28% | 42 | 30% | 0.72 |
| Mean sPCDAI score at randomization | 17.0 | 15.6 | 17.2 | 16.4 | 16.9 | 14.6 | 0.86 |
| Physician Global Assessment at randomization | |||||||
| Quiescent | 69 | 23% | 37 | 24% | 32 | 23% | 0.53 |
| Mild | 100 | 34% | 48 | 31% | 52 | 37% | |
| Moderate | 80 | 27% | 41 | 26% | 39 | 28% | |
| Severe | 8 | 3% | 6 | 4% | 2 | 1% | |
| Mean Baseline PROMIS Fatigue Score (SD) | 47.6 | 15.2 | 47.4 | 15.5 | 47.8 | 14.9 | 0.83 |
| Mean Baseline PROMIS Pain Score (SD) | 46.9 | 14.3 | 46.5 | 14.5 | 47.4 | 14.1 | 0.60 |
| Prior Treatment | |||||||
| Prior azathioprine or mercaptopurine therapy (n, %) | 36 | 12% | 18 | 12% | 18 | 13% | 0.75 |
| Prior methotrexate (n, %) | 47 | 16% | 26 | 17% | 21 | 15% | 0.70 |
| Current Treatment | |||||||
| Any Steroid at Randomization (n, %) | 120 | 41% | 64 | 41% | 56 | 40% | 0.90 |
| Anti-TNF (n, %) | |||||||
| Infliximab | 212 | 71% | 110 | 71% | 102 | 72% | 0.73 |
| Adalimumab | 85 | 29% | 46 | 29% | 39 | 28% | |
| Baseline Labs | |||||||
| Mean Sed rate (ESR) highest within 42 days of randomization (SD) | 18.6 | 18.4 | 20.4 | 19.3 | 16.6 | 17.3 | 0.11 |
| Mean Alb worst within 42 days of randomization (SD) | 3.8 | 0.6 | 3.8 | 0.5 | 3.9 | 0.6 | 0.40 |
| Mean Hemoglobin (Hgb) lowest within 42 days of randomization (SD) | 12.1 | 2.2 | 11.8 | 1.8 | 12.4 | 2.7 | 0.06 |
| CRP at randomization greater than 2x upper limit of normal (n, %) | 47 | 19% | 27 | 21% | 20 | 16% | 0.34 |
Primary endpoint
Overall, 88/297 participants (30%) experienced study-defined treatment failure [57/212 (27%) of infliximab initiators and 31/85 (36%) of adalimumab initiators]. A total of 40/156 participants (26%) in the combination therapy group and 48/141 participants (34%) in the monotherapy group experienced treatment failure (Table 2). The most common component of the composite endpoint experienced by study participants was hospitalization for active IBD after week 25. A breakdown of the number of participants who experienced each component of the composite endpoint, stratified by treatment assignment and anti-TNF agent used is provided as Supplemental Table 2.
Table 2:
Treatment Failure in Participants Treated with Anti-TNF in Combination with Methotrexate versus Anti-TNF Monotherapy
| Treatment failures (%) | Unadjusted HR (95% CI) | Adjusted HR* (95% CI) | |
|---|---|---|---|
| Overall (n=297) | 88 (30%) | 0.69 (0.45–1.05) | 0.69 (0.45–1.07) |
| Infliximab (n=212) | 57 (27%) | 0.93 (0.55–1.56) | 0.85 (0.50–1.45) |
| Adalimumab (n=85) | 31 (36%) | 0.40 (0.19 to 0.81) | 0.42 (0.19–0.90) |
Overall analyses adjusted for baseline CRP > twice upper limit of normal and race. Infliximab analyses adjusted for baseline CRP > twice upper limit of normal, ESR > 20, region and race. Adalimumab analyses adjusted for baseline short Pediatric Crohn’s Disease Index.
Kaplan Meier analysis of the overall population (Figure 2A) showed a non-significant trend towards lower event rates in the combination therapy (HR 0.69, 95% CI 0.45 to 1.05, p=0.08). Figure 2B and 2C show Kaplan Meier curves after stratification by anti-TNF (infliximab and adalimumab). Among infliximab initiators, there was no difference between combination therapy and monotherapy (HR 0.93, 95% CI 0.55 to 1.56, p=0.78). Among adalimumab initiators, combination therapy significantly outperformed monotherapy (HR 0.40, 95% CI 0.19 to 0.81, p=0.01). Effect estimates were essentially unchanged after adjustment (Table 2).
Figure 2:
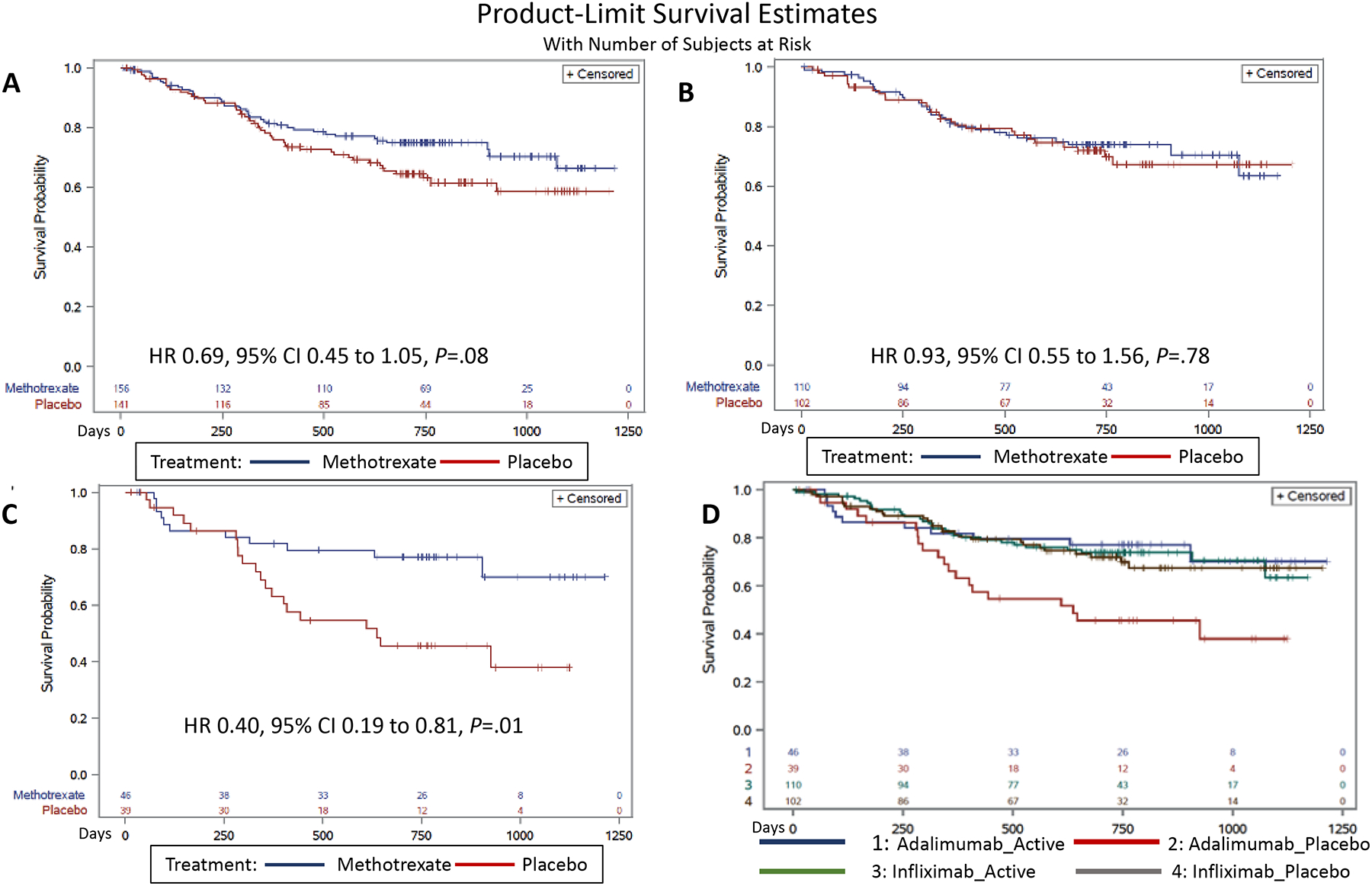
Figure 2A shows the Kaplan Meier analysis of the time-to-event in the overall population. Figure 2B shows the Kaplan Meier analysis among infliximab initiators and 2C shows the Kaplan Meier analysis among adalimumab initiators. Figure 2D shows Kaplan Meier curves broken out by both anti-TNF agent and combination versus monotherapy.
Pre-specified subgroup analyses
The results of pre-specified subgroup analyses are shown in Supplemental Table 3. We observed a larger magnitude of treatment effect in participants with colonic Crohn’s disease and elevated sed rate at baseline and a similar trend among those with elevated baseline CRP. Additionally, we observed a trend towards a smaller magnitude of treatment benefit in patients who underwent anti-TNF dose adjustment.
Post-hoc Analyses
In a per-protocol analysis where patients who discontinued study methotrexate or placebo for non-medical reasons were censored after 30 days, effect estimates were stronger and statistically significant in the overall study population (HR 0.65, 95% CI 0.43–0.99) and among adalimumab users (HR 0.35, 95% CI 0.17–0.73). In an analysis only including events due to lack of effectiveness (66/88 participants with treatment failure), effect estimates were also stronger and statistically significant overall (HR 0.56, 95% CI 0.3–0.9) and among adalimumab users (HR 0.23, 95% CI 0.09–0.57). Discontinuations due to toxicity were no different overall, and after stratification by anti-TNF.
Figure 2D shows Kaplan Meier curves broken out by both anti-TNF agent and combination versus monotherapy. Among participants treated with monotherapy, adalimumab-treated patients had higher event rates than those receiving infliximab (HR 2.19, 95% CI 1.23 to 3.89, p=0.008). Event rates in the infliximab combination therapy group or adalimumab combination therapy group were no different than infliximab monotherapy.
Secondary endpoints
We observed no clinically or statistically significant differences in PROMIS measures of Pain Interference and Fatigue domain when weeks 52 and 104 were averaged, or at either time alone (Table 3).
Table 3.
Differences in PROMIS Pain Interference and Fatigue between Combination Therapy and Monotherapy Groups
| Overall | Pain Interference | Fatigue | ||
|---|---|---|---|---|
| Effect estimate | p | Effect estimate | p | |
| Week 52 | −1.36 | 0.33 | 0.59 | 0.64 |
| Week 104 | −0.70 | 0.69 | 0.88 | 0.64 |
| Infliximab | ||||
| Week 52 | −1.29 | 0.43 | 0.52 | 0.79 |
| Week 104 | −1.40 | 0.52 | 0.05 | 0.98 |
| Adalimumab | ||||
| Week 52 | −1.56 | 0.55 | 0.82 | 0.79 |
| Week 104 | 0.81 | 0.78 | 2.93 | 0.34 |
Effect estimate is mean difference in T scores between the active and placebo groups. Negative values indicate lower levels of the measured domain in active versus placebo groups. Minimally important differences in PROMIS measures are in the range of 3–5 based on studies in other populations.
Of 151 infliximab users (71%) with available serum, 61 (40%) had positive ADA. Differences between groups (47% monotherapy versus 34% combination therapy) did not reach statistical significance (RR 0.72, 95% CI 0.49–1.07). Infliximab users with positive versus negative ADA were no more likely to experience treatment failure (44% vs 39%, p =0.71). Of 61 adalimumab users (72%) with available serum, 11 (18%) had positive ADA. This proportion was higher in the monotherapy group (21% versus 15%) but did not reach statistical significance (RR 0.71, 95% CI 0.24–2.07) (Supplemental Table 4). Adalimumab users with positive ADA were more likely to experience treatment failure compared to those with negative ADA (64% vs 36%, p =0.03).
Safety
A total of 118 (76%) combination therapy patients experienced ≥1 AEs, compared with 96/141 (68%) monotherapy patients. Forty-four percent of combination therapy patients experienced an AE that was possibly or definitely related to treatment, compared to 33% of monotherapy patients. However, participants in the monotherapy arm were more likely to experience a SAE (16% versus 12%) (Table 4).
Table 4.
Summary of Adverse Events (at Participant Level)
| Event | All Patients (n=297) | Combination Therapy (Active) (n=156) | Monotherapy (Placebo) (n=141) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Adverse Event | 214 | 70% | 118 | 73% | 96 | 67% |
| Serious Adverse Event | 40 | 13% | 18 | 11% | 22 | 15% |
| Related Adverse Event | ||||||
| Possibly Related | 113 | 37% | 68 | 42% | 45 | 31% |
| Definitely Related | 9 | 3% | 6 | 4% | 3 | 2% |
| Possibly or Definitely Related | 115 | 38% | 69 | 43% | 46 | 32% |
Supplemental Tables 5–7 describe categories of AEs observed in > 2% of the study population, all SAEs, and laboratory abnormalities. Nausea/vomiting, elevated liver enzymes, and infection SAEs were more commonly reported in patients receiving combination therapy. Conversely gastrointestinal symptoms more prevalent in the monotherapy arm.
Discussion
In the largest double-blind, randomized trial to date in PCD, we found that anti-TNF combination therapy with low dose oral methotrexate outperformed monotherapy for adalimumab-treated patients but not infliximab-treated patients, resulting in a 2-fold reduction in the occurrence of events indicating treatment failure. We observed slightly more AEs in the combination therapy group, as expected, but fewer SAEs. Overall, these findings suggest improved effectiveness of combination therapy in adalimumab-treated patients with a tolerable safety profile.
Our findings reinforce and extend those of prior trials in adult patients. While the SONIC trial suggested that combination therapy with infliximab and azathioprine was more efficacious than infliximab monotherapy,13 the COMMIT trial showed no clinical benefit of combination therapy with methotrexate.14 Our pediatric study confirms the absence of a clinical benefit of combination therapy with infliximab and methotrexate. Our pragmatic design allowed proactive TDM and higher doses of infliximab rather than the fixed dosing (5mg/kg) utilized in prior adult studies. Thus, it is possible that with optimized use of infliximab we have reached a ceiling of effectiveness above which combination therapy doesn’t add benefit.
The improved effectiveness of combination therapy among adalimumab-treated patients was notable. Prior studies of adalimumab combination therapy were less rigorous and inconclusive. A single center, open-label, randomized trial of adalimumab with and without azathioprine in adult CD patients showed no benefit of combination therapy.26 In a post-hoc analysis of a small (n=78) pediatric trial that compared proactive versus reactive TDM, investigators reported a numeric trend towards longer steroid-free remission among patients treated with combination therapy, though only 7 combination therapy patients received methotrexate.27 Our multicenter, double-blind, placebo-controlled trial provides robust and compelling data in favor of adalimumab and methotrexate combination therapy.
Our study was not designed to compare infliximab to adalimumab; nor was it designed to evaluate the role of proactive TDM. However, proactive TDM is endorsed in the ImproveCareNow Mode Care Guidelines16 and was considered standard of care at study sites during the time of our study. In our study population, 54% of infliximab patients and 44% of adalimumab patients had ≥ 1 recorded anti-TNF dose or interval change during follow-up and 45% of infliximab and 40% of adalimumab patients had ≥ 1 standard of care therapeutic drug monitoring test in the first year since randomization (Supplemental Table 8). Thus, the observed benefit of combination therapy among adalimumab users was demonstrated in the setting of standard of care TDM. Of note, anti-TNF dose or interval adjustment and TDM were more frequent in infliximab-treated patients than adalimumab-treated patients, likely due to ease of obtaining trough levels during infusions and more flexible dosing. It is possible that lower rates of treatment failure among infliximab users in our study may be related to more intensive TDM. Indeed, prior studies have suggested a benefit of proactive TDM in adalimumab-treated patients28 although more definitive studies are needed. Although not statistically significant, we observed a trend towards a smaller magnitude of treatment benefit in patients who underwent anti-TNF dose adjustment, raising the possibility that more aggressive TNF dosing may have similar effectiveness to combination therapy.
Among both infliximab and adalimumab users we observed non-significantly lower rates of immunogenicity in the combination versus monotherapy groups. This trend is consistent with prior adult studies14 and adds substantially to the pediatric literature on this topic.27 Prevention of ADA may partially explain the benefits of combination therapy among adalimumab users. However, our study and the prior adult study showed no clinical benefit of infliximab and methotrexate, despite lower rates of ADA development. Therefore, preventing immunogenicity cannot fully account for the benefits of combination therapy.9 Indeed, some patients in our study who developed ADA continued to maintain steroid free remission and other patients who experienced treatment failure did so in the absence of ADA. Future research to evaluate the significance of anti-drug antibodies, including neutralizing and non-neutralizing antibodies, especially in pediatric populations, will be important.
We did not observe any differences in PROs of Pain Interference or Fatigue. Prior data demonstrate these PROs are reliable, valid and responsive.20, 21 We speculate that failure to observe differences between treatment groups may be related to analyzing these PROs at fixed time points rather than at the time of treatment failure. Patients experiencing treatment failure likely switched therapy and improved by the pre-defined timepoints in our study. In a prior blinded analysis of PROs assessed closer to the time of treatment failures, we observed higher Pain Interference and Fatigue in those who experienced treatment failure compared to those who remained outcome-free.21 While PRO measurement at fixed time points limited our ability to observe treatment-related differences, it reassuringly indicates that patients experiencing treatment failure with anti-TNF may improve with subsequent therapy. Future studies of PCD that utilize PROs should focus on analyzing PRO trajectories over multiple timepoints rather than focusing on pre-specified time points.
Key strengths of our study include the rigorous randomized, double-blind design and the pragmatic nature of our trial, including broad eligibility criteria, flexible and adaptive dosing of anti-TNF and study medications, and inclusion of a diverse group of study centers. Thus, our study findings should be broadly generalizable to real-world care of patients facing the treatment decision of combination or monotherapy. We also incorporated robust input from parents and patients throughout all phases of the study, ensuring that the study question, design, and outcomes were all patient/family-centered (Supplemental Methods).
The most notable study limitation is that slow recruitment compounded by the COVID-19 pandemic prevented us from reaching our recruitment target. Thus, failure to detect a difference between combination and monotherapy in our overall study population may reflect Type 2 error. However, stratified analyses by specific TNF provide compelling data that 1) even with a larger sample size, it is unlikely there would have been a significant difference among infliximab initiators, and 2) treatment effects among adalimumab initiators were readily apparent, even with a smaller sample size. Consistent with pragmatic trial design17, adherence was encouraged but not strictly monitored. Thus, our ITT results reflect real-world effectiveness rather than optimal efficacy. Had we excluded those with poor adherence, the effect size among adalimumab users would likely be, similar to the per-protocol analysis. In an effort to include all patients initiating anti-TNF, we did not require colonoscopy prior to enrollment and thus could not confirm active intestinal inflammation in all participants. We also recognize that baseline measures of disease activity are imperfect and there was missing data for some participants. Nevertheless, randomization should have accounted for any differences between treatment groups. There is also the possibility that use of infliximab versus adalimumab may vary by site and that site case mix and/or other practices may be associated with patient outcomes. We did not include endoscopy or other measures of mucosal healing (i.e., calprotectin or imaging) as trial endpoints. As a pragmatic trial, we prioritized inclusion of outcomes routinely assessed in clinical care. Emerging data indicates that evaluation of mucosal healing at a pre-specified time point is not yet standard of care, even in adult patients.29 In our study, only 38% of participants underwent colonoscopy during follow up (41% had calprotectin measurement). To the extent such testing was differentially performed in symptomatic patients, the use of available data would have introduced substantial bias. However, our primary endpoint indirectly reflects mucosal healing. Among 66 participants with loss of effectiveness, 39 (59%) underwent colonoscopy of which 85% were found to have active intestinal inflammation and 31 (47%) had fecal calprotectin measurement with a median value of 814 (μg/g).
In conclusion, our study findings suggest strong consideration of using combination therapy for PCD patients initiating adalimumab but not infliximab. Dissemination and implementation of these findings should lead to improved outcomes in this patient population, including consideration of de-implementation of combination therapy in infliximab treated patients. The evaluation and comparison of additional strategies to further optimize response to adalimumab, including proactive therapeutic drug monitoring, warrant additional research.
Supplementary Material
BACKGROUND AND CONTEXT.
Anti-Tumor Necrosis Factor (TNF) inhibitors, including infliximab and adalimumab, are a mainstay of pediatric Crohn’s disease (PCD) therapy; however, non-response and loss of response is common. Combination therapy with methotrexate may improve response.
NEW FINDINGS
We conducted a randomized, double-blind, multicenter, pragmatic clinical trial to compare anti-TNF in combination with low dose oral methotrexate to anti-TNF monotherapy in children with PCD. Among infliximab initiators, there were no differences between combination and monotherapy. Among adalimumab initiators, combination therapy was associated with longer time to failure Combination therapy resulted in more adverse events but fewer serious adverse events.
LIMITATIONS
Slow recruitment compounded by the COVID-19 pandemic prevented us from reaching our recruitment target. Thus, some of our analyses were underpowered. As a pragmatic trial, we prioritized inclusion of outcomes routinely assessed in clinical care. Therefore, we could include endoscopy or other measures of mucosal healing (i.e., calprotectin or imaging) as trial endpoints.
CLINICAL RESEARCH RELEVANCE
These findings suggest strong consideration of using methotrexate combination therapy for PCD patients initiating adalimumab but not infliximab. Future research evaluating other strategies to optimize anti-TNF therapy and focusing on outcomes of mucosal healing and are necessary.
BASIC RESEARCH RELEVANCE
This randomized controlled trial demonstrates that combination therapy with adalimumab and methotrexate results in fewer treatment failures than adalimumab monotherapy. Future research to identify clinical, genetic, immunologic, and microbiome-related predictors of response and loss of response to anti-TNF therapy will further inform precision medicine approaches to guide care.
Acknowledgements
We recognize the contributions of the many site coordinators and investigators who worked tirelessly to recruit and follow study participants and the study pharmacist at McKesson. We greatly appreciate the manufacturing of matched placebos by Temple CGMP Services. We also recognize the many patient, parent, and other stakeholders who contributed to various aspects of study design and implementation. Finally, we wish to thank all study participants and their parents, guardians, and other family members.
Funding
This study was funded by grants from the Patient Centered Outcomes Research Institute (PCS-1406–18643), the Helmsley Charitable Trust, and National Institute of Arthritis and Musculoskeletal and Skin Diseases (U19AR069525). Biosample supplies and shipping costs and anti-Drug Antibody testing were provided in kind by Progenika Biopharma, a Grifols Company. Anti-Drug Antibody testing was also provided in kind by Esoterix Specialty Laboratory, Labcorp.
Declaration of Interests:
MDK has consulted for Abbvie, Janssen, Pfizer, Takeda, and Lilly, is a shareholder in Johnson & Johnson, and has received research support from Pfizer, Takeda, Janssen, Abbvie, Lilly, Genentech, Boehringer Ingelheim, Bristol Myers Squibb, Celtrion, and Arenapharm.
DAW – no disclosures
HHF has consulted for Alivio, BMS, Boehringer, ExeGi Pharma, Finch, Fresenius Kabi, Gilead, Janssen, Otsuka, Pfizer, Pure Tech, Ventyx and has received research support form Allakos, Artizan, NovoNordisk, Pfizer.
AMF – no disclosures
JA – no disclosures
RFA – no disclosures
JEA – no disclosures
DMB – no disclosures
JAB – no disclosures
KB – no disclosures
CBT – no disclosures
MEB – no disclosures
BMB – no disclosures
WBB has common stock holdings in the following publicly traded companies: Pfizer, Merck, Abbott Laboratories, Viatris, and Johnson & Johnson.
JMC – no disclosures
KC – no disclosures
RBC has consulted for Janssen Research & Development and is a member of the scientific advisory board for Janssen Biotech.
CMD – no disclosures
JMD – no disclosures
DRE – no disclosures
EE - no disclosures
CBF – no disclosures
JAG – no disclosures
JEG – no disclosures
ASG – no disclosures
AI – no disclosures
TWJ has received research support from Abbvie.
JLK – no disclosures
SK – no disclosures
MEK – no disclosures
IHL – no disclosures
TML – no disclosures
EAL has received research support from Pfizer, Inc.
PAM – no disclosures
PM – no disclosures
ZMR – no disclosures
JM is on the Speaker’s Bureau for AbbVie and on the scientific medical advisory board for PSI Inc.
KO – no disclosures
LO - no disclosures
PJP – no disclosures
HP – no disclosures
KTP – no disclosures
DSP has received research support from Janssen and Abbvie.
LP – no disclosures
MR – no disclosures
CMS – no disclosures
KCS – no disclosures
JRS – no disclosures
MS – no disclosures
KAS – no disclosures
SJS – no disclosures
JAS – no disclosures
JSS – no disclosures
JT – no disclosures
PW – no disclosures
MZ – no disclosures
MW – no disclosures
SS is a member of the advisory board for Abbvie, Inc.
AB has consulted for Takeda, Best Doctors, Eli Lilly, Fresenius Kabi, and has received research support from Janssen, Abbvie, Takeda, Buhlmann, Arena, Eli Lilly, Bristol Myers Squibb, PROCISE diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing
De-identified study data, data dictionary, study protocol, statistical analysis plan, and informed consent form will be made available within 12 months of publication in accordance with PCORI’s data sharing policy. Information regarding the PCORI-designated repository and submission of data requests can be found at https://www.pcori.org/about/governance/policy-data-management-and-data-sharing
References
- 1.Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci 2013;58:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. Journal of Crohn’s and Colitis 2013;7:322–337. [DOI] [PubMed] [Google Scholar]
- 3.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology 2008;135:1907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debruyn JC, Soon IS, Hubbard J, et al. Nationwide temporal trends in incidence of hospitalization and surgical intestinal resection in pediatric inflammatory bowel diseases in the United States from 1997 to 2009. Inflamm Bowel Dis 2013;19:2423–32. [DOI] [PubMed] [Google Scholar]
- 5.Longobardi T, Jacobs P, Bernstein CN. Work losses related to inflammatory bowel disease in the United States: results from the National Health Interview Survey. Am J Gastroenterol 2003;98:1064–72. [DOI] [PubMed] [Google Scholar]
- 6.Cohen RD. The quality of life in patients with Crohn’s disease. Aliment Pharmacol Ther 2002;16:1603–9. [DOI] [PubMed] [Google Scholar]
- 7.Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology 2008;135:1106–13. [DOI] [PubMed] [Google Scholar]
- 8.Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. [DOI] [PubMed] [Google Scholar]
- 9.Schultheiss JPD, Mahmoud R, Louwers JM, et al. Loss of response to anti-TNFα agents depends on treatment duration in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2021;54:1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007;56:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol 2013;108:40–7; quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah ED, Coburn ES, Nayyar A, et al. Systematic review: hepatosplenic T-cell lymphoma on biologic therapy for inflammatory bowel disease, including data from the Food and Drug Administration Adverse Event Reporting System. Aliment Pharmacol Ther 2020;51:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 14.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014;146:681–688 e1. [DOI] [PubMed] [Google Scholar]
- 15.Crandall WV, Margolis PA, Kappelman MD, et al. Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics 2012;129:e1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ImproveCareNow Model Care Guidelines.
- 17.Ford I, Norrie J. Pragmatic Trials. New England Journal of Medicine 2016;375:454–463. [DOI] [PubMed] [Google Scholar]
- 18.Berger VW, Ivanova A, Knoll MD. Minimizing predictability while retaining balance through the use of less restrictive randomization procedures. Stat Med 2003;22:3017–28. [DOI] [PubMed] [Google Scholar]
- 19.Schuchard J, Kappelman M, Grossman A, et al. P041 DEVELOPMENT OF PROMIS PEDIATRIC PATIENT-REPORTED OUTCOME SHORT FORMS FOR FATIGUE AND PAIN INTERFERENCE IN CHILDREN WITH CROHN’S DISEASE. Gastroenterology 2020;158:S101–S102. [Google Scholar]
- 20.Schuchard J, Carle AC, Kappelman MD, et al. Interpreting Patient-Reported Outcome Scores: Pediatric Inflammatory Bowel Disease as a Use Case. Acad Pediatr 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller TL, Schuchard J, Carle AC, et al. Use of Patient-Reported Outcomes Measurement Information System Pediatric Measures as Clinical Trial Endpoints: Experience from a Multicenter Pragmatic Trial in Children with Crohn’s Disease. J Pediatr 2022;242:86–92.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marini JC, Sendecki J, Cornillie F, et al. Comparisons of Serum Infliximab and Antibodies-to-Infliximab Tests Used in Inflammatory Bowel Disease Clinical Trials of Remicade®. Aaps j 2017;19:161–171. [DOI] [PubMed] [Google Scholar]
- 23.Pratt J, Jeffers D, King EC, et al. Implementing a Novel Quality Improvement-Based Approach to Data Quality Monitoring and Enhancement in a Multipurpose Clinical Registry. EGEMS (Wash DC) 2019;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014;1:CD001431. [DOI] [PubMed] [Google Scholar]
- 25.Lipstein EA, Breslin M, Dodds CM, et al. Integrating shared decision making into trial consent: A nested, cluster-randomized trial. Patient Educ Couns 2021;104:1575–1582. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab Monotherapy and a Combination with Azathioprine for Crohn’s Disease: A Prospective, Randomized Trial. J Crohns Colitis 2016;10:1259–1266. [DOI] [PubMed] [Google Scholar]
- 27.Matar M, Shamir R, Turner D, et al. Combination Therapy of Adalimumab With an Immunomodulator Is Not More Effective Than Adalimumab Monotherapy in Children With Crohn’s Disease: A Post Hoc Analysis of the PAILOT Randomized Controlled Trial. Inflamm Bowel Dis 2020;26:1627–1635. [DOI] [PubMed] [Google Scholar]
- 28.Assa A, Matar M, Turner D, et al. Proactive Monitoring of Adalimumab Trough Concentration Associated With Increased Clinical Remission in Children With Crohn’s Disease Compared With Reactive Monitoring. Gastroenterology 2019;157:985–996.e2. [DOI] [PubMed] [Google Scholar]
- 29.Yang JY, Lund JL, Pate V, et al. Utilization of Colonoscopy Following Treatment Initiation in U.S. Commercially Insured Patients With Inflammatory Bowel Disease, 2013–2019. Inflamm Bowel Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified study data, data dictionary, study protocol, statistical analysis plan, and informed consent form will be made available within 12 months of publication in accordance with PCORI’s data sharing policy. Information regarding the PCORI-designated repository and submission of data requests can be found at https://www.pcori.org/about/governance/policy-data-management-and-data-sharing


